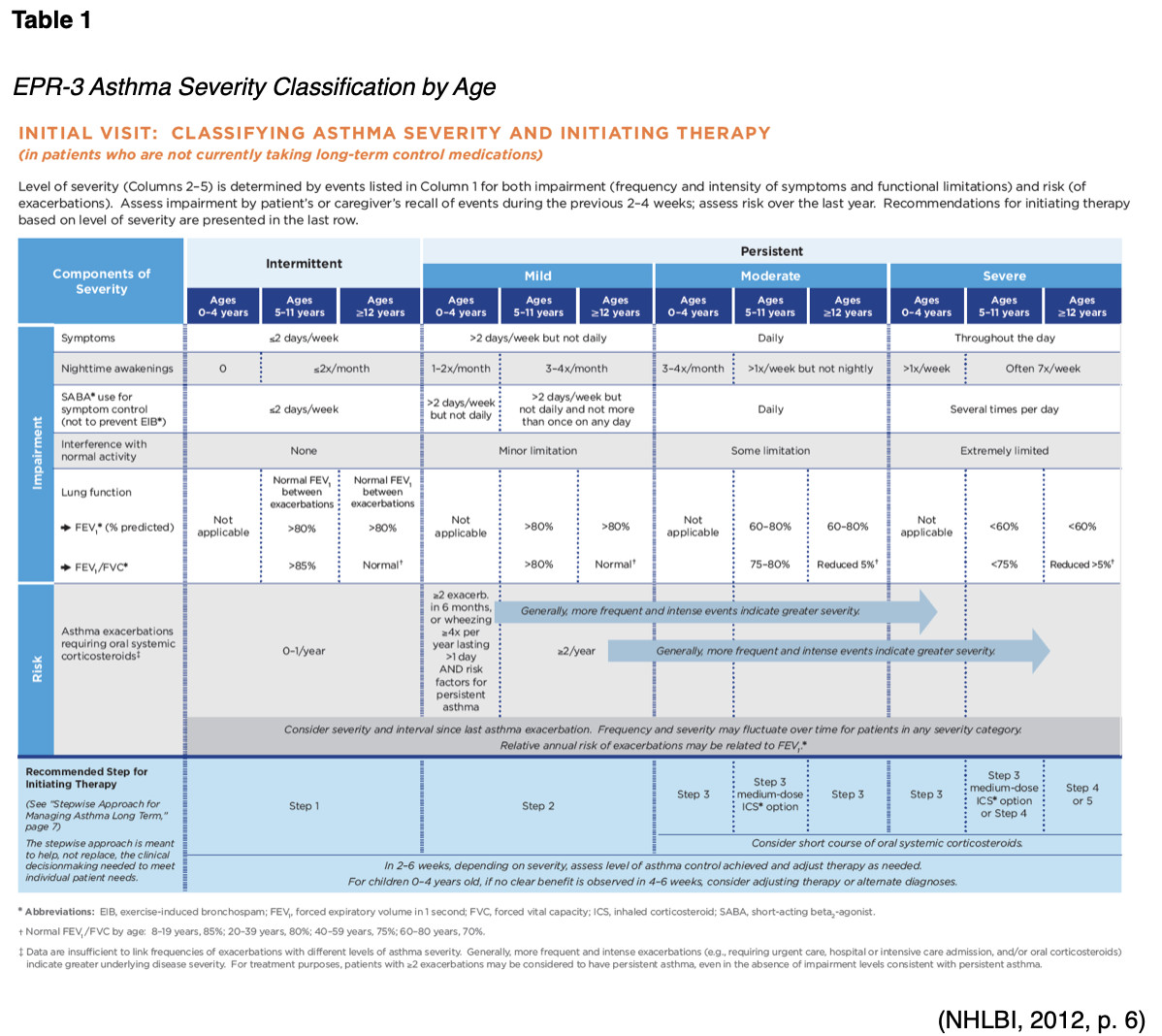
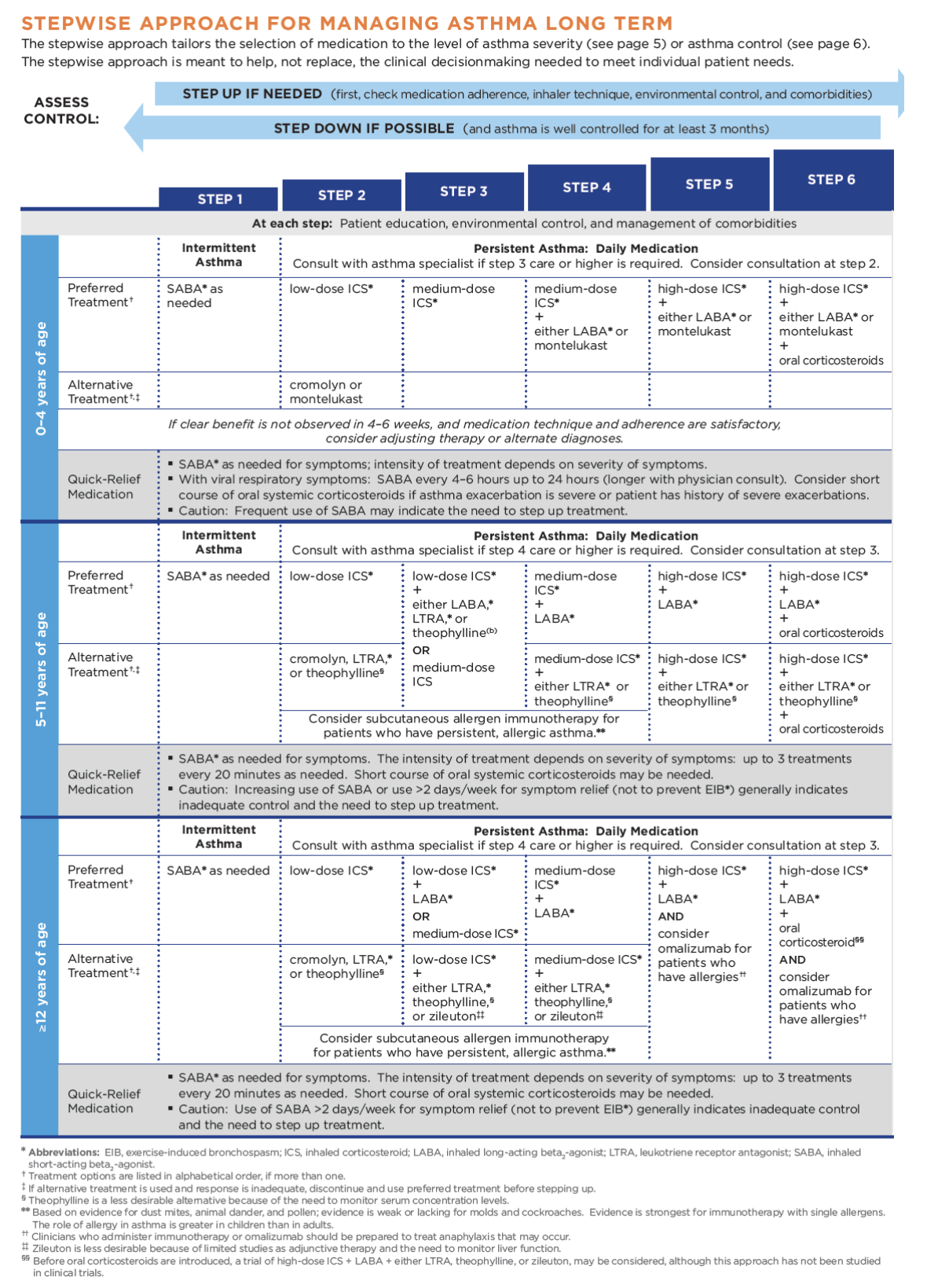
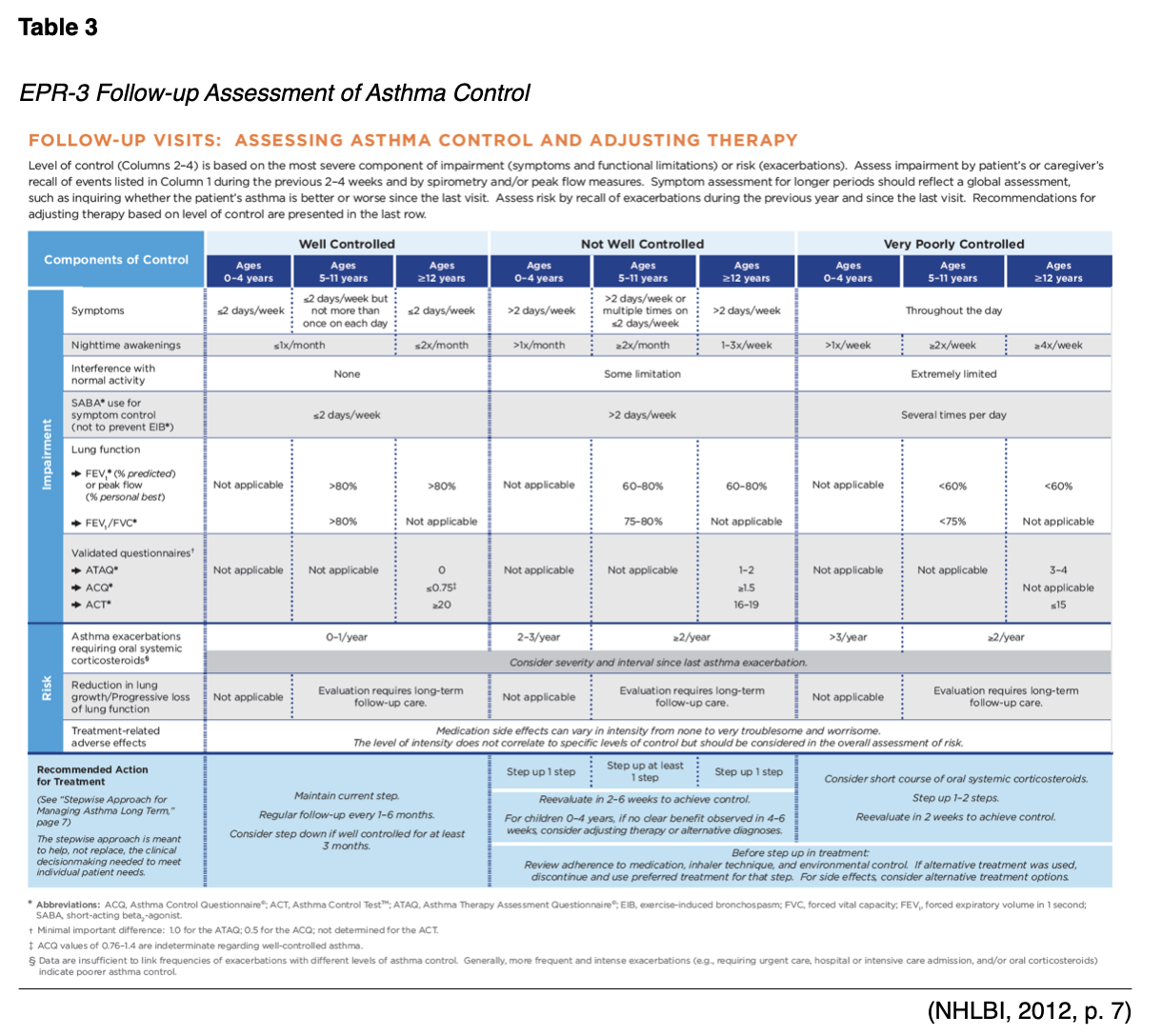
The purpose of this activity is to ensure that all APRNs within primary care and emergency medicine fields are aware of and able to implement the most up-to-date information regarding the assessment and pharmacological management of asthma into their practice.
