About this course:
The purpose of this course is to familiarize the learner with some of the most common geriatric syndromes (malnutrition, insomnia, self-neglect, depression, and hearing loss), outlining their diagnosis and evidence-based management.
Course preview
The purpose of this course is to familiarize the learner with some of the most common geriatric syndromes, outlining their diagnosis and evidence-based management.
At the conclusion of this course, the advanced practice registered nurse (APRN) will be prepared to:
- explain the etiology, diagnosis, and evidence-based treatment guidelines for malnutrition in older adult patients
- discuss the diagnosis of and evidence-based treatment guidelines for insomnia in older adult patients
- describe the evaluation, diagnosis, and prevention processes as well as management guidelines for self-neglect by older adult patients
- review the diagnosis and evidence-based treatment guidelines for depression in older adult patients
- identify the diagnosis and evidence-based treatment guidelines for hearing loss in older adult patients
When caring for older adults (65+), healthcare professionals need to account for various unique considerations. The medical team must be prepared to care for these patients, as the population of Americans over the age of 65 is expected to more than double between 2000 and 2030, increasing from 34.8 million to more than 70.3 million. Best-practice and evidence-based geriatric protocols should be developed and utilized in hospitals, rehabilitation centers, long-term care (LTC) facilities, home-care agencies, and community clinics; these protocols should be introduced in nursing education programs to enhance familiarity. APRNs must function in tandem with the rest of the interdisciplinary team, as the Institute of Medicine (now the National Academy of Medicine) highlighted collaboration as a vital component of care in their Retooling for an Aging America: Building the Health Care Workforce report in 2008. The primary goals of geriatric care should be to promote well-being and optimize each patient’s quality of life (QOL) through continued maintenance of function, dignity, and self-determination (Brown-O’Hara, 2013; Ward & Reuben, 2020).
Characteristics of Geriatric Syndromes
Geriatric syndromes often do not fall into a particular disease category (e.g., congestive heart failure within cardiology or chronic obstructive pulmonary disease within pulmonology) despite being common among older patients. These conditions may affect patients’ QOL, decrease function, put their ability to live independently at risk, lead to disability, and impact mortality. Their causes are often multifactorial (Brown-O’Hara, 2013). While numerous geriatric syndromes exist, this activity will highlight 5 of the most common: malnutrition, insomnia, self-neglect, depression, and hearing loss.
Malnutrition
Malnutrition affects older adults more often compared to younger adults and also exerts a greater impact on their health outcomes. It can interfere with function, increase healthcare utilization, and lengthen postoperative hospital stays for surgical patients. A 2016 meta-analysis indicated that the prevalence of malnutrition among older adults varies by setting—6% of outpatients, 17.5% of skilled nursing facility (SNF) patients, 22% of hospitalized patients, 28.7% of LTC patients, and 29% of rehabilitation/sub-acute care patients, although the data mostly consisted of studies conducted in Europe (Ritchie & Yukawa, 2020). Malnutrition in an older adult patient may initiate a cycle of frailty, as it leads to a decrease in lean muscle mass, which reduces strength, aerobic capacity, gait speed, and activity level, resulting in functional decline and progressive frailty (Agarwal, 2020). Risk factors for malnutrition in older adults include anorexia, acute delirium, a higher body mass index (BMI), the presence of a pre-existing infection or cancer, and the need for assistance while eating. Weight loss may be due to inadequate intake or anorexia, sarcopenia (loss of lean muscle mass and strength), or the inflammatory effects of a disease (e.g., cachexia). Inadequate intake in older adults can result from social isolation, financial limitations, and medical or psychological conditions. Age-related physiologic changes (e.g., decreased sensitivity to smells and taste, delayed gastric emptying, early satiety due to adjustments in digestive hormones) contribute to an expected reduction in appetite with age. Illness, medications, and chronic conditions (e.g., dementia, depression) also contribute to anorexia (Ritchie & Yukawa, 2020). Polypharmacy in older adults can also prompt or worsen malnutrition, as it has been associated with a decreased intake of fiber, minerals, and fat-soluble and B vitamins and an increase in cholesterol, glucose, and sodium intake (Saljoughian, 2019).
The mnemonic MEALS ON WHEELS summarizes some of the common causes for malnutrition and unintentional weight loss in adults, which include the following:
- medications (e.g., antiepileptic drugs [AEDs], digoxin [Lanoxin], anticholinergics, angiotensin converting enzyme [ACE] inhibitors, antibiotics, chemotherapeutic agents)
- emotional problems (e.g., mood disorders such as depression or anxiety)
- anorexia
- late-life paranoia or alcoholism
- swallowing disorders (e.g., odynophagia [painful swallowing], dysphagia)
- oral factors (e.g., dental carries/abscess, ill-fitting dentures, xerostomia)
- no money (financial limitations e.g., economic hardship, food deserts, lack of transportation to obtain food)
- wandering (by dementia patients)
- hyperthyroidism or hyperparathyroidism
- entry problems or malabsorption
- eating problems (e.g., upper extremity or jaw weakness due to stroke or tremor)
- low-salt or low-cholesterol diet
- shopping and food preparation problems (e.g., food deserts, lack of transportation to obtain food; Agarwal, 2020, Tables 1 & 2)
Various medical conditions can also contribute to weight loss, such as malignancy (most commonly), gastrointestinal conditions (e.g., peptic ulcer disease, chronic pancreatitis, inflammatory bowel disease), cardiac conditions (e.g., heart failure, coronary artery disease), pulmonary conditions (e.g., chronic obstructive pulmonary disease [COPD], interstitial lung disease), infectious conditions (e.g., tuberculosis [TB], bacterial endocarditis), neurologic conditions (e.g., stroke, dementia, Parkinson’s disease), endocrine disorders (e.g., diabetes mellitus [DM], thyroid dysfunction), renal conditions (e.g., uremia, nephrotic syndrome), psychiatric conditions (e.g., depression, alcohol use disorder), or rheumatic conditions (e.g., polymyalgia rheumatica). Malnutrition and weight loss may also be related to a deficiency in thiamine, vitamin B12, vitamin C, or zinc (Agarwal, 2020, Table 3).
Diagnosis
Malnutrition diagnostic criteria were proposed in a 2012 joint statement from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition (ASPEN). The list includes 6 criteria, 2 or more of which must be present to establish a diagnosis of malnutrition:
- insufficient energy (caloric) intake
- weight loss
- loss of lean muscle mass
- loss of subcutaneous adipose tissue
- fluid accumulation, either generalized or localized, that may conceal weight loss
- decreased strength, as evidence by handgrip strength testing (Ritchie & Yukawa, 2020)
The Global Leadership Initiative on Malnutrition (GLIM) introduced updated criteria in 2018. This diagnosis requires the presence of at least one phenotypic criterion (non-volitional weight loss, low BMI, or reduced muscle mass) and one etiologic criterion (reduced food intake or absorption, or underlying inflammation due to chronic or acute disease or injury). This eliminated the diagnostic criteria related to subcutaneous adipose tissue, fluid accumulation, and decreased strength (Ritchie & Yukawa, 2020).
Current weight and recent weight loss shou
...purchase below to continue the course
Aside from weight loss over time, body fat and lean muscle mass can be estimated using bioelectrical impedance or anthropometric measures. Bioelectrical impedance can vary with hydration, so a patient’s hydration status should be taken into consideration and remain consistent between serial measurements if possible. These devices are also available for bedbound and wheelchair-bound patients. The patient’s mean upper arm circumference or mid-arm circumference is measured at the mid-point between the olecranon process and the acromion on the left arm. A measurement below 22 cm in women or 23 cm in men suggests chronic energy deficiency. A complete history should also include the patient’s report of their appetite, dietary patterns (number, timing, contents, and size of meals and snacks in an average day), satiety, and recent changes or patterns in any of these factors. This history may be completed through a dietitian consult. Laboratory screening may help identify any metabolic or inflammatory conditions. Basic labs typically include a complete blood count (CBC), a basic metabolic profile, thyroid-stimulating hormone (TSH), and c-reactive protein (CRP). Vitamin B12 and 25-hydroxyvitamin D levels may be required if a deficiency is suspected based on the presence of risk factors (patients with a history of H. pylori infection; those who are institutionalized, homebound, obese, or those with limited sun exposure). Imaging studies, including x-rays of the patient’s chest and abdomen, and advanced imaging studies such as computed tomography (CT) of the patient’s chest, abdomen, and pelvis are not universally recommended but may be indicated in certain scenarios if the patient’s history and physical exam indicate an underlying condition that has not been conclusively identified. While upper endoscopy studies may be indicated for patients with early satiety not otherwise explained, colonoscopy is typically not useful, as colon cancer does not typically present with initial weight loss except in cases of obstruction or extensive metastases (Ritchie & Yukawa, 2020).
Management
If malnutrition is related to a reversible cause, this condition should be addressed first or alongside any nutritional support. For example, a patient with a vitamin B12 deficiency (or an older adult with low-normal levels) should be encouraged to take a daily B12 supplement and ensure 10-15 mcg of dietary B12 daily. The potential benefits of vitamin D supplementation regarding bone fracture risk reduction and frailty are known. Daily caloric requirements should be determined through a consultation with a dietitian or by applying the estimated energy requirement (EER) formula. This formula uses the patient’s age, height, weight, sex, and physical activity coefficient (PAC) to calculate caloric intake. The PAC is 1.0 for sedentary patients, 1.12 for low-activity individuals, 1.27 for active individuals, and 1.45 for very active patients. In terms of energy expenditure, low activity is equivalent to walking 2 miles/day at a pace of 3-4 miles/hour, while active is defined as 7 miles/day, and very active is equivalent to 17 miles/day. Based on these components, the EER formula is as follows:
For females: 354.1 - (6.91 x age in years) + PAC x ([9.36 x weight in kg] + [726 x height in m])
For males: 661.8 - (9.53 x age in years) + PAC x ([15.91 x weight in kg] + [539.6 x height in m])
The National Academy of Medicine, which makes recommendations for the daily intake of macronutrients, suggests 0.80 g/kg of body weight in daily protein intake for adults over the age of 50. Studies indicate that a protein intake higher than this may preserve lean muscle mass and strength and decrease the risk of disability. Like older adults diagnosed with frailty, malnourished older adults should not adhere to dietary restrictions (e.g., low-salt or low-fat), as this may impede intake. This includes a short-term reprieve from low-sugar restrictions for older adults with DM who are nutritionally at risk. Shopping or feeding assistance should be provided if needed. Dietary advice should be tailored to the patient’s taste to encourage intake, including ethnic and cultural preferences. As noted with frail older adults, liquid nutritional supplements provide only a modest (approximately 2%) increase in weight gain across research studies involving older adults, with no improvement in mortality for community-dwelling adults and no improvement in function across multiple settings. Nutritional supplementation may facilitate some improvement in mortality risk for hospitalized undernourished patients over the age of 75 (Ritchie & Yukawa, 2020).
One randomized trial of 41 sarcopenic geriatric patients found improvements across multiple outcomes (e.g., lean body mass, grip strength, albumin, MNA score) when given an amino acid supplement, but this data should be confirmed on a larger scale before being adopted in clinical practice. Vitamin D supplementation with 600-800 IU daily of cholecalciferol (vitamin D3) is recommended for those with a serum 25-hydroxyvitamin D level of 20-30 ng/mL, while higher doses may be required for those with levels under 20 ng/mL. As calcium absorption decreases significantly with age (decreasing by as much as one-third between the ages of 70 and 90), the recommended dietary allowance (RDA) of calcium intake for those over age 50 is 1,200 mg/day. Mineral and vitamin supplementation (i.e., a daily multivitamin with minerals) may aid in managing malnutrition until an underlying cause can be identified. Long-term, the use of a multivitamin/multimineral supplement may be beneficial for certain high-risk older adults (e.g., those in LTC settings) who are not meeting their micronutrient needs due to poor intake if intake cannot be increased or improved via other means. The patient’s dietary intake should be monitored to avoid exceeding the tolerable upper intake level of these nutrients (Ritchie & Yukawa, 2020).
Pharmacologic appetite stimulants should be prescribed cautiously to malnourished older patients. Megestrol acetate (Megace) has only been shown to increase weight in patients with anorexia or cachexia related to cancer or AIDS. These patients should be monitored closely for adverse effects, such as edema, worsening congestive heart failure, venous thromboembolism, corticoadrenal dysfunction, and weakness. Studies indicate an elevated mortality risk among older SNF residents. Dronabinol (Marinol) is a cannabinoid that only improved appetite in AIDS patients with cachexia and appears to be less effective than megestrol acetate (Megace) for cancer patients. It has not been well-studied in older adults and may cause significant central nervous system side effects. For patients with coexisting or underlying depression, mirtazapine (Remeron) is an antidepressant that may cause more weight gain than SSRIs, which should be avoided for malnourished older adults with depression. Ghrelin is an endogenous growth hormone secretagogue that can help stimulate appetite and increase muscle mass in healthy older adults with weight loss and no other underlying conditions, but additional safety and efficacy studies are needed (Ritchie & Yukawa, 2020).
