The purpose of this activity is to enable the learner to understand bariatric surgery as an option to treat obesity and how to care for and treat patients during the perioperative period.
...purchase below to continue the course
s removal of approximately 80% of the stomach. The remaining stomach is tubular-shaped, resembling a banana (ASMBS, 2019a; Mayo Clinic, 2018c). By limiting the stomach size, the amount of food that can be consumed is considerably decreased, thereby prompting weight loss. Hormonal changes are also triggered to aid in weight loss and help to reverse some of the associated complications of obesity, including hypertension, cardiac disease, and blood sugar control. This procedure is not reversible (Mayo Clinic, 2018c).
The surgical process of a sleeve gastrectomy is demonstrated in Figure 2.
Figure 2
Sleeve Gastrectomy

(Comesaña, 2018)
Advantages of the sleeve gastrectomy procedure include:
- Rapid and substantial weight loss very similar to the Roux-En-Y gastric bypass.
- Weight loss maintenance is typically greater than 50% of initial weight over three to five years and comparable to gastric bypass maintenance.
- No bypass of the food stream (as in the gastric bypass) and requires no foreign object (as in the adjustable gastric band).
- A short recuperation and hospital stay (typically two days).
- Changes in gut hormones that improve satiety, decrease appetite, and suppress hunger (ASMBS, 2019a; Mayo Clinic, 2018c).
Disadvantages of the sleeve gastrectomy procedure include:
- The procedure is non-reversible.
- A higher early complication rate than the adjustable gastric band.
- Potential for long-term vitamin deficiencies (ASMBS, 2019a; Mayo Clinic, 2018c).
Adjustable Gastric Band
The adjustable gastric band, or "the band," as it is often called, utilizes an inflatable band placed around the upper portion of the stomach to create a small pouch above the band, leaving the residual stomach below the band. The device works by limiting the amount of food that can be ingested. The pouch becomes full quickly, and hunger is satisfied. The size of the pouch can be adjusted by filling the band with sterile saline that is injected via a port placed under the skin. The pouch size is decreased over time with repeated injections into the port. There is no malabsorption of food with this procedure. This procedure can be reversed in most cases (ASMBS, 2019a). The adjustable gastric band procedure is demonstrated in Figure 3.
Figure 3
Gastric Banding
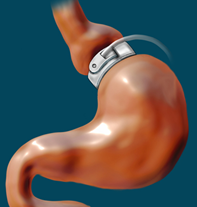 (Gray, 2007)
(Gray, 2007)
Advantages of the adjustable gastric band procedure include:
- Promotes eight loss of 40-50% of EBW, and while less than other procedures, is a significant weight loss for most individuals.
- No incision within the stomach or rerouting of the intestines.
- The procedure is reversible and adjustable.
- The lowest rate of early postoperative complications and mortality among all bariatric procedures.
- The lowest risk of vitamin or mineral deficiencies of all bariatric procedures (ASMBS, 2019a).
Disadvantages of the adjustable gastric band procedure include:
- Slower weight loss than other bariatric procedures.
- Decreased early weight loss compared to other bariatric procedures.
- A foreign device is implanted into the body.
- The band can migrate, erode into the stomach, or develop mechanical problems, including tube or port malfunctions.
- The patient can develop esophageal dilation (expansion of the tissue), dysmotility (lack of movement), or esophagitis (inflammation) if they overeat.
- The highest rate of re-operation secondary to complication of all bariatric procedures (ASMBS, 2019a).
Biliopancreatic Diversion with Duodenal Switch
The biliopancreatic diversion with duodenal switch is the least common weight-loss surgery among those discussed within this module. This surgery involves two steps, starting with a sleeve gastrectomy. As described above, approximately 80% of the stomach is removed, and a banana-shaped tubular stomach is maintained. The pyloric sphincter, which allows food to pass into the small intestine, also remains. The second part of the surgery bypasses most of the small intestine. During this procedure, the duodenum (first portion of the small intestine), is divided just past the pyloric sphincter. The lower portion of the small intestine is then connected to the outlet of the newly created stomach pouch. When the patient eats, food goes through the tubular stomach and empties directly into the lower portion of the small intestine, bypassing about 75% of the small intestine. Consequently, food does not mix with the natural pancreatic enzymes or bile until it is further along in small intestine, resulting in decreased absorption of nutrients and calories (ASMBS, 2019a; Mayo Clinic, 2018a). The biliopancreatic diversion with duodenal switch is demonstrated in Figure 4.