...purchase below to continue the course
ms and/or use of reliever medication more than twice per week, nighttime awakenings, and any activity limitation in the last four weeks (GINA, 2018). Other asthma symptom control tools that have been well-validated and tested include the following:
- Royal College of Physicians' Three Question Tool- a categorical tool that asks about activity limitation, daytime/daily symptoms, and difficulty sleeping in the last 30 days.
- Asthma Control Questionnaire (ACQ)- a numerical self-assessment of morning symptoms, limitation in activity, nighttime awakenings, SOB, wheezing, use of reliever medication (in ACQ-6), and pre-bronchodilator FEV1 (in ACQ-7); final score ranges 0-6, <0.75 is well controlled, 0.75-1.5 yellow/grey zone, >1.5 is poorly controlled; may be used in adults and pediatrics.
- Asthma Control Test (ACT)- a numerical test that includes patient report of level of control, limitation in activity, nighttime awakenings, SOB, and use of reliever medication; scores range from 5-25, 20-25 is well controlled, 16-19 not well controlled, <16 very poor control; childhood ACT (c-ACT) is available.
- Asthma Control Scoring System- a numerical tool that includes daytime/daily symptoms, limitation in activity, nighttime awakenings, use of reliever medication, PEF% predicted, FEV1% predicted, change in PEF% predicted, and sputum eosinophilia (optional).
- Primary Care Asthma Control Screening Tool (PACS)- five simple yes/no questions regarding symptoms occurring more than once weekly for the last month.
- 30-second Asthma Test- a self-report of symptoms based on five yes/no questions.
- Test for Respiratory and Asthma Control in Kids (TRACK) - for use in pediatrics, includes a recent history of exacerbations.
- Composite Asthma Severity Index (CASI)- for use in pediatrics, includes a recent history of exacerbations (Bostantzoglou et al., 2015; GINA, 2018; NHLBI, 2012).
Controller Medications
GINA (2018) guidelines also include a stepwise treatment algorithm that is very similar to EPR-3's. The primary difference between the two is that GINA's algorithm includes just five steps and lacks the age-group breakdown (their algorithm is applicable to all patients age six and up), although they do specify dosage differences based on age (see Table 4 below). Both groups recommend stepping down treatment after three months of good control or stepping up treatment in the case of continued symptoms/exacerbations. They both point out the importance of confirming correct inhaler technique and medication adherence, as well as treating comorbidities such as GERD, obesity, or smoking prior to stepping up treatment, as all of these conditions may worsen or mask asthma symptoms and lead to inappropriate treatment (GINA, 2018; NHLBI, 2012).

ICS is the primary controller medication used in both algorithms for persistent asthma. They function by reducing bronchial inflammation, preventing exacerbations, and often relieve cough (Bostantzoglou et al., 2015). They were first developed in 1973 due to the significant side effects seen with systemic steroids (de Groot et al., 2015). They are traditionally dosed daily or BID regardless of symptoms. Thrush is a common side effect of ICS, but the risk of thrush can be reduced significantly with diligent oral hygiene after medication administration and the use of a spacer or chamber (see Figure 1 below), which also helps with medication delivery when using a metered-dose inhaler (MDI) (NHLBI, n.d.). In older patients, ICS may also increase the rate of bone mineral loss and cause skin thinning, bruising, and adrenal suppression (Bostantzoglou et al., 2015). ICS has also been shown to slow growth rates in children by an average of 1 cm, although this is both non-progressive and not entirely predictable (NHLBI, 2012). GINA guidelines recommend a lower dose of ICS earlier, as opposed to a higher dose, with greater side effects, later. They indicate that an increase in FEV1 should be seen within days of starting ICS and usually plateaus in roughly two months, while PEF readings usually increase to PB level after about two weeks of treatment, with variability diminishing after three months of treatment. They also list nasal spray corticosteroid as an alternative in patients with allergic rhinitis and a proven allergy in lieu of ICS (GINA, 2018). Eosinophilic asthmatics typically require a higher dose of ICS for inflammation management, regardless of what may seem like acceptable symptom control (Bostantzoglou et al., 2015). In some patients, because inflammation can affect the entire respiratory tract, ICS may be insufficient. Systemic steroids carry with them significantly greater adverse effects (de Groot et al., 2015). Dunican and Fahy (2017) discuss an area of research that is potentially upcoming, which involves the development of a six gene expression biomarker signature to predict steroid responsiveness in asthma patients. This biomarker is able to select patients with airway inflammation secondary to T-helper 2 cells that respond better to corticosteroid treatment with more accuracy than sputum or blood eosinophil counts (Dunican & Fahy, 2017).

