About this course:
The purpose of this course is to review the pathophysiology of AMI and explore the current AMI diagnosis and treatment guidelines by the ACC, AHA and ESC.
Course preview
Learning Objectives: Upon completion of this activity, the nurse should be able to:
- Discuss the pathophysiology, causes, and risk factors of acute myocardial infarction (AMI).
- Consider the diagnostic process for AMI.
- Describe the evidence-based treatment guidelines for AMI.
- Discuss the nursing management of patients with AMI.
- Review the post-discharge care that is recommended for AMI patients, including the top ten primary prevention techniques recommended by the American College of Cardiology and the American Heart Association.
The purpose of this course is to review the pathophysiology of AMI and explore the current AMI diagnosis and treatment guidelines according to the American College of Cardiology, American Heart Association, as well as the European Society of Cardiology.
An acute myocardial infarction (AMI), commonly referred to as a heart attack, occurs when ischemia causes irreversible tissue necrosis within the myocardium. It is the leading cause of death worldwide (The Centers for Disease Control and Prevention [CDC], 2017). There is one AMI in the US every 42 seconds. It is a frequent cause of hospital admission in the US and is associated with significant mortality and morbidity. Survivors of AMI are at increased risk for recurrent cardiovascular events, which carries a significant cost burden on the health care system (Ibanez et al., 2018).
According to the CDC (2018), 790,000 AMIs occur annually in the US, and 210,000 of these incidents occur in individuals who have previously had an AMI. Upon completion of this module, the learner will be able to discuss the pathophysiology and causes of AMI, consider the diagnosis of an AMI, describe the national evidence-based treatment guidelines for AMI, and discuss how to manage and apply the nursing process in caring for patients with AMI.
Pathophysiology of AMI
An AMI, or type 1 spontaneous MI, indicates irreversible myocardial injury resulting in tissue necrosis of a significant portion (generally greater than 1 cm) of the myocardium (the muscular tissue of the heart, see Figure 1). The term "acute" denotes infarction that is less than three to five days old. AMIs may be of the nonreperfusion type, indicating that the obstructed blood flow is permanent, or of the reperfusion type, indicating that the lack of blood flow is long enough in duration (typically hours) to induce cell death but is later reversed or restored (Urden et al., 2018).

AMIs generally affect a segment or region of the myocardium secondary to occlusion of an epicardial artery (see Figure 1). In contrast, concentric subendocardial necrosis (necrosis of the inner layer of the heart, or the endocardium, and inner portion of the myocardium) may result from global ischemia and reperfusion in cases of prolonged cardiac arrest with resuscitation. Areas of myocardial infarction may be subepicardial (affecting the outer portion of the myocardium and epicardium) if there is thromboembolic occlusion of smaller vessels originating from coronary thrombi. In the majority of patients, obstructive coronary artery disease (CAD) can be found on angiography (Urden et al., 2018).
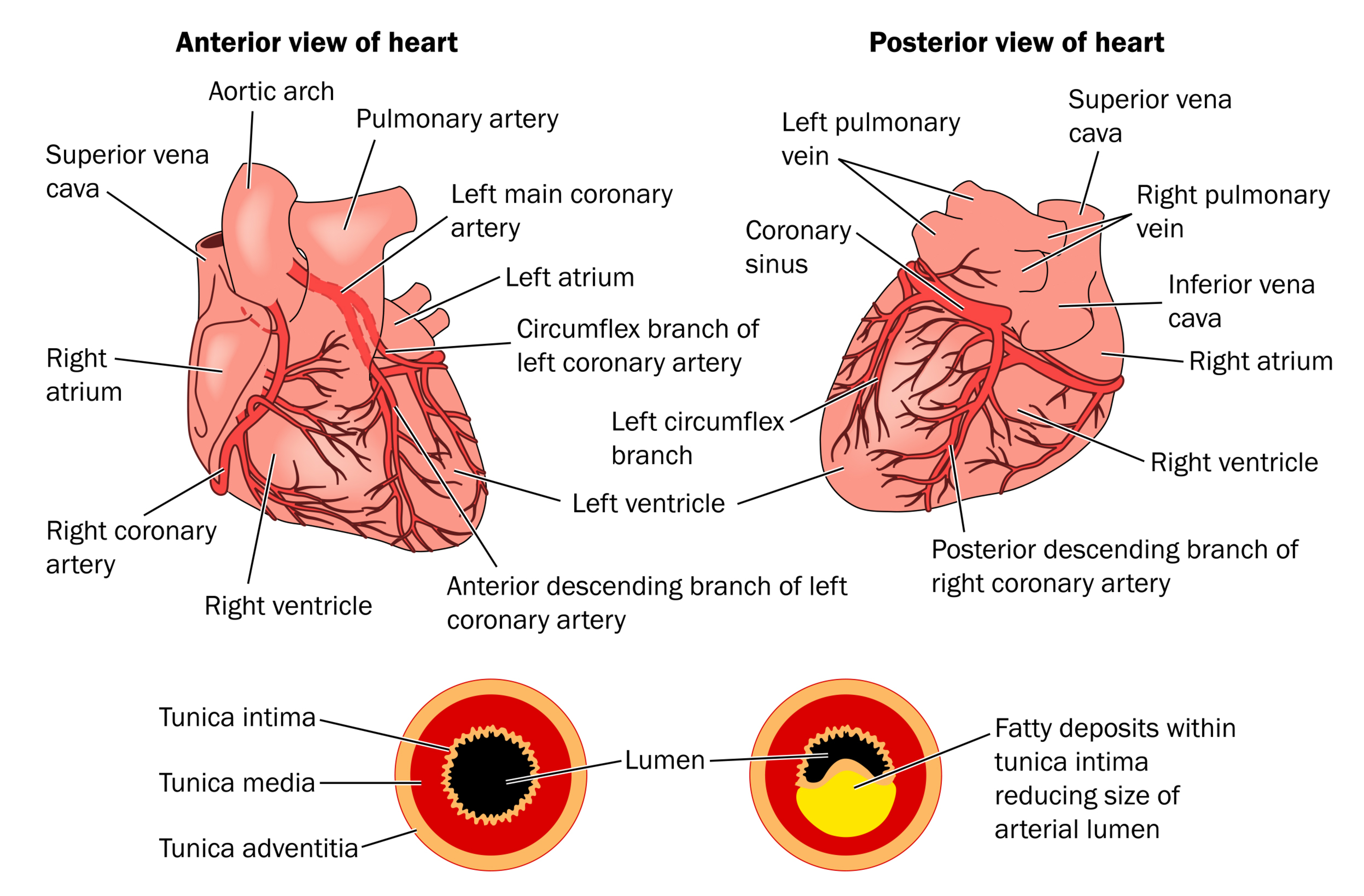
The area of infarct occurs in the distribution of the occluded vessel (see Figure 2). Left main coronary artery occlusions generally result in a large anterolateral infarct, whereas occlusion of the left anterior descending coronary artery causes necrosis limited to the anterior wall. There is often an extension to the anterior portion of the ventricular septum with proximal left coronary occlusions (Urden et al., 2018).
In hearts with a right coronary dominance (the right artery supplying the posterior descending branch), a right coronary artery occlusion causes a posterior inferior infarct, a proximal obtuse marginal thrombus will cause a lateral wall infarct only, and the distal circumflex is a small vessel. With a left coronary dominance (only about 15% of the population), a proximal circumflex occlusion will infarct the posterior wall (Urden et al., 2018).
Any anatomic variation due to microscopic collateral circulation, which is not evident at autopsy, plays a large factor in the size and distribution of the necrosis. Unusual patterns of supply to the posterior wall, such as wraparound left anterior descending or posterior descending artery supplied by the obtuse marginal artery, may also result in unexpected areas of infarct in relation to the occluded proximal segment (Urden et al., 2018).
Figure 2
Coronary Arteries and CAD
Causes of Acute Angina and AMI
AMIs almost always happen when the blood supply to the heart muscle has been obstructed. In most cases, it is an acute event, resulting from the sudden rupture of an atherosclerotic plaque in the wall of a coronary artery in a person with typical CAD, the leading cause of AMIs (Urden et al., 2018; CDC, 2017). AMIs occur most often early in the morning, which may be related to circadian variations in sympathetic tone (Zafari, 2019). The most common conditions that may lead to an AMI include:
- Acute coronary syndrome (ACS) associated with typical CAD is, by far, the most common cause of AMI. It may lead to unstable angina, ST-elevation MI (STEMI), or non-ST-elevation MI (NSTEMI).
