About this course:
The purpose of this module is to examine blood clotting during the pregnancy and postpartum period in healthy women and those with inherited thrombophilia disorders, outlining evidence-based prevention strategies, thromboprophylaxis, and treatment to enhance APRN practice and improve maternal and fetal outcomes.
Course preview
The purpose of this module is to examine blood clotting during the pregnancy and postpartum period in healthy women and those with inherited thrombophilia disorders, outlining evidence-based prevention strategies, thromboprophylaxis, and treatment to enhance APRN practice and improve maternal and fetal outcomes.
By the completion of the module, the APRN should be able to:
- Examine the pathophysiologic maternal adaptations to pregnancy, as well as the etiology of venous thromboembolism development during the pregnancy and postpartum period.
- Describe the specific risk factors, clinical considerations, and complications of venous thromboembolism during pregnancy and the postpartum period, as well as strategies for risk reduction and prevention.
- Discuss the evidence-based guidelines regarding thromboprophylaxis, as well as the therapeutic management of venous thromboembolism during pregnancy and postpartum.
- Outline the prescribing recommendations regarding the use of anticoagulation therapy during pregnancy and postpartum, including dosing regimens, side effects, and contraindications to pharmacologic therapy.
- Discuss the most common inherited thrombophilia disorders in pregnancy, their clinical considerations, complications, prevention, and treatment recommendations according to the evidence-based guidelines.
Disclaimer: For an enhanced understanding of the concepts presented within this module, please refer to the NursingCE.com course entitled "Blood Clotting and Bleeding Disorders," which provides a comprehensive overview of the physiology of normal blood clotting as well as the pathologic mechanisms of common blood clotting and bleeding disorders.
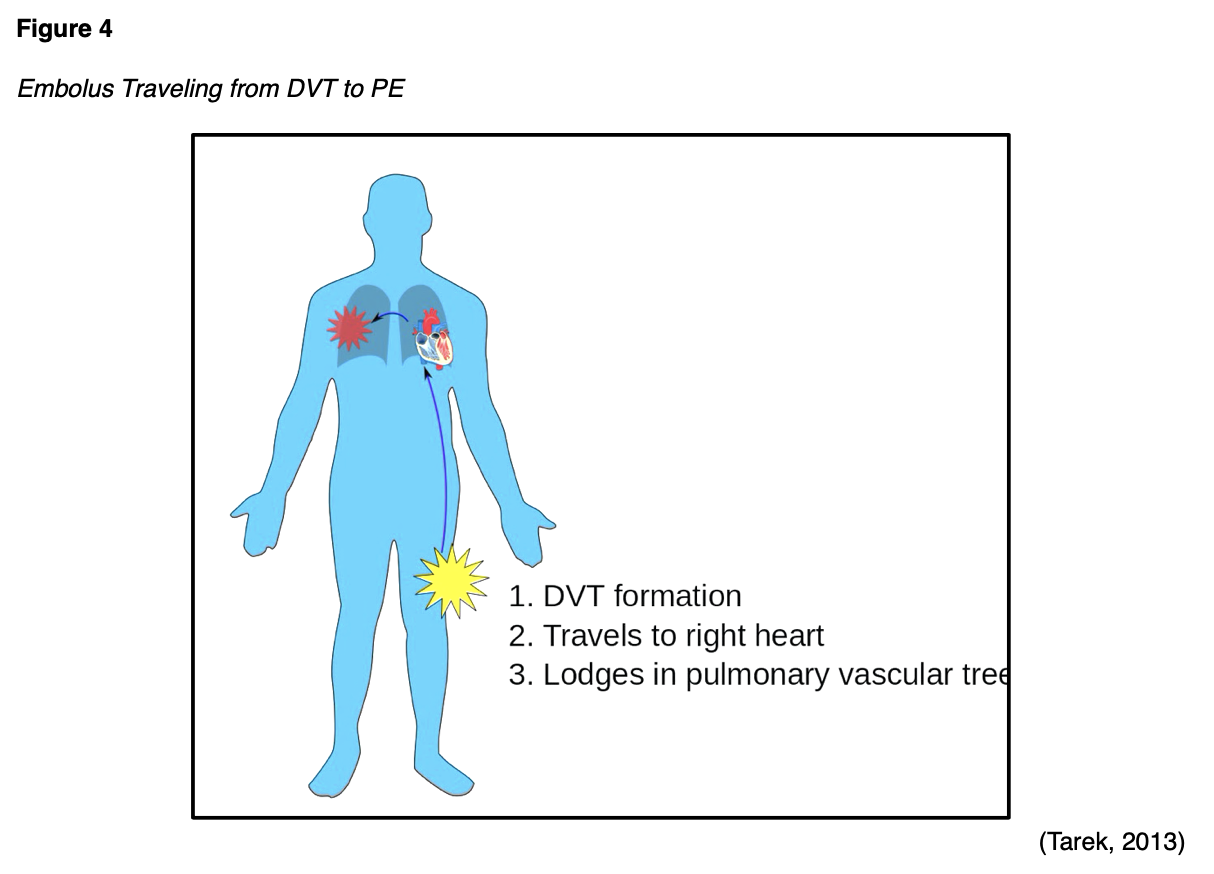
It is well-cited that pregnancy and postpartum is a hypercoagulable state, increasing the risk for venous thromboembolism (VTE), which includes superficial vein thrombosis (SVT), deep vein thrombosis (DVT), and pulmonary embolism (PE). An SVT is a type of blood clot (thrombus) that forms in a superficial vein near the surface of the body, usually in the lower extremities. A DVT is a stationary thrombus that forms in the deep veins of the legs or arms and is much more dangerous than an SVT. As demonstrated in Figure 1, a DVT can develop into a PE, a life-threatening complication in which the thrombus (or a piece of the thrombus) detaches from the vessel wall, travels to the lungs, and becomes lodged in the lung's blood vessels (The Centers for Disease Control and Prevention [CDC], 2020b).

According to the CDC (2020b), women are five times more likely to develop a DVT when pregnant, and the risk extends throughout pregnancy, childbirth, and three months postpartum. Women who have underlying disorders of excessive blood clotting (also called thrombophilias) are at an even higher risk for VTE and its associated morbidity and mortality. Given the potentially lethal sequela of VTEs, there has been increased focus and clinical attention toward VTE prevention and treatment in pregnancy over the last decade. The American Society of Hematology (ASH) 2018 Guidelines and Thromboprophylaxis Recommendations in Pregnancy are among the most well-established and widely utilized evidence-based practice standards. Endorsed by the Association of Women's Health, Obstetric and Neonatal Nurses (AWHONN), the guidelines outline the recommendations for screening and prophylaxis, taking into consideration the complexities surrounding the diagnosis and treatment of pregnancy-associated VTE to safeguard the well-being of both the fetus and mother (Bates et al., 2018). Incorporating the ASH guidelines, this module will examine the prevention, risk reduction, and management of blood clotting during pregnancy and postpartum in healthy women and women with thrombophilia disorders (CDC, 2020b).
Part I: Pregnancy & Blood Clotting
According to the ASH, VTE occurs in 1.2 of every 1,000 deliveries. While some clinical studies report the frequency of VTE is comparable across all three trimesters, extending up to 12 weeks postpartum, several studies demonstrate a rising risk of VTE with each passing trimester (Bates et al., 2018). The majority of studies agree that the peak incidence occurs in the immediate postpartum period, with the greatest risk within the first six weeks after delivery. This is followed by a gradual decline in risk, which becomes equivalent to a nonpregnant state by 12 weeks postpartum (Abe et al., 2019; Bates et. al., 2018; Malhotra & Weinberger, 2020; Tlamcani et al., 2018). Despite the relatively low incidence, VTE complications are a leading cause of maternal mortality. According to the National Blood Clot Alliance (NBCA, n.d.b), PE is the most common cause of pregnancy-related death. In the US, PEs account for nearly 10% of maternal deaths, or approximately 1.5 deaths per 100,000 live births (CDC, 2020b). Maternal morbidity and mortality secondary to PE are more common among women who deliver by cesarean section (C-section), as a C-section nearly doubles the risk for VTE (NBCA, n.d.b). Racial and ethnic disparities in pregnancy-related deaths have persisted across several decades. Currently, African American women have a 3 to 4 times higher pregnancy-related mortality ratio than White women. Compared to White and Asian women, Black women are at higher risk of developing a VTE and are at increased risk of death from VTE-related complications (Abe et al., 2019).
Pathophysiology
Blood clotting, commonly referred to as coagulation, is a vital mechanism that protects against blood loss due to bleeding. The coagulation process is mediated by a complex interplay of vascular mechanisms, enzymes, hormones, proteins, and cells. Hemostasis, or the cessation of bleeding, refers to the body's physiological response to stop bleeding from an injured blood vessel to prevent blood loss. The coagulation process is divided into two stages: primary hemostasis (which results in the formation of a weak platelet plug) and secondary hemostasis (which stabilizes the weak platelet plug, evolving into a secure and insoluble fibrin clot; Garmo et al., 2020). Pregnancy and postpartum are conditions characterized by Virchow's triad: venous stasis, endothelial injury, and hypercoagulability (McCance & Heuther, 2019).
Venous Stasis
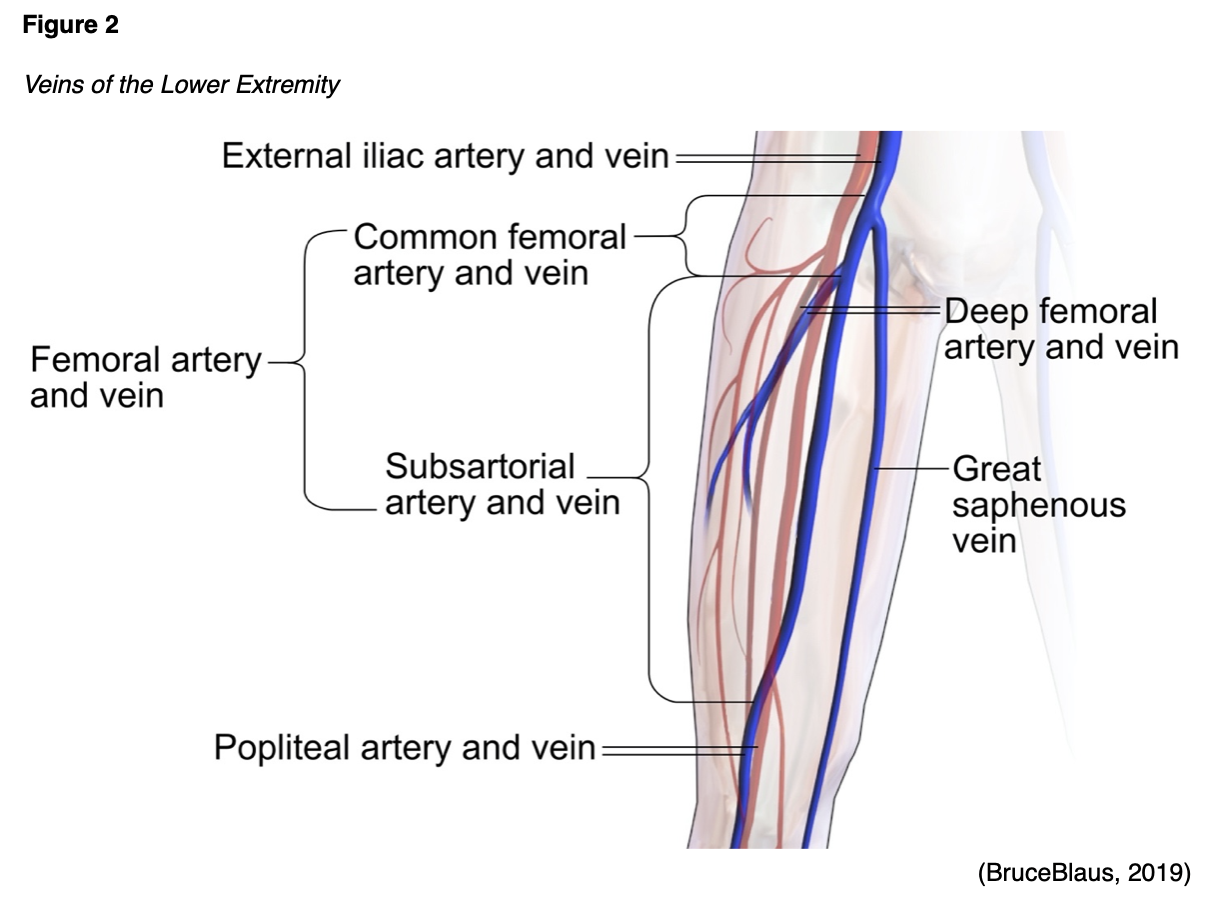
Blood tends to flow at a slower rate during pregnancy in response to the pressure induced on the mother's blood vessels from the demands of the growing fetus. This causes changes in the vascular capacitance, or the blood vessel's ability to increase the volume of blood it holds without increasing the blood pressure. Around 25 weeks' gestation, a reduction of venous flow velocity by approximately 50% occurs in the legs. The sluggish return of blood predisposes the pregnant woman to injury of the lower extremity veins and the subsequent development of VTE (Malhotra & Weinberger, 2020). As the fetus continues to grow, the uterus increases in size, causing increased pressure and compression of the iliac veins (large veins within the pelvis) and the major veins returning blood from the legs. Among pregnant and postpartum women, the left lower extremity and the iliofemoral region is the most common site for DVT, responsible for up to 82% of cases. The rationale for this is based on the anatomic location of the enlarging uterus that favors compression of the left common iliac vein. Figure 2 is a graphic depiction of the anatomy of the veins in the lower extremity (Devis & Knuttinen, 2017).
