About this course:
The purpose of this activity is to provide nurses with the best and most up to date science regarding the pathophysiology, symptomatology, prevention, and management of COVID-19.
Course preview
Course created on August 10, 2020
Top Things a Nurse Should Know about COVID-19
Course Objectives
At the conclusion of this activity, the learner should be prepared to:
- Discuss the basic pathophysiology of COVID-19, as well as risk factors, signs, and symptoms.
- Discuss the best ways in which nurses can help their communities cope with the COVID-19 pandemic.
- Recognize the daily strategies nurses can use to boost their own immunity in light of this most recent pandemic.
- Describe the current state of the global pandemic in late summer 2020, and what we have learned since early 2020.
- Discuss the prevention methods currently recommended and proven to be effective, and who is at the highest risk of contracting COVID or struggling to recover from the virus.
- Describe the current diagnostic process for COVID-19, including an update on real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing as well as point-of-care (POC) and antibody testing.
- Develop a clear picture of how this pandemic is affecting pediatric patients, including a brief overview of Multisystem Inflammatory Syndrome in Children (MIS-C).
- Discuss the most effective treatment options that have been developed as of late summer 2020 in the treatment of COVID-19.
- Highlight the ongoing efforts to clarify information regarding immunity and the scientific race to develop a vaccine.
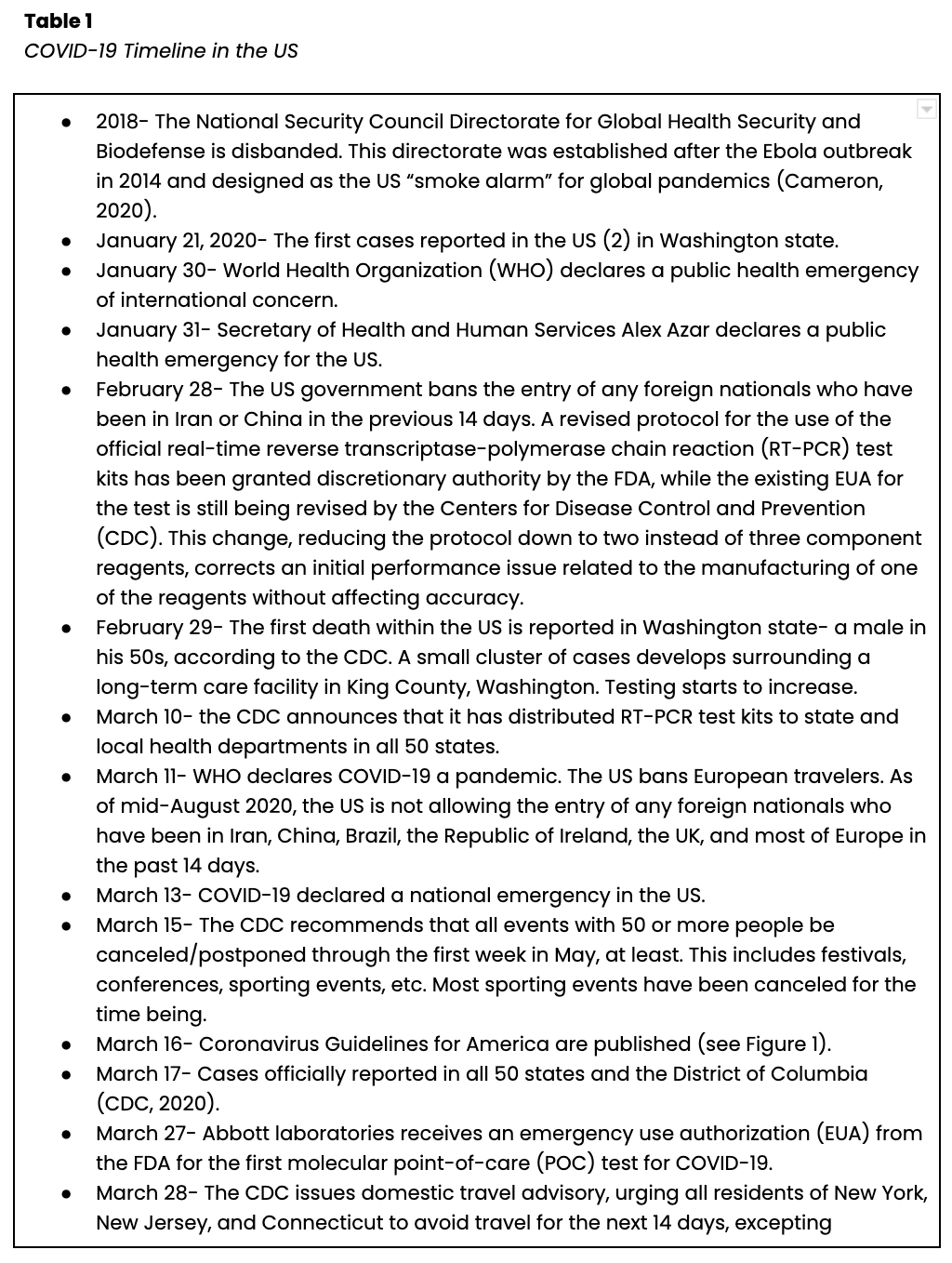
The Coronavirus Infectious Disease 2019 (or COVID-19) has dominated 2020. Since this is a novel or new virus, what do we really know? The purpose of this activity is to provide nurses with the best and most up-to-date science currently available regarding the pathophysiology, symptomatology, prevention, and management of COVID-19 as of early August 2020. See Table 1 for a brief overview of the timeline of COVID-19 in the US.




Pathophysiology and Transmission/Prevention
COVID-19 is a respiratory virus that is related to the severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS) coronaviruses. COVID-19 is also referred to as SARS-CoV-2. The current outbreak was believed to have started in an outdoor market in Wuhan, a city in Hubei Province, China. Imported, person-to-person, and community spread of the virus (people infected with the virus who are not sure how or where they were exposed) has been confirmed outside of China, including in the US (CDC, 2020). Currently, the US has the most confirmed cases and deaths in the world, and almost twice as many cases as the second-highest country (currently Brazil; JHU, 2020).
The virus appears to bind to the angiotensin-converting enzyme 2 (ACE2) receptors in the bronchus, alveoli, mucosa, and conjunctiva (Milton, 2020). Most of the spread is person-to-person, and people are contagious while they are symptomatic. However, research suggests that asymptomatic individuals may be highly contagious, as well as those within 48 hours of developing symptoms (pre-symptomatic), based on peak RT-PCR detection on days -2 and -1, which indicates active viral shedding. Infectious disease specialists discuss the contagiousness of a given pathogen using its reproduction number (R0, pronounced “r-naught”) which is a mathematical estimate of the average number of additional people infected by each infected individual. Currently, SARS-CoV-2 appears to be more contagious and deadlier than influenza (R0 of 1.3), more contagious than TB (which has an R0 less than 2, requiring prolonged close contact for person-to-person transmission), but less contagious than the measles (R0 greater than 10, requiring only incidental contact for person-to-person transmission) or varicella. Current research suggests an R0 of 2.5 for COVID, but experts believe that with robust contact tracing, widespread testing, enhanced hand hygiene, and universal public face shield use, the R0 could be less than or equal to 1 (Edmond, 2020; Langelier, 2020; Milton, 2020). Droplet transmission is the most common, although research indicates that airborne transmission via small respirable particles (aerosols or droplet nuclei) over short distances occurs (CDC, 2020; Edmond, 2020). Dr. E Nardell (2020), professor at the Harvard Medical School in the Department of Immunology and Infectious Disease, cites multiple documented instances of circumstantial evidence for the spread of COVID via droplet nuclei, including an outbreak in Washington State after a choir practice session, related to a Wuhan restaurant, and involving the residents of a Hong Kong apartment building (Nardell, 2020). Dr. Milton (2020), a professor and physician at the Institute for Applied Environmental Health, found that droplets of 10 µm or less can remain suspended in indoor air for minutes to hours and can travel up to 65 meters with adequate indoor air movement. The severity of infection appears to correlate with both the dose and route of exposure (Milton, 2020). Infectious disease professor and physician at the University of Iowa, Dr. Edmond (2020) cites an overall risk of infection for the general public of 5%; the risk of infection following a casual interaction in public with an infected person is 0.5%, the risk after sharing a meal with an infected person is 7%, and the risk of infection following household contact with an infected person ranges from 10-40%. In June, Long and colleagues (2020) published findings on 37 asymptomatic patients in China with RT-PCR-confirmed SAR-CoV-2 infection. They found a median duration of viral shedding of 19 days (range 6-45 days) in asymptomatic patients, which was significantly longer than the corresponding cohort of symptomatic patients (14 days). An earlier larger study of 191 patients found a median of 20 days of viral shedding amongst survivors, while a small study in Singapore (n=18) found viral nucleic acid material in nasopharyngeal aspirates for at least 24 days after symptom onset (Long et al., 2020).
Kissler and colleagues (2020) used data collected through May of 2020 to predict the transmission dynamics of SARS-CoV-2. Based on models of related strains of betacoronavirus (OC43 and HKU1), the authors suggest that prolonged and intermittent social distancing, expanded critical care capacity, and an effective therapeutic regimen would help to hasten the acquisition of herd immunity. Despite all that we have learned since the beginning of the year, some unanswered questions related specifically to SAS-CoV-2 remain, such as whether or not it will vary seasonally, the presence and extent of cross-immunity with closely related viruses (as is the case with OC43, HKU1, and SARS-CoV-1), and the duration of individual immunity. Infection with OC43 and HKU1 results in roughly 40 weeks of immunity (although SARS-CoV-1 immunity can be longer), which, if replicated by SARS-CoV-2, may result in annual cyclical outbreaks. Of note, their predictions are based on a hypothetical mathematical model. They were also premised on a hospitalization rate of just 3% and a critical care rate
...purchase below to continue the course
For now, the CDC recommends universal public social distancing, remaining at least six feet away from all people other than immediate family members, and the use of cotton face coverings when venturing out for essentials, such as trips to the grocery store. Face coverings are not recommended for those under the age of two or those with difficulty breathing. These face coverings should not be medical grade (these should be reserved only for healthcare providers [HCPs]). These face coverings are intended to prevent asymptomatic individuals from unknowingly spreading the virus, not as protection for the individual wearing the mask (CDC, 2020). A research group at the Institute for Applied Environmental Health determined that facemasks were able to eliminate all coarse particles (larger than 5 µm) and reduced fine particle spread by two-thirds (Milton, 2020). A mechanical engineering group at Florida Atlantic University studied the distance-traveling capacity of droplet nuclei using a simulator and found an average of 8-12 feet traveled, which was extended with increased air movement in the environment. They then applied various coverings to assess their effect on the distance and found that a cotton, single-layer bandana tied around the nose/mouth reduced the distance of the droplet nuclei to four feet, a multi-layer folded handkerchief reduced it to a single foot, and a stitched, two-layer cotton mask that was fitted to the wearer's face reduced the distance to two to three inches. Their studies indicated that all masks leak (especially at the top, around the nose). They found that masks built with exhalation ports (one-way valves in the center of the mask, over the mouth, to allow for more comfortable exhalation of air) significantly reduced the effectiveness of the source control (protection for the surrounding individuals; Verma, 2020).
Dr. Edmond finds that wearing a face shield may have advantages for HCPs, as they tend to be less hot, more comfortable, easier to breathe, and easier to disinfect. They also prevent face touching better than masks, add protection for the eyes, and allow for better visualization of facial expression and lip reading for the hearing impaired. However, they may obstruct vision and create a glare depending on the material used and may be heavier and more difficult to fit properly. A proper fit involves a face shield that extends below the chin, nearly to the ears on either side, and flush with the forehead (without leaving a gap). Face shields have proven exposure control (protection for the wearer from surrounding individuals) for HCPs, but their ability to function as source control (protection for surrounding individuals from the wearer) is less predictable (Edmond, 2020). Verma’s group (2020) found that face shields were less effective than fabric masks at controlling smaller droplet nuclei due to larger gaps around the edges of the face. Dr. Edmond cites a study which demonstrated that face shields can reduce particle spread at a distance of 18 inches by 68% when testing particles that are 3.4 µm or by 96% in simulations using 8.5 µm particles. A face shield also completely blocked any contamination at 20 inches using a simulated 5 µm spray. He recommends a combination of facemasks/respirators and face shields for all HCPs if available, and for the general public, he recommends whichever covering an individual or group will most reliably wear (Edmond, 2020).
