About this course:
The purpose of this module is to provide nurses with up-to-date information on the causes and treatments of chronic heart failure.
Course preview
Introduction
The American Heart Association (AHA) estimates that approximately 6.2 million Americans 20 years of age and older have heart failure, and although survival rates for heart failure (HF) are improving, it remains a serious disease (Benjamin et al., 2019). Patients with HF have an increased risk of developing atrial fibrillation, cognitive decline and dementia, chronic kidney disease (CKD), diabetes, stroke, and venous thromboembolism. Heart failure has an adverse effect on the quality of life that is considered comparable to or worse than that caused by chronic obstructive pulmonary disease (COPD) or the need for chronic hemodialysis, and the five-year mortality rate after a diagnosis of heart failure has been reported to be as high as 50- 70% (The Centers for Disease Control and Prevention [CDC], 2019; Gupta, Andersson, Vasan & Wang, 2017; Mann & Chakinala, 2018).
In the United States, the prevalence of HF between men and women is similar. HF affects African Americans more often and more severely than Caucasians and is predominantly a disease of older adults. The lifetime risk for developing HF has been estimated to be 20-46%, depending on gender and race (Butler, Anker, & Packer, 2019; Gupta et al., 2017).
Definition and Categories
Heart failure is a syndrome caused by a functional or structural inability of the ventricles to eject blood or fill with blood in an amount that can satisfy metabolic demands (Mann & Chakinala, 2018; Quesada & Klein, 2017).
There are two basic types of HF, and they are classified based on ejection fraction (EF). Ejection fraction is the percentage of blood contained within the ventricles that is pumped out during systole. Heart failure with reduced ejection fraction (HFrEF) is defined as HF with an EF of 40% or less; HF with preserved ejection fraction (HFpEF) is defined as HF with an EF of ≥50% (Ahmad, Butler & Borlaug, 2017).
Heart failure has been categorized as diastolic or systolic; left-sided or right-sided; preserved, mid-range, or reduced EF; acute or chronic; and high-output. These distinctions are not consistently used or always helpful. Separating HF into categories based on the cardiac cycle, the ventricle that is involved, or by the EF is, in some ways, inaccurate and artificial (Butler et al., 2019; Gaggin & Dec, 2017; Quesada & Klein, 2017). Opinions differ as to whether mid-range HF is truly a distinct form of the disease (Butler et al., 2019; Lyu et al., 2019). There is considerable overlap in the etiologies and clinical characteristics of these different types of HF (Gupta et al., 2017; Mann & Chakinala, 2018;), and HFrEF is the only type of HF for which there are well established clinical guidelines and proven effective treatments (Butler et al., 2019; Gaggin & Dec, 2017). Textbooks and the medical literature typically limit their discussion of heart failure to HFrEF, HFpEF, high-output heart failure, and acute versus chronic, and this module will as well.
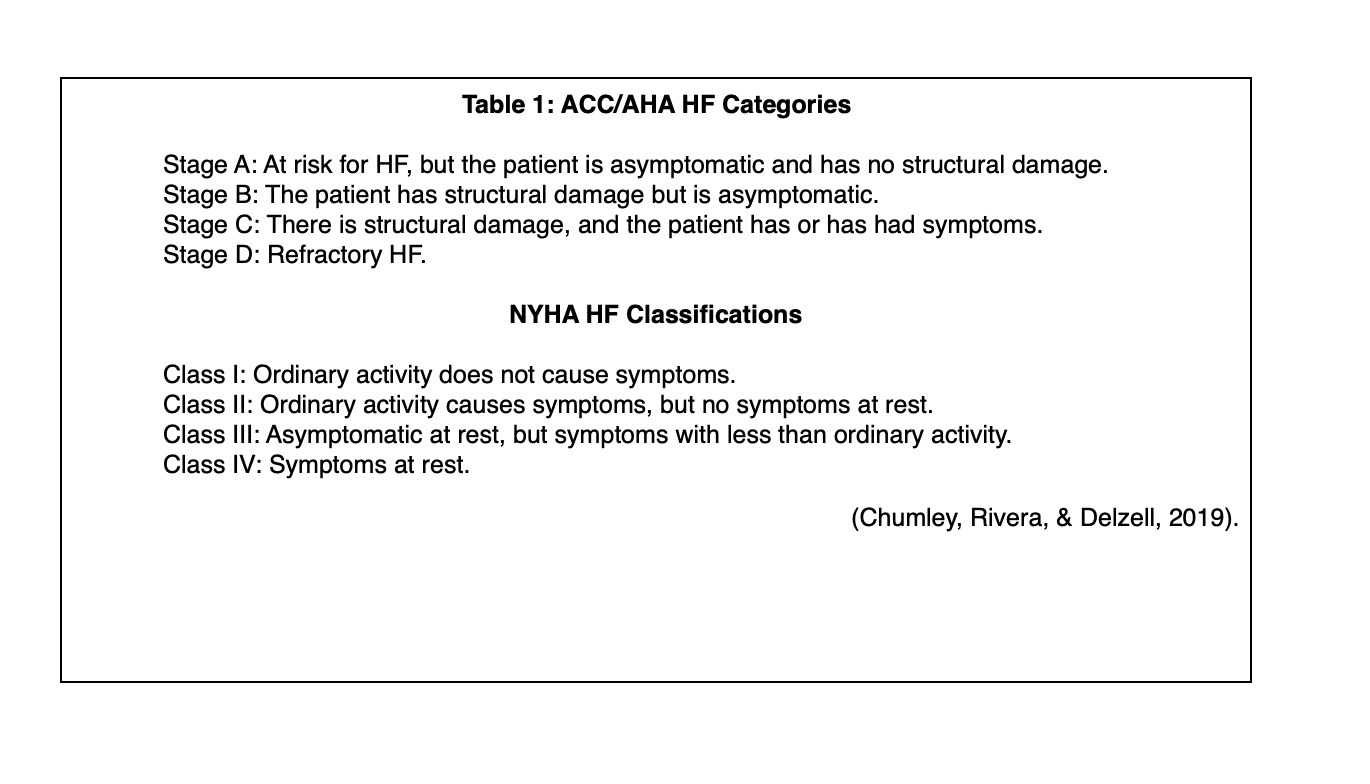
Heart failure is also categorized (see Table 1 below) based on signs, symptoms, and the functional impact of the disease using systems developed by the New York Heart Association (NYHA) and the American College of Cardiology (ACC)/AHA (Chumley, Rivera, & Delzell, 2019).
Cardiac Output, Stroke Volume, and Heart Failure
Heart failure limits the ability of the heart to pump enough blood to satisfy metabolic demands, and a basic understanding of cardiac function is necessary for understanding HF.
Cardiac output (CO) is the volume of blood that is pumped from the heart in one minute. Stroke volume is the volume of blood that is pumped from the heart with each ventricular contraction; CO is the product of heart rate and stroke volume. Stroke volume - and by implication left ventricular function - is determined by three variables:
1. Preload (or end-diastolic volume): the amount of blood in the ventricle before systole. Preload can also be defined as the resting length of the myocardial muscle fibers before systole.
2. Myocardial contractility: the force of a cardiac contraction at a given preload.
3. Afterload: the impedance that must be overcome for blood to be pumped from the ventricles (Mohrman & Heller, 2018).
A normal heart can produce a stroke volume to supply the metabolic demands of the organs and tissues and increase the stroke volume when needed. The ventricles can expand/stretch to accommodate a wide range of blood volume, and when preload is increased, the myocardium responds by increasing the force of myocardial contraction. But in patients with HF, the myocardium has been damaged. This a) impairs the contractile ability of the myocardium, resulting in systolic dysfunction, or b) prevents the myocardium from stretching enough to accommodate the volume of blood that is needed to the ventricles, resulting in impaired diastolic filling and function. Fibrosis, hypertrophy, and other changes to the myocardium diminish the filling capacity of the ventricles, and damage to the myocardium causes systolic dysfunction and impaired pumping force. If the damage to the heart is not sudden and severe - and in most cases of HF it is not - compensatory mechanisms can restore cardiac function, but they can also cause injury to the heart, and in most patients, this means that HF is a progressive disease characterized by declining cardiac function (Mann & Chakinala, 2018).