Endothelial Injury
When the blood vessel wall (endothelium) is i
...purchase below to continue the course
Hypercoagulability
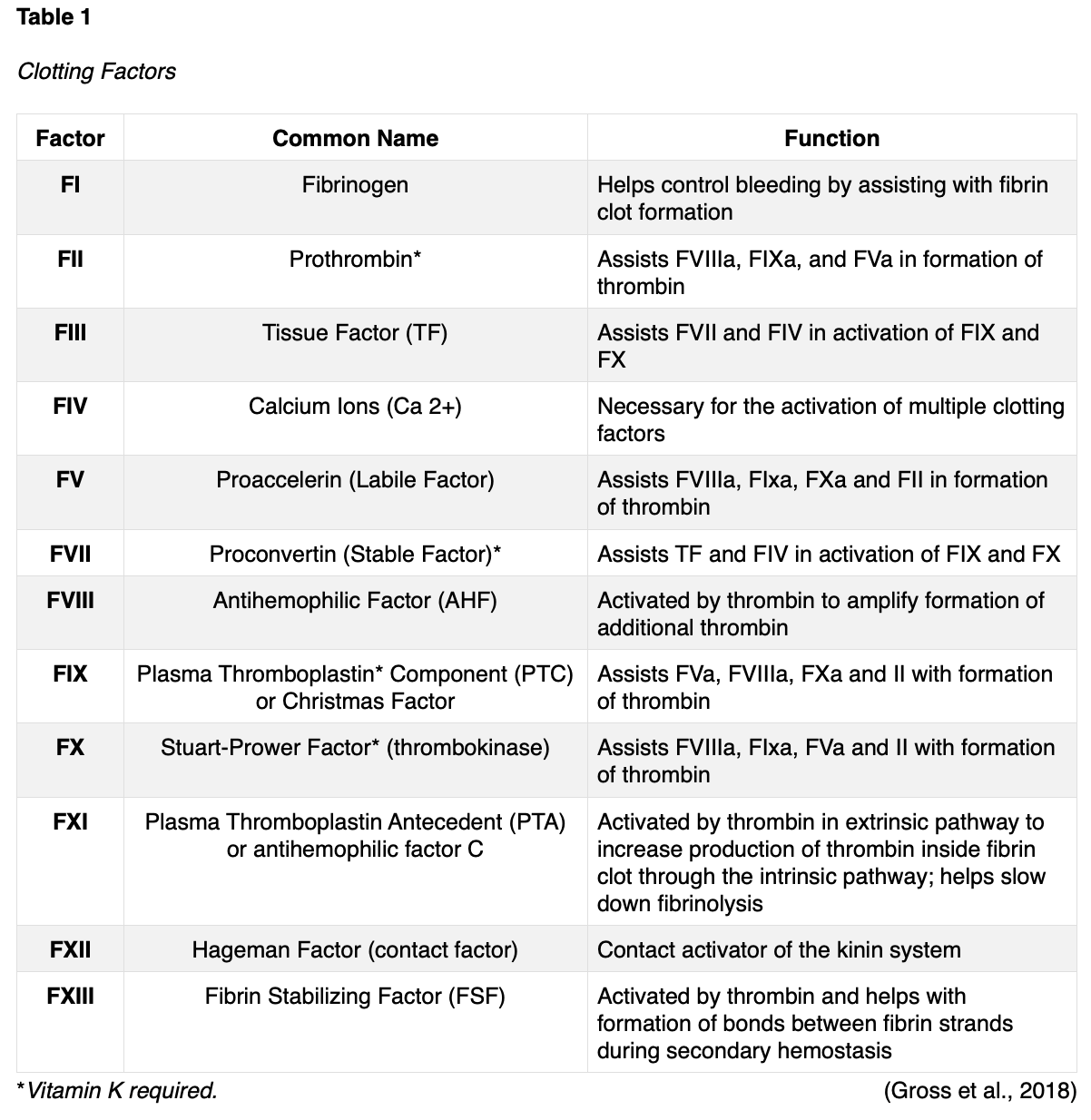
Pregnancy naturally induces a state of increased hypercoagulability in which the blood clots more easily through a set of physiologic changes. The hypercoagulable state is considered an evolutionary protective mechanism to prepare for childbirth and protect against hemorrhaging during delivery. When a woman reaches the term of the pregnancy, approximately 500 ml of blood flows through the placenta every minute. Therefore, without rapid and effective hemostasis, postpartum hemorrhage would ensue, and the mother could die from blood loss. Unfortunately, these changes also predispose women to VTE events (Abe et al., 2019). The physiologic mechanisms of hemostasis ensure appropriate clot formation via complex interactions between clotting factors within the coagulation cascade, platelets, and vascular endothelium. The fibrinolytic system prevents excessive coagulation via the removal of fibrin and unnecessary blood clots (Bauer, 2020). During pregnancy, the majority of coagulation factors increase, generating a prothrombotic state, and heightening the tendency to develop abnormal blood clots. In addition to the rise in clotting factors, there is also an increase in von Willebrand factors (vWF), which is the primary mediating factor for platelet adhesion, acting like a 'glue' and binding the platelets with enough strength to withstand stress and prevent detachment during normal hemostasis. Platelets circulating in the blood travel to the site of injury and adhere to the wall of the vessel, sticking together to form a temporary platelet plug. Platelets mainly bind to collagen in tissues at the site of vessel wall injury, as exposed vWF at the damaged vessel site ignites platelet aggregation. Therefore, the increased vWF circulating within the blood during pregnancy, leads to increased platelet activity and clotting. Table 1 provides a review of the 12 clotting factors, their primary roles, and interactions under physiologic conditions to better understand the changes in clotting factors associated with pregnancy. As a reminder, there is no clotting FVI, and clotting factors are primarily dependent upon vitamin K and calcium ions (Ca2+), which are vital for the acceleration of blood clotting (Gross et al., 2018). D-dimer is a protein fragment that is produced when the body is forming or breaking down blood clots. Under physiologic conditions, the D-dimer level is usually undetectable, or at very low levels. The levels increase when there is significant formation and breakdown of fibrin clots in the body. The test measures the amount of the substance released into the bloodstream when fibrin proteins in a blood clot dissolve. Due to the hypercoagulable state of pregnancy, the D-dimer is routinely elevated during pregnancy (Lim et al., 2018).
During pregnancy and postpartum the hypercoagulable state is attributed to a combination of increased levels of clotting factors, decreased levels of certain natural blood thinning proteins, and venous stasis. Specifically, the following major physiologic changes in hemostasis occur:
- Clotting factors I, II, VII, VIII, IX, X, and XII increase by 20% to 200%,
- Plasma fibrinogen (Factor I) concentration increases by 50%,
- Increase in vWF and platelet reactivity,
- Decline in anticoagulant activity
- Gradual and significant decrease in protein S, up to 50% by pregnancy term, and persisting for at least two months postpartum,
- Progressive increase in resistance to activated protein C,
- Steady decline in fibrinolytic capacity, which can lead to a hypo-fibrinolytic state (impaired ability to breakdown old blood clots) by the third trimester (Bates et al., 2018; Bauer, 2020; Tlamcani et al., 2018).
Risk Factors
In addition to the pathophysiological changes associated with pregnancy, several additional factors can further heighten the risk of VTEs during pregnancy. Often, there are multiple risk factors present in a single individual simultaneously. The most well-established risk factors are listed in Table 2 (Longo, 2019).

Signs and Symptoms
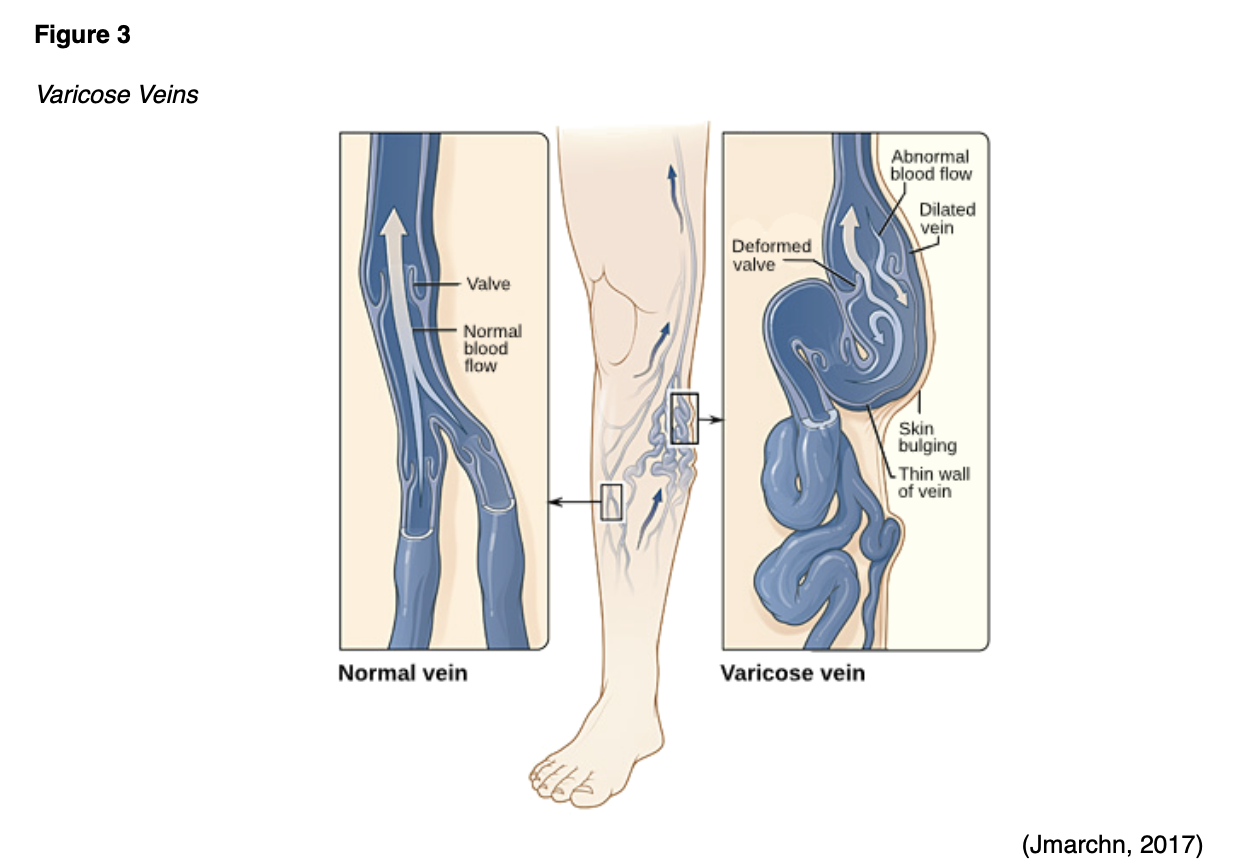
The APRN must remain vigilant to the potential signs and symptoms of VTE, as early identification and intervention are essential to mitigate complications and reduce mortality. The symptoms of VTE can overlap with the symptoms of pregnancy, which can impede early diagnosis. SVT is characterized by vein inflammation due to a thrombus directly below the skin's surface, and the most common symptoms include pain, tenderness, irritation, and erythema along the superficial vein. SVT is usually associated with varicose veins, which are enlarged, swollen, and damaged veins that appear as bulging, palpable, and bluish cords, as demonstrated in Figure 3 (Devis & Knuttinen, 2017; NBCA, n.d.b).
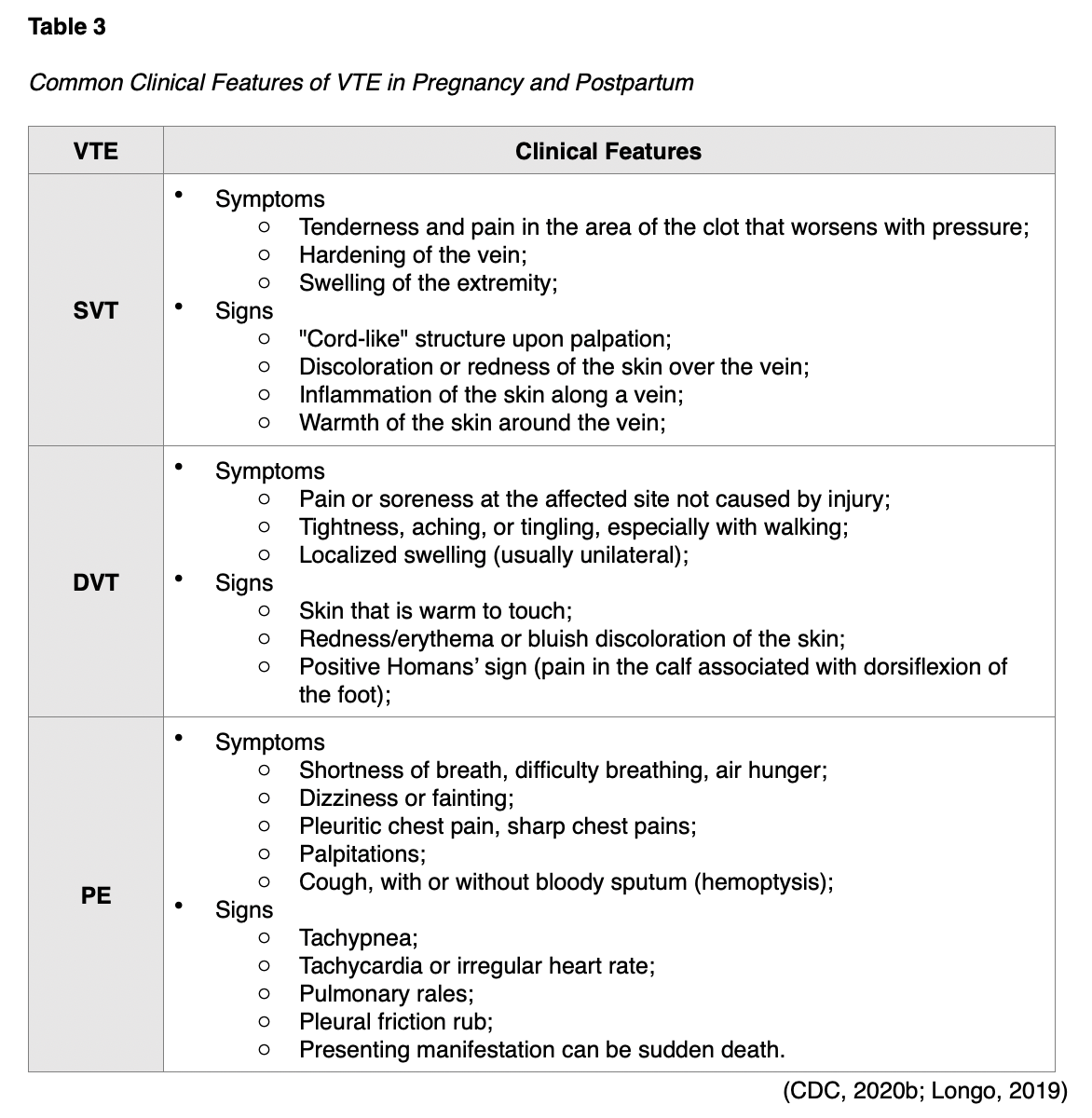
The most common symptoms of DVT include swelling and extremity discomfort, although these vary depending upon the location and the body part affected. The incidence of an isolated DVT in the iliac veins is higher during pregnancy, which can present with abdominal pain, back pain, or swelling of the entire leg (Devis & Knuttinen, 2017). The clinical features of the three types of VTE are outlined in Table 3 (Malhotra & Weinberger, 2020).