Figure 4
Biliopancreatic Diversion
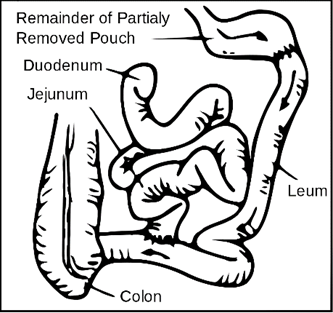
(Stevenfruitsmaak, 2009)
Advantages of the biliopancreatic diversion with duodenal switch procedure include:
- EWL is 60-70% of EBW or higher at the five-year follow-up, which is higher than other bariatric surgeries.
- It allows patients to eat more "normal" meals after a few months.
- Enhances gut hormones to reduce appetite and improve satiety.
- Most effective at reducing the rate of diabetes in comparison to other bariatric surgeries.
- Fat absorption is reduced up to 70% due to delayed pancreatic enzyme activity (ASMBS, 2019a; Mayo Clinic, 2018a).
Disadvantages of the biliopancreatic diversion with duodenal switch procedure include:
- There is a higher complication rate and increased risk of mortality with this procedure than with other bariatric surgeries.
- There is a greater potential for vitamin and mineral deficiencies than after other procedures.
- A more extended hospital stay after surgery when compared to the gastric bypass or gastric band procedures.
- Compliance with diet and follow-up visits are crucial with this procedure to avoid serious complications from protein and vitamin deficiencies (ASMBS, 2019c; Mayo Clinic, 2018a).
Benefits and Risks of Bariatric Surgery
Evidence regarding the benefits of weight loss surgery dates back to the 1960s. The last few decades have been accompanied by a significant rise in weight-loss surgeries as an increase in obesity occurs. Through multiple studies, all bariatric procedures provided better long-term weight management than non-surgical weight loss options such as diet, exercise, or other supportive services. There is a decreased risk of mortality and morbidity in patients with morbid obesity after bariatric surgery when compare to conventional weight loss methods. Studies show the weight loss associated with bariatric surgery leads to decreased OSA, osteoarthritis, stress incontinence, dyslipidemia, hypertension, coronary artery disease, acute cardiovascular events, diabetes, obesity-related cancers, and death (Albaugh & Abumrad, 2018; ASMBS, 2019c; Mayo Clinic, 2019).
The risks associated with bariatric surgeries include those complications related to general anesthesia. Immediate postoperative complications include bleeding, bowel obstruction, or bowel perforation. Long-term complications include ulcerations from the gastric band or nutritional deficits from lack of absorption with all bariatric procedures (Albaugh & Abumrad, 2018; ASMBS, 2019c; Mayo Clinic, 2019).
Criteria for Bariatric Surgery Clearance
Bariatric surgery is not ideal for all patients, and it can be costly. Patients considering bariatric surgery should be counseled to contact their health insurance company directly to determine coverage and to discuss payment options with their healthcare provider’s billing department for any costs not covered by their health insurance. Furthermore, the physical, mental, and social aspects of the surgery require that providers conduct extensive screening processes to clear a patient for the procedure. Generally, these procedures are considered for anyone with a BMI over 40, or someone with a BMI between 35 and 39 along with comorbidities such as severe OSA, hypertension, type 2 diabetes, or other serious weight-related health problems. Occasionally, a patient with a BMI between 30 and 34 may be considered if the weight-related health problems are considered serious (Mayo, 2019). Candidate approval for bariatric surgery should be based on prior weight loss attempts, the presence and impact of comorbidities, overall health status for surgery consideration, and the ability to comply with pre- and postoperative instructions (Schlottmann et al., 2018). A complete assessment and focus on comorbidities such as cardiac and respiratory concerns can create a safer environment for intraoperative care. Closer postsurgical monitoring may be required for those with identified concerns prior to surgery (ASBMS, 2019; Schlottman et al., 2018).
