About this course:
The learner will review and strengthen their knowledge in caring for a critically ill patient with acute respiratory distress syndrome, while examining the roles of the intensive care unit and the American Association of Critical-Care Nurses, enhancing skill knowledge in the use of mechanical ventilators, artificial airways, and tracheostomy suction and cares.
Course preview
Overview of Intensive Care Units
Many hospitals in the United States have an intensive or critical care unit, but they are not found worldwide, especially in developing nations. Many countries that would be considered lower to middle range income have started these units or are interested in providing this type of care to the people they serve. These departments care for critically ill patients resulting from many medical/surgical disorders or situations. The number of patients in the intensive care unit (ICU) is dependent on the number of available beds assigned to that department, and typically there would be a unit for adults including geriatrics, one for neonates, and one for pediatrics. Not all smaller hospitals will have a unit for neonates or pediatrics, which may require a transfer of the patient to a larger facility. The number of beds is traditionally lower than is seen in other departments, and the staff to patient ratio is much lower because of the higher acuity and needs of these patients (Marshall et al., 2017).
The medical care and nursing care in these units is more specialized, which allows for specialized monitoring and treatment options for patients that are suffering from organ dysfunction, which can result in multiple organ failure. Highly skilled life-saving care is required in these units as they are defined by the level of care provided. A level 1 ICU includes oxygen therapy, cardiac and vital sign monitoring, and more intensive nursing care in comparison to other floors. Level 2 ICUs offer a higher level of care, which may include invasive monitoring and basic life support for a short period. Finally, level 3 ICUs provide intensive, invasive monitoring and life support technology. They are typically found in larger metropolitan areas and are likely to support smaller nearby or regional hospitals, taking transfers from these hospitals to provide a higher level of care when needed. The type of patients that are cared for in an ICU varies but could include surgical patients, cardiac cases, respiratory disease, trauma victims, and many others (Marshall et al., 2017).
Acute Respiratory Distress Syndrome (ARDS) Overview
The cardinal feature of ARDS is sustained or refractory hypoxemia and decreased pulmonary compliance. The patient will likely experience dyspnea with pulmonary edema. Imaging will often indicate the presence of infiltrates frequently described as having a "ground glass" appearance. Patients can experience ARDS as a result of an existing lung disorder or with no prior history of respiratory disease as a result of an acute lung injury. The source of the acute lung injury could be an aspiration event, therapeutic or illicit medication(s), an inhalation injury, an infection such as sepsis, a traumatic injury, a medical disorder such pancreatitis, disseminated intravascular coagulation, or multiple blood transfusions amongst others (Grossman & Porth, 2014; Ignatavicius, Workman & Rebar, 2018; National Heart, Lung, & Blood Institute [NHLBI], 2019).
ARDS was initially given the name adult respiratory distress syndrome in 1967 when it was first diagnosed in an adult patient. It was later recognized that this disorder also occurred in children, requiring a name change to acute respiratory distress syndrome. This also replaced other names that include shock lung, wet lung, acute lung injury, noncardiac pulmonary edema, and stiff lung. There is a difference between acute lung injury (ALI) and ARDS. In 1994, during an American-European Consensus Conference, it was established that ALI and ARDS would be distinguished based on the level of hypoxemia the patient exhibited, with ARDS having a higher level of hypoxemia. This would be evaluated with objective data using the PO2 to FiO2 ratio. This was important to assist healthcare providers in determining the correct diagnosis to implement new treatment options as well as tools for prevention and continued research. This definition was updated in 2011 and is known as the Berlin definition, which further categorizes patients with ARDS into three categories. These categories include mild, moderate, and severe ARDS, with specific parameters for healthcare providers to utilize in the diagnostic process (Grossman & Porth, 2014; Ignatavicius et al., 2018; Ranieri et al., 2012).
It is challenging to know the exact number of cases that occur, but the ARDS Foundation estimates there are approximately 150,000 new cases annually in the United States. Some healthcare providers believe this estimate is low and that the actual numbers are higher as ARDS often exists with another disorder, which is more likely to be the disorder that is reported. It is estimated that approximately 42% of cases of ARDS are fatal (Ignatavicius et al., 2018).