Signs, Symptoms, and Patient Presentation
The incubation period can last up to 14 days, with a median of four to five days for most patients, and 97.5% of patients develop symptoms within 11.5 days. The primary symptoms include fever (83-99% of patients), cough (59-82% of patients), fatigue (44-70% of patients), anorexia (40-84% of patients), shortness of breath (31-40% of patients), sputum production (28-33% of patients), and myalgias (11-35% of patients). Approximately 2% of the reported cases in China were in patients under the age of 20, while the majority occurred in patients aged 30-69 (77.8%). Guan and colleagues (2020) identified a median age of 47 based on over 1000 patients reviewed from December through January. They also found that 58% were male. The numbers within China (including results from Guan and colleagues) indicate that roughly 81% of those infected develop mild or moderate disease, 14% develop severe disease, 5% require intensive care, 2.3% required mechanical ventilation, and 1.4% died. These numbers have changed over time, and the official case fatality rate published in the largest cohort trial in China was 2.3%. A certain percentage of those infected may also be asymptomatic, but the definitive proportion is unknown. A recent study found that as many as 50% of confirmed cases within a skilled nursing facility (SNF) were asymptomatic or pre-symptomatic at the time of testing (CDC, 2020; Guan et al., 2020). Most patients present with the aforementioned common symptoms, although research based on a self-reported symptom tracker also found that up to 65% of COVID patients report anosmia (loss of smell) or dysgeusia (altered sense of taste; Menni et al., 2020). Others report gastrointestinal symptoms prior to the onset of respiratory symptoms. If dyspnea is going to develop, it typically presents in five to eight days, acute respiratory distress syndrome (ARDS, 3-17% of patients) typically presents in 8-12 days, and most intensive care unit (ICU) admissions occur on days 10-12. Most (83%) hospitalized patients present with lymphopenia (or lymphocytopenia, a low level of lymphocytes). Those with more severe cases typically present with laboratory abnormalities, including neutrophilia (increased level of neutrophils), increased liver enzymes (ALT and AST), increased lactate dehydrogenase (LDH), increased c-reactive protein (CRP), and increased ferritin levels. A chest x-ray typically demonstrates bilateral air space consolidation, whereas CT scans often show bilateral peripheral ground-glass opacities (CDC, 2020). This finding was identified in 56% of patients reviewed by Guan and colleagues (2020), although 156 patients had no abnormal findings on their chest imaging studies. Long and colleagues (2020) found that asymptomatic patients have lower levels of inflammatory cytokines as compared to symptomatic patients, but 11 of the 37 asymptomatic patients had elevated levels of CRP, and 21 of 37 had abnormal findings on chest imaging (Long et al., 2020).
Severe and critical cases are resulting in respiratory distress and death, although the numbers regarding severity and death rates are still difficult to assess accurately at this point (CDC, 2020). Of confirmed cases in the US, the JHU tracking system reports that approximately 7% have required hospitalization (JHU, 2020). By contrast, the CDC estimates that 10-33% of adult patients and 5-20% of pediatric patients in the US are requiring hospitalization, while 1.4-4.5% of adults and 2% or less of pediatric cases have required intensive care (CDC COVID Response Team, 2020). The worldwide case fatality rate/ratio (the number of deaths divided by the number of confirmed cases, or CFR) currently stands at 3.75% (over 732,000 deaths and 19.9 million confirmed cases), with the US CFR reported as roughly 3.3-3.5% (163,047 deaths and 5 million confirmed cases). This equates to 48.37 deaths per 100,000 population in the US and is similar to the CFR reported in Brazil and Germany, worse than New Zealand and Australia, and better than Italy. Experts remark that this number is based on confirmed cases, not actual infections, which is limited by the amount of testing that is taking place in any given country or area (JHU, 2020; Wilson, 2020). As a point of comparison, the estimated influenza hospitalization rate in the US was 1.4% in 2018-19, while the death rate was 0.1% or two deaths per 100,000 population. COVID-19 is currently responsible for more American deaths than the four preceding influenza seasons combined (2015-16 through 2018-19; CDC, 2020). The infection fatality rate or ratio (IFR), which is calculated using the number of infections as the denominator in lieu of the number of confirmed cases, is estimated in the US at 0.5-0.8% (Wilson, 2020). The WHO estimates that the worldwide IFR may converge at 0.5-1% (WHO, 2020).
Some COVID patients have developed coagulation abnormalities, placing them at increased risk for both venous and arterial thrombosis. Most of these patients have presented with mild thrombocytopenia (reduction in platelet count), elevated D-dimer, increased fibrin degradation products, and prolonged prothrombin time (PT). So far, elevated D-dimer levels have been the most strongly associated with an increased risk of death in this subset of COVID patients. The most common complication has been the development of venous thromboembolism (VTE; i.e., deep vein thrombosis [DVT] or pulmonary embolism [PE]), but other observed complications include thrombosis of the toes, clotting of catheters, myocardial ischemia/injury with ST-segment elevation, and large vessel strokes. While the pathophysiology of these complications is still being explored, early estimates point towards hypoxia, systemic inflammation, and elevated levels of inflammatory cytokines, all of which contribute to activation of the coagulation pathway (CDC, 2020).
Coagulopathy has been seen more commonly in patients requiring critical care and those with ARDS. The prevalence is not fully understood but was estimated at 16.7% of critical care patients in a French study, 22.2% in an Italian study, 27% in a Dutch study, 69% in a French study using routine lower extremity doppler ultrasound on 26 consecutive ICU admissions, and just 3.3% in a New York study involving 393 ICU patients. The NIH does not recommend screening outpatients for coagulation markers, and while they state that this laboratory data is commonly measured in hospitalized patients, there is insufficient data to recommend for or against using this data to help guide management. They recommend against routine screening for DVT in hospitalized patients with COVID-19 in the absence of symptoms. They also recommend against the prophylactic use of anticoagulant or antiplatelet therapy in outpatients and suggest that these only be initiated and continued beyond discharge in hospitalized adults based upon the current existing standards of care. In the instance of rapid respiratory or cardiac deterioration in a COVID-19 patient, the possibility of VTE development should be evaluated. Those who develop VTE related to COVID-19 should be treated with therapeutic doses of anticoagulant therapy per the same current standards of care for VTE not related to COVID-19. Intravenous or subcutaneous medications with a shorter half-life and fewer drug-drug interactions are typically preferred over oral anticoagulants (NIH, 2020).
Of the 449 patients admitted to an ICU in Wuhan, China from January to February of this year and included in the study, 99 received therapeutic anticoagulation therapy with low molecular weight heparin (LMWH, i.e., enoxaparin [Lovenox]) or similar for at least seven days. The authors found no significant reduction in the 28-day mortality rate amongst all of those on anticoagulation therapy. However, those patients with sepsis-induced coagulopathy (SIC, per the International Society of Thrombosis and Haemostasis) score of greater than or equal to 4 who were treated had a significantly lower mortality rate as compared to those who were untreated (40% versus 64.2%). The SIC score is based on a patient's prothrombin time-international normalized ratio (PT-INR), platelet count, and a sequential organ failure assessment (SOFA) score. The SOFA score, originally described over 20 years ago by an international working group related to sepsis care, takes into account oxygenation (PaO2/FiO2 ratio), coagulation (platelet count), liver function (bilirubin), cardiac function (mean arterial pressure), neurological function (Glasgow Coma Scale), and renal function (creatinine). Similar results were seen in the subset of patients with a D-dimer above 3.0 µg/mL (32.8% mortality rate versus 52.4%; Tang et al., 2020). A normal D-dimer level is less than 0.5 µg/mL according to the American Board of Internal Medicine (2020). This indicates that carefully selected patients who may be at higher risk of coagulopathy could benefit from therapeutic treatment with anticoagulation medication such as LMWH (Tang et al., 2020).
An observational study at Mount Sinai in New York looked for trends amongst 2,773 patients admitted to the ICU from March to April of this year for COVID-19. Results indicate no significant difference in mortality rate amongst those who received anticoagulation therapy and those who did not (22.5% versus 22.8%). Of note, this study was not randomized and was limited by a lack of details regarding patient characteristics, indications for treatment initiation, and other concurrent treatments that were given (Paranjpe et al., 2020). Those on extracorporeal membrane oxygenation (ECMO) or continuous renal replacement therapy, or those with thrombosis of catheters or extracorporeal filters, should be managed using antithrombotic therapy per the same current standards of care for thrombi not related to COVID-19. Pregnant women requiring these treatments should be managed similarly to non-COVID pregnant patients requiring anticoagulant therapy. Unfractionated heparin, LMWH, and warfarin (Coumadin) are safe in breastfeeding patients if/when required and indicated. COVID patients on warfarin (Coumadin) who are unable to obtain consistent monitoring of their PT-INR due to isolation precautions should be considered for transition to a direct oral anticoagulant (DOAC). DOACs should be avoided in those with mechanical heart valves, ventricular assist devices, valvular atrial fibrillation, antiphospholipid antibody syndrome, and breastfeeding mothers. Additional randomized trials regarding the role of anticoagulation therapy in the management of COVID-19 are ongoing (NIH, 2020).
Pediatric patients have been difficult to assess in relation to COVID-19. As mentioned earlier, initial reports from China indicated very few pediatric patients affected and a relatively mild course with significantly reduced risk of hospitalization, intubation, or mortality. This remains (mostly) true, but pediatric patients have not been as simple and straightforward as previously hoped. In April, Lu and colleagues (2020) reported on pediatric cases in China. From January 28 through February 26, 1,391 tests were performed, resulting in 171 confirmed pediatric cases during that time. Approximately 90% of the cases were confirmed to be transmitted from a family cluster, while 8.8% were felt to be community-acquired. The median age was 6.7 years. Symptoms included cough (48.5% of cases), fever (41.5% of cases), and a finding of ground-glass opacity on chest CT (32.7% of cases). 15.8% of children included in the study were asymptomatic without normal imaging, and 7% were asymptomatic but with significant abnormalities on imaging studies. Three patients required mechanical ventilation, and one child died (Lu et al., 2020). The state of California reported in late July that their official number of confirmed pediatric cases (under the age of 18) is roughly 10% of their adult confirmed cases. The CDC estimates that 1.7% of all confirmed cases in the US are in patients under the age of 18, and 15% of these are infants under one year. Between 5-20% of pediatric patients require hospitalization, and 62% of these are infants; 0.58-2% of pediatric cases are requiring intensive care, with very few deaths. Pediatric patients present similar to adults, with 73% complaining of a fever, cough, and/or shortness of breath. 57% of pediatric patients are male, and 23% have an underlying condition (CDC COVID Response Team, 2020; Rutherford, 2020). A study amongst high school students in Israel found that only 66/153 patients (43%) were symptomatic, while 76% of adult staff members in the study were symptomatic (Stein-Zamir et al., 2020).
According to Bunyavanich and colleagues (2020), the low number of pediatric cases is likely due to a reduced expression of the ACE2 receptor in children, which typically increases gradually throughout the lifespan. An Iceland study reported that after over 800 children between the ages of 0-9 were tested, they found no positive nasopharyngeal swabs (Rutherford, 2020). A Lancet study by Qiu and colleagues (2020) in March found that 67% of children are likely exposed by a family member, 11% in the community, and 22% by both, but these were based on very small sample size (n=36). A similar study preprinted by Zhu and colleagues (2020) found that less than 10% of the 31 household clusters of COVID included in their study had an identified index case that was under the age of 18. Fontanet and colleagues (2020) preprinted a retrospective study indicating a 40% seropositivity amongst teens (age 15-17) two to three weeks after schools closed due to an outbreak, while they only found an 11% seropositivity rate amongst their household contacts. A symptomatic pediatric patient in France was followed and determined to have no positive cases of transmission after negative tests were confirmed amongst over 100 school contacts (Danis et al., 2020). The National Center for Immunization Research and Surveillance (Rutherford, 2020) in New South Wales reported on 18 cases (nine students and nine staff) in 15 different primary and high schools from March to mid-April, resulting in 863 close contacts that were tested; the study found no adult secondary cases, and only two pediatric secondary cases.
Despite these seemingly positive findings regarding pediatric patients and their lack of infectiousness, Heald-Sargent and colleagues (2020) tested 145 patients with mild to moderate disease and less than seven days of symptoms using a nasopharyngeal swab for molecular testing. They found a significantly lower median PCR amplification cycle threshold (CT) in the youngest group (age under 5, n= 46), indicating a higher viral nucleic acid content in the respiratory tract as compared to the older groups (ages 5-17, n=51; age 18-65, n=48). This study did not definitely link the amount of viral genetic material to infectiousness, however (Heald-Sargent et al., 2020). Outbreaks have also been identified recently in groups of pediatric patients. An overnight summer camp in Georgia with 600 campers and counselors is one example. A teen counselor was sent home after developing symptoms, and campers were dismissed/the camp was closed a few days later. Despite quick action to dismiss campers, 260 of the 344 individuals tested were found to be positive. The camp reports that they required a negative molecular test of counselors and campers within 12 days of arrival, asked counselors to wear masks, enhanced their cleaning of common areas, and staggered the use of their common areas by the campers, but did not require campers to wear masks (Rabin, 2020). Similarly, a high school (7-12th grade) in Israel with 1,190 students and 162 staff members reopened on May 18. Due to a heatwave, masks were not required on May 19-21; the school detected its first positive case on 5/26 and a second unrelated positive case on 5/27. The school was dismissed, and all students/staff were encouraged to be tested. Of the 1,161 students tested, 153 (13%) were found to be infected. Of the 151 staff members tested, 25 (16.6%) were found to be infected. In addition, more than 80 additional cases amongst friends and family members of students/staff were also linked to the outbreak (Stein-Zamir et al., 2020).
