About this course:
The purpose of this module is to describe the pathophysiology of cardiovascular disease (CVD) in type 2 diabetes mellitus (T2DM), review the national guidelines for optimum cardiovascular (CV) health in diabetes, and describe the American Diabetes Association (ADA) evidence-based prescribing recommendations.
Course preview
Upon completion of this module, the APRN will be able to:
- Describe the pathophysiology of cardiovascular disease in type 2 diabetes mellitus.
- List national guideline recommendations for optimal cardiovascular health in diabetes mellitus.
- Discuss evidence-based recommendations for diabetic patients with cardiovascular disease, including an overview of recommended medications, dosing, and side effects.
CVD is the leading cause of death among men and women in the US, with approximately 647,000 deaths each year (The Centers for Disease Control and Prevention [CDC], 2019). According to the National Diabetes Statistics Report (2020), 34.1 million adults, or 13.1% of the US adult population, have diabetes (CDC, 2020). Patients with T2DM are disproportionately affected by CVD when compared to those without T2DM, as CVD is the leading cause of morbidity and mortality in these patients. According to the American Heart Association (AHA, 2018), adults with T2DM are two to four times more likely to die from CVD than those without diabetes. Nearly 70% of patients with T2DM who are 65 years or older die from some form of CVD. Even with the therapeutic effects of antihypertensive and lipid-lowering medications, most patients with T2DM will die from CV events (Rodriguez et al., 2017). Einarson and colleagues (2018) conducted a systematic review of scientific evidence regarding the prevalence of CVD in T2DM across ten years (2007 to 2017). They concluded that worldwide, CVD affects more than 30% percent of individuals diagnosed with T2DM, accounts for nearly 50% of all deaths in patients with T2DM, and coronary artery disease (CAD), stroke, and myocardial infarction (MI) are cited as the chief offenders (Einarson et al., 2018). To provide optimal care to this high-risk patient population, APRNs must understand the basis for the underlying pathophysiological processes, strategies for and challenges associated with managing CVD risk in T2DM, and the evidence-based prescribing guidelines for lowering CV risk.
Pathophysiology of CVD in T2DM
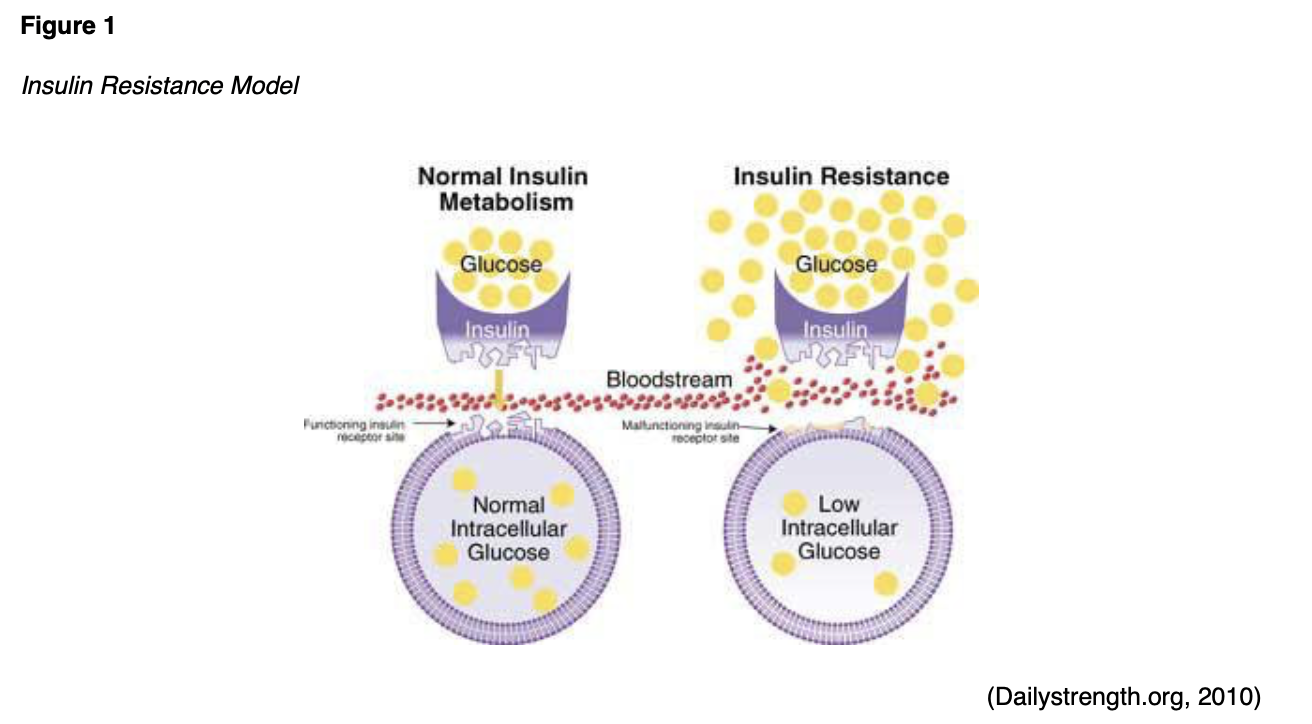
T2DM is a complex, chronic metabolic condition that impacts the way the body metabolizes glucose. The condition is characterized by the body's inability to generate an adequate supply of insulin to maintain balanced glucose levels, or through insulin resistance mechanisms, where the body is unable to effectively utilize the insulin that it already produces. This process is demonstrated in Figure 1 (Mayo Clinic, 2019b).
CVD, or heart disease, refers to a cluster of conditions affecting the heart and blood vessels, most commonly induced by atherosclerosis, which is the buildup of atheroma (or fatty plaque) within the arteries. As shown in Figure 2, as atherosclerosis progresses, plaque growth within the arteries accumulates, causing damage, narrowing, or blockage of the arteries, which can result in serious consequences (Mayo Clinic, 2018).

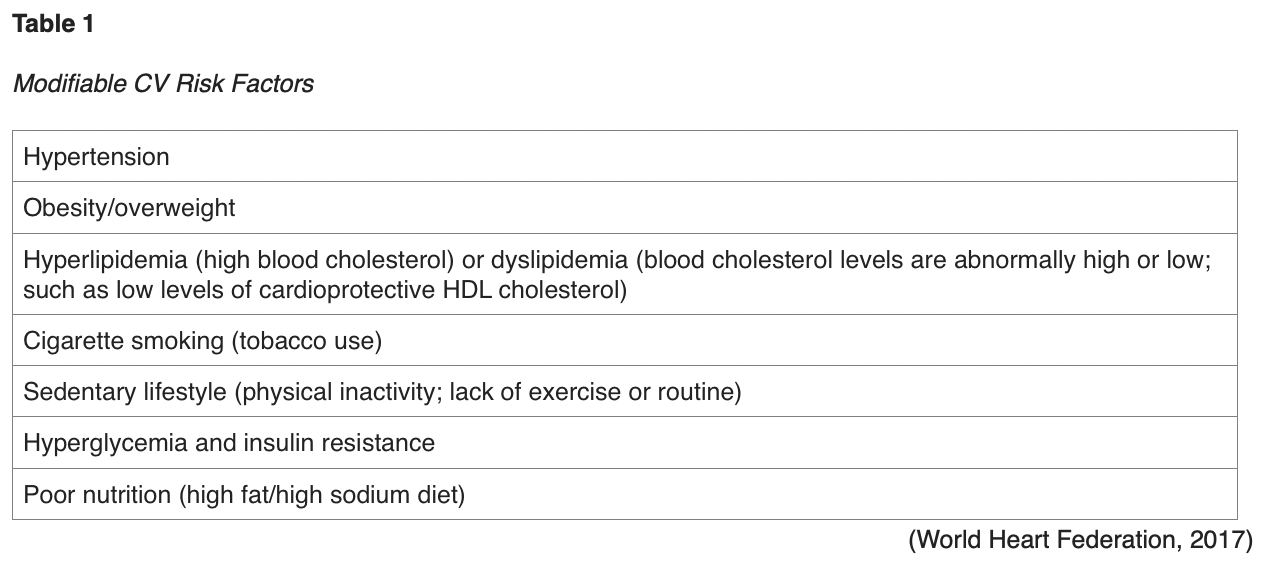
Atherosclerotic cardiovascular disease (ASCVD) is the largest contributor to T2DM-associated mortality, and the risk for developing ASCVD is increased in the setting of uncontrolled modifiable CV risk factors, as listed in Table 1. These risk factors place patients with T2DM at increased risk for cardiac events (Hudspeth, 2018).
