About this course:
The purpose of this module is to provide an overview of anemia in the adult patient, outlining the classification of the various types of anemia, most common etiologies, and a systematic approach to diagnosis, evaluation, and management.
Course preview
Introduction
Anemia is a global public health problem with serious health implications and contributes to increased morbidity and mortality (Le, 2016). Anemia is a blood disorder marked by a deficiency in the mass of circulating red blood cells, characterized by a decline in the hemoglobin (Hb) concentration and/or red blood cells to lower than the normal range. Hemoglobin is the protein inside red blood cells that binds oxygen (Chaparro & Suchdev, 2019). Therefore, the fundamental physiologic manifestation of anemia is reduced oxygen-carrying capacity of the blood resulting in tissue hypoxia (McCance & Heuther, 2014). The American Society of Hematology (ASH, 2019a) defines anemia as a hemoglobin level of less than 13.5 g/dL in adult males and less than 12 g/dL in adult females, but these values often vary based on discrepancies in laboratory reference ranges, age, ethnicity, geographic location, nutritional status, and overall health (ASH, 2019a). The World Health Organization (WHO) poses a working definition of anemia as a hemoglobin level less than the normal mean minus two standard deviations (Cappellini & Motta, 2015).
Epidemiology
Anemia is the most common blood disorder in the world, affecting one-third of the world's population and more than 3 million Americans (Chaparro & Suchdev, 2019). The Centers for Disease Control and Prevention (CDC, 2018) conducts the National Health and Nutrition Examination Survey (NHANES) every two years, compiling data on cross-sectional health, nutrition, and health behavior among civilians in the United States. Five NHANES surveys between 2003 and 2012, inclusive of 41,026 individuals, demonstrated that an estimated 5.6% of the US population met the criteria for anemia. Rates of anemia in men increased at relatively constant and gradual intervals alongside rising age during this period, while women endured peaks within two distinct age groups; 40–49 years and 80–85 years. The data portrays the highest incidence of anemia in Black women aged 80–85 years at 35.6%, which is 6.4 times higher than the population average (Le, 2016).
Anemia varies with gender, ethnicity, and physiological status. Studies have shown that anemia is three times more common in African Americans than in Caucasians (Kochanek, Murphy, Xu & Arias, 2019). Anemia is most frequently diagnosed among adults aged 65 and older, with a global prevalence of approximately 17%, which continues to rise with age. Within the older adult population, the etiology of anemia is commonly due to a combination of underlying disease processes and health conditions. Those with chronic medical conditions such as kidney disease, cancer, thyroid or liver disease, inflammatory bowel disease, or autoimmune conditions such as rheumatoid arthritis are at heightened risk of developing anemia (ASH, 2019a). Approximately 40% of older adults admitted to the hospital are affected by anemia, and the incidence is even higher (47%) among those who reside in nursing homes (Stauder & Thein, 2014). Nearly 50% of patients older than 80 years are affected by anemia in both hospital inpatient and outpatient settings (Stauder et al., 2018). Additional at-risk groups include pregnant women, children, and females of reproductive age (Le, 2016).
In 2017, the age-adjusted death rate for anemia in the US was 1.4 per 100,000 people or 5,382 deaths. Of these deaths, 3,800 were Non-Hispanic White individuals (1,681 males and 2,119 females), followed by 1,078 Non-Hispanic Black individuals (488 males and 590 females). Among all ethnicities and age groups, females have a higher rate of death associated with anemia than males (Kochanek et al., 2019). Globally, iron deficiency anemia (IDA) is the most prevalent type of anemia affecting more than one-fifth of the world population (McCance & Heuther, 2014). Within the US, the incidence of iron deficiency is approximately 1% in men and at least 11% in women, but often higher (DeLoughery, 2017). Furthermore, the prevalence of anemia is expected to rise dramatically over the next decade due to an advancing aging population in Western societies (Bach, Schruckmayer, Sam, Kemmler, & Stauder, 2014).
Pathophysiology
To understand anemia, it is first essential to acquire a baseline understanding of the components of blood, the physiologic basis of red blood cell production, and the mechanisms of blood cell destruction (Longo, 2019).
Components of Blood
Blood consists of both liquid and solid components. Plasma is the liquid part and is comprised of primarily water. It functions to carry nutrients, proteins, and hormones throughout the body, as well as transport waste products to the kidneys and digestive tract for removal. The solid constituents of the blood include three types of blood cells: white blood cells, red blood cells, and platelets. White blood cells (WBCs) are components of the immune system and are comprised of five specific subtypes that work to fight infection and other illnesses. WBCs have variable lifespans, as some live for only 24 hours, but the average WBC lifespan is 13 to 20 days. Mature red blood cells (RBCs), or erythrocytes, carry hemoglobin, a protein that transports oxygen from the lungs to all the tissues within the body. The body relies on oxygen as a critical component for all cellular functioning and processes. Hemoglobin also carries waste products (mainly carbon dioxide) from the tissues back to the lungs, where waste is expelled through breathing. Erythrocytes appear as biconcave discs of uniform shape and size that lack organelles and granules. They have an average lifespan of 120 days and are pink in appearance due to their high content of hemoglobin. Hematocrit reflects the percentage, by volume, of RBCs in a given volume of blood. Platelets are essential blood cell fragments that help blood clot in response to an injury, laceration, or blunt trauma. Platelets gather at the site of an injury to seal small cuts or breaks in blood vessels and work in conjunction with proteins called clotting factors to stop bleeding. Platelets have an average lifespan of 7 to 10 days (Longo, 2019).
Hematopoiesis
Hematopoiesis is the ongoing process of blood cell production in the human body. It occurs in the liver and spleen of the fetus, but after birth, it occurs primarily in the bone marrow (Longo, 2017). Extramedullary hematopoiesis is the formation of blood cells at sites other than the bone marrow. While extramedullary hematopoiesis is normal for the fetus inside the womb, if this occurs after birth, it is usually a sign of disease. Hematopoiesis is regulated through a series of steps and involves the biochemical stimulation of undifferentiated cells to undergo mitotic cell division (i.e., proliferation) and cell maturation (i.e., differentiation). Hematopoiesis continues throughout the lifespan, functioning to maintain homeostasis within the body in response to infection or injury. When there is an increase in the destruction of circulating cells, such as during acute bleeding, the process of hematopoiesis accelerates to generate more cells and compensate for the loss. In long-term dysfunction, as in chronic illness, there is a greater increase in the rate of hematopoiesis than in acute conditions such as hemorrhage (McCance & Heuther, 2014).
While several components comprise circulating blood, with each serving specific roles, every cell type originates from hematopoietic stem cells. The stem cells grow, multiply, and differentiate under the control of cytokines and growth factors. It is during the differentiation process that the stem cells follow distinctive paths to maturity and travel down committed lines of blood cells. Each line of blood cells differentiates or matures to perform a specific function. The average human body requires nearly 100 billion new blood cells per day, which is why hematopoietic stem cells are self-renewing, or have the abi
...purchase below to continue the course
Erythropoiesis
Erythropoiesis is the process by which erythrocytes develop within the bone marrow. Erythropoiesis begins with the development of erythroid progenitor cells, which are precursors to future erythrocytes. Erythropoietin (EPO) is the primary regulatory hormone for red blood cell production and is required for the maintenance, growth, and development of erythroid progenitor cells (Longo, 2019). EPO is produced by healthy kidneys and is the hormone that communicates to the bone marrow to make more red blood cells. Under the influence of EPO, cells proliferate and differentiate into specialized pro-erythroblasts, which then pass through a series of stages to become reticulocytes. Reticulocytes are immature erythrocytes, and can be measured to assess how well the bone marrow is compensating for anemia. A normal reticulocyte count is 1% of an individual's total red blood cell count. Approximately 1% of the body's circulating erythrocyte mass is generated every 24 hours. Therefore, reticulocytes serve as a good indicator of erythropoietic activity, indicating how well new red blood cells are being produced. On average, reticulocytes mature into erythrocytes within 24 to 48 hours (McCance & Heuther, 2014). Refer to Figure 1 below for a graphic depiction of erythrocyte development.
