About this course:
The purpose of this course is to provide an overview of the most up-to-date science available regarding the clinical manifestations, disease severity, and evidence-based treatment approaches for patients with COVID-19, as well as a summary of the COVID-19 vaccines for APRNs.
Course preview
The purpose of this course is to provide an overview of the most up-to-date science available regarding the clinical manifestations, disease severity, and evidence-based treatment approaches for patients with COVID-19, as well as a summary of the COVID-19 vaccines.
Following the completion of this course, APRNs will be able to:
- outline COVID-19 mortality in the US, discuss the pathogenesis of COVID-19, including risk factors, transmission, prevention, and the most common signs and symptoms
- review the clinical features of COVID-19 based on disease severity (mild, moderate, severe, critical), and common laboratory, biomarker, and radiographic findings
- outline screening and testing for COVID-19 infections, and antibody testing
- discuss the management of COVID-19 based on the highest level of evidence available to date and provide an outline of the various treatment options, their clinical considerations, indications, side effects, and monitoring parameters
- discuss mechanisms of COVID-19 vaccination, the types of COVID-19 vaccines available in the US, their mechanism of action, side effects, and indications for use in special populations (pregnancy, lactation, and immunocompromised conditions)
- discuss the most updated information available regarding the emerging covid-19 variants
Disclaimer: This course provides an update to the material offered in the August 2020 COVID-19 NursingCE course. Since COVID-19 is a rapidly evolving global health emergency, all statistics and estimates cited in this course are subject to change after this activity’s release date. For learners interested in more in-depth information regarding the historical timeline, pathophysiology, risk factors, risk reduction, and clinical features of COVID-19, please refer to the COVID-19 Pandemic course published on our site in August of 2020. In order to allow for readability and understanding of the concepts discussed in this course, some information may be briefly restated in Part 2 as a memory refresher.
The SARS-CoV-2 (COVID-19) outbreak continues to wreak havoc globally, spreading rapidly and inducing significant morbidity and mortality. As of February 1, 2021, COVID-19 has been confirmed in over 210 countries, has infected more than 102 million people worldwide, and reached a death toll surmounting 2.2 million. To ensure the delivery of the most efficient and effective care and improve clinical outcomes, all APRNs must remain well-informed on the emerging data and evidence regarding this deadly disease (World Health Organization [WHO], 2021b).
COVID-19 Mortality in the US
While COVID-19 has emerged in most parts of the world, the US is among the most severely affected countries. The exponential rise in COVID-19 cases has been catastrophic, overwhelming the health care system and leading to extensive loss of life. In a December 2020 article published by JAMA, a poignant comparison helped put the daily US death toll into perspective. According to the authors, “The daily US mortality rate for COVID-19 deaths is equivalent to the September 11, 2001, attacks, which claimed 2988 lives, occurring every 1.5 days, or 15 Airbus 320 jetliners, each carrying 150 passengers, crashing every day” (Woolf et al., 2020, p. 123). COVID-19 has become a leading cause of death in the US, affecting all ages across the lifespan, demonstrating that no group is ‘safe’ from contracting, and ultimately dying from, this virus. In response to the global pandemic, the National Center for Health Statistics (NCHS, 2021) releases provisional death data every week. As of February 10, 2021, a total of 443,107 deaths are reported within the 50 states, and the District of Columbia confirmed or presumed COVID-19 based on death certificates (i.e., coded with ICD–10 code U07.1). Approximately 64% died in a hospital or other inpatient healthcare setting, about 21.0% died in a nursing home or long-term care facility. The remaining are unspecified; presumed to have died in their homes or other indeterminate location. The distribution of COVID-19 deaths in the US has notable disparities according to race and Hispanic origin. Individuals who identify as non-Hispanic White comprise the highest percentage of deaths (61%), followed by those who identify as Hispanic (18%) and non-Hispanic Black (16%); despite these groups comprising only 18.5% and 13.4% of the US population, respectively (US Census Bureau, 2019). Native Hawaiian/Pacific Islanders and American Indian/Alaskan Natives have the lowest mortality rates (0.2% and 1%, respectively). The most frequently listed comorbidities with COVID-19 deaths include influenza and pneumonia (43.5%), hypertension (19.8%), diabetes (15.6%), Alzheimer’s disease and other dementias (14.8%), and sepsis (9%; NCHS, 2021).
Background
Coronaviruses (CoV) are a large group of single-stranded RNA viruses. They have a central core of genetic material surrounded by a lipid envelope with protein spikes, which gives it the crown’s appearance shown in Figure 1. In Latin, a crown is called ‘corona,’ and thus, the terminology coronavirus evolved (Azer, 2020; Parasher, 2020).
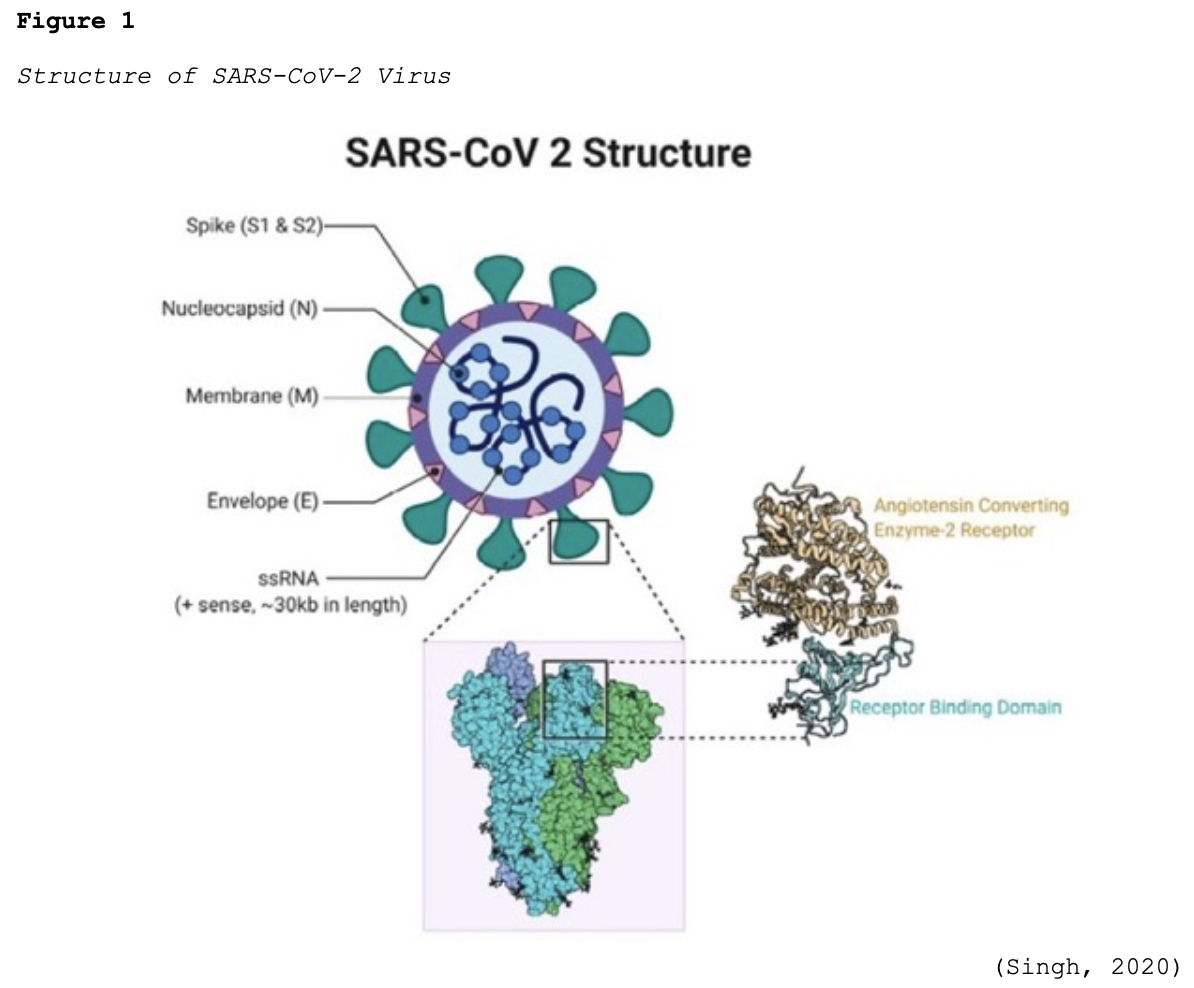
In humans, CoV target the respiratory tract and most notably include severe acute respiratory syndrome (SARS-CoV), which emerged in 2003, Middle East respiratory syndrome (MERS-CoV) in 2012, and now the novel SARS-CoV-2 (COVID-19). COVID-19 has already demonstrated a higher degree of lethality in humans when compared to these earlier outbreaks. While there are different types of CoV that are known to cause illness in animals and humans, COVID-19 had never been detected in humans before December 2019 in Wuhan, China. Early reports described a cluster of pneumonia cases in this region, which was subsequently traced to an outdoor seafood and live animal market located in Wuhan. On January 30, 2020, the WHO declared the COVID-19 outbreak a public health emergency of international concern. To date, the animal reservoir remains under investigation (Azer, 2020; Parasher, 2020; WHO, 2021a).
For a more detailed account of the background and timeline of events, refer to the COVID-19 Pandemic NursingCE Course.
Immune System Overview
To understand how COVID-19 evades the body’s defense system and causes illness, it is first critical to ensure a foundational understanding of the immune system. The immune system is a collection of cells, tissues, and organs that work together to defend the body against attacks by pathogens or foreign invaders (such as microbes, viruses, bacteria, and parasites). The immune system strives to prevent invasion and protect against illness and infection by seeking out and destroying pathogens. The key to a healthy immune system is its ability to distinguish between the body’s own cells (self) and foreign cells (non-self). The cells of the immune system launch an attack when they encounter anything that appears foreign. Any substance capable of triggering an immune response is called an antigen. An antigen can be a virus, bacteria, or any infectious organism, and all antigens carry marker molecules that identify them as foreign. White blood cells (WBCs) are the components of the immune system which work to fight infection and other illnesses. WBCs comprise five specific subtypes (neutrophils, monocytes, macrophages, eosinophils, and basophils). Each WBC serves a specific function in mediating the inflammatory and immune response to infection. WBCs have variable lifespans; while some may live for only 24 hours, the average WBC lifespan is 13 to 20 days. There are two main types of immune responses: innate and adaptive immunity (Longo, 2019; McCance & Heuther, 2019).
Innate Immunity
Also known as nat
...purchase below to continue the course
Adaptive Immunity
Adaptive immunity or acquired immunity is the second line of defense and is highly specific, responding individually to each pathogen it encounters. The adaptive immune system is activated if an invading pathogen somehow breaches innate immune mechanisms. Due to adaptation, the acquired immune system responds comparatively slower than the innate immune system. The adaptive immune system boasts immunologic memory and specificity, meaning it “remembers” prior antigens and can develop a repeat specified response. There are three types of adaptive immunity: humoral immunity, cell-mediated immunity, and T-regulatory cells. The immune system organs are positioned strategically throughout the body. They are referred to as lymphoid organs because they house macrophages and lymphocytes, the two key mediators of the adaptive immune system. Macrophages engulf and digest germs and dead cells, leaving behind antigens for the body to identify as dangerous, triggering the stimulation of antibodies. There are two main types of lymphocytes: B-Lymphocytes (B-cells) and T-lymphocytes (T-cells). B-cells mediate the production of antibodies that attack antigens left behind by the macrophages. B-cells bind directly with unique proteins on the invading antigen's surface and then hand the baton to the T-cells, who have the job of attacking the target cells. T-cells attack cells in the body that have already been infected. They lyse the infected cells, provide immunity against most pathogens, and aid in antibody production. Humoral immunity is mediated by B-cells and results in the production of immunoglobulins (Ig), otherwise known as antibodies. T-cells and their cytokine products facilitate cell-mediated immunity, which does not involve antibodies. Instead, cell-mediated immunity includes cytotoxic T-cells (usually CD8) and helper T-cells (usually CD4). T-regulatory cells, also known as suppressor T-cells, display the markers CD4 and CD25 and limit other immune effector cells’ activity. Ultimately, their primary role is to prevent damage to normal tissues and lessen the inflammatory response. Vaccination (to be discussed later) is an example of acquired immunity (Longo, 2019; McCance & Heuther, 2019).
Pathogenesis of COVID-19
Structurally, SARS-CoV-2 is comprised of four proteins: the spike (S), membrane (M), envelop (E), and nucleocapsid (N). As demonstrated in Figure 1, the S protein protrudes furthest from the viral surface and is considered one of the central points for host attachment and penetration. The S protein contains two functional subunits (i.e., S1 and S2). S1 is responsible for binding to the host cell receptor, and S2 facilitates the fusion between the viral and host cellular membranes. Based on scientific understanding, the virus’s high infectivity is related to mutations in its receptor binding and the acquisition of a furin cleavage (division or separation) site in the S protein. Furin is a protease (otherwise known as an enzyme) that breaks down proteins and peptides. It is widely expressed in various organs and tissues throughout the human body. Numerous studies have demonstrated that SARS-CoV-2 uses two chief host proteins to gain entry and activate the viral replication process: the angiotensin-converting enzyme 2 (ACE2) and the cell surface transmembrane protease serine 2 (TMPRSS2). TMPRSS2 activates the S protein and cleaves the ACE2 receptor to enable the attachment and entry of SARS-CoV-2 into the cell. The ACE2 receptors are the predominant binding receptors for the virus and are highly expressed throughout the upper and lower respiratory tracts, particularly the alveolar cells (e.g., bronchus, alveoli, mucosa). The respiratory tract is the main target of COVID-19, with post-mortem evidence of severe pulmonary histopathologic changes and diffuse lung damage. However, extra-pulmonary effects have also been well-established, as the virus can penetrate and infect the hepatic, renal, gastrointestinal, cardiovascular, and nervous systems (Azer, 2020; McCance & Heuther, 2019; Parasher, 2020; WHO, 2021a).
Disease Progression
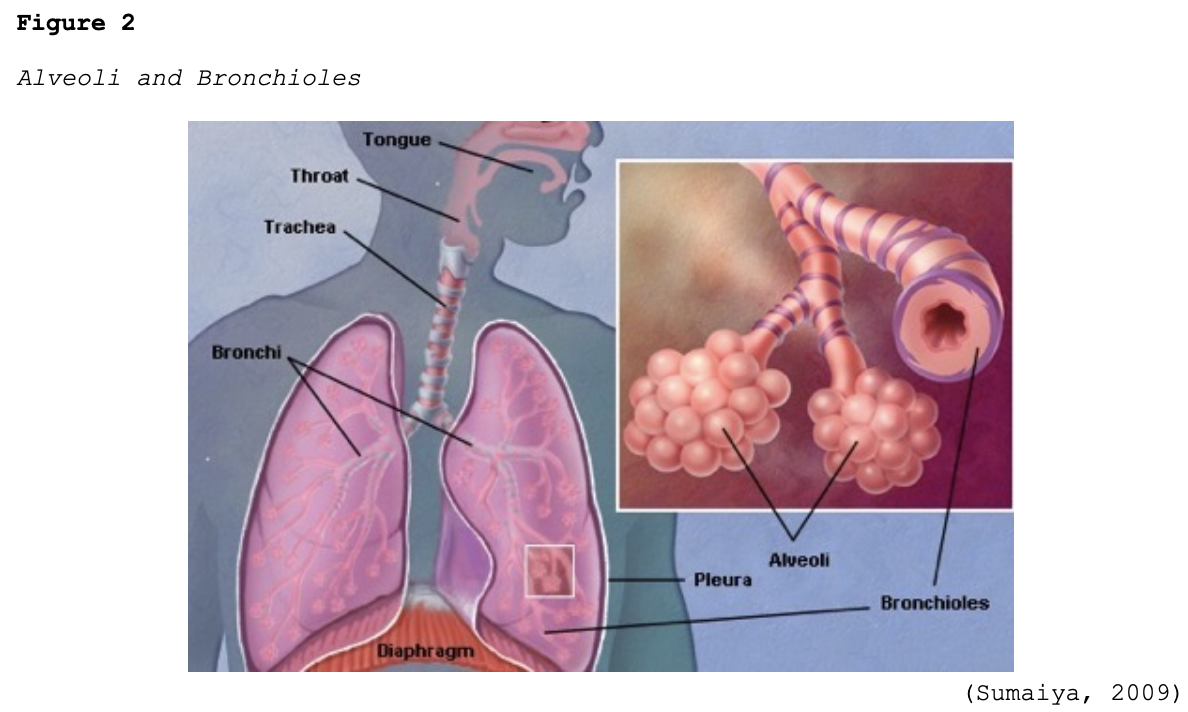
Early-stage COVID-19 infection starts with the bronchial epithelial cells (type I and II pneumocyte [alveoli] cells) and the capillary epithelial cells. As shown in Figure 2, each sac of air (or alveolus) is the site of gas exchange. As depicted in Figure 3, the alveoli are wrapped with capillaries, the sites where red blood cells release carbon dioxide (CO2) and acquire oxygen (O2). Type I cells are thinner and allow for the direct passage of O2, whereas type II cells secrete surface surfactant, a substance that lines the alveolus and prevents the air sacs from collapsing (Azer, 2020; McCance & Heuther, 2019; Parasher, 2020).
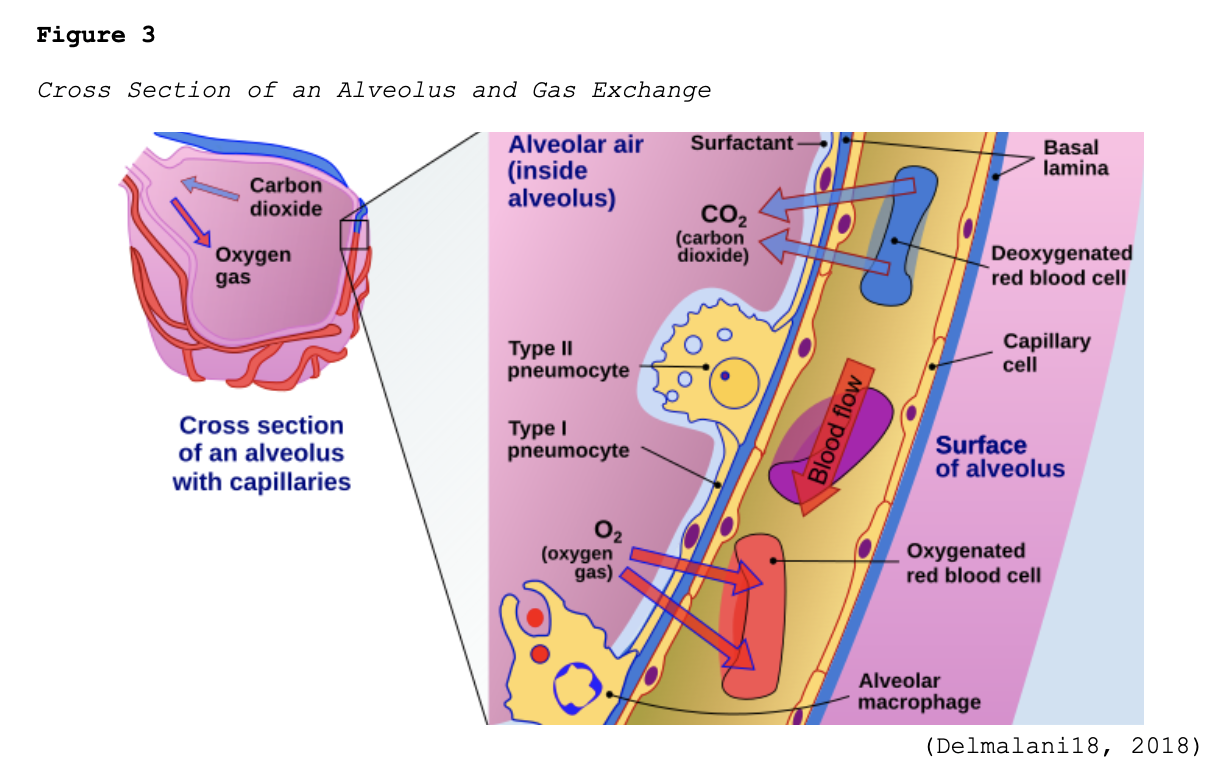
The S proteins primarily bind to the ACE2 receptors on the type II alveolar cells. Once the virus enters the host via this receptor, it releases its ribonucleic acid (RNA, or viral material) into the cell, igniting the replication process. After they are infected, the type II cells respond by releasing inflammatory signals, which direct the recruitment of WBCs, particularly macrophages, and ignite the immune response. Since one infected host cell can generate hundreds of new virions, this process rapidly disseminates, infecting more cells, and inducing widespread infection. Viral replication leads to the upregulation of cytokines (inflammatory mediators) as part of the host’s immune response. Interleukin (IL)-6 is a pro-inflammatory cytokine produced by various cell types, including lymphocytes and monocytes. Infection with SARS-CoV-2 triggers the bronchial epithelial cells to release IL-6, inducing inflammation, pyrexia (fever), vasodilation, and increased vascular permeability, recruiting more immune cells to the site of injury. Fluid accumulates inside the alveolus, diluting the surface surfactant and triggering its collapse. Upon arrival at the infection site, recruited neutrophils release reactive oxygen species (ROS) to destroy the infected host cells, but not before the virions quickly replicate and disseminate to penetrate neighboring cells. Over time, the virus can infiltrate ACE2-bearing cells throughout extra-pulmonary organs, such as the blood vessels and kidneys (Azer, 2020; Johnson et al., 2020, 2021; Nikhra, 2020; Parasher, 2020; Qiao et al., 2021; Yoshikawa et al., 2021; Xing et al., 2020).
As type I and II cells are destroyed, more alveoli collapse, and gas exchange is impaired. Less O2 enters the bloodstream, and more fluid enters the alveolus, leading to hypoxemia and pulmonary edema. In severe cases, the hyperactivated immune response (commonly referred to as cytokine storm) starts to damage non-infected cells and tissues. If enough type I and II cells are destroyed, systemic inflammatory response syndrome (SIRS) ensues, resulting in widespread inflammation and damage to various organ systems. COVID-19-associated SIRS can be associated with heightened cytokine release, as evidenced by elevated blood levels of IL-6, C-reactive protein (CRP), D-dimer, and ferritin. Late-stage COVID-19 is characterized by alveolar interstitial thickening, increased vascular permeability, and vascular endothelial injury; this can prompt the activation of the coagulation cascade. Systemic infection or inflammation can impair healthy coagulation mechanisms, leading to microthrombus and venous thromboembolism (VTE; e.g., deep vein thrombosis [DVT] and pulmonary embolism [PE]) formation (Azer, 2020; Longo, 2019; Nikhra, 2020; Parasher, 2020; Yoshikawa et al., 2021).