Intermittent ICS dosing is defined as varying in dose, frequency, or duration of administration, such as initiating a temporary course of ICS or temporarily increasing the dose. An executive summary in preparation for the upcoming update to EPR-3 regarding intermittent ICS use and LAMA use was published by Sobieraj et al. (2017) regarding the efficacy of intermittent ICS use by age group. They reviewed 54 randomized controlled trials and two observational studies as part of their process. In patients aged four and under with recurrent wheezing, they found intermittent ICS use with SABA (vs. SABA alone) reduced the risk of exacerbation requiring oral steroids based on moderate strength of evidence and improved QOL based on low strength of evidence. In these same patients, intermittent ICS was found to reduce the risk of exacerbation requiring oral steroids, hospitalization, or rescue medication use versus regularly scheduled ICS controller use. There was insufficient evidence regarding intermittent ICS use versus non-pharmacological therapy or no therapy in this age group. In patients age 5-11 with persistent asthma, they found intermittent ICS use did not affect QOL or rescue medication use versus ICS controller use based on low strength of evidence, and there was insufficient evidence to assess the effect on the other outcomes in this age group. In patients with persistent asthma aged 12 and older, they found that the use of intermittent ICS dosing, either alone or with ICS controller dosing, versus ICS controller dosing alone, was found not to affect the risk of exacerbation based on low strength of evidence. However, the use of intermittent and controller ICS versus controller dosing alone was found to decrease the number of asthma-related outpatient visits based on low strength of evidence (Sobieraj et al., 2017).
Typically, the second controller medication introduced in the third step of both algorithms discussed above is a LABA such as salmeterol (Serevent, Advair), vilanterol (Breo Ellipta) or formoterol (Perforomist, Symbicort, Dulera). These work as bronchodilators by relaxing the smooth muscles that surround the airway by selectively stimulating ß-2 adrenergic receptors. Vilanterol’s (Breo Ellipta) half-life is 16-21 hours (allowing once-daily dosing) with an onset of about 10 minutes, formoterol's (Perforomist, Symbicort, Dulera) half-life is 10 hours with an onset of fewer than five minutes, and salmeterol's (Serevent, Advair) half-life is just 5.5 hours with an onset of about 15 minutes. They should not be used as monotherapy, but always in conjunction with ICS, and (other than formoterol) not for acute symptom relief. EPR-3 recommends a maximum daily dose of 100 mcg of salmeterol (Serevent, Advair) or 24 mcg of formoterol (Perforomist, Symbicort, Dulera). The effect of adding LABA to low-dose ICS in adult asthma patients who are not well-controlled with low-dose ICS alone results in better control than doubling the ICS dose, but the use of LABA alone has been shown to increase the risk of exacerbation and is not recommended. They work especially well in patients with a lot of wheezing, SOB, and nocturnal symptoms or those patients found to be in the non-eosinophilic clinical phenotype characterized by severe symptoms but minimal inflammation (Bostantzoglou et al., 2015; Lynn & Kushto-Reese, 2015; NHLBI, 2012).
GINA guidelines recommend increasing to moderate ICS doses in patients age 5-11 in the third step, but in adults suggests adding a LABA to low-dose ICS. ICS/LABA, as the initial maintenance controller treatment, has been shown to reduce symptoms and improve lung function but does not reduce the risk of exacerbation and is more expensive than ICS alone (GINA, 2018). In 2016, the New England Journal of Medicine (NEJM) published two single safety studies conducted by GlaxoSmithKline (GSK), and AstraZeneca mandated by the FDA secondary to safety concerns (increased risk of mortality) that arose after LABAs began to be used on a widespread basis. The first, by GSK, compared a combination of fluticasone/salmeterol (Advair) with fluticasone (Flovent) alone. Over 11,000 patients over the age of 11 were studied. They found a total of 74 serious asthma events (hospitalization or intubation), with 36 in the combination group and 38 in the control group. There were no deaths in this study. The risk of a severe exacerbation was 21% lower in the combination group (Stempel et al., 2016). The second, by AstraZeneca, was a comparison of budesonide/formoterol (Symbicort) versus budesonide (Pulmicort) alone. Based on over 11,000 patients over the age of 11, the study found no increased risk of death or serious event with the use of ICS plus LABA. Patients with a known history of previous life-threatening asthma event were notably excluded from this study (more than four exacerbations or two hospitalizations in the last year, more than 10-year pack history of smoking, history of unstable asthma in the last seven days, previous history of intubation or hypercapnia requiring non-invasive ventilatory support). There were 43 patients with serious events in the combination group and 40 in the control group. Of note, this study did report two deaths in the combination group. The results showed an over 16% decrease in the risk of exacerbation in the combination group versus the control group (Peters et al., 2016).
In 2018, the NEJM published a compilation study regarding the safety of LABA based on the four large prospective randomized controlled clinical trials mandated by the FDA. These trials compared ICS with a combination of ICS and LABA in over 36,000 patients. This included the two trials above, as well as Merck's trial regarding mometasone/formoterol (Dulera) and a similar trial by Novartis (who terminated their study early after they discontinued their inhaled formoterol product Foradil in 2015, not for safety reasons). The combination of LABA and ICS was not found to increase the risk of hospitalization, intubation, or death, but did show a decreased relative risk for exacerbation, similar to the individual studies' findings. Unfortunately, this decrease in risk was not universal as it was not seen as strongly in adolescents, African Americans, or Asian Americans. Despite the lack of clear evidence that this combination is dangerous, there remains a Black Box warning on all ICS-LABA combination products that patients should be made aware of (Busse et al., 2018). Weiler et al. (2016) stated in the practice parameter published regarding the treatment of patients with exercise-induced bronchospasms (EIB) that while quick-onset SABA or LABA medications are the most effective, tolerance was common if used daily.