Figure 1
The Development of An Erythrocyte
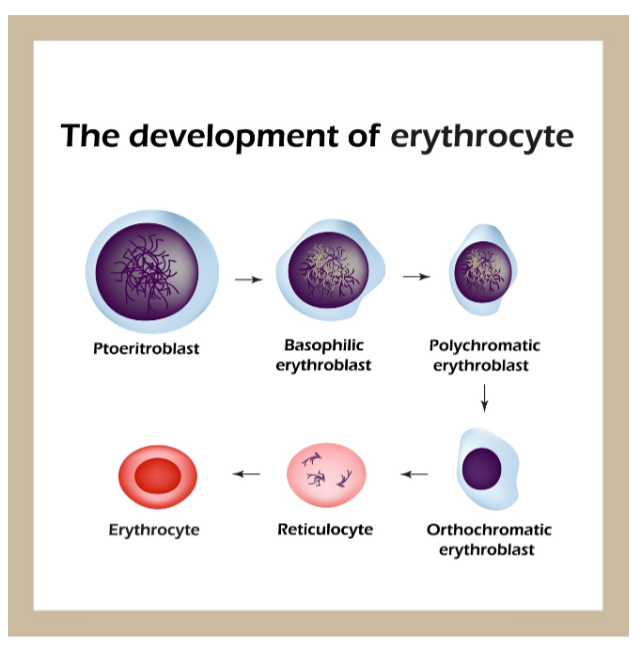
Since erythrocytes have an average lifespan of 120 days, normal RBC production strives for daily replacement of 0.8-1.0% of all circulating red cells in the body. In general, when the hemoglobin concentration falls below 12 g/dL, plasma EPO levels increase in proportion to the severity of the anemia. As individuals age, the level of EPO needed to sustain normal hemoglobin levels appears to increase, which is why there is a higher prevalence of anemia in the older adult population. In addition to EPO, sufficient iron, vitamin B12, folate, as well as other vitamins and minerals must be available for the body to make enough healthy RBCs and hemoglobin. Therefore, the critical elements of erythropoiesis include EPO production, iron availability, the proliferative capacity of the bone marrow, and effective maturation of erythrocytes; a defect in any of these critical components can lead to anemia (Adamson & Longo, 2018).
Etiology
The physiologic basis of red cell production and destruction provides an understanding of the mechanisms that can lead to anemia. Anemia has a complex array of etiologies and is not a disease in itself, but instead a manifestation of an underlying disorder (Cappellini & Motta, 2015). Anemia occurs only in the presence of clinical injury severe enough to disrupt the normal hematological hemostatic mechanisms and exceed the body's hematological reserves (Ignatavicius & Workman, 2015). There are numerous etiologies of anemia, but most can be grouped into three major categories; (1) blood loss (acute or chronic bleeding), (2) deficient erythropoiesis (inadequate production of RBC), or (3) hemolysis (excessive destruction or breakdown of red blood cells) (Le, 2016). Anemia may also be caused by a combination of these three mechanisms (McCance & Heuther, 2014).
Blood Loss
Blood loss occurs when the body loses too many RBCs, and it can be acute or chronic. With acute blood loss, anemia does not develop for at least several hours, as the compensatory mechanisms of the body kick in to offset the anemia. Compensation for reduced blood volume during hemorrhage causes the interstitial fluid in the cells to diffuse into the intravascular space. This expands plasma volume to maintain adequate blood volume, inducing hemodynamic alterations such as increased venous return, preload, and stroke volume. These mechanisms function to increase cardiac output and maintain adequate oxygen delivery to tissues and organs. Concurrently, the viscosity (thickness) of the blood decreases, causing dilution of the RBC mass and resulting in anemia. In severe cases of bleeding or in situations where timely and adequate intervention is lacking, the cardiac compensatory mechanisms fail, leading to congestive heart failure (CHF). The resulting tissue hypoxia leads to compensatory mechanisms of the pulmonary system, inducing symptoms of increased rate and depth of respiration, tachycardia, and consequential clinical sequelae. When greater than 30% of the blood volume is acutely lost, most individuals are unable to compensate with their usual mechanisms and often have signs of postural hypotension and tachycardia. If the blood volume loss is greater than 40%, symptoms of hypovolemic shock develop, which may include confusion, diaphoresis, hypotension, tachycardia, and dyspnea. These patients have significant deficits in vital organ perfusion, hypoxia, and require immediate volume replacement to avoid fatal outcomes (Longo, 2019). Some common causes of acute blood loss includes trauma, injuries, surgery, gastrointestinal bleeding, and childbirth (McCance & Heuther, 2014). Chronic blood loss leads to anemia over time if the blood loss is more rapid than the body's ability to restore or replace the loss, or if accelerated erythropoiesis depletes the body's iron stores. Chronic blood loss can be caused by cancer, heavy menstrual cycles, or gastrointestinal ulcers (McCance & Heuther, 2014).
Deficient Erythropoiesis
Deficient erythropoiesis occurs when the body makes too few RBCs. Anemias due to decreased erythropoiesis are called hypoproliferative anemias and are accompanied by reticulocytopenia, which is an abnormal decrease of reticulocytes evident on the peripheral blood smear. The RBC indices, particularly the mean corpuscular volume (MCV), can help narrow down the differential diagnosis of deficient erythropoiesis and help determine what further testing is necessary. Causes of decreased production of erythrocytes may include altered hemoglobin synthesis (iron deficiency anemia, thalassemia, anemia of chronic disease), altered deoxyribonucleic acid (DNA) synthesis due to deficient nutrients (pernicious anemia, folate deficiency anemia), stem cell dysfunction (aplastic anemia, myeloproliferative leukemia), or bone marrow infiltration (carcinoma, lymphoma) (McCance & Heuther, 2014).
Hemolysis
Hemolytic anemia occurs when red blood cells are destroyed faster than they are produced (refer to Figure 2). The body attempts to compensate for the premature RBC destruction by increasing levels of EPO (Cappellini & Motta, 2015). Hemolysis can occur within the blood vessels or in the lymphoid tissues that filter the blood, such as in the spleen and liver. Therefore, patients with this condition may demonstrate splenomegaly or enlargement of the spleen. When the spleen is enlarged, RBCs are destroyed at a more rapid rate. Hemolysis generally leads to increased reticulocyte production, unless iron or other essential nutrients are depleted (Cappellini & Motta, 2015). Hemolysis can occur in response to intrinsic (inherited) abnormalities of the RBCs or extrinsic (acquired) factors. Inherited causes of hemolytic anemia include defects in the RBC membranes, enzymatic pathways, or hemoglobin synthesis, such as in thalassemia and sickle cell anemia. Acquired causes of hemolytic anemia are usually immunologic, such as RBC destruction due to autoantibodies (immune-mediated hemolysis from blood transfusion reactions), allergic reactions (drug-induced hemolytic anemia), infection/inflammatory responses (bacterial infections, disseminated intravascular coagulation [DIC]), or exposure to toxic drugs or chemicals (McCance & Heuther, 2014).