- Coronary artery spasms (otherwise known as Prinzmetal angina) can lead to tissue ischemia within the myocardium with longer durations, although the duration of angina is typically less.
- Microvascular angina (otherwise known as cardiac syndrome X) occurs within the smaller cardiac vessels and may lead to an AMI.
- Stress cardiomyopathy (otherwise known as broken heart syndrome) occurs as a result of stressful life events, causing endothelial tissue dysfunction that can lead to heart failure.
- Viral myocarditis is an infection directly affecting the heart muscle. It produces extensive localized inflammation in the cardiac muscle, interrupting the local blood supply.
- Blood clotting disorders, such as Factor V Leiden, predispose individuals to abnormal blood clotting. This can lead to acute thrombosis of a coronary artery even without any underlying CAD, and subsequently, myocardial infarctions.
- Coronary artery embolism is a blood clot, usually originating within one of the chambers of the heart that breaks free and becomes lodged in a coronary artery, interrupting the blood supply to part of the heart muscle. Several conditions predispose to blood clot embolization, such as atrial fibrillation, dilated cardiomyopathy, prosthetic heart valves. These patients are usually treated with anticoagulants to prevent the formation of these clots.
- The risk of premature heart attacks tends to have a genetic link. Evidence of familial hypercholesterolemia or Factor V Leiden places a patient at higher risk for CAD and cardiac events (Urden et al., 2018).
Risk Factors for Atherosclerosis
The nonmodifiable risk factors include age, sex, family history of premature CAD, and the presence of male-pattern baldness. The vast majority of risk factors are modifiable, and include:
- Smoking/tobacco use
- Hypercholesterolemia, hypertriglyceridemia, and dyslipidemia
- Diabetes mellitus
- Hypertension
- Obesity, especially abdominal obesity
- Psychosocial stress
- Sedentary lifestyle
- Dietary lack of fruits/vegetables
- Poor oral hygiene
- Anxiety/”type A” personality
- Elevated homocysteine level
- Peripheral vascular disease (Zafari, 2019).
Tables
...purchase below to continue the course


Diagnosis of AMI
The first step in diagnosing an AMI is identifying the presenting symptoms, which include persistent, intense, substernal chest pain that lasts at least 30 minutes and classically radiates to the neck, jaw, shoulder, or left arm. A history of CAD should increase the suspicion of an AMI. Chest discomfort, especially pressure that is described as squeezing, aching, burning, or sharp, is also common. Less typical symptoms may include shortness of breath, nausea/vomiting, fatigue, palpitations, malaise, lightheadedness/syncope, coughing, wheezing, profuse sweating, or epigastric feelings of fullness, indigestion, or gas. Women tend to present with atypical symptoms more often than men, and usually develop atherosclerotic disease seven to ten years later than men. Vital sign assessment usually indicates tachycardia with or without an arrhythmia, tachypnea, and elevated blood pressure. In patients with a right ventricular AMI or severe left ventricular dysfunction may present with symptoms of hypotension and cardiogenic shock. AMI diagnosis should occur within 10 minutes of the patient’s first medical contact, according to the 2017 ESC guidelines (Ibanez et al., 2018; Zafari, 2019).
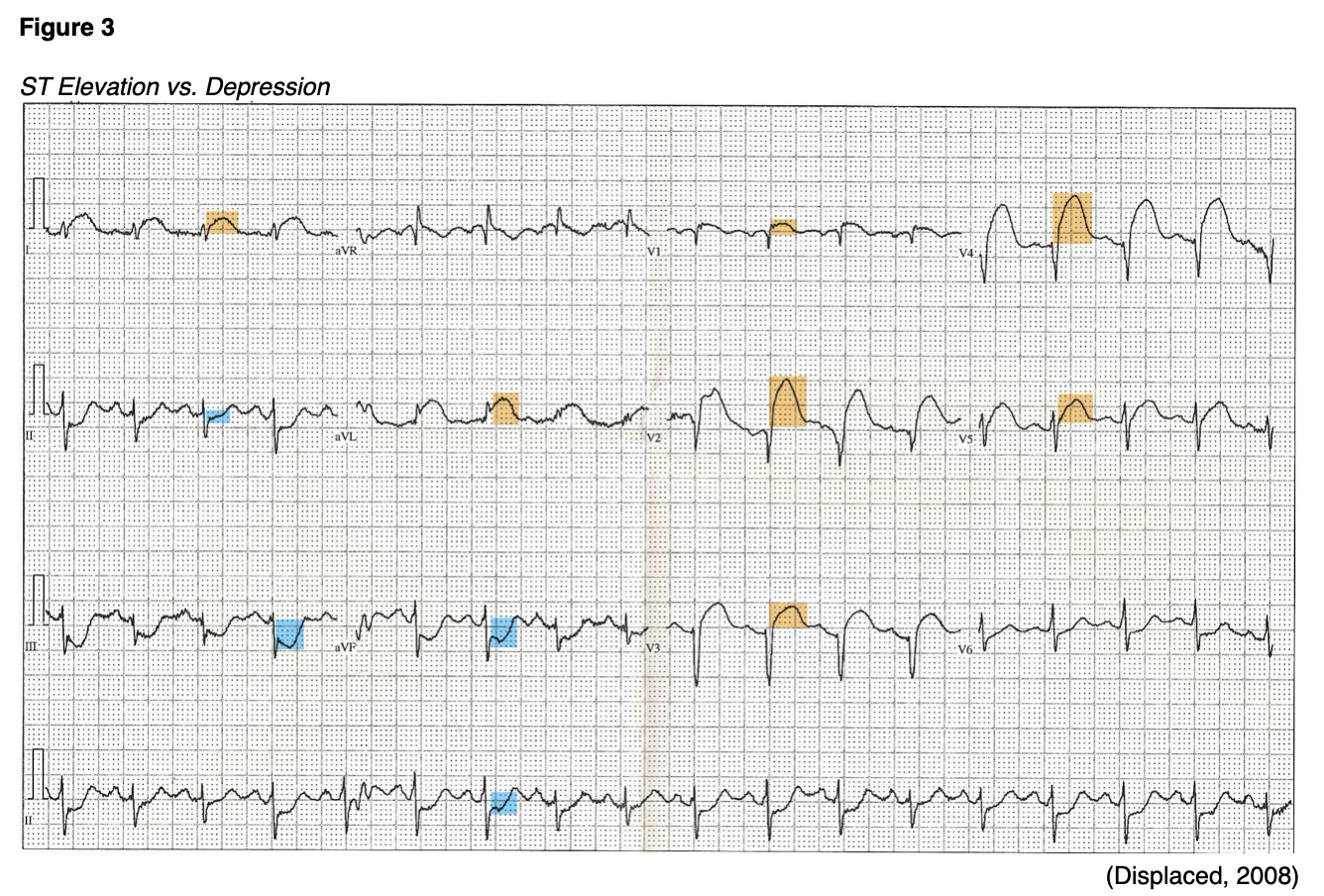
Electrocardiography (ECG) monitoring should be initiated as soon as possible when an AMI is suspected in order to establish a rhythm baseline, assess for arrhythmias, and allow immediate treatment. AMIs are classified into those which cause ST-segment elevation (STEMI) on ECG and those that do not (NSTEMI). The electrocardiogram tracings below (see Figure 3) illustrate ST-segment elevation in anterior leads (highlighted in orange) and ST-segment depression in inferior leads (highlighted in blue). The ESC defines ongoing coronary artery occlusion as at least a 2.5 mm elevation in the ST-segment in at least two contiguous leads in men under 40, and at least 2 mm in men over 40. In women, the ESC defines it as an elevation of at least 1.5 mm in leads V2-V3 or 1 mm in the other leads. The right precordial leads (V3R and V4R) should be monitored in patients with suspicion of inferior AMI, as a right ventricular infarct (defined as a 1 mm or greater ST-segment elevation in V3R or V4R) is found in roughly one-third of inferior AMIs. Management for these patients is often complicated, so this finding is significant (Ibanez et al., 2018; Zafari, 2019). A combination of at least 0.5 mm of ST-segment depression and a positive terminal (inverted) T-wave (especially in leads V1-V3) can indicate myocardial ischemia corresponding to the left circumflex artery. Obstruction of the left main coronary artery may present as hemodynamic instability coupled with ST-segment depression greater than 1 mm in eight or more surface leads along with ST-segment elevation in aVR and/or V1. Bundle branch block (BBB) and pacemakers can make diagnosis more difficult. Diagnosis in patients with left BBB is difficult unless marked ST-segment changes are present. Right BBB makes it difficult to detect transmural (affecting the entire thickness of the myocardium, spreading from the subendocardium to the epicardium) ischemia. The presence of a pacemaker and ACS symptoms may require urgent angiography to confirm the diagnosis and begin therapy. Some patients present without ST-segment changes initially, although awareness of hyperacute T-waves may be an early clue. If symptoms persist, ECG monitoring should be repeated with consideration of extension into leads V7-V9 (Ibanez et al., 2018).
