About this course:
The purpose of this course is to familiarize the learner with some of the most common geriatric syndromes, outlining their diagnosis and evidence-based management.
Course preview
The purpose of this course is to familiarize the learner with some of the most common geriatric syndromes, outlining their diagnosis and evidence-based management.
At the conclusion of this course, the APRN will be prepared to:
- discuss the risk factors, prevention, diagnosis, and evidence-based treatment guidelines for pressure injuries in older adult patients
- describe the evaluation, diagnosis, and evidence-based treatment guidelines for incontinence in older adult patients
- review the evidence-based guidelines for the prevention of falls and management of syncope and vertigo in older adult patients
- identify the evidence-based guidelines regarding the evaluation and management of functional decline, failure to thrive, and frailty in older adult patients
- recognize the diagnosis of, prevention of, and evidence-based treatment guidelines for acute delirium, as differentiated from chronic dementia in older adult patients
When caring for older adults (65+), the healthcare professional should account for various unique considerations. The healthcare team must be prepared to care for these patients, as the population of Americans over the age of 65 is expected to more than double between 2000 and 2030, increasing from 34.8 million to more than 70.3 million. Best-practice and evidence-based geriatric protocols should be developed and utilized in hospitals, rehabilitation centers, long-term care (LTC) facilities, home-care agencies, and community clinics; these same protocols should be introduced in nursing education programs to enhance familiarity. Advanced practice nurses (APRNs) must function in tandem with the rest of the interdisciplinary team. In fact, the Institute of Medicine (now the National Academy of Medicine) highlighted collaboration as a vital component of care in their Retooling for an Aging America: Building the Health Care Workforce report in 2008. The primary goals of geriatric care should be to promote well-being and optimize the patient’s quality of life (QOL) through continued maintenance of function, dignity, and self-determination (Brown-O’Hara, 2013; Ward & Reuben, 2020).
The Most Common Geriatric Syndromes
Geriatric syndromes do not fall into a particular disease category (e.g., congestive heart failure within cardiology or chronic obstructive pulmonary disease within pulmonology), despite being common among older patients. These conditions impact patients’ QOL, ability to function and live independently, cumulative level of disability, and potentially mortality. The causes of such conditions are often multifactorial (Brown-O’Hara, 2013). While numerous geriatric syndromes exist, this activity will highlight 5 of the most common: pressure injuries, incontinence, falls, functional decline or frailty, and delirium.
Pressure Injuries
The National Pressure Injury Advisory Panel (NPIAP) recommends the term pressure injury instead of the outdated pressure ulcer. Roughly 2.5 million pressure-induced injuries are treated in acute care hospitals in the US each year. These wounds develop when external forces are applied to the skin at a sufficient magnitude and duration to cause tissue injury (Berlowitz, 2020b). Pressure injuries can be chronic and lead to bacteremia, sinus tracts that connect with the bowel or bladder, heterotrophic calcification, systemic amyloidosis, and squamous cell carcinoma (Berlowitz, 2020a). A patient’s tissue morphology and capacity for repair affect their tissue’s resiliency. Shearing forces (gravity’s effect on friction) may also contribute to injury and result in more severe tissue damage. Pressure above the arteriolar pressure (32 mm Hg) limits or prevents the supply of blood—and thus oxygen and nutrients—to tissues. Studies utilizing animal models indicate that irreversible tissue damage results over 2 or more hours with pressure applied over 70 mm Hg. Muscle tissue is the most susceptible to damage, while the dermis is the most resilient. High-stage injuries begin as deep tissue damage adjacent to the bone-muscle interface and do not progress from Stages 1 through 4. The pressure is highest over bony prominences, which bear the patient’s weight (Berlowitz, 2020b). Moisture increases the coefficient of friction, tissue deformation, and shear forces, making deep tissue injury more likely (Berlowitz, 2020b, 2020c).
Risk Factors and Prediction
The primary independent risk factors for pressure-induced injury are immobility, malnutrition, reduced tissue (skin) perfusion, and sensory loss, all of which often describe an older adult patient. Immobility may be prolonged or temporary. Reduced perfusion may be related to hypovolemia, hypotension, vasomotor failure, peripheral artery disease, or vasoconstriction, as associated with certain medications, cardiovascular shock, and heart failure. The loss of sensation may be due to neurological injury (e.g., stroke, spinal cord injury, peripheral neuropathy), dementia, or delirium (Berlowitz, 2020b).
Risk prediction for pressure injury is often complicated. Global measures of disease severity and overall comorbidities such as the Comprehensive Severity Index (CSI), the Acute Physiology and Chronic Health Evaluation (APACHE), and the Laboratory-Based Acute Physiology Score, version 2 (LAPS2) have all been associated with increased risk of pressure injury in certain patient populations (skilled nursing facilities, intensive care units [ICUs], and hospitalized patients, respectively). The published guidelines from NPIAP and their European counterpart suggest the use of a prediction tool, such as the Norton or Braden scales, despite little evidence that the use of these validated tools results in fewer pressure injuries as compared to a comprehensive nursing assessment including the patient history and physical examination with regular (daily) skin inspections. The Norton scale includes 5 subscales (physical condition, mental condition, activity, mobility, and incontinence) which are each given a score between 1 and 4 and then added. Ranging from 5 to 20, a score at or below 14 is considered at-risk. The Braden scale includes 6 subscales (sensory perception, moisture, activity, mobility, nutrition, and friction/shear). Most categories are scored between 1 and 4, except for friction/shear, which has a maximum score of 3. Scores range between 6 and 23, and a score below 19 is considered at-risk. Unfortunately, these scales have a relatively low rate of interobserver reliability unless performed by trained staff, leading to a sensitivity ranging from 0.70-0.90, and specificity ranging from 0.60-0.80. Two studies indicated that the activity and mobility subscores of the Norton scale are sufficient to predict risk, and inclusion of the remaining 3 characteristics may reduce the scale’s predictive performance. Research has identified that the Braden scale is less useful in ICU and surgical patients due to the high rate of immobility in these populations (Berlowitz, 2020b).
Outside of a formal tool, a retrospective study identified low serum albumin, fecal incontinence, and a recent fracture as predictors among immobile (chair- or bed-bound) hospitalized patients. Given the medical preference for prospective as opposed to retrospective data, several studies have attempted to identify risk factors in various different healthcare settings. One such study of over 300 hospital admissions found that pressure injuries correlated with immobility as well as recent stroke and impaired nutrition. A larger study (n=1192) found an increased risk in older patients admitted to the hospital for an acute medical condition with non-blanching erythema or skin trauma at the time of admission, diabetes mellitus (DM), and low hemoglobin. A nationwide survey monitoring over 1,500 LTC facility residents found increased risk among patients with a recent history of pressure injury, a high
...purchase below to continue the course
Prevention
Numerous methods can prevent pressure injuries. Pressure redistribution can be performed through the use of support surfaces and devices. Reactive support surfaces can change their weight distribution when a load is applied and may be powered or nonpowered. In contrast, an active support surface is powered and designed to change its distribution regardless of the applied load. An overlay is made to be placed on top of an existing surface, a mattress is a support surface used with an existing bed frame, and integrated bed systems combine a support surface with a bed frame. Regardless of components, a support system should incorporate features proven to prevent pressure injuries such as air fluidization (i.e., a fluid-like medium achieved by forcing air through beads), alternating pressure (i.e., cyclic changes in pressure redistribution), lateral rotation (i.e., rotation on a longitudinal axis), low air loss (i.e., airflow designed to manage the heat and humidity of the skin), and multiple zones (i.e., areas of the system with unique pressure redistribution characteristics). Other factors that should be considered include cost and ease of use. A Cochrane review of support surfaces found that postoperative injuries were reduced with the use of a pressure-relieving overlay, especially a micropulse overlay, on the operating room table. Foam overlays may cause adverse skin changes, and a meta-analysis combining 3 randomized clinical trials (RCTs) found that Australian Medical Sheepskin may relieve pressure when used on top of a hospital mattress. Research indicates with moderate certainty that pressure injury risk is reduced with the use of a powered active air surface versus a standard hospital mattress and that dynamic support surfaces may be cost-effective for high-risk patient cohorts. Alternative high-specification foam mattresses may provide a singular alternative to standard foam mattresses without the need for an additional purchase. In chair-bound patients, research supports the use of full-seat cushions over donut-shaped cushions. Cushions composed of air, viscous fluid and foam, or gel and foam are superior to standard segmented foam cushions (Berlowitz, 2020c).
Patient positioning and repositioning are crucial for injury prevention, although most recommendations are based on theoretical rationale, as the available data are of relatively low quality. Pillows or foam (e.g., wedges) may prevent breakdown at the knees and ankles in patients with lower extremity immobility. Heel protectors can prevent breakdown at the heels, or pillows should be placed under the lower legs to “float” the heels. The head of the bed should be kept at or below 30 degrees to avoid pressure on the greater trochanter when side-lying and to prevent injury due to sliding or friction when supine. Patients should be repositioned gently to maintain microcirculation and decrease interface pressure. Chair-bound patients should be repositioned hourly using seat tilting, wheelchair pushups, or monitoring devices as a reminder if needed. Bed-bound patients can typically be repositioned in 2-hour intervals, with adjustments based on the mattress quality/construction, use of a support surface, the patient’s activity level, ability to reposition themselves, presence of existing tissue damage/injury, and other risk factors. The patient should be repositioned repeatedly from their back (supine) to their side and then to the other side; an assistive device should be utilized to reduce friction and shear as needed. The evidence supporting the use of mechanized beds designed for continuous rotation along a longitudinal axis is insufficient at this time to recommend their use (Berlowitz, 2020c).
Supportive patient interventions also prevent pressure injury development. The most important of these is to improve the patient’s mobility by using an early mobilization program, physical therapy (PT), providing pharmacological treatment for severe spasticity, and limiting the use of sedatives or other medications contributing to immobility. Skin perfusion can be optimized by increasing cardiac contractility and avoiding hypotension, hypovolemia, and the use of vasoconstrictive agents. Patients with severe peripheral artery disease should be evaluated by a vascular surgeon for consideration of revascularization. Skin assessments should be thorough (e.g., an inspection of skin color and palpation for skin temperature, turgor, moisture status, and integrity), well-documented, and occur daily. The skin should be kept dry and cleaned with a pH-balanced cleanser and warm (not hot) water to avoid irritation. Vigorous massage over bony prominences should be avoided. Moisturizers containing fatty acids should be used to avoid excessive dryness and scaling, protect against friction and pressure, and reduce hyperproliferative skin growth, especially over the sacrum. Excess heat increases the likelihood of injury by transferring the heat to deeper tissues, while excess moisture increases friction, thereby contributing to shear forces on deeper tissues. Incontinence can be managed using absorbent pads or loosened adult briefs to allow air circulation along with consistent cleansing in patients with intact skin integrity, but catheterization may be needed while treating an acute wound. Multilayer silicone foam dressings applied over a bony prominence may help prevent pressure injury formation. Nutritional assessments should be performed to ensure adequate nutrition, especially regarding caloric and protein intake. Unless contraindicated, patients should maintain a daily protein intake of 1.2-1.5 g/kg, and any nutritional deficiencies should be addressed. Continuous pressure mapping may have limited benefit, as evidence for its effectiveness outside of an ICU setting is lacking (Berlowitz, 2020c).
Diagnosis and Staging
Superficial moisture-induced injuries, skin tears, tape burns, perineal dermatitis, or excoriation injuries should not be diagnosed or labeled as pressure injuries. Wounds should be assessed for size (e.g., depth, width, and length), the presence of sinus tracts, necrotic tissue, or exudate. All findings should be documented vigilantly, including granulation, which indicates wound healing. Photographs are often helpful for staging. Infection delays wound healing and may be indicated by local signs (e.g., erythema, warmth, tenderness, purulent drainage, or odor) or systemic indications (e.g., fever, leukocytosis). Wound infections may contain resistant pathogens, necessitating culture and sensitivity testing to ensure appropriate treatment (Berlowitz, 2020a).