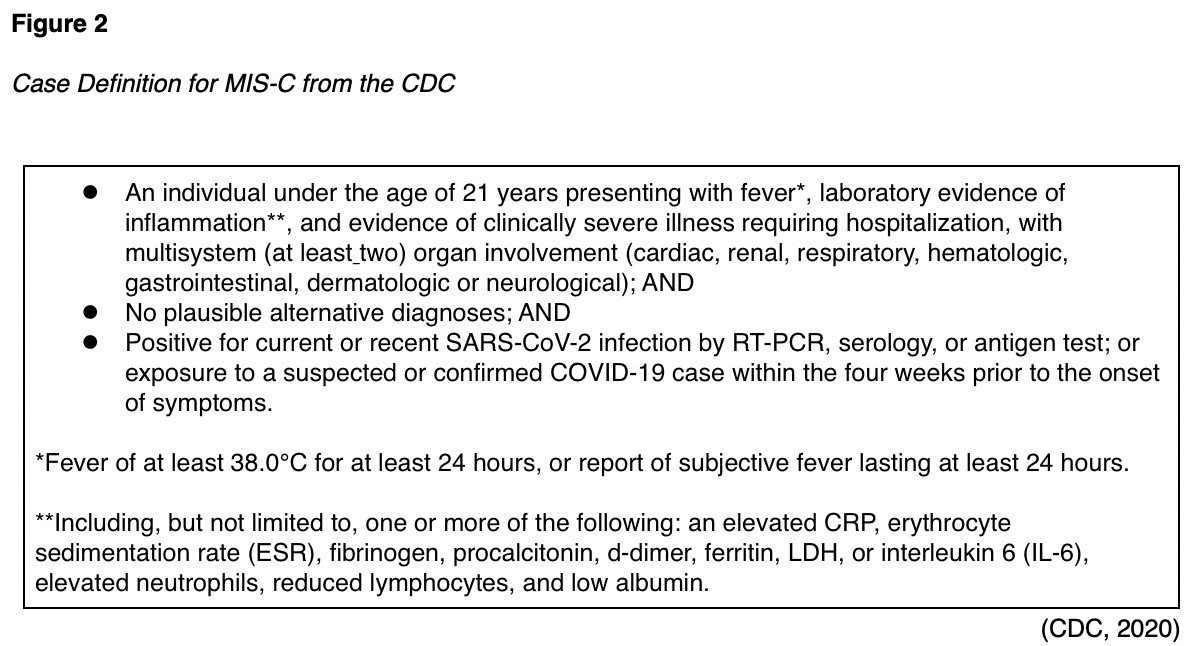
An additional concern amongst pediatric patients is the emergence of a delayed or secondary Multisystem Inflammatory Syndrome (MIS-C, see Figure 2). Reports from New York and across the US were printed simultaneously in the NEJM in July. Dufort and colleagues (2020) focused on cases within the state of New York between March 1 and May 10. They found 191 potential cases, 95 confirmed cases, four suspected cases, and two deaths. All cases reported fever and chills. Nearly all patients (97%) presented with tachycardia, 80% reported gastrointestinal symptoms, 60% presented with a rash, 56% with conjunctival injection, 53% with myocarditis, and 27% with mucosal changes. Elevated levels of CRP, D-dimer, and troponin were found in 100%, 91%, and 71% of cases, respectively. Eighty percent of patients required intensive care, and 62% required vasopressor support (Dufort et al., 2020). Feldstein and colleagues (2020) published a description of 186 cases of MIS-C in the US between mid-March and May 20. They found an increased incidence in areas that had experienced earlier outbreaks. They found a median age of 8.3 years, 62% of cases were male, and 73% were previously healthy, with no underlying or comorbid conditions. 70% of the children tested positive via RT-PCR or antibody serology assay, while the remaining 30% were epidemiologically linked. Of the 73 patients who tested positive using molecular RT-PCR, only 27 also tested positive for the presence of antibodies. Of the 186 children profiled, 88% of cases were hospitalized, 80% required intensive care, 20% required mechanical ventilation, 17% required noninvasive ventilation support, and four resulted in death. Symptoms affected multiple organ systems, including the gastrointestinal system (92% of cases), the cardiovascular system (80% of cases), the hematologic system (76% of cases), the mucocutaneous system (74% of cases), and the respiratory system (70% of cases). Fifteen cases (8%) were found to have coronary artery aneurysms, and Kawasaki-like features were present in 74 (40%) of the 186 cases reviewed. Ninety percent of the patients reported a fever lasting four or more days, 92% had inflammatory marker elevations, an elevated b-type natriuretic peptide (BNP) was found in 73% of patients, and an elevated troponin level in 50%. The most frequent treatment course included supportive care along with intravenous immunoglobulin (IVIG, in 144 [77%] of cases) and/or corticosteroids (in 91 [49%] of cases). Fourteen patients were determined to have had acute symptoms of COVID-19 prior to developing MIS-C, with a median of 25 days from symptom onset. Experts believe many of the symptoms are related to dysregulation in the immune system resulting in host tissue damage, similar to theories regarding why adult patients seem to worsen clinically during the second week of their illness (Feldstein et al., 2020). Levin (2020) describes over 1,000 cases of MIS-C worldwide. He describes an average time from infection to symptom onset in MIS-C patients of two to four weeks, a prevalence of 2 in 100,000 persons under the age of 21 (versus the global COVID prevalence rate at the time of 322 in 100,000 persons), and a 2-4% global mortality rate. He also corroborates the theory that MIS-C is related to an aberrant immune response conducted via T- or B-lymphocytes (Levin, 2020).
Risk Factors
According to the CDC, those at increased risk for contracting the virus include individuals residing in areas with a higher rate of community spread, HCPs, family members and close contacts of those infected, as well as those traveling from affected areas. A prospective observational study based on the self-reported COVID Symptom Study in the US and the UK from March through April compared over 2 million individuals in the database with over 99,000 HCPs. They found that the hazard ratio for the HCPs was 11.61 compared to the general public, which was decreased to 3.4 when adjusted for selection bias and testing frequency. This resulted in 2,747 positive cases per 100,000 HCPs versus just 242 positive cases per 100,000 members of the general public (Nguyen et al., 2020). Racial and ethnic minority patients have been affected at a higher rate than white patients, owing to decades of inequalities in wealth and access to education, healthcare, housing, and occupational opportunities (CDC, 2020). Pan and colleagues (2020) performed a systematic review to identify ethnicity trends in COVID patients. Database and medical journal articles they reviewed found no association with ethnicity. Only 34 out of the 209 preprint articles they reviewed reported on ethnicity; 13 of these reported an increased risk of COVID infection in Black, Asian, and Minority Ethnic (BAME) populations and 12 reported worse clinical outcomes for BAME patients diagnosed with COVID. They identified 12 grey literature articles that reported on ethnicity using original data, seven of which reported significant worsening of clinical outcomes for BAME patients (Pan et al., 2020). Similarly, a study conducted with 1,293 pregnant patients found positive RT-PCR results in 10.4% of Latin American/Hispanic American patients, 9.7% of African American patients, while only 2% of European American and 0.9% of Asian American patients tested positive (Flannery et al., 2020). A large (7,807 participants) population-based study found age over 50, male sex, and low-medium socioeconomic status were all positively associated with a risk of infection (Merzon et al., 2020)
Older adults, residents of skilled nursing facilities and long-term care facilities, and individuals with underlying health conditions (i.e., heart disease, pulmonary disease, diabetes) appear to be at increased risk for severe disease requiring hospitalization and respiratory support (CDC, 2020). A population-based study found a positive association between age over 50 and the need for hospitalization in COVID-19 patients (Merzon et al., 2020). A systematic review and meta-analysis including nearly 2,000 patients correlate older age (average age of 62 years versus 46 years), male sex, and a presenting symptom of dyspnea with more severe disease necessitating admission to the ICU (Jain & Yuan, 2020). Two recent systematic reviews and meta-analyses found that underlying conditions correlated with more severe disease. The first, conducted using studies published prior to March 5, determined that a diagnosis of COPD prior to COVID transmission correlated strongest with ICU admission and severe disease, followed by cardiovascular disease and hypertension (Jain & Yuan, 2020). The second, using studies through April, found that while just 41% of all COVID cases were identified as having a comorbid condition, as many as 74% of fatal cases had at least one comorbid condition. They looked more closely at the conditions of hypertension, diabetes, and respiratory conditions. They found that 14% of total cases were diagnosed with hypertension prior to infection, yet this group made up 48% of fatal cases. Similar trends were seen with diabetes (10% of total cases and 25% of fatal cases) and respiratory conditions (3.65% of total cases and 11% of fatal cases; Gold et al., 2020). There is insufficient data on whether or not someone can be reinfected after recovering, but it is thought to be unlikely (CDC, 2020). However, there have been isolated case reports of patients recovering and then testing positive for the virus again. These patients remain asymptomatic despite testing positive a second time, and experts are unsure if their testing was falsely negative or if they were re-exposed. They are also doubtful that they are infectious, but this remains uncertain (Feng & Cheng, 2020).
Multiple studies have attempted to establish other risk factors related to either transmission of SARS-CoV-2 or the severity of the disease. Two of the most commonly cited theories include the potential effect of an individual's blood type, including rhesus factor (Rh), and 25-hydroxyvitamin D (25[OH]D) level. We will briefly review the four most commonly cited studies regarding blood type in chronological order. In March, Zhao and colleagues (2020) released their study findings in preprint form (prior to peer review) based on over 2,000 patients. They found an increased risk of infection in those patients with type A blood and a reduction in infection risk amongst patients with type O blood (Zhao et al., 2020). The following month, Zietz and Tatonetti (2020) released their findings in preprint form. Their observational study was based on over 7,700 patients and corroborated that patients with type O blood had a reduced risk of infection (p= 0.064). They also found a reduced risk of infection (p= 0.021) and of death (p= 0.029) related to infection in those with Rh-negative blood, an increased risk for infection (p= 0.032) but a reduced risk of death (p=0.069) in those with B blood type, and a slightly increased risk of intubation (p= 0.015) or death in those with type AB blood, although the small sample size prevented this from reaching statistical significance (p= 0.15). In contrast to the Zhou study from March, they found that patients with type A blood had a reduced risk of intubation (p=0.016) and no significant change in risk of infection (Zietz & Tatonetti, 2020). In June, Ellinghaus and colleagues (2020) published a genome-wide association study based on 1,980 patients with severe disease in Italy and Spain. They found an increased risk of severe disease in those with type A blood, a decreased risk in those with type O blood, and felt that these differences were related to genetic differences between the patient groups (Ellinghaus et al., 2020). Finally, Latz and colleagues (2020) published their findings in July based on over 7,600 patients tested between early March and mid-April. Of the patients tested, 1,289 of them were confirmed positive for SAR-CoV-2, 484 (37.5%) were hospitalized, 123 (9.5%) were admitted to the ICU, 108 (8%) were intubated, and 89 (7%) eventually died. On univariate analysis, they found no statistically significant correlations. After multivariate analysis, their results indicate that there is no increased or decreased risk of infection (based on positive test result) in those with type A blood, higher risk in those with type B or AB, and lower risk in those with type O blood. Multivariate analysis also corroborated earlier findings that those with Rh-positive blood types had an increased risk of infection. No clinically significant correlation was found between blood type and intubation or death, even after multivariate analysis (Latz et al., 2020).
Regarding vitamin D, a large population-based study involving 7,807 participants found that an abnormally low 25(OH)D level was independently associated with an increased risk for infection and hospitalization. They found a lower mean plasma 25(OH)D in the subset of COVID-positive patients (n=782) versus COVID-negative participants (19 ng/mL versus 20.55 ng/mL; Merzon et al., 2020). Another observational study performed in South Asia found that patients with more severe disease had reduced 25(OH)D levels as compared to others. A United Kingdom Biobank study found no association between 25(OH)D levels and COVID infection in multivariate models. Unfortunately, other studies on vitamin D as either prophylaxis or to reduce the disease severity have produced mixed results with no clear indication for its use. A randomized and prospective study is still needed to establish this causality with confidence, as seasonality (time of year) and the social class of the participants were potential confounders not yet addressed (McNamara, 2020).