The term AMI should only be used when there is evidence of myocardial injury, which is defined as “an elevation of cardiac troponin I (cTn) values. At least one value should be above the 99th percentile upper reference limit with necrosis in a clinical setting consistent with myocardial ischemia” (Ibanez et al., 2018, p.124). The 2017 ACC/AHA Measure Set recommends cTn measurement at initial presentation and three to six hours after symptom onset. Troponins are measured using a venous blood sample. They are very sensitive and specific to diagnose myocardial necrosis as they are components of the cell contractile apparatus between myosin and actin. Additional cTn levels (beyond six hours) are recommended in patients with normal initial cTn levels, but symptoms or ECG findings that are suspicious. Elevated levels of cTn can be detected two to four hours after an ischemic cardiac event and remain elevated for up to 14 days, but sensitivity is low in the first six hours of symptoms. Many emergency departments are equipped with point of care devices that can detect cardiac enzymes in blood within seconds (Jneid et al., 2017; Urden et al., 2018). Previously, cardiac enzyme panels included creatinine kinase (CK), creatinine kinase myocardial B fraction (CK-MB), and myoglobin. However, due to its superior sensitivity and specificity, cTn is the only biomarker that is recommended to be used for AMI diagnosis per the ESC and ACC. In the past, patients with normal CK-MB levels despite elevated cTn were diagnosed with unstable angina or minor myocardial injury. The current recommendations now classify patients with a sufficiently elevated cTn level (even a single level above the established cutoff) as NSTEMI. The other markers may still be used in some institutions to establish a diagnosis or monitor for additional tissue ischemia/necrosis over time. The initiation of treatment is not dependent on cardiac markers. Current ACC/AHA guidelines recommend that patients with ACS symptoms on presentation and ST-segment elevation on ECG should be treated immediately regardless of cTn results (Schrelber, 2018). Additional testing for patients with a suspected AMI includes a complete blood count (CBC), comprehensive metabolic panel (CMP), and a lipid profile (Zafari, 2019).
In cases of symptom relief after administration of nitroglycerin (Nitrostat, NitroMist, NTG), another 12-lead ECG should be obtained. Complete normalization of the ST-segment elevation along with symptom relief is suggestive of coronary spasms without associated MI (Ibanez et al., 2018). Please see Table 3 below for clinical recommendations on the diagnosis of AMI from the ESC.

Treatment Guidelines for AMI
Prehospital Care
A common acronym used to recall the appropriate initial treatment for AMI is MONA, which corresponds with morphine, oxygen, nitroglycerin (Nitrostat, NitroMist, NTG), and aspirin. Patients with ACS symptoms who are initially being managed by EMS should also receive intravenous access. Morphine is typically dosed at 2-4 mg and given intravenously. This can be repeated every 5-10 minutes until relief is achieved; the patient should be monitored for hypotension, vomiting, or respiratory depression. Supplemental oxygen should be administered if pulse oximetry readings indicate decreased oxygen saturation. Nitroglycerin (Nitrostat, NitroMist, NTG) works as a systemic vasodilator to reduce the venous return and subsequently, the workload of the heart; it can be given using sublingual tabs, 0.3-0.6 mg, or one to two sprays sublingually every five minutes with up to two repeat doses after the first. The patient should be monitored for hypotension and headache. The ACC/AHA (2017) performance measures recommend oral chewable non-enteric coated aspirin administration at the initial presentation, except for those patients with a significant hypersensitivity or gastrointestinal intolerance, who should be given a loading dose of clopidogrel (Plavix). See Table 8 (below) for additional details regarding the dosing and duration of aspirin for STEMI and NSTEMI patients according to the 2013 ACC/AHA recommendations. If an ECG can be obtained by EMS personnel indicating ST-segment elevation, the ESC goes so far as to recommend that fibrinolytics be given in the prehospital setting when possible to shorten the time to treatment; prehospital fibrinolysis is not common practice in the US. Alternatively, the ACC/AHA guidelines recommend that EMS squads should route AMI patients directly to PCI-capable hospitals (Ibanez et al., 2018; Jneid et al., 2017; Zafari, 2019).
Symptom Relief
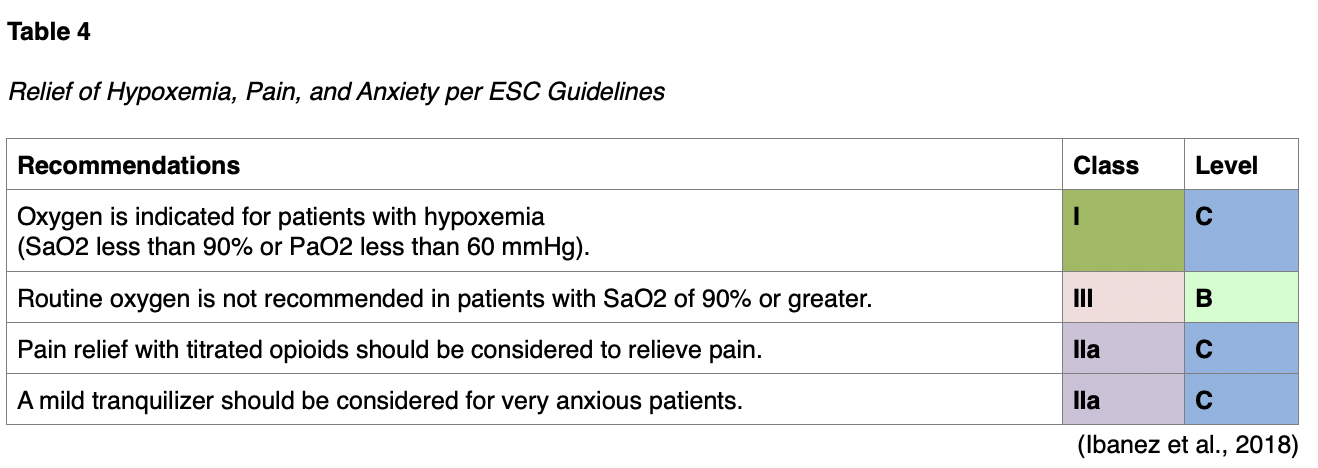
Symptomatic relief is of primary concern, including pain as well as breathlessness and anxiety. Pain is associated with sympathetic activation, which causes vasoconstriction and increases the workload of the heart. Table 4 (below) illustrates some of the recommended medications, such as morphine or other opioids. Of note, it is important to remember that morphine and oxygen can cause adverse effects and medication interactions and should therefore only be given when clinically relevant (Ibanez et al., 2018; Zafari, 2019)
Reperfusion Therapy
In patients with myocardial ischemia, reperfusion therapy should begin as soon as possible. If it can be completed within 12 hours of symptom onset and 120 minutes from STEMI diagnosis, primary percutaneous coronary intervention (PCI) is the preferred reperfusion strategy in patients with STEMI. Primary PCI is defined as being performed with a balloon, stent, or other approved device on the infarct-related artery without previous fibrinolytic therapy. Preferably, this should be done in a high-volume center. There is some level of controversy regarding the time delay to primary PCI versus opting instead for more immediate treatment with fibrinolytic therapy. The data is mixed, but the ACC/AHA recommends if the time from first medical contact to PCI device time is expected to be more than 120 minutes, then fibrinolytic therapy is recommended instead (unless contraindicated). The ideal time-lapse from first medical contact to PCI device time per the ACC/AHA is 90 minutes or less. Table 5 below highlights the recommendations for reperfusion therapy according to ESC time goals, while Figure 4 illustrates the difference in the ACC/AHA treatment guidelines for patients seen at PCI-capable facilities versus facilities that are not PCI-capable (Ibanez et al., 2018; Jneid et al., 2017).
