About this course:
The purpose of this module is to provide an overview of colorectal cancer, its risk factors, clinical features, treatment options, and screening guidelines to enrich nursing knowledge of the condition, patient education, and nursing practice.
Course preview
This module aims to provide an overview of colorectal cancer, its risk factors, clinical features, treatment options, and screening guidelines to enrich nursing knowledge of the condition, patient education, and nursing practice.
By the completion of this learning activity, the nurse should be able to:
- discuss the epidemiology of colorectal cancer in the US and recognize screening guidelines for both average-risk and high-risk individuals
- explain the pathophysiology, primary classifications, and subtypes of colorectal cancer, and identify the risk factors and signs and symptoms of the disease
- review the various treatment modalities for colorectal cancer, including surgery, radiation, chemotherapy, and the evolving role of targeted treatments and immunotherapy
- describe the possible side effects, monitoring parameters, and precautions of the different treatments and important components of patient education
The latest data released by the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program ranks colorectal cancer (CRC) as the third leading cause of cancer death in both men and women in the US. When genders are combined, it moves up in rank to second (Siegel et al., 2020). The recent unexpected and tragic death of celebrity icon Chadwick Boseman has directed attention toward the hidden perils of CRC, prompting increased awareness of the disease and screenings’ importance. CRC originates in the colon (large intestine) or the rectum (terminal portion of the colon) and is collectively referred to as colon cancer. Since colon cancer and rectal cancer share many similar features, they are often grouped as one condition; however, it is essential to recognize these as distinct disease entities in terms of their cancer staging, treatment, and survival patterns. The National Comprehensive Cancer Network (NCCN) offers separate evidence-based guidelines for each disease. Figure 1 summarizes the parallels and differences between the two conditions (NCCN, 2020a, 2020b).

Note. Original figure based on information found in NCCN, 2020a and 2020b.
Epidemiology
The American Cancer Society (ACS, 2020a) estimates there will be 147,950 new cases of CRC (104,610 colon and 43,340 rectal) diagnosed in the US this year. The lifetime risk of CRC is approximately 1 in 23 (4.4%) among males and 1 in 25 (4.1%) for females. Recognizing that rectal cancer is commonly misclassified as colon cancer, SEER statistics describe combined mortality analysis, with an estimated 53,200 deaths from CRC in 2020. The majority of cases are diagnosed in adults 50 years of age and older, as incidence steadily climbs with age, as demonstrated in Figure 2. Over the last two decades, the age at diagnosis has steadily declined, shifting from a median onset of 72 years in 2000 to the current median age of 66 years (66 years for males and 69 years for females; Siegel et al., 2020).
The ACS (2020a) broadly defines racial and ethnic groups using the following terminology: non-Hispanic blacks (NHB), American Indians/Alaska Natives (AI/AN), non-Hispanic whites (NHW), Hispanic/Latino (Hispanic), and Asian/Pacific Islanders (API). CRC incidence and mortality are highest among NHB, followed closely by AI/AN, and lowest in APIs. Between 2012 and 2016, CRC incidence was 20% higher in NHB than NHW and 50% higher than APIs (ACS, 2020a). Between 2013 and 2017, CRC death rates in NHB were nearly 40% higher than NHWs and twice as much as APIs. Proposed rationales for these racial disparities include socioeconomic status, education, and lifestyle factors (i.e., smoking, diet, and obesity). NHB individuals are less likely to receive timely follow-up of a positive screening test and high-quality colonoscopy, contributing to higher mortality (Siegel et al., 2020).
In the US, ANs are at disproportionately high-risk for advanced cancers at the time of diagnosis. The reasons are mostly unknown, but theories include a higher prevalence of risk factors such as diets high in animal fat, vitamin D deficiency, and an increased overall incidence of diabetes, obesity, and smoking. ANs have a higher prevalence of Helicobacter pylori (H. pylori), a bacterium associated with inflammation and cancer of the stomach, which may also be associated with CRC. Finally, there is a notable inadequacy of available endoscopic services throughout Alaska (ACS, 2020a; Siegel et al., 2020). According to a recent study by Berkowitz et al. (2018), Alaska has the nation’s lowest CRC screening rates. The most important predictor of CRC survival is the stage at the time of diagnosis. The five-year survival rates are as follows:
- 90% for patients with localized-stage disease (no sign of cancer spread outside the colon or rectum)
- 71% for patients with regional-stage disease (cancer has spread outside the colon or rectum to nearby structures or lymph nodes)
- 14% for patients diagnosed with distant-stage (cancer has spread to distant organs or lymph nodes (ACS, 2020a; Siegel et al., 2020).

Risk and Protective Factors
Roughly 55% of CRC cases are attributable to modifiable risk factors (see Table 1) and, thus, are potentially preventable. Advancing age, lifestyle habits, inflammatory bowel disease (ulcerative colitis and Crohn’s disease), type 2 diabetes mellitus (T2DM), environmental factors, and inherited and acquired genetic susceptibilities are associated with increased CRC risk. People who engage in healthy and active lifestyle behaviors have up to a 52% lower risk of CRC compared to those who do not engage in these behaviors. Those who are physically active have a 25% lower risk of developing proximal and distal colon tumors than sedentary people. Men who are obese have about a 50% higher risk of colon cancer and a 25% higher risk of rectal cancer, whereas women who are obese have about a 10% increased risk of colon cancer and no apparent increased risk of rectal cancer (ACS, 2020b). In the US, an estimated 13% of CRCs are attributed to excessive alcohol consumption and 12% to current or former tobacco use; the risk in current smokers is about 50% higher than in never smokers (Islami et al., 2018).

While Table 1 catalogs modifiable risk factors supported by clinical evidence, several other proposed risks are correlated with the disease but currently lack definitive evidence. Some of the most common are diet, vitamin D, and acetylsalicylic acid (Aspirin) use.
Diet
Diet is believed to play a dual role in preventing and promoting CRC through its influence on the immune response and inflammation; however, the precise impact of specific foods on cancer occurrence is not definitively established beyond those listed in Table 1. Nevertheless, the ACS (2020a) describes diets high in refined carbohydrates and processed sugar as posing an increased risk for adenomas and CRC. While diets rich in whole grains and fiber are associated with a reduced risk of CRC due to potentially less carcinogen exposure in higher stool volume and faster transit time, evidence from randomized controlled trials remains inconclusive (ACS, 2020a). Two 2017 meta-analyses determined that whole grains served a more substantial role in risk reduction than fiber intake, revealing a 5% risk reduction for CRC for every 30 grams/day of whole grains consumed (Schwingshackl et al., 2017; Vieira et al., 2017). &nb
...purchase below to continue the course
Vitamin D
Vitamin D has received a lot of attention over recent years regarding its potentially protective role against CRC; however, research findings remain inconclusive. Pooled data from 17 cohort studies determined that higher circulating blood levels of vitamin D (up to 100 nmol/L) were associated with a statistically significant, substantially lower CRC risk in women only. Correspondingly, the findings also revealed a 37% increased risk of CRC in those with vitamin D deficiency (McCullough et al., 2019).
Acetylsalicylic acid (Aspirin)
While there is a broad research base supporting the long-term regular use of acetylsalicylic acid (Aspirin) and other nonsteroidal anti-inflammatory drugs (NSAIDs) as protective factors against CRC, the ACS (2020a) confers this risk reduction to be stronger only among those younger than 70 and without excess body weight. Acetylsalicylic acid (Aspirin) users who develop CRC appear to have less aggressive tumors and improved survival than non-aspirin users. The ACS (2020a) currently does not recommend using NSAIDs for cancer prevention in the general population due to the potentially serious side effect of gastrointestinal (GI) bleeding. The US Preventive Services Task Force (USPSTF, 2016a) reports the regular use of acetylsalicylic acid (Aspirin) is more likely to have a beneficial effect when begun between the ages of 50 and 59, and benefits only become apparent after 10 years of consistent use.
Non-Modifiable Risk Factors
While 60% of CRC-related deaths can be mitigated through proper screening and surveillance practices, research demonstrates that at least one in three people is not up to date with CRC screenings. Non-modifiable risk factors also serve critical roles in the evolution of this disease, such as a personal history of chronic inflammatory bowel disease, a family history of CRC or adenomas (precancerous polyps), and inherited gene mutations. While most CRC cases are sporadic (60 to 70%), meaning healthy cells and genes mutate from genetic and environmental exposures over one’s lifetime, up to 30% of people diagnosed with CRC have a family history of the disease. Individuals who have a first-degree relative (i.e., parent or sibling) with CRC have two to four times the risk of developing the disease than people without this family history. This risk increases further for those with first-degree relatives diagnosed before age 50 or those with multiple-affected relatives. According to ACS (2020a), most “CRC clustered in families is believed to reflect interactions between lifestyle factors and the cumulative effect of relatively common genetic variations that increase disease risk, referred to as high prevalence/low penetrance mutations” (p. 13). Nearly 10% of CRCs are hereditary, meaning they are caused by an inherited gene mutation or syndrome (ACS, 2020a; Yurgelun et al., 2017). The most common inherited causes of CRC include Lynch syndrome, familial adenomatous polyposis (FAP), and inheritance of BRCA1/BRCA2 gene mutations (ACS, 2020a, 2020b).
Lynch Syndrome
Lynch syndrome is also called hereditary non-polyposis colorectal cancer (HNPCC) and is the most common hereditary risk factor for CRC. It also poses an increased risk for several other types of cancer, including ovarian, endometrial (uterine), brain, stomach, and breast. Approximately 5 in 100 people with CRC have Lynch syndrome. Around 8% of CRCs in people younger than 50 years are due to Lynch syndrome, and the median age at CRC diagnosis is 61 (ACS, 2020a). Individuals with Lynch syndrome tend to develop colon polyps (benign growth in the colon) at younger ages and typically develop cancer in their 40s or 50s. Affected women have an overall higher risk of developing cancer than their male counterparts. In the US, it is estimated that 1 in 279 individuals (1.2 million people) have a gene mutation associated with Lynch syndrome; however, most are undiagnosed since the identification of this mutation depends on a cancer diagnosis (ACS, 2020a). Individuals inherit lymph syndrome in an autosomal dominant pattern, which means one inherited copy of each cell’s altered gene is sufficient to increase cancer risk. Changes in the MLH1, MSH2, MSH6, or PMS2 genes are most commonly found in Lynch syndrome. Under physiologic conditions, these genes are responsible for repairing any potential errors during DNA replication (the process during which DNA is copied in preparation for cell division); collectively, they are known as mismatch repair (MMR) genes. Since mutations in any of these genes impede the cell’s ability to repair the DNA replication errors, abnormal cells continue to divide. Over time, the accumulated DNA replication errors can lead to uncontrolled cell growth and an increased propensity for cancer development (US National Library of Medicine [NLM], 2020b).