Complications of VTE
As demonstrated in Figure 4, a large/severe PE can completely obstruct blood flow and induce sudden death. They can also cause hypoxia or low oxygen levels in the blood, leading to permanent damage to the lungs or other organs in the body due to insufficient oxygen (McCance & Heuther, 2019). PE-associated mortality during pregnancy and postpartum are attributed to delayed diagnosis, delayed or inadequate treatment, and insufficient thromboprophylaxis (Devis & Knuttinen, 2017).
Additional complications associated with pregnancy-related VTE include increased risk for a recurrent VTE event, post-thrombotic syndrome (PTS), and chronic thromboembolic pulmonary hypertension (CTEPH). VTEs can also obstruct blood flow to the fetus and result in fetal demise, or neurological and developmental complications from hypoxia following birth (Devis & Knuttinen, 2017). PTS is the most common, long-term, and potentially debilitating complication of a DVT. PTS is characterized by pain and swelling in the affected extremity, which can be mild to severe, and occurs in 20-50% of patients with a DVT. PTS typically presents within three to six months of DVT diagnosis, but has been reported up to two years later, even when appropriate treatment is implemented. The clinical manifestations are similar to those of primary venous insufficiency, but the degree and severity can vary. Symptoms are generally limited to the affected leg and include swelling, pain, heaviness, and fatigue, and can be constant or intermittent. They are usually aggravated by standing or walking and improve with elevation and rest. Patients often exhibit leg redness, dusky cyanosis when the leg is in the dependent position, varicose veins, edema, and hyperpigmentation. In its most severe form, PTS can lead to venous ulcers and permanent disability. PTS is primarily diagnosed based on clinical manifestations, as there is no gold standard biomarker or test to establish the diagnosis. Approximately 60% of patients with PTS recover fully without any residual symptoms, whereas 10% develop chronic symptoms (Kahn, 2016).
CTEPH is a rare complication of a PE, reported in up to 4% of patients within the two years following the initial PE diagnosis (Abe et al., 2019). CTEPH is a condition in which abnormally high blood pressure in the arteries of the lungs causes the right side of the heart to work harder than normal, leading to heart failure. This rare and progressive condition is caused by undissolved blood clots that develop into scar tissue and impede flow in the lungs' small blood vessels. Since the most common symptom of CTEPH is shortness of breath, which is a symptom of a PE, and numerous other conditions, early diagnosis of the condition is difficult. CTEPH can be cured with a surgical intervention called pulmonary thromboendarterectomy (PTE), during which the clots are removed from the arteries in the lungs. A newer treatment, called balloon pulmonary angioplasty (BPA), involves a tiny balloon that is inflated inside the pulmonary artery to widen it. If the condition is left untreated, most patients with CTEPH will die within five years (Sahay et al., 2020).
Diagnostic Workup
All pregnant women presenting with any signs or symptoms suspicious for DVT should undergo objective diagnostic testing as soon as possible since sudden death from PE is a possibility. Unless contraindicated, the ASH recommends the initiation of anticoagulation treatment when the clinical suspicion is high, until the diagnosis of DVT is ruled out (Bates et al., 2018). For suspected DVT, compression duplex ultrasound (CDUS) with doppler evaluation of the iliofemoral region is the recommended first-line diagnostic tool (Bates et al., 2018; Devis & Knuttinen, 2017; Khan et al., 2017). In patients with an initial negative ultrasound with imaging of the iliac veins, the ASH suggests additional investigations with serial CDUS or magnetic resonance venography (MRV). A single CDUS may not be sufficient to rule out DVT, as up to 24% of DVTs in pregnancy are found on serial testing (Bates et al., 2018).
Ultrasound is a safe, noninvasive imaging modality that uses sound waves to generate images of internal body structures. CDUS involves a two-part process. First, ultrasound images are obtained by placing a small probe (transducer) and ultrasound gel against the skin. The transducer produces sound waves at very high frequencies, which exceed the threshold of human hearing. These high-frequency sound waves travel from the probe through the gel and into the body and are used to generate images on a computer. While imaging the deep veins of the leg, the sonographer tries to collapse or compress the veins. If a vein cannot be compressed, it is likely due to a clot being present, preventing the vein from collapsing. The ability to flatten a vein completely with compression is the most useful way to be certain that a clot is not present. The second part of the CDUS utilizes Doppler ultrasound, a specialized technique that evaluates blood flow through the arteries and veins in the body. Images are captured in real-time, which allows for the evaluation of the structures and movement of the body's internal organs, including blood flow through the vessels. The Doppler technique focuses on detecting abnormalities in blood flow, with the absence of blood flow confirming the diagnosis of a DVT (NBCA, n.d.c; Needleman et al., 2018). Serial CDUS involves repeating the test within seven days and is associated with a low rate of missed DVT; only 5 in 1,000 women with negative results are projected to present with symptomatic VTE during follow-up. CDUS has a sensitivity of 97% and a specificity of 94% for the diagnosis of femoropopliteal DVTs among the general population (Needleman et al., 2018). In patients with a negative or inconclusive CDUS with increased clinical suspicion for a VTE, consideration should be given toward MRV, as it can be useful in determining the true extent of a DVT into the abdomen or pelvis (Bates et al., 2018; Devis & Knuttinen, 2017). MRV is considered the second-line or alternative option for additional investigation when the initial CDUS is negative. MRV is a type of MRI scan that specifically examines the veins; however, there is limited evidence suggesting MRV of the pelvic veins might detect pelvic thrombosis not identified with ultrasonography (Bates et al., 2018).
In patients with suspected PE, ventilation-perfusion (V/Q) lung scanning is preferred over computed tomography pulmonary angiography (CTPA), as radiation exposure for the fetus should be limited. The fetal radiation dose is lower with V/Q scan, and it is considered safer for women with regards to breast-absorbed radiation dose, as it minimizes the potential impact on breast cancer risk. However, V/Q scans may not be readily accessible in all healthcare settings. In settings without access to V/Q scanners, the ASH cites a CTPA as an acceptable alternative. CTPA may also be indicated in women with abnormal chest radiographs or preexisting lung disease (Bates et al., 2018). Since the D-dimer assay test is typically positive (elevated) during uncomplicated and healthy pregnancies, it is not considered a reliable component of the diagnostic workup for VTE during pregnancy (Devis & Knuttinen, 2017). The ASH guidelines warn against a high rate of false-positive results with D-dimer testing in pregnant patients (Lim et al., 2018).
Risk Assessment and Prevention
As in the nonpregnant population, thrombosis during pregnancy can be provoked or unprovoked. This is key information to acquire during the risk assessment, as it significantly impacts management. Unprovoked VTE implies that no identifiable risk factor or underlying cause is evident or identifiable. In contrast, a provoked thrombus is caused by a known event, such as surgery, trauma, or prolonged immobility. Unprovoked events are considered more serious in pregnant and nonpregnant patients, and often require a specialized diagnostic workup and treatment, usually under the care of a hematologist. Despite the increased risk of VTE during pregnancy, the pregnancy period in itself is not considered a provoked event (Lip & Hull, 2020). In most patients, blood clots can be prevented. As part of a collaborative effort called the "Stop the Clot, Spread the Word" campaign, the CDC (2020a) joined forces with the NBCA (n.d.d) to create a checklist for women to assess their risk for blood clots during pregnancy and postpartum. The campaign strives to enhance awareness of the signs and symptoms of blood clots and educate women on their risk factors so that they can take appropriate preventative action to mitigate their risk. The campaign encourages women to complete the checklist, identify any potential risks that may apply to them, and discuss the information with their healthcare provider. A copy of the checklist is shown in Figure 5 (CDC, 2020a; NBCA, n.d.d).

The CDC (2020b) provides general recommendations for all women who are pregnant or postpartum to diminish their risk of developing a VTE, which are summarized below:
- Remain active up to delivery, and after delivering the baby, as movement is the key to preventing blood clots. If sitting for long periods, exercise the legs every one to two hours;
- Get out of bed and walk as soon as possible after delivery, even after a C-section;
- Stay well hydrated; drink 10 glasses of liquid daily during the pregnancy, and 12-13 glasses of liquid daily while breastfeeding;
- For patients on bedrest, adequate hydration and compression devices are essential (CDC, 2020b).
Anticoagulation Therapy
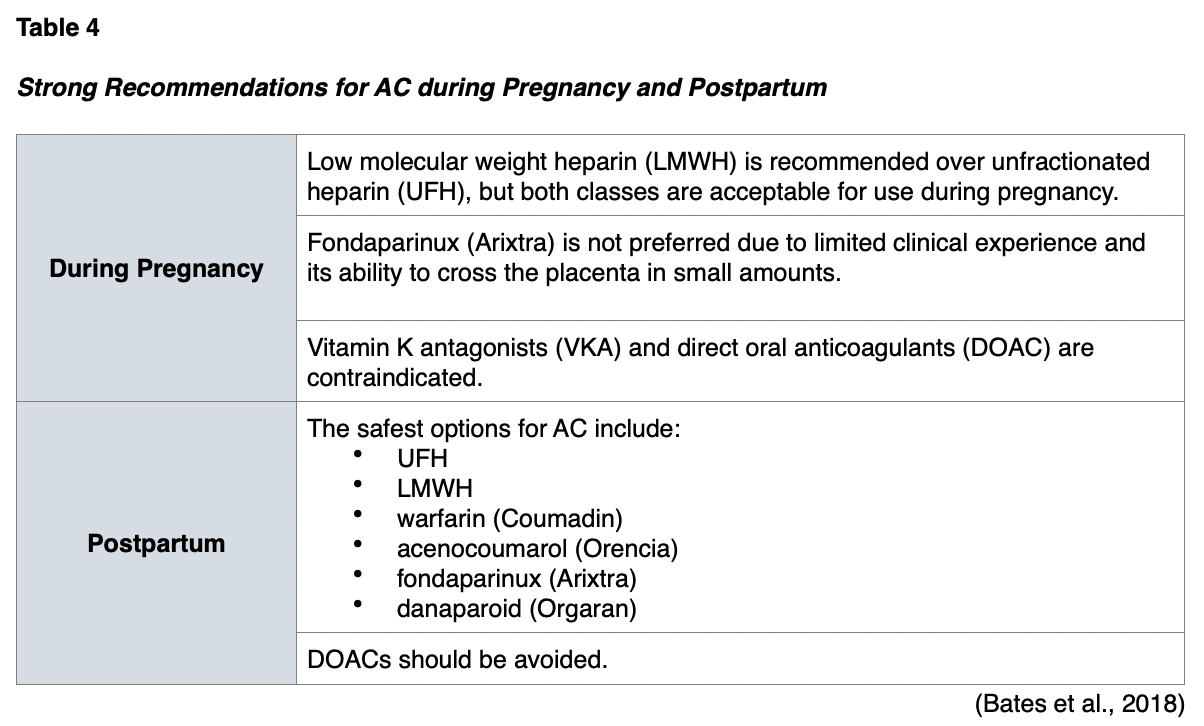
Anticoagulation (AC) is the standard treatment for acute VTE events during pregnancy and postpartum and may also be used for prophylaxis in certain high-risk patients. Anticoagulants, or "blood thinners," reduce the risk of morbidity and mortality associated with thrombotic events by helping prevent and/or dissolve existing blood clots and slow down the body's process of generating new blood clots (Witt et al., 2018). In pregnancy, the choice of AC is primarily based on safety, efficacy, and adherence to therapy. There are fewer options for AC in pregnancy than in nonpregnant adults, as consideration must be given to the health and well-being of the fetus and the mother, as well as the risk for excretion to the newborn via breastmilk. Table 4 highlights the key recommendations for AC therapy during pregnancy and postpartum, supported by the highest level of clinical evidence (Bates et al., 2018).
Unfractionated Heparin (UFH)
UFH is a rapidly acting anticoagulant agent that collaborates with antithrombin to block clot formation. UFH binds to antithrombin and enhances its ability to inhibit FXa and FIIa quickly. While UFH does not break down clots, it functions to prevent new ones from forming, allowing the body to dissolve any existing thrombi gradually. UFH is administered via subcutaneous injection or as a continuous IV infusion. Dosing is determined by body weight, and patients require frequent monitoring while on treatment with UFH to ensure appropriate dosing and safety. Since UFH does not rely heavily on the kidneys for its excretion, it is considered the treatment of choice for severely obese or severely underweight patients or those with underlying renal dysfunction (NBCA, n.d.e). The primary advantages of UFH include its rapid onset and its relatively rapid termination following discontinuation of the medication. While the definitive half-life of UFH depends on the dose, the average half-life is about 30 to 90 minutes in most healthy adults (Longo, 2019). Protamine sulfate (Prosulf) is commonly used for the reversal of the anticoagulation effect of UFH. In nonpregnant patients on UFH who develop life-threatening bleeding, the ASH recommends stopping the anticoagulant and administering protamine sulfate (Prosulf; Witt et al., 2018). However, according to the Prescribers' Digital Reference (PDR, n.d.), the use of protamine sulfate (Prosulf) was classified as pregnancy risk category C by the US Food & Drug Administration (FDA), indicating that it is not known if the drug can cause fetal harm when administered to pregnant women.