The ESC makes a Class I recommendation for radial access (versus femoral) during primary PCI as well as placement of a new-generation drug-eluting stent versus balloon angioplasty (Ibanez et al., 2018). The ACC/AHA cautions to avoid the use of these newer stents in patients with an increased risk of bleeding, an anticipated invasive or surgical procedure, or that may struggle with compliance due to financial or social barriers. A bare-metal stent may be used instead (Zafari, 2019). An anticoagulant during the procedure as well as a P2Y12 (platelet) inhibitor, dosed before or at the time of primary PCI and continued for one year, is also a Class I recommendation of the ESC and ACC/AHA (Ibanez et al., 2018; Jneid et al., 2017). Unfractionated heparin should be given at a loading dose of 60 IU/kg and maintained for 48 hours or until PCI. Enoxaparin (Lovenox) can be given subcutaneously at a dose of 1 mg/kg every 12 hours for the duration of the hospital stay or until PCI. Enoxaparin (Lovenox) results in more efficient and predictable effects yet a slightly higher risk of bleeding. Bivalirudin (Angiomax) is another option, a direct thrombin inhibitor that may be used for PCI patients with similar efficacy to heparin (Zafari, 2019). For antiplatelet therapy, the ESC prefers ticagrelor (Brilinta) or prasugrel (Effient) over clopidogrel (Plavix) if available and indicated. The prognosis for right bundle branch block and ischemia is poor. In these instances, emergent coronary angiography and PCI should be considered when persistent ischemic symptoms occur in the presence of right bundle branch block. PCI should be done promptly in patients with ongoing ischemic symptoms and atypical ECG findings suggestive of an isolated posterior AMI or left main coronary artery occlusion (Ibanez et al., 2018). Cardiac rupture is the most significant lethal complication of PCI; although rare (less than 2%), this potential complication should be discussed with the patient/family prior to the procedure and included on consent forms (Zafari, 2019).
In NSTEMI patients, the ACC/AHA recommends an early (within 12-24h) invasive strategy including diagnostic angiography with intent to perform revascularization if indicated in stabilized patients with ACS without ST-segment elevation if there is an elevated risk for clinical events based on risk stratification score. This includes older patients, women with elevated troponin, patients with a prior history of coronary bypass grafting, as well as patients presenting with heart failure, refractory angina, or hemodynamic or electrical instability. This is not recommended in patients with hepatic failure, renal failure, pulmonary failure, cancer, or those patients with acute chest pain, a low likelihood of ACS, and normal troponin levels. If conservative treatment without PCI is elected, medical therapy (anticoagulants, antiplatelet agents, beta-blockers, statins, and possible angiotensin-converting enzyme inhibitor [ACEI]) is optimized and noninvasive cardiovascular imaging is recommended (Jneid et al., 2017; Zafari, 2019).


Coronary artery bypass grafting (CABG) may be indicated in patients with cardiogenic shock, high-risk anatomy, or in cases of failed PCI or mechanical complications of PCI (see below). It can also be considered in patients with left main disease if the patient's anatomy is not favorable for PCI, and their surgical risk is low (Zafari, 2019). CABG is a Class II recommendation from the ESC in cases of ongoing ischemia if PCI cannot be performed (Ibanez et al., 2018).
Fibrinolytic Therapy
Guidelines indicate that fibrinolytics should be administered as soon as possible in order to optimize effectiveness. If the fibrinolytic therapy is indicated, the goal per the ESC is to administer the bolus within 10 minutes of the diagnosis of STEMI, while the ACC/AHA recommends administration within 30 minutes of hospital arrival (Ibanez et al., 2018; Jneid et al., 2017).
The ACC/AHA guidelines recommend against the administration of fibrinolytic therapy to patients with ST-segment depression except when associated with ST-segment elevation in the aVR lead, and a true posterior AMI is suspected. Despite mixed data on the topic, the clinical consensus is that fibrinolysis should be attempted in STEMI patients with symptoms lasting longer than 12 hours when primary PCI is not feasible (Jneid et al., 2017). The Class I recommendations from the ESC include the use of a fibrin-specific plasminogen activator such as tenecteplase (TNK-tPA, TNKase), alteplase (tPA, Activase), or reteplase (rPA, Retavase) in combination with aspirin and clopidogrel (Plavix). Enoxaparin (Lovenox) is also recommended as the anticoagulant of choice (versus unfractionated heparin) until revascularization (if performed) or for at least 48 hours up to eight days following an AMI. Caution should be used with enoxaparin (Lovenox) use in patients over 75 or those with renal impairment. Bivalirudin (Angiomax) may be used in those with a history of heparin-induced thrombocytopenia. Following administration, patients should be transferred immediately to a PCI-capable facility, with routine early PCI recommended 2-24 hours after administration. The ESC define fibrinolysis as being unsuccessful if less than 50% of the ST-segment alteration is not resolved within 60-90 minutes of fibrinolysis administration and recommend rescue PCI in these patients. The ESC recommends emergency angiography and PCI in patients who develop heart failure, cardiogenic shock, recurrent ischemia, or artery reocclusion (Ibanez et al., 2018; Zafari, 2019).
Absolute contraindications to fibrinolysis include:
- Any prior intracranial hemorrhage
- Known structural cerebral vascular lesion
- Known intracranial neoplasm (primary or metastatic)
- Ischemic stroke within the past three months (except for acute stroke within 4.5 hours)
- Suspected aortic dissection
- Active bleeding or bleeding diathesis (excluding menses)
- Significant closed-head or facial trauma within three months
- Intracranial or intraspinal surgery within two months
- Severe uncontrolled hypertension (unresponsive to emergency therapy)
- For streptokinase (no longer marketed in the US): Prior treatment within the previous six months (Zafari, 2019).
Relative contraindications include:
- History of chronic, severe, poorly controlled hypertension
- Systolic pressure above 180 mm Hg or diastolic pressure above 110 mm Hg
- History of prior ischemic stroke more than three months ago
- Dementia
- Known intracranial pathology not covered in absolute contraindications
- Traumatic or prolonged CPR (longer than 10 minutes)
- Recent (within two to four weeks) internal bleeding
- Noncompressible vascular punctures
- Pregnancy
- Active peptic ulcer disease
- Current use of anticoagulants: the higher the INR, the higher the risk of bleeding
- For streptokinase (no longer marketed in the US): Prior exposure (more than 5 days previously) or prior allergic reaction to these agents (Zafari, 2019).
Cerebral hemorrhage, the most lethal complication of fibrinolytic therapy, is more common in patients of advanced age, lower weight, prior cerebrovascular disease, hypertension on admission, and female sex (Zafari, 2019).
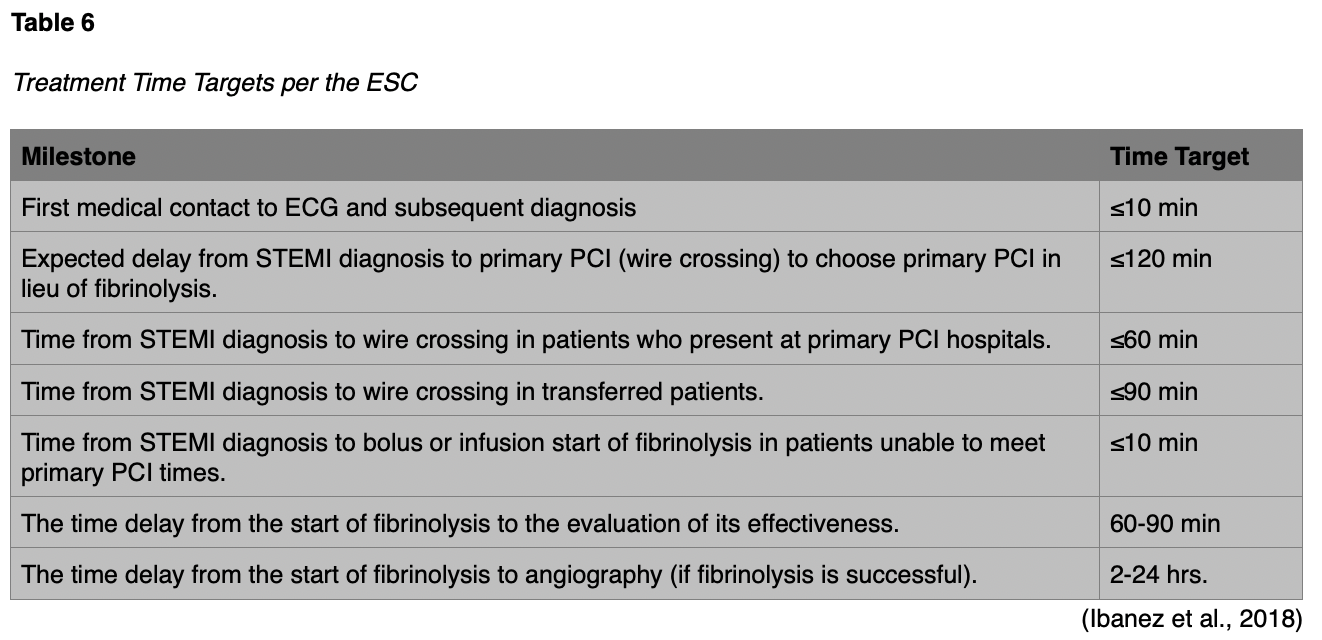
Treatment Time Targets
Specific time targets are necessary in order to set guideline suggestions for prompt treatment. Many studies by various national and international groups have come to a consensus on agreed-upon time frames. Please see Table 6 below for a listing of those targets.