Figure 2
Hemolysis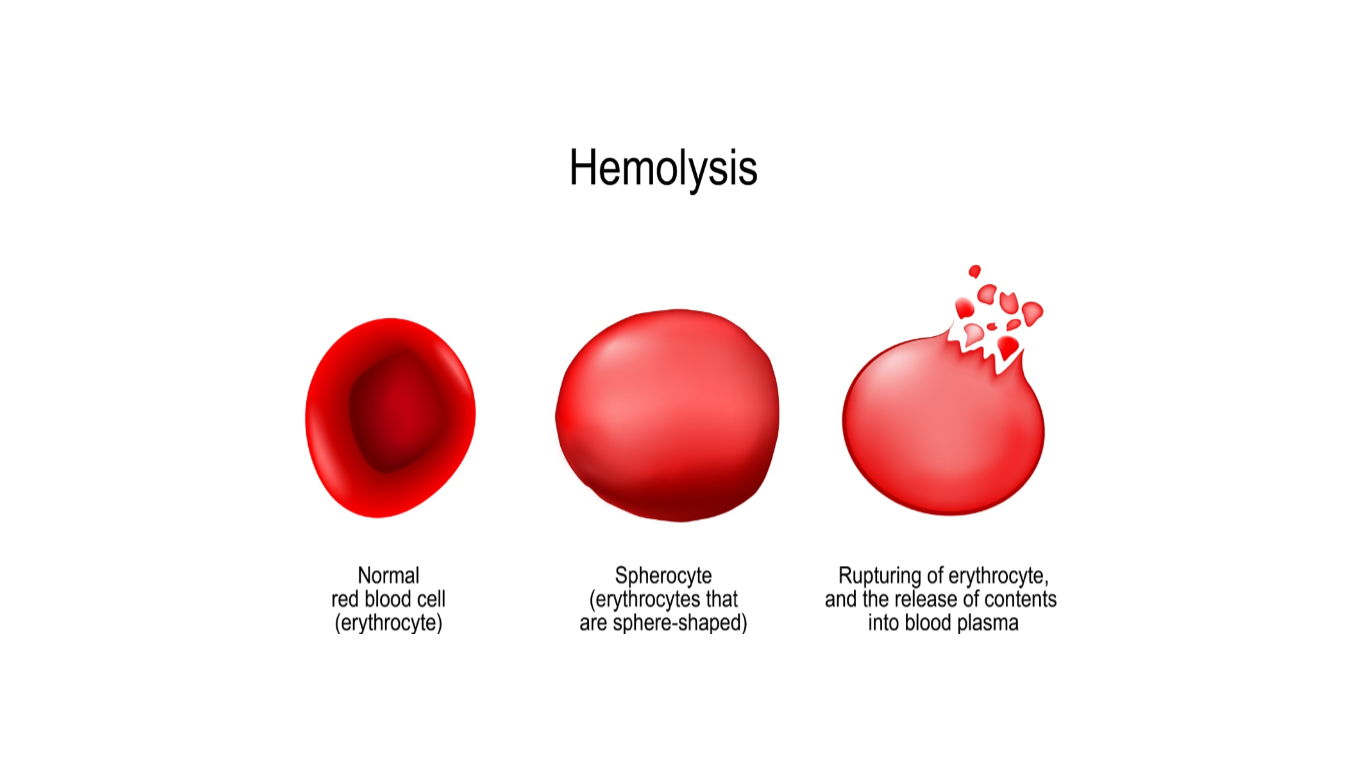
Clinical Presentation of Anemia
Anemia is most commonly recognized by abnormal screening laboratory tests when the hemoglobin and/or hematocrit is reduced below the normal range and is often suspected based on history and physical examination findings (Adamson & Longo, 2018).
Health History
Obtaining a detailed health history is critical as it provides important clues to the diagnosis and etiology of the condition. A patient who presents with symptoms of deep, sighing respirations with activity and a sensation of a rapid heart rate are vital indicators of decreased oxygen-carrying capacity of the blood. Patients should be asked about acute blood loss, blunt trauma, injury, or abnormal bruising. Healthcare professionals should inquire about any rectal bleeding, including bright red blood per rectum, as well as black or tarry stools. Female patients should be questioned on menstrual cycle regularity and heaviness of flow, as well as any other abnormal vaginal bleeding (Ignatavicius & Workman, 2015).
The clinical presentation of anemia can be highly variable based on the severity and the body's ability to compensate for hypoxia. Symptoms can range from mild fatigue to dyspnea on exertion or reduced cognitive performance (Le, 2016). Anemia that is mild and develops gradually is usually easier for the body to compensate for and may induce symptoms that are present only with physical exertion. However, as the anemia becomes more prominent, and hemoglobin levels continue to decline, symptoms often become more pronounced, and alterations of specific organs and compensatory effects are more apparent (McCance & Heuther, 2014). Symptoms are generally more prominent in patients with preexisting limitations in cardiopulmonary reserve and in those in which the anemia develops very rapidly (Braunstein, 2019).
Signs and Symptoms
The initial signs and symptoms of anemia are often apparent in the cardiovascular system. Common signs and symptoms reported by patients include generalized fatigue or overall loss of energy, weakness, dyspnea on exertion, chest pain, dizziness, difficulty concentrating, headaches, lightheadedness, cold intolerance, leg cramps, and insomnia (Adamson & Longo, 2018). More severe, but less common symptoms include angina, syncope, vertigo, amenorrhea (loss of menstrual cycle), loss of libido (loss of sex drive), and pulsatile tinnitus (ringing or thumping sound in the ear). In the most severe cases, heart failure or symptoms of shock can develop in those who have progressed to severe tissue hypoxia or hypovolemia. In patients with coronary artery disease, anginal symptoms are common (Braunstein, 2019).
Physical Examination
On physical exam, anemic patients may have very few abnormal findings, or they may exhibit several. A complete cardiac examination should be performed to evaluate for murmur or enlargement of the heart, which may provide evidence of the duration and severity of the anemia. A hemic murmur (an early systolic murmur) may be present in response to an increase in blood flow over the heart valves. Irregular and rapid heart rate is common, in addition to neurological findings such as paresthesia (stocking-glove neuropathy), cold hands and feet, or imbalance. The skin, mucous membranes, lips, nail beds, and conjunctivae may appear pale (pallor). Additional skin changes such as jaundice (yellowing of the skin), scleral icterus (yellowing of the whites of the eyes), spider nevi, purpura, palmar erythema, and nail defects may be evident. Also, patients may develop coarseness of the hair, weight loss, and malnutrition, thinning of the lateral aspects of the eyebrows, an unusually prominent venous pattern on the abdominal wall, and splenomegaly. As highlighted earlier, splenomegaly may be evident in patients with hemolytic anemia as a consequence of RBC destruction. However, splenomegaly may also develop in response to anemias of chronic inflammatory etiology, such as connective tissue disease, myeloproliferative disorder, infection, or cancer. These conditions can suppress bone marrow activity and enlarge the spleen. In patients who present following blunt trauma, abdominal distention may suggest acute hemorrhage or splenic rupture (Braunstein, 2019).
Diagnostic Tests
When anemia is suspected or identified on routine laboratory tests, a more comprehensive work up to evaluate the etiology of anemia is indicated. The initial anemia work-up often consists of a series of blood tests, which may include several or all of the following, as listed in Table 1. Reference ranges are compiled from the American Board of Internal Medicine (ABIM, 2019).
Table 1
Anemia: Common Laboratory Tests with Reference Ranges
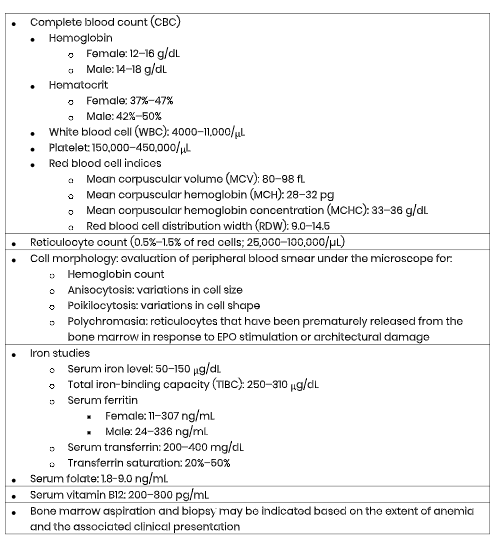
(ABIM, 2019; Braunstein, 2019; Longo, 2019).
Subsequent diagnostic tests are ordered and performed based on the results from the above tests and the patient's clinical presentation. Clinicians may order a stool guaiac test to evaluate for the presence of blood in stools when acute blood loss is suspected. A heme-positive stool guaiac is a common sign of anemia due to gastrointestinal bleeding. An ultrasound of the spleen may also be ordered to evaluate for splenomegaly (Chaparro & Suchdev, 2019).