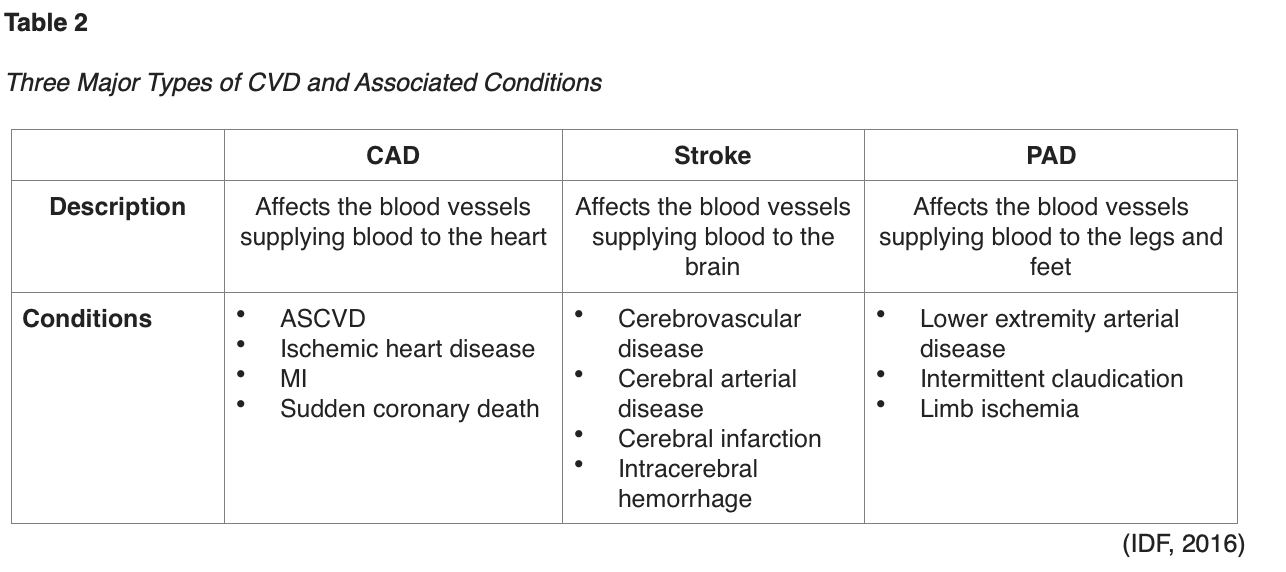
Atherosclerotic lesions are formed through complex interactions of various factors, and T2DM accelerates all of these interactions (Katakami, 2018). The CV damage occurs over time and is the byproduct of a series of metabolic changes within the large blood vessels (macrovascular) and small blood vessels (microvascular) of tissues and organs. These changes begin to occur during the prediabetes stage, which elucidates why more than half of patients already have evidence of CVD at the time of T2DM diagnosis (Ignatavicius et al., 2018). As defined in Table 2, the International Diabetes Federation (IDF) cites the three most common forms of CVD to be direct consequences of macrovascular tissue damage: CAD, stroke, and peripheral arterial disease (PAD). The American Diabetes Association (ADA) released the 2020 clinical practice recommendations regarding CVD and risk management, defining ASCVD as "coronary heart disease (CHD), cerebrovascular disease, or peripheral arterial disease presumed to be of atherosclerotic origin" (ADA, 2020, p. S111). Further, the ADA cites heart failure (HF) as another major cause of morbidity and mortality from CVD, with a twofold higher risk in T2DM patients, as hypertension often serves as the precursor to this condition. Patients with T2DM are at increased risk for all of these conditions, the onset is typically at earlier ages than those without T2DM, and they affect women more commonly than men (ADA, 2020).
The pathogenesis of CVD in T2DM is a complex process mediated by several underlying cellular, molecular, and genetic processes. Cell-signaling defects in metabolic and inflammatory pathways affect the endothelium, liver, skeletal muscle, and beta cells of the pancreas. As portrayed in Figure 3, these defects likely have genetic components but are also largely influenced by environmental factors such as obesity, sedentary lifestyles, tobacco use, and certain medications (Rodriguez-Araujo et al., 2018). Patients with T2DM often have traditional risk factors for CVD, such as obesity, hypertension, dyslipidemia, and sedentary lifestyles, which creates a clear pathway for ASCVD development and its consequences. The following three theories explain the mechanism of vascular complications in patients with T2DM:
- Chronic hyperglycemia leads to permanent basement membrane thickening, tissue damage, and organ destruction, and is a chief cause of premature development of macrovascular complications;
- Glucose toxicity directly or indirectly impairs the functional cell integrity;
- Chronic ischemia in small blood vessels causes tissue hypoxia (Ignatavicius et al., 2018, p. 2542).

Endothelial cells are essential to maintaining homeostasis within the vasculature. These cells are tasked with generating and releasing various biochemical substances within the body to control and maintain the function and integrity of the vessels. Through the release of dilator and constrictor substances, endothelial cells serve critical roles in sustaining the balance between a series of mechanisms:
- oxidation and antioxidation;
- inflammation and anti-inflammation within the vascular walls;
- proliferation and antiproliferation of vascular smooth muscle cells;
- dilatation and contraction of vessels;
- coagulation and fibrinolysis of blood (Katakami, 2018).
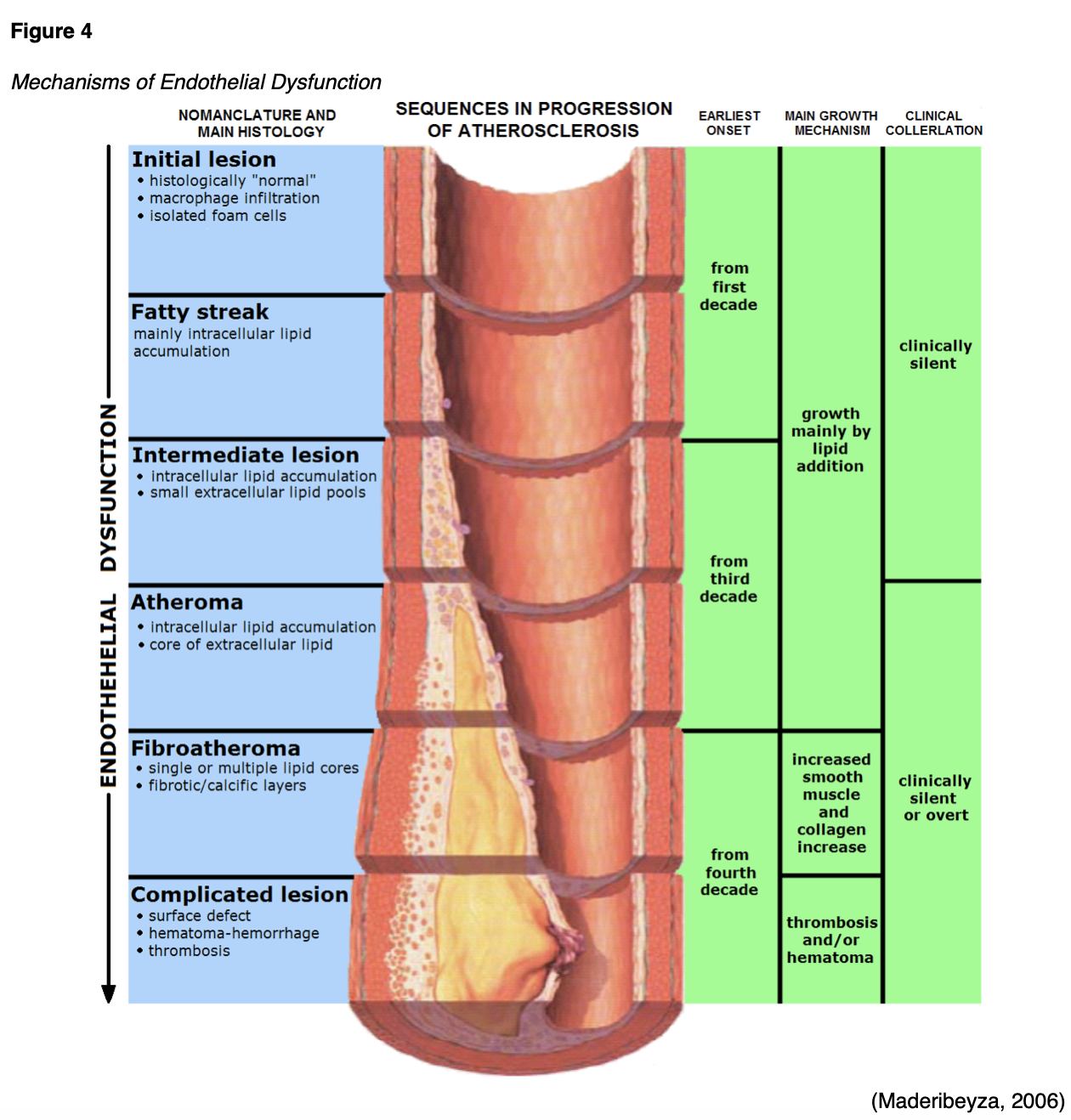
Endothelial dysfunction is the principal complication in T2DM, serving as an independent risk marker for atherosclerosis and CV events. In the setting of T2DM, various mechanisms can disrupt normal functioning and provoke adverse metabolic events within the endothelial cell, such as hyperglycemia, excess free fatty acid release, insulin resistance, increased levels of low-density lipoprotein (LDL) cholesterol in the blood, oxidative stress, and tobacco use. As demonstrated in Figure 4, endothelial dysfunction promotes leukocyte and platelet adhe
...purchase below to continue the course
Chronic hyperglycemia and insulin resistance serve important roles in the initiation of vascular complications of diabetes and involve a number of mechanisms, such as: (1) increased formation of advanced glycation end products (AGEs) and activation of the receptor for advanced glycation end products (RAGE) AGE-RAGE axis, (2) oxidative stress, and (3) inflammation (Fishman et al., 2018). Insulin serves an essential dual-action role in maintaining homeostasis of the vasculature, as it stimulates endothelial cell production of nitric oxide (NO), which is a vasodilator that exerts antiaggregatory effects on smooth muscle. Insulin also mediates the release of endothelin (ET-1), which is a strong vasoconstrictor. Under physiological conditions, the actions of insulin are mediated by a vasoprotective signaling pathway called phosphoinositide 3-kinase (PI3K)/Akt. However, when insulin resistance develops, the actions of insulin become mediated by a pathological signaling pathway known as the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK). The MAPK/ERK pathway mediates inflammation, vasoconstriction, and vascular smooth muscle cell proliferation, thereby contributing to the development of the consequential CV byproducts of insulin resistance (Janus et al., 2016). Patients with insulin resistance have higher rates of hyperglycemia, which accelerates plaque formation and accumulation. Acute hyperglycemia reduces NO bioavailability and endothelial-dependent vasodilation, whereas chronic hyperglycemia promotes atherogenesis and accelerates the progression of atherosclerosis (Katakami, 2018). The relationship between hyperglycemia, increased insulin secretion, and the resulting consequences are portrayed in Figure 5.