Nursing Assessment and Monitoring
Nurses play a vital role in the management of an AMI. They must respond rapidly and efficiently to patients who are experiencing symptoms of acute MI to assess the patient's circulation, airway, and breathing. In addition, they must quickly work to assess the level of consciousness; administer sublingual nitroglycerin (Nitrostat, NitroMist, NTG) and aspirin, if indicated; obtain a 12-lead ECG, and notify the physician. The incidence of sudden death is very high during the first hour of an AMI, so it is essential to monitor the patient closely and be prepared for an emergency (Urden et al., 2018).
One of the most crucial responsibilities of the nurse when caring for a patient with a suspected AMI is a pain assessment. Chest pain can occur secondary to pulmonary edema, congestive heart failure, pericarditis, pneumothorax, and unstable angina. A systematic method for assessing chest pain should be utilized; be sure to ask about precipitating factors, quality, duration, location, and radiation.
MI-associated chest pain is often precipitated by activity but does not resolve with rest, is typically very intense, and may be described as pressure or burning. It often begins substernal/in the center of the chest and radiates to the left arm, neck, jaw, shoulder, or back. If the patient is stable, the nurse should perform a focused assessment, including:
- Review of the presenting symptoms/illness.
- Overview of general cardiac history (previous diagnoses, surgeries, interventions, diagnostic studies, medications/herbs/vitamins).
- Family history, specifically of CAD, hypertension, peripheral artery disease, stroke, or diabetes mellitus.
- Survey of lifestyle risk factors for CAD (Urden et al., 2018).
The patient’s vital signs should be monitored closely, with a target systolic blood pressure range of 100-140 mm Hg. All patients should be placed on continuous cardiac monitoring after the initial 12-lead ECG using a three or five lead system to monitor for indications of worsening or developing ischemia (Zafari, 2019).
When a patient experiences an AMI, healthcare professionals focus most of their attention on meeting the eminent physical needs of the patient. However, an AMI is an extremely stressful experience for the patient and family. The emotional stress can have a profound effect on physiological functions as well. During times of anxiety and apprehension, the sympathetic nervous system is activated (the "fight or flight" response). The heart rate increases, cardiac contractility becomes stronger, blood vessels constrict, and, initially, cardiac output increases. These responses, in turn, increase the myocardial oxygen demand in a compromised patient. As the heart demands more oxygen and the supply diminishes, the patient may experience more chest pain and other signs of hemodynamic instability. These changes then create more fear and anxiety in the patient. The healthcare team should attempt to make the environment less stressful. If possible, schedule lab tests, ECGs, x-rays, and other diagnostic tests to be done within the same time frame. Rest is an essential part of the recovery process and allowing for uninterrupted periods of rest and sleep is helpful. Reducing bright lights and noise is also important. During patient transfers between units, avoid having large groups at the bedside. One or two members of the healthcare team calmly and confidently admitting the patient usually helps to decrease patient and family anxiety. During the admission process, explain each procedure, treatment, and piece of equipment to the patient, offering reassurance that the patient is closely monitored (Urden et al., 2018).
Complications Associated with AMI
Cardiac Arrest/Ventricular Fibrillation (VF)
Many deaths occur early after a STEMI outside of the hospital setting. The primary lethal arrhythmia that occurs is ventricular fibrillation (VF). VF results in an ineffective quivering of the ventricles and no cardiac output. Treatment includes basic life support (circulation, airway, and breathing), defibrillation, and advanced cardiac life support. The sooner the VF is treated, the greater the chance of survival for the patient. All medical and paramedical personnel caring for patients with suspected AMI outside of the hospital should have access to defibrillation equipment and training in cardiac life support. Continuous cardiac monitoring should be implemented for all inpatients with known or suspected AMI after completing the initial 12 lead ECG (Ibanez et al., 2018).
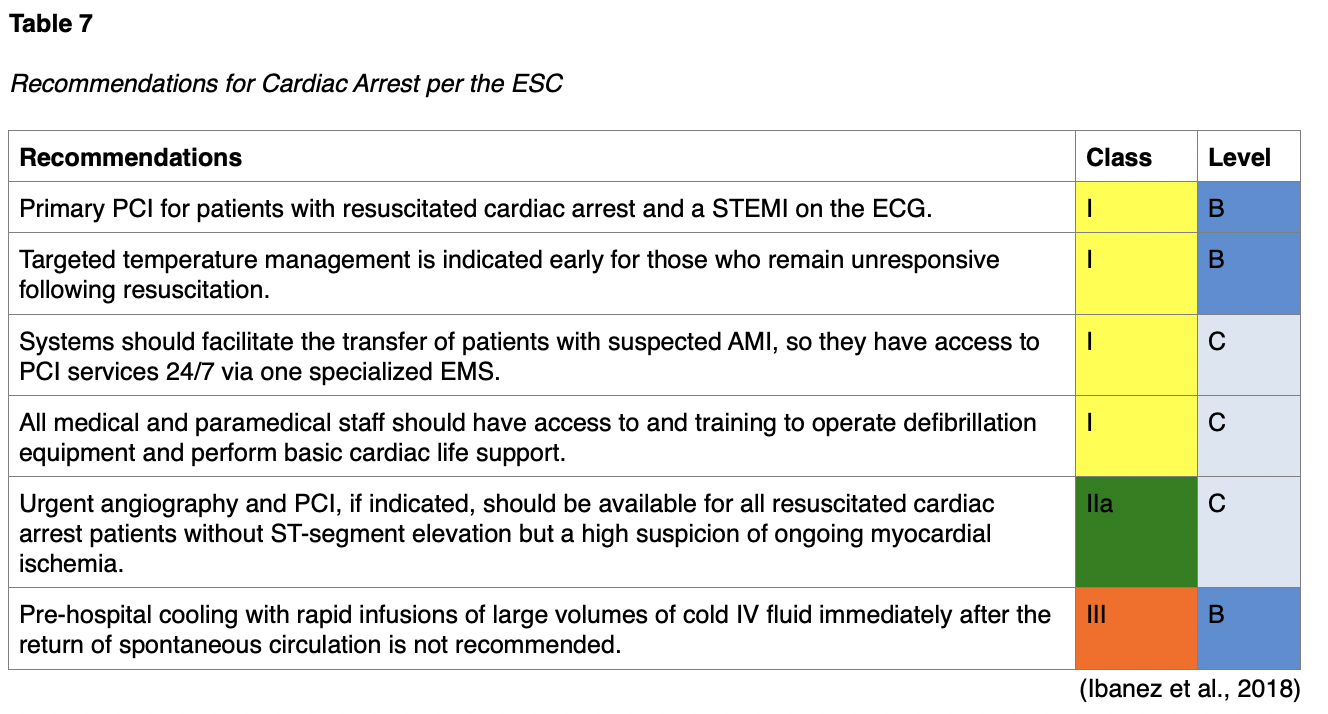
Unconscious patients admitted to critical care units after out-of-hospital cardiac arrests are at high risk for death, and neurologic deficits are common among those who survive. ESC recommendations for cardiac arrest are listed below in Table 7 and include guidelines for PCI, temperature control, and medical management in patients post-cardiac arrest (Ibanez et al., 2018). The ACC/AHA recommends immediate angiography (and PCI if indicated) in patients who are resuscitated after an out-of-hospital arrest if ECG shows STEMI, as well as initiating therapeutic hypothermia as soon as possible in comatose patients with a history of ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) (Jneid et al., 2017).