Familial Adenomatous Polyposis (FAP)
There are a few types of polyposis syndromes associated with increased risk for CRC, but FAP is the most common and accounts for approximately 1% of all CRCs. People with FAP tend to develop multiple benign polyps in their colon as early as their 20s and 30s. The number of polyps increases to thousands with advancing age, and unless the colon is removed, these polyps will develop into cancer. The median age for CRC in patients with FAP is 39 years. There are two genes associated with FAP that carry different inheritance patterns. Mutations in the APC gene can cause FAP and attenuated FAP, in which polyp growth is delayed. The average age of CRC diagnosis for patients with attenuated FAP is 55 years. Mutations in the APC gene affects the cell’s ability to maintain healthy growth and function, leading to cellular overgrowth. Most people with mutations in the APC gene will develop CRC, but the number of polyps and the time frame in which they become malignant depends on the location of the mutation in the gene. FAP resulting from mutations in the APC gene is inherited in an autosomal dominant pattern. Nurses should counsel patients with FAP on the importance of vigilant surveillance with frequent colonoscopy screenings as recommended by their physician. Surgery is often the standard of care for cancer prevention in patients with FAP syndromes once polyp growth accelerates beyond control with colonoscopy screenings (ACS, 2020a; NLM, 2020a).
BRCA1/BRCA2 gene mutations
All individuals have BRCA1/2 genes, which act as tumor suppressor genes to prevent cancer by regulating cellular growth and division. Mutations in these genes prevent them from working correctly, thereby increasing the propensity toward cancer development. Most commonly associated with breast, ovarian, and prostate cancers, BRCA1/2 mutations are being explored regarding their role in increasing the risk for several other types of cancers (The Centers for Disease Control and Prevention [CDC], 2020b). Regarding CRC, the absolute risk associated with BRCA1 and BRCA2 gene mutations is not definitively established (ACS, 2020a). Yurgelun and colleagues (2017) have determined that approximately 1% of CRC patients have heritable mutations in BRCA1/2. Further, a 2018 systematic review and meta-analysis investigating the association between CRC and BRCA1 and BRCA2 mutations found an association limited to BRCA1 mutations only, not BRCA2. They found that individuals with BRCA1 mutations have about a 50% increased risk of the disease than individuals without the mutation (Oh et al., 2018).
Early Detection and Screening
Since a precancerous polyp can take 10 to 15 years to progress into cancer, there is a unique opportunity for cancer prevention and early detection with screenings at defined intervals. Screening offers substantial opportunities to lessen CRC incidence and mortality. CRC screening aims to identify disease in the precancerous or early stage of cancer when it is small, localized, and curable. Various cancer screening guidelines are put forth by credible organizations and grounded in clinical research, evidence, and expert consensus. While there are some variations between the sets of guidelines, they are relatively consistent in their recommendations on CRC screening. The ACS is one of the most widely utilized, comprehensive, evidence-based resources for cancer care; they publish an annual report that summarizes CRC screening recommendations (ACS, 2020a).
Nurses’ Role in Cancer Prevention and Screening
One of the most powerful tools that nurses have is their ability to develop deep professional relationships with patients and their family members. Nurses are in a unique position to convey the importance of cancer prevention across healthcare settings. To effectively counsel patients on CRC risk reduction practices, it is important that nurses understand the basis for CRC screening and possess adequate knowledge of the various types of screening tests available. Nurses can help patients understand their options and select a test based on their individual preferences, such as ease of access and cost factors. Nurses must also recognize and understand the various features that place patients at increased risk for the disease to ensure they are counseled appropriately (Kahl, 2018).
Screening Modalities. There are multiple options for CRC screening, all of which are associated with a significant reduction in CRC incidence, although colonoscopy is the most accurate and commonly used screening test. CRC screening modalities are divided into two major categories; high-sensitivity stool-based test (collected at home) or visual examination (performed at health care facilities), as described in Table 2. The ACS (2020a) and the US Preventive Services Task Force (USPSTF, 2016b) do not endorse one test over another; they instead stress that all recommended tests can help save lives.

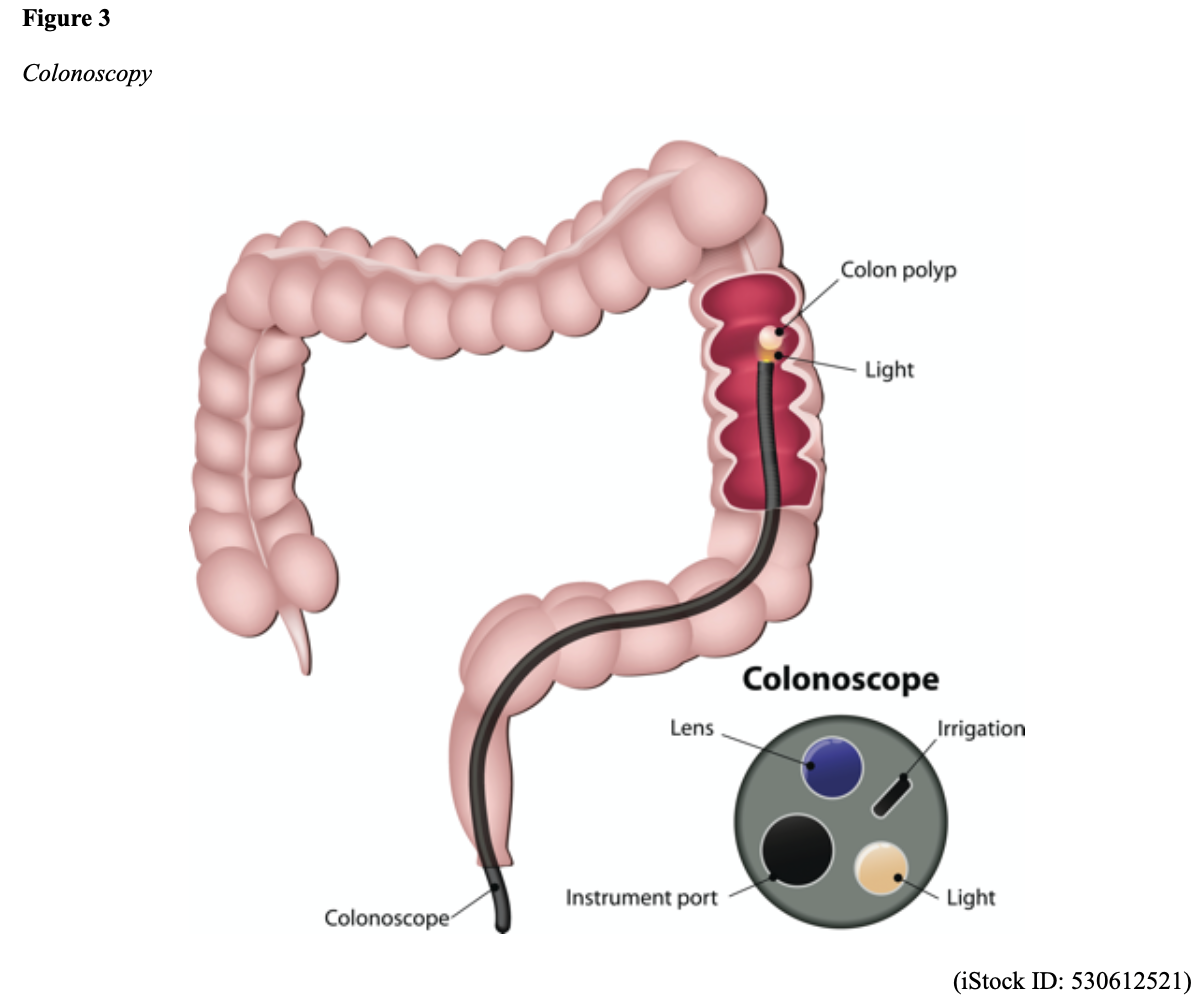
Limitations. Nurses should educate patients on the limitations and potential harms of CRC screening tests. All screening tests carry a risk for false-positive and false-negative results. While stool-based tests are noninvasive and less costly, they are more likely to miss polyps or cancers. Patients should also be advised that any positive findings on non-colonoscopy screening tests will need to be followed up with a colonoscopy. Delays in the follow-up of abnormal results are associated with an increased risk of advanced disease and mortality. Colonoscopy is also subject to error and may miss adenomas. Although rare, colonoscopy poses a higher risk of complications such as bowel tears, infection, and sepsis (ACS, 2020a).
Summary of Colorectal Cancer Screening Recommendations
The ACS (2020a) screening recommendations are based on risk category: average risk and increased or high-risk. The ACS explicitly defines individuals at high-risk to include those with one or more of the following characteristics listed in Box 1-1. By default, individuals are considered to be at average risk if they do not have any of the characteristics listed in Box 1-1.

Average Risk Screening Recommendations
The ACS recently lowered the age to initiate CRC screening from 50 to 45 years due to increasing incidence in younger populations. Currently, the ACS (2020a) recommends that adults aged 45 years and older undergo regular CRC screening with either a stool test or a visual exam. Screening intervals are based on individual patient factors and findings at the time of the initial screening test. Individuals who are in good health and with a life expectancy of greater than ten years should continue regular CRC screening through 75. The USPSTF (2016b) recommends screening for CRC starting at age 50 years and continuing until age 75 years; however, the guideline is currently undergoing revision. Collectively, the guidelines recommend that for individuals aged 76 through 85, the decision to be screened should be based on a person’s preferences, life expectancy, overall health, and prior screening history. The ACS (2020a) recommends that those over 85 should no longer undergo CRC screening.
High-Risk for CRC
People at increased or high-risk for CRC should start screening before age 45. For patients with one first-degree relative diagnosed with CRC before age 60, or two first-degree relatives diagnosed at any age, screening should be performed at age 40 or 10 years younger than the earliest age of diagnosis in the family, whichever comes first. Finite screening intervals for those at increased or high-risk for CRC vary based on the individual patient and screening test results. The USPSTF and ACS do not put forth screening guidelines specifically for those in higher-risk categories due to the high variability between these individuals (ACS, 2020a; USPSTF, 2016b).