Approach to the Classification of Anemia
Complete Blood Count (CBC)
When evaluating the patient with anemia, the components of the CBC provide essential clues toward the classification and origin of the anemia. The hemoglobin and hematocrit usually exist in a 1:3 ratio, so that 1 g of hemoglobin is equivalent to 3% of hematocrit. The body's hydration status largely influences the hematocrit, so in severe dehydration, the hematocrit is falsely elevated, whereas, in overhydration, the hematocrit is falsely reduced. The MCV is a measure of the average size of the erythrocytes. When the MCV is low, the RBC size is abnormally small and is called microcytosis or microcytic anemia. When the MCV is high, it is called macrocytosis, or macrocytic anemia. The MCH refers to the average amount (content) of hemoglobin found in each RBC or the color of the cell. Since hemoglobin provides the RBCs with its characteristic red color, the suffix –"chromic" is used. Therefore, an RBC with a normal MCH has a typical red color and is called "normochromic," whereas an RBC with a low MCH is pale in color and termed "hypochromic." The MCHC is the average weight (concentration) of hemoglobin based on the volume of RBCs. Variations in MCH and MCHC values can indicate defects in hemoglobin synthesis. The RDW is a measure of how many RBCs vary in size and volume. It reflects the degree of variation in RBC size and is often reported as anisocytosis on the RBC morphology results. The RDW is elevated when there is a wide variation in RBC size, which indicates that the cells were produced under varying conditions. Minor variation in cell size is normal, so the RDW is only considered increased when it is greater than 15%. Elevated RDW is commonly seen with iron-deficiency anemia (Longo, 2019).
Reticulocytosis
As noted earlier, reticulocytes are immature erythrocytes. When anemia develops, the body's normal response is to increase reticulocyte production (reticulocytosis) to increase the body's hemoglobin level. Therefore, reticulocytosis is a normal response when the hemoglobin level declines. If reticulocytosis is not present in the setting of anemia, consideration should be given to bone marrow failure or lack of EPO. The reticulocyte production index (RPI) is an indicator of how rapidly new RBCs are being produced and how quickly they mature. The RPI is a formula calculation using a correction factor. In summary, a value of greater than 3 indicates adequate hematological and bone marrow responses to anemia, whereas an RPI less than 2 indicates an inadequate response (McCance & Heuther, 2014).
Iron Studies
Routine iron studies include folate (folic acid), serum iron, serum ferritin, total iron-binding capacity (TIBC), and iron saturation. Folate is an essential vitamin for erythrocyte production and maturation and comes solely from dietary intake (McCance & Heuther, 2014). Serum iron refers to the total iron concentration in circulation. It is reflective of total iron intake over the last 24 to 48 hours, and it can be falsely elevated due to a high level of dietary iron ingestion or oral iron supplementation. The serum ferritin is the body's major iron storage protein. The first biochemical change in iron deficiency is a low ferritin level, which occurs before serum iron is decreased and before morphologic changes are seen in RBCs. Serum ferritin is essential in iron deficiency anemia and anemia of chronic disease, as in these conditions, serum ferritin is low. Serum ferritin reflects the body's iron stores if the patient does not have liver disease or an acute inflammatory reaction. About 15 to 20% of iron is stored within ferritin, but serum ferritin does not reflect bone marrow stores. The TIBC is a measure of transferrin, which is a plasma protein that combines with iron. When more transferrin is available for binding, the TIBC level increases, reflecting iron deficiency. Iron saturation is a percentage calculated by dividing the serum iron level by the TIBC (Longo, 2019).
Differences in Cellular Morphology
Anemias are commonly classified by their causes or according to changes in their cellular morphology, such as the size, shape, or hemoglobin content of the erythrocytes. Anemias are generally classified as microcytic, normocytic, or macrocytic, as described below (McCance & Heuther, 2014).
Microcytic Anemia
Microcytic anemias usually result from deficient or defective hemoglobin synthesis. Since hemoglobin is a major contributor to cell size, microcytosis is generally seen in patients who have anemia due to impaired hemoglobin synthesis, such as iron deficiency anemias. This can be confirmed by testing of iron stores. Since hemoglobin gives RBCs their red color, small (microcytic) RBCs are generally hypochromic (pale), with a low MCH. Additional causes of microcytic anemia include lead poisoning and thalassemia (Longo, 2019).
Normocytic Anemia
A normal RDW and normochromic indices characterize normocytic anemias. In this case, the cells are made under normal functioning conditions, and there is sufficient EPO and hemoglobin, and no defects in the synthesis of hemoglobin. The most common causes of normocytic anemia include acute blood loss and anemia of chronic disease (Longo, 2019).
Macrocytic Anemia
Macrocytic anemia, or macrocytosis, is reflected by a higher than normal MCV and resulting abnormally large cells. Macrocytosis is most commonly caused by impaired RNA and DNA synthesis. Vitamin B12 and folate deficiencies significantly contribute to RNA and DNA in developing erythrocytes. Therefore, a deficiency in vitamin B12 or folate can lead to macrocytic anemia. Since hemoglobin synthesis is not affected, macrocytic cells are typically normochromic, which means they maintain their healthy, characteristic red color. Aside from vitamin B12 and folate deficiency, additional causes of macrocytic anemia include alcohol use disorder, copper deficiency, and liver disease. Some patients with hypothyroidism have macrocytic RBC indices (Longo, 2019).
It is important to recognize that while the above explanations provide a basic guide to the preliminary classification of anemia, many cases of anemia have variable findings on the peripheral smear. For example, anemia of chronic disease is not always normocytic, as it can also present as microcytic. The variability in these findings is based on the duration, severity, and pathophysiology of the underlying disease process. Anemias due to myelodysplastic syndromes can be normocytic or macrocytic. Anemias due to endocrine disorders (such as hypothyroidism) can be either normocytic or macrocytic (Braunstein, 2019).
Types of Anemia: Clinical Features and Management
There are several types of anemia, and the distinctions between each type are important to understand, as the presentation, symptoms, and treatment can vary widely.
Iron Deficiency Anemia (IDA)
While iron deficiency is the most common type of anemia worldwide, it is also the most treatable (DeLoughery, 2017). Iron is a component of hemoglobin and is required for normal erythropoiesis, along with several other biologic processes within the body. Since iron is recyclable, the body maintains a balance between iron contained within hemoglobin and iron stored for future hemoglobin synthesis (McCance & Heuther, 2014). It takes years of inadequate oral iron intake in adults before IDA develops. While IDA can be caused by a diet lacking adequate iron, folate, or other essential vitamins or minerals, there are other causes of iron-deficiency that extend beyond inadequate dietary intake. Poor absorption of iron and nutrients due to malabsorption disorders or other gastrointestinal issues such as inflammatory bowel disease is a known cause. Chronic blood loss from gastrointestinal bleeding, oozing gastritis, gastric or duodenal ulcers, or gastrointestinal malignancies often leads to iron deficiency anemia, as the bleeding depletes the body's iron stores. IDA is common in H. pylori infections, as the infection impairs iron absorption (DeLoughery, 2017).
Women of childbearing age are at risk due to iron losses through menstruation, with an average loss equivalent to 16 mg of iron per menstrual cycle, or higher for those with menorrhagia (excessive bleeding during menstruation) (DeLoughery, 2017). Those at highest risk are African American females of childbearing age living in urban poverty. Iron deficiency is also common during pregnancy and delivery. About 50% of pregnant women do not have enough iron in their bodies. Iron deficiency anemia has been found to increase the likelihood of pre-term labor, low birth weight, intrauterine growth retardation, and death in utero (Breymann, 2015).
Furthermore, premature birth is the most common cause of infant death. Both premature birth and low birth weight raise the baby's risk for health and developmental problems at birth and throughout childhood. When anemia is severe, it can also lead to maternal mortality (Le, 2016). According to the WHO, anemia is associated with 40% of maternal deaths worldwide (Breymann, 2015). During pregnancy, the body needs more iron to support the growth of the fetus. Pregnant women require almost twice as much iron as women who are not pregnant. Most guidelines recommend almost doubling the iron consumption to approximately 30 mg/d, which is the amount readily met by most prenatal vitamins. Therefore, pregnant women are advised to take prenatal vitamins with iron daily. The American College of Obstetricians and Gynecologists has estimated that 5% of women who give birth lose 1,000 mL of blood or more during delivery. Therefore, women should be tested for iron-deficiency anemia four to six weeks after childbirth (Breymann, 2015). Since the leading site of iron absorption is within the duodenum of the intestines, surgeries that bypass this part of the bowel commonly lead to a higher incidence of IDA as a consequence of decreased absorption and decreased intestinal transit time (McCance & Heuther, 2014). This most frequently occurs following bariatric surgery, particularly gastric bypass, as the incidence of IDA can range from 25-50% within this population (DeLoughery, 2017).
