About this course:
The purpose of this module is to provide an overview of the pathophysiology of the three primary types of diabetes, the risk factors, and the current diagnostic and management guidelines.
Course preview
Objectives:
- Articulate the pathophysiology of the primary forms of diabetes.
- Discuss the risk factors for diabetes.
- Explore the diagnostic guidelines for diabetes.
- Define evidence-based care for the management of diabetes.
- Recognize complications of diabetes and opportunities to decrease risk.
Each year in the US, about 1.4 million individuals are diagnosed with diabetes, and while the incidence of diabetes has declined every year since 2008, the prevalence has gradually increased since 1980. In 2015, there were an estimated 30 million individuals in the US with some form of diabetes, both diagnosed and undiagnosed (The Centers for Disease Control and Prevention [CDC], 2017). In 2017, the overall cost was calculated to be in excess of $327 billion dollars and appears to be rising annually, with the average medical expenditure cost per person near $17,000 (American Diabetes Association [ADA], 2018). It is easy to see the economic impact on the healthcare system as well as the personal impact on the individual diagnosed with diabetes.
Statistical Data in the US
There is a tremendous amount of data gathered each year related to diabetes. It is important to understand individuals and populations at risk to provide targeted education and prevention information. Among individuals aged 18 years or older in the US, when categorized by ethnic group, Native Americans and Alaska Natives had the highest rate of diagnosed diabetes between 2013 and 2015, totaling 15.1%. The next highest groups were African Americans (prevalence rate of 12.7% of US adults), Hispanic or Latin Americans (12.1%), Asian Americans (8%), and European Americans (7.4%). When US adults with diabetes between 2013-15 were categorized by education level, those patients with less than a high school diploma had the highest prevalence rate at 12.6%. Those with a high school diploma had a prevalence rate of 9.5%, followed by those with more than a high school diploma at 7.2% (CDC, 2017).
While the national median indicates that just over 9% of the adult population in the US is diagnosed with diabetes, the state with the highest prevalence is Mississippi at 13.6%, and the lowest is Colorado at 6.4%. Most of the south has double-digit prevalence rates, including Texas, Louisiana, Arkansas, Mississippi, Alabama, Georgia, South Carolina, Kentucky, and Tennessee. In 2015, approximately 193,000 of the diagnosed diabetics were children under the age of 20. Type 1 (T1DM) and type 2 diabetes (T2DM) are rising in children with an increase of 4.8% in T2DM versus a 1.8% increase in T1DM. European American children were diagnosed with T1DM at a rate of 27 per 100,000, versus African American children at a rate of 19 per 100,000 and Native American children at a rate of 6.5 per 100,000. Conversely, Native American children were diagnosed with T2DM at a rate of 46.5 per 100,000, African American children at a rate of 32.6 per 100,000, and European American children at a rate of 3.9 per 100,000 (CDC, 2017).
In addition to the high number diagnosed with some form of diabetes, the CDC estimates that over 84 million US adults over age 18 (almost 34%) have prediabetes; a significant number remain undiagnosed and not aware of their risk or condition. This leaves a significant opportunity for education, screening, and prevention measures. Public service announcements put out by the CDC in 2016 focused on getting individuals to check their risk in a campaign called “Know Where you Stand, DoIHavePrediabetes.org”. It consists of a one-minute screening quiz that will identify prediabetes risk as well as local resources to find teaching and support to decrease risk (CDC, 2017; Zand et al., 2018).
In 2017, the reported cost of medication and supplies to treat diabetes was $29.3 billion. An additional $37.3 billion was spent on cardiovascular-related illnesses associated with diabetes. Patients over 65 years of age contribute greatly to the growing economic impact of diabetes. Studies have shown that the average hospital stay is longer for patients with diabetes as compared to those without. There is also increased utilization of all healthcare services among patients with diabetes. There was a projected 40.3 million hospital days incurred by patients with diabetes in 2017, and about one-fourth of all nursing/residential facility days are incurred by patients with diabetes. Almost half of all physician office visits, emergency department visits, hospital outpatient visits, and medication prescriptions incurred by patients with diabetes are attributed to their diabetic diagnoses. In addition to the monetary cost of diabetes, diabetes also imposes a negative impact on quality of life, is associated with disability leading to inability to work/lost productivity, premature death, pain, and suffering of the patient and their loved ones (ADA, 2018).
Pathophysiology and Risk Factors of Diabetes
Diabetes mellitus (DM) is a chronic disease impacting multiple body systems due to abnormal insulin production, impaired insulin utilization, or both. If inadequately treated, DM can lead to severe complications; DM is the leading cause of end-stage renal disease, blindness, and non-traumatic lower-limb amputations. DM is a major contributing factor to hypertension (HTN, elevated blood pressure), cardiovascular disease (CVD), and stroke, which all lead to premature death (Lewis et al., 2014).
The cause of diabetes is a combination of genetic, autoimmune, and environmental factors, including viruses and obesity. Normal insulin metabolism occurs through the continuous release of insulin by the ß (beta)-cells in the islets of Langerhans of the pancreas (Figure 1). Insulin synthesis begins with its precursor, proinsulin. Proinsulin is split by enzymes to make insulin and C-peptide in equal amounts. This byproduct, C-peptide, is useful when assessing pancreatic ß-cell function as it can be measured in the urine and blood. The average amount of insulin secreted daily by a healthy adult is 40-50 U or 0.6 U/kg of body weight. Insulin acts as an anabolic or storage hormone in the body. The insulin secreted with food intake promotes glucose transport into the cell to be used for energy by unlocking receptor sites in the skeletal muscle and adipose tissue. Skeletal muscles and adipose tissue are considered insulin-dependent; the brain, liver, and blood cells do not depend on insulin and only require an adequate supply of glucose for normal functioning. While liver cells (hepatocytes) are not insulin-dependent, they do have “insulin receptor sites that facilitate hepatic uptake of glucose and its conversion to glycogen” (Lewis et al., 2014, p. 1154). As blood glucose (BG) increases after a meal or food intake, glucose is stored as glycogen in the liver and muscle tissue. Concurrently, the secretion of insulin inhibits gluconeogenesis (the production of glucose from non-sugar substances), enhances the deposition of adipose tissue, and increases protein synthesis. The reduced insulin that occurs overnight (or during periods of fasting) causes the liver to release glucose, the muscles to release proteins, and the adipose tissue to release fat (Lewis et al., 2014).
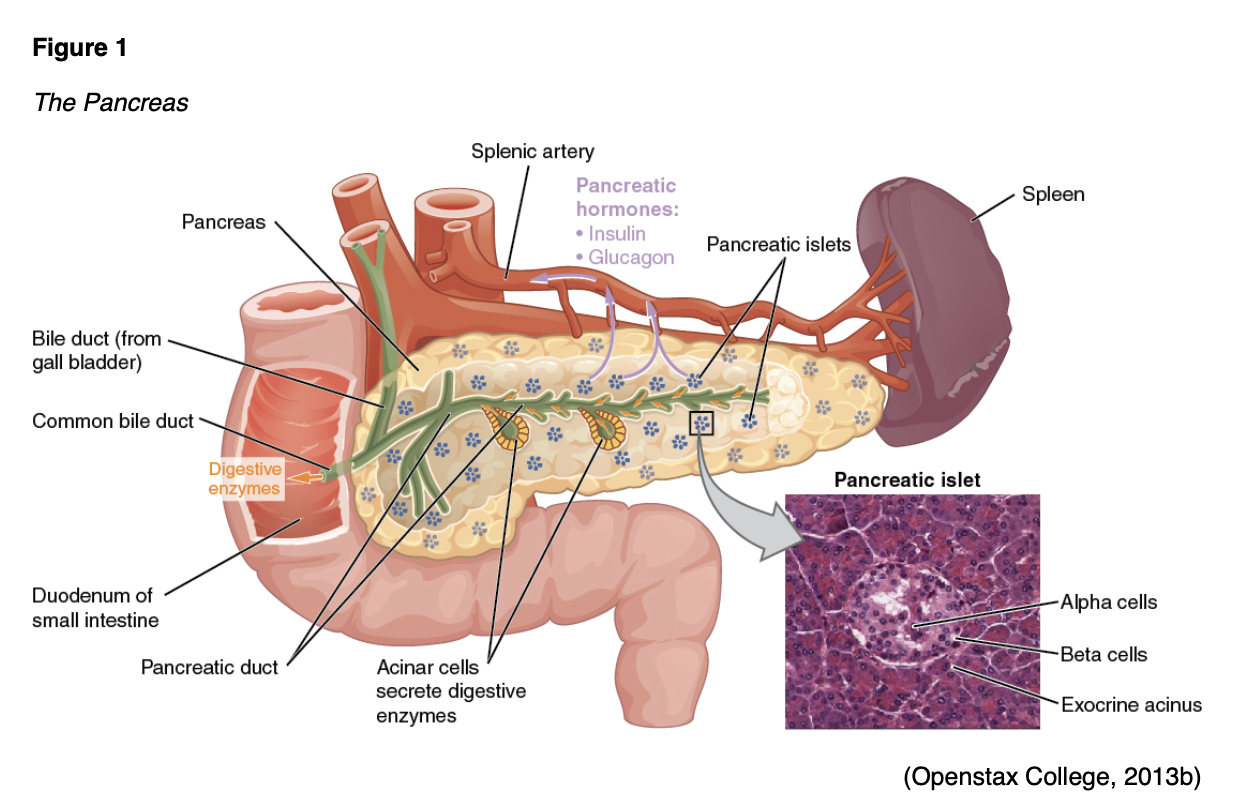
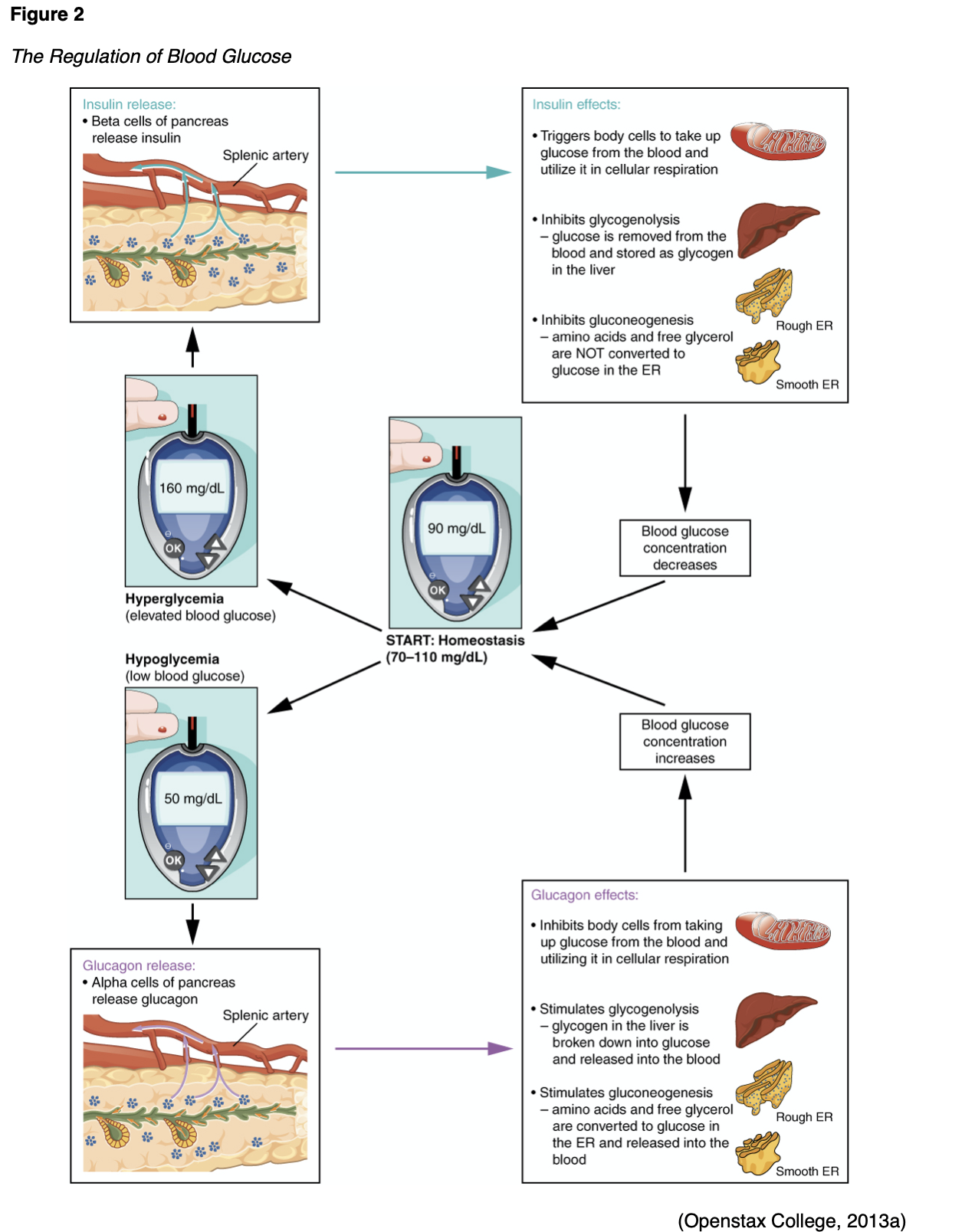
Counter-regulatory hormones such as glucagon, epinephrine, growth hormone, and cortisol work to oppose the effects of insulin (Figure 2). They increase BG by stimulating the production of glucose and liver output and decrease the movement of glucose into the cells. Insulin secretion is designed to maintain a stable BG level of 70-120 mg/dL. The normal range of BG levels is typically maintained by regulating the release of glucose for energy during periods of fasting, food intake, and the production and release of insulin and the counter-regulatory hormones (L
...purchase below to continue the course
Type 1 DM is characterized by the autoimmune destruction of the pancreatic ß-cells leading to the total absence of insulin production. There is typically a genetic predisposition compounded by exposure to a virus that contributes to this autoimmune condition. Autoantibodies to the islet cells cause a decrease in the normal function before other symptoms of T1DM appear. The genetic component to T1DM is largely (40-50%) related to the human leukocyte antigens (HLAs), also called the major histocompatibility complex (MHC). Certain HLA genotypes, the combination of HLA alleles inherited from two genetic parents, increases the risk of developing T1DM. When these at-risk individuals are exposed to viral infections, the ß-cells of the pancreas can be destroyed. Twin studies indicate that T1DM is found in both identical twins in roughly 50% of cases (ADA, n.d.d; Lewis et al., 2014).
T1DM affects children and adults and is usually diagnosed in children, teens, and young adults; about 5-10% of all diabetics have T1DM (CDC, 2020g). Peak incidence is between the ages of 11 and 13, and rarely before the age of four. Those diagnosed after the age of 30 typically present with a less aggressive and gradual onset of ketosis without ketoacidosis, referred to as LADA. It is more common in males than in females. Genetic factors constitute a strong component in the development of T1DM, with less than a 0.5% risk in the general population (Khardori, 2020). Fathers with T1DM have a 1 in 17 risk of passing it along to their child (or 5.8%). Mothers with T1DM have a lower risk of passing along T1DM to their children: 1 in 25 if the mother is under 25 years old, and 1 in 100 if the mother is over the age of 25. Young adults considering having children should be educated on this risk (ADA, n.d.d).
As previously discussed, there are other risk factors/triggers for T1DM, including viral illnesses, autoimmune responses, and unknown or simply poorly understood factors. While diet and lifestyle do not cause T1DM, they are important components in management (CDC, 2020b, f). Allen and colleagues performed a systematic review and meta-analysis in 2018 and found a statistically significant increase in the rate of T1DM diagnosis during childhood after proven maternal viral infections, but no increase in autoantibodies to islet cells.
Risk factors for T1DM:
- Genetic predisposition
- Having a parent, brother, or sister with T1DM.
- European Americans are more likely to develop T1DM than African Americans or Hispanic/Latin Americans (CDC, 2020b, f).
- Viral illnesses
- Enteroviruses,
- Mumps,
- Cytomegalovirus (CMV),
- Rotavirus,
- Influenza,
- Congenital rubella (Allen et al., 2018).
- Autoimmune factors
- May be associated with other organ-specific autoimmune diseases, prompting the recommendation that T1DM patients undergo regular screening for autoimmune thyroid disease and celiac disease (ADA, 2020). T1DM is more commonly diagnosed in patients with other autoimmune diseases, including Graves’ disease, Hashimoto thyroiditis, and Addison disease (Khardori, 2020).
- Idiopathic factors
- Hereditary component but no evidence of autoimmunity, not HLA-associated (ADA, 2020).
Another form of T1DM, idiopathic diabetes, is inherited and does not have an autoimmune component. There are only a small number of people with this type of diabetes; it is most common in individuals of Hispanic, African, or Asian descent. Similar to above, these patients do not produce sufficient insulin, but when tested for antibodies do not have any ß-cell autoantibodies or other autoimmune markers. A third form of T1DM is latent autoimmune diabetes in adults (LADA). LADA is a slow-developing autoimmune form of T1DM that usually occurs in people over 35 years of age and typically presents without obesity (ADA, 2020; Lewis et al., 2014).
Educating patients on their risk and encouraging screenings can decrease the risk of T2DM as well as the risk of the complications of diabetes. Zand et al. (2018) note that individuals with prediabetes have an increased risk of cardiovascular disease and stroke. Healthcare providers can offer education and support to decrease the risk associated with prediabetes, including lifestyle management. There are modifiable and non-modifiable risk factors for prediabetes. Modifiable risk factors include:
- Overweight/obesity,
- Reduced physical activity (less than three times per week),
- Smoking, and
- HTN.
Non-modifiable risk factors include:
- Age (over 45);
- Ethnicity (African American, Hispanic, Native American or Alaska Native);
- Family history of T2DM (mother, father, or sibling); and
- Personal history of hyperglycemia, gestational diabetes, or giving birth to a child who weighed more than nine pounds (CDC, 2020b; Zand et al., 2018).