Pathophysiology
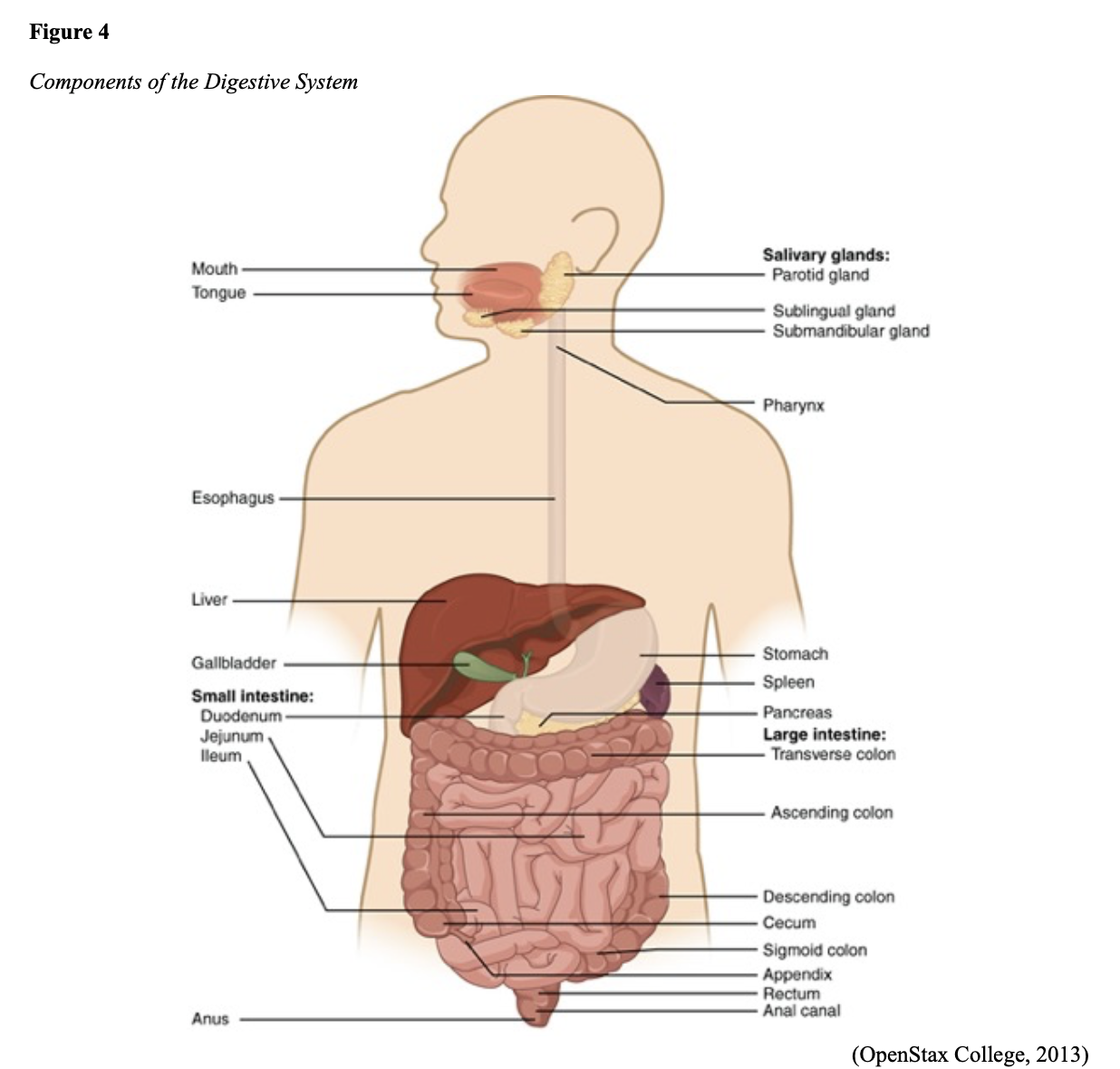
The GI tract extends from the mouth to the anus and consists of several organs, as demonstrated in Figure 4. It carries out several digestive processes to perform two primary functions: breaking down food to assimilate nutrients and eliminating waste. Food is processed and mixed with an enzyme called salivary amylase in the mouth before the esophagus propels the food into the stomach. The stomach churns the food and combines it with enzymes, mucus, gastric acids, and other secretions. The food is stored in the stomach until phasic contractions help propel it into the small intestine, where most nutrient absorption occurs. Biochemicals and enzymes secreted by the pancreas and liver break down the food particles into smaller, absorbable nutrients. The nutrients pass through the small intestine walls into blood vessels and lymphatics before reaching the large intestine, where fluid absorption continues. Liquid wastes are transported to the kidneys, where they are excreted from the body in urine. Solid waste travels into the rectum and is eliminated through the anus (McCance & Heuther, 2019).
The Large Intestine
The large intestine is the final passageway of undigested food particles and, as shown in Figure 5, is subdivided into four regions: the cecum, colon, rectum, and anus. It also includes the appendix. Its primary function is to complete the absorption of any residual nutrients and water, synthesize specific vitamins, form feces (waste), and eliminate it. The cecum is the cul-de-sac, or small pouch at the beginning of the large intestine. It receives partially digested food (known as chyme) from the small intestine and mixes it with bacteria to continue digestion and form feces before transporting it into the colon. The colon is about five feet in length and comprises four connecting regions: the ascending colon, transverse colon, descending colon, and sigmoid colon. The ascending colon receives the feces from the cecum, bacteria digest the waste, and the intestinal wall absorbs water, nutrients, and vitamins into the bloodstream. The hepatic flexure is located on the right side of the body near the liver and creates a sharp, right-angle bend in the colon, marking the beginning of the transverse colon. The transverse colon is the longest region of the colon, and most nutrient absorption and feces formation occur in this region. The splenic flexure is the connection point between the transverse colon and the descending colon, forming a sharp, right-angle bend on the abdomen’s left side. The descending colon walls absorb water and any remaining nutrients from the feces, dehydrating the stool in preparation for elimination. The sigmoid colon is the curved, S-shaped section of the colon that transports and stores the residual fecal matter until it is transported to the rectum. The rectum is the final straight portion of the large intestine and stores fecal matter until the body is ready to eliminate the waste through the process of defecation. The rectum connects the colon to the anus, the GI tract’s final segment. The anus is a short tube at the end of the rectum terminating to the body’s exterior that feces pass through during defecation (Innerbody Research, 2017; McCance & Heuther, 2019).

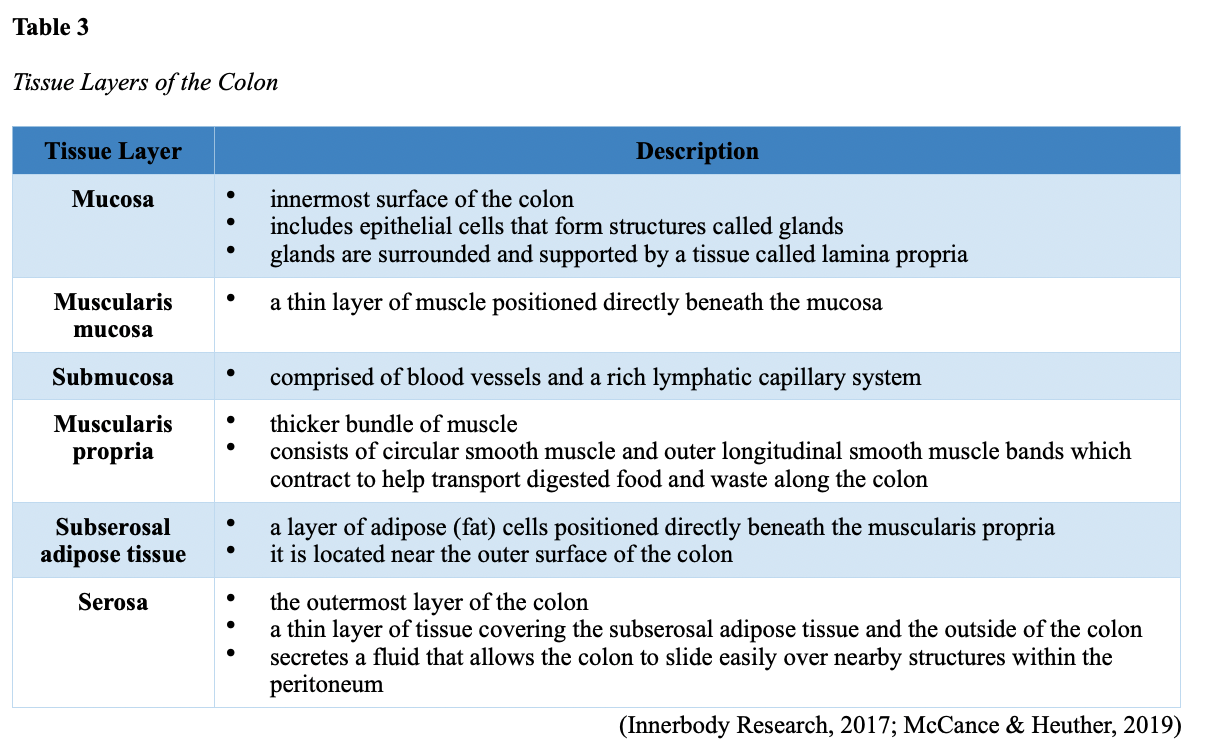
Layers of the Intestinal Wall
The six layers of tissue that comprise the colon wall are displayed in Figures 6 and 7 and then described below in Table 3.