Risk Factors Associated with ARDS
Many factors may increase the risk of developing ARDS, such as various types of infection, products that patients are exposed to, lifestyle habits, family history, genetic make-up, other medical diagnoses, ethnic background and gender. Of all the potential risk factors, the most common risk factor associated with ARDS is infection. The infection may be minor or significant, ranging from a common viral infection to sepsis (American Lung Association [ALA], 2019a; Ignatavicius et al., 2018; NHLBI, 2019).
Exposures that can increase the risk of ARDS includes living or working in an area that has significant air pollution, first or second-hand smoke inhalation, and heavy use of alcohol or illegal drugs. There is a lower incidence of ARDS in Caucasian patients, and males are more likely to develop ARDS in the pediatric population but not necessarily in the adult population. Genetics may increase the risk, but it may also contribute to how the lung tissue and immune system respond to direct or indirect injuries that can contribute to ARDS. As previously mentioned, medical disorders such as pancreatitis can also increase the risk of ARDS (ALA, 2019a; Ignatavicius et al., 2018; NHLBI, 2019).
Etiology and Pathophysiology
In a healthy individual, the process of gas exchange should take place efficiently and effectively with little effort by the individual. The gas exchange involves the intake of oxygen from the atmosphere via inhalation and the removal of carbon dioxide via exhalation. The process of inhalation starts in the neurons of the brain, which sense the need to exchange gases. The neurons signal the skeletal muscles in the diaphragm to contract and initiate inhalation. Once the air is inhaled, it will be conducted through the upper and lower airways deep into the respiratory tract to the alveoli, which are the terminal air spaces. Each adult has approximately 300 million alveoli to facilitate the gas exchange process (Grossman & Porth, 2014; Wilkinson, Treas, Barnett, & Smith, 2016).
The alveoli consist of two different types: type I and type II alveolar cells. Type I cells are thin squamous cells that make up 95% of the surface area and are unable to complete cell division. Type II cells are equally as numerous as type I cells, but they only contribute about 5% of the surface area. It is within these cells that surfactant is produced, which decreases the surface tension. When the surface tension is at an average level, it allows for more natural lung expansion during inhalation. Surfactant produced by type II cells is also a part of the immune regulation within the lungs (Grossman & Porth, 2014).
Conditions that lead to the development of ARDS can be direct or indirect. If the injury does not directly involve the lungs, an inflammatory response is triggered that spreads to the lungs. Because both direct and indirect injuries result in inflammation, the symptoms are similar. The inflammation impacts the alveolar-capillary membrane, which is impermeable except to very small molecules in healthy patients. Due to the inflammation, the membrane allows larger molecules to pas
...purchase below to continue the course
As fluid enters the alveoli, which are typically dry, the ARDS patient accumulates more fluid and proteins in the lung than usual. This shift of fluid from the intravascular space into the alveoli impedes surfactant production, which is necessary to facilitate decreased surface tension resulting in more natural contraction and relaxation of the alveolar space. This also allows a hyaline membrane to form, which becomes resistant to gas exchange. As these changes occur, the patient will start to have trouble breathing which will worsen as the lung tissue stiffens allowing for decreased ability to inflate the lungs properly (Grossman & Porth, 2014; Ignatavicius et al., 2018).
These changes lead to impaired gas exchange resulting in refractory hypoxemia that does not resolve even with high levels of supplemental oxygen. One role of surfactant is to help the alveoli retain their shape and ability to remain open. Therefore, the alveoli will begin to collapse without a sufficient amount of surfactant resulting in further impaired gas exchange and eventually the total sum of the damage and dysfunction leads to fibrosis (Grossman & Porth, 2014; Ignatavicius et al., 2018).
The exact etiology of ARDS is not clear; however, an inflammatory response both at the local level and the systemic level does occur. Patients who develop ARDS frequently have another disorder already in progress and typically one that is causing a capillary leak in another part of the body, such as in pancreatitis. It is understood that neutrophils play an active role in ARDS and are present early in the disease. Because the neutrophils are highly active in ARDS, they are releasing products into the bloodstream that further perpetuate an inflammatory response (Grossman & Porth, 2014; Ignatavicius et al., 2018).