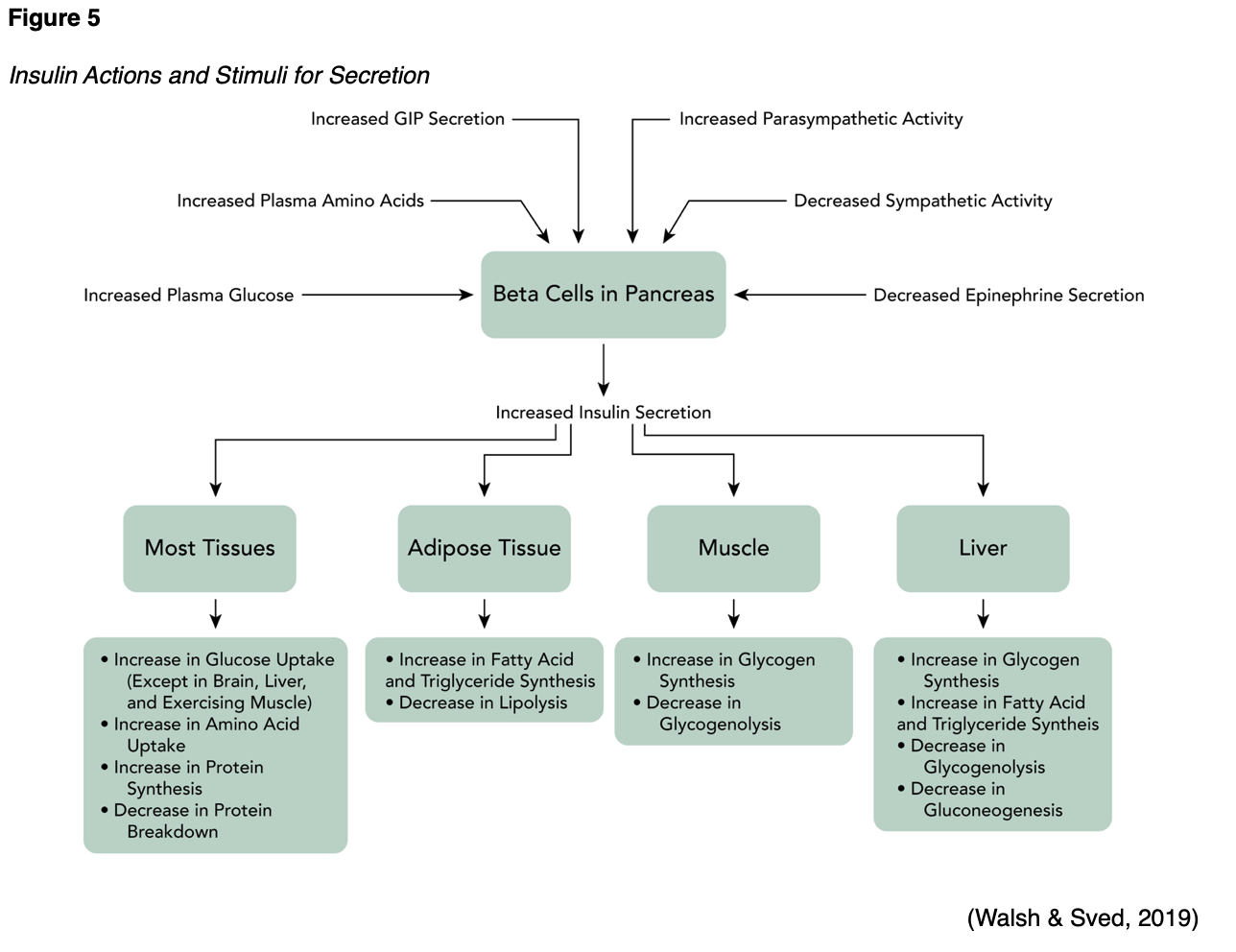
As displayed in Figure 5, T2DM and dyslipidemia commonly occur together. Lipid abnormalities affect up to 70% of patients with T2DM (ADA, 2020). Atherosclerosis is also prompted by local inflammation in the vascular wall that is induced by hyperlipidemia, specifically high levels of LDL. Patients with T2DM have a higher prevalence of lipid abnormalities in the peripheral venous circulation, increased amounts of atherosclerotic plaque accumulation, and smaller coronary artery lumen diameter than those without T2DM. Hyperlipidemia within the atherosclerotic plaque results in the recruitment and migration of monocytes and other immune and inflammatory cells into the vascular subendothelial layer. Recruited monocytes differentiate into macrophages or dendritic cells. Activated macrophages express scavenger receptors to facilitate engulfment of both native and oxidized LDL cholesterol, and along with other inflammatory cells, increase the production of chemokines and cytokines. These mechanisms operate to promote atherosclerotic lesion progression within the inflammatory cycle (Janus et al., 2016; Low Wang et al., 2016).
National Guidelines for Optimal CV Health in T2DM
Research has demonstrated that preventing or slowing the progression of CVD in patients with T2DM is based on controlling individual CV risk factors. Studies have consistently demonstrated that lowering the glycated hemoglobin, or hemoglobin A1C (A1C) level, in patients with T2DM has only a minimal effect on reducing CV risks when performed in isolation. Correcting and controlling multiple CV risk factors simultaneously has been shown to markedly reduce CVD mortality in patients with T2DM (Abdul-Ghani et al., 2017). Over the last decade, research has demonstrated a decline in CVD-associated morbidity and mortality when aggressive risk factor modifications are concurrently implemented (ADA, 2020). The ADA's Standards of Care in Diabetes (2020), a national resource for the optimal management of diabetes, includes annual updates on the evidence-based prevention and management of diabetes and diabetes-related complications. Embedded within these standards are specific guidelines on CVD and risk management in patients with T2DM, strongly emphasizing the concurrent control of hyperglycemia, hypertension, and hyperlipidemia as the central targets (ADA, 2020).
CVD Risk Assessment
To improve patient outcomes and effectively manage CV risk in the context of T2DM, the American College of Cardiology (ACC, n.d.) and ADA (2020) both cite the following as essential components:
- Expand the narrow focus of glucose control to include a systematic assessment of all CV risk factors (Table 1) at least annually and perform aggressive risk reduction with an emphasis on the close monitoring and control of the ABCs of CV risk (Table 3).
- Encourage and implement an individualized, patient-centered, and collaborative approach to reducing CV risk through shared-decision making and open communication between clinicians and patients.
- Employ the ACC/AHA ASCVD risk calculator to better stratify ASCVD risk and help guide therapy. This risk calculator, otherwise referred to as the Risk Estimator Plus, is an online tool that estimates the 10-year risk of a first ASCVD event, accounting for a diagnosis of T2DM as a risk factor (ACC, n.d.; ADA, 2020).

In addition to the above, the following CV risks should also be assessed at least annually:
- Smoking: smoking affects microcirculation and accelerates CV complications. Smoking cessation is strongly advised.
- Family history of premature CAD: this is a non-modifiable risk factor for both DM and CVD.
- Chronic kidney disease (CKD): BP control should be maintained to reduce the risk of kidney disease, a common complication of uncontrolled hypertension, and diabetes.
- Presence of albuminuria (protein in the urine): albuminuria is a biomarker for CVD and coronary events (ADA, 2020).
Treatment/Management
The ACC (2018) cites six steps for optimizing CV risk reduction among patients with T2DM when developing each patient's individualized treatment plan:
- Educate patients with T2DM about CV risks (beyond poor glucose control) that might contribute to and accelerate CV damage (Table 1).
- Empower patients to take action by setting personal goals for lowering CV risk.
- Create an individualized plan to assess and manage CV risk on an ongoing basis and revisit this plan over time to reevaluate, update, and make necessary changes to promote compliance.
- Ensure adequate glycemic control is obtained by developing a realistic plan for lifestyle changes and adhering to prescribed glucose-lowering medications.
- Consider prescribing novel antihyperglycemic agents when appropriate (discussed in the next section).
- Assess adherence and identify hurdles, such as cost of treatment, side effects, personal preferences, or treatment complexity (ACC, n.d.).
Lifestyle Management
Lifestyle management for T2DM and CVD risk reduction should begin at the initial contact with the patient, continue throughout all subsequent evaluations, including during the assessment for complications and management of comorbid conditions. Lifestyle strategies should be implemented across the spectrum of care, preventatively, and as part of the treatment plan in collaboration with any medications prescribed. The combination of lifestyle and pharmacologic interventions enhances treatment efficacy, aids in the control of CV risk factors, thereby more successfully reducing morbidity and mortality. Clinicians and patients should engage in shared decision-making to determine appropriate goals and targets across the spectrum of their diabetes care. Heart-healthy lifestyle interventions are advised for all patients with T2DM as a means of reducing CV risk. The ADA (2020) recommends the following lifestyle recommendations:
- Glucose monitoring is key for achieving glycemic targets for many patients with T2DM. Self-monitoring of blood glucose (SMBG) may help with self-management and medication adjustment, and diabetes self-management education (DSME) should be patient-centered and help guide clinical decisions.