Causes and Pathophysiology of Heart Failure
Heart Failure with Reduced Ejection Fraction
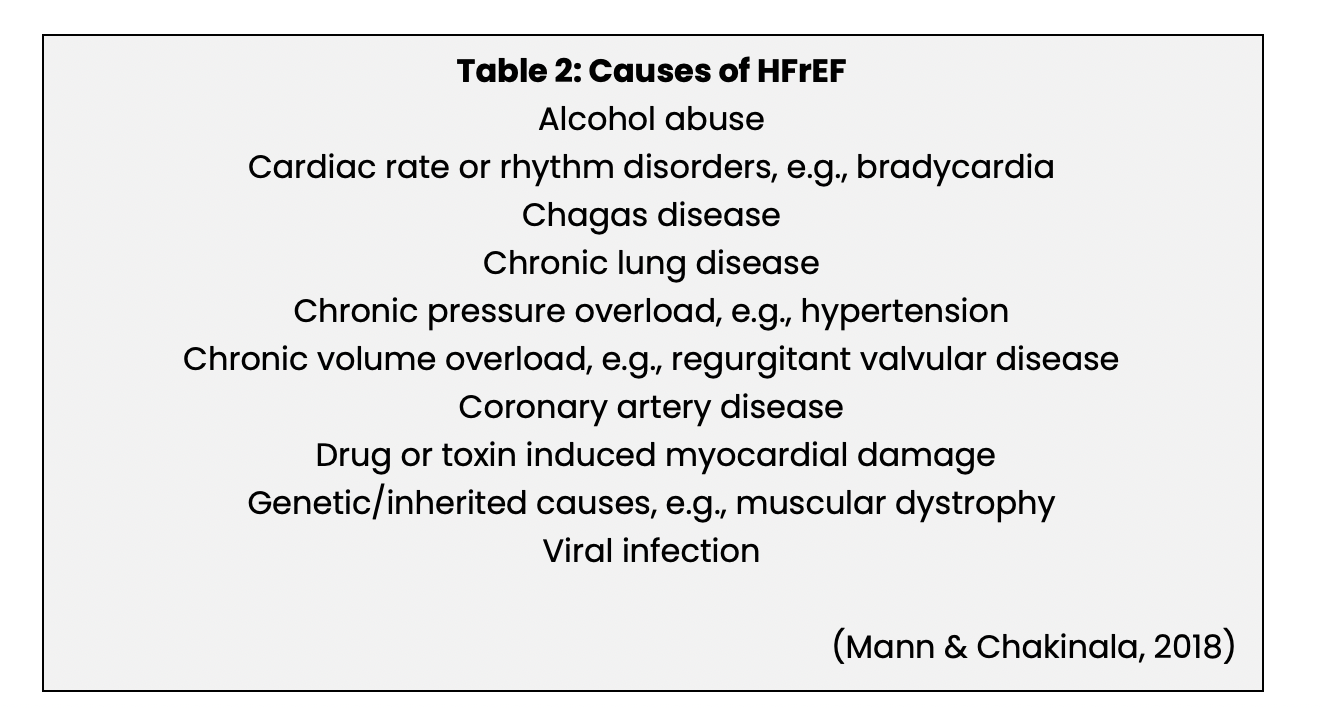
The primary pathophysiology underlying HFrEF is acute or chronic damage to the myocardium and impaired systolic function with insufficient contractile force to produce an adequate stroke volume. The most commonly occurring causes of HFrEF are listed in Table 2. Most cases of HFrEF are caused by ischemic heart disease or hypertension, but in approximately 20-30% of patients, the cause is not identified; these cases are referred to as non-ischemic or idiopathic cardiomyopathy (Butler et al., 2019; Mann & Chakinala, 2018).
Heart failure with reduced ejection failure is a complex disease that is characterized by three basic steps: a) the initiating disease/event; b) the development of compensatory mechanisms, and; c) progression of the disease and development of signs and symptoms.
Patients who have HFrEF are initially asymptomatic or minimally symptomatic and may be so for many years due to multiple compensatory mechanisms that can restore ventricular function and CO. These compensatory mechanisms improve the CO, stroke volume, vascular tone, and pump function, and although they are discussed separately, they overlap and influence each other.
Compensatory Mechanisms in Heart Failure
- Renin-angiotensin-aldosterone system activation: this causes peripheral vasoconstriction, sodium and water retention, and helps maintain CO and tissue perfusion.
- Increased sympathetic output and tone: the baroreceptors in the aorta, carotid arteries, and left ventricle activate the vasomotor center in the brain stem in response to decreased CO and fall in blood pressure. Sympathetic tone is increased, CO is restored, and peripheral vasoconstriction helps maintain normal blood pressure. Increased sympathetic tone also causes increased release of antidiuretic hormone (ADH), increasing water resorption by the kidneys and increasing intravascular volume. <
...purchase below to continue the course
These compensatory mechanisms are effective. However, renin-angiotensin-aldosterone system activation, increased sympathetic activation and tone, and the other adaptations to the myocardial injury of HF can and do cause myocardial damage and dysfunction, e.g., apoptosis and necrosis of myocardial cells, dilated ventricles, an abnormal response to adrenergic stimulation, and abnormal myocardial metabolism. The compensatory mechanisms are initially beneficial, but over time, they weaken the heart, and the patient develops symptomatic HF (Mann & Chakinala, 2018; Gaggin & Dec, 2017).
Heart Failure with Preserved Ejection Fraction
The primary cause of HFpEF is the inability of the ventricles to fill with blood. The underlying dysfunction of the ventricles in HFpEF is very complex and may include myocardial stiffness and elevated diastolic pressure, resulting in congestion, pulmonary hypertension, and decreased ability to produce enough CO to meet metabolic demands. Common causes of HFpEF include cardiomyopathy, pericardial disease, and valvular disease (Gaggin & Dec, 2017).
Heart failure with preserved ejection fraction is a complex and heterogeneous disease. For example, systemic inflammation that damages the myocardial blood vessels, initiated by diseases like CKD, diabetes, hypertension, and obesity, is a common and important mechanism of injury of HFpEF. However, normal aging and myocardial ischemia from coronary artery disease are also contributing factors. In addition, although HFpEF is primarily characterized by left ventricular stiffness and impaired relaxation of the left ventricle, this is not the only myocardial dysfunction of the disease (Butler et al., 2019; Gaggin & Dec, 2017; Quesada & Sanchez, 2017; van de Wouw et al., 2019).
High-Output Heart Failure
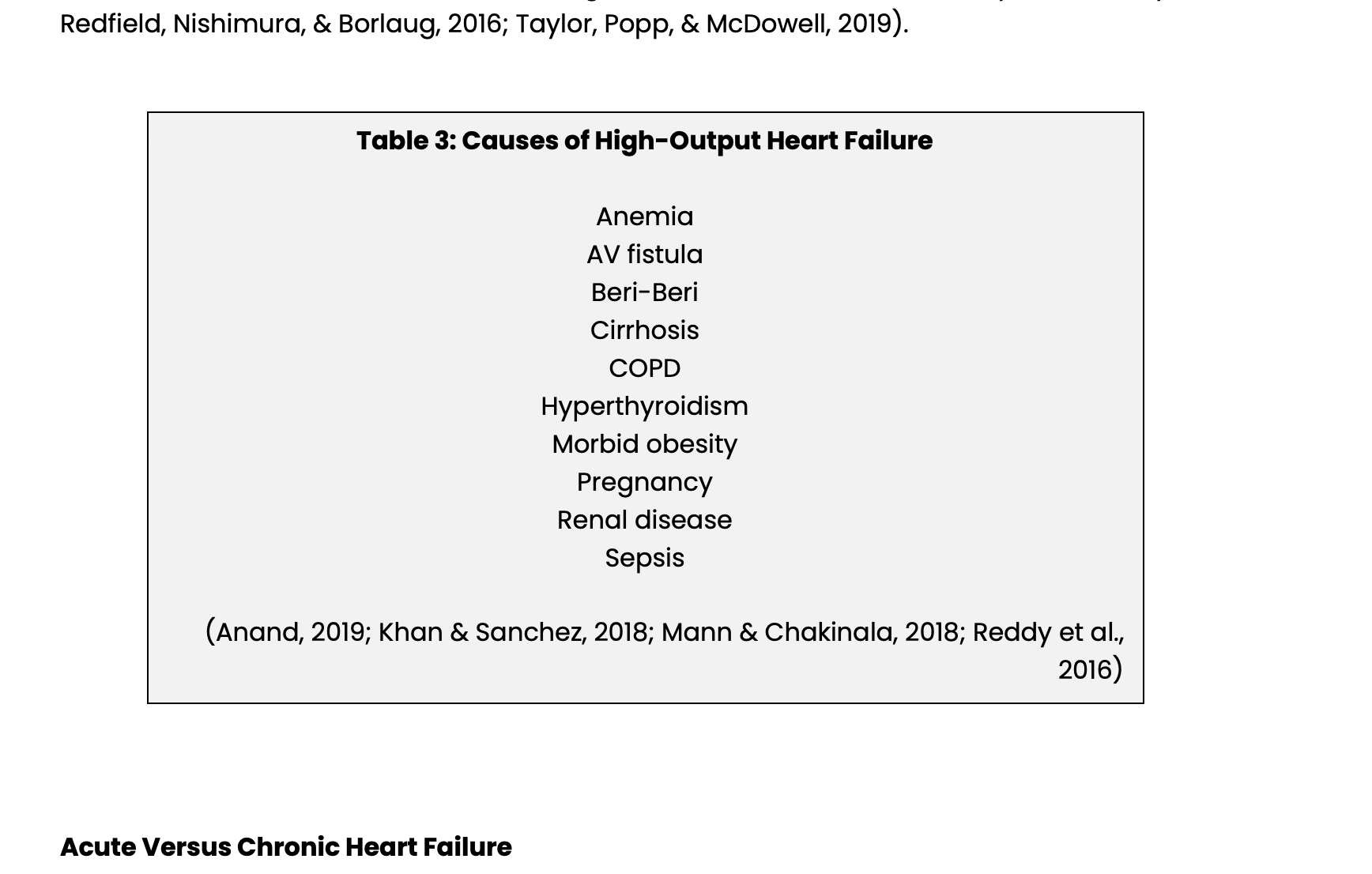
High-output heart failure is similar to HFrEF and HFpEF, but there is usually no intrinsic damage to the heart, EF is normal, and CO is high yet insufficient for metabolic demands. High-output HF is characterized by decreased peripheral resistance with poor tissue perfusion, increased metabolic demands, myocardial dysfunction, or a combination of these three. The causes of high-output HF are listed below in Table 3. For some of these diseases and conditions, e.g., anemia, AV fistulas, and pregnancy, high-output HF only develops if there is structural heart disease or a particularly severe form of the disease, e.g., thyroid storm but in others like cirrhosis, COPD, and morbid obesity HF is relatively common and may involve changes in the vascular bed, damage to the myocardium, and increased metabolic demand (Anand, 2109; Mann & Chakinala, 2018; Mogos et al., 2018; Onishi, 2017; Reddy, Melenovsky, Redfield, Nishimura, & Borlaug, 2016; Taylor, Popp, & McDowell, 2019).