The NPIAP classification system for wound staging was last revised in 2016 and remains the most commonly used option in the US. This system can be used to describe a pressure injury during the initial assessment but should not be applied to traumatic injuries, moisture-related injuries, incontinence injuries, adhesive injuries, or dermatitis. Reverse staging, or adjusting the staging as the injury heals, is not recommended by the NPIAP. Prior to Stage 1, an area of injury may develop altered sensation, temperature, or firmness. The NPIAP stages include (Berlowitz, 2020a) the following:
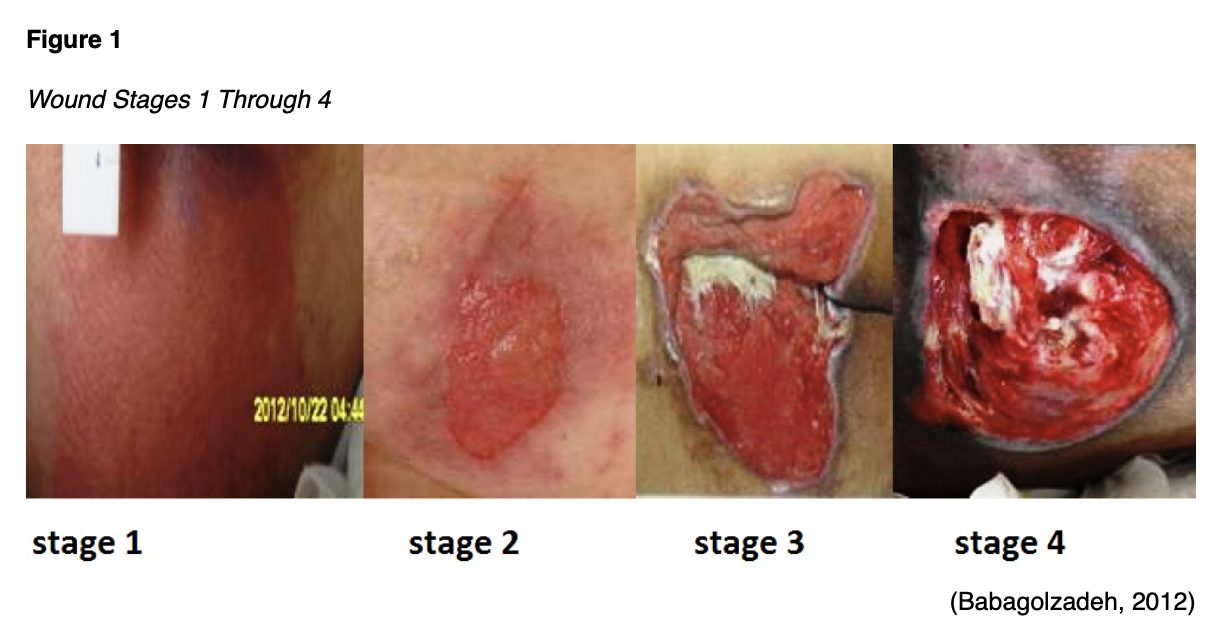
- Stage 1 wounds contain intact skin with an area of non-blanchable erythema (see Figure 1).
- Stage 2 wounds demonstrate exposed dermis due to partial-thickness loss of skin. The wound bed is pink or red and may contain a serum-filled blister. No adipose or deeper tissues are visible, and no eschar, slough, or granulation tissue is present (see Figure 1).
- Stage 3 wounds involve full-thickness loss of skin, exposing the underlying adipose tissue but not fascia, muscle, tendon/ligament, or bone. Granulation tissue and epibole (rolled edges) may be evident, along with eschar or slough. These wounds should be assessed for undermining and tunneling (see Figure 1).
- Stage 4 wounds result from full-thickness skin and tissue loss, exposing the underlying fascia, muscle, tendon/ligament, cartilage, or bone. Epibole, undermining, and tunneling are common, and slough or eschar may be present (see Figure 1).
- Unstageable wounds are full-thickness wounds (Stage 3 or 4) that are currently obscured by eschar or slough, making the depth of the injury indeterminant.
The NPIAP system also allows for the diagnosis of a deep tissue pressure injury, which is characterized by a discolored (deep red or purple) area of intact or broken skin that is preceded by temperature changes and discomfort. The skin may appear firm, spongy, or boggy compared to surrounding tissue. This injury indicates underlying deep tissue damage secondary to intense or prolonged pressure and typically evolves rapidly, revealing a full-thickness pressure injury. Deep tissue pressure injuries can be especially difficult to detect in patients of darker skin tone. Pressure injuries should be categorized based on their deepest point. The amount of subcutaneous adipose tissue can drastically affect the depth of wounds in different areas of the body (Berlowitz, 2020a).
Management
The first step in managing pressure injuries is to eliminate any additional pressure via enhanced prevention techniques such as pressure redistribution and support surfaces. Patient positioning should avoid or minimize pressure on an existing wound. Smokers should be encouraged to quit, with assistance offered and rationale provided regarding the deleterious effect of tobacco smoking on wound healing. Wound care may necessitate debridement (i.e., if necrotic tissue develops) or adjunctive therapies such as negative pressure wound therapy (NPWT or wound vac placement). The patient’s pain level should be assessed and aggressively managed, and psychosocial support should be provided. Pain may be intermittent (e.g., during dressing changes or debridement), continuous, or cyclic, and opioid analgesics may be required for moderate to severe pain. Topical local anesthetics may offer limited benefit but can be trialed, along with topical opioids and nonsteroidal anti-inflammatory drug (NSAID)-releasing dressings. The patient’s progress should be assessed regularly and meticulously documented. Healing scales may be used for documentation purposes, such as the Pressure Ulcer Scale for Healing (PUSH) tool, the Pressure Sore Status Tool (PSST), the Sessing Scale, or the Wound Healing Scale. Documentation should also include the status of the dressing, status of the area surrounding the wound, pain and pain control adequacy, and possible complications. Any clinically evident infection within a pressure injury should be cultured and treated based on sensitivity assays. The care team should include a dietitian to optimize nutrition, especially to promote sufficient caloric and protein intake. Nutritional supplementation may be necessary if oral intake is insufficient, but data do not support its use in patients without nutritional deficiencies. The use of supplemental vitamin C and zinc is common despite a lack of evidence regarding their efficacy (Berlowitz, 2020a).
Stage 1 injuries are typically managed by covering them with a transparent dressing for protection and intensifying the prevention techniques discussed previously. Uninfected Stage 2 injuries require little to no debridement (except for a ruptured blister), but a moist wound bed must be maintained. Therefore, wet-to-dry dressings are avoided in exchange for semi-occlusive (e.g., transparent film) or occlusive (e.g., hydrocolloids or hydrogels) dressings to encourage the digestion of any necrotic tissue by the enzymes naturally found within the wound bed. Dressings are both protective against contamination and helpful in establishing the optimal level of moisture. Excessive moisture in a wound bed leads to maceration and inhibits cell proliferation, while desiccation slows epithelial cell migration. Absorptive dressings include foams and alginates. Dry wounds are best treated using saline-moistened gauze, transparent films, hydrocolloids, and hydrogels (Berlowitz, 2020a).
Stage 3 or 4 injuries and unstageable wounds typically require debridement of necrotic tissue. Stable eschar (dry, adherent, and without fluctuance or erythema) on the heel or an ischemic limb should not be softened or debrided due to proximity to the bone. Stage 3 or 4 wounds with granulation tissue should not be debrided. Necrotic tissue promotes bacterial growth and impairs wound healing. Debridement can be done enzymatically, mechanically, or surgically. Surgical debridement is preferred for areas with extensive necrosis or thick eschar, while minor tissue slough is amenable to mechanical, enzymatic, or biological debridement. Once healthy granulation tissue is present and necrotic tissue has been removed, debridement should be discontinued. In certain circumstances, surgical management of pressure injuries using a skin graft, skin flap, or myocutaneous flap may be indicated, particularly for patients with relatively low surgical risk and whose QOL may improve significantly with rapid wound closure. This decision should be based on patient preference, risk of recurrence, and treatment goals. Prior to surgical closure, the wound must be fully debrided of devitalized tissue and free of infection; the patient’s nutrition should be optimized. Despite this, complications are common (35% in one study monitoring over 1240 individuals), including wound infection, dehiscence, postoperative anemia necessitating blood transfusion, sepsis, urinary tract infection, and pneumonia. In wounds contaminated regularly by fecal material, a colostomy may be considered, although this has questionable efficacy and is associated with a high rate of complications (Berlowitz, 2020a).
Adjunctive therapies include NPWT, electrical stimulation, therapeutic ultrasound, hyperbaric and topical oxygen, and topical growth factors. NPWT increases blood flow and granulation tissue, decreases edema, improves patient comfort, and decreases the labor intensity of wound care. Several small studies indicate enhanced healing with electrical stimulation during which a direct current is applied to the wound bed once or twice daily via a wound overlay. This applied current promotes the proliferation and migration of fibroblasts. Electromagnetic therapy and pulsed radiofrequency energy therapy have shown limited or no evidence of benefit for wound healing. Therapeutic ultrasound evidence is limited, but two RCTs using high-frequency (1 MHz) ultrasound demonstrated a significant reduction in wound surface area versus controls. Hyperbaric oxygen therapy is often advocated despite insufficient evidence of sustained benefit for pressure injuries and a risk of adverse effects, including pneumothorax and seizures. Becaplermin gel (Regranex) is a platelet-derived growth factor applied topically to enhance wound healing that appears to be cost-effective. Platelet-rich plasma (PRP) may enhance wound healing as well (Berlowitz, 2020a).
Treatment plans should address monitoring, establish reasonable treatment goals, and outline a timeline. Over 1,000 LTC patients with pressure injuries were included between 2 studies and followed for 2 years. More than 70% of Stage 2 and 50% of Stage 3 injuries healed within 6 months, while 77% of Stage 4 injuries resolved by 2 years. Non- or slow-healing wounds should be evaluated for infection or reversible causes of ischemia (DM, vascular insufficiency). The PUSH tool, as mentioned earlier, is a validated and easy-to-use healing metric that aligns with the NPIAP system (Berlowitz, 2020a).
For additional information regarding the assessment, diagnosis, and treatment of pressure injuries, please refer to the NursingCE course on Pressure Injuries.
Incontinence
Urinary incontinence is defined as the involuntary leakage of urine. While up to 50% of adult women experience urinary incontinence (38% of women over the age of 60), only 25% to 60% of symptomatic community-dwelling women seek care. In comparison, 21% of men over 65 experience incontinence symptoms, and only 1 in 5 of these seek medical care for the condition. This may be due to embarrassment, lack of knowledge regarding treatment options, and fear of surgery. Although incontinence does not impact mortality, it does affect QOL. Incontinence is associated with depression, anxiety, work impairment, social isolation, sexual dysfunction, perineal infections, falls, fractures, and increased caregiver burden. Evidence suggests these effects may be even greater for male patients. Outside of age, other risk factors for incontinence include obesity, parity and mode of delivery, family history, ethnicity/race, smoking, caffeine intake, DM, stroke, depression, vaginal atrophy, fecal incontinence, hormone replacement therapy, genitourinary surgery, and radiation. The prevalence of incontinence in cognitively impaired adults is 10% to 38% (Clemens, 2019; Lukacz, 2020a).
Types of Urinary Incontinence
Stress incontinence is defined as the leakage of urine from increased intraabdominal pressure (e.g., exertion, sneezing, coughing, laughing) without an urge to void. The risk of stress incontinence increases with high-impact activities, such as running and jumping, and the volume of urine varies. It often affects younger women and is caused by urethral hypermobility or intrinsic sphincteric deficiency (ISD). Urethral hypermobility involves insufficient support of the pelvic floor and vaginal connective tissue to the urethra/bladder neck; this may be caused by high-impact activity or trauma related to vaginal delivery. ISD is the loss of mucosal and muscular tone to keep the urethra closed secondary to neuromuscular damage. This damage may be related to multiple pelvic or incontinence surgeries and can result in severe leakage with minimal abdominal pressure increase. ISD is more difficult to treat and tends to have less favorable surgical outcomes (Lukacz, 2020a). While the condition is less common in men, stress incontinence may develop after mechanical damage to the urethral sphincter during prostate surgery (Clemens, 2019).
Urge incontinence involves the involuntary leakage of urine immediately after or during an urge to void. It is also referred to as overactive bladder (OAB) if accompanied by nocturia and urinary frequency. The risk of urge incontinence rises with impaired functional status, recurrent urinary tract infections (UTIs), and bladder symptoms in childhood (e.g., enuresis). It is more common in men and older women. Leakage can range in amount and is likely related to detrusor overactivity (i.e., uninhibited contractions during bladder filling), although this has been detected in as many as 21% of healthy, continent older adults. Detrusor overactivity may be related to neurological injury, bladder abnormalities, or an increased or altered bladder microbiome but is often idiopathic. Urge incontinence in men may also be related to bladder outlet obstruction (BOO) as a component of lower urinary tract symptoms (LUTS) secondary to benign prostatic hypertrophy (BPH). Men with urge incontinence may describe hesitancy, straining, and an intermittent or slow stream. Patients with symptoms of both stress and urge incontinence have mixed incontinence, which is rare in men but more common in women (Clemens, 2019; Lukacz, 2020a).
Overflow incontinence is a sequela of incomplete bladder emptying leading to continuous urinary leakage or dribbling. It is associated with a weak or inconsistent urinary stream, hesitancy, frequency, and nocturia. If the bladder becomes too full, symptoms of either stress or urinary incontinence emerge. Overflow incontinence is typically related to detrusor underactivity or BOO. While detrusor contractility and efficiency diminish with age, severe underactivity occurs in only 5% to 10% of older adults. The contractility of the detrusor muscle can become significantly impaired in patients with severe acute sustained overdistention of the bladder, Fowler’s syndrome, fibrosis, reduced estrogen levels, peripheral neuropathy, or spinal cord damage affecting the spinal detrusor efferent nerves. These patients will typically have no warning or trigger with urine loss, and leakage may occur with activity or position changes. In women, BOO typically occurs due to urethral compression which may be secondary to fibroids, pelvic organ prolapse, or overcorrection of the urethra following pelvic floor surgery. It may also result from urethral stricture, an external mass or tumor, or uterine incarceration of a retroverted uterus. Patients often describe stress or urge incontinence symptoms with an intermittent or slow stream, hesitancy, incomplete emptying, and straining. A small subset of women with overflow incontinence can develop the condition due to detrusor hyperactivity with impaired contractility (DHIC), a combination of detrusor hyperactivity (similar to above in urge incontinence) with impaired contractility of the bladder, causing incomplete emptying (Lukacz, 2020a). In men, BOO is often related to prostate hypertrophy, as described above, as well as urethral stricture disease, neurologic disorders, or some medications (Clemens, 2019).
