About this course:
The purpose of this module is to educate nurses on the various types of diagnostic radiology imaging tests, ensuring a satisfactory understanding of the risks, benefits, and important clinical considerations of each type of test, the use of contrast media, management of contrast allergic reactions, and radiation safety.
Course preview
By the completion of this module, the nurse should be able to:
- Define radiation and the three types of radiation dosing, distinguish between non-ionizing and ionizing radiation, and review the essential components of radiation safety.
- Identify the general risks of medical imaging tests with radiation exposure, special considerations to follow in pregnancy and lactation, and list the amount of radiation exposure associated with several of the most common diagnostic radiology imaging tests.
- Review the different types of contrast media agents, with regards to their clinical considerations, including risks, contraindications, and monitoring parameters, and outline the signs and symptoms of allergic reactions to iodinated contrast, including proper management and premedication regimens as guided by the American College of Radiology.
- Describe the different types of diagnostic radiology tests, key patient teaching points, including how to prepare for the test, what to expect during the test, how long the test should take, as well as the associated benefits.
Diagnostic radiology refers to a sector of medicine that includes various kinds of medical imaging technologies widely utilized throughout the US healthcare system, with millions of patients undergoing imaging evaluations on a daily basis. These are noninvasive and minimally invasive medical procedures used to view the inside of the body as a means to assess, diagnose, monitor, and treat a wide array of medical conditions. Several of these tests also serve as the backbone of preventative medicine and routine health maintenance, including cancer prevention, screening, and early detection. While diagnostic radiology is a valuable and critical component to the proper diagnosis of numerous medical conditions, they are not without risks. Many of these tests are linked to potential harms that must be considered prior to ordering the test, as well as a high cost burden to patients and society. To provide optimal patient care, nurses must acquire an appropriate understanding of each type of imaging modality, as well as a keen awareness of the clinical risks, benefits, and management of adverse events (Elsayes & Oldham, 2014).
Part I. Overview of Diagnostic Radiology
Radiology is considered one of the most technologically advanced fields in medicine, dating back to 1895 when Wilhelm Conrad Rontgen first discovered the x-ray, using his wife as his patient. Subsequently, Henri Becquerel discovered radioactivity in 1896, followed by the discovery of radium in 1898 by Marie and Pierre Curie. The field has expanded exponentially over the last few centuries and continues to rely on the collaboration of scientists, medical physicists, radiologists, and imaging technologists. Medical physicists ensure the safe and optimal use of radiological imaging modalities in patients. Diagnostic radiologists are physicians who undergo specialized training in the analysis and interpretation of medical imaging to draw conclusions useful for the diagnosing, treating, and managing of acute and chronic medical conditions and injuries. Interventional radiologists are physicians who undergo specialized training in the use of medical imaging to guide minimally invasive surgical procedures that diagnose, treat, and cure many kinds of conditions. Radiology technologists, also referred to as radiographers, have specialized training in performing digital imaging procedures under the direct supervision of a nurse or physician (Elsayes & Oldham, 2014).
According to the International Atomic Energy Agency (IAEA), a radiograph, or medical image "is a pictorial representation of a measurement of an object or function of the body" (IAEA, 2014, p. 55). The overall objective of medical imaging is to make a particular area within the patient's body visible. Radiographs are created by sending an x-ray beam, which is generated by an x-ray tube, through a patient. As described in the most simplistic form, the image produced represents a shadow of the underlying structures that the x-ray beam passes through. Each medical imaging method is devised to reveal distinctive characteristics of the body, and the variability in imaging quality and visibility of structures can differ considerably. Some of the most common factors contributing to the inconsistencies in the resulting images include the quality and characteristics of the imaging equipment, the skill of the operator (including positioning and placement of the patient), specific patient characteristics (such as body habitus, prior surgeries, presence of scar tissue, or prior radiation therapy), as well as the timing of the imaging in relation to the injury or medical condition in question (IAEA, 2014).
Radiation
Radiation is energy in the form of particles or waves emitted by both natural and synthetic sources. Comprised of two forms, ionizing and non-ionizing, radiation surrounds us in our daily lives. As demonstrated in Figure 1, radiation exists on the electromagnetic (EM) spectrum, ranging from lower energy microwaves to higher energy gamma rays. EM energy travels in waves, and the strength of the radiation depends on the frequency (how rapidly the waves move up and down) and the distance the wavelength is traveling. In general, the smaller the wavelength, the higher the energy of the radiation (The Centers for Disease Control and Prevention [CDC], 2015d).
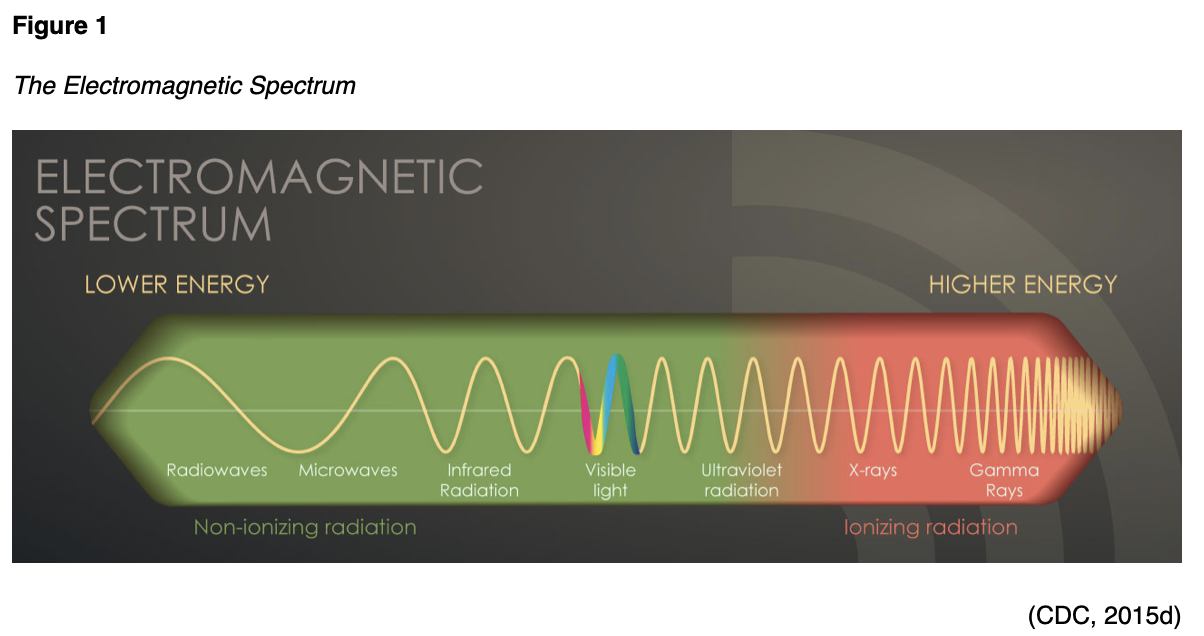
Non-ionizing radiation is the most prevalent form of radiation that is freely available at low levels within our environment. It can heat substances, yet it does not have the ability to remove electrons from atoms and molecules. The most common types of non-ionizing radiation include radiofrequency (RF) waves in many communication and electronic devices, kitchen microwaves, visible light, and lower energy forms of ultraviolet (UV) radiation. Intense, direct exposure to RF waves and microwaves can result in tissue damage due to heat. In contrast, overexposure to UV rays can result in skin burns, premature skin aging, eye damage, and skin cancer. The vast majority of skin cancers are directly related to intense, prolonged, and unprotected exposure to UV radiation from the sun and tanning beds. The line dividing ionizing and non-ionizing radiation becomes somewhat blurred in the UV section of the EM spectrum demonstrated in Figure 1. Radiation in the UV section at lower energies is considered non-ionizing, whereas, at higher energies, extreme UV radiation becomes more harmful, rendering it ionizing radiation (CDC, 2015d).
Ionizing radiation removes electrons from atoms and molecules, causing the atom to become ionized (or charged), thereby enabling the wavelengths to pass through air, water, and tissue. Ionizing radiation is considered a carcinogen, or a substance capable of causing cancer, as it can penetrate the human body (Agency for Healthcare Research and Quality [AHRQ], 2019). When absorbed by living tissue, ionizing radiation has the potential to induce harm, especially when exposed at high levels. Most humans are exposed to low levels of ionizing radiation daily, and these radiation sources may be natural or manmade (CDC, 2015a). Exposure can occur through construction and building materials, terrestrial radiation (radiation from the earth), cosmic radiation (radiation from space), and air travel (CDC, 2015d). Some people are exposed to higher amounts of natural background radiation, such as those who live at higher mountain elevations, as well as those who engage in frequent air travel (American College of Radiology [ACR], 2018). X-rays are the most classic example of ionizing radiation; they penetrate the body to visualize underlying bony structures. Ionizing radiation has enough energy to alter molecules within the cells of the human body, ta
...purchase below to continue the course
- The cell will repair itself and restore its normal function;
- The cell will remain altered, or only partially repair the damage, thereby heightening the risk for future cancer development;
- The cell will die, and your body will recover; however, if there is widespread cell death, this can increase the risk for organ failure (CDC, 2015b).
Risks with Medical Imaging
Many types of diagnostic radiology imaging tests and procedures involve exposure to ionizing radiation, which increases the risk of harmful effects. Some of the most common examples of imaging tests associated with the highest ionizing radiation exposure include x-rays, computed tomography (CT scans), fluoroscopy, and nuclear medicine scans. Ionizing radiation can penetrate deep into the body, and repeated exposure from imaging tests increases the risk for cancer later in life (CDC, 2019). Certain populations are more vulnerable to the harmful health effects of radiation exposure, such as infants and young children. Younger age groups have more cells that are dividing rapidly and tissues that are growing, thereby placing them at higher risk for long-term effects. Also, young people have a longer lifespan ahead of them, which gives cancer more time to develop (CDC, 2015b). Older adults and the elderly are also considered a vulnerable population and at heightened risk from lifelong radiation exposure, impaired organ function, and other factors of aging that already place them at increased risk for cancer development. Further, individual sensitivity is another important factor regarding radiation, as for unknown reasons, some individuals appear to be more sensitive to its effects than others (CDC, 2015b).
Pregnancy and Lactation
Diagnostic imaging during pregnancy and lactation is a controversial topic with various opinions. Pregnant women are also considered another vulnerable population, due to the potential for harming the fetus at various stages of development. However, data remains uncertain and inconsistent on the suspected risks in utero and to the newborn with radiation exposure. The ACR (2018) describes radiation exposure at levels less than 100 mGy as having "probably no effect" or "potential effects are scientifically uncertain and probably too subtle to be clinically detectable." However, at dose levels greater than 100 mGy, there is the potential for "possible spontaneous abortion, possible malformations increasing in likelihood as the dose increases, and risk for diminished IQ or of mental retardation, increasing in frequency and severity with increasing dose" (ACR, 2018, p. 3). In general, the most common documented adverse effects from high-dose radiation exposure on the developing fetus include growth restriction, microcephaly, and intellectual disability. The American College of Obstetricians and Gynecologists [ACOG] Committee on Obstetric Practice (2017) makes the following recommendations regarding diagnostic imaging procedures during pregnancy and lactation:
- Ultrasound and magnetic resonance imaging (MRI) are the imaging techniques of choice for the pregnant patient. Still, they should only be used when their use is strongly predicted to provide medical benefits to the mother or fetus.
- The majority of radiation exposure through CT scan or nuclear medicine imaging is provided at a dose that is considered much lower than the exposure associated with fetal harm. If these techniques are deemed medically necessary, they should not be withheld solely based on pregnancy.
- Iodinated contrast: it generally is recommended that contrast only be used if required to obtain essential additional diagnostic information that will affect the care of the fetus or mother during the pregnancy. Breastfeeding can be continued without interruption following the use of iodinated contrast materials.
- Gadolinium contrast: the use of gadolinium contrast with MRI should be limited, and it should be used only if it significantly improves the predicted diagnostic outcomes for the fetus or mother. Breastfeeding should not be interrupted following gadolinium administration.
- Radioactive iodine: iodine-123 (I-123) and iodine-131 (I-131) readily crosses the placenta, and use should be avoided in pregnancy. If a thyroid scan is essential, it is recommended that the radioactive isotope technetium 99m is used.
- Since specific nuclear materials excreted into breast milk can have deleterious effects on the infant, consultation with experts in breastfeeding and nuclear medicine are recommended when these compounds are used in lactating women (ACOG, 2017).
Radiation Dose
The health effects associated with ionizing radiation are strongly linked to the dose. The radiation dose or the amount of radiation is the critical factor when evaluating the risk for future unintended health consequences, as the risks are dose-dependent. However, the amount of ionizing radiation that is absorbed by the body is what induces harm to health. At high doses, radiation can be lethal; at lower doses, it can lead to serious health consequences (CDC, 2015b). The radiation dose is not the same as a dose of standard medication. There are three principal ways in which radiation doses are measured as absorbed dose, equivalent dose, and effective dose, as described in Table 1.