Following an AMI, prompt revascularization is recommended to correct any myocardial ischemia, as this is commonly the underlying cause of recurrent VF. Electrolyte imbalances (especially hypokalemia or hypomagnesemia) should be corrected. An intravenous beta-blocker may be ordered for polymorphic VF. Radiofrequency catheter ablation may also be considered for recurrent VF. Long term, an implantable cardioverter-defibrillator is typically recommended for patients that are at least six weeks out from their AMI with symptomatic heart failure (NYHA class II-III) and a left ventricular ejection fraction (LVEF) of 35% or less despite optimal medical therapy for at least three months. This or a wearable cardioverter defibrillator may be considered in certain high-risk patients less than 40 days out from their AMI (Ibanez et al., 2018).
Coronary Artery Reocclusion
A small number of patients will experience reocclusion of the artery after fibrinolytic therapy, even when preventative measures are taken. While the clot in the artery has been dissolved, the atherosclerotic plaque is still present; if anticoagulation is inadequate, another thrombus may form. Symptoms may include chest pain, nausea, diaphoresis, and ST-segment elevation, similar to those experienced with the original presentation. With this in mind, it is crucial to monitor the patient closely and be aware of changes indicative of reocclusion. Repeat administration of a fibrinolytic agent is not recommended. The ESC recommends emergency angiography and PCI (if indicated) in these patients (Class I/Level B). To prevent this emergency, ESC guidelines recommend transfer to a PCI-capable facility after fibrinolysis for routine early angiography with subsequent PCI if indicated 2-24 hours after fibrinolytic administration (Ibanez et al., 2018; Urden et al., 2018).
Heart Failure and Cardiogenic Shock
Congestive heart failure following an AMI can range from mild to severe, depending on the extent of ventricular damage. Heart failure occurs as a result of myocardial tissue damage and the subsequent decrease in the efficiency of the ventricle(s) as a pump. In right-sided failure, the compromised right ventricle causes fluid to back up into the peripheral circulation. In left-sided heart failure, fluid backs up into the pulmonary circulation. Signs of heart failure include shortness of breath; hypoxia; production of pink, frothy sputum; hypotension; oliguria; confusion or changes in the level of consciousness, and tachycardia (Urden et al., 2018).
Treatment of heart failure depends primarily on the severity. Typical management includes supplemental oxygen (if pulmonary edema and SaO2 is less than 90%). The ESC recommends starting an ACEI and beta-blocker as soon as hemodynamically stable, as well as a mineralocorticoid receptor antagonist (MRA) in those with an LVEF under 40% and no significant renal failure or hyperkalemia. A loop diuretic, a nitrate, morphine or similar opiate (to relieve dyspnea and anxiety), and/or inotropic agents to improve cardiac contractility may be considered in these patients if needed. These patients will be quite ill and may require transfer to the critical care unit. Mechanical ventilation and intubation may also be necessary if the patient develops hypoxemia or hypercapnia and becomes acidotic and/or exhausted, although non-invasive ventilation should be attempted first (Ibanez et al., 2018; Urden et al., 2018).
Patients with heart failure can rapidly decline into cardiogenic shock. Cardiogenic shock occurs when 40% or more of the myocardium has been affected by the infarction. Because the heart is incapable of contracting with sufficient force, the vital organs and peripheral tissues cease to function as a result of ischemia. The patient may experience pulmonary congestion, diaphoresis, cool extremities, and mental confusion. Treatment for cardiogenic shock is aggressive and can include fluid replacement or diuresis/ultrafiltration, inotropic/vasopressor agents, and an intra-aortic balloon pump in the case of mechanical complications; this is an invasive device used to decrease ventricular workload and improve coronary artery perfusion (Ibanez et al., 2018; Urden et al., 2018). Monitoring for these patients should include invasive blood pressure monitoring (arterial line) and blood gas assessment to determine if/when respiratory support is required; hemodynamic monitoring with a pulmonary artery catheter and doppler echocardiography may be considered. The ACC/AHA and ESC make a Class I recommendation for primary PCI therapy in patients with STEMI and cardiogenic shock or acute severe heart failure, regardless of time delay from symptom onset if the anatomy is suitable; CABG is recommended in those with unsuitable anatomy or in the case of failed PCI. Fibrinolytic therapy is recommended in those patients who are unsuitable for either CABG or PCI. After fibrinolysis, these patients should be transferred immediately to a PCI-capable hospital for coronary angiography irrespective of time delay (Ibanez et al., 2018; Jneid et al., 2017). Unfortunately, death occurs in about 85% of patients who develop cardiogenic shock. Therefore, nursing interventions should include assisting patients and families in working through the end of life issues (Urden et al., 2018).
Arrhythmias
Successful thrombolysis can cause a variety of cardiac arrhythmias, such as atrial fibrillation (AF), ventricular tachycardia (VT), premature ventricular contractions (PVCs), accelerated idioventricular rhythm, and sinus bradycardia (Urden et al., 2018). Of all AMI patients, 90% develop an arrhythmia, 25% in the first 24 hours. Most are self-limited and benign. The risk of VF and other serious arrhythmias is greatest in the first hour (Zafari, 2019). These are generally accepted as normal consequences of coronary reperfusion, and treatment is not necessary unless the patient becomes unstable (Urden et al., 2018). The use of antiarrhythmic medications in STEMI patients is difficult, as their evidence for benefit is limited, and they have been shown to increase the risk of early mortality. Prompt revascularization is recommended to correct any myocardial ischemia, as this is commonly the underlying cause (Ibanez et al., 2018)
Atrial Fibrillation (AF)
Up to 21% of STEMI patients are affected by AF, either new onset or pre-existing (known or unknown). If stable, no treatment other than anticoagulation may be necessary. Long term anticoagulation may be necessary based on the CHA2DS2-VASc score. Rate control can be achieved with the use of intravenous beta-blockers (if no acute heart failure or hypotension) or intravenous digoxin (Lanoxin, if acute heart failure and hypotension present), rhythm control with amiodarone (Pacerone, Cordarone, if acute heart failure present, but without hypotension). The use of ACEIs or angiotensin receptor blocker (ARBs) and statin therapy may also reduce the rate of new-onset AF after AMI. If unstable, cardioversion can be considered if adequate rate control is not achieved with pharmacological agents and in the presence of ongoing ischemia, hemodynamic compromise, or heart failure, but there is frequent early recurrence. Intravenous amiodarone (Pacerone, Cordarone) can also be used to promote electrical cardioversion or decrease the risk for early recurrence after cardioversion. Digoxin (Lanoxin) is not recommended for converting AF to sinus rhythm or rhythm control; calcium channel blockers and beta-blockers are also not recommended for converting AF to sinus rhythm (Ibanez et al., 2018).
Ventricular Tachycardia (VT)
Patients who have suffered an AMI may experience VT or v-tach. This ventricular arrhythmia can be benign or life-threatening. Patients may be asymptomatic, or they may experience shortness of breath, chest discomfort, palpitations, and syncope. Prompt revascularization is recommended to correct any myocardial ischemia, as this is commonly the underlying cause of recurrent VT. Electrolyte imbalances (especially hypokalemia or hypomagnesemia) should be corrected. Intravenous beta-blockers and/or amiodarone (Pacerone, Cordarone) is recommended for polymorphic VT (Ibanez et al., 2018). If the patient is unstable, electrical cardioversion may be conducted in an attempt to convert the myocardium to sinus rhythm. This arrhythmia is most common in patients who have experienced an anterior or anterolateral AMI (Urden et al., 2018). Intravenous amiodarone (Pacerone, Cordarone) may also be used for recurrent VT if repeated cardioversion is not successful. Patients may be given lidocaine (Xylocaine) if amiodarone (Pacerone, Cordarone) is contraindicated or to manage recurrent VT not responding to cardioversion, beta-blockers, amiodarone, and overdrive stimulation. If cardioversion is unsuccessful, transvenous catheter pace termination or overdrive pacing should be considered. Radiofrequency catheter ablation should also be considered for recurrent VT or VF. Long term, an implantable cardioverter-defibrillator is recommended for patients that are at least six weeks out from their AMI with symptomatic heart failure (NYHA class II-III) and an LVEF of 35% or less despite optimal medical therapy for at least three months. In certain high-risk patients, this or a wearable cardioverter defibrillator may be considered in patients less than 40 days out from their AMI (Ibanez et al., 2018).