In the GINA treatment guidelines (2018), low-dose ICS-formoterol (Symbicort, Dulera) is listed below steps 3-5 as an option for reliever medication. This method, they report, has been shown to reduce exacerbations and hospitalizations in patients over the age of 11 (and likewise in patients 4-11, but they remark that this practice is not approved for this age group in many countries) (GINA, 2018). This newer treatment concept that has emerged recently in asthma is termed single maintenance and reliever therapy (SMART) (Sobieraj et al., 2018b). It is also referred to as maintenance and reliever therapy (MART), dynamic dosing, or adjustable maintenance dosing (AMD) (Dinakar et al., 2014; Tang et al., 2018). The basic concept is as follows: the traditional instructions to asthma patients involve one or two inhalers for daily control/maintenance, and separate reliever medication in another inhaler to be used PRN. Alternatively, the SMART method instructs patients to utilize one inhaler, a combination of ICS and formoterol, which is a rapid-onset LABA, for both maintenance and PRN symptom control. Dinakar et al. (2014) discussed this concept as a recommended strategy in adult patients with mild to moderate asthma to both avoid and exit from a state of increased asthma symptoms, termed the "yellow zone". They recommended the use of a single inhaler, containing both ICS and a LABA, as they found this to be the most convenient for the patient as well as the most well-studied. They did not believe this would be advisable in children or severe asthmatics. They also pointed out that this method of dosing is currently considered off-label in the US (Dinakar et al., 2014). Sobieraj et al. (2017) discussed this strategy within the executive summary regarding intermittent ICS use and LAMA use. They found in patients age 5-11 with persistent asthma, the use of SMART versus ICS and LABA as controller medication versus a higher ICS controller dose reduced the risk of exacerbation based on low strength of evidence. In patients with persistent asthma over the age of 11, when compared with regularly-scheduled ICS controller dosing, they found that the use of SMART reduced the risk of exacerbation and improved spirometry results based on moderate strength of evidence, as well as reducing the use of rescue medication based on low strength of evidence. When SMART was compared with ICS controller medication at a higher dose, there was still a reduced risk of exacerbation based on low strength of evidence in the SMART group. When SMART was compared with ICS plus LABA as controller medication, they found a reduced risk of exacerbation (based on high strength of evidence), improved asthma control scores (based on moderate strength of evidence), and reduced use of reliever medication (based on low strength of evidence). When SMART was compared with ICS plus LABA as controller medication at a higher ICS dose, they again found a reduced risk of exacerbations in the SMART group based on high strength of evidence. Finally, when they compared the outcomes of patients using SMART versus the conventional best practices of ICS as a controller medication with or without LABA, they found a reduced risk of exacerbation, reduced use of reliever medication, and improved asthma control scores in the SMART group all based on moderate strength of evidence (Sobieraj et al., 2017). The SYGMA studies, published in 2018 in the NEJM, were conducted by AstraZeneca to look at SMART treatment results utilizing their Symbicort inhaler for an entire year. O’Byrne et al. (2018) looked at the PRN use of budesonide-formoterol (Symbicort) in 3,836 patients older than 11 with mild asthma. They compared:
- SMART Group: PRN budesonide-formoterol (Symbicort) and BID placebo
- Control Group 1: PRN terbutaline (not currently available as an inhaler in the US) and BID placebo
- Control Group 2: PRN terbutaline and BID budesonide (Pulmicort)
The results indicated that the SMART Group had an improved number of "well-controlled asthma weeks", and a reduced number of exacerbations as compared to Control Group 1. The SMART Group had a similar number of exacerbations compared to Control Group 2 and was found to be non-inferior based on ACQ-5 scores and FEV1 but with a significant 17% decrease in daily steroid dosage (O'Byrne et al., 2018). The second SYGMA study again reviewed 4,176 patients over the age of 11 with diagnosed mild asthma. They compared PRN budesonide-formoterol (Symbicort) and BID placebo (SMART group) with PRN terbutaline (not currently available as an inhaler in the US) and BID budesonide (Pulmicort) (control group). They found no significant difference in the annual severe asthma exacerbation rate between the groups. They did find that the SMART group utilized 75% less steroids as compared to the control group. They did see slightly better ACQ-5 scores and improved lung function in the control group, but these findings were below the level of clinical relevance (Bateman et al., 2018). Tang et al. (2018) point out that while up to 70% of asthma patients are categorized as mild, between 40-50% of these are uncontrolled, and 25% have had a severe exacerbation in the last 12 months. They postulated that this is due to an overreliance on SABA, which is likely secondary to a combination of the comfort level of the patient's first medication and the quick symptom relief it provides. They found that if SMART therapy is utilized in mild asthma patients, it leads to fewer exacerbations, as well fewer hospitalizations and ED visits secondary to reduced inflammation (which SABAs do not affect), improved adherence, and overall less exposure to corticosteroids (Tang et al., 2018). Finally, a systematic review and meta-analysis of SMART therapy using budesonide/formoterol (Symbicort) in dry powder inhaler (DPI) evaluated 16 randomized clinical trials involving 22,748 patients over the age of four with persistent asthma ranging from mild to severe. All studies included the use of SABA as reliever medication in the control group. Amongst patients over the age of 11, they found a significantly reduced risk of exacerbation compared with ICS-LABA controller use, even when a higher dose of ICS was used in the control group. Similar results were seen when SMART was compared with ICS alone as a controller medication, either at the same or at higher doses. Amongst the 341 patients ages 5-11, they saw the same reduced risk of exacerbation but based on a smaller sample size (Sobieraj et al., 2018b). GINA guidelines (2018) specify a maximum of 72 μg of formoterol in one 24-hour period.