A thrombus can develop anywhere within the cardiovascular system, with the majority classified as venous (VTE; within a vein) or arterial thrombosis (AT; within an artery). DVT is the most common type of VTE, typically arising in the large veins of a lower extremity. PE is the most severe complication of a DVT, in which the thrombus detaches from the vessel wall and circulates within the bloodstream. This circulating thrombus causes an abrupt blockage of a pulmonary vessel. PE can obstruct blood flow and induce sudden death in some patients. It can also cause hypoxia (low O2 levels in the tissues), rendering permanent damage to the lungs or other organs due to insufficient O2 supply (Longo, 2019; McCance & Heuther, 2019). A recently published meta-analysis including 27 studies and 3,342 patients demonstrated a 16.5% incidence of PE in COVID-19 patients, more commonly found in those admitted to intensive care unit (ICU) settings. While the analysis also revealed a 14.8% incidence of DVT, more than 50% of those with PE lacked any evidence of DVT (Suh et al., 2020). While VTE in COVID-19 patients is well described in the literature, data on AT remain limited. ATs almost always arise from the heart (i.e., aorta or coronary arteries) but may also occur in the brain's cerebral arteries. An AT or arterial emboli can cause tissues to become starved of blood and O2, leading to necrosis and infarction. In a systematic review, Cheruiyot and colleagues (2021) determined that ATs occur in approximately 4% of critically ill COVID-19 patients, most commonly presenting symptomatically and affecting multiple arteries.
For more information on the mechanisms of thrombosis and their complications, refer to the following NursingCE courses:
- Venous Thromboembolism
- Blood Clotting and Bleeding Disorders
- Stroke Prevention, Thrombolytic Therapy, and Rehab
Transmission
COVID-19 is a highly transmissible virus, infecting people across the lifespan, with the number of new cases rising across the world with each passing day. The latest epidemiology of COVID-19 indicates that most infections are spread through close contact via respiratory droplets, in which the virus gains entry through the nose, mouth, or eyes. According to the WHO (2020a) and the Centers for Disease Control and Prevention (CDC, 2020e), the transmission of COVID-19 is a combination of three major factors: how, when, and where, which are summarized in Table 1.

Respiratory droplets are produced through breathing and exhalation (e.g., sneezing, speaking, coughing). The droplet size is associated with the duration of time it remains suspended in the air (i.e., larger droplets fall out of the air rapidly and stay close to the source, whereas smaller droplets can remain suspended for up to several hours and travel further). Airborne transmission occurs when smaller droplets and particles remain suspended in the air over long distances (i.e., greater than six feet) and time (i.e., typically hours). The evidence available as of February 2021 indicates that while the mode of COVID-19 transmission is primarily via respiratory droplets, airborne transmission can occur when specific conditions are met. According to the CDC (2020c), documented instances of COVID-19 airborne transmission have occurred in circumstances that facilitate the build-up of suspended small respiratory droplets, such as:
- the presence of an infectious person producing respiratory droplets for an extended time (i.e., 30 minutes to multiple hours) in an enclosed space or area with inadequate ventilation
- enough viral particles were present to cause infections in people who were more than six feet away
- prolonged exposure to respiratory particles caused by expiratory exertion (i.e., by yelling, singing, exercising) in the absence of a mask (CDC, 2020d)
As of February 2021, there is insufficient evidence to support the spread of COVID-19 to people who enter a space hours after an infectious person was there (Alsved et al., 2020; CDC, 2020e; Morawska & Milton, 2020).
Risk Factors
There are two major groups at heightened risk of developing a more severe clinical course and poorer prognosis: older adults (older than 60 years, with mortality increasing incrementally alongside advancing age) and individuals with underlying comorbidities. According to the CDC (2020c), adults (at any age) with the following comorbid conditions are at increased risk of severe illness from COVID-19:
- type 2 diabetes mellitus (T2DM)
- heart conditions, such as hypertension, heart failure, coronary artery disease, or cardiomyopathies
- cancer and other immunocompromised states (i.e., weakened immune system) from solid organ transplant or immunosuppressive therapy (such as long-term steroid use)
- chronic liver disease
- chronic kidney disease
- chronic obstructive pulmonary disease (COPD)
- down syndrome
- obesity (body mass index [BMI] of ≥30 kg/m2)
- sickle cell disease
- tobacco use
- pregnancy (CDC, 2020d)
Risk factors associated with more severe COVID-19 infections during pregnancy include advanced maternal age, high BMI, chronic hypertension, and pre-existing diabetes. While children and young adults have also been affected, they appear to be at a lower risk of a severe clinical course and mortality. Children with underlying medical conditions are at increased risk for severe illness when compared to their healthy counterparts, but information on which specific medical conditions remains limited at this time (Azer, 2020; CDC, 2020d; Parasher, 2020; Tong et al., 2020; WHO, 2021a).
Risk Reduction
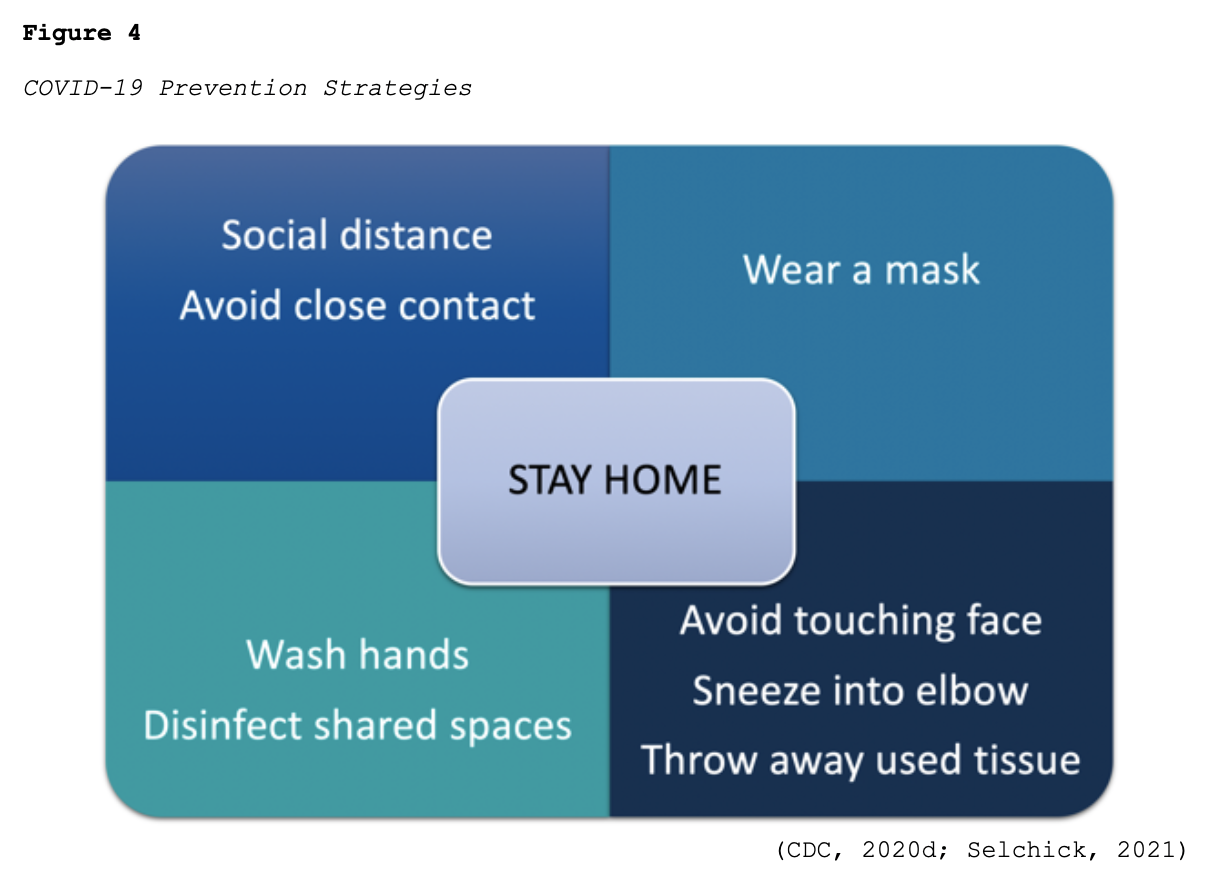
While much of the information surrounding COVID-19 has changed as the COVID-19 pandemic has evolved, best practices regarding protecting oneself and reducing the virus’s spread have remained relatively constant. According to the CDC (2020c), the three most important ways to slow the spread of COVID-19 is for all persons (over the age of 2 years) to wear a mask, stay at least six feet from people not in the same household, and avoid crowds. Figure 4 provides a brief overview of best practices regarding preventing the spread of COVID-19.
Clinical Features
The incubation period (the time between being exposed to the virus [becoming infected] and symptom emergence) has a median time of 4 to 5 days; however, it can extend up to 14 days based on the latest evidence. While the signs and symptoms of COVID-19 can vary widely, the most common presenting symptoms include the following:
- fever (83 to 99% of patients)
- dry cough (59 to 82% of patients)
- fatigue (44 to 70% of patients)
- anorexia (40 to 84% of patients)
- shortness of breath (SOB; 31 to 40% of patients)
- myalgias (11 to 35% of patients; CDC, 2020c; WHO 2020b, 2021a)
Other less common and nonspecific symptoms include sore throat, nasal congestion, gastrointestinal (GI) symptoms (e.g., nausea, vomiting, or diarrhea), weakness, and headache. Loss of taste (ageusia) or smell (anosmia) preceding the onset of respiratory symptoms has also been reported (Wiersinga et al., 2020; WHO, 2020b, 2021a). In a systematic review and meta-analysis by Tong and colleagues (2020), analyzing ten studies, there was a 43.93% prevalence of ageusia and a 52.73% prevalence of anosmia in COVID-19 patients, most of which presented early in the clinical course of infection. Research has also demonstrated that the presenting symptoms of COVID-19 vary in special populations. Among those who are older (>75 years) or immunocompromised, atypical symptoms such as fatigue, reduce mobility, anorexia, reduced alertness, delirium, and agitation, in the absence of a fever, are more common (CDC, 2020c; WHO, 2020b, 2021a).
Although limited information is available on the impact of the COVID-19 on pregnant women and newborns, pregnant women are a high-risk group for severe COVID-19 infections. Pregnant women require meticulous assessment due to the physiologic adaptations of pregnancy, which can overlap with symptoms of COVID-19, such as fatigue, GI changes, and dyspnea (WHO, 2020b). In a systematic review and meta-analysis involving 77 studies, Allotey and colleagues (2020) found a 1 in 10 (10%) prevalence rate of COVID-19 infections in pregnant or recently pregnant women attending or admitted to a hospital for all causes. Their analysis revealed the most common clinical manifestations of COVID-19 in pregnancy included fever (40%) and cough (39%). However, compared with non-pregnant counterparts, pregnant and recently pregnant women were less likely to manifest fever and myalgias and were more likely to require admission to an ICU and invasive ventilation. Lymphopenia (35%) and elevated C-reactive protein levels (49%) were the most common abnormal laboratory findings in infected pregnant women. In a sample size of 11,580 women across 26 studies, 73 pregnant women with confirmed COVID-19 infections died from any cause. The authors also reported severe COVID-19 infections in 13% of pregnant and recently pregnant women, ranging from 6% to 21% across 21 studies, including 2271 women. Four percent of pregnant women with COVID-19 were admitted to an ICU, and 3% required invasive ventilation. Spontaneous preterm birth rates were higher in women with COVID-19, but the rates were still low and resulted in negligible risks. The study demonstrated that stillbirth and neonatal death rates were low in women with suspected or confirmed COVID-19, and there were no differences observed in other maternal outcomes (Allotey et al., 2020). Research continues to evolve in this population regarding the impact on neonates’ development, as long-term evidence regarding neonate and maternal outcomes is unavailable (WHO, 2020b, 2021a).
Screening and Testing for COVID-19
There are two kinds of diagnostic tests available for COVID-19: viral tests and antibody tests. Viral tests evaluate for the presence of current infection, and antibody tests are used to identify a prior infection (CDC, 2021b, 2021d).
Viral Test
There are two categories of viral tests available: molecular real-time reverse transcriptase-polymerase chain reaction (RT-PCR) tests and antigen tests. RT-PCR tests (also called nucleic acid amplification tests [NAATs]) detect the presence of the virus’s genetic material and are more accurate but typically require longer processing times. Antigen tests detect specific proteins on the virus’s surface; although they characteristically provide results faster than molecular tests, they carry a higher false-negative rate (i.e., a higher chance of missing an active infection). Correspondingly, positive antigen test results are usually accurate, but negative results may require confirmation with an RT-PCR test. The CDC was the first in the US to develop a diagnostic assay test for COVID-19 (CDC, 2020b). An emergency use authorization (EUA) was initially granted on February 4, 2020, and virus samples became available to commercial developers for validation at the end of February 2020. An EUA is issued by the US Food & Drug Administration (FDA) to allow access to critical medications and medical products that may help during a public health emergency. An EUA is different from standard medical testing, medication, or vaccination approval and must meet the following criteria (FDA, 2020a, 2021a):
- The product will be used for a serious or life-threatening disease or condition.
- Based on the totality of scientific evidence available, it is reasonable to believe the product may be effective.
- The known and potential benefits of the product outweigh the known and potential risks of the product.
- There are no adequate FDA-approved alternatives available.
Since March 10, 2020, when the CDC distributed the original RT-PCR test kits to state and local health departments across the US, there has been tremendous growth and development of COVID-19 testing kits. The FDA (2020a, 2021a) has continued to work with commercial labs and test developers to streamline the testing process and make more tests available. To date, innumerable test kits have been developed and authorized under the FDA’s EUA regulations. Most of the initial tests required collecting samples with nasopharyngeal (NP) swabs, as shown in Figure 5.
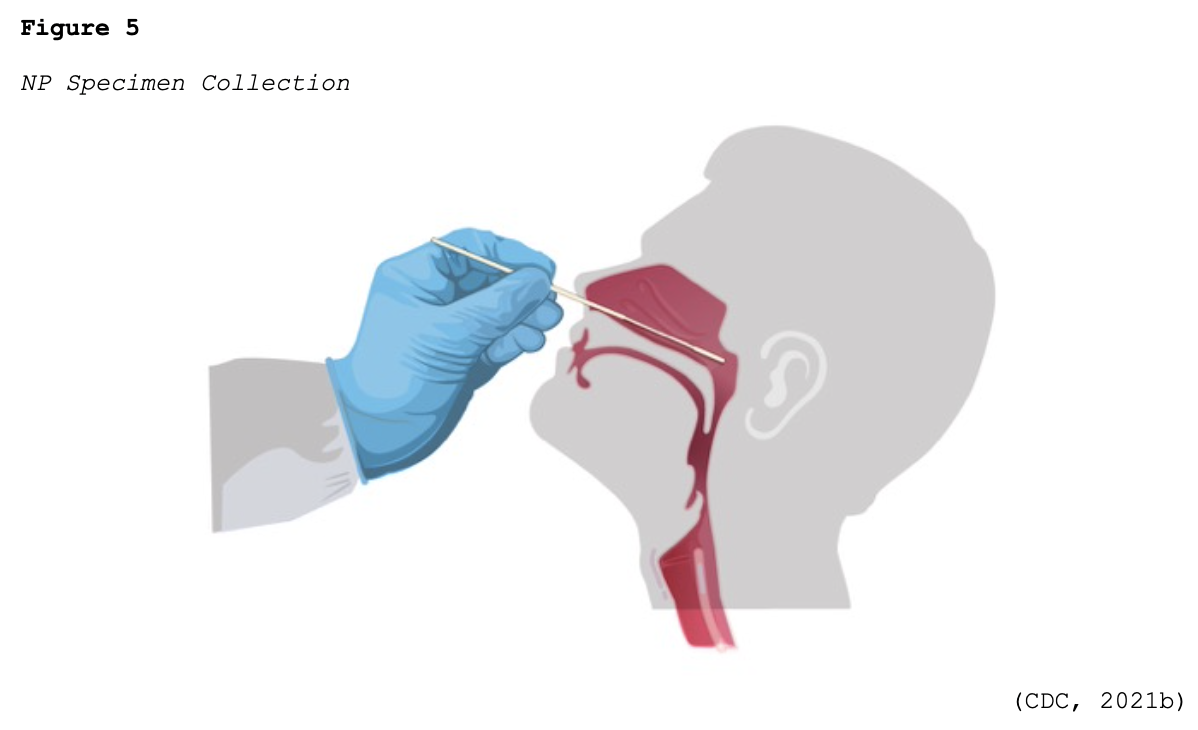
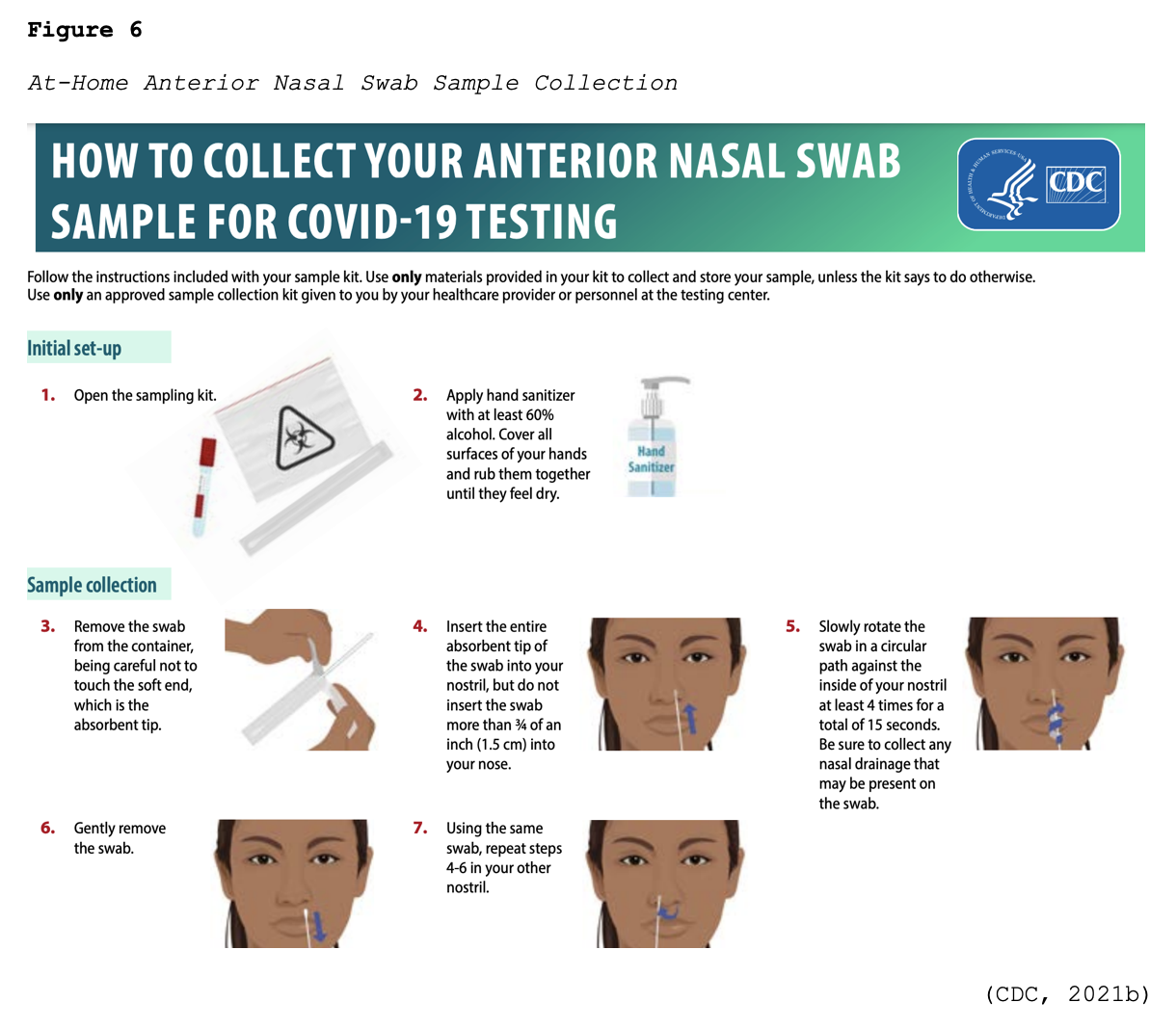
Many viral tests are still performed using NP swabs, but manufacturers have extended the sampling pool to allow for a sample collection from the anterior nostril, nasal mid-turbinate, throat, or buccal mucosa. Rapid, point-of-care (POC) diagnostic tests are available as molecular and antigen tests. They use a mucus sample from the nose or throat, and results may be available in minutes. At-home testing kits allow for samples to be collected as an anterior nasal swab in which the individual is advised to insert the absorbent tip of the swab no further than ¾ of an inch (1.5 cm) into the nose. As demonstrated in Figure 6, the swab is then rotated in a circular path against the inside at least four times for 15 seconds. The procedure is then repeated in the other nostril using the same swab (CDC, 2021b, 2021d).
Test selection differs based on institution, geographic location, and availability. Aside from the at-home test kits, most healthcare workers and patients cannot choose which test they receive. The consensus as of February 2021 is that a definitive diagnosis of an active COVID-19 infection requires confirmation with an RT-PCR test. An updated list of available COVID-19 tests approved by the FDA for EUA is available for review on the FDA website: https://www.fda.gov (FDA, 2021a).
Viral Testing Recommendations. The recommendations for testing individuals for current infection with COVID-19 have evolved. There are various recommendations based on each individual’s level of risk, exposure, employment setting, test availability, and state and local guidelines. According to the CDC recommendations, which were last updated on January 21, 2021, the following individuals should have a viral test:
- “people who have symptoms of COVID-19
- people who have had close contact (within 6 feet of an infected person for a cumulative total of 15 minutes or more over a 24-hour period) with someone with confirmed COVID-19.
- people who have taken part in activities that put them at higher risk for COVID-19 because they cannot socially distance as needed, such as travel, attending large social or mass gatherings, or being in crowded indoor settings
- people who have been asked or referred to get testing by their healthcare provider, local or state health department
- if you do get tested or take an at-home test because you have COVID-19 symptoms or have had a close contact with someone who has it, you should self-quarantine at home pending test results and follow the advice of your healthcare provider or a public health professional” (CDC, 2021d, para. 1)
The CDC (2021d) and many individual states also offer online self-checker tools to help individuals decide when to seek testing and appropriate medical care.