Testing
In addition to the original RT-PCR test kit developed by the CDC, commercial labs have developed their own tests to allow for local testing, and most of these tests are conducted using nasopharyngeal swabs. Despite being told initially that all symptomatic patients could get tested, most local and state health departments were initially forced to be selective regarding testing patients, especially early on in the pandemic. The CDC suggested that symptomatic hospitalized patients and symptomatic HCPs be given first priority in testing to ensure proper isolation precautions. The CDC recommended that secondary priority for testing be granted to symptomatic older adults, symptomatic patients with underlying medical conditions that predispose them to worse outcomes, symptomatic long-term care facility residents, and symptomatic first responders. The third level of priority for testing included all symptomatic individuals who did not fit into one of the previously mentioned categories, especially critical infrastructure employees. This third level also included asymptomatic HCPs and first responders, and those with mild symptoms in high-risk communities. The CDC listed asymptomatic patients (screening) as a nonpriority for testing, although an EUA was granted for this indication in late July to LabCorp. Asymptomatic patients are now being performed regularly prior to entrance into hospitals and prior to elective procedures; availability of asymptomatic testing for the public varies by area. Primary care, urgent care, and pediatric clinics should be aware of their local resources, including who to contact and how to facilitate testing for any patients that meet the criteria. Infectious disease departments and state or local health departments should be notified of any persons under investigation (PUI) to facilitate contact tracing (CDC, 2020).
Abbott (and other similar POC tests) purports that they can deliver a positive result in as little as five minutes, and a negative in as little as 13 minutes using nucleic acid amplification testing (NAAT). The obvious advantage of this system is the ability to test patients in urgent care and physician offices. Their ID NOW platform is already in use for influenza, strep A and respiratory syncytial virus (RSV) testing in offices across the US. Combined with their lab-based RT-PCR molecular test, Abbott predicted being able to complete 5 million tests in April (Abbott Laboratories, 2020). Unfortunately, reports from the US Department of Health and Human Services (HHS) and FEMA documents indicate that on March 30 only 5,500 test cartridges and 780 new ID NOW machines were to be shipped to 55 state and local labs across the US (Kaiser Health News, 2020).
As of the end of July, and according to infectious disease professor and physician at the University of California at San Francisco Chaz Langelier (2020), 193 tests have received EUA from the FDA for acute/active COVID testing. This includes 158 molecular tests, which can be further broken down into two subcategories. The original test, RT-PCR, takes about 45-180 minutes to obtain results in the lab and may, at times, result in a wait of 48 hours and up to two weeks to return results to the patient. These tests have the highest sensitivity and are considered the gold standard. Within the first five days of symptoms, their sensitivity can be greater than 90%, decreasing to less than 70% at symptom-day ten as the viral load decreases. POC tests based on NAAT can return results in an office setting within 15 minutes, eliminating the wait time for the patient during which they could be unintentionally infecting those around them. Their sensitivity varies between 50-80% depending on the manufacturer. Two antigen-based tests have been approved, which also return results within 15 minutes, with a sensitivity of 80%. Laminar flow assays can be done quickly and easily, based on the Crisper Cas technology to detect antigens or nucleic acid, but with an undetermined sensitivity. Dr. Langelier proposes a potentially effective strategy based on community surveillance with daily/highly frequent, inexpensive, and quick testing be done, with less concern for the test's sensitivity in order to increase frequency and efficiency. His data suggests that eliminating even a 48-hour waiting period for positive test results would reduce an infected patient's infectiousness by 32%. More sensitive testing using the original RT-PCR technology would then be reserved for high-risk settings, such as hospitals or institutional settings (Langelier, 2020). The WHO (2020) recommends the use of chest imaging (in the form of computed tomography [CT] or chest x-ray) to confirm a diagnosis and initiate treatment/isolation in symptomatic patients if test results are delayed.
Antibodies are one of our immune system's primary defenses against infection. The human body produces five different subtypes of antibodies, named after their Greek letter heavy chain components (mu, gamma, alpha, epsilon, and delta). Immunoglobulin M (IgM) is the principal antibody of the primary immune response and are typically detected first after an infection is identified. IgM serves to activate the complement system early and accounts for just 5-10% of all antibodies produced. Immunoglobulin G (IgG) is the principal antibody of the secondary immune response and comprises between 75-80% of the body's total immunoglobulin pool. They also have the longest half-life, between 20-24 days, on average. Immunoglobulin A (IgA) accounts for just 5-15% of total antibodies and is found predominantly in the mucous secretions (i.e., tears, saliva, and milk). Immunoglobulin E (IgE) antibodies are scarcely found within human serum but are found primarily on the cell surface of basophils and mast cells. Although not fully understood, they are thought to function in the allergic response and against parasitic infections. The function of Immunoglobulin D (IgD) is largely unknown; they account for less than 1% of our antibodies and are found on the cell surface of our B lymphocytes (McComb, 2019). A study conducted at Emory Hospital in Georgia assessed 44 patients (RT-PCR confirmed cases) using assays to detect the receptor-binding domain (RBD) of the spike (S) glycoprotein of IgG antibodies and neutralizing antibodies. All 44 patients had positive antibody titers within six days of RT-PCR diagnosis, and most titers were positive eight days after symptom onset. The antibodies obtained from 40 out of the 44 patients had in-vitro neutralization capacity based on a reduction in virally infected foci via a focus reduction neutralization titer assay. The RBD and neutralization titers were positively correlated, according to the study's authors. The group also found evidence of IgA and IgM antibodies at lower levels (Suthar et al., 2020). In their previously mentioned study on 37 asymptomatic patients in China with RT-PCR-confirmed SAR-CoV-2 infection, Long and colleagues (2020) found that 81% of asymptomatic and 84% of symptomatic patients had positive IgG antibody titers; 62% of asymptomatic and 78% of symptomatic patients had positive IgM titers. They also found significantly lower levels of IgG antibodies in the asymptomatic patients versus the corresponding cohort of symptomatic patients. During the eight-week convalescent phase of this study, 93% of asymptomatic patients had a median decline in their IgG titer of 71% (as compared to 97% of symptomatic patients with a median decline of 76%) and 40% of asymptomatic patients became seronegative for IgG antibodies, versus just 13% of symptomatic patients. The authors identify previous exposure to similar strains of coronavirus (i.e., SARS-CoV or MERS) as possible confounding factors limiting this study (Long et al., 2020). Based on a July study in the NEJM, IgG antibodies were detected in 34 confirmed or epidemiologically linked and mildly symptomatic COVID-19 patients in two or three serial enzyme-linked immunosorbent assay (ELISA) assays to quantify the receptor-binding domain activity of IgG antibodies. The study found the quantity detected at the two or three time points correlated with a mean half-life of approximately 36 days (95% CI 26-60 days). This is much longer than the average of 2024 days for IgG antibodies in general. The authors suggest, based on this information, that humoral immunity may not be long-lasting following mild illness (Ibarrondo et al., 2020). Most experts expect that IgG and neutralizing antibodies decrease in quantity within two to three months of exposure (Long et al., 2020).
As of the end of July, there are currently 33 serology antibody tests authorized for use by the FDA, with varying degrees of sensitivity and specificity (Langelier, 2020). The CDC (2020) recommends that antibody testing not be used for the diagnosis of acute infections. They cite a primate study that established protection from re-infection and believe that this indicates that recurrence of infection is very unlikely and that at least short-term immunity is conferred to patients following their recovery, corresponding to the reduction in viral load. They recommend serology antibody testing in patients who present 9-14 days after symptom onset to support clinical assessment, or those presenting with a post-infectious syndrome (i.e., MIS-C). The CDC recommends using a test with a published specificity of at least 99.5% in order to augment the positive predictive value of this testing due to the low prevalence of SARS-CoV-2 (varying between 5-25%). Tests are designed in various formats, some using a POC technology and a simple fingerstick. Tests may utilize ELISA, chemiluminescent immunoassay (CLIA), chemiluminescent microparticle immunoassay (CMIA), or lateral flow technology. All authorized tests assess for either IgG or IgM antibodies, and specifically for the S protein (the protein found on the virus surface and determined to be essential for virus entry and replication), the RBD of the S-protein (the most conserved portion), or the nucleocapsid (N) phosphoprotein (found more abundantly than the S-protein, and more conserved across all coronavirus strains). Antibody titers should not be used to establish immunity, relax isolation precautions, or make decisions regarding cohorting or return to school/work environments. A positive antibody titer does not indicate the need for isolation precautions in an asymptomatic patient (CDC, 2020). Qualitative antibody tests are not able to establish future risk until more information is known regarding long-term immunity and is typically not covered by insurance. Researchers are able to utilize the antibody test results to determine patterns of incidence, prevalence, mortality rate, and to assess the effectiveness of various public health efforts (Gronvall, 2020). While most of these tests are simply qualitative (positive/negative), Siemens has developed two "semi-quantitative" tests currently authorized (Frellick, 2020). Based on manufacturer testing as well as some independent testing done by the FDA, Table 2 describes the calculated sensitivity, specificity, and 95% confidence interval (CI) of various antibody tests currently authorized for use in the US.

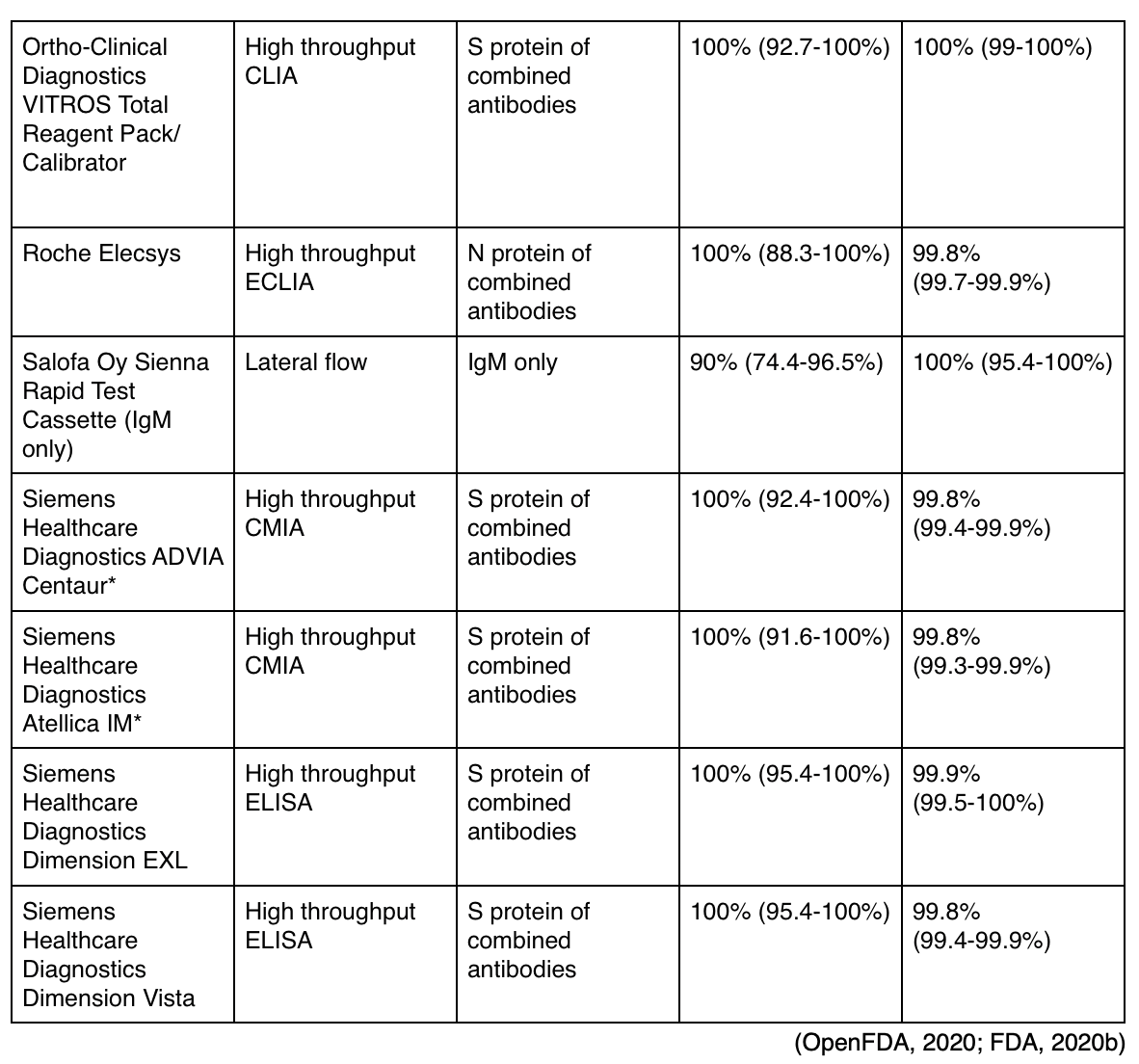
* Newly authorized semi-quantitative tests that may estimate the number of antibodies present (Frellick, 2020).