Cancer Development
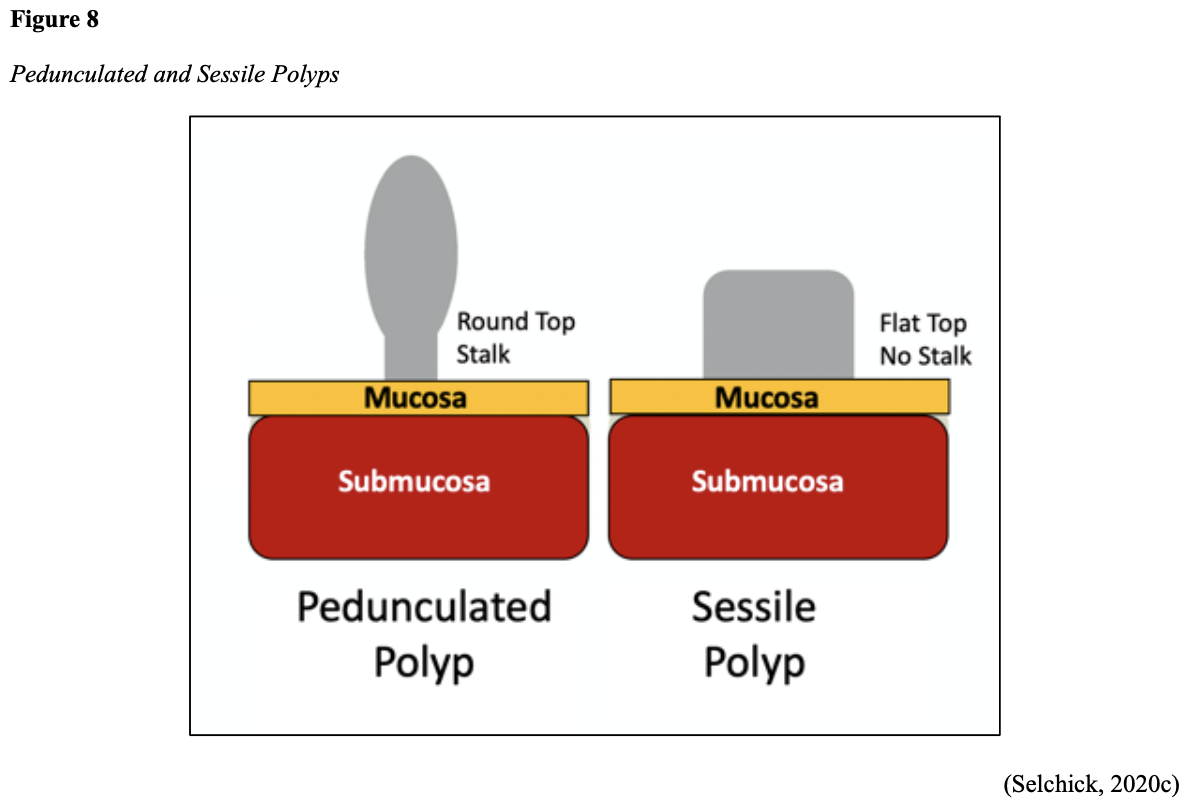
Cancer growth is a multistep process involving several genetic and environmentally induced alterations in cellular growth and differentiation that occur over time. While most CRCs arise from polyps, most polyps are not cancerous. There are two types of polyps strongly associated with cancer; hypoplastic and adenomatous (adenoma) polyps. Hypoplastic polyps associated with cancer are typically large (> 1 cm), present in multiples (>20), and located on the right side of the colon (ascending colon). Adenomas are more closely associated with CRC, and there are two chief growth patterns: tubular and villous. Most adenomas have a combination of both and are therefore called tubulovillous adenomas. Adenomas that are smaller (< 1.25 cm) typically have a tubular growth pattern, whereas larger adenomas generally have a villous growth pattern. Larger adenomas and those with a villous growth pattern are more likely to have cancerous cells within them (ACS, 2017a). Adenoma subtypes are also characterized by shape; pedunculated and sessile. As displayed in Figure 8, pedunculated polyps have a mushroom-like appearance as they have a round top attached to the surface by a narrow stalk. Sessile polyps are more common than pedunculated, are flatter, and do not have a stalk. These do not protrude as much from the colon wall, making them harder to identify. The three major types of sessile polyps include papillary, villous, or serrated. Serrated is a term used to describe any polyp with a saw-tooth pattern (McCance & Heuther, 2019; NCCN, 2020a, 2020b).
CRC Subtypes
CRC is not a solitary tumor type, as its pathogenesis can vary widely from slow-growing tumors to more aggressive disease. Histologically, the majority of CRCs are adenocarcinomas. The prefix ‘adeno’ means glands, and ‘carcinoma’ denotes a type of cancer that starts in the cells that form glands producing mucus to line the inner surfaces of intestines. Adenocarcinomas produce abnormal glands that can infiltrate the submucosa, muscle layer, and invade the surrounding tissues. In the submucosa, the cancerous cells can infiltrate lymphatic vessels and spread to the nearby lymph nodes or penetrate veins and metastasize (spread) to the liver or other organs. There are two less common subtypes of adenocarcinoma, which include mucinous and signet ring cell. Although rare, other types of CRC include primary colorectal lymphomas, GI stromal tumors, leiomyosarcomas, carcinoid tumors, and melanomas. Combined, these account for less than 5% of all cases. CRC subtypes are outlined further in Table 4 (ACS, 2017b; Macrae & Bendell, 2020).

Signs and Symptoms of CRC
Most early-stage CRCs do not have any obvious warning signs and are most often identified during screenings. Symptoms of CRC are typically due to the expansion of the tumor into the lumen or adjacent structures (Macrae & Bendell, 2020). Nurses can serve a central role in detecting early signs and symptoms of CRC. Nurses routinely triage patient symptoms, by telephone, in primary care offices, or the emergency department. Regardless of the setting, a well-informed nurse can recognize and respond to warning signs to facilitate a timely diagnosis (Kahl, 2018).
Some of the most common symptoms of CRC include the following:
- ongoing abdominal pain, cramping, gas pains, bloating, or fullness,
- rectal bleeding or blood in the stools (bright red, black, or tarry stools),
- new-onset anemia,
- unintentional weight loss, and
- excessive fatigue or unusual tiredness.
Rectal tumors can affect the stools’ caliber, causing narrower stools than usual, rectal pain, and bleeding. Tenesmus is also common, which is a continual inclination to evacuate the bowels and a sensation that the bowel does not empty completely. Some patients may present with a palpable mass on the digital rectal exam (DRE; CDC, 2020a; Macrae & Bendell, 2020).
Diagnosis
To definitely diagnose CRC, a tissue biopsy is required. The biopsy may be performed during a colonoscopy or as part of a surgical procedure. Nurses are critical in helping patients navigate the healthcare system as they undergo diagnostic testing. Nurses must ascertain adequate knowledge to explain the clinical significance of the testing, simplify medical terminology, effectively respond to questions, and clarify any misconceptions (Macrae & Bendell, 2020; Yarbro et al., 2018).
Carcinoembryonic antigen (CEA)
CEA is the most widely used and diagnostically sensitive tumor marker for patients with CRC. Tumor markers are substances that may be produced by cancer or by the body’s response to cancer’s presence but are also made in smaller quantities by healthy cells. Serum CEA is not sensitive enough to be used as a screening test for CRC. Research has demonstrated that the sensitivity of CEA for the diagnosis of CRC is only around 50%. CEA’s specificity is low as it is a nonspecific marker and can also be elevated in other cancerous processes such as pancreatic cancer or breast cancer. CEA is often elevated in noncancerous conditions such as gastritis, liver disease, peptic ulcer disease, diabetes, and several other acute or chronic inflammatory conditions. CEA levels are significantly higher in smokers than in nonsmokers. A normal CEA level is below 2.5 ng/mL in nonsmokers and below 5.0 ng/mL in smokers (Macrae & Bendel, 2020).
Diagnostic Imaging
Imaging tests are performed to evaluate the extent of the tumor, infiltration into surrounding structures, or distant metastasis to other sites in the body. In the US, about 20% of patients have distant metastatic disease at the time of presentation. The most common metastatic sites for CRCs are the regional (neighboring) lymph nodes, liver, lungs, and peritoneum. Many patients need to undergo a computed tomography [CT] of the chest, abdomen, and pelvis, or magnetic resonance imaging [MRI] of the abdomen and pelvis as part of their diagnostic workup (NCCN, 2020a). Nurses educate patients about the purposes, indications, and required preparation for diagnostic tests; thus, an accurate understanding of these tests is essential (Olson et al., 2019).
CT Scan
CT scans use a series of x-rays and computer technology to create cross-sections of the internal body structures. The CT scanner resembles a large doughnut, and the patient is positioned on a table that slides into the machine. Patients are advised to lie flat and remain still during the test for enhanced quality of images. The X-ray beam moves in a circle around the body to generate several different views. CT scans use ionizing radiation as their imaging method and may be performed with or without intravenous contrast administration (International Atomic Energy Agency [IAEA], n.d.). Iodine-based contrast may be administered intravenously (IV), orally (PO), or both. If contrast is used, patients are typically required to fast or remain nothing by mouth (NPO) for several hours before the scan. Oral contrast is administered about two hours before the examination and is most useful in visualizing the abdomen and pelvis structures; this is particularly useful when evaluating colon cancers. Patients should be informed that they may feel warm or flushed almost immediately following contrast injection and may develop a metallic taste in the mouth. These symptoms are transient, harmless, and only last seconds to minutes before they resolve (RadiologyInfo.org, 2018).
MRI
An MRI is distinct from a CT scan as it does not use x-rays or pose radiation exposure to patients and is considered a very safe imaging test. MRIs utilize strong electromagnetic fields (EM) and radio waves to measure the water content of tissues as a means to generate detailed images of internal organs and structures. An MRI image comes mainly from the protons in fat and water molecules within the body. The MRI scanner is essentially a large magnet, measured in a unit called Telsa (T). Most modern MRI scanners are 1.5 to 3T. To put this into context, an MRI of 3T strength is about 60,000 times stronger than the earth’s magnetic field. During an MRI, an electric current creates a temporary magnetic field within the patient’s body, and radio waves are sent from and received by a transmitter and receiving device within the machine. These signals are used to generate images of the body’s scanned area (US Food & Drug Administration [FDA], 2018). While CT scans are superior at visualizing anatomical structures, internal organs, and the skeleton, MRI scans are superior to CT scans at identifying the difference between normal and abnormal soft tissue. MRIs can be performed on nearly any body part, and each scan follows a specific protocol depending upon the clinical concern; they may be performed without or without contrast administration. Gadolinium-based contrast agents (GBCAs) are rare earth metals administered intravenously to enhance the contrast of the MRI images. Patients who are undergoing MRI scans of the abdomen or soft tissue pelvic structures are usually advised to remain NPO for six hours before the scan (Ibrahim et al., 2020).
Principles of Pathologic Review
A biopsy sample undergoes a series of tests to determine cancer’s pathologic features, evaluate its behavior, and select the best treatment options. It is important for nurses to understand the fundamental elements of the pathology report, including tumor grade and common CRC gene and biomarker analyses as a means to enhance understanding of CRC treatment options. Knowledge of the basic principles of pathology can also provide a foundational background necessary when educating patients and responding to questions regarding their pathology results (Yarbro et al., 2018).
Tumor grade
Tumor grade is a measurement of how different the cancer cells look compared to healthy cells under the microscope. The grade helps predict how likely the cancer is to grow and metastasize.
- Grade 1 is well-differentiated (appears similar to healthy cells) and the least aggressive.
- Grade 2 is moderately-differentiated, appears less like healthy cells, and is an intermediate grade (more aggressive than grade 1).
- Grade 3 is poorly differentiated or undifferentiated (does not resemble healthy cells at all), is high-grade, most aggressive, and tends to grow and spread more quickly (ACS, 2017b).
Gene and Molecular Biomarker Analyses
KRAS and NRAS
RAS is a family of genes that include KRAS and NRAS genes, which serve an important role in the treatment of metastatic CRC (mCRC). Patients with mutations in the KRAS and NRAS genes do not respond to certain treatments recommended for mCRC (NCCN, 2020a, 2020b).
BRAF V600E
Less than 10% of CRCs have a mutation called BRAF V600E. This mutation is considered a poor prognostic sign, as it causes cancer cells to grow and spread more quickly. BRAF mutations can be targeted with specific therapeutics termed BRAF inhibitors (NCCN, 2020a, 2020b).
Mismatch Repair (MMR)/Microsatellite Instability (MSI)
Most CRCs do not have high MSI levels or changes in MMR genes; changes in these genes are most common in those with Lynch syndrome. MMR testing helps guide if the patient should be tested for Lynch syndrome and determines if specific treatments (such as immunotherapy drugs) are likely to be effective. While MMR deficiency can be reported as microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), it is important for nurses to understand that these are interpreted to have the same meaning (NCCN, 2020a, 2020b).