Symptoms of IDA usually present gradually, and the earliest signs often include weakness, fatigue, and shortness of breath with physical activity or even mild exertion. As the condition progresses, poor circulation can lead to changes within the epithelial cells, causing the nails to become thin, brittle, and ridged. On physical exam, koilonychia may be evident, which is the upward curvature of the nails (Braunstein, 2019). Patients may exhibit pallor of the skin and conjunctivae. Some patients report dryness and soreness of the mouth with cracks at the corners. In more severe cases, the tongue may become red and painful; the degree of pain is often associated with the severity of the IDA. A common manifestation of IDA is a condition called pica, which is a hunger for non-food substances such as dirt, ice, or paper (Camaschella, 2015). Other clinical manifestations include gastritis, irritability, neuromuscular alterations, headache, numbness, tingling, and vasomotor symptoms (McCance & Heuther, 2014).
The recommendations for dietary intake of iron is 8 mg daily for adult men and 18 mg for premenopausal women. Pregnant women are advised to increase daily iron intake to at least 27 mg (DeLoughery, 2017). Food has two kinds of iron: heme and non-heme. Heme iron is found in meat, fish, and poultry and is considered the best source of dietary iron as it is most readily absorbed and utilized by the body. The majority of adults absorb up to 30% of the heme iron they consume, and it boosts iron levels much more effectively than non-heme iron sources. Non-heme (or non-meat) sources of iron are poorer in iron stores and less effectively absorbed. Some examples include plant-based foods such as vegetables, fruits, nuts, and iron-fortified cereals. While these are still critical components to a healthy, well-balanced diet, the iron contained within these foods is not absorbed as completely. Adults only absorb between 2-10% of iron from non-heme food sources (American National Red Cross, 2019). Therefore, it is much more difficult to ingest large enough quantities to meet iron requirements if only utilizing non-heme sources (DeLoughery, 2017).
To devise treatment for IDA, the priority is to identify any source of blood loss and correct it, as treatment will be ineffective in the setting of ongoing bleeding. IDA may be treated with oral or intravenous iron replacement therapy. For IDA caused by nutritional deficits, oral iron replacement therapy (IRT) is highly effective and considered first-line treatment. Ferrous sulfate (Slow Fe) is the most common oral iron replacement prescribed, as it is the most readily absorbed, best tolerated, effective, and inexpensive (McCance & Heuther, 2014).
The recommended daily dose of oral IRT for adults is 100 to 200 mg of ferrous sulfate (Slow Fe), administered in divided daily doses. Oral IRT is considered the gold standard of treatment for mild to moderate anemia and is safe for use in pregnant women (Breymann, 2015). In patients undergoing oral IRT, serum ferritin measurements are useful in monitoring the response to therapy and in determining the time when iron therapy should be discontinued, as a normal hemoglobin level does not necessarily indicate that the body’s iron stores have been replenished. It is recommended that serum ferritin assays are performed at three to four-week intervals until the serum ferritin rises above 50 ng/ml, which indicates adequate body iron stores of around 400mg. While on therapy, patients often demonstrate a rapid decline in fatigue and dyspnea on exertion, as well as other symptoms. A treatment duration of three to six months is generally required for the repletion of iron stores and the normalization of serum ferritin levels (Camaschella, 2015). Patients should be advised to take oral IRT without food, although vitamin C has been shown to enhance iron absorption when taken simultaneously. Therefore, some prescribers recommend patients take their oral IRT with ½ an orange or with 4oz of orange juice, among other food sources rich in vitamin C. Side effects of oral IRT include nausea, constipation, diarrhea, or a metallic taste. Patients should be advised that their stools may appear darker or even black, but this is a common side effect and does not indicate the presence of blood in stools (DeLoughery, 2017).
Additional nursing considerations regarding oral IRT include counseling patients on common drug interactions. Patients should be advised to separate oral IRT and antacids by at least two hours due to decreased iron absorption. Antacids neutralize the acid in the stomach and contain ingredients such as aluminum, calcium, magnesium, or sodium bicarbonate, which act as bases to counteract stomach acid and neutralize the pH. Similarly, patients should avoid caffeine within two hours of oral IRT due to decreased iron absorption. Oral IRT may reduce the efficacy of certain antibiotics such as fluoroquinolones (ciprofloxacin [Cipro], levofloxacin [Levaquin]) and tetracyclines (doxycycline [Doxy-100, Vibramycin]). Additionally, some antihypertensives such as ACE inhibitors (lisinopril [Zestril], ramipril [Altace]), and thyroid hormones (levothyroxine [Synthroid]) may also be affected. Many of these medications need to be separated from IRT by at least six hours to ensure there is no interaction. Nurses should counsel patients on the importance of speaking with their prescriber or pharmacist for specific dosing guidelines (Itano, 2016).
For the management of more severe IDA in the setting of cardiovascular symptoms such as heart failure or angina, it is recommended that these patients receive blood transfusions. This approach rapidly corrects the cardiovascular compromise and associated hypoxia, in conjunction with correcting the IDA. One unit of packed red blood cells is the equivalent of approximately 200 mg of iron. By administering a blood transfusion, the iron does not have to be absorbed by the gastrointestinal system, thereby enhancing systemic absorption and enhancing its efficacy. In some cases, IDA may be treated with parenteral (intravenous) iron replacement. This may be the treatment choice for patients who cannot tolerate or have failed oral IRT, those with malabsorption disorders, inflammatory bowel disease, or those with more severe IDA (Longo, 2019). Further, intravenous iron replacement is preferred when a rapid increase in hemoglobin level is needed, such as in patients with chronic kidney disease (CKD) who are receiving dialysis. It is more effective and increases hemoglobin levels quicker than oral IRT, as it bypasses the issues associated with iron absorption within the gastrointestinal system (Camaschella, 2015).
There are several parenteral formulations for iron replacement, but the most common include sodium ferric gluconate (Ferrlecit), iron sucrose (Venofer), ferumoxytol (Feraheme), and ferric carboxymaltose (Injectafer). Traditionally, intravenous iron infusions have posed a risk for hypersensitivity reactions and anaphylaxis, primarily related to previously utilized high-molecular-weight iron dextran (DexFerrum, INFeD). However, these newer agents are biochemically structured to pose significantly less risk of hypersensitivity reaction and are therefore used more widely. Patients receiving intravenous iron replacement should be counseled on potential side effects of nausea, vomiting, pruritus, flushing, and headache. While the risk for hypersensitivity allergic reactions is reduced with the newer iron formulations, patients may instead experience infusion reactions with flu-like symptoms within the 24 to 48-hour time period following the infusion. Symptoms may include myalgias, arthralgias, and pain in the chest and back. For patients who experience any acute flushing, discomfort, or abnormal reactions during the infusion, nurses are advised to stop the infusion immediately and monitor the patient closely. When the patient is clinically stable, and the infusion can be safely resumed, the rate of the infusion should be slowed. Premedication with an antihistamine such as diphenhydramine (Benadryl) is not advised due to the risk for hypotension and tachycardia (Camaschella, 2015).
Another treatment option for patients with IDA includes the use of erythropoiesis-stimulating agents (ESAs), such as epoetin alfa (Procrit) and darbepoetin alfa (Aranesp). These are agents approved by the US Food & Drug Administration (FDA, 2017) for the treatment of anemia resulting from CKD and cancer chemotherapy, as well as due to specific treatments for HIV. ESAs are colony-stimulating factors that function similarly to the body's natural hormone, EPO, by stimulating the bone marrow to make red blood cells. They are administered by subcutaneous injection into the arm, thigh, or abdomen. Data compiled from numerous randomized clinical trials have indicated that ESAs are associated with an increased risk for tumor progression, tumor recurrence, and shortened overall survival in patients with certain types of cancer. In addition, the prescribing information of these injectable ESAs reports an increased risk of myocardial infarction, stroke, venous thromboembolism, thrombosis of vascular access, and death. As a result, the FDA placed injectable ESAs on a Risk Evaluation and Mitigation Strategy (REMS) program to ensure that prescribers and patients both understand the risks and benefits associated with the use of ESAs. However, as of 2017, the REMS requirement was lifted. The risks remain equally as important, and healthcare providers and nurses must continue to discuss the risks and benefits of using ESAs with patients before initiating use and periodically during therapy (FDA, 2017).