Aside from uncontrolled bleeding events, additional potential side effects of UFH include redness and irritation at the injection site, elevations in liver enzymes, reduced bone mineral density measures within four to seven years following treatment, and increased risk for osteoporotic fractures. The most clinically relevant and devastating adverse effect of the UFH is heparin-induced thrombocytopenia (HIT). HIT is a potentially fatal acquired disorder that occurs in response to the administration of UFH. Affected patients form antibodies against heparin and the platelet factor-4 (PF4) complex. Immune complexes of HIT antibodies and PF4/heparin bind to the surface of platelets and activate them. Activated platelets adhere to the blood vessel lining, promote clotting activity, and clump together, causing overuse of platelets, thereby inducing thrombocytopenia. Damage to the blood vessel wall and platelet clumping associated with HIT can lead to blood clots formation despite the presence of UFH. UFH administration is the most common cause of the condition, but LMWH agents and fondaparinux (Arixtra) also carry a potential risk, albeit less than UFH. The prevalence of HIT is up to 5% of patients receiving some form of heparin therapy, and if left untreated, 50-89% of affected patients will develop a thrombosis. In most cases, UFH is administered for seven to ten days before the onset of HIT symptoms, and the clinical presentation can be variable. The most common clinical signs include bleeding, bruising, ecchymosis, new thrombosis formation, or an increase in the size of a current thrombosis despite being on anticoagulation therapy (Indiana Hemophilia & Thrombosis Center [IHTC], 2020). The risk for HIT in pregnancy appears rare; however, most data is limited to case reports. Further, the safety and efficacy of therapeutic agents used for HIT are not well established in pregnancy (Chaudhary et al., 2015).
Since UFH does not cross the placenta, it is considered an acceptable form of AC in pregnancy. While it is less expensive than LMWH, HIT is 10-fold more common with UFH than LMWH (NBCA, n.d.e). Although the overall risk for HIT during pregnancy appears low, the risk has been premised on limited clinical data. Since the preponderance of available data in nonpregnant patients demonstrates a higher risk of HIT with UFH prophylaxis over LMWH, the ASH only recommends UFH in circumstances where LMWH is not an option; such as due to limited accessibility, high cost, or other contraindications (Bates et al., 2018). UFH may be preferred over LMWH in pregnant women with severe renal insufficiency, as its metabolism is renal and hepatic (Bauer, 2020). UFH does not pass into breast milk due to its large molecular size and negative charge and is therefore considered an acceptable option in women who are lactating (Bates et al., 2018).
Low Molecular Weight Heparin (LMWH)
LMWHs are subcutaneous injections that work by inhibiting thrombin and factor Xa. Some of the most common LMWH agents include dalteparin (Fragmin), enoxaparin (Lovenox), nadroparin (Fraxiparin), and tinzaparin (Innohep). LMWH metabolism is exclusively renal and is dosed based on the patient's body weight. ASH guidelines recommend using the patient's actual body weight when calculating the dose, including those with obesity and a body mass index (BMI) above 30. The dose of LMWH should be adjusted in patients with CrCl under 30mL/min, or an alternative agent such as UFH should be considered (Witt et al., 2018). LMWHs are preferred over UFH due to an enhanced maternal safety profile. They carry a lower risk of HIT and are less likely to be associated with reduced bone mineral density. LMWHs do not cross the placenta and are therefore safe for the developing fetus. LMWH has clinical data demonstrating that it is excreted into breastmilk in small amounts, but with limited bioavailability. Therefore, LMWH is unlikely to be absorbed by a breastfeeding infant and are considered acceptable for use in lactation (Bates et al., 2018; 2020). With regards to cost effectiveness, economic evaluations have determined that from a health care payer perspective, the use of LMWH for VTE prophylaxis has similar or lower costs than the use of UFH (Fowler et al., 2014).
Contraindications to LMWH include major acute bleeding or a history of HIT within the prior 100 days (National Comprehensive Cancer Network [NCCN], 2020). The ASH advises against the routine monitoring of anti-Xa levels to guide dosing as monitoring demonstrates no difference in recurrent VTE or bleeding risk. Currently, anti-Xa tests are unreliable, and there is no established therapeutic range for LMWH in pregnancy. Further, monitoring is not routinely available in all centers and is associated with a higher cost of care as well as more frequent blood tests and clinic visits without corresponding benefits (Bates et al., 2018; 2020).
Fondaparinux (Arixtra)
Fondaparinux (Arixtra) is a factor Xa inhibitor that is chemically related to LMWH. While it is more effective than LMWH in most studies, it carries an increased risk of bleeding. It works as a synthetic selective factor Xa inhibitor and is most commonly used to manage superficial thrombophlebitis, which can progress to a VTE if left untreated (Nisio et al., 2018). Despite these benefits, fondaparinux (Arixtra) crosses the placenta, and therefore, LMWHs are preferred during pregnancy. However, no data demonstrate that fondaparinux (Arixtra) is excreted in breastmilk and is considered a safe option in women who are breastfeeding (Bates et al., 2018; 2020).
Direct Oral Anticoagulants (DOAC)
DOACs are a relatively novel group of AC that works by directly inhibiting specific proteins within the clotting cascade. DOACs are classified into two basic categories; direct thrombin inhibitors and factor Xa inhibitors. Dabigatran (Pradaxa) is a direct thrombin inhibitor that exerts its anticoagulant effects by binding directly to thrombin, thereby inhibiting both soluble and fibrin-bound thrombin. Since thrombin enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents thrombus development. Rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa) are oral direct factor Xa inhibitors, which work by selectively and reversibly blocking the activity of FXa, thereby preventing clot formation. They affect FXa presence within the bloodstream and within a preexisting clot, but they do not affect platelet aggregation. The 2016 American College of Chest Physicians (ACCP) and 2017 European Society of Cardiology (ESC) guidelines recommend the use of DOACs over other medications as they have been proven to be non-inferior in terms of efficacy with an improved safety profile based on a reduced risk of major bleeding as compared to warfarin (Coumadin). They also carry the added advantage of a rapid onset of action and a predictable pharmacokinetic profile, which negates the need for monitoring and dose adjustments (Tritschler et al., 2018).
Since DOACs are relatively new, there remains limited clinical data regarding their safety, tolerability, and efficacy during pregnancy and postpartum. Dabigatran (Pradaxa) and other factor Xa inhibitors have demonstrated their ability to cross the placenta and are not recommended during pregnancy. Further, reproductive effects in humans remain definitively unknown at this time. Case reports suggest there is low excretion of rivaroxaban (Xarelto) into breastmilk. Due to the limited clinical experience and the potential for adverse bleeding outcomes in infants, the ASH strongly advises against the use of DOACs in breastfeeding women (Bates et al., 2018; 2020).
Vitamin K Antagonists (VKA)
VKAs are the oldest class of oral anticoagulants and prevent coagulation by suppressing the synthesis of vitamin K-dependent factors. They act as antagonists to vitamin K, impairing the liver's ability to process vitamin K into clotting factors, thereby curbing blood clotting. The goal of VKA therapy is to decrease the clotting tendency of blood, but not to prevent clotting entirely. There are several types of VKAs, which include warfarin (Coumadin), acenocoumarol (Ascumar), phenprocoumon (Marcoumar), and coumatetralyl (Racumin); however, warfarin (Coumadin) is the most widely used and well-studied oral anticoagulant in the US (Tritschler et al., 2018). As a class, VKAs are not acceptable options for AC during pregnancy. They cross the placenta and carry a potential risk for teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficits (Bates et al., 2018; 2020). Exposure during early pregnancy can cause embryopathy, whereas exposure later in pregnancy can cause intracranial hemorrhage (Bauer, 2020).
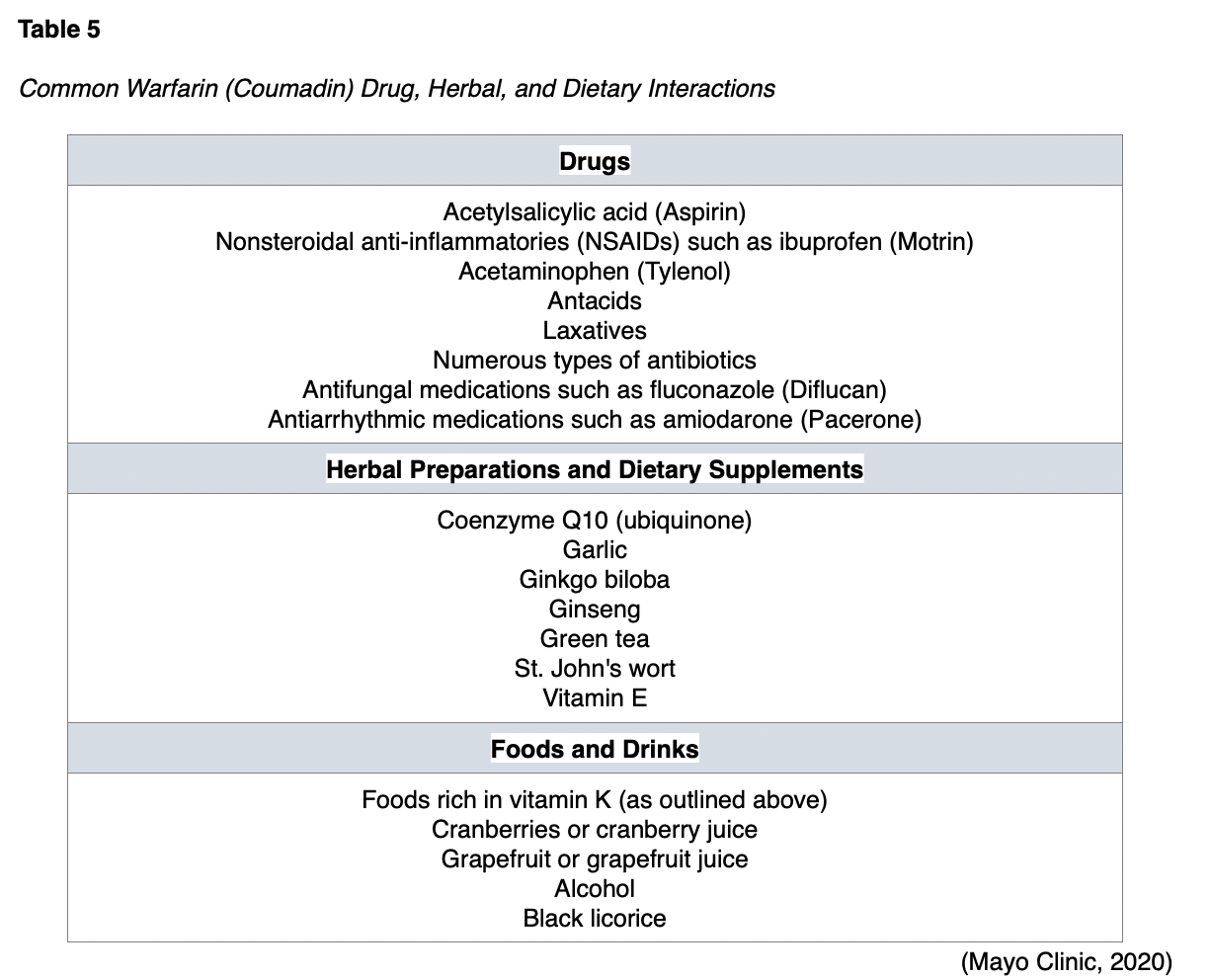
Warfarin (Coumadin) and acenocoumarol (Ascumar) are the only two VKAs considered acceptable for use in women who are breastfeeding, as they are unlikely to be secreted in breastmilk. Small clinical studies demonstrate no detectable levels of these two agents in breastmilk (Bates et al., 2018; 2020). However, both medications should be avoided within 48 hours of vaginal deliveries and within 72 hours of C-section deliveries due to heightened risk for bleeding. While the use of warfarin (Coumadin) and acenocoumarol (Ascumar) are considered acceptable in breastfeeding, they are usually reserved for patients who are not candidates for the other AC options. VKAs require more frequent monitoring and special consideration due to their side effect profile and high risk for drug and food interactions. Whereas patients on LWMHs do not require any routine monitoring of therapeutic levels, patients on warfarin (Coumadin) therapy require ongoing surveillance of blood to ensure therapeutic levels of the drug are maintained. Vitamin K is found in many foods such as green leafy vegetables and certain oils. If the consumption of vitamin K-rich foods increases, the patient will require higher warfarin (Coumadin) doses to maintain therapeutic levels. Similarly, the contrary is also true; with reduced intake of foods rich in vitamin K, the warfarin (Coumadin) dose may need to be reduced to prevent bleeding. Therefore, patients should be advised to maintain a consistent diet regarding the amount of vitamin K intake weekly. Similarly, if patients skip a dose (or take an extra dose) of warfarin (Coumadin), the warfarin (Coumadin) level may be altered for several days, as both vitamin K and warfarin (Coumadin) levels rise gradually. In addition, there are several other special considerations regarding dietary and medication interactions while on warfarin (Coumadin) therapy. Various medications, nutritional supplements, and herbal preparations can interfere with the metabolism of warfarin (Coumadin), leading to increased risk for bleeding or reduced efficacy of the medication, thereby increasing the risk for blood clot formation. Therefore, patients must be counseled on the importance of reporting any new medications or dietary preparations before starting to ensure that there are no potential interactions. Some of the most common interactions are listed in Table 5 (Bauer, 2020; Mayo Clinic, 2020).
Thromboprophylaxis
While the physiologic changes of pregnancy increase the risk of thrombosis, they are not enough in themselves to serve as an indication for pharmacological intervention in the general population (Bauer, 2020). While most women do not require thromboprophylaxis during pregnancy, all women should be subjected to vigilant clinical surveillance throughout pregnancy. Thromboprophylaxis is typically reserved for those considered to be at greater risk (Malhotra & Weinberger, 2020). VTE thromboprophylaxis can be mechanical (i.e., intermittent pneumatic compression (IPC) devices or graduated compression stockings) or pharmacological. IPC devices are cuffs placed around the legs, filled with air, that squeeze the legs. This mechanism facilitates increased blood flow through the veins of the legs to help prevent blood clots. There are many types of IPC devices, and most are used in the hospital setting (University of Rochester Medical Center, 2020). Graduated compression stockings are specialized stockings, usually prescribed by a clinician, that are available in a variety of compression strengths measured in mmHg as listed below. The stockings are tightest around the foot and gradually loosen around the calf. The stockings compress the lower extremity to help increase blood flow to the heart from the lower extremities
- 15-20 mmHg (mild) – treats mild spider veins, slight varicose veins, achy legs;
- 20-30 mmHg (moderate) – treats leg fatigue and heaviness, moderate spider veins, pronounced varicose veins;
- 30-40 mmHg (firm) – treats severe varicose veins, post sclerotherapy and prevention of post-thrombotic syndrome (Blood Clot Recovery Network, 2014).