Antibody Testing
Antibody tests do not diagnose an active infection; they instead detect the antibodies created by the immune system in response to a prior COVID-19 infection. Antibody production following a COVID-19 infection varies, and it can take several weeks to months to generate enough antibodies to be detected in a test. As of February 2021, it is indeterminate how long antibodies stay in the body following a COVID-19 infection; however, researchers are exploring this phenomenon. Dan and colleagues (2021) analyzed antibody production in 188 patients with confirmed COVID-19 infections and found durable immune responses in the majority, with immunoglobulin G (IgG) antibodies to the S protein relatively stable for up to 8 months. The memory B-cells were also more abundant at six months than 1-month post symptom onset, endorsing the concept that immunity takes time to develop. At 8 months, 95% of individuals were still positive for at least three out of five SARS-CoV-2-specific immune memory responses (Dan et al., 2021). Choe and colleagues (2021) found similar findings at 8 months after asymptomatic or mild COVID-19 infection in a relatively younger (< 65 years) population. While this study was small (n= 58 individuals), the seropositive rates were high, ranging from 69% to 91.4% at 8 months post-infection (Choe et al., 2021). In a more extensive study including 12,219 healthcare workers, a prior COVID-19 infection that generated antibody responses offered protection from reinfection for most people in the 6 months following infection (Lumley et al., 2020). More research is needed to definitively determine the long-term durability of natural immunity across populations.
Infection Protection and Control (IPC)
IPC is a critical and fundamental aspect of the clinical management of suspected or confirmed positive patients. APRNs should immediately implement appropriate infection control measures, including universal masking of all persons in healthcare facilities (e.g., wearing a mask at all times) and appropriate use of personal protective equipment (PPE). To uphold IPC measures, APRNs should follow the following guidelines, as outlined by the WHO (2021a):
- screening and triage for early recognition of suspected COVID-19 patients and rapid implementation of control measures
- screening all individuals at the first point of contact in the healthcare facility
- separate suspected or confirmed COVID-19 patients to a well-ventilated, isolated area away from other contacts, and maintain at least 1 m distance between patients
- apply standard precautions for all patients at all times, which include (but are not limited to),
- hand and respiratory hygiene
- appropriate use of PPE
- universal masking is required for all persons
- proper environmental cleaning and safe waste management
- apply contact and droplet precautions for suspected or confirmed COVID-19 patients
- wear gloves, a clean, long-sleeved gown, medical mask, and eye protection (i.e., goggles or face shield) and remove PPE when leaving the patient care area (WHO, 2021)
For more information on PPE and standard procedures for donning and removing PPE, refer to the Personal Protective Equipment NursingCE course.
Isolation
Isolation is intended for persons who are sick with COVID-19 and are advised to separate from others to prevent spreading the virus. Those who test positive for COVID-19 should remain at home, in a separate space from household contacts (if possible), and use a separate bathroom (if available). Isolation practices should continue until all of the following are met:
- at least 10 days since symptoms first appeared, and
- at least 24 hours without a fever (without the use of fever-reducing medications), and
- once other symptoms of COVID-19 are improving (ageusia and anosmia can persist for weeks or months after recovery and do not require an extension of the isolation period; CDC, 2021c).
Individuals who test positive for COVID-19 but are asymptomatic should isolate for 10 days following the positive test. Following recovery from a COVID-19 infection, some people may continue to test positive for 3 months or longer without evidence of transmission to others. At this time, the CDC does not recommend the routine re-testing of individuals recovering from an acute infection. People should only be tested again if they develop new symptoms or have come in close contact with another person who tested positive for COVID-19 in the last 14 days (CDC, 2021c).
Quarantine
Quarantine is intended to reduce the risk that persons exposed to COVID-19 by a close contact may unknowingly transmit the infection to others by separating them. The CDC (2020f) defines close contact as any of the following:
- persons within 6 feet of someone who has COVID-19 for a total of 15 minutes or more,
- persons who provided care at home to someone who is sick with COVID-19
- persons with direct physical contact with someone who has COVID-19 (i.e., hugged, kissed, or shared eating/drinking utensils)
- persons exposed to the respiratory droplets of someone who has COVID-19 (i.e., sneezing, coughing; CDC, 2020f).
While the CDC recommends a quarantine period of 14 days for close contacts of infected individuals, local public health authorities typically determine and establish the quarantine options for their jurisdictions. Healthcare workers are often subject to another layer of quarantine guidelines as per their employing institution policy. There have been several discussions regarding safe options of maintaining IPC and reducing transmission of COVID-19 but shortening the quarantine period. In early December 2020, the CDC released a statement that quarantine could end after day 10 if no symptoms have been reported during daily monitoring but carries an approximate 1 to 10% risk of residual post-quarantine transmission (CDC, 2020f).
Disease Severity
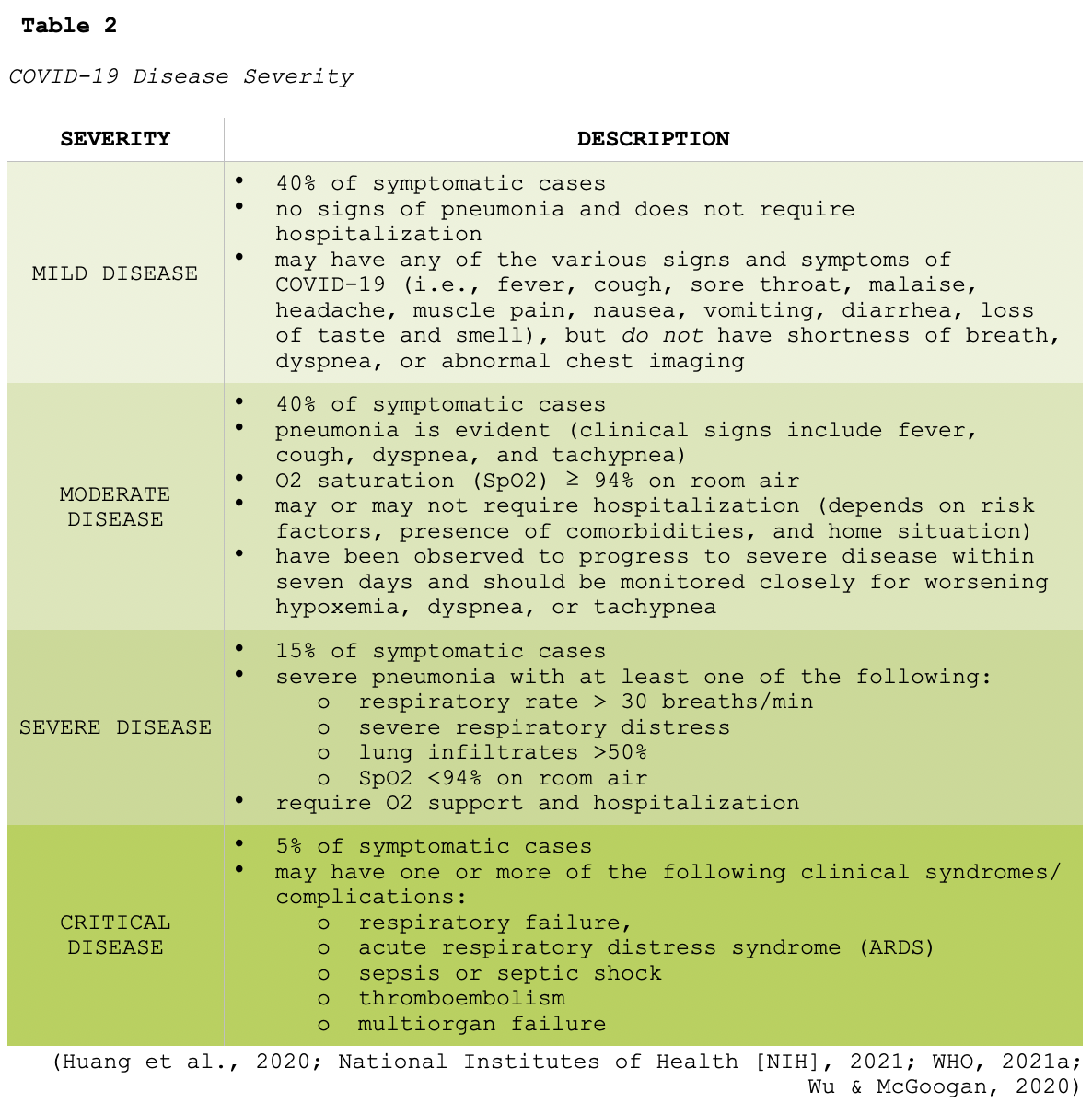
In addition to underlying comorbidities and the risk factors outlined earlier, the severity of infection appears to correlate with both the dose and route of exposure. The severity of COVID-19 refers to symptomatic individuals only and has been subdivided into four major categories, as described in Table 2. The largest cohort described to date included more than 44,000 people with COVID-19 in China. Findings demonstrated that illness severity ranges from mild to critical, with the estimated incidence outlined in Table 2 (Wu & McGoogan, 2020).
Research has demonstrated an overall higher incidence of severe and critical disease in men than women. Wu and colleagues (2020) examined 201 patients with COVID-19 admitted to a hospital in China within a 4-week period. Greater than 60% (128) were men, 41.8% (84) developed ARDS. Among the patients with ARDS, 52.2% (44) died. Their findings reinforced that older age was associated with a greater risk of ARDS development and subsequent death. High fever was associated with a higher likelihood of ARDS but a lower likelihood of death. Additional research examining the role of advanced age, ARDS, and mortality has credited the grim outcomes to a less vigorous immune response in older adults (Wu et al., 2020). Murk and colleagues (2021) analyzed the ICD-10-CM diagnosis codes of 288 patients with confirmed COVID-19 infection. The median age was 65 years, 53.4% were admitted to the hospital, and 4.7% were admitted to an ICU. They found that the highest absolute risk among all patients included pneumonia (27.6%), respiratory failure (22.6%), acute renal failure (11.8%), and sepsis (10.4%). Among those admitted to the ICU, the absolute risk levels were significantly higher and included respiratory failure (75.3%), ARDS (26%), ARF (50.7%), sepsis (54.1%), and encephalopathy (24.9%; Murk et al., 2021). Wiersinga and colleagues (2020) similarly demonstrated up to a 25% risk of venous and arterial thromboembolic complications in hospitalized patients. They also reported cardiac injuries (elevated troponin, heart failure, myocarditis, dysrhythmias) occurring in up to 17% of patients.
Common Laboratory and Radiographic Findings
Many of the defining features of COVID-19 can be based on clinical grounds; however, several laboratory and radiologic findings common in patients with COVID-19 infections may be useful for evaluating disease severity and guiding treatment (NIH, 2021).
Labs and Biomarkers
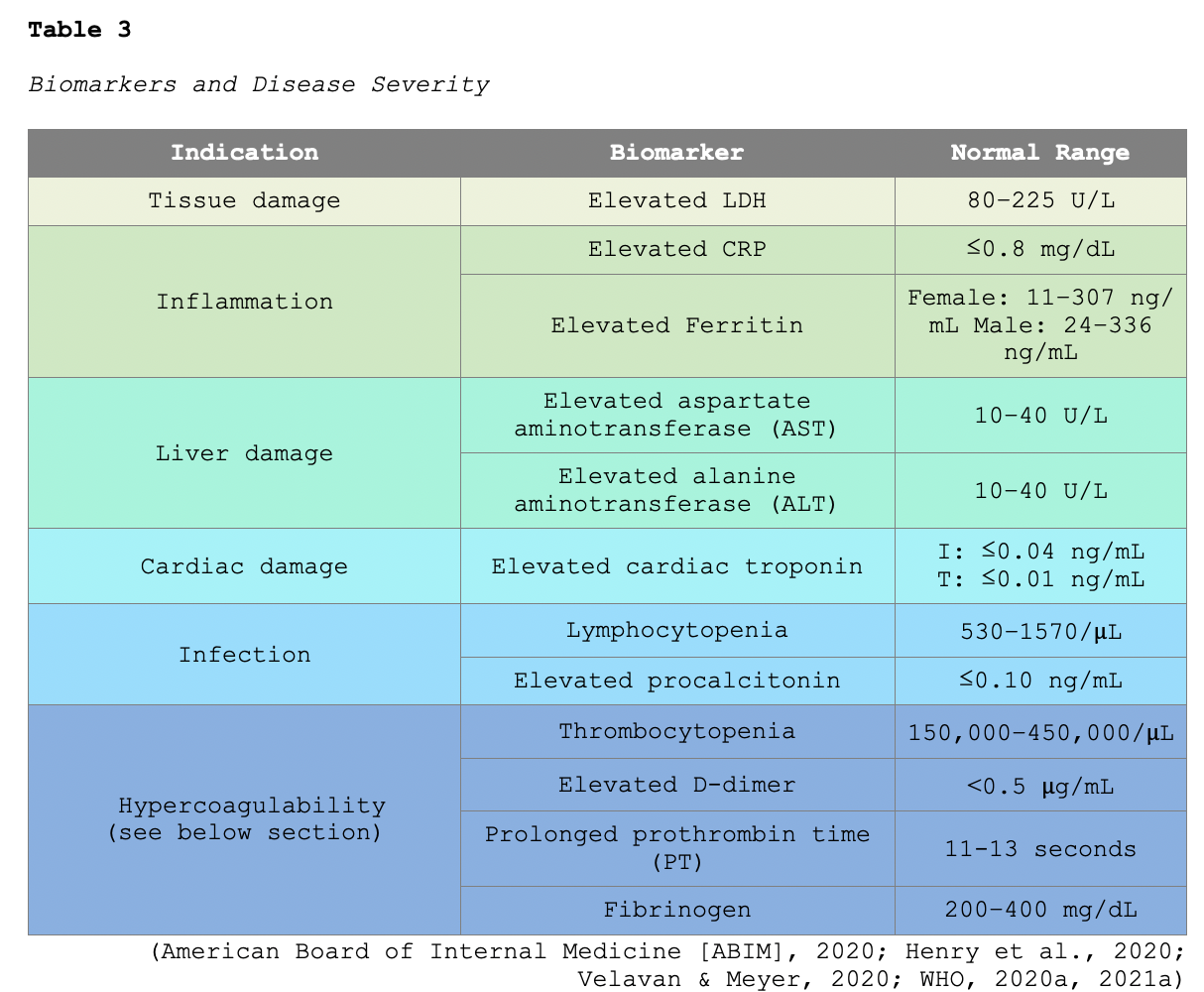
Lymphopenia is one of the most common laboratory findings, present in up to 83% of hospitalized patients with COVID-19. Multiple systematic reviews have reported an association between several biomarkers and disease severity. In particular, elevated liver function enzymes (LFTs), lactate dehydrogenase (LDH), CRP, ferritin levels, and procalcitonin are associated with greater illness severity. Table 3 provides an overview of well-cited markers correlating with disease severity (CDC, 2020c; Guan et al., 2020; Velavan & Meyer, 2020).
Hypercoagulability
As described earlier, coagulopathy is a well-documented clinical sequela of COVID-19 infections. A study of 184 ICU patients with COVID-19 revealed a 31% incidence of thrombotic complications (Klok et al., 2020). Some patients may demonstrate signs of a hypercoagulable state, thereby predisposing them to potentially life-threatening thrombotic events. According to the NIH (2021) and the American Society of Hematology (ASH, 2021), the most frequent signs of coagulopathy identified in hospitalized patients with COVID-19 include the following: elevated fibrinogen and D-dimer levels, mild prolongation of PT, mild thrombocytopenia (platelet count ~100,000μL [normal platelet range 150,000 to 450,000/μL]), and a parallel rise in markers of inflammation including CRP and ferritin. Several studies have also demonstrated that elevated D-dimer levels are strongly associated with increased mortality (ABIM, 2020; CDC, 2020c; Tang et al., 2020). D-dimer is a protein fragment produced when the body forms or breaks down fibrin (blood clots). Under physiologic conditions, the D-dimer level is typically not detectable or present at only very low levels. D-dimer levels increase when there are a significant formation and breakdown of fibrin clots within the body. Therefore, the D-dimer test measures the amount of the substance released into the bloodstream when fibrin proteins in a blood clot dissolve. The D-dimer test is commonly used as a screening test for VTE, with a negative (low or undetectable) result indicating a low likelihood that a thrombus is present. However, the test is nonspecific, and a positive D-dimer result (elevated level) cannot definitively predict whether or not a blood clot is present in the body. Further, the D-dimer test historically has a high false-positive rate, particularly in postoperative and pregnant patients (American Association for Clinical Chemistry, 2020; Lim et al., 2018).
Fibrinogen is a soluble plasma protein that is an essential component of the coagulation cascade that helps control bleeding by assisting with fibrin formation. The coagulation cascade's ultimate goal is to convert fibrinogen into fibrin, a non-soluble plasma protein (blood clot). When an injury is detected, fibrinogen, along with the platelets, creates a weak platelet plug that temporarily protects against further bleeding. PT assesses how well the coagulation factors in the coagulation cascade are functioning collectively (Gross et al., 2018). In a multicenter prospective cohort study including 150 COVID-19 patients, 64 patients (> 40%) endured thrombotic complications, and most of them (> 95%) had elevated D-dimer and fibrinogen levels. Further, despite the use of anticoagulation, a high percentage of patients with ARDS developed life-threatening thrombotic complications (Helms et al., 2020). While DVT and PE are the most common thrombotic complications in COVID-19 infections, other manifestations associated with hypercoagulability have been reported, such as clotting of implanted catheter devices, microvascular thrombosis of the toes (i.e., “COVID toes”), and cerebral vascular ischemia (CDC, 2020c; Cheruiyot et al., 2021).
Radiographic Findings
Chest imaging (i.e., chest x-ray [CXR] and computed tomography [CT]) may be useful in obtaining information regarding disease severity but have variable findings. While some patients have unremarkable chest imaging early in the illness, abnormalities on chest imaging most commonly present bilaterally. Classic features of COVID-19 on CXR include bilateral airspace consolidation, peripheral bilateral ground-glass opacities, interstitial thickening of the lung(s), or lung consolidation zones. Abnormal CT findings are not specific for COVID-19, and studies have found conflicting results. Some studies demonstrate normal CT scan results early in the course of illness, whereas others may pick up residual findings from a prior COVID-19 infection. Given the variability in chest imaging findings, the American College of Radiology (ACR, 2020) recommends that CT imaging is reserved for symptomatic, hospitalized patients with specific pulmonary indications. They advise against the use of chest imaging for screening or as a first-line test for the diagnosis of COVID-19 infection; a normal chest CT does not exclude a COVID-19 infection (ACR, 2020).
Management
Data surrounding the treatment of COVID-19 continues to evolve and is premised on the severity of the disease and individual patient factors. Various treatment algorithms have been devised to help clinicians navigate the most effective treatment options for patients in each category. There is variation between guidelines based on the evolving evidence and the availability of resources across geographic regions and institutions. The American Society of Health System Pharmacists (ASHP, 2021) has been instrumental in accumulating evidence blocks as the data on pharmacological therapies evolve. The ASHP maintains an online resource regarding progressing recommendations, which are updated regularly. In addition, the NIH (2021) compiled the COVID-19 Treatment Guidelines, an electronic resource for clinicians to ensure timely access to the most updated clinical evidence regarding treatment strategies. The NIH guidelines are updated frequently by a panel of experts appointed based on their clinical experiences and prior expertise in developing treatment-based guidelines. All guideline recommendations are based on scientific evidence and expert opinion and are endorsed by most panel members (NIH, 2021).
The NIH (2021) guidelines support the therapeutic management of patients with COVID-19 based on their proposed understanding that two primary processes drive the pathogenesis of the disease: (1) viral replication early in the course of illness, followed by (2) an exaggerated immune/inflammatory response to the virus that leads to tissue damage. Based on these concepts, the guidelines endorse the use of antiviral therapies early in the course of the disease and immunosuppressive and/or anti-inflammatory agents in later stages. The NIH recommendations are the highest level of evidence available regarding the treatment of COVID-19 patients to date and will be explored in this section. The primary treatment categories for COVID-19 infections include monoclonal antibodies, convalescent plasma, antiviral therapies, and corticosteroids. Except for remdesivir (Veklury), all of the pharmacologic agents discussed in this module are considered investigational and not approved by the FDA to treat COVID-19; however, several agents have received an EUA from the FDA. It is well-established that antibiotics are not effective against viral infections yet continue to be prescribed for patients with COVID-19. There is a paucity of data supporting the association with respiratory bacterial coinfection requiring antibiotics (NIH, 2021). In a recent study including 806 COVID-19 patients, Rawson and colleagues (2020) found that only 8% of patients experienced a bacterial/fungal co-infection during hospital admission. Their findings reinforce the need to minimize concomitant broad-spectrum antimicrobial therapy. Evidence-based guidelines regarding appropriate antibiotic stewardship interventions specific for COVID-19 infections are lacking but urgently needed (Rawson et al., 2020).
Treatment Overview Based on Disease Severity
Management of asymptomatic infection is based on monitoring since it is indeterminate what percentage of individuals will progress to clinical disease. Some asymptomatic individuals have been reported to demonstrate objective radiographic findings consistent with COVID-19 pneumonia; however, most do not. Patients with mild illness may exhibit various signs and symptoms as outlined earlier but are stable for outpatient (ambulatory) or telemedicine follow-up. Treatment is primarily centered on alleviation of symptoms using supportive therapies. No specific imaging or laboratory evaluations are routinely indicated in otherwise healthy patients with mild COVID-19 treated on an ambulatory basis. However, older patients and those with underlying comorbidities are at higher risk for disease progression and require heightened monitoring and surveillance. As noted in Table 2, pulmonary disease can progress rapidly in patients with moderate illness, and therefore more cautious surveillance is advised. If secondary bacterial pneumonia is suspected, empiric antibiotics are recommended. However, the patient should be evaluated closely by the treating clinician, and antibiotics should be de-escalated and discontinued as soon as there is no clinical evidence of bacterial infection. The use of monoclonal antibodies is primarily reserved for symptomatic patients with mild or moderate disease who are not hospitalized and meet the specific inclusion requirements listed (see the monoclonal antibodies section below). Patients with severe and critical illness require hospitalization, as they have more prominent symptoms and are at higher risk for more rapid clinical deterioration, necessitating inpatient monitoring. O2 therapy should be administered immediately for even mild respiratory distress signs, using a nasal cannula or a high-flow O2 device according to individual patient requirements. As with moderate illness, if bacterial pneumonia or sepsis is suspected, empiric antibiotics are indicated. However, patients should still be evaluated daily, and antibiotics should be de-escalated and discontinued as soon as there is no clinical evidence of bacterial infection. Critically ill patients require ICU care and are at the highest risk for severe complications and fatal outcomes, as described in Table 2. Further, these patients are at increased risk for exacerbation of underlying comorbidities and cardiac, hepatic, renal, central nervous system, or thrombotic sequelae. Table 4 provides a brief overview of the NIH (2021) panel recommendations for pharmacologic therapies based on disease severity. Each category will be explored at greater length in the upcoming sections.