Those approved for use yet listed by the FDA as having specificity ratings below the recommended 99.5% include the following:
- Abbott Alinity i (IgG),
- Assure Tech Assure Rapid Test Device (IgM only),
- Autobio Rapid Test (IgG only),
- Beijing Wantai Biological Pharmacy Enterprise Rapid Test,
- Biohit Healthcare (Hefei) Antibody Test Kit,
- Cellex qSARS Rapid Test,
- DiaSorin LIAISON IgG,
- Diazyme DZ-Lite IgG,
- Emory Medical Laboratories RBD IgG,
- Hangzhou Laihe Biotech LYHER Combo Test Kit (IgG only),
- Healgen Rapid Test Cassette (IgG only),
- InBios Detect ELISA (IgM only),
- Salofa Oy Sienna Rapid Test Cassette (IgG only),
- Vibrant America Labs, Vibrant Assay,
- Wadsworth New York Microsphere Immunoassay (FDA, 2020b).
The FDA recommends against using the following unauthorized antibody tests:
- Abacus Pharma International Antibody Rapid Test,
- Abbexa IgG/IgM Rapid Test Kit,
- Accudiagnostics IgM/IgG Test Kit,
- Alfa Scientific Designs Inc. IgG/IgM Antibody Test,
- Atlas-Link (Beijing) Nova IgG/IgM Antibody Rapid Test,
- Aurora Biomed Inc IgG/IgM Rapid Test,
- Biolidics Ltd IgG/IgM Detection Kit (Colloidal Gold),
- Biomedomics IgM/IgG Rapid Test Kit,
- ChemBio DPP IgM/IgG System,
- Chemtron Biotech, Inc. Rapid IgM/IgG Antibody Screen Test,
- GenBody Inc IgM/IgG,
- GP Getein Biotech, Inc. One-Step Test (Colloidal Gold),
- Guangzhou Fenghua Bioengineering Co, Ltd. IgM/IgG Rapid Test Kit,
- H-Guard (China) Co., Ltd. IgM/IgG Test Kit (Colloidal Gold),
- MEDsan GmbH MED san Biological Health Solutions IgM/IgG Rapid Test,
- Nanjing Vazyme Medical Technology Co. Ltd. Vazyme IgG/IgM Detection Kit,
- Phamatech Rapid Test,
- SD Biosensor, Inc. Standard Q IgM/IgG Duo,
- Shanghai Fosun Long March Medical Science Co., Ltd. Fosun IgG/IgM Rapid Detection Kit,
- TESTSEALABS IgG/IgM Test Cassette,
- Tianjin Beroni Biotechnology Co., Ltd. IgG/IgM Antibody Detection Kit,
- Tianjin New Bay Bioresearch C. #1 Quick Pac II IgG/IgM Test,
- W. H. P. M., Inc. Covisure IgM/IgG Rapid Test,
- Wuhan Easy Diagnosis Biomedicine Co. Ltd. IgM/IgG Antibody Test Kit,
- Zhongshan Bio-Tech Co Ltd IgM/IgG (GICA),
- Zhuhai Livzon Diagnostic Inc. IgM/IgG Diagnostic Kit,
- Zhuhai Livzon Diagnostic, Inc. Livzon IgM/IgG Diagnostic Kit Lateral Flow (OpenFDA, 2020).
Management of Mild-Moderate Disease
Supportive care is still the most effective treatment for mild-moderate COVID-19. Acetaminophen (Tylenol) can be used for fever and body aches; however, ibuprofen (Motrin) should be avoided if possible. Preliminary information has emerged from a study of patients in Italy, which has seen more severe cases than in any other part of the world. According to a recent study published in The Lancet (Fang et al., 2020), it was determined that one of the common factors in many of the Italian cases was that the majority of patients took ibuprofen (Motrin) at home. Based on this and unclear laboratory evidence, researchers are not certain if ibuprofen (Motrin) and other nonsteroidal anti-inflammatory drugs may accelerate the multiplication of the virus, leading to a more severe course of the disease (Fang et al., 2020). Patients without breathing difficulty are encouraged to self-quarantine for ten days (and at least 24 hours past final symptom resolution) and recover at home if appropriate supportive caregivers are available. Patients at home should be encouraged to rest, hydrate, and maintain adequate and balanced nutrition. Patients should be placed in a separate bedroom/bathroom away from other family members, and appropriate personal protective equipment (PPE, at minimum gloves and facemask) should be provided to the family. Patients recovering at home should not leave their homes except to attend medical appointments if telemedicine is not an available or appropriate alternative. Hand hygiene and cough etiquette are compulsory, and if possible, the patient should maintain a facemask when around family members. Dishes, towels, and other household items should not be shared and should be thoroughly washed with soap and hot water after being used by the patient. High-touch surfaces such as counters, doorknobs, light switches, toilets, and phones should be disinfected daily. Those with family members at high risk (over 65, young children, pregnant women, immunocompromised patients, chronically ill) should be isolated outside of the home when possible (CDC, 2020). At the beginning of the pandemic, hydroxychloroquine (Plaquenil) was thought to hold promise as an effective treatment option. Commonly used for malaria, rheumatoid arthritis, and systemic lupus erythematous, the medication was given off-label for COVID-19. Since then, two separate outpatient trials were conducted to assess the effectiveness of oral hydroxychloroquine (Plaquenil) for five to seven days versus placebo and azithromycin (Zithromax); they found no significant clinical benefit on day 14, and this regimen is therefore not recommended (Luetkmeyer, 2020). Another double-blind, randomized trial was published in August, including 821 patients who received either hydroxychloroquine (Plaquenil) or placebo within four days of moderate to high-risk exposure to assess its effectiveness as a prophylactic. It was dosed at 800 mg initially, followed by 600 mg six to eight hours later, and 600 mg daily for four days. Study administrators found no benefit at 14 days (11.8% of the test group became ill, versus 14.3% of the control group). They found a significantly increased rate of side effects reported in the hydroxychloroquine (Plaquenil) group but no serious adverse reactions (Boulware et al., 2020). An oral RNA polymerase inhibitor, favipiravir, is currently the subject of a phase III clinical trial and may provide a modest benefit to outpatient COVID patients. EIDD 2801 is another oral RNA polymerase inhibitor being explored, especially as an additive treatment with remdesivir (Luetkemeyer, 2020).
To enhance the safety of HCPs and other patients, patients with respiratory symptoms should be instructed to call ahead and be processed through a nurse-directed triage protocol. Masks should be worn throughout their visit whenever possible. Patients being seen for well-child visits or unrelated complaints should be rescheduled when possible or physically/temporally separated from those with respiratory symptoms. EMS personnel should contact facilities prior to arrival when transporting patients with respiratory symptoms to obtain transport protocol instructions for arrival. Facilities should consider limiting entry points and encourage all patients to adhere strictly to hand hygiene, cough etiquette, and facemasks, ensuring alcohol-based hand sanitizer, tissues, and disposable facemasks are available. Triage centers may be set up outside the facility or in ancillary buildings for screening purposes. PUIs should be quickly triaged, given a facemask, and immediately isolated in a closed examination room, personal vehicle, or otherwise separated from other patients by at least six feet. Those with known or suspected COVID-19 who are admitted to a facility should be placed in a private room, and providers should utilize standard precautions as well as donning a respirator, gown, gloves, and eye protection when entering the room (this is most similar to a combination of both contact and droplet transmission-based isolation precautions; see Figure 3). A test-based strategy regarding the discontinuation of transmission-based precautions is no longer recommended due to prolonged viral shedding, limiting the utility of this method in most cases. Patients with mild to moderate disease and no underlying immunocompromise may be released from transmission-based precautions or in-home isolation after at least ten days have passed since symptom onset, at least 24 hours of remaining afebrile (no fever) and symptoms have improved. The same criteria should be used to discontinue precautions in severely ill patients, with the exception that at least 20 days must have passed since symptom onset (CDC, 2020).

Many hospitals continue to report shortages of PPE, such as N95 respirators, facemasks, and gowns. See Figure 4 for clarification regarding types of PPE. The CDC has established optimization strategies for hospitals dealing with inadequate PPE supplies. Contingency plans should be utilized by all healthcare facilities at this time, which include maximizing engineering and administrative controls to prevent transmission, canceling all elective procedures and appointments, and reserving all available PPE for HCPs. This may include reducing the length of stay of hospitalized patients. Reusable PPE should be used and processed/disinfected for reuse in lieu of disposable options, such as other classes of filtering facemask respirators: elastomeric respirators or powered air-purifying respirators (PAPRs). Expired PPE can be used beyond its published shelf life for training and demonstration purposes. Finally, facilities should consider using respirators or facemasks/eye protection beyond a single patient. This may include extended use of N95s (without removing the mask) for a cohort of patients all infected with COVID-19. They also propose the limited reuse of N95 masks, which includes the donning/doffing of a single mask up to five times by the same HCP. This has been established as low risk when contact transmission is not a significant concern (such as in tuberculosis) but has not been well-established in COVID-19. Respirators should be hung up or stored in paper bags between uses (CDC, 2020). Additional safety considerations for the extended use/limited reuse of N95s include:
- Discard N95 respirators following use during aerosol-generating procedures.
- Discard N95 respirators contaminated with blood, respiratory or nasal secretions, or other bodily fluids from patients.
- Discard N95 respirators following close contact with any patient co-infected with an infectious disease requiring contact precautions.
- Consider the use of a cleanable face shield (preferred3) over an N95 respirator and/or other steps (e.g., masking patients, use of engineering controls), when feasible to reduce surface contamination of the respirator.
- Hang used respirators in a designated storage area or keep them in a clean, breathable container such as a paper bag between uses. To minimize potential cross-contamination, store respirators so that they do not touch each other, and the person using the respirator is clearly identified. Storage containers should be disposed of or cleaned regularly.
- Clean hands with soap and water or an alcohol-based hand sanitizer before and after touching or adjusting the respirator (if necessary, for comfort or to maintain fit).
- Avoid touching the inside of the respirator. If inadvertent contact is made with the inside of the respirator, discard the respirator and perform hand hygiene as described above.
- Use a pair of clean (non-sterile) gloves when donning a used N95 respirator and performing a user seal check. Discard gloves after the N95 respirator is donned, and any adjustments are made to ensure the respirator is sitting comfortably on your face with a good seal (CDC, 2020).
Figure 4
Facemasks versus Respirators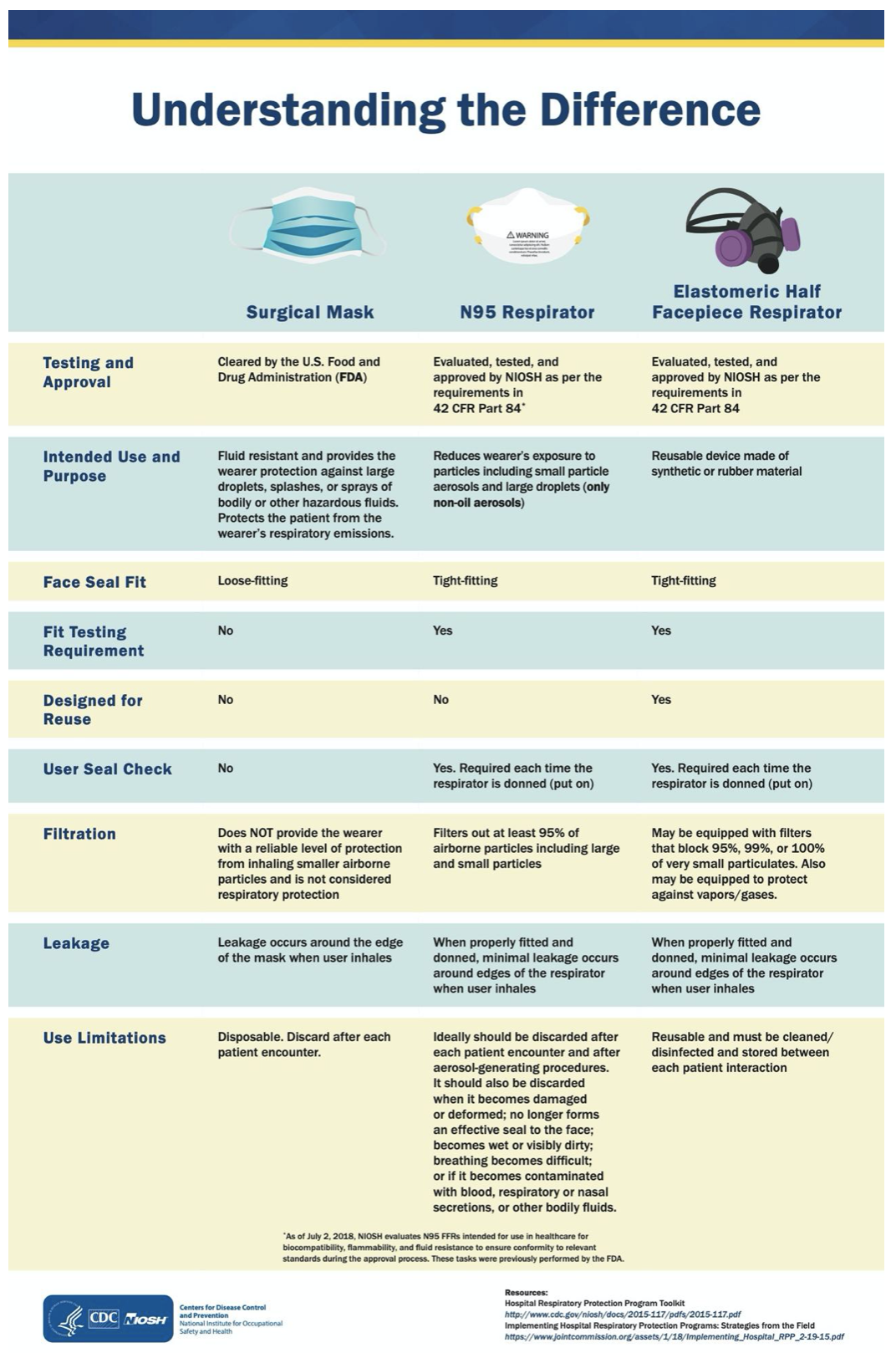
(CDC, 2020)
The second tier of optimization strategies, crisis alternate strategies, which apply to facilities with severe PPE shortages, can be accessed through the CDC website. In the direst of scenarios, when no N95s remain in a facility, the CDC recommends restricting those HCPs at high risk from direct patient care and encourages convalescent HCPs to volunteer for the care of COVID-19 patients. Ventilated headboards may help reduce the risk to HCPs in the room, in addition to expedient patient isolation rooms using portable high-efficiency particulate air (HEPA) fans. Surgical masks should be used when no respirators are available, and homemade cloth masks may be used if surgical masks are unavailable (CDC, 2020).