Other underlying etiologies for incontinence in female patients include genitourinary syndrome of menopause or vaginal atrophy due to low estrogen levels, leading to a diminished mucosal seal, urethritis, loss of compliance, and irritation. Chronic or acute UTIs can contribute to incontinence during and immediately after the resolution of the infection. Less common urological or gynecologic causes for urinary incontinence in women include urogenital fistulas, urethral diverticula, and ectopic ureters (Lukacz, 2020a). Men may describe post-void dribbling (PVD), which is the leakage of a small amount of urine retained in the urethra immediately after voiding. It is often but not always described in men with other LUTS (Clemens, 2019).
Systemic causes of incontinence include spinal cord dysfunction, stroke, Parkinson’s disease, and normal pressure hydrocephalus. Diabetic neuropathy can contribute to overflow incontinence. Bladder cancer and invasive cervical cancer may present with urinary incontinence. Functional incontinence involves an intact genitourinary system with storage and emptying capabilities, but these patients have a limited ability to toilet themselves due to mobility issues, manual dexterity limitations, or cognitive impairment. Among patients with known cognitive impairment, incontinence is caused or exacerbated by comorbid conditions and medications. Environmental and reversible factors that may exacerbate incontinence include alcohol intake (decreases contractility), caffeine intake (increases contractility), constipation or stool impaction, and certain medications. The list of medications that may contribute to incontinence is lengthy and includes the following:
- antihistamines (first-generation H1 antagonists such as chlorpheniramine [Chlor-Trimeton], hydroxyzine [Vistaril], and diphenhydramine [Benadryl]) decrease contractility via anticholinergic effects
- decongestants such as pseudoephedrine (Sudafed) and phenylephrine (Sudafed PE) increase urethral sphincter tone
- benzodiazepines impair micturition via muscle relaxation
- opioids reduce the sensation of bladder fullness and increase urethral sphincter tone
- antimuscarinics designed to treat overactive bladder (oxybutynin [Ditropan], solifenacin [Vesicare], tolterodine [Detrol], trospium [Sanctura], fesoterodine[Toviaz], and others) decrease contractility via anticholinergic effects
- spasmolytics such as dicyclomine (Bentyl), hyoscyamine (Levsin), glycopyrrolate (Robinul), and others decrease contractility via anticholinergic effects
- antiparkinson medications such as benztropine (Cogentin) and trihexyphenidyl (Artane) decrease contractility via anticholinergic effects
- ACE inhibitors decrease the contractility of the bladder and cause a chronic cough
- alpha-agonists such as midodrine (Orvaten, ProAmatine) and various vasopressors increase urethral sphincter tone
- alpha-1 blockers such as doxazosin (Cardura), tamsulosin (Flomax), and terazosin (Hytrin) decrease urethral sphincter tone
- antiarrhythmics disopyramide (Norpace) and flecainide (Tambocor) decrease bladder contractility via local anesthetic or anticholinergic effects
- diuretics increase urine production, bladder contractility, or rate of emptying
- antidepressants such as serotonin-norepinephrine reuptake inhibitors (SNRIs) like duloxetine (Cymbalta) increase urethral sphincter tone, while tricyclic antidepressants (TCAs) such as amitriptyline (Elavil) and clomipramine (Anafranil) decrease contractility via anticholinergic effects
- antipsychotics such as chlorpromazine (Thorazine), fluphenazine (Prolixin), clozapine (Clozaril), olanzapine (Zyprexa), and risperidone (Risperdal) decrease contractility via anticholinergic effects, stimulate alpha-1 receptors, and have central dopaminergic effects
- skeletal muscle relaxants such as orphenadrine (Norgesic, Norflex) and tizanidine (Zanaflex) decrease contractility via anticholinergic effects
- oral estrogen therapy increases urinary incontinence
- beta-3 agonist mirabegron (Myrbetriq) decreases contractility (Lukacz, 2020a)
The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (known as the Beers Criteria, or BC) was last updated in 2019. The list includes 88 medications or classes of medications. In addition to many of the listed medication warnings, the 2019 BC explicitly warns against combining loop diuretics with alpha-1 blockers for older patients due to an increased risk of incontinence (AGS Beers Criteria Update Expert Panel, 2019; Fixen, 2019).
Evaluation and Assessment of Urinary Incontinence
A thorough history and physical examination should be completed first, evaluating the patient for potentially reversible causes of incontinence as well as underlying conditions and medications that may be contributing. Patients at high risk for incontinence should be screened regularly as opposed to waiting for the patient to bring up the subject. Symptoms should be characterized, including the severity, duration, frequency, and volume of the incontinence. The patient should be asked about any associated hesitancy, precipitating factors, nocturia, intermittent or slow stream, incomplete emptying, continuous leakage, and straining. The 3 Incontinence Questionnaire (3IQ) is a short, standardized form that can distinguish between stress, urge, and mixed incontinence with a sensitivity of 0.75 and a specificity of 0.77. Evaluating a patient’s post-void residual (PVR, or the remaining volume of urine in the bladder after voiding) may help distinguish between overflow incontinence due to detrusor muscle underactivity and mixed urinary incontinence in female patients. A history of systemic symptoms such as fevers, pelvic or flank pain, dysuria, and hematuria may indicate a UTI. Intake of caffeine and alcohol should be assessed, and patients who ingest either or both may want to consider reducing or eliminating them to assess for an associated improvement in their incontinence symptoms. Patients should be asked to consider and then identify the aspects or symptoms that are most bothersome in order to prioritize care based on patient concerns. A voiding diary may help identify patterns related to urinary frequency, volume, and large amounts of fluid intake. Free versions can be found online, or clinics can construct their own based on the information desired. Most are completed over 72 hours and include a column for cataloging the time of day, any fluid intake with the amount, and the volume of voids with an assessment of associated urgency or leaks. Many specialty clinics will request that referred patients complete this diary prior to their initial appointment for the sake of efficiency. This allows the data to be readily available for review during the patient’s clinic assessment, but presupposes that (a) clinic staff can give the patient a copy of the instructions and form electronically, (b) the patient has the ability to print the form at home, and (c) the patient has access to a drugstore where they can purchase a plastic disposable “hat” or jug with which to measure their urine output (Lukacz, 2020a).
During the examination, female patients should be asked to consent to a pelvic exam if their symptoms do not immediately indicate stress versus urge incontinence, if there is suspicion of urinary retention, if they present with systemic symptoms, or if there is evidence of pelvic pathology. A neurological exam may be indicated for any patient who presents with sudden-onset incontinence, especially urge incontinence, or associated neurological symptoms. The exam typically includes an evaluation of bilateral lower extremity strength, reflexes (with long-track signs), and sensation, including perineal sensation. Poor strength with hyperreflexia may indicate an upper motor neuron lesion (i.e., brain or spinal cord), while reduced strength, areas of numbness or paresthesias, and diminished reflexes suggest a lower motor neuron lesion (i.e., exerting pressure on the spinal nerves, not the cord). A urinalysis should be performed on all patients, with a follow-up urine culture indicated for symptomatic patients or those with abnormal urinalysis results (Lukacz, 2020a). Male patients should be asked to consent to a rectal exam to assess the size and consistency of their prostate. The decision to include prostate-specific antigen (PSA) testing should be made via shared decision-making after reviewing the risks and benefits. All patients should undergo a brief abdominal exam evaluating for an overextended bladder or an abdominal mass causing interference or pressure on the bladder (Clemens, 2019).
Additional testing may be indicated. A bladder stress test is an option for female patients with symptoms of stress incontinence. With a comfortably full bladder, the patient is asked to stand and cough or complete a Valsalva maneuver while the examiner observes the opening of the urethra (in women, by separating the labia) for leaking caused by the increased pressure. In patients with mobility impairment or cognitive dysfunction, this test may be easier to perform in the dorsal lithotomy position (laying on the back with feet elevated and abducted out). A positive test (leaking with stress) has a high predictive value for stress incontinence, while a negative result may be related to low urine volume or voluntary inhibition of leakage (Lukacz, 2020a).
If there is clinical concern for urinary retention or overflow incontinence, an uncertain diagnosis, or no significant resolution of incontinence symptoms with initial treatment, then PVR assessment may be indicated. Patients with neurological disease, recurrent UTIs, DM with associated peripheral neuropathy, severe constipation, or pelvic organ prolapse may benefit from PVR assessment. If the residual volume of urine is under 50 mL or below one-third of the voided volume, the value is typically considered within normal limits. A residual volume greater than 150-200 mL warrants additional testing. While PVR can easily be assessed using catheterization, an ultrasound bladder scan is less invasive and more comfortable for the patient. Urodynamic testing with cystometry may be beneficial for a small subset of patients, but for the vast majority of patients with incontinence, this testing is invasive, costly, and likely unnecessary. It has not been proven to improve outcomes in patients with uncomplicated stress incontinence. This testing may be beneficial for patients with overflow incontinence secondary to neurologic conditions or DM, complicated stress incontinence, or mixed incontinence. This test assesses bladder function by objectively indicating the pressure and volume of fluid present during bladder filling, storage, and voiding. In rare circumstances, patients may require a referral to a subspecialist for a urethral mobility evaluation. Other indications that a patient may benefit from a specialist referral include:
- associated abdominal or pelvic pain in the absence of a UTI
- recurrent UTIs (3 in a year or 2 in 6 months)
- hematuria in the absence of a UTI, prostate nodule/induration/asymmetry, or elevated PSA with risk factors for malignancy
- lifelong or severe (multiple heavy pads/briefs daily) incontinence
- suspected vesicovaginal fistula
- urethral diverticula, pelvic mass, or severe prolapse (beyond the hymen) on exam
- new neurologic symptoms with incontinence
- history of pelvic reconstructive surgery or pelvic/prostate irradiation
- persistently elevated PVR volume (> 300 mL), despite successful treatment of underlying causes
- overflow incontinence due to an underlying neurological condition or DM
- chronic catheterization or difficulty inserting a catheter (Clemens, 2019; Lukacz, 2020a)
Management of Urinary Incontinence
The first step in the management of incontinence, once it has been diagnosed and correctly characterized, is to establish treatment priorities based on the patient’s goals of care and managing expectations. The goals should include an improvement in QOL by focusing on the patient’s most bothersome symptoms, but rarely is full continence achieved. Tools to assist the assessment of symptom impact may help focus treatment goals and track efficacy, such as the International Consultation on Incontinence Questionnaire, the King’s Health Questionnaire, Pelvic Floor Distress Inventory, Pelvic Floor Impact Questionnaire, the OAB Questionnaire, and the Patient Global Impression of Improvement (PGII) or Severity (PGIS). Most patients with incontinence, regardless of the cause or type, utilize disposable undergarments or incontinence pads. In the US, these products are easily accessible but may be expensive over time and do not address the underlying cause of incontinence. These products can also lead to contact dermatitis or skin breakdown if they are not changed frequently enough. Men may also utilize an external condom catheter if able, as many find this option preferable to pads/briefs (see Figure 2). These patients should undergo urodynamic testing to assess bladder storage pressures and avoid consequent renal damage. Indwelling or intermittent bladder catheterization is associated with a high risk for infection. As a result, it is reserved for limited instances with few alternatives (Clemens, 2019; Lukacz, 2020c).
Treatment should begin with conservative therapies prior to more invasive or aggressive options. External contributing factors should be addressed first, such as medical conditions or medications that are exacerbating the patient’s incontinence. This should include assessment and treatment for constipation if present (Lukacz, 2020c). Older patients typically experience decreased gastric motility, which may predispose them to constipation and require the use of a bulk-forming laxative such as psyllium (Nguyen et al., 2020). Lifestyle modifications that may be beneficial for those with incontinence include weight loss for obese patients; smoking cessation; avoidance of alcoholic, caffeinated, and carbonated beverages; and maintaining a fluid intake of no more than 64 ounces/day. Patients with nocturia symptoms should avoid drinking within several hours of their bedtime. Pelvic floor (Kegel) exercises can be especially helpful for male and female patients with stress, urge, or mixed incontinence. Exercises should be completed in sets of 8-12 contractions, held for 8-10 seconds each, and repeated 3 times per day. A pelvic floor PT may be helpful for patients who struggle with technique (properly isolating pelvic floor muscles), as they can incorporate biofeedback, electrical stimulation, pulsed magnetic stimulation, or vaginal weights to increase strength. Patients with urge incontinence or those who experience stress incontinence at higher volumes may benefit from bladder training. A bladder diary should be used to identify the patient’s shortest voiding interval, which serves as the initial point for training. Patients should be instructed to void based on the clock at that interval (e.g., every hour). Distraction, mental relaxation, and quick flicks (i.e., rapid-fire pelvic floor contractions) should be used if urgency develops between intervals. Once the patient can successfully avoid leaking for a full day, the interval is increased by 15 minutes, with an end goal of voiding every 3-4 hours while awake without urgency or incontinence episodes. This process may take weeks to accomplish (Lukacz, 2020c). Hypnotherapy and acupuncture are both relatively safe complementary therapies that may be beneficial, although the research regarding their effectiveness for the treatment of incontinence is minimal (Lukacz, 2020b).