The ADA (2020) recommends screening for risk factors in the general population (asymptomatic adult patients). Testing should be considered in those at risk, including those with a body mass index (BMI) of 25 kg/m2 or greater who also have one or more of the following: HTN, physical inactivity, history of cardiovascular disease, HDL less than 35 and/or a triglyceride level greater than 250 mg/dl, first-degree relative with diabetes, high-risk ethnicity (African Americans, Pacific Islanders, Latinos, Native Americans) and women with a history of polycystic ovarian syndrome (PCOS). Annual screening for prediabetes/diabetes is recommended for patients prescribed antipsychotic medications. Risk-based screening is recommended after the age of ten in children who are overweight/obese (BMI above 85th percentile) with additional risk factors. The ADA risk test is a formal assessment tool of risk available on the ADA’s website. Those patients with prediabetes (impaired glucose tolerance [IGT], impaired fasting glucose [IFG], or a hemoglobin A1C of 5.7-6.4%) should be tested for diabetes annually. Urine glucose testing is minimally sensitive but can be used for screening prior to more invasive testing. Women with a history of gestational diabetes should be tested for T2DM at 4-12 weeks postpartum using the oral glucose tolerance test (OGTT) and at least every three years for the remainder of their life. Adult patients with average or low risk should begin screening at age 45. For any abnormal screenings, a second test should be performed prior to initiating treatment. A second abnormal test confirms a diagnosis of prediabetes. Intervals of screening are debated, but the ADA recommends that patients with normal testing be retested at least every three years (Abdallah et al., 2019; ADA, 2020).
The pathophysiology of T2DM differs from T1DM based on the continued production of endogenous insulin by the pancreas. With T2DM, the insulin is either generated in insufficient quantities, used poorly by the tissues, or both. The most common risk factor for T2DM is obesity, especially abdominal adiposity (Lewis et al., 2014). Genetic mutations that increase the risk of obesity and insulin resistance are found in individuals with T2DM. Twin studies have identified that when one twin has T2DM, the risk is approximately 3 in 4 for the other twin (ADA, n.d.d) There are four major metabolic abnormalities connected to the development of T2DM:
- Insulin resistance, or the gradual decline in the normal reaction of skeletal muscle and adipose cells to insulin;
- A decrease in the pancreas’s ability to produce insulin;
- Inappropriate glucose production by the liver;
- Altered production of hormones and cytokines by adipose tissue (Lewis et al., 2014).
Risk factors for T2DM include prediabetes, as well as the aforementioned risk factors listed for prediabetes itself (CDC, 2019d, 2020b). While T2DM is typically found in those over 45 years of age, there has been an increase in the incidence of T2DM amongst children, teens, and young adults in recent years due to obesity and poor lifestyle choices, including inactivity. While T1DM is almost entirely outside the individual's control, T2DM is largely determined by modifiable risk factors related to the individual's lifestyle choices (CDC, 2019d, 2020b; Wood & Peters, 2018). T2DM can be prevented or delayed with modifications to lifestyle, including exercising at least three times per week and a healthy diet based on complex, low GI carbohydrates (ADA, 2020).
Another risk factor for developing T2DM is metabolic syndrome. The individual diagnosed with metabolic syndrome has at least three of the following components:
- Hyperglycemia,
- Abdominal obesity,
- Hypertension,
- High triglycerides,
- Decreased levels of high-density lipoproteins (HDLs; Lewis et al., 2014).
Gestational diabetes (GDM) is similar to T2DM; however, this develops during pregnancy in women without a preexisting diagnosis of diabetes. Approximately 2-10% of all pregnancies in the US are affected by GDM. The pregnant woman's body is unable to make enough insulin due to the hormonal changes and weight gain associated with pregnancy. Insulin resistance may develop due to these changes, and the body's increased insulin requirements. All women have an increased need for insulin during late pregnancy, but women with GDM require more insulin throughout the entire pregnancy, necessitating treatment (CDC, 2019a). Risk factors include:
- Previous pregnancy with GDM,
- Previous birth with a baby over nine pounds,
- Obesity,
- Over 25 years of age,
- Family history of T2DM,
- PCOS, and
- Ethnic backgrounds, including Native American, African American, Hispanic American, Alaska Native, Native Hawaiian, or Pacific Islander (CDC, 2019a).
Diabetes can occur due to other medical conditions or due to treatment for other diseases if the ß-cells within the pancreas are damaged, injured, or destroyed. Risk factors could include Cushing’s syndrome, hyperthyroidism, recurrent pancreatitis, cystic fibrosis, hemochromatosis, and parenteral nutrition. Medications that can cause diabetes include corticosteroids, thiazides, phenytoin, and atypical antipsychotics such as clozapine (Clozaril). Diabetes caused by any of the previous may resolve when the underlying condition is treated, or the medication is discontinued (Lewis et al., 2014).
Presentation, Diagnosis, and Management of Diabetes
Prediabetes
An individual is said to have prediabetes if they have IGT, IFG, or both. The ADA (2020) defines IGT as a BG level between 140 mg/dL to 199 mg/dL (7.8-11 mmol/L) two hours after the ingestion of a 75 g oral glucose solution (a two-hour OGTT). This type of test is primarily used in screening pregnant women, but it is also highly sensitive at predicting patients with T2DM. The patient should fast for at least eight hours prior to the OGTT. The ADA defines fasting as no caloric intake for at least eight hours. They define IFG as a fasting plasma glucose (FPG) level between 100 and 125 mg/dL (5.6-6.9 mmol/L). They note that the World Health Organization (WHO) and others have a slightly different definition for IFG (110-125 mg/dL or 6.1-6.9 mmol/L). The hemoglobin A1C (or simply A1C) indicates the percentage of the total hemoglobin that has glucose attached to it, in mmol/mol. A hemoglobin A1C greater than 5.7% but less than 6.5% also indicates prediabetes. Prediabetes is not meant to be a diagnosis unto itself, but these individuals are at an increased risk of developing T2DM. Further, damage to their vessels may already be occurring, yet they are often asymptomatic. This serves as a powerful rationale for individuals to have annual physicals with routine BG screenings (ADA, 2020).
Treatment/Management of Prediabetes
The goal of treatment and/or management of the those diagnosed with prediabetes is to prevent or delay the development of T2DM, as well as the micro- and macrovascular complications related to diabetes. Evidence has proven that lifestyle changes such as increased physical activity and dietary modifications are effective ways to decrease this risk in patients with prediabetes, as well as control BG levels in patients with T2DM (Abdallah et al., 2019). The ADA (2020) suggests referral to an intensive behavioral lifestyle intervention program modeled on the CDC National Diabetes Prevention Program (National DPP) with an overall goal of weight loss (lose and then maintain a 7% reduction of initial body weight, at a rate of two to three pounds per week) and routine physical activity (at least 150 min/week). This program allows for flexibility in selecting dietary and exercise options. The program focuses on decreasing calories, increasing exercise and physical activity, and maintaining healthy lifestyle behaviors along with social and psychological challenges that motivate the individual. The National DPP also assigns a trained coach to help the participants manage stress, stay motivated, and problem-solve to ensure success. Further goals geared toward the prevention of T2DM may be additional strength/resistance training and the treatment of other cardiovascular disease risk factors. These interventions increase the chance of avoiding the complications of prediabetes and subsequent T2DM. Those with confirmed prediabetes should be tested for T2DM annually per the ADA (Abdallah, 2019; ADA, 2020; CDC, 2019b, 2020e)
As previously mentioned, dietary management is a significant part of minimizing risk with prediabetes. A specific diet is not recommended, but rather a varied diet that includes whole grains, nuts, berries, yogurt, coffee, and tea is associated with a reduced risk of diabetes. The Mediterranean Diet and Dietary Approaches to Stop Hypertension (DASH) Diet have both been linked to reducing the incidence of DM regardless of weight loss. Red meat and sugary beverages should be avoided as well as high-carbohydrate diets (Abdallah et al., 2019; ADA, 2020; CDC, 2020e).
The APRN has many opportunities for promoting success in patients with prediabetes through education, promotion of self-care, screenings, and encouraging continued monitoring by the healthcare team. Studies also identified the efficacy of technology to track exercise, diet, and progress with personal goals (Abdallah et al., 2019; Beauvais et al., 2018; Grock et al., 2017). One option for diabetic education is a program called Diabetes Self-Management Education and Support (DSMES) which are services that will help the patient diagnosed with prediabetes or T2DM learn how to care for themselves with the new diagnosis (ADA, 2020; CDC, 2018b). There are other diabetic education programs across the country that can be found on the American Association of Diabetes Educators (AADE) website. These programs strive to help patients prevent diabetes, or after diagnosed, manage the day-to-day challenges of living with diabetes and support their management of long-term implications (AADE, 2019).
Metformin (Glucophage) is a biguanide, a group of medications that work by lowering glucose production in the liver and improving the way the body utilizes insulin (Mayo Clinic, 2019f). The ADA recommends consideration of metformin (Glucophage) therapy for patients with prediabetes to prevent or delay the onset of T2DM. The APRN should consider use of this medication in patients with a BMI above 35 kg/m2, those over the age of 60, and women with a history of GDM; although the medication is not currently approved by the US Food and Drug Administration (FDA) for this indication (ADA, 2020).
Type 1 Diabetes (T1DM)
Common signs and symptoms of T1DM include:
- Polydipsia (excessive thirst);
- Polyphagia (excessive eating due to increased hunger);
- Polyuria (increased urination), especially nocturnal enuresis in children;
- Unintentional weight loss;
- Blurred vision;
- Excessive fatigue/lassitude;
- Nausea, vomiting, or stomach pains; and
- Diabetic ketoacidosis (DKA), with or without Kussmaul respirations (Khardori, 2020).
The initial presentation of T1DM may be DKA. This patient may not realize they have diabetes until they have advanced symptoms that require them to seek medical care. Symptoms of DKA may include:
- Decreased alertness,
- Dry skin and mouth,
- Flushed face,
- Polydipsia/polyuria lasting for a day or more,
- Fruity-smelling breath,
- Headache,
- Muscle stiffness or aches,
- Nausea/vomiting or stomach pain (MedlinePlus, 2018b)
In DKA, the liver breaks down body fat for energy due to the lack of glucose available for the cells, producing ketones as a byproduct. These ketones build up and cause metabolic acidosis. A compensatory symptom of Kussmaul respirations (deep and labored breathing) may occur in an attempt to decrease the acidity in the body by reducing carbon dioxide. If untreated, DKA will lead to coma or death (CDC, 2020c; Gallo de Moraes & Surani, 2019).
Diagnostic Tests for T1DM
According to the presentation of a T1DM patient (e.g., emergency in DKA or office visit due to symptoms such as polyuria, polyphagia, polydipsia, and or weight loss), diagnostic tests may vary. Patients should have the following for a diagnosis of T1DM:
- Random BG or urine glucose
- These are the quickest options for testing when a patient presents with symptoms. If either of these tests is high/abnormal (i.e., BG greater than 200 mg/dL), the APRN should proceed with the following tests for more precise diagnosis (ADA, 2020; Juvenile Diabetes Research Foundation [JDRF], n.d.b).
- FPG
- BG greater than 126 mg/dL after at least eight hours without caloric intake.
- Hemoglobin A1C
- An A1C of 6.5% or above.
- Two-hour OGTT
- BG greater than 200 mg/dL two hours after 75 g glucose solution.
- Urine ketones
- The presence of ketones suggests T1DM versus T2DM (ADA, 2020).
- C-Peptide
- This test measures how much C-peptide is in a person’s blood. Peptide levels typically mirror insulin levels in the body. Low levels of C-peptide and insulin can point to T1DM (JDRF, n.d.b).
- Antibody testing: islet cell antibodies (ICAs) can be found in as many as 85% of T1DM patients, and most also have anti-insulin antibodies. Antibodies against glutamic acid decarboxylase (GAD, an enzyme found in pancreatic ß-cells) is the most common type of ICA (Khardori, 2020).
For abnormal screenings of BG or A1C, a second test is typically performed in order to confirm a diagnosis of T1DM. Antibody testing and ketone presence are more precise diagnostic methods, and a diagnosis can be made without subsequent revalidation of the tests, and classic signs/symptoms of diabetes at the time of presentation may negate the need to repeat testing as well (ADA, 2020).
Treatment/Management of T1DM
Hemoglobin A1C monitoring should be performed with any type of diabetes and prediabetes. The A1C is a blood test that will give an average of the BG level over the prior three months. While it is the most commonly used test to diagnose prediabetes and diabetes, it can also determine the effectiveness of the treatment plan and if modifications should be made. The ADA recommends A1C monitoring at least twice per year in patients with stable glycemic control, and up to quarterly in those not meeting treatment goals or transitioning therapy strategies/modalities. Point of care testing provides more timely feedback for more efficient treatment changes. Increased A1C levels are linked to an increase in diabetic complications such as retinopathy, nephropathy, and neuropathy (CDC, 2020c; ADA, 2020).
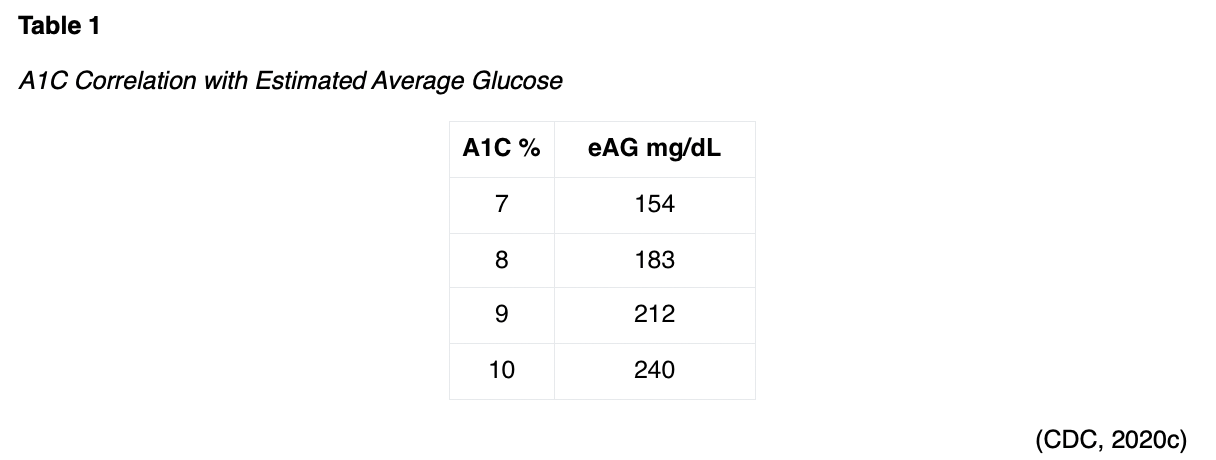
A1C results are reported as estimated averages rather than mg/dL (displayed on glucometers). Table 1 indicates the A1C percentage as it correlates to the estimated average glucose (eAG), which the patient would see on their glucometer.
The A1C may be inaccurately high or low due to kidney failure, liver disease, or severe anemia. Certain medications can interfere with A1C levels, including opioids and some HIV medications. Blood loss or transfusions, early or late pregnancy, and certain blood disorders such as sickle cell anemia or thalassemia may all interfere with the accuracy of A1C results (CDC, 2020c; Mayo Clinic, 2019f).
A1C goals are typically 7% or lower. In certain circumstances, a goal of less than 6.5% may be achievable if it can be done safely (without significant hypoglycemia or other adverse effects). Personal goals may be adjusted due to age or medical conditions. Each patient’s A1C goal should be determined between the individual and their healthcare provider or healthcare team. For individuals who experience frequent hypoglycemia, a higher goal may be established to avoid severe lows. Younger people tend to have lower A1C goals since they have many years to live with diabetes. Older individuals may have higher goals due to multiple health conditions or to avoid hypoglycemic episodes. A goal of less than 8% may be acceptable in those patients with a history of hypoglycemia episodes, limited life expectancy, advanced macro- or microvascular complications, or extensive comorbid conditions (ADA, 2020; CDC, 2020c).
In addition to the A1C goals, BG monitoring (called self-monitoring of blood glucose [SMBG]) should be performed throughout each day. The maintenance of normal (70-120 mg/dL) BG levels supports a decrease in vascular damage and future complications associated with diabetes. BG should typically be checked at the following times:
- Upon awakening,
- Preprandial (before a meal or snack),
- Postprandial (two hours after a meal), and
- At bedtime (ADA, 2020).
The ADA also recommends checking BG levels using SMBG prior to exercise, when the patient suspects hypoglycemia, after treating hypoglycemia (until euglycemic/normoglycemic again), and before/while performing important tasks (i.e., driving; ADA, 2020). Individual targets for BG will vary, but a preprandial goal is typically 80-130 mg/dl. The postprandial goal should be less than 180 mg/dl. The individual, along with their healthcare team, will develop targets for the pre- and postprandial BG levels as part of the treatment plan. Logging BG levels will allow the patient to discuss trends with their healthcare provider and determine opportunities for further improvement and management of their overall BG levels. Many glucometers perform this function automatically now, syncing their data with smartphone apps and websites to help visualize time spent below, in, or above BG range. The patient should also note associated food intake and activity when logging BG levels within a paper log or an app/program. The cost of testing supplies and medications is a concern for many diabetics. These can be very expensive, and often insurance policies do not cover the cost. The healthcare team can help with resources that may offer low-cost or free supplies to the diabetic in need (CDC, 2020c).
The CDC offers the following suggestions for patient education regarding the safe and appropriate use of a glucometer for SMBG:
- Ensure that the glucometer is clean and adequately charged/has properly functioning batteries if applicable.
- The patient should remove a single test strip and then immediately reseal the container to ensure that the remaining test strips are not damaged by ambient moisture/humidity or extreme temperatures.
- Perform proper handwashing with soap/warm water for at least 30 seconds, massaging the hands to get blood into the intended finger. Dry well.
- The patient should use a lancet to prick the finger once, then squeeze from the base of the finger to extract a large drop of blood.
- Place the drop of blood onto the test strip, then insert the test strip into the glucometer.
- The results will appear (in mg/dL) in a few seconds, depending on the specific glucometer being used. If not done automatically, this should be recorded immediately.
- The lancet and strip should be disposed of properly based on the state and local regulations regarding medical waste/sharps. The APRN should be sure to include in the patient education the importance of not sharing or reusing supplies such as lancets and test strips, even with family members (CDC, 2020c).