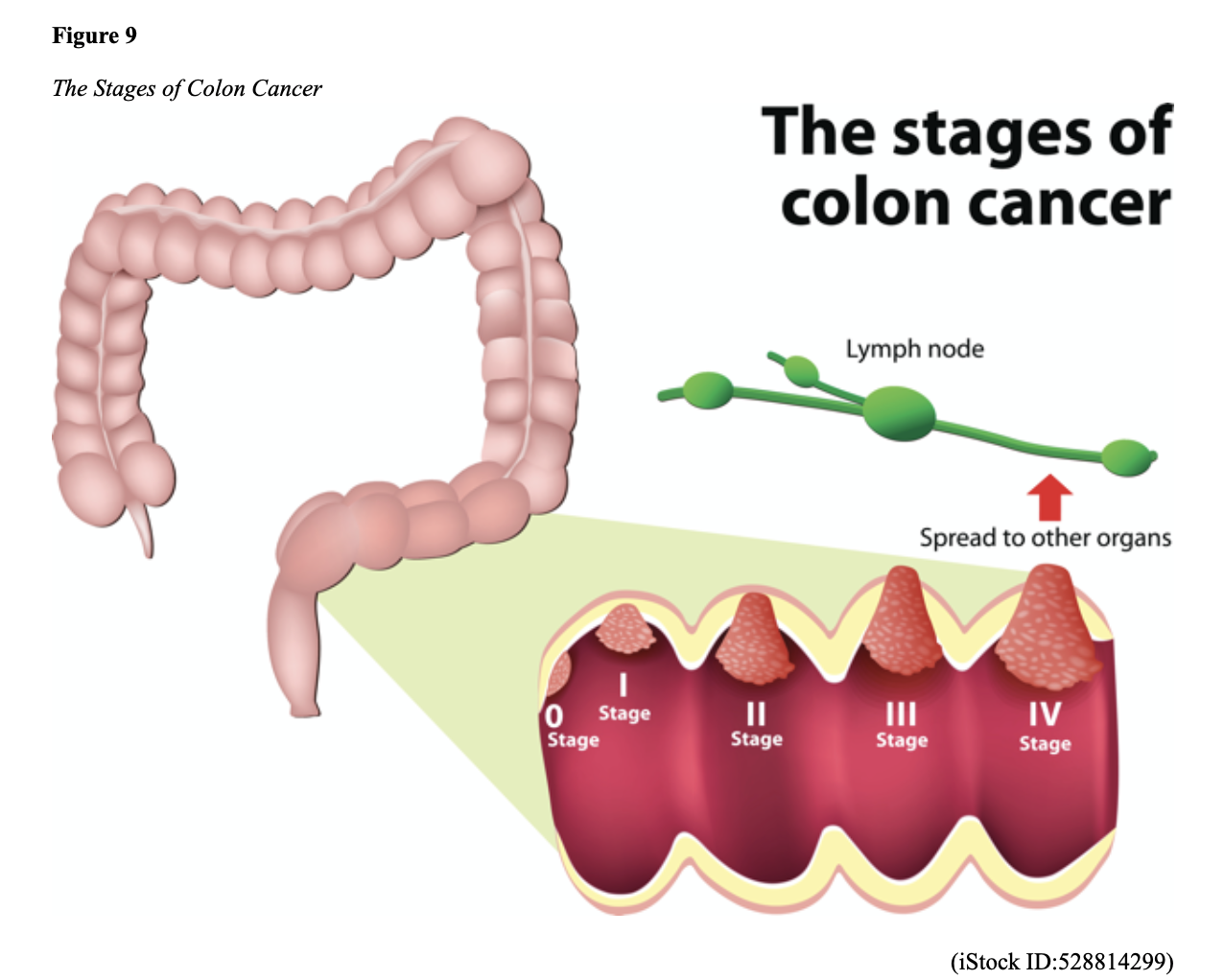
Cancer Staging
CRCs are staged based on the location of origin; the depth of invasion of cancer into the intestinal wall is also an important staging parameter. There are four stages of CRC, stage 1 (the cancer is localized) to stage IV (cancer has spread to distant sites in the body), as demonstrated in Figure 9
Treatment Modalities
Treatment for CRC is often multimodal in which more than one therapy is combined and administered simultaneously (concurrently) or sequentially. CRC treatments are grouped into two major categories of localized and systemic therapies, as demonstrated in Figure 10. The most optimal treatment modality depends on several factors, including the pathologic features and cancer stage. A synopsis of each treatment subcategory will be described within this section (ACS, 2020c; NCCN, 2020b).
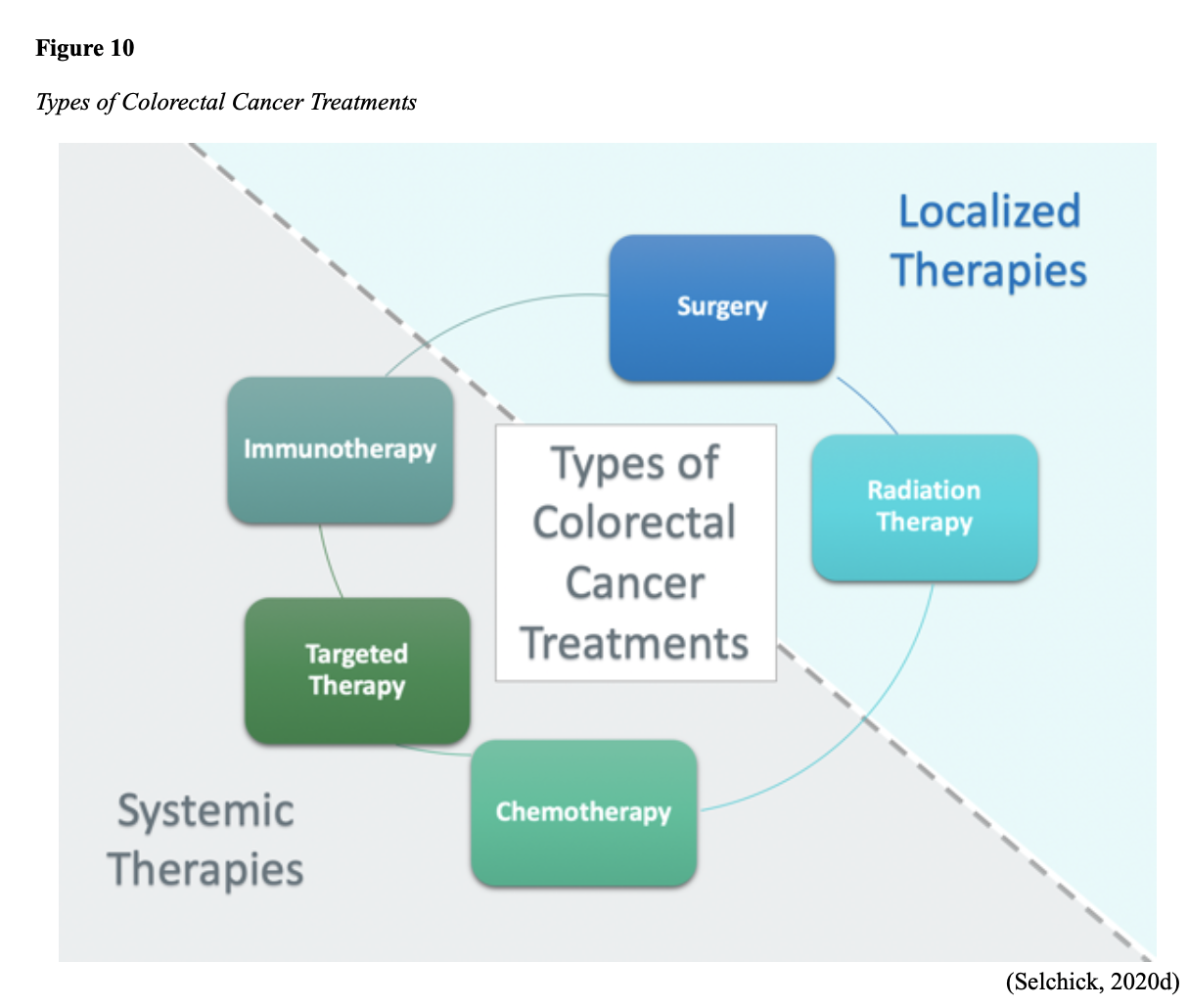
Localized Therapies
Treatments explicitly directed at the tumor without affecting the rest of the body are referred to as localized therapies. There are two major types of localized treatment options for CRC; surgery and radiation therapy. These treatments are most useful for early-stage cancers that have not metastasized beyond the area of origin. Surgery is the mainstay treatment for early-stage colon and rectal tumors. Since there are fundamental distinctions between surgical interventions for colon tumors and rectal tumors, they will be reviewed separately (ACS, 2020c; NCCN, 2020a).
Surgical Intervention for Colon Cancer
Polypectomy. Precancerous lesions and some early-stage colon cancers can be effectively removed with a polypectomy, a relatively noninvasive procedure to remove a polyp that is most often performed during a colonoscopy. As demonstrated in Figure 11, the polyp is removed with a snare, a wire loop device designed to slip over the polyp. Upon closure of the device, the polyp is removed at the stalk. Snares can burn through the base of the polyp (electrocautery). The goal of polypectomy is to remove the entire lesion in one piece and avoid leaving residual cancerous cells behind, which could replicate, grow, and spread over time (ACS, 2020c).

Colectomy. A colectomy is a surgical procedure in which all or part of the colon is removed. If only a portion of the colon is removed, the procedure is called a hemicolectomy. The extent of the surgery depends on the degree of cancer presence. Lymphadenectomy is usually performed during a colectomy, which involves removing nearby lymph nodes (ACS, 2020c). At the end of the procedure, the remaining two ends of the colon are adjoined. Figure 12 demonstrates a transverse colectomy in which part of the bowel was removed.

Colostomy. If the cancer is more advanced or the colon becomes obstructed by the tumor, more extensive surgery is required. If a large portion of the colon is removed, the surgeon may not be able to reattach the colon. In these situations, a stoma is created in which the end of the intestine is brought to the surface of the abdominal wall to provide an outlet for waste products (stool) to leave the bowel. A colostomy may be temporary or permanent, and the type of colostomy may vary based on the stoma’s location, as shown in Figure 13. An ileostomy (not pictured) is formed when the end of the small intestine (the ileum) is connected to a stoma. A collection bag is attached to the skin to hold the stool, as displayed in Figure 14 (ACS, 2020c).


Surgical Intervention for Rectal Cancer
The type of surgery depends on the anatomical location and extent of rectal cancer. Early rectal cancers are usually removed by polypectomy, whereas more advanced rectal tumors may require local excision in which healthy surrounding tissue is removed alongside the cancerous tissue (NCCN, 2020b).