Acute Versus Chronic Heart Failure
Acute heart failure is distinguished from chronic heart failure by a sudden onset and the need for immediate treatment. Acute myocardial infarction (AMI), papillary muscle or chordae tendineae rupture, or sudden onset of atrial fibrillation are common causes of acute onset HF, but it can also occur in patients who have chronic HF (Gaggin & Dec, 2017).
Risk Factors for Heart Failure
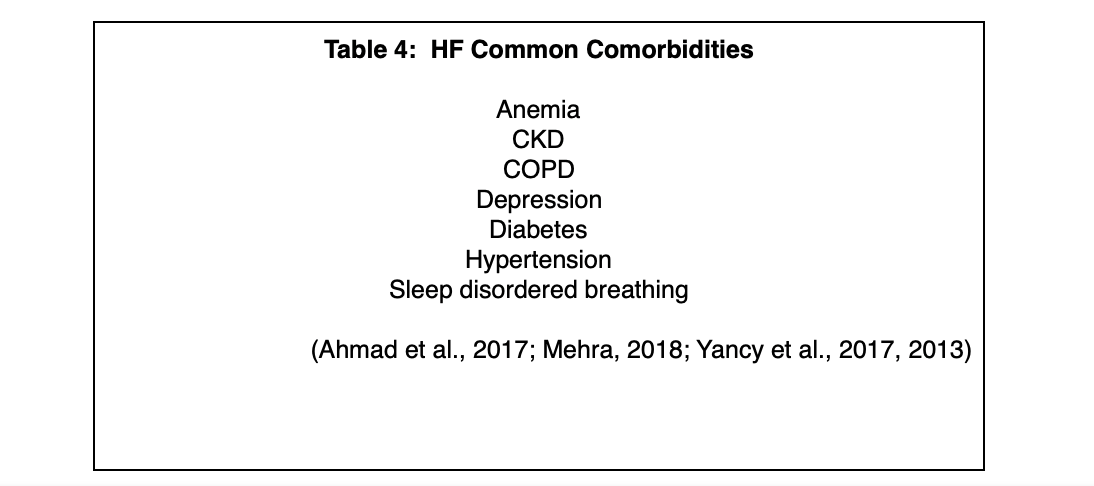
Common risk factors for HF include advanced age, CKD, COPD, coronary artery disease, diabetes, hypertension, obesity, and smoking. These are common behaviors and diseases, and they have a significant impact on the development of HF, likely because many of them cause systemic inflammation, a process that has been implicated in the development of HF. The lifetime risk for HF in people who have blood pressure above 160/90 mm Hg is almost doubled, and for hypertensive patients, the greatest benefit of lowering blood pressure is reducing the risk of HF (Butler et al., 2019; Gupta et al., 2017). Hyperglycemia, insulin resistance, and diabetes independently increase the risk for HF, and the prevalence of HF in patients who have diabetes is four times that of the general population (Gupta et al., 2017; Rosano, Vitale, & Seferovic, 2017). The lifetime risk for HF in people who have are obese (BMI ≥ 30 kg/m2) is double that of people who have a BMI of < 25 kg/m2 (Butler et al., 2019). For a list of frequently seen comorbidities as well as common signs and symptoms of HF, please see Tables 4 and 5 below.
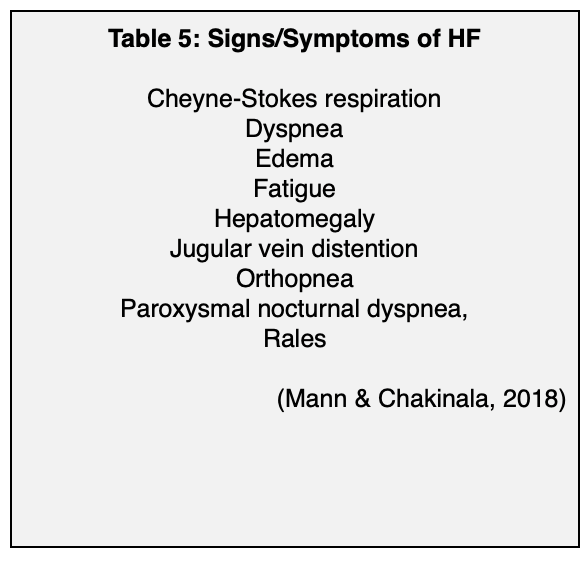
Diagnosis
Heart failure is a clinical diagnosis and a diagnosis of exclusion that is based on a clinician’s assessment of the patient’s risk factors and signs and symptoms (see Table 5 above). Laboratory studies, imaging tests, and other tests can be done to confirm the diagnosis, determine the cause, and assess the degree of impairment (Mann & Chankinala, 2018).
For new patients, blood glucose, BUN and creatinine, complete blood count, a lipid panel, liver function tests, and serum electrolytes should be measured (Mann & Chakinala, 2018). Measurement of natriuretic peptides, B-type natriuretic peptide (BNP) and n-terminal pro-BNP (NT-proBNP), can be done to determine the presence of HF and the level of left ventricular function (Ahmad et al., 2017; Mann & Chakinala, 2018). BNP is a hormone, and NT-proBNP is a prohormone. They are released from the ventricles (and to a lesser degree from the atria) when volume overload and myocardial stretching are present (Ahmad et al., 2017). Measurement of BNP and NT-proBNP is recommended by the ACC, the AHA, and the Heart Failure Society of America (HFSA) as a way to confirm or exclude the presence of HF, determine the severity of the disease, and establish a prognosis (Yancy et al., 2017). The 2017 HF treatment guidelines from the ACC/AHA/HFSA also recommend that the natriuretic peptides should be measured before the patient leaves the hospital to establish a post-discharge prognosis (Yancy et al., 2017).
There are other cardiac causes of elevated natriuretic hormones, such as atrial fibrillation, left ventricular hypertrophy, and pericardial disease. In addition, there are many conditions/diseases that can cause elevated BNP and NT-proBNP levels, including (but not limited to) advanced age, anemia, female gender, the use of an angiotensin-neprilysin inhibitor, obstructive sleep apnea, and renal impairment. Obesity can cause false low levels (Ahmad et al., 2017, 2017; Mann & Chakinala, 2018; Yancy et al., 2017).
A 12-lead ECG should be done to determine the cardiac rhythm and assess for the presence of left ventricular hypertrophy and heart damage. The primary assessment tool for HF is a two-dimensional echocardiogram. This test provides information about ventricular function, ventricular size, valve function, abnormal wall movement, and other data. Magnetic resonance imaging can also be used to provide some of the same information and is considered the gold standard for determining left ventricular mass and volume. Other assessment tools include transesophageal echocardiography and stress echocardiography; these should be used in specific clinical situations, e.g., detecting a thrombus or in patients who have congenital heart disease. A chest x-ray is typically a part of the assessment for HF (Ahmad et al., 2017; Mann & Chakinala, 2018; Price et al., 2017).
Treatment of Heart Failure with Reduced Ejection Fraction
The goals of treatment of HFrEF are a) decrease the severity of the signs and symptoms, b) halt or slow the progression of the disease, c) find and treat the underlying cause, and d) treat comorbidities and risk factors (Mann & Chakinala, 2018).
Drug Therapy for Heart Failure with Reduced Ejection Fraction
The ACC and the HFSA published guidelines in 2017 for drug therapy for the management of patients who have HF, primarily HFrEF (Yancy et al., 2017). The 2017 guidelines are an update of the 2013 ACC/AHA guidelines, and the current guidelines outline a basic approach to treating HF in all patients who have NYHA class I-IV HF or ACC/AHA stage C heart failure. The ACC/HFSA guidelines divided care for HFrEF into six categories: general guidelines as well as five specific patient populations; in these patients, it should be assumed that the basic therapy is used as well (Yancy et al., 2017).
1) Treatment for Patients with HFrEF, NYHA Class I-IV or ACC/AHA Stage C
All patients who have NYHA class I-IV or ACC/AHA stage C HF should be treated with a drug that works through the renin-angiotensin system: an angiotensin-converting enzyme inhibitor (ACEI), an angiotensin receptor II blocker (ARB), or an angiotensin-neprilysin inhibitor (ARNI), and a beta-blocker. This basic approach is often called guideline-directed medical therapy (GDMT). ACEIs and the ARBs reduce blood pressure, help modify left ventricular remodeling, and decrease intravascular volume. Treatment with an ACEI or an ARB has been proven to reduce the morbidity and mortality, the rate of hospitalization (ARBs), and progression of HF in patients with HFrEF who have been symptomatic, regardless of severity, and those with and without coronary artery disease (Yancy et al., 2017).
For treating HFrEF, there is no significant difference between the individual ACEIs, and both ACEIs and ARBs seem to be equally effective for long-term management (Ahmad et al., 2017). ACEIs can decrease urinary excretion of potassium, cause hypotension, and decrease renal perfusion, so they should be used cautiously if the patient has serum potassium above 5.0 mEq/L, hypotension, or renal impairment; these cautions apply to the ARBs, as well (Yancy et al., 2017).