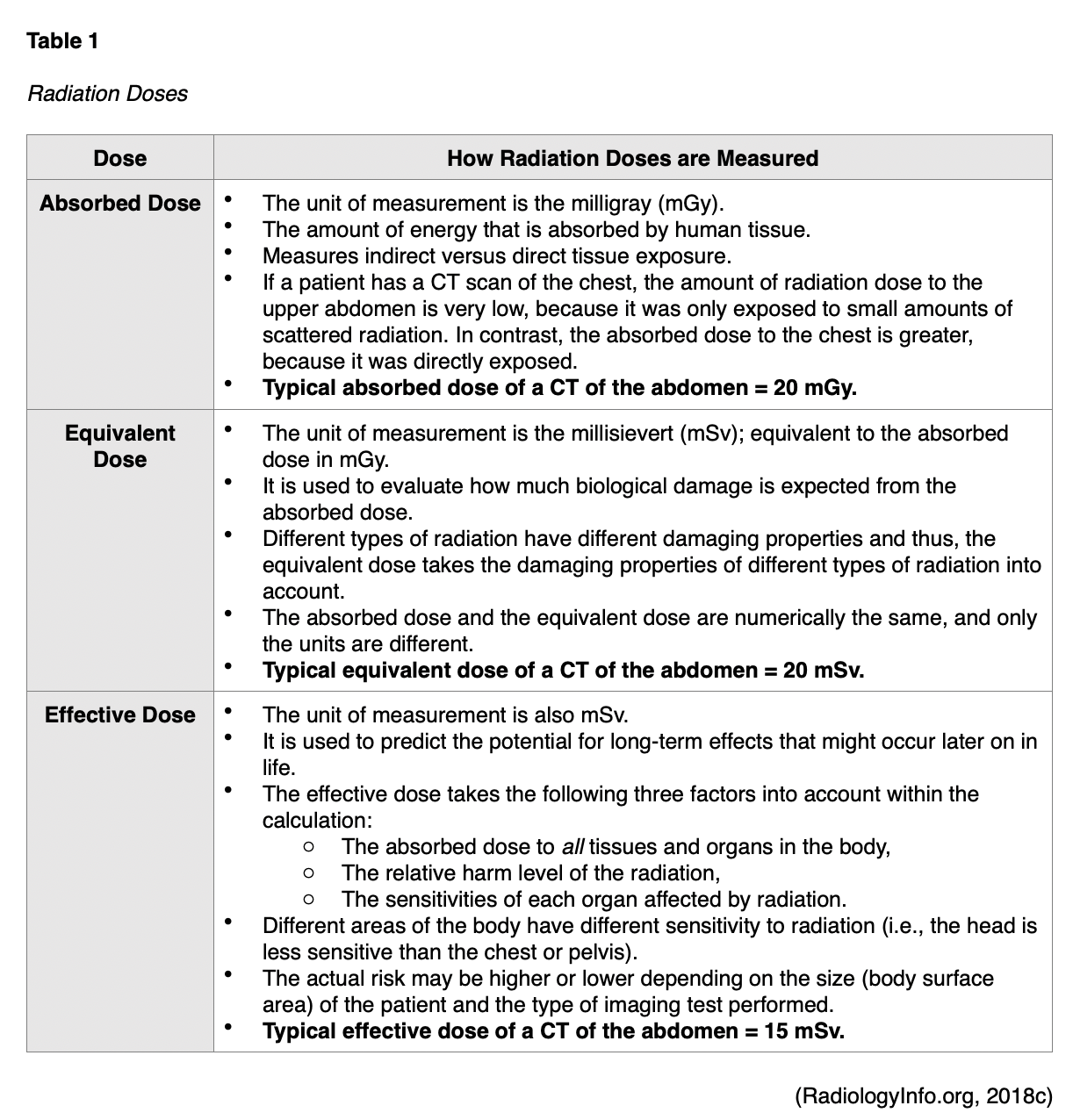
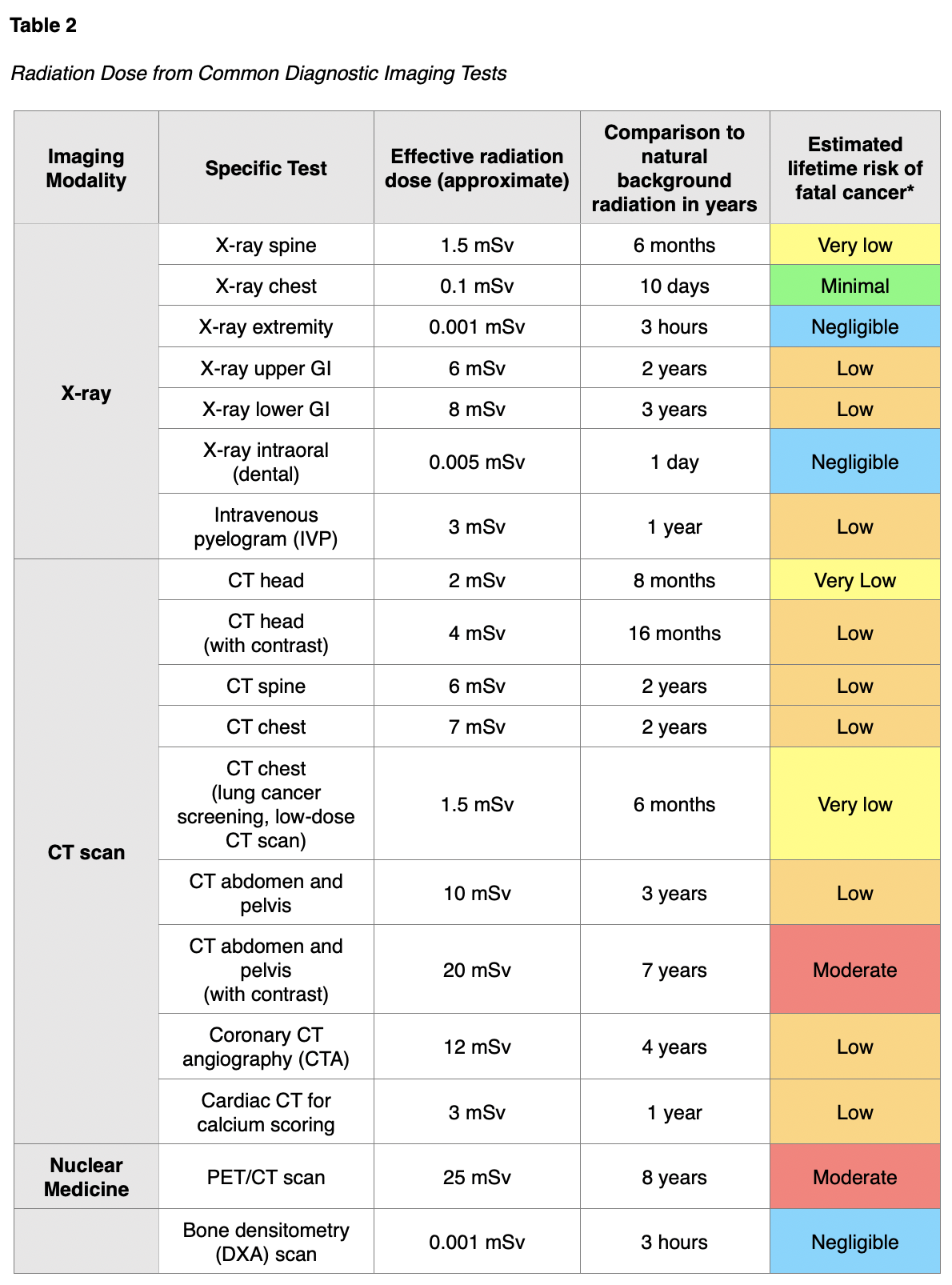
The absorbed dose and equivalent dose are used to evaluate the short-term effect of radiation on tissue, which ranges from weeks to months. When diagnostic imaging is performed correctly, there are generally no short-term effects; therefore, the absorbed and equivalent doses are less meaningful in clinical practice. For the majority of patients, the effective dose is the most important and useful dose quantity, since it applies to long-term effects (RadiologyInfo.org, 2018c). The speed at which the dose is administered also has implications on health and long-term effects. For example, if a person receives the radiation dose over an extended period, the impact on health will not be as severe as if the dose were received all at once (CDC, 2015b). The absorbed radiation dose varies widely based on the different types of examinations. Among the most common medical imaging tests, nuclear medicine scans such as the positron emission tomography-computed tomography (PET/CT) scan have the highest associated radiation exposure. A PET/CT scan is comparable to eight years of natural background radiation and poses a moderately increased (1 in 1,000 to 1 in 500) estimated lifetime risk of dying from fatal cancer. Table 2 provides a detailed overview of the radiation dose associated with common diagnostic radiology imaging examinations, as well as a classification of risk levels. Further, the radiation dose varies based on the operator and the facility equipment (RadiologyInfo.org, 2015).

Radiation Safety
While the potential for increased risk of adverse health effects from diagnostic imaging is clearly described, there is no universally recognized threshold for specific radiation dose and associated effects. Therefore, it has been argued that there is "no safe level" of radiation exposure. To safeguard patients, the priority is to ensure that the potential risks of ionizing radiation are continuously weighed against the benefits derived from performing the imaging test or procedure. This is a clinical decision that should be made between the ordering provider and the patient with full disclosure of the potential risks of imaging from radiation exposure in comparison with the predicted benefits (ACR, 2018). The US Department of Labor Occupational Safety and Health Administration (OSHA, n.d.), outlines standards for controlling ionizing radiation hazards and preventing radiation exposure to both healthcare workers and patients. Diagnostic radiology departments within hospitals, as well as free-standing diagnostic radiology facilities, must implement radiation protection programs that are managed by a radiation safety officer (RSO), who is a qualified expert, such as a radiologist or a medical physicist. The radiology equipment is housed in designated areas within hospitals, usually on the ground floor, and secured behind lead doors to reduce exposure to employees and patients. Radiologists and radiology technicians must undergo specialized training in radiation safety practices, which are governed by both state and federal law. OSHA standards require appropriate radiation caution signage to alert individuals to radiation usage in designated areas, such as the x-ray caution sign displayed in Figure 2 (OSHA, n.d.)

A central concept of radiation protection programs is keeping each healthcare worker's occupational radiation dose as limited as possible by implementing "As Low as Reasonably Achievable" (ALARA) programs. ALARA is premised on three chief components of time, distance, and shielding as follows:
- Time: minimize the time spent in areas with elevated radiation levels;
- Distance: maximize the distance from sources of radiation, as a worker's radiation dose decreases as the distance from the source increases;
- Shielding: Use shielding for radiation sources (i.e., placing an appropriate shield between sources of radiation and the worker), Inserting the proper shielding (e.g., lead, concrete, or special plastic shields depending on the type of radiation) between a worker and a radiation source will greatly reduce or eliminate the dose received by the worker. Shielding also refers to lead doors and geographic locations within the facility to reduce exposure to other individuals who are not directly working within that environment (CDC, 2015c; OSHA, n.d.).

As shown in Figure 3, shielding patients with lead aprons and lead coverings during diagnostic imaging tests has been the standard practice in diagnostic imaging since its endorsement by the US Code of Federal Regulations in 1976. This practice originated due to concerns related to hereditary risks with fertility (such as mutations in germ cells affecting future generations), and gonadal shielding was advised and implemented for all x-rays. Shielding has historically been justified as protection from hereditary risk, but not as an overall reduction in risk from radiation exposure. To date, no hereditary effects from radiation have ever been observed or concluded in humans (Marsh & Silosky, 2019).
However, over recent years, a growing body of evidence has demonstrated that patient shielding provides negligible or no benefit. Some studies have begun to establish evidence that shielding actually carries a substantial risk of increasing the absorbed radiation dose and compromises the diagnostic efficacy of the acquired images. Part of these risks is related to advancements in diagnostic imaging technologies. Modern x-ray imaging systems have an automatic exposure control, in which a sensor detects when the target dose is obtained. In fluoroscopy procedures, automatic brightness control algorithms function as a feedback loop, so that the x-ray tube output is constantly adjusted to ensure quality images are consistently obtained. Therefore, with these new technologies, if a lead shield is applied and enters the field of view, the radiographic tube will automatically adjust and drastically increase the radiation output to try to penetrate the shield. This heightens the risk of increased absorbed radiation by the patient. Furthermore, studies continually demonstrate that gonadal shielding is often positioned incorrectly, obscuring the image of the intended body part, and thus increasing repeat imaging rates (Marsh & Silosky, 2019). The focus on the discontinuation of patient shielding practices has become increasingly prominent in radiology literature over the last few years. On April 2, 2019, the American Association of Physicists in Medicine (AAPM, 2019) released a position statement supporting the discontinuation of patient gonadal and fetal shielding in routine practice. In May 2019, the ACR submitted a letter to the AAPM, endorsing their position on patient gonadal and fetal shielding. The ACR is in the process of adapting this new change into their guidelines, with the objective that this recommendation is universally adopted and becomes the standard of care across diagnostic radiology (ACR, 2019a).
Part II. Contrast Media in Medical Imaging
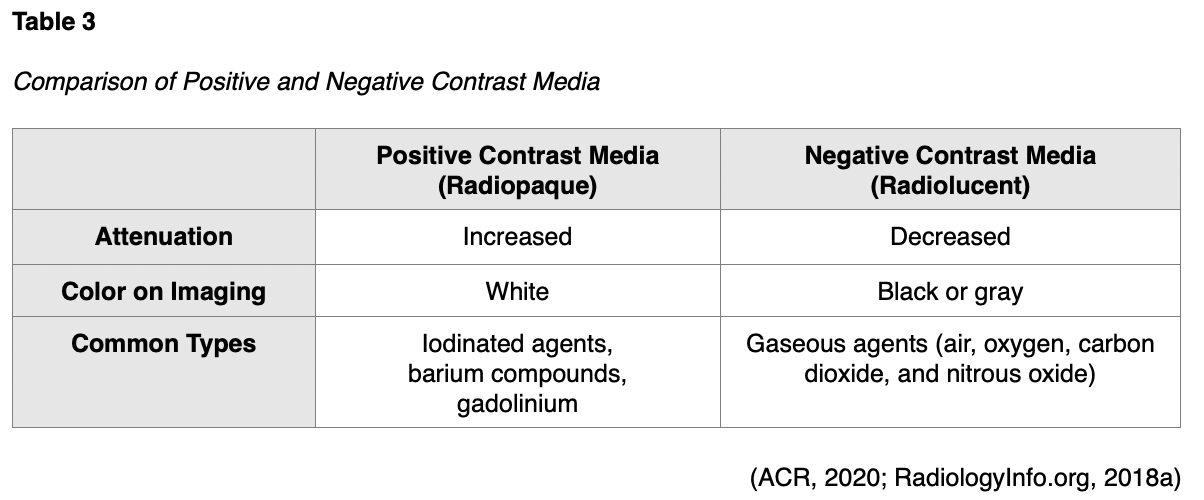
For various types of diagnostic radiology tests, contrast media agents are administered to temporarily change the appearance of body structures on radiographic images as a means of improving the visibility of specific body structures. Contrast media alters the absorption of the x-rays to enhance the contrast (or difference) between distinct anatomical structures, thereby generating a higher quality image of the underlying pathology. There are several types of contrast agents, and they are generally categorized as either positive or negative, as displayed in Table 3. Positive contrast media agents include barium compounds, gadolinium, and iodinated agents, which absorb more x-rays than surrounding tissue due to their high density. Positive contrast media leads to increased attenuation of x-rays and appears white on the resulting images. Negative contrast media agents are primarily gaseous agents, such as air, oxygen, carbon dioxide, and nitrous oxide, which absorb fewer x-rays than surrounding tissue due to their low density. Negative agents lead to decreased attenuation of x-rays and appear black or gray on the resulting images (ACR, 2020; RadiologyInfo.org, 2018a).
Contrast agents can be administered orally, intravenously (IV), intra-arterially (IA), or instilled directly into body cavities such as the urinary tract or gastrointestinal (GI) tract, including rectally through an enema (Spampinato et al., 2017). While contrast agents are used to enhance medical images, and their value is widely recognized, they are still considered pharmaceutical agents and therefore are not without risks. Since adverse effects from contrast media agents can range from minor side effects to life-threatening clinical emergencies, nurses must be well versed on the potential risks these agents pose. Nurses must ascertain a keen awareness of the signs of impending adverse reactions as a means to respond promptly and effectively to reduce morbidity and mortality (ACR, 2020).
Iodinated Contrast Agents
Iodine is a naturally occurring chemical element that is found in many types of contrast agents. Iodine-based or iodinated contrast agents (ICAs) enhance x-ray and CT images and are most commonly administered by injection through veins, arteries, or other body cavities. ICAs are the most widely utilized category of contrast agents, with millions of doses of ICAs administered each year in the US (Spampinato et al., 2017). While ICAs are considered safe and effective when administered correctly, there are several clinical risks and potential harms. One of the most concerning and well-established risks associated with ICAs is contrast-induced nephropathy (CIN) in patients with impaired renal function. This condition is characterized by an acute kidney injury (AKI), or an acute worsening in renal function within 48 hours following intravenous contrast administration. Risk factors for contrast-associated AKI include eGFR, diabetes mellitus, exposure to other nephrotoxic agents, hypotension, hypovolemia, congestive heart failure, and impaired kidney perfusion (Davenport et al., 2020).
Patients with type II diabetes mellitus who are taking metformin (Glucophage), an oral antihyperglycemic agent, as well as other biguanides, are at risk for a significant adverse reaction to ICA known as lactic acidosis. Lactic acidosis is characterized by a buildup of lactic acid in the bloodstream (greater than 2 mmol/L), which can lead to the development of severe symptoms including extreme exhaustion, muscle cramps, body weakness, abdominal pain, diarrhea, headache, jaundice, and breathing changes. Since the kidneys are primarily responsible for excreting metformin (Glucophage), patients who are taking this medication who have underlying renal insufficiency are at heightened risk for this severe complication. Therefore, the US Food & Drug Administration (FDA, 2016), recommends the discontinuation of metformin (Glucophage) and metformin-containing products, 24 hours prior to ICA administration in patients with underlying renal impairment with an eGFR between 30 and 60 mL/minute/1.73 m2 (FDA, 2016).
Allergic Reactions
ICAs pose a risk for allergic reactions, which can have variable presentations ranging from mild to severe, and delayed, as outlined in Table 4. Patients can also have an allergic reaction at initial exposure to the ICA, mediated by anaphylaxis mechanisms, in which symptoms develop within minutes of exposure. Anaphylactic reactions are usually unpredictable but are the most clinically significant reactions to ICAs, as they involve the release of histamine and other biologic mediators. Most reactions occur within the first 5 to 60 minutes following ICA administration. However, delayed reactions can occur between one hour and one week following the injection. Delayed reactions are more common in young adults, women, and patients with a history of a contrast allergy, and they tend to be cutaneous in nature (Beckett et al., 2015).