The ADA (2020) recommends evaluating patient glucometer technique regularly (similar to asthma patients and inhaler technique), especially in patients not currently meeting treatment goals. They also caution that external factors such as extreme temperatures and patient factors such as oxygen saturation, uric acid, ascorbic acid (vitamin C), galactose (milk sugar), xylose (a monosaccharide used in some foods as a sweetener), and even acetaminophen (Tylenol) may affect glucometer accuracy (ADA, 2020).
Alternatively, continuous glucose monitors (CGM) measure interstitial glucose rather than plasma glucose and have recently become important in the simplification of care for many patients with T1DM, and some selected patients with T2DM as well. The units have improved in terms of accuracy and affordability since their initial introduction to the market. They provide a readout called an ambulatory glucose profile (AGP) to assist patients and providers with data interpretation. They require additional patient education and initial training to ensure proper use. Many patients still use SMBG to calibrate their CGMs or confirm readings when discordant from symptoms. CGMs may help lower A1C levels and reduce hypoglycemia in T1DM adult patients who have hypoglycemia unawareness or multiple episodes of hypoglycemia as well as those not meeting glycemic targets with SMBG. They should be considered and discussed in all children or adolescents with T1DM. CGMs can either be real-time (providing measurements of BG continuously with user alarms for preset BG thresholds or level changes) or intermittently scanned (these measure continuously but only display results when prompted by reader/smartphone). Intermittent CGMs should be scanned often, at a minimum of every eight hours. Blinded CGMs may also be used by specialized clinics for brief periods (10-14 days) to temporarily analyze and correct patterns and trends of hypo- and hyperglycemia, but do not display results for the patient in real-time (ADA, 2020).
One of the more challenging aspects of managing diabetes is troubleshooting consistent irregularities. One common irregularity in T1DM patients is early morning hyperglycemia (before breakfast). While likely related back to counter-regulatory hormones, there may be two separate underlying mechanisms at play, leading to two disparate solutions. The dawn phenomenon refers to hyperglycemia related to the normal daily release of cortisol, growth hormone, and catecholamines (i.e., epinephrine, dopamine), which occurs between 3 and 8 am in all individuals. This leads to the release of glucose from the liver as well as insulin resistance. Alternately, the Somogyi effect (or rebound effect/phenomenon) occurs after insulin doses at bedtime, which causes hypoglycemia and triggers the release of counter-regulatory hormones (i.e., cortisol, glucagon, growth hormone, and epinephrine) which cause BG to increase for the same reason described above. If a patient reports consistent hyperglycemia upon waking, they should be asked to check their BG between 2 and 3 am for several days in a row. If their BG is consistently low at this time, this indicates that the Somogyi effect is the likely culprit. Providers should consider decreasing the patient’s evening insulin dose, adjusting the administration time to earlier, changing the type of insulin, or adding an additional bedtime snack. Bedtime insulin doses should always be adjusted, especially increased, in very small increments to avoid this phenomenon. If the patient’s BG is normal or elevated at this time, then it is more likely related to the dawn phenomenon. To prevent this, patients should be counseled to avoid carbohydrates before bed, and providers may consider adjusting the evening insulin dose to later (right before bed). Patients with early morning hyperglycemia may require management with a CGM and an insulin pump to help stabilize their BG levels. Of note, the scientific evidence regarding both of these phenomena is not very solid. Small studies have found either no evidence of hypoglycemia during the night in patients with hyperglycemia upon waking, or no increase in growth hormone, cortisol, or glucagon levels in those patients that did experience rebound hyperglycemia following an episode of hypoglycemia (ADA, n.d.e; Leonard, 2019; Schaefer & Sarachik, 2018). However, a 2015 study by Minicucci and colleagues found that among 85 participants with T1DM who were monitored for a total of 255 nights collectively, 61% experienced hypoglycemia overnight, and 82% experienced hyperglycemia upon waking. The conclusions were that roughly 60% of the study participants have CGM patterns indicating the Somogyi effect and 13% indicating the dawn phenomenon (Minicucci et al., 2015).
The patient with T1DM is typically diagnosed by adolescence or young adulthood but will be impacted by the disease for their entire lifespan. Therefore, it is vital that steps are taken to avoid the long-term complications associated with diabetes. Complications can be prevented or delayed by consistently maintaining BG levels within acceptable ranges. The poorly controlled diabetic is at heightened risk for micro and macro-vascular damage to vessels throughout the body (ADA, n.d.b). The APRN should help focus the patient’s efforts on preventing damage through careful regulation of BG levels. This is accomplished through eating a healthy, balanced diet focused on low-GI foods and proper administration of insulin to meet the demands of dietary consumption. In addition, the T1DM patient should work with their healthcare team to individualize a treatment plan that works for their lifestyle, resources, and preferences (Wood & Peters, 2018). Additional details regarding the specific complications seen in patients with diabetes will be explored later in this module.
Diet/Carbohydrate Counting. While all individuals should consume a healthy diet combined with an exercise regime, it is vital for people with diabetes. The patient with diabetes and/or prediabetes should consume a balanced diet for good nutrition. A diet consisting of the four food groups with a low intake of empty carbohydrates is optimal. Proper nutritional intake, control of BG levels, and maintaining a healthy weight can decrease the impact of diabetes on the body (JDRF, n.d.b). Nutrition, physical activity, and medication are major factors in determining the BG level. The combination, amount, and timing of foods/meals all impact the BG level, in addition to exercise and medication (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], 2016). Newly diagnosed diabetic patients often feel as if they will not be able to eat the foods they like or enjoy. It is important for the APRN to help educate patients on eating smaller portions as part of a healthy diet that meets their nutritional needs and their personal taste. As previously mentioned, the National DPP, DASH, and Mediterranean diets have all been proven beneficial. The food groups that should be included in any healthy eating plan are:
- Vegetables, including non-starchy and starchy options. Suggested foods are carrots, greens, tomatoes, broccoli, carrots, squash, and peppers in the non-starchy group. Starchy options would include potatoes and corn;
- Fruits, including bananas, grapes, oranges, apples, berries, and melons;
- Grains, primarily whole grains, including wheat, rice, oats, quinoa, cornmeal, and barley, as well as seeds;
- Protein, including lean meats, nuts, eggs, dried beans, tofu, and fish, such as tuna or salmon;
- Nonfat or low-fat dairy products, including yogurt, cheese, and milk;
- Heart-healthy fats, including avocados and oils that are liquid at room temperature such as olive oil or canola oil (JDRF, n.d.b; NIDDK, 2016).
Certain foods and drinks that should be avoided or severely limited in patients with diabetes include:
- Fried foods;
- High-sodium foods;
- High-sugar foods, including ice cream and candy;
- High-sugar beverages, including juices, sweetened coffees, sports drinks, or soft drinks;
- Alcohol should be used in moderation, as it is high in glucose. Women should drink no more than one drink per day and men no more than two drinks per day. Alcohol can cause hypoglycemia in patients who use insulin or diabetes medications that increase the amount of insulin the body produces. Food should be ingested simultaneously with alcohol to avoid hypoglycemia (NIDDK, 2016).
Most patients with diabetes should be taught to count carbohydrates by calculating the carbohydrate content of their meals and administer insulin based on the grams of carbohydrates being consumed. Carbohydrates are converted into glucose in the body after ingesting, and amounts can be found easily on nutritional facts food labels (NIDDK, 2016). The patient should calculate their intake and match it with an appropriate dose of insulin. For the patient who continues to have an elevated BG even after their bolus of insulin, a correction bolus can be given to bring their BG within the desired range. This same process of calculating carbohydrate intake and adjusting insulin bolus administration can be achieved with an insulin pump (University of California San Francisco [UCSF] Medical Center, n.d.). An alternative to carbohydrate counting is the plate method, which teaches diabetic patients to use a 9-inch plate and split it into halves. One half of the plate should be filled with non-starchy vegetables. The remaining half should be split in half again, filled with a lean protein on one side and a grain or other starch in the final quadrant. If the patient’s meal plan allows, they may also eat a small piece/bowl of fresh fruit and drink a small glass of milk. This method applies best to lunch and dinner. This method helps many patients with portion size, but if additional teaching is required, many diabetic educators will correlate portions with everyday objects. Examples include comparing a serving of meat to the palm pf the patient’s hand or a deck of cards, a serving of cheese to six dice, a serving (1/2 cup) of rice or pasta to a rounded handful or a tennis ball, one pancake or waffle to a DVD, and a serving of peanut butter (2 tbsp) to a ping-pong ball (NIDDK, 2016).
The term glycemic index (GI) is used by some in diabetic nutrition as well as evidence-based practice related to managing diabetes. The GI measures the manner in which carbohydrate-containing foods raise BG. Food choices are ranked on how they compare to a specific reference food (i.e., white bread and glucose). High-GI foods (70-100, i.e., white bread, corn flakes, high fructose corn syrup, mashed potatoes, bagels, and waffles) will raise the individual’s BG level more than a food with medium (56-69, i.e., basmati rice, couscous, raisins, and cranberry juice) or low-GI foods (less than 55, i.e., legumes, fruits, starchy vegetables, whole grains). When meal plans are being developed, it is best to choose low- or medium-GI foods. Occasionally eating a high-GI food can be offset by eating it with a low-GI food, allowing individuals to eat the foods they prefer while also limiting the impact on their BG (Wood & Peters, 2018).
The GI of a particular food is affected by numerous factors, such as fat and fiber content, which typically lower the GI of a food. Other factors that impact the GI of food are the cooking or processing method. Also, ripeness and storage of food may impact the GI. The riper the fruit, the higher the GI. The more cooked or processed a food is, the higher the GI, which is another rationale for eating whole foods that are less processed. The GI takes into consideration the type of carbohydrate in a food, but not the quantity of the carbohydrate. Portion sizes are important for both the GI as well as weight management. Nutrition (protein, vitamin, and mineral content) should be taken into consideration as well. Some nutritionally dense foods may have a high GI. A balance of all these aspects should be considered in order to provide the optimal nutritional intake and support a steady BG level. While the GI of a particular food is not necessary to know in order to calculate the carbohydrate count, it is helpful to recognize that certain carbohydrates will affect the BG more than others (Wood & Peters, 2018). For further guidance on helping diabetic patients manage their nutrition, patients and care providers should explore the American Diabetic Associations’ website.
Exercise. Health and wellness are impacted by physical activity, as exercise improves BG control, well-being, cardiovascular fitness, muscle strength, and insulin sensitivity in diabetic patients and may lead to weight loss. Adults with T1DM and T2DM should exercise for at least 150 min/week but should be made aware of the effect of physical activity on their BG levels. Even mild exercise and physical activity can cause a decline in BG, or hypoglycemia, depending on the activity type/timing. Exercise can cause hypoglycemia through an increased need for glucose related to the increase in metabolic activities. An individual’s BG will respond based on the BG level prior to starting the activity, the intensity of the activity, the length of time of the activity, and any modifications to insulin intake. A pre-exercise snack, frequent BG checks (before, during, and after exercise), and possible reduction in insulin should be encouraged to prevent hypoglycemia. The ADA recommends a pre-exercise snack with approximately 15 g of carbohydrates if the patient’s BG level is less than 100 mg/dL prior to exercising, especially if longer than 30 minutes of exercise is planned. Individuals with an insulin pump may be able to lower their basal rate during the physical activity to avoid hypoglycemia. In the event of severe hypoglycemia during exercise, patients should have an emergency management plan in place and travel with juice or glucose tabs while exercising away from home. Timing, incorporating short sprints, and performing resistance exercise immediately before aerobic exercise are additional strategies to avoid hypoglycemia. Nocturnal hypoglycemia after exercise can be avoided by decreasing basal insulin, eating a bedtime snack, and the use of real-time CGM. This also applies to children and adolescents with diabetes, who may have unpredictable and longer periods of activity. The ADA recommends that preschoolers, children, and adolescents engage in at least 60 minutes of physically active play each day. Parents should be counseled to monitor BG levels frequently and be prepared to give children snacks with 5-15 g of carbohydrates (depending on age/size) for every 30 minutes of active play (ADA, n.d.c; Colberg et al., 2016).
Occasionally, high-intensity exercise (i.e., high-intensity interval training [HIIT], sprinting, powerlifting) can also cause hyperglycemia due to elevated stress hormones in patients with diabetes, especially in those with T1DM. Reducing the insulin dosage prior to exercise may worsen this, as can a high-carbohydrate meal prior to exercise. This risk can be mitigated by interspersing intense activity with periods of moderate-intensity aerobic exercise, as well as a period of resistance training prior to aerobic training. If a patient’s BG is high (above 250 mg/dL) prior to exercise, they should utilize a home test to check their blood or urine for ketones. If the ketone screen is positive (above 1.5 mmol/L), then exercise should be postponed or avoided. If there are no ketones in the blood/urine, they can proceed with exercising. Hyperglycemia that occurs after exercise should be managed with an aerobic cooldown or conservatively corrected with insulin (50% of a typical correction dose), as overcorrection increases the risk of nocturnal hypoglycemia. Efforts should be taken to develop consistent routines and understand the impact of insulin, food, and exercise on the individual’s BG levels. By recognizing this impact and working with the healthcare team, the best outcomes for exercise can be achieved. Devices such as CGMs can give even more precise information related to the impact of exercise on the body’s BG level over time. These devices are currently only recommended as an adjunct to SMBG during and after exercise due to inconsistent studies regarding their accuracy (ADA, n.d.c; Colberg et al., 2016).
The T1DM patient should proceed with caution for certain high-risk activities such as scuba diving and skydiving. T1DM patients should obtain clearance from their physician prior to scuba diving for the first time. Additional precautions include diving with a slightly higher (rather than lower) BG level as well as diving with glucose gels (to be used if the BG level falls while submerged) and a partner that is aware of the patient’s diagnosis. Skydiving causes a release of adrenaline, which can increase BG levels. If an insulin pump or other diabetes device is used, care must be used to secure it during either activity (Wood & Peters, 2018).
Pharmacological Treatment. Exogenous insulin will be needed for the patient with T1DM for life as there is no endogenous insulin available. Daily needs for insulin will vary in each patient based on illness, stressors, type and quantity of food intake, and activity level, amongst other factors. The goal of any exogenous insulin regimen is to mimic how the body normally releases endogenous insulin. Unfortunately, insulin cannot be absorbed orally and is therefore injected subcutaneously, infused intravenously, or inhaled into the lungs (CDC, 2020c; UCSF Medical Center, n.d.). See Table 2 for examples of different types of insulins currently available.
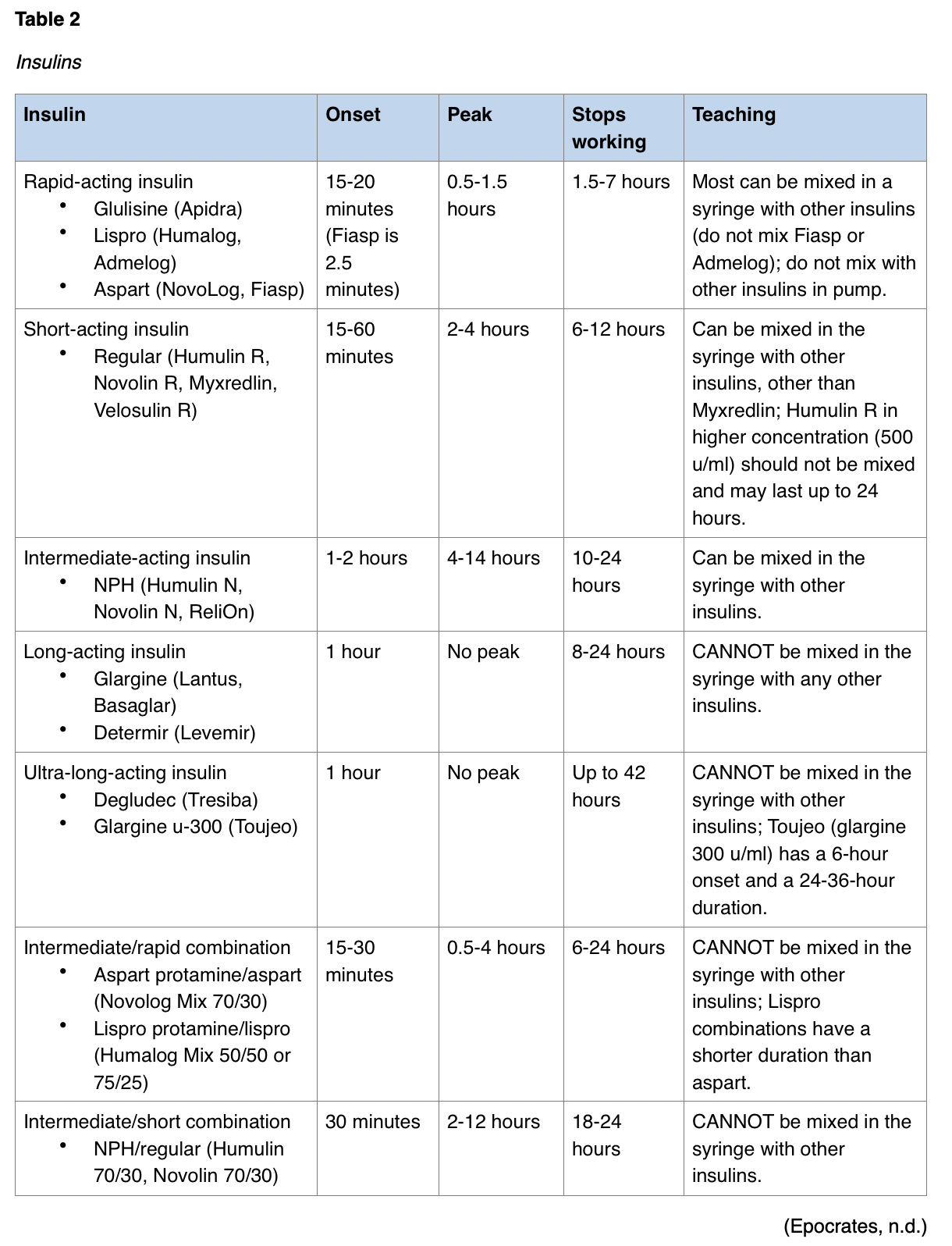
As seen in Table 2, insulin can be premixed in various combinations for convenience or individuals who have difficulty drawing insulin from two bottles. This can be helpful to elderly patients or those suffering from limited eyesight or manual dexterity (ADA, n.d.e, 2020). Insulin pens have a prefilled cartridge and attachment site for a needle. The pen is typically constructed with a dial to adjust the dosage of insulin delivered. Insulin pens contain only a single type of insulin, which eliminates the possibility of mixing two types of insulin into a single injection. Insulin via a dry powdered inhaler, Afrezza, has been introduced as an alternative to injections. This insulin is introduced via the lungs and absorbed into the bloodstream within seconds. This insulin advertises an onset of 12 minutes, peaks within 30-60 minutes, and lasts up to 4.5 hours (ADA, n.d.e; Epocrates, n.d.). Patient education for insulin administration should include information on the insulin regime, including the type of insulin, times of administration, methods of administration, and adverse reactions to monitor for. Site rotation should be included to preserve subcutaneous tissue integrity (ADA, 2020). The injection site affects the speed of absorption for insulin; abdominal injections are absorbed the fastest, followed by the upper arm and finally the thighs/buttocks. The APRN should encourage patients to vary the exact location but to be consistent regarding the use of the same area of the body each day/time. Patients should be educated according to the insulin's strength, which may vary. While the standard and most common insulin strength is u-100 (100 units per mL), there are u-300 (glargine u-300 [Toujeo]) and u-500 (Humulin R u-500) options available. The older version (u-40) is no longer common other than in veterinary medicine (ADA, n.d.e).