Transanal excision (TAE). TAE is an option for early-stage rectal tumors located near the anal opening that have not spread to the anus or sphincter. During this procedure, the surgeon cuts through the layers of the rectal wall to remove the tumor as well as some surrounding healthy tissue. TAE typically allows patients to retain bowel function and eliminates the need for a permanent colostomy (ACS, 2020c).
Low anterior resection (LAR). LAR is a more extensive surgical approach reserved for advanced rectal tumors located in the upper portion of the rectum. The cancerous portion of the rectum is removed, and the lower portion of the colon is then reattached to the remaining part of the rectum. Patients may need a temporary colostomy following a LAR, but most will regain the normal functioning of their bowels (ACS, 2020c).
Proctectomy with colo-anal anastomosis. Surgical removal of the entire rectum is called a proctectomy. Most stage II and III tumors located in the middle or lower third portion of the rectum are treated with proctectomy, followed by a total mesorectal excision (TME). A TME involves the removal of all surrounding lymph nodes. The colon is then reattached to the anus in a procedure called a colo-anal anastomosis. Most patients will regain normal function of their bowels over time (ACS, 2020c).
Abdominoperineal resection (APR). APR is similar to a LAR, but it is a more extensive surgery. APR is typically indicated for Stage II and Stage III rectal tumors that have invaded the sphincter muscles or the levator muscles (the muscles that control urinary flow). In an APR, the entire anus is removed, necessitating a permanent colostomy (ACS, 2020c).
Pelvic Exenteration. Pelvic exenteration is a much more radical and complex surgical approach in which the rectum and any affected nearby organs are removed, such as the prostate or bladder. Patients require a permanent colostomy, and if the bladder is removed, a urostomy (opening through the skin to allow the elimination of urinary waste). This surgical technique is not commonly performed as it is associated with significant morbidity (ACS, 2020c).
Surgical Risks and Side Effects
The risks and side effects of surgery depend on the size and degree of cancer invasion, the extent of surgery, and the structures removed. All surgeries and invasive procedures are accompanied by risks, such as adverse reactions to anesthesia, bleeding, infection, perforation of the bowel (a hole in the intestines), and life-threatening sepsis. Patients may have complications with newly formed colostomies, such as malfunctioning of the stoma, skin breakdown, and leaking at the site. Adjusting to a colostomy can be challenging, and patients may struggle with caring for and managing the device. Nurses serve a vital role in helping patients acclimate to the physical care of their new stoma and colostomy device, which can pose extensive challenges for CRC survivors. Patients are often affected by the physical and psychological aspects of body image distortion and lifestyle adjustments. Studies have demonstrated that nurses are key in identifying and meeting patients’ psychosocial needs and managing negative emotions toward colostomy care. Compassionate and knowledgeable nursing care focused on managing psychosocial needs, reducing anxiety, and addressing depression is helpful in alleviating patients’ stress responses and promotes healthy coping and adjustment (Jin et al., 2019). Sexual dysfunction is common among males who undergo LAR or APR as the nerves that supply blood to the penis may be injured during the surgery. Males may be unable to achieve an erection or orgasm, which can negatively impact interpersonal relationships, quality of life, psychological health, self-esteem, and wellbeing (ACS, 2020c). Nurses are tasked with addressing these issues with sensitivity, empathy, and compassion, fostering a safe and nonjudgmental environment for patients to openly express their concerns. Nurses can offer psychosocial support and connect patients with resources, support groups, and medical specialists (Yarbro et al., 2018).
Radiation Therapy
Radiation therapy is a type of localized treatment that delivers a precisely measured amount of high-energy, highly focused rays of ionizing radiation to the tumor while providing as little injury as possible to surrounding tissue. Radiation causes cellular damage to cancer cells, leading to biological changes in the DNA, rendering cells incapable of reproducing or spreading. All healthy cells and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed; however, healthy cells can repair themselves and remain functional. The total dose of radiation is hyper-fractionated, which means it is delivered to the tumor in small divided doses, or fractions, rather than all at once. Hyper-fractionation allows healthy cells a chance to recover between treatments. The total number of fractions (doses) administered depends on the tumor size, location, reason for treatment, patient’s overall health, performance status, goals of therapy, as well as consideration of any other concurrent therapies the patient is receiving (Nettina, 2019). Radiation therapy plays a central role in treating many types of CRCs and can be delivered externally or internally; some patients may receive both (ACS, 2020c).
External beam radiation therapy (EBRT) delivers radiation from a source outside the body and is the most common type of radiation therapy used for CRC. Traditionally, radiation beams were only able to match the tumor’s height and width, exposing more healthy tissue to the consequences of radiation. Over recent decades, 3-D conformational radiation therapy (3D-CRT) became the mainstay EBRT for CRCs. 3D-CRT is credited with the ability to reshape the radiation beam to match the shape of the tumor. Further advancements in imaging technology have led to more precise treatment mechanisms that allow even more of the radiation beam to reach the tumor. Intensity-modulated radiation therapy (IMRT) is a newer, highly conformal form of radiation that further reduces unintended exposure to healthy tissues. While 3D-CRT and IMRT are very similar in that they both target the tumor while sparing healthy tissue, IMRT allows for modulation of the radiation beam’s intensity, delivering a higher radiation dose to a precise location. The enhanced targeting technology of IMRT allows for the delivery of higher radiation doses to the site of disease, thereby enhancing clinical outcomes and limiting side effects (Hathout et al., 2017). Stereotactic body radiation therapy (SBRT) is a technique in which extremely high biological doses of radiation are administered over a few short treatments. The target area is affected to a higher degree over a shorter period with minimal impact on healthy tissue (Jingu et al., 2018).
Brachytherapy and intraoperative radiation therapy (IORT) are among the most well-cited internal modalities. Brachytherapy involves implanting a wire, seed, pellet, or catheter into the body within or near the tumor. IORT is an intensive form of radiation administered directly into the target area during surgery while sparing the surrounding healthy tissues. The role of IORT is limited but may be considered as an additional radiation boost for patients with large tumors or those with recurrent tumors. IORT is also a viable treatment option for patients with CRC that has spread to the liver, causing predominant hepatic disease (Hathout et al., 2017; NCCN, 2020b).
Radiation Side Effects
Nurses caring for patients undergoing radiation therapy serve multifaceted roles in monitoring for and managing side effects. Radiation side effects depend on the specific area(s) of the body exposed and the dose received. Superficial skin irritation at treatment site is common and can include redness, blistering, and inflammation, giving skin the appearance of a sunburn. GI symptoms are common due to the tumor’s anatomical location and the impact of the radiation on surrounding tissues and structures. Common symptoms of GI toxicity include nausea, vomiting, diarrhea, anorexia, bowel incontinence, rectal irritation, rectal bleeding, blood in stools, and pain with defecation. Radiation to these regions can also lead to fluid volume deficit, electrolyte disturbances, weight loss, and malnutrition. Some patients may require placement of a feeding tube to ensure adequate nutrition and prevent cachexia. Nutrition is a core component of all forms of cancer treatment and largely influences the patient’s tolerance to therapy and toxicities. The placement of a percutaneous endoscopic gastrostomy (PEG) tube may be necessary for some patients. Radiation for CRC can sometimes indirectly affect underlying structures located within the radiation field, such as the bladder or prostate, causing cystitis (inflammation of the bladder), dysuria (painful urination), hematuria (blood in the urine), urinary incontinence, and loss of pelvic floor muscular strength. Systemic effects may include fatigue, weakness, dehydration, scarring, fibrosis, and adhesion formation (the tissues impacted by radiation stick together; ACS, 2020c; Yarbro et al., 2018).
For more information regarding nursing implications in radiation therapy, refer to the Oncology Nursing Part 1: Surgical and Radiation Oncology Nursing CE Course and earn five ANCC credits.
Systemic Therapies
Chemotherapy
Chemotherapy, also called cytotoxic or antineoplastic therapy, refers to a group of high-risk, hazardous medications that destroy cancer cells throughout the body. Chemotherapy works by interfering with the normal cell cycle, impairing DNA synthesis and cell replication, thereby preventing cancer cells from dividing and multiplying (Yarbro et al., 2018). Chemotherapy serves a prominent role in treating CRC and can be used at various time points during the disease trajectory. Neoadjuvant chemotherapy intends to shrink a tumor so that the surgical intervention is less extensive. Neoadjuvant chemoradiation (concurrent chemotherapy and radiation therapy) is the gold standard for locally advanced rectal cancer, followed by surgical resection and then adjuvant chemotherapy (additional chemotherapy following surgery). Chemotherapy acts as a radiosensitizer, thereby rendering cancer cells more vulnerable to the toxic effects of radiation. After surgery, adjuvant chemotherapy aims to prevent cancer recurrence, reduce micro-metastases, and eradicate any remaining cancer cells. Palliative chemotherapy aims to relieve or delay cancer symptoms, enhance comfort, reduce symptom burden, and improve quality of life. CRC chemotherapy can include oral and/or intravenous formulations and typically consist of at least two or three medications. Some of the most common chemotherapy agents used for CRC are listed below (Itano, 2016; Olsen et al., 2019).
- 5-fluorouracil (5-FU)
- capecitabine (Xeloda)
- irinotecan (Camptosar)
- oxaliplatin (Eloxatin)
- trifluridine and tipiracil (Lonsurf) (ACS, 2020c; NCCN, 2020a, 2020b).
Chemotherapy Side Effects
Side effects of chemotherapy are inevitable due to the nonspecific nature of cytotoxic therapy; it simultaneously impacts healthy cells along with cancerous cells. However, side effects vary based on the drug type, dosage, duration of treatment, and specific patient factors. Not all patients respond in the same way, and not all chemotherapy agents pose the same risks. Assessment and education are the most critical components to ensuring timely recognition, intervention, and management of side effects as experienced by each patient. Many side effects, such as nausea, can be primarily thwarted by implementing appropriate prevention strategies and medications. As a group, the most common side effects include reduced blood counts (anemia, thrombocytopenia, neutropenia), fatigue, nausea, anorexia, alopecia (hair loss), mucositis (mouth sores), diarrhea, skin changes, and peripheral neuropathy (damage to the sensory nerves). Table 6 highlights a few of the unique side effects and important patient teaching points for each medication (Nettina, 2019; Olsen et al., 2019).

Targeted Therapy
Targeted agents are a highly specialized treatment modality devised to attack specific parts of cancer cells. They work to prevent tumor development or to shrink existing tumors. Proteins (called growth factor receptors) connect the external and internal cellular environments and are essential for healthy cell growth and development. Alterations in genes lead to changes in these proteins, disrupting normal cellular processes and igniting an environment for cancer growth. While each type of targeted therapy has a distinct mechanism of action, they all interfere with the cancer cell’s ability to grow, divide, repair, and/or communicate with other cells. By directing their effects at tumor cell growth through specific targets, these therapies are considered less toxic to normal cells and tissues than traditional chemotherapy agents. However, cancer cells have the potential to become resistant to them, as they only block specific pathways of cancer growth. The most common targeted therapies used for the treatment of CRCs will be described in this section (Sengupta, 2017).