Regarding overnutrition in older adults, the risks associated with being overweight or obese appear to diminish with older age. Most studies indicate that being overweight does not increase the risk for mortality. Factors such as cardiorespiratory fitness, strength, and abdominal circumference may be more important risk indicators in this age group as opposed to BMI alone. Obese older adults with sarcopenia have increased risks of mortality, falls, and cognitive impairment. Weight loss may provide other benefits for obese older adults, such as reduced disability and improved physical function, improved cholesterol and blood glucose, and enhanced QOL. It can also improve symptoms of pain related to arthritis or fatigue due to obstructive sleep apnea (OSA). If appropriate, weight loss in older adults should be slow, cautious, and achieved through regular exercise and a balanced diet consisting of whole foods—especially fruits and vegetables—as opposed to caloric restrictions. Supplementation with calcium and vitamin D may also limit the loss of muscle mass and bone mineral density that affects older adults who experience significant weight loss, even when such weight loss is intentional. A high-protein diet may preserve muscle mass and may be especially important for older obese adults with concomitant sarcopenia (Ritchie & Yukawa, 2020).
Insomnia
Insomnia affects between one-third and two-thirds of American adults, with 10%-15% indicating their insomnia is chronic and associated with daytime consequences. Insomnia can contribute to impaired daytime performance, increase the risk of comorbidities, and decrease QOL (Bonnet & Arand, 2021; Matheson & Hainer, 2017). It is more common among women and older adults and persists longer in these individuals (Bonnet & Arand, 2019, 2021). Other environmental risk factors include unemployment, being single due to divorce/separation or the death of a spouse, and lower socioeconomic status. Having a pre-existing psychiatric disorder also increases a patient’s risk, as approximately one-half of patients with chronic insomnia have a mood disorder, substance use disorder (SUD), or posttraumatic stress disorder (PTSD). Other sleep disorders—including OSA, restless leg syndrome (RLS), and circadian sleep-wake rhythm disorders—often co-occur with insomnia. Medical conditions such as pulmonary disease, hypertension (HTN), DM, cancer, chronic pain, and heart failure are also associated with insomnia. Insomnia frequently accompanies neurodegenerative conditions such as Parkinson’s disease and dementia. Certain medications can contribute to insomnia, especially stimulants, antidepressants, and corticosteroids. The use of alcohol or tobacco or the ingestion of caffeine also correlates with insomnia in adults (Bonnet & Arand, 2021).
Diagnosis
Given insomnia’s prevalence, screening by primary care providers should be integral to the patient’s preventative care and wellness plan. Patients with chronic insomnia seldom present with sleep difficulty as their primary complaint. Screening can occur easily and informally, with a straightforward inquiry regarding the patient's recent sleep habits (Reynolds & Cone, 2018). Short-term insomnia occurs for less than 3 months and is typically associated with a particular medical or psychosocial stressor. Treatment for short-term insomnia is usually not necessary, as symptoms typically resolve when the stressor is eliminated. Chronic insomnia lasts longer than 3 months and occurs at least 3 times a week. These patients report difficulty falling or staying asleep, despite adequate opportunity and circumstances for sleep; the sleeplessness must also be associated with daytime functional impairment, which can include fatigue, inattentiveness, mood disturbances, lack of motivation, increased rate of errors, behavioral disturbances, and increased worry regarding sleep. The patient's anxiety regarding the consequences of lost sleep tend to increase as bedtime approaches and lengthens the period attempting to fall asleep, creating a cycle of worry and poor sleep. Symptoms usually wax and wane, with reports of a good night followed by a couple of bad nights of sleep. Patients with chronic insomnia typically report taking longer than 30 minutes to fall asleep at night (a healthy average is 10-20 minutes for an adult) and spending more than 30 minutes awake during the night. Early morning awakening in chronic insomnia is defined as waking up more than 30 minutes prior to the desired time (Bonnet & Arand, 2019).
Chronic insomnia should be differentiated from delayed sleep-wake phase disorder (DSWPD), which is a significant circadian sleep-wake rhythm disorder that is most prominent in adolescence. These patients similarly report difficulty falling asleep at traditional times and struggle to wake in the morning at an appointed time. In contrast, patients with DSWPD find that when they are on vacation or allowed to sleep based on their desired timeline (e.g., going to bed late on the weekends), they can sleep well. Older adults are prone to advanced sleep-wake phase disorder (ASWPD), in which their circadian rhythm is shifted earlier. They tend to fall asleep early (1900) and wake at early (0300). Similarly, ASWPD can be differentiated from chronic insomnia by asking the patient about their ability to fall asleep if they go to bed when they are tired (e.g., in the early evening). Those with ASWPD typically report no difficulty falling asleep at an earlier time. In contrast to patients with DSWPD and ASWPD, chronic insomnia patients report difficulty sleeping regardless of timing. Chronic insomnia should also be carefully distinguished from short sleep duration, which is a genetic predisposition to a decreased sleep requirement for adequate functioning, and chronic sleep insufficiency, which is a volitional sleep restriction or lack of adequate opportunity to sleep (Bonnet & Arand, 2019).
The diagnosis of insomnia is made clinically based on the patient's report of symptoms and history. The sleep history should entail how long the problem has persisted, how often symptoms occur, the number of awakenings, the length of awakening, the bedtime, duration until sleep onset, awakening time, nap times and length, sleep environment, and any associated daytime symptoms. A validated questionnaire such as the Pittsburgh Sleep Quality Index or the Sleep Problems Questionnaire may be the most reliable method for obtaining this history, as these provide a score that can be used to indicate significant sleep disturbance (Bonnet & Arand, 2019). The Epworth Sleepiness Scale can also be used to assess the severity of sleepiness (Reynolds & Cone, 2018). Most patients with chronic insomnia will exaggerate the amount of time it takes them to fall asleep and underestimate their total sleep time when compared to objective measures such as polysomnography (PSG) or actigraphy. Despite this phenomenon, the patient’s perception of their sleep disturbance is the primary diagnostic indicator used in clinical practice. A set of diagnostic criteria for chronic insomnia called the International Classification of Sleep Disorders – Third Edition (ICSD-3), was last revised in 2014 and is summarized in Table 1 (American Academy of Sleep Medicine [AASM], 2014; Bonnet & Arand, 2019).
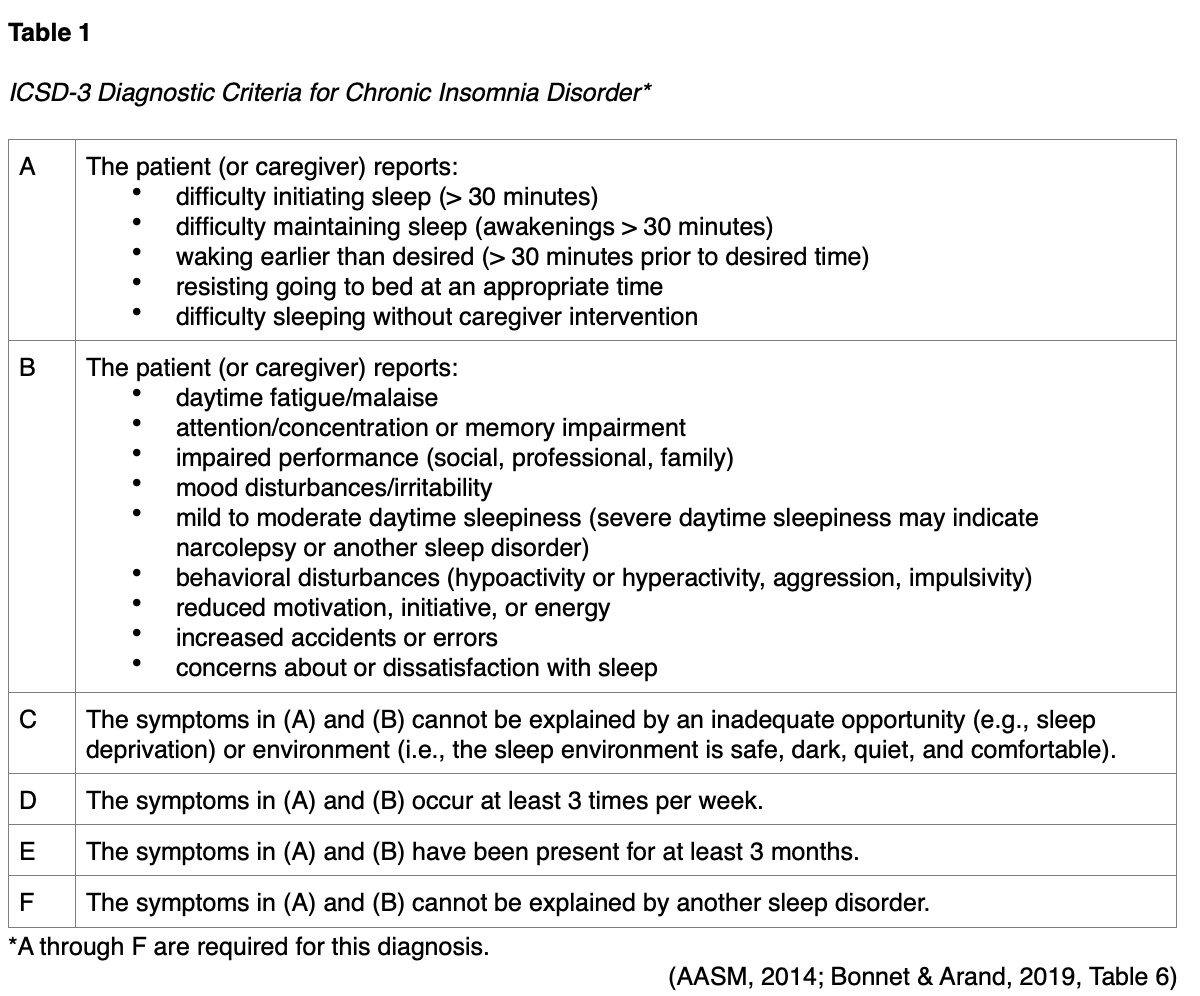
Similarly, the DSM-5 lists the following diagnostic criteria for chronic insomnia (APA, 2013, p. 362; Reynolds & Cone, 2018):
- The patient reports dissatisfaction with current sleep quality or quantity, which may involve sleep initiation, maintenance, or early morning awakening.
- The patient reports associated daytime dysfunction or distress, affecting their social, occupational, educational, academic, behavioral, or other important areas of functioning.
- These symptoms occur at least 3 times a week.
- These symptoms occur for at least 3 months.
- These symptoms occur despite adequate opportunity for sleep.
- These symptoms are not better explained by and do not occur exclusively during a sleep-wake disorder (e.g., narcolepsy, OSA, circadian rhythm sleep-wake disorder, or parasomnia).
- Other organic causes for insomnia have been ruled out (e.g., medications, comorbid conditions).
Symptom duration helps indicate if insomnia is episodic or acute (symptoms lasting for at least 1 month but under 3 months), persistent or chronic (symptoms lasting for 3 months or longer), or recurrent (2 or more episodes within a year; APA, 2013).
Alternatively, a sleep diary may help track symptoms with greater accuracy, as events are reported in real-time and are less prone to reporting errors from the patient. This may be especially helpful for patients with highly variable sleep patterns or those who cannot give an accurate history. Sleep diaries may also aid in tracking symptom changes to assess treatment plan effectiveness. The Consensus Sleep Diary is a 21-item validated sleep log that should be completed within an hour of awakening (items 1-10) and right before bed (items 11-15). Substances (e.g., caffeine, alcohol), medications, and medical conditions that may be contributing to the patient’s sleep dysfunction should be identified via a complete history and physical and optimized if needed. Contributing factors may include:
- psychiatric conditions (e.g., depression/anxiety, PTSD, SUD)
- pulmonary conditions (e.g., COPD, asthma)
- rheumatologic conditions (e.g., arthritis, fibromyalgia/chronic fatigue syndrome, chronic pain)
- cardiovascular conditions (e.g., heart failure, ischemic heart disease, nocturnal angina, HTN)
- neurological conditions (e.g., Alzheimer’s disease, dementia, Parkinson’s disease, peripheral neuropathy, stroke, brain tumor, TBI, headache syndromes, narcolepsy, and fatal familial insomnia)
- other sleep disorders (e.g., RLS, periodic limb movement disorder, OSA, DSWPD, ASWPD, jet lag, shift work disorder, non-24-hour sleep-wake rhythm disorder, irregular sleep-wake rhythm disorder)
- other medical conditions (e.g., hyperthyroidism, nocturia, gastroesophageal reflux, DM, cancer, pregnancy, menopause, Lyme disease, HIV infection, pruritis)
- medications known to cause sleep disturbance (e.g., stimulants, CNS depressants, bronchodilators, antidepressants, beta-antagonists, diuretics, corticosteroids; Bonnet & Arand, 2019, Table 1)
Due to a high correlation with anxiety and depression, patients with insomnia may benefit from screening for these conditions using a validated tool such as the Patient Health Questionnaire – 2 (PHQ-2), PHQ-9, the generalized anxiety disorder 7-item scale (GAD-7), or the State-Trait Anxiety Inventory. The only laboratory or diagnostic testing necessary for insomnia is confirmation of a contributing medical condition, as explained above. Patients with persistent treatment-resistant insomnia or signs and symptoms of other sleep or neurological disorders, such as severe daytime sleepiness indicative of narcolepsy or circadian rhythm sleep-wake disorders, should be referred to a sleep medicine specialist for additional evaluation. A PSG is only indicated clinically for patients with other suspected sleep disorders, such as OSA. The multiple sleep latency test (MSLT) is only indicated for suspected narcolepsy, as evidenced by extreme daytime sleepiness, which is not common in chronic insomnia. Actigraphy uses a noninvasive wristwatch-style accelerometer to track sleep and motor activity over several days. It is only indicated for those with a suspected sleep-wake rhythm disorder (Bonnet & Arand, 2019). The ability to track treatment effectiveness is crucial for chronic insomnia, and this may be done using a sleep diary or a validated tool such as the Insomnia Severity Index (ISI) score (Schroeck et al., 2016).