Cough is a common and troublesome side effect of ACEIs, occurring in up to 37% of all patients; ARBs are much less likely to cause cough (Vukadinović et al.,2019; Yancy et al., 2017). ACEIs can cause angioedema, which is a potentially deadly adverse effect, causing sudden and severe airway compromise. It is very uncommon, occurring in less than 1% of patients, more often in African American and female patients (Yancy et al., 2017). ARBs can cause angioedema, but much less so than the ACEIs; although there have been concerns about angioedema and cross-reactivity between the ACEIs and ARBs, using an ARB for someone who developed angioedema from an ACEI is considered acceptable (Rasmussen et al., 2019; Yancy et al., 2017).
ARNIs, particularly valsartan/sacubitril (Entresto), lower blood pressure, change the neurohormonal response to HF, and promote diuresis, and they been more effective than some ACEIs for treating HFrEF (Drazner, 2019; Leong, McMurray, Joseph, & Yusuf, 2019). The ACC/AHA/HFSA guidelines recommend that if a patient can tolerate an ACEI or an ARB, “replacement with an ARNI is recommended to further reduce morbidity and mortality.” The use of ARNIs for patients who have HFrEF is a Class 1 recommendation in the ACC/AHA/HFSA guidelines, although direct comparisons between the ARBs and the ARNIs have not been made (Yancy et al., 2017). ARNIs can cause hypotension, hyperkalemia, and decreased renal function. The risk of angioedema with the use of an ARNI is comparable to that of an ACEI (Shi et al., 2019). For this reason, ARNIs are contraindicated in patients who have had ACEI-related angioedema. Concomitant use of an ARNI and an ACEI is contraindicated, and an ARNI should not be used within 36 hours of the last dose of an ACEI (Yancy et al., 2017).
Beta-blockers help to ameliorate the long-term adverse effects of the compensatory adrenergic stimulation that occurs in chronic HF, and beta-blockers in combination with an ACEI or an ARB reduce the risk of mortality and hospitalization in patients who have HFrEF. Bisoprolol (Zebeta), carvedilol (Coreg), and metoprolol (Lopressor, Toprol XL) are preferred as these drugs have the strongest evidence for efficacy. Unfortunately, they can cause bradycardia, dizziness, dyspnea, and fatigue, and the adverse effects can make using the target dose difficult. Beta-blockers can be safely used in patients who have COPD and/or diabetes (Ahmad et al., 2017; Bocchi & Salemi, 2019; Cleland et al., 2018; Joseph, Swedberg, Leong, Yusuf, 2019).
2) Patients with NYHA Class II-IV HF and Renal Dysfunction
If the patient’s creatinine clearance (CrCL) is above 30 mL and serum potassium is less than 5.0 mEq/L, therapy with an aldosterone antagonist should be initiated (Yancy et al., 2017). The aldosterone antagonists eplerenone (Inspra) and spironolactone (Aldactone, CaroSpir) have been proven to reduce mortality and hospitalizations in patients who have HFrEF. Hyperkalemia has been reported to occur in 5-17.5% of HF patients taking an aldosterone antagonist. Patients who have CKD, diabetes mellitus, or are taking an ACEI or an ARB have a higher risk for this adverse effect, and their renal function and serum potassium should be closely monitored. Doses and dosing schedules of the aldosterone antagonist, the ACEI, and the ARB may need to be adjusted (Ahmad et al., 2017; Durstenfeld, Katz, Park, Blecker, 2019; Mehra, 2018; Vukadinović et al., 2018).
3) Patients with NYHA Class III-IV HF and African Americans
In this patient population, hydralazine (Apresoline) and nitrates should be started if an adequate response has not been attained with an ACEI or an ARB and a beta-blocker (Yancy et al., 2013, 2017). Hydralazine is a direct vasodilator of the arterioles. Nitrates, e.g., isosorbide mononitrate (Imdur), dilate the peripheral veins, and to a lesser degree, the peripheral arteries. This combination has long been established as an effective treatment for HFrEF and can be found in combination products such as isosorbide dinitrate/hydralazine (BiDil). They can cause dizziness and headaches, and most of these drugs must be taken three to four times a day.
ACEIs and ARBs are less effective at reducing blood pressure in African Americans, perhaps due to differences in the renin-angiotensin-aldosterone system or drug metabolism. ACEIs are specifically less effective in African Americans who have HFrEF (Al-Mohammad, 2019). In contrast, research has shown that hydralazine and nitrates decrease the mortality and hospitalization rate in African Americans with HFrEF, and their use for Africans Americans who have HFrEF is a Class I recommendation in current guidelines. Hydralazine and nitrates are less effective than ACEIs for non-African Americans who have HFrEF, and this combination is usually used only when these patients cannot tolerate an ACEI or an ARB (Al-Mohammad, 2019; Yancy et al., 2017; Ziaeian, Fonarow, Heidenrich, 2017).
4) Heart Failure NYHA Class II-III, EF ≤ 35%, > 1 Year Survival and 40 Days Post-AMI
These patients have an increased risk for sudden cardiac death, and they should be treated with an implantable cardioverter defibrillator (ICD) (Yancy et al., 2013, 2017).
5) Heart Failure NYHA Class II-IV, EF ≤ 35%, NSR with QRS ≥ 150 msec and an LBBB Pattern
These patients should be treated with cardiac resynchronization therapy or a cardiac resynchronization device (Yancy et al., 2013, 2017).
6) Heart Failure NYHA Class II-III, NSR, Heart Rate ≥ 70 bpm and Receiving a Maximum Tolerated Dose of a Beta-Blocker
Patients who are receiving GDMT and fit the above criteria should be treated with ivabradine (Corlanor) (Yancy et al., 2017). Increased heart rate as a compensatory mechanism increases the metabolic needs of the myocardium, causes inefficient myocardial contraction, and is a poor prognostic sign in HF patients. Ivabradine (Corlanor) inhibits the If current in the sinoatrial node and reduces heart rate. The Systolic Heart Failure with the If Inhibitor Trial (SHIFT) study found that ivabradine (Corlanor) reduces cardiovascular death and hospitalization from HF (Bocchi& Salemi, 2019). There is also evidence that ivabradine (Corlanor) can reverse left ventricular remodeling and improve symptoms and quality of life. Potential adverse effects include atrial fibrillation, bradycardia, hypotension, and visual disturbances. Ivabradine (Corlanor) is contraindicated in patients who have acute, decompensated heart failure. Other contraindications include clinically significant bradycardia or hypotension, heart blocks, pacemaker dependence, severe hepatic impairment, sinoatrial block, sick sinus syndrome, and concomitant use of strong CYP3A4 inhibitors (Bocchi & Salemi, 2019; Koruth, Lala, Pinney, Reddy & Dukkipati, 2017; Tardif et al., 2011).
Other Drugs Used to Treat Heart Failure
Digoxin (Lanoxin) and HFrEF
Digoxin (Lanoxin) has been used for centuries to treat HF. The DIG trial, the largest randomly controlled trial of its use in HF patients, showed that digoxin (Lanoxin) reduced hospitalizations and readmissions in HF patients but not mortality (Malik et al., 2019). The use of digoxin (Lanoxin) as a treatment for HF has decreased considerably, due in part to the availability of other therapies and research that may indicate an increased mortality risk. Despite this, the 2013 ACC/AHA guidelines and other sources do recommend its use in HF patients who are receiving GDMT but remain symptomatic (Ahmad et al., 2017; Malik et al., 2019; Mehra, 2018; Yancy et al., 2013).
Loop Diuretics and HFrEF
Fluid retention and hypervolemia, along with the resulting dyspnea and decreased exercise tolerance, are common in patients who have HF. Diuretics are recommended for HF patients who have these signs and symptoms. Loop diuretics, such as bumetanide (Bumex), furosemide (Lasix) and torsemide (Demadex), are mentioned in the 2013 guidelines but not the 2017 guidelines. The 2013 guidelines recommend using a loop diuretic for all patients who have fluid retention or a history of fluid retention to provide symptomatic relief and improve exercise tolerance. However, there is no evidence that loop diuretics improve survival or clinical outcomes (Ahmad et al., 2017; Mehra, 2018; Täger et al., 2019). There is no conclusive evidence that one loop diuretic is superior to another (Miles et al., 2019). Despite these issues, loop diuretics are commonly used to treat HFrEF and Mehra (2018) writes that “diuretics are essential at the outset to achieve volume control before neurohormonal therapy is likely to be well tolerated or titrated.”
Fluid and electrolyte losses are a common adverse effect of loop diuretics. For this reason, close monitoring of body weight and serum electrolytes is mandatory in HFrEF patients on loop diuretics. Diuretic resistance, defined as fluid retention and failure to decongest with a diuretic, is a common effect of long-term use of these drugs, occurring in approximately 25-30% of all HF patients (Masella et al., 2019).
Statins and Omega-3 Polyunsaturated Fatty Acid (PUFA) Supplements
The relationship between cholesterol, statins, and HF is complex. Low serum cholesterol is a poor prognostic indicator in HF patients. Although the mechanisms driving this seemingly counter-intuitive phenomenon are not fully understood, the current thinking is that low serum cholesterol is caused by severe HF; low serum cholesterol does not cause a poor outcome (Lee, Sattar, McMurray, & Packard, 2019). Statins can reduce the risk of developing HF secondary to atherosclerosis and AMI. In patients already diagnosed with HF, statins do not improve survival or clinical outcomes and should not be routinely given to HF patients. If a patient who is diagnosed HFrEF is already taking a statin or needs to start a statin for other reasons, there is no contraindication to continuing its use or starting the drug (Al-Gobari, Agrinier, Soundant, Burnand, & Thilly, 2019; Lee et al., 2019; Mehra, 2018; Yancy et al., 2013).