Most T1DM patients are maintained on a basal/bolus dosing regimen. A basal dose of insulin is one that will deliver continuous BG control. This can be attained via a long-acting or ultra-long-acting insulin or via an insulin pump with rapid-acting insulin that is administered in continuous small doses. The basal dose is typically administered at the same time every day and does not change with increased BG levels; it is intended to mimic the normal pancreas in a healthy individual, which constantly secretes a basal dose of insulin to manage BG levels. A bolus dose of insulin is then administered throughout the day (i.e., with meals) to counteract the intake of food or with elevated BG levels (UCSF Medical Center, n.d.). The ADA (n.d.e) recommends taking regular insulin (Humulin R, Novolin R) approximately 30 minutes before eating to optimize its effectiveness in relation to the glucose influx in the blood. The Diabetes Control and Complications Trial (DCCT) provided evidence that three or more insulin injections per day (described as intensive therapy, or INT) provided the optimal glycemic control and improved outcomes for T1DM patients, even as many as 30 years after the start of the original trial. INT patients achieved lower A1C levels, as well as reduced rates of retinopathy, nephropathy, and neuropathy. The two primary adverse events reported during the initial study included hypoglycemia and weight gain in the INT group (Nathan & DCCT/EDIC Research Group, 2014). Variations of INT include multiple daily injections (MDI) and continuous subcutaneous insulin infusion (CSII, or insulin pump therapy; ADA, 2020).
CSII, or insulin pump therapy, is a convenient way for T1DM patients to reduce the insulin injections required to manage their blood sugar. These computerized pumps deliver insulin into the subcutaneous tissue via a catheter that is inserted and then taped into place (an infusion set). They are programmed to deliver a basal rate and allow for bolus dosing per the user's input at mealtimes or in response to elevated BG levels as needed. However, pumps may be difficult for technologically challenged patients, and most cannot be worn while swimming, which may be problematic for pediatric patients during the summertime. An insulin pump should be considered in patients with T1DM who are interested in this form of management, very active, have frequent hypoglycemia episodes, those with gastroparesis (delayed gastric motility and absorption), and women who are planning a pregnancy (ADA, n.d.e). Some insulin pumps are even designed to communicate with and respond to a compatible CGM, although this technology is new and just in early development stages. The combination of real-time CGM and a compatible insulin pump is called a sensor-augmented pump (SAP), and studies thus far indicate that this may improve glycemic variability and reduce the rate of hypoglycemia in patients with T1DM. Unfortunately, pediatric and adolescent populations have high rates of noncompliance with SAP, reducing its effectiveness significantly in this group of patients (Tumminia et al., 2015).
Another treatment option for diabetes is pramlintide (Symlin), an injection of synthetic hormone that resembles amylin, a hormone that is released into the bloodstream, much like insulin after a meal. Amylin has been found to be deficient in patients with diabetes. This drug delays gastric emptying, blunts pancreatic secretion of glucagon, and promotes a feeling of fullness. The FDA has approved this drug for the treatment of T1DM and T2DM, to be given via subcutaneous inject with meals in conjunction with insulin. Further positive outcomes with pramlintide (Symlin) include weight loss and decreased insulin dose (MedlinePlus, 2018c).
Surgical Management of T1DM. There exists currently one curative option for T1DM: a pancreas transplant. Many patients have experienced positive results from a pancreas transplant, while others have not had the same success. This variability has prevented the procedure from being utilized more commonly; it is currently reserved for those with significant complications related to T1DM. In those with advanced kidney disease, the pancreas transplant is often combined with a kidney transplant as well. About 10% of all pancreas transplants are done in those with T2DM who have a combination of low insulin production but also low insulin resistance. The primary benefit of a pancreas transplant is the ability to maintain euglycemia, which is a normal concentration of glucose in the blood, without taking exogenous insulin. The long-term damages caused by diabetes are prevented or delayed, and nerve damage from diabetes is slowed or even reversed after a transplant. The primary risk of a pancreas transplant is the body's rejection of the foreign organ and requisite immunosuppressant drugs that must be taken to avoid such a rejection. Surgical risks include blood clots, infection, bleeding, and urinary complications. As mentioned above, immunosuppressant medications are necessary to lower the chance of rejection; these drugs increase the risk of infections, cancer, and opportunistic diseases. Other side effects from antirejection medications include osteoporosis, hypercholesterolemia, hypertension, gastrointestinal (GI) symptoms, sensitivity to light, weight gain, acne, swollen gums, and hair growth/loss. In patients that have undergone pancreas transplant, signs and symptoms of rejection include abdominal pain/increased tenderness at the transplant site, fever, hyperglycemia, vomiting, and oliguria. Diet and lifestyle recommendations described above should be continued in transplant recipients to ensure long-term health and well-being (decreased urination; Mayo Clinic, 2019b).
Due to the high risk of rejection of the pancreas, there has been research on and success with islet transplants. Islet cells, which produce insulin, are destroyed in T1DM. Only 1-2% of the pancreas is made up of islet cells, so the transplantation of islet cells conveys significantly less risk of rejection. Islet cells are taken from a donor pancreas and injected into the portal vein of the recipient, possibly repeatedly. The new islet cells should start producing insulin gradually and thus reduce or eliminate the need for exogenous insulin. Very close monitoring of the BG in the initial phase of transplantation is important to maintain euglycemia. Islet transplantation is much less invasive and costly than a pancreas transplant but is currently only being performed as part of clinical trials. Phase 3 clinical trials indicated that roughly 90% of recipients had attained an A1C under 7% at one year and 70% at two years after transplant; approximately half of the trial recipients did not require exogenous insulin at one year and 40% at two years. Recipients reported improved quality of life and better overall health. Procedural risks include rejection and transplant failure but otherwise are limited to pain, bleeding, and blood clots. At this time, immunosuppressant drugs are still needed to avoid rejection, but research is ongoing to potentially eliminate that need (Mayo Clinic, 2019b; NIDDK, 2018d).
Type 2 Diabetes (T2DM)
Due to its slow, insidious onset, T2DM is often silent and without symptoms for years prior to being diagnosed. Similar to patients with prediabetes, these individuals may be unaware there is a problem until they have been exposed to abnormally elevated BG levels for extended periods of time. T2DM is often discovered on a routine check-up, annual physical, pre-employment screening, or when the patient develops a wound that won’t heal, repeated vaginal infections, or other infections of increased frequency. Further presenting symptoms may be blurred vision, polyuria, polydipsia, polyphagia, numbness or tingling of the hands or feet, or dry skin. Individuals who have increased risk factors should be screened at routine intervals for early intervention and recognition of the disease as outline in the previous section regarding (ADA, n.d.b; CDC, 2019d).
Diagnostic Tests for T2DM
The most common diagnostic tests for T2DM are those listed above for the diagnosis of T1DM; the exceptions are that ketone testing, C-peptide, and antibody testing are not typically applicable in T2DM patients, although antibody testing may be indicated to differentiate between T1DM and T2DM. For abnormal screenings of BG or A1C, a second test is typically performed (may be repeated on the same sample) prior to confirming the diagnosis of T2DM. To diagnose T2DM, other historical data and symptoms should be considered (ADA, 2020).
Treatment/Management of T2DM
Type 2 DM is primarily managed by the patient on a day-to-day basis with education, training, and support from the healthcare team. Management includes eating a healthy diet along with physical activity at least three times per week as described previously, and if needed, monitoring of BG levels as well as the administration of medication. The APRN should encourage patients to consistently maintain a healthy diet, including lots of vegetables, complex, low-GI carbohydrates, whole grains, nuts and fruits, lean proteins, low-fat dairy products, and heart-healthy fats whenever possible. Further opportunities to maintain glycemic control are limiting (or avoiding) alcohol and sugary drinks, portion control, increased water intake, and consistency in both dietary intake and exercise (CDC, 2019d; Mayo Clinic, 2019f).
The initial diagnosis of T2DM should prompt a referral to other members of the healthcare team, including a dietician, diabetes educator, and/or mental health providers, as appropriate. Other referrals may include an ophthalmologist, dentist, podiatrist, and potentially a bariatric doctor for patients also diagnosed with obesity (BMI above 40 kg/m2). The entire family and any direct caregivers should be involved in diabetic education. Long and short-term goals will be established with the primary healthcare provider, dietician, and/or diabetic educator. Goals should be reviewed at each subsequent visit and updated as appropriate. Smoking cessation must be discussed if applicable and support provided by the healthcare team. Comorbidities such as hypertension and hypercholesterolemia must be adequately managed in patients with T2DM. Vaccinations should be kept up-to-date and include hepatitis B vaccines and flu vaccines since diabetes lowers the immune system. Managing stress with a regular exercise regimen, adequate sleep, and relaxation techniques such as meditation and yoga can help improve overall health and well-being in patients with T2DM. It is very important for the patient with T2DM to be active in their treatment plan; they should be encouraged to voice preferences and concerns with their healthcare team (ADA, 2020; CDC, 2019d; Mayo Clinic, 2019f).
Providers should seek out additional resources for patient education, such as the DSMES toolkit found on the CDC website. This program provides diabetic education and support to patients and their families and is often eligible for reimbursement by Medicare, some state Medicaid agencies, and many private insurers. For example, Medicare Part B members are eligible for 10 hours of diabetes education during their first year after diagnosis, followed by two additional hours of education every year following. There were over 4,000 programs nationwide as of 2016, and details regarding local recognized/accredited programs can be found on the CDC, ADA, and AADE websites. Less than 5% of Medicare patients and less than 7% of privately insured patients with diabetes have participated, despite the fact that studies indicate that participation leads to a positive impact on lifestyle changes, decreases in A1C levels, prevention or delay of complication, improved quality of life, and reduced hospitalizations. Unfortunately, access continues to be an issue, as recognized/accredited programs are currently located in 56% of counties nationwide (CDC, 2018b).
SMBG may provide limited clinical benefit (does not significantly reduce A1C levels in studies) in those T2DM patients not using insulin. For some of these patients, SMBG may provide valuable insight into the effect of diet, exercise, and medication on BG levels and be helpful while adjusting diet/exercise or other medications (especially those that may cause hypoglycemia). T2DM patients who do not require INT and are able to be maintained on just basal insulin with or without oral medications may achieve lower A1C levels with SMBG (especially when assessing fasting BG levels to inform dose adjustments). T2DM patients on INT should abide by the very same guidelines for SMBG or CGM, as described above for T1DM patients, with minor modifications to customize for each individual patient. When used properly, CGM may reduce A1C levels and episodes of hypoglycemia in T2DM patients on insulin who are not meeting glycemic targets. Similar to T1DM, most T2DM patients should have an A1C goal of less than 7%, including children and adolescents. A goal of less than 6.5% may be appropriate if it can be achieved without significant hypoglycemia, and 7.5% may be necessary if the risk of hypoglycemia is increased (ADA, 2020).
Pharmacological Treatment. Many patients with T2DM are able to avoid oral and subcutaneous hypoglycemic medications and/or insulin with diet, exercise, and the other lifestyle modifications discussed above. In other patients, these measures are inadequate, and medications are added when needed to avoid complications related to consistently elevated BG levels (CDC, 2019d). Oral medications to lower BG levels are typically the first-line pharmacological treatment; it is crucial that patients understand that these medications work best when combined with diet and exercise. Metformin (Glucophage) is typically the first medication prescribed for T2DM and sometimes for weight loss in prediabetes (Mayo Clinic, 2019f). This is especially appropriate in metabolically stable patients with A1C levels under 8.5% and normal renal function. Metformin (Glucophage) is also commonly used in women with PCOS to induce ovulation; this medication should be stopped by the end of the first trimester once pregnancy is confirmed. Young T2DM patients with BG levels above 250 mg/dL and an AIC level above 8.5% who are symptomatic (polydipsia, polyuria, nocturia, and weight loss) should be started on basal insulin while metformin (Glucophage) is titrated (ADA, 2020). When metformin (Glucophage), diet, and exercise are not successful in controlling BG levels, another oral or injectable medication should be added. Sulfonylureas and meglitinides can both cause hypoglycemia, and patients should be educated regarding the signs/symptoms of hypoglycemia when prescribed these medications specifically. Thiazolidinediones are typically not the first-choice treatment due to their side effect profile and are used only in unique cases where BG control is not attained with other drug categories. Dipeptidyl-peptidase 4 (DPP-4) inhibitors often have only a modest effect on BG levels. Sodium-glucose transporter 2 (SGLT2) inhibitors may reduce the risk of acute myocardial infarction or stroke (Mayo Clinic, 2019f).

Not all individuals are able to control their BG with oral medications. GLP-1 receptor agonists are non-insulin injectable medications that slow down digestion and thus decrease BG levels. They may reduce BP and promote weight loss but have potential adverse effects such as nausea and increased risk of pancreatitis. These drugs may be taken twice daily, daily, or weekly. Examples are exenatide (Byetta, Bydureon), dulaglutide (Trulicity), semaglutide (Ozempic), and liraglutide (Victoza). Liraglutide (Victoza) and semaglutide (Ozempic) have been associated with a decreased risk of acute myocardial infarction or stroke in those patients at increased risk (ADA, n.d.e; Mayo Clinic, 2019f). The ADA specifically recommends starting the GLP-1 receptor agonist liraglutide (Victoza) in patients with T2DM over the age of 9 who are not meeting glycemic targets on metformin (Glucophage) and lifestyle interventions. This is not appropriate in patients with a past medical or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 (ADA, 2020).
Insulin should be added to the treatment plan when a T2DM patient is unable to adequately control their BG levels with lifestyle modifications and oral medications and/or non-insulin injectable medications. Initially, a single injection of long-acting insulin such as glargine (Lantus) or detemir (Levemir) may be used in combination with other oral or subcutaneous medications. Some patients with T2DM eventually worsen and require multiple injections of insulin daily. Other medications that may be required for T2DM patients include antihypertensive medications to control blood pressure (BP) and protect renal function, low-dose aspirin to reduce cardiovascular risk, and/or cholesterol-lowering medications to manage hypercholesterolemia (ADA, n.d.e; Mayo Clinic, 2019f).
Weight Loss. Diet, exercise, and behavioral therapy is the recommended method to reduce body weight (goal of at least 5%) in patients with T2DM and a BMI above 25 kg/m2. It should be explained to patients that a weight loss of at least 5% of their body weight will benefit BG control and reduce CV risk factors/complications. Sessions should be intensive (at least 16 sessions over a period of six months), with a goal of a 500-750 calorie daily deficit. Motivation and willingness to lose weight should be assessed first, as interventions are not successful in patients not eager to lose weight. Diets should be individualized based on personal taste, cultural factors, food availability, and macronutrient needs based on activity level and lifestyle. Weight maintenance programs are recommended to help patients sustain their short-term goals for over one year with monthly support and weekly weight assessments (ADA, 2020).
Patients with a BMI over 27 kg/m2 who are unable to achieve their desired weight loss goals with diet, exercise, and behavioral therapy should be invited to consider pharmacological agents approved by the FDA for weight loss, which have been shown to lead to improved glycemic control in T2DM patients. Phentermine (Adipex-P, Lomaira) is approved for short-term (less than 12 weeks) use only. It may also be combined with topiramate (Qsymia). Orlistat (Xenical, Alli) is available over-the-counter or by prescription (in a stronger dosage form) and works by inhibiting the action of lipase and thus reducing the absorption of dietary fat intake. Naltrexone/bupropion (Contrave) contains an antidepressant which blocks the reuptake of norepinephrine and dopamine but has a black box warning for increased risk of suicidal ideation/behavior. Liraglutide (Saxenda, Victoza) is a GLP-1 receptor agonist that reduces appetite. Individuals with T2DM who have a BMI over 40 kg/m2 may be referred for weight loss (bariatric) surgery. Adults with a BMI between 35 and 39 kg/m2 could also be considered for weight loss surgery if they are unable to lose weight and decrease their medical comorbidities (including hyperglycemia) with diet, exercise, behavioral counseling, and pharmacological methods. BG levels are often drastically improved by the weight loss that occurs after bariatric surgery. The consideration of risks such as long-term nutritional deficiencies, osteoporosis, and even death must be discussed with the patient by the healthcare team (ADA, 2020; Epocrates, n.d.; Mayo Clinic, 2019f).
Gestational Diabetes (GDM)
Patients with GDM are often asymptomatic. It is typically diagnosed during routine screening or early screening based on known risk factors. All pregnant women are screened in the US between 24-28 weeks. For a patient who was diagnosed with GDM with a previous pregnancy or otherwise at increased risk, screening should take place at their first prenatal visit with further testing at 24-28 weeks pregnancy if the initial testing was negative. Both of these tests are intended for pregnant women without a prior history of diabetes (ADA, 2020; CDC, 2019a).