Management
All contributing conditions and medications should be addressed in order to manage chronic insomnia successfully. For episodic insomnia, patient education and reassurance are typically adequate. Basic sleep hygiene principles should be explained to all patients with insomnia, such as establishing a consistent bedtime routine, avoiding substances (e.g., caffeine after lunch or alcohol and nicotine before bed) or activities (e.g., vigorous exercise within 2 to 3 hours of sleep) that interfere with sleep, avoiding daytime sleeping (e.g., no naps, especially longer than 1 hour and later in the day), and optimizing the sleep environment (e.g., temperature, darkness, limitation of ambient noise). A stable bedtime and wake time should be adhered to throughout the week, with little to no variation on weekends and weekdays. The sleep environment should be kept quiet, dark, and cool. A white noise machine or earplugs can reduce ambient noise, while blackout curtains or an eye mask can minimize ambient light overnight. The use of technological devices (tablets, smartphones, TV) before bed may impact circadian rhythms and shift sleep timing later. Checking the time repeatedly while trying to fall asleep is counterproductive, increasing cognitive arousal and prolonging wakefulness, and should be avoided. Some patients remove their bedside alarm clock for this reason. Large meals or rich, heavy foods should be avoided right before bed, but an evening meal should be eaten to avoid hunger. Regular physical activity can facilitate sleep, especially if performed 4-6 hours before bedtime (but not within 2-3 hours of that time, as described above; Reynolds & Cone, 2018; Winkelman, 2021). A recent randomized clinical trial (RCT) involving 320 older adults found a statistically significant improvement in sleep efficiency, wake time after sleep onset, and the number of awakenings in both the traditional exercise (brisk walking combined with strengthening exercises) and tai chi treatment arms of the study compared to the control group. Both of the intervention groups completed 3 hour-long exercise or tai chi sessions per week throughout the 12-week study. These improvements were maintained through the study’s 24-month follow-up (Siu et al., 2021).
For older adults with chronic insomnia, cognitive behavioral therapy for insomnia (CBT-I) is considered the first-line treatment prior to pharmacological interventions. This multi-component approach targets common behaviors and thoughts that interfere with sleep and can be easily taught to patients by nonpsychiatric clinicians familiar with the components. Alternatively, CBT-I may be delivered face-to-face in individual or group settings over 4-8 sessions. In addition to the sleep hygiene principles described above, sleep restriction is another common component of CBT-I, in which the time spent in bed is limited to the approximate time of desired sleep (e.g., 8 hours in bed). This time should be individualized based on the patient’s 2-week sleep diary. The time spent asleep should be added to half of the time spent awake in bed, yielding the new prescribed sleep restriction. This time should never be below 5 hours. Once the patient sleeps for at least 80% of the prescribed time for 7 consecutive days, the prescription should be increased by 20 minutes for the following week. An example of this calculation is as follows:
Larry reports sleeping 3 hours a night on average over the last 2 weeks in his sleep diary. He reports an average rest (awake) time in bed of 7 hours.
His prescribed sleep restriction time would be: 3 + (7 x ½) = 6.5 hours.
CBT-I also includes avoiding other activities in bed (e.g., reading, watching TV) other than sleeping and sex. Patients are encouraged to go to bed when they feel most sleepy and to get out of bed if they start feeling anxious while lying awake. If anxious and unable to sleep, the patient should leave the room for approximately 10-15 minutes and return to bed when they feel sleepy again. A scheduled wake time should be adhered to consistently, regardless of the amount of sleep achieved. This concept, called stimulus control, is designed to repair the cognitive association between the patient’s bed, bedtime, and sleep. The cognitive components of CBT-I typically focus on anxious thoughts associated with sleep quality and quantity, realistic sleep quantity expectations, accurate attribution of daytime dysfunction and symptoms, and relaxation techniques (e.g., progressive muscle relaxation, diaphragmatic breathing, meditation). The relaxation techniques decrease sympathetic stimulation and physical arousal at bedtime and strive to facilitate calmness and awareness by reconnecting the mind and body. Common misconceptions that should be addressed for patients with chronic insomnia include the belief that (a) sleep must occur for 8 hours uninterrupted to be sufficient, (b) sleep initiation should occur within 10 minutes, and (c) initial tiredness upon awakening indicates poor sleep quality. This cognitive retraining typically takes the most time and may require a referral to a psychiatric provider or sleep center (Reynolds & Cone, 2018; Winkelman, 2021). A recent study by McCurry and colleagues found that CBT-I delivered via telephone was effective. This RCT was completed in Washington State and involved 282 participants 60 years of age and above with chronic osteoarthritis pain. The researchers found that over half (56%) of the study participants remained in remission (as evidenced by an ISI score of < 8) at 1 year (McCurry et al., 2021). For most patients, nonpharmacological treatments should be given for 6 weeks to improve insomnia symptoms before escalating treatment to include a pharmacologic component (Reynolds & Cone, 2018).
Many over-the-counter (OTC) sleep aids are available in the US. OTC antihistamines, which are often used by younger adults for short-term insomnia and even occasionally for chronic symptoms, typically have significant anticholinergic effects, leading to confusion, constipation, urinary retention, sedation, blurry vision, and other side effects in older adults. Dietary supplements like exogenous melatonin are not regulated in the US, making the use of these products less reliable. Melatonin is available as immediate- or extended-release pills, dissolvable tablets, transdermal patches, and liquids and should be dosed a few hours prior to bedtime for the appropriate effect. It functions as an agonist at melatonin receptor sites, decreasing evening arousal of the suprachiasmatic nucleus, and is typically dosed at 1-5 mg. The most commonly reported side effects include vivid dreams or nightmares, dizziness, daytime sleepiness, headaches, stomach cramps, and mood symptoms (depressed or irritable mood), but it is generally considered safe. Evidence for melatonin’s effectiveness in treating sleep-onset insomnia is weak. A 2020 systematic review indicated a small but statistically significant improvement in sleep latency and total sleep time with melatonin use. The study was inconclusive regarding whether these effects were clinically significant. There is no evidence that melatonin is effective in maintaining sleep later in the night or early morning (Reynolds & Cone, 2018; Neubauer, 2020). Research indicates that the production of melatonin typically decreases with age, which may account for the increased prevalence of insomnia in older adults. For this reason alone, some primary care practices (and the American Academy of Family Physicians [AAFP]) recommend a trial of melatonin or a melatonin agonist, which will be discussed later (Matheson & Hainer, 2017). Diphenhydramine (Benadryl) and melatonin are not recommended by the AASM for the treatment of chronic insomnia in adults based on weak evidence. Valerian and L-tryptophan—other OTC products that are often marketed for sleep—are also not recommended by the AASM for the treatment of chronic insomnia in adults based on weak evidence. Specifically, all 4 products have shown a lack of efficacy in reaching clinical significance. While most of these options appear to carry roughly equivalent harms and benefits, the potential harms of L-tryptophan outweighed the benefits (Sateia et al., 2017).
For those with severe distress and dysfunction related to their insomnia, the short-term use of medication may be indicated alongside CBT-I (Winkelman, 2021). The AASM published a clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults in 2017. Eszopiclone (Lunesta), zolpidem (Ambien), and temazepam (Restoril) are recommended by the AASM for the treatment of insomnia in adults with both sleep-onset and maintenance symptoms. They recommended zaleplon (Sonata) and triazolam (Halcion) for the treatment of insomnia in adults with sleep-onset symptoms (Sateia et al., 2017). Unfortunately, most sleep-inducing medications exacerbate existing age-related impairments (e.g., gait instability, urinary dysfunction, sedation, cognitive dysfunction) and are inappropriate for most older adults. Additionally, older adults may have altered drug metabolism, leading to increased serum concentrations of these medications during the day (Winkelman, 2021). Older adults have an increased risk for experiencing adverse drug reactions such as delirium and falls due to sleep medications. Benzodiazepines (e.g., estazolam [ProSom], flurazepam [Dalmane], temazepam [Restoril], triazolam [Halcion], and quazepam [Doral]) and the newer nonbenzodiazepine benzodiazepine receptor agonists (non-BZRAs; e.g., eszopiclone [Lunesta], zaleplon [Sonata], and zolpidem [Ambien]) are all considered potentially inappropriate and included in the most recent and prior versions of the American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (Beers Criteria, or BC). This is due to the risk of impaired cognition, delirium, falls, or motor vehicle accidents. Non-BZRAs may be used with extreme caution in carefully selected older adults, and dosing should be based on the prescribing information for dosing for older adults (typically the lowest available dose). Zolpidem (Ambien) also specifies in its prescribing info that it should be tapered at a rate of 25% every 1-2 weeks when being discontinued to avoid withdrawal symptoms (Neubauer, 2020). A 2016 review of the efficacy and safety of medications used for sleep in older adults concluded that the use of non-BZRAs should be limited based on the current evidence. Non-BZRAs can cause cognitive deficits and serious injury (e.g., fracture) despite their improved side effect profile when compared to traditional benzodiazepines (Schroeck et al., 2016). Antidepressants that are used off-label for insomnia in younger adults (e.g., trazodone [Desyrel]) may have unintended hypotensive effects in older adults and should be considered with extreme caution (Neubauer, 2020). In addition, trazodone (Desyrel) is not recommended by the AASM for the treatment of chronic insomnia in adults based on weak evidence (Sateia et al., 2017).
Dual orexin receptor antagonists (DORA; e.g., lemborexant [DayVigo] and suvorexant [Belsomra]) reduce the wake drive to facilitate sleep and are categorized as schedule IV controlled substances (very low abuse potential). They have been tested for older adults with cognitive impairment, and suvorexant (Belsomra) has been approved for patients with mild to moderate Alzheimer’s disease, but both are contraindicated in patients with narcolepsy. For this reason, these medications are typically considered first-line treatment for older adults with persistent chronic insomnia despite CBT-I or are used short-term while CBT-I is being initiated. The patient should have at least 7 hours available for sleep after taking a DORA. Lemborexant [DayVigo] should be dosed at 5 mg immediately before bed, while suvorexant (Belsomra) should be dosed at 10-15 mg within 30 minutes of bedtime for older adults. A recent meal may delay their onset of action. They typically display similar potency to non-BZRAs, especially for patients with sleep-maintenance symptoms. Their use should also be avoided in patients with severe hepatic impairment or those taking moderate or strong inhibitors of CYP3A4 (e.g., clarithromycin [Biaxin], ketoconazole [Nizoral], voriconazole [Vfend], indinavir [Crixivan], darunavir [Prezista]). The primary side effect of concern is next-day sleepiness due to a longer half-life, and higher cost may be a limitation for many patients (Neubauer, 2020). Suvorexant (Belsomra) is recommended by the AASM to treat insomnia in adults with sleep maintenance symptoms (Sateia et al., 2017). Rosenberg and colleagues (2019) recently published their double-bind RCT involving more than 1,000 adults over the age of 55 with insomnia. They compared the use of 5 or 10 mg of lemborexant (DayVigo) to 6.25 mg of zolpidem extended-release (Ambien XR) or placebo for a month. Their study results indicated a significant improvement in sleep latency, efficiency, and wake time after sleep onset when lemborexant (DayVigo) was compared to placebo, as well as a decreased wake time after sleep onset in the second half of the night when lemborexant (DayVigo) was compared to zolpidem extended-release (Ambien XR; Rosenberg et al., 2019).
Also, 8 mg of ramelteon (Rozerem), a melatonin receptor agonist, within 30 minutes of bedtime may be used as a first-line treatment for those who describe isolated sleep-onset symptoms (difficulty falling asleep without nighttime or early awakenings). It functions as a melatonin receptor agonist and has no abuse potential (i.e., it is not a controlled substance). It typically has a less potent effect (increased sleep time of < 10 minutes) but is also considered a safer option than DORAs for those with mildly delayed circadian rhythms. Patients should avoid eating a high-fat meal with or just prior to taking ramelteon (Rozerem). Aside from somnolence, the most commonly reported side effects include dizziness, nausea, and worsening insomnia. Ramelteon (Rozerem) should be avoided in those taking fluvoxamine (Luvox) and those with severe hepatic impairment (Neubauer, 2020). Ramelteon (Rozerem) is recommended by the AASM for the treatment of insomnia in adults with sleep-onset symptoms (Sateia et al., 2017). Melatonin receptor agonists are especially helpful for shift workers and others with circadian rhythm disturbances. Tasimelteon (Hetlioz) is only available in the US via restricted distribution and is approved for patients with underlying non-24-hour sleep-wake rhythm disorder due to blindness or sleep disturbance related to Smith-Magenis syndrome (a genetic developmental disability syndrome; Reynolds & Cone, 2018). Of note, ramelteon (Rozerem) is also recommended for sleep-onset insomnia in adults by the AAFP, although they note that it is “only modestly effective” (Matheson & Hainer, 2017).