Omega-3 PUFA supplements, commonly known as fish oil, may decrease mortality and the rate of hospitalization in HF patients. The AHA considers their use a Class IIA recommendation (the weight of evidence/opinion is in favor of the intervention). There is no conclusive evidence that omega-3 PUFA supplementation can prevent the development of HF (Sakamoto, Saotome, Iguchi, & Maekawa, 2019; Siscovick et al., 2017).
Preventing HF Progression and Treating Risk Factors
Lifestyle Interventions
Regular exercise, smoking cessation, and weight loss are recommended for patients who have HF to reduce the risk of hospitalization, decrease mortality, and improve functional status and quality of life (Ahmad et al., 2017; Aune, Schlesinger, Norat, & Riboli, 2019; Haf, Toukhsati, Cameron, Yates, & Hare, 2018; Mahajan, Stokes, Elliott, Munawar, & Sanders, 2019; Toukhsati et al., 2019; Yancy et al., 2013).
A restricted sodium diet is typically prescribed for HF patients (Ahmad et al., 2017; Yancy et al., 2013). However, a 2019 review by Khan, Jones and Butler concluded that the quality of evidence supporting this intervention is suboptimal; although the typical recommendation is to consume 1500-3000 mg of sodium a day, there is no consensus on how much sodium HF patients should have in their diet. In addition, very low sodium intake may activate the sympathetic and renin-angiotensin-aldosterone systems and be harmful to HF patients, increasing mortality and readmission rates (Ahmad et al., 2017; Khan et al., 2019).
Treating Comorbidities
Anemia
Anemia and iron deficiency contribute to increased mortality, increased hospitalization, and early readmission in HF patients (Jacob et al., 2019; Mistry, Hosoya, Kohut, & Ford, 2019). The increased risk of mortality associated with anemia and iron deficiency occurs in patients with HFrEF, HFpEF, and mid-range HF (Savarese et al., 2019). Anemia in HF has many causes, e.g., chronic inflammation, reduced iron intake, increased need for hemoglobin. Anemia caused by HF medication therapy is uncommon (Cunha, Rocha, Menezes Falcão, 2018).
Intravenous iron supplementation has many beneficial effects for anemic HF patients (Zhou, Xu, Xu, & Qian, 2019).The 2017 guidelines state that IV iron supplementation may improve quality of life and functional ability in patients who have NYHA class II or III HF and a ferritin level under 100 ng/mL or a ferritin level 100-300 ng/mL and transferrin saturation less than 20%. The use of erythropoietin-stimulating agents is not recommended, and oral iron supplementation has not been shown to be effective for HF patients (Lewis et al., 2017; Yancy et al., 2017).
Atrial Fibrillation
Patients who have HF and atrial fibrillation are treated with anticoagulants to prevent thromboembolic events and with cardioversion, medications, or invasive procedures to attain rate and rhythm control (Lip, Heinzel, Gaita, Gonzalez-Juanatey, & Pieske, 2016).
Chronic Kidney Disease
Heart failure and CKD are inextricably linked. The two together are often referred to as cardiorenal syndrome; approximately 50% of patients who have HF have CKD (van de Wouw et al., 2019). The two diseases share common risk factors such as diabetes mellitus, dyslipidemia, and hypertension (Tuegel & Bansal, 2017). Cardiovascular disease is one of the most common causes of morbidity and mortality in CKD patients, and CKD is associated with a worse outcome in HF patients (Carubelli, Metra, & Lund, 2018).
Treatment of patients who have HF and CKD is difficult. There is relatively little research on this clinical issue. Many of the drugs used to treat HF, e.g., ACEIs, ARNIs, ARBs, and mineralocorticoid receptor antagonists, can have adverse effects on kidney function and cause hyperkalemia. To compound this issue, HF patients often have a decreased response to diuretics. ACEIs, ARNIs, ARBs, mineralocorticoid receptor antagonists, and loop diuretics have been used safely and successfully in patients who have HF and CKD, but there is limited data available; renal function and serum potassium must be closely monitored (Carubelli et al., 2018).
Depression
Depression is very common in HF patients and is associated with an increased risk for morbidity (He, Zhou, Ma, Wei, & Fu, 2019; Peng, Fang, Huang, & Qin, 2019). Psychotherapies like cognitive behavioral therapy in combination with antidepressant medications have been used with some success and failures, but there is no standardized approach to treating depression in this population (Mehra, 2018).
Diabetes
Type 2 diabetes mellitus is an independent risk factor for HF, and HF patients who have diabetes have an increased risk of mortality and hospitalization (Bashier et al., 2019). Insulin, metformin (Glucophage), sulfonylureas (glimepiride [Amaryl] and glyburide [DiaBeta]), thiazolidinediones (pioglitazone [Actos] and rosiglitazone [Avandia]), and DDP-4 inhibitors (saxagliptin (Onglyza) and sitagliptin [Januvia]) are drugs that are commonly used to treat type 2 diabetes, and their use in patients who have HF can be problematic. Insulin and sulfonylureas may or may not worsen HF (Bell & Goncalves, 2019).
Lactic acidosis is a very uncommon yet potentially deadly adverse effect of metformin (Glucophage). The prescribing information for metformin contains a boxed warning that states that a hypoxic state (e.g., acute heart failure) is a risk factor for metformin-induced lactic acidosis. Metformin-induced lactic acidosis is very uncommon, and the available research suggests that HF does not increase the risk of this adverse effect (Trinkley, Anderson, Nair, Malone, & Saseeen, 2018).
The thiazolidinediones can cause fluid retention, worsen existing HF, and have been associated with new-onset of HF (Arnold et al., 2019). The American Diabetes Association (2019) recommends that thiazolidinediones should be avoided in patients who have symptomatic HF.
Research indicates that the DDP-4 inhibitors can worsen heart failure, increase the risk for hospitalization and mortality, and may have an adverse effect on cardiac remodeling (Packer, 2018, 2019).
The alpha glucosidase inhibitors (acarbose [Precose] and miglitol [Glyset]) and glucagon-like peptide 1 (GLP-1) agonists (dulaglutide [Trulicity], exenatide [Byetta, Bydureon], liraglutide [Victoza, Saxenda], lixisenatide [Adlyxin], and semaglutide [Ozempic, Rybelsus]) do not have adverse effects on HF (Bell & Goncalves 2019).
Hypertension
The 2017 ACC/AHA/HFSA guidelines recommend that GDMT should be used to reach a systolic blood pressure under130 mm Hg. Beneficial treatment options include an ACEI, an ARB, an ARNI, and mineralocorticoid receptor antagonist (Yancy et al., 2017).
Sleep Disordered Breathing
Obstructive sleep apnea, Cheyne’s-Stokes breathing, and other sleep disorders are a common complication of HF. The 2017 ACC/AHA/HFSA guidelines (Yancy et al.) recommend that a formal sleep assessment is reasonable for patients who have NYHA class II-IV HF and excessive daytime sleepiness or for whom there is a suspicion of the presence of a sleep disorder. Positive airway pressure during sleep and adaptive servo-ventilation can improve oxygenation, left ventricular EF, and the apnea-hypopnea index in patients with HFrEF or HFpEF, but these therapies may not positively affect mortality or the progression of the disease (Hernandez, Jeon, Denegri-Galvan, Ortega-Loayza, & Kaw, 2019; Mehra, 2018)
Treatment of Heart Failure with Preserved Ejection Fraction
HFpEF is a heterogeneous disease, and the pathophysiologic mechanisms that cause and drive it are not completely understood. There are no evidence-based, effective therapies for treating patients who have HFpEF (Ahmad et al., 2017; Naing, Forrester, Kangaharan, Muthumala, & Playford, 2019). The medications that have been successful for HFrEF have not worked for HFpEF and have not been effective at reducing the mortality rate related to HFpEF (Gaggin & Dec, 2017; Zheng et al., 2018). The current treatment recommendations for HFpEF are a) treat volume overload with diuretics, b) treat the underlying cause and manage comorbidities c) blood pressure control, if needed, and d) lifestyle interventions. Of these interventions, treating comorbidities and lifestyle interventions have been the most successful. If a specific treatment issue is not mentioned, readers can assume that there is no information on the topic or that the treatment recommendations for HFrEF apply (Ahmad et al., 2017; Gazewood, 2017; Mehra, 2018; Naing et al., 2019; Yancy et al., 2013).
Volume Overload
Diuretics are recommended to treat volume overload in patients who have HFpEF; however, research has concluded that they are often no more effective than placebo and do not improve the prognosis or decrease mortality (Ahmad et al., 2017; Bonsu, Arunmanakul, & Chaiyakunapruk, 2018; Matsushita et al., 2019a; Zheng et al., 2018). Chrysant and Chrysant (2019) concluded that the sodium-glucose cotransporter-2 (SGLT2) inhibitors that are used to treat type 2 diabetes might be useful to treat HFpEF as these drugs cause osmotic diuresis, decreased plasma volume, and increased sodium and water excretion. This includes canagliflozin (Invokana), dapagliflozin (Farxiga), empagliflozin (Jardiance), and ertugliflozin (Steglatro) (Chrysant & Chrysant, 2019).