- Two-step strategy (originally recommended by an NIH panel in 2013)
- Without fasting, the patient drinks a 50 g glucose solution with a BG level check one hour later. If the BG is higher than 130-40 mg/dL (depending on the professional organization making the recommendation) at one hour, it indicates a need for additional testing.
- The second step is indicated only in women with abnormally high BG level in step #1. Similar to below, the pregnant patient fasts overnight and presents for an FPG. The patient then drinks a 100 g glucose solution, and BG checks are done hourly for three hours. Two of the four BG readings must be abnormally high to constitute a positive test (95 mg/dL fasting, 180 mg/dL at one hour, 155 mg/dL at two hours, and 140 mg/dL at three hours).
- The American College of Obstetricians and Gynecologists (ACOG) recommends this strategy but states that positive test results in either step #1 or #2 should infer a diagnosis of GDM, not both.
- One-step strategy (derived from the International Association of the Diabetes and Pregnancy Study Groups [IADPSG] and currently recommended by the ADA)
- After fasting overnight, an FPG is drawn prior to starting the test. A 75 g glucose solution is ingested, and then the patient’s BG is checked every hour for three hours.
- If any of the three BG levels are higher than the established threshold, the patient is diagnosed with GDM. The fasting threshold is 92 mg/dL, the one-hour threshold is 180 mg/dL, and the two-hour threshold is 153 mg/dL (ADA, 2020).
Treatment/Management of GDM
Primary care providers should educate non-pregnant women of childbearing age about the importance of weight loss and regular exercise to reduce their risk of developing GDM. Pregnant mothers with diabetes (GDM or pre-existing) should be instructed to check their BG routinely; both fasting and postprandial BG checks are recommended. Fasting BGs should be below 95 mg/dL, and postprandial BGs should be below 140 mg/dL at one-hour, or below 120 mg/dL at two-hours. Alternately, CGM may be utilized to improve A1C levels and neonatal outcomes, especially in pregnant women with T1DM. The A1C target during pregnancy is less than 6% but may be increased to 7% to avoid hypoglycemia. Lifestyle modification, including diet (based on referrals to a nutritionist/dietary consult) and exercise, is recommended as the basis for the treatment of diabetes of any type during pregnancy. Weight loss during pregnancy is not encouraged. Insulin is the first-line pharmacological therapy for GDM if lifestyle modifications do not achieve glycemic targets. It is also the first-line treatment in pregnant women with pre-existing T2DM or T1DM. Metformin (Glucophage) and glyburide (DiaBeta) are both considered safe secondary alternatives, but patients should be warned that both of these medications cross the placenta to the fetus. Other oral/injectable medications currently lack long-term safety data in pregnant patients and should be avoided. Close monitoring of the fetus for optimal growth and development is required. Following delivery, insulin requirements will quickly decrease to roughly 50% of previous requirements, and the patient and care team should be cautious to avoid hypoglycemia immediately postpartum. To reduce the risk of developing GDM in future pregnancies, the patient should be encouraged to lose weight and engage in regular physical activity prior to trying to conceive again. In addition, early BG screening should take place with future pregnancies (ADA, 2020; CDC, 2019a).
Secondary Diabetes
Secondary diabetes is elevated BG levels caused by an illness, toxin, or medication. This type of diabetes occurs due to reduced insulin sensitivity or secretion and constitutes approximately 1-2% of all diagnosed cases of diabetes (Parks-Chapman & Schub, 2018). While there are several individual disease processes and medications that can cause secondary diabetes, some examples can be found in Table 4.

The signs and symptoms of secondary diabetes are consistent with those previously listed for T1DM and T2DM, although symptoms depend on the severity of the insulin insensitivity and hyperglycemia. If the cause is not obvious, this type of diabetes can be difficult to diagnose. These patients may present with DKA (similar to T1DM) or hyperglycemic hyperosmolar syndrome (HHS, similar to T2DM) or more minor symptoms, including polydipsia, polyphagia and/or polyuria (Parks-Chapman & Schub, 2018).
Diagnostic Tests for Secondary Diabetes
Diagnostic tests for secondary diabetes may vary based on the etiology of the condition, although most patients will be diagnosed in a similar fashion to T1DM and T2DM. Starting with the random BG level, additional testing (i.e., antibody testing) may be needed to determine the optimal treatment plan for the patient with secondary diabetes (Parks-Chapman & Schub, 2018). This diagnostic work-up and parameters mirror those discussed above for T1DM and T2DM. A thorough history and physical is indicated to determine any pharmacological or physical history that could indicate secondary diabetes (ADA, 2020; JDRF, 2019b).
Treatment/Management of Secondary Diabetes
Immediate treatment to lower the BG level should be initiated with either oral antidiabetic drugs and/or insulin. For secondary diabetes caused by a medication, changing the medication regime should be considered if possible, which eliminates the need for antidiabetic medications and other management. When due to a medical condition, treatment should focus on rectifying the underlying cause. For some patients with secondary diabetes, lifelong treatment will be needed. Diabetic education should be given, as well as education regarding nutrition, medication regimen, and lifestyle modifications needed for all newly diagnosed diabetic patients (Parks-Chapman & Schub, 2018).
Complications of DM
Prediabetes Complications
As previously stated, prediabetes carries an increased risk of cardiovascular disease and other complications, and most patients are unaware of their diagnosis; thus, routine screening is crucial to identify those at risk and start early interventions that are both cost and therapeutically effective. Dietary modifications and increased physical activity can likely reverse the risk of developing diabetes and the need for future costly interventions such as oral/subcutaneous hypoglycemic medications and/or insulins (ADA, 2020; CDC, 2019c).
GDM Complications
Women with GDM have an increased chance of developing T2DM at some point in their life. Their risk can be lowered by maintaining a healthy weight, eating a healthy diet, and routine exercise. The mother is also at an increased risk for HTN and a cesarean delivery due to the potential for macrosomia, or excessive birth weight (CDC, 2019a). In order to decrease the risk of preeclampsia (HTN, peripheral swelling, and proteinuria during pregnancy), pregnant patients with T1DM and T2DM should be prescribed low-dose aspirin by the end of the first trimester. Pregnant diabetic patients with HTN (above 135/85 mmHg consistently) should be treated with a goal no lower than 120/80 mmHg, but angiotensin-converting enzyme inhibitors (ACE inhibitors) and angiotensin receptor blockers (ARBs) should be avoided or stopped at conception (ADA, 2020). Complications for the baby born to a mother with GDM include:
- Macrosomia, over nine pounds at birth,
- Hypoglycemia at birth,
- Prematurity leading to respiratory issues, and
- Development of T2DM later in life (CDC, 2019a).
Patients should be counseled on the potential complications requiring interventions in the neonatal intensive care unit (NICU; CDC, 2019a). For additional information regarding diabetes management in pregnant patients, please see the NursingCE course entitled Diabetes in Pregnancy.
Diabetes Complications
Hyper/Hypoglycemia
Hyperglycemia occurs when the BG is higher than the target levels. Symptoms include polyuria, polydipsia, dry mouth, blurred vision, fatigue, and nausea. Hyperglycemia can occur for a number of reasons including but not limited to:
- Illness,
- Stress,
- Ingestion of high-GI or large quantities of food,
- Missed doses of medication, and
- Decreased activity.
T1DM patients should have access to a home ketone testing kit, using either urine or blood sample, and test for ketones if the BG is above 240 mg/dL. The APRN should instruct patients to call their healthcare provider if their BG level remains above 240 mg/dL for more than two consecutive checks (CDC, 2020c; Mayo Clinic, 2019f).
Hypoglycemia (level 1) occurs when the BG falls below 70 mg/dl. The ADA defines level 2 hypoglycemia as a BG under 54 mg/dL and level 3 as hypoglycemia associated with altered mental or physical status. While it is more common with T1DM, hypoglycemia may occur with T2DM after taking insulin or certain medications that stimulate more insulin production (i.e., sulfonylureas), more physical activity than normal, and/or upon skipping a meal. The individual suffering from hypoglycemia may exhibit the following signs and symptoms:
- Sweating,
- Shakiness,
- Weakness,
- Hunger,
- Irritability,
- Dizziness,
- Headache,
- Blurred vision,
- Heart palpitations,
- Slurred speech,
- Drowsiness,
- Anxiety/nervousness, or
- Confusion (ADA, 2020; CDC, 2020c; Mayo Clinic, 2019f).
The APRN should educate patients to recognize hypoglycemia quickly, and that management should include:
- A glucose tablet (15-20 g) if available. Alternatively, the patient should be instructed to eat or drink a simple sugar such as fruit juice, hard candy, or regular soda. The amount should be the equivalent of 15-20 g of sugar.
- Retest BG in 15 minutes to ensure it is above 70 mg/dl. If not, repeat the step above. Avoid foods high in fat as glucose absorption will be delayed in the presence of fat.
- Once the BG level is above 70 mg/dl, the individual should eat a meal or snack containing a complex carbohydrate and protein such as cheese and whole-grain crackers to avoid another episode of hypoglycemia.
- For the patient who loses consciousness during hypoglycemia (level 3), caregivers/family members should be instructed to administer a glucagon injection or simple sugar inside the gums to regain consciousness, then follow step 3 as above (ADA, 2020; CDC, 2020c; Mayo Clinic, 2019f).
It is important for APRNs to educate the patient and caregivers about the risk of this life-threatening condition. Hypoglycemia can be avoided through early recognition, intervention, and prevention. Patients on insulin with hypoglycemia unawareness, a single level-3 event, or a pattern of unexplained level-2 events should be instructed to increase their BG targets for at least several weeks to partially reverse the hypoglycemia unawareness and reduce future risk (ADA, 2020; CDC, 2020c).
Diabetic Ketoacidosis (DKA)
Diabetic ketoacidosis (DKA) is a complication that can lead to a diabetic coma or death. DKA is a hyperglycemic crisis that occurs primarily with T1DM but can occur in some cases with T2DM. Due to a lack of insulin to transport glucose into the cells for energy, the body breaks down fat for energy, which produces ketones. These ketones build up in the blood and make it more acidic. The rising ketones lead to DKA. Increased mortality and morbidity occur with DKA (ADA, n.d.b; Alshammari et al., 2017). The signs and symptoms of DKA are:
- Thirst or dry mouth;
- Frequent urination;
- Hunger;
- Elevated BG levels;
- Elevated ketones in the urine;
- Fruity-smelling breath;
- Increased respiration (Kussmaul's);
- Fatigue;
- Dry, flushed skin;
- Nausea, vomiting, or abdominal pain;
- Confusion (ADA, n.d.b).
For any of these symptoms, the patient should be instructed to seek healthcare immediately. The causes of DKA are:
- Not enough insulin is taken or increased need due to illness,
- Not enough food intake,
- Overnight hypoglycemia causing a breakdown of fat (ADA, n.d.b).
DKA may be the first sign of T1DM in the patient who is unaware of their disease, or it may be brought on by infection, failure to use insulin, or an acute medical condition involving the cardiac, endocrine, or GI system. Stressors to the body from medical procedures such as surgery can also lead to DKA. Medications that impact carbohydrate metabolism or volume status may trigger an episode of DKA, including diuretics, corticosteroids, antipsychotics, or beta-blockers (Alshammari et al., 2017).
Emergency management of DKA is based on the restoration of circulating volume and the reduction of BG levels (Alshammari et al., 2017). For the treatment of DKA, the ADA recommends initiation of 0.9% normal saline, a crystalloid fluid to replace the volume loss caused by polyuria. The fluid replacement goal is 15-20 mL/kg of body weight within the first hour, as early intervention is critical to reversing the risk associated with DKA (ADA). An insulin drip will be initiated in the early phases of treatment; the greatest fall in BG typically occurs in the first one to two hours. Short-acting and rapid-acting insulins are equally effective in the treatment of DKA. As BG levels fall, D5W should be infused to prevent hypoglycemia. (Alshammari et al., 2017). Potassium levels should be monitored throughout the acute phases to assess for hypokalemia and replacement potassium given as appropriate. Most acid-base imbalances are corrected with the initiation of fluid and insulin; however, the patient's electrolytes should be monitored throughout treatment and managed accordingly (Alshammari et al., 2017; Rouf et al., 2018; Sindi et al., 2017).
Sick Days and Insulin
Diabetic patients experiencing a sick day should check their BG levels every three to four hours. T1DM patients should further check their urine for ketones if their BG is significantly elevated (above 250 mg/dL). It is important to drink consistently during sick days to avoid dehydration, about 8-12 ounces of water per hour (CDC, 2020d). Patients should attempt to continue to drink, eat, and take their usual short-acting, intermediate, and/or premixed insulin dose; their bolus dose(s) should match their carbohydrate intake. If the patient is unable to eat or drink, patients should be instructed to take a correction factor dose every three to four hours. A correction factor dose is given to decrease the BG when it goes too high. Each patient should have an individualized correction dose determined by their healthcare provider and based on their current BG reading, but typically it is 50% of their traditional bolus dosing or 1 unit for each 50 mg/dL if their BG is less than 250 mg/dL. This should be adjusted based on various factors, including age, weight, or activity level. Patients that are unable to eat for longer than one day should contact their primary care provider to check in for further instructions if needed (Wood & Peters, 2018). If patients are unable to keep fluids down for more than four hours or those with moderate to high ketones in their urine should consider seeking medical attention at an emergency room (CDC, 2020d).
Hyperglycemic Hyperosmolar Syndrome (HHS)
HHS is a life-threatening condition that primarily occurs in T2DM patients if their BG level rises above 600 mg/dL. Some patients may present with HHS initially, previously unaware of their T2DM diagnosis. In diagnosed T2DM patients, this condition may be preceded by illness or infection and is typically accompanied by extreme dehydration and an altered level of consciousness. It may also be caused by missing doses of T2DM medications or taking a medication that decreases the effectiveness of insulin or diuretics. Their glucometer may simply indicate "high" upon a check of the BG. They may present with a dry mouth, extreme thirst, confusion, weakness, nausea, weight loss, fever, hypotension, tachycardia, and seizures; if untreated, the patient may become comatose. Lab values typically indicate no acidosis present, as the patients with T2DM are still making enough insulin to avoid ketogenesis. The lack of high ketone levels and marked metabolic acidosis is the primary difference between HHS and DKA. Despite this, the patient with HHS may have some ketones in their blood and/or urine if the condition is severe enough. The dehydration is related to the severe hyperglycemia, as the kidneys attempt to correct the elevated BG levels by increasing urine output. The APRN should instruct patients and caregivers to seek immediate care with any signs or symptoms of HHS. Emergent treatment for HHS mimics that of DKA, with a focus on intravenous fluid replacement and insulin to reduce BG levels. Potassium replacement should be based on comprehensive metabolic profile results and institution algorithms. Shock, cerebral edema, lactic acidosis, and blood clot formation are known complications to be aware of in patients being treated for HHS (Mayo Clinic, 2019f; MedlinePlus, 2018a).
Cardiovascular Disease
CVD is the leading cause of death among diabetes patients; patients with diabetes have double the risk of CVD as compared to non-diabetic patients and are affected earlier than non-diabetic patients. This is due to the effect of hyperglycemia on the vessels and nerves that feed and control the heart. Hypertension and hypercholesterolemia are both more common in patients with diabetes and should be diligently controlled to reduce the risk of CVD. Other ways to reduce the risk of CVD include a diet rich in fruits/vegetables, lean protein, whole grains, and lots of water. Diabetic patients should avoid processed foods, trans fats, sugary drinks, and alcohol. Patients that are overweight should attempt to lose 5% of their body weight in order to reduce their risk, but benefits may be obtained with as little as 3%, and additional benefits may be seen with a weight loss of 7%. Adult patients should exercise at a moderate intensity at least days per week, for a total of at least 150 minutes per week. Smoking cessation should be strongly encouraged if applicable (CDC, 2019c). For additional information regarding CVD in patients with diabetes, please see the NursingCE course entitled Cardiovascular Health and Prescribing for the Diabetic Patient.
Neuropathy
Diabetic neuropathy is defined as nerve damage related to the cumulative chronic effects of hyperglycemia. Age and the length of time that the patient has had diabetes are nonmodifiable risk factors for neuropathy, while glycemic control, being overweight, hypertension, hypercholesterolemia, advanced kidney disease, alcohol use disorder, and smoking are all modifiable factors that also increase the risk. The nerves may become damaged as a direct result of hyperglycemia, and/or secondary to reduced vascular supply. As many as one-half of all patients with diabetes have peripheral neuropathy, up to 30% have autonomic neuropathy, and up to 25% have some focal neuropathy in the wrist. Symptoms typically develop gradually over the years, although some may present suddenly (NIDDK, 2018b).
Peripheral neuropathy typically causes pain, tingling, numbness, or weakness in the feet and legs, and may also affect the arms and hands. The pain may be described as pins and needles sticking into the feet or hands. There may be burning or shooting pain in the feet or hypersensitivity to touch even with socks, shoes, gloves, or bed linens. Most patients describe symptoms that are bilateral and worse at night. Numbness and weakness may be part of the presentation of neuropathy as well. This may affect gate, balance, and muscle tone, and increase a patient's risk for falls. The decreased sensation may lead to blisters/ulcers on the toes or feet that develop or worsen without the patient's awareness. Severe cases may lead to Charcot's foot, a deformity related to damaged tissues/bones in the feet. Examinations for patients with diabetes should include an annual assessment of peripheral sensation, including vibration, light touch, and temperature, as well as an assessment of lower extremity strength, gait, and balance. Additionally, a visual assessment of the patient’s feet is required, and many patients require regular care from a podiatrist. Vitamin B12 deficiency, which may occur secondary to metformin (Glucophage) use, may worsen symptoms of peripheral neuropathy and should be ruled out in patients taking metformin (Glucophage). Physical therapy should be encouraged to help prevent falls and improve gait. Topical anesthetics such as lidocaine (Lidoderm) may be helpful to some and can be prescribed in a patch for easy application with minimal adverse effects. Antidepressants and anticonvulsant medications may be helpful in the symptomatic management of peripheral neuropathy, although there is no therapeutic cure for the condition. Pregabalin (Lyrica) and its precursor gabapentin (Neurontin) were originally designed to treat seizures but were found to be helpful in the treatment of neuropathic pain (NIDDK, 2018b). Certain tricyclic antidepressants (TCAs; i.e., nortriptyline [Pamelor], desipramine [Norpramin], imipramine [Tofranil], and amitriptyline [Elavil]) may be helpful in some patients by blocking the reuptake of norepinephrine and serotonin. They often cause drowsiness, increased appetite, weight gain, dry mouth, urinary retention, constipation, and may cause orthostatic hypotension and cardiac arrhythmias (Mayo Clinic, 2019e). These were included in the 2015 American Geriatric Society Beers Criteria of potentially inappropriate medication in older adults due to their anticholinergic effects and should be avoided in patients over 65 if possible (Terrery & Nicoterri, 2016). Selective serotonin reuptake inhibitors (SSRIs) traditionally used for depression, such as citalopram (Celexa) and paroxetine (Paxil), may be helpful as well as serotonin-norepinephrine reuptake inhibitors (SNRIs) duloxetine (Cymbalta) or venlafaxine (Effexor, NIDDK, 2018b). These four medications may cause nausea, headache, dry mouth, insomnia, and sexual dysfunction, as well as place the patient at risk for serotonin syndrome due to excessive serotonin levels. Serotonin syndrome may present with agitation, anxiety, confusion, high fever, sweating, fluctuating BP, and tachycardia and requires emergent medical attention as it can be lethal (Mayo Clinic, 2019c, 2019d).