The ASH guidelines do not provide recommendations on the use of compression devices as a prevention strategy, as recommendations are primarily focused on AC therapy. The perspective on the use of compression stockings in pregnancy varies across the literature base. Many sources cite the safe and effective use of IPC devices or graduated compression stockings following cesarean section in hospitalized women during pregnancy. However, the efficacy of mechanical thromboprophylaxis in the outpatient setting during pregnancy or the postpartum period is not well-described due to a lack of clinical evidence (Malhotra & Weinberger, 2020). In women without underlying thrombophilia who have a history of a VTE, but are not currently taking AC, the ASH makes specific recommendations regarding the need for pharmacological thromboprophylaxis. For an enhanced understanding of the clinical decision-making tool demonstrated in Figure 6, the ASH specifically cites hormonal risk factors as pregnancy, postpartum, and hormonal contraception, and nonhormonal provoking risk factors to include surgery, trauma, and prolonged immobilization (Bates et al., 2018).
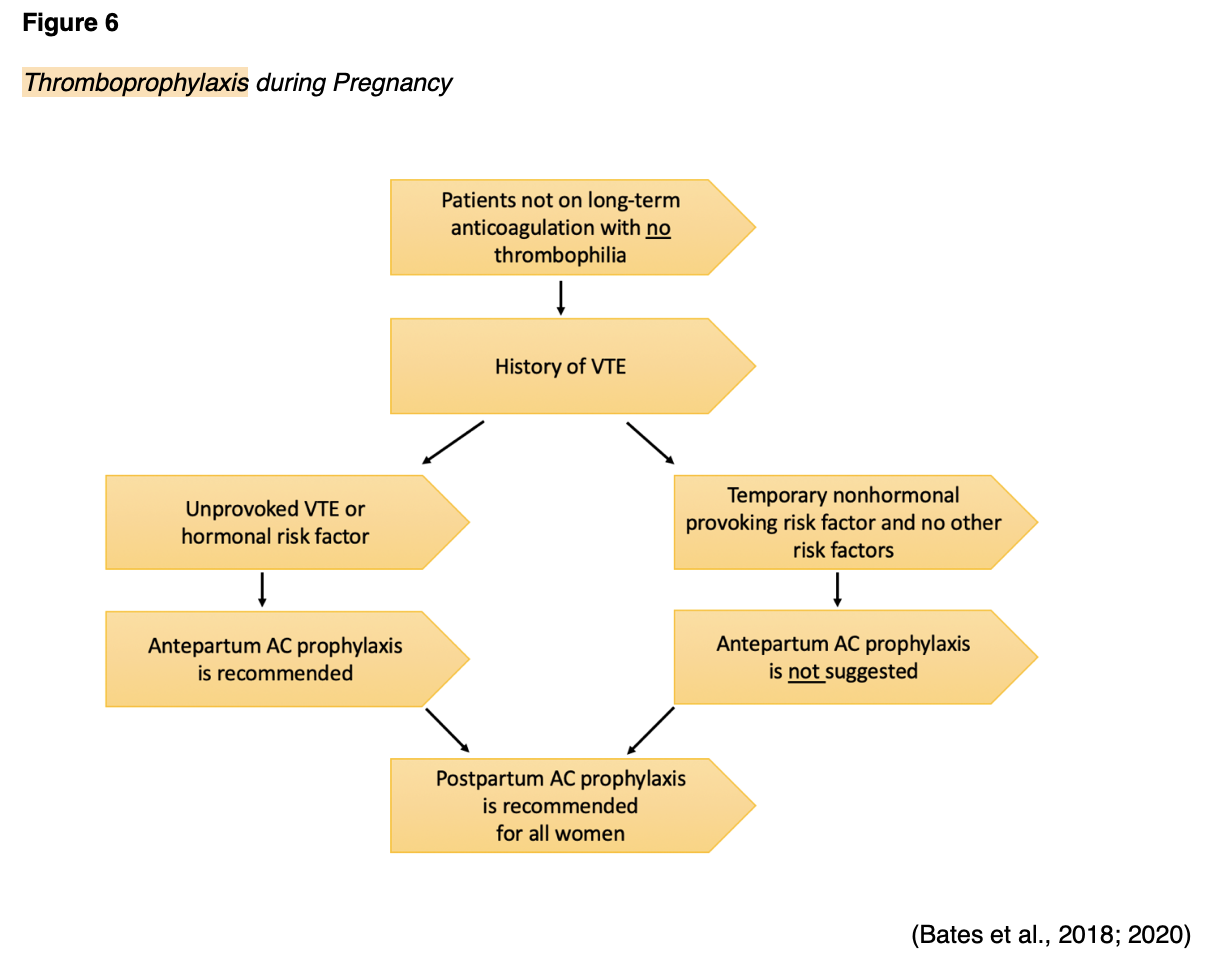
In summary, the ASH recommendations demonstrated in Figure 6 include the following:
- Women with unprovoked VTE should receive both antepartum (during pregnancy) prophylaxis and postpartum prophylaxis,
- Women with provoked VTE and hormonal risk factor(s) should receive both antepartum prophylaxis and postpartum prophylaxis,
- Women with provoked VTE and temporary nonhormonal risk factors do not require antepartum prophylaxis, but should receive postpartum prophylaxis (Bates et al., 2018).
For pregnant women who require AC thromboprophylaxis, the ASH recommends standard-dose LMWH during the antepartum period, and either standard or intermediate-dose during the postpartum period.
- Standard-dose regimens:
- Enoxaparin (Lovenox) 40 mg SC once daily;
- Dalteparin (Fragmin) 5,000 units SC once daily;
- Tinzaparin (Innohep) 4,500 units SC daily or 75 units/kg/day for patients at extremes of weight (i.e. severely underweight or severely obese).
- Intermediate-dose regimens:
- Enoxaparin (Lovenox) 40 mg SC every 12 hours or 80 mg once daily;
- Dalteparin (Fragmin) 5,000 units SC every 12 hours or 10,000 units once daily;
- Tinzaparin (Innohep) 10,000 units SC daily (Bates et al., 2018; 2020).
The American College of Obstetricians and Gynecologists (ACOG, 2018) recommends the following for thromboprophylaxis using UFH:
- 5,000–7,500 units SC every 12 hours in first trimester;
- 7,500–10,000 units SC every 12 hours in the second trimester;
- 10,000 units SC every 12 hours in the third trimester (unless the activated partial thromboplastin time [aPTT] is elevated) (ACOG, 2018, p. e27).
Treatment of Acute VTE in Pregnancy
Most patients can be safely managed on an outpatient basis and do not need to be hospitalized. However, outpatient management may not be appropriate for high-risk patients who meet any of the following criteria:
- Abnormal vital signs or hemodynamic instability,
- Severe analgesic requirements,
- Massive DVT that is potentially limb-threatening,
- Advanced gestational age,
- Maternal comorbidities,
- Contraindications to LMWH,
- Lack of home support, illiteracy, or evidence of complex difficulty with understanding the essential aspects of AC, patient education, and management (Bates et al., 2018).
Treatment with therapeutic AC markedly reduces mortality in patients with acute VTE, as well as reduces the risk of recurrent VTE and PTS in patients with DVTs. Therapeutic AC is essentially a higher dose than thromboprophylaxis to ensure adequate AC. Prior to prescribing therapeutic AC and to ensure safety, it is recommended that the patient undergo baseline laboratory testing inclusive of the following:
- Complete blood count (CBC),
- Renal and hepatic function panel,
- Coagulation profile to measure the blood's clotting capacity including prothrombin time (PT), international normalized ratio (INR), and aPTT (NCCN, 2020).
PT is measured in seconds and refers to the amount of time it takes for the plasma portion of the blood to clot. The INR is a standardized number that is calculated from the PT result. Usually, the INR is reported and monitored in patients treated with AC therapy. One of the most common reasons for performing PT/INR testing is to monitor warfarin (Coumadin) levels. Higher than normal PT/INR levels indicate a higher risk for bleeding events due to the body's impaired ability to clot blood. While on warfarin (Coumadin), it is essential that the PT/INR does not exceed therapeutic thresholds. The aPTT test is commonly performed alongside the PT/INR when evaluating patients with suspected bleeding or blood clotting disorders. While the PT test assesses how well all the coagulation factors in the extrinsic and common pathways of the coagulation cascade are functioning collectively, the aPTT evaluates the clotting factors within the intrinsic and common pathways (American Association of Clinical Chemistry [AACC], 2019b).
Acute SVT
SVTs are traditionally treated with nonsteroidal anti-inflammatories such as Ibuprofen (Motrin), compression stockings, and warm compresses. While SVT is generally considered a benign condition that can be managed conservatively, it can be complicated by extension of the superficial thrombus into the deep venous system. According to the ASH, clinicians may consider AC therapy in patients with acute SVT where there are risk factors. The ASH suggests that LMWH be prescribed for pregnant women with proven SVT; however, they acknowledge that there is low certainty of the evidence for a net health benefit from using AC for acute SVT (Bates et al., 2018; 2020).
Acute DVT
The ASH strongly recommends using LMWH as first-line therapy for the management of acute DVT, supporting the use of either once-daily or twice-per-day dosing regimens with LMWH; studies demonstrate no clear difference between dosing regimens. However, twice-per-day dosing is considered more burdensome for patients, and consideration should be given to patient compliance to dosing schedule when deciding upon which regimen to prescribe. The most commonly used therapeutic AC agent is enoxaparin (Lovenox), administered at a dose level of 1 mg/kg subcutaneous injection every 12 hours or 1.5mg/kg subcutaneous injection once daily. Additional options for therapeutic LMWH include the following:
- Dalteparin (Fragmin) 200 units/kg SC daily, or dalteparin (Fragmin) 100 units/kg SC every 12 hours;
- Tinzaparin (Innohep) 175 units/kg SC daily (ACOG, 2018).
The ASH advises against routine monitoring of anti-FXa levels in patients on therapeutic AC as there is no data to support this practice, and there is no validated therapeutic range for LMWH (Bates et al., 2018; 2020).
Acute PE
For pregnant women with acute PE who are hemodynamically stable, the ASH recommends following the same therapeutic AC measures as for acute DVT. The ASH also suggests against adding thrombolytic therapy to therapeutic AC; there is weak evidence to support its clinical benefit. In pregnant women with acute PE and life-threatening hemodynamic instability, the ASH suggests adding thrombolytic therapy to AC, although the evidence is limited. In this setting, the addition of thrombolytic therapy is associated with a 50% reduction in mortality, despite an increased risk for bleeding events (Bates et al., 2018, 2020). Refer to the next section for further information on thrombolytic therapy and clinical considerations during pregnancy.
AC with delivery
In pregnant women receiving LMWH at therapeutic doses for acute VTE, scheduled childbirth is recommended with discontinuation of the LMWH 24 hours prior to the induction. This recommendation serves to reduce the risk of maternal bleeding and hemorrhage during childbirth. Patients with proximal DVT or PE diagnosed two to four weeks prior to delivery are considered high-risk for recurrent VTE with a prolonged interruption in AC. In these patients, AC should be converted to UFH and discontinued four to six hours before expected delivery or anticipated need for epidural insertion. The aPTT level should be drawn four hours after UFH is stopped to ensure normalization (Bates et al., 2018; 2020).
Postpartum AC
Women with pregnancy-associated VTE typically require AC for at least six weeks postpartum, but many clinicians opt to continue through 12 weeks until the risk for VTE normalizes to the nonpregnant state (Bates et al., 2018; 2020).
Thrombolytic Therapy
Thrombolytic therapy is a specific type of AC that involves the use of potent AC medication geared toward directly dissolving the thrombus. Thrombolytic therapy is generally reserved for patients with serious complications related to DVT or PE, and who have a low risk of serious bleeding complications. Thrombolytic therapy is most effective when initiated shortly after the diagnosis of a DVT or PE (Pai & Douketis, 2020). The goals of thrombolytic therapy are to reduce thrombus burden. For massive PE, the objective is to reduce mortality and recurrent PE, alleviate symptoms, prevent CTEPH, and improve quality of life. For acute DVT, the goal is to alleviate symptoms, prevent PTS, improve quality of life, and in some patients, to preserve the limb (Vedantham et al., 2016).
There are several thrombolytic drugs administered systemically via the intravenous route, and the most common types include streptokinase (Streptase), urokinase (Kinlytic), recombinant tissue plasminogen activator (rt-PA, alteplase), and tenecteplase (TNKase). These agents actively dissolve the thrombus by converting plasminogen into plasmin. While each agent has specific considerations, warnings, and precautions, all carry a similar risk of bleeding. Absolute contraindications to thrombolytic therapy include intracranial hemorrhage, malignant intracranial neoplasm, active bleeding, or stroke within the prior three months (Ucar, 2019). In pregnant women, thrombolytic therapy poses a higher risk for maternal hemorrhage than other types of AC, as well as increased risk for fetal complications such as serious bleeding events, neurocognitive deficits, and fetal demise (Ho et al., 2018). The ASH guidelines warn clinicians to carefully balance the risks and benefits of using thrombolytic therapy and make the following conditional recommendations based on only low-quality evidence available in this population:
- In pregnant women with an acute PE and life-threatening hemodynamic instability, the ASH suggests administering systemic thrombolytic therapy in addition to AC therapy;
- In women who are hemodynamically stable, the ASH suggests against systemic thrombolytic therapy;
- In women with acute lower extremity DVT, the ASH suggests against adding catheter-directed thrombolysis (CDT) to AC (Bates et al., 2018).
Catheter-directed Thrombolysis (CDT)
Catheter-directed thrombolysis (CDT) is a specific type of thrombolytic therapy primarily indicated for acute iliofemoral DVT, or an extensive, multilevel DVT. As demonstrated in Figure 7, CDT is a minimally invasive targeted therapy geared toward dissolving an obstructive thrombus. CDT often involves the use of x-ray imaging to help guide the thrombolytic medication to the site of the thrombus to dissolve the blockage. However, x-rays carry radiation exposure and pose risks to the fetus premised on the age of gestation, which must be considered. The actual risk depends on how far along the pregnancy is, the type of x-ray imaging, its accompanying degree of radiation exposure, and the area of the body under examination (RadiologyInfo.org, 2019).