Stress Incontinence Management. Treatment for stress incontinence varies depending on the underlying pathology. Female patients with urethral hypermobility require additional support for the urethra, while patients with ISD may benefit from increased blood flow and increased urethral coaptation achieved with pelvic floor exercises and/or surgery (Lukacz, 2020a). Women with vaginal atrophy due to genitourinary syndrome of menopause (GSM) may benefit from topical vaginal estrogen. These are available as creams, rings, gelcaps, or tablets that are typically dosed twice weekly; the ring is dosed every 3 months. Patients may not see results for up to 3 months. Oral (systemic) therapy with estrogen may worsen incontinence and is not recommended (Lukacz, 2020c). Estrogen therapy may be especially effective for patients with stress incontinence related to ISD secondary to increased blood flow (Lukacz, 2020a). Estrogen should be used with caution in patients with a history of estrogen-receptor positive breast cancer, as use is controversial. The decision to use vaginal estrogen preparations should be made in coordination with the patient’s oncologist (The American College of Obstetricians and Gynecologists, 2016). Continence pessaries (Figure 3) are common support devices that are traditionally fitted by a urologic continence specialist or gynecologist, although a disposable over-the-counter option (Impressa) has recently become available. These devices are especially helpful for patients who experience stress incontinence during specific activities (e.g., exercise). No pharmacological treatment options for stress incontinence have been approved by the US Food and Drug Administration (FDA). Duloxetine (Cymbalta) is an antidepressant that may improve stress incontinence by increasing urethral sphincter tone but with a high rate of associated adverse events. While not approved for this indication in the US, it is approved in many European countries. It functions by stimulating pudendal motor neuron receptors, and some studies have shown it may be effective in men with stress incontinence. Imipramine (Tofranil) is a tricyclic antidepressant with insufficient evidence to support its use in patients with stress or mixed incontinence. Some men with stress incontinence in combination with normal bladder capacity and storage function can utilize a penile clamp. These can cause discomfort with consistent use and are chiefly implemented when needed, such as attending an event or eating at a restaurant (Clemens, 2019; Lukacz, 2020c).
Figure 3
Continence Pessaries

A mid-urethral sling is a minimally invasive surgical treatment option with a high curative rate for female patients with stress incontinence and may also be beneficial in patients with persistent mixed incontinence. The surgical risks associated with this treatment option should be taken into consideration and fully explained to the patient. A similar surgical approach has also been adapted for the treatment of stress incontinence in men, which is referred to as a perineal sling. They compress and mobilize the urethra using a synthetic mesh placed transversely through the obturator foramen. Artificial urinary sphincters are the most effective long-term corrective option for men with stress incontinence. The silicone cuff, balloon reservoir, and pump are implanted surgically with success rates ranging from 59% to 90%. Unfortunately, surgical revision may be necessary due to urethral erosion, infection, or device malfunction. Erosion is more common following catheterization, so this should be kept to a minimum, the smallest possible catheter should be utilized, and the sphincter should be deactivated prior to catheterization. Transurethral radiofrequency collagen denaturation is a minimally invasive device-based option that has been proposed but has insufficient evidence to establish its effectiveness. A urethral bulking agent (UBA) may be an option for women with persistent incontinence related to ISD who fail to improve or cannot tolerate surgery and men with mild stress incontinence or who are not surgical candidates. These periurethral injections may be associated with urinary retention and an increased risk of UTI, as well as pain at the injection site. Injections typically need to be repeated to maintain efficacy. There is some evidence that electroacupuncture therapy over 6 weeks may reduce incontinence frequency and volume, but the poor availability of this procedure in the US is significantly limiting (Clemens, 2019; Lukacz, 2020c).
Urge Incontinence Management. Pharmacotherapy for the management of urge incontinence/OAB or urge-predominant mixed incontinence consists of two classes: antimuscarinics and beta-3 adrenergic medications (e.g., mirabegron [Myrbetriq], vibegron [Gemtesa]). This includes treating non-neurogenic OAB in men unrelated to obstruction, reducing bladder contractions triggered by acetylcholine. Antimuscarinics are less expensive, as they are available in a generic form and often attempted first for this reason. They function by increasing bladder capacity and decreasing urgency by blocking acetylcholine’s stimulation of the muscarinic receptors. Several options are available in the US at varying strengths, including darifenacin (Enablex), fesoterodine (Toviaz), oxybutynin (Ditropan, Oxytrol), solifenacin (Vesicare), tolterodine (Detrol), and trospium (Sanctura). Dosing should begin as low as possible and titrated up every 2 to 3 weeks based on patient response and side effects. The use of extended-release formulas may improve adherence and minimize side effects. Anticholinergic side effects are most common with antimuscarinics, including urinary retention, dry mouth, constipation, dizziness, blurred vision, tachycardia, drowsiness, and impaired cognition. Darifenacin (Enablex) and solifenacin (Vesicare) are more selective, which may decrease side effects. Those at high risk or with retention symptoms should have a baseline PVR and occasional repeat testing to assess for urinary retention. If the baseline PVR is elevated, antimuscarinics are not the ideal primary therapeutic choice and should be trialed cautiously. Antimuscarinics are contraindicated in patients with uncontrolled tachyarrhythmias, gastric retention, narrow angle-closure glaucoma, and myasthenia gravis. Antimuscarinics may not be suitable for older patients with dementia due to their anticholinergic effects; however, trospium (Sanctura) does not cross the blood-brain barrier and may not impact cognition substantially. Symptom improvement may take 4 weeks, and the full effect may not appear for 12 weeks. Adherence should be assessed before changing therapies or adjusting medications (Lukacz, 2020b; McVary, 2021; McVary & Saini, 2021).
Mirabegron (Myrbetriq) or vibegron (Gemtesa) may be used as a secondary option for patients with OAB who cannot take an antimuscarinic or do not find them effective. They may also be used in combination with an antimuscarinic in some cases. Due to their favorable side effect profile, they can be a primary treatment for some with OAB, especially for patients with elevated PVRs or concurrently taking a cholinesterase inhibitor (to avoid increasing the anticholinergic effects). They activate the bladder’s beta-3 adrenergic receptors, causing relaxation of the detrusor and increasing bladder capacity. Mirabegron (Myrbetriq) should be dosed at 25 mg to start, increasing to 50 mg after 2-6 weeks if the response is inadequate. Vibegron (Gemtesa) is available only as a 75 mg tablet. Some monitoring with PVRs to assess for urinary retention may be necessary, although beta-3 adrenergic receptor agonists are not associated with retention as frequently or severely as antimuscarinics. Mirabegron (Myrbetriq) is contraindicated in patients with uncontrolled or severe hypertension, and blood pressure (BP) should be monitored after starting the medication to assess for hypertension. Both medications should be avoided in patients with severe renal (eGFR < 15 mL/minute) or hepatic impairment (Child-Pugh class C), as they have not been tested in this subset of patients (Lukacz, 2020b; McVary, 2021; McVary & Saini, 2021).
Management of persistent urge incontinence/OAB should be referred to a subspecialist. More aggressive treatment options include botulinum toxin injections into the detrusor muscle. This option is associated with a higher risk of urinary retention, which should be monitored carefully; injections must be repeated every 6-9 months to maintain effectiveness. It is typically avoided for patients with a history of retention or recurrent UTIs for this reason. Some women with detrusor muscle overactivity may benefit from percutaneous tibial nerve stimulation. Weekly 30-minute sessions over 12 weeks are followed by monthly maintenance sessions, conferring a low risk for side effects. However, this therapy has minimal evidence regarding long-term efficacy. Sacral neuromodulation is an OAB management technique that consists of surgically placing a neuromodulator at the S3 foramen to deliver electrical stimulation to the sacral nerve roots after a test phase. Although the procedure is considered minimally invasive, surgical complications and device malfunction or failure can occur. Additional surgical treatment options for urge incontinence/OAB that does not respond to other treatments include augmentation cystoplasty (bladder augmentation or enlargement), urinary diversion, or placement of a suprapubic catheter (Lukacz, 2020b; McVary & Saini, 2021).
Urge incontinence in males related to BOO secondary to BPH is common, as previously mentioned. If symptoms are mild to moderate, pharmacological treatment is typically initiated using an alpha-blocker, 5-alpha reductase inhibitor, or a combination. Approved alpha adrenergic-receptor antagonists include terazosin (Hytrin), doxazosin (Cardura), tamsulosin (Flomax), alfuzosin (Uroxatral), and silodosin (Rapaflo). These agents function by relaxing the smooth muscles at the prostate and along the bladder neck. Terazosin (Hytrin) and doxazosin (Cardura) may not be ideal choices for older males, as they are associated with increased likelihood of dizziness and hypotension due to less specific targeting in their pharmacotherapeutics. To avoid these effects, the medication doses should be titrated carefully. If the incontinence is directly related to BPH, a 5-alpha reductase inhibitor may be considered. This should be confirmed via rectal exam, PSA (> 1.5 ng/mL), or transrectal ultrasound prior to starting pharmacologic treatment. In the US, the approved options in this class include finasteride (Proscar, Propecia) and dutasteride (Avodart). These medications convert testosterone to dihydrotestosterone. The full therapeutic effect can take 6-12 months, and the agent should be continued indefinitely if effective. Most patients experience a reduction in PSA by roughly half. A baseline PSA level should be checked prior to starting therapy and can be used to monitor treatment efficacy. These medications may reduce the risk of prostate cancer but also make detection more difficult, thus increasing the risk of high-grade prostate cancer developing without detection. These medications may cause sexual dysfunction. They can contribute to abnormal fetal development and should be avoided in pregnant patients. For some patients with severe BPH, combination therapy with doxazosin (Cardura) and finasteride (Proscar, Propecia) or tamsulosin (Flomax) with dutasteride (Avodart) has been more effective than monotherapy with either drug. In men with a combination of BPH and erectile dysfunction, phosphodiesterase type 5 (PDE5) inhibitors may relieve symptoms of both conditions simultaneously. Common side effects include headaches, flushing, heartburn, nasal congestion/sinusitis, and myalgias, especially back pain. These drugs are typically not combined with alpha-blockers due to the compound risk of hypotension. For BPH treatment, tadalafil (Cialis, Adcirca, Alyq) should be dosed at 5 mg daily (McVary, 2021; McVary & Saini, 2021).
Men with treatment-resistant urge incontinence related to BPH should be referred to a surgical specialist for further discussions regarding more aggressive treatment options. Minimally invasive options exist, including microwaves or radiofrequency ablation to decrease the size of the prostate gland. Laser vaporization, transurethral resection, and open prostatectomy are additional surgical options used commonly in the US, the details of which are beyond the scope of this activity (McVary & Saini, 2021).
Fecal Incontinence
Fecal or anal incontinence is the involuntary loss of solid/liquid feces or flatus. It impacts the patient’s QOL and ability to live independently, which may then lead to financial implications related to an increased level of assistance required for ADLs and the potential for a forced change in the patient’s living arrangements or setting. Fecal incontinence can also have profound professional and consequently financial implications for younger patients who are unable to continue working due to their condition. This leakage may be associated with a perceived urge to defecate (urge incontinence) or a lack of awareness (passive incontinence). Beyond advanced age, additional risk factors include diarrhea, fecal urgency, urinary incontinence, DM, and hormone replacement therapy. Fecal incontinence may result from anal sphincter weakness related to neurologic disorders (e.g., DM or spinal cord dysfunction), infiltrative disorders (e.g., systemic sclerosis), anal trauma (e.g., post-childbirth, postoperative). It may also be due to rectal compliance issues (e.g., related to ulcerative or radiation proctitis, proctectomy), decreased rectal sensation (e.g., related to neurologic dysfunction secondary to DM, Parkinson’s disease, spinal cord injury), altered stool consistency (e.g., stool impaction), or a combination of these etiologies. It may also be idiopathic, which occurs most commonly in middle-aged and older women. Certain medical conditions, such as thyroid dysfunction, can alter the frequency or number of bowel movements. Smoking can affect colonic transit, yet another reason to facilitate smoking cessation (O’Donnell, 2020; Robson & Lembo, 2020a).
Patients should be explicitly asked about any changes in bowel and bladder habits, as most will not voluntarily disclose incontinence issues unless directly asked. When evaluating a patient with fecal incontinence, the history and physical examination should include an inspection of the perianal area, a digital rectal exam, and an assessment of the patient’s anocutaneous reflex. Stool studies should be performed in patients with diarrhea to evaluate the underlying pathology (e.g., C. difficile infection). Basic laboratory studies should be considered to rule out DM, thyroid disease, and celiac disease. Endoscopy exams should be considered to assess for malignancy in those presenting with persistent diarrhea, bleeding, obstruction, or other risk factors (e.g., family history). While a flexible sigmoidoscopy is acceptable for patients under 40 at average risk for colon malignancy, a colonoscopy should be performed for all patients over 40 or those at increased risk for malignancy or inflammatory bowel disease. Although less supported by evidence on sensitivity and specificity, a fecal immunochemical test may be used as a screening test for malignancy in low-risk patients without the inconvenience of dietary restrictions and bowel preparation. Providers should consider anorectal manography and/or endorectal ultrasound or magnetic resonance imaging (MRI) for patients who do not respond to initial treatment to assess for functional sphincter weakness, abnormal rectal sensation, or structural anomalies (e.g., sphincter damage, muscle atrophy). Anal manometry assesses resting and squeezing pressures of the anal sphincter, rectal sensation, and capacity. Barium defecography may be needed, especially for patients considering surgical intervention, to assess for enterocele, rectocele, rectal prolapse, anal sphincter length, anorectal angle, and pelvic descent. This test visualizes the pelvic floor during relaxation and contraction using barium and psyllium fiber that is injected into the rectum (O’Donnell, 2020; Robson & Lembo, 2020a).