Monoclonal Antibodies
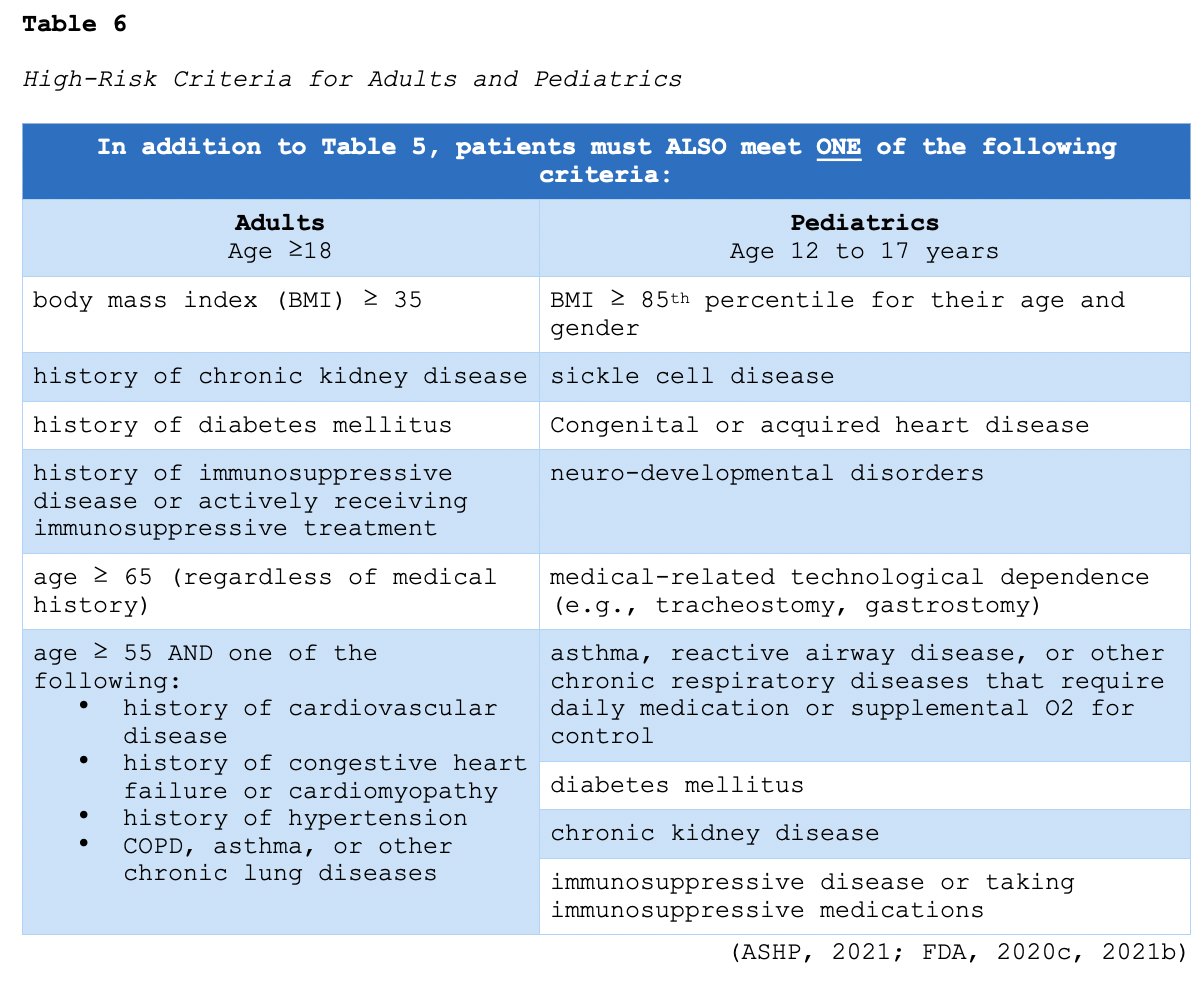
Monoclonal antibodies are widely used in the management of cancer, infectious diseases, and autoimmune conditions. They are synthetically engineered versions of antibodies that attach to a specific target site. In the laboratory, scientists analyze specific antigens on infected cells' surfaces to find a protein (target) to match the antigen. Using proteins from animals or humans, they create an antibody that can attach to the target antigen, similar to how a key fits into a lock. This technology allows treatment to focus on the specified cells, thereby limiting harm to healthy cells. Monoclonal antibody therapy can only be performed for diseases in which the antigens (and the respective antibodies) have been identified. These drugs work on infected cells in the same way natural antibodies work, identifying and binding to the target cells and then alerting the immune system to their presence (Sengupta, 2017). Monoclonal antibodies have been designed to target and bind to the S protein of SARS-CoV-2, thereby preventing it from interacting with the ACE2 receptor. This action precludes the virus from entering the host cells and replicating. To date, bamlanivimab (LY-CoV555) and the combination of casirivimab/imdevimab are the most widely studied monoclonal antibodies in patients with COVID-19. They have not demonstrated clinical benefit in patients hospitalized due to COVID-19. Preliminary clinical data suggest that these agents may be associated with worse clinical outcomes when administered to hospitalized patients requiring high-flow O2 or mechanical ventilation. The FDA issued an EUA for single-agent bamlanivimab (LY-CoV555) as well as the combination of casirivimab/imdevimab for the treatment of mild to moderate COVID-19 in patients meeting the specified criteria outlined in Tables 5 and 6. The objective of monoclonal therapy is to reduce the clinical course and thwart the progression of mild or moderate COVID-19 infections to severe or critical disease (ASHP, 2021; FDA, 2020c, 2021b). Based on this understanding, bamlanivimab (LY-CoV555) and casirivimab/imdevimab are not authorized for use in patients who meet any of the following criteria:
- are hospitalized due to COVID-19,
- require O2 therapy due to COVID-19,
- require an increase in baseline O2 flow rate due to COVID-19 in patients on chronic O2 therapy due to an underlying, non-COVID-19 related comorbidity (ASHP, 2021; FDA, 2020c, 2021b).
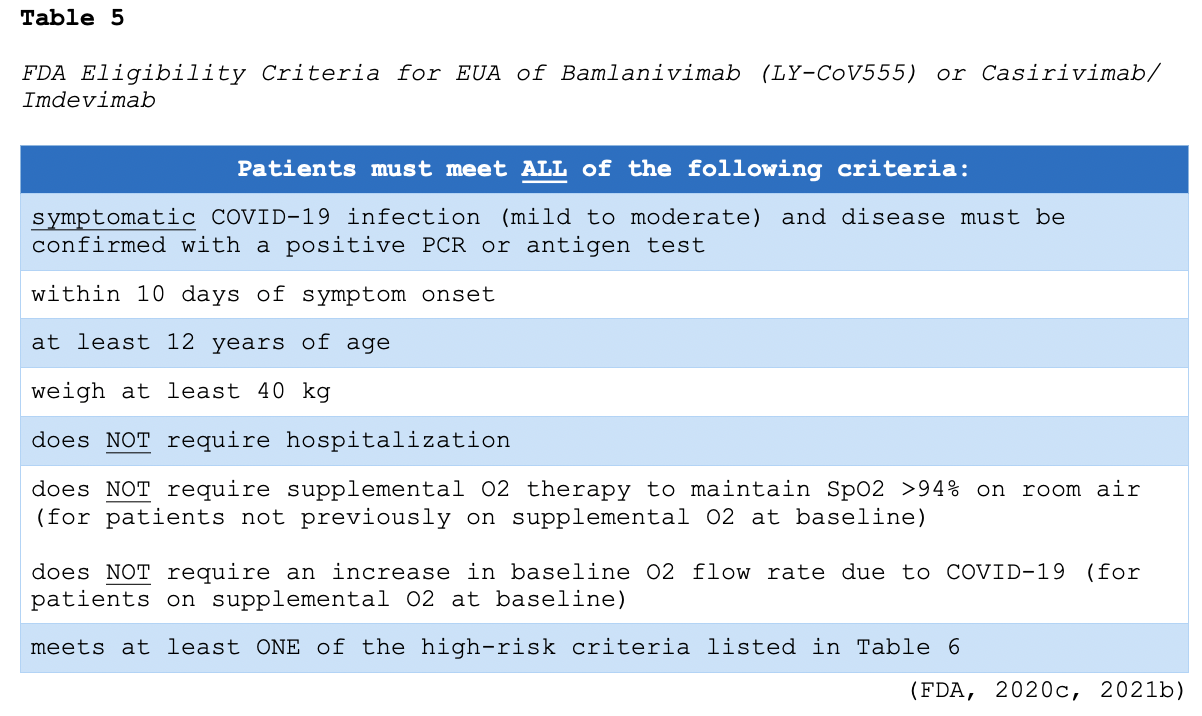
There are no comparative data to determine whether there are differences in the efficacy or safety between bamlanivimab (LY-CoV555) and casirivimab/imdevimab as of February 2021 (NIH, 2021). Bamlanivimab (LY-CoV555) is a single 700 mg intravenous (IV) infusion administered over 60 minutes. The EUA dosage for casirivimab and imdevimab is 1200 mg (each) infused concurrently over 60 minutes. Patients must be monitored during the administration of either infusion and observed for at least 1 hour after the infusion is complete (FDA, 2020c, 2021b). Hypersensitivity reactions (HSR) are common with the administration of monoclonal antibodies. An HSR occurs when the immune system is overstimulated by a foreign substance and creates antibodies that cause an exaggerated response. While data is limited to clinical trials, HSRs have been reported with varying degrees of severity. Early manifestations of HSR can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, and anxiety. Symptoms may suddenly progress to life-threatening hypotension, bronchospasm, angioedema (swelling of the oral cavity, lips, and/or tongue), and anaphylaxis. Therefore, APRNs must remain hypervigilant for signs of HSR and ensure they are prepared to intervene immediately. If an HSR is suspected, the infusion should be stopped immediately. In addition to closely monitoring patients, clinical interventions may include applying supplemental O2, administering normal saline, and other emergency medications as indicated. In the event of life-threatening symptoms, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) may need to be administered by IV push or subcutaneous injection until emergency personnel arrives (Nettina, 2019).
Infusion-related reactions (IRR) have also been observed, and some clinical manifestations can overlap with HSRs. The most common signs of IRRs include chills (rigors), fever, nausea, headache, hypotension, rash (urticaria or pruritus), myalgias, and dizziness. Most IRRs require the infusion to be slowed or stopped for the administration of appropriate medications to manage the symptoms and control the reaction (e.g., meperidine [Demerol] for rigors), after which the infusion is usually completed successfully. To date, over 1350 subjects have been exposed to bamlanivimab (LY-CoV555) across clinical trials. The safety of bamlanivimab (LY-CoV555), as reported by the FDA (2021b), is based on data from a phase 2 trial of 465 non-hospitalized patients with COVID-19. Mild adverse events occurred in 23% of the bamlanivimab (LY-CoV555) subjects compared to 26% of the placebo-treated subjects, and the most commonly reported adverse event was nausea. Across ongoing clinical trials, only one case of anaphylaxis has been reported. Further, only one IRR was cited as moderate and included facial edema (FDA, 2021b). The safety of casirivimab/imdevimab is based on analysis of one phase 1/2 trial of 799 non-hospitalized subjects with COVID-19. The adverse events collected were mainly limited to IRR and HSR of at least moderate severity. Serious adverse events were reported in 12 subjects and included pneumonia, hyperglycemia, nausea and vomiting, and dyspnea. A single anaphylactic reaction was reported, which required the use of epinephrine. Moderate IRRs were reported in 4 subjects and included pyrexia, chills, urticaria, pruritus, abdominal pain, nausea, vomiting, and flushing. Of these, 2 subjects required permanent discontinuation of the infusion (ASHP, 2021; FDA, 2020c).
The NIH (2021) panel’s recommendations regarding the use of bamlanivimab (LY-CoV555) or casirivimab/imdevimab are as follows:
- There is insufficient data to recommend either for or against the use of bamlanivimab (LY-CoV555) or casirivimab/imdevimab to treat outpatients with mild to moderate COVID-19.
- Bamlanivimab (LY-CoV555) or casirivimab/imdevimab should not be used as the standard of care treatment for patients with COVID-19.
- Patients at the highest risk for COVID-19 progression should be prioritized for treatment with either of these antibodies due to limited supply.
Antiviral Treatments
Antiviral therapies have been a source of interest among researchers as they are proposed to inhibit viral entry via the ACE2 receptor and TMPRSS2 and block viral membrane fusion. Since research has demonstrated that viral replication is most active early in the course of illness, antiviral therapy seems to have the most significant therapeutic impact when administered before the onset of a hyperinflammatory state (NIH, 2021).
Remdesivir (Veklury)
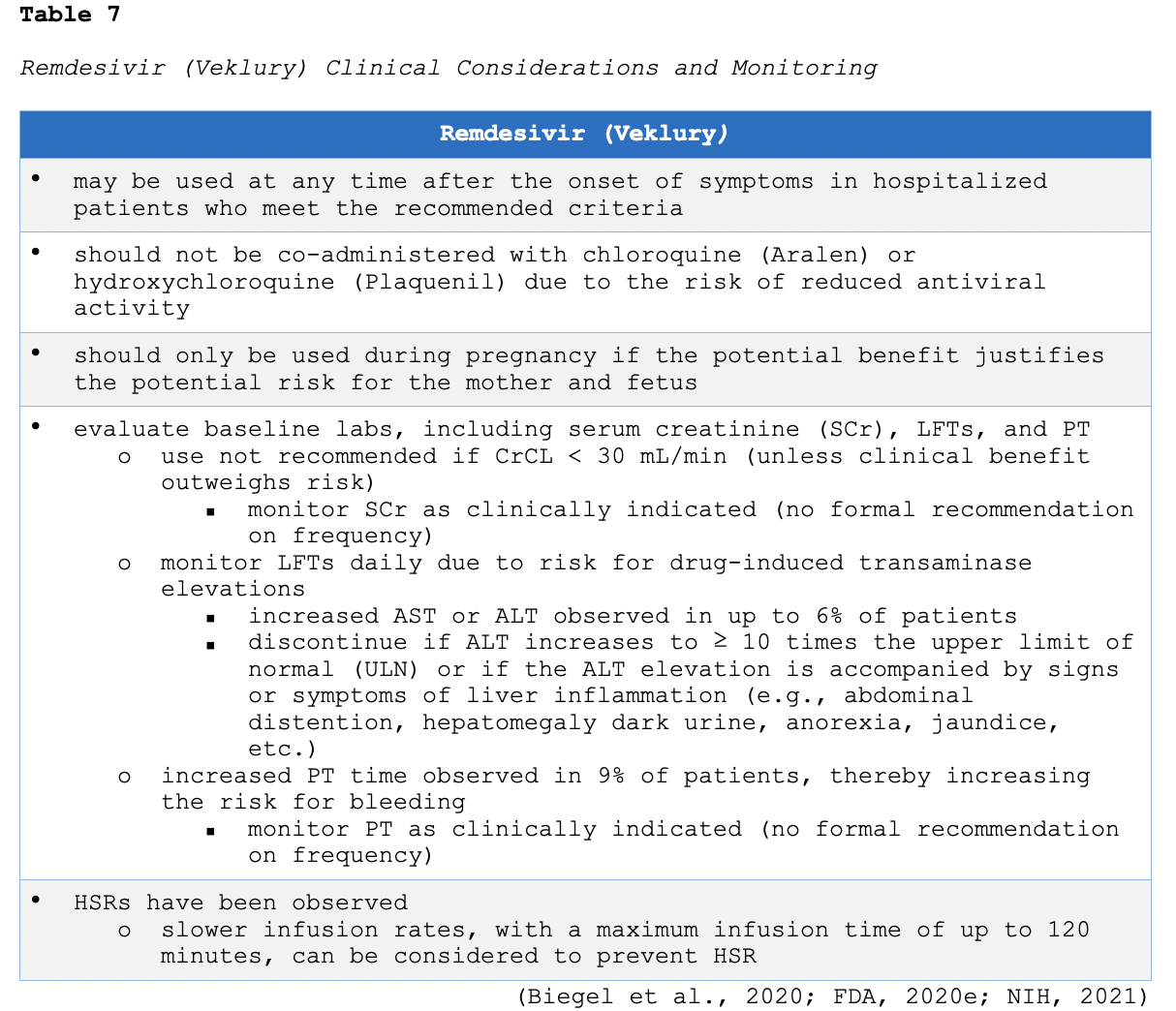
As of February 2021, remdesivir (Veklury) is the only FDA-approved drug for the treatment of COVID-19 in hospitalized adults and children aged 12 and older who weigh at least 40 kg (FDA, 2020e). As outlined in Table 4, remdesivir (Veklury) is only recommended for use in hospitalized patients who require supplemental O2. In the ACTT-1 trial, a total of 1062 patients underwent randomization (541 assigned to remdesivir [Veklury] and 521 to placebo). The greatest benefit was seen among hospitalized patients with COVID-19 who required supplemental O2 but not high-flow O2, invasive ventilation, noninvasive ventilation, or ECMO at baseline. Further, treatment with remdesivir (Veklury) beyond 5 days duration did not appear to improve outcomes. The study revealed that remdesivir (Veklury) was superior to placebo in shortening the time to recovery in adults hospitalized with evidence of lower respiratory tract infection (Biegel et al., 2020). The recommended administration of the remdesivir (Veklury) is a single loading dose of 200 mg on day 1, followed by once-daily maintenance doses of 100 mg from days 2 through 5. According to the FDA (2020d), if a patient does not demonstrate clinical improvement, treatment may be extended for up to a maximum duration of 10 days. The clinical considerations, monitoring, and possible adverse events of remdesivir (Veklury) are summarized in Table 7.
Chloroquine (Aralen) or Hydroxychloroquine (Plaquenil)
Since early in the COVID-19 pandemic, a significant amount of information has circulated regarding the potential efficacy of chloroquine (Aralen) and hydroxychloroquine (Plaquenil) in treating COVID-19 infections. Chloroquine (Aralen) is FDA-approved for the treatment of malaria, and hydroxychloroquine (Plaquenil) is FDA-approved for the treatment of malaria, lupus erythematosus, and rheumatoid arthritis (RA). Researchers hypothesized that these agents demonstrated antiviral activity in some in vitro systems. Based on preliminary evidence, the FDA issued an EUA for both agents. However, several randomized trials have failed to demonstrate any clinical benefit in COVID-19 patients prescribed chloroquine (Aralen) or hydroxychloroquine (Plaquenil). Neither agent has shown to lessen the clinical course's severity or reduce upper or lower respiratory tract viral loads. Further, a few studies examined the benefit of chloroquine (Aralen) or hydroxychloroquine (Plaquenil) used in combination with azithromycin (Z-Pak). Azithromycin (Z-pak) is a commonly prescribed macrolide antibiotic used to treat various bacterial infections, sexually transmitted infections, and other indications. Gautret and colleagues (2020) examined this combination in 80 mildly infected COVID-19 patients hospitalized in France and found that all patients (except one) improved clinically and were discharged rapidly (median of 5 days). However, their study was noncontrolled, non-comparative, and low power. Their findings were not able to be replicated in randomized controlled trials with adequate power. In a multicenter, randomized, and open-label controlled trial including 504 patients with mild-to-moderate COVID-19 infections, Cavalcanti and colleagues (2020) found the use of hydroxychloroquine (Plaquenil), alone or in combination with azithromycin (Z-Pak), did not improve clinical status or outcomes (at 15 days) when compared to the standard of care. Further, they found a significantly higher incidence of cardiac toxicity (i.e., QT interval prolongation) and LFT elevations in patients receiving hydroxychloroquine (Plaquenil) with or without azithromycin (Z-Pak) than those not receiving either agent (Cavalcanti et al., 2020).
Effective June 15, 2020, the FDA revoked its EUA for chloroquine (Aralen) and hydroxychloroquine (Plaquenil), stating that the agents were unlikely to provide clinical benefit. Correspondingly, the risk of adverse effects from these agents (i.e., cardiac dysfunction, skin rash, retinopathy, and major drug-drug interactions) is higher than the potential benefit (FDA, 2020f). To date, the NIH (2021) and ASHP (2021) strongly recommend against the use of chloroquine (Aralen) or hydroxychloroquine (Plaquenil) in hospitalized and non-hospitalized patients, either with or without azithromycin (Z-Pak). The NIH (2021) only supports the use of chloroquine (Aralen) or hydroxychloroquine (Plaquenil) in COVID-19 patients as part of a clinical trial (ASHP, 2021; NIH, 2021).
Ivermectin (Stromectol)
Ivermectin (Stromectol) is an anthelmintic (anti-parasitic) agent used to treat various conditions, such as scabies. Ivermectin (Stromectol) is widely used in animals to prevent heartworm disease and treat parasitic infections. As of February 2021, the use of this medication is being evaluated as a potential treatment for COVID-19, as it has been shown to inhibit the replication of SARS-CoV-2 in cell cultures. It has also demonstrated anti-inflammatory properties in vivo animal studies. However, clinical trial results have demonstrated mixed results. Some studies have shown no clinical benefit or potential worsening of the disease following ivermectin (Stromectol) administration; others have reported a greater reduction in inflammatory markers and shorter time to viral clearance. In a recently published retrospective cohort study by Rajter and colleagues (2021), 280 hospitalized patients with COVID-19 were enrolled; 173 were treated with ivermectin (Stromectol), and 107 were not. The overall mortality was lower in the ivermectin (Stromectol) group (15%) than in the control group (25.2%). Further, among patients with severe pulmonary involvement (defined as requiring FIO2 ≥ 50%, noninvasive ventilation, or invasive ventilation at the time of study enrollment), mortality was significantly lower among ivermectin-treated patients (38.8%) compared to the non-ivermectin treated group (80.7%). However, there were several limitations cited within this study; the effect of ivermectin (Stromectol) on viral load was not evaluated, and the impact of confounding variables in these patients (i.e., time from diagnosis to initiation of treatment and differences in drugs used for standard care) are unknown (Rajter et al., 2021). To date, published data from randomized, controlled clinical trials to support the use of ivermectin (Stromectol) in the treatment or prevention of COVID-19 is not available (ASHP, 2021). The NIH (2021) panel last updated their recommendation on ivermectin (Stromectol) on January 14, 2021, describing an insufficient quantity of evidence-based data to advise for or against the use of the drug in patients with COVID-19. Furthermore, the FDA (2020d) released a warning concerning the possible inappropriate use of ivermectin (Stromectol) products intended for animals as an attempt to self-medicate for the treatment of COVID-19. In summary, the NIH (2021) panel recommends against the use of ivermectin (Stromectol) for the treatment of COVID-19, except in a clinical trial (NIH, 2021).