Medical clearance for bariatric surgery should include a thorough history that explores the patient’s weight history, dietary history, social history, psychological history (particularly eating disorders, physical abuse, or substance abuse), physical activity, review of medications, and psychosocial behaviors/factors that might impact weight loss or future success due to noncompliance. The clinical examination should include assessments of laboratory values, including electrolytes, vitamins, iron, folate and calcium levels, a basic metabolic panel, complete blood count, and lipid profile. Patients with type 2 diabetes should achieve glycemic control before surgery, thus decreasing the risk of postoperative complications (Schlottmann et al., 2018).
Since cardiac complications often co-exist with obesity, it is vital to optimize all comorbidities before surgery to prevent postoperative complications. All patients who are evaluated for bariatric surgery should have a 12-lead electrocardiogram, followed by a cardiac stress test, either with a treadmill or scintigraphic imaging if possible. These may not be possible in patients with morbid obesity due to the weight limitations of testing equipment, difficulty in accurately interpreting images due to body habitus, and the physical capabilities of the patient. In these patients, pharmacological stress testing may be done, with or without ultrasound contrast agents to determine cardiac function as accurately as possible (Schlottmann et al., 2018).
Airway complications are a prevalent concern in patients with morbid obesity due to mechanical restriction. Preoperative pulmonary function testing may help identify patients at high-risk for pulmonary complications following the surgery. Screening tools such as the Stop, STOP-BANG (SB), Epworth Sleepiness Scale (ESS), or 4-Variable screening tool (4-V) are available to help identify patients at risk for OSA (Singh & Mims, 2015). Patients who are identified as at risk for OSA during this preoperative testing should be referred for evaluation with an overnight polysomnography test (sleep study) and may need to begin treatment with a continuous positive airway pressure (CPAP) machine during sleep. Patients who present with daytime hypercapnia, also known as obesity hypoventilation syndrome (OHS), may also benefit from CPAP therapy or bi-level positive pressure (Bi-Pap) preoperatively as well. Routine spirometry testing may be done for those with a high risk for pulmonary complications, and preoperative optimization with a pulmonologist may be warranted to improve patient outcomes during and after the surgery (Schlottman, 2018).
Preoperative psychological evaluation and clearance is significant to the patient's success post-surgery. The long-term outcomes of surgery are based on the patient’s ability to follow a strict postoperative diet and lifestyle regimen. This will also prepare the patient for the emotional adjustments that will be needed as their weight is reduced. A comprehensive behavioral assessment should include a history of any eating or behavioral disorders, any current or history of anxiety or mood disorders, all current or past mental health conditions or treatments, and an evaluation of the patient's motivation for weight loss. Perhaps more important than the physical ability to withstand the bariatric surgery is the patient’s psychological capacity to learn, comply, and adapt to the changes that will occur during the perioperative cycle. Social support systems should be identified during the psychological evaluation as the best outcomes are related to the presence of family members, friends, and a community that is supportive of the patient’s weight-loss journey with bariatric surgery. This evaluation will also identify any existing barriers to postoperative success through a lack of social or emotional support in these same areas (Schlottman, 2018).
A registered dietician should complete a nutritional evaluation. This assessment will focus on preoperative weight loss efforts, a nutritional assessment of current eating behaviors, and education on postoperative eating behaviors that will need to be strictly adhered to. Some controversy exists over the need for preoperative weight loss for long-term success; studies show that preoperative nutritional counseling is vital to the success of postoperative dietary compliance (Dagan et al., 2017). During the nutritional assessment, a careful evaluation of micro-and macronutrient measurements should be completed, and the nutritionist or RD should educate the patient regarding the post-operative diet components before ALL bariatric surgical procedures. However, for the patient undergoing one of the malabsorptive bariatric procedures such as the gastric bypass or the biliopancreatic diversion with duodenal switch, a more extensive perioperative nutritional evaluation should be done to offset anticipated deficiencies since the portion of intestines is removed that allows for the easiest absorption of vitamins and minerals. This patient may have deficiency of iron, calcium, magnesium and vitamins, including B12 (Schlottman, 2018).