Severe Disease Treatment
Treatment options for those with severe disease have expanded tremendously in recent months. Lopinavir-ritonavir (Kaletra) had disappointing results in a recent trial in China but is currently being investigated in a WHO study. Hydroxychloroquine (Plaquenil) and chloroquine (Aralen) are oral medications used for the treatment of malaria and other inflammatory conditions, including chemoprophylaxis, rheumatoid arthritis, and systemic lupus erythematous. Both drugs exhibited in-vitro activity against SARS-CoV-2 and other coronaviruses. Initial studies indicated that hydroxychloroquine (Plaquenil) appeared to have a higher potency against SARS-CoV-2. A study in China indicated benefit from chloroquine (Aralen) versus a comparison group. Both medications have known safety risks, such as prolonged QT syndrome in patients with hepatic or renal dysfunction and immunosuppression (CDC, 2020). In July, a randomized controlled trial, this one open-label, was published regarding the use of hydroxychloroquine (Plaquenil) with or without azithromycin (Zithromax). They enrolled 667 hospitalized patients with either confirmed or suspected COVID-19 infection requiring no more than 4 liters of supplemental oxygen. The use of hydroxychloroquine (Plaquenil) 400 mg twice daily (BID) for seven days was compared to hydroxychloroquine (Plaquenil) 400 mg BID and azithromycin (Zithromax) 500 mg daily for seven days and the control group (usual supportive care). The authors found no significant effect of either treatment regimen on the clinical status of the final 504 patients at 15 days, rated on a seven-level ordinal scale. They did observe an increased incidence of QTc prolongation and elevated liver enzymes in both treatment groups (Cavalcanti et al., 2020). In total, four randomized clinical trials regarding the use of hydroxychloroquine (Plaquenil) in hospitalized patients found no significant clinical benefit (Luetkmeyer, 2020).
Although experts initially advised against the use of corticosteroids in COVID patients due to concern for prolonged viral replication. However, the use of dexamethasone (Decadron) has emerged as an effective treatment option in severely or critically ill COVID patients. In July, the RECOVERY Collaborative Group (2020) published their findings of a controlled, open-label, randomized trial of dexamethasone (Decadron), administered orally or intravenously at 6mg daily for ten days (n= 2104), versus usual supportive care (n=4321). Overall, the mortality rate at 28 days was reduced in the treatment group (22.9%) versus the control group (25.7%). When broken down into patient subtypes, those patients on mechanical ventilation had a significant reduction in the 28-day mortality rate when treated with dexamethasone (Decadron, 29.3% versus 41.4% in the control group). Those patients requiring noninvasive supplemental oxygen saw a less significant reduction in the 28-day mortality rate (23.3% versus 26.2% in the control group). Those patients not requiring supplemental oxygen did not clinically benefit from the use of dexamethasone (Decadron) and saw an increase in the 28-day mortality rate from 14% (with usual care) to 17.8% with treatment. For this reason, dexamethasone (Decadron) is not recommended in those patients not requiring supplemental oxygen or respiratory support (RECOVERY Collaborative Group, 2020). This medication also has the added advantage of being very accessible and inexpensive. It is thought to function by inhibiting the inflammatory pathways and thus helping to prevent ARDS seen in many severe COVID cases. For this reason, it is thought to be most beneficial during the later phase of the disease characterized by inflammatory tissue damage, versus the viral replication phase seen earlier (Rubin et al., 2020). The NIH currently recommends the use of dexamethasone (Decadron) in patients mechanically vented (A1) or those on supplemental oxygen (B1). Similarly, the Infectious Diseases Society of America (IDSA) recommends with moderate certainty that dexamethasone (Decadron) or similar corticosteroid be used in those COVID patients with severe disease with hypoxemia (oxygen saturation below 94%) but not in those without hypoxemia (Luetkemeyer, 2020).
Remdesivir (GS-5734), an investigational intravenous broad-spectrum antiviral produced by Gilead, may be an effective treatment option. Preclinical data suggested effectiveness against MERS and SARS-CoV, and it demonstrated early in-vitro activity against SARS-CoV-2 (CDC, 2020). It has been granted EUA by the FDA and is currently recommended by the NIH for hospitalized patients with oxygen saturation levels below 94%. The ACTT-1 study enrolled over 1,000 hospitalized patients randomized to receive remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to nine days) or placebo for up to 10 days. The primary outcome of median time to recovery was 11 days in the treatment group as compared to 15 days in the control group. A secondary measure, the mortality rate at 14 days, was reduced to 7.1% in the test group, as compared to 11.9% in the control group. The study found that clinical benefit was most significant in the cohort of patients requiring supplemental oxygen (median seven days versus nine), with minimal clinical benefit in those patients not requiring oxygen (median five versus six days), or those on high-flow oxygen/noninvasive respiratory support (16 versus 22 days). They found no clinical benefit for those patients on mechanical ventilation or ECMO. Serious adverse events were reported in 21% of those participants in the treatment group versus 27% of the control group participants. 45% of enrolled participants identify as an ethnicity/race other than "white" (Beigel et al., 2020). The NIH is currently recommending remdesivir for use in COVID patients hospitalized and requiring oxygen, but they do not recommend its use for those on high-flow oxygen, noninvasive respiratory support, mechanical ventilation, or ECMO (Luetkmeyer, 2020). Of note, remdesivir is difficult to obtain at times and can be very expensive (Rubin et al., 2020). An inhaled formulation is currently under review for potential use in outpatients (Luetkemeyer, 2020).
What Can we do Now, as Nurses?
- Prepare, do not panic.
- As always, hand hygiene. Diligent. Thorough. Consistent.
- Know the signs and symptoms of COVID-19: fever, cough, myalgia, fatigue, and SOB, which typically present four to five days following exposure to the virus (but may be as much as 14 days).
- Avoid travel when possible:
Most of the world is currently categorized as Level 3 (high risk- avoid nonessential travel, with potential restrictions upon re-entry into the US, see US Department of State for country details). Some countries have also limited the entry of US residents, or established required period(s) of quarantine.
Level 1 (low risk) countries currently include Bonaire, Fiji, New Zealand, Saba, St Barthelemy, St Eustatius, and Thailand.
Very low risk (no travel health notice) currently includes the British Virgin Islands, Brunei, Cayman Islands, Dominica, Falkland Islands, French Polynesia, Greenland, Laos, Macau SAR, Mauritius, New Caledonia, Taiwan, and Timor-Leste.
- If you can, stay in- call elderly neighbors and family members to check on them and ensure they have any supplies that they need. Avoid dining in restaurants, gyms, coffee shops, etc., and pick-up or take-out instead. If possible, order groceries, dry goods, and paper products to be delivered or brought out to the car to avoid wandering through grocery stores, and only leave the house for essentials or to report to essential employment.
- Boost your immune system naturally- get adequate sleep/rest, eat a balanced diet high in all essential vitamins and minerals, ensure sufficient hydration, and limit stress (more on this below).
- Educate yourself- go to cdc.gov and click on COVID-19 for the latest information.

Protect Yourself
Nurses and other HCPs are subject to fatigue, burnout, and other adverse effects of a system that is often deficient in resources yet overflowing with expectations and responsibilities. The Agency for Healthcare Research and Quality (AHRQ; Zipperer, 2020) is an agency within the HHS focused on the safety, quality, accessibility, equitability, and affordability of healthcare in the US. Safety concerns for HCPs during this pandemic are varied, but burnout and fatigue are exacerbated and accelerated under these extraordinary conditions. A study conducted with HCPs in China early in the pandemic found that explicit, evidence-based infection-control guidelines, equipment that was customized, and facilities designed specifically for the management of COVID patients significantly mitigated psychological burnout in HCPs. Additionally, a group of Italian HCPs found that establishing an organizational infrastructure and the addition of opportunities for peer support aided in the removal of the stigma of HCPs asking their colleagues for help when overwhelmed. Another identified concern is the lack of trust amongst members of the healthcare team. Many team members may be new, unseasoned, or inexperienced for several reasons, and without first establishing trust, the team members are reluctant to fully trust their colleagues. The AHRQ has published specific suggestions regarding the promotion of safety during this extreme pandemic. They suggest shift team huddles targeted at sharing vital information to update new team members, role clarification, and task assignments, as well as debriefings at the conclusion of shifts/scenarios to share insight, educate, and improve. TeamSTEPPS© or similar systems may help facilitate these team-building efforts. HCPs report a feeling of psychological stress and a lack of safety related to under-trained team members, compounded by the anxieties of caring for patients in an uncertain situation and the unprecedented loss of life. The AHRQ recommends mitigating this by establishing a culture of safety, encouraging team members to openly voice their concerns, organizational support of employee safety (i.e., providing adequate PPE), and a continuous supply of positive feedback. Many HCPs cite a lack of sufficient time for patient and self-care under normal circumstances within the workday, and this has certainly been exacerbated by the COVID pandemic in most workplaces. Administration and leadership should ensure that all team members have access to breaks, as well as adequate food and hydration during a shift. Human factors engineering can also facilitate safety, through increased signage (i.e., reminders regarding equipment location/usage, PPE use, and hand hygiene), workflow engineering (review workflows to help identify potential failures and optimize design using healthcare failure mode and effects analysis), checklists (i.e., for communication optimization, to educate and orient new team members, and to help guide crisis situations), and simulations to educate team members in a low-risk environment (Zipperer, 2020).
Aside from utilizing appropriate PPE when caring for our patients and diligent hand hygiene, the best way to protect ourselves and our families is to boost our body's defense system. Our immune system will be responsible for handling that for us. Harvard Health Publishing (2014) lays it out in plain language: choose a healthy lifestyle. This includes not smoking, eating fruits and vegetables, exercising regularly, maintaining a healthy weight, drinking alcohol in moderation only, getting adequate sleep, and minimizing stress. Healthy people continuously generate more lymphocytes than is needed, so there is no need to boost the level of any one cell type. Micronutrient deficiencies (i.e., zinc, selenium, iron, copper, folic acid, and vitamins A, B6, C, and E) have been shown to alter immune responses in animals and may have the same effect on the human immune response. Megadoses of a single vitamin do not appear to enhance immunity. Exercise not only promotes overall health, supporting a healthy immune system, but it also enhances circulation, allowing immune cells and other necessary nutrients to move through the body more easily. Age naturally reduces our body's immune response, likely due to reduced T-cells secondary to thymus atrophy and/or bone marrow inefficiency (Harvard Health Publishing, 2014).
While many supplements will advertise immune-boosting effects, most have not been proven in large clinical trials. Black elderberry has shown some antiviral promise. A 2001 in-vitro study by Barak and colleagues found that elderberry extract increased the production of inflammatory cytokines, especially tumor necrosis factor (TNF-alpha). A 2004 study in Norway, including 60 people with influenza, found that 15 ml of elderberry syrup taken four times daily shortens the average length of symptoms from seven/eight days to two/four days. Another study of 64 people indicates that 175 mg of elderberry (lozenge) for two days decreases flu symptoms such as fever, headache, muscle aches, and nasal congestion. Finally, an Australian study in 2016, including 312 subjects, indicates that 300 mg of elderberry extract taken three times daily results in symptoms that are less severe and of a shorter duration. Uncooked berries and leaves of the black elderberry plant should be avoided due to the risk of cyanide and lectins, which may cause gastrointestinal symptoms (Mandl, 2018). Similarly, some small studies have indicated that zinc nasal gel or oral lozenges, when administered within 24 hours of the onset of cold symptoms, typically caused by the rhinovirus, can shorten the duration and reduce the severity of cold symptoms (Hulisz, 2004). Given their general antiviral effects, these two may help shorten the duration or reduce the severity of other viral infections, such as COVID-19, although this is purely speculative and has never been studied as this is a novel (new) virus.