For older adults who describe difficulty with sleep maintenance or mixed symptoms (i.e., both sleep-onset and sleep-maintenance difficulty), a low dose (3-6 mg QHS) of doxepin (Silenor) may also be considered a first-line treatment option. This tricyclic antidepressant (TCA) functions by antagonizing the histamine receptors centrally, with high selectivity for the postsynaptic receptor, creating a sedating effect. It has no abuse potential and is not a controlled substance. Doxepin (Silenor) should be initiated at 3 mg within 30 minutes of bedtime and not within 3 hours of a meal. It should not be used concurrently with a monoamine oxidase inhibitor (MAOI). Side effects, other than the intended somnolence include nausea and other anticholinergic effects. Little improvement was noted across several RCTs between the 3 mg and 6 doses (an increase in 30 versus 38 minutes of sleep/night), and those with hepatic impairment should be maintained at 3 mg (Matheson & Hainer, 2017; Neubauer, 2020). The AASM and AAFP recommend doxepin (Silenor) to treat insomnia in adults with sleep maintenance symptoms due to improved sleep outcomes and a lack of significant adverse effects (Matheson & Hainer, 2017; Sateia et al., 2017).
Regardless of the medication used, the lowest effective dose should be prescribed, and refills should not be given unless tolerability and required need have been established. These medications should not be combined with alcohol, opioids, or other CNS depressants or sedatives (Neubauer, 2020). Irrespective of treatment, patients with insomnia should be scheduled for follow-up every 4-8 weeks, depending on their symptom severity, until symptoms stabilize. Sleep hygiene practices and some learned components of CBT-I can be continued indefinitely, as long as they are perceived as beneficial by the patient. Rarely, patients may require pharmacological treatment for their insomnia long-term, and these patients should be seen to ensure medication is being used properly and to assess for effectiveness every 6 months. Patients with persistent symptoms despite treatment or those with symptoms requiring pharmacological treatment beyond 6 months should be referred to a specialist (Reynolds & Cone, 2018). Insomnia is not only a risk factor for depression, but persistent insomnia is also associated with persistent depression (Espinoza & Unutzer, 2019).
Self-Neglect
Self-neglect among older adults is defined as the “refusal or failure to provide oneself with care and protection in areas of food, water, clothing, hygiene, medication, living environments, and safety precautions” (Dong, 2017, p. 1). In the US, self-neglect is determined to be the underlying cause for roughly 40% of neglect cases reported to Adult Protective Services. Prevalence is difficult to estimate due to a paucity of research on the topic and variable operational definitions and methods of measurement. The Chicago Health and Aging Project (CHAP) found a self-neglect prevalence of 21% among African American participants and only 5.3% among European Americans across 5,519 total study participants. While the 2010 Elder Justice Act (EJA) defines self-neglect as “the inability, due to physical or mental impairment or diminished capacity, to perform essential self-care,” numerous conceptual definitions have been developed since that time, and no universal operational definition exists. Risk factors are equally difficult to define but generally include cognitive impairment (e.g., diminished executive or global functioning), physical disability, and psychological distress (e.g., depressed mood). Self-neglect also appears to occur more commonly among older adults with limited family or social support and engagement. Self-neglect not only impacts well-being but also increases healthcare resource utilization and mortality risk. It typically leads to poor adherence to the medical plan of care and nutritional deficiencies (Dong, 2017).
Screening and Assessment
The American Medical Association and the American Academy of Neurology both recommend screening individuals over 65 for potential abuse (Halphen, 2021). A screening tool may help HCPs identify vulnerable older adults. Screening can be difficult to perform clinically, as most scales contain an in-home assessment portion. HCPs must be aware that in most states, reporting suspected elder abuse, including self-neglect, is mandatory for medical providers. HCPs should know the specific regulations of their state and locality regarding the expectations of reporting suspected abuse of adults over the age of 65 (Dong, 2017). This can typically be done confidentially and does not require the permission of the patient (Halphen, 2021). Currently, there are 2 available screening tools for self-neglect that have been tested psychometrically: The Chicago Self-Neglect Scale (CSNS) and the Texas Self-Neglect Scale (TSNS). The CSNS assesses for self-neglect through 27 items in 6 domains: hoarding, personal hygiene, a house in need of repair, unsanitary conditions, and inadequate utilities. The TSNS uses 5 categories: living condition, financial status, physical/medical status, mental status, and social interaction/support (Dong, 2017). The Elder Self-Neglect Assessment (ESNA) evaluates a patient’s living conditions, as well as their physical and mental health, social network, and financial concerns (Baruth & Lapid, 2017). Additionally, the Self-neglect Severity Scale (SSS) incorporates the patient’s hygiene, function, and living environment. A full history and physical exam (e.g., a comprehensive geriatric assessment [CGA]) should be completed if suspicion exists, with a focus on functional and cognitive ability. Direct observation should be prioritized over a patient’s response to questioning. Warning signs of physical injuries include skin tears, pressure injuries, fractures, and wounds that are not properly cleaned or dressed. The HCP should also note evidence of malnutrition or dehydration, such as hypernatremia, elevated blood urea nitrogen (BUN), low cholesterol, decreased lymphocyte count, and elevated hemoglobin (Halphen, 2021).
An older adult’s lack of awareness regarding self-neglect behaviors may indicate a diminishing capacity for decision-making. The capacity to make decisions requires an understanding of the current situation or problem, a grasp of the proposed solution(s) and their associated risks and benefits, and the ability to communicate that understanding to those around them. This ability may falter during the decision-making process or in the execution. In general, an HCP should assume older adults have decision-making capacity until indicated otherwise, even when their decision may not align with the HCP’s expectations. Brief screening tools to assess decision-making capacity include the Aid to Capacity Evaluation, the Hopkins Competency Assessment Test, and Understanding Treatment and Disclosure. The results of a CGA, as mentioned above, will also aid the HCP in assessing a patient's decision-making ability without infringing on their autonomy (Dong, 2017). The patient’s prior history of decision-making should also be taken into account to help determine whether recent decisions align or deviate from previous patterns (Baruth & Lapid, 2017). Neuropsychological testing may be required if the patient’s decision-making capacity remains in question, and the legal system is ultimately responsible for determining a person’s capacity for self-care and self-protection (Halphen, 2021).
If an older adult is no longer capable of making decisions, the HCP should advocate for the maximum preservation of the patient’s rights and decision-making based on the patient’s best interests (Dong, 2017). This balance between the medical concepts of beneficence (acting in the patient’s best interest), nonmaleficence (do no harm), and the patient’s right to self-determination and autonomy are difficult to navigate for even the most experienced and conscientious HCPs. If a patient is determined to be incapable of decision-making, then a surrogate decision-maker should be appointed immediately based on the patient’s advance directives, if available (Baruth & Lapid, 2017). While legal provisions vary by state for adult guardianship, the following conceptual model describes 6 key domains:
- the presence of a medical condition that may lead to disability
- cognitive impairment (e.g., memory, communication, attention, or executive function)
- difficulty with everyday functioning (e.g., the provision of food, shelter, and protection)
- consideration for the patient’s preferences and values
- the risk of harm present and the level of supervision required
- consideration of the feasibility of a less-restrictive option besides guardianship (Halphen, 2021)
Prevention and Management
Unfortunately, randomized clinical trials regarding the best methods to prevent and manage self-neglect are lacking (Dong, 2017). Most programs designed to prevent self-neglect focus on training HCPs to recognize the signs of neglect and encouraging a more positive attitude toward older adults within a community or culture. Training should include all HCPs, not just those specifically caring for older adults, as most HCPs interact with older adults in some capacity, and have the ability to intervene on their behalf. This training should also encompass reporting elder abuse, including local statutes and common barriers to reporting (e.g., lack of recognition regarding when and why to report, lack of knowledge about how and where to report suspected cases, and awareness of the legal protections for those who report suspected cases of neglect in good faith). Public education campaigns often target community members who interact with older adults daily (e.g., grocery store clerks, police officers, mail carriers) with similar information, such as risk factors, warning signs, and the process of filing a report. The management of self-neglect, similar to its assessment, should involve a multidisciplinary team with solid patterns of communication, collaboration, and shared decision-making. This team should include HCPs, a licensed social worker, community educational programs, and financial service agencies (Dong, 2017). Any underlying conditions identified during the evaluation should be managed appropriately based on evidence-based guidelines, such as depression or delirium. Through discussions regarding goals of care and shared decision-making, the HCP should find commonality whenever possible to encourage a sense of teamwork; the patient and the healthcare team should then collaborate on achieving these goals first. Safety interventions should focus on areas with the greatest possible harm reduction (Baruth & Lapid, 2017).
Depression and Loneliness
Depression often affects older adults (with a prevalence of 2% to 10% in community-dwelling older adults), but is not a normal part of aging. Sadness, stress, and grief are expected reactions to some of the common life events that occur after the age of 65, such as experiencing the departure of grown children; selling a home to downsize; retiring; losing family members and loved ones; declining social, cognitive, or physical functioning due to age or illness; and decreasing independence due to disability. The prevalence of depression increases with comorbid medical conditions and within healthcare settings, climbing as high as 50% in SNF residents. Risk factors for late-life depression include female sex, social isolation, previously married status (i.e., separated, divorced, or widowed), lower socioeconomic status, comorbid medical conditions, chronic pain, insomnia, functional impairment, and cognitive impairment. Depressed mood amplifies a patient’s disabilities, decreases their QOL, increases their consumption of healthcare resources, and increases their risk of SUD. Although adults over age 65 account for roughly 13% of the US population, they comprise nearly 24% of completed suicides. The suicide rate is highest among older men, especially over the age of 85. Acute indications of suicide risk include hopelessness, insomnia, agitation, restlessness, poor concentration, psychotic symptoms, SUD, and untreated pain (Espinoza & Unutzer, 2019).
For additional information regarding the assessment and prevention of suicide, please refer to the NursingCE course entitled Suicide.
Assessment and Diagnosis
Depression may be harder to diagnose in older adults, as their symptoms may vary. Older adults often exhibit fatigue, sleep disturbances, irritability, confusion, or inattention. Chronic medical conditions (e.g., Alzheimer’s disease, Parkinson’s disease, heart disease, stroke, and cancer) can increase the risk of depression or cause depressive symptoms. This is especially true of vascular depression, which is more common in older adults with other vascular conditions, such as cardiovascular or cerebrovascular disease. Certain medications can also provoke symptoms of depression (NIA, 2017). Older adults with depression may present with no response to standard medical treatment for an unrelated condition, poor motivation to participate in their medical care, somatic symptoms that are more severe than expected, or decreased engagement with the healthcare team. For those older than 85, dysphoric mood is a less reliable indicator of depression (Espinoza & Unutzer, 2019).
A validated assessment tool should be used for depression screening, such as the Two-Question Screener/PHQ-2. This tool asks about feeling down, depressed, or hopeless and a lack of interest or pleasure in doing things previously enjoyed (anhedonia). If positive, the remaining 7 questions that make up the PHQ-9 can be used to improve the specificity of the screen. The Geriatric Depression Scale is specifically designed for use in older adults (Ward & Reuben, 2020). Major depressive episode (MDE) and major depressive disorder (MDD) can be diagnosed clinically based on the criteria in the APA’s DSM-5. For a patient to be diagnosed, they should demonstrate a minimum of 5 of the 9 symptoms listed in Criteria A for at least 2 consecutive weeks, including a depressed mood or anhedonia. Other than a depressed mood, the remaining symptoms can be recalled using the mnemonic SIG E CAPS, which stands for sleep, interest, guilt, energy, concentration, appetite, psychomotor agitation/retardation, and suicidal ideations. For patients with fewer symptoms that last for at least 2 years, a diagnosis of persistent depressive disorder or dysthymia should be considered (APA, 2013).
Grief, which is also common among older adults, can be difficult to distinguish from MDD or MDE. Grief is characterized by feelings of emptiness and loss, and dysphoria occurs in varying intensity (associated with reminders of the departed) but typically decreases over time. These “waves” or “pangs” of grief are interspersed by periods of humor or positive emotions. Thoughts tend to focus on the deceased and joining them, but suicidal ideations are uncommon. While guilt is common regarding actions or lack of actions regarding the deceased, self-esteem is preserved. By contrast, dysphoria in MDE is consistent, and thoughts are largely self-critical and pessimistic. Feelings of worthlessness and thoughts of suicide are common (APA, 2013).
For additional information regarding the assessment and diagnosis of depression, please refer to the NursingCE courses on Depression and Care Considerations for Older Adults: The Complete Geriatric Assessment.
Management
The undertreatment of depression can have devastating effects. In a systematic review and meta-analysis of older adults with depression living in the community or being managed through a primary care office, only 4% to 37% of patients received some treatment. At the 2-year follow-up, only 33% of patients were well, while 33% reported persistent depression and 21% were deceased (Kok & Reynolds, 2017).