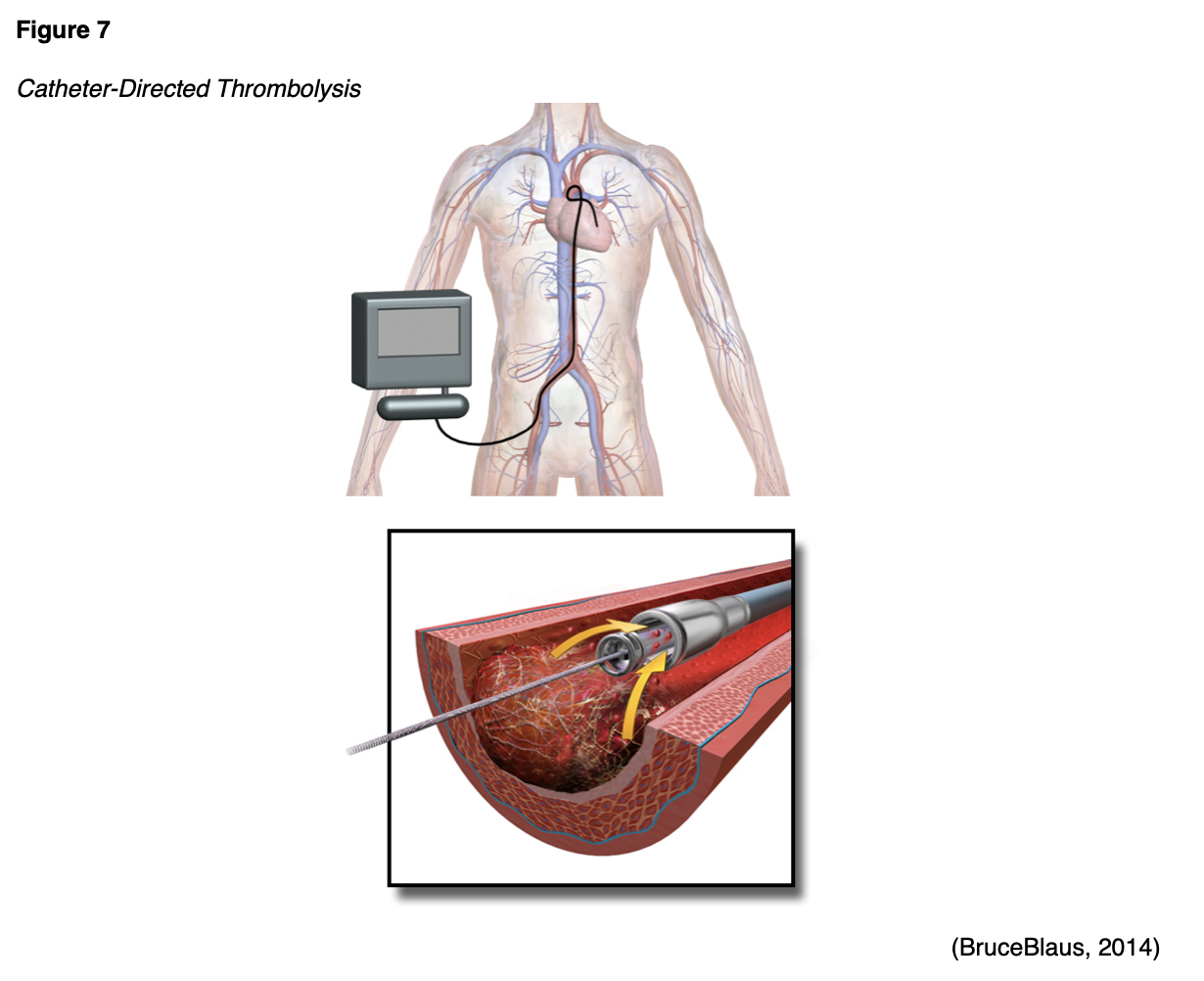
There is a lack of solid evidence regarding the clinical considerations and use of CDT in pregnant women. The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial included 300 nonpregnant patients with DVT involving the femoral vein who were randomized to receive CDT with AC or AC only. Patients were followed for 24 months, and CDT did not appear to reduce the risk of PTS in nonpregnant patients. The ATTRACT study cites potential fetal harms associated with radiation exposure and risk for major bleeding (Kearon et al., 2019). Pregnancy-specific data is limited to case series with low certainty of the evidence, so it is difficult to draw substantive conclusions regarding benefit. Therefore, at this point, CDT should be reserved for patients with severe, limb-threatening DVT (Bates et al., 2018; Kearon et al., 2019).
Part II: Thrombophilia Disorders and Pregnancy
Thrombophilia disorders are characterized by an increased propensity or predisposition for VTE. During pregnancy, the thrombogenic potential of these disorders is associated with a heightened risk for VTE due to the maternal physiologic adaptations to pregnancy (Colucci & Tsakiris, 2020). According to the American College of Obstetricians and Gynecologists (ACOG, 2018), it remains controversial whether there is an association between inherited thrombophilias and uteroplacental thrombosis leading to adverse pregnancy outcomes, such as fetal loss, severe pregnancy-induced high blood pressure (preeclampsia), intrauterine growth restriction (IUGR), stillbirth, and placental abruption. Despite the lack of definitive evidence, the potential association has led to increased screening for thrombophilias before and during pregnancy. Thrombophilia disorders may be inherited or acquired, and the most common conditions affecting pregnancy are listed in Table 6 (Colucci & Tsakiris, 2020).

Factor V Leiden (FVL)
A mutation in the FV gene causes FVL. FVL gene mutations cause factor V to be inactivated more slowly than normal, allowing the clotting process to remain active longer than usual. As a result, this increases the patient's risk for VTE (Genetic and Rare Diseases Information Center [GARD], 2017). Although the condition increases the risk of VTE, only about 10% of affected individuals ever develop abnormal clots, with DVT and PE among the most common. The chance of developing abnormal blood clots depends on whether the individual has inherited one or two copies of the FVL mutation in each cell. Those who inherit two copies of the mutation (one from each parent) are at higher risk of developing a thrombus than those who inherit one copy of the mutation. The majority of individuals affected by the condition have one "normal" factor V gene and one with the FVL gene mutation (US National Library of Medicine [NLM], 2020a). FVL can affect pregnancy outcomes as it can cause blood clots to form in the vessels of the placenta, which can lead to recurrent pregnancy loss (RPL). The American Society of Reproductive Medicine has defined RPL as two or more failed pregnancies before the 20th week of pregnancy. According to a systematic review and meta-analysis by Eslami and colleagues (2020), clinical studies have demonstrated that FVL mutation increases the risk of VTE 80 times in homozygote carriers (FVL homozygotes) and seven times in heterozygote (FVL heterozygote). FVL also increases the risk of preeclampsia (Eslami et al., 2020).
Methylenetetrahydrofolate Reductase (MTHFR)
MTHFR is an enzyme that converts dietary folate (methylenetetrahydrofolate) to its active form (methyltetrahydrofolate). Folate is essential to generate DNA and modify proteins within the body. All healthy individuals carry two copies of MTHFR, which are involved in breaking down the amino acid homocysteine into methionine. Genetic variations in the MTHFR gene can lead to decreased function or inactivation of this enzyme, which results in increased homocysteine levels; especially in those who are deficient in folate. Hyperhomocysteinemia is characterized by increased homocysteine levels in the blood and is associated with a marked increase in the risk for VTE. Elevated homocysteine levels carry prothrombotic properties, causing vascular injury, platelet accumulation, and increased risk for forming occlusive thrombosis. Approximately 10% of first episodes of VTE are due to elevated homocysteine levels (MedlinePlus, 2018). Patients who inherit the condition usually have a mutation in MTHFR, causing increased homocysteine production (Mandava, 2018). Studies suggest that women with two MTHFR variants are twice as likely to have a child with a neural tube defect (NTD); however, the risk is incredibly low, around 0.14% (Dean, 2016).
While a MTHFR mutation is cited as a potential risk factor for VTE in pregnancy, several studies have failed to demonstrate a statistical association between MTHFR mutation and VTE (GARD, 2019). According to ACOG (2018), homozygosity for the MTHFR gene mutation is the most common cause of hyperhomocysteinemia. However, by themselves, MTHFR mutations do not appear to convey an increased risk of VTE in pregnant women as elevated homocysteine levels are considered a weak risk factor of VTE (ACOG, 2018). Further, the incidence of VTE associated with MTHFR has been shown to be significantly reduced through dietary intake of folate (ObG Project, n.d.). Current clinical guidelines do not recommend changes in prenatal care based upon MTHFR gene variant status. All women of childbearing age should take the standard dose of folic acid supplementation (400 mcg per day) for NTD risk reduction in the newborn (Dean, 2016). There are many controversies surrounding the clinical significance of MTHFR gene mutation and its impact on RPL and adverse pregnancy outcomes. Several studies have demonstrated a correlation between MTHFR and recurrent miscarriages and adverse pregnancy outcomes, including IUGR, preeclampsia, preterm labor, preterm premature rupture membranes (PPROM), ablation placenta, and stillbirth. However, the evidence is limited, and the impact of MTHFR on pregnancy outcomes requires additional investigation (ObG Project, n.d.; Turgal et al., 2018).
Prothrombin G20210A (prothrombin gene mutation [PGM])
Patients with PGM produce excess amounts of prothrombin (FII), which leads to an abundance of thrombin in the circulation, thereby increasing the tendency to form VTE. Research suggests that PGM is associated with an increased risk of RPL and may also increase the risk of other complications during pregnancy, such as preeclampsia, slow fetal growth, and placental abruption. However, the majority of women with PGM have normal pregnancies (NLM, 2020c).
Protein C and Protein S Deficiency
Protein C and protein S are part of the body's innate anticoagulation system. Therefore, a deficiency in one or both of these proteins, or if either protein's functioning is impaired, clot formation can occur unregulated. Patients with deficiencies in either or both proteins are at increased risk for VTE events (NLM, 2020b). Inherited protein C deficiency is caused by a genetic mutation in the PROC gene. The degree of deficiency can be mild to severe. In the rare instance that a patient inherits two mutated copies of the protein C gene, the disease can be very severe, heightening the risk for intracranial thromboembolism in infants and VTE during childhood. Although rare, newborns who are homozygous for protein C deficiency are at risk for neonatal purpura fulminans, a life-threatening condition involving severe clotting throughout the body. Inherited protein S deficiency is caused by a genetic mutation in the PROC1 gene and may occur in conjunction with protein C deficiency. Patients who inherit two mutated copies are at higher risk for developing a more severe form of purpura fulminans, which can lead to hemorrhagic tissue necrosis or disseminated intravascular coagulation (DIC) (AACC, 2019a). Deficiencies in either or both of these proteins are associated with placental thrombosis, hypoperfusion, fetal loss, and stillbirth (ObG Project, 2018).
Antithrombin Deficiency (ATD)
Antithrombin (AT) is also part of the body's natural anticoagulation system, serving as the primary inhibitor of thrombin. Patients with inherited ATD have reduced levels of AT circulating within their blood and are at a lifelong predisposition to developing VTEs. The majority of patients affected by inherited ATD endure their first VTE before age 40, usually in the form of a DVT or SVT. About 40% of patients with ATD develop a PE, which makes the condition particularly dangerous (NBCA, n.d.a). Women with ATD are at particularly high risk for developing clots during pregnancy or after delivery. According to the National Organization for Rare Diseases (NORD, 2018), the incidence of VTE during pregnancy in women with ATD can extend up to 50%. Women are also at a marginally increased risk for fetal loss in the absence of appropriate treatment, most likely due to thrombus formation in the placenta, obstructing blood and oxygen to the fetus (NBCA, n.d.a).
Antiphospholipid Antibody Syndrome (APS)
APS is the most common acquired thrombophilia disorder during pregnancy. APS is a systemic autoimmune disorder characterized by thrombosis and heightened risk for RPL secondary to the presence of antiphospholipid antibodies (aPL) (Lockwood & Lockshin, 2020). The body develops aPL that attack and damage phospholipids, which are present on the lining of blood vessels and play an important role in maintaining the integrity of cell wall membranes. When these antibodies attack phospholipids, the blood cells become damaged, degrade, and lead to thrombosis formation. Arterial and/or VTE development is the hallmark of the condition. Patients who have APS are also at higher risk for thrombocytopenia. The antibodies either destroy the platelets, or the platelets are used up during the clotting process; this heightens the risk for mild to serious bleeding events (National Heart, Lung, and Blood Institute [NHLBI], n.d.).
APS can affect up to 5% of women during pregnancy and is considered an established acquired thrombophilia risk factor leading to increased risk for RPL (Eslami et al., 2020). Aside from RPL, pregnant women with APS are at increased risk IUGR, placental insufficiency, preeclampsia, premature birth, and stillbirth. Pregnant women with APS can still have successful pregnancies, as treatment during pregnancy reduces the frequency of thrombosis and associated adverse pregnancy outcomes (Lockwood & Lockshin, 2020). Although APS is the most common acquired thrombophilia of pregnancy, interestingly, neither the ASH and ACOG guidelines outline thromboprophylaxis or management of the condition. Some sources cite that APS should be suspected in patients who develop one or more unprovoked venous or arterial thrombotic events, especially young patients. APS should also be considered in patients who endure specific adverse outcomes related to pregnancy, such as fetal death after ten weeks' gestation, premature birth due to severe preeclampsia or placental insufficiency, or multiple embryonic losses under ten weeks' gestation (Erkan & Ortel, 2020). Nevertheless, the ACOG (2018) guidelines only briefly mention testing for the presence of the acquired antibodies, but only in the setting of RPL or stillbirth; otherwise, it is not routinely included in the thrombophilia workup (ACOG, 2018).
Risk Assessment
It is estimated that 5 to 8% of the US population has a genetic risk factor known to predispose VTE. Knowledge of the risk for a VTE and its associated complications help guide clinical decision-making, prevention, and management during the pregnancy and postpartum period (Colucci & Tsakiris, 2020). Inherited thrombophilia disorders are further broken down into high-risk types and low-risk types, as denoted in Table 7. Studies consistently demonstrate the highest incidence of VTE in women with high-risk variants (homozygotes) of inherited thrombophilia (Malhotra & Weinberger, 2020).