Convalescent Plasma
Convalescent plasma therapy has been used in the treatment of several viral diseases with varying degrees of efficacy. Plasma is the largest portion of the blood and comprises water, salt, enzymes, proteins, and antibodies. The plasma of many patients who have recovered from COVID-19 contains antibodies against the virus. Administering COVID-19 antibody-rich convalescent plasma to those currently infected with the virus is hypothesized to provide short-term passive immunity (possibly by viral neutralization), thereby contributing to a faster recovery. On August 23, 2020, the FDA (2021c) issued an EUA for convalescent plasma in hospitalized patients with COVID-19. The EUA was issued based on the clinical benefit derived from using convalescent plasma in historical outbreaks of respiratory viruses and promising evidence demonstrated in small clinical trials with COVID-19 patients. While there is some evidence suggesting possible benefits of COVID-19 convalescent plasma in the treatment of COVID-19, the specific role is unclear, and additional data are needed from well-controlled, adequately powered, and randomized trials. Appropriate criteria for selecting patients to receive COVID-19 convalescent plasma and the optimal time during the disease to receive the therapy have not been established. As of February 2021, data suggests the clinical benefit may be greatest when high-titer convalescent plasma (plasma that is rich in antibody levels) is administered early in the course of illness (ASHP, 2021). A randomized, controlled study in China, including 103 adults with severe or life-threatening COVID-19, found no significant difference in time to clinical improvement, mortality, or time to hospital discharge in patients treated with high-titer COVID-19 convalescent plasma when compared to standard of care (Li et al., 2020). Joyner and colleagues (2020) analyzed pooled data from a total of 804 COVID-19 patients across 12 studies to evaluate the safety and efficacy of convalescent plasma in hospitalized adults with severe or life-threatening COVID-19. The risk of death was substantially reduced (57% reduction in mortality) among hospitalized COVID-19 patients transfused with convalescent plasma compared to matched patients receiving standard therapy. However, the findings are not yet peer-reviewed. The authors acknowledge several limitations within this analysis (i.e., aggregating mortality data across study populations that varied by dose and timing of convalescent plasma administration, geographic region, and duration of follow-up; Joyner et al., 2020).
According to the FDA (2021c), COVID-19 convalescent plasma administration should begin with one high-titer COVID-19 plasma unit (about 200 mL). The administration of additional convalescent plasma units should be based on the prescribing provider's medical judgment and the patient’s clinical response. Health care providers are advised to administer the COVID-19 convalescent plasma according to standard hospital procedures and institutional medical and nursing practices for other blood product transfusions. Patients with impaired cardiac function and underlying heart failure should receive a smaller volume and/or a more prolonged transfusion time (FDA, 2021c). While side effects of COVID-19 convalescent plasma are still being studied, adverse event monitoring has shown a low overall incidence. The risks do not appear to exceed those associated with plasma transfusions in general. In an expanded access program in the US, 20,000 hospitalized adults with COVID-19 received a convalescent plasma transfusion. The incidence of serious adverse events was low and was limited to transfusion reactions (<1%), thrombotic events (<1%), and cardiac events (3%). The majority of cardiac events were considered to be unrelated to the plasma itself and instead associated with the patient’s underlying cardiac dysfunction (e.g., exacerbation of congestive heart failure from fluid overload; Klassen et al., 2020). Known side effects and hazards extrapolated from other plasma transfusions include “transfusion-transmitted infections (e.g., HIV, hepatitis B, hepatitis C), allergic reactions, anaphylactic reactions, febrile nonhemolytic reactions, transfusion-related acute lung injury (TRALI), transfusion-associated cardiac overload (TACO), and hemolytic reactions. Hypothermia, metabolic complications, and posttransfusion purpura have also been described” (FDA, 2021c, p 3). At this time, the NIH (2021) panel states there are insufficient data to recommend for or against the use of convalescent plasma in patients with COVID-19. Adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use (NIH, 2021).
Dexamethasone (Decadron) and Other Corticosteroids
Dexamethasone (Decadron) is a long-acting, systemic glucocorticoid that has potent anti-inflammatory effects. Despite initial concerns regarding the potential for prolonged viral replication, the use of dexamethasone (Decadron) has emerged as an effective treatment option in severely or critically ill COVID-19 patients. Dexamethasone (Decadron) has demonstrated efficacy in preventing an extended cytokine response and accelerating the resolution of pulmonary and systemic inflammation in patients with pneumonia. However, in the RECOVERY trial (Horby et al., 2020), there was a possibility of harm in patients who did not require respiratory support at enrollment and received dexamethasone (Decadron). In this trial, 2104 patients were assigned to receive dexamethasone (6 mg once daily for up to 10 days) and 4321 to receive usual care. A total of 482 patients (22.9%) in the dexamethasone group and 1110 patients (25.7%) in the usual care group died within 28 days after randomization. In the dexamethasone (Decadron) group, the incidence of death was lower (29.3%) than the usual care group (41.4%) among patients receiving invasive mechanical ventilation and among those receiving O2 without invasive mechanical ventilation (23.3% vs. 26.2%). Researchers concluded that the use of dexamethasone (Decadron) resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or O2 alone at randomization but not among those with mild disease (receiving no respiratory support at the time of enrollment; Horby et al., 2020).
In October 2020, the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group (Sterne et al., 2020) performed a prospective meta-analysis of studies using systemic corticosteroids (i.e., dexamethasone [Decadron], hydrocortisone [Cortef], or methylprednisolone [SoluMedrol]). This meta-analysis pooled data from 7 randomized clinical trials in 12 countries that evaluated systemic corticosteroids' efficacy in 1703 critically ill patients with COVID-19. The administration of systemic corticosteroids was associated with lower all-cause mortality at 28 days compared with usual care or placebo (222 deaths among 678 patients who received corticosteroids [32.7%] compared to 425 deaths among 1025 patients who received usual care [41.5%]). They found that a higher dosage of corticosteroids was not associated with a more significant benefit than a lower dosage. Also, there was no increased risk of severe adverse effects associated with corticosteroid use (Sterne et al., 2020). The NIH (2021) recommends dexamethasone (Decadron) 6 mg PO or IV daily for up to 10 days of treatment or at hospital discharge (whichever is sooner). An oral formulation of dexamethasone (Decadron) achieves up to 86% bioavailability; however, IV should be considered in patients unable to tolerate oral intake or those with impaired gastric absorption. Further guidance on the appropriate populations for the use of dexamethasone (Decadron) is outlined in Table 4 (NIH, 2021).
Immunomodulators
Immunomodulators, including interferons (IFNs) and interleukin (IL)-inhibitors, have been proposed as potential treatment modalities for COVID-19 infections (NIH, 2021).
IFNs
IFNs are a family of cytokines with antiviral properties that have been proposed as a potential treatment for COVID-19 due to their in vitro and in vivo antiviral properties. However, prior clinical studies have demonstrated no therapeutic benefit of IFNs in patients with other CoV infections (i.e., MERS-CoV and SARS-CoV). As of February 2021, only limited clinical trial data is available specifically evaluating the efficacy of IFNs in treating COVID-19 infections. In an open-label, randomized study of hospitalized adults with severe COVID-19, Rahmani and colleagues (2020) randomly assigned 80 patients to either the IFN group or the control groups (40 patients to each group). Patients in the IFN group received IFN beta-1b (250 mcg injection every other day for 2 weeks) along with the standard agents given to the control group (i.e., antiviral therapy and hydroxychloroquine [Plaquenil]). There was no difference in duration of hospitalization, intubation rate, or all-cause 28-day mortality between the two groups, demonstrating no clinical benefit derived from the IFN (Rahmani et al., 2020). Some data suggests that IFNs may have antiviral activity early in the course of infection. In a retrospective cohort study by Wang and colleagues (2020), 446 hospitalized patients with COVID-19 were analyzed, and 242 (54.5%) received IFN (48.4% of patients received early IFN therapy, 6% received late IFN therapy, and 46% received no IFN). Researchers found that early initiation of IFN therapy (within the first 5 days of hospitalization) was associated with reduced mortality. In contrast, late IFN therapy was associated with increased mortality and delayed recovery (Wang et al., 2020). Still, there remains insufficient data to assess the potential benefit of IFN use during early disease compared to the significant risk of toxicities associated with IFN. At this time, the NIH (2021) panel recommends against the use of IFNs for the treatment of patients with severe or critical COVID-19, except in a clinical trial. The NIH emphasizes the lack of available data to recommend either for or against the use of IFN to treat early, mild, and moderate COVID-19 infections (NIH, 2021).
Interleukin-6 (IL-6) Inhibitors
Since COVID-19 infections are associated with heightened cytokine release, it is hypothesized that modulating the levels of IL-6 or its effects may alter the course of the disease. Therefore, IL-6 inhibitors have been a source of interest for researchers, particularly sarilumab (Kevzara) and tocilizumab (Actemra). These drugs are approved for several indications, such as moderate to severe RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). While they have been evaluated in the use of patients with COVID-19 who have signs of systemic inflammation, they are not yet approved for use in COVID-19 at this time. As of February 2021, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) is the largest trial available that has investigated the use of tocilizumab (Actemra) and sarilumab (Kevzara) in patients with COVID-19. Although this study has not yet been peer-reviewed, its preprint results are promising. In this trial, 803 patients with suspected or confirmed COVID-19 were randomly assigned to receive an IL-6 inhibitor (353 participants received tocilizumab [Actemra] and 48 received sarilumab [Kevzara]) or the standard of care (402 participants). The preliminary report notes that the use of either tocilizumab (Actemra) or sarilumab (Kevzara) reduced both mortality and time to ICU discharge and increased the number of organ support-free days compared to placebo (Gordon et al., 2021). Although these findings are promising, the NIH (2021) panel states there are insufficient data to recommend either for or against the use of tocilizumab (Actemra) to treat COVID-19 at this time. However, some of the NIH (2021) panel members advised that they would administer a single dose of tocilizumab (Actemra) in addition to dexamethasone (Decadron) to patients exhibiting rapid progression of respiratory failure. Since too few patients in the REMAP-CAP trial received sarilumab (Kevzara), it is not recommended (NIH, 2021). Severe and potentially fatal infections (including tuberculosis, invasive fungal, bacterial, viral, protozoal, and other opportunistic infections) have been reported in patients receiving tocilizumab (Actemra). Further, reactivation of prior infections such as HIV, hepatitis B, and tuberculosis have also been reported. Other possible side effects of tocilizumab (Actemra) include neutropenia, thrombocytopenia, transaminitis, and lipid abnormalities (ASHP, 2021; NIH, 2021).
Baricitinib (Olumiant)
The FDA (2020b) has granted EUA for baricitinib (Olumiant) to treat COVID-19 in some instances. Baricitinib (Olumiant) is an oral Janus kinase (JAK) inhibitor used to treat moderate to severe RA. Its mechanism in treating COVID-19 infections is premised on the belief that it disrupts regulators of endocytosis, which may help reduce viral entry and inflammation. It is also thought to interfere with intracellular virus particle assembly and have antiviral activity (ASHP, 2021). The EUA was issued on November 19, 2020, and permits baricitinib (Olumiant) use only in combination with remdesivir (Veklury) in hospitalized patients aged 2 years or older requiring supplemental O2, invasive mechanical ventilation, or ECMO. It is administered as a once-daily oral tablet. While the optimal duration of therapy is unknown, the FDA recommends a total treatment duration of 14 days or until hospital discharge, whichever comes first (FDA, 2020b). The EUA is based on data from the Adaptive Covid-19 Treatment Trial (ACTT-1), which was a randomized, double-blind, placebo-controlled trial comparing baricitinib (Olumiant) in combination with remdesivir (Veklury) to remdesivir (Veklury ) alone. A total of 1033 patients were enrolled, and 515 were randomly assigned to combination treatment and 518 to control. Patients in the combination arm had a median recovery time of 7 days compared to 8 days in the control arm. Patients receiving high-flow O2 or noninvasive ventilation at the time of enrollment had a median recovery time of 10 days on the combined treatment compared to 18 days on the control. The 28-day mortality was lower in the combination group (5.1%) than the control group (7.8%). Further, the combination was associated with fewer serious adverse events (Kalil et al., 2020). The NIH (2021) panel states that data are insufficient to recommend either for or against the use of baricitinib (Olumiant) in combination with remdesivir (Veklury) for the treatment of COVID-19 in hospitalized patients when corticosteroids can be used. However, in rare circumstances when corticosteroids cannot be used, the panel recommends the use of baricitinib (Olumiant) in combination with remdesivir (Veklury) for the treatment of hospitalized, non-intubated patients requiring supplemental O2 (NIH, 2021).
Anticoagulation (AC) in Patients with COVID-19
Various organizations, including the NIH, CDC, and ASH, have published interim guidance for AC management in hospitalized patients with COVID-19. Several studies have shown that therapeutic-dosing of AC in critically ill patients with COVID-19 is associated with a lower risk of VTE but a higher risk of major bleeding events. The consensus amongst the guidelines is that hospitalized patients with COVID-19 should receive prophylactic-doses of AC to prevent VTE unless there are contraindications. However, due to a lack of evidence supported by randomized controlled trials, several questions regarding the specifics of AC use in this setting remain unanswered, such as the safest type of AC, duration of AC, and intensity of therapy. The NIH (2021) panel recommends using prophylactic-dose AC over therapeutic-dose AC in patients with COVID-19 related critical illness who do not have suspected or confirmed VTE. Several studies have suggested that patients with COVID-19 related critical illness have improved clinical outcomes with prophylactic AC. Still, the optimal intensity of AC and its effect on clinical outcomes is uncertain. The ASH (2021) recommends the routine monitoring of platelet, PT, D-dimer, and fibrinogen in hospitalized patients with COVID-19, attributing worsening parameters, specifically the D-dimer, as a predictor that more aggressive critical care may be needed. However, they do not define a specified time interval or frequency of monitoring these laboratory values. As of February 2021, there is no data in non-hospitalized patients with COVID-19 to support the evaluation and monitoring of coagulation markers, as VTE risk is low (ASH, 2021).
The ASH (2020) formed a multidisciplinary, international panel to develop guidelines for AC in patients with COVID-19. They recommend prophylactic-intensity AC with low molecular weight heparins (LMWH; see Table 8) for all acutely ill hospitalized COVID-19 patients who do not have a suspected or confirmed VTE, in the absence of bleeding (unless the risk of bleeding outweighs the risk of thrombosis). They specifically discourage the use of therapeutic-dose AC in patients without any other indication warranting therapeutic-doses of AC. These recommendations apply despite abnormalities detected on coagulation tests, and LMWHs should only be held if platelet count declines to less than 20,000 or the fibrinogen level drops to less than 50 mg/dL. Further, the ASH specifically notes that an abnormal PT level is not a contraindication for thromboprophylaxis. When pharmacological thromboprophylaxis is contraindicated, mechanical thromboprophylaxis is recommended; combined pharmacologic and mechanical prophylaxis is not advised. LMWH is also recommended over unfractionated heparin (UFH). In the event of heparin-induced thrombocytopenia (HIT), a potentially life-threatening disorder that occurs in response to the administration of UFH, fondaparinux (Arixtra) is recommended (ASH, 2021). For hospitalized, critically ill patients, the NIH (2021) recommends LMWH or UFH over oral AC agents due to their shorter half-lives, ability to be administered intravenously or subcutaneously, and fewer drug interactions.

The NIH (2021) recommends against prophylactic AC in non-hospitalized (ambulatory) patients with COVID-19. The NIH endorses the recommendation that hospitalized non-pregnant adults with COVID-19 receive prophylactic dose AC. They cite insufficient evidence to recommend either for or against the use of AC at higher than the prophylactic dose in hospitalized COVID-19 patients. Further, hospitalized patients with COVID-19 should not routinely be discharged from the hospital while on VTE prophylaxis in the absence of a diagnosed VTE. Patients with COVID-19 who experience a thromboembolic event or highly suspected of having thromboembolic disease (in the absence of access to confirmatory diagnostic imaging) should be managed with therapeutic doses of AC therapy. For hospitalized COVID-19 patients who experience rapid deterioration of pulmonary, cardiac, or neurological function or the sudden, localized loss of peripheral perfusion, the possibility of thromboembolic disease should be urgently evaluated and considered. In pregnant patients hospitalized with severe COVID -19, prophylactic-dose AC is recommended if there are no contraindications to its use. If AC therapy is prescribed before diagnosing COVID-19 in a pregnant patient, it should be continued (NIH, 2021).
Adjunctive Therapies and Concomitant Medications
Vitamin C
Vitamin C (ascorbic acid) is a water-soluble vitamin and antioxidant that is believed to benefit patients with severe and critical illness based on its anti-inflammatory properties, influences on cellular immunity, and vascular integrity. Research demonstrates that people may need more vitamin C in states of oxidative stress. Therefore, the role of vitamin C supplementation has been evaluated in numerous disease states, including sepsis and cancer. Since severe and critical COVID-19 infections can cause sepsis and ARDS, the potential role of high doses of vitamin C in lessening inflammation and vascular injury in patients with COVID-19 has been a topic of interest that remains under investigation (Wei et al., 2020). While there are several ongoing clinical trials as of February 2021, there are no completed controlled trials of vitamin C in patients with COVID-19. Available observational data are lacking, and findings remain inconclusive. The NIH (2021) panel states there are insufficient data to recommend either for or against the use of vitamin C for the treatment of COVID-19 in non-critically ill patients.
Vitamin D
Vitamin D is a fat-soluble vitamin found in certain foods and is also produced endogenously when ultraviolet rays from the sunlight triggers its synthesis through the skin. Vitamin D enables normal bone mineralization by promoting calcium absorption and maintaining adequate serum calcium and phosphate concentrations. It also serves as an anti-inflammatory, modulating various cellular processes, such as cell growth, neuromuscular and immune function, and glucose metabolism (NIH Office of Dietary Supplements, 2020). The rationale for vitamin D use is based on its immunomodulatory effects that could protect against COVID-19 infection or decrease illness severity (NIH, 2021). A large population-based study involving 7,807 participants found an abnormally low 25(OH)D level was independently associated with an increased risk for infection and hospitalization. Researchers found a lower mean plasma 25(OH)D in the subset of COVID-positive patients (n=782) versus COVID-19-negative participants (19 ng/mL versus 20.55 ng/mL; Merzon et al., 2020). In a randomized, double-blind, placebo-controlled clinical trial by the National Health, Lung, and Blood Institute PETAL Clinical Trials Network (Ginde et al., 2019), high doses of vitamin D were administered to critically ill patients (but not COVID-19) with vitamin D deficiency. Findings revealed no reduction in the length of the hospital stay or the mortality rate compared to placebo. Further, high levels of vitamin D are linked to adverse effects of hypercalcemia and nephrocalcinosis. The definitive role of vitamin D supplementation in preventing or treating COVID-19 remains unknown, and observational studies continue to evaluate vitamin D's role in preventing and treating COVID-19. To date, the NIH (2021) panel states there are insufficient data to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.
Zinc
Prior studies have demonstrated that increased intracellular zinc concentrations impair replication in several RNA viruses (not COVID-19; NIH, 2021). The relationship between zinc and COVID-19, including how zinc deficiency affects the severity of COVID-19 and whether zinc supplements can improve clinical outcomes, remains under investigation (Calder et al., 2020). Zinc has also been shown to enhance cytotoxicity and induce apoptosis when used in vitro with chloroquine (Aralen). Based on this rationale, Carlucci and colleagues (2020) examined the role of zinc when used in combination with hydroxychloroquine (Plaquenil) and azithromycin (Z-Pak) in a retrospective review of hospitalized patients with COVID-19. This study included 932 patients (411 received zinc and 521 did not receive zinc). Although it is not yet peer-reviewed, results demonstrate that patients who received zinc had higher absolute lymphocyte counts and lower troponin and procalcitonin levels than those who did not receive zinc. Further, zinc supplementation was associated with a decreased mortality rate and a lower transfer rate to hospice; however, this finding did not apply to those admitted to the ICU. This study has several limitations that must be considered; patients were not randomized, and statistical methods did not account for confounding variables. Furthermore, patients who were still hospitalized after analyses concluded were not followed, preventing a complete comparison of clinical outcomes (Carlucci et al., 2020). Furthermore, long-term zinc supplementation is associated with risks, as it can cause copper deficiency, which can induce potentially irreversible neurologic manifestations (e.g., myelopathy, paresthesia, ataxia, spasticity). The NIH (2021) panel states there are insufficient data to recommend either for or against zinc use for the treatment of COVID-19. Moreover, the panel specifically recommends against using zinc supplementation above the recommended dietary allowance (11 mg daily for men and 8 mg for non-pregnant women; Calder et al., 2020; NIH, 2021).
ACE-inhibitors (ACEI) and Angiotensin Receptor Blockers (ARB)
Since early findings demonstrated the role of the ACE2 receptor as a major binding site and driver of pathogenesis in COVID-19 infections, ACEIs and ARBs have been a topic of interest regarding their hypothetical harms. Since the expression of ACE2 may be increased in patients treated with ACEI (e.g., lisinopril [Zestril] or enalapril [Vasotec]) or ARBs (e.g., losartan [Cozaar] or olmesartan [Benicar]), concerns regarding the increased expression of ACE2 as a potential facilitator of COVID-19 infections has sparked much concern. There is only limited data evaluating the effect of these drugs on COVID-19 infections, including a lack of data demonstrating beneficial or adverse outcomes in COVID-19 patients receiving ACEI or ARB therapy. In a large, population-based case-control study in Italy, 6272 case-patients with COVID-19 were matched to 30,759 controls according to sex, age, and the municipality of residence to evaluate the association between the use of ACEIs, ARBs, and the risks of COVID-19. The use of ARBs or ACEIs did not show any beneficial or harmful effect on patients with COVID-19 or among patients who had a severe or fatal course of the disease. The authors noted that the use of ACEIs and ARBs was more frequent among patients with COVID-19 than among controls due to their higher prevalence of cardiovascular disease; however, there was no evidence that ACEIs or ARBs affected the risks associated with COVID-19 or prompted more severe disease. The authors concluded that there was no evidence that treatment with ACEIs or ARBs significantly altered the course of infection or resulted in more severe disease (Mancia et al., 2020). As of February 2021, the consensus among the American Heart Association (AHA), American College of Cardiology (ACC), and the NIH (2021) is for the continuation of treatment with these medications in patients who have previously been prescribed them for cardiovascular disease (or other indications). Correspondingly, they recommend against the use of ACE inhibitors or ARBs for the treatment of COVID-19 infections. There is a lack of clinical evidence demonstrating that ACEIs or ARBs impact the susceptibility of individuals to COVID-19 infections or on clinical outcomes at this time, and further study is needed (ASHP, 2021; NIH, 2021).