Sinus Bradycardia and High Degree AV Block
Bradycardia is a slowing of the heart rhythm. Heart blocks occur as a result of problems in the atrioventricular (AV) node of the conduction system. Electrical impulses are not conducted from the atrium to the ventricles, which can cause a decrease in cardiac output (Urden et al., 2018). Second degree AV blocks are more common with inferior wall AMIs. The patient may experience hypotension and syncope. If symptomatic, intravenous epinephrine, vasopressin (Vasostrict), or atropine (AtroPen) is recommended (Class I) by the ESC (Ibanez et al., 2018). To correct the arrhythmia, patients may need transcutaneous (external) pacing or surgery to implant a pacemaker (Urden et al., 2018). Angiography with the potential for revascularization is recommended if reperfusion therapy has not already been done (Ibanez et al., 2018).
Mechanical Complications
Although rare, mechanical complications from AMI can be lethal and may occur in the first few days following STEMI. Primary PCI has reduced the rate of these complications, but nurses still need to be aware and watchful for indications. Patients may present with sudden onset hypotension, recurrence of chest pain, pulmonary congestion, a new murmur (indicates mitral regurgitation or ventricular septal defect), or jugular venous distension. If the symptoms listed above present, an immediate echocardiogram is warranted to rule out a mechanical complication such as free wall rupture, ventricular septal rupture, papillary muscle rupture, or aneurysm/pseudoaneurysm. Surgical repair (CABG) may be required. Aneurysms are also a potential complication of AMI. Females are more prone to an aneurysm, as well as patients with single-vessel disease, total occlusion of the left anterior descending artery, and those with no previous history of angina. Left ventricular aneurysms may present with signs/symptoms of heart failure, ventricular arrhythmias, or recurrent embolization (Ibanez et al., 2018).
Pericarditis
The nurse should also be watchful for pericarditis (early or late) or pericardial effusion following an AMI, as these are also potential complications. (Ibanez et al., 2018). Incidence has decreased with the use of PCI and thrombolysis but remains roughly 10%, and typically develops within the first 24-96 hours. It is due to inflammation of the pericardial tissue adjacent to the infarcted myocardium. Symptoms may include pleuritic chest pain and an audible pericardial friction rub (Zafari, 2019). The ACC/AHA recommends against the use of corticosteroids or nonsteroidal anti-inflammatory drugs for the treatment of pericarditis following AMIs secondary to increased risk of major adverse events (Jneid et al., 2017).
Left Ventricular Mural Thrombus
Occurring in 20-40% of post-AMI patients (and in up to 60% of anterior AMI patients treated with anticoagulants), a left ventricular mural thrombus (a blood clot on the wall of the left ventricle) can lead to systemic embolization. Anticoagulant therapy decreases the risk of this complication and is recommended (Zafari, 2019).
Post-AMI Care
The ACC/AHA (Jneid et al., 2017) and ESC (Ibanez et al., 2018) guidelines contain information regarding the care of patients following an AMI. All AMI patients with atherosclerotic cardiovascular disease under the age of 75 should be placed on a high-intensity statin indefinitely if they are able to tolerate it. A moderate-intensity statin may be used if the patient is unable to tolerate the higher intensity dose, and patients over the age of 75 should be considered on a case-by-case basis, based on individual risks and benefits of treatment. The ACC/AHA and ESC make the following suggestions regarding ongoing care and secondary prevention in STEMI patients:
- LVEF should be assessed in all STEMI patients prior to discharge.
- Noninvasive stress testing is recommended prior to discharge in all STEMI patients who did not undergo angiography and are not considered high-risk.
- Daily aspirin should be prescribed to all AMI patients indefinitely after successful PCI.
- Daily aspirin should be prescribed indefinitely after successful fibrinolysis, along with clopidogrel 75 mg daily (dual antiplatelet therapy [DAPT]) for at least two weeks and up to one year.
- A beta-blocker should be prescribed during and after hospitalization, if not contraindicated. Preferably, metoprolol (Toprol), carvedilol (Coreg), or bisoprolol (Zebeta) should be used, as these have been shown to reduce the mortality risk in patients with heart failure.
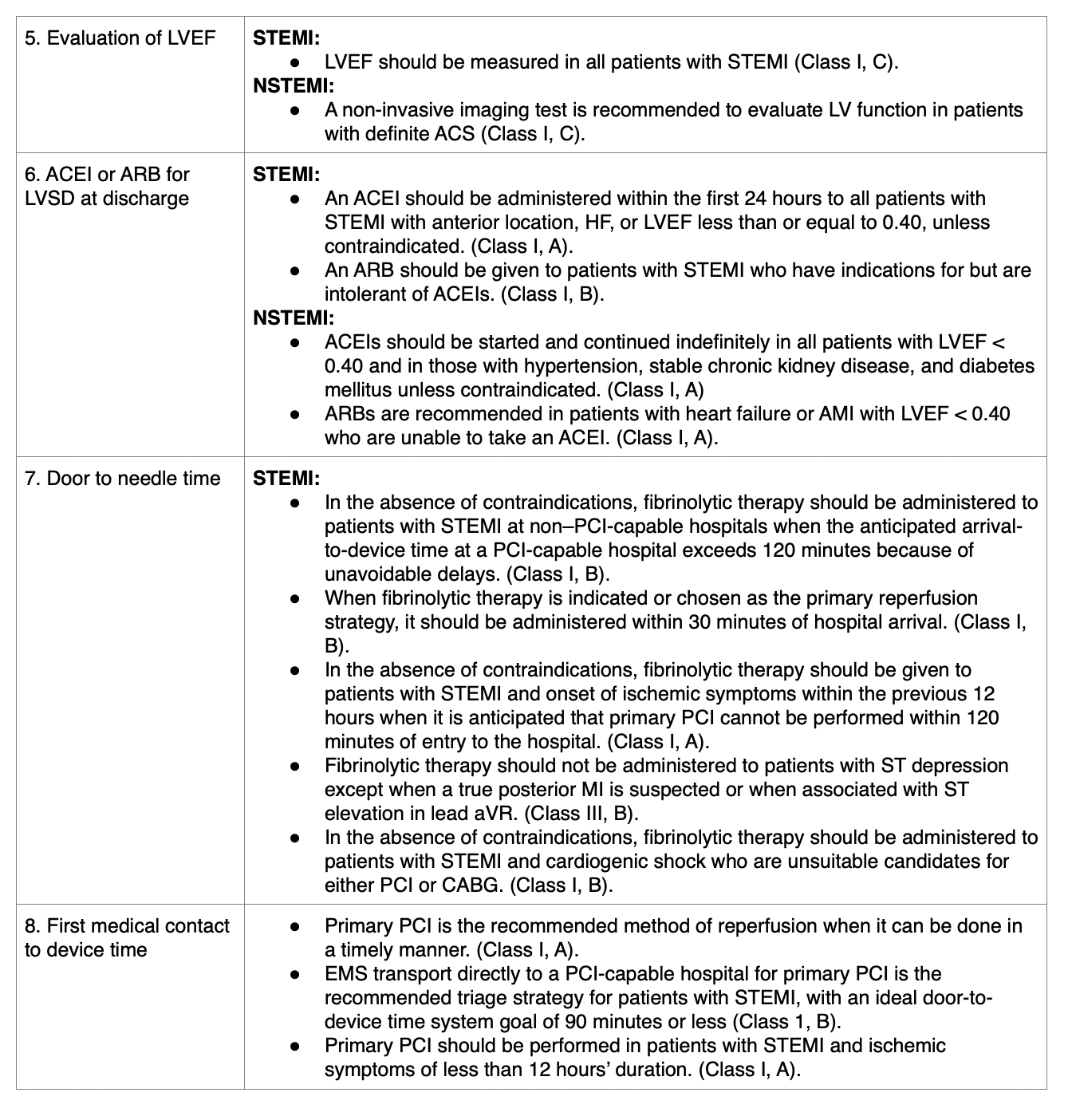
- An ACEI should be started within 24 hours in all STEMI patients with: an anterior AMI, heart failure, or LVEF less than 40%. An ARB may be given if the patient is intolerant.
- DAPT with a P2Y12 inhibitor, such as clopidogrel (Plavix), prasugrel (Effient), or ticagrelor (Brilinta), should be prescribed for one year to all patients with a stent placed during PCI unless contraindicated (Ibanez et al., 2018; Jneid et al., 2017; Zafari, 2019).
Recommendations for NSTEMI patients include:
- Noninvasive imaging is recommended to evaluate LVEF in all NSTEMI patients prior to discharge.
- Noninvasive stress testing is recommended prior to discharge in low- to intermediate-risk NSTEMI patients free of ischemia.