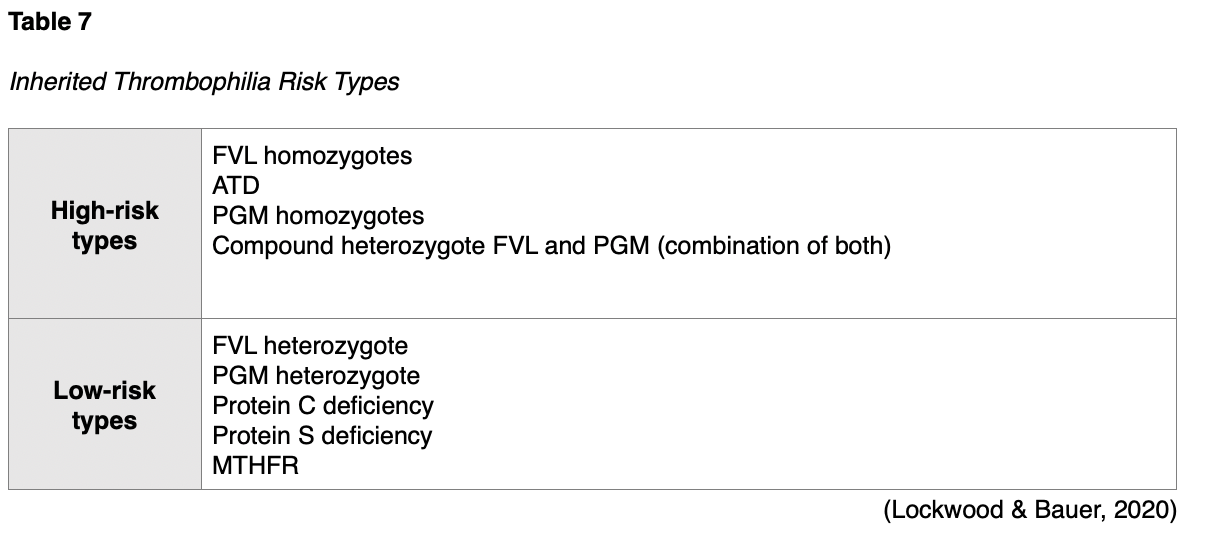
Cross and colleagues (2017) performed a systematic review and meta-analysis evaluating pregnancy, thrombophilia, and the risk for a first VTE. They concluded that women with a positive family history for VTE have a 3.7-fold to 8.5-fold increased risk of pregnancy-associated VTE. Inherited thrombophilia increases the risk of pregnancy-associated VTE up to 34-fold. History of a prior VTE indicates an increased risk for another VTE with subsequent pregnancy (Bates et al., 2018). In women with FVL homozygote, the ACOG (2018) cites a 2.2% to 14.0% risk of VTE per pregnancy without a prior VTE, whereas the risk increases to 17% in women who have had a previous VTE. While the ASH and ACOG guidelines collectively acknowledge the limited evidence available to guide the screening for and management of thrombophilias in pregnancy, both support the need for the following critical information to confer individual risk:
- Personal history of a prior VTE (in either pregnant or non-pregnant state),
- Family history of prior VTE in a first-degree relative (parents, siblings, or children),
- Risk level (high versus low) of the underlying thrombophilia disorder (ACOG, 2018; Bates et al., 2018).
Screening for Thrombophilia Disorders
ACOG cautions against the routine screening for thrombophilia disorders in pregnancy, as screening is "useful only when results will affect management decisions and it is not useful in situations in which treatment is indicated for other risk factors" (p. e23). ACOG supports the targeted assessment for inherited thrombophilia in only the following clinical situations and if testing results are suspected to influence management:
- A personal history of VTE, with or without a recurrent risk factor, and no prior thrombophilia testing;
- A first-degree relative with a history of a high-risk inherited thrombophilia.
For all other clinical scenarios, ACOG advises against routine thrombophilia testing. Screening is not recommended for women with a history of fetal loss or adverse pregnancy outcomes since there is insufficient evidence that prophylaxis during pregnancy would prevent recurrence in these patients (ACOG, 2018).
Diagnostic Workup
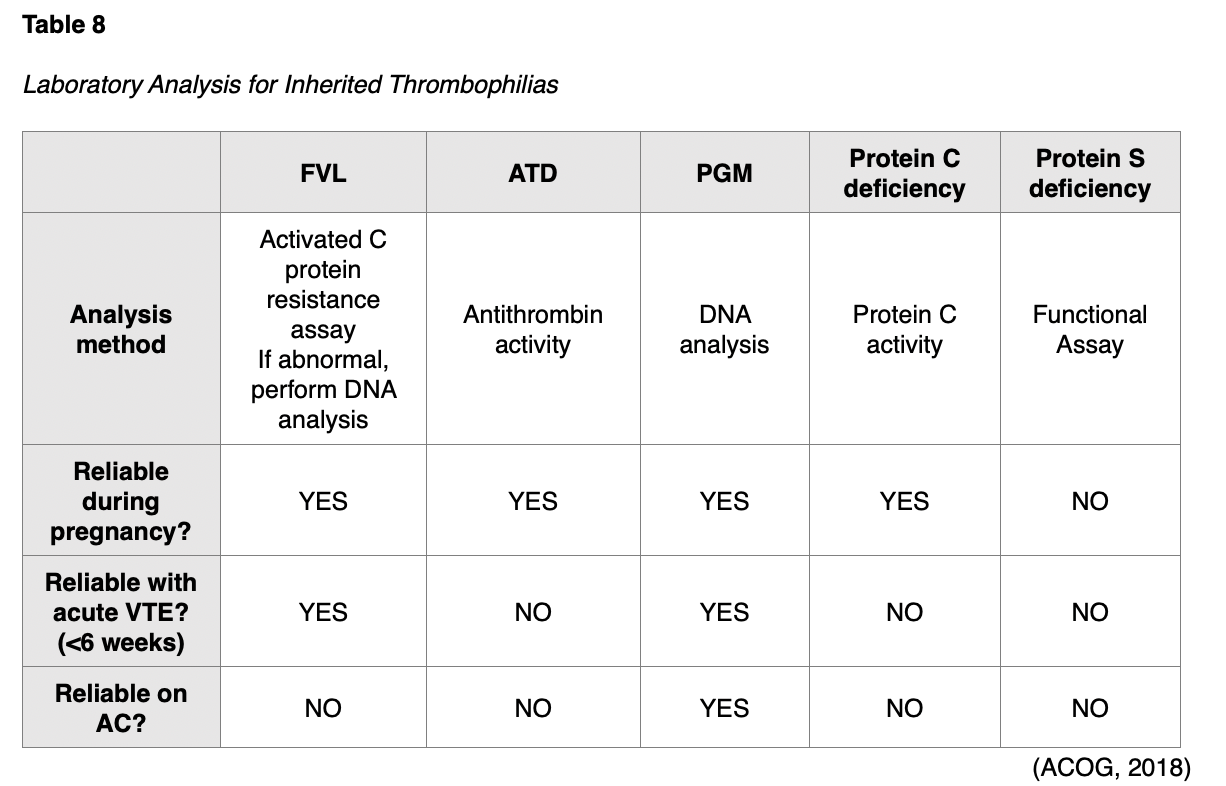
For women who meet the criteria to undergo screening for thrombophilia, the recommended screening panel includes the following:
- FVL mutation,
- Prothrombin G20210A mutation,
- Antithrombin deficiency,
- Protein C deficiency,
- Protein S deficiency,
- Antiphospholipid antibodies (ACOG, 2018).
The optimal time for performing these tests is at least six weeks following the thrombotic event and ideally when the patient is not pregnant, as certain testing may not be reliable during pregnancy. Further, AC and hormonal therapies can also lead to unreliable findings. For instance, when evaluating for ATD, clinicians should be aware that warfarin (Coumadin) can increase AT levels. Therefore, a normal level in the setting of warfarin (Coumadin) therapy does not definitively rule out the presence of ATD (NBCA, n.d.a). Table 8 designates the testing method and clinical considerations for performing the diagnostic workup. Since current evidence demonstrates a lack of association between MTHFR and negative pregnancy outcomes, including the increased risk for VTE, ACOG advises against screening for MTHFR mutation analysis (ACOG, 2018).
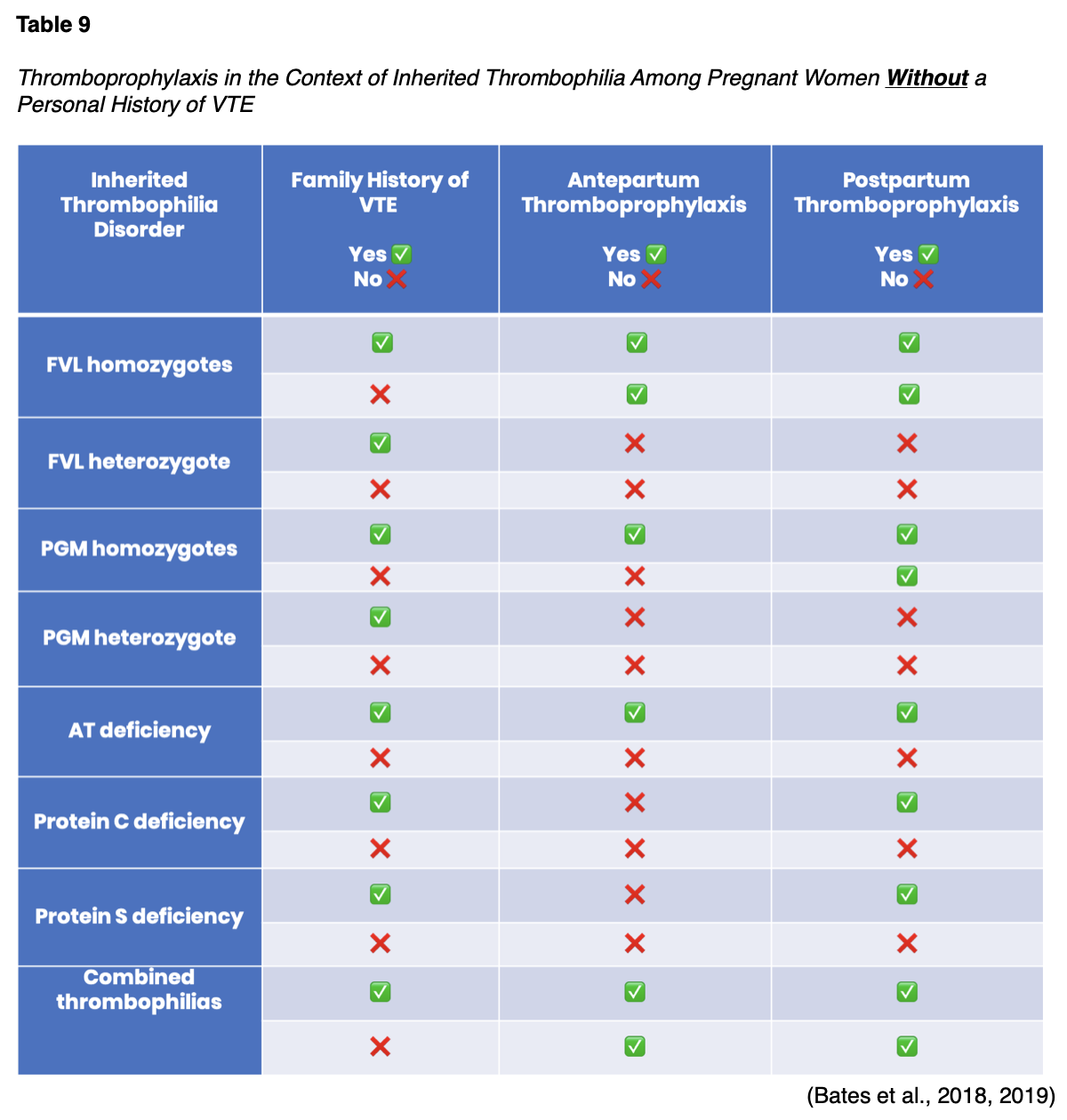
Thromboprophylaxis in the Context of Inherited Thrombophilia
All pregnant women with an inherited thrombophilia disorder should undergo an individualized risk assessment. Women with a personal history of VTE should consistently receive both antepartum and postpartum thromboprophylaxis. Women who are currently receiving long-term AC should continue on AC throughout the antepartum and postpartum period, as long as it is acceptable. Thromboprophylaxis dosing follows the same recommendations as described earlier in women without underlying thrombophilia disorders (Bates et al., 2018). The management of pregnant women without a personal history of VTE is more complex and relies heavily on the risk level of the specific inherited thrombophilia in conjunction with the family history of VTE. Table 9 outlines ASH recommendations for pregnant women with underlying thrombophilia disorders who have not had a prior VTE. All of the recommendations in Table 9 are conditional recommendations due to limited and low-quality evidence, except for the following, which are considered strong recommendations:
- In pregnant women with protein C deficiency who have a positive family history of VTE, the ASH strongly recommends postpartum thromboprophylaxis;
- In pregnant women with protein S deficiency who have a positive family history of VTE, the ASH strongly recommends postpartum thromboprophylaxis;
- In pregnant women with AT who have a positive family history of VTE, the ASH strongly recommends postpartum thromboprophylaxis (Bates et al., 2018, 2019, 2020).
Closing Thoughts
The guidelines regarding the appropriate management of blood clotting during the pregnancy and postpartum period are fluid and continually changing as new evidence and clinical data are unveiled. The exact effects of inherited thrombophilia on pregnancy outcomes remain controversial. Many women with a personal or family history of VTE or a diagnosis of an inherited thrombophilia disorder require specialized care under the direction of a high-risk obstetrician and/or a hematology specialist.