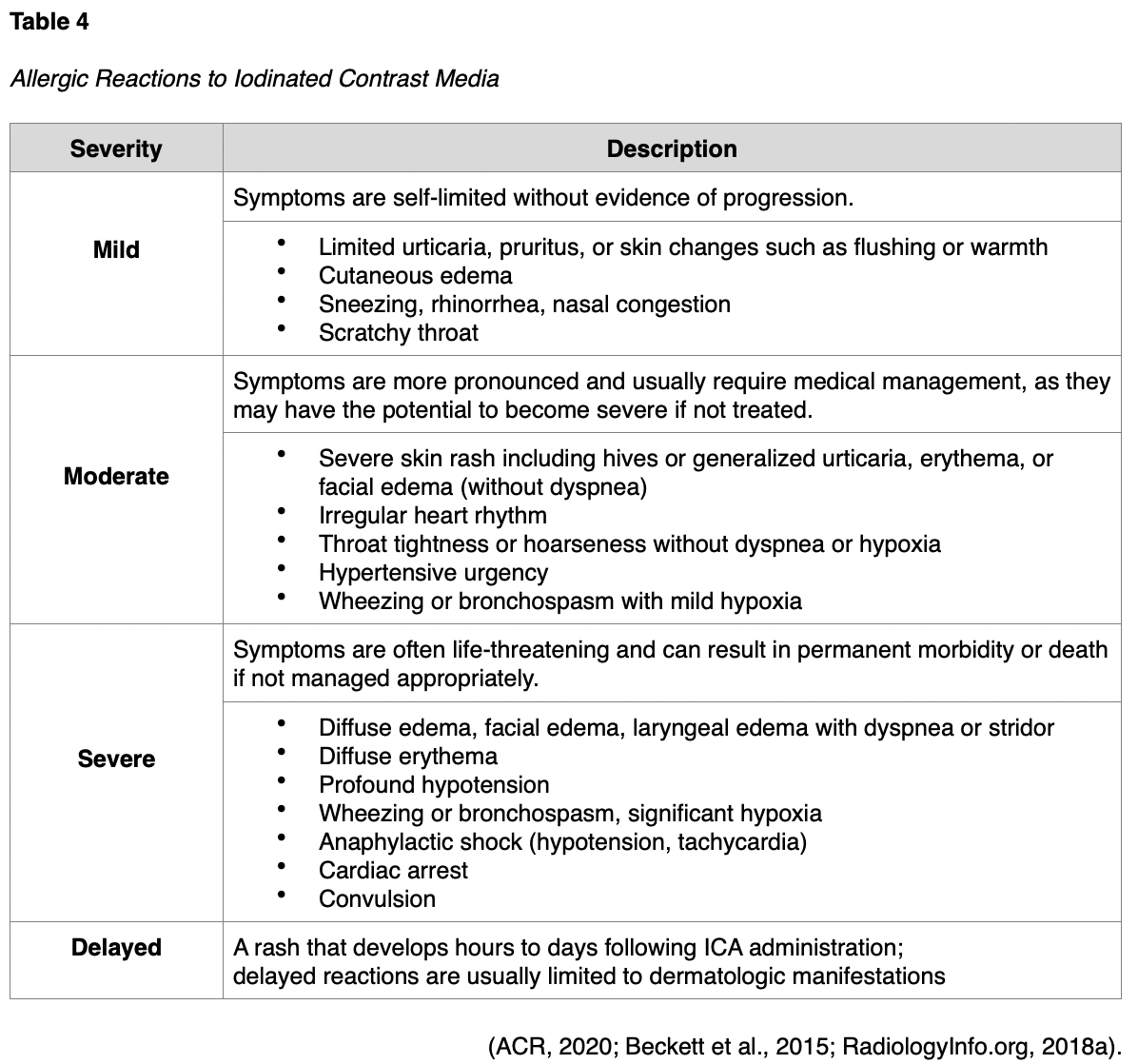
While ICA reactions are sporadic and often unpredictable, there are some risk factors associated with an increased risk of reaction to ICA. The ACR (2020) advises that prior allergic-like or unknown type of reactions to the same class of contrast medium is considered the most significant risk factor for predicting future adverse events. Additional risk factors for ICA allergic-like reactions include the following:
- Patients with a prior allergic reaction (or an unknown-type of reaction) to an ICA are at an approximately five-fold increased risk of developing a future allergic-like reaction with subsequent exposure to contrast;
- Patients with unrelated allergies are at a two- to three-fold increased risk for an allergic-like reaction;
- Patients with a history of asthma are at increased risk for allergy and may be more likely to develop bronchospasm, but those with well-controlled asthma may not be at increased risk;
- Patients with multiple allergies (such as dermatitis and urticaria) have at least a three-fold increased risk for a severe reaction to contrast media;
- Patients with mild allergies (such as seasonal allergies) are at unclear risk (ACR, 2020; Beckett et al., 2015).
Despite the popular misconception, it is now well-established and confirmed that there is no link between shellfish allergy and allergy to ICAs. Patients with shellfish or povidone-iodine (Betadine) allergies are at no higher risk from iodinated contrast than patients with other allergies. Furthermore, there is no cross-reactivity between the different classes of contrast; therefore, a patient with an ICA allergy is not at heightened risk for a gadolinium-based allergy (ACR, 2020; Beckett et al., 2015).
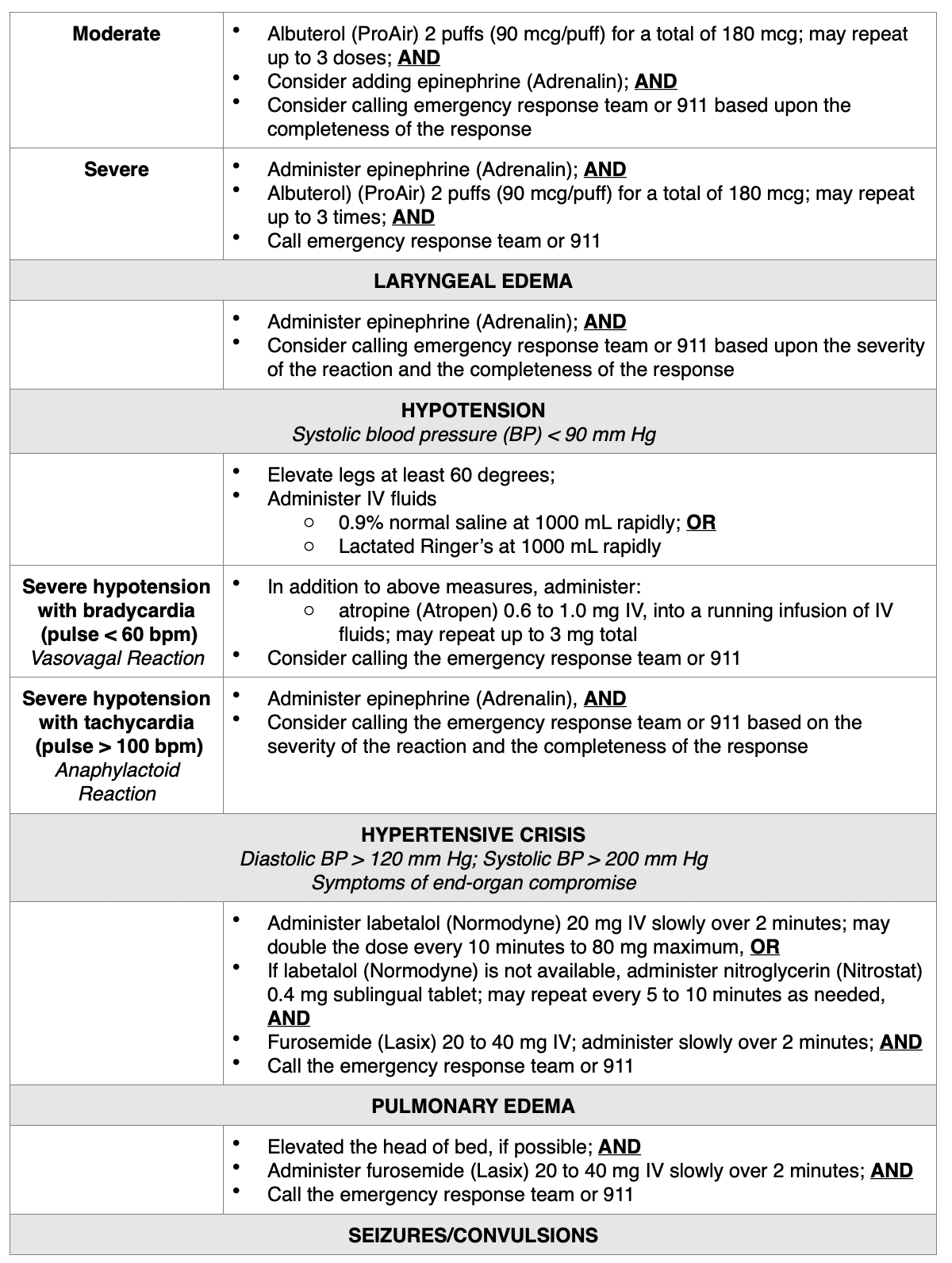
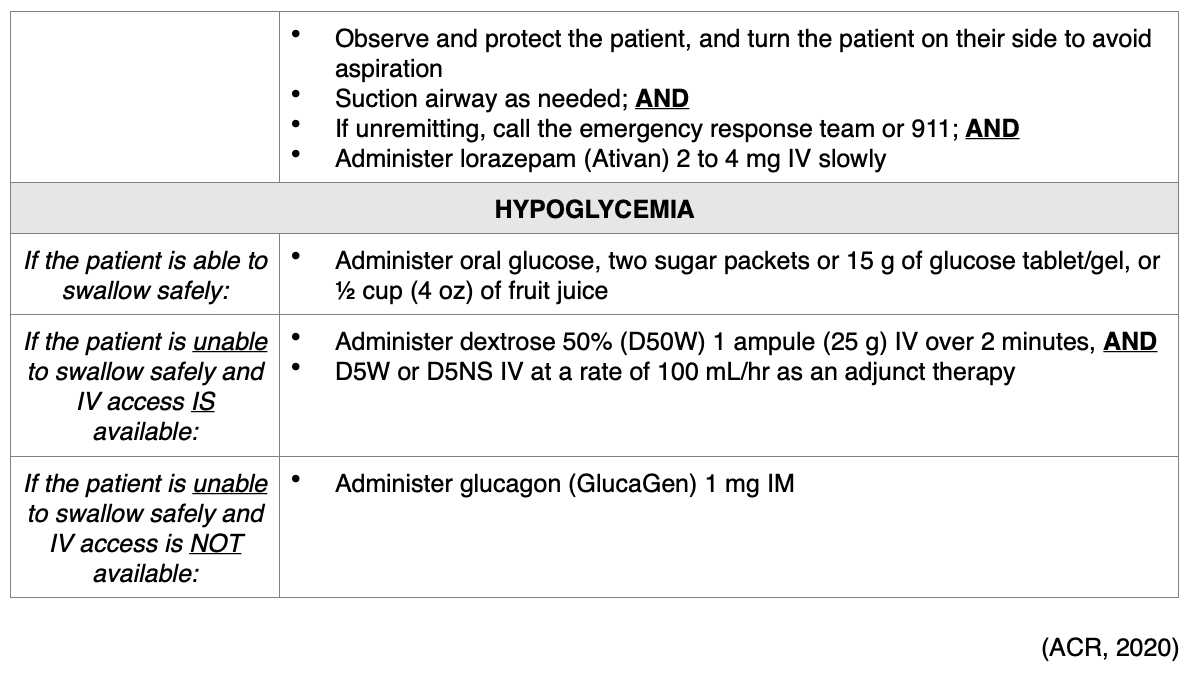
Treatment of Allergic Reactions. According to ACR (2020), all facilities in which ICAs are injected must be equipped with emergency supplies, and nurses must be trained in basic life support in the event of a life-threatening allergic reaction. While the majority of allergic reactions are mild, self-limiting, and do not progress in severity, they can sometimes become more severe. Therefore, it is advised that any patient who is displaying signs of reaction should be observed for a minimum of 20 to 30 minutes, or longer if needed, to ensure clinical stability and recovery. Mild reactions may require symptomatic treatment, but often do not require any treatment at all. Moderate reactions are usually not life-threatening, and severe reactions are rare but can be potentially fatal. Severe reactions require prompt recognition and action to prevent morbidity and death (Beckett et al., 2015). For all types of reactions, nurses must ensure that IV access is maintained, observe vital signs, and assess the patient's appearance (ACR, 2020). In addition to alerting the on-site clinician, nurses are advised to prioritize a focused assessment, evaluate the patient's level of consciousness, the appearance of their skin, quality of phonation, lung auscultation, blood pressure, and heart rate. These key assessment points help quickly determine the severity of the reaction so that effective treatment can be promptly and successfully administered. Table 5 provides a concise account of the guidelines of additional interventions for managing various types of acute reactions to ICAs in adult patients (ACR, 2020).

Premedication Regimen for Patients with an ICA Allergy. While well-controlled studies and definitive data are not available on the efficacy of premedication in preventing an allergic reaction in high-risk patients, the ACR (2020) believes that premedication reduces the likelihood of a reaction in high-risk patients. While there is no formal consensus on the exact premedication regimen, oral premedication is preferable to IV premedication due to lower cost, increased convenience, and greater evidence support in the literature. The majority of data exists on the use of oral methylprednisolone (Medrol Dosepak) or oral prednisone (Deltasone), with or without oral diphenhydramine (Benadryl) (ACR, 2020).
Gadolinium
Gadolinium-based contrast agents (GBCAs) are the primary form of contrast used to enhance the quality of magnetic resonance imaging (MRI) scans. Administered intravenously, GBCAs work by altering the magnetic properties of neighboring water molecules, thereby enhancing the quality of the MRI images. MRI is distinct from other radiology imaging exams as they do not operate using x-rays or ionizing radiation. Instead, they function on a high strength magnetic field, magnetic field gradients, radio waves, and a computer to generate images to demonstrate if a disease process, pathological condition, or injury is present. Similar to ICAs reviewed in the prior section, osmolarity and viscosity of the GBCAs can also vary widely and are generally higher for the ionic than the non-ionic agents. Gadolinium belongs to a class of metal ions, and if used alone, it is toxic to humans. Gadolinium present in GBCAs has undergone a process called chelation, in which other chemical ions are mixed with the gadolinium to prevent it from harming the body. The majority of GBCAs used in the US are chelates of gadolinium, have a positive magnetic susceptibility, and function to produce an increased signal on the resulting images. The GBCA is injected into the patient before the MRI and is primarily eliminated from the body via the kidneys, with a small amount of liver excretion (Ibrahim et al., 2020).
GBCAs are generally well-tolerated and are much less likely to produce an allergic reaction than ICAs. The incidence of adverse events is low, ranging from 0.07% to 2.4%, and most reactions are mild and physiologic, including coldness, warmth, and pain at the injection site, nausea, vomiting, headache, paresthesia, and dizziness. Allergic-like reactions are uncommon, ranging from 0.004% to 0.7%, and symptoms are generally very mild and transient, usually limited to hives, other skin reactions, or itchy eyes (RadiologyInfo.org, 2018a). While manifestations of allergic-like reactions to GBCA can be similar to those of ICAs, they are rare, with life-threatening anaphylactic reactions occurring at a low rate of 0.001% or 0.01%. While the ACR (2020), acknowledges that fatal reactions to GBCAs are possible, they are extraordinarily rare. When managing GBCA-related allergic reactions, clinicians should follow the same ICA guidelines outlined previously in Table 6 (ACR, 2020).
Barium Sulfate
Barium sulfate contrast agents (BSCAs) are the preferred agents for evaluating and visualizing the GI tract and can only be administered through the GI tract, which includes the oral and rectal routes (ACR, 2020). They are comprised of a suspension of insoluble barium sulfate particles that are not absorbed by the gut and are ideal for illuminating the GI tract since they have excellent coating properties of the GI mucosa. They are the most common type of orally administered contrast material and enhance the visualization of the GI tract in CT scans of the abdomen and pelvis and specific types of MRI scans. They are also commonly used for fluoroscopic studies, such as an upper gastrointestinal (UGI) series or a small bowel follow-through (SBFT) examination. Barium enemas are also used therapeutically in some instances to remove feces from the bowel, such as in the event of severe constipation or blockages, as means to prevent surgical intervention and reduce the risk for bowel perforation (RadiologyInfo.org, 2018a).
While the ACR (2020) states explicitly that there are no absolute contraindications for the use of BSCAs, they recommend avoiding their use in patients who are suspected or known to have bowel perforations or prior allergy to barium (ACR, 2020). Barium sulfate is generally well tolerated when administered orally, although some patients describe a mildly unpleasant taste. When barium sulfate is administered through the rectum using an enema, patients may experience temporary abdominal fullness and mild discomfort. It is generally recommended to ensure the BSCA is warmed to room temperature, which helps enhance patient tolerability and reduce spasms of the colon (RadiologyInfo.org, 2018a). Adverse reactions to BSCAs are rare and are almost always mild in presentation. The most common symptoms include nausea, vomiting, abdominal cramping, or discomfort, which can occur during or after the examination. Following the completion of a BSCA procedure, nurses should instruct patients to increase oral fluid intake to help facilitate and expedite the removal of the contrast material from the body, which is expelled through the feces. Patients should also be advised that their stool may be white for a few days until all the contrast material is eliminated. It is common for some patients to experience changes in their routine bowel habits for the first 12 to 48 hours, such as diarrhea or constipation (RadiologyInfo.org, 2018a).
Allergic-like reactions to BSCA are very uncommon, with an incidence rate of 1 in 750,000 examinations. If an allergic-like reaction does occur, the manifestations are usually very mild and limited to transient rashes, urticaria, pruritus, or mild bronchospasm. Moderate to severe allergic reactions are exceedingly rare, occurring in about 1 out of 2.5 million patient exposures. Clinical manifestations of moderate to severe allergic reactions may include more extensive dermatologic changes, respiratory symptoms, and vascular events such as hypotension. Rarely, angioedema of the stomach and small bowel, and toxic epidermal necrolysis (TEN) have been described (ACR, 2020). Direct barium toxicity has been reported in patients who received oral and rectal BSCAs, although this is an infrequent occurrence. The etiology is based on the concept that any barium that dissociates from the stable barium compound may be absorbed through the bloodstream and lead to toxicity. Symptoms of barium toxicity usually present rapidly and are limited to the GI tract, including watery diarrhea, nausea, and vomiting. The treatment is limited to supportive therapy, rehydration, and managing any electrolyte disturbances associated with fluid volume deficit (ACR, 2020).