Signs and Symptoms
As patients develop dyspnea and have changes in their chest x-ray, they could be diagnosed with pneumonia or heart failure with fluid in the lungs. However, these patients do not respond to typical treatment and continue to be more dyspneic and remain acutely ill. The patient with ARDS experiences shortness of breath, dyspnea, and cough. Some may be febrile, with associated tachycardia and tachypnea. As patients develop a cough, it will likely be productive and lead to fatigue. Some patients will complain of chest pain that may be more noticeable upon inhalation. The patient may experience hypoxemia and some degree of cyanosis. The nurse should monitor for changes in mental status such as confusion, drowsiness, restlessness, and other indicators of hypoxemia. The nurse should assess and follow vital signs regularly. Ongoing evaluation of body temperature may be useful as patients who are febrile early in the development of ARDS often have a lower mortality rate as compared to those patients who have average or subnormal temperatures early in the disorder (Grossman & Porth, 2014; Ignatavicius et al.; NHLBI, 2019).
While doing a physical assessment on this patient, the nurse would not only want to auscultate for lung sounds but to observe for sternal retractions or any signs of an increased effort to breathe. It is important to note that the nurse will not necessarily hear abnormal lung sounds because the inflammatory process is creating excess fluid in the interstitial space but not in the airways. The nurse will assess and continue to monitor the oxygen saturation of the patient and carefully document the saturation level when the supplemental oxygen was initiated as well as the response. This should also be done if the level or type of oxygen device is changed. The nurse should also continue to closely monitor the respiratory effort being made by the patient as well as changes in vital signs (Grossman & Porth, 2014; Ignatavicius et al., 2018; Wilkinson et al., 2016).
Diagnosis
ARDS can be challenging to diagnose and often masquerades as another disorder or disease initially, which makes prompt treatment difficult at times. The provider starts with a medical history, which should focus on previous respiratory conditions, heart disease, recent infections, or recent travel that could have resulted in some contamination and other relevant information. The provider will complete a thorough physical assessment looking carefully for any signs of change in fluid status or inflammatory response (Grossman & Porth, 2014; Ignatavicius et al., 2018; NHLBI, 2019).
Based on the findings during the history and physical, the provider will order diagnostic studies to assess the patient's condition further. The provider will likely order:
- arterial blood gases to determine the degree of hypoxemia and functional gas exchange,
- a complete blood count to evaluate for signs of infection,
- a complete or basic metabolic profile to assess electrolyte and fluid status as well as renal and liver function, and
- C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR) to assess for inflammation.
The provider may also order a urinalysis and urine culture to look for possible infection of the urinary and renal system. An EKG and other cardiac tests might be ordered if the patient has a previous history of cardiac disease or has symptoms suggestive of cardiac dysfunction (Grossman & Porth, 2014; Ignatavicius et al., 2018; NHLBI, 2019).
A chest x-ray will be completed to assess for the presence of fluid in the lungs or other abnormalities such as the appearance of what looks to be “ground glass” or an area of "white-out" on the x-ray. The chest x-ray can also be used to assess for infection, although a CT scan may be ordered to further evaluate for infection in the lungs as well as the abdomen. The provider may order a bronchoscopy to directly assess the lungs, take biopsies, and potentially collect a specimen for culture (Grossman & Porth, 2014; Ignatavicius et al., 2018; NHLBI, 2019).
Based on the results of the health history, physical assessment, patient condition, and the diagnostic study results, the healthcare provider will make a diagnosis that indicates ARDS or another disorder. Therefore, there is not a single diagnostic tool that allows for the diagnosis of ARDS, but rather an accumulation of studies and the physical condition of the patient (Grossman & Porth, 2014; Ignatavicius et al., 2018; NHLBI, 2019).