Management of Fecal Incontinence. Initial management should consist of basic supportive care, including perianal skincare. The skin should be kept clean and dry with premoistened wipes, avoiding astringent cleaners and excessive wiping; a barrier cream should also be applied. Incontinence pads may be used, and certain patients may benefit from a regular defecation program (e.g., patients with cognitive impairment or functional limitations). Foods that exacerbate symptoms should be avoided, such as incompletely digested sugars (e.g., fructose, lactose, sugar substitutes) and caffeine. A food and stool diary should be completed over 2 to 3 months to help patients identify personal triggers. Smoking cessation should be encouraged. Medications should also be reviewed to identify drugs that may be contributing to fecal incontinence: metformin (Glucophage) and proton pump inhibitors (PPIs) typically loosen stool consistency, while calcium channel blockers (CCBs) and nitrates reduce sphincter tone (O’Donnell, 2020; Robson & Lembo, 2020b).
Medical therapy targets reducing stool frequency and optimizing stool consistency, and it should always begin with treating any underlying conditions if present and identified. Patients with fecal impaction should be disimpacted. Adding a bulking agent (e.g., psyllium [Metamucil], methylcellulose [Citrucel]) to the diet is beneficial for patients with loose stools at lower volumes. Loperamide (Imodium) or diphenoxylate/atropine (Lomotil) can alleviate the symptoms of liquid stools by slowing intestinal motility and improving sphincter tone. Bismuth subsalicylate (Pepto Bismol) and cholestyramine (Questran) bind bile acids, which may be especially helpful for patients with a history of cholecystectomy or ileocolonic resection. Amitriptyline (Elavil), a TCA, inhibits sphincter relaxation and gastric motility but is typically not used for older adults due to its anticholinergic side effects. The use of suppositories or enemas to evacuate the rectum on a schedule reduces incontinence episodes, especially in patients with neurogenic bowel related to spinal cord injury. The role of phenylephrine gel (Preparation H) applied to an intact anal sphincter to improve resting tone is unclear and may be associated with dermatitis, a burning sensation, and headaches. For patients with passive fecal incontinence, injectable anal bulking agents (e.g., dextranomer in hyaluronic acid [Deflux, Solesta]) may improve anal resting pressure and promote continence (O’Donnell, 2020; Robson & Lembo, 2020b).
Many nonpharmacological management options are available for fecal incontinence. Anal plugs to reduce incontinence episodes have limited efficacy and are poorly tolerated by most patients. If anorectal manometry indicates external anal sphincter weakness or decreased rectal sensation, biofeedback may be a beneficial treatment that is painless, non-invasive, low-risk, and inexpensive (Robson & Lembo, 2020b). Unfortunately, it requires a significant commitment from the patient over several months to obtain results. Biofeedback attempts to retrain the muscles of the pelvic floor and abdominal wall to increase strength, endurance, and sensation (O’Donnell, 2020). It has not been shown to help patients with isolated internal sphincter weakness, behavioral or psychiatric disorders, neurogenic bowel, decreased rectal storage, or major structural damage to continence mechanisms. Radiofrequency ablation attempts to create thermal lesions at the anorectal junction under local anesthesia, with limited evidence regarding its efficacy. Posterior tibial nerve stimulation appears to be less effective for patients with fecal incontinence. If confirmed on anorectal ultrasound/MRI, anatomic external sphincter injury (e.g., after vaginal delivery) can be surgically repaired in some cases with anal sphincteroplasty. Unfortunately, functional gains tend to dissipate with time after sphincteroplasty. Sacral nerve electrical stimulation may be considered for patients with structurally intact yet defective anal sphincters if biofeedback and sphincteroplasty are either unavailable or ineffective. Just as sacral neuromodulation was described above for urinary incontinence, stimulation is beneficial for those with neurologic dysfunction or status-post lower anterior resection. Some studies approach an 80% success rate (O’Donnell, 2020; Robson & Lembo, 2020b).
Finally, for refractory symptoms, colostomy, dynamic graciloplasty, or an artificial anal sphincter are currently the most aggressive surgical management options. Colostomy procedures permanently reroute or divert the fecal stream. Dynamic graciloplasty is associated with considerable morbidity, and the implantable pulse generator that stimulates the transposed gracilis muscle around the anal canal is not currently available in the US. An artificial sphincter—the Neosphincter by Acticon—consists of an implanted cuff, a pressure-regulating balloon, and a control pump implanted in the labium or scrotum that the patient squeezes to permit defecation. These are often effective but are associated with a high rate of complications such as infection, device erosion, or malfunction. Other implantable devices (e.g., a balloon that senses and alerts the patient about an imminent bowel movement, a silastic inflatable cuff, and a magnetic anal sphincter) are less commonly utilized (Robson & Lembo, 2020b).
Falls
For more information on the epidemiology, risk factors, and individual assessment of fall risk in older patients, please see the corresponding section in our Comprehensive Geriatric Assessment course. In brief, most falls are multifactorial in etiology. Since many people wrongly assume that falls are an inevitable component of aging, falls frequently go unreported if the patient is not asked directly and if there is no significant associated injury. Often, providers who care for a patient following a fall focus solely on addressing the related injuries rather than the underlying causes of the fall. Therefore, in lieu of a discussion on the management of injuries suffered during a fall, which consists of a straightforward utilization of orthopedic injury management guidelines, we will instead discuss fall prevention in community-dwelling adults and fall complication prevention. Based on systematic reviews of hundreds of RCTs involving tens of thousands of patients, the following were found to reduce the risk of falls consistently (Kiel, 2020).
Studies indicate that fall prevention programs should be individualized based on the relevant risk factors identified during the assessment and multifactorial (i.e., encompassing multiple factors and interventions collectively). Exercise is the most consistent intervention that reduces the rate and risk of falls in older patients by as much as 23%. Activities should specifically focus on increasing strength and improving balance. Tai chi is especially beneficial due to its ability to integrate balance, strength, and movement. Exercise regimens that are progressive and incorporate resistance training are also evidence-based. This includes gait and balance training, strength training, movement (e.g., dance), and aerobic exercises. Exercise programs should be tailored to patient preference, and a PT consultation may provide helpful insight for a patient with little experience exercising. Exercise may be less beneficial (but still not considered harmful) for sedentary female patients over the age of 70 with physical performance impairment (Kiel, 2020).
Deprescribing high-risk medications (e.g., psychotropic medications such as benzodiazepines, sedatives, and antipsychotics) has proven effective in reducing the rate of falls (Kiel, 2020). The 2019 BC includes the following medications that should be avoided in older patients at risk for falls: SNRIs (newly added since the 2015 list), selective serotonin reuptake inhibitors (SSRIs), TCAs, antiepileptic drugs (AEDs), antipsychotics, benzodiazepines, nonbenzodiazepine receptor agonists (non-BZRAs or Z-drugs, such as zolpidem [Ambien], zaleplon [Sonata], and eszopiclone [Lunesta]), and opioids, specifically mu-opioid receptor agonists (AGS Beers Criteria Update Expert Panel, 2019; Fixen, 2019). Some studies indicate that while universal vitamin D supplementation does not help prevent falls, supplementation (about 1,000 international units [IU] daily) in high-risk older adults (i.e., those with limited sun exposure, malabsorption, obesity, slow gait speed, or impaired balance) may be beneficial. A home safety assessment with an occupational therapist (OT) is beneficial for reducing the rate and risk of falls. These assessments should be followed by safety recommendations, such as installing handrails on stairs and grab bars in bathrooms, ensuring clear and adequate lighting, optimizing slip-resistant surfaces, removing throw rugs and other trip hazards, and adding nonslip mats in bathrooms. Nonslip shoes worn during winter may help reduce the rate of outdoor falls. Some patients may benefit from single-lens eyewear (versus multifocal) while walking outdoors. Interventions that do not appear to reduce the risk of falls include ophthalmology assessments to improve vision, mobility training, and the use of an assistive device (e.g., walker or cane). Education regarding falls as a stand-alone intervention was also not effective (Kiel, 2020).
The Centers for Disease Control and Prevention (CDC, 2020) developed the Stopping Elderly Accidents, Deaths, and Injuries (STEADI) tool kit in 2019 with recommendations for providers on developing a multifactorial and individualized plan to reduce fall risk in older patients. The tool offers resources for patients/caregivers, providers, and pharmacists to work collectively to reduce fall risk for patients over 65. The algorithm provides succinct guidance regarding the 3-step process to screen patients for fall risk, assess for modifiable risk factors, and intervene to address the risk factors identified. They recommend a final follow-up with patients in 30-90 days (CDC, 2020). A small study (n=36) based on the CDC algorithm utilized exercise, home safety visits, and an individualized medication plan to identify and limit polypharmacy. While the results did not find a statistically significant reduction in falls in the intervention group compared to baseline, they did confirm a statistically significant reduction in falls in the intervention group compared to the control group over 12 months (Frith et al., 2019).
Interventions designed to treat comorbidities also decrease the risk of falls related to those particular conditions. For example, patients with syncope related to carotid sinus hypersensitivity have a reduced fall rate when a cardiac pacemaker is implanted. For patients with cataracts, surgical correction of at least one eye can lower the risk of falls and the risk of hip fracture. For older adults with a body mass index (BMI) under 20 kg/m2 who had been recently hospitalized, oral nutritional supplementation for 3 months reduced the number of falls and the number of individuals reporting a fall. Treatment for postural hypotension (e.g., reduced antihypertensive medications, increased fluids, compression stockings, corticosteroid fludrocortisone [Florinef], or alpha-agonist midodrine [Amatine]) has been effective in reducing fall risk. For those with disabling foot pain, podiatry care that is comprehensive and multifaceted (e.g., consultation, orthotics, footwear subsidy, exercises, fall education) led to a 36% reduction in falls. Even osteoporosis treatment via denosumab (Prolia, Xgeva) was linked to a 21% reduction in falls in a meta-analysis of RCTs (Kiel, 2020).
Some interventions have been effective in preventing the complications of falls when they do happen. Osteoporosis screening using an assessment of known risk factors followed by appropriate treatment decreases the incidence of hip fractures. After a fall, prolonged time on the floor (i.e., an inability to get up after falling) is associated with a serious injury, hospital admission, and transition to an LTC facility. While call alarm systems designed to summon assistance after falls or emergencies (e.g., LifeAlert) are promoted to prevent long periods on the floor, their efficacy is unproven, as the vast majority are not used after a fall. Hip protectors have not been proven effective, likely related to poor patient compliance with these devices, as well as significant variation in device thickness, stiffness, and geometry (Kiel, 2020).
Syncope
Syncope is the transient loss of consciousness due to inadequate cerebral flow. While 10% of syncope cases are idiopathic, most can be categorized as (a) reflex (neurally-mediated, e.g., vasovagal, carotid sinus, or micturition, defecation, swallowing, or coughing-triggered syncope), (b) orthostatic, (c) related to a cardiac arrhythmia (e.g., AV block, sinus node pause, ventricular tachycardia, bigeminy, or supraventricular tachycardia), or (d) related to structural cardiopulmonary disease (e.g., aortic stenosis, cardiomyopathy, atrial myxoma, pulmonary embolism [PE] or stenosis). Approximately one-half of syncope cases in older patients are considered reflex syncope, in which HR and BP are inappropriately modified. Conditions that mimic syncope should be ruled out first, such as seizures, sleep disturbances, intoxication, and some psychiatric conversion reactions (Benditt, 2020b).
To effectively evaluate a patient with syncope, the history (including the number, frequency, duration, provocative factors, and associated symptoms of episodes) and a physical examination should be completed, including a careful carotid sinus massage and a 12-lead electrocardiogram (ECG). Patients with vasovagal syncope typically describe a prodromal sensation of lightheadedness, feeling warmth or cold, sweating, palpitations, pallor, nausea, blurry vision, and auditory changes. Vital signs should be obtained when the patient is supine, seated, and standing to assess for orthostatic hypotension (evidenced by a decrease in SBP of at least 20 mm Hg over 5 minutes, or at least 30 mm Hg in a patient with hypertension). An audible heart murmur may indicate aortic stenosis, hypertrophic cardiomyopathy, or myxoma, while tachycardia in combination with tachypnea may indicate a PE. Arrhythmias are rarely identified on the ECG and may require longer monitoring (e.g., Holter). If structural cardiopulmonary disease is suspected, it should be confirmed with a transthoracic echocardiogram (TTE). Focal neurological symptoms (hemiparesis, dysarthria, diplopia, vertigo) should warrant a full neurological workup to assess for a potential neurological cause of the patient’s loss of consciousness (Benditt, 2020a).
Medications causing or contributing to syncopal episodes should be eliminated, replaced, reduced, or adjusted in schedule or timing. Antihypertensives should be reviewed for dose reduction or elimination in these patients, especially those with carotid sinus hypersensitivity. Patients with orthostatic hypotension related to hypovolemia should avoid diuretics. Orthostatic hypotension may be due to medications (e.g., vasodilators or negative chronotropics) that should be eliminated, replaced, or reduced (Benditt, 2019). The 2019 BC indicates that acetylcholinesterase inhibitors should be avoided based on high-quality evidence due to the risk of bradycardia, which could lead to syncope. Nonselective peripheral alpha-1 blockers (doxazosin [Cardura], prazosin [Minipress], and terazosin [Hytrin]) should be avoided due to their increased risk of orthostatic hypotension. Tertiary TCAs and certain antipsychotics (chlorpromazine [Thorazine], thioridazine [Mellaril], and olanzapine [Zyprexa]) should be avoided due to the risk of bradycardia or orthostatic hypotension based on high-quality evidence (AGS Beers Criteria Update Expert Panel, 2019; Fixen, 2019).