Microbubble Contrast Agents
Microbubble contrast agents (MCAs) are most commonly used to enhance the images of ultrasonography, particularly exams of the heart. MCAs are microscopic-sized, highly compressible gas-filled microbubbles that are coated with a phospholipid or protein outer layer (Wang et al., 2018). MCAs have a high degree of "echogenicity", or the ability to reflect ultrasound waves; structures with higher echogenicity will appear brighter than surrounding areas on the resulting ultrasound images (RadiologyInfo.org, 2018a). These agents should be administered through slow intravenous infusion or bolus injection. Since the microbubbles dissolve rapidly, usually within 15 minutes, the ACR (2020) recommends real-time assessment with images obtained over a 10-minute period, before the microbubbles spontaneously rupture and dissolve. As the microbubbles dissolve, they release a gas that is mostly eliminated by the lungs through exhalation. This is considered a useful and practicable alternative to ICAs or GBCAs for patients with AKI, CKD, or allergies (RadiologyInfo.org, 2018a).
MCAs are considered to have a reasonably favorable safety profile, as the rate of adverse events is less than that of ICAs or GBCAs. The ACR (2020) cites a severe reaction rate of 0.01% (n=8) in a large investigational study involving more than 78,000 doses conducted in 2008. Of these, only four were anaphylactoid reactions, and there were no fatalities reported (Wei et al., 2008). Adverse events are usually mild and physiologic. The most common symptoms include headache, flushing, warmth, nausea, and altered taste, and the majority of severe reactions occur within 30 minutes of MCA administration. The risk for severe cardiopulmonary reactions is increased in patients who have a preexisting and unstable cardiac condition, such as an acute myocardial infarction or congestive heart failure. There is no reported renal toxicity with MCAs. MCAs should not be used intra-arterially and are contraindicated in patients with prior allergic reactions to these agents (ACR, 2020).
Gaseous Contrast Agents
Gaseous agents (such as oxygen [02] and carbon dioxide [CO2]) can also be used as contrast agents in specific types of imaging tests. Similar to MCAs used in ultrasonography, these agents are also absorbed rapidly by the body and eliminated through exhalation. Research is limited on these contrast agents, and the ACR (2020) manual of contrast media omits any reference to or discussion of these agents entirely. While air and oxygen can be dangerous due to the risk for gas emboli during specific procedures, CO2 does not pose a risk of gas emboli and can be used with relative safety. CO2 has the most research data supporting its clinical benefits and use as an intravascular contrast agent. It is used as an alternative to ICA or GBCAs in patients with renal dysfunction or allergies to ICA. It is inexpensive, readily available, has a low molecular weight when compared to ICAs, and is less viscous than blood. These characteristics make it an ideal agent to image small collateral vessels and a wide variety of vascular beds and chambers, as it displaces the blood in the vessels and acts as a negative contrast agent. However, administering CO2 as a contrast agent requires extreme care as it is a colorless, odorless, and significantly compressible gas. It is also less sensitive and specific than ICAs or GBCAs (Ali et al., 2017; Gupta et al., 2020).
Part III. Diagnostic Imaging Tests with Radiation Exposure
Imaging studies can be divided into two main categories: planar and cross-sectional. Planar studies produce two-dimensional (2D) images and include basic diagnostic radiology testing such as x-rays and mammography. Cross-sectional imaging techniques address the three-dimensional (3D) aspects of human anatomy by capturing more detailed images, often referred to as "slices." This type of imaging technology can then create a composite analysis of 2D slices to provide a 3D visualization of the anatomy. Cross-sectional imaging includes CT scan, ultrasound, and MRI (Elsayes & Oldham, 2014).
X-ray
X-ray is the most common and readily available type of diagnostic imaging test. Often referred to as a plain film, an x-ray is a quick, noninvasive, and painless imaging modality that produces images of the structures inside the body. In a standard x-ray, a beam of energy is aimed at the intended body part, and a plate is placed behind the body part to capture the variations of the energy beam. As demonstrated in Figure 4, a conventional x-ray is the simplest example of how ionizing radiation produces a 2D image. For optimal imaging quality, the positioning of the x-ray detector should be as close to the body part as possible. The radiation beams should be perpendicular (situated at a right angle) to the body part, as this helps to minimize the magnification and enhance the sharpness of the resulting image, thereby producing a clearer result (Elsayes & Oldham, 2014).
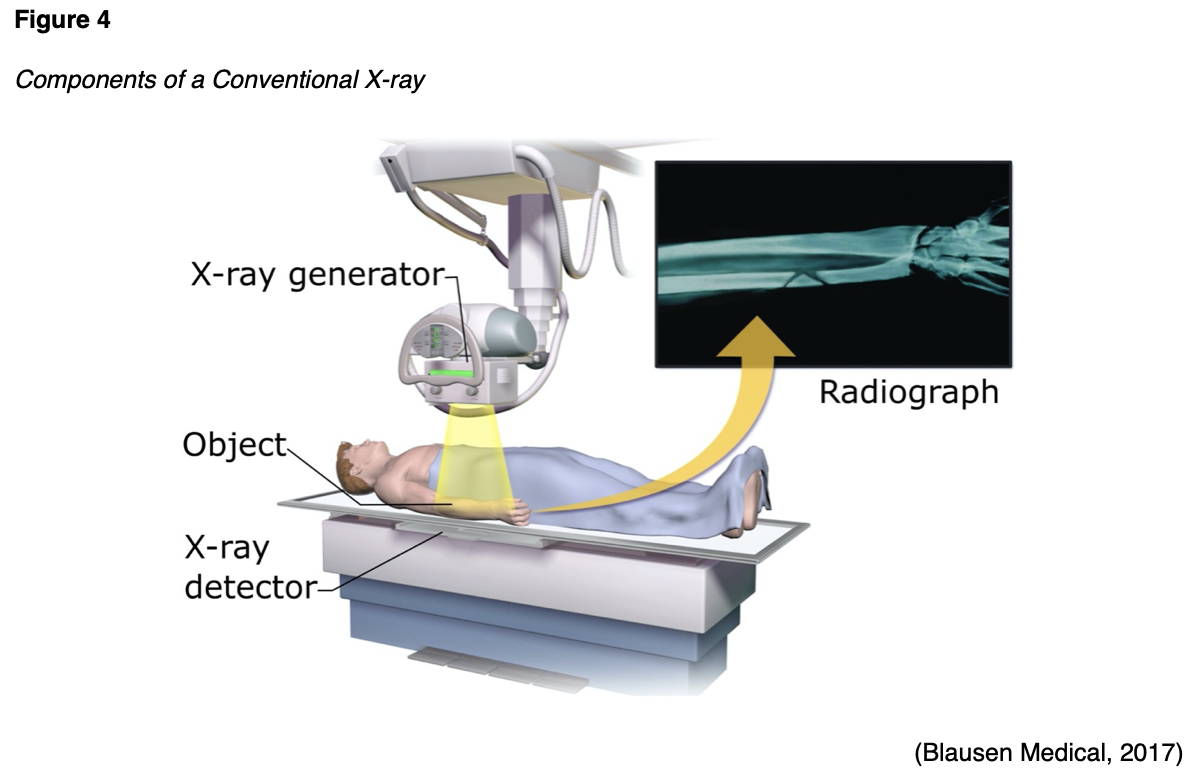
X-rays are widely utilized throughout various healthcare domains with several indications. They are particularly useful for diagnosing acute bone fractures and cardiopulmonary conditions, such as cardiac enlargement, pneumonia, or pleural effusion (fluid inside the lung). X-rays are commonly used in dental care as a means of identifying cavities and underlying dental pathology. They are also useful for diagnosing arthritis, identifying foreign bodies or bowel obstructions, and may be used to assist in fluoroscopy procedures (IAEA, n.d.b). Patients are required to remove any clothing or jewelry, particularly metal, which may interfere with the procedure. This standard applies to all forms of diagnostic imaging tests and procedures. The patient should be educated that they will either lie, sit, or stand still while the x-ray machine takes images. To obtain the highest quality imaging results, patients may be asked to move into specific positions, and several images may be taken from different viewpoints, but the entire process generally takes less than 15 minutes (Elsayes & Oldham, 2014).
Fluoroscopy
Fluoroscopy is a medical imaging test used to study the motion of internal body structures. According to the CDC (2016), fluoroscopy uses an x-ray beam that passes continuously through the body to create an image, and the image is projected on a monitor, which allows clinicians to evaluate the movement of internal organs or devices in real-time. Medical imaging tests using fluoroscopy are generally noninvasive diagnostic tests performed to evaluate specific areas of the body. Fluoroscopy can help evaluate the bones, muscles, joints, as well as solid organs, such as the heart, lung, or kidneys. It can be used alone as a diagnostic procedure or in combination with other procedures. Fluoroscopy is used in many types of diagnostic testing and procedures, and the amount of exposure to ionizing radiation during fluoroscopy depends on the test being performed and the equipment used (Johns Hopkins Medicine, n.d.b).
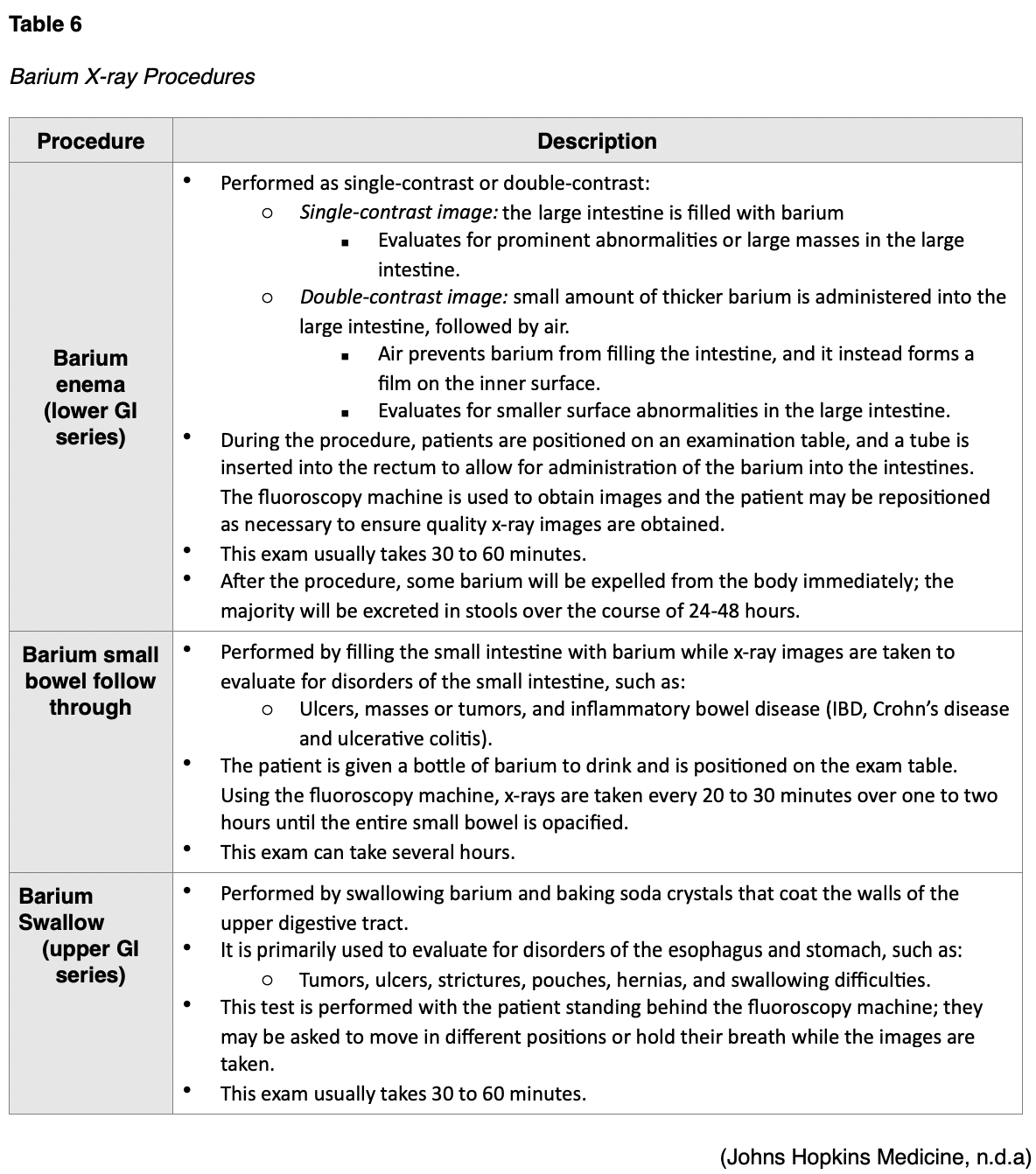
Barium X-rays
Barium x-rays are used to diagnose underlying pathology within the GI tract, which may include ulcers, inflammation, tumors, hernias, or strictures. After the barium is administered, fluoroscopy allows the radiologist to see the movement of the barium through the GI tract. There are three types of barium x-ray procedures, which include the barium enema, or lower GI series, the barium small-bowel follow-through, and the barium swallow or upper GI series. Barium x-ray tests are typically performed as an outpatient procedure, and the defining features and details of each test are described in Table 6. Prior to any of these procedures, patients usually require a bowel preparation to empty the large intestine and any feces that can obscure the x-ray images, and preparation instructions generally include some variation of the following:
- Adherence to a special liquid diet for up to two days prior to the test;
- A bowel cleanse (laxatives, suppositories);
- Remain NPO after midnight before the procedure (Johns Hopkins Medicine, n.d.a).
Following all barium x-ray procedures, patients should be advised to increase the consumption of foods high in fiber as well as oral hydration to facilitate bowel movements. Bowel movements may be white or lighter in color for one to two days following the procedure. Further, patients should be counseled to contact their provider if they are unable to pass gas or if they do not have a bowel movement for more than two days following the procedure, as they may require a laxative or enema to assist with eliminating the barium (Johns Hopkins Medicine, n.d.a).
Intravenous Pyelogram
Intravenous pyelogram (IVP) is a fluoroscopic procedure that uses iodinated contrast material to assess for abnormalities within the kidneys, ureters, and bladder. The contrast is injected into the patent intravenously, and it travels to and collects within the kidneys and urinary tract. The contrast makes these areas appear bright white on the resulting x-ray images, thereby allowing the radiologist to identify any underlying pathology. This test is commonly performed to evaluate the etiology of hematuria or flank pain generating from the kidneys, as well as to evaluate for the presence of kidney stones. Typically performed as an outpatient procedure, patients will need to empty their bladder immediately before the scan to allow for the best quality images. Following contrast administration, the patient will lie flat on an exam table, and a series of x-ray images are taken while the kidneys are processing the contrast. Patients may be asked to lie on their side for enhanced images depending on the underlying issue. The exam usually takes up to one hour in duration, but in patients with impaired or sluggish renal function, the exam may take up to four hours. Following the procedure, patients are advised to increase oral hydration to flush the contrast out of the renal system (RadiologyInfo.org, 2019a).