Preoperative gastrointestinal (GI) imaging or an upper GI radiographic series should be completed to determine pre-existing anatomical or physiological abnormalities that may be present. As the GI tract will be modified during bariatric surgery, this provides a preoperative view of the esophagus and gastric anatomy including esophageal clearance and the presence or size of a hiatal hernia. An abdominal ultrasound is recommended to assess for biliary tract pathology. If undiagnosed cholelithiasis is present, the rapid weight loss induced by bariatric surgery can be the impetus for gallstones to become symptomatic. The ultrasound should also assess for steatosis (abnormal retention of fats), fibrosis (abnormal amounts of scar tissue), or the presence of nonalcoholic steatohepatitis (inflammation with concurrent fat accumulation) of the liver. An esophagogastroduodenoscopy (EGD) should be done in the preoperative evaluation of a bariatric surgical candidate. The pharynx, esophagus, stomach, and duodenum are evaluated using a flexible endoscope. This procedure can be used to rule out any underlying GI disease, severe esophagitis, Barrett's esophagus, or a malignancy of the upper GI tract (Schlottman, 2018).
While there is controversy over the pre-operative weight loss, other studies show that patients with a preoperative weight loss requirement have a higher percentage of EWL at six months post-surgery. The healthcare team will determine the individual goals for the bariatric patient prior to surgery. One advantage of preoperative weight loss is abdominal fat and liver volume are reduced and can improve access to the stomach during laparoscopic procedures, shortening the operative time (Schlottman, 2018).
The major contraindications to bariatric surgery are psychological features that might render a patient unable to cope with the impact of the surgery or physical conditions or comorbidities that put the patient at extremely high risk, such as cardiac conditions or other anesthesia risks. Most patients benefit from the procedure and subsequent weight loss, thereby making their clearance for surgery likely (Schlottman, 2018). See Table 1 for preoperative checklist for bariatric surgery.
Table 1
Preoperative Checklist for Bariatric Surgery

(ASMBS, 2013)
Perioperative Care for the Bariatric Patient
Preoperative Nursing Care
The nurse should focus on the comprehensive patient and discuss goal setting for postsurgical weight loss. The patient and their healthcare team should jointly develop goals based on the patient’s individual situation and willingness to comply with future restrictions (ASBMS, 2019; Schlottman, 2018). The nurse should focus on the following during preoperative care:
1. Patient care needs:
- The individual physical risk factors identified and developing a plan to offset any anticipated risk.
- Developing relationships with the healthcare team to improve compliance post-surgery.
- Discussing strengths and weaknesses of the patient and their personal support system (ASBMS, 2019a; Lim, 2018).
2. Education on:
- The specific procedure to be performed and the associated risks.
- Discharge planning for surgery.
- The importance of dietary compliance and follow-up with healthcare providers.
- What and how much can be eaten after surgery.
- Understanding the connection between food and triggers to overeating as well as behavioral changes that can lead to a healthier lifestyle.
- The importance of exercise to mentally and physically prepare for surgery and maintain after surgery (ASBMS, 2019a).
- Education should occur prior to surgery and be reinforced as they are alert enough to understand after surgery (ASBMS, 2019b).
Postoperative Nursing Care
During the surgery and in the postoperative period, patient care needs will focus on maintaining the respiratory and cardiac functioning, pain management, and early recognition of surgical complications. In the early postoperative cycle, the nurse should focus on the patient's pain, safety, and hydration. Patient care needs:
- Maintenance of airway/breathing through the use of incentive spirometry or other respiratory therapies.
- Continuous monitoring of vital signs.
- Administration of IV fluids and any other ordered medications.
- Regular monitoring for bleeding.
- Pain management.
Venous thromboembolism event (VTE) prophylaxis (ASBMS, 2019b).
See Table 2 for early postoperative care.
Table 2
Early Postoperative Care
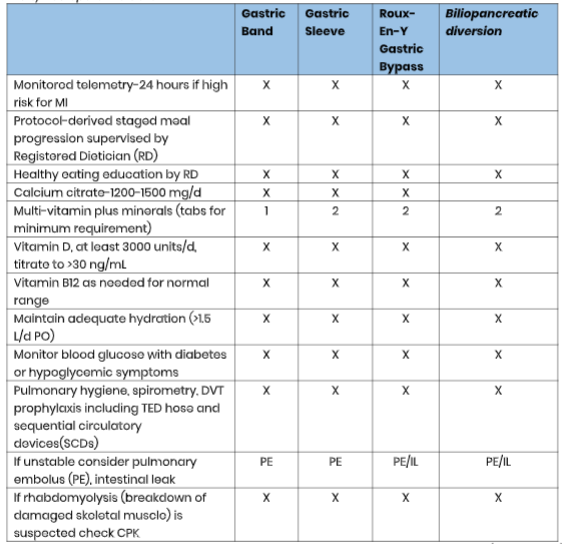 (ASMBS, 2013)
(ASMBS, 2013)
Nursing should provide education on:
- The importance of reporting pain to the nurse before it becomes severe and taking pain medication both routinely and as needed during the immediate postoperative cycle.