EPR-3 (NHLBI, 2012) and GINA (2018) guidelines both list leukotriene modifiers such as montelukast (Singulair), zafirlukast (Accolade), or zileuton (Zyflo) as alternative options for persistent asthma treatment in patients age five and above. They are available as once-daily oral tablets, and montelukast (Singulair) is also available in a granule packet. They work by blocking the leukotriene portion of the inflammatory cascade (NHLBI, n.d.). GINA specifies that these oral medications may be an appropriate daily controller choice in patients who experience intolerable adverse effects related to ICS or who may have concomitant allergic rhinitis as well as an alternative in EIB. It is an alternative adjunct with ICS but has been shown to be less effective than adding LABA. It can also be considered an optional adjunct therapy in patients with aspirin-sensitive asthma (GINA, 2018). Bostantzoglou et al. (2015) point out that this medication type may be more beneficial in the non-eosinophilic clinical phenotype characterized by severe symptoms but minimal inflammation. EPR-3 (NHLBI, 2012) specifies that montelukast (Singulair) may be used in children as young as one year, while zafirlukast (Accolade) should not be used in patients under five and zileuton (Zyflo) should not be used in patients under 12. They further specify that both zafirlukast (Accolade) and zileuton (Zyflo) require liver function monitoring regularly. Zileuton (Zyflo) also has a slightly different mechanism of action, working as a 5-lipoxygenase inhibitor to interfere with leukotriene formation while montelukast (Singulair) and zafirlukast (Accolade) both function as selective leukotriene receptor antagonists (LTRAs). They also suggest LTRAs as an alternative in the treatment of EIB (NHLBI, 2012). In the American Association of Allergy, Asthma, and Immunology (AAAAI) practice parameter on EIB, they mention leukotriene modifiers as a reasonable option and suggest they be used intermittently but warn that protection may be incomplete (Weiler et al., 2016). A study comparing montelukast (Singulair) to placebo in 189 patients with mild to moderate asthma previously on SABA alone failed to improve FEV1 significantly after daily oral dosing for 12 weeks (Korenblat et al., 2017).
Tiotropium bromide (Spiriva) was FDA approved in 2014 as the only LAMA for use in asthma patients over the age of 11 in the US (although others are approved for the treatment of COPD). It functions as an antagonist to acetylcholine receptors, causing bronchodilation, with a half-life of 25 hours. GINA states that in adolescent or adult patients with a history of exacerbations not well-controlled on low-dose ICS and LABA, the addition of tiotropium (Spiriva) can be considered as the fourth step. It has been shown to modestly improve lung function and increase time to severe exacerbation (GINA, 2018). Sobieraj et al. (2017) evaluated the research regarding the use of LAMA as part of the executive summary in preparation for the forthcoming EPR-4. They found that in patients over the age of 11 with uncontrolled persistent asthma, the addition of LAMA to ICS versus placebo decreased the risk of exacerbation and improved spirometry results. They also compared the combination of LAMA and ICS versus doubling the dose of ICS and found no significant effect. They compared adding LAMA to ICS versus adding LABA and similarly found no significant effect. Finally, they reviewed what they termed "triple treatment", which included ICS, LABA, and LAMA and found that the addition of LAMA improved FEV1 based on high strength of evidence and improved asthma control scores based on low to moderate strength of evidence but saw no significant effect on risk of exacerbation or hospitalization (Sobieraj et al., 2017). Separately, a systematic review and meta-analysis of LAMA use published in 2018 included 15 randomized clinical trials and over 7,000 patients over the age of 11, including 789 between the ages of 12-17. They found similarly that the addition of LAMA to ICS versus placebo reduced the risk of exacerbation requiring systemic steroids and improved spirometry results. They found no significant difference between ICS-LABA and ICS-LAMA. Very similar to the results above, when they compared ICS-LABA with triple therapy (ICS/LABA/LAMA), they found an improvement in FEV1 as well as QOL scores but saw no decrease in exacerbation risk (Sobieraj et al., 2018a).
Theophylline (Theo-24) is an oral medication that functions by antagonizing adenosine receptors and increasing cyclic adenosine monophosphate (cAMP), thereby causing bronchodilation. It can be especially helpful with nocturnal symptoms (Lynn & Kushto-Reese, 2015). It is limited in its utility by the necessity of checking serum drug levels periodically to ensure a therapeutically appropriate serum concentration (NHLBI, n.d.). EPR-3 (NHLBI, 2012) recommends theophylline (Theo-24) as an alternative to low-dose ICS in step 2 treatment, an alternative adjunct to ICS or ICS-LABA in steps 3, 4, and 5, or in step 6 in conjunction with ICS-LABA in an attempt to avoid a course of oral corticosteroids. It is available in liquid, capsule, or sustained-release tablet form. It is typically dosed at 10 mg/kg per day initially (NHLBI, 2012). GINA guidelines (2018) offer theophylline (Theo-24) as an alternative initial controller medication in adolescents and adults with initial presentation of asthma symptoms and/or SABA use more than twice weekly but note that this is typically less effective than low-dose ICS. They also mention the use of short-acting theophylline (Theo-24) as a reliever medication but note that it has a slower onset of action than most other SABAs and a higher risk of adverse effects. GINA guidelines also list theophylline (Theo-24) as an adjunct treatment option with ICS and/or ICS-LABA in adolescents and adults but do not recommend its use in children. Lastly, they note that the use of theophylline (Theo-24) may worsen GERD symptoms via relaxation of the lower esophageal sphincter (GINA, 2018).