Autonomic neuropathy affects the nerves that control internal organs, such as the heart, GI tract, bladder, sexual organs, sweat glands, and eyes. Damage to these nerves can also lead to a poor sense of hypoglycemia, known as hypoglycemia unawareness. The traditional early symptoms of hypoglycemia, such as confusion, dizziness, hunger, irritability, or nervousness, are not experienced. This may potentially lead to severe hypoglycemia with loss of consciousness. Other symptoms of autonomic neuropathy may vary depending on which nerves are damaged but include hypotension (especially orthostatic hypotension), tachycardia or variable heart rate, reduced sensation of angina during myocardial ischemia events, GI symptoms (bloating, fullness, nausea/vomiting, constipation, nocturnal diarrhea, fecal incontinence, or dysphagia [difficulty swallowing]), a poor sensation of bladder fullness, urinary incontinence, sexual dysfunction (erectile dysfunction [ED], retrograde ejaculation, vaginal dryness, etc.), night sweats, gustatory sweating (increased sweating while eating), anhidrosis (reduced sweating), delayed pupillary response to light, or difficulty driving at night (NIDDK, 2018b).
If concerned, initial tests for autonomic neuropathy include assessing the patient's heart rate and BP after lying down or sitting for 5-10 minutes, immediately upon standing, and then again after several minutes of standing (this is known as checking orthostatic blood pressure). A decrease of more than 20 mm/Hg in systolic or 10 mm Hg in diastolic or the presence of symptoms (dizziness, loss of balance) indicates orthostatic hypotension. Tilt table testing may also be indicated for some patients (Mayo Clinic, 2020). Patients with confirmed autonomic neuropathy affecting heart rate and BP should be encouraged to increase their fluid intake and potentially salt (sodium) intake if hypotensive. Increased physical exercise may help improve symptoms as well as compression stockings to improve blood flow. They may find it helpful to raise the head of their bed while sleeping and should be instructed to stand from a lying or seated position very gradually and carefully, with support as needed from caregivers, furniture, or an assistive device (cane, walker) if indicated. The most important management tool for autonomic neuropathy is enhanced glycemic control (NIDDK, 2018b).
Patients with bladder or sexual symptoms indicative of autonomic neuropathy affecting the bladder or sexual function should be referred to a specialist, such as a urologist or a gynecologist. For bladder issues, the urologist will assess the patient and likely perform urodynamic testing, including a cystometrogram (CMG). Patients with urinary incontinence (UI) should be encouraged to avoid constipation, instructed in timed voiding and bladder training, and referred for pelvic floor PT if indicated (NIDDK, 2018a, 2018b). Numerous products ranging from pads to washable absorbent underwear and incontinence briefs may be helpful. Tablets can also be taken by mouth to reduce the odor of urine and enhance patients' comfort regarding incontinence odors. Skincare around the peroneal area in patients who are incontinent should include frequent cleansing, protective creams, and regular visual inspections for irritation/infection (NIDDK, 2018e).
Those with stress incontinence should be encouraged to lose weight if overweight or obese, as this may alleviate some of the symptoms. Frequency, urge incontinence, or urinary retention may be reported as well. Elevated BG levels and urinary retention contribute to more frequent UTIs (NIDDK, 2018a, 2018b). Some female patients with stress incontinence may benefit from a consultation for a pessary to be inserted into the vagina or may attempt to use disposable internal bladder supports (Impressa) that can be purchased over the counter and inserted into the vagina for up to 12 hours to support the urethra and prevent leaking. A longer-term solution for stress incontinence is injectable bulking agents that are injected near the urinary sphincter to prevent leaks. Stress incontinence in men can sometimes be treated with penile clamps/clips. Severe urinary retention may require intermittent self-catheterization (NIDDK, 2018e).
Diabetics with UI should be conscious and aware of how much, what, and when they drink. Avoiding liquids right before bed will help with nocturia, and avoiding caffeine, alcohol, and carbonated beverages may help as well. Pharmacological options for urge incontinence/overactive bladder include:
- Antimuscarinics (also called anticholinergics) may be helpful for those with urge incontinence, such as oxybutynin (Ditropan), solifenacin (Vesicare), and tolterodine (Detrol), but should be avoided in those with delayed gastric emptying or gastroparesis. These work by binding to muscarinic receptors in the detrusor, blocking acetylcholine and thus suppressing involuntary contractions of the bladder. They often cause traditional anticholinergic side effects, such as a dry mouth.
- β3-adrenergic agonists, such as mirabegron (Myrbetriq), work by relaxing the smooth muscles within the bladder wall and may be combined with solifenacin (Vesicare).
- TCAs such as imipramine (Tofranil) and doxepin (Silenor) are not indicated for first-line treatment but may decrease bladder contractility.
- Botulinum toxin injections (Botox) is a neurotoxin that can be injected into the detrusor muscle, temporarily blocking nerve impulses and relaxing muscle contractions, but often causing urinary retention as a side effect, requiring temporary, intermittent catheterization.
- Hormones such as estrogen (Premarin) may be useful in female patients with atrophic urethritis to reduce detrusor activity but is not typically first-line treatment (NIDDK, 2018e).
Women with persistent UI ineffectively treated with medications may be candidates for electrical nerve stimulation. Male patients with diabetes and an enlarged prostate may be prescribed an alpha-blocker or 5-alpha reductase inhibitor. Alpha-blockers (i.e., doxazosin [Cardura], prazosin [Minipress], terazosin [Hytrin]) work by blocking the effects of norepinephrine on alpha-1 adrenergic receptors and thus causing smooth muscle relaxation. Alpha-blockers may cause hypotension, dizziness, or headache due to the reduction in BP. 5-alpha reductase inhibitors (i.e., finasteride [Proscar], dutasteride [Avodart]) block the enzymatic conversion of testosterone into dihydrotestosterone. They may cause impotence, decreased libido, affect ejaculation, and gynecomastia. Surgical resection of the prostate may be required if lifestyle and medications are ineffective (NIDDK, 2018e).
Surgical options for incontinence include:
- In patients with overflow incontinence, surgery may be recommended to unblock or restore the urethra (in men, this commonly involves a transurethral resection of the prostate or TURP);
- In women with stress incontinence, a bladder sling procedure may be performed to insert material (typically mesh, but may also be a donor or autologous tissue graft) between the vagina and urethra to provide additional support, or a bladder neck suspension to attach the bladder neck to the ligament along the pubic bone;
- In men with stress incontinence, an artificial urinary sphincter can be implanted. This involves a fluid-filled cuff that is placed around the urethra and connected to a saline reservoir/balloon in the abdomen and controlled by a pump inserted into the scrotum to allow for urination when needed.
- A male version of the sling procedure is also available, during which synthetic mesh is placed under the urethra like a hammock and is most effective in patients with mild to moderate stress incontinence (NIDDK, 2018e).
One study noted the emotional toll that sexual dysfunction can have on the wellbeing of a patient with diabetes, correlating sexual dysfunction with depressive symptoms, anxiety, and diabetes-specific distress. It confirmed a significant need for sexual function assessments by healthcare professionals during routine and follow-up visits (Ventura et al., 2017). Hyper and hypoglycemic episodes during sex can greatly affect sexual satisfaction. The NIDDK (2018a) recommends monitoring blood sugar closely around the times that sexual intimacy is expected, particularly if those times are around the administration of insulin or other diabetic medications. It is noted that diabetic-related sexual dysfunction treatment should consist of a multidisciplinary approach, including urology, gynecology, endocrinology, and psychiatry (Kizilay et al., 2017).
Men with diabetes are three times more likely to develop ED than those without diabetes. Men may also develop a condition known as Peyronie’s disease or penile curvature. This curvature is caused by scar tissue called "plaque" in the penis, making it curve when erect. Curves in the penis can cause pain and discomfort during intercourse. ED can co-exist with Peyronie's disease, making sex even more difficult for the patient. Low testosterone can also occur with diabetic men, which can cause or worsen other sexual problems. Low testosterone can cause ED and decreased libido (sex drive). Men with diabetes are often overweight and less active, which can further contribute to low testosterone levels. ED can be treated with medication, so it is important for APRNs to inquire about sexual concerns with patients and encourage open communication. Testosterone therapy can improve the patient’s sex drive and energy levels but should be used judiciously due to the detrimental side effects of steroid usage. The main course of treatment for sexual disorders in the patient with diabetes is phosphodiesterase type 5 inhibitors (Kizilay et al., 2017; NIDDK, 2018a). Many medications are approved by the FDA for sexual dysfunction, particularly erectile dysfunction. This includes sildenafil (Viagra), avanafil (Stendra), tadalafil (Cialis), and vardenafil (Levitra). These medications block phosphodiesterase 5, the enzyme that breaks down cyclic guanosine monophosphate, which is required for an erection. This action only occurs in the presence of nitrous oxide, which is released during sexual arousal. Therefore, phosphodiesterase inhibitors only work to achieve an erection in the presence of arousal. These medications come with significant adverse effects, such as headache, flushing, dizziness, along with an FDA black box warning of the potential for sudden vision and hearing loss. These medications are also contraindicated with the use of nitrates. The APRN should inquire about the use of herbal supplements and alternative medications when assessing the patient. For example, Yohimbe is a natural remedy used for ED that can cause a hypertensive crisis when taken with tyramine-containing foods (cured meats, red wine, caffeine, aged cheeses, overripe fruit, etc.). Other supplements (i.e., “horny goat weed”) should also be noted, as they are not regulated by the FDA and can contain ingredients that may be harmful to the patient. Other treatments for ED include intraurethral suppositories, intracavernosal injections, vacuum tumescence therapy, or penile-prosthesis surgery. A surgical penile prosthesis is generally reserved for those patients that are not responsive to other therapies (Townsend & Morgan, 2017).
Women’s sexual problems may be related to low sexual desire, vaginal dryness, and painful sex related to decreased blood flow to the genitals and hormonal changes from diabetes. This decreased blood flow can lead to reduced or no sensation in the genital area, an inability to have an orgasm, and an inability to become aroused or stay aroused. Dryness can lead to pain and discomfort with intercourse. Mental health implications of having diabetes can also be involved in the decreased sexual desire and response by female patients. Furthermore, women may have increased vaginal yeast infections due to elevated BG and decreased immunity, leading to painful intercourse (NIDDK, 2018a). Hormonal therapy, such as estrogen replacement therapy, has been controversial due to increased mortality during randomized studies. However, new research indicates no link to increased mortality due to cancer or cardiovascular disease with postmenopausal hormone therapy. It is important to note that the women surveyed in this longitudinal study had no incidence of cancer, which can be a contraindication against hormone replacement therapy (Jensen, 2019). It is important to note that estrogen therapy has not been proven to increase sexual desire; however, it does help decrease vaginal dryness and dyspareunia. Applications of this medication include vaginal creams, rings, and tablets. Patients who cannot partake in hormonal treatment due to a history of hormone-receptive cancers, or who do not care for hormone therapy, may be treated with water-based lubricants and moisturizers to help with dryness and discomfort (Thornton et al., 2018).
Both sexual and bladder health can be improved by:
- Maintaining therapeutic BG levels as determined by goals set with healthcare providers,
- Routine physical activity,
- Maintaining a healthy weight as determined by goals set with healthcare providers,
- Quitting smoking,
- Seeking mental health interventions for any emotional or psychological symptoms (NIDDK, 2018a).
Significant others of the patient with diabetes should also be part of the conversation and an active member of the healthcare team to offer the greatest amount of support and understanding possible. Such personal concerns as sexual and bladder health can be embarrassing and cause anxiety in many patients, and a support system that involves their partner can be helpful in dealing with the issues of concern (NIDDK, 2018a).
Delayed emptying of the stomach, or gastroparesis, can occur due to autonomic neuropathy that damages the vagus nerve. The vagus nerve controls the muscular activity of the stomach and intestines, leading to gastric motility. Slower movement of food through the digestive tract may lead to gastroesophageal reflux (GERD), heartburn, nausea, vomiting of undigested food, abdominal bloating, decreased appetite, erratic BG levels, abdominal pain, and early or prolonged satiety when eating. With gastroparesis, food stays in the stomach longer and leads to elevated BG and increased difficulty maintaining euglycemia (ADA, n.d.b). Nutrients are not well absorbed, and patients with vomiting are at risk of dehydration and electrolyte disturbances (NIDDK, 2018c). Further, food that stays in the stomach too long can lead to fermenting and bacterial overgrowth. The food can also harden into solid masses called bezoars that can lead to nausea, vomiting, and obstruction, blocking the passage of food into the small intestines. Patients with gastroparesis should be instructed to avoid large amounts of indigestible cellulose found in celery, pumpkin, prunes, raisins, leeks, beets, persimmons, and sunflower-seed shells to reduce their risk of developing a bezoar (ADA, n.d.b; Mayo Clinic, 2019a). Patients with diabetes should avoid medications that may slow gastric emptying, such as narcotics, certain antidepressants (amitriptyline [Elavil], nortriptyline [Pamelor], and venlafaxine [Effexor]), anticholinergics, antimuscarinics (used to treat overactive bladder, such as oxybutynin [Ditropan]), and pramlintide (Symlin). Gastroparesis is typically diagnosed based on an endoscopy, gastric emptying scintigraphy, and/or gastric emptying breath test. A wireless motility capsule, or SmartPill, may also be an option for some patients. Patients diagnosed with diabetic gastroparesis should have a dietary consultation with a trained and experienced nutritionist to plan a diet that is low in fat and fiber and divided into five or six small meals throughout the day. Patients should be trained to take small bites, chew food thoroughly, eat softer foods that are well-cooked, and drink plenty of water and other non-carbonated fluids. Liquid meal replacements may be helpful. They should avoid carbonated beverages and alcohol. A walk or similar gentle physical activity after eating may aid in gastric motility, and they should avoid lying down for at least two hours after eating. A dietary supplement such as a daily multivitamin may help restore any nutritional deficiencies. Some patients with gastroparesis will switch to postprandial insulin injections (in lieu of the traditional preprandial injection). Certain medications may assist with increasing gastric motility:
- Metoclopramide (Reglan) may increase gastric muscular contractions and reduce nausea/vomiting;
- Erythromycin (Erythrocin) is an antibiotic that may be used to increase muscle contractions within the GI tract;
- Ondansetron (Zofran), prochlorperazine (Compazine), promethazine (Phenergan), and OTC antiemetics such as bismuth subsalicylate (Pepto-Bismol) may be used to treat nausea and vomiting associated with gastroparesis, but do not increase gastric emptying;
- Mirtazapine (Remeron) is an antidepressant that may help alleviate nausea/vomiting, but may not increase gastric emptying;
- Non-narcotic pain medications may be used to treat abdominal pain (narcotics should be avoided, as previously mentioned; NIDDK, 2018c).
In severe cases where malnutrition develops, tube feedings into the small intestine may be required. This can be done through a nasal or oral tube temporarily, or a surgically placed jejunal, or "J" tube, if longer-term feeding is required. Parenteral nutrition may be necessary if the jejunal tube is not effective. A venting gastrostomy is a surgical procedure in which a tube is placed into the stomach to relieve pressure and allow stomach contents to flow out when too full. A gastric electrical stimulator (GES) can be placed subcutaneously in the lower abdomen to electrically stimulate the muscles lining the stomach to decrease nausea and vomiting related to diabetic gastroparesis (NIDDK, 2018c).
Focal neuropathies are less common than peripheral or autonomic neuropathies; they affect a single nerve, most often in the hand/wrist, head, torso, or leg. Entrapments are the most common type of focal neuropathy in which a nerve becomes compressed when passing through a small space between bones and other tissues. Diabetic patients are at increased risk for nerve entrapment. One example is carpal tunnel syndrome, in which the median nerve is entrapped/compressed by the transverse carpal ligament in the wrist, causing progressively worsening tingling, numbness, or pain into the thumb, index, and middle fingers. Other focal neuropathies include:
- Cubital tunnel syndrome (ulnar entrapment at the elbow, causing symptoms that radiate down into the pinky and ring fingers),
- Entrapment of the common peroneal nerve (causing neuropathy symptoms into the lateral lower leg and big toe), and
- Cranial neuropathies (causing various symptoms depending on the affected nerve, such as unilateral eye pain, double vision/difficulty focusing the eyes, or unilateral facial paralysis [Bell’s palsy]).
Nerve conduction studies and/or electromyograms (EMGs) may be done to confirm a focal neuropathy. Various splints or braces may help maintain a neutral position across a joint (such as the wrist or elbow) to allow the nerve time to heal and inflammation to subside. Anti-inflammatory medications such as corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) may help relieve the symptoms temporarily, but if chronic irritation and inflammation persist due to entrapment, then surgical decompression with an orthopedic surgeon may become necessary in severe cases (NIDDK, 2018b).