- The important of early ambulation to decrease complications, but always asking for assistance before getting out of bed to avoid injury.
- The use of an incentive spirometer and other respiratory treatments to avoid postoperative pneumonia.
- The importance of having nothing by mouth initially followed by a gradual dietary introduction of liquids, progressing to a pureed diet.
- The patient’s medication regime (ASBMS, 2019b).
The nurse should provide dietary education for the bariatric surgery patient in the early days after surgery. Important education points should include:
- Start with a clear liquid diet before progressing to thicker liquids.
- After two weeks, the patient may start a blended, pureed diet. Liquid supplements or powders can meet the protein requirements during this period.
- Eat small meals every two hours.
- Foods should be high in protein and low in carbohydrates, calories, fat, and sugar.
- Fluids should be consumed between meals, with a goal of one cup of fluids between each small meal, six to eight times per day, for a total of 64 ounces of fluids daily.
- Maintain a log of all food consumed along with protein intake, with a protein goal of 65-75 grams daily post-surgery (this may take some time to achieve).
- Chew food thoroughly and take small bites.
- Do not use straws or chew ice, as this introduces air into the stomach and causes pain.
- Caloric intake for the first two months should remain around 300-600 calories per day with a focus on thin and thicker liquids, not to exceed 1000 calories.
- Avoid rice, bread, raw vegetables, fresh fruits, and carbonated or sugary beverages.
- Avoid pork or steak that is hard to chew. Ground meats are best.
- Avoid alcohol, as this is absorbed into the patient’s system more rapidly and can cause an increased effect on blood alcohol levels.
- Liquid vitamin supplements are best. Others should be crushed or cut into small pieces.
- A multivitamin with selenium, copper, zinc, 400 mcg of folic acid, and 18 mg of iron are advised. The patient should take two tablets daily for at least three months after surgery followed by one table daily for life.
- Take a calcium supplement with 1200 to 2000 mg daily.
- Take a vitamin D supplement with 800 to 1000 IU daily.
- Take an oral or sublingual vitamin B complex supplement with 500 mcg daily (ASBMS, 2019b).
Further education to the family as supportive agents is encouraged as well (ASBMS, 2019b).
Complications of Bariatric Surgery
Due to the fact that most bariatric procedures are performed laparoscopically, surgical risk is lower than from traditional open abdominal surgeries including wound infections and delayed healing. The risk involved with general anesthesia and rerouting of the GI tract is unchanged. Postsurgical risks include VTE, bleeding, and bowel obstruction (Albaugh & Abumrad, 2018).
Anesthesia risks are a concern with any surgery, but the risk for patients with severe obesity is increased. Improved anesthesia and surgical management have decreased this risk, and appropriate cardiac and pulmonary function testing during the preoperative evaluation for bariatric surgery can reduce the risk further still (Albaugh & Abumrad, 2018).
The risk for blood clots or venous thromboembolism (VTE) is a primary concern postoperatively for patients with morbid obesity. Early ambulation after surgery is the most effective method for preventing VTE. Mechanical and pharmacological prophylaxis should be ordered as well, including lower extremity compression with TED hose and sequential circulatory devices (SCDs) and pharmacologic prophylaxis. The use of low molecular weight heparins is suggested in the bariatric patient such as enoxaparin (Lovenox), parnaparin (Baflux), or dalteparin (Fragmin) over unfractionated heparin (Heparin Sodium). Evidence shows a decrease in VTE with the use of low molecular weight heparins and no increased postoperative bleeding rates among this group. Bleeding precautions should be implemented, and the nurse should monitor for bruising or bleeding and avoid invasive procedures (Imberti et al., 2014).