Epidermal growth factor receptor (EGFR) Inhibitors. EGFR is a protein found on some normal cells’ surface, which causes cells to divide when the epidermal growth factor binds to it. EGFR is found at abnormally high levels in certain types of CRCs, and activation of the EGFR accelerates tumor growth. EGFR inhibitors are a class of medications that impede the activation of EGFR, thereby blocking cancer growth. Cetuximab (Erbitux) and panitumumab (Vectibix) are monoclonal antibodies widely utilized in CRC treatment. They selectively bind to the extracellular component of the EGFR, thereby preventing cellular growth. Scientists analyze specific antigens on cancer cells (target) to determine a protein to match the antigen and then create a specialized antibody to precisely attach to the target antigen like a key fits a lock. The antibodies bind to the antigen and mark it for destruction by the immune system. Monoclonal antibodies work on cancer cells in the same way natural antibodies work by identifying and binding to the target cells and then alerting other cells in the immune system to the presence of the cancer cells (Sengupta, 2017). Cetuximab (Erbitux) is a chimeric monoclonal antibody, primarily made of a mouse (murine) protein with a (lesser) human protein component. It binds to extracellular EGFR resulting in inhibition of cell growth and induction of apoptosis. Panitumumab (Vectibix) is a fully human monoclonal antibody that inhibits ligand binding to the EGFR receptor resulting in inhibition of cell growth. There is a higher risk of infusion-related reactions with cetuximab (Erbitux) due to its higher murine content, which can induce rigors, chills, and fevers. Rarely, life-threatening hypersensitivity reactions can occur. The most common side effects of EGFR inhibitors include skin manifestations, particularly an acne-like rash on the face and chest. Other side effects can include fatigue, diarrhea, and headache (ACS, 2020c).
Vascular endothelial growth factor (VEGF) inhibitors. VEGF is a signaling protein that stimulates angiogenesis (the formation of new blood vessels) in both healthy and cancerous cells. Blood vessels carry oxygen and nutrients to the tissue for growth and survival. Tumors need blood vessels to grow and spread. Anti-angiogenesis is the process of inhibiting the formation of new blood vessels by blocking the VEGF receptors. Angiogenesis inhibitors (VEGF-inhibitors) target the blood vessels that supply oxygen to the tumor cells, ultimately causing them to starve by cutting off their nutrient supply. VEGF-inhibitors such as bevacizumab (Avastin) work by cutting off blood supply to cancer cells by interfering with the VEGF receptor, so tumors stay small and eventually die due to starvation. Bevacizumab (Avastin) is a humanized monoclonal antibody that binds to and inhibits the activity of human VEGF to its receptors, thereby blocking proliferation and formation of new blood vessels that supply tumor cells. Bevacizumab (Avastin) is commonly used as part of combination therapy for the treatment of CRC. VEGF inhibitors’ potential side effects include bleeding events, headache, hypertension, and proteinuria (protein spilling out in the urine due to increased pressure in the kidneys; Olsen et al., 2019).
Nurses should educate patients on the risk of hypertension while on treatment with VEGF-inhibitors. Patients may require blood pressure management with antihypertensives or may need an adjustment to their current antihypertensive medication regimen. Patients should also be counseled on ways to reduce their blood pressure through compliance with medications, dietary adjustments such as following a heart-healthy diet high in fiber, fruits and vegetables, and low in sodium, and engaging in regular cardiovascular exercise. VEGF-inhibitors are contraindicated within six weeks of surgery (preoperatively or postoperatively) due to an increased risk for major bleeding events, delayed wound healing, and fistula (an abnormal connection between two hollow spaces within the body). VEGF-inhibitors carry a boxed warning for bowel perforation (a hole in the intestines). Nurses must ensure patients are aware of this rare but serious side effect. Patients must report any sudden onset of severe and diffuse abdominal pain, an abdomen that is unusually firm or hard to touch, sudden onset bloating, nausea, vomiting, or rectal bleeding (Olsen et al., 2019).
BRAF V600E inhibitors. BRAF inhibitors are only indicated for patients with a mutation in the BRAF V600E gene. As mentioned earlier, BRAF-mutated disease is an aggressive subtype of CRC that carries a poorer prognosis, although the mechanism remains poorly understood. BRAF inhibitors have been extensively studied in their management of malignant melanomas; however, their activity in CRC is limited (Caputo et al., 2019; Korphaisarn & Kopetz, 2016). The most common BRAF-inhibitors used for CRCs include encorafenib (Braftovi), vemurafenib (Zelboraf), and dabrafenib (Tafinlar). These oral medications attack the abnormal BRAF protein directly. When given with cetuximab (Erbitux) or panitumumab (Vectibix), the combination has been shown to shrink or reduce the progression of metastatic CRC and increase survival (Ducreux et al., 2019; NCCN, 2020a, 2020b). Common side effects include skin rash, abdominal pain, diarrhea, anorexia, nausea, joint pain, and fatigue. BRAF inhibitors increase the risk for the development of new squamous cell skin cancers (SCC). Nurses should educate patients on monitoring for and reporting any new skin lesions (ACS, 2020c).
Immunotherapy
Immunotherapy is a novel group of cancer treatments that stimulate the immune system to recognize and destroy cancer cells. Immunotherapy aims to produce antitumor effects by modifying the actions of the body’s natural host defense mechanisms to become more sensitive to cancer cells. Immune-based treatments work differently than chemotherapy as they are highly specialized in their activity (Miliotou & Papadopoulou, 2018). Immune therapy has emerged as an effective treatment strategy for patients with metastatic MSI-H or MMR-deficient (dMMR) disease who have disease progression (or whose cancer has grown) despite the use of standard chemotherapy. Immune checkpoint inhibitors work by blocking the receptors that cancer cells use to inactivate immune cells (specifically, T-cells). When this signal is blocked, T-cells can better differentiate between healthy cells and cancer cells, thereby augmenting the cancer cells’ immune response. Checkpoint inhibitors are categorized into two categories: (1) programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) inhibitors, and (2) cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitors (Sasikumar & Ramachandra, 2018). Pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy) are the three immunotherapy drugs approved for metastatic CRC (Olsen et al., 2019).
Immunotherapy Side Effects
The most common side effects of immunotherapy include fatigue, nausea, anorexia, cough, diarrhea, arthralgias, myalgias, skin rash, pruritus, and flu-like symptoms. All immunotherapy agents carry boxed warnings for immune-mediated adverse reactions (irAEs), which can be fatal if left untreated. An immune response can impact any organ system, inducing nonspecific inflammation throughout the body, ranging from mild inflammatory effects to life-threatening reactions that develop without warning. The incidence of irAEs across clinical trials ranged from as low as 15% to as high as 90% (Winer et al., 2018). Some of the most commonly reported irAEs include hepatitis (inflammation of the liver), endocrinopathies (inflammation to the thyroid and adrenal glands), nephritis (inflammation of the kidneys), and uveitis (inflammation of the eye). Less common but more severe and potentially fatal irAEs include pneumonitis (inflammation of the lung tissue), enterocolitis (inflammation of the bowel), and Stevens-Johnson syndrome (SJS), a rare, severe disorder of the skin and mucous membranes (Sasikumar & Ramachandra, 2018). Among the immunotherapy agents, PD-L1 inhibitors/PD-1 inhibitors are generally the best tolerated and pose a lower risk for irAEs. Compared to drugs that target PD-1 or PD-L1, serious side effects are much more likely with CTLA-4 agents such as ipilimumab (Yervoy; Stenger, 2018). There is an evolving role for combination therapy, in which immunotherapy is given in tandem with one another to provide a synergistic effect; however, this combination poses an even greater risk for severe irAEs. Immunotherapy is also given in combination with cytotoxic chemotherapy agents at times, further complicating their already complex side effect profiles (Winer et al., 2018).
Nursing care of the patient receiving immunotherapy requires cautious triage and continuous meticulous assessment to identify signs of potential irAEs, as a timely diagnosis is critical to ensure prompt response and reduce morbidity. Most irAEs are reversible with immunosuppressive steroid treatment but must be graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5 (v5.0) and managed per specific medication guidelines (NCI, 2020). Patient education is vital. Nurses must teach patients and caregivers about the importance of self-assessment and the immediate reporting of any symptoms. With pneumonitis, symptoms can range from mild cough and dyspnea to severe shortness of breath and life-threatening hypoxia. GI toxicity can range from mild diarrhea and abdominal cramping to severe colitis, which can be fatal if not managed. Skin toxicity may present initially as mild pruritus or dermatitis and can progress to SJS. SJS is characterized by a painful systemic red rash that leads to blistering and sloughing of the skin’s top layer. Life-threatening endocrinopathies can cause an abundance of symptoms that may vary widely, such as extreme weakness, excessive fatigue or lethargy, electrolyte disturbances, thyroid inflammation, and pituitary dysfunction (Olsen et al., 2019; Winer et al., 2018).