Reinfection
To date, limited data exist regarding reinfection with COVID-19 after recovery from a prior infection. While cases of reinfection have been reported, it is relatively rare. It is unclear which individuals are at the highest risk for reinfection and how long people who have recovered from COVID-19 are protected against reinfection. Studies have demonstrated mixed results regarding the duration of antibody protection following a COVID-19 infection, and the efficacy of natural antibodies in preventing reinfection is still being investigated (CDC, 2020c). A recent large multi-center prospective study determined that a prior COVID-19 infection provided up to 83% protection for at least 5 months (Hall et al., 2021).
Vaccination
To end the COVID-19 pandemic, a large proportion of the world needs to develop immunity to the virus. The safest and most effective way to accomplish this is through vaccination. Vaccines work by impersonating the infectious bacteria or viruses that inflict disease to prompt the body to create antibodies that fight the condition upon exposure. Vaccination is a prime example of adaptive immunity, as it prompts the creation of antibodies against a pathogen to prevent the acquisition of the illness later. If that particular pathogen tries to invade the body, the adaptive immune system responds more quickly, producing additional antibodies to address the infection and thwart illness. Vaccination stimulates the body’s immune system to build up defenses against the infectious organism without inducing the disease. The most common types of vaccines contain a weakened version or inactivated parts of a particular organism to trigger an immune response. Typically, the vaccine is comprised of a minuscule, weakened, and a non-dangerous fragment of the organism that includes specific parts of the antigen. This tiny amount is enough for the body to learn how to build the specific antibody in the event of an encounter with the real antigen later. More novel vaccines contain the blueprint for producing antigens rather than the antigen itself. Regardless of whether the vaccine is made up of the antigen itself or the blueprint so that the body will produce the antigen, the recipient does not acquire the illness from the vaccine. Some vaccines require multiple doses, given weeks or months apart. This is sometimes needed to allow for the production of long-lived antibodies and the development of long-standing memory cells. Table 9 provides a comparison of the major types of vaccines used throughout the US (CDC, 2018; McCance & Heuther, 2019; WHO, 2020b).
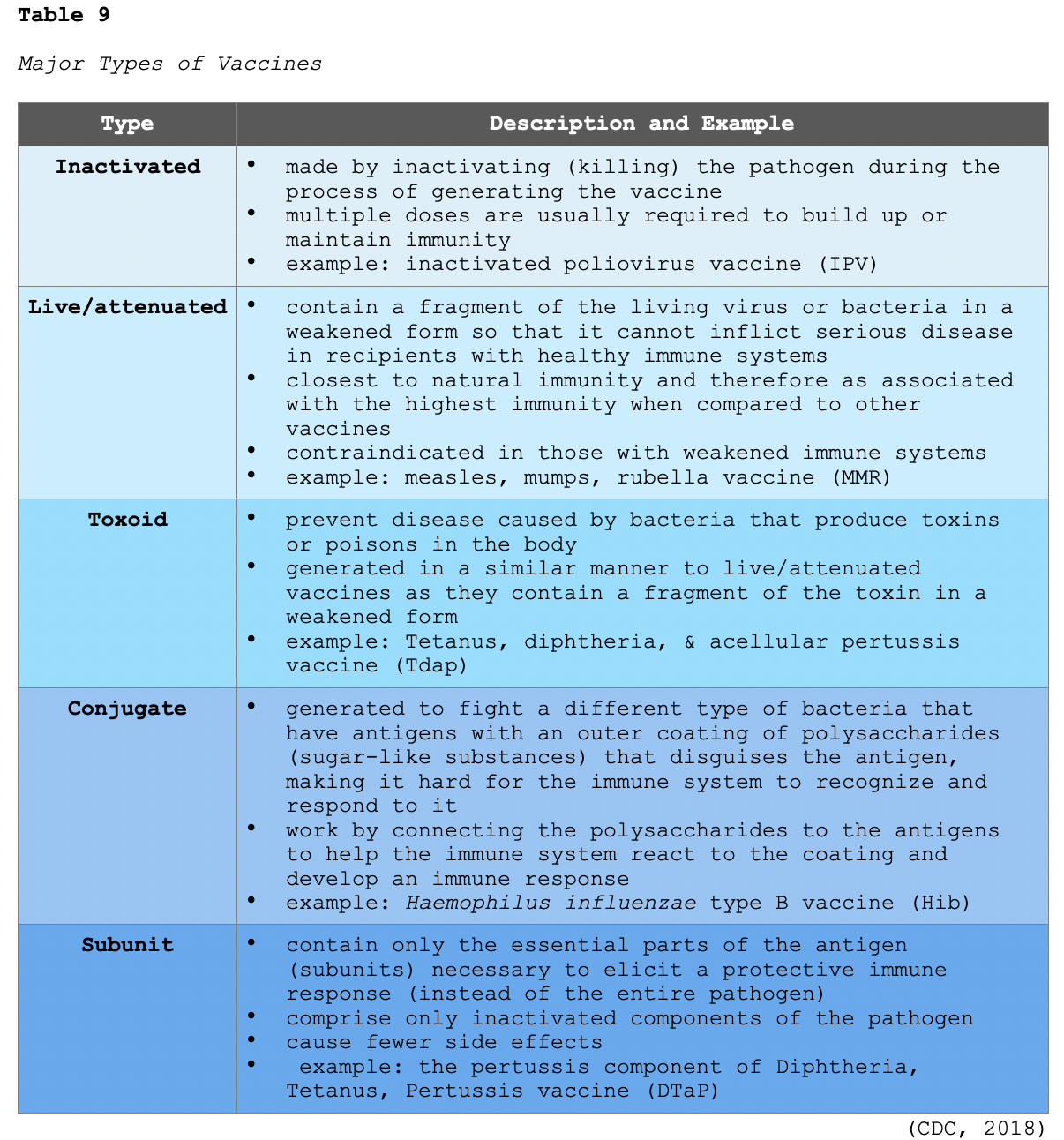
Vaccine Development and FDA Regulation
Vaccine development is traditionally a prolonged, multi-step, and intricate process that takes at least 10 years of research and testing before the vaccine is brought to market. The FDA is the regulatory authority that oversees the safety, effectiveness, and quality of vaccines before approving them for use in the US. The year 2020 has been marked by significant uncertainty, turmoil, and tragedy brought forth by the COVID-19 virus; but is also characterized by the fastest vaccine developed in the US to date. Once a COVID-19 vaccine receives an EUA, the FDA mandates ongoing safety monitoring and reporting. Healthcare providers must adhere to the following conditions of an EUA:
- providing the recipient/caregiver the Fact Sheet for Recipients (similar to a vaccine information statement [VIS] for licensed vaccines), which communicates vaccine benefits and risks to the recipient via hard copy or electronic means
- reporting vaccine administration data to CDC
- reporting vaccine administration errors and specified adverse events to Vaccine Adverse Effect Reporting System (VAERS), a nationwide online system led by the US Department of Health and Human Services (HHS) that collects reports of adverse events following vaccination (CDC, 2020b; HHS, n.d.).
As an added measure of data collection, the FDA has also developed v-safe, a novel, voluntary, smartphone-based application tool that uses text messaging and internet-based surveys to facilitate the collection of additional COVID-19 vaccination-related side effects. This tool generates automated daily check-ins for COVID-19 vaccine recipients, allowing them to directly report any side effects in real-time. It allows for the estimation of rates of local and systemic reactions and clinically important adverse effects following vaccination. V-safe also provides recipients with reminders on obtaining their second COVID-19 vaccine dose (CDC, 2020b; HHS, n.d.).
COVID-19 Vaccines
As of February 1, 2021, two very similar vaccines were approved for EUA in the US; Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273). A third vaccine by Johnson & Johnson/JNJ-78436735 (Ad26.COV2.S) received FDA approval on February 27, 2021. Researchers worldwide are testing more than 65 vaccines in clinical trials on humans, and 20 have reached the final stages of testing. Several other vaccines are approved for early emergency use in other countries, such as Gamaleya (Sputnik V) in Russia and Astrazeneca ChAdOx1 nCoV-19 (AZD1222) in India. Before delving into the details of the COVID-19 vaccines available in the US, the next section dispels some of the most common COVID-19 vaccine misconceptions (CDC, 2020a, 2020b; FDA, 2021d; Vizient, 2021; Zimmer et al., 2021).
Vaccine Myths and Realities (CDC, 2020b; Sax, 2021)
- Myth: COVID-19 vaccines were ‘rushed,’ so they should not be trusted.
- Reality: You cannot rush safety trials; researchers and drug manufacturers were allowed to focus solely on this one task, and the government removed several of the bureaucratic inefficiencies that most commonly slow down the process.
- Myth: You can get a COVID-19 infection from the vaccine.
- Reality: The vaccine cannot cause an infection with the SARS-COV-2 virus because it is not an inactivated (attenuated) version of the virus.
- Myth: The vaccine can change your DNA.
- Reality: The vaccine can't change, interact with, or affect human DNA (i.e., genetic material) since it is a messenger ribonucleic acid (mRNA) vaccine that never enters the nucleus of the cells where the DNA is stored.
- Myth: The vaccine can cause genetic changes in pregnant women and women of childbearing age, leading to fetal harm and infertility.
- Reality: Since the COVID-19 vaccines do not enter the nucleus and do not alter human DNA, they cannot cause genetic changes in the woman, fetus, infant or impact future pregnancies.
- Myth: Once you’ve been vaccinated, you cannot spread the virus and no longer need to wear a mask or practice social distancing.
- Reality: It is not clear if vaccine recipients are capable of spreading the virus to others. As of February 2021, data only demonstrates that the vaccine effectively prevents serious symptoms in the vaccine recipient. It remains crucial that vaccine recipients continue to follow all of the CDC guidelines to prevent the spread of COVID-19, including wearing masks, handwashing, and social distancing.
- Myth: You don’t need to be vaccinated if you had a prior COVID-19 infection.
- Reality: The duration and effectiveness of natural immunity after a COVID-19 infection is unclear and varies between individuals but has shown to be decline over time. Research demonstrates that the COVID-19 vaccines are skilled at prompting a significant immune response to the target protein responsible for preventing the infection, thereby enhancing a directed immune response. Therefore, the CDC recommends that anyone who had a prior COVID-19 infection should be vaccinated following a 90-day symptom-free period.
Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA Vaccines
Disclaimer: For the remainder of this module, the Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) COVID-19 vaccines will be referred to as ‘Pfizer’ and ‘Moderna,’ respectively.
Pfizer and Moderna not only developed the first two COVID-19 vaccines in the US, but their agents are very similar with regards to their chemical structure, technology, and mechanism of building immunity. Both vaccines use novel messenger RNA (mRNA) to generate immunity to COVID-19, which has been studied and broadly tested by scientists for more than a decade but has never before been brought to market. Immunity generated from mRNA vaccines is distinct from traditional vaccines as they do not contain a weakened or inactivated version of the COVID-19 virus; thus, there is no potential risk of infection or insertional mutagenesis. These vaccines contain only the genetic material for a specific protein, and no genetic material enters the nucleus of the cells. When the vaccine is injected, the mRNA is taken up by macrophages near the injection site. The mRNA provides a blueprint (i.e., instructions) to the immune system cells, teaching them how to create the S protein unique to SARS-CoV-2. The S protein subsequently appears on the macrophages' surface, triggering the body’s immune response and directing the production of antibodies that recognize and react to the S protein. Once the mRNA has completed its task, it is rapidly degraded by enzymes and removed from the body. This enzymatic degradation process occurs naturally in the body to remove dead, unwanted, or damaged cells (CDC, 2020b; Sax, 2021).
Both the Pfizer and Moderna vaccines have shown impressive and near equivalent efficacy across clinical trials. The Pfizer vaccine demonstrated nearly 95% efficacy at preventing symptomatic COVID-19 infection about 10 days following receipt of the second dose. Data demonstrated that the vaccine appeared to be relatively equally effective across demographic groups (i.e., age, racial and ethnic groups). The Moderna vaccine was about 94% effective in preventing symptomatic COVID-19 infection at 10 to 14 days following the second dose. Efficacy appeared to be marginally lower in individuals aged 65 and older; however, the numbers could have been influenced by the fact that there were fewer cases in that age group during the clinical trial. High efficacy (≥ 86%) was observed across age, sex, race, and ethnicity categories and persons with underlying medical conditions. Both vaccines require a two-dose series, and the specifics regarding dosing and administration schedules are outlined in Table 10 (CDC, 2020a, 2020b, 2021a; Vizient, 2021).
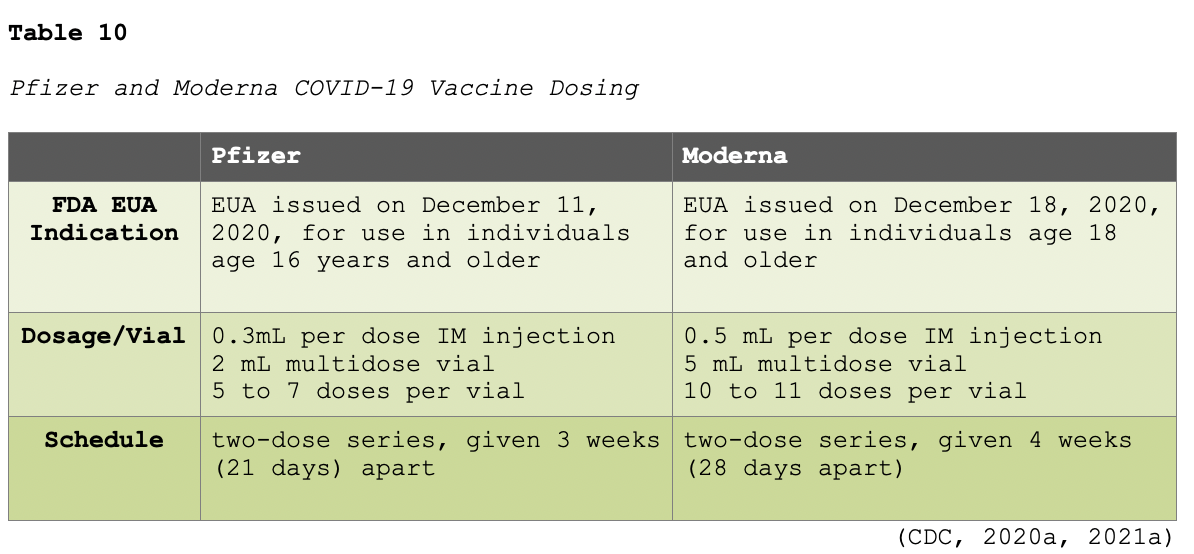
According to the CDC (2021a), second doses administered up to 4 days earlier than recommended are considered valid (i.e., Moderna may be administered on days 24 to 27; Pfizer may be administered on days 17 to 20). However, efforts should be made to administer the second dose as close to the recommended interval as possible. If adhering to the recommended timelines is not feasible, the second dose may be scheduled for administration up to 6 weeks (42 days) after the first dose. Further, both doses are necessary for protection as the efficacy of a single dose has not been formally studied in clinical trials (CDC, 2021a). As of February 2021, there are few absolute contraindications to mRNA vaccines, which include the following:
- severe allergic reaction (e.g., anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components,
- an immediate allergic reaction of any severity to a prior dose of an mRNA COVID-19 vaccine or any of its components (including polyethylene glycol [PEG], or polysorbate)
- PEG is an ingredient in both mRNA COVID-19 vaccines structurally related to polysorbate, and cross-reactive hypersensitivity between the compounds may occur (CDC, 2021a; Vizient, 2021).
The CDC (2021a) defines an immediate allergic reaction as any HSR or symptoms consistent with urticaria, angioedema, respiratory distress (e.g., wheezing, stridor), or anaphylaxis that occurs within 4 hours of administration. Patients meeting any of the above criteria should not be vaccinated with the second dose and should instead be referred to an allergist/immunologist (CDC, 2021a; Vizient, 2021). Anaphylactic reactions in persons receiving mRNA COVID-19 vaccines outside of clinical trials have been reported. The CDC (2020a) recommends that healthcare professionals become familiar with identifying immediate-type allergic reactions and have a plan in place to respond to these emergencies. The following medications and equipment should be readily available at all sites where mRNA vaccines are administered:
- at least three prefilled syringes of epinephrine or autoinjectors
- antihistamines
- histamine-1 (H1) blocker, such as diphenhydramine (Benadryl)
- histamine-2 (H2) blocker, such as famotidine (Pepcid)
- antihistamines may be given as adjunctive therapy but should not be used as the initial or sole treatment for suspected anaphylaxis
- pulse oximeter
- supplemental O2
- bronchodilators (e.g., albuterol [ProAir])
- IV fluids
- intubation kit (CDC, 2020a, 2021a)
Table 11 compares the side effect profiles of the Pfizer and Moderna vaccines.
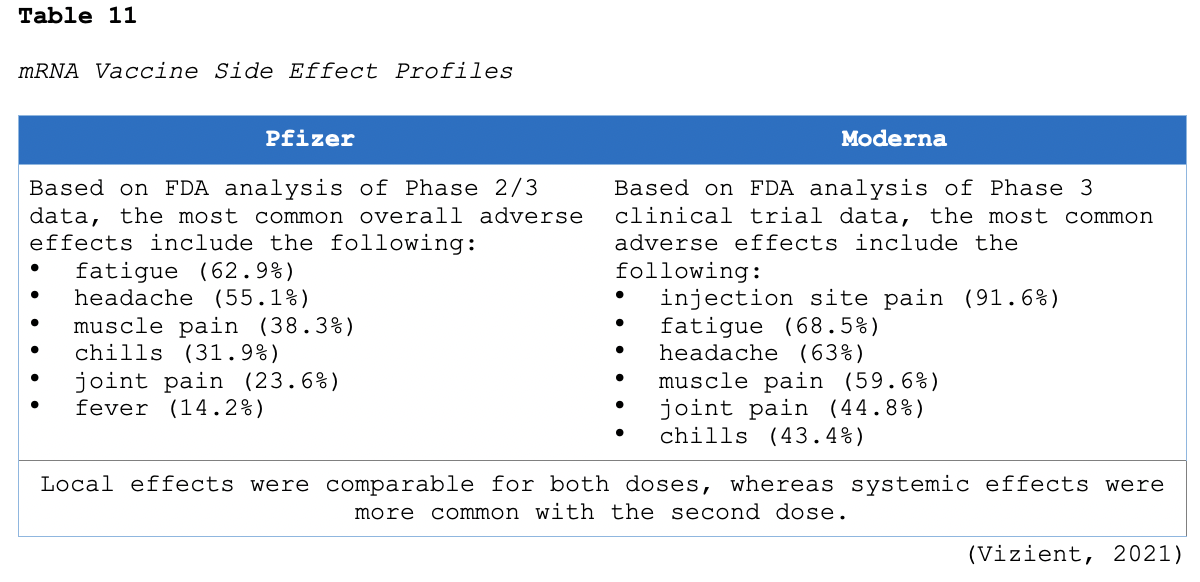
Receipt of either the Pfizer or Moderna vaccine will not affect the results of RT-PCR or antigen COVID-19 tests performed after vaccination. These tests are used to detect active disease and not immunity, and the vaccine does not cause the virus or induce viral replication. Therefore, if a patient who has previously received the vaccine subsequently has a positive RT-PCR or other antigen test, they should be treated as an active COVID-19 infection. Since the vaccine is intended to induce an immune response, an antibody test may be positive in someone who has been vaccinated. However, the time from vaccine administration to measurable levels of antibodies has not yet been determined. Information regarding the durability of protection following receipt of either mRNA vaccine is not yet available. Data from the Moderna trial suggests that antibodies were highest for about 4 months, with titers slightly declining over time. Since there is no information on how long the vaccines will be protective as of February 2021, there is no recommendation for booster doses following completion of the two-dose vaccination series (CDC, 2020a; Sax, 2021).
Clinical Considerations for mRNA Vaccine Use in Special Populations
Prior History of COVID-19 Infection
Data from clinical trials indicate that mRNA COVID-19 vaccines can safely be given to persons with evidence of a prior COVID-19 infection. Vaccination should be offered to persons regardless of a history of a prior symptomatic or asymptomatic COVID-19 infection. Viral RT-PCR or antigen testing for an acute COVID-19 infection or serologic testing to assess a prior infection is not recommended before vaccination (CDC, 2020a, 2021a).
Current COVID-19 Infection
Vaccination of individuals with known current COVID-19 infection should be deferred until the person has recovered from the acute illness (resolution of symptoms) and criteria have been met to discontinue the isolation period. This recommendation applies to persons who develop a COVID-19 infection before receiving any vaccine doses and those who develop an acute infection after their first dose but before receipt of the second dose. There is no recommended minimum time interval between infection and vaccination. As of February 2021, evidence suggests that the risk of COVID-19 reinfection is lowest in the first 3 months (90 days) after initial infection but may increase with time due to waning immunity. Thus, while vaccine supply remains limited, persons with recent documented COVID-19 infection may choose to temporarily delay vaccination, if desired, during this 90-day period. However, these individuals should be informed of the risk of reinfection with waning immunity and the continued need to complete the vaccination series despite prior COVID-19 infection. For vaccinated persons who subsequently develop a COVID-19 infection, prior receipt of an mRNA COVID-19 vaccine should not affect treatment decisions or the timing of treatments (including the use of monoclonal antibodies, convalescent plasma, antiviral therapy, or corticosteroid administration; CDC, 2020a, 2021a; NIH, 2021; Vizient, 2021).
Post-exposure Prophylaxis
It is important to note that mRNA vaccines are not recommended for outbreak management or post-exposure prophylaxis. In other words, the mRNA vaccines are not indicated nor considered effective in preventing the development of infection in a person with a known exposure in the days before vaccination. It is deemed unlikely that the first dose of an mRNA COVID-19 vaccine would provide an adequate immune response within the incubation period to allow for effective post-exposure prophylaxis and therefore is not recommended at this time (CDC, 2020a, 2021a).