Caregivers should be educated regarding safety measures they can take when they witness a syncopal episode. This includes gently and safely lowering the patient to the ground, placing them supine and elevating their legs, assessing their vital signs, and calling for assistance if required. Patients with vasovagal syncope should be educated regarding the benign nature of the diagnosis, avoidance of individual triggers (e.g., prolonged standing, straining), and the identification of warning signs. Otherwise, management should include physical isometric counterpressure maneuvers that the patient starts as soon as the prodromal symptoms begin. The most common maneuvers include lower extremity muscle tensing, crossing of the legs, maximal handgrip, or arm tensing. These may also help patients with orthostatic hypotension. Patients with carotid sinus hypersensitivity should also avoid accidental mechanical stimulation of the carotid sinuses. Patients with orthostatic hypotension related to hypovolemia should be counseled on the importance of remaining adequately hydrated. Patients with arrhythmias may be candidates for medication, procedural (e.g., ablation), or device therapy (e.g., cardiac pacemaker or implantable cardioverter-defibrillator [ICD]) under the supervision of a cardiologist. Aortic valve replacement may be required for patients with severe aortic stenosis and associated persistent syncopal episodes. In some cases of recurrent syncope, driving restrictions should be instituted (Benditt, 2019).
Vertigo
Vertigo is a symptom, not a diagnosis; it is most commonly experienced as transient spinning dizziness, an illusion of movement. It is typically caused by damage or dysfunction of the labyrinth, vestibular nerve, or central vestibular structures in the brainstem leading to asymmetry in the vestibular system, which is summarized in Figure 4. This sensation may be associated with nausea, vomiting (unless mild and brief), and postural or gait instability. Vestibular dysfunction may also cause a tilt illusion, drop attacks (a sudden feeling of being pushed or pulled to the ground), spatial disorientation, oscillopsia (i.e., a visual illusion of environmental motion and blurred vision with head movement), and poor balance. Vertigo related to vestibular dysfunction is typically not permanent and continuous but episodic and diminished as the vestibular system adapts. Benign paroxysmal positional vertigo (BPPV) typically presents with discrete but recurrent episodes lasting under 1 minute, and a history of head trauma is common. A patient with vertigo related to a migraine or transient ischemia of the labyrinth or brainstem will present with a single vertigo episode lasting minutes to hours. Migraine patients will typically describe concurrent photophobia, headaches, sonophobia, and a history of migraines. Meniere disease and recurrent vestibulopathy both cause recurrent vertiginous episodes lasting minutes to hours, often accompanied by tinnitus and loss of hearing secondary to a peripheral lesion of the inner ear. Vestibular neuritis, multiple sclerosis, or infarction of the brainstem or cerebellum causes severe, prolonged vertigo episodes that can continue for days. A patient who has suffered a vertebrobasilar stroke will typically present with other indications of ischemia—such as dysarthria, diplopia (double vision), dysphagia (difficulty swallowing), or weakness—and often has risk factors for stroke such as hypertension, tobacco use, DM, and vascular disease. Head movement will provoke or worsen vertigo symptoms. Episodes of BPPV are often triggered by a specific motion, such as extending the neck or rolling over in bed. Vertigo that worsens with coughing or sneezing may indicate a perilymphatic fistula or a superior canal fistula. A family history of vertigo can point to a rare familial channelopathy (Furman & Barton, 2020).

Nystagmus—repetitive, involuntary, rapid eye movements—is the physical manifestation of a patient’s report of vertigo. Nystagmus on physical exam suggests vertigo associated with a vestibular lesion, while pronounced nystagmus in combination with mild vertigo indicates a potential brainstem lesion. Specific features of nystagmus can often specify the location and underlying cause of vertigo. A neurological exam to assess balance, gait, cranial nerve function, and hearing should be done. An abnormal hearing test supports the diagnosis of Meniere disease. The Dix-Hallpike maneuver may be performed to confirm vertigo and nystagmus in those with BPPV related to canalithiasis of the posterior semicircular canal (dysfunction in the posterior canal of the lower ear). In this maneuver, the neck is extended while the patient sits in an upright position on a bed. The neck is turned 45 degrees to the side, and the patient is reclined quickly into the supine position with the head hanging off the edge of the bed. This position is held for 30 seconds while observing for nystagmus. The patient should then be returned to the seated position and observed for nystagmus for another 30 seconds. The maneuver should then be repeated on the alternate side. A peripheral vestibular lesion (inner ear or vestibular nerve) is typically confirmed with a head impulse test or head thrust test. In this assessment, the patient sits with their head turned slightly (10 degrees or so) and their gaze fixed on a distant target. The examiner turns the patient’s head approximately 15 degrees quickly and without warning. A healthy patient will maintain their gaze on the target during and after the head turn, while a deficient vestibulo-ocular reflex on the side of the head turn will result in the gaze chasing the head turn followed by a saccade back to the target. This test may help separate vestibular dysfunction from nonvestibular dizziness or central vertigo (e.g., cerebellar infarct). Alternative tests may include assessing the patient’s nystagmus for direction changes or a test of skew, which looks for the vertical misalignment of both eyes. Advanced imaging (MRI, MR angiography [MRA], or computed tomography [CT]) should be performed to rule out a lesion or vascular event in patients with suspected central vertigo (Furman & Barton, 2020).
Vertigo should be managed by addressing the underlying pathology when possible. Acute symptomatic treatment should be used for no more than 48 hours, as these options may affect the patient’s ability to compensate and recover long-term. Such treatments involve various drug classes:
- first-generation antihistamines (dimenhydrinate [Dramamine], diphenhydramine [Benadryl], or meclizine [Antivert])
- antiemetics (e.g., ondansetron [Zofran], promethazine [Phenergan], metoclopramide [Reglan], or prochlorperazine [Compazine])
- benzodiazepines (e.g., alprazolam [Xanax], clonazepam [Klonopin], diazepam [Valium], or lorazepam [Ativan])
As previously mentioned, many of these medications are potentially inappropriate and should be used with extreme caution in older patients. Patients with peripheral vestibular disorders (e.g., BPPV, vestibular neuritis, Meniere disease), and even some with central vestibular disorders (e.g., vestibular migraine, infarction or ischemia of the cerebellum or brainstem, multiple sclerosis), should be referred to a PT specializing in vestibular therapy. This is likely more effective when initiated early, as it supports the central nervous system’s ability to compensate. The main tenets of vestibular rehabilitation are that activity promotes adaptation and facilitates strategic substitution, while inactivity leads to secondary physical and psychological effects. Exercises are typically tailored to address the specific condition (e.g., acute versus chronic peripheral vertigo, bilateral injury, central vertigo) as indicated (Furman & Barton, 2018).
Functional Decline, Frailty, and Failure to Thrive
Functional capacity refers to a patient’s ability to perform basic, instrumental, and advanced activities of daily living. This can include everything from toileting, grooming, and eating to cooking, driving, and managing finances. As a patient loses the ability to perform these various tasks, they are described as experiencing a functional decline (Ward & Reuben, 2020). Frailty in older adults is defined by weight loss, malnutrition, and inactivity (Agarwal, 2020). Older frail adults have an increased risk of adverse outcomes and report worsened symptoms such as fatigue and weakness. Frailty prevalence estimates vary between 4% and 16% of community-dwelling adults over 65. Risk factors for frailty in the US patient population include older age, lower educational level, smoking, hormone replacement therapy, African American or Hispanic American ethnicity, unmarried status, depression, antidepressant use, and intellectual disability (Walston, 2020). Female patients and those with lower incomes, more comorbidities, and poorer overall health are also at increased risk (Voelker, 2018). Failure to thrive (FTT) in older patients is a syndrome of global decline consisting of weight loss, anorexia (decreased appetite), poor nutrition, and inactivity that is often accompanied by dehydration, symptoms of depression, impaired immunity, and decreased cholesterol. As opposed to the FTT syndrome seen in pediatric patients who cannot achieve an expected functional level, older adults with the same constellation of symptoms are unable to maintain their previously acquired functional status. These terms may function interchangeably or be used to describe points along a continuum between the independence and virility of middle age and the full dependence and decline experienced at the end of life. Others consider physical frailty to be a required component of an FTT diagnosis typically accompanied by psychical disability and neuropsychiatric impairment; however, these elements are not required to establish a diagnosis. FTT and frailty are often related to adverse effects of medication(s) or medical comorbidities and are compounded by psychosocial factors (Agarwal, 2020). Prior to establishing a treatment plan for a patient with frailty or FTT, the patient and their caregiver(s) should engage in an extensive conversation to establish their goals of care, and the treatment plan should be based on the patient’s priorities. In this context, a thorough Comprehensive Geriatric Assessment (CGA) can efficiently and effectively guide an older adult's care via shared decision-making and clear goals. For example, pain management may be a key focus in the care plan for many patients diagnosed with frailty or FTT (Walston, 2020). Please see the NursingCE course Care Considerations for Older Adults: The Comprehensive Geriatric Assessment for more details on this process.
Evaluation
All patients with FTT should undergo a thorough medication review to assess for and address polypharmacy. If a particular medication with potential for adverse effects is identified, the situation should be discussed with the patient, including the risks and benefits of continuing the medication, alternatives for replacing the medication, or the process of deprescribing the medication. This is more common with medications that cause drowsiness or lethargy and those considered inappropriate by the AGS Beers Criteria (e.g., anticholinergics). If a medication is replaced or deprescribed, the patient should be monitored closely for signs or symptoms of withdrawal in the short-term, as well as improvement in FTT symptoms after the medication has been stopped. Please see the NursingCE course Care Considerations for Older Adults: Polypharmacy and Prescribing for additional details regarding prescribing and deprescribing medications safely for older adult patients (Agarwal, 2019).
The most important aspect of managing older adults with FTT is to fully and correctly identify all underlying and contributing conditions, such as malignancy, depression, and thyroid dysfunction. Please see the NursingCE course Care Considerations for Older Adults: The Comprehensive Geriatric Assessment for additional details regarding the assessment and diagnosis of these conditions. Next, the management plan should include optimization and adequate treatment of the diagnosed condition(s). For these patients, FTT should be considered a symptom that will resolve when the underlying condition is adequately addressed. For example, a patient diagnosed with depression who initiates structured psychotherapy plus an antidepressant medication may experience an improvement in their FTT symptoms. Based on their side effect profiles, mirtazapine (Remeron), citalopram (Celexa), and venlafaxine (Effexor) are less likely to contribute to anorexia and weight loss, while TCAs, bupropion (Wellbutrin), fluoxetine (Prozac), and sertraline (Zoloft) should be avoided for these patients. For those with severe depression, electroconvulsive therapy (ECT) has also been effective (Agarwal, 2019). Please see the section on Depression in the NursingCE course Care Considerations for Older Adults: Management of Common Geriatric Syndromes for APRNs, Part 2 for additional information about the management of social isolation and mood disorders in older adults.
There are instances of FTT in older patients already receiving optimal care for their existing medical condition(s), or no underlying etiologies are identified. In these cases, FTT is managed symptomatically according to the patient’s goals of care in collaboration with a multidisciplinary team. This team may consist of a clinician, nurse, PT, OT, SLP, licensed social worker (LSW), dietitian, and dentist where appropriate. Findings from a comprehensive history and physical examination help identify complications of inactivity, malnutrition, and FTT (e.g., pressure injuries, venous thromboembolism, and chronic subcutaneous infections), which should also be addressed according to the evidence-based guidelines associated with each condition (Agarwal, 2019). The next section will focus on the symptomatic management of older adults diagnosed with frailty and FTT.
A consultation with various specialists will contribute to a comprehensive treatment plan for a patient diagnosed with FTT or frailty and ensure the underlying contributing factors are correctly identified and addressed. This should include a referral to a speech and language pathologist (SLP) and dietitian for patients with unintentional weight loss or malnutrition to distinguish between difficulty with chewing, swallowing issues, or potential aspiration. The SLP may recommend incorporating certain food textures, swallowing exercises and techniques, and body positioning to optimize function while eating. A dietitian may provide meal planning based on the individual’s caloric needs in light of their activity level, comorbid conditions, and nutritional status. A referral to PT and OT may be most beneficial for patients with inactivity, apraxia, cognitive decline, or a limited ability to perform ADLs independently. These providers can recommend and facilitate the delivery and proper utilization of assistive devices, grab bars, and other safety equipment, along with a treatment plan that addresses the patient’s most significant deficits. Patients with global deficits may be recommended for enrollment in a comprehensive rehabilitation program. An LSW can address social isolation, patient and family education, advanced care planning, and referrals to community or mental health resources (Agarwal, 2020).
Management
FTT symptoms should be addressed, particularly weight loss, physical frailty, and neuropsychological impairment. Interventions for weight loss should address any impediments to intake (e.g., mechanical, social, financial, dietary). A dental consultation to manage oral pain or adjust dentures should be included. A dietitian consultation should consist of suggested meal plans with adequate caloric and protein intake using creative alternatives when necessary for patients who report taste alterations, food intolerances, or other special circumstances. An SLP should be consulted to address any dysphagia, chewing issues, or texture modifications. An OT can assist with food preparation, transportation details to obtain groceries, and exercises to address any upper extremity limitations related to weakness or tremors. Assistance with shopping or feeding may be required for some patients, and strategies should be explored with the patient and caregiver. Social interaction during mealtimes is beneficial, along with frequent small meals. The patient should be encouraged to eat whenever they are hungry and avoid limiting their diet based on previous education; for example, low-salt or low-fat modifications can be discontinued (Agarwal, 2019). The nutrient density of foods can be optimized by adding whey or milk powder, egg whites, or tofu to increase the protein content or by adding olive oil or avocado to increase fat content (Ritchie & Yukawa, 2020). Caloric supplements have not been proven to improve QOL, mood, or functional status despite evidence that they provide a 2% increase in weight and a small reduction in mortality in older adults with FTT. The AGS does not recommend the appetite stimulant megestrol acetate (Megace) and the antihistamine cyproheptadine (Periactin) due to a lack of evidence that these medications improve QOL or reduce mortality in older adults with FTT, in addition to their unfavorable associated risks (thrombotic events, fluid retention, increased mortality; Agarwal, 2019). Additional information regarding the diagnosis and management of malnutrition can be found in the NursingCE course Care Considerations for Older Adults: Management of Common Geriatric Syndromes for APRNs, Part 2.