Retinopathy and Other Eye Complications
Individuals with diabetes have a 40% increased risk of developing glaucoma; this risk increases as they age. It is typically higher in patients with T1DM as compared to those with T2DM due to the lifelong nature of the disease. Cataracts are 60% more common in the diabetic patient. The age of onset is often younger, and the progression of cataracts is often faster in patients with diabetes. While cataract removal can correct the issue, there are further complications that can occur after cataract removal, including glaucoma and retinopathy, a general term for all the disorders of the retina caused by diabetes. Retinopathy may be nonproliferative or proliferative (Figure 3).
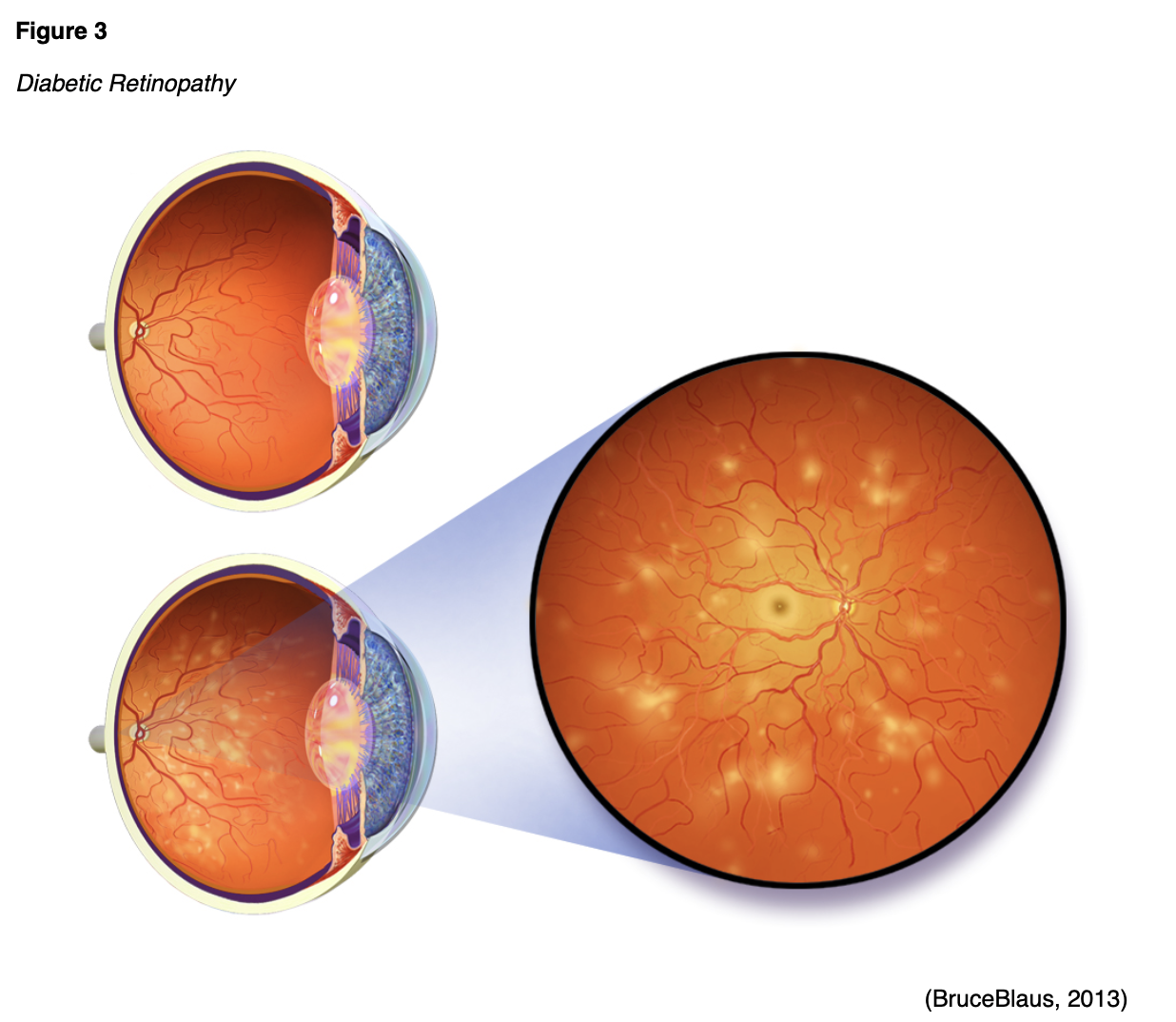
- Nonproliferative retinopathy is the most common. Capillary blood flow to the back of the eye is blocked, which leads to the capillaries swelling and forming pouches. This type of retinopathy may progress to proliferative as more blood vessels are damaged.
- Proliferative retinopathy develops over a period of years as more blood vessels are blocked. New blood vessels start growing in the retina, but these tend to be weak and leak blood, leading to vitreous hemorrhage. The progressive damage can also cause scar tissue to form, pulling the retina out of place and causing a retinal detachment (ADA, n.d.b).
Both types of retinopathy can be corrected with precision laser treatment. Medications can be injected into the eye to slow the growth of new blood vessels and reduce leakage. Surgical intervention with a vitrectomy may also be considered. This procedure is done by removing the vitreous humor gel that fills the eye cavity to gain better access to the retina. This allows the removal of scar tissue, laser repair of retinal detachments, and treatment of macular holes. Despite the availability of medical and surgical procedures to correct retinopathy, prevention via vigilant BG and BP control should be the first line of treatment (ADA, n.d.b).
Renal Complications
Renal complications are one of the costliest aspects of long-term diabetes. In 2016, the cost of treating chronic kidney disease (CKD) by Medicare beneficiaries alone was over $79 billion dollars. The kidneys are very vascular with tiny vessels that filter out the waste products from the blood. Due to microvascular damage, the kidneys may gradually lose the ability to filter out waste, eventually necessitating dialysis. Roughly a third of diabetic patients develop CKD. Factors that contribute to kidney disease include HTN, poor glycemic control, and genetics. By maintaining therapeutic BG levels and controlling HTN, patients are able to decrease their risk of kidney damage. Diabetic patients are also at increased risk for UTIs and should be instructed to report any symptoms immediately. Unfortunately, kidney disease is often asymptomatic until advanced stages, so urinalysis to check for proteinuria in diabetic patients is key to screening for kidney damage. Diabetic kidney disease also increases the risk of hyperkalemia, so this should be monitored closely as well. Kidney disease may present with fluid retention (pitting edema, sudden weight gain), poor sleep, poor appetite, nausea, weakness, and difficulty concentrating. Control of BP and BG can also slow the progression of kidney disease. Patients who stay well-hydrated and eat a low-protein and low-sodium diet may be able to both reduce their BP and the workload of their kidneys, especially patients with macroalbuminuria (elevated protein in the urine). Unfortunately, once end-stage renal disease occurs, the only definitive treatment options include dialysis and renal transplant (ADA, n.d.b; CDC, 2019c, 2020a). For additional information regarding kidney disease, please see the NursingCE course entitled Chronic Kidney Disease.
Hypertension and Stroke
While 1 in 3 Americans have HTN, the prevalence rises to 2 in 3 among patients with diabetes. HTN increases the risk of stroke, cardiac disease, and kidney disease. By lowering the BP, the risk of these complications declines or is delayed. The ADA identifies the ideal BP as 120/80 or less. Blood pressure should be checked at each follow-up visit to help patients to know their numbers. The APRN should counsel patients regarding the following tips to reduce their BP:
- Eat more whole-grain bread and cereals,
- Eat a low-sodium diet,
- Maintain a healthy weight,
- Limit or avoid alcohol intake (ADA, n.d.b).
APRNs should choose antidiabetic medications carefully, especially in patients with comorbid HTN. As previously mentioned, SGLT2 inhibitors and, to a lesser extent, GLP-1 receptor agonists may cause a reduction in BP in T2DM patients (ADA, n.d.e; Mayo Clinic, 2019f). If medications are required to reduce BP (130/80 and above per the AHA, 140/90 per the ADA), ACE inhibitors such as captopril (Capoten) and enalapril (Vasotec) have been shown to reduce BP and slow kidney disease without elevating BG in patients with diabetes (ADA, n.d.b). Hyperkalemia is a concern for diabetic kidney disease patients, especially on ACE inhibitors or ARBs, and this should be monitored closely. Despite this, the current practice guidelines recommend an ACE inhibitor or an ARB for diabetic patients with albuminuria. Thiazide-like diuretics such as HCTZ (Hydrodiuril) can be effective in reducing volume as well as reducing systemic potassium levels. With advanced renal disease and an eGFR of 30 mL/min/1.73 m 2 or less, a long-acting loop diuretic is preferred, such as torsemide (Demadex). The ADA recommends treating hypertension to a goal of less than 140/90 in most patients, less than 130/80 in those at increased risk of CVD (DeBoer et al., 2017; Whelton et al., 2017). For additional information regarding hypertension, please see the NursingCE courses entitled Hypertension: Diagnosis and Hypertension: Management.
Patients with diabetes have a 1.5 times greater risk of stroke than healthy individuals. Further risk factors for stroke include the following modifiable risk factors:
- Overweight,
- High LDL cholesterol or low HDL cholesterol,
- Lack of exercise/activity,
- Tobacco use,
- Alcohol use.
The APRN should encourage diabetic patients to lower their stroke risk with regular physical activity, smoking cessation, maintaining a healthy weight, and managing any existing hypercholesterolemia and hypertension. Similar to other diabetic complications, the patient’s overall risk can be lowered by maintaining therapeutic BG levels (ADA, n.d.b). For additional information regarding stroke, please see the NursingCE course entitled stroke.
Skin Complications
Diabetes impacts every part of a patient's body, including their skin. Most skin conditions can be prevented or easily managed with proper treatment. Good skincare and BG control can decrease this risk. Skin conditions found amongst diabetic patients include diabetic dermopathy, which presents with brown scaly or shiny round/oval lesions found on the anterior distal surface of the lower legs that are usually painless and do not require specific treatment. Necrobiosis lipoidica diabeticorum (NLD) presents similar to dermopathy, but the lesions are larger, and there are typically fewer of them. They initially present as dull, red, and raised, but then progress to shiny lesions with a violet border than may become itchy, painful, or crack open. NLD is rare, and treatment is only required to prevent/treat a secondary infection if the lesions break open. Bullosis diabeticorum, or diabetic blisters, are rare and typically present on the fingers, hands, toes, and feet (occasionally on the forearm or lower legs). They are painless with no surrounding redness and are most often seen in diabetic patients with neuropathy. They will typically heal within a few weeks without any specific treatment required, but BG control should be improved to facilitate healing and prevent future blisters from developing. Eruptive xanthomatosis is associated with hypercholesterolemia and presents with itchy, firm yellow bumps on the skin surrounded by a red halo. It is most often seen on the hands, feet, arms, legs, or buttocks in young male patients with T1DM. As with diabetic blisters, lesions will typically resolve with improved glycemic control. Digital sclerosis occurs in about one-third of patients with T1DM and is characterized by tight, thick skin that may reduce mobility and flexibility on the back of the hands, the fingers, the toes, and the forehead. The only treatment for sclerosis is improved glycemic control. Generalized or disseminated granuloma annulare may present with red-brown or skin-colored patches of small papules arranged in rings or arcs (ADA, n.d.b). Granuloma patches may respond to topical steroid treatment, but improved glycemic control is preferred (Oakley, 2015). While most of these conditions are quite rare, the most common skin problems amongst patients with diabetes are bacterial or fungal infections or itching (ADA, n.d.b).
Bacterial infections are typically caused by Staphylococcus bacteria, which may cause styes, boils, folliculitis, carbuncles, or nail infections. These infections may present with skin that is red, warm, swollen, and tender to the touch, and typically require topical or systemic antibiotics. Fungal infections in diabetic patients are most often caused by Candida albicans. Common varieties include jock itch (groin region), ringworm, athlete’s foot, or vaginal infections. Fungal infections are often very red, moist, and itchy, and may have tiny blisters or scales surrounding it. They often occur in moist skin folds, such as under the breasts, between fingers/toes, around the nails, in the corners of the mouth, in the armpits, and the groin. Mild infections can typically be treated with a topical antifungal ointment (ADA, n.d.b).
Itching is common in diabetics due to dry, cracked skin and poor circulation (typically seen bilaterally in the lower legs). Itching may also be related to a fungal infection, which often causes itching. Most itching can be controlled by instructing patients to limit the frequency of bathing, use a mild soap, and always apply a moisturizer to their skin after bathing. When applying a moisturizing lotion, the nurse should be careful to educate the patient to avoid applying lotion between the toes as this can increase the moisture and cause further problems with infections (ADA, n.d.b).
Acanthosis nigricans are hyperpigmented, velvety plaques seen most often in patients that are very overweight. They appear most often on the sides of the neck, armpits, or groin, but may also be seen on the hands, elbows, and knees. While topical treatments such as retinoids (0.1% tretinoin [Retin-A] or adapalene [Differin]), vitamin D analogs (Calcipotriene), and chemical peels work for some, results can be variable. Oral retinoids such as isotretinoin (Accutane) and acitretin (Soriatane) may be effective, as well as certain oral antidiabetic medications such as metformin (Glucophage) and thiazolidinediones. Ultimately, addressing the underlying condition with weight loss and improved glycemic control is the most effective treatment option available (Patel et al., 2018).
Wound Care. Diabetic patients are at a higher risk of developing wounds or other skin conditions that lead to wounds, and their wound healing time is prolonged due to the complications of diabetes. Elevated BG levels delay healing, and bacteria thrive in the high-glucose environment. Poor circulation further impedes healing in the diabetic patient, leading to the risk of amputation for even minor wounds in the lower extremities (CDC, 2019c). Maintaining euglycemia is vital to improving the chances of wound healing in diabetic patients. More aggressive wound treatments such as negative pressure wound therapy, hyperbaric oxygen therapy, silver cream, extracorporeal shockwave therapy, or higher-cost dressing treatments (biological skin equivalent) may be needed to ensure healing and avoid further complications (Ahangar et al., 2018). For more on wound care, refer to the NursingCE course entitled Interdisciplinary Wound Care.
Foot Care. The APRN should educate patients on the implications of elevated BG levels on their feet and legs. Skin changes can cause the feet to become dry and cracked. Diabetic neuropathy may be contributing to reduced sensation in the feet. Blisters, sores, or cuts may not be felt, or burning hot water may go unnoticed. Due to poor circulation, ulcers may develop, and the healing process will be delayed; this may eventually lead to an infection that could put the patient at risk of amputation (ADA, n.d.b; CDC, 2019c). Patients should be instructed to do the following daily to avoid this outcome:
- Inspect the feet daily for cuts, cracks, redness, edema, blisters, calluses, splinters, or dark spots. The primary healthcare provider should be notified if foot lesions do not heal within one day.
- Discuss care of corns and calluses with a podiatrist or primary healthcare provider if needed.
- Wash feet daily in warm water and dry completely. Avoid hot water as burns can occur more easily due to neuropathy and reduced temperature sensation.
- Toenails should be filed or cut straight across. The edges should be filed to avoid sharp edges. Soak feet first to soften toenails. Avoid cutting toenails too short or rounded, which may lead to ingrown toenails.
- Avoid lotion between the toes as this can lead to skin breakdown or fungal infections. However, the heels and top of feet should be moisturized to prevent cracking or dry skin, which can cause break down.
- Cotton socks should be worn daily to avoid blisters or sores from shoes. Socks should be clean and dry without seams, if possible.
- Good-fitting shoes should be worn to avoid friction and pressure sores. New shoes should be broken in slowly by wearing them one to two hours daily for two weeks.
- Do not go barefoot in or out of the house. Slippers or shoes should be worn to avoid injury to the feet.
- Protect feet from extreme hot or cold.
- Elevate the feet when sitting to promote blood flow. Avoid crossing legs and wiggle toes and move ankles up and down for five minutes three to four times per day.
- Stop smoking as this causes damage to the circulatory system that can lead to poor circulation to the feet and legs.
- Manage BG levels, BP, and cholesterol levels to promote circulation and decrease vascular damage.
- Routine exercise is vital to promote circulation to the lower extremities and decrease damage from diabetes.
- Schedule an annual podiatrist evaluation (CDC, 2019c).
Oral Health
An increased BG leads to increased sugar in the saliva as well, increasing the risk for dental cavities and gum disease, which can then lead to more challenging BG management or tooth loss. Diabetic patients should be encouraged to brush their teeth at least twice daily, floss at least once daily, and get dental cleanings with a check-up at least every six months. Patients with diabetes who have dentures should remove and clean them at least daily (CDC, 2019c).
Mental Health and Diabetes
Diabetes is a lifelong chronic illness that will require daily monitoring, treatment, and acceptance. There is little chance that mental health will not be impacted and that an adjustment period will not be needed as part of the process by anyone being diagnosed with a life-altering disease. Mental health issues can lead to depression and non-compliance with treatment for diabetes or other comorbid conditions. "Untreated mental health issues can make diabetes worse, and problems with diabetes can make mental health issues worse" (CDC, 2018a). Due to this mind-body connection, it is very important for the APRN to educate the patient regarding signs or symptoms of depression or stress, such as:
- Sadness;
- Anhedonia (loss of interest in activities of interest previously enjoyed);
- Over or under-eating;
- Insomnia or sleeping continuously;
- Difficulty making decisions;
- Extreme fatigue;
- Hopelessness, irritability, anxiety, or guilt;
- Aches, pains, or digestive problems not associated with other conditions;
- Having thoughts of suicide or death (CDC, 2018a).
A condition known as diabetes distress, which is overwhelming feelings of frustration, worry, fatigue, or discouragement related to the demands of having diabetes, affects a large number of diabetics throughout the course of their life due to management of their disease. It is very similar in characteristics to anxiety or depression, but traditional antidepressant medications do not seem to improve the symptoms. Interventions that have been linked to improvement include:
- Treatment with a mental health counselor familiar with patients being treated for chronic health conditions and their unique needs;
- Session(s) with a diabetic educator to strategize and problem solve concerns;
- A focus on small steps, such as one to two specific diabetic management goals;
- Participation in a local or virtual support group for diabetes patients; and
- Regular consultations with an endocrinologist (CDC, 2018a).