Persons who Previously Received Passive Antibody Therapy
As of February 2021, there are no data on the safety and efficacy of mRNA COVID-19 vaccines in persons who received monoclonal antibodies or convalescent plasma as part of COVID-19 treatment. Based on the estimated half-life of such therapies as well as evidence suggesting that reinfection is uncommon in the 90 days after initial infection, vaccination should be deferred for at least 90 days as a precautionary measure until additional information becomes available to avoid the potential interference of the antibody therapy with vaccine-induced immune responses. This recommendation applies to persons who receive passive antibody therapy before receiving any vaccine doses, as well as those who receive passive antibody therapy after the first dose but before the second dose, in which case the second dose should be deferred for at least 90 days following receipt of the antibody therapy. For persons receiving antibody therapies for indications not related to COVID-19 (i.e., for autoimmune conditions or cancer therapy), the administration of mRNA COVID-19 vaccines either simultaneously with or at any interval before or after receipt of an antibody-containing product is considered unlikely to substantially impair the development of a protective antibody response. Therefore, there is no recommended minimum interval between non-COVID-19-related antibody therapies and receipt of one of the mRNA COVID-19 vaccines (CDC, 2020a, 2021a)
Pregnancy and Lactation
Pregnancy alone (apart from occupational-related risk for those in healthcare or other front-line workers) places women in a high-priority group and thereby eligible for vaccination. Pregnancy has been identified as a condition that increases the risk of serious illness with a COVID-19 infection, including hospitalization, ventilation requirement, and death. The American College of Obstetricians and Gynecologists (ACOG, 2021) and the CDC (2020a) collectively support and recommend vaccination in women of reproductive age during pregnancy and lactation based on the established priority criteria. ACOG (2021) last updated their statement on February 4, 2021, and summary of their major recommendations are as follows:
- Since the mRNA vaccines are not live virus vaccines and they do not enter the nucleus or alter human DNA, they cannot cause any genetic changes in the woman, fetus, infant or impact future pregnancies.
- Pregnancy testing should not be a requirement before receiving one of these mRNA COVID-19 vaccines.
- It is not necessary to delay pregnancy until after completing both doses of these mRNA COVID-19 vaccines.
- If a woman learns that she is pregnant after the first dose of the vaccine, she should still complete the series and receive her second dose on schedule.
- ACOG recommends that mRNA COVID-19 vaccines not be withheld from pregnant individuals who meet the vaccination criteria based on the CDC's recommended priority groups.
- COVID-19 vaccines should be offered to lactating individuals similar to non-lactating individuals when they meet the criteria for receiving the vaccine based on the CDC's prioritization groups.
Cancer and Other Immunocompromised Conditions
COVID-19 continues to place a significant burden on health systems across the world. Patients with cancer have increased susceptibility to COVID-19 and face reduced access to care due to competition and finite resources. The preliminary recommendations from the National Comprehensive Cancer Network (NCCN, 2021) COVID-19 Vaccination Advisory Committee were released on January 22, 2021. Large cohort studies have demonstrated that cancer patients are at high-risk for COVID-19 infection and associated complications. There is a clear need for vaccinating cancer patients to avoid excess morbidity and mortality in this at-risk population. Therefore, the NCCN (2021) advises that individuals with active cancer or with active, recent (less than 6 months), or planned cancer treatment should be considered among the highest priority groups to receive COVID-19 vaccination. The NCCN (2021) recommendations include the following:
- Cancer patients must be appropriately educated that although these vaccines have shown to be safe and effective in the general population, their safety profile and effectiveness among cancer patients is unknown.
- Patients with active cancer on treatment, those planned to start treatment, and those less than 6 months posttreatment should be prioritized for vaccination and should be immunized when vaccination is available to them
- this does not apply to those receiving only hormonal therapies
- immunization is recommended for these groups, with the understanding that there are limited safety and efficacy data in these patients
- Reasons to delay vaccination in this population are similar to those outlined for the general public (i.e., recent infection with COVID-19), and cancer-specific factors (i.e., those receiving intensive cytotoxic chemotherapy should delay until recovery of the absolute neutrophil count [ANC] as per the discretion of the treating clinician).
- Vaccination should be delayed for at least 3 months following hematopoietic cell transplantation or engineered cellular therapy (i.e., chimeric antigen receptor T-cell therapy [CAR-T]) to maximize the efficacy of the vaccine.
- Caregivers and household/close contacts of persons meeting the above criteria should be immunized when possible.
Persons with other immunocompromising conditions such as HIV and those prescribed non-cancer immunosuppressive agents might be at increased risk for severe COVID-19 infection. The CDC (2021a) recommends that these persons are vaccinated with no contraindications. However, these individuals must be appropriately counseled on the unknown vaccine safety and efficacy profiles in immunocompromised persons and the potential for a reduced immune response to the vaccine (CDC, 2021a).
JNJ-78436735 (Ad26.COV2.S) Vaccine
Janssen Pharmaceuticals is a Belgium-based division of Johnson & Johnson that has been instrumental in developing the JNJ-78436735 (Ad26.COV2.S) vaccination. As noted above, the vaccination received EUA from the FDA on February 27, 2021 and has already begun distribution throughout the US. They expect to supply 100 million doses to the US by mid-2021. The JNJ-78436735 (Ad26.COV2.S) vaccine is based on the virus’s genetic instructions for building the S protein; however, it is distinct from the Pfizer-BioNTech and Moderna mRNA vaccines since it uses double-stranded DNA (instead of single-stranded RNA) to store the instructions. With this vaccine, a gene for the coronavirus S protein was added to another virus called adenovirus 26. Adenoviruses are a group of common viruses that cause cold or flu-like symptoms. Utilizing recombinant, replication-defective adenovirus type 26 vector leveraging technology, the company modified a version of the human adenovirus. Due to genetic alterations, the adenovirus vector can enter cells but cannot replicate once inside them or cause illness. Once the double-stranded DNA is injected, the host cells generate the S protein, stimulating the body’s immune response to produce unique antibodies. The JNJ-78436735 (Ad26.COV2.S) only requires a single dose, not a two-dose series. The vaccine was studied in the Phase 3 ENSEMBLE study, a randomized, double-blind, placebo-controlled clinical trial in adults 18 years old and older. The trial was conducted in eight countries and enrolled more than 45,000 individuals. It included a diverse and broad population, with significant representation of Black, Hispanic/Latin, Native American, and Alaskan Native participants. The study enrolled 44% of participants in the US, 55% male, and 41% of participants had comorbidities associated with an increased risk for progression to severe COVID-19, including obesity (28.5%), type 2 diabetes (7.3%), hypertension (10.3%), and HIV (2.8%) (US National Library of Medicine, 2021). According to a press release, the Phase 3 ENSEMBLE clinical trial demonstrated that the investigational single-dose vaccine met all primary and secondary endpoints, assessed at day 14 and 28-time points. The company released the following findings regarding the efficacy of the JNJ-78436735 (Ad26.COV2.S) vaccine:
- 72% effective in the US
- 66% effective overall in preventing moderate to severe COVID-19, 28 days after administration
- 85% effective overall in preventing severe disease and demonstrated complete protection against COVID-19 related hospitalization and death as of day 28
- protection against severe disease across multiple virus variants, including the SARS-CoV-2 Variant from the B.1.351 lineage observed in South Africa (Johnson & Johnson, 2021a, 2021b).
The most frequent overall adverse effects cited in phase 1/2 trials include fatigue, headache, myalgia, and injection-site pain. Most events were grade 1 or 2 in severity, and systemic effects were more common in those aged 55 and younger than adults aged 65 and older. Information regarding its safety and efficacy in special populations, such as pregnant women and immunocompromised patients, is not yet available (Johnson & Johnson, 2021a, 2021b; Vizient, 2021).
ChAdOx1 nCoV-19 (AZD1222) Vaccine (Oxford-Astrazeneca)
The ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccine is a product of the University of Oxford and AstraZeneca’s combined efforts. While not yet approved for use in the US, it has received an EUA in Britain since December 2020 and other parts of Europe. On January 3, 2021, India authorized the use of a version of this vaccine called Covisheild. The ChAdOx1 nCoV-19 (AZD1222) vaccine is very similar to the JNJ-78436735 (Ad26.COV2.S) vaccine. It is a replication-defective vectored vaccine using an adenovirus vector. Researchers initially genetically engineered an adenovirus that typically infects monkeys, and they found that it protected animals from COVID-19 infections by raising antibodies and other immune defenses against the virus. Phase 2/3 trials were initially launched in the UK and India, followed by additional trials in Brazil, South Africa, and the US. The pharmacology and mechanism of mounting immunity are identical to the JNJ-78436735 (Ad26.COV2.S) vaccine described earlier. However, it is a two-dose vaccination series with the second dose administered between 4 and 12 weeks following the first. In phase 2/3 UK trials, the most common side effects included pain and tenderness at the injection site, fatigue, headache, fevers, and myalgias (ASHP, 2021; Voysey et al., 2021a, 2021b).
In November 2020, they announced that the vaccine had promising efficacy based on a study of the first 131 cases of COVID-19 trials in the UK and Brazil. However, while all volunteers received both doses, the first dose was only half-strength in some cases. Their findings demonstrated surprising results, as an initial half-strength dose led to 90% efficacy, while the two standard-dose shots led only to 62% efficacy. The lower dose resulted from a mistake in how the vaccines were measured and were not part of the original trial plan. Further, the half-strength was only administered to volunteers under 55 years, thereby obstructing the data's accurate extrapolation. Accounting for the mistake, the strength of the preliminary results was indeterminate. Recently, AstraZeneca released a statement announcing that the two doses (separated by 12 weeks) had an efficacy rate of 82.4%. On February 1, 2021, data from the phase 3 efficacy trials were released in a preprint with the Lancet, describing the safety of the two-dose vaccination series and the durability of the antibody levels by day 90. Further, efficacy was higher among those who received the second dose at 12 weeks than 6 weeks. Additionally, they reported that the vaccine protected people from getting sick and reduced the transmission of COVID-19 (Voysey et al., 2021a, 2021b). South Africa had planned to distribute 1 million doses of the ChAdOx1 nCoV-19 (AZD1222) vaccine but announced their decision to halt these plans on February 7, 2021. A small trial failed to demonstrate that the vaccine protected against an emerging variant of COVID-19 (B.1.351), widespread in the area (American Association for the Advancement of Science, 2021). Many questions remain unanswered regarding the ChAdOx1 nCoV-19 (AZD1222) vaccine, its side effects, the durability of antibodies, and its use in special populations (The New York Times, 2021; Zimmer et al., 2021).
NVX-CoV2373 (Novavax) Vaccine
The NVX-CoV2373 (Novavax) is a recombinant nanoparticle vaccine created by Novavax, a small, Maryland-based pharmaceutical company. NVX-CoV2373 is a protein-based subunit vaccine that works differently than the other COVID-19 vaccines. It comprises a synthetically engineered version of the S protein and an adjuvant (i.e., saponin-based Matrix-M). The adjuvant signals the immune system to spring into action, thereby enhancing the immune response to produce large quantities of neutralizing antibodies. This technology is not new, as it has been effectively used for the hepatitis B (HepB) and human papillomavirus vaccines (HPV), but it does take longer to develop. The NVX-CoV2373 (Novavax) vaccine does not contain live virus particles, and thus, it cannot replicate or cause infection with COVID-19. The vaccine demonstrated 89.3% efficacy in the UK phase 3 trial, which enrolled 15,000 participants between 18 and 84 years, including 27% over the age of 65. In a report released on January 28, 2021, the NVX-CoV2373 (Novavax) is cited as the first COVID-19 vaccine to demonstrate clinical efficacy against the UK and South Africa variants. Data from South Africa’s phase 2b trial showed a 60% risk reduction against COVID-19 in a region when over 90% of cases are attributable to the prevalent South Africa COVID-19 variant (Novavax, 2021a). In the US and Mexico, the phase 3 PRE-fusion protein subunit Vaccine Efficacy Novavax Trial (PREVENT-19) is underway and has already enrolled more than 16,000 participants; it expects to achieve the targeted enrollment of 30,000 by mid-February 2021. PREVENT-19 is a randomized, placebo-controlled, observer-blinded study evaluating the efficacy of NVX-CoV2373 in adults aged 18 and older, including at least 25% of participants aged 65 and older (Novavax, 2021b). The vaccine requires a two-dose series, administered 21 days (3 weeks) apart. Participants from phase 1/2 trials endured minimal adverse effects, with the most common including injection site pain, muscle pain, fever, rash, and nausea. Data revealed no serious adverse events, and systemic reactions were mild (Keech et al., 2020).
Emerging Variants: Novel Strains of COVID-19
Multiple COVID-19 variants emerged in the Fall of 2020 and continue to circulate the globe. While less is known about these variants, they are reported to be more transmissible versions of COVID-19. It remains unknown if patients with prior COVID-19 infections are less susceptible to becoming infected with one of these novel variants. It is also indeterminate if any of the COVID-19 vaccines provide adequate protection against the novel strains. The CDC is collaborating with other public health agencies to monitor the spread and evolution of these emerging variants. Although data remains limited at this time, Table 12 provides a brief synopsis of the identified variants as of February 9, 2021 (CDC, 2021e).
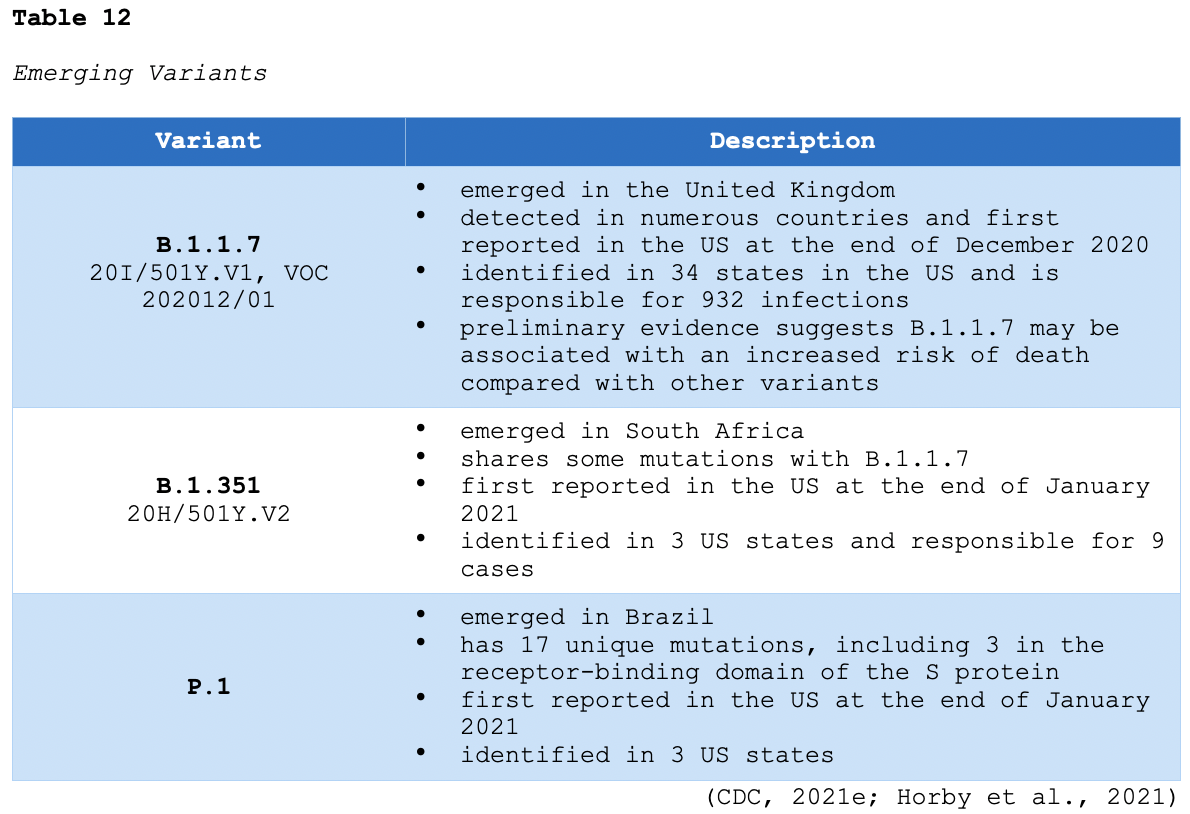
Information regarding these emerging variants is limited to case reports due to the small number of individuals infected. However, they appear to be evolving rapidly and have shown to spread more quickly, demonstrating enhanced transmissibility alongside a potentially higher mortality rate. They may cause either milder or more severe disease, but as noted in Table 12, the B.1.1.7 variant is associated with an increased risk of death compared to other variants. Additional studies are urgently needed to confirm this finding. It also remains unknown if these variants cause decreased susceptibility to COVID-19 therapeutic agents available as of February 2021, such as monoclonal antibodies and COVID-19 convalescent plasma. Further, the efficacy of COVID-19 vaccines against these variants is unknown. However, evading natural or vaccine-induced immunity is a possibility and requires urgent exploration (CDC, 2021; Horby et al., 2021).
References
Allotey, J., Stallings, E., Bonet, M., Yap, M., Chatterjee, S., Kew, T., Debenham, L., Llavall, A. C., Dixit, A., Zhou, D., Balaji, R., Lee, S. I., Qiu, X., Yuan, M., Coomar, D., van Wely, M., van Leeuwen, E., Kostova, E., Kunst, H., … Thangaratinam, S. (2020). Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ, 370(3320), 1-14. https://doi.org/10.1136/bmj.m3320
Alsved, M., Matamis, A., Bolin, R., Richter, M., Bengtsson, P. E., Fraenkel, C. J., Medstrand, P., & Londahl, J. (2020). Exhaled respiratory particles during singing and talking. Aerosol Science and Technology, 54(11), 1245-1248. https://doi.org/10.1080/02786826.2020.1812502
American Association for Clinical Chemistry. (2020). D-dimer. https://labtestsonline.org/tests/d-dimer
American Association for the Advancement of Science. (2021). South Africa suspends use of AstraZeneca’s COVID-19 vaccine after it fails to clearly stop virus variant. https://doi.org/10.1126/science.abg9559
American Board of Internal Medicine. (2020). ABIM laboratory test reference ranges – January 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American College of Obstetricians and Gynecologists. (2021). Vaccinating pregnant and lactating patients against COVID-19. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
American College of Radiology. (2020). ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
American Society of Health System Pharmacists. (2021). Assessment of evidence for COVID-19-related treatments: Updated 01/29/2021. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx
American Society of Hematology. (2020). ASH guidelines on the use of anticoagulation in patients with COVID-19. https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19
American Society of Hematology. (2021). COVID-19 and VTE/anticoagulation: Frequently asked questions. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
Azer, S, A. (2020). COVID-19: Pathophysiology, diagnosis, complications, and investigational therapeutics. New Microbes and New Infections, 37(100738), 1-8. https://doi.org/10.1016/j.nmni.2020.100738
Biegel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., Hohmann, E., Chu, H. Y., Luetkemeyer, A., Kline, S., de Castilla, D. L., Finberg, R. W., Dierberg, K., Tapson, V., Hsieh, L., Patterson, T. F., Paredes, R., Sweeney, D. A., Short, W. R., … Lane, H. C. (2020). Remdesivir for the treatment of COVID-19- Final report. New England Journal of Medicine, 383, 1813-1826. https://doi.org/10.1056/NEJMoa2007764
Calder, P. C., Carr, A. C., Gombart, A. F., & Eggersdorfer, M. (2020). Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients, 12(4), 1181. https://doi.org/10.3390/nu12041181
Carlucci, P. M., Ahuja, T., Petrilli, C., Rajagopalan, H., Jones, S., & Rahimian, J. (2020). Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. Journal of Medical Microbiology, 69 (10), 1228-1234. https://doi.org/10.1099/jmm.0.001250
Cavalcanti, A. B., Zampieri, F. G., Rosa, R. G., Azevedo, L. C., Veiga, V. C., Avezum, A., Damiani, L. P., Marcadenti, A., Kawano-Dourado, L., Lisboa, T., Junqueira, D. L., de Barros e Silva, P. G., Tramujas, L., Abruei-Silva, E. O., Laranjeira, L. N., Soares, A. T., Echenique, L. S., Pereira, A. J., Freitas, F. G., … Berwanger, O. (2020). Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. New England Journal of Medicine, 383, 2041-2053. https://doi.org/10.1056/NEJMoa2019014
Centers for Disease Control and Prevention. (2018). Understanding how vaccines work. https://www.cdc.gov/vaccines/hcp/conversations/downloads/vacsafe-understand-color-office.pdf
Centers for Disease Control and Prevention. (2020a). COVID-19 vaccination: Clinical resources for each COVID-19 vaccine. https://www.cdc.gov/vaccines/covid-19/index.html
Centers for Disease Control and Prevention. (2020b). Immunization courses: Webcast and self-study. https://www.cdc.gov/vaccines/ed/courses.html#covid-19
Centers for Disease Control and Prevention. (2020c). Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
Centers for Disease Control and Prevention. (2020d). People with certain medical conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Centers for Disease Control and Prevention. (2020e). Scientific brief: SARS-CoV-2 and potential airborne transmission. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html#:~:text=%E2%80%A2%20Larger%20droplets%20some%20of,source%20on%20air%20currents
Centers for Disease Control and Prevention. (2020f). When to quarantine. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html
Centers for Disease Control and Prevention. (2021a). Interim clinical considerations for use of mRNA covid-19 vaccines currently authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
Centers for Disease Control and Prevention. (2021b). Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
Centers for Disease Control and Prevention. (2021c). Isolate if you are sick. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/isolation.html
Centers for Disease Control and Prevention. (2021d). Test for current infection (viral test). https://www.cdc.gov/coronavirus/2019-ncov/testing/diagnostic-testing.html
Centers for Disease Control and Prevention. (2021e). US COVID-19 cases caused by variants. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant-cases.html
Cheruiyot, I., Kipkorir, V., Ngure, B., Misiani, M., Munguti, J., & Ogene’o, J. (2021). Arterial thrombus in coronavirus disease 2019 patients: A rapid systematic review. Ann Vasc Surg, 70, 273-281. https://doi.org/10.1016/j.avsg.2020.08.087
Choe, P. G., Kim, K., Kang, C. K., Suh, H. J., Kang, E., Lee, S. Y., Kim, N. J., Yi, J., Park, W. B., & Oh, M. (2021). Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerging Infectious Diseases, 27(3). https://doi.org/10.3201/eid2703.204543
Dan, J. M., Mateus, J., Kato, Y., Hastie, K. M., Yu, E. D., Falithi, C. E., Grifoni, A., Ramirez, S. I., Haupt, S., Frazier, A., Nakao, C., Rayaprolu, V., Rawlings, S. A., Peters, B., Krammers, F., Simon, V., Saphire, E. O., Smith, D. M., Weiskopf, D., … Crotty, S. (2021). Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science, 1-23. https://doi.org/10.1126/science.abf4063
Delmalani18. (2018). Cross section of an alveolus and gas exchange [image]. https://commons.wikimedia.org/wiki/File:Cross_section_of_an_alveolus_and_capillaries_showing_diffusion_of_gases.svg
Gautret, P., Lagier, J., Parola, P., Hoang, V. T., Meddeb, L., Sevestre, J., Mailhe, M., Doudier, B., Aubry, C., Amrane, S., Seng, P., Hocquart, M., Eldin, C., Finance, J., Vieira, V. E., Tissot-Dupon, H. T., Honore, S., Stein, A., Million, M., … Raoult, D. (2020). Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least six-day follow-up: A pilot observational study. Travel Medicine of Infectious Diseases, 34, 101663. https://doi.org/10.1016/j.tmaid.2020.101663
Ginde, A. A., Brower, R. G., Caterino, J. M., Finck, L., Banner-Goodspeed, V. M., Grissom, R. G., Hayden, D., Hough, C. L., Hyzy, R. C., Khan, A., Levitt, J. E., Park, P. K., Ringwood, N., Rivers, E. P., Self, W. H., Shapiro, N. I., Thompson, B. T., Yealy, D. M., Talmor, D., … National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. (2019). Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. New England Journal of Medicine, 381(26), 2529-2540. https://doi.org/10.1056/NEJMoa1911124
Gordon, A.C., Mouncey, P. R., Al-Beidh, F., Rowan, K. M., Nichol, A. D., Arabi, Y. M., Annane, D., Beane, A., van Bentum-Puijk, W., Berry, L. R., Bhimani, Z., Bonten, M. J., Bradbury, C. A., Brunkhorst, F. M., Buzgau, A., Cheng, A. C., Detry, M. A., Duffy, E. J., Estcourt, L. J., Fitzgerald, M., Goossens, H., Haniffa, R.,. … Derde L. P. (2021). Interleukin-6 receptor antagonists in critically ill patients with COVID-19- Preliminary report. medRxiv, https://doi.org/10.1101/2021.01.07.21249390
Gross, P. L., Weil, P., & Rand, M. L. (2018). Harper’s illustrated biochemistry. (31st ed.). McGraw-Hill.