Research and Development
The scientific community is working feverishly to develop a vaccine. While there are several trials ongoing to develop a vaccine, the three most advanced trials include:
- NIH/Moderna- RNA format vaccine, phases I and II complete, III is scheduled to be complete by 10/22, including 30,000 participants in a community-based randomized trial
- Oxford/AstraZeneca- chimpanzee adenovirus format, phases I and II complete, III is scheduled to be complete by 8/21, including 10,000 participants in a community-based randomized trial
- Cansino- adenovirus 5 format, phases I and II complete, phase III enrolling (Ernst, 2020).
There are at least three other trials currently enrolling phase I or II trials by Pfizer (in conjunction with BioNTech), John & Johnson (in conjunction with Harvard), and Novavax. Of note, none of the trials have thus far allowed for the enrollment of pregnant patients. Once developed, the CDC Advisory Committee on Immune Practices will make recommendations regarding who should be vaccinated first. It is currently expected to recommend that the highest risk healthcare, national security, and other essential workers receive it first. Others that should be given relative priority include those with underlying conditions, ethnic minority patients, older adults, residents of long-term care facilities, hospital staff (housekeeping and food service), and teachers (Ernst, 2020). Some experts are also suggesting the use of the longstanding oral polio vaccine to elicit a response by stimulating the innate immune system, as it is a live attenuated vaccine with a long history of proven safety (Chumakov et al., 2020).
Other treatment options that have been considered include inhaled interferon beta-1b or IL-6 antagonists. COVID is known to reduce interferon type 1 activity. An open-label phase II trial with 100 patients treated with inhaled interferon beta-1b for 14 days found a 79% reduction in disease progress towards intubation or death, leading to twice the chance of recovery for those treated patients versus control. Subcutaneous and home trials for this medication are still pending, including a combination trial with remdesivir. Two IL-6 antagonists (tocilizumab [Actemra] and sarilumab [Kevzara]) were tested in phase III randomized, controlled trials, and found no significant clinical difference with their use (Luetkemeyer, 2020).
The NIH and officials at Eli Lilly announced in August that two large placebo-controlled joint trials would be opening soon to assess the first monoclonal antibody (LY-CoV555) for the treatment of COVID. It consists of lab-produced antibodies derived from cloned cells. ACTIV-2 will include 200 outpatients as a phase II trial, while ACTIV-3 is a phase III trial enrolling 300 hospitalized COVID patients, excluding any patients with end-stage organ failure (Brunk, 2020; Luetkemeyer, 2020).
Moving Forward
The current plan for containment of this virus in the US is largely self-quarantine and social distancing. Most areas temporarily closed schools, restaurants, gyms, theatres, and bars to force the social distancing, and are now in various stages of reopening, depending on the state and the local area. Parents are having to make gut-wrenching decisions about taking a leave of absence from their jobs in order to stay home with children, hiring childcare, or allowing their children to return to crowded schools with a mask. Those in the service industry endured weeks or even months without pay. The US Congress has passed multiple relief bills since March to provide relief to families and economic stimulus to the country. As previously mentioned, the CDC continues to recommend that the public utilize cloth masks when forced to leave their house to perform essential functions to prevent asymptomatic individuals from unintentionally spreading the virus to others (CDC, 2020). As of August 10, 2020, more than 5 million cases have been reported in the US (19.9 million worldwide) to a dashboard managed by the Johns Hopkins School of Engineering, and more than 160,000 people have died (732,000 worldwide; JHU, 2020). The WHO Director-General is encouraging everyone to remember that while social distancing may help, the backbone of the COVID-19 response should be testing, isolation, and contact tracing. His message: Test, test, test. Test every suspected case.” (Chappell, 2020, para. 30). Kissler and colleagues (2020) used mathematical modeling to predict the transmission of SARS-CoV-2. They indicated that the most effective model, one using moderate-intensity temporary social distancing in 20-week periods, should continue through 2022 in order to maintain a prevalence below the national critical care threshold, or early 2022 if that critical care capacity could somehow be doubled. They cautioned that widespread surveillance would be required in order to time the social distancing measures appropriately. They suggest that a vaccine, new and more effective treatment regimens, and aggressive contract tracing and quarantine measures could also potentially accelerate this timeline. (Kissler et al., 2020).
*(Most of this information is up to date as of August 10, 2020)
References
Abbott Laboratories. (2020). Detect COVID-19 in as little as five minutes. https://www.abbott.com/corpnewsroom/product-and-innovation/detect-covid-19-in-as-little-as-5-minutes.html
American Board of Internal Medicine. (2020). ABIM laboratory test reference ranges. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American Journal of Managed Care. (2020). A timeline of COVID-19 developments in 2020. AJMC. https://www.ajmc.com/view/undefined
Barak, V., Halperin, T., & Kalickman, I. (2001). The effect of Sambucol, a black elderberry-based natural product, on the production of human cytokines: I. Inflammatory cytokines. European Cytokine Network. 12(2), 290-6.
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., Hohmann, E., Chu, H. Y., Luetkemeyer, A., Kline, S., Lopez de Castilla, D., Finberg, R. W., Dierberg, K., Tapson, V., Hsieh, L., Patterson, T. F., Paredes, R., Sweeney, D. A., Short, W. R., … Lane, H. C. (2020). Remdesivir for the treatment of Covid-19: Preliminary report. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMoa2007764
Boulware, D. R., Pullen, M. F., Bangdiwala, A. S., Pastick, K. A., Lofgren, S. M., Okafor, E. C., Skipper, C. P., Nascene, A. A., Nicol, M. R., Abassi, M., Engen, N. W., Cheng, M. P., LaBar, D., Lother, S. A., MacKenzie, L. J., Drobot, G., Marten, N., Zarychanski, R., Kelly, L. E., … Hullsiek, K. H. (2020). A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMoa2016638
Brunk, D. (2020). Two monoclonal antibody trials for COVID-19 launched. Medscape. http://www.medscape.com/viewarticle/935170
Bunyavanich, S., Do, A., & Vicencio, A. (2020). Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA, 323(23), 2427–2429. https://doi.org/10.1001/jama.2020.8707
Cameron, B. (2020). I ran the White House pandemic office. Trump closed it. https://www.washingtonpost.com/outlook/nsc-pandemic-office-trump-closed/2020/03/13/a70de09c-6491-11ea-acca-80c22bbee96f_story.html
Cavalcanti, A. B., Zampieri, F. G., Rosa, R. G., Azevedo, L. C. P., Veiga, V. C., Avezum, A., Damiani, L. P., Marcadenti, A., Kawano-Dourado, L., Lisboa, T., Junqueira, D. L. M., de Barros e Silva, P. G. M., Tramujas, L., Abreu-Silva, E. O., Laranjeira, L. N., Soares, A. T., Echenique, L. S., Pereira, A. J., Freitas, F. G. R., … Berwanger, O. (2020). Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMoa2019014
The Centers for Disease Control and Prevention. (2020). Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html
The Centers for Disease Control and Prevention COVID-19 Response Team. (2020). Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep, 69(14), 422–426. http://dx.doi.org/10.15585/mmwr.mm6914e4
Chappell, B. (2020). Coronavirus: US tells Americans to home-school; The WHO urges: ‘Test, test, test’. NPR. https://www.npr.org/sections/health-shots/2020/03/16/816428320/coronavirus-u-s-enters-quarantine-life-as-many-schools-and-businesses-close
Chumakov, K., Benn, C. S., Aaby, P., Kottilil, S., & Gallo, R. (2020). Can existing live vaccines prevent COVID-19? Science, 368(6496), 1187–1188. https://doi.org/10.1126/science.abc4262
Danis, K., Epaulard, O., Bénet, T., Gaymard, A., Campoy, S., Botelho-Nevers, E., Bouscambert-Duchamp, M., Spaccaferri, G., Ader, F., Mailles, A., Boudalaa, Z., Tolsma, V., Berra, J., Vaux, S., Forestier, E., Landelle, C., Fougere, E., Thabuis, A., Berthelot, P., … Mounayar, A.-L. (2020). Cluster of coronavirus disease 2019 (COVID-19) in the French Alps, February 2020. Clinical Infectious Diseases, 71(15), 825–832. https://doi.org/10.1093/cid/ciaa424
Dufort, E. M., Koumans, E. H., Chow, E. J., Rosenthal, E. M., Muse, A., Rowlands, J., Barranco, M. A., Maxted, A. M., Rosenberg, E. S., Easton, D., Udo, T., Kumar, J., Pulver, W., Smith, L., Hutton, B., Blog, D., & Zucker, H. (2020). Multisystem inflammatory syndrome in children in New York state. New England Journal of Medicine, 383(4), 347–358. https://doi.org/10.1056/NEJMoa2021756
Edmond, M. (2020). COVID-19 & face shields: Can we reduce community transmission of COVID-19 with face shields? What are the main advantages and disadvantages? https://www.vumedi.com/video/covid-19-face-shields-can-we-reduce-community-transmission-of-covid-19-with-face-shields-what-are-th/
Ellinghaus, D., Degenhardt, F., Bujanda, L., Buti, M., Albillos, A., Invernizzi, P., Fernández, J., Prati, D., Baselli, G., Asselta, R., Grimsrud, M. M., Milani, C., Aziz, F., Kässens, J., May, S., Wendorff, M., Wienbrandt, L., Uellendahl-Werth, F., Zheng, T., … Karlsen, T. H. (2020). Genomewide association study of severe Covid-19 with respiratory failure. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMoa2020283
Ernst, J. (2020). SARS-CoV-2 vaccines August update: Is immunity short-lived & should we worry? What are the recent estimates on vaccine efficacy & how to prioritize which group will receive it first? https://www.vumedi.com/video/sars-cov-1-vaccines-august-update-is-immunity-short-lived-should-we-worry-what-are-the-recent-estima/
Fang, L., Karakiulakis, G., & Roth, M. (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet. https://doi.org/10.1016/S2213-2600(20)30116-8
Feldstein, L. R., Rose, E. B., Horwitz, S. M., Collins, J. P., Newhams, M. M., Son, M. B. F., Newburger, J. W., Kleinman, L. C., Heidemann, S. M., Martin, A. A., Singh, A. R., Li, S., Tarquinio, K. M., Jaggi, P., Oster, M. E., Zackai, S. P., Gillen, J., Ratner, A. J., Walsh, R. F., … Randolph, A. G. (2020). Multisystem inflammatory syndrome in U.S. children and adolescents. New England Journal of Medicine, 383(4), 334–346. https://doi.org/10.1056/NEJMoa2021680
Feng, E. & Cheng, A. (2020). Mystery in Wuhan: Recovered Coronavirus patients test negative ... then positive. https://www.npr.org/sections/goatsandsoda/2020/03/27/822407626/mystery-in-wuhan-recovered-coronavirus-patients-test-negative-then-positive
Flannery, D. D., Gouma, S., Dhudasia, M. B., Mukhopadhyay, S., Pfeifer, M. R., Woodford, E. C., Gerber, J. S., Arevalo, C. P., Bolton, M. J., Weirick, M. E., Goodwin, E. C., Anderson, E. M., Greenplate, A. R., Kim, J., Han, N., Pattekar, A., Dougherty, J., Kuthuru, O., Mathew, D., … Hensley, S. E. (2020). SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Science Immunology, 5(49). https://doi.org/10.1126/sciimmunol.abd5709
Fontanet, A., Tondeur, L., Madec, Y., Grant, R., Besombes, C., Jolly, N., Pellerin, S. F., Ungeheuer, M.-N., Cailleau, I., Kuhmel, L., Temmam, S., Huon, C., Chen, K.-Y., Crescenzo, B., Munier, S., Demeret, C., Grzelak, L., Staropoli, I., Bruel, T., … Hoen, B. (2020). Cluster of COVID-19 in northern France: A retrospective closed cohort study. MedRxiv, 2020.04.18.20071134. https://doi.org/10.1101/2020.04.18.20071134