- Reduce excess body weight through caloric restriction (losing 5% of body weight can benefit glycemic control, lipids, and blood pressure).
- Follow the Dietary Approaches to Stop Hypertension (DASH) eating pattern to reduce intake of sodium (less than 2,300 mg/day).
- Increase dietary consumption of fruits and vegetables (eight to ten servings/day).
- Moderate alcohol intake (no more than two servings/day for men, and no more than one serving/day for women).
- Increase physical activity (150 minutes or more of moderate-to-vigorous intensive aerobic activity each week) (ACC, n.d.; ADA, 2020).
Evidence-Based Prescribing Recommendations
Unless specified otherwise, the prescribing recommendations in this section are adapted from the ADA (2020), which utilizes the following ABCE evidence-grading system to demonstrate the level of evidence of each recommendation:
- A - clear evidence from well-conducted, generalizable randomized controlled trials that are adequately powered.
- B – supportive evidence from well-conducted cohort studies.
- C - supportive evidence from poorly controlled or uncontrolled studies.
- E - expert consensus or clinical experience (ADA, 2020).
While lifestyle interventions and modifications are essential, gaining control over T2DM and reducing CV risk usually requires adjunctive pharmacologic therapy. CV risk reduction in T2DM is largely premised on four categories: (1) antiplatelet therapy, (2) antihypertensive medications, (3) lipid-lowering agents, and (4) antihyperglycemic drugs.
Antiplatelet Therapy
Antiplatelet therapy may be used to thin the blood, so it is less likely to clot and clog blood vessels, thereby reducing the risk of stroke or MI. The most common and well-studied form of antiplatelet therapy is low dose acetylsalicylic acid (ASA), or aspirin. For patients with documented acetylsalicylic acid (ASA) allergy, P2Y12 inhibitors, such as clopidogrel (Plavix) are recommended alternatives (ADA, 2020). Acetylsalicylic acid (ASA) has demonstrated efficacy in reducing CV morbidity and mortality in high-risk patients who have endured a prior MI or stroke and is strongly recommended for secondary prevention. In primary prevention, among patients with no prior CV events, the benefits are less clear, and its use is more controversial. The risks associated with acetylsalicylic acid (ASA) therapy, or second-line antiplatelet treatments such as P2Y12 inhibitors, must be carefully considered and balanced against the benefits (ADA, 2020).
Aspirin. Acetylsalicylic acid (ASA) is an over the counter (OTC) nonsteroidal anti-inflammatory drug (NSAID) that is widely used to treat several conditions such as fever, pain, and inflammation. Acetylsalicylic acid (ASA) functions to reduce CV risk by blocking the enzyme that makes prostaglandins (cyclooxygenase), thereby reducing concentrations of prostaglandins, and lowering levels of pain, inflammation, and body temperature. Since acetylsalicylic acid (ASA) is a potent inhibitor of both prostaglandin synthesis and platelet aggregation, it inhibits platelets for the entire cell lifespan of seven to ten days. Therefore, it decelerates the blood's clotting action by reducing the clumping of platelets. Acetylsalicylic acid (ASA) inhibits the function of platelets in a manner different from other NSAIDs such as ibuprofen (Motrin), as its antithrombotic effects last longer, making it the ideal agent for MI and stroke reduction (MedicineNet, n.d.). There is a risk for bleeding events in patients taking acetylsalicylic acid (ASA), particularly gastrointestinal (GI) bleeding. The drug is listed within Beers Criteria and should be used with caution (or avoided) in older adults due to its bleeding effects. The risk for GI bleeding is heightened in individuals who are aged 60 or older, have a history of stomach ulcers, bleeding disorders, as well as those who are taking other types of anticoagulants (blood thinners). Further, those who consume three or more alcoholic beverages per day are at heightened risk for bleeding events. Aside from GI bleeding, the most common side effect is tinnitus (ringing in the ears). Enteric-coated formulations of acetylsalicylic acid (ASA) are considered safer with regards to the risk for GI bleeding, as they are designed to pass through the stomach and not disintegrate until it reaches the small intestine. Acetylsalicylic acid (ASA) may cause a severe allergic reaction, causing hives, facial swelling, shock, or asthma (wheezing). Children, adolescents, and young adults under the age of 21 who have or are recovering from chickenpox or flu-like syndromes should not take aspirin due to risk for a rare but serious illness known as Reye's syndrome (Mayo Clinic, 2019a).
P2Y12 Inhibitors. P2Y12 inhibitors are a group of antiplatelet drugs that may be used in place of acetylsalicylic acid (ASA) for patients with an allergy or other contraindication. Clopidogrel (Plavix) is the most widely used and studied P2Y12 inhibitor for reducing CV risk, but other medications in this class may also be considered, such as ticlopidine (Ticlid), ticagrelor (Brilinta), and rasugrel (Effient). Clopidogrel (Plavix) works by binding to the P2Y12 receptor on platelets, preventing adenosine diphosphate (ADP) from activating platelets. The tolerability and side effects of clopidogrel (Plavix) are similar to that of acetylsalicylic acid (ASA), as it also poses a risk for bleeding events, particularly GI bleeding and ulcers. Ticlopidine (Ticlid) carries an added risk of neutropenia (a decline in the white blood cell count), which heightens the risk for acquiring an infection, and thrombotic thrombocytopenic purpura (TTP), an immune disorder that destroys platelets and occurs in about one out of every 250,000 people (MedicineNet, n.d.). Ticagrelor (Brilinta) may worsen kidney function and induce shortness of breath, and therefore is not advised in patients with T2DM who have underlying renal dysfunction (MedlinePlus, 2018). The ADA endorses the following antiplatelet prescribing recommendations:
- Acetylsalicylic acid (ASA) is not recommended in patients under 50 years of age with T2DM who are at low risk of ASCVD (have no major ASCVD risk factors), as the benefit is low and the risk of bleeding is higher (A).
- Acetylsalicylic acid (ASA) 75 to 162 mg/day may be considered as a primary prevention strategy in patients with T2DM who are at increased CV risk, following a comprehensive discussion with the patient on the potential benefits compared with the increased risk of bleeding (A).
- Use acetylsalicylic acid (ASA) 75 to 162 mg/day as a secondary prevention strategy in patients with T2DM who have a history of ASCVD (A).
- In patients with ASCVD and aspirin allergy, clopidogrel (Plavix), 75 mg/day should be used (B).
- For a year following an acute cardiac event, dual antiplatelet therapy with low-dose aspirin and a P2Y12 inhibitor is reasonable (A) and may have benefits extending beyond this one-year period (B) (ADA, 2020).
Antihypertensive Medications
Treatment of hypertension to maintain the BP below 140/90 mmHg reduces CV events as well as microvascular complications in patients with T2DM. Several studies have demonstrated that antihypertensive therapy reduces ASCVD events, HF, and microvascular complications in patients with T2DM. Treatment should focus on controlling BP to achieve individualized targets utilizing drug classes demonstrated to reduce CV events, specifically in patients with T2DM. These agents include angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium channel blockers (CCBs), as outlined in Table 4. ACEIs and ARBs are considered first-line antihypertensive agents in patients with T2DM, as they not only function to lower the blood pressure but also have an effect on reducing the risk of kidney dysfunction from microvascular complications. The ADA (2020) cautions that BP targets more aggressive than < 140/90 mmHg are not likely to improve CV outcomes among most patients with T2DM and are more likely to contribute to adverse effects and costs. Therefore, a balance between the potential benefits and risks must be considered prior to prescribing more intensive antihypertensive therapy (ADA, 2020).