Emergency Preparedness for Diabetics
Diabetics should always be prepared with sufficient amounts of medication and supplies to maintain their care. During natural disasters, emergencies, or other hazards, the diabetic must be prepared to manage their care for at least a week. The CDC (2020f) and ADA (n.d.a) have extensive Emergency Preparedness resources with specific advice for patients to:
- Have at least one week’s supply of water, food, medications, and syringes.
- Have at least one week’s supply of test strips and extra batteries for a glucometer and equipment.
- Identify themselves as a diabetic upon arrival to a shelter or emergency facility.
- Know their rights related to the Americans with Disabilities Act and Section 504 of the Rehabilitation Act that requires shelters to accommodate them with a service dog, use of sharps, or anything related to diabetes care.
- Maintain fluid intake to avoid dehydration.
- Monitor for hyper and hypoglycemia.
- If food is limited, they should adjust medications accordingly.
- They should monitor closely for infection (ADA, n.d.a; CDC, 2020f).
Future Opportunities for the Diabetic
Cell therapy is an emerging hope toward a cure for diabetes, particularly in T1DM patients. The replacement of missing or damaged insulin-producing islet cells could potentially recover normal insulin production and cure patients. The Diabetes Research Institute is working toward a bioengineered "mini-organ where insulin cells are encapsulated within a protective barrier". The first trials were completed in 2016 in Europe with a 41-year-old male with T1DM since the age of 11. Insulin therapy was discontinued after the procedure and continued to be successful for one year at the time of publication of the study. Currently, several US drug companies are developing their own cell therapy approaches for diabetes (Diabetes Research Institute Foundation, 2016; Fernandez, 2019; JDRF, n.d.a).
Another opportunity to cure diabetes is through immunotherapy. With T1DM, islet β-cells are progressively destroyed by the immune system. After diagnosis, as much as 10% of the insulin-producing cells are still producing insulin. If the immune process could be interrupted or stopped early, the remaining beta cells could potentially be saved and continue to produce insulin, potentially providing a cure through prevention. A Belgium company is currently working on a clinical trial that uses immunotherapy to target the immune cells that attack and destroy pancreatic cells (Fernandez, 2019; JDRF, n.d.a).
Finally, an artificial pancreas is being researched that would replace the body’s pancreatic function. This treatment is a fully automated system that measures BG levels, and injects the right amount of insulin, mimicking a healthy pancreas. This option replaces the human components of measuring BG and would, in theory, maintain a steadier glycemic control. Challenges with this treatment option are the need for a faster form of insulin that to make swift changes in the BG level as well as complex algorithms that must be developed to consider all aspects of the normal pancreas function to regulate BG levels (Fernandez, 2019; JDRF, n.d.a).
Conclusions
While the information related to diabetes can be overwhelming for a member of the healthcare team, it is significantly more overwhelming for a newly diagnosed patient with this life-changing disease. Resources abound, but the patient must become an active participant in their treatment and utilize the resources at their disposal; their healthcare team must actively assist in that process. For those who have fewer financial resources, it may be challenging to obtain the necessary supplies. With such a multifactorial condition as diabetes, an entire team is needed to coordinate the care to meet the patient’s nutritional, medical, and mental health needs.
References
Abdallah, M. A., Ahmed, K. M., Stevens, D., & Griebeler, M. (2019). Screening for impaired glucose tolerance (prediabetes) and prevention of type 2 diabetes. South Dakota Medicine: The Journal of the South Dakota State Medical Association, 72(2), 67–73.
Ahangar, P., Woodward, M., & Cowin, A. J. (2018). Advanced wound therapies. Wound Practice & Research, 26(2), 58–68.
Allen, D. W., Kim, K. W., Rawlinson, W. D., & Craig, M. E. (2018). Maternal virus infections in pregnancy and type 1 diabetes in their offspring: Systematic review and meta‐analysis of observational studies. Reviews in Medical Virology, 28(3), 1. https://doi.org/10.1002/rmv.1974
Alshammari, A. A., Alahdal, L. M., Jawi, J., Alnofaie, H. A., Aldossari, N. A., & Alassaf, H. M. A. (2017). First line management of adult diabetic ketoacidosis patients. The Egyptian Journal of Hospital Medicine, 67(2), 571–577. https://doi.org/10.12816/0037808
American Association of Diabetes Educators. (2019). Find a diabetes education program in your area. https://www.diabeteseducator.org/living-with-diabetes/find-an-education-program
American Diabetes Association. (n.d.a). Caring for people with diabetes in emergency situations. Retrieved June 25, 2020, from https://www.diabetes.org/resources/disaster-relief/caring-people-diabetes-emergency
American Diabetes Association. (n.d.b). Complications. Retrieved June 25, 2020, from https://www.diabetes.org/diabetes/complications
American Diabetes Association. (n.d.c). Exercise and type 1. Retrieved June 25, 2020, from https://www.diabetes.org/fitness/get-and-stay-fit/exercise-and-type-1
American Diabetes Association. (n.d.d). Genetics of diabetes. Retrieved June 25, 2020, from https://www.diabetes.org/diabetes/genetics-diabetes
American Diabetes Association. (n.d.e). Insulin and other injectables. Retrieved June 25, 2020, from https://www.diabetes.org/diabetes/medication-management/insulin-other-injectables
American Diabetes Association. (2018). Economic costs of diabetes in the US in 2017. Diabetes Care, 41(5), 917–928. https://doi.org/10.2337/dci18-0007
American Diabetes Association. (2020). Standards of medical care in diabetes—2020. Diabetes Care, 43 (S1), 10-38. https://doi.org/10.2337/dc20-Sint
Beauvais, A., Wardian, J. L., & Sauerwein, T. J. (2018). Attitudes regarding technology and weight loss in patients with diabetes and prediabetes in a military health system. Diabetes, 67(1), 2. https://doi.org/10.2337/db18-2069-P
BruceBlaus. (2013). Diabetic retinopathy [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Blausen_0312_DiabeticRetinopathy.png
The Centers for Disease Control and Prevention. (2017). Diabetes report card. https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf
The Centers for Disease Control and Prevention. (2018a). Diabetes and mental health. https://www.cdc.gov/diabetes/managing/mental-health.html
The Centers for Disease Control and Prevention. (2018b). Diabetes self-management education and support (DSMES) toolkit. https://www.cdc.gov/diabetes/dsmes-toolkit/background/index.html
The Centers for Disease Control and Prevention. (2019a). Gestational diabetes. https://www.cdc.gov/diabetes/basics/gestational.html
The Centers for Disease Control and Prevention. (2019b). National diabetes prevention program. https://www.cdc.gov/diabetes/prevention/info-hcp.htm
The Centers for Disease Control and Prevention. (2019c). Prevent complications. https://www.cdc.gov/diabetes/managing/problems.html
The Centers for Disease Control and Prevention. (2019d). Type 2 diabetes. https://www.cdc.gov/diabetes/basics/type2.html
The Centers for Disease Control and Prevention. (2020a). Chronic kidney disease. https://www.cdc.gov/kidneydisease/basics.html
The Centers for Disease Control and Prevention. (2020b). Diabetic risk factors. https://www.cdc.gov/diabetes/basics/risk-factors.html
The Centers for Disease Control and Prevention. (2020c). Manage blood sugar. https://www.cdc.gov/diabetes/managing/manage-blood-sugar.html
The Centers for Disease Control and Prevention. (2020d). Managing sick days. https://www.cdc.gov/diabetes/managing/flu-sick-days.html
The Centers for Disease Control and Prevention. (2020e). Prediabetes. https://www.cdc.gov/diabetes/basics/prediabetes.html
The Centers for Disease Control and Prevention. (2020f). Preparedness: Be prepared! https://www.cdc.gov/diabetes/managing/preparedness.html
The Centers for Disease Control and Prevention. (2020g). Type 1 diabetes. https://www.cdc.gov/diabetes/basics/type1.html
Colberg, S. R., Sigal, R. J., Yardley, J. E., Riddell, M. C., Dunstan, D. W., Dempsey, P. C., Horton, E. S., Castorino, K., & Tate, D. F. (2016). Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39(11), 2065-2079. https://doi.org/10.2337/dc16-1728
deBoer, I.H., Bangalore, S., Benetos, A., Davis, A.M., Michos, E.D., Muntner, P., Rossing, P., Zoungas, S., & Bakris, G. (2017). Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care, 40(9), 1273-1284. https://doi.org/10.2337/dci17-0026
Diabetes Research Institute Foundation. (2016). First type 1 diabetes patient in Europe is free from insulin therapy after undergoing diabetes research institute’s biohub transplant technique. https://www.prnewswire.com/news-releases/first-type-1-diabetes-patient-in-europe-is-free-from-insulin-therapy-after-undergoing-diabetes-research-institutes-biohub-transplant-technique-300282581.html
Epocrates. (n.d.). Insulin product comparisons: Full insulin table. Retrieved on June 12, 2020 from Epocrates® mobile app.
Fernandez, C.R. (2019). The future of diabetes treatment: Is a cure possible? https://labiotech.eu/features/diabetes-treatment-cure-review/
Gallo de Moraes, A., & Surani, S. (2019). Effects of diabetic ketoacidosis in the respiratory system. World Journal of Diabetes, 10(1), 16–22. https://doi.org/10.4239/wjd.v10.i1.16
Grock, S., Ku, J.H., Kim, J., & Moin, T. (2017). A review of technology-assisted interventions for diabetes prevention. Current Diabetes Reports, 17(11), 107. https://doi.org/10.1007/s11892-017-0948-2
Jenson, J. T. (2019). Hormone therapy and mortality: No overall effect? Internal Medicine Alert, 41(4). https://doi.org/10.1111/1471-0528.15433
Juvenile Diabetes Research Foundation (n.d.a). Curing type I diabetes. Retrieved June 12, 2020, from https://www.jdrf.org/impact/research/curing-t1d/
Juvenile Diabetes Research Foundation. (n.d.b). Type I diabetes basics. Retrieved June 12, 2020 from https://www.jdrf.org/t1d-resources/about/
Khardori, R. (2020). Type 1 diabetes mellitus. https://emedicine.medscape.com/article/117739-overview#a5
Kizilay, F., Fali, H. E., & Serefoglu, E. C. (2017). Diabetes and sexuality. Sexual Medicine Reviews, 5(1), 45-51. https://doi.org/10.1016.j.sxmr.2016.07.002
Leonard, J. (2019). Somogyi effect: Causes and prevention. https://www.medicalnewstoday.com/articles/317998#:~:text=Somogyi%20effect%20vs.&text=The%20dawn%20phenomenon%20or%20%E2%80%9Cdawn,an%20increase%20in%20growth%20hormones.
Lewis, S. Dirksen, S., Heitkemper, M., & Bucher, L. (2014). Medical-surgical nursing: Assessment and management of clinical problems. Elsevier/Mosby.
Mayo Clinic. (2019a). Bezoars: How do they happen? https://www.mayoclinic.org/diseases-conditions/gastroparesis/expert-answers/bezoars/faq-20058050
Mayo Clinic. (2019b). Pancreas transplants. https://www.mayoclinic.org/tests-procedures/pancreas-transplant/about/pac-20384783
Mayo Clinic. (2019c). Selective serotonin reuptake inhibitors (SSRIs). https://www.mayoclinic.org/diseases-conditions/depression/in-depth/ssris/art-20044825
Mayo Clinic. (2019d). Serotonin and norepinephrine reuptake inhibitors (SNRIs). https://www.mayoclinic.org/diseases-conditions/depression/in-depth/antidepressants/art-20044970
Mayo Clinic. (2019e). Tricyclic antidepressants and tetracyclic antidepressants. https://www.mayoclinic.org/diseases-conditions/depression/in-depth/antidepressants/art-20046983
Mayo Clinic. (2019f). Type 2 diabetes. https://www.mayoclinic.org/diseases-conditions/type-2-diabetes/diagnosis-treatment/drc-20351199
Mayo Clinic. (2020). Orthostatic hypotension. https://www.mayoclinic.org/diseases-conditions/orthostatic-hypotension/diagnosis-treatment/drc-20352553
MedlinePlus. (2018a). Diabetic hyperglycemic hyperosmolar syndrome. https://medlineplus.gov/ency/article/000304.htm
MedlinePlus. (2018b). Diabetic ketoacidosis. https://medlineplus.gov/ency/article/000320.htm
MedlinePlus. (2018c). Pramlintide injection. https://medlineplus.gov/druginfo/meds/a605031.html
Minicucci, W.J., Maia, F. F., Neto, A. M., & Zantut-Wittmann, D. E. (2015). Somogyi effect as the most common cause of fasting hyperglycemia in T1D patients. Diabetology & Metabolic Syndrome, 7(Supp 1), A62. https://doi.org/10.1186/1758-5996-7-S1-A62
Nathan, D. M. & DCCT/EDIC Research Group. (2014). The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care, 37(1), 9-16. https://doi.org/10.2337/dc13-2112
National Institute of Diabetes and Digestive and Kidney Diseases. (2016). Diabetes diet, eating, & physical activity. https://www.niddk.nih.gov/health-information/diabetes/overview/diet-eating-physical-activity
National Institute of Diabetes and Digestive and Kidney Diseases. (2018a). Diabetes, sexual and bladder problems. Retrieved from https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/sexual-bladder-problems?dkrd=/health-information/diabetes/overview/preventing-problems/sexual-urologic-problems
National Institute of Diabetes and Digestive and Kidney Diseases. (2018b). Diabetic neuropathy. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/nerve-damage-diabetic-neuropathies/all-content
National Institute of Diabetes and Digestive and Kidney Diseases. (2018c). Gastroparesis. https://www.niddk.nih.gov/health-information/digestive-diseases/gasrtoparesis/all-content
National Institute of Diabetes and Digestive and Kidney Diseases. (2018d). Pancreatic islet transplantation. https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatment/pancreatic-islet-transplantation
National Institute of Diabetes and Digestive and Kidney Diseases. (2018e). Treatments for bladder control problems (urinary incontinence). https://www.niddk.nih.gov/health-information/urological-diseases/bladder-control-problems/treatment
Oakley, A. (2015). Granuloma annulare. DermNet NZ. https://dermnetnz.org/topics/granuloma-annulare/
Openstax College. (2013a). The regulation of blood glucose levels [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:1822_The_Homostatic_Regulation_of_Blood_Glucose_Levels.jpg
Openstax College. (2013b). The pancreas [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:1820_The_Pancreas.jpg
Patel, N. U., Roach, C., Alinia, H., Huang, W. W., & Feldman, S. R. (2018). Current treatment options for acanthosis nigricans. Clinical, cosmetic and investigational dermatology, 11, 407–413. https://doi.org/10.2147/CCID.S137527
Rouf, R., Rahim, M., & Latif, Z. A. (2018). Requirement of intravenous fluid and insulin in the management of diabetic ketoacidosis to overcome the crisis: Experience in a specialized hospital. Journal of Medicine, 19(1), 18–21.
Schaefer, A. & Sarachik, J. (2018). What is the Somogyi effect? https://www.healthline.com/health/diabetes/what-is-the-somogyi-effect
Sindi, B. Z., Alazwari, N. M., Khateeb, A. M., Alzaher, A. A., Alkhawajah, Almutairi, A. K., Al-Mutairi, N. A., Alghanim, Z. R., Nemer, A. A., & Khatib, H. A. (2017). Management of diabetic ketoacidosis in patients with diabetes type I. Egyptian Journal of Hospital Medicine, 69(4), 2278–2285.
Terrery, C. L., & Nicoteri, J. A. (2016). The 2015 American Geriatric Society Beers Criteria: Implications for nurse practitioners. The Journal for Nurse Practitioners, 12(3), 192-200. https://doi.org/10.1016/j.nurpra.2015.11.027
Thornton, K., Chervenak, J., & Neal-Perry, G. (2018). Menopause and sexuality. Endocrinology and Metabolism Clinics of North America, 44(3), 649-661. https://doi.org/10.1016/j.ecl.2015.05.009
Tumminia, A., Sciacca, L., Frittitta, L., Squatrito, S., Vigneri, R., Le Moli, R., & Tomaselli, L. (2015). Integrated insulin pump therapy with continuous glucose monitoring for improved adherence: Technology update. Patient Preference and Adherence, 9, 1263–1270. https://doi.org/10.2147/PPA.S69482
Townsend, M.C., & Morgan, K.I. (2017). Schizophrenia spectrum and other psychotic disorders. Essentials of Psychiatric Mental Health Nursing, (7th ed.) F.A. Davis Company.
University of California, San Francisco Medical Center (n.d.). Diabetes education online: Designing an insulin regimen. Retrieved on June 18, 2020 from https://dtc.ucsf.edu/types-of-diabetes/type1/treatment-of-type-1-diabetes/medications-and-therapies/type-1-insulin-therapy/designing-an-insulin-regimen/#intense
Ventura, A. D., Browne, J.L., Pouwer, F., Speight, J., & Byrne, M. (2017). Emotional well-being factors associated with sexual dysfunction in adults with type 1 or type 2 diabetes: Results from diabetes MILES-Australia. International Journal of Sexual Health, 30(3), 237-249. https://doi.org/10.1080/19317611.2018.1470591
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Colling, K. J., Himmelfarb, C. D., DePalma, S. M., Gidding, S., Jameson, K. A., Jones, D.W., MacLaughlin, E. J., Muntner, P., Ovbiagele, B., Smith, S. C., Spencer, C. C., Stafford, R. S., Taler, S. J., Thomas, R. J., Williams, K. A., Williamson, J. D., & Wright, J. T. (2017). 2017 Clinical practice guideline: ACC/AHA/AAPA/ABC/ACPM/AGS/AphA/ASH/ASPC/MA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension (71), e13-115. https://doi.org/10.1161/HYP.0000000000000065
Wood, J. & Peters, A. (2018). The type 1 diabetes self-care manual. The American Diabetes Association. https://doi.org/10.2337/9781580406208
Zand, A., Ibrahim, K., & Patham, B. (2018). Prediabetes: Why should we care? Methodist Debakey Cardiovascular Journal, 14(4), 289-297. https://doi.org/10.14797/mdcj-14-4-289.