Blood Pressure Control
ACEIs, ARNIs, ARBS, beta-blockers, and hydralazine and nitrates are used to treat hypertension in patients who have HFrEF, but there is no evidence that these drugs are effective for treating hypertension in patients who have HFpEF (Ahmad et al., 2017; Khan, Fonarow, Khan, Greene & Butler, 2017; Meyer & LeWinter, 2019; Nambiar, Silverman, Vanburen, LeWinter,& Meyer, 2019; Solomon et al., 2019; Tsujimoto & Kajio, 2019). The 2017 ACC/AHA/HFSA guidelines did not update the ACCF/AHA 2013 recommendations; the 2013 guidelines simply state that systolic and diastolic blood pressure should be controlled in accordance with published clinical guidelines, and the use of beta-blockers, ACEIs, and ARBs is reasonable for these patients (Yancy et al., 2013, 2017).
Pulmonary Hypertension
Pulmonary hypertension is a common feature of HFpEF (Obokata et al., 2019). The phosphodiesterase inhibitor sildenafil (Viagra, Revatio) is FDA-approved for the treatment of pulmonary arterial hypertension, but the 2013 RELAX trial and others have found that sildenafil (Viagra, Revatio) does not improve functional capacity, quality of life, cardiac structure or cardiac function, amongst other parameters (Liu et al., 2017).
Statins and Omega-3 PUFA Supplements
Systemic inflammation that damages the myocardial blood vessels, initiated by diseases like CKD, diabetes, hypertension, and obesity, is a common and important mechanism of injury of HFpEF (Aoki, 2019; Butler et al., 2019; van de Wouw et al., 2019). The anti-inflammatory effects of the statin drugs are an important part of their mechanism of action, and there is clear evidence that statins can reduce mortality and improve clinical outcomes in patients who have HFpEF (Lee et al., 2018; Marume, Takashio, Nagai, Tsuijita & Anzai, 2019).
There is very little published information about the benefits of omega-3 PUFA supplementation for patients who have HFpEF, but they can decrease the interstitial fibrosis that is part of the myocardial damage of HFpEF (Sakamoto et al., 2019; Siscovik et al., 2017). The 2013 ACCF/AHA guidelines describe their use in HFpEF as reasonable (Yancy et al., 2013).
Lifestyle Interventions
Smoking, a sedentary lifestyle, and obesity are risk factors - obesity being a key risk factor - for developing HFpEF (Chrysant & Chrysant, 2019; Matsushita et al., 2019b; Naing et al., 2019; Tadic & Cuspidi, 2019). Exercise, weight loss, and presumably smoking cessation can improve physical functioning and quality of life for patients who have HFpEF (Ahmad et al., 2017; Leggio, Fusco, Loreti, Limongelli, & Padua, 2019; Tadic & Cuspidi, 2019).
Atrial Fibrillation
Atrial fibrillation is less common in patients with HFpEF than HFrEF, but it is still a complication with potentially serious consequences (Sartipy, Dahlström, Fu, & Lund, 2017). Patients who have HF and atrial fibrillation are treated with anticoagulants to prevent thromboembolic events and with cardioversion, medications, or invasive procedures to attain rate and rhythm control (Lip et al., 2016).
Chronic Kidney Disease
There are no proven, effective therapies for treating CKD in patients who have HFpEF, and because CKD in these patients is so closely associated with diabetes, hypertension, and obesity, treatment of these comorbidities is the primary goal (Carubelli et al., 2018; Tuegel & Bansal, 2017; van de Wouw et al., 2019)
Diabetes
Type 2 diabetes is common in patients with both primary types of HF, but diabetes in patients who have HFpEF seems to affect the heart differently, with different patterns of cardiac remodeling (Chirinos et al., 2019). In addition, patients who have HFpEF are more likely to develop diabetic microvascular complications (i.e., diabetic nephropathy and diabetic retinopathy) than patients who have HFrEF, perhaps as one of the results of the systemic inflammation that is part of HFpEF (Shah et al., 2018; Tromp et al., 2019). There are no specific recommendations for the treatment of type 2 diabetes in patients who have HFpEF.
Nursing Considerations for Patients with Heart Failure
Heart failure is a complex disease that affects patients in many ways. Heart failure patients often have serious comorbidities, their physical and psychological quality of life is adversely affected, and self-care requires a considerable amount of time and knowledge. Patient education, and continued re-education, is necessary to maintain health and provide good outcomes. When working with patients with HF, these basic issues will need to be covered during patient education:
- Decreased exercise tolerance
- Depression
- Fatigue
- Medication adverse effects
- Nutrition
- Weight gain and weight loss
Nursing care should be individualized for each patient, but issues common to all HF patients include assisting with activities of daily living as needed (fatigue and decreased exercise tolerance are common), intake/output monitoring, monitoring for adverse drug effects, monitoring for trends in signs and symptoms (improving, stable, worsening), assessing gas exchange status, observing for signs and symptoms of depression, and monitoring for/treating common comorbidities.
References
Ahmad, T, Butler, J., & Borlaug, B. (2017). Chapter 70: The diagnosis and management of chronic heart failure. In Valentin Fuster, Robert A. Harrington, Jagat Narula, Zubin J. Eapen (Eds). Hurst's the heart, 14th ed. [Online edition]. New York, NY: McGraw-Hill Education.
Al-Gobari, M., Agrinier, N., Soudant, M., Burnand, B., & Thilly, N. (2019). Effects of statins to reduce all-cause mortality in heart failure patients: Findings from the EPICAL2 Cohort Study. Am J Cardiovasc Drugs, 19(5) 497-508. doi: 10.1007/s40256-019-00346-4.
Al-Mohammad, A. (2019). Hydralazine and nitrates in the treatment of heart failure with reduced ejection fraction. ESC Heart Fail, 6(4) 878-883. doi: 10.1002/ehf2.12459.
American Diabetes Association (2019). Cardiovascular disease and risk management: Standards of medical care in diabetes. Diabetes Care, 42, (Supplement 1), S103-S123. doi: 10.2337/dc19-S010.
Anand, I.S. (2019). High-output heart failure revisited. J Am Coll Cardiol, 68(5), 483-486. doi: 10.1016/j.jacc.2016.05.036.
Aoki, T. (2019). Beneficial prognostic effects of statins in heart failure with preserved ejection fraction (HFpEF) patients: HFpEF as a manifestation of systemic disease. Circ J, 25, 277-278. doi: 10.1253/circj.CJ-18-1268.
Arnold, S.V., et al. (2019). Understanding contemporary use of thiazolidinediones. Circ Heart Failure,12(6), e005855. doi: 10.1161/CIRCHEARTFAILURE.118.005855.
Aune, D., Schlesinger, S., Norat, T., & Riboli, E. (2019). Tobacco smoking and the risk of heart failure: A systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol, 26(3), 279-288. doi: 10.1177/2047487318806658.
Bashier, A., Bin Hussain, A., Abdelgadir, E., Alawadi, F., Sabbour, H., & Chilton, R. (2019). Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndr, 11(80), 1-28. doi: 10.1186/s13098-019-0476-0.
Bell, D.S.H., & Goncalves, E. (2019). Heart failure in the patient with diabetes: Epidemiology, etiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab, 21(6), 1277-1290. doi: 10.1111/dom.13652.
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M.S., Callaway, C.W. …….Virani, S.S. (2019). Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation, 139(10) e56-e3528. doi: 10.1161/CIR.0000000000000659.
Bocchi, E.A. & Salemi, V.M.C. (2019). Ivabradine for treatment of heart failure. Expert Opin Drug Saf, 18(6), 393-402. doi: 10.1080/14740338.2019.1612873.
Bonsu, K.O., Arunmanakul, P., & Chaiyakunapruk, N. (2018). Pharmacological treatments for heart failure with preserved ejection fraction-a systematic review and indirect comparison. Heart Fail Rev, 23(2), 147-156. doi: 10.1007/s10741-018-9679-y.
Butler, J., Anker, S.D., & Packer, M. (2019). Redefining heart failure with a reduced ejection fraction. JAMA. doi: 10.1001/jama.2019.15600.
Carubelli, V., Metra, M., & Lund, L.H. (2018). Negotiating renal dysfunction when treating patients with heart failure. Expert Rev Cardiovasc Ther, 16(2), 113-122. doi: 10.1080/14779072.2018.1422178.
The Centers for Disease Control and Prevention. (2019). Heart failure fact sheet. Retrieved from https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm
Chirinos, J. A., Bhattacharya, P., Kumar, A., Proto, E., …...Zamani, P.,. (2019). Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc, 8(4), e011457. doi: 10.1161/JAHA.118.011457.
Chrysant, S.G., & Chrysant, G.S. (2019). Obesity-related heart failure with preserved ejection fraction: New treatment strategies. Hosp Pract, 47(2), 67-72. doi: 10.1080/21548331.2019.1575662.
Chumley, H.S., Rivera, N., & Delzell Jr, J.E. (2019). Chapter 50: Heart failure. In Richard P. Usatine, Mindy A. Smith, E.J. Mayeaux, Jr., & Heidi S. Chumley (Eds). The Color atlas and synopsis of family medicine, (3rd ed.) New York, NY: McGraw-Hill Education.
Cleland, J.G.F, Bunting, K.V., Flather, M.D., Altman, D.G., Holmes, J., Coats, A. J. S., … Kotecha, D. (2018). Beta-blockers for heart failure with reduced ejection fraction, Mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J, 39(1), 26-35. doi: 10.1093/eurheartj/ehx564.