Megaloblastic Anemia
Megaloblastic anemia is a condition in which the bone marrow produces unusually large, thick, immature red blood cells called megaloblasts. Megaloblasts are structurally abnormal and are usually oval-shaped instead of round like healthy RBCs (McCance & Heuther, 2014). This condition results from a defect in DNA synthesis that affects rapidly dividing cells within the bone marrow. The two most common causes of megaloblastic anemia are deficiencies in the essential nutrients cobalamin (vitamin B12) or folate (vitamin B9) (Longo, 2019).
Pernicious Anemia (Vitamin B12 Deficiency)
Pernicious anemia is a type of vitamin B12 deficiency that develops from impaired uptake of vitamin B12 due to a lack of a substance known as intrinsic factor (IF). IF is a glycoprotein produced by the gastric parietal cells (stomach lining) which helps the body absorb dietary vitamin B12 in the intestine. Vitamin B12 is a water-soluble vitamin that is essential for proper RBC formation, neurological function, and DNA synthesis. It is found in animal products, such as meat, eggs, milk, poultry, and fish, as well as fortified cereals (McCance & Heuther, 2014). While the term pernicious anemia was initially used to refer only to vitamin B12 deficiency resulting from a lack of IF, vitamin B12 deficiency due to other causes is often called pernicious anemia as well (National Heart, Lung, and Blood Institute [NHLBI], n.d.a).
When vitamin B12 is ingested orally, it binds to IF. Nuclear maturation and DNA synthesis in RBCs occurs through a series of biochemical reactions requiring the synergistic activities between vitamin B12, folic acid, and IF. When deficiencies in any of these essential nutrients exist, DNA synthesis within the RBC is impaired, leading to distinctive changes in the RBCs and bone marrow (Longo, 2019). If left untreated, pernicious anemia can cause permanent damage to nerves and other organs, increase the risk for developing stomach cancer, and is eventually fatal, usually due to heart failure (McCance & Heuther, 2014).
Vitamin B12 deficiency is most commonly caused by the loss of gastric parietal cells, malabsorption, or inadequate dietary intake. Pernicious anemia usually develops slowly over several years or even decades, as the median age at the time of diagnosis is 60 years old. Signs and symptoms of vitamin B12 deficiency may be vague and can include fatigue, weakness, mood swings, and gastrointestinal ailments such as anorexia, nausea, abdominal pain, and weight loss. Patients often report a reduced sense of touch or describe peripheral neuropathy such as stocking-glove paresthesia, "pins and needles," or numbness and tingling sensations in the hands, fingers, feet, or toes. The neurological manifestations are due to nerve demyelination and, consequently, neuronal death. The tongue can become sore, and on physical examination, patients may have evidence of a smooth, beefy red tongue. The skin may be light yellow due to a combination of pallor and icterus, and hepatomegaly (enlargement of the liver) may be present. Patients may also present with a wobbly gait and difficulty walking, or clumsiness and stiffness of the arms and legs. They may exhibit ataxia and loss of positioning and vibrational sense on neurological exam (Braunstein, 2019). Dementia is another sign associated with vitamin B12 deficiency anemia, as an increased prevalence of vitamin B12 deficiency is reported in patients with Alzheimer's disease (McCance & Heuther, 2014).
Treatment for pernicious anemia is relatively straightforward and requires the prompt initiation of vitamin B12 therapy. While oral and injectable options for treatment exist, injectable formulations are preferred mainly due to their heightened efficacy and enhanced absorption over oral agents. Only about 10 mcg of a 500 mcg oral supplement is absorbed in healthy adults (National Institute of Health [NIH], 2019). Vitamin B12, usually in the form of cyanocobalamin, is administered as an intramuscular injection. There are varied recommendations for the treatment of pernicious anemia in adults, but in general, evidence-based guidelines recommend treating with an initial dose of cyanocobalamin 1000 mcg intramuscular injection. Some sources recommend repeat injections daily for six to seven days or until clinical improvement and reticulocyte response is seen on laboratory testing. Other courses advise an initial injection of cyanocobalamin 1000 mcg, followed by monthly injections (Longo, 2014).
Patients generally respond briskly to injectable vitamin B12 therapy with reticulocytosis occurring within five to seven days, and anemia resolving typically within two months. Symptoms often improve rapidly alongside treatment; however, neurological manifestations can be prolonged or permanent in some cases depending upon the severity. It is important to test patients for concomitant folate deficiency (to be reviewed below), as replacement with folic acid should co-occur with vitamin B12 therapy to ensure optimal outcomes (McCance & Heuther, 2014). Important nursing considerations during the first week of vitamin B12 replacement therapy is to monitor for hypokalemia. Hypokalemia is common during B12 replacement therapy for severe anemia and is due to intracellular potassium shifts during reticulocytosis. Oral potassium replacement is generally sufficient to correct hypokalemia. Patients should additionally be counseled on the importance of strict compliance with treatment, as pernicious anemia cannot be cured. Therefore, patients require lifelong maintenance dosing with cyanocobalamin 1000 mcg monthly (Longo, 2019).
Folate Deficiency Anemia
Folate (folic acid) is a water-soluble B complex vitamin found within food and is an essential vitamin for RBC production and maturation. The human body depends on dietary intake of folate, with the average adult requiring 50 to 200 mcg/day. Folate is absorbed within the small intestine and transported to the liver where it is stored. Pregnant and lactating women need increased folic acid intake, as folic acid transfers through the placenta to the fetus. Folate deficiency anemia during pregnancy is teratogenic, which means it contributes to significant abnormalities in the fetus by interfering with healthy development and can lead to neural tube defects. Therefore, the fetus has higher folate requirements than the mother, rendering pregnancy as a maternal folate-depleting condition, with repeated pregnancies posing the potential to deplete maternal folate stores. Folate deficiency is such a significant contributor to neural tube defects that folate has been added to grains in the US to prevent these congenital disabilities (Khan & Jialal, 2018).
The most common cause of folate deficiency is inadequate dietary intake and chronic malnourishment. Therefore, this condition is frequently seen among those living in poverty, as well as chronic alcoholics and the elderly. Less commonly, folate deficiency is caused by impaired absorption due to celiac disease or other gastrointestinal malabsorption disorders. The symptoms of folate deficiency anemia are very similar to those of pernicious anemia, except for the neurological manifestations which generally do not occur with this condition. Some clinical manifestations specific to folate deficiency include severe cheilosis (scales and fissures of the lips and corners of the mouth), as well as inflammation and ulceration of the mouth and tongue. Also, gastrointestinal symptoms are common, such as increased flatulence, watery diarrhea, and dysphagia (difficulty swallowing) (McCance & Heuther, 2014).
It is strongly recommended that all women of childbearing age consume folate-rich foods and receive at least 0.4 mg per day of supplemental folic acid to prevent pregnancy-related complications and fetal abnormalities in the event of an unplanned pregnancy. It is also strongly recommended that pregnant women take a daily prenatal vitamin, which usually contains enough folic acid to meet day-to-day requirements. For those women who cannot tolerate prenatal vitamins due to nausea or other side effects, they may safely take oral supplementation with folic acid 1 mg daily, in addition to increasing folate-rich foods within their diet. In general, oral folic acid supplementation (1 mg to 5 mg) daily is sufficient to treat folate deficiency in otherwise healthy adults (Khan & Jialal, 2018). However, persons with alcoholism may require up to 5 mg of folic acid supplementation daily (McCance & Heuther, 2014).
Folic acid supplementation at a dose of 1 mg daily is usually sufficient to prevent folic acid deficiency in specific high-risk patient populations, such as those who are undergoing bariatric surgery or those with chronic alcohol use. All patients with folate deficiency should be encouraged to eat a diet rich in green leafy vegetables and fruits and should be counseled on the importance of compliance with daily supplementation. Response to folic acid therapy is usually rapid, with reticulocytosis peaking at seven to ten days after initiation of treatment. The hematocrit level is estimated to increase by 4-5% each week during therapy and usually reaches normal limits within one month. If left untreated, folate deficiency can lead to neuropsychiatric manifestations such as depression, irritability, insomnia, cognitive decline, and psychosis (Khan & Jialal, 2018).