Cromolyn sodium (Intal) is an anti-inflammatory medication that is delivered via nebulizer, which functions as a mast cell stabilizer, blocking the cellular response to inhaled antigens that can trigger asthma exacerbations. A significant limitation of cromolyn (Intal) is the need for a nebulizer and the need to be dosed four times daily in order to be effective. It is listed in EPR-3 as an alternative controller medication in step 2, but not recommended in children under two. It is also one of the options that may be used to pre-treat EIB prior to exercise (NHLBI, 2012). Notably, there was no mention of the use of cromolyn (Intal) or other mast cell stabilizers in GINA (2018).
Reliever Medications
Reliever or rescue medications are used to treat asthma exacerbations or sudden onset of symptoms on an as-needed basis. Albuterol (Pro-Air, Ventolin) and levalbuterol (Xopenex) are bronchodilators that selectively stimulate ß-2 adrenergic receptors, similar to the previously mentioned LABAs, relaxing the airway smooth muscles. Their half-life is 2.7-6 hours. Both are available as MDIs or can be used in nebulizers, and albuterol (Pro-Air, Ventolin) can also be found in oral tablet form, extended-release oral tablet, or liquid syrup. Terbutaline (Bricanyl) is a SABA that is available in tablet form as well as a subcutaneous injection with a half-life of three to four hours. It is approved for use in patients over the age of five up to three times per day. EPR-3 and GINA guidelines suggest the use of SABAs like albuterol (Pro-Air, Ventolin) on an as-needed basis in patients with mild intermittent asthma. Formoterol (Perforomist, Symbicort, Dulera), as mentioned above, is a quick-onset but long-acting ß-agonist with a 10-hour half-life. Ipratropium bromide (Atrovent) is a SAMA that creates bronchodilation by antagonizing acetylcholine receptors. It has a half-life of two hours and is available as a nebulizer solution that can be mixed with Albuterol (Pro-Air, Ventolin) or levalbuterol (Xopenex) or an MDI that can be used independently. Both guidelines suggest utilizing the patient’s report of reliever use frequency to help gauge the level of symptom control in asthma patients (GINA, 2018; NHLBI, 2012).
Asthma Action Plan
A crucial component of comprehensive care, an AAP serves as a patient’s road map for the management of symptoms at home on a daily basis. In 2014, Dinakar et al. discussed the use of the AAP in the management of acute loss of control, termed the "yellow zone". AAPs utilize a traffic light analogy, and thus the yellow zone is a loss of control that is mild or moderate in nature and not severe enough to require an ED visit. An AAP helps the patient monitor their symptoms and their lung function to first identify where they are in the plan (green, yellow, or red zone) and the appropriate actions to take based on that self-assessment. They recommend that all asthma patients, regardless of age, be given a written or electronic AAP that is reviewed, and if needed, edited or updated at each follow-up visit. They define the green zone as less than two days per week of symptoms (cough, wheeze) or reliever medication use. They define the yellow zone as an increase in symptoms, increased use of reliever medication, a PEF reading that is decreased 15% from previous readings or less than 80% of PB, or the presence or increase in nocturnal symptoms. SABA use should be initiated if the patient finds themselves in the yellow zone at two to four puffs every four to six hours. If this persists longer than twelve hours, the patient should then contact their provider. If the patient is on a low-to-moderate daily dose of ICS, they could also be instructed on the AAP to increase their ICS dose up to four times per 24 hours. For pediatric patients under the age of six with recurrent wheezing and risk factors for subsequent asthma, they recommend considering initiation of high-dose ICS or montelukast (Singulair) at the first sign of wheezing illness to reduce the intensity. For patients with mild to moderate asthma, they recommend the symptom-driven use of a combination ICS-LABA inhaler to manage yellow zone symptoms, as described above in SMART therapy (Dinakar et al., 2014). GINA guidelines state that all adolescent and adult patients should be educated on and given an AAP (GINA, 2018, p.77). See Figure 2 below for a sample plan from the NHLBI, recommended by the EPR-3 guidelines:

It is important to note that while current guidelines all recommend written AAPs, some studies question whether or not they improve outcomes in pediatric patients. A 2016 review by Kelso questions their effectiveness. His literature review found minimal evidence of improved outcomes, and in fact found a higher cost of care in AAP users secondary to increased medication and health care service usage. As an alternative, he suggests time spent educating patients and caregivers regarding the asthma diagnosis itself as well as goals of care and signs/symptoms that should prompt follow-up action. He also suggests reinforcing this with written information (Kelso, 2016).