Cunha, G.J.L., Rocha, B.M.L., & Menezes Falcão, L. (2018). Iron deficiency in chronic and acute heart failure: A contemporary review on intertwined conditions. Eur J Intern Med, 521-7. doi: 10.1016/j.ejim.2018.04.013. Epub 2018 Apr 19.
Drazner, M.H. (2019). Angiotensin receptor-neprilysin inhibition (ARNI) therapy and reverse remodeling in heart failure with reduced ejection fraction. JAMA, 322(11), 1051-1053. doi: 10.1001/jama.2019.12662.
Durstenfeld, M.S., Katz, S.D., Park, H., & Blecker, S. (2019). Mineralocorticoid receptor antagonist use after hospitalization of patients with heart failure and post-discharge outcomes: a single-center retrospective cohort study. BMC Cardiovasc Disord, 19(194), 1-9. doi: 10.1186/s12872-019-1175-3.
Gaggin, H.K., & Dec, G.W. (2017). Chapter 68: Pathophysiology of heart failure. In Valentin Fuster, Robert A. Harrington, Jagat Narula, & Zubin J. Eapen (Eds). Hurst's the heart, (14th ed.) New York, NY: McGraw-Hill Education.
Gazewood, J.D., & Turner, P.L. (2017). Heart failure with preserved ejection fraction: Diagnosis and management. Am Fam Physician, 96(9), 582-588.
Gupta, D.K, Andersson, C., Vasan, R.S., & Wang, T.J. (2017). Chapter 69: The epidemiology of heart failure. In Valentin Fuster, Robert A. Harrington, Jagat Narula, & Zubin J. Eapen (Eds). Hurst's the heart, 14th ed.. New York, NY: McGraw-Hill Education.
Haf, J., Toukhsati, S.R., Cameron, J.D., Yates, R., & Hare, D.L. (2018). Association between the 6-minute walk test and exercise confidence in patients with heart failure: A prospective observational study. Heart Lung, 47, (1), 54-60. doi: 10.1016/j.hrtlng.2017.09.006.
He, W., Zhou, Y., Ma, J., Wei, B., & Fu, Y. (2019). Effect of antidepressants on death in patients with heart failure: A systematic review and meta-analysis. Heart Fail Rev. 2019 Sep 16. doi: 10.1007/s10741-019-09850-w.
Hernandez, A.V., Jeon, A., Denegri-Galvan, J., Ortega-Loayza, F., & Kaw, R. (2019). Use of adaptive servo ventilation therapy as treatment of sleep-disordered breathing and heart failure: A systematic review and meta-analysis. Sleep Breath. doi: 10.1007/s11325-019-01882-8.
Jacob, C., Altevers, J., Barck, I., Hardt, T., Braun, S., & Greiner, W. (2019). Retrospective analysis into differences in heart failure patients with and without iron deficiency or anaemia. ESC Heart Fail, 6(4), 840-855. doi: 10.1002/ehf2.12485.
Joseph, P., Swedberg, K., Leong, D.P., & Yusuf, S. (2019). The evolution of β-blockers in coronary artery disease and heart failure (Part 1/5). J Am Coll Cardiol, 74(5), 672-682. doi: 10.1016/j.jacc.2019.04.067.
Khan, M.S., Fonarow, G.C., Khan, H., Greene, S.J., & Butler, J. (2017). Renin-angiotensin blockade in heart failure with preserved ejection fraction: A systematic review and meta-analysis. ESC Heart Fail, 4(4), 402-408. doi: 10.1002/ehf2.12204.
Khan, M.S., Jones, D.W., & Butler, J. (2019). Salt, no salt or less salt for heart failure patients? Am J Med,. doi: 10.1016/j.amjmed.2019.07.034.
Khan, M.K., & Sanchez, A. (2018). Chapter 11: Congestive heart failure and management drugs. In Adel Elmoselhi (Ed). Cardiology: An integrated approach. [Online edition]. New York, NY: McGraw-Hill Education. Retrieved from www.UCHC.edu
Koruth, J.S., Lala, A., Pinney, S., Reddy, V.Y., & Dukkipati, S.R. (2017). The clinical use of ivabradine. J Am Coll Cardiol, 70(14), 1777-1784. doi: 10.1016/j.jacc.2017.08.038.
Lee, M.S., Duan, L., Clare, R., Hekimian, A., Spencer, H., & Chen W. (2018). Comparison effects of statin use on mortality in patients with heart failure and preserved versus reduced left ventricular function. Am J Cardiol, 122(3), 405-412. doi: 10.1016/j.amjcard.2018.04.027.
Lee, M.M.Y., Sattar, N., McMurray, J.J.V., & Packard, C.J. (2019). Statins in the prevention and treatment of heart failure: a review of the evidence. Curr Atheroscler Rep, 21(41). doi: 10.1007/s11883-019-0800-z.
Leggio, M., Fusco, A., Loreti, C., Limongelli, G., & Padua, L. (2019). Effects of exercise training in heart failure with preserved ejection fraction: An updated systematic literature review. Heart Fail Rev, 2019 Aug 9. doi: 10.1007/s10741-019-09841-x. [Epub ahead of print]
Lewis, G. D., Malhotra, R., Hernandez, A.F., McNulty, S.E., Braunwald, E. (2017). Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. JAMA, 317(19), 1958-1966. doi: 10.1001/jama.2017.5427.
Lip, G. Y., Heinzel, F.R., Gaita, F., Gonzalez-Juanatey, J.R., & Pieske, B. (2016). European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace, 18(1),12-36. doi: 10.1093/europace/euv191.
Liu, L.C., Hummel, Y.M., van der Meer, P., Berger, R.F., Damman, K., Hoendermis, E.S. (2017). Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur J Heart Fail, 19(1), 116-125. doi: 10.1002/ejhf.662.
Lyu, S., Litian, Y., Huiqiong, T., Shaoshuai, L., Jun, Z. (2019). Clinical characteristics and prognosis of heart failure with mid-range ejection fraction: insights from a multi-centre registry study in China. BMC Cardiovasc Disord, 19(209). doi: 10.1186/s12872-019-1177-1.
Mahajan, R., Stokes, M., Elliott, A., Munawar, D.A., & Sanders, P. (2019). Complex interaction of obesity, intentional weight loss and heart failure: A systematic review and meta-analysis. Heart. doi: 10.1136/heartjnl-2019-314770.
Malik, A., Masson, R., Singh, S., Wu, W.C., Packer, M., & Ahmed, A. (2019). Digoxin discontinuation and outcomes in patients with heart failure and reduced ejection fraction. Journal of the American College of Cardiology, 74(5), 617-627. doi: 10.1016/j.jacc.2019.05.064.
Mann, D.L., & Chakinala, M. (2018). Chapter 252 Heart failure: Pathophysiology and diagnosis. In J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, Joseph Loscalzo (Eds), Harrison’s principles of internal medicine, (20th ed.) [Online edition]. New York, NY: McGraw-Hill Education.
Marume, K., Takashio, S., Nagai, T., Tsuijita, K., & Anzai, T. (2019). Effect of statins on mortality in heart failure with preserved ejection fraction without coronary artery disease. Circ J, 83(2), 357-367. doi: 10.1253/circj.CJ-18-0639.
Masella, C., Viggiano, D., Molfino, I, Zacchia, M., & Simeoni, M. (2019). Diuretic resistance in cardio-nephrology: Role of pharmacokinetics, hypochloremia, and kidney remodeling. Kidney Blood Press Res, 44(5), 915-927. doi: 10.1159/000502648.
Matsushita, K., Harada, K., Miyazaki, T., Miyamoto, T., Kohsaka, S., Iida, K., & Takayama, M. (2019a). Different prognostic associations of beta-blockers and diuretics in heart failure with preserved ejection fraction with versus without high blood pressure. J Hypertens, 37(3), 643-649. doi: 10.1097/HJH.0000000000001932.
Matsushita, K., Kazumasa, H., Miyazaki, T., Miyamoto, T., Kohsaka, S., Iida, K., & Takayama, M. (2019b). Younger- vs older-old patients with heart failure with preserved ejection fraction. Journal of the American Geriatrics Society, 67(10), 2123-2128. doi: 10.1111/jgs.16050.
Mehra, M.R. (2018). Chapter 253: Heart failure: Management. In J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, Joseph Loscalzo (Eds), Harrison’s principles of internal medicine, 20th ed. [Online edition]. New York, NY: McGraw-Hill Education.
Meyer, M., & LeWinter, M.M. (2019). Heart rate and heart failure with preserved ejection fraction: Time to slow β-blocker use? Circ Heart Fail, 12(8), e006213. doi: 10.1161/CIRCHEARTFAILURE.119.006213.
Miles, J.A., Hanumanthu, B.K., Patel, K., Chen, M., Siegel, R.M., & Kokkinidis, D.G. (2019). Torsemide versus furosemide and intermediate-term outcomes in patients with heart failure: an updated meta-analysis. J Cardiovasc Med (Hagerstown), 20(6), 379-388. doi: 10.2459/JCM.0000000000000794.
Mistry, R., Hosoya, H., Kohut, A., & Ford, P. (2019). Iron deficiency in heart failure, an underdiagnosed and undertreated condition during hospitalization. Ann Hematol, 98(10),2293-2297. doi: 10.1007/s00277-019-03777-w.