Guan, W., Ni, Z., Hu, Y., Liang, W., Ou, C., He, J., Liu, L., Shan, H., Lei, C., Hui, D. S., Du, B., Li, L., Zeng, G., Yuen, K., Chen, R., Tang, C., Wang, T., Chen, P., Xiang, J., … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Med, 382, 1708-1720. https://doi.org/10.1056/NEJMoa2002032
Hall, V., Foulkes, S., Charlett, A., Atti, A., Monk, E. J., Simmons, R., Wellington, E., Cole, M. J., Saei, A., Oguti, B., Munro, K., Wallace, S., Kirwan, P. D., Shrotri, M., Vusirikala, A., Rokadiya, S., Kall, M., Zambon, M., Ramsay, M., Brooks, T., Brown, C. S., Chand, M. A., … Hopkins, S. (2021). Do antibody-positive healthcare workers have lower SARS-CoV-2 infection rates than antibody-negative healthcare workers? Large multicentre prospective cohort study (the SIREN study), England: June to November 2020. medRxiv, 1-35. https://doi.org/10.1101/2021.01.13.21249642
He, W., Yi, G. Y., & Zhu, Y. (2020). Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J Med Virol, 92(11), 2543-2550. https://doi.org/10.1002/jmv.26041
Helms, J., Tacquard, C., Severac, F., Leonard-Lorant, I., Ohana, M., Delabranche, X., Merdji, H., Clere-Jehl, R., Schenck, M., Gandet, F., Fafi-Kremer, S., Castelain, V., Schneider, F., Grunebaum, L., Anglés-Cano, E., Sattler, L., Mertes, P., Meziani, F., & the members of Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis. (2020). High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Medicine, 46, 1089-1098. https://doi.org/10.1007/s00134-020-06062-x
Henry, B. M., de Oliveira, M. H., Benoit, S., Plebani, M., & Lippi, G. (2020). Hematologic, biochemical, and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clinical Chemistry and Laboratory Medicine, 58(7), 1021-1028. https://doi.org/10.1515/cclm-2020-0369
Horby, P., Lim, W. S., Emberson, J.R., Mafham, M., Bell, J. L., Linsell, L., Staplin, N., Brightling, C.,
Ustianowski, A., Elmahi, E., Prudon, B., Green, C., Felton, T., Chadwick, D., Rege, K., Fegan, C., Chappell, L. C., Faust, S. N., Jaki, T., & the members of The RECOVERY Collaborative Group. (2020). Dexamethasone in hospitalized patients with COVID-19- Preliminary report. New England Journal of Medicine, 1-11, https://doi.org/10.1056/NEJMoa2021436
Horby, P., Huntley, C., Davies, N., Edmunds, J., Ferguson, N., Medley, G., Hayward, A., Cevik, M., & Semple, C. (2021). SAGE meeting paper: NERVTAG note on B.1.1.7 severity. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/955239/NERVTAG_paper_on_variant_of_concern__VOC__B.1.1.7.pdf
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z. Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497-506. https://doi.org/10.1016/S0140-6736(20)30183-5
Johnson & Johnson. (2021a). Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its Phase 3 ENSEMBLE trial. https://www.prnewswire.com/news-releases/johnson--johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial-301218035.html
Johnson & Johnson. (2021b). Johnson & Johnson announces submission of application to the US FDA for emergency use authorization of its investigational single-shot Janssen COVID-19 vaccine candidate. https://www.jnj.com/johnson-johnson-announces-submission-of-application-to-the-u-s-fda-for-emergency-use-authorization-of-its-investigational-single-shot-janssen-covid-19-vaccine-candidate
Johnson, B. A., Xie, X., Kalveram, B., Lokugamage, K. G., Muruato, A., Zou, J., Zhang, X., Juelich, T., Smith, J. K., Zhang, L., Bopp, N., Schindewolf, C., Vu, M., Vanderheiden, A., Swetnam D., Plante, J. A., Aguilar, P., Plante, K. S., Lee, B., … Menachery, V. D. (2020). Furin cleavage site is key to SARS-CoV-2 pathogenesis. bioRxiv [The Preprint Server for Biology]. https://doi.org/10.1101/2020.08.26.268854
Johnson, B. A., Xie, X., Bailey, A. L., Kalveram, B., Lokugamage, K. G., Muruato, A., Zou, J., Zhang, X., Juelich, T., Smith, J. K., Zhang, L., Bopp, N., Schindewolf, C., Vu, M., Vanderheiden, A., Winkler, E. S., Swetnam, D., Plante, J. A., Aguilar, P., ... Menachery, D. (2021). Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature, 1-12. https://doi.org/10.1038/s41586-021-03237-4
Joyner, M. J., Bruno, K. A.., Klassen, S. A., Kunze, K. L., Johnson, P. W., Lesser, E. R., Wiggins, C. C., Senefeld, J. W., Klompas, A. M., Hodge, D. O., Shepherd, J. R., Rea, R. F., Whelan, E. R., Clayburn, A. J., Spiegel, M. R., Baker, S. E.., Larson, K. F., Ripoll, J. G., Andersen, K. J., … Wright, R. S. (2020). COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clinic Proceedings, 95(9), 1888-1897. https://doi.org/10.1016/j.mayocp.2020.06.028
Kalil, A. C., Patterson, T. F., Mehta, A. K., Tomashek, K. M., Wolfe, C. R., Ghazaryan, V., Marconi, V. C., Ruiz-Palacios, G. M., Hsieh, L., Kline, S., Tapson, V., Lovine, N. M., Jain, M. K., Sweeney, D. A., El Sahly, H. M., Branche, A. R., Pineda, J. R., Lye, D. C., Sandkovsky, U., ... Biegel, J. H. (2020). Baricitinib plus remdesivir for hospitalized adults with COVID-19. New England Journal of Medicine, 1-13, https://doi.org/10.1056/NEJMoa2031994
Keech, C., Albert, G., Cho, I., Robertson, A., Reed, P., Neal, S., Plested, J. S., Zhu, M., Cloney-Clark, S., Zhou, H., Smith, G., Patel, N., Frieman, M. B., Haupt, R. E., Logue, J., McGrath, M., Weston, S., Piedra, P. A., … Glenn, G. M. (2020). Phase 1-2 trial of SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine, 383, 2320-2332. https://doi.org/10.1056/NEJMoa2026920
Klassen, S. A., Senefeld, J. W., Johnson, P. W., Carter, R. E., Wiggins, C. C., Shoham, S., Grossman, B. J., Henderson, J. P., Musser, J. M., Salazar, E., Hartman, W. R., Bouvier, N. M., Liu, S. T., Pirofski, L., Baker, S. E., van Helmond, N., Wright, R. S., Fairweather, D., Bruno, K. A. … Joyner, M. J. (2020). Evidence favoring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. https://doi.org/10.1101/2020.07.29.20162917
Klok, F. A., Keuip, M. J., van der Meer, N. J., Arbous, M. S., Gommers, D. A., Kant, K. M., Kaptein, F. H., van Paassen, J., Stals, M. A., Huisman, M. V., & Enderman, H. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research, 191, 145-147. https://doi.org/10.1016/j.thromres.2020.04.013
Li, L., Zhang, W., Hu, Y., Tong, X. Zheng, S., Yang, J., Kong, Y., Ren, L., Wei, Q., Mei, H., Hu, C., Tao, C., Yang, R., Wang, J., Yu, Y., Guo, Y., Wu, X., Xu, Z., Zeng, L., … Liu, Z. (2020). Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA, 324(5), 460-470. https://doi.org/10.1001/jama.2020.10044
Lim, W., Gal, G. L., Bates, S. M., Righini, M., Haramati, L. B., Lang, E., Kline, J. A., Chasteen, S., Snyder, M., Patel, P., Bhatt, M., Patel, P., Braun, C., Begum, H., Wiercioch, W., Schunemann, H. K., & Mustafa, R. A. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Advances, 2(22), 3226–3256. https://doi.org/10.1182/bloodadvances.2018024828
Longo, D. L. (2019). Harrison’s hematology and oncology. (3rd ed.). McGraw-Hill Education.
Lumley, S.F., O’Donnell, D., Stoesser, N. E., Matthews, P. C., Howarth, A., Hatch, S. B., Marsden, B. D., Cox, S., James, T., Warren, F., Peck, L. J., Ritter, T. G., de Toledo, Z., Warren, L., Axten, D., Cornall, R., Jones, E. Y., Stuart, D. I., Screaton, G., … Eyre, D. W. (2020). Antibodies to SARS-CoV-2 are associated with protection against reinfection. medRxiv, 1-27. https://doi.org/10.1101/2020.11.18.20234369
Mancia, G., Rea, F., Ludergnani, M., Apolone, G., & Corrao, G. (2020). Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. New England Journal of Medicine, 382, 2431-2440. https://doi.org/10.1056/NEJMoa2006923
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
Merzon, E., Tworowski, D., Gorohovski, A., Vinker, S., Cohen, A. G., Green, I., & Morgenstern, M. F. (2020). Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. The FEBS Journal, 287, 3693-3702. https://doi.org/10.1111/febs.15495
Morawska, L., & Milton, D. K. (2020). It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clinical Infectious Disease, 71(9), 2311-2313. https://doi.org/10.1093/cid/ciaa939
Murk, W., Gierado, M., Fralick, M., Weckstein, A., Klesh, R., & Rassen, J. A. (2021). Diagnosis-wide analysis of COVID-19 complications: An exposure-crossover study. CMAJ, 193(1), https://doi.org/10.1503/cmaj.201686
National Center for Health Statistics. (2021). COVID-19 mortality overview. https://www.cdc.gov/nchs/covid19/mortality-overview.htm
National Comprehensive Cancer Network. (2021). Preliminary recommendations of the NCCN COVID-19 vaccination advisory committee: Version 1.0. https://www.nccn.org/covid-19/pdf/COVID-19_Vaccination_Guidance_V1.0.pdf
National Institutes of Health. (2021). Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/
National Institutes of Health Office of Dietary Supplements. (2020). Vitamin D: Fact sheet for health professionals. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice. (11th ed.). Wolters Kluwer
Novavax. (2021a, February 14). Press release: Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
Novavax. (2021b, February 14). What is the Novavax vaccine, and how does it work? https://qz.com/1950365/what-is-the-novavax-vaccine-and-how-does-it-work/
The New York Times. (2021, February 13). COVID-19: The world has changed. So has the playbook for the Olympics. https://www.nytimes.com/live/2021/02/03/world/covid-19-coronavirus
Nikhra, V. (2020). Exploring pathophysiology of COVID-19 infection: Faux espoir and dormant therapeutic options. International Journal of Clinical Virology, 4, 34-40. https://doi.org/10.29328/journal.ijcv.1001013
Nisio, M. D., Wichers, I., & Middeldorp, S. (2018). Treatment of lower extremity superficial thrombophlebitis. JAMA, 320(22), 2367–2368. https://doi.org/10.1001/jama.2018.16623
Parasher, A. (2020). COVID-19: Current understanding of its pathophysiology, clinical presentation, and treatment. Postgraduate Medical Journal, 0, 1-9. https://doi.org/10.1136/postgradmedj-2020-138577
Prescribers’ Digital Reference. (2021). Fondaparinux sodium - Drug summary. https://www.pdr.net/drug-summary/Arixtra-fondaparinux-sodium-170.2712
Qiao, Y., Wang, X., Mannan, R., Pitchiaya, S., Zhang, Y., Wotring, J. W., Xiao, L., Robinson, D. R., Wu, Y., Tien, J. C., Cao, X., Simko, S. A., Apel, I. J., Bawa, P., Kregel, S., Narayanan, S. P., Raskind, G., Ellison, S. J., Parolia, A., … Chinnaiyan, A. M. (2021). Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. PNAS, 118(1), https://doi.org/10.1073/pnas.2021450118
Rahmani, H., Davoudi-Monfared, E., Nourian, A., Khalili, H., Hajizadeh, N., Jalabadi, N. Z., Fazeli, M. R., Ghazaeian, M., & Yekaninejad, M. S. (2020). Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. International Immunopharmacology, 88(106903), 1-9. https://doi.org/10.1016/j.intimp.2020.106903
Rajter, J. C., Sherman, M. S., Fatteh, N., Vogel, F., Sacks, J., & Rajter, J. (2021). Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019. Chest Journal, 159(1), 85-92. https://doi.org/10.1016/j.chest.2020.10.009
Rawson, T. M., Moore, L. S., Zhu, N., Ranganathan, N., Skolimowska, K., Gilchrist, M., Satta, G., Cooke, G., & Holmes, A. (2020). Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clinical Infectious Diseases, 71(9), 2459-2468. https://doi.org/10.1093/cid/ciaa530
Sax, P. E. (2021). Covid-19 vaccine—Frequently asked questions. The New England Journal of Medicine. https://www.nejm.org/covid-vaccine/faq
Selchick, F. (2021). COVID-19 prevention strategies [image].
Sengupta, S. (2017). Cancer nanomedicine: Lessons for immune-oncology. Trends Cancer, 3(8), 551-560. https://doi.org/10.1016/j.trecan.2017.06.006.
Singh, R. B. (2020). Structure of SARS-CoV-2 virus [image]. https://commons.wikimedia.org/wiki/File:Struktura_SARS-CoV_2.jpg
Sterne, J. A., Murthy, S., Diaz, J. V., Slutsky, A. S., Villar, J., Angus, D. C., Annane, D., Azevedo, L. C., Berwanger, O., Cavalcanti., A. B., Dequin, P., Du, B., Emberson, J., Fisher, D., Giraudeau, B., Gordon, A. C., Grandholm, A., Green C., Haynes, R., … the members of WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. (2020). Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA, 324(13), 1330-1341. https://doi.org/10.1001/jama.2020.17023
Suh, Y. J., Hong, H., Ohana, M., Bompard, F., Revel, M., Valle, C., Gervaise, A., Poissy, J., Susen, S., Hekimian, G., Artifoni, M., Periard, D., Contou, D., Delaloye, J., Sanchez, B., Fang, C., Garzillo, G., Robbie, H., & Yoon, S. H. (2020). Pulmonary embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis. Radiology, 298(2), E70-E80. https://doi.org/10.1148/radiol.2020203557
Sumaiya. (2009). Anatomy of alveoli and bronchioles [image]. https://commons.wikimedia.org/wiki/File:Lungs_Anatomy.jpg
Tang, N., Li, D., Wang, X., & Sun, Z. (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 18(4), 844-847. https://doi.org/10.1111/jth.14768
Tong, J.Y., Wong, A., Zhu, D., Fastenberg, J.H., & Tham, T. (2020) The prevalence of olfactory and gustatory dysfunction in COVID19 patients: A systematic review and meta-analysis. Otolaryngol Head Neck Surg, 163(1), 3-11. https://doi.org/10.1177/0194599820926473
Yoshikawa, A., Munkdelger, J., & Bychkov, A. (2021). Infectious viral covid-19. University of Washington Medicine. https://www.pathologyoutlines.com/topic/lungnontumorcovid.html
US Census Bureau. (2019). Quick facts: United States population estimates July 1, 2019, (v2019). https://www.census.gov/quickfacts/fact/table/US/PST045219
US Department of Health and Human Services. (n.d.). Report an adverse event to VAERS. Retrieved January 24, 2021, from https://vaers.hhs.gov/reportevent.html
US Food & Drug Administration. (2020a). Coronavirus testing basics. https://www.fda.gov/media/138094/download
US Food & Drug Administration. (2020b.) Fact sheet for health care providers: Emergency use authorization (EUA) of baricitinib. https://www.fda.gov/media/143823/download
US Food & Drug Administration. (2020c). Fact sheet for health care providers: Emergency use authorization (EUA) of casirivimab and imdevimab. https://www.fda.gov/media/143892/download
US Food & Drug Administration. (2020d). FDA letter to stakeholders: Do not use ivermectin intended for animals as treatment for COVID-19 in humans. https://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-ivermectin-intended-animals-treatment-covid-19-humans
US Food & Drug Administration. (2020e). Highlights of prescribing information: VEKLURY®(remdesivir) for injection, for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf
US Food & Drug Administration. (2020f). Letter revoking EUA for chloroquine phosphate and hydroxychloroquine sulfate, 6/15/2020. https://www.fda.gov/media/138945/download
US Food & Drug Administration. (2021a). Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
US Food & Drug Administration. (2021b). Fact sheet for health care providers: Emergency use authorization (EUA) of bemlanivimab. https://www.fda.gov/media/143603/download
US Food & Drug Administration. (2021c). Fact sheet for health care providers: Emergency use authorization (EUA) of convalescent plasma for treatment of hospitalized patients with COVID-19. https://www.fda.gov/media/141478/download
US Food & Drug Administration. (2021d). Janssen COVID-19 vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine
US National Library of Medicine. (2021). A study of Ad26.COV2.S for the prevention of SARS-CoV-2-mediated COVID-19 in adult participants (ENSEMBLE). https://clinicaltrials.gov/ct2/show/NCT04505722
Velavan, T. P., & Meyer, C. G. (2020). Mild versus severe COVID-19: Laboratory markers. International Journal of Infectious Diseases, 95, 304-307. https://doi.org/10.1016/j.ijid.2020.04.061
Vizient. (2021). COVID-19 vaccine candidates. https://www.vizientinc.com/-/media/documents/sitecorepublishingdocuments/public/covid19_sidebyside_vaccinecompare.pdf
Voysey, M., Clemens, S. A., Madhi, S. A., Weckx, L. Y., Folgetti, P. M., Aley, P. K., Angus, B., Baillie, V. L., Barnabas, S. L., Bhorat, Q. E., Bibi, S., Briner, C., Cicconi, P., Collins, A. M., Colin-Jones, R., Cutland, C. L., Darton, T. C., Dheda, K., Duncan, C. J., …the members of the OXFORD COVID Vaccine Trial Group. (2021a). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, 397(10269). 99-111. https://doi.org/10.1016/S0140-6736(20)32661-1
Voysey, M., Clemens, S. A., Madhi, S. A., Weckx, L. Y., Folgetti, P. M., Aley, P. K., Angus, B., Baillie, V. L., Barnabas, S. L., Bhorat, Q. E., Bibi, S., Briner, C., Cicconi, P., Clutterbuck, E., Collins, A., Cutland, C., Darton, T., Dheda, K., Douglas, A. D., … the members of the OXFORD COVID Vaccine Trial Group. (2021b). Single dose administration, and the influence of the timing of the booster on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine. Preprints with The Lancet, 1-37. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3777268
Wang, N., Zhan, Y., Zhu, L., Hou, Z., Liu, F., Song, p., Qiu, F., Wang, X., Zou, X., Wan, D., Qian, X., Wang, S., Guo, Y., Yu, H., Cui, M., Tong, G., Xu, Y., Zheng, Z., lu, Y., & Hong, P. (2020). Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVDI-19 patients. Cell Host & Microbe, 28, 455-464. https://doi.org/10.1016/j.chom.2020.07.005
Wei., X., Wang, Z., Liao, X., Guo, W., Wen, J., Qin, T., & Wang, S. (2020). Efficacy of vitamin C in patients with sepsis: An updated meta-analysis. Eur J Pharmacol, 868, 172889. https://doi.org/10.1016/j.ejphar.2019.172889
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J., & Prescott, H. C. (2020). Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA, 324(8), 782-793. https://doi.org/10.1001/jama.2020.12839
Witt, D. M., Nieuwlaat, R. Clark, N. P., Ansell, J., Holbrook, A., Skov, J., Shehab, N., Mock, J., Myers, T., Dentali, F., Crowther, M.A., Agarwal, A., Bhatt, M., Khatib, R., Riva, J. J., Zhang, Y., & Guyatt, G. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Advances, 2(22). 3257-3291.https://doi.org/10.1182/bloodadvances.2018024893
Woolf, S. H., Chapman, D. A., & Lee, J. H. (2020). COVID-19 as the leading cause of death in the United States. JAMA, 325(2), 123-124. https://doi.org/10.1001/jama.2020.24865
World Health Organization. (2020a). Clinical management of patients with COVID-19. https://openwho.org/courses/clinical-management-COVID-19-general-considerations
World Health Organization. (2020b). How do vaccines work? https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work
World Health Organization. (2021a). COVID-19 clinical management. https://www.who.int/publications/i/item/clinical-management-of-covid-19
World Health Organization. (2021b). WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., Huang, H., Zhang, L., Zhou, X. Du, C., Zhang, Y., Song, J., Wang, S., Chao, Y., Yang, Z., Xu, H., Zhou, X., Chen, D., Xiong, W., … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med, 180(7), 934-943. https://doi.org/10.1001/jamainternmed.2020.0994
Wu, Z., & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease (COVID-19) outbreak in China. JAMA, 323(13), 1239-1242. https://doi.org/10.1001/jama.2020.2648
Xing, Y., Li, X., Gao, X., & Dong, Q. (2020). Natural polymorphisms are present in the furin cleavage site of the SARS-CoV-2 spike glycoprotein. Frontiers in Genetics, 11(783). https://doi.org/10.3389/fgene.2020.00783
Zimmer, C., Corum, J., & Wee, S. (2021). Coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html