Bleeding related to the surgery is possible, although rare. Most facilities perform a postoperative upper GI series to rule out bleeding or leakage in gastric bypass, sleeve gastrectomy, and biliopancreatic diversion with duodenal switch patients. Symptoms indicating bleeding could include hematemesis, severe left upper quadrant pain, back and shoulder pain, intractable hiccups, severe nausea with retching and melena. For a suspected bleed, the nurse should contact the primary healthcare provider immediately (Lim, 2018). See Table 3 for the algorithm for suspected bleeding post-surgery.
Table 3
Post-Surgical Bleed Algorithm

(Kitamura et al., 2015)
Bowel obstruction is an unlikely but potentially serious complication of bariatric surgery. This is most often associated with the gastric bypass procedure, but it can occur with other procedures. The obstruction can be related to adhesions, internal hernia, intussusception (one section of the intestine telescopes inside of another and causes a blockage), and intraluminal clots or strictures. There may be defects of the mesentery bowel that lead to an obstruction. The patient typically complains of severe abdominal pain, nausea and vomiting. Abdomen will typically be tender to palpation, but without rebound tenderness. The patient with intussusception may present with currant jelly stools, abdominal mass or peritonitis. Regardless of the cause, surgical intervention is typically required to correct it (Clapp, 2015).
Bariatric Surgery Short and Long-Term Effects
The most significant impact of bariatric surgery, both long- and short-term, is weight loss. The weight loss leads to increased activity, improved overall health, as well as increased self-esteem. Studies show that more than 90% of individuals who undergo weight loss surgery maintain 50% of their EWL after surgery, and more than 80% maintain greater than 50% of their EWL after surgery, indicating long-term success with these procedures. Obesity population studies demonstrate that individuals undergoing bariatric surgery have a lower risk of mortality than their counterparts who do not have surgery. One study showed as much as an 89% reduction in mortality over five years for those patients who elect to proceed with bariatric surgery versus patients that do not (ASBMS, 2019a). Improvement in the following co-existing diseases is prevalent:
- Hypertension
- Type 2 diabetes
- OSA
- Osteoarthritis
- Asthma
- Gastroesophageal reflux disease
- Fatty liver disease
- Urinary stress incontinence
- Venous stasis
- Infertility
- Hyperlipidemia (ABSMS, 2019)
Most patients report improved sleep, activities of daily living, and overall improved quality of life after their surgery, and many wonder why they did not do it earlier. Depression, anxiety, and other mental health illnesses often improve as well. Sexual function, relationships, and social interactions frequently improve for the patient, and improved employability is commonly reported (ASBMS, 2019).
Bariatric Surgery in Adolescents and Children
Children and adolescents are also affected by obesity. Data shows that up to 80% of children with obesity will be affected by obesity in adulthood. They are often affected by the same weight-related conditions as adults, including hypertension, OSA, type 2 diabetes, and asthma. Additionally, children and adolescents are often bullied or victims of weight bias, inducing depression and anxiety. While non-invasive treatments such as lifestyle modification, pharmacotherapy, and behavioral therapy are preferred, some cases require more drastic measures, including bariatric surgery. The most common procedures in children and adolescents is the gastric bypass, adjustable gastric band, and the sleeve gastrectomy procedures. The goal of bariatric surgery among this population is to provide the most significant benefit with the lowest risk (ASMBS, 2019b). Criteria for this age group are:
- BMI 35 or higher with major comorbidities (type 2 diabetes, moderate to severe OSA, pseudotumor cerebri, or fatty liver disease).
- BMI 40 or greater with less severe comorbidities (hypertension, hyperlipidemia, mild or moderate OSA) (ASBMS, 2019b).
Risks and Benefits for the Adolescent
There appear to be short-term benefits to bariatric surgery in this population with improved depression, quality of life, and fewer eating disturbances. Negative psychosocial risks could exist but are not well documented. Vitamin and nutritional deficiencies may exist, both short- and long-term, and must be monitored closely to ensure proper growth and development during adolescence (ASMBS, 2019b).