Physical exercise offers many benefits for adults over the age of 60 with depression, especially cardiovascular activities such as walking or swimming. Although high-quality RCTs are lacking and the benefits seem modest, systematic reviews are positive (Kok & Reynolds, 2017). Bright light therapy, using pale blue 7500 lux, appears to be well-tolerated and may be beneficial for some adults with depression. If available, home-based interventions may help those with limited mobility and mild depression. The involvement of family members in the education and care of patients with depression can improve outcomes, as they not only assist in reinforcing and encouraging treatment adherence but also provide valuable observational insight to the healthcare team (Espinoza & Unutzer, 2019).
Yoga is a mind and body practice founded in ancient Indian philosophy. It centers on achieving a relaxation response through spirituality and meditation. Meditation is especially beneficial for reducing stress and depressive symptoms. Studies have shown that yoga and meditation have positive benefits for people with depression and various mental health conditions (Mental Health America, 2016; National Center for Complementary and Integrative Health, 2019). Sharma and colleagues (2017) evaluated the feasibility, efficacy, and tolerability of Sudarshan Kriya yoga (SKY) as an adjunctive intervention for patients with MDD. SKY is a breathing-based meditative technique that focuses on slow, medium, or fast rhythmic breathing cycles. It has been reported to decrease cortisol, increase prolactin, and improve antioxidant status in practitioners. The researchers’ findings demonstrated that SKY helped alleviate severe depression in people who did not fully respond to antidepressant treatments (Sharma et al., 2017).
Both psychotherapy and somatic treatments (e.g., antidepressant medication) are considered first-line and equally efficacious for older adults with depression of any severity. The choice between options (or a combination) should depend on various factors, such as contraindications, availability, cost, patient preference, and characteristics of the condition (severity, type, and chronicity). Pharmacotherapy is recommended for those with moderate to severe disease, while a combination of pharmacotherapy and psychotherapy appears to be most effective for those with chronic disease (Espinoza & Unutzer, 2019). A collaborative care model can produce improved outcomes, using patient education and care managers or non-physician mental health professionals to integrate the psychiatric and primary care components of care. These programs improve not only depression but also general medical outcomes and reduce mortality. Depression-specific case management can reduce depressive symptoms and mortality (Espinoza & Unutzer, 2019).
Psychotherapy is effective yet underutilized; it can be difficult to access (at times) depending on location and often not/poorly covered by medical insurance. It can be done individually, in groups, or as a couple or a family. It can be performed in a private office, in a senior center, or within outpatient or day treatment programs. Multiple RCTs and meta-analyses have demonstrated a significant, clinically moderate to large effect. Options for psychotherapy include cognitive-behavioral therapy (CBT), interpersonal therapy (IPT), and problem-solving therapy. They are typically delivered over a 2- to 4-month period. While CBT is the most widely studied, research indicates that IPT is equally efficacious, and problem-solving therapy can be beneficial (Espinoza & Unutzer, 2019). Psychotherapy may be adequate for the treatment of depression that is mild to moderate (i.e., with a PHQ-9 score of below 10; Kok & Reynolds, 2017).
CBT. CBT is a type of psychotherapy with strong clinical evidence supporting its use as an effective treatment for depression. CBT helps patients assess and restructure negative thinking patterns associated with depression. Through CBT, the patient can recognize negative thoughts and learn positive and effective coping strategies. CBT is time-limited and typically consists of 8 to 16 sessions. Patients track their thoughts and activities to identify the affective and behavioral consequences. They subsequently learn techniques to change their way of thinking and activities to improve their mood. CBT has demonstrated efficacy across diverse populations, including civilians, veterans, active service members, and family members suffering from depression. CBT can also be administered via computer programs, a process referred to as computer-based CBT (CCBT; Stein, 2020; Stone et al., 2017). An RCT of older adults with an anxiety disorder also found that CBT in a primary care setting effectively decreased worry and moderately improved depressive symptoms (Espinoza & Unutzer, 2019).
Mindfulness-Based Cognitive Therapy (MBCT). MBCT integrates CBT interventions with mindfulness-based skills to help patients attend to the present moment in a non-judgmental, accepting manner. Unlike CBT, MBCT does not seek to modify or eliminate dysfunctional thoughts. Instead, it helps patients become more detached and observe their thoughts objectively, without necessarily attempting to change them. MBCT employs meditation, imagery, experiential exercises, and relaxation techniques. Mindfulness is not appropriate as first-line therapy for severe depression but rather as an adjunct or complementary therapy or an alternative for mild symptoms in motivated patients (MacKenzie & Kocovski, 2016).
IPT. IPT focuses on improving problems within personal relationships as a core component of depression. While an event or a relationship may not always cause depression, depression affects relationships and can create interpersonal problems. IPT is a short-term treatment that teaches patients to evaluate their interactions to understand and improve how they relate to others. IPT is derived from attachment theory, and it treats depression by focusing on improving interpersonal functioning and exploring relationship-based difficulties. IPT specifically targets 4 primary areas: interpersonal loss, role conflict, role change, and interpersonal skills (IPT Institute, n.d.; Stone et al., 2017).
Pharmacotherapy. Antidepressant medications are the pharmacological treatment of choice for depression. While mild-to-moderate depression can often be treated with therapy alone, moderate-to-severe cases of depression often require the addition of medication (PHQ-9 score of 10 or above; Kok & Reynolds, 2017). Medication therapy aims to help reduce or control the symptoms of depression. The bulk of medications that are currently approved by the US Food and Drug Administration (FDA) for treating depression target the 3 neurotransmitters historically associated with depression: serotonin, norepinephrine, and dopamine. Most agents need to be initiated at low doses, tapered up slowly when starting, and tapered down before discontinuing. Antidepressants should not be abruptly stopped due to the risk of withdrawal and the subsequent return of depressive symptoms. If they are stopped abruptly, withdrawal-like symptoms can include dizziness, headaches, flu-like syndrome (tiredness, chills, muscle aches), agitation, irritability, insomnia, nightmares, diarrhea, and nausea. Regardless of the medication prescribed, patients must be counseled that antidepressants may take 4-6 weeks to have an effect and 12-16 weeks to achieve their full benefits (National Alliance on Mental Illness [NAMI], 2017; National Institute of Mental Health [NIMH], 2016b; US Department of Veterans Affairs [VA], 2016). Older adults should be treated with monotherapy when possible to avoid drug-drug interactions and polypharmacy. While the initial dosing should be adjusted (typically by cutting the initial adult dosage in half), most older adults should be maintained within the same therapeutic dosage range as younger adults (Espinoza & Unutzer, 2019).
All of the major medication classes commonly prescribed for depression are included in the 2019 BC list, including SNRIs, SSRIs, and TCAs. TCAs can exert anticholinergic effects, such as sedation, leading to orthostatic hypotension and falls. SSRIs are included on the list, and SNRIs were recently added, but only for older patients with a history of falls. SSRIs and SNRIs both increase the risk of patient falls, hyponatremia, and syndrome of inappropriate antidiuretic hormone secretion. The BC list cites a study that showed a 48% increase in fall risk for older adults taking an antidepressant. They also caution against combining 3 or more CNS-active medications (e.g., antidepressants, antipsychotics, AEDs, and benzodiazepines). The AGS encourages healthcare providers to “avoid antidepressants unless safer alternatives are not available,” specifying that the evidence does not appear to support the safety of a particular antidepressant over others (AGS Beers Criteria Update Expert Panel, 2019; Fixen, 2019).
In 2004, the FDA required a warning to be printed on the labels of all antidepressant medications regarding the risk of increased suicidality among children and adolescents taking these medications. The warning was expanded in 2007 to include all young adults, especially those under the age of 25, stating that these individuals may experience an increase in suicidal thoughts or behaviors during the first few weeks of taking an antidepressant. Before starting the medication, the individual may have been too paralyzed by depression to make a suicide plan. As a result, the risk of suicide rises while the depressive symptoms start to improve. Researchers found evidence that individuals taking antidepressant medication may have an even higher risk of suicide than individuals whose depression is improving for other reasons (Fornaro et al., 2019). The FDA also requires manufacturers to provide a Patient Medication Guide (MedGuide), which is given to individuals receiving these medications to advise them of the risks and precautions that can reduce the risk of suicide. Furthermore, clinicians are advised to ask patients about suicidal thoughts prior to prescribing antidepressants to young persons (FDA, 2018). Studies have shown a similar trend in older adults, with a mild increase in the suicide rate among men over age 65 during the first month of treatment with SSRIs (Espinoza & Unutzer, 2019).
SSRIs include citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac), fluvoxamine (Luvox CR), paroxetine (Paxil), and sertraline (Zoloft; NAMI, 2017). Compared to TCAs, these drugs have fewer adverse cardiac effects, typically require only 1-2 dose increases to reach their targeted dose, and have a reduced risk of fatal overdose. Unfortunately, they involve more drug-drug interactions than TCAs, which is a concern among older adults, who are more prone to polypharmacy (Kok & Reynolds, 2017). Research indicates that the improved side effect profile of SSRIs is clinically significant, as it leads to a higher rate of voluntary patient withdrawal when patients are given TCAs versus SSRIs. For this reason, in combination with the anticholinergic effects of TCAs, SSRIs are typically the first-choice antidepressant for older adults. This is not related to increased efficacy with SSRI use. In older adults, SSRIs can also cause parkinsonism, restlessness (akathisia), anorexia, sinus bradycardia, and hyponatremia (Espinoza & Unutzer, 2019). SSRIs can increase serotonin levels in the body, posing a risk of serotonin syndrome, which is characterized by agitation, anxiety, confusion, high fevers, sweating, tremors, a lack of coordination, dangerous fluctuations in blood pressure, and rapid heart rate. Serotonin syndrome is a potentially life-threatening condition for which patients must seek immediate medical attention (NAMI, 2017).
A dose-related increase in the risk for fragility fractures has been reported with SSRI use. Sertraline (Zoloft), citalopram (Celexa), and escitalopram (Lexapro) are generally well-tolerated (i.e., non-sedating with a low risk of insomnia) and may be good first choices for older adults with depression. Escitalopram (Lexapro) should be initiated at 5 mg daily, with a goal of 5-20 mg/day. Citalopram (Celexa) should be initiated at 10 mg daily, with a goal of 10-20 mg/day. Due to an increased risk for QT interval prolongation and arrhythmias, citalopram (Celexa) should not be dosed for older adults above 20 mg/day. Sertraline (Zoloft) should be initiated at 12.5-25 mg every morning, with a goal of 25-200 mg/day; it has been reported to cause diarrhea more often than other SSRIs. Fluoxetine (Prozac) should be initiated at 5-10 mg every morning, with a goal of 5-60 mg/day. It may have an activating effect, which may be beneficial for patients with fatigue. It also has a longer half-life, which may lengthen the period to reach a steady-state but typically involves a lower need to taper when discontinuing. All other SSRIs should be tapered when discontinuing to avoid withdrawal symptoms. Paroxetine (Paxil) should be initiated at 10 mg every evening, with a goal of 10-40 mg/evening (Espinoza & Unutzer, 2019, Table 6). A 2010 meta-analysis by Serretti and Mandelli found that paroxetine (Paxil) was more likely to lead to weight gain. Fluvoxamine (Luvox) should be initiated at 25 mg every evening, with a goal of 25-200 mg/evening. Paroxetine (Paxil) and fluvoxamine (Luvox) may be good choices for those with insomnia, although fluvoxamine (Luvox) and fluoxetine (Prozac) have more significant drug interactions (Espinoza & Unutzer, 2019, Table 6). A longitudinal study involving over 2,300 adults (average age 55) found an association between SSRI use and weight gain, especially for those who ate a Western diet, had a sedentary lifestyle, and smoked (Shi et al., 2017).
SNRIs include duloxetine (Cymbalta), venlafaxine (Effexor), desvenlafaxine (Pristiq), and levomilnacipran (Fetzima). Like SSRIs, SNRIs can cause serotonin syndrome (NAMI, 2017). SNRIs are typically a second choice for those who respond to or tolerate treatment with an SSRI. They may be especially beneficial for those with chronic pain. Few studies directly compare SSRIs and SNRIs, although a study has indicated that frail patients may not tolerate venlafaxine (Effexor) as well as sertraline (Zoloft). Both venlafaxine (Effexor) and duloxetine (Cymbalta) may cause diastolic HTN at higher doses, and extended-release formulas of venlafaxine (Effexor) appear to cause fewer gastrointestinal symptoms and agitation. Venlafaxine (Effexor) and desvenlafaxine (Pristiq) may have an activating effect, which may be beneficial for those with fatigue. Venlafaxine (Effexor) should be initiated at 18.75-37.5 mg daily, with a goal of 75-225 mg/day for ER formulas and 75-150 mg twice daily for immediate-release formulas. Desvenlafaxine (Pristiq) should be initiated at 50 mg every morning and maintained at this dosage. It should be dosed at 50 mg every other day for those with a creatine clearance (CrCl) below 30 mL/min. Duloxetine (Cymbalta) should be initiated at 10-20 mg every day, with a goal of 20-60 mg/day. It is mildly sedating and poses a reduced risk of insomnia but more significant drug interactions (Espinoza & Unutzer, 2019).