References
Bostantzoglou, C., Delimpoura, V., Samitas, K., Zervas, E., Kanniess, F., & Gaga, M. (2015). Clinical asthma phenotypes in the real world: Opportunities and challenges. Breathe, 11(3), 186–193. https://doi.org/10.1183/20734735.008115
Bateman, E. D., Reddel, H. K., O’Byrne, P. M., Barnes, P. J., Zhong, N., Keen, C., Jorup, C., Lamarca, R., Siwek-Posluszna, A., & FitzGerald, J. M. (2018). As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. New England Journal of Medicine, 378(20), 1877–1887. https://doi.org/10.1056/NEJMoa1715275
Busse, W. W., Bateman, E. D., Caplan, A. L., Kelly, H. W., O’Byrne, P. M., Rabe, K. F., & Chinchilli, V. M. (2018). Combined analysis of asthma safety trials of long-acting B2-agonists. New England Journal of Medicine, 378 (26), 2497-2505. https://doi.org/10.1056/NEJMoa1716868
de Groot, J. C., ten Brinke, A., & Bel, E. H. D. (2015). Management of the patient with eosinophilic asthma: A new era begins. ERJ Open Research, 1(1), 00024–02015. https://doi.org/10.1183/23120541.00024-2015
Dinakar, C., Oppenheimer, J., Portnoy, J., Bacharier, L. B., Li, J., Kercsmar, C. M., Bernstein, D., Blessing-Moore, J., Khan, D., Lang, D., Nicklas, R., Randolph, C., Schuller, D., Spector, S., Tilles, D., & Wallace, D. (2014). Management of acute loss of asthma control in the yellow zone: A practice parameter. Annals of Allergy, Asthma & Immunology, 113(2), 143–159. https://doi.org/10.1016/j.anai.2014.05.017
Dunican, E. M., & Fahy, J. V. (2017). Asthma and corticosteroids: Time for a more precise approach to treatment. European Respiratory Journal, 49(6), 1701167. https://doi.org/10.1183/13993003.01167-2017
Global Initiative for Asthma. (2018). Global strategy for asthma management and prevention. www.ginasthma.org
Hsu, J., Sircar, K., Herman, E., & Garbe, P. (2018). EXHALE: A technical package to control asthma. National Center for Environmental Health, The Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/pdfs/EXHALE_technical_package-508.pdf
Kelso, J. M. (2016). Do written asthma action plans improve outcomes? Pediatric Allergy, Immunology, and Pulmonology, 29(1), 2-5. https://doi.org/10.1089/ped.2016.0634
Korenblat, P., Kerwin, E., Leshchenko, I., Yen, K., Holweg, C. T. J., Anzures-Cabrera, J., Martin, C., Putnam, W. S., Governale, L., Olsson, J & Matthews, J. G. (2017). Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respiratory Medicine, 134, 143-149. https://doi.org/10.1016/j.rmed.2017.12.006
Lynn, S. J., & Kushto-Reese, K. (2015). Understanding asthma pathophysiology, diagnosis, and management. American Nurse Today, 10(7), 49–51. https://www.myamericannurse.com/wp-content/uploads/2015/07/ant7-Asthma-622.pdf
National Heart, Lung, and Blood Institute. (n.d.). Asthma. Retrieved January 21, 2020, from https://www.nhlbi.nih.gov/health-topics/asthma
National Heart, Lung, and Blood Institute. (2007). Asthma action plan. (NIH Publication No. 07-5251). https://www.nhlbi.nih.gov/health-topics/all-publications-and-resources/asthma-action-plan
National Heart, Lung, and Blood Institute. (2012). Asthma care quick reference: Diagnosing and managing asthma (NIH Publication No. 12-5075). https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthma_qrg_0_0.pdf
O'Byrne P. M., FitzGerald J. M., Bateman E. D., Barnes, P., Zhong, N., Keen, C., Jorup, C., Lamarca, R., Ivanov, S., & Reddel, H. K. (2018) Inhaled combined budesonide-formoterol as needed in mild asthma. New England Journal of Medicine, 378, 1865-76. https://doi.org/10.1056/NEJMoa1715274
Peters, S. P., Bleecker, E. R., Canonica, G. W., Park, Y. B., Ramirez, R., Hollis, S., … Martin, U. J. (2016). Serious asthma events with budesonide plus formoterol vs. budesonide alone. New England Journal of Medicine, 375(9), 850–860. https://doi.org/10.1056/NEJMoa1511190
Sobieraj, D. M., Baker, W. L., Weeda, E. R., Nguyen, E., Coleman, C. I., White, C. M., Lazarus, S. C., Blake, K. V., & Lang, J. E. (2017). Intermittent inhaled corticosteroids and long-acting muscarinic antagonists for asthma. Agency for Healthcare Research and Quality. https://doi.org/10.23970/AHRQEPCCER194
Sobieraj, D. M., Baker, W. L., Nguyen, E., Weeda, E. R., Coleman, C. I., White, C. M., Lazarus, S. C., Blake, K. V., & Lang, J. E. (2018a). Association of inhaled corticosteroids and long-acting muscarinic antagonists with asthma control in patients with uncontrolled, persistent asthma: A systematic review and meta-analysis. JAMA, 319(14), 1473–1484. https://doi.org/10.1001/jama.2018.2757
Sobieraj, D. M., Weeda, E. R., Nguyen, E., Coleman, C. I., White, C. M., Lazarus, S. C., Blake, K. V., Lang, J. E., & Baker, W. L. (2018b). Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: A systematic review and meta-analysis. JAMA, 319(14), 1485-96. https://doi.org/10.1001/jama.2018.2769
Stempel, D. A., Raphio, I. H., Kral, K. M., Yeakey, A. M., Emmett, A. H., Prazma, C. M, Buaron, K. S., Pascoe, S. J. (2016). Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. New England Journal of Medicine 374, 1822-1830. https://doi.org/10.1056/NEJMoa1511049
Tang, W., Sun, L., & FizGerald, J. M. (2018). A paradigm shift in the treatment of mild asthma? Journal of Thoracic Disease, 10(10), 5655–5658. https://doi.org/10.21037/jtd.2018.09.127
US Food and Drug Administration. (2012). MDI with attached spacer device [image]. https://www.flickr.com/photos/fdaphotos/7349095984/in/photostream/
Weiler, J. M., Brannan, J. D., Randolph, C. C., Hallstrand, T. S., Parsons, J., Silvers, W., Storms, W, Zeiger, J., Bernstein, D. I., Blessing-Moore, J., Greenhawt, M., Khan, D., Lang, D., Nicklas, R. A., Oppenheimer, J, Portnoy, J. M., Schuller, D. E., Tilles, S. A., & Wallace, D. (2016). Exercise-induced bronchoconstriction update—2016. Journal of Allergy and Clinical Immunology, 138(5), 1292-1295.e36. https://doi.org/10.1016/j.jaci.2016.05.029
World Health Organization. (2019). Asthma. https://www.who.int/news-room/q-a-detail/asthma