Informed consent among children is not possible, but they should be part of a formal agreement called "assent." This involves the child in the decision-making process and agrees with the surgery without any coercion from the family or the medical staff. The clinical team must carefully decide about the adolescent’s ability to make decisions cognitively, socially, and emotionally. It is optimal to have a supportive family and a willing, committed child on the same page regarding their future health decisions. In situations where this does not exist, the healthcare provider must ensure that all efforts are made to ensure the child's best interests are being considered by all parties involved (ASMBS, 2019b).
Future Opportunities in Bariatrics
While already in practice with some healthcare organizations, the intragastric balloon is a more recent weight-loss procedure that does not require surgery. The procedure is an option for those who are overweight/obese when diet and exercise have not proven successful. Much like the bariatric surgeries, a commitment to lose weight is needed to be considered for the intragastric balloon. The patient must make permanent healthy choices in their diet and add an exercise regime to have long-term success with the procedure (Mayo Clinic, 2018b). This procedure is an option for individuals who have:
- A BMI between 30 and 40.
- A willingness to commit to a healthy lifestyle and follow the medical plan, including behavioral therapy.
- No previous esophageal or stomach surgeries (Mayo Clinic, 2018b).
The intragastric balloon can be placed in an outpatient procedure in an endoscopy suite using sedatives and avoiding general anesthesia. The balloon functions by making the patient feel full faster, thereby decreasing the amount of food that is digested. The balloon also appears to change the hormones that control the appetite, improving satiety. Weight loss depends on the amount of corresponding change to lifestyle habits. The typical amount of weight loss is 7-15% of body weight in the first six months after placement. For those receiving concurrent behavioral therapy, the average weight loss increases to 29% of their EBW, compared to only 14% of EBW among the group that received behavioral therapy alone (Mayo Clinic, 2018b).
Minor complications for the intragastric balloon include pain and nausea after the insertion that subsides within hours to days and can be treated with oral medications. A serious risk includes balloon deflation, allowing it to move through the digestive system, causing a blockage, and requiring a surgery or procedure to retrieve it. Other possible risks include ulcerations or perforations in the stomach that could require surgical repair (Mayo Clinic, 2018b).
Other non-surgical weight loss devices that are approved by the US Food and Drug Administration (FDA) include the Electrical Stimulation System (Maestro Rechargeable System), which blocks nerve activity between the brain and the stomach. The FDA notes that the system is no longer marketed as of September 2018 but is still in use. The Gastric Emptying System (AspireAssist) is a third option approved by the FDA. This device is a tube inserted between the stomach and outside of the abdomen with a port to drain food after eating. The Oral Removable Palatal Space Occupying Device (Sensor Monitored Alimentary Restriction Therapy [SMART] Device) is worn during meals to limit the bite size. The Ingested, Transient, Space Occupying Device (Plenity) is an ingested material that briefly occupies space in the stomach (FDA, 2019).
References
Albaugh, V.L. & Abumrad, N.N. (2018). Surgical treatment of obesity. F1000Research, 7(F1000), Rev-617. https://doi.org/10:12688/f1000research.13515.1
American Society of Metabolic and Bariatric Surgical Procedures. (2013). Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. https://asmbs.org/resources/clinical-practice-guidelines-for-the-perioperative-nutritional-metabolic-and-nonsurgical-support-of-the-bariatric-surgery-patient
American Society of Metabolic and Bariatric Surgical Procedures. (2019a). Bariatric surgery procedures. https://asmbs.org/patients/bariatric-surgery-procedures#bypass