Tricyclic and tetracyclic antidepressants are an older class of medications, consisting of amitriptyline (Elavil), nortriptyline (Pamelor), clomipramine (Anafranil), imipramine (Tofranil), and desipramine (Norpramin). They also inhibit norepinephrine and serotonin reuptake but carry significantly more adverse effects than SSRIs or SNRIs (NAMI, 2017). TCAs may be fatal in overdose, have significant drug-drug interactions, and are potentially cardiotoxic. Anticholinergic side effects include dry mouth, constipation, urinary retention, blurry vision, and orthostatic hypotension. Due to these effects, they are contraindicated in men with prostatic disease or patients with narrow-angle glaucoma. They are also no longer considered first- or second-line treatment options for depression. They may be effective for those with melancholic or delusional depression, and they may prevent relapse after electroconvulsive therapy. Nortriptyline (Pamelor) should be started at 10 mg every evening, increasing to a maximum of 100 mg daily in 1 dose or 2 divided doses. The established serum concentration is 50-150 ng/mL. Nortriptyline (Pamelor) tends to be mildly sedating, making it more useful for anxious patients with insomnia when taken at bedtime. Desipramine (Norpramin) should be started at 10 mg every morning, increasing to a maximum of 150 mg daily in 1 dose or 2 divided doses. The established serum concentration is 125-300 ng/mL. It tends to have a mild stimulant effect, which may be beneficial for patients with low energy. Mirtazapine (Remeron) is a tetracyclic antidepressant that is occasionally used to treat depression in older adults, especially those suffering from anorexia or insomnia related to their mood disorder, as it tends to be sedating and lead to weight gain. It can also cause dry mouth and constipation. Mirtazapine (Remeron) should be initiated at 7.5 mg every evening with a dosing goal of 15-60 mg per evening. It can also be beneficial for those with essential tremors or parkinsonism and nausea related to chemotherapy (Espinoza & Unutzer, 2019). A 2010 meta-analysis by Serretti and Mandelli found amitriptyline (Elavil) and mirtazapine (Remeron) were among the most likely antidepressants to contribute to weight gain.
MAOIs were the first type of antidepressant medications developed. They impair serotonin’s metabolism and block monoamine oxidase, an enzyme that breaks down excess tyramine in the body. Tyramine is an amino acid that occurs naturally in the body (and certain foods) to help regulate blood pressure. MAOIs include tranylcypromine (Parnate), phenelzine (Nardil), isocarboxazid (Marplan), and a transdermal skin patch (selegiline [Emsam]). They may be effective for reverse neurovegetative depression, mixed anxiety-depressive states, and panic disorders. Due to the risk of serious adverse effects, the use of MAOIs for the treatment of depression should be reserved for patients who have failed all other treatment options or those who have previously been started on and tolerated them. MAOIs have dangerous drug and food interactions that can lead to serotonin syndrome and hyperadrenergic crisis. In particular, clinicians must warn patients to avoid foods containing high levels of tyramine, such as aged cheese (aged cheddar, swiss, parmesan, and blue); cured, smoked, or processed meats (pepperoni, salami, hotdogs, bologna, bacon, corned beef, smoked fish); pickled or fermented foods (sauerkraut, kimchi); sauces (soy sauce, miso, teriyaki); soybean products; and alcoholic beverages (beer, red wine, liquors). Drug interactions with other medications that increase serotonin levels, such as triptans to treat migraines, can cause serotonin syndrome (Espinoza & Unutzer, 2019; NAMI, 2017; NIMH, 2016b). The most common adverse effects of antidepressants are displayed in Table 2.
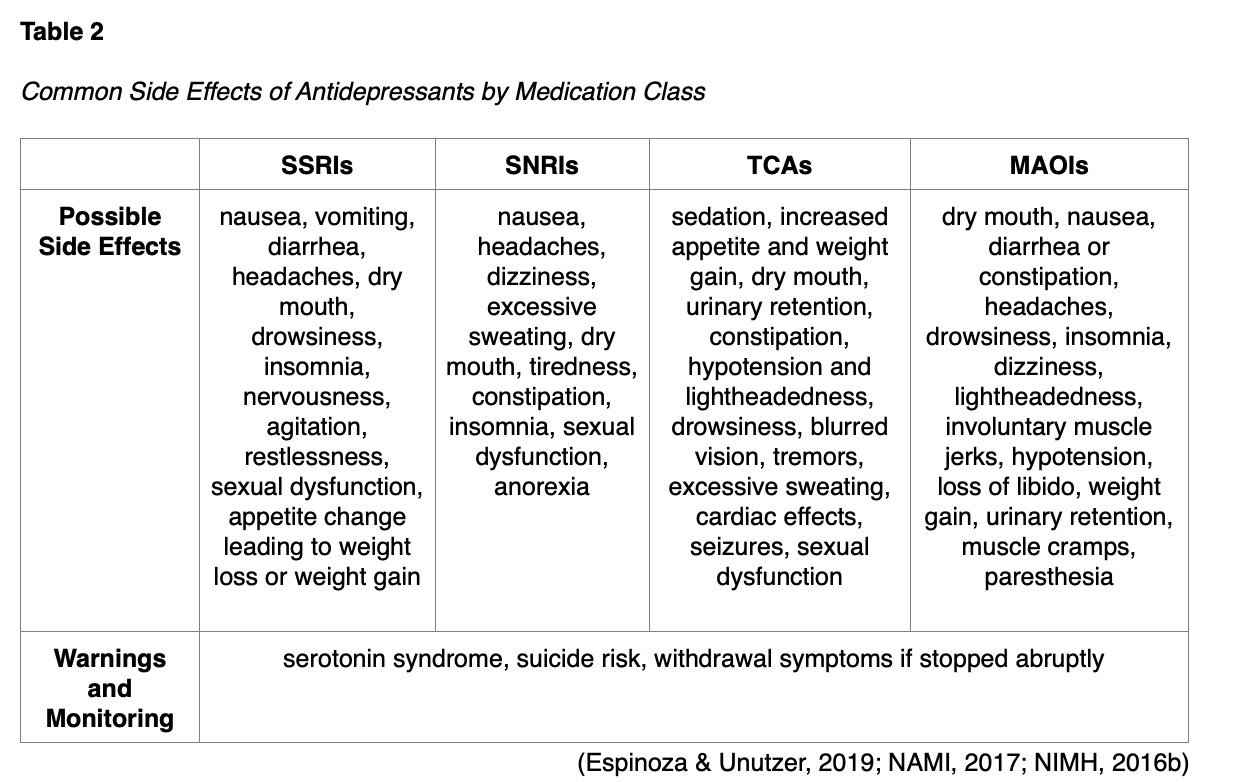
There are also a few uncategorized atypical antidepressants. Bupropion (Wellbutrin) blocks the reuptake of norepinephrine and dopamine and can be used for depression, seasonal affective disorder, and smoking cessation. Bupropion (Wellbutrin) affects mostly dopamine in the brain and does not confer a risk of serotonin syndrome (NAMI, 2017; NIMH, 2016b). It should be initially dosed at 75 mg in the morning and increased to twice daily (midafternoon) toward a goal of 150 mg twice daily. Bupropion (Wellbutrin) should be avoided by those with a seizure history or bulimia nervosa and should not be used concurrently with CNS depressants (e.g., benzodiazepines). It tends to have a stimulating effect, which may be beneficial for patients with lethargy or fatigue but should be avoided for those with agitation. Its dopaminergic activity may also be beneficial for those with a diagnosis of Parkinson’s disease. It can also cause diastolic HTN at higher doses in older adults (Espinoza & Unutzer, 2019). A 2010 meta-analysis by Serretti and Mandelli found Bupropion (Wellbutrin) was among the most likely antidepressants to contribute to weight loss.
Trazodone (Oleptro) antagonizes serotonin and alpha-1 adrenergic receptors and blocks the reuptake of serotonin (NAMI, 2017; NIMH, 2016b). It should initially be dosed at 12.5-25 mg about 30-60 minutes before bedtime, with a goal of increasing the dose to 25-100 mg daily before bed. It tends to be highly sedating, making it beneficial in lower dosages as an adjunctive treatment for depressed patients reporting insomnia. It can also cause orthostatic hypotension, nausea, cognitive impairment, residual daytime fatigue, and priapism (Espinoza & Unutzer, 2019). Similarly, nefazodone (Serzone) antagonizes serotonin receptors and blocks the reuptake of norepinephrine and serotonin. It has been removed from markets around the world due to hepatotoxicity concerns. It may cause sedation or drowsiness, making it useful for certain depressed patients who report insomnia, anxiety, or agitation. Significant drug-drug interactions may occur with macrolide antibiotics, antiarrhythmics, and certain psychotropics. Both trazodone (Oleptro) and nefazodone (Serzone) have been associated with hyponatremia (Espinoza & Unutzer, 2019). Vilazodone (Viibryd) is an SSRI and a partial serotonin receptor agonist (NAMI, 2017; NIMH, 2016b). It should initially be dosed at 10 mg daily with food, with a goal of increasing the dose to 20-40 mg daily with food. It may cause side effects such as diarrhea, nausea, vomiting, dizziness, or insomnia but has a lower incidence of sexual dysfunction and weight gain (Espinoza & Unutzer, 2019). Vortioxetine (Trintellix) is a newer medication for depression that inhibits serotonin reuptake and acts as a mixed antagonist/agonist of specific serotonin receptors (NAMI, 2017; NIMH, 2016b).
Once any medication has been started, patients should be contacted within 2 weeks and seen in the office in 2-4 weeks to discuss tolerance, side effects, efficacy, complications, and dose adjustments if appropriate. The usual course of treatment for an initial MDE or episode of MDD is 6-12 months once remission is achieved. This treatment period may need to be extended to prevent relapse, which occurs at a higher rate in older adults compared to younger adults. Those with dysthymia or multiple relapses may require long-term treatment Espinoza & Unutzer, 2019). Most research findings support treatment lasting for a year before discontinuation is considered. Multiple relapses likely indicate the need for a longer treatment course, lasting up to 24 months in those with 2 episodes of depression and up to 36 months in those with 3 or more episodes of depression, to reduce the risk of relapse (Kok & Reynolds, 2017).
As previously stated, older adults with depression and unintended weight loss should be treated with structured psychotherapy and antidepressant medication to reverse their weight loss and improve mood and QOL (Agarwal, 2019). Certain SSRIs should be avoided for older adults with depression who are malnourished, as they may contribute to weight loss. Mirtazapine (Remeron), amitriptyline (Elavil), citalopram (Celexa), paroxetine (Paxil), and venlafaxine (Effexor) are less likely to contribute to anorexia and weight loss, while TCAs, bupropion (Wellbutrin), and certain SSRIs—especially fluoxetine (Prozac) and sertraline (Zoloft)—should be avoided by these patients (Agarwal, 2019; Ritchie & Yukawa, 2020; Serretti & Mandelli, 2010). Despite a small sample size (362 patients), in 2015 Uguz and associates found weight gain occurred in more than half of patients taking antidepressants. Weight gain in the study occurred with citalopram (Celexa), escitalopram (Lexapro), paroxetine (Paxil), sertraline (Zoloft), duloxetine (Cymbalta), mirtazapine (Remeron), and venlafaxine (Effexor). The only medication not linked to weight gain in this study was fluoxetine (Prozac; Uguz et al., 2015). Psychostimulants, at low doses, may prove beneficial for some older adults with severe depression and associated apathy. Methylphenidate (Ritalin, Concerta) may be initiated cautiously for this group at a dose of 2.5 mg in the morning and titrated up if needed to a maximum of 5-40 mg daily. Caution should be used regarding dosing, as methylphenidate (Ritalin, Concerta) leads to weight loss when given in doses of 20 mg daily or higher. When combined with an SSRI, methylphenidate may improve outcomes and lead to a faster improvement in mood versus either treatment alone. Aripiprazole (Abilify), a dopamine and serotonin receptor partial agonist and serotonin receptor antagonist that is typically used as an antipsychotic, may be combined with an SNRI for those with treatment-resistant depression. Side effects of aripiprazole (Abilify) include weight gain, akathisia, and parkinsonism. While quetiapine (Seroquel) has been proven effective in treating MDD, the side effects are substantial and extensive, making this treatment option unattractive. Typical side effects include sedation, dry mouth, weight gain, and extrapyramidal symptoms (Agarwal, 2019; Espinoza & Unutzer, 2019). Lithium (Lithobid) has also been used as adjunctive therapy for severe treatment-resistant depression with some success in older adults, with an overall response rate of 42% (Espinoza & Unutzer, 2019; Kok & Reynolds, 2017).
Brain Stimulation. For patients with severe depression, electroconvulsive therapy (ECT) is also effective (Agarwal, 2019). ECT is a brain stimulation technique that may be appropriate for those with severe depression (i.e., determined to be causing significant functional impairment or life-threatening) who have not responded to pharmacologic treatments. Other brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), and vagus nerve stimulation (VNS), are used for the treatment of medication-resistant depression. However, no RCTs are available to show a significant benefit for older adults (Espinoza & Unutzer, 2019).