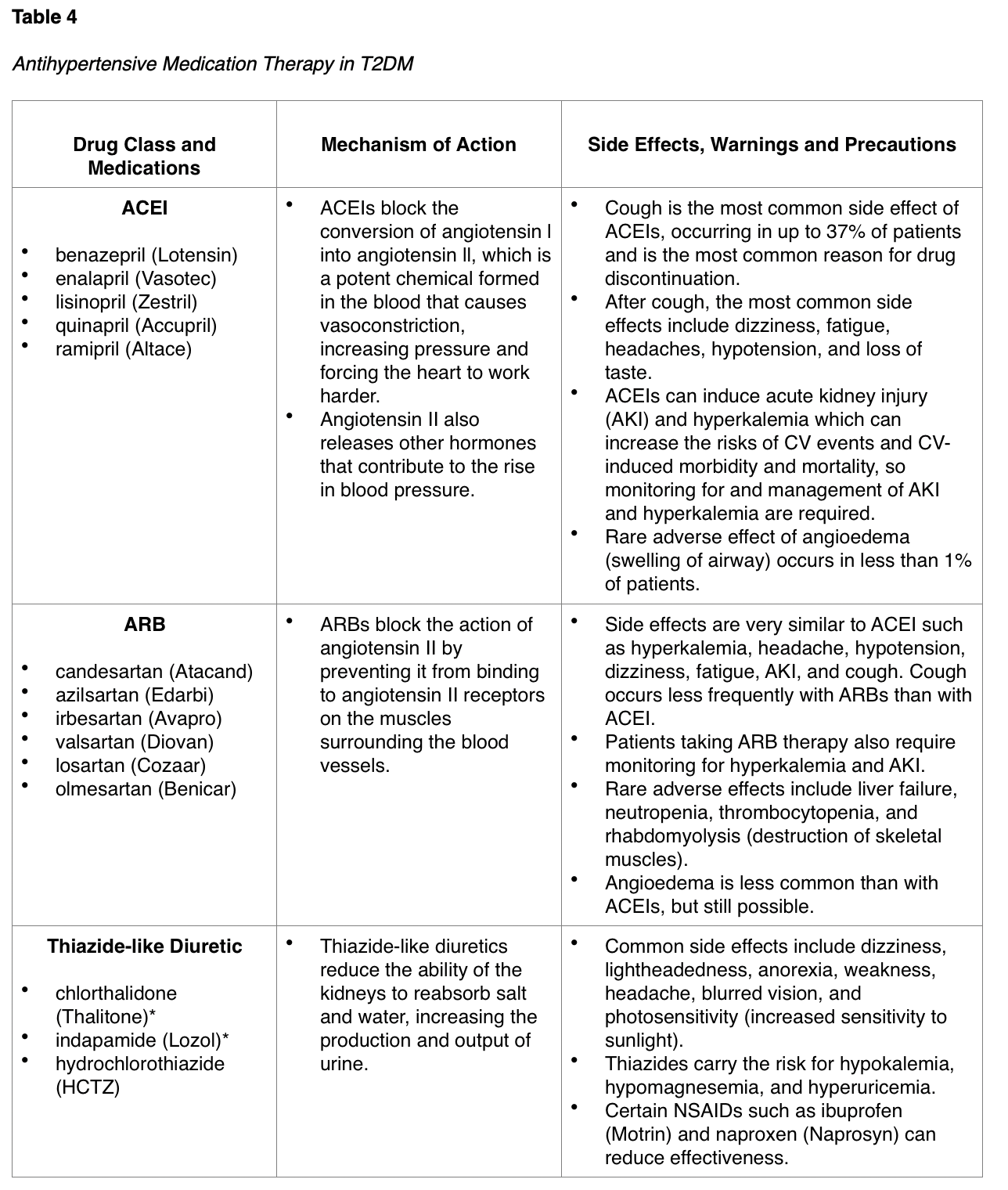
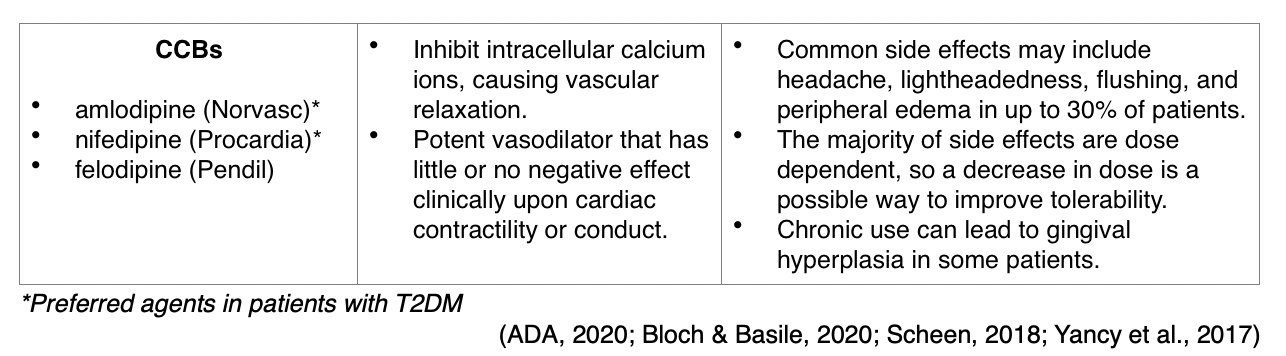
The ADA endorses the following antihypertensive prescribing recommendations, which are intended to be used in conjunction with continued lifestyle interventions:
- In patients with known ASCVD, consider prescribing an ACEI or an ARB to reduce the risk of CV events (B).
- In patients with BP of at least 140/90 mmHg, prompt initiation and timely titration of one pharmacologic agent is advised to achieve blood pressure goals (A).
- In patients with BP above 160/100 mmHg, prompt initiation and timely titration of two drugs or a single-pill combination of drugs demonstrated to reduce CV events in patients with diabetes is advised to achieve blood pressure goals (A).
- Multi-drug therapy is usually necessary to achieve BP targets, but combinations of ACEI and ARBs or combinations of ACEI or ARBs with direct renin inhibitors should not be used (A).
- An ACEI or ARB, at the maximum, tolerated dose indicated for BP treatment, is the recommended first-line treatment for hypertension in patients with T2DM and albuminuria (urinary albumin-to-creatinine ratio [UACR] of greater than 29 mg/g), and if one class is not tolerated, the other should be substituted (B).
- Patients who are being treated with ACEI, ARB, or diuretic therapy should have serum creatinine, eGFR, and serum potassium levels monitored at least annually (B) (ADA, 2020).
The initial treatment of hypertension depends upon the severity of hypertension and clinical risk factors. As demonstrated in the treatment decision algorithm displayed in Figure 6, the following basic prescribing guidelines apply when initiating antihypertensive therapy in patients with T2DM:
- For initial BP between 140/99 mmHg and 159/99 mmHg, begin single-drug therapy with an ACEI, ARB, thiazide diuretic, or CCB.
- For initial BP above 159/99 mmHg, begin dual-drug therapy with two antihypertensive medications.
- In patients with albuminuria, initial treatment should include an ACEI or ARB to reduce the risk of progressive kidney disease.
- In patients without albuminuria, ACEIs or ARBs do not provide superior cardioprotection when compared to thiazide-like diuretics or CCBs (ADA, 2020).
- Certain types of thiazide diuretics can contribute to hyperglycemia and metabolic dysfunction in patients with T2DM. The preferred thiazide agents include chlorthalidone (Thalitone) and indapamide (Lozol), as they carry a reduced risk for these metabolic effects than thiazide diuretics such as hydrochlorothiazide (HCTZ) (Scheen, 2018).
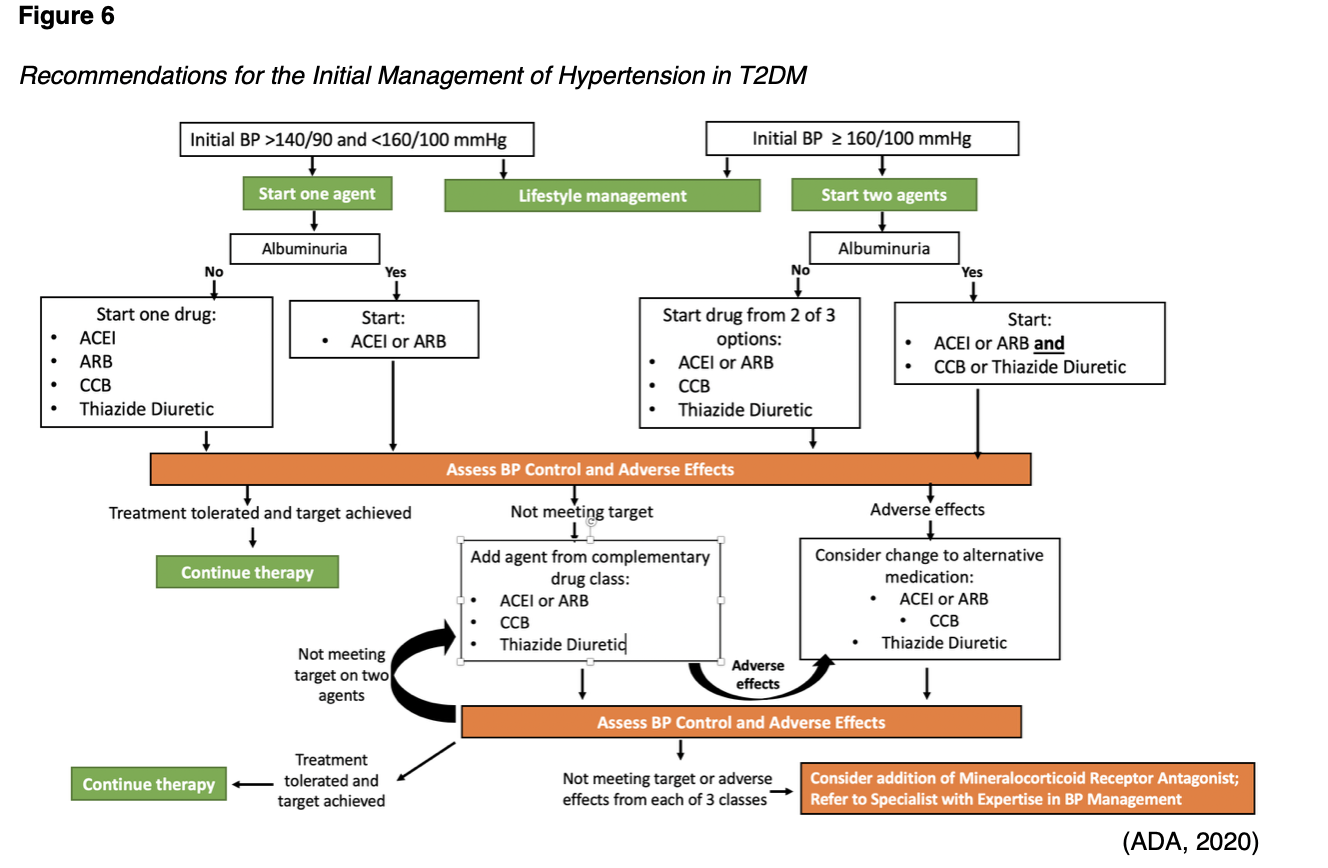
For patients with resistant hypertension (which is defined as a BP above 139/89 mmHg despite three classes of antihypertensive medications, including a thiazide-like diuretic) adding a mineralocorticoid receptor antagonist (MRA) should be considered. However, prior to diagnosing resistant hypertension, clinicians should confirm the absence of any barriers that may be impairing adherence to the existing antihypertensive medication regimen, as well as the potential for secondary hypertension. The ADA (2020) makes the following recommendation for patients with confirmed resistant hypertension:
- MRAs should be reserved for the management of resistant hypertension and these agents should be added to the patient’s existing treatment with an ACEI or ARB, thiazide-like diuretic, and CCB (B) (ADA, 2020).