Mogos, M.F, Piano, M.R., McFarlin, B.L., Salemi, J.L., Liese, K.L., & Briller, J.E. (2018). Heart failure in pregnant women: A concern across the pregnancy continuum. Circ Heart Fail, 11, (1), e004005. doi: 10.1161/CIRCHEARTFAILURE.117.004005.
Mohrman, D.E., & Heller, L.J. (2018). Chapter 1: Overview of the cardiovascular system. Cardiovascular Physiology, 9the ed. New York, NY: McGraw-Hill Education:2018. Online edition.
Naing, P., Forrester, D., Kangaharan, N., Muthumala, A., Mon Myint, S., & Playford, D. (2019). Heart failure with preserved ejection fraction: A growing global epidemic. Aust J Gen Pract, 48(7), 465-471.
Nambiar, L., Silverman, D., Vanburen, P., LeWinter, M., & Meyer, M. (2019). Beta-blocker cessation in stable outpatients with heart failure with a preserved ejection fraction. Journal of Cardiac Failure, 25(8), s58-s59. doi: 10.1016/j.cardfail.2019.08.020.
Obokata, M., Kane, G.C., Reddy, Y.N., Melenovsksy, V., Olseon, T.P. (2019). The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J. doi: 10.1093/eurheartj/ehz626.
Packer, M. (2018). Worsening heart failure during the use of DPP-4 inhibitors: Pathophysiological mechanisms, clinical risks, and potential influence of concomitant antidiabetic medications. JACC Heart Fail, 6(6), 445-451. doi: 10.1016/j.jchf.2017.12.016.
Packer, M. (2019). Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Clues from laboratory models and clinical trials. Circ Res, 122(7), 928-932. doi: 10.1161/CIRCRESAHA.118.312673.
Peng, Y., Fang, J., Huang, W., & Qin, S. (2019). Efficacy of cognitive behavioral therapy for heart failure. Int Heart J, 60(3), 665-670. doi: 10.1536/ihj.18-408.
Price, S., Platz, E., Cullen, L., Tavazzi, G., Christ, M., Cowie, M.R., Mueller, C. (2017). Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol, 14(7), 427-440. doi: 10.1038/nrcardio.2017.56.
Quesada, O., & Klein, L. (2017) Chapter 26: Heart failure with reduced ejection fraction. In Michael H. Crawford, ed. Current diagnosis & treatment: Cardiology, (5th ed.). New York, NY: McGraw-Hill education. Online edition.
Rasmussen, E.R., Pottegård, A., Bygum, A., von Buchwald, C., Homøe, P., & Hallas, J. (2019). Angiotensin II receptor blockers are safe in patients with prior angioedema related to angiotensin-converting enzyme inhibitors - a nationwide registry-based cohort study. J Intern Med, 285(5), 553-561. doi: 10.1111/joim.12867.
Reddy, Y.N.V., Melenovsky, V., Redfield, M.M., Nishimura, R.A., & Borlaug, B.A. (2016). High-output heart failure: A 15-Year Experience. J Am Coll Cardiol, 68(5), 473-482. doi: 10.1016/j.jacc.2016.05.043.
Rosano, G.M., Vitale, C., & Seferovic, P. (2017). Heart failure in patients with diabetes mellitus. Card Fail Rev, 3(1), 52-55. doi: 10.15420/cfr.2016:20:2.
Sakamoto, A., Saotome, M., Iguchi K., & Maekawa. Y. (2019). Marine-derived omega-3 polyunsaturated fatty acids and heart failure: Current understanding for basic to clinical relevance. Int J Mol Sci, 20(16), pii: E4025. doi: 10.3390/ijms20164025.
Sartipy, U., Dahlström, U., Fu, M., & Lund, L.H. (2017). Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail, 5(8), 565-574. doi: 10.1016/j.jchf.2017.05.001.
Savarese, G., Jonsson, Å., Hallberg, A.C., Dahlström, U., Edner, M., & Lund, L.H. (2019). Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int J Cardiol. doi: 10.1016/j.ijcard.2019.08.049.
Shah, S. J., Lam, C.S.P., Svedlund, S., Saraste, A., Hage, C., Tan, R.S., & Lund, L.H. (2018). Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J, 39(37), 3439-3450. doi: 10.1093/eurheartj/ehy531.
Shi, V., Senni, M., Streefkerk, H., Modgill, V., Zhou, W., & Kaplan, A. (2019). Angioedema in in heart failure patients treated with sacubitril/valsartan (LCZ696) or enalapril in the PARADIGM-HF study. Int J Cardiol, 264, 118-123. doi: 10.1016/j.ijcard.2018.03.121.
Siscovick, D.S., Barringer, T.A., Fretts, A.M., Wu, J.H.Y, Lichtenstein, A.H., Costello, R.B.,…Mozaffarian, D. (2017). Omega-3 polyunsaturated fatty acid (Fish Oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation,135(15) e867–e884. doi: 10.1161/CIR.0000000000000482.
Solomon, S. D., McMurray, J.J.V., Anand, I.S., Phil, D., Ge, J., Lam, C.S.P., & Lefkowitz, M.P. (2019). Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med, 381, 1609-1620. doi: 10.1056/NEJMoa1908655.
Tadic, M., & Cuspidi, C. (2019). Obesity and heart failure with preserved ejection fraction: A paradox or something else? Heart Fail Rev, 24(3), 379-385. doi: 10.1007/s10741-018-09766-x.
Täger, T., Frohlich, H., Grundtvig, M., Seiz, M., Schellberg, D., Goode, K., & Frankenstein, L. (2019). Comparative effectiveness of loop diuretics on mortality in the treatment of patients with chronic heart failure: A multicenter propensity score matched analysis. Int J Cardiol, 289, 83-90. doi: 10.1016/j.ijcard.2019.01.109.
Taylor, G.M., Pop, A.M.C., & McDowell, E.L. (2019). High-output congestive heart failure: A potentially deadly complication of thyroid storm. , 2019(6): omz045. doi: 10.1093/omcr/omz045.
Toukhsati, S. R., Mathews, S., Sheed, A., Freijah, I., Moncur, L., Cropper, P., & Hare, D.L. (2019). Confirming a beneficial effect of the six-minute walk test on exercise confidence in patients with heart failure. Eur J Cardiovasc Nurs. doi: 10.1177/1474515119876784.
Trinkley, K.E., Anderson, H.D., Nair, K.V., Malone, D.C., & Saseen J.J.. (2018). Assessing the incidence of acidosis in patients receiving metformin with and without risk factors for lactic acidosis. Ther Adv Chronic Dis. 9(9), 179-190. doi: 10.1177/2040622318779760.
Tromp, J., Lim, S.L., Tay, W.T., Teng, T.K., Chandramouli, C., Ouwerkerk, W., & Lam, C.S.P.. (2019). Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care, 42(11), 1792-1799. doi: 10.2337/dc18-2515.
Tsujimoto, T., & Kajio, H. (2019). Use of nitrates and risk of cardiovascular events in patients with heart failure with preserved ejection fraction. Mayo Clin Proc, 94(7), 1210-1220. doi: 10.1016/j.mayocp.2018.11.032.
Tuegel, C., & Bansal, N. (2017). Heart failure in patients with kidney disease. Heart, 103(23), 1848-1853. doi: 10.1136/heartjnl-2016-310794.
van de Wouw, J., Broekhuizen, M., Sorop, O., Joles, J.A., Verhaar, M.C., Duncker, D.J., & Merkus, D. (2019). Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: A focus on microcirculatory factors and therapeutic targets. Front Physio, 10, 1108. doi: 10.3389/fphys.2019.01108.
Vukadinović, D., Vukadinović, A.N., Lavall, D., Laufs, U., Wagenpfeil, S., & Böhm, M., (2019).Rate of cough during treatment with angiotensin-converting enzyme inhibitors: A meta-analysis of randomized placebo-controlled trials. Clin Pharmacol Ther, 105(3), 652-660. doi: 10.1002/cpt.1018.
Vukadinović, D., Lavall, D., Vukadinović, A.N., Pitt, B., Wagenpfeil, S., & Bohm, M. (2018). True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: A meta-analysis. Am Heart J, 188, 99–108. doi: 10.1016/j.ahj.2017.03.011.
Yancy, C.W., Jessup, M., Bozhurt, B., Butler, J., Casey, D.E., Colvin, M.M.,…Westlake, C. (2017). 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol, 70(6), 776-803. doi: 10.1016/j.jacc.2017.04.025.
Yancy, C.W., Jessup, M., Bozkurt, B., Butler, J., Casey, D.E., Drazner, M.H., Wilkoff, B.L. (2013). 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 62(16), e147–239. doi: 10.1016/j.jacc.2013.05.019.
Zheng, S.L., Chan, F.T., Nabeebaccus, A.A., Shah, A.M., McDonagh, T., Okonko, D.O., & Avis, S. (2018). Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart, 104(5), 407-415. doi: 10.1136/heartjnl-2017-311652.
Zhou, X., Xu, W., Xu, Y., & Qian, Z. (2019). Iron supplementation improves cardiovascular outcomes in patients with heart failure. Am J Med, 132(8), 955-963. doi: 10.1016/j.amjmed.2019.02.018.
Ziaeian, B., Fonarow, G.C., & Heidenreich, P. A. (2017). Clinical effectiveness of hydralazine-isosorbide dinitrate in African American patients with heart failure. JACC Heart Fail, 5(9), 632-639. doi: 10.1016/j.jchf.2017.04.008.