ECT involves transmitting short electrical impulses into the brain. These controlled electric currents provoke a brief period of seizure-like activity. ECT is typically performed in a series of 4-6 treatments before an improvement can be expected, with a total of 6-12 treatments administered over 2-6 weeks; monthly maintenance treatments are sometimes required. The patient is placed under general anesthesia for each treatment and can resume normal activity about an hour following the procedure. ECT can have significant adverse effects, such as headaches, muscle pain, nausea, confusion, and memory loss. It is only utilized for severe depression, depression with psychosis, or bipolar disorder that has not responded to medication and psychotherapy with more conventional methods. For patients with uncomplicated severe depression, ECT can lead to improved mood in 80% of cases. Patients need to understand both the potential risks and benefits of ECT before beginning treatment (NIMH, 2016a). ECT effectiveness ranges from 60%-80% for older adults with MDD and is especially beneficial in cases of severe depression or for patients with psychotic features, severe malnutrition, or treatment nonadherence (Kok & Reynolds, 2017).
Hearing Loss
Presbycusis, or age-related hearing loss, typically affects higher frequencies (i.e., above 2 kHz) and is quite common with increasing age. The prevalence of presbycusis is approximately 43% in individuals over the age of 65, over 50% in adults over 75, and more common in men than women. There is a genetic component to age-related hearing loss, and dietary factors (e.g., a high-fat diet) may be associated. Other risk factors include:
- Caucasian race
- low socioeconomic status
- previous loud noise exposure
- previous exposure to ototoxins (e.g., aminoglycosides, heavy metals, certain chemotherapeutic agents)
- previous history of otologic infection(s)
- smoking
- HTN
- DM
- vascular disease
- immunologic disorders
- hormonal factors (Blevins, 2020)
Hearing loss may be either conductive or sensorineural. Conductive hearing loss involves dysfunction in the external or middle ear, affecting the ability to transmit vibrations mechanically from the environment to the inner ear (Blevins, 2020). This dysfunction may be due to cerumen impaction, infection, foreign body occlusion, squamous cell carcinoma, congenital microtia, otosclerosis, cholesteatoma, or trauma to the tympanic membrane or temporal bone, among others (Wahid et al., 2021). Sensorineural hearing loss is typically related to an inner ear disorder, disrupting the transduction of sound information into usable neural signals. It is rarely related to the vestibulocochlear nerve, despite the term “nerve deafness” (Blevins, 2020). The condition may be hereditary or due to labyrinthitis, Meniere disease, viral cochleitis, vascular insult, autoimmune disorder, excessive noise or ototoxin exposure, vestibular schwannoma (acoustic neuroma), or trauma to the inner ear (Wahid et al., 2021).
Presbycusis often consists of sensorineural hearing loss due to dysfunction of the cochlear hair cells and, to a lesser degree, the spiral ganglion cells in the vestibulocochlear nerve. Presbycusis may be categorized histopathologically as sensory (a loss of high-frequency sounds due to loss of hair cells), metabolic (a loss of low-frequency sounds due to loss of stria vascularis), or neural (variable hearing loss due to loss of ganglion cells). Many researchers have proposed that this classification system is overly simplistic and thus invalid, although it continues to be used regularly, as presbycusis varies clinically. Anatomical studies indicate that patients with presbycusis often exhibit degeneration of the hair cells (predominantly the outer hair cells), the stria vascularis, and the spiral ganglion cells, combining the three histopathological categories detailed above. The resulting hearing loss typically presents bilaterally and gradually over years. It can be associated with tinnitus and vertigo and may contribute to falls. Over time, the middle and lower frequencies (0.5 to 2 kHz) may be affected, including those associated with human speech, which typically occurs at frequencies between 500 Hz and 4 kHz at a volume of about 50 dB. Consonants, which convey much of the meaning of speech, tend to involve higher frequencies and softer decibels, while vowels tend to be at lower frequencies and louder volumes. Due to the insidious nature of presbycusis (and the negative stigma), a patient will often wait for years before presenting with hearing loss in order to obtain evaluation and assistance at the insistence of family members. If untreated, presbycusis can lead to social isolation, depression, and interpersonal or family stress (Blevins, 2020).
Patients with presbycusis often can often hear when someone is speaking, as the majority of the energy of the sound wave is carried by the lower and middle frequencies. However, the higher frequencies carry the consonant sounds and the majority of speech information. For this reason, patients often describe being unable to understand the speech. These difficulties are exacerbated in the presence of background noise and also make it more difficult for patients to hear women’s (higher-pitched) voices as opposed to those of men. A paradoxical report of hypersensitivity to loud noises can also be common in older adults with presbycusis as a result of “recruitment,” causing a narrowing of the patient’s dynamic range. This makes adjusting hearing aids and other devices difficult, as the upper output limit must be carefully considered to avoid discomfort. This is also why shouting at a patient with presbycusis is often counterproductive, as it only augments the low vowel frequencies and creates discomfort for the patient (Blevins, 2020).
Tinnitus is commonly reported by patients with presbycusis and may be described as steady and bilateral ringing, static, rushing, or musical bells/chirping. A report of pulsing may indicate a vascular disorder, and asymmetrical symptoms suggest the need for further testing by an audiologist or otolaryngologist. Patients with vestibular end-organ dysfunction or presbyastasis may experience vertigo and falls. This loss of vestibular function is only amplified by pre-existing peripheral neuropathy, arthritis, peripheral vascular disease, or reduced visual acuity. The presence of these conditions makes compensation nearly impossible (Blevins, 2020).
Assessment and Diagnosis
The American Speech-Language-Hearing Association recommends audiometric testing for all adults over the age of 50 every 3 years. However, this screening requires extensive resources and time. The United States Preventive Services Task Force (USPSTF) has maintained that they cannot recommend for or against the routine screening of asymptomatic adults over the age of 50 for hearing loss. However, a patient's report of progressive hearing loss (or reports from a spouse or caregiver) should prompt an assessment. Other historical pieces of information include concurrent symptoms, exposure to ototoxins (including medications), occupational exposure to loud noises, and family history. Unilateral or asymmetrical symptoms may indicate otitis media, trauma, a tumor, or asymmetrical exposure to higher decibels (e.g., firearms or power tools are often utilized consistently on a person’s dominant side). A sudden or rapid loss of hearing should also elicit a more in-depth evaluation, as this is not indicative of presbycusis. The gradual onset of unilateral sensorineural hearing loss may indicate Meniere disease or vestibular schwannoma. A screening tool, such as the Hearing Handicap Inventory for the Elderly – Screening (HHIE-S), may also be used to collect this information. A brief physical examination, including otoscopy, should be done to rule out excessive or impacted cerumen or another foreign body occlusion. A tone-emitting otoscope can be used for screening. A whisper test and a 512 Hz tuning fork can differentiate sensorineural from conductive hearing loss. Then, audiogram testing should be performed to confirm presbycusis, assess its severity, and clarify management (Blevins, 2020; Weber, 2021).
Any occlusion or infection in the external canal, including cerumen impaction, should be ruled out prior to performing the whisper or Rinne tests, which are detailed below. The eardrum and middle ear should be examined to confirm they are intact (not perforated) and free from inflammation or infection (Kong & Fowler, 2021). Additional laboratory testing may be indicated to rule out metabolic abnormalities associated with hearing loss. A blood glucose test may indicate DM, while a CBC would help the examiner rule out anemia. A TSH would indicate the presence of thyroid dysfunction, and a syphilis test may be required if this sexually transmitted infection (STI) is suspected based on other historical or examination findings. Rheumatologic or autoimmune serology tests (rheumatoid factor and antinuclear antibody) may also be needed to rule out Sjogren’s syndrome for patients reporting dry eyes or mouth (Weber, 2021).
Whisper, Weber, and Rinne Tests. The whispered voice test is a simple assessment that can be performed in an office setting. One systematic review showed it offered a sensitivity of 90%-100% and a specificity of 70%-87%. The examiner should stand behind the patient (to prevent lip reading) and occlude the right ear canal while simultaneously rubbing the tragus on the same side to further prevent hearing in the right ear. The examiner whispers a short sequence of letters or numbers and asks the patient to repeat them. The exam is then repeated while occluding the left ear (Weber, 2021).
The Weber and Rinne tests attempt to differentiate between a patient’s ability to hear via air and bone conduction. They are not screening tests for hearing loss but rather can further characterize this condition. An individual with normal hearing will report symmetrical hearing (Weber) and enhanced air conduction via the tympanic membrane that is louder than bone conduction via the skull and cochlea (Rinne). The Weber test involves striking a tuning fork (the examiner grasps the handle/stem between the index finger and thumb and strike the tines against their knee or elbow at one-third of the distance from the end of the tines/prongs) and pressing the handle on the patient’s nose, forehead, or top of the head to assess for symmetry (see Figure 1). For patients describing unilateral hearing loss, the examiner should note which side they report loss of hearing prior to the test. Patients should be asked if they hear the tuning fork more on the left, on the right, or equally in both ears. Patients who report hearing the tuning fork equally in both ears have hearing that is symmetrical and intact. For those with unilateral sensorineural hearing loss, the sound will be heard louder on the contralateral or “good” side. As previously mentioned, unilateral sensorineural hearing loss may indicate Meniere disease or vestibular schwannoma. Patients with unilateral conductive hearing loss will report hearing the tuning fork vibration louder in their affected or “bad” ear. Those with bilateral conductive hearing loss typically report no lateralization (Wahid et al., 2021; Weber, 2021).
During a Rinne test, the patient covers their contralateral ear canal while the handle of a struck (vibrating) tuning fork is placed against their mastoid bone behind the ear. The patient indicates when the sound is no longer audible. The bifurcated end of the tuning fork is then placed perpendicularly at 3-4 cm from the external auditory canal (see Figure 2). If the patient reports they can hear the sound again for a time (typically twice as long), the test is normal (as air conduction = bone conduction x2) or positive. If the patient reports that bone conduction extends beyond air conduction (i.e., when the vibrating tuning fork is moved in front of the external auditory canal, the patient denies being able to hear it again), this is an abnormal or negative test result. The latter case typically indicates a conductive hearing loss on that side. This condition is most often due to external or middle ear pathology. A patient with profound or total sensorineural one-sided hearing loss may present with a false-negative test result due to sound waves from the tuning fork on the affected side being transmitted through the skull to the unaffected side. Any abnormal findings on the whisper, Weber, or Rinne tests should be followed up with audiometry testing for confirmation prior to diagnosis (Kong & Fowler, 2021; Weber, 2021).
Audiometry. An audiogram indicates a patient’s ability to hear tones and understand words. In this test, the patient is presented with tones of varying frequencies (250 Hz to 8 kHz) through a pair of headphones while sitting in a soundproof booth. An individual with intact hearing should be able to perceive tones below 25 dB. A pure-tone air conduction average is calculated using the patient’s average decibel scores at 500, 1000, and 2000 Hz. A patient with presbycusis typically has a “downward slope” to their tone threshold, requiring tones at higher frequencies to be transmitted at higher decibels to be audible. Older patients with high-frequency thresholds above 40 dB should be referred for an amplification trial with an audiologist. Tone testing may be repeated with a bone oscillator held against the mastoid to assess bone conduction. A significant gap between air and bone conduction indicates conductive hearing loss (Blevins, 2020; Weber, 2021).
Speech audiometry has 2 components: the speech reception threshold (SRT) and the word discrimination score. The SRT is the softest level at which the patient can correctly repeat half (50%) of the prompted words. These words are two syllables (e.g., airplane, armchair, or pancake) in which both syllables are stressed. The SRT can confirm the air conduction tone testing above, as it is typically equal to the patient’s pure tone average ± 6 dB. The word recognition or discrimination score is based on the patient’s ability to understand a standardized list of words at a given volume (usually 40 dB above the patient’s SRT established above) and repeat them. A score of 90% or above indicates normal hearing, while a low score indicates advanced neural degeneration. Higher scores also predict a favorable response to amplification. Word discrimination testing can help audiologists locate the specific location of a lesion (Blevins, 2020; Weber, 2021).
Additional testing may include pneumoscopy, which is performed to assess the tympanic membrane’s mobility in response to applied air pressure. Tympanometry objectively measures any changes in the acoustic impedance of the middle ear due to air pressure changes, so it can be considered a “hard copy” of pneumoscopy. This test may indicate fluid behind the tympanic membrane, a retracted tympanic membrane, stiffness due to myringosclerosis or otosclerosis, or an overly compliant tympanic membrane indicating ossicular chain discontinuity. Stapedial reflex testing and auditory brainstem response testing are available but rarely performed for adults with hearing loss except in specific circumstances (Weber, 2021). Advanced imaging studies, such as an MRI or CT, are rarely needed for patients with diagnosed presbycusis. These measures should only be considered to rule out space-occupying lesions or other central pathologies for patients who present with asymmetrical/unilateral tinnitus, vertigo, cranial nerve deficits, or significant asymmetry in their hearing loss (Blevins, 2020). A CT scan of the temporal bone is most appropriate for a patient with conductive hearing loss due to an unknown etiology, while an MRI with gadolinium may help identify the underlying cause of unilateral/asymmetrical or fluctuating sensorineural hearing loss (Weber, 2021).
Management
Despite its prevalence, there is no direct treatment for presbycusis. Effective communication with a hearing-impaired adult requires using a lower-pitched voice, facing the patient so that they can read the speaker’s lips, and speaking slowly and clearly. Engaging the patient in active conversation or asking them to paraphrase what was said may help ensure understanding. Once the diagnosis of hearing loss has been made, treatment options to help the patient manage their symptoms and optimize their function include hearing aids, assistive listening devices, auditory rehabilitation, and cochlear implants for those with refractory hearing loss (Blevins, 2020).