A 2018 meta-analysis and systematic review by Kojima and colleagues reviewed the effect of the Mediterranean diet specifically on older adults and frailty. They looked at 4 studies, comprising 5,789 community-dwelling adults with a mean age over 60 years. When scored on dietary adherence, participants’ enhanced adherence to the Mediterranean diet was associated with a significantly lower-incident frailty risk without significant heterogeneity (Kojima et al., 2018). The Mediterranean diet is rich in plant foods (e.g., fruits, vegetables, legumes, whole grains, seeds, olives, and tree nuts). It includes moderate fish intake as the primary source of protein, limiting or avoiding red or processed meats. Olive oil is the primary source of fat, along with avocados and other “healthy” fats. Dairy products are somewhat limited, but small amounts of wine with meals are encouraged. It is anti-inflammatory, which may explain its effectiveness. The fish content in the diet supplies vitamin B12, and other antioxidants (e.g., vitamins A, C, B6, D, and folate) are provided in abundance. The Mediterranean diet is also associated with cultural and lifestyle elements, encourages the social and community aspects of food, and emphasizes the health benefits of 30 minutes of moderate physical activity daily (Voelker, 2018).
In addition to the dietary approaches discussed above, interventions to address physical frailty include strength and aerobic exercise plus vitamin D supplementation. Exercise improves function, mobility, and gait and prevents disability and falls. Exercise can also improve bone mineral density and increase a patient’s overall well-being. An exercise plan should be made through a PT referral for those with any physical limitations or disability and according to the patient’s wishes if they are terminally ill. The method with the most robust evidence of effectiveness is progressive resistance training, which increases muscle mass, gait speed, and exercise tolerance. Vitamin D supplementation may help those with sarcopenia (age-related loss of lean muscle mass) and a serum 25-hydroxyvitamin D level below 20 ng/mL. Supplementation may still provide a low-risk intervention with a modest benefit for patients with sarcopenia and a normal vitamin D level. As mentioned above, vitamin D supplementation may reduce the risk of falls, promote the maintenance of muscle strength, and enhance balance. The daily intake of vitamin D should be at least 800-1000 IU in older adults. Anabolic agents (testosterone replacement), growth hormone, growth hormone stimulants (e.g., ghrelin), and dehydroepiandrosterone sulfate supplementation are not recommended due to significant known risks and a current lack of evidence for efficacy. A multidimensional program incorporating cognitive training, education on physical activity, and nutritional counseling decreased the risk of developing or persisting comorbidities in over 1,600 community-dwelling older adults in a French study. Along with exercise and nutritional supplementation, cognitive training was effective in ameliorating multiple frailty components according to a systematic review of 21 RCTs regarding frailty prevention. An outpatient program by Medicare entitled the Program of All-inclusive Care for the Elderly (PACE) aims to overcome environmental challenges to improve function and keep patients living at home. The interdisciplinary PACE team consists of a geriatric practitioner, nurses, PTs, OTs, and LSWs. The program is customized and can include home visits, therapy sessions, transportation assistance, self-care aides, or adult daycare (Agarwal, 2019; Walston, 2020).
Patients with frailty or FTT related to dementia tend to respond positively to environments with increased support and supervision, as well as increased social interaction. Advanced dementia typically renders the patient unable to feed themselves, chew, or swallow. Feeding may be provided with assistance for comfort, but a discussion with the family regarding the risks of aspiration is warranted. Advance care planning in the early stages of dementia should guide shared decision-making with advanced dementia patients regarding the use of supplemental tube feeding (Agarwal, 2019).
Frail older adults who are hospitalized have an increased risk of institutionalization and report a decreased QOL. However, certain acute geriatric care units are attempting to improve this experience. Geriatric Evaluation and Management Units (GEMUs, in US Department of Veterans Affairs [VA] hospitals) or based on the Acute Care of the Elderly model (ACE, found in private hospitals) are typically staffed by clinicians who assume primary care of the patient, thereby streamlining the implementation of recommendations. This places a skilled team of professionals (e.g., nurses, PT, OT, SLP, LSW) who tailor care to the older adult population, enhancing therapy consistency. This care model is designed to prevent the prolonged functional decline that is typically seen after acute hospitalizations in frail older adults (Walston, 2020; Ward & Reuben, 2020).
For older adults with FTT nearing the end of their life, the focus of care should shift from improving outcomes to comfort and palliation (Agarwal, 2019). Screening and intervention should be less aggressive and should no longer target non-life-threatening conditions. FTT is no longer recognized by the Centers for Medicare and Medicaid Services (CMS) as a qualifying diagnosis for hospice care. Instead, hospices are instructed to bill for the underlying primary disease state. However, clinical decline worsens the prognosis of any underlying condition, and typically include clinical features such as weight loss not related to reversible causes, inadequate oral intake due to intractable dysphagia, and progressive decline in Karnofsky Performance Scale status, among other factors. Additionally, progressive inanition (exhaustion related to malnutrition) is a criterion that may indicate a life expectancy at or below 6 months. Consultation with a palliative care or hospice provider is recommended in this subset of patients (Agarwal, 2019; Walston, 2020).
Delirium
Cognitive impairment incidence increases with age, especially after 85. Most dementia patients exhibit a steady cognitive decline over months with intact attention and remote memory despite impaired short-term memory, judgment, confusion, and disorientation. Dementia patients may experience paranoia and hallucinations, although these episodes are rare (Lippmann & Perugula, 2016). By contrast, cognitive impairment associated with attention deficit that develops over hours or days may indicate delirium, which can be difficult to distinguish from dementia. A key feature that defines delirium (which is also known as an acute confusional state) is the high likelihood that the acute cognitive change is related to a medical condition, medication, substance use, or withdrawal. It typically persists for days but may last for months (termed persistent) if the underlying cause is not correctly identified and treated quickly; symptoms often worsen throughout the day, peaking in the evening. For older patients who are acutely ill, cognitive and behavioral changes may be the only obvious symptom of their underlying illness. Psychomotor disturbances (e.g., hypoactivity or hyperactivity), sleep disturbances, emotional disturbances, hallucinations, and delusions often accompany delirium. Those with underlying brain disorders (e.g., dementia, Parkinson’s disease, or stroke) have an increased risk of delirium. Other risk factors include age and sensory impairment. Patients may initially appear easily distracted; in more advanced cases, they may present as lethargic, drowsy, or nearly comatose. These adults often exhibit memory loss, disorientation, and speech or language abnormalities. Among older adults hospitalized for a medical condition, almost 30% experience delirium at some point during their hospitalization (Francis & Young, 2020). Delirium symptoms may persist for up to a year, especially in those with underlying dementia. The mortality rate among patients with delirium is roughly twice that of patients without delirium in the 6 to 12 months following their illness. In a long-term study, only one-third of delirium patients were still living independently 2 years after diagnosis (Francis, 2019).
Diagnosis
Brief screening tools have been developed to help identify potential cases of delirium with high sensitivity and specificity, such as the Confusion Assessment Method (CAM), the 3-Minute Diagnostic Assessment (3D-CAM), the 4 As Test, and the Family CAM. The CAM or the CAM-ICU can assist in identifying delirium in about 5 minutes, with a validated sensitivity of at least 94% and a specificity of at least 90%. Inattention and an acute and fluctuating course of cognitive decline must be evident for a positive screen, along with either disorganized thinking (e.g., disorientation) or an altered level of consciousness (e.g., somnolence, hypervigilance, or stupor). The Intensive Care Delirium Screening Checklist (ICDSC) may also be used for critically ill patients. The 3D-CAM contains 3 orientation items, 4 attention items, 3 symptom probes, and 10 observational items with a sensitivity of 95% and a specificity of 94%. The 4 As Test is brief, but its sensitivity and specificity are lower (84-89%) than for the other tests described. If not already included in the above screening, the patient’s attention can be assessed with the mini-mental state exam (MMSE), serial 7s (repeatedly adding by a factor of 7), backward spelling of the word “world,” digit span (asking a patient to repeat a series of random digits from memory), or the vigilance “a” test (reading a list of random letters, asking the patient to indicate every time they hear the letter “a” listed). They may also be asked to recite the months of the year or the days of the week backward. Routine use of the modified Richmond Agitation and Sedation Scale (mRASS) for screening purposes in patients with suspected delirium outside of the postoperative recovery room and ICU is not recommended (Francis & Young, 2020; Oh et al., 2017).
Delirium diagnosis should be based on the characteristics defined by the American Psychological Association (APA, 2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). These consist of the following (APA, 2013, p. 596):
- Disturbances appear in attention (inability to direct, focus, shift, or maintain) and awareness (disorientation to the environment).
- This disturbance develops quickly (i.e., over hours to days), tends to fluctuate throughout the day, and represents a dramatic shift from baseline.
- Findings are accompanied by a disturbance in at least a single area of cognition (e.g., memory, orientation, language, perception, or visuospatial ability).
- The disturbances are not better explained by a neurocognitive disorder and do not occur with a reduced level of consciousness or arousal (i.e., coma).
- The patient’s history findings, physical exam results, or laboratory data indicate that these disturbances are related to a medical condition, substance intoxication or withdrawal, exposure to a toxin, or a combination of etiologies.
The assessment should account for the patient’s baseline level of functioning if known. Caregivers and family members can help establish this baseline if prior cognitive testing results are not immediately available. A recent history of illness, a new medication, or substance abuse should be established. Targeted laboratory testing will facilitate identification of the underlying condition, which may include a basic metabolic profile (BMP), complete blood count (CBC), urinalysis/urine culture, toxicology screen, drug levels of narrow therapeutic range medications (e.g., digoxin [Lanoxin], lithium [Lithobid]), arterial blood gas panel, liver function testing, thyroid-stimulating hormone (TSH), and a vitamin B12 level. If an obvious cause is still not identified, advanced imaging of the brain via CT or electroencephalogram (EEG) may be indicated. Differential diagnoses to consider for a patient with suspected delirium include dementia with sundowning, stroke, traumatic brain injury (TBI), Wernicke aphasia, bitemporal dysfunction, Anton syndrome of cortical blindness and confabulation, bifrontal brain lesion (e.g., due to tumor or trauma), nonconvulsive status epilepticus, and primary psychiatric illnesses such as depression, bipolar depression with mania, or schizophrenia. Older patients who contract meningitis may present with delirium, as opposed to the classic triad of fever, headache, and meningismus (a state of meningeal irritation causing neck stiffness or hypersensitivity to light), requiring a lumbar puncture for confirmation (Francis & Young, 2020). Differentials that should be ruled out or considered include other mental health conditions (e.g., psychotic disorders or mood disorders with psychotic features), acute stress disorder, malingering and factitious disorder, and dementia or a similar neurocognitive disorder (APA, 2013).
Prevention
The 2019 BC recommends avoiding certain high-risk medications for older patients based on moderate-quality evidence regarding delirium risk. This includes benzodiazepines, nonbenzodiazepine receptor agonists (non-BZRAs or Z-drugs, such as zolpidem [Ambien], zaleplon [Sonata], and eszopiclone [Lunesta]), anticholinergics, antipsychotics, corticosteroids, and meperidine [Demerol]. The level of evidence supporting the avoidance of histamine 2-receptor antagonists (H2 blockers) was reduced to low quality (AGS Beers Criteria Update Expert Panel, 2019; Fixen, 2019). Certain antibiotics (e.g., fluoroquinolones, penicillins, macrolides, aminoglycosides, cephalosporins, sulfonamides, metronidazole [Flagyl], linezolid [Zyvox], rifampin [Rifadin]), antifungals (e.g., amphotericin B [Amphocin]), antivirals (e.g., acyclovir [Zovirax]), and antimalarials may induce or prolong delirium. Otherwise, the prevention of delirium can be difficult. Additional medications that are considered high-risk for delirium development include AEDs (e.g., carbamazepine [Tegretol], phenytoin [Dilantin], levetiracetam [Keppra]), SSRIs, TCAs, beta-blockers, diuretics, antiarrhythmics, muscle relaxants, dopamine agonists (e.g., levodopa [Sinemet], ropinirole [Requip], amantadine [Symmetrel]), antiemetics, antispasmodics, barbiturates, and cholinesterase inhibitors (e.g., donepezil [Aricept]; Francis, 2019, Table 1).