Mammography
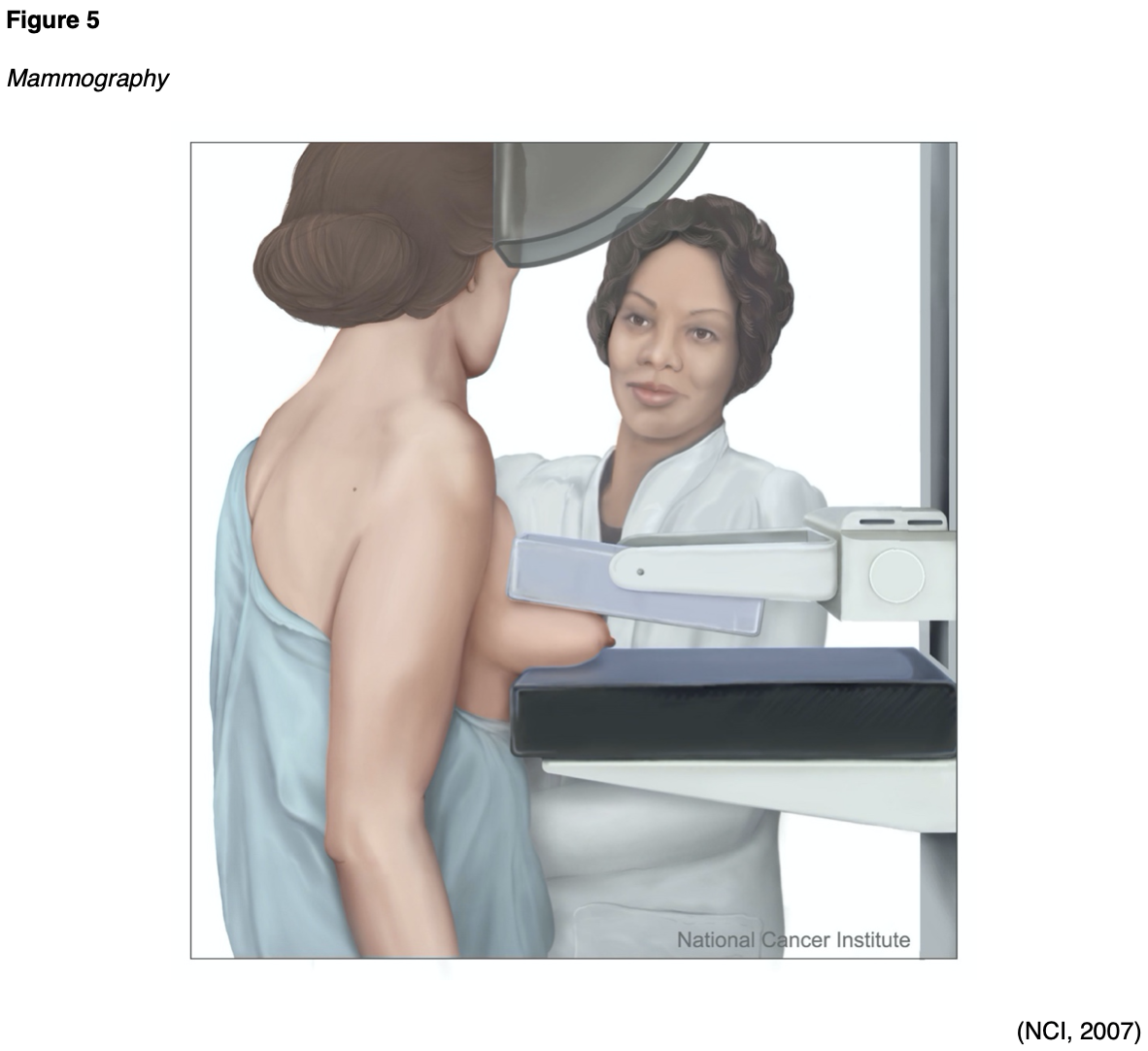
Mammography is a type of breast imaging test that uses low-dose x-rays to view the breast tissue as a means to identify abnormalities that are suspicious for breast cancer. A mammogram is one of the most widely used cancer screening tools that has been successful in identifying early breast cancer in asymptomatic women and preventing breast cancer deaths. Approximately 33 million screening mammography exams are performed each year. Since the introduction of screening mammography in the late 1980s, the breast cancer death rate in the US has decreased by nearly 40% (Arleo et al., 2017). Randomized clinical trials have demonstrated that routine screening mammography can reduce the number of deaths from breast cancer in women aged 40 to 74 years (National Cancer Institute [NCI], 2019). In modern practice, screening mammography is central to detecting precancerous and cancerous breast lesions in women who have no symptoms. Diagnostic mammography is performed in a patient who presents with abnormalities, such as a palpable breast lump, nipple discharge, or skin changes. Diagnostic mammography may also be advised following an abnormal screening mammogram as a means of obtaining enhanced and dedicated images of the area of concern. In many patients, a diagnostic mammogram is performed in combination with an ultrasound of the breast tissue (RadiologyInfo.org, 2019b).
As demonstrated in Figure 5, a mammogram is performed by compressing the breast tissue within a machine that looks like a rectangular metal box, which spreads out the breast tissue and eliminates motion. The patient is instructed to stand facing the mammography machine during the exam, one breast is placed on the flat surface at a time, and a lever called a compression paddle is lowered to squeeze the breast tissue. Compression of the breast tissue is essential to ensure that all of the breast tissue can be visualized, and to allow the x-ray beam to penetrate through the breast tissue. Further, compressing the breast tissue also allows for the use of lower doses of radiation when a thinner amount of breast tissue is imaged. Once the images are acquired, they are subsequently processed on a special computerized system. The patient will be advised to remain very still during the test and, at times, may be asked to hold their breath to reduce motion and artifact when the x-ray is acquiring the images. Most women describe the exam as uncomfortable due to the pressure on the breast tissue from the compression paddle, but it is generally not considered a painful test. A mammogram usually takes about 30 minutes, and patients can resume normal activities immediately following the test (Mayo Clinic, 2019a; RadiologyInfo.org, 2019b).
Standard mammography imaging has evolved significantly over the last few decades, with the development of computer-aided detection (CAD), digital mammography, and breast tomosynthesis (RadiologyInfo.org, 2019b). CAD is a form of artificial intelligence that was devised as a means to help identify possible abnormalities on the mammogram that may otherwise have been missed by highlighting them for the radiologist. While CAD is increasingly used, the efficacy of the system remains controversial due to mixed results from research studies. Its clinical benefits and efficacy continue to be studied; however, its use does not replace the need for a qualified radiologist to interpret mammogram images directly (Katzen & Dodelzon, 2018). Some women may opt to undergo digital mammography. This modality is similar to a digital camera and produces higher quality images using lower amounts of radiation. Breast tomosynthesis is also referred to as 3-D mammography and is an advanced imaging modality that can capture several images of the breast from different angles. The images are then compiled into 3-D images. The radiation dose for breast tomosynthesis is slightly higher than conventional mammography. However, large population-based studies have demonstrated improved breast cancer screening detection rates and reduced need for additional views for women with dense breasts. Although breast tomosynthesis is a newer imaging modality, some of its clinical benefits include the following:
- Earlier detection of small breast cancer that may not have been seen on a conventional mammogram due to clearer images;
- Particularly helpful in patients with dense breasts;
- Increased likelihood of detecting multiple breast tumors during one imaging test and pinpointing the size, shape, and specific location of the abnormalities;
- Reduced number of unnecessary biopsies or additional imaging tests (RadiologyInfo.org, 2019b).
CT Scans
CT scans, sometimes referred to as computed axial tomography (CAT) scans, use a series of x-rays and computer technology to create cross-sections of the inside of the body, including bones, blood vessels, organs, and soft tissues. The x-ray detector moves in a circle around the body to generate several different views of the same body structure being evaluated. It sends x-rays through the body during each scan rotation to form a complete picture in much greater detail than a conventional x-ray. CT scans use ionizing radiation as their imaging method and may be performed with or without intravenous contrast administration. CT scans are used to diagnose injuries from trauma such as bone fractures, infections, internal bleeding, tumors, masses, and cancers, and are also used to guide biopsies (Elsayes & Oldham, 2014). There are numerous indications for performing CT scans. CT scans may be performed of the face, sinuses, orbits, or neck, and are generally performed to evaluate for suspected infection (sinusitis, orbital infection) or mass (malignant or benign tumors) within these locations. A CT scan of the chest is commonly performed to evaluate the lung parenchyma as well as the mediastinum for the presence of pulmonary nodules, masses, pleural effusions, or other signs of lung disease. CT imaging of the abdomen and pelvis may be performed for suspected appendicitis, diverticulitis, abscess, or small bowel obstruction. CT angiography (CTA) is a type of CT scan performed to evaluate the blood vessels in a particular area for narrowing, obstruction, or thrombosis. Most commonly, a CTA scan is ordered to evaluate for suspected pulmonary embolism (pulmonary CTA), aortic dissection (thoracic aorta CTA), and brain aneurysm (intracranial CTA). Patients should be advised that they will be required to lie flat and remain still on a table that slides into the scanning machine, which resembles a large doughnut, as displayed in Figure 6. The machine will rotate around the patient to take all the necessary images. The test takes about 10 to 15 minutes to complete (AHRQ, 2019; IAEA, n.d.a).
Nuclear Medicine Imaging
Nuclear medicine imaging is capable of visualizing how the body is functioning at the cellular and molecular levels. It uses small quantities of radioactive tracers (radiotracers) to diagnose and treat disease. The radiotracers are most commonly injected into a vein but may also be taken orally or inhaled. The radiotracer travels through the body, releasing energy in the form of gamma rays. It gets absorbed by specific tissues and organs. It is then detected by the external scanning device to provide information on organ function and cellular activity (Society of Nuclear Medicine & Molecular Imaging [SNMMI], n.d.). The radiotracers are comprised of molecules that are bonded tightly to a radioactive atom, and these molecules vary greatly depending on the purpose of the scan (National Institute of Biomedical Imaging and Bioengineering [NIBIB], 2019). Radiotracers must meet FDA standards for safety due to radiation exposure. The use of radioactive materials for nuclear medicine is regulated by the Nuclear Regulatory Commission (NRC), the FDA, and individual states to ensure the safety of patients, healthcare professionals, and the general public. Each nuclear medicine imaging test uses a specific radioactive agent (SNMMI, n.d.).
Positron Emission Tomography/Computed Tomography (PET/CT)
PET/CT imaging uses the radiotracer fluorodeoxyglucose (FDG) to create 3D images and is combined with CT scans to better locate areas of abnormal cell activity. There are a few types of PET/CT scans, but the most common is a full-body PET/CT, which evaluates the internal structures from the mid-portion of the skull through the thigh region and is combined with a low dose CT scan. This is a hybrid imaging modality; the resulting images acquired from each test are fused using advanced computerized technology to create enhanced images of higher quality. The imaging obtained by the PET scan illustrates the spatial distribution of metabolic or biochemical activity in the body, which is then more precisely aligned with the anatomic imaging obtained by the CT scan. PET/CT scans provide superior information for evaluating tissues, the staging and restaging of cancers, and are useful for monitoring the effectiveness of cancer treatments. Since cancer cells take in glucose faster than normal tissue, the FDG tracer is a superior radiotracer for evaluating cancerous tissue, as FDG is a compound similar in chemical composition to glucose. The FDG accumulates in areas of the body that are most metabolically active, which helps differentiate between physiologic uptake (healthy tissue) and pathologic uptake (diseased tissue) (Thayalan, 2014). The FDG radiotracer is injected into the patient's bloodstream, and the PET/CT scan creates images that show the distribution of the radiotracer throughout the body and determine if abnormalities are present. Highly active cancer cells show higher levels of uptake of FDG, whereas brain cells affected by dementia consume smaller amounts of glucose, indicated by lower FDG uptake (Memorial Sloan Kettering Cancer Center [MSKCC], 2019).
For 24 hours prior to the PET/CT scan, patients are advised to avoid strenuous activities such as running or cycling, as these activities can impair the quality of the images obtained. Since the PET/CT scan measures the uptake of glucose, strenuous activity can lead to increased uptake of the radiotracer in strained and recovering muscles, which increases the likelihood of false-positive results. Patients are advised to fast for six to eight hours before the scan and follow a low glucose, low carbohydrate diet for 24 hours prior to the scan. On the day of the scan, the patient's fasting blood glucose (FBG) will be obtained, and it should be between 70 and 199 mg/dL for the highest quality imaging results. If the FBG is too high, the scan will be of poor quality, thereby interfering with the clinical benefit and accuracy of the results. Typically, patients with FBG levels of 200 mg/dL or above will be referred back to the ordering provider for glucose management, and their scan will be rescheduled. All patients should be advised to avoid taking any antihyperglycemic medications, including insulin, within four hours of the scheduled scan. If insulin or diabetic medications are taken too close to the FDG injection time, too much FDG will collect in the muscles instead of throughout the tissues where it should flow. As demonstrated in Figure 7, the PET/CT scanner resembles a CT scanner, which looks like a large doughnut. The patient will lay down on the table, usually in the supine position, and should be advised to lay very still. The exam table will move slowly through the scanning ring, and the test usually takes 25 to 40 minutes (Thayalan, 2014).

Following the scan, patients should be advised that some security equipment can detect radioactivity, setting off radiation alarms, such as radioactive detectors in police cars and airport security. Therefore, most patients are given a card stating that they had a test performed with a radioactive tracer. Patients should also be advised to limit close contact with infants and pregnant women for at least 12 hours following the scan (MSKCC, 2019).
Single Photo Emission Computed Tomography (SPECT)
SPECT is a nuclear medicine imaging test that is very similar to PET/CT scans as it integrates CT technology and a radiotracer administered via injection. The primary distinction between PET/CT and SPECT imaging is the type of radiotracer that is used. In SPECT imaging, the radiotracer stays within the bloodstream rather than being absorbed by tissues and organs. SPECT imaging is largely focused on areas where blood flows; it is used to evaluate blood flow to surrounding tissues and organs, and how the organs are functioning. SPECT studies are most commonly used to diagnose or evaluate heart and brain disorders but may also be used to assess certain types of bone disorders. With regards to the heart, SPECT imaging can be used to detect blockages within the coronary arteries, detect damage to the myocardium (heart muscle) resulting from a heart attack, and determine if the heart is pumping blood adequately, particularly when it is stressed. With brain functioning, SPECT studies may be ordered to evaluate for dementia, as well as the location and etiology of a stroke by visualizing how blood flows through veins and arteries in the brain. It can be used to diagnose areas of ischemia (blood deprivation) within the brain following a stroke or as a result of a tumor. These studies are also used in epilepsy, for detecting and identifying seizure activity, or to evaluate areas of bone healing and assess for hidden bone fractures, such as spondylolysis (a stress fracture in the spine) (Mayo Clinic, 2019c).
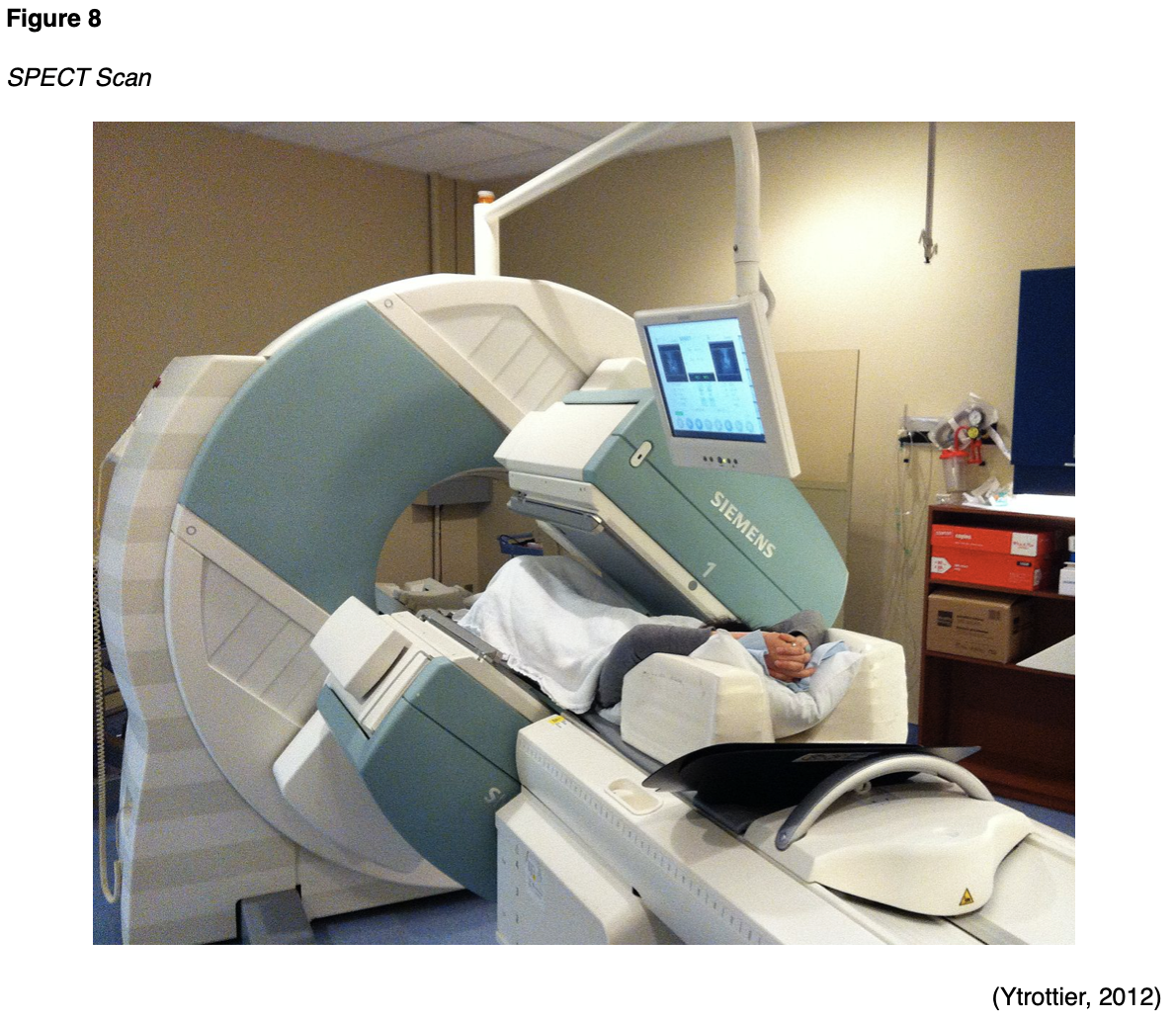
During a SPECT scan, the patient is usually placed in the supine position on the examination table and asked to lie very still for the duration of the test. Figure 8 depicts a patient undergoing a total body SPECT scan to evaluate their bones. After the radiotracer is injected, a special device called a gamma camera rotates around the patient. The device accumulates pictures which are then used to construct 3D images of the distribution of the radiotracer. The resulting images disclose information regarding blood flow and target organ function (SNMMI n.d.). Unlike PET/CT scans, which primarily use FDG as the radiotracer, SPECT studies can use various types of radioisotopes depending upon the disease suspected and the area of study. Patients are advised to fast for four to six hours prior to the scan, which can take up to two and a half hours to complete. Following the scan, patients are advised to maintain increased oral hydration for about two days to flush the radioactive material from the body. Otherwise, there are generally no special discharge instructions (American Heart Association [AHA], 2015c).