Numerous studies have demonstrated that primary aldosteronism and hyperaldosteronism are common mechanisms in patients with resistant hypertension. MRAs, also referred to as aldosterone antagonists, have well-established benefits with regards to their efficacy in the management of resistant hypertension. MRAs are shown to be effective in acting on the renin-angiotensin-aldosterone pathway, providing CV and renal protection. Spironolactone (Aldactone) and eplerenone (Inspra) are the two most extensively studied MRAs in the management of resistant hypertension. MRAs are classified as potassium-sparing diuretics, as they function to prevent the body from absorbing too much salt and avert potassium excretion. These drugs bind to the androgen receptors to prevent their interaction with testosterone. Therefore, common side effects include gynecomastia, breast pains, erectile dysfunction, and menstrual irregularities, occurring in up to 9% of patients, and are reversible upon discontinuation of the drug (Yugar-Toledo et al., 2017). These side effects are most common with spironolactone (Aldactone), the prototype of MRAs, which has been the subject of numerous studies over time with demonstrated efficacy. Spironolactone (Aldactone) is much more potent than eplerenone (Inspra), a second-generation selective MRA. Eplerenone (Inspra) carries a higher affinity for the mineralocorticoid receptor and lower affinity for androgen receptors than spironolactone (Aldactone). While it poses a lower risk of adverse effects, it is more expensive (Dudenbostel & Calhoun, 2017). MRAs can also reduce albuminuria and have additional CV risk reduction benefits. The most common adverse effect among all MRAs is hyperkalemia, so monitoring of serum creatinine and potassium levels is strongly advised (ADA, 2020). Additional side effects include drowsiness, lightheadedness, blurred vision, nausea, vomiting, diarrhea, headache, increased thirst, and orthostatic hypotension (Yugar-Toledo et al., 2017). Newer data have suggested that MRAs may additionally provide preferential benefits in treating obesity-related hypertension, particularly in the setting of high dietary sodium intake (Dudenbostel & Calhoun, 2017).
Statins or Other Lipid-Lowering Therapy
The most prevalent pattern of dyslipidemia in patients with T2DM includes low levels of HDL cholesterol combined with elevated LDL and triglyceride levels. There is significant evidence to support the critical importance of reducing LDL levels as one of the most effective ways to reduce ASCVD. Statins are the preferred first-line pharmacologic therapy for most patients with T2DM due to their well-established benefits on lowering LDL cholesterol and cardioprotective factors (ADA, 2020). They work by inhibiting hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, which is the enzyme in the cholesterol biosynthesis pathway. While statins are highly effective at lowering LDL, they are associated with fair levels of toxicity. The most common adverse effect and reason for discontinuation of statin therapy is statin-associated muscle symptoms (SAMSs); up to 72% of all statin adverse events are muscle related. SAMSs can present as myalgia, myopathy, myositis with elevated creatinine kinase, or in its most severe form, rhabdomyolysis. Other side effects include joint and abdominal pain, neurological and neurocognitive effects, hepatotoxicity, and renal toxicity (Ward et al., 2019).
There are two dosing regimens recommended by the ADA, which are listed in Table 6. High-intensity statin therapy is cited for achieving greater than 50% reduction in LDL cholesterol, and moderate-intensity statin regimens typically achieve 30–49% reductions in LDL cholesterol. Low-dose statin therapy is generally not recommended in patients with T2DM, but at times may be the maximal dose of statin that a patient can tolerate. For patients who do not tolerate the intended intensity of statin, the maximally tolerated statin dose should be used (ADA, 2020).

While there are other lipid-lowering agents available, the evidence for the use of drugs that target these lipid fractions is not nearly as rigorous or extensive as the literature surrounding statin therapy, particularly with regards to efficacy in patients with T2DM. In patients that are not achieving individualized lipid profile targets on statin therapy or those with intolerance to an increased dosage of statin, the ADA (2020) recommends combination therapy with ezetimibe (Zetia) or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor such as evolocumab (Repatha) or alirocumab (Praluent) (ADA, 2020).
Ezetimibe (Zetia). Ezetimibe (Zetia) belongs to a class of drugs called cholesterol absorption inhibitors, as it works by inhibiting the absorption of dietary cholesterol in the intestines to lower LDL levels in the blood (Danaf et al., 2016). In a randomized controlled trial, ezetimibe (Zetia) 10 mg was added to moderate-intensity simvastatin (Zocor) 40 mg and compared with simvastatin (Zocor) 40 mg alone. Among patients with T2DM, this combination demonstrated a significant reduction in major CV events with an absolute risk reduction of 5% and a relative risk reduction of 14% over single-agent simvastatin (Zocor) (Giugliano et al., 2018). Ezetimibe (Zetia) may be taken at the same time as statins, and a commonly cited benefit is that it can be helpful in reducing the dose of statin and consequently the associated risk for muscle injury and SAMSs. It has been proven tolerable across clinical trials; the most common side effects reported include drowsiness, diarrhea, sinus congestion, and joint pain. Ezetimibe (Zetia) should be avoided in patients with moderate to severe liver dysfunction due to the rare risk of liver failure. Other rare adverse effects include allergic reaction, rhabdomyolysis, pancreatitis, and a severe skin rash characterized by red, blistering, and peeling skin (Rosenson, 2020).
PCSK9 inhibitors. PCSK9 inhibitors are approved for patients with inadequately treated levels of LDL while on other agents. PCSK9 is an enzyme encoded for by the PCSK9 gene that is predominantly produced in the liver. PCSK9 binds to the LDL receptor on the surface of hepatocytes (liver cells), leading to the destruction of the LDL receptor and higher levels of LDL in the plasma. PCSK9 inhibitors, such as evolocumab (Repatha) and alirocumab (Praluent), are humanized monoclonal antibodies that bind to free plasma PCSK9, promoting the destruction of this enzyme. This leaves less free PCSK9 available to bind to the LDL receptors and results in lower LDL plasma levels. These medications can lower LDL levels by as much as 60% in patients concurrently on statin therapy and have shown clinical benefits in reducing the rates of stroke and MI (Stroes et al., 2019). They are approved for patients with ASCVD or familial hypercholesterolemia who are receiving maximally tolerated doses of statin therapy but require additional lowering of LDL levels (ADA, 2020). These medications can only be administered by subcutaneous injection and are not available in oral preparations. The most common reported side effects are injection site reactions that are usually mild and limited to erythema, pain, and bruising. There are no reports of these medications inducing muscle breakdown or liver impairment; however, hypersensitivity reactions have been reported, including rash, pruritus, and urticaria. There is concern that PCSK9 inhibitors can potentially influence hepatitis C infectivity, but this is not yet confirmed, and more studies are needed to establish this as a definitive risk factor. Medication dosing is as follows:
- evolocumab (Repatha) 140 mg subcutaneous injection every two weeks, or 420 mg subcutaneous injection once monthly.
- alirocumab (Praluent) 75 mg subcutaneous injection every two weeks is the starting dose, with LDL levels measured within four to eight weeks. The maintenance dose is 75 mg to 100 mg every two weeks, depending on response to therapy. Alternatively, patients may be given 300 mg subcutaneous injection every four weeks (Stroes et al., 2019).
The ADA (2020) endorses the following recommendations for patients with hyperlipidemia in the setting of T2DM.
- Intensify lifestyle therapy and optimize glycemic control for patients with elevated triglyceride levels (greater than 149 mg/dL) and/or low HDL cholesterol (less than 40 mg/dL for men, or less than 50 mg/dL for women) (C).
- For patients of all ages with T2DM and established ASCVD disease, high-intensity statin therapy should be added to lifestyle therapy (A).
- Obtain a lipid profile at initiation of statin therapy or other lipid lowering therapy, 4–12 weeks after initiation or a dose change, and annually thereafter to monitor the response to therapy and inform medication adherence (E).
- For patients with ASCVD considered to be at very high risk, if LDL is greater than 69 mg/dL on maximum tolerated statin dose, consider adding additional LDL-lowering therapy such as ezetimibe (Zetia) (A).
- For patients who do not tolerate the intended statin intensity, the maximally tolerated statin dose should be used (E).
- For patients over 75 years of age who are already taking statin therapy, it is reasonable to continue statin treatment (B).
- For patients over 75 years of age, it may be reasonable to initiate statin therapy after discussion of potential benefits and risks (C).
- Statin therapy is contraindicated in pregnancy (B).
- Statin plus fibrate combination therapy has not been shown to improve ASCVD outcomes and is generally not recommended (A).
- Statin plus niacin combination therapy has not been shown to provide additional CV benefit above statin therapy alone, may increase the risk of stroke with additional side effects, and is generally not recommended (A).