Orientation protocols, which provide access to outside windows, clocks, calendars, and as-needed verbal reorientation, may help prevent delirium in institutionalized older adults, especially immediately after transitions between settings and in unfamiliar locations. Cognitive stimulation may help, especially for patients with underlying dementia, but sensory overstimulation should be avoided. Cognitive stimulation can be achieved through simple reminiscence or engaging conversation. Uninterrupted sleep during nighttime is crucial, ideally in a quiet, dark environment to facilitate restorative sleep. Acutely ill patients appear to benefit from early mobilization with PT and OT consultation for safety. Patients with hearing or vision impairment should receive aids or devices to facilitate accurate sensation and processing. Pain, dehydration, hypoxemia, and infection are common contributors to delirium and should be avoided or managed appropriately in older adults. Combining 3 or more of the above interventions into a multicomponent program is most effective in preventing delirium, especially for non-ICU hospitalized patients. Unfortunately, these programs may be less effective for patients in LTC facilities and those with cancer or terminal illness. The evidence does not support the routine use of any pharmacological agents to prevent delirium (Francis, 2019; Oh et al., 2017).
Management
A physical exam should be performed to determine the underlying condition, including a neurological exam. Once identified, the underlying medical condition should be appropriately treated. The most common conditions that contribute to delirium include metabolic encephalopathy related to fluid and electrolyte disturbances, infection, organ failure, or hypoglycemia, drug toxicity (which may occur even with “therapeutic” levels of drugs such as digoxin [Lanoxin] or lithium [Lithobid]), or withdrawal from alcohol or sedatives. In cases of sedative or alcohol withdrawal, benzodiazepines such as lorazepam (Ativan) can be dosed at 0.5-1 mg as needed and tapered over several days to avoid severe withdrawal, delirium tremens, and seizures. Otherwise, medical care for older adults with delirium should be interdisciplinary and focus on sustaining hydration, optimizing nutrition, and maintaining mobility and range of motion (Francis, 2019). The prevention measures listed above to promote reorientation, sleep, cognitive stimulation, and vision or hearing deficit correction should also be instituted as nonpharmacological treatment (Oh et al., 2017). Any incontinence or pain should be managed as in other older patients, with care to avoid opioids if possible and meperidine [Demerol] especially. Nonopioid medications such as acetaminophen (Tylenol) are preferred, and pre-emptive pain management may be beneficial. A nerve block is an example of a pain-management technique that is frequently used postoperatively; it may reduce the incidence of delirium and help manage pain effectively in patients immediately following surgery. Long-acting opioids such as methadone (Dolophine) may also provide some benefit versus shorter-acting agents. The risk of skin breakdown and pressure injuries or aspiration pneumonitis should be assessed and minimized. This care may need to continue for months and may extend beyond the acute hospital environment. Delirious patients with disruptive or agitated behavior may respond to frequent reassurance, verbal orientation, and physical touch, especially when provided by family members or familiar persons. If present, delusions and hallucinations should not be confirmed but also not denied. Physical restraints should be a last resort for these patients, as they increase agitation and delirium duration; restraints also reduce mobility and increase the risk of pressure injuries and aspiration. Vigilant supervision may be more effective if available (Francis, 2019). Healthy sleep patterns should be encouraged as described above, as well as melatonin supplementation or the addition of 8 mg of oral ramelteon (Rozerem) at bedtime if needed (Oh et al., 2016). Ramelteon (Rozerem) works as a melatonin receptor agonist, activating melatonin receptors in the brain (Neubauer, 2020). Additional sleep hygiene strategies can be found in the Insomnia section in the NursingCE course Care Considerations for Older Adults: Management of Common Geriatric Syndromes for APRNs, Part 2. Ethically, the healthcare team must provide care with implied consent in life-threatening situations only. In all other instances, the patient’s ability to make decisions and consent to medical care should be consistently and carefully documented. If a patient cannot consent to care, informed consent should be obtained from a surrogate decision-maker (Francis, 2019).
Scales to assess the severity of delirium have been developed with some success, such as the CAM-Severity score (CAM-S), the Delirium Rating Scale – Revised-98 (DRS-R-98), or the Memorial Delirium Assessment Scale. These scales can help establish prognosis, stratify risk, and assess response to treatment (Oh et al., 2017). For severe cases of agitation with a threat of self-harm, antipsychotics may be used off-label, as they are not indicated for the management of delirium. Haloperidol (Haldol) may be given in 0.5-1 mg doses as needed (oral, intramuscular, or intravenous [IV]), up to a maximum of 5 mg daily. It should not be dosed continuously or prophylactically but only short-term. Less substantial evidence and experience support the use of newer antipsychotics, such as quetiapine (Seroquel), risperidone (Risperdal), ziprasidone (Geodon), and olanzapine (Zyprexa). However, these agents have fewer side effects when studied in other settings, and they have shown similar efficacy in small studies. Doses should be kept low to avoid extrapyramidal symptoms, especially with haloperidol (Haldol). These medications may increase a patient’s mortality and stroke risk, and IV haloperidol (Haldol) is also associated with QT prolongation, which should be closely monitored (Francis, 2019). A 2016 systematic review conducted by Neufeld and colleagues evaluated the use of antipsychotics across 19 studies. Researchers concluded that evidence does not currently support antipsychotic use for preventing or treating delirium in hospitalized older adults due to a lack of significant impact on delirium incidence, duration, or severity. However, they found no association with mortality (Neufeld et al., 2016). In most cases, the management of delirium with benzodiazepines is not recommended. An older adult with severe delirium near the end of their life (often termed terminal or preterminal delirium) may benefit from palliative sedation with a short-acting benzodiazepine that can be titrated to effect such as midazolam (Nayzilam). The use of methadone (Dolophine) for those with refractory pain and terminal delirium may be effective (Francis, 2019).
References
Agarwal, K. (2019). Failure to thrive in older adults: Management. UpToDate. Retrieved February 25, 2021, from https://www.uptodate.com/contents/failure-to-thrive-in-older-adults-management
Agarwal, K. (2020). Failure to thrive in older adults: Evaluation. UpToDate. Retrieved February 25, 2021, from https://www.uptodate.com/contents/failure-to-thrive-in-older-adults-evaluation
The American College of Obstetricians and Gynecologists. (2016). The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2016/03/the-use-of-vaginal-estrogen-in-women-with-a-history-of-estrogen-dependent-breast-cancer
American Geriatrics Society Beers Criteria Update Expert Panel. (2019). American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 67(4), 674–694. https://doi.org/10.1111/jgs.15767
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association.
Babagolzadeh. (2012). Wound stages [image]. https://commons.wikimedia.org/wiki/File:Wound_stage.jpg
Benditt, D. (2019). Syncope in adults: Management. UpToDate. Retrieved February 19, 2021, from https://www.uptodate.com/contents/syncope-in-adults-management
Benditt, D. (2020a). Syncope in adults: Clinical manifestations and initial diagnostic evaluation. UpToDate. Retrieved February 19, 2021, from https://www.uptodate.com/contents/syncope-in-adults-clinical-manifestations-and-initial-diagnostic-evaluation
Benditt, D. (2020b). Syncope in adults: Epidemiology, pathogenesis, and etiologies. UpToDate. Retrieved February 19, 2021, from https://www.uptodate.com/contents/syncope-in-adults-epidemiology-pathogenesis-and-etiologies
Berlowitz, D. (2020a). Clinical staging and management of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 11, 2021, from https://www.uptodate.com/contents/clinical-staging-and-management-of-pressure-induced-skin-and-soft-tissue-injury
Berlowitz, D. (2020b). Epidemiology, pathogenesis, and risk assessment of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 11, 2021, from https://www.uptodate.com/contents/epidemiology-pathogenesis-and-risk-assessment-of-pressure-induced-skin-and-soft-tissue-injury
Berlowitz, D. (2020c). Prevention of pressure-induced skin and soft tissue injury. UpToDate. Retrieved February 11, 2021, from https://www.uptodate.com/contents/prevention-of-pressure-induced-skin-and-soft-tissue-injury
Brain Made Simple. (2021). Structures of the vestibular system [image]. https://brainmadesimple.com/vestibular-function/
Brown-O’Hara, T. (2013). Geriatric syndromes and their implications for nursing. Nursing2021, 43(1), 1–3. https://doi.org/10.1097/01.NURSE.0000423097.95416.50
BruceBlaus. (2016). Condom catheter drainage [image]. https://commons.wikimedia.org/wiki/File:Condom_Cather_Drainage.png
Centers for Disease Control and Prevention. (2020). STEADI - Older adult fall prevention. https://www.cdc.gov/steadi/index.html
Fixen, D. R. (2019). 2019 AGS Beers Criteria for older adults. Pharmacy Today, 25(11), P42-54. https://doi.org/10.1016/j.ptdy.2019.10.022
Francis, J. (2019). Delirium and acute confusional states: Prevention, treatment, and prognosis. UpToDate. Retrieved March 2, 2021, from https://www.uptodate.com/contents/delirium-and-acute-confusional-states-prevention-treatment-and-prognosis
Francis, J., & Young, G. B. (2020). Diagnosis of delirium and confusional states. UpToDate. Retrieved March 2, 2021, from https://www.uptodate.com/contents/diagnosis-of-delirium-and-confusional-states
Frith, K. H., Hunter, A. N., Coffey, S. S., & Khan, Z. (2019). A longitudinal fall prevention study for older adults. The Journal for Nurse Practitioners, 15(4), P295-300. https://doi.org/10.1016/j.nurpa.2018.10.012
Furman, J. M., & Barton, J. J. S. (2018). Treatment of vertigo. UpToDate. Retrieved February 19, 2021, from https://www.uptodate.com/contents/treatment-of-vertigo
Furman, J. M., & Barton, J. J. S. (2020). Evaluation of the patient with vertigo. UpToDate. Retrieved February 19, 2021, from https://www.uptodate.com/contents/evaluation-of-the-patient-with-vertigo
Kiel, D. P. (2020). Falls: Prevention in community-dwelling older persons. UpToDate. Retrieved February 9, 2021, from https://www.uptodate.com/contents/falls-prevention-in-community-dwelling-older-persons
Kojima, G., Avgerinou, C., Iliffe, S., & Walters, K. (2018). Adherence to Mediterranean diet reduces incident frailty risk: Systematic review and meta-analysis. Journal of the American Geriatrics Society, 66(4), 783-788. https://doi.org/10.1111/jgs.15251
Lippmann, S., & Perugula, M. L. (2016). Delirium or dementia? Innovations in Clinical Neuroscience, 13(9-10), 56–57.
Lukacz, E. S. (2020a). Evaluation of females with urinary incontinence. UpToDate. Retrieved February 16, 2021, from https://www.uptodate.com/contents/evaluation-of-females-with-urinary-incontinence
Lukacz, E. S. (2020b). Treatment of urgency incontinence/overactive bladder in females. UpToDate. Retrieved February 17, 2021, from https://www.uptodate.com/contents/treatment-of-urgency-incontinence-overactive-bladder-in-females
Lukacz, E. S. (2020c). Treatment of urinary incontinence in females. UpToDate. Retrieved February 16, 2021, from https://www.uptodate.com/contents/treatment-of-urinary-incontinence-in-females
McVary, K. T. (2021). Medical treatment of benign prostatic hyperplasia. UpToDate. Retrieved February 18, 2021, from https://www.uptodate.com/contents/medical-treatment-of-benign-prostatic-hyperplasia
McVary, K. T., & Saini, R. (2021). Lower urinary tract symptoms in men. UpToDate. Retrieved February 18, 2021, from https://www.uptodate.com/contents/lower-urinary-tract-symptoms-in-men
Neubauer, D. N. (2020). Pharmacotherapy for insomnia in adults. UpToDate. Retrieved March 5, 2021, from https://www.uptodate.com/contents/pharmacotherapy-for-insomnia-in-adults
Neufeld, K. J., Yue, J., Robinson, T. N., Inouye, S. K., & Needham, D. M. (2016). Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: A systematic review and meta-analysis. Journal of the American Geriatrics Society, 64(4), 705-714. https://doi.org/10.1111/jgs.14076
Nguyen, T., Wong, E., & Ciummo, F. (2020). Polypharmacy in older adults: Practical applications alongside a patient case. The Journal for Nurse Practitioners, 16(3), 205–209. https://doi.org/10.1016/j.nurpra.2019.11.017
O’Donnell, K. F. (2020). Fecal incontinence: A stepwise approach to primary care management. The Journal for Nurse Practitioners, 16(8), P586-589. https://doi.org/10.1016/j.nurpa.2020.05.006
Oh, E. S., Fong, T. G., Hshieh, T. T., & Inouye S. K. (2017). Delirium in older persons: Advances in diagnosis and treatment. JAMA, 318(12):1161–1174. https://doi.org/10.1001/jama.2017.12067
Ritchie, C., & Yukawa, M. (2020). Geriatric nutrition: Nutritional issues in older adults. UpToDate. Retrieved March 2, 2021, from https://www.uptodate.com/contents/geriatric-nutrition-nutritional-issues-in-older-adults
Robson, K. M., & Lembo, A. J. (2020a). Fecal incontinence in adults: Etiology and evaluation. UpToDate. Retrieved February 18, 2021, from https://www.uptodate.com/contents/fecal-incontinence-in-adults-etiology-and-evaluation
Robson, K. M., & Lembo, A. J. (2020b). Fecal incontinence in adults: Management. UpToDate. Retrieved February 18, 2021, from https://www.uptodate.com/contents/fecal-incontinence-in-adults-management
Voelker, R. (2018). The Mediterranean diet’s fight against frailty. JAMA, 319(19):1971–1972. https://doi.org/10.1001/jama.2018.3653
Walston, J. D. (2020). Frailty. UpToDate. Retrieved February 25, 2021, from www.uptodate.com/contents/frailty
Ward, K. T., & Reuben, D. B. (2020). Comprehensive geriatric assessment. UpToDate. Retrieved February 5, 2021, from www.uptodate.com/contents/comprehensive-geriatric-assessment