Thyroid Scintigraphy and Radioactive Iodine Uptake (RAIU) Test
There are two types of nuclear medicine imaging tests of the thyroid; thyroid scintigraphy, also called the thyroid scan, and radioactive iodine uptake (RAIU) test. Both scans use a small amount of radioactive iodine, usually in the form of I-123, as the thyroid gland is the only tissue within the body that absorbs and holds onto iodine. The radiation emitted by I-123 is harmless to thyroid cells, and it can be detected externally through the use of thyroid scanning. There are rare instances where I-131 may be used with RAIU scans, but I-131 destroys thyroid cells and is commonly reserved for the treatment of thyroid disorders such as overactive thyroid, thyrotoxicosis, and thyroid cancers, which are beyond the scope of this module (ACR, 2019b).
The thyroid scan is ordered to acquire information about the thyroid function and evaluate for abnormalities within the gland, such as nodules, masses, or inflammation (ACR, 2019b). When undergoing a thyroid scan, the I-123 is either injected into a vein within thirty to sixty minutes of the scan or administered orally in the form of a pill or liquid. With oral administration, the I-123 must be given approximately four to six hours before the scan, as this allows the radioactive iodine the opportunity to reach and saturate the thyroid gland. The thyroid scan is painless, and patients are usually positioned lying flat (supine) on an examination table with their head tilted back to extend their neck. A scanner will take images of the thyroid from at least three different angles, and the patient will be asked to lie very still. It takes about 30 minutes to complete a thyroid scan (RadiologyInfo.org, 2019c).
The RAIU scan is performed to evaluate the function of the gland or determine the etiology of an overactive thyroid gland (hyperthyroidism). It may also be used to plan treatment for patients who have thyroid cancer. The RAIU uses a specialized probe to measure how much tracer the thyroid gland absorbs from the blood. In most cases, the RAIU scan is performed alongside with the thyroid scan to show if the radiotracer is evenly spread in the gland. According to the ACR (2019b), while the RAIU scan does have overlapping indications with the thyroid scan, it is considered most useful in the following clinical situations:
- "Differentiating hyperthyroidism from other forms of thyrotoxicosis (such as subacute or chronic thyroiditis and thyrotoxicosis factitial [exogenous thyrotoxicosis]);
- Assessing the necessity and calculating iodine-131 sodium iodide administered activity for patients to be treated for hyperthyroidism" (ACR, 2019b, p.2).
Agents that contain iodine can decrease iodine uptake in the thyroid gland and lead to inaccurate test results. Iodine is hidden in many types of commonly used supplements, over-the-counter agents, and certain prescription medications. Therefore, before the test, a comprehensive medication reconciliation should be performed, and patients must be informed to discontinue thyroid hormones, anti-thyroid medications, and any other medication or dietary supplement that contains iodine. Each medication or supplement has a specified period in which it should be discontinued before the scan, as outlined by the ordering clinician (ACR, 2019b).
In the one to two weeks leading up to the radioactive iodine administration, patients are generally advised to consume a low-iodine diet, avoiding the highest sources of dietary iodine which include salt, grains, cereals, fish, poultry, and milk products (American Thyroid Association [ATA], 2020). The RAIU scan requires administration of the radioactive iodine in liquid or capsule form, and the RAIU scan occurs at two distinct time points: usually at four to six hours following radiotracer administration, and then again at 24 hours. During an RAIU test, the patient is usually seated in an upright position, and a small device called a radioactive detector (uptake probe) is placed against the patient's neck. The uptake probe takes measurements of radioactive iodine uptake, and a gamma camera is used to record pictures of the thyroid gland. Both instruments work to detect and record the distribution of the radioactive material within the thyroid. The RAIU test usually takes several minutes. For patients who undergo both the thyroid scan and the RAIU scan, the oral route is generally preferred, as it can be used for both tests and does not require a second dose of the radiotracer (RadiologyInfo.org, 2019c). Following the test, patients should be advised that the majority of radioactive material is cleared from the body within one to two days. No special precautions need to be taken as I-123 is harmless to thyroid cells. The ACR (2019b) states it is safe to use radioactive iodine in patients who report iodinated contrast allergies or seafood allergies, as the reaction is to the compound containing iodine and not the iodine itself (ACR, 2019b).
Skeletal Scintigraphy (Bone Scan)
Skeletal scintigraphy is also called a bone scan, and it is a type of nuclear medicine imaging test that uses a small amount of radioactive tracer to diagnose and evaluate various bone disorders and conditions (RadiologyInfo.org, 2020). A bone scan can evaluate for bone fractures, primary or metastatic bone neoplasms, bone infections, and other underlying bone pain (ACR, 2017). As demonstrated in Figure 9, a radiotracer is injected into the vein and travels through the bloodstream, emitting radiation in the form of gamma rays. These are detected by a specialized gamma camera and fused with computer images to derive an overall picture of the bones (RadiologyInfo.org, 2020).

A bone scan is capable of detecting changes at the molecular level, thereby allowing the early detection of bone disorders, even at the earliest stages of the disease. Abnormal areas within the bone will take up more or less of the radiotracer, thereby producing brighter or darker areas on the resulting images (RadiologyInfo.org, 2020). In preparation for a bone scan, patients should be counseled to avoid bismuth-containing medications such as Bismuth subsalicylate (Pepto-Bismol) for at least four days before the scan, as these medications can interfere with the results. In addition, patients should be screened for recent x-ray tests utilizing barium contrast material, which can also skew the results. Barium should similarly be avoided for at least four days before the bone scan. The radiotracers used with bone scans take a few hours to circulate through the body and bind to the bones to produce the highest quality images. Patients should be advised that there will be a two to four-hour period between the injection administration and the time in which the scan will take place. During this time, patients will need to consume several glasses of water (usually four to eight) to facilitate the removal of any excess radiotracer from the body. Patients will also be instructed to empty their bladder prior to the scan, as any residual tracer in the bladder can obscure the view of the underlying pelvic bones (RadiologyInfo.org, 2020). Patients will be asked to lie very still on the examination table during the test. A total body bone scan usually takes about one hour to complete. Following the scan, patients may resume normal activities and are advised to increase oral hydration for one to two days to help facilitate the removal of any residual radiotracer circulating in their system. It generally takes 48 hours for all of the radioactive tracer material to be excreted from the body (Cancer.net, 2018).
Bone Densitometry Scan (DXA)
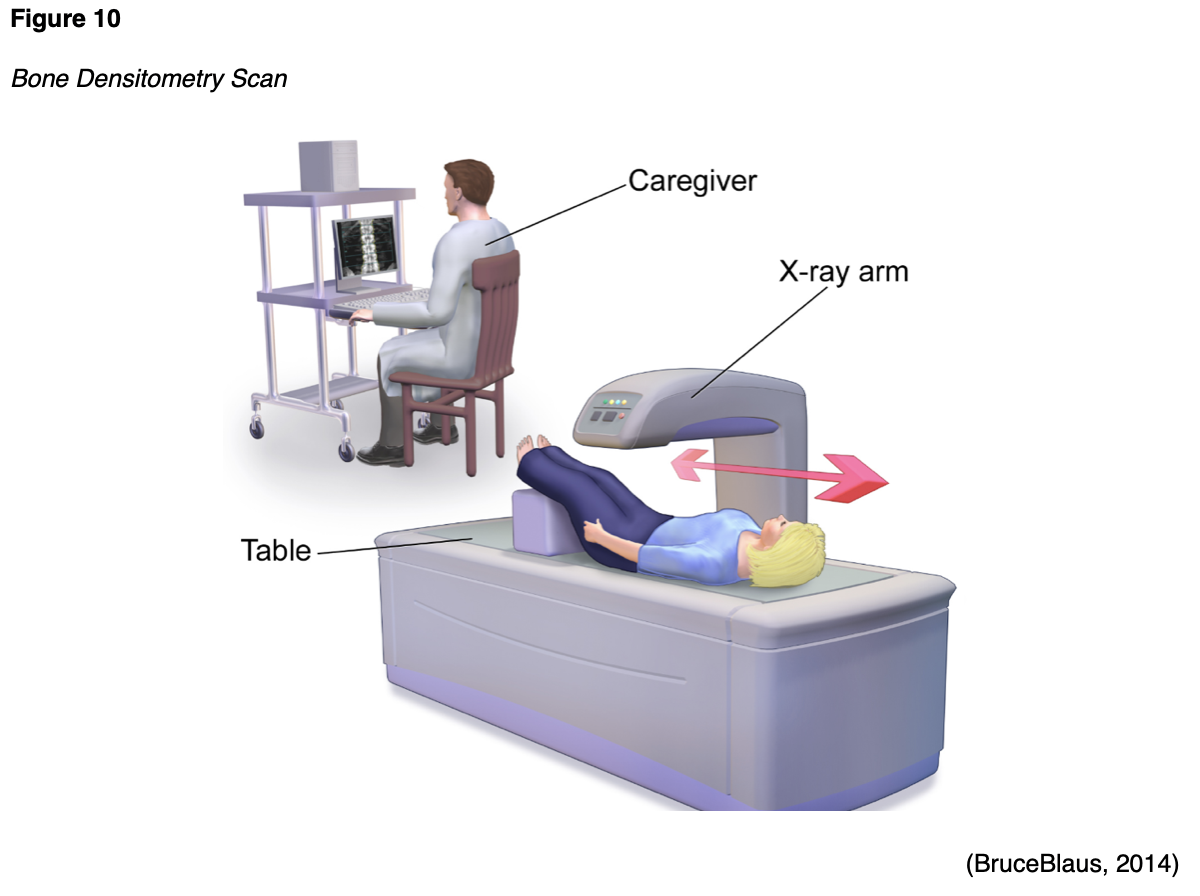
A bone densitometry scan is also referred to as a dual-energy x-ray absorptiometry (DXA) scan, which is vastly different than a bone scan. A DXA scan evaluates the bone mineral density (BMD), or the health and strength of the bones, and assesses fracture risk. It is considered the gold standard test to screen for, diagnose, and monitor osteoporosis, which is a chronic, systemic disease characterized by low BMD, weakening of the bones, and deterioration of bone tissue and architecture (Porter & Varacallo, 2019). Osteopenia is the precursor condition to osteoporosis and is characterized by a lower than normal BMD that is not severe enough to meet the criteria for osteoporosis. People who have osteopenia are at higher risk for developing osteoporosis, but when identified early through screening with a DXA scan, and appropriate action is taken, progression to osteoporosis can be successfully averted (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS], 2019). The DXA scan is a quick, noninvasive, and painless test. As demonstrated in Figure 10, the patient is instructed to lie or sit down for less than ten minutes while the machine scans the body. The test exposes the patient to a small amount of radiation, but less than the amount associated with a chest x-ray (ACOG, 2018).
Multigated Acquisition Scan (MUGA)
A MUGA scan may also be called radionuclide ventriculography (RNV) or radionuclide angiography (RNA), and it is a type of nuclear imaging test that evaluates how well the heart is functioning; particularly the left ventricular ejection fraction (LVEF). The LVEF measures the amount of blood that is pumped out of the heart with each contraction and is expressed as a percentage. The normal LVEF in an adult is 50% to 75%. This test may be performed for various reasons but is most commonly performed as part of a cardiology workup for chest pain, in follow up to an abnormal electrocardiogram (EKG) or echocardiogram scan, or for monitoring patients with heart failure. MUGA scans are also used for monitoring patients undergoing chemotherapy that is toxic to the heart, chest wall radiation, or other cancer treatment regimens that can impair the heart function (AHA, 2015b). During the MUGA scan, small electrodes are placed on the patient's chest, arms, and legs, similar to an EKG, and these are used to track the patient's heartbeat and heart rhythm during the test. A radioactive tracer is injected into a vein, and a gamma camera takes images of the heart at designated time points during each heartbeat. A MUGA scan may be performed at rest, in which the patient lies on the table while the gamma camera takes photographs of the heart. Alternatively, it may be performed as an exercise or "stress" test, in which the patient walks on a treadmill or rides a stationary bicycle to obtain peak activity level and is subsequently asked to stop and lie on the table while the gamma camera takes pictures of the heart. The test takes approximately one to two hours to complete, and patients can generally resume normal activities following the test. Patients are advised to drink plenty of water to help flush the radioactive materials through the renal system after the scan (AHA, 2015b).
Part IV. Diagnostic Imaging Tests without Radiation Exposure
Magnetic Resonance Imaging (MRI)
MRI is a widely utilized diagnostic imaging modality, with an estimated 30 million scans performed in the US each year. MRIs are distinct from other forms of diagnostic imaging as they do not use x-rays or pose radiation exposure to patients and are considered a very safe imaging test. MRIs utilize strong electromagnetic fields (EM) and radio waves to measure how much water is contained in different tissues within the body as a means to generate detailed images of internal organs and tissues (FDA, 2018). The signal in an MRI image comes mainly from the protons in fat and water molecules within the body. MRI is superior to CT scan at identifying the difference between normal and abnormal soft tissue. MRIs can be performed to image nearly any body part; each scan follows a specific protocol depending upon the clinical concern and may be performed without or without GBCAs (Ibrahim et al., 2020).
MRI scans are performed for numerous indications and are considered one of the most frequently performed imaging tests of the brain and spinal cord to diagnose aneurysms, multiple sclerosis, strokes, herniated discs, and tumors. MRI scans can also be performed to evaluate the heart and blood vessels, structural abnormalities in the aorta, the thickness of the heart walls, and damage caused by heart disease. MRI is used extensively in orthopedics to evaluate for bone and joint conditions, such as torn ligaments or cartilage. In women with dense or fibrotic breasts, MRI may be the preferred screening modality in place of mammography due to superior evaluation of dense soft tissues (Mayo Clinic, 2019b).