Novel Antihyperglycemic Medications and CV Risk Reduction
Glucose control is the cornerstone of T2DM management with regards to reducing target organ damage and limiting complications. It is well-established that metformin (Glucophage) is the first line antihyperglycemic treatment for T2DM and also helps lower the risk of CVD as a byproduct. The ADA (2020) states that metformin (Glucophage) should be continued for glucose-lowering as long as the eGFR remains above 30 mL/min; however, it should be avoided in unstable or hospitalized patients with HF. In 2008, the US Food & Drug Administration (FDA) issued guidance for CV outcome trials to be performed for all new medications for patients with T2DM due to concerns for increased CV risk. Over the last decade, several large randomized controlled trials have reported statistically significant reductions in CV events with the use of the novel antihyperglycemic medications listed below. Both classes reduce the risk of major adverse CV events to a comparable degree in patients with T2DM and established ASCVD across large meta-analyses (ADA, 2020).
Sodium-glucose cotransporter-2 (SGLT2) inhibitors. SGLT2 inhibitors are FDA-approved for use with other diabetic medications to lower blood sugar in adults with T2DM and include empagliflozin (Jardiance), canagliflozin (Invokana) and dapagliflozin (Farxiga). These agents work by causing the kidneys to excrete excess sugar through the urine and, over time, have an impact on reducing A1C levels. SGLT2 inhibitors have demonstrated a reduction in the progression of kidney disease across numerous clinical trials but have limited efficacy in patients with eGFR under 45 mL/ min/1.73 m2. They carry secondary benefits of weight loss, reductions in systolic BP, and circulating fluid levels (edema), all of which reduce the stress on the CV and renal systems. Since these agents cause increased diuresis, the most common side effects include dehydration, hypotension, syncope, and falls. They carry the rare but serious side effect of necrotizing fasciitis of the perineum, which is a severe infection of the genitals and surrounding area. These agents are also associated with an increased risk of yeast infection and urinary tract infection in females. There are ongoing investigations into case reports of SGLT2 inhibitor-associated diabetic ketoacidosis (DKA) (American Association of Clinical Endocrinologists [AACE]/American College of Endocrinologists [ACE], 2020). There are a few variances in efficacy and side effect profiles between the three agents as follows:
- Empagliflozin (Jardiance) is the most well-established with regards to CV risk reduction across clinical trials and significantly reduces the risk of death from MI and stroke in adults with T2DM and CVD. In the EMPA-REG OUTCOME trial, it was associated with significantly lower rates of all-cause and CV death, as well as a lower risk of hospitalization for HF (Zinman et al., 2015). Empagliflozin (Jardiance) is dosed as a 100 to 300 mg tablet taken by mouth once daily prior to breakfast (AACE/ACE, 2020).
- Canagliflozin (Invokana) carries an increased risk of leg and foot amputation. The CANVAS trial demonstrated that over a year's time, the risk of amputation for patients in the trial was equivalent to 5.9 out of every 1,000 patients versus 2.8 out of every 1,000 patients treated with placebo (Neal et al., 2017). For this reason, it should not be prescribed to patients with PAD or a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers. In addition, canagliflozin (Invokana) is associated with an increased risk of bone fractures associated with decreased bone mineral density (FDA, 2018). Canagliflozin (Invokana) is dosed as a 5 to 10 mg tablet taken by mouth once daily prior to breakfast (AACE/ACE, 2020).
- Dapagliflozin (Farxiga) is dosed as a 10 to 25 mg tablet taken by mouth once daily prior to breakfast (AACE/ACE, 2020). In the DECLARE-TIMI trial, Dapagliflozin (Farxiga) reduced CV death and HF hospitalizations; however, it did not significantly lower the combined risk of CV death and nonfatal MI or stroke (Wiviott et al., 2019).
Glucagon-like peptide 1 receptor agonists (GLP-1RAs). Clinical trial data have demonstrated CV risk reduction in patients with T2DM taking liraglutide (Victoza), semaglutide (Ozempic), and dulaglutide (Trulicity). These agents work by interfering with the functioning of GLP-1, an incretin peptide hormone released from the ileum and colon after intake. GLP-1 receptor activation subsequently stimulates insulin release, inhibits glucagon secretion, slows gastrointestinal transit, and suppresses appetite (Brown & Everett, 2019). They are typically reserved for those who require two or more antidiabetic agents in order to reach and maintain their target A1C level. GLP-1RAs have significant A1C-lowering properties, with an average A1C reduction of 1.5%. GLP-1RAs also pose secondary benefits, including weight loss and lowering of lipids and blood pressure, which can be cardioprotective. GLP-1RAs approved for CV risk reduction in patients with T2DM are only available as injectable medications. Therefore, mild injection site reactions are routinely reported, manifested as discomfort, inflammation, redness, or bruising at the injection site. GLP-1RAs can delay gastric emptying, causing fullness and nausea or vomiting and should, therefore, be used with caution in patients with gastroparesis, those with a history of gastric bypass, or severe gastroesophageal reflux disease (GERD). Patients should be educated on the importance of staying well hydrated and consuming smaller meals to avoid fullness and vomiting (AACE/ACE, 2020). There are a few variances in efficacy and side effect profiles between the three agents, as follows:
- Dulaglutide (Trulicity) is dispensed as a single-dose pen and prefilled syringe that needs to be refrigerated and protected from sunlight. Dulaglutide (Trulicity) dosing guidelines recommend starting with a 0.75 mg SC injection once every week, followed by dosing titration up to 1.5 mg weekly if inadequate glycemic response. Nausea is a commonly reported side effect, reported in up to 12.4% of patients taking the 0.75 mg dose, which increases to 21.1% in patients taking the 1.5 mg dose. The average weight loss on dulaglutide (Trulicity) is 2.5 kg. The drug also carries a low risk of sinus tachycardia in up to 6% of patients.
- Liraglutide (Victoza) and semaglutide (Ozempic) are similar agents, as they are dispensed as multi-dose pens, and must be refrigerated prior to first use, but can subsequently be stored at room temperature thereafter. Their dosing regimens are also distinct, as Liraglutide (Victoza) is administered as a 0.6 mg subcutaneous (SC) injection daily for seven days, followed by 1.2 mg SC daily. If inadequate glycemic response, dosing guidelines recommend increasing the dose to 1.8 mg SC daily. Semaglutide (Ozempic) is administered at a dose of 0.25 mg SC daily for four weeks, followed by an increase to 0.5 mg once weekly. The maximum dose of semaglutide (Ozempic) is 1.0 mg once weekly. Nausea occurs in up to 20% of patients taking these medications, and semaglutide (Ozempic) is associated with greater weight loss than liraglutide (Victoza) (4.5 kg versus 2.5 kg). Liraglutide (Victoza) is FDA-approved to reduce the risk of CV mortality, nonfatal MI, and nonfatal stroke in adults with T2D and CVD (Munoz, 2018).
The ACC (n.d.) has established guidelines for when to consider adding a SGLT2-inhibitor or GLP-IRA to a patient’s glucose-lowering regimen. In general, these medications are intended to be used in conjunction with ADA’s (2020) evidence-based CV risk reduction prescribing guidelines.
- At the time of T2DM diagnosis in patients with clinical ASCVD.
- At the time of ASCVD diagnosis in patients with T2DM.
- In patients with T2DM who are not meeting glycemic targets and have clinical ASCVD.
- At the time of hospital discharge following admission for an ASCVD-related or T2DM-related clinical event.
- Consider initiating these medications for primary prevention in patients with T2DM who have additional risk factors for CVD, such as:
- Age over 65 years.
- Poorly controlled hypertension (BP above 140/90 mmHg).
- Hyperlipidemia (LDL above 100 mg/dl or non-HDL above 130 mg/dl).
- Ongoing tobacco use.
- Chronic kidney disease stage III or higher (ACC, n.d.).
The ADA (2020) endorses the following antihyperglycemic prescribing recommendations to help guide clinical decision-making when choosing between an SGLT2-inhibitor or an GLP-1RA:
- In patients with T2DM who have established ASCVD, established kidney disease, or multiple risk factors, either group will effectively reduce the risk of major CV events, however SGLT2 inhibitors may be slightly better in those with diabetic kidney disease and HF (A).
- In patients with T2DM and established HF, an SGLT2 inhibitor may be considered to reduce risk of HF hospitalization (C).
Older Adults
Special consideration is required when prescribing and monitoring therapies in the older adult population to reduce adverse effects and increase compliance with therapy. The ADA (2020) makes the following prescribing recommendations among older adults with T2DM:
- In those at increased risk for hypoglycemia, medication classes with a low risk of hypoglycemia are preferred (B).
- Overtreatment of T2DM is common, contributes to hypoglycemia, and should be avoided (B).
- Deintensification (simplification) of complex treatment regimens can reduce the risk of hypoglycemia and polypharmacy, and promote compliance, by lowering the dose or discontinuing some medications (B).
- The cost of medications as pertaining to insurance coverage and co-payments must be considered to reduce the risk of cost-related nonadherence (B) (ADA, 2020).