Anemia of Chronic Disease (ACD)
Anemia of chronic disease is the second most common type of anemia worldwide, following IDA. It is usually multifactorial in etiology, as diagnosis requires the presence of a chronic inflammatory condition, autoimmune disease, or other chronic illnesses such as kidney disease, hypothyroidism, or cancer. The classic features include microcytic or normocytic anemia and a low reticulocyte count. Serum iron is usually low or normal, while ferritin can be normal or elevated. ACD may partly be due to reduced EPO response in the bone marrow, as well as the suppression of bone marrow from medications used to treat chronic conditions. In cancer treatment, bone marrow is suppressed due to chemotherapy, and as a result, normal RBC death occurs without the production of new RBCs (Le, 2016).
Anemia is highly prevalent among patients with CHF, as the two conditions frequently co-exist. Anemia in the setting of CHF often leads to more severe symptoms, significantly worsening functional capacity, and reduced survival rates (Le, 2016). Further, the most frequent comorbid conditions in patients with CHF is CKD. CHF and CKD share many common causes (i.e., hypertension), clinical features (i.e., impaired performance status due to deconditioning), and risk factors (i.e., older age, obesity, poor lifestyle choices). The major factors contributing to CHF-related anemia involve CKD, renin-angiotensin system, iron deficiency, chronic inflammation, and hemodilution (McCance & Heuther, 2014).
In CHF, the reduced cardiac output from impaired heart function leads to hypoxia, which causes reduced renal perfusion and subsequent renal damage. While hypoxia initially stimulates EPO production as a compensatory mechanism, patients with CKD and especially those on dialysis, often have anemia due to a lack of EPO. When the glomerular filtration rate declines to less than 30 to 40 mL/min, renal EPO synthesis declines. As highlighted earlier, EPO is generated by healthy kidneys and fuels red blood cell growth. When the kidneys fail, they no longer produce enough EPO, and without enough EPO, there are fewer red blood cells generated to carry oxygen to tissues within the body (McCance & Heuther, 2014). Treatment should focus on reversing the underlying disorder, and injectable ESAs may be indicated. Therefore, before starting any treatment for anemia in these patients, it is vital to first identify and treat any other causes of anemia, such as active bleeding, hemolysis, vitamin B12 or folate deficiency, or malignant processes (Le, 2016).
Aplastic Anemia
Aplastic anemia is a critical condition that develops from the failure of the bone marrow to produce essential cells leading to a deficiency in circulating RBCs. Its etiology can be traced back to an injury to an immature stem cell, which may be related to exposure to ionizing radiation or toxic agents, viral infection, or it may be idiopathic. The incidence of aplastic anemia is relatively rare, with 2 to 5 cases per 1,000,000 people per year. It most commonly occurs in young adults between the ages of 15 and 25, and adults older than 60 (McCance & Heuther, 2014). The onset can be rapid, and presentation depends on which cell type is most affected. The CBC often reveals pancytopenia, which is a reduction in the RBCs, WBCs (leukopenia), and platelets (thrombocytopenia). Many patients present with abnormal bleeding or hemorrhage due to unusually low platelet count and have a high risk of death related to infection or bleeding. Aside from bleeding, common symptoms may include fever, fatigue, weakness, as well as dyspnea and hypoxemia in more severe cases. On physical examination, patients often exhibit pallor, petechiae, purpura, ecchymosis, and ulceration of oral mucosa. Splenomegaly and neurological manifestations are not commonly seen (McCance & Heuther, 2014). A bone marrow biopsy is indicated to diagnose this condition accurately, and findings often demonstrate the replacement of marrow forming cells with adipose (fat) cells (Ignatavicius & Workman, 2015).
The management of aplastic anemia is more complex than other types of anemias. Patients are treated with supportive therapies as needed based on laboratory values and clinical symptoms. They often require blood transfusions for anemia and platelet transfusions for thrombocytopenia and to control bleeding. Immunosuppressive treatment with medications such as steroids (i.e., prednisone [Rayos]) or those used to protect against organ transplant-rejection (i.e., cyclosporine [Sandimmune]) may be prescribed to suppress the immune system’s attack on the bone marrow. Infrequently, splenectomy (surgical removal of the spleen) may be necessary for some patients. Allogeneic hematopoietic stem cell transplantation (Allo-HSCT) is the only potential cure for aplastic anemia. An Allo-HSCT is a type of bone marrow transplant that uses stem cells taken from another person (a donor source) that are transplanted into the patient to replace defective stem cells with healthy, functioning stem cells (Sun et al., 2018).
Sickle Cell Anemia
Sickle cell anemia is a hereditary disorder that affects the red blood cells, causing the hemoglobin to become defective and misshaped. In sickle cell anemia, the hemoglobin is called hemoglobin S (HgbS) and widely replaces normal hemoglobin, inducing a clinical sequela of sickle-shaped red blood cells that wreak havoc within the body. These cells tend to break apart easily, clump together, stick to the walls of blood vessels and block the flow of blood. When the blood vessels are clogged, this cuts off oxygen to vital organs and tissues, inducing severe pain and other serious health complications. See Figure 3 for a graphic representation of this process. These sickle-shaped cells also get trapped inside the spleen due to their irregular shape and are subsequently destroyed. The body is not able to replace the damaged cells at the rate in which they are destroyed, resulting in anemia (Agrawal et al., 2014).
Figure 3
Sickle-Cell Anemia
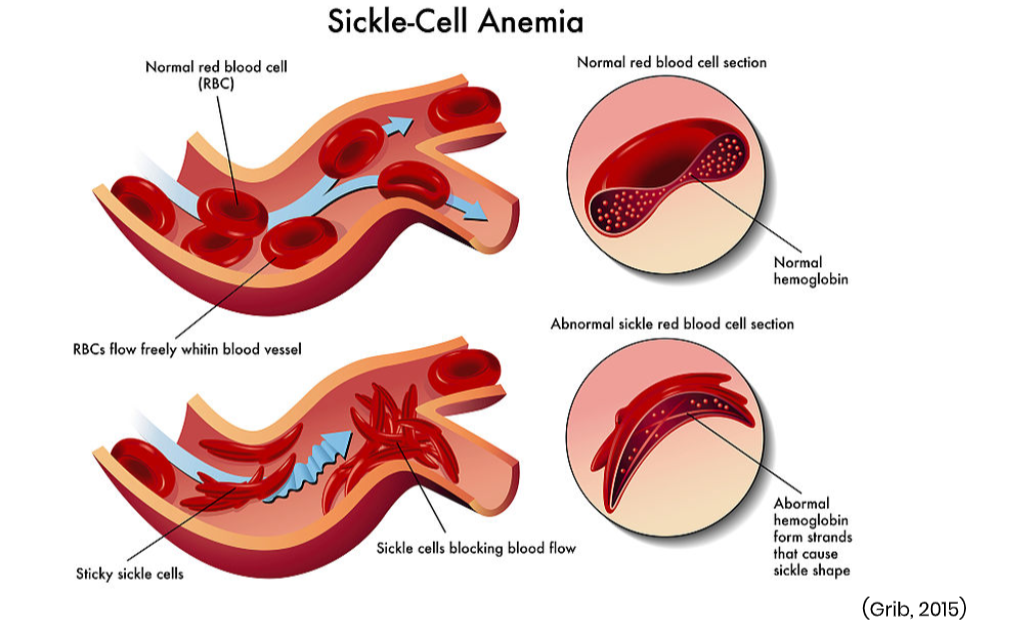
Sickle cell anemia most often occurs among African Americans, at a rate of about 1 in every 365 African American births and affecting 100,000 individuals within the US. Aside from anemia, patients with sickle cell anemia often report symptoms of fatigue and episodes of severe pain, especially in the joints, limbs, and abdomen. Joint pain can resemble arthritis pain, and chronic neuropathic pain is also common. Patients may require frequent hospitalization due to dehydration and pain crises, which can last for hours to days. Patients with sickle cell anemia are at heightened risk for blood clots, stroke, and are highly susceptible to life-threatening infections (ASH, 2019b).