For a more detailed review of the principles of chemotherapy, immunotherapy, and specific nursing implications, refer to the following NursingCE courses:
- Oncology Nursing Part 2: Chemotherapy and Oncologic Emergencies (5 contact hours)
- Oncology Medication Administration for LPNs and RNs (7 ANCC contact hours)
References
American Cancer Society. (2017a). Understanding your pathology report: Colon polyps (sessile or traditional serrated adenomas). https://www.cancer.org/treatment/understanding-your-diagnosis/tests/understanding-your-pathology-report/colon-pathology/colon-polyps-sessile-or-traditional-serrated-adenomas.html
American Cancer Society. (2017b). Understanding your pathology report: Invasive adenocarcinoma of the colon. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/understanding-your-pathology-report/colon-pathology/invasive-adenocarcinoma-of-the-colon.html
American Cancer Society. (2020a). Colorectal cancer facts & figures 2020-2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
American Cancer Society. (2020b). Colorectal cancer risk factors. https://www.cancer.org/cancer/colon-rectal-cancer/causes-risks-prevention/risk-factors.html
American Cancer Society. (2020c). Treating colon cancer. https://www.cancer.org/content/dam/CRC/PDF/Public/8607.00.pdf
Berkowitz, Z., Zhang, X., Richards, T. B., Nadel, M., Peipins, L. A., & Holt, J. (2018). Multilevel small-area estimation of colorectal cancer screening in the United States. Cancer Epidemiology Biomarkers & Prevention, 27(3), 245-253. https://doi.org/10.1158/1055-9965.EPI-17-0488
BruceBlaus. (2013). The large intestine [image]. https://commons.wikimedia.org/wiki/File:Blausen_0604_LargeIntestine2.png
BruceBlaus. (2014). Colostomy types [image]. https://upload.wikimedia.org/wikipedia/commons/8/88/Blausen_0247_Colostomy.png
Cancer Research UK. (2014a). Part of the bowel removed with a transverse colectomy [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_the_part_of_the_bowel_removed_with_a_transverse_colectomy_CRUK_319.svg
Cancer Research UK. (2014b). Stoma with colostomy bag [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_a_colostomy_with_a_bag_CRUK_061.svg
Caputo, F., Santini, C., Bardasi, C., Cerma, K., Casadei-Gardini, A., Spallanzani, A., Andrikou, K., Cascinu, S., & Gelsomino, F. (2019). BRAF-mutated colorectal cancer: Clinical and molecular insights. International Journal of Molecular Sciences, 20(21), 5369. https://doi.org/ 10.3390/ijms20215369
The Centers for Disease Control and Prevention. (2020a). Basic information about colorectal cancer. https://www.cdc.gov/cancer/colorectal/basic_info/
The Centers for Disease Control and Prevention. (2020b). The BRCA1 and BRCA2 genes. https://www.cdc.gov/genomics/disease/breast_ovarian_cancer/genes_hboc.htm
Ducreux, M., Chamseddine, A., Laurent-Puig, P., Smolenschi, C., Hollebecque, A., Dartigues, P., Samallin, E., Boige, V., Malka, D., & Gelli, M. (2019). Molecular targeted therapy of BRAF-mutant colorectal cancer. Therapeutic Advances in Medical Oncology, 11, 1-15. https://doi.org/10.1177/1758835919856494
Goran, T. (2014). Layers of the intestinal wall [image]. https://commons.wikimedia.org/wiki/File:Layers_of_the_GI_Tract_english.svg
Hathout, L., Williams, T. M., & Jabbour, S. K. (2017). The impact of novel radiation treatment techniques on toxicity and clinical outcomes in rectal cancer. Curr Colorectal Cancer Rep, 13(1), 61-72. https://doi.org/10.1007/s11888-017-0351-z
Ibrahim, M. A., Hazhirkarzar, B., & Dublin, A. B. (2020). Magnetic resonance imaging (MRI) gadolinium. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482487/
Innerbody Research. (2017). Cecum. [image]. https://www.innerbody.com/image_dige08/dige44.html
International Atomic Energy Agency. (n.d.). Computed tomography – what patients need to know. Retrieved October 16, 2020, from https://www.iaea.org/resources/rpop/patients-and-public/computed-tomography
Islami, F., Sauer, A. G., Miller, K. D., Siegel, R. L., Fedewa, S. A., Jacobs, E. J., McCullough, M. L., Patel, A. V., Ma, J., Soerjomatarm, I., Flanders, W. D., Brawley, O. W., Gapstur, S. M., & Jemal, A. (2018). Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: Cancer J Clin, 68(1), 31-54. https://doi.org/10.3322/caac.21440
Itano, J. K. (2016). Core curriculum for oncology nursing. (5th ed.). Elsevier
Jin, Y., Zhang, J., Zheng, M., Bu, X., & Zhang, J. (2019). Psychosocial behavior reactions, psychosocial needs, anxiety and depression among patients with rectal cancer before and after colostomy surgery: A longitudinal study. Journal of Clinical Nursing, 28(19-20), 3547-3555. https://doi.org/10.1111/jocn.14946
Jingu, K., Matsushita, H., Yamamoto, T., Umezawa, R., Ishikawa, Y., Takahashi, N., Katagiri, Y., Takenda, K., & Kadoya, N. (2018). Stereotactic radiotherapy for pulmonary oligometastases from colorectal cancer: A systematic review and meta-analysis. Technology in Cancer Research & Treatment, 17, 1-7. https://doi.org/10.1177/1533033818794936
Kahl, K. L. (2018). Nurses play integral role in newly issues colorectal screening guidelines. https://www.oncnursingnews.com/web-exclusives/nurses-play-integral-role-in-newly-issued-colorectal-screening-guidelines
Klinikum, O. (2013). Method of removing a polyp with a snare [image]. https://commons.wikimedia.org/wiki/File:Ortenau_Klinikum_Darmspiegelung.jpg
Korphaisarn, K., & Kopetz, S. (2016). BRAF-directed therapy in metastatic colorectal cancer. Cancer J, 22(3), 175-178. https://doi.org/10.1097/PPO.0000000000000189
Macrae, F. A., & Bendell, J. (2020). Clinical presentation, diagnosis, and staging of colorectal cancer. UpToDate. https://www.uptodate.com/contents/clinical-presentation-diagnosis-and-staging-of-colorectal-cancer
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
McCullough, M. L., Zoltick, E. S., Weinstein, S. J., Fedirko, V., Wang, M., Cook, N. R., Eliassen, A. H., Zeleniuch-Jacquotte, A., Agnoli, C., Albanes, D., Barnett, M. J., Buring, J. E., Campbell, P. T., Clendenen, T. V., Freedman, N. D., Gapstur, S. N., Giovannucci, E. L., Goodman, G. G., Haiman, C. A., ... Smith-Warner, S. A. (2019). Circulating vitamin D and colorectal cancer risk: An international pooling project of 17 cohorts. Journal of National Cancer Institute, 111(2), 158-169. https://doi.org/10.1093/jnci/djy087
Miliotou, A. N., & Papadopoulou, L. C. (2018). CAR T-cell therapy: A new era in cancer immunotherapy. Current Pharm Biotechnology, 19(1), 5-18. https://doi.org/10.2174/1389201019666180418095526.
National Cancer Institute. (2020). Common terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
National Comprehensive Cancer Network. (2020a). NCCN clinical practice guidelines in oncology (NCCN guidelines®) colon cancer: Version 4.2020-June 15, 2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
National Comprehensive Cancer Network. (2020b). NCCN clinical practice guidelines in oncology (NCCN guidelines®) rectal cancer: Version 6.2020- June 25, 2020. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice. (11th ed.). Wolters Kluwer.
Oh, M., McBride, A., Yun, S., Bhattacharjee, S., Slack, M., Martin, J. R., Jeter, J., & Abraham, I. (2018). BRCA1 and BRCA2 gene mutations and colorectal cancer risk: Systematic review and meta-analysis. J Natl Cancer Institute, 110(11), 1178-1189. https://doi.org/10.1093/jnci/djy148
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice. (1st Ed.). Oncology Nursing Society
Olson, A., Rencic, J., Cosby, K., Rusz, D., Papa, F., Croskerry, P., Zierler, B., Harkless, G., Giuliano, M. A., Schoenbaum, S., Colford, C., Cahill, M., Gerstner, L., Grice, G. R., & Graber, M. L. (2019). Competencies for improving diagnosis: An interprofessional framework for education and training in health care. Diagnosis, 6(4), 335-341. https://doi.org/10.1515/dx-2018-0107
OpenStax College. (2013). Components of the digestive system [image]. https://commons.wikimedia.org/wiki/File:2401_Components_of_the_Digestive_System.jpg
RadiologyInfo.org. (2018). Contrast materials. https://www.radiologyinfo.org/en/pdf/safety-contrast.pdf
Sasikumar, P. G. & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. https://doi.org/10.1007/s40259-018-0303-4.
Selchick, F. (2020a). Age-specific CRC incidence rates in US per 100,000 individuals [image].
Selchick, F. (2020b). Comparison of colon and rectal cancers [image].
Selchick, F. (2020c). Pedunculated and sessile polyp [image].
Selchick, F. (2020d). Types of colorectal cancer treatment [image].
Sengupta, S. (2017). Cancer nanomedicine: Lessons for immune-oncology. Trends Cancer, 3(8), 551-560. https://doi.org/10.1016/j.trecan.2017.06.006.
Schwingshackl, L., Schwedhelm, C., Hoffman, G., Knuppel, S., Preterre, A. L., Iqbal, K., Bechthold, A., De Henauw, S., Michels, N., Devleesschauwer, B., Boeing, H., & Schlesinger, S. (2017). Food groups and risk of colorectal cancer. International Journal of Cancer, 142(9), 1748-1758. https://doi.org/10.1002/ijc.31198
Siegel, R. L., Miller, K. D., Sauer, A. G., Fedewa, S. A., Butterly, L. F., Anderson, J. C., Cercek, A., Smith, R. A., & Jemel, A. (2020). Colorectal cancer statistics, 2020. CA Cancer J Clin, 70(3), 145-164. https://doi.org/10.3322/caac.21601
Stanford Health Care. (n.d.). Colorectal cancer. Retrieved September 23, 2020, from https://stanfordhealthcare.org/medical-conditions/cancer/colorectal-cancer.html
Stenger, M. (2018). Ipilimumab in combination with nivolumab for MSI-H or dMMR metastatic colorectal cancer. https://ascopost.com/issues/september-10-2018/ipilimumab-in-combination-with-nivolumab-for-colorectal-cancer/
US Food & Drug Administration. (2018). MRI (magnetic resonance imaging). https://www.fda.gov/radiation-emitting-products/medical-imaging/mri-magnetic-resonance-imaging
US National Library of Medicine. (2020a). Familial adenomatous polyposis. https://ghr.nlm.nih.gov/condition/familial-adenomatous-polyposis
US National Library of Medicine. (2020b). Lynch syndrome. https://ghr.nlm.nih.gov/condition/lynch-syndrome#:~:text=Inheritance%20Pattern,sufficient%20to%20increase%20cancer%20risk
US Preventative Services Task Force. (2016a). Final recommendation statement: Aspirin use to prevent cardiovascular disease and colorectal cancer: Preventative medication. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin-to-prevent-cardiovascular-disease-and-cancer
US Preventative Services Task Force. (2016b). Final recommendation statement: Colorectal cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Vieira, A. R., Abar, L., Chan, D. S. M., Vingeliene, S., Polemiti, E., Stevens, C., Greenwood, D., & Norat, T. (2017). Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Annals of Oncology, 28(8), 1788-1802. https://doi.org/10.1093/annonc/mdx171
Winer, A., Bodor, J. N., & Borghaei, H. (2018). Identifying and managing the adverse effects of immune checkpoint blockade. Journal of Thoracic Disease, 10(Supple 3); S480-S489. https://doi.org/10.21037/jtd.2018.01.111
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice. (8th ed.). Jones & Bartlett Learning.
Yurgelun, M. B., Kulke, M. H., Fuchs, C. S., Allen, B. A., Uno, H., Hornick, J. L., Ukaegbu, C. I., Brais, L. K., McNamara, P. G., Mayer, R. J., Schrag, D., Meyerhardt, J. A., Ng, k., Kidd, J., Singh, N., Hartman, A., Wenstrup, R. J., & Syngal, S. (2017). Cancer susceptibility gene mutations in individuals with colorectal cancer. Journal of Clinical Oncology, 35(10), 1086-1095. https://doi.org/10.1200/JCO.2016.71.0012