References
Abdul-Ghani, M., DeFronzo, R. A., Del Prato, S., Singh, R., & Ryder, R. E. J. (2017). Cardiovascular disease and type 2 diabetes: Has the dawn of a new era arrived? Diabetes Care, 40(7), 813-820. https://doi.org/10.2337/dc16-2736
American Association of Clinical Endocrinologists and American College of Endocrinologists. (2020). Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endocrine Practice, 26 (1), 107-139. https://doi.org/10.4158/CS-2019-0472
American College of Cardiology. (n.d.). Type 2 diabetes and cardiovascular risk toolkit. https://www.acc.org/~/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Tools%20and%20Practice%20Support/Quality%20Programs/CV%20Risk%20in%20Diabetes%20Initiative/B19040_Diabetes_and_CV_Risk_Discussion_Guide_Final.pdf
American Diabetes Association. (2020). Cardiovascular disease and risk management: Standards of medical care in diabetes-2020. Diabetes Care, 41(Suppl. 1), S111-S134. Https://doi.org/10.2337/dc20-s010
American Heart Association. (2018). Cardiovascular disease & diabetes. http://www.heart.org/HEARTORG/Conditions/More/Diabetes/WhyDiabetesMatters/%20Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp
Back, S. H., & Kaufman, R. J. (2012). Causation of T2DM [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Causation_for_T2D.JPG
Bloch, M. J., & Basile, J. (2020). Major side effects and safety of calcium channel blockers. UpToDate. https://www.uptodate.com/contents/major-side-effects-and-safety-of-calcium-channel-blockers
Brown, J. M., & Everett, B. M. (2019). Cardioprotective diabetes drugs: What cardiologists need to know. Cardiovascular Endocrinology Metabolism, 8(4), 96-105. https://doi.org/10.1097/XCE.0000000000000181
The Centers for Disease Control and Prevention. (2019). Heart disease facts. https://www.cdc.gov/heartdisease/facts.htm
The Centers for Disease Control and Prevention. (2020). National diabetes statistics report 2020. https://www.diabetesresearch.org/file/national-diabetes-statistics-report-2020.pdf
Dailystrength.org. (2010). Insulin resistance model [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Insulinresistance.jpg
Danaf, J. A., Martin, S. S., & Blumenthal, R. S. (2016). Ezetimibe: The lower the LDL-C the better (even for total cardiovascular events). https://www.acc.org/latest-in-cardiology/articles/2016/03/09/06/50/ezetimibe-the-lower-the-ldlc-the-better
Dudenbostel, T., & Calhoun, D. A. (2017). Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. American Journal of Hypertension, 30(2), 103-109. https://doi.org/10.1093/ajh.hpw105
Einarson, T. R., Acs, A., Ludwig, C., & Panton, U. H. (2018). Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence across the world in 2007-2017. Cardiovascular Diabetology, 17(18), 1-19. https://doi.org/10.1186/s12933-018-0728-6
Fishman, S. L., Sonmez, H., Basman, C., Singh, V., & Poretsky, L. (2018). The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Molecular medicine, 24(59), 1-12. https://doi.org/10.1186/s10020-018-0060-3
Giugliano, R. P., Cannon, C. P., Blazing, M. A., Nicolau, J. C., Corbalan, R., Spinar, J., Park, J. G., White, J. A., Bohula, E. A., Braunwald, E. (2018). Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results from IMPROVE-IT (improved reduction of outcomes: Vytorin efficacy international trial). Circulation, 137(15), 1571-1582. https://doi.org/10.1161/CIRCULATIONAHA.117.030950
Hudspeth, B. (2018). The burden of cardiovascular disease in patients with diabetes. American Journal of Managed Care, 24. https://www.ajmc.com/journals/supplement/2018/reducing-rick-cv-patients-with-diabetes/the-burden-of-cardiovascular-disease-in-patients-with-diabetes
Ignatavicius, D. D., Workman, M. L., & Rebar, C. R. (2018). Medical-surgical nursing: Concepts for interprofessional collaborative care (9th ed.). Elsevier
International Diabetes Federation. (2016). Diabetes and cardiovascular disease – executive summary. https://www.idf.org/our-activities/care-prevention/cardiovascular-disease/cvd-report.html#sub-content-tab-nav
Janus, A., Szahidewicz-Krupska, E., Mazur, E., & Doroszko, A. (2016). Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators of Inflammation, 1-10. http://dx.doi.org/10.1155/2016/3634948
Katakami, N. (2018). Mechanism of atherosclerosis and cardiovascular disease in diabetes mellitus. Journal of Atherosclerosis and Thrombosis, 25(1), 27-39. https://doi.org/10.5551/jat.RV17014
Low Wang, C. C., Hess, C. N., Hiatt, W. R., & Goldfine, A. B. (2016). Clinical update: Cardiovascular disease in diabetes mellitus. Circulation, 133(24), 2459-2502. https://doi.org/10.1161/CIRCULATIONAHA.116.022194
Maderibeyza. (2006). Mechanisms of endothelial dysfunction [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Endo_dysfunction_Athero.PNG
Mayo Clinic. (2018). Heart disease. https://www.mayoclinic.org/diseases-conditions/heart-disease/symptoms-causes/syc-20353118
Mayo Clinic. (2019a). Daily aspirin therapy: Understand the benefits and risks. https://www.mayoclinic.org/diseases-conditions/heart-disease/in-depth/daily-aspirin-therapy/art-20046797
Mayo Clinic. (2019b). Type 2 diabetes. https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/symptoms-causes/syc-20351193
MedicineNet. (n.d.). Aspirin vs. plavix (clopidogrel) differences, side effects, and uses. https://www.medicinenet.com/aspirin_vs_plavix/article.htm#aspirin_vs_plavix_clopidogrel_quick_comparison_of_differences
MedlinePlus. (2018). Antiplatelet drugs – P2Y12 inhibitors. https://medlineplus.gov/ency/patientinstructions/000100.htm
Munoz, K. (2018). GLP-1 receptor agonists for type 2 diabetes currently available in the U.S. http://www.diabetesincontrol.com/wp-content/uploads/2014/09/GLP-1-Chart-Nov-1-2018.pdf
The National Heart, Lung, and Blood Institute. (2013). Atherosclerosis [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Coronary_heart_disease-atherosclerosis.PNG
Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., Desai, M., & Matthews, D. R. (2017). Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine, 277(7), 644-657. https://doi.org/10.1056/NEJMoa1611925
Rodriguez, V., Weiss, M., Weintraub, H., Goldberg, I., & Schwartzbard, A. (2017). Cardiovascular disease leads to a new algorithm for diabetes treatment. Journal of Clinical Lipidology, 11(5), 1126-1133. https://doi.org/10.1016/j.jacl.2017.07.004
Rosenson, R. S. (2020). Low density lipoprotein cholesterol lowering with drugs other than statins and PSCK9 inhibitors. UpToDate. https://www.uptodate.com/contents/low-density-lipoprotein-cholesterol-lowering-with-drugs-other-than-statins-and-pcsk9-inhibitors?search=zetia&source=search_result&selectedTitle=1~38&usage_type=default&display_rank=1
Scheen, A. J. (2018). Type 2 diabetes and thiazide diuretics. Curr Diab Rep, 18(2), 6. https://doi.org/10.1007/s11892-018-0976-6
Stroes, E. S., Stiekema, L. C., & Rosenson, R. (2019). PCSK9 inhibitors: Pharmacology, adverse effects, and use. https://www.uptodate.com/contents/pcsk9-inhibitors-pharmacology-adverse-effects-and-use?search=PCSK9%20inhibitors:%20Pharmacology,%20adverse%20effects,%20and%20use&source=search_result&selectedTitle=1~27&usage_type=default&display_rank=1
US Food & Drug Administration. (2018). Sodium-glucose cotransporter-2 (SGLT2) inhibitors. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/sodium-glucose-cotransporter-2-sglt2-inhibitors
Walsh, D., & Sved, A. (2019). Insulin actions and stimuli for secretion [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Insulin_-_actions_and_stimuli_for_secretion.png
Ward, N. C., Watts, G. F., & Eckel, R. H. (2019). Statin toxicity. Circulation Research, 124(2). https://doi.org/10.1161/CIRCRESAHA.118.312782
Wiviott, S. D., Raz, I., Bonaca, M. P., Mosenzon, O., Kato, E. T., Cahn, A., Silverman, M. G., Zelniker, T. A., Kuder, J. F., Murphy, S. A., Bhatt, D. L., Leiter, L. A., McGuire, D. K., Wilding, J. P. H., Ruff, C. T., Guase-Nilsson, I. A. M., Fredriksson, M., Johansson, P. A.M, Langkilde, A. M., Sabatine, M. S. (2019). Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine, 380(4), 347-355. https://doi.org/10.1056/NEJMoa1812389
World Heart Federation. (2017). Cardiovascular risk factors. https://www.world-heart-federation.org/resources/risk-factors/
Yancy, C. W., Jessup, M., Bozhurt, B., Butler, J., Casey, D. E., Colvin, M. M.., Drezner, M. H., Filippatos, G. S., Fonarow, G. C., Givertz, M. M., Hollenberg, S. M., Lindenfeld, J., Masoudi, F. A., McBride, P. E., Peterson, P. N., Stevenson, L. W., & Westlake, C. (2017). 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology, 70(6), 776-803. https://doi.org/10.1016/j.jacc.2017.04.025.
Yugar-Toledo, J. C., Modolo, R., de Faria, A. P., & Moreno, H. (2017). Managing resistant hypertension: Focus on mineralocorticoid receptor antagonists. Vasc Health Risk Manag, 13, 403-411. https://doi.org/10.2147/VHRM.S138599
Zinman, B., Wanner, C., Lachin, J. M., Fitchett, D., Bluhmki, E., Hantel, S., Mattheus, M., Devins, T., Johansen, O. D., Woerle, H. J., Broedl, U. C., & Inzucchi, S. E. (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine, 373, 2117-2128. https://doi.org/10.1056/NEJMoa1504720