The use of appropriately fit hearing aids reduces withdrawal, depression, and the emotional impact of presbycusis and improves patients’ QOL. Concerns include patients who are uncomfortable with the cosmetic and social implications of wearing hearing aids, their high cost, high levels of static or noise, and an improper fit within the meatus. Patients may also produce too much cerumen, clogging the device. As a result, patients should be evaluated by an experienced audiologist in order to be matched with the most appropriate device for their needs and anatomy. Most options can be trialed prior to purchase. Many patients with tinnitus related to presbycusis report a reduction in this bothersome symptom with well-fitting hearing aids. Follow-up visits and referrals to rehabilitation services may enhance the patient’s chance of success and maximize the benefit from their hearing aids (Blevins, 2020).
Auditory rehabilitation incorporates and integrates sensory management, patient education/instruction, perceptual training, and counseling to improve the function and QOL of those with presbycusis. It may involve active listening training to augment the patient’s conversational listening skills from a cognitive standpoint. Auditory rehabilitation may also include speech reading (i.e., lip-reading) and other communication-enhancement skills. Patients can learn to assess a speaker’s facial expressions, lip contours, and body language. They are typically counseled to enhance environmental factors whenever possible by phasing out competing sound sources and optimizing the lighting to enhance visualization skills. Patients are encouraged to select restaurants and other venues based on acoustics and to position themselves to hear others better by sitting them on the side with superior hearing (if their hearing loss is unilateral or asymmetrical). Most importantly, and yet sometimes most difficult, rehabilitation programs reinforce to patients that they must consistently inform their companions of their hearing loss. This allows others to optimize their communication patterns and facilitates communication. Despite observational reports, there is little evidence that auditory rehabilitation significantly improves communication for those with hearing loss (Blevins, 2020).
Assistive auditory devices are often utilized by those with presbycusis to enhance hearing and improve QOL or convenience. Options include flashing lights that correspond with doorbells or phone chimes to alert an individual with hearing loss when someone is at their door or their phone is ringing. Telecoils transmit sounds directly from a phone to an individual’s hearing aid. High-fidelity frequency-modulation systems are often useful in theaters or large lecture halls with troublesome acoustics. Closed captioning for television or movies is another example of an assistive listening technique (Blevins, 2020).
Cochlear implantation may be considered for patients who do not report significant improvement with hearing aids and have been diagnosed with severe bilateral presbycusis. This involves the surgical placement of an electrode array in the inner ear to stimulate the remaining cochlear neurons, bypassing the damaged cochlea (Blevins, 2020).
References
Agarwal, K. (2019). Failure to thrive in older adults: Management. UpToDate. Retrieved February 25, 2021, from https://www.uptodate.com/contents/failure-to-thrive-in-older-adults-management
Agarwal, K. (2020). Failure to thrive in older adults: Evaluation. UpToDate. Retrieved February 25, 2021, from https://www.uptodate.com/contents/failure-to-thrive-in-older-adults-evaluation
American Academy of Sleep Medicine. (2014). International classification of sleep disorders – (3rd edition). American Academy of Sleep Medicine.
American Geriatrics Society Beers Criteria Update Expert Panel. (2019). American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 67(4), 674–694. https://doi.org/10.1111/jgs.15767
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association.
Baruth, J. M., & Lapid, M. I. (2017). Capacity determinations and elder self-neglect. AMA Journal of Ethics, 19(10), 1047-1050. https://doi.org/10.1001/journalofethics.2017.19.10.corr2-1710
Blevins, N. H. (2020). Presbycusis. UpToDate. Retrieved March 15, 2021, from https://www.uptodate.com/contents/presbycusis
Bonnet, M., & Arand, D. L. (2019). Evaluation and diagnosis of insomnia in adults. UpToDate. Retrieved March 3, 2021, from https://www.uptodate.com/contents/evaluation-and-diagnosis-of-insomnia-in-adults
Bonnet, M., & Arand, D. L. (2021). Risk factors, comorbidities, and consequences of insomnia in adults. UpToDate. Retrieved March 3, 2021, from https://www.uptodate.com/contents/risk-factors-comorbidities-and-consequences-of-insomnia-in-adults
Brown-O’Hara, T. (2013). Geriatric syndromes and their implications for nursing. Nursing2021, 43(1), 1–3. https://doi.org/10.1097/01.NURSE.0000423097.95416.50
Dong, X. Q. (2017). Elder self-neglect: Research and practice. Clinical Interventions in Aging, 12, 949-54. https://doi.org/10.2147/CIA.S103359
DrSaltMD. (2019). The Weber test [image]. https://commons.wikimedia.org/wiki/File:Weber_Test.jpg
Espinoza, R. T., & Unutzer, J. (2019). Diagnosis and management of late-life unipolar depression. UpToDate. Retrieved March 11, 2021, from https://www.uptodate.com/contents/diagnosis-and-management-of-late-life-unipolar-depression
Fixen, D. R. (2019). 2019 AGS Beers Criteria for older adults. Pharmacy Today, 25(11), 42-54. https://www.pharmacytoday.org/article/S1042-0991(19)31235-6/fulltext
Fornaro, M., Anastasia, A., Valchera, A., Carano, A., Orsolini, L., Vellante, F., Rapini, G., Olivieri, L., Di Natale, S., Perna, G., Martinotti, G., Di Giannantonio, M., & De Berardis, D. (2019). The FDA “black box” warning on antidepressant suicide risk in young adults: More harm than benefits? Frontiers in Psychiatry, 10. https://doi.org/10.3389/fpsyt.2019.00294
Fox 52. (2018). The Rinne test [image]. https://commons.wikimedia.org/wiki/File:Rinneversuch.svg
Halphen, J. M. (2021). Elder abuse, self-neglect, and related phenomena. UpToDate. Retrieved March 8, 2021, from https://www.uptodate.com/contents/elder-abuse-self-neglect-and-related-phenomena
IPT Institute. (n.d.). (IPT) interpersonal psychotherapy. Retrieved February 22, 2021, from https://iptinstitute.com/about-ipt/
Kok, R. M., & Reynolds, C. F. (2017). Management of depression in older adults: A review. JAMA. 317(20), 2114-2122. https://doi.org/10.1001/jama.2017.5706.
Kong, E. L., & Fowler, J. B. (2021). Rinne test. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK431071/
MacKenzie, M. B., & Kocovski, N. L. (2016). Mindfulness-based cognitive therapy for depression: Trends and developments. Psychology Research and Behavior Management, 9, 125-132. https://doi.org/10.2147/PRBM.S63949
Matheson, E., & Hainer, B. L. (2017). Insomnia: Pharmacologic therapy. American Family Physician. 96(1), 29-35. https://www.aafp.org/afp/2017/0701/p29.html
McCurry, S. M., Zhu, W., Von Korff, M., Wellman, R., Morin, C. M., Thakral, M., Yeung, K., & Vitiello, M. V. (2021). Effect of telephone cognitive behavioral therapy for insomnia in older adults with osteoarthritis pain: A randomized clinical trial. JAMA Internal Medicine. https://doi.org/10.1001/jamainternmed.2020.9049
Mental Health America. (2016). Complementary and alternative medicine for mental health. https://www.mhanational.org/sites/default/files/MHA_CAM.pdf
National Alliance on Mental Illness. (2017). Types of medication. https://nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Types-of-Medication
National Center for Complementary and Integrative Health. (2019). Depression and complementary health approaches. https://www.nccih.nih.gov/health/providers/digest/depression-and-complementary-health-approaches
National Institute of Mental Health. (2016a). Brain stimulation therapies. https://www.nimh.nih.gov/health/topics/brain-stimulation-therapies/brain-stimulation-therapies.shtml
National Institute of Mental Health. (2016b). Mental health medications. https://www.nimh.nih.gov/health/topics/mental-health-medications/index.shtml
Nestle Nutritional Institute. (n.d.). Mini nutritional assessment (MNA). Retrieved March 3, 2021, from https://www.mna-elderly.com/forms/mini/mna_mini_english.pdf
Neubauer, D. N. (2020). Pharmacotherapy for insomnia in adults. UpToDate. Retrieved March 5, 2021, from https://www.uptodate.com/contents/pharmacotherapy-for-insomnia-in-adults
Reynolds, M. E., & Cone, P. H. (2018). Managing adult insomnia confidently. The Journal for Nurse Practitioners, 14(10), 718-724.e1. https://doi.org/10.1016/j.nurpra.2018.08.019
Ritchie, C., & Yukawa, M. (2020). Geriatric nutrition: Nutritional issues in older adults. UpToDate. Retrieved March 2, 2021, from https://www.uptodate.com/contents/geriatric-nutrition-nutritional-issues-in-older-adults
Rosenberg, R., Murphy, P., Zammit, G., Mayleben, D., Kumar, D., Dhadda, S., Filippov, G., LoPresti, A., & Moline, M. (2019). Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: A phase 3 randomized clinical trial. JAMA Network Open, 2(12), e1918254. https://doi.org/10.1001/jamanetworkopen.2019.18254
Saljoughian, M. (2019). Polypharmacy and drug adherence in elderly patients. US Pharmacist, 44(7), 33-36. https://www.uspharmacist.com/article/polypharmacy-and-drug-adherence-in-elderly-patients
Sateia, M. J., Buysse, D. J., Krystal, A. D., Neubauer, D. N., & Heald, J. L. (2017). Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine, 13(02), 307–349. https://doi.org/10.5664/jcsm.6470
Schroeck, J. L., Ford, J., Conway, E. L., Kurtzhalts, K. E., Gee, M. E., Vollmer, K. A., & Mergenhagen, K. A. (2016). Review of safety and efficacy of sleep medicines in older adults. Clinical Therapeutics, 38(11), 2340–2372. https://doi.org/10.1016/j.clinthera.2016.09.010
Serretti, A., & Mandelli, L. (2010). Antidepressants and body weight: A comprehensive review and meta-analysis. Journal of Clinical Psychiatry, 71(10), 1259–1272. https://doi.org/10.4088/jcp.09r05346blu
Sharma, A., Barrett, M. S., Cucchiara, A. J., Gooneratne, N. S., & Thase, M. E. (2017). A breathing-based meditation intervention for patients with major depressive disorder following inadequate response to antidepressants: A randomized pilot study. Journal of Clinical Psychiatry, 78(1), e59-e63. https://dx.doi.org/10.4088%2FJCP.16m10819
Shi, Z., Atlantis, E., Taylor, A. W., Gill, T. K., Price, K., Appleton, S., Wong, M. L., & Licinio, J. (2017). SSRI antidepressant use potentiates weight gain in the context of unhealthy lifestyles: Results from a 4-year Australian follow-up study. BMJ Open, 7(8), e016224. https://doi.org/10.1136/bmjopen-2017-016224
Siu, P. M., Yu, A. P., Tam, B. T., Chin, E. C., Yu, D. S., Chung, K.-F., Hui, S. S., Woo, J., Fong, D. Y., Lee, P. H., Wei, G. X., & Irwin, M. R. (2021). Effects of tai chi or exercise on sleep in older adults with insomnia: A randomized clinical trial. JAMA Network Open, 4(2), e2037199. https://doi.org/10.1001/jamanetworkopen.2020.37199
Stein, M. B. (2020). Psychotherapy and psychosocial interventions for posttraumatic stress disorder in adults. UpToDate. Retrieved March 1, 2021, from https://www.uptodate.com/contents/psychotherapy-and-psychosocial-interventions-for-posttraumatic-stress-disorder-in-adults#H1772362614
Stone, D., Holland, K., Bartholow, B., Crosby, A., Davis, S., & Wilkins, N. (2017). Preventing suicide: A technical package of policy, programs, and practices. https://www.cdc.gov/violenceprevention/pdf/suicidetechnicalpackage.pdf
Uguz, F., Sahingoz, M., Gungor, B., Aksoy, F., & Askin, R. (2015). Weight gain and associated factors in patients using newer antidepressant drugs. General Hospital Psychiatry, 37(1), 46–48. https://doi.org/10.1016/j.genhosppsych.2014.10.011
US Department of Veterans Affairs. (2016). Management of major depressive disorder. https://www.healthquality.va.gov/guidelines/MH/mdd/
US Food & Drug Administration. (2018). Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/suicidality-children-and-adolescents-being-treated-antidepressant-medications
Wahid, N. W. B., Hogan, C. J., & Attia, M. (2021). Weber test. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK526135/
Ward, K. T., & Reuben, D. B. (2020). Comprehensive geriatric assessment. UpToDate. Retrieved February 5, 2021, from www.uptodate.com/contents/comprehensive-geriatric-assessment
Weber, P. C. (2021). Evaluation of hearing loss in adults. UpToDate. Retrieved March 15, 2021, from www.uptodate.com/contents/evaluation-of-hearing-loss-in-adults
Winkelman, J. W. (2021). Overview of the treatment of insomnia in adults. UpToDate. Retrieved March 5, 2021, from https://www.uptodate.com/contents/overview-of-the-treatment-of-insomnia-in-adults