Frellick, M. (2020). FDA OKs two blood tests to estimate SARS-CoV-2 antibodies. Medscape. http://www.medscape.com/viewarticle/935090
Gold, M. S., Sehayek, D., Gabrielli, S., Zhang, X., McCusker, C., & Ben-Shoshan, M. (2020). COVID-19 and comorbidities: A systematic review and meta-analysis. Postgraduate Medicine, 1–7. https://doi.org/10.1080/00325481.2020.1786964
Gronvall, G. (2020). COVID-19 antibody testing update: Why does the accuracy of SARS-CoV-2 antibody tests vary so much? How much does the serosurvey strategy impact the interpretation of results? https://www.vumedi.com/video/covid-19-antibody-testing-update-why-does-the-accuracy-of-sars-cov-2-antibody-tests-vary-so-much-how/
Guan, W., Ni, Z., Hu, Y., Liang, W., Ou, C., He, J., Liu, L., Shan, H., Lei, C., Hui, D. S. C., Du, B., Li, L., Zeng, G., Yuen, K.-Y., Chen, R., Tang, C., Wang, T., Chen, P., Xiang, J., … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. https://doi.org/10.1056/NEJMoa2002032
Harvard Health Publishing. (2014). How to boost your immune system. https://health.harvard.edu/staying-healthy/how-to-boost-your-immune-system
Heald-Sargent, T., Muller, W. J., Zheng, X., Rippe, J., Patel, A. B., & Kociolek, L. K. (2020). Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatrics. https://doi.org/10.1001/jamapediatrics.2020.3651
Hulisz, D. (2004). Efficacy of zinc against common cold viruses: An overview. Journal of the American Pharmacists Association 44(5), 594-603. https://doi.org/ 10.1331/1544-3191.44.5.594.hulisz
Ibarrondo, F. J., Fulcher, J. A., Goodman-Meza, D., Elliott, J., Hofmann, C., Hausner, M. A., Ferbas, K. G., Tobin, N. H., Aldrovandi, G. M., & Yang, O. O. (2020). Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMc2025179
Jain, V., & Yuan, J.-M. (2020). Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: A systematic review and meta-analysis. International Journal of Public Health, 65(5), 533–546. https://doi.org/10.1007/s00038-020-01390-7
Johns Hopkins University. (2020). Coronavirus resource center. https://coronavirus.jhu.edu/map.html
Kaiser Health News. (2020). Trump touted Abbott’s quick COVID-19 test. HHS document shows only 5,500 are on the way for entire US. https://khn.org/news/trump-touted-abbotts-quick-covid-19-test-hhs-document-shows-only-5500-are-on-way-for-entire-u-s/
Kissler, S. M., Tedijanto, C., Goldstein, E., Grad, Y. H., & Lipsitch, M. (2020). Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science (New York, N.y.). https://doi.org/10.1126/science.abb5793
Langelier, C. (2020). COVID-19 & testing: Can more frequent and faster testing with cheaper, less sensitive tests control the pandemic? When do we need it? Can pool testing increase capacity? https://www.vumedi.com/video/covid-19-testing-can-more-frequent-and-faster-testing-with-cheaper-less-sensitive-tests-control-the-/
Latz, C. A., DeCarlo, C., Boitano, L., Png, C. Y. M., Patell, R., Conrad, M. F., Eagleton, M., & Dua, A. (2020). Blood type and outcomes in patients with COVID-19. Annals of Hematology, 1–6. https://doi.org/10.1007/s00277-020-04169-1
Levin, M. (2020). Childhood multisystem inflammatory syndrome—A new challenge in the pandemic. New England Journal of Medicine, 383(4), 393–395. https://doi.org/10.1056/NEJMe2023158
Long, Q.-X., Tang, X.-J., Shi, Q.-L., Li, Q., Deng, H.-J., Yuan, J., Hu, J.-L., Xu, W., Zhang, Y., Lv, F.-J., Su, K., Zhang, F., Gong, J., Wu, B., Liu, X.-M., Li, J.-J., Qiu, J.-F., Chen, J., & Huang, A.-L. (2020). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine, 1–5. https://doi.org/10.1038/s41591-020-0965-6
Lu, X., Zhang, L., Du, H., Zhang, J., Li, Y. Y., Qu, J., Zhang, W., Wang, Y., Bao, S., Li, Y., Wu, C., Liu, H., Liu, D., Shao, J., Peng, X., Yang, Y., Liu, Z., Xiang, Y., Zhang, F., … Wong, G. W. K. (2020). SARS-CoV-2 infection in children. New England Journal of Medicine, 382(17), 1663–1665. https://doi.org/10.1056/NEJMc2005073
Luetkemeyer, A. (2020). COVID-19 treatments August update: Can hydroxychloroquine be used? What are the recommendations for prioritizing limited supplies of remdesivir? How to use corticosteroids and when? https://www.vumedi.com/video/covid-19-treatments-august-update-can-hydroxychloroquine-be-used-what-are-the-recommendations-for-pr/
Mandl, E. (2018). Elderberry: Benefits and dangers. Healthline. https://www.healthline.com/nutrition/elderberry#risks-and-side-effects
McComb, S., Thiriot, A., Akache, B., Krishnan, L., & Stark, F. (2019). Introduction to the immune system. In Methods in molecular biology (Clifton, N.J.) (Vol. 2024, pp. 1–24). https://doi.org/10.1007/978-1-4939-9597-4_1
McNamara, D. (2020). Low vitamin D linked to increased COVID-19 risk. Medscape. http://www.medscape.com/viewarticle/934835
Menni, C., Valdes, A. M., Freidin, M. B., Sudre, C. H., Nguyen, L. H., Drew, D. A., Ganesh, S., Varsavsky, T., Cardoso, M. J., El-Sayed Moustafa, J. S., Visconti, A., Hysi, P., Bowyer, R. C. E., Mangino, M., Falchi, M., Wolf, J., Ourselin, S., Chan, A. T., Steves, C. J., & Spector, T. D. (2020). Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Medicine, 26(7), 1037–1040. https://doi.org/10.1038/s41591-020-0916-2
Merzon, E., Tworowski, D., Gorohovski, A., Vinker, S., Cohen, A. G., Green, I., & Morgenstern, M. F. (2020). Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. The FEBS Journal, n/a(n/a). https://doi.org/10.1111/febs.15495
Milton, D. (2020). COVID-19 droplet & aerosol transmission: What's the difference & why is this distinction crucially important? https://www.vumedi.com/video/covid-19-droplet-aerosol-transmission-whats-the-difference-why-is-this-distinction-crucially-importa/
Nardell, E. (2020). SARS-CoV-2 transmission myths: Is SARS-CoV-2 airborne transmitted? Why is it important to know the difference between respiratory droplets and droplet nuclei? https://www.vumedi.com/video/sars-cov-2-transmission-myths-is-sars-cov-2-airborne-transmitted-why-is-important-to-know-the-differ/
National Institutes of Health. (2020). Antithrombotic therapy in patients with COVID-19. COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/
National Public Radio. (2020). US coronavirus deaths top 140,000 as world sets daily record in new cases. NPR.Org. https://www.npr.org/sections/coronavirus-live-updates/2020/07/18/892677119/world-sets-daily-record-in-new-coronavirus-cases
Nguyen, L. H., Drew, D. A., Graham, M. S., Joshi, A. D., Guo, C.-G., Ma, W., Mehta, R. S., Warner, E. T., Sikavi, D. R., Lo, C.-H., Kwon, S., Song, M., Mucci, L. A., Stampfer, M. J., Willett, W. C., Eliassen, A. H., Hart, J. E., Chavarro, J. E., Rich-Edwards, J. W., … Zhang, F. (2020). Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. The Lancet Public Health, 0(0). https://doi.org/10.1016/S2468-2667(20)30164-X
OpenFDA. (2020). Independent evaluations of COVID-19 serological tests. https://open.fda.gov/apis/device/covid19serology/
Pan, D., Sze, S., Minhas, J. S., Bangash, M. N., Pareek, N., Divall, P., Williams, C. ML., Oggioni, M. R., Squire, I. B., Nellums, L. B., Hanif, W., Khunti, K., & Pareek, M. (2020). The impact of ethnicity on clinical outcomes in COVID-19: A systematic review. EClinicalMedicine, 23, 100404. https://doi.org/10.1016/j.eclinm.2020.100404
Paranjpe, I., Fuster, V., Lala, A., Russak, A. J., Glicksberg, B. S., Levin, M. A., Charney, A. W., Narula, J., Fayad, Z. A., Bagiella, E., Zhao, S., & Nadkarni, G. N. (2020). Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. Journal of the American College of Cardiology, 76(1), 122–124. https://doi.org/10.1016/j.jacc.2020.05.001
Qiu, H., Wu, J., Hong, L., Luo, Y., Song, Q., & Chen, D. (2020). Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. The Lancet Infectious Diseases, 20(6), 689–696. https://doi.org/10.1016/S1473-3099(20)30198-5
Rabin, R. C. (2020). The coronavirus infected hundreds at a Georgia summer camp. The New York Times. https://www.nytimes.com/2020/07/31/health/coronavirus-children-camp.html
The RECOVERY Collaborative Group. (2020). Dexamethasone in hospitalized patients with Covid-19: Preliminary report. New England Journal of Medicine, 0(0), null. https://doi.org/10.1056/NEJMoa2021436
Rubin, E. J., Baden, L. R., & Morrissey, S. (2020). Dexamethasone and Covid-19. New England Journal of Medicine, 383(4), e52. https://doi.org/10.1056/NEJMe2025927
Rutherford, G. (2020). COVID-19 July update: Why are children less likely to get infected and to transmit compared to adults? How is COVID-19 transmitted in households and schools? https://www.vumedi.com/video/covid-19-july-update-why-are-children-less-likely-to-get-infected-and-to-transmit-compared-to-adults/
Stein-Zamir, C., Abramson, N., Shoob, H., Libal, E., Bitan, M., Cardash, T., Cayam, R., & Miskin, I. (2020). A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Eurosurveillance, 25(29), 2001352. https://doi.org/10.2807/1560-7917.ES.2020.25.29.2001352
Suthar, M. S., Zimmerman, M., Kauffman, R., Mantus, G., Linderman, S., Vanderheiden, A., Nyhoff, L., Davis, C., Adekunle, S., Affer, M., Sherman, M., Reynolds, S., Verkerke, H., Alter, D. N., Guarner, J., Bryksin, J., Horwath, M., Arthur, C., Saakadze, N., … Wrammert, J. (2020). Rapid generation of neutralizing antibody responses in COVID-19 patients. MedRxiv, 2020.05.03.20084442. https://doi.org/10.1101/2020.05.03.20084442
Tang, N., Bai, H., Chen, X., Gong, J., Li, D., & Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis: JTH, 18(5), 1094–1099. https://doi.org/10.1111/jth.14817
Taylor, D. B. (2020). A timeline of the coronavirus pandemic. The New York Times. https://www.nytimes.com/article/coronavirus-timeline.html
US Food and Drug Administration. (2020a). Coronavirus (COVID-19) update: FDA authorizes first diagnostic test for screening of people without known or suspected COVID-19 infection. FDA; FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-diagnostic-test-screening-people-without-known-or
US Food and Drug Administration. (2020b). EUA authorized serology test performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
Verma, S. (2020). Airborne transmission & face masks: How do different types of masks protect against various ranges of transmitted droplets? How far can aerosolized droplets travel in the air from an uncovered cough & for how long? https://www.vumedi.com/video/airborne-transmission-face-masks-how-do-different-types-of-masks-protect-against-various-ranges-of-t/
Whitehouse.gov. (2020). The president’s coronavirus guidelines for America. https://www.whitehouse.gov/wp-content/uploads/2020/03/03.16.20_coronavirus-guidance_8.5x11_315PM.pdf
Wilson, F. (2020). What does the case fatality rate really tell us? Medscape. http://www.medscape.com/viewarticle/934641
World Health Organization. (2020). Estimating mortality from COVID-19. https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19
Zhao, J., Yang, Y., Huang, H., Li, D., Gu, D., Lu, X., Zhang, Z., Liu, L., Liu, T., Liu, Y., He, Y., Sun, B., Wei, M., Yang, G., Wang, X., Zhang, L., Zhou, X., Xing, M., & Wang, P. G. (2020). Relationship between the ABO blood group and the COVID-19 susceptibility. MedRxiv, 2020.03.11.20031096. https://doi.org/10.1101/2020.03.11.20031096
Zhu, Y., Bloxham, C. J., Hulme, K. D., Sinclair, J. E., Tong, Z. W. M., Steele, L. E., Noye, E. C., Lu, J., Chew, K. Y., Pickering, J., Gilks, C., Bowen, A. C., & Short, K. R. (2020). Children are unlikely to have been the primary source of household SARS-CoV-2 infections. MedRxiv, 2020.03.26.20044826. https://doi.org/10.1101/2020.03.26.20044826
Zietz, M., & Tatonetti, N. P. (2020). Testing the association between blood type and COVID-19 infection, intubation, and death. MedRxiv. https://doi.org/10.1101/2020.04.08.20058073
Zipperer, L. (2020). COVID-19: Team and human factors to improve safety. https://psnet.ahrq.gov/primer/covid-19-team-and-human-factors-improve-safety