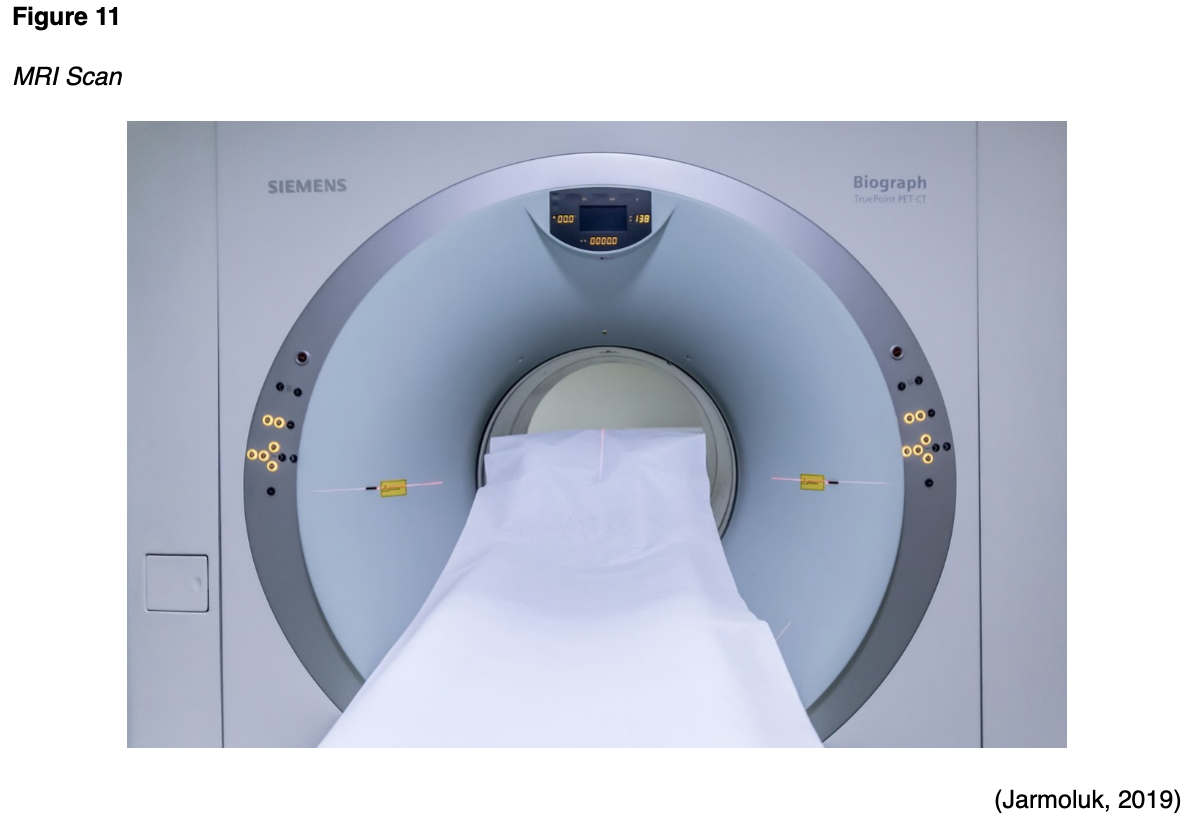
Patients undergoing MRI scans of the abdomen or soft tissue pelvic structures are usually advised to remain NPO for six hours before the scan. Prior to an MRI of the prostate, or to evaluate for a vaginal/rectal fistula, a bowel preparation with an enema may be necessary. However, for all other MRIs, there are generally no special instructions that need to be followed prior to the scan. The MRI scanner is essentially a large magnet, and they can vary in strength, measured in a unit called Tesla (T). Most modern MRI scanners are 1.5 to 3T. To put this into context, an MRI that is of 3T strength is about 60,000 times stronger than the earth's magnetic field. During an MRI, an electric current creates a temporary magnetic field within the patient's body, and radio waves are sent from and received by a transmitter and receiving device within the machine. These signals are used to generate images of the scanned area of the body (FDA, 2018). Patients are advised to lie very still on the table, as motion can create artifact and reduce the clarity of the images. The table slides into the MRI machine, which is somewhat similar in appearance to a CT scanner, as demonstrated in Figure 11. However, an MRI scanner is deeper and narrower than a CT scanner and poses a heightened risk for claustrophobia and anxiety in some patients, who may require a sedative before the scan to ensure relaxation and to manage anxiety. Patients should be advised that they will hear loud noises that sound like thumping and banging throughout the examination, but these are harmless sounds produced by the machine; some patients prefer to wear earplugs during the scan (Ibrahim et al., 2020). An MRI scan can take anywhere from 20 to 90 minutes, depending on the part of the body being imaged (FDA, 2018).
While there is no risk for radiation exposure with MRIs, there are serious risks for potential injury. Due to the powerful EM fields, the MRI machine can propel magnetic objects toward the center of the machine at dangerous speeds, including medically implanted hardware, such as cardiac pacemakers and infusion pumps. The RF field can also cause tissue heating and burning, particularly in the presence of implanted devices, which can also heat internally (Ghadimi & Sapra, 2019). All patients must undergo screening evaluations before testing to assess for the presence of metal or any type of implanted hardware. The FDA has received reports of serious adverse events associated with implantable pumps in the MRI setting, such as pump malfunction, including bolus dose, overdose, or pump failure, and some of these injuries have been fatal. According to the FDA (2017), only implantable infusion pumps labeled "MR Conditional" may be safely used within an MRI environment, and only under the specified conditions of safe use as outlined by the manufacturer of the individual device. During the screening, patients must also be asked if they have ever welded without eye protection or had any facial injury with metal. If the patient responds yes, then an orbit x-ray must be taken to ensure there is no hidden metal in the orbits prior to the MRI (Ghadimi & Sapra, 2019).
Given the strength of the EM fields, the below list is comprised of the most common absolute contraindications to MRI. However, it should be noted that given advancements in technology and newer medical devices, some of these may be compatible. It is critical to refer to the manufacturer's instruction of the device when determining safety and compatibility with MRI imaging.
- Cardiac implantable electronic devices (CIED) such as pacemakers, implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices;
- Metallic intraocular foreign bodies;
- Implantable neurostimulation systems;
- Cochlear implants;
- Catheters with metallic components (Swan-Ganz catheter);
- Metallic fragments such as bullets, shotgun pellets, and so forth;
- Cerebral artery aneurysm clips;
- Magnetic dental implants;
- Tissue expanders;
- Medication patches, hearing aids, body piercing, external drug delivery pumps (insulin pumps), and artificial limbs are all contraindicated and must be removed prior to the MRI (Ghadimi & Sapra, 2019).
Additional consideration must be given to the following situations prior to performing the MRI scan:
- Patients with programmable shunts must be informed that they have to reprogram their shunt with their provider following the MRI;
- Patients with inferior vena cava (IVC) filters that are of unknown composition, must wait six weeks following implantation and can only be scanned with a 1.5T MRI scan;
- Patients with Harrington rods (stainless steel surgical device) can only undergo an MRI on 1.5T scanners;
- Tattoos should be greater than six weeks old, and ice packs or padding should be used against any tattoo that is in contact with the bore of the scanner or the MRI coil, and patients must be educated to immediately report any warm sensation that develops around the tattoo site (Ghadimi & Sapra, 2019).
Ultrasound
Ultrasound is a safe, noninvasive imaging modality that uses soundwaves to generate images of internal body structures. Also referred to as sonography, ultrasound does not use x-rays or ionizing radiation and can be used for diagnostic and therapeutic indications. Ultrasound images are obtained by placing a small probe (transducer) and gel on the skin. The transducer produces soundwaves at very high frequencies, which exceed the threshold of human hearing. These high-frequency soundwaves travel from the transducer through the gel and into the body, and then the soundwaves are used to generate images on a computer. Images are captured in real-time, which allows for the evaluation of the structures and movement of the body's internal organs, including blood flow through vessels. Transducers may be placed external to the body on the skin, such as with a fetal ultrasound, which is demonstrated in Figure 12. To optimize image quality, some transducers can also be placed directly inside the body via the vagina, GI tract, or blood vessels. As shown in Figure 13, a transducer is placed inside the vaginal canal of a nonpregnant female to enhance the visualization of the uterus and ovaries (NIBIB, 2016).
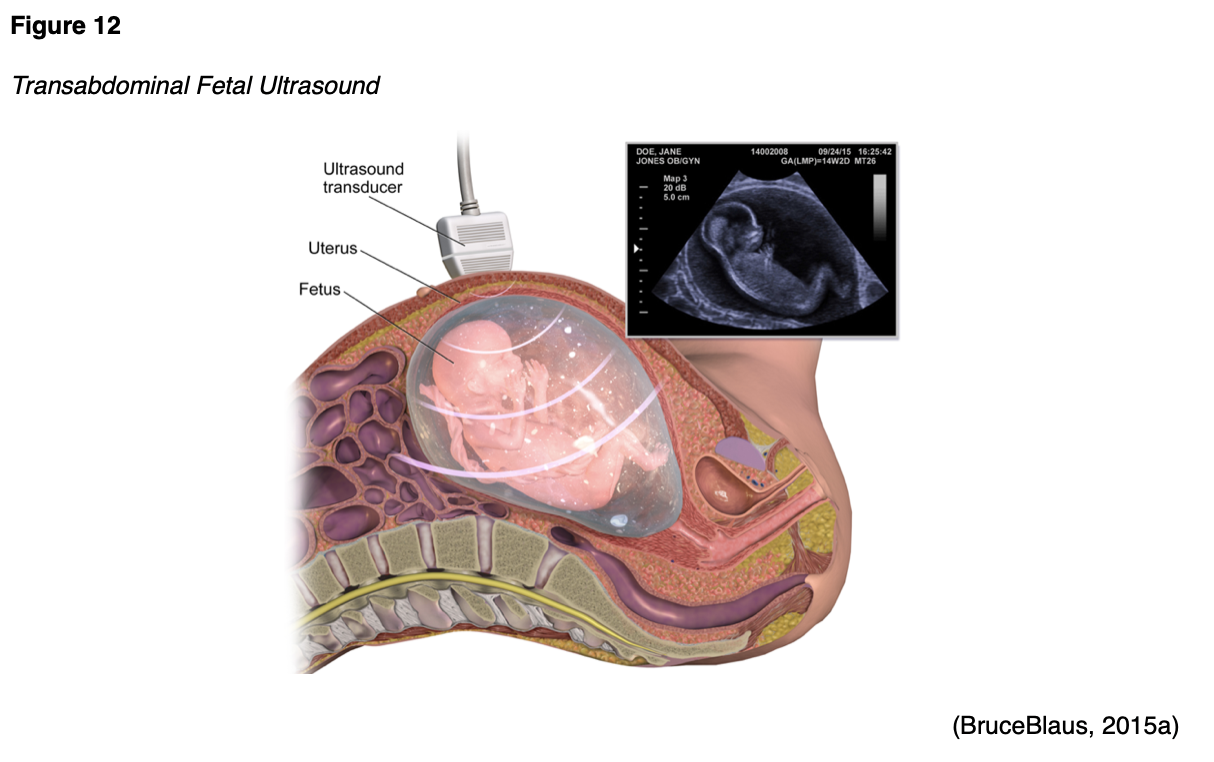
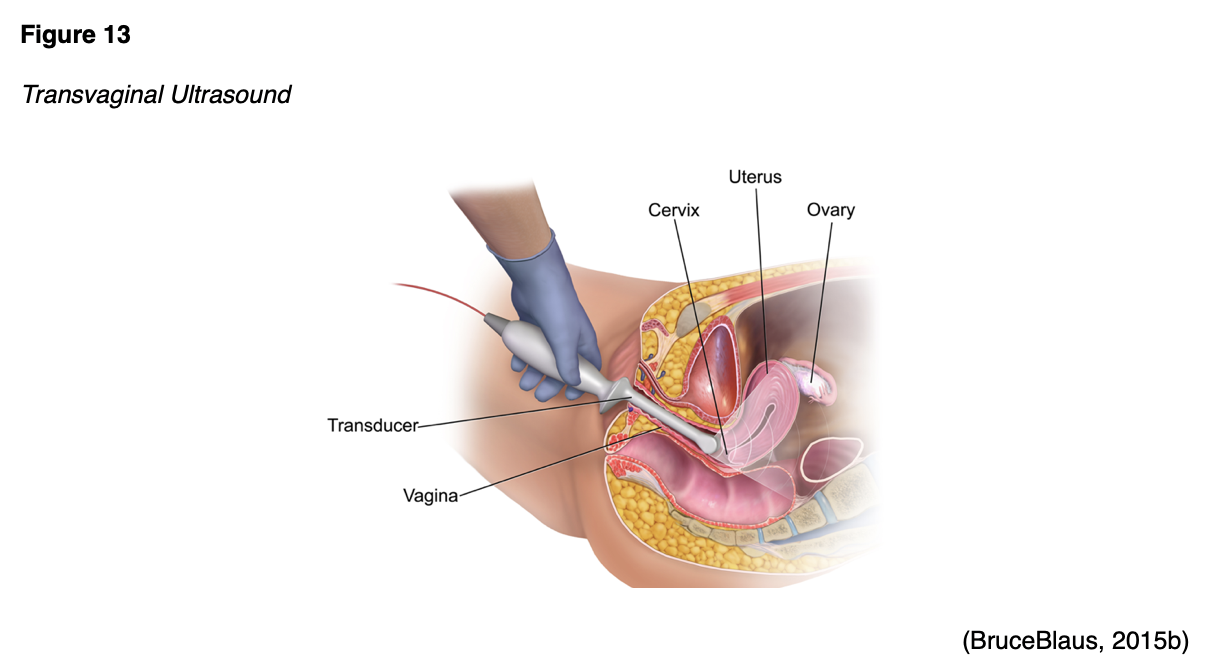
Conventional ultrasound displays images in flat sections of the body, but advancements in technology have led to the development of 3-D ultrasound that allows for the formatting of sound wave data into 3-D images. The most widespread use of a 3-D ultrasound is to assess a developing fetus, and the distinction between the resultant images generated from a conventional sonogram versus a 3-D sonogram is demonstrated in Figure 14 (NIBIB, 2016).
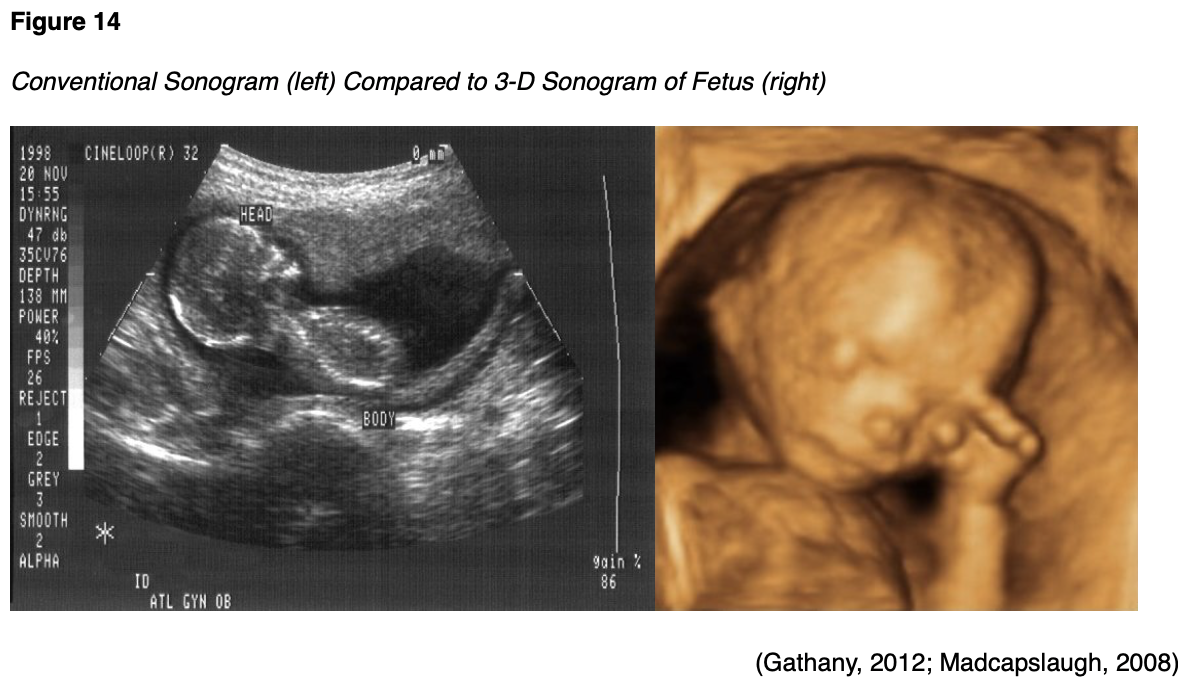
Ultrasound can be performed for diagnostic or therapeutic reasons. Therapeutic ultrasound interacts with tissues in the body to modify or destroy them. For example, therapeutic ultrasound may be used for ablative functions such as dissolving blood clots or delivering drugs to specific locations within the body. Diagnostic ultrasound is used to evaluate the internal organs and structures, movement, and velocity of tissue or blood, as well as any other abnormalities (RadiologyInfo.org, 2018b). Doppler ultrasound is a specialized technique that evaluates blood flow through the arteries and veins in the body and can be used to evaluate for blockages to blood flow (such as thrombosis, deep vein thrombosis, or other blood clots), narrowing of vessels (such as narrowing of the carotid arteries which increases the risk for stroke); tumors or masses; or reduced or absent blood flow to organs, such as the testes or ovaries (RadiologyInfo.org, 2018b).
Most ultrasounds require no special preparation prior to the exam. However, ultrasounds ordered to evaluate the abdominal structures such as the gallbladder generally require patients to fast for four to eight hours before the scan. For ultrasounds evaluating the bladder, as well as many prenatal ultrasounds, the patient may be asked to drink four to six glasses of water prior to your exam and avoid urinating so that the bladder is full and distended for optimal viewing (NIBIB, 2016).
Echocardiogram
An echocardiogram is an ultrasound of the heart. It is a safe and effective cardiac imaging test that does not pose any radiation exposure. Utilizing the same sound wave technology as outlined in the above section, an echocardiogram provides critical information about the heart's functioning and structures, including the valves, chambers, walls, and blood vessels. Similar to a MUGA scan, an echocardiogram also provides information regarding the heart's blood pumping strength, through measurements of the LVEF. As demonstrated in Figure 15, the test is usually performed with the patient lying on their back or their left side. Some patients will also be connected to an EKG to monitor their heartbeat and heart rhythm during the exam. The transducer and gel are applied to the chest and provide real-time images of the heart in motion. There are generally no risks associated with the test, and there are no special preparation instructions (AHA, 2015a).