Sickle cell disease symptoms can begin by four months of age; early diagnosis is critical. All newborns in the US are now tested for the disease. Sickle cell disease can be identified before birth by testing a sample of amniotic fluid or tissue from the placenta. Unfortunately, there remains no standard curative treatment for sickle cell anemia. Treatment focuses on alleviation of pain, management of anemia with blood transfusions as needed, and prevention of infection and other complications. Some patients are treated with a medication called hydroxyurea (Hydrea), which is a myelosuppressive agent and the only drug proven to reduce the frequency of painful episodes in patients with sickle cell. Research reveals that the use of hydroxyurea (Hydrea) can decrease the rate of painful episodes by 50%. It works by making the red blood cells more flexible, thereby reducing pain and the need for blood transfusions. The most common side effects of hydroxyurea (Hydrea) include bone marrow suppression (i.e., neutropenia), anorexia, nausea, vomiting, elevation in liver function enzymes, and infertility (Agrawal et al., 2014).
Thalassemias
Thalassemias are inherited blood disorders in which the body produces an inadequate amount of normal hemoglobin. Since the condition is caused by a defect in two major proteins that make up normal hemoglobin, the alpha-globin, and beta-globin, the two major types of thalassemia are named after the defects within these proteins. Alpha thalassemia is related to a mutation in the alpha-globin protein, whereas beta-thalassemia related to a gene defect in the beta-globin protein. In general, the condition is inherited in an autosomal recessive manner. Alpha thalassemia major is the most severe form of alpha thalassemia, and requires inheritance of a defective gene from both parents. Infants who are diagnosed with this condition usually die shortly before or after birth. Most people affected by beta-thalassemia have mutations in both copies of the beta globulin gene, are considered carriers, and do not have symptoms. Males and females are affected in equal proportions, and the most severe forms are usually diagnosed in early childhood and are lifelong conditions (Longo, 2019).
Beta thalassemia major is the most severe form of beta-thalassemia and is often referred to as ‘Cooley's Anemia.' Signs and symptoms usually occur within the first two years of life and are often accompanied by severe anemia and other health problems, including a pale and listless appearance, anorexia and weight loss, and dark urine secondary to hemolysis. Other clinical manifestations include slowed growth, delayed puberty, jaundice, splenomegaly, hepatomegaly, or cardiomegaly. Many of these patients also experience bone problems, such as osteoporosis, which is a condition in which the bones become weak, brittle, and break easily. The clinical severity of symptoms varies depending on the degree to which the globin synthesis is affected. Patients with mild forms generally do not require treatment, and mild thalassemias often do not shorten their lifespan. Those with more severe forms often require frequent blood transfusions to manage moderate to severe anemia. Regular blood transfusions can cause iron overload, a condition in which iron builds up in the blood. This can damage organs and tissues, especially the heart and liver, and is managed with iron chelation therapy, treatments to remove excess iron from the body. Further, severe thalassemia can cause early death due to heart failure (NHLBI, n.d.b).
Closing Thoughts
There are many forms of anemia that extend well beyond the scope of this educational module. Given that anemia is the most common blood disorder, affecting more than 3 million Americans, it is a highly prevalent occurrence across all healthcare settings. Therefore, nurses and other healthcare professionals must understand the importance of a systematic approach to diagnosis, work-up, and management (ASH, 2019a). It is crucial to understand that patients may present with anemia in several ways and that anemia is a manifestation of an underlying condition. Identifying and correcting the underlying etiology is the priority intervention before initiating treatment (Longo, 2019).
References
Adamson, J. W., & Longo, D. L. (2018). Harrison's principles of internal medicine (20th ed.). McGraw-Hill Education.
Agrawal, R. K., Patel, R, K., Shah, V., Nainiwal, L., & Trivedi, B. (2014). Hydroxyurea in sickle cell disease: Drug review. Indian Journal of Hematology & Blood Transfusion, 30(2), 91-96. https://doi.org/10.1007/s12288-013-0261-4
American Board of Internal Medicine. (2019). ABIM Laboratory Test Reference
Ranges - January 2019. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American National Red Cross. (2019). Iron rich foods.
https://www.redcrossblood.org/donate-blood/blood-donation-process/before-during-after/iron-blood-donation/iron-rich-foods.html
American Society of Hematology. (2019a). Anemia.
https://www.hematology.org/Patients/Anemia
American Society of Hematology. (2019b). Sickle cell disease.
https://www.hematology.org/Patients/Anemia/Sickle-Cell.aspx
Bach, V., Schruckmayer, G., Sam, I., Kemmler, G., & Stauder, R. (2014). Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging, 9, 1187-1196. https://doi.org/10.2147/CIA.S61125
Braunstein, E. M. (2019). Evaluation of anemia.
https://www.merckmanuals.com/professional/hematology-and-oncology/approach-to-the-patient-with-anemia/evaluation-of-anemia
Breymann, C. (2015). Iron deficiency anemia in pregnancy. Seminars in
Hematology, 52(4), 339-347. https://doi.org/10.1053/j.seminhematol.2015.07.003
Camaschella, C. (2015). Iron-deficiency anemia. NEJM, 327(19), 1832-1843. https://doi.org/10.1056/NEJMra1401038
Cappellini, M. D., & Motta, I. (2015). Anemia in clinical practice- definition and classification: Does hemoglobin change with aging? Semin Hematol, 52(4), 261-269. https://doi.org/10.1053/j.seminhematol.2015.07.006
Centers for Disease Control and Prevention. (2018). NHANES questionnaires,
datasets, and related documentation. https://wwwn.cdc.gov/nchs/nhanes
Chaparro, C. M., & Suchdev, P. S. (2019). Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Annals of the New York Academy of Sciences, 1450, 15-31. https://doi.org/10.1111/nyas.14092
Deloughery, T. G. (2017). Iron deficiency anemia. Med Clin North Am, 101(2), 319-332. https://doi.org/10.1016/j.mcna.2016.09.004
Grib, D. (2015). Sickle-cell anemia [image]. https://commons.wikimedia.org/wiki/File:Risk-Factors-for-Sickle-Cell-Anemia (1)2.jpg
Itano, J. K. (2016). Core curriculum for oncology nursing (5th ed.). J. Brant, F. Conde, & M. Saria, (Eds.). Elsevier.
Khan, K., & Jialal, I. (2018). Folic acid (folate) deficiency. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK535377/
Kochanek, K. D., Murphy, S. L., Xu, J., & Arias, E. (2019). Deaths: Final data for 2017. National Vital Statistics Reports, 68(9), 1-77. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf
Le, C. H. (2016). The prevalence of anemia and moderate-severe anemia in the
US population. (NHANES 2003-2012). PLOS One, 11(11), 1-14. https://doi.org/10.1371/journal.pone.0166635.
Longo, D. L. (2019). Harrison’s hematology and oncology. (3rd ed.). McGraw-Hill Education.
McCance, K. L., & Heuther, S. E. (2014). Pathophysiology: The biologic basis for disease in adults and children. (7th ed.). Mosby Elsevier.
National Heart, Lung, and Blood Institute. (n.d.a). Pernicious anemia. Retrieved October 1, 2019, from https://www.nhlbi.nih.gov/health-topics/pernicious-anemia
National Heart, Lung, and Blood Institute. (n.d.b). Thalassemias. Retrieved
October 3, 2019 from https://www.nhlbi.nih.gov/health-topics/thalassemias
National Institute of Health. (2019). Vitamin B12.
https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/#en8
Stauder, R., & Thein, S. L. (2014). Anemia in the elderly: Clinical implications and new therapeutic concepts. Hematologica, 99 (7), 1127-1130.
Stauder, R., Valent, P., & Theurl, I. (2018). Anemia at older age: Etiologies, clinical implications, and management. Blood, 131(5), 505-514. https://doi.org/10.1182/blood-2017-07-746446
Sun, Q., Wu, B., Zhu, Z., Sun, C., Xu, J., Long, H., …Song, C. (2018). Allogeneic hematopoietic stem cell transplant for severe aplastic anemia: Current state and future directions. Curr Stem Cell Res Ther, 13(5), 350-355. https://doi.org/10.2174/1574888X12666170227151226.
U.S. Food & Drug Administration. (2017). Information on erythropoiesis-
stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-erythropoiesis-stimulating-agents-esa-epoetin-alfa-marketed-procrit-epogen-darbepoetin