References
Abe, K., Kuklina, E. V., Hooper, W. C., & Callaghan, W. M. (2019). Venous thromboembolism as a cause of severe maternal morbidity and mortality in the United States. Seminars in Perinatology, 43(3), 200-204. https://doi.org/10.1053/j.semperi.2019.03.004
American Association for Clinical Chemistry. (2019a). Protein C and protein S. https://labtestsonline.org/tests/protein-c-and-protein-s
American Association of Clinical Chemistry. (2019b). Prothrombin time and international normalized ratio (PT/INR). https://labtestsonline.org/tests/prothrombin-time-and-international-normalized-ratio-ptinr
American College of Obstetricians and Gynecologists. (2018). ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists. Obstetrics & Gynecology, 132(1), e18-e34. https://doi.org/10.1097/AOG.0000000000002703
Bates, S. M., Rajasekhar, A., & McLintock, C. (2019). Venous thromboembolism (VTE) in the context of pregnancy. http://ashpocketguides.hematology.org/#/app/guides/26/pages/
Bates, S. M., Rajasekhar, A., Middeldorp, S., McLintock, C., Rodger, M. A., James, A. H., Vazquez, S. R., Greer, I. A., Riva, J. J., Bhatt, M., Schwab, N., Barrett, D., LaHaye, A., & Rochwerg, B. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Advances, 2(22), 3317-3359. https://doi.org/10.1182/bloodadvances.2018024802
Bates, S., Rochwerg, B., Riva, J., Bhatt, M., & Schwab, N. (2020). Should antithrombotic therapy (unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) vs. no antithrombotic intervention be used for pregnant women with acute VTE? Gradepro GDT. https://guidelines.gradepro.org/profile/FC27E96F-2A38-D41D-9C2D-0E39F7EBBE93
Bauer, K. (2020). Maternal adaptations to pregnancy: Hematologic changes. UpToDate. https://www.uptodate.com/contents/maternal-adaptations-to-pregnancy-hematologic-changes
Blood Clot Recovery Network. (2014). Your guide to compression stockings. https://bloodclotrecovery.net/?s=compression+stockings
BruceBlaus. (2014). Catheter-directed thrombolysis [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Blausen_0024_Angiojet.png
BruceBlaus. (2019). Veins of the lower extremity [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Common_femoral_and_subsartorial_artery_and_vein.jpg
The Centers for Disease Control and Prevention. (2020a). Blood clot risk checklist for pregnant women. https://www.cdc.gov/ncbddd/dvt/materials/pregnancy-blood-clot-risk-checklist.html
The Centers for Disease Control and Prevention. (2020b). Expecting or recently had a baby? Learn about blood clots. https://www.cdc.gov/ncbddd/dvt/infographics/blood-clot-pregnancy-info.html
The Centers for Disease Control and Prevention. (2020c). Venous thromboembolism (blood clots) and pregnancy. https://www.cdc.gov/ncbddd/dvt/pregnancy.html
Chaudhary, R. K., Nepal, C., Khanal, N., Pathak, R., Giri, S., & Bhatt, V. R. (2015). Management and outcome of heparin-induced thrombocytopenia in pregnancy: A systematic review. Cardiovascular & Hematological Agents in Medicinal Chemistry, 13(2), 92-97. https://pubmed.ncbi.nlm.nih.gov/26695420/
Colucci, G., & Tsakiris, D. A. (2020). Thrombophilia screening revisited: An issue of personalized medicine. Journal of Thrombosis and Thrombolysis, 49, 618-629. https://doi.org/10.1007/s11239-020-02090-y
Cross, F. N., Nasserinejad, K., Duvekot, J. J., Kruip, M. J., Meijer, K., & Leebeek, F. W. (2017). Pregnancy, thrombophilia, and the risk of a first venous thrombosis: Systematic review and bayesian meta-analysis. BMJ, 359, j4452. https://doi.org/10.1136/bmj.j4452
Dean, L. (2016). Methylenetetrahydrofolate reductase deficiency. Medical Genetics Summaries. https://www.ncbi.nlm.nih.gov/books/NBK66131/
Devis, P., & Knuttinen, M. G. (2017). Deep vein thrombosis in pregnancy: Incidence, pathogenesis and endovascular management. Cardiovascular Diagnosis & Therapy, 7(Suppl 3), S309-S319. http://dx.doi.org/10.21037/cdt.2017.10.08
Erkan, D., & Ortel, T. (2020). Diagnosis of antiphospholipid syndrome. UpToDate. https://www.uptodate.com/contents/diagnosis-of-antiphospholipid-syndrome
Eslami, M. M., Khalili, M., Soufizomorrod, M., Abroun, S., & Razi, B. (2020). Factor V leiden 1691G > A mutation and the risk of recurrent pregnancy loss (RPL): Systematic review and meta-analysis. Thrombosis Journal, 18(11), 1-16. https://doi.org/10.1186/s12959-020-00224-z
Fowler, R. A., Mittman, N., Geerts, W., Heels-Ansdell, D., Gould, M. K., Guyatt, G., Krahn, M., Finfer, S., Pinto, R., Chan, B., Ormanidhi, O., Arabi, Y., Qushmaq, I., Rocha, M. G., Dodek, P., McIntyre, L., Hall, R., Fergusen, N. D., Mehta, S., … Cook, D. (2014). Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA, 312(20), 2135-2145. https://doi.org/10.1001/jama.2014.15101
Garmo, C., Bajwa, T., & Burns, B. (2020). Physiology, clotting mechanism. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK507795/
Genetic and Rare Diseases Information Center. (2017). Factor V Leiden thrombophilia. https://rarediseases.info.nih.gov/diseases/6403/factor-v-leiden-thrombophilia
Genetic and Rare Diseases Information Center. (2019). MTHFR gene variant. https://rarediseases.info.nih.gov/diseases/10953/mthfr-gene-mutation#ref_13395
Gross, P. L., Weil, P., & Rand, M. L. (2018). Harper’s illustrated biochemistry. (31st ed.). McGraw-Hill.
Ho, V. T., Dua, A., Lavingia, K., Rothenberg, K., Rao, C., & Desai, S. S. (2018). Thrombolysis for venous thromboembolism during pregnancy: A literature review. Vascular and Endovascular Surgery, 52(7), 527-534. https://doi.org/10.1177/1538574418777822
Indiana Hemophilia & Thrombosis Center. (2020). Heparin-induced
thrombocytopenia. https://www.ihtc.org/heparin-induced- thrombocytopenia/
Jmarchn. (2017). Varicose veins [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Varicose_veins-en.svg
Kahn, S. R. (2016). The post-thrombotic syndrome. Hematology, 2016(1), 413-418. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6142466/
Kearon, C., Gu, C., Julian, J. A., Goldhaber, S.Z., Comerota, A. J., Gornik, H. L., Murphy, T. P., Lewis, L., Kahn, S. R., Kindzelski, A. L., Slater, D., Geary, R., Winokur, R., Natarajan, K., Dietzek, A., Leung, D. A., Kim, S., & Vedantham, S. (2019). Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: Analysis from a stratified randomized trial. Thrombosis and Haemostasis, 119(4), 633-644. https://doi.org/10.1055/s-0039-1677795
Khan, F., Vaillancourt, C., & Bourjelly, G. (2017). Diagnosis and management of deep vein thrombosis in pregnancy. BMJ, 357, j2344. https://www.bmj.com/content/357/bmj.j2344
Lim, W., Gal, G. L., Bates, S. M., Righini, M., Haramati, L. B., Lang, E., Kline, J. A., Chasteen, S., Snyder, M., Patel, P., Bhatt, M., Patel, P., Braun, C., Begum, H., Wiercioch, W., Schunemann, H. K., & Mustafa, R. A. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Advances, 2(22), 3226–3256. https://doi.org/10.1182/bloodadvances.2018024828
Lip, G., & Hull, R. D. (2020). Overview of the treatment of lower deep vein thrombosis (DVT). UpToDate. https://www.uptodate.com/contents/overview-of-the-treatment-of-lower-extremity-deep-vein-thrombosis-dvt
Lockwood, C. J., & Bauer, K. A. (2020). Inherited thrombophilias in pregnancy. UpToDate. https://www.uptodate.com/contents/inherited-thrombophilias-in-pregnancy
Lockwood, C. J., & Lockshin, M. D. (2020). Antiphospholipid syndrome: Pregnancy implications and management in pregnant women. UpToDate. https://www.uptodate.com/contents/antiphospholipid-syndrome-pregnancy-implications-and-management-in-pregnant-women
Longo, D. L. (2019). Harrison’s hematology and oncology. (3rd ed.). McGraw-Hill Education.
Malhotra, A., & Weinberger, S. E. (2020). Deep vein thrombosis in pregnancy: Epidemiology, pathogenesis, and diagnosis. UpToDate. https://www.uptodate.com/contents/deep-vein-thrombosis-in-pregnancy-epidemiology-pathogenesis-and-diagnosis
Mandava, P. (2018). Homocystinuria/homocyteinemia. https://emedicine.medscape.com/article/1952251-overview#a1
Mayo Clinic. (2020). Warfarin side effects: Watch for interactions. https://www.mayoclinic.org/diseases-conditions/deep-vein-thrombosis/in-depth/warfarin-side-effects/art-20047592
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
MedlinePlus. (2018). Homocysteine test. https://medlineplus.gov/lab-tests/homocysteine-test/
National Blood Clot Alliance. (n.d.a). Antithrombin deficiency. Retrieved July 17, 2020, from https://www.stoptheclot.org/news/antithrombin-deficiency/
National Blood Clot Alliance. (n.d.b). Expecting or recently had a baby? Don’t let a blood clot spoil your joy. Retrieved July 4, 2020, from https://www.stoptheclot.org/spreadtheword/pregnancy/
National Blood Clot Alliance. (n.d.c). How is DVT diagnosed? Retrieved July 11, 2020, from https://www.stoptheclot.org/learn_more/signs-and-symptoms-of-blood-clots/how_dvt_is_diagnosed/
National Blood Clot Alliance. (n.d.d). Stop the clot, spread the word. Retrieved July 5, 2020, from https://www.stoptheclot.org/spreadtheword/
National Blood Clot Alliance. (n.d.e). Unfractionated heparin (UFH). Retrieved July 14, 2020, from https://www.stoptheclot.org/about-clots/blood-clot-treatment/unfractionated-heparin/#:~:text=2020%20NYC%20Marathon-,Unfractionated%20Heparin%20(UFH),body%2C%20to%20block%20clot%20formation
National Comprehensive Cancer Network. (2020). NCCN guidelines version 1.2020 cancer-associated venous thromboembolic disease. https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf
National Heart, Lung, and Blood Institute. (n.d.). Antiphospholipid antibody syndrome. Retrieved July 17, 2020, from https://www.nhlbi.nih.gov/health-topics/antiphospholipid-antibody-syndrome
National Organization for Rare Diseases. (2018). Antithrombin deficiency. https://rarediseases.org/rare-diseases/antithrombin-deficiency/
Needleman, L., Cronan, J. J., Lilly, M. P., Merli, G. J., Adhikari, S., Hertzberg, B. S., DeJong, R., Streiff, M. B., & Meissner, M. H. (2018). Ultrasound for lower extremity deep venous thrombosis: Multidisciplinary recommendations from the society of radiologists in ultrasound consensus conference. Circulation, 137, 1505-1515. https://doi.org/10.1161/CIRCULATIONAHA.117.030687
Nisio, M. D., Wichers, I., & Middeldorp, S. (2018). Treatment of lower extremity superficial thrombophlebitis. JAMA, 320(22), 2367–2368. https://doi.org/10.1001/jama.2018.16623
ObG Project. (n.d.) MTHFR Polymorphism testing – the evidence isn’t there. Retrieved July 18, 2020, from https://www.obgproject.com/2017/07/23/mthfr-polymorphism-testing-evidence-isnt/
ObG Project. (2018). ACOG guidance on thrombophilia in pregnancy. https://www.obgproject.com/2018/07/18/acog-guidance-on-thrombophilia-in-pregnancy/
Pai, M., & Douketis, J. D. (2020). Patient education: Deep vein thrombosis (DVT) (Beyond the basics). UpToDate. https://www.uptodate.com/contents/deep-vein-thrombosis-dvt-beyond-the-basics
Prescribers’ Digital Reference. (n.d.). Protamine sulfate - drug summary. Retrieved July 11, 2020, from https://www.pdr.net/drug-summary/Protamine-Sulfate-protamine-sulfate-2769
RadiologyInfo.org. (2019). Catheter-directed thrombolysis. https://www.radiologyinfo.org/en/info.cfm?pg=thrombo
Sahay, S., Levine, D. J., & Peters, J. (2020). Chronic thromboembolic pulmonary hypertension. https://foundation.chestnet.org/lung-health-a-z/chronic-thromboembolic-pulmonary-hypertension-cteph/
SportEX Journals. (2011). DVT [Image]. Flickr. https://www.flickr.com/photos/sportex/6634103129
Tarek. (2013). Embolus traveling from DVT to PE [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Embolus_travel_from_DVT_to_PE.svg
Tlamcani, I., El Mouh, N., Amrani, K., & Hassani, M. A. (2018). Pregnancy and hemostasis: From physiology to pathological states. Clinical Research in Hematology, 1(1), 1-7. https://asclepiusopen.com/clinical-research-in-hematology/volume-1-issue-1/8.php
Tritschler, T., Kraaijpoel, N., Gal, G. L., & Wells, P. S. (2018). Venous thromboembolism: Advances in diagnosis and treatment. JAMA, 320(15), 1583–1594. https://doi.org/10.1001/jama.2018.14346
Turgal, M., Gumruk, F., Karaagaoglu, E., & Beksac, M. S. (2018). Methylenetetrahydrofolate reductase polymorphisms and pregnancy outcome. Geburtshilfe Frauenheilkd, 78(9), 871-878. https://doi.org/10.1055/a-0664-8237
Ucar, E. Y. (2019). Update on thrombolytic therapy in acute pulmonary thromboembolism. Eurasian Journal of Medicine, 51(2), 186-190. https://doi.org/10.5152/eurasianjmed.2019.19291
University of Rochester Medical Center. (2020). DVT prevention: Intermittent pneumatic compression devices. https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=135&contentid=328#:~:text=Intermittent%20pneumatic%20compression%20%28IPC%29%20devices%20are%20used%20to,your%20legs%20%20and%20helps%20prevent%20blood%20clots
The US National Library of Medicine. (2020a). Factor V Leiden thrombophilia. https://ghr.nlm.nih.gov/condition/factor-v-leiden-thrombophilia#definition
The US National Library of Medicine. (2020b). Protein C deficiency. https://ghr.nlm.nih.gov/condition/protein-c-deficiency#diagnosis
The US National Library of Medicine. (2020c). Prothrombin thrombophilia. https://ghr.nlm.nih.gov/condition/prothrombin-thrombophilia
Vedantham, S., Piazza, G., Sista, A. K., & Goldenberg, N. A. (2016). Guidance for the use of thrombolytic therapy for the treatment of venous thromboembolism. J Thromb Thrombolysis, 41, 68-80. https://doi.org/10.1007/s11239-015-1318-z
Witt, D. M., Nieuwlaat, R. Clark, N. P., Ansell, J., Holbrook, A., Skov, J., Shehab, N.,
Mock, J., Myers, T., Dentali, F., Crowther, M.A., Agarwal, A., Bhatt, M., Khatib, R., Riva, J. J., Zhang, Y., & Guyatt, G. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Advances, 2(22). 3257-3291. https://doi.org/10.1182/bloodadvances.2018024893