American Society of Metabolic and Bariatric Surgical Procedures. (2019b). Childhood and adolescent obesity. https://asmbs.org/patients/adolescent-obesity
American Society of Metabolic and Bariatric Surgical Procedures. (2019c). Story of obesity surgery. https://asmbs.org/resources/story-of-obesity-surgery
BruceBlaus. (2014). Roux-En-Y Gastric Bypass [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Blausen_0776_Roux-En-Y_01.png
Centers for Disease Control and Prevention. (2015). Healthy weight. https://www.cdc.gov/healthyweight/assessing/bmi/index.html
Clapp, B. (2015). Small bowel obstruction after laparoscopic gastric bypass with non-closure of mesenteric defects. Journal of the Society of Laparoendoscopic Surgeons, 19(1). https://doi.org/10.4293/JSLS.2014.00257
Comesaña, A.R. (2018). Gastrectomilia. [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:GVL.png#globalusage
Dagan, S.S., Goldenshluger, A., Globus, I., Schweiger, C., Kessler, Y., Sandbank, G.K., Ben-Porat, T., & Sinaj, T. (2018). Advances in Nutrition, 8(2), 382-394. https:doi.org/ 10.3945/an.116.014258
Gray, J.P. (2007). Gastric Banding. [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Adjustable_Gastric_Band.png
Imberti, D., Baldini, E., Pierfranceschi, M., Nicolini, A, Cartelli, C., DePaoli, M., Boni, M., Filippucci, E., Cariani, S., & Bottani, G. (2014). Prophylaxis of venous thromboembolism with low molecular weight heparin in bariatric surgery: A prospective, randomised pilot study evaluating two doses of parnaparin (BAFLUX Study).Obesity Surgery, 24(2), 284-291. https://doi.org/10.1007/s11695-013-1105-x
Kitamura, R.K., Lee, J., & Katz, L.B. (2017). The management of GI bleeding after gastric bypass surgery. International Journal of Surgery Research and Practice, 2(2), 1-3. https://clinmedjournals.org/articles/ijsrp/international-journal-of-surgery-research-and-practice-ijsrp-2-026.pdf
Lim, R.B. (2018). Patient education: Weight loss surgery and procedures (Beyond the basics). In UpToDate. https://www.uptodate.com/contents/weight-loss-surgery-and-procedures-beyond-the-basics
Mayo Clinic. (2017). Gastric bypass (Roux-en-Y). https://www.mayoclinic.org/tests-procedures/gastric-bypass-surgery/about/pac-20385189
Mayo Clinic. (2018a). Biliopancreatic diversion with duodenal switch. https://www.mayoclinic.org/tests-procedures/biliopancreatic-diversion-with-duodenal-switch/about/pac-20385180
Mayo Clinic. (2018b). Intragastric balloon. https://www.mayoclinic.org/tests-procedures/intragastric-balloon/about/pac-20394435
Mayo Clinic. (2018c). Sleeve gastrectomy. https://www.mayoclinic.org/tests-procedures/sleeve-gastrectomy/about/pac-20385183
Mayo Clinic. (2019). Bariatric surgery. https://www.mayoclinic.org/tests-procedures/bariatric-surgery/about/pac-20394258
Office of Disease Prevention and Health Promotion. (2019). Nutrition, physical activity, and obesity. In Healthy People 2020. https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Nutrition-Physical-Activity-and-Obesity/data#NWS-9
Penn Medicine. (2019). How to calculate your weight loss. https://www.pennmedicine.org/updates/blogs/metabolic-and-bariatric-surgery-blog/2019/march/how-to-calculate-your-weight-loss
Schlottmann, F., Nayyar, A., Herbella, F. A. M., & Patti, M. G. (2018). Preoperative evaluation in bariatric surgery. Journal of Laparoendoscopic & Advanced Surgical Techniques, 28(8), 925–929. https://doi.org/10.1089/lap.2018.0391.
Singh, J. & Mims, N. (2015). Screening tools for the obstructive sleep apnea for the cardiovascular clinician. https://www.acc.org/latest-in-cardiology/articles/2015/07/14/11/04/screeing-tools-for-the-obstructive-sleep-apnea-for-the-cardiovascular-clinician
Stevenfruitsmaak. (2009). Biliopancreatic Diversion. [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Biliopancreatic_diversion.svg
US Food and Drug Administration. (2019). Weight-loss and weight-management devices. https://www.fda.gov/medical-devices/products-and-medical-procedures/weight-loss-and-weight-management-devices
World Population Review. (2019). Most obese countries, 2019. http://worldpopulationreview.com/countries/most-obbnhbese-countries/
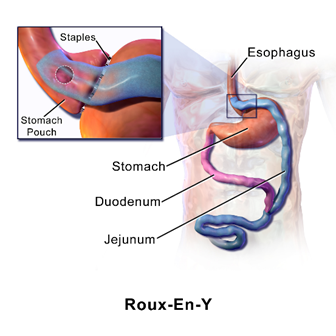 (BrusBlaus, 2014)
(BrusBlaus, 2014)
 (Gray, 2007)
(Gray, 2007)

 (ASMBS, 2013)
(ASMBS, 2013)