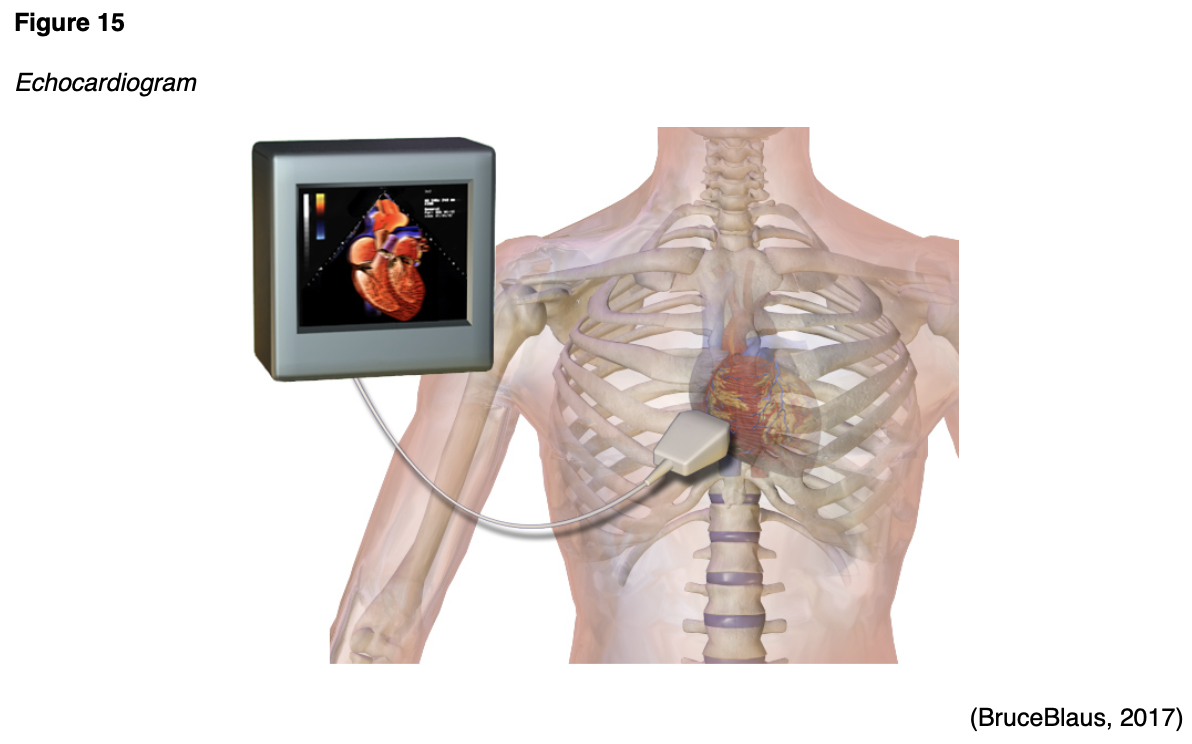
References
Agency for Healthcare Research and Quality. (2019). Radiation safety. https://psnet.ahrq.gov/primer/radiation-safety#Radiation-Risks-Associated-With-Diagnostic-Imaging
Ali, F., Mangi, M. A., Rehman, H., & Haluski, E. (2017). Use of carbon dioxide as an intravascular contrast agent: A review of current literature. World Journal of Cardiology, 9(9), 715-722. https://doi.org/10.4330/wjc.v9.i9.715
American Association of Physicists in Medicine. (2019). AAPM position statement on the use of patient gonadal and fetal shielding (PP 32-A). https://www.aapm.org/org/policies/details.asp?id=468&type=PP¤t=true
American College of Obstetricians and Gynecologists. (2017). ACOG committee opinion: Guidelines for diagnostic imaging during pregnancy. Obstetrics & Gynecology, 130(4), e210-e216. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2017/10/guidelines-for-diagnostic-imaging-during-pregnancy-and-lactation.pdf
American College of Obstetricians and Gynecologists. (2018). Osteoporosis. https://www.acog.org/Patients/FAQs/Osteoporosis?IsMobileSet=false
American College of Radiology. (2017). ACR–SPR practice parameters for the performance of skeletal scintigraphy (bone scan). https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Skeletal-Scint.pdf
American College of Radiology. (2018). ACR-SPR practice parameters for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Pregnant-Pts.pdf
American College of Radiology. (2019a). ACR endorses AAPM position on patient gonadal and fetal shielding. https://www.acr.org/Advocacy-and-Economics/Advocacy-News/Advocacy-News-Issues/In-the-June-8-2019-Issue/ACR-Endorses-AAPM-Position-on-Patient-Gonadal-and-Fetal-Shielding
American College of Radiology. (2019b). ACR-SNMMI-SPR practice parameter for the performance of scintigraphy and uptake measurements for benign and malignant thyroid disease. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Thy-Scint.pdf
American College of Radiology. (2020). ACR manual on contrast media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
American Heart Association. (2015a). Echocardiogram (echo). https://www.heart.org/en/health-topics/heart-attack/diagnosing-a-heart-attack/echocardiogram-echo
American Heart Association. (2015b). Radionuclide ventriculography or radionuclide angiography (MUGA scan). https://www.heart.org/en/health-topics/heart-attack/diagnosing-a-heart-attack/radionuclide-ventriculography-or-radionuclide-angiography-muga-scan
American Heart Association. (2015c). Single photon emission computed tomography (SPECT). https://www.heart.org/en/health-topics/heart-attack/diagnosing-a-heart-attack/single-photon-emission-computed-tomography-spect
American Thyroid Association. (2020). Low iodine diet. https://www.thyroid.org/low-iodine-diet/
Anderson Air Force Base. (2008). Radiation safety practice: x-ray with protective lead apron shielding. [image]. https://www.andersen.af.mil/News/Articles/Article/415997/base-dental-clinic-improves-capabilty-in-areas-of-time-and-efficiency-by-going/
Arleo, E. K., Hendrick, R. E., Helvie, M. A., & Sickles, E. A. (2017). Comparison of recommendations for screening mammography using CISNET models. Cancer, 3673-3680. https://acsjournals.onlinelibrary.wiley.com/doi/epdf/10.1002/cncr.30842
Beckett, K. R., Moriarity, A. K., & Langer, J. M. (2015). Safe use of contrast media: What the radiologist needs to know. Radiographics, 35(6), 1738-1750. https://doi.org/10.1148/rg.2015150033
Blausen Medical. (2017). Components of conventional x-ray [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Projectional_radiography_components.jpg
Blausen Medical Communications, Inc. (2013). Bone scan [image]. https://commons.wikimedia.org/wiki/File:Blausen_0098_BoneScan.png
BruceBlaus. (2014). Bone densitometry scan [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Blausen_0095_BoneDensitometryScan.png
BruceBlaus. (2015a). Fetal ultrasound [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Fetal_Ultrasound.png
BruceBlaus. (2015b). Vaginal ultrasound [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Vaginal_Ultrasound.png
BruceBlaus. (2017). Echocardiogram [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Echocardiogram.png
Brudersohn. (2012). PET/CT scan [image]. Wikimedia. https://en.m.wikipedia.org/wiki/File:PET-CT_Siemens_Biograph01.jpg
Cancer.net. (2018). Bone scan. https://www.cancer.net/navigating-cancer-care/diagnosing-cancer/tests-and-procedures/bone-scan
The Centers for Disease Control and Prevention. (2015a). Electromagnetic spectrum: Ionizing radiation. https://www.cdc.gov/nceh/radiation/ionizing_radiation.html
The Centers for Disease Control and Prevention. (2015b). Health effects of radiation: Health effects depend on the dose. https://www.cdc.gov/nceh/radiation/dose.html
The Centers for Disease Control and Prevention. (2015c). Radiation safety. https://www.cdc.gov/nceh/radiation/safety.html
The Centers for Disease Control and Prevention. (2015d). What is radiation? The electromagnetic spectrum. https://www.cdc.gov/nceh/radiation/spectrum.html
The Centers for Disease Control and Prevention. (2016). Radiation in medicine fluoroscopy. https://www.cdc.gov/nceh/radiation/fluoroscopy.html
The Centers for Disease Control and Prevention. (2019). Radiation in medicine: Medical imaging procedures. https://www.cdc.gov/nceh/features/medical-imaging-procedures/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffeatures%2Fmedical-imaging-procedures%2Findex.html
Davenport, M. S., Perazella, M. A., Yee, J., Dillman, J. R., Fine, D., McDonald, R. J., Rodby, R. A., Wang, C. L., & Weinreb, J. C. (2020). Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American college of radiology with the national kidney foundation. Radiology, 294(3), 660-668. https://doi.org/10.1148/radio.2019192094
Elsayes, K. M., & Oldham, S. A. A. (2014). Introduction to diagnostic radiology. McGraw-Hill Education.
Gathany, J. (2012). Conventional Sonogram (left) Compared to 3-D Sonogram of Fetus (right) [image]. http://www.publicdomainfiles.com/show_file.php?id=13526946615346
Ghadimi, M., & Sapra, A. (2019). Magnetic resonance imaging (MRI), contraindications. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK551669/
Gupta, A., Dosekun, A. K., & Kumar, V. (2020). Carbon dioxide-angiography for patients with peripheral arterial disease at risk of contrast-induced nephropathy. World Journal of Cardiology, 12(2), 76-90. https://doi.org/10.4330/wjc.v12.i2.76
Ibrahim, M. A., Hazhirkarzar, B., & Dublin, A. B. (2020). Magnetic resonance imaging (MRI) gadolinium. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482487/
International Atomic Energy Agency. (n.d.a.). Computed tomography – what patients need to know. Retrieved April 17, 2020 from https://www.iaea.org/resources/rpop/patients-and-public/computed-tomography
International Atomic Energy Agency. (n.d.b). X-rays - what patients need to know. Retrieved April 18, 2020 from https://www.iaea.org/resources/rpop/patients-and-public/x-rays#1
International Atomic Energy Agency. (2014). Diagnostic radiology physics: A handbook for teachers and students. IAEA Publications. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1564webNew-74666420.pdf
Jarmoluk. (2019). MRI scan [image]. https://www.needpix.com/photo/1153705/mri-magnetic-resonance-imaging-diagnostics-hospital-the-test-research-medical-health-the-disease
Johns Hopkins Medicine. (n.d.a). Barium x-rays (upper and lower GI). Retrieved April 16, 2020 from https://www.hopkinsmedicine.org/health/conditions-and-diseases/barium-xrays-upper-and-lower-gi
Johns Hopkins Medicine. (n.d.b). Fluoroscopy procedure. Retrieved April 16, 2020 from https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/fluoroscopy-procedure
Katzen, J., & Dodelzon, K. (2018). A review of computer aided detection in mammography. Clinical Imaging, 52, 305-309. https://doi.org/10.1016/j.clinimag.2018.08.014
Kolla, B. C., Roy, S. S., Duval, S., Weisdorf, D., Valeti, U., & Blaes, A. (2017). Cardiac imaging methods for chemotherapy-related cardiotoxicity screening and related radiation exposure: Current practice and trends. Anticancer Research, 37(5), 2445-2449. https://doi.org/10.21873/anticanres.11584
Madcapslaugh. (2008). Conventional Sonogram (left) Compared to 3-D Sonogram of Fetus (right) [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:4dsonogram.jpg
Marsh, R. M., & Silosky, M. (2019). Patient shielding in diagnostic imaging: Discontinuing a legacy practice. AJR, 212(4), 755-757. https://doi.org/10.2214/AJR.18.20508
Mayo Clinic. (2019a). Mammogram. https://www.mayoclinic.org/tests-procedures/mammogram/about/pac-20384806
Mayo Clinic. (2019b) MRI. https://www.mayoclinic.org/tests-procedures/mri/about/pac-20384768
Mayo Clinic. (2019c). SPECT scan. https://www.mayoclinic.org/tests-procedures/spect-scan/about/pac-20384925
Memorial Sloan Kettering Cancer Center. (2019). About your PET-CT with FDG tracer. https://www.mskcc.org/cancer-care/patient-education/pet-ct#section-5
National Cancer Institute. (2007). Mammography [image]. https://commons.wikimedia.org/wiki/File:MammographyinprocessGraphic.jpg
National Cancer Institute. (2019). Breast cancer risk in American women. https://www.cancer.gov/types/breast/risk-fact-sheet#r1
National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2019). Osteoporosis. https://www.niams.nih.gov/health-topics/osteoporosis#tab-overview
National Institute of Biomedical Imaging and Bioengineering. (2016). Ultrasound. https://www.nibib.nih.gov/science-education/science-topics/ultrasound
National Institute of Biomedical Imaging and Bioengineering. (2019). What is nuclear medicine? https://www.nibib.nih.gov/sites/default/files/Nuclear%20Medicine.pdf
NithinRao. (2009). CT scan [image]. https://commons.wikimedia.org/wiki/File:Ct-scan.jpg
Porter, J. L., & Varacallo, M. (2019). Osteoporosis. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK441901/
PxHere. (n.d.). Caution x-ray equipment [image]. https://pxhere.com/en/photo/1054776
RadiologyInfo.org, Radiological Society of North America, & American College of Radiology. (2015). Radiation dose to patients from common imaging examinations. https://radltd.com/files/Dose-Reference-Card_web_62215.pdf
RadiologyInfo.org. (2018a). Contrast materials. https://www.radiologyinfo.org/en/pdf/safety-contrast.pdf
RadiologyInfo.org. (2018b). General ultrasound. https://www.radiologyinfo.org/en/info.cfm?pg=genus
RadiologyInfo.org (2018c). What is radiation dose? https://www.radiologyinfo.org/en/info.cfm?pg=safety-hiw_09
RadiologyInfo.org. (2019a). Intravenous pyelogram (IVP). https://www.radiologyinfo.org/en/info.cfm?pg=ivp
RadiologyInfo.org. (2019b). Mammography. https://www.radiologyinfo.org/en/info.cfm?pg=mammo
RadiologyInfo.org. (2019c). Thyroid scan and uptake. https://www.radiologyinfo.org/en/info.cfm?pg=thyroiduptake
RadiologyInfo.org. (2020). Skeletal scintigraphy (bone scan). https://www.radiologyinfo.org/en/info.cfm?pg=bone-scan
Society of Nuclear Medicine & Molecular Imaging. (n.d.). Fact sheet: What is nuclear medicine and molecular imaging. Retrieved on April 17, 2020 from https://www.snmmi.org/AboutSNMMI/Content.aspx?ItemNumber=5648
Spampinato, M. W., Abid, A., & Matheus, M. G. (2017). Current radiographic iodinated contrast agents. Magnetic Resonance Imaging Clinics of North America, 25(4), 697-704. https://doi.org/10.1016/j.mric.2017.06.003
Thayalan, K. (2014). The physics of radiology and imaging. Jaypee Brothers Medical Publishers (P) Ltd
U.S. Department of Labor Occupational Safety and Health Administration. (n.d.). Ionizing radiation control & prevention. https://www.osha.gov/SLTC/radiationionizing/prevention.html#PPE
U.S. Food & Drug Administration. (2016). FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/media/96771/download
U.S. Food & Drug Administration. (2017). Safety concerns with implantable infusion pumps in the magnetic resonance (MR) environment: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/safety-concerns-implantable-infusion-pumps-magnetic-resonance-mr-environment-fda-safety
U.S. Food & Drug Administration. (2018). MRI (magnetic resonance imaging). https://www.fda.gov/radiation-emitting-products/medical-imaging/mri-magnetic-resonance-imaging
Wang, S., Hossack, J. A., & Klibanov, A. L. (2018). Targeting of microbubbles – contrast agents for ultrasound molecular imaging. J Drug Target, 26(5-6), 420-434. https://doi.org/10.1080/1061186X.2017.1419362
Wei, K., Mulvagh, S. L., Carson, L., Davidoff, R., Gabriel, R., Grimm, R. A., Wilson, S., Fane, L., Herzog, C. A., Zoghbi, W. A., Taylor, R., Farrar, M., Chaudhry, F. A., Porter, T R., Irani, W., & Lang, R. M. (2008). The safety of Definity and Optison for ultrasound image enhancement: A retrospective analysis of 78,383 administered contrast doses. Journal of American Society of Echocardiography, 21(11),1202-1206. https://doi.org/10.1016/j.echo.2008.07.019
Ytrottier. (2012). SPECT scan [image]. https://commons.wikimedia.org/wiki/File:SPECT_CT.JPG