- Daily aspirin should be prescribed to all AMI patients indefinitely after successful PCI.
- Beta-blocker therapy in those NSTEMI patients with stabilized heart failure and reduced systolic function if not contraindicated. Preferably, metoprolol (Toprol), carvedilol (Coreg), or bisoprolol (Zebeta) should be used, as these have been shown to reduce the mortality risk in patients with heart failure.
- An ACEI should be continued indefinitely in those NSTEMI patients with LVEF less than 40%, hypertension, diabetes mellitus, chronic kidney disease. An ARB is recommended in those with heart failure or ACEI intolerance.
- DAPT with a P2Y12 inhibitor, such as clopidogrel (Plavix), prasugrel (Effient), or ticagrelor (Brilinta) should be prescribed for one year to all patients with NSTE-ACS without contraindications who are treated with either an early invasive or ischemia-guided strategy (Ibanez et al., 2018; Jneid et al., 2017; Zafari, 2019).
Refer to Table 8 for additional details regarding ACC/AHA performance measures for STEMI/NSTEMI patients.
In 2004, the ACC/AHA published a Class I recommendation for the prescription of formal cardiac rehabilitation for all patients with recent ACS or NSTEMI, recent revascularization, unstable angina, s/p CABG, or heart failure with reduced LVEF (Jneid et al., 2017). Aerobic training within cardiac rehabilitation should be included, with 30 minutes of exercise at a frequency of three or more times per week. Lifestyle modifications, including the adoption of a low-fat and low-salt diet, smoking cessation, maintaining vaccinations, and increasing physical activity, have all been shown to reduce the risk of recurrent AMI (Zafari, 2019). In 2019, the ACC/AHA published the following ten key messages regarding the primary prevention of cardiovascular disease, which may be useful for patient discharge planning and education:
- A healthy lifestyle over a lifetime is the most important way to prevent atherosclerotic vascular disease, heart failure, and atrial fibrillation.
- A team-based care approach is an effective strategy for CVD prevention. Clinicians should evaluate the social determinants of health that affect individuals to inform treatment decisions.
- Adults aged 40-75 years being evaluated for CVD prevention should undergo 10-year atherosclerotic CVD (ASCVD) risk estimation and have a clinician-patient risk discussion before being started on pharmacotherapy (e.g., antihypertensive therapy, a statin, or aspirin). The presence or absence of additional risk factors and/or the use of coronary artery calcium (CAC) scanning can help guide decisions about preventive interventions in select individuals.
- All adults should consume a healthy diet that emphasizes consumption of vegetables, fruits, nuts, whole grains, lean vegetable or animal protein, and fish, and minimizes the intake of trans fats, processed meats, refined carbohydrates, and sweetened beverages. In the setting of overweight and obesity, counseling and caloric restriction are recommended to achieve and maintain weight loss.
- Adults, including those with type 2 diabetes mellitus (T2DM), should engage in at least 150 minutes per week of accumulated moderate-intensity physical activity or 75 minutes per week of vigorous-intensity physical activity.
- For adults with T2DM, lifestyle changes (e.g., improving dietary habits, achieving exercise recommendations) are crucial. If medication is indicated, metformin is first-line therapy, followed by consideration of a sodium-glucose cotransporter 2 inhibitor (SGLT2) or a glucagon-like peptide-1 receptor agonist (GLP-1).
- At every healthcare visit, assess all adults for tobacco use. Assist tobacco users and strongly advise them to quit.
- Aspirin should be used infrequently in the routine primary prevention of ASCVD because of a lack of net benefit.
- Statin therapy is the first-line treatment for the primary prevention of ASCVD in patients with elevated low-density lipoprotein cholesterol (LDL-C) levels (≥190 mg/dL), those with diabetes mellitus who are aged 40-75 years, and those determined to be at sufficient ASCVD risk after a clinician-patient risk discussion.
- Nonpharmacological interventions are recommended for all adults with elevated blood pressure or hypertension. When pharmacologic therapy is required, target the blood pressure to generally be below 130/80 mmHg (Arnett et al., 2019).
Patient and Family Education/Support for AMI
Nurses play an important role in educating the patient and family. Initial information should be simple and concise and focus on what to expect. It may also be necessary to educate the patient and family about thrombolytic agents and PCI so that informed decisions can be made. Nurses need to remember to use familiar terms when describing medications and procedures. For example, the nurse may want to describe the thrombolytic agent as a medicine used to dissolve clots in the arteries of the heart. Nurses should also explain that these drugs may cause bleeding complications. Most importantly, nurses should offer emotional support and attempt to relieve anxiety. It is appropriate in most cases to begin a more detailed education once the patient's condition has stabilized when the fear and anxiety have subsided. This is a good time to begin explaining what happened. It may be helpful to use pictures and diagrams of the heart and provide educational information that the patient can take home and review again at a later time (Urden et al., 2018).
The nurse should take into account the patient's learning style, learning challenges, and home language. Give information in a way that will be most beneficial to the patient. Provide written materials in the patient's native language and use a translation service or professional translator if needed. To address individual learning needs, nurses can offer the patient multiple choices, such as a booklet or a 15-minute video. Always remain open to queries and don't give the patient and family a sense that they are asking unimportant questions (Urden et al., 2018).
The next step in the patient education process usually occurs once the patient transfers from an intensive care unit to a monitored floor or intermediate care area. Patients and families may be less anxious at this point and may begin making plans for discharge. Patients may also begin to ask questions about lifestyle changes. Keep in mind that each individual may have different ideas about what caused the AMI. One patient may attribute it to smoking while another patient may think it was brought on by workplace stress. The nurse should discuss the patient's perceptions and talk about making lifestyle changes specific to these perceptions, keeping in mind that patients will usually have more motivation to change the things that are most meaningful to them (Urden et al., 2018).
Discharge teaching is an essential part of nursing care and helps prevent readmissions. The nurse should give the patient verbal and written instructions about medications, smoking cessation, exercise and daily activities, ability to return to work, and dietary changes. Most patients will also be discharged with medication prescriptions, including anticoagulants, beta-blockers, ACEIs, and a lipid-lowering drug. It is helpful to provide verbal and written instructions about the medications. It is also valuable to assess if the patient has the necessary resources to acquire medications. If not, a social worker or case manager may be able to assist the patient. The patient and family also should be given specific instructions regarding what to do if chest pain reoccurs (Urden et al., 2018).


Reference List
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., Himmelfarb, C. D., Khera, A., Lloyd-Jones, D., McEvoy, J. W., Michos, E. D., Miedema, M. D., Muñoz, D., Smith Jr, S.C., Virani, S. S., Williams Sr, K. A., Yeboah, J., & Ziaeian, B. (2019). ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140, E596-646. https://doi.org/10.1161/CIR.0000000000000678
Centers for Disease Control and Prevention. (2017). Heart attack facts and statistics. https://www.cdc.gov/heartdisease/heart_attack.htm.
Displaced. (2008). 12-lead EKG ST-elevation tracing color-coded [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:12_Lead_EKG_ST_Elevation_tracing_color_coded.jpg
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., Caforio, A. L. P., Crea, F., Goudevenos, J. A., Halvorsen, S., Hindricks, G., Kastrati, A., Lenzen, M. J., Prescott, E., Roffi, M., Valginigli, M., Varenhorst, C., Vranckx, P., & Widimský, P. (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 39(2), 119-177. https://doi.org/10.1093/eurheartj/ehx393.
Jneid, H., Addison, D., Bhatt, D. L., Fonarow, G. C., Gokak, S., Grady, K. L., Green, L. A., Heidenreich, P. A., Ho, P. M., Jurgens, C. Y., King, M. L., Kumbhani, D. J., & Pancholy, S. (2017). AHA/ACC clinical performance and quality measures for adults with ST-elevation and non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circulation: Cardiovascular Quality and Outcomes, 10, e000032. https://doi.org/10.1161/HCQ.0000000000000032.
OpenStax College (2013). Heart wall [image]. https://commons.wikimedia.org/wiki/File:2004_Heart_Wall.jpg
Schrelber, D. (2018). Cardiac markers. Medscape. https://emedicine.medscape.com/article/811905-overview
Urden, L., Stacy, K., & Lough, M. (2018). Cardiovascular alterations. In Critical care nursing: Diagnosis and management, (8th ed., pp. 184-199). Elsevier.
Zafari, A. M. (2019). Myocardial infarction. Medscape. https://emedicine.medscape.com/article/155919-overview