Treatment
ARDS is divided into three separate phases: the exudate phase, the fibroproliferative phase, and the resolution phase. In the exudate phase, which occurs early in the onset of ARDS, the patient has early symptoms such as dyspnea and tachypnea. While the symptoms may not appear complex, the internal changes are already causing the alveoli to fill with fluid leading to atelectasis. The goal of treatment in this stage is to administer supplemental oxygen to correct or maintain the current saturation, provide supportive care, and treat any underlying etiology such as infection. The purpose of this is to prevent the condition from worsening and advancing to the next stage (Ignatavicius et al., 2018).
In the fibroproliferative phase, the patient experiences increasing lung injury that causes pulmonary hypertension and possible fibrosis of the injured lung tissue. The body will attempt to repair the damage and stop the inflammatory process. During this stage, a more significant portion of the lung is affected, which further impacts the process of gas exchange. It is during this phase that the patient may experience dysfunction in other organs, which is referred to as multiple organ dysfunction (MODS). Treatment in this phase is directed towards improving or maintaining oxygenation, continuing to deliver supportive care, as well as trying to prevent further complications (Ignatavicius et al., 2018).
The patient usually enters the resolution phase around 14 days after the initial symptoms began. Hopefully, the initial injury that caused the ARDS is resolving; if not, the patient may succumb to the disorder or develop a chronic disease as a result of the ARDS. The patient may or may not have fibrosis as well as other long-term effects (Ignatavicius et al., 2018).
The patient with ARDS needs supportive care and management of oxygenation during each phase. Some patients will need to be intubated and require mechanical ventilation. If the patient requires mechanical ventilation, they will need either positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP). The safest and best practice standards would indicate that this process should be provided using "open lung" and lung-protective ventilation strategies. These strategies recommend using low tidal volumes (6 ml/kg of body weight) (Ignatavicius et al., 2018).
When using PEEP, the recommendation is to start at 5 cm of water and increase as needed to keep oxygen saturation at an appropriate level. One of the goals during mechanical ventilation is to get the alveoli that are not functioning to participate in gas exchange by using a method of ventilation that is pressure-controlled versus volume-controlled. One potential disadvantage is that PEEP can result in a tension pneumothorax, so the nurse needs to assess lung sounds on an hourly basis and use suction to maintain a patent airway as often as needed. Diminished or absent breath sounds may indicate a tension pneumothorax. Other types of ventilation may be indicated in ARDS cases that are more moderate to severe such as airway pressure-release ventilation (APRV) or high-frequency oscillatory ventilation (HFOV) (Ignatavicius et al., 2018).
Patient positioning is helpful as it can help improve the lung condition, but researchers are not in agreement as to which position is best. The two positions that seem to be most helpful are the prone position and elevation of the head, or the fowler’s position. The prone position is frequently recommended as this shifts pressure off the dependent portions of the lung, allowing for better ventilation. As the patient is moved from a prone position to a Fowler’s position, there is a noted rise in arterial oxygenation compared to just supine positioning. This is felt to be attributed to an increase in lung volume redistribution as the patient moves from the prone to a Fowler’s position. In the prone position, the chest wall is less compliant, but the upright position allows for increased chest wall compliance and increased volume resulting in higher oxygen levels. When a patient is placed in a supine position, the dependent lung can be negatively affected by the weight of the other organs. Prone positing can be a difficult task to complete, but the use of specialized beds and a collaborative approach can assist with this (Aoyama et al., 2019; Ignatavicius et al., 2018).
Patients with ARDS will likely need antibiotics when an infection is suspected or has been definitively diagnosed. Healthcare providers may also order medications to help reduce the levels of inflammation. ARDS may be the result of a primary condition such as pancreatitis or other disorder, which will be treated with the appropriate medications. Some providers are using vitamins C and E as well as surfactant replacement drugs to assist in the treatment of ARDS. The patient may also need fluid and electrolyte therapy to maintain balance, but fluid resuscitation should be limited to limit edema. A more conservative approach to fluid infusion will often lead to a better response and quicker recovery. Some patients will also need diuretic therapy to assist with the level of edema or treat some cardiac disorders (Ignatavicius et al., 2018).
Other medications that could be indicated are proton pump inhibitors or histamine-2 receptor antagonists to help reduce gastric acid and the risk of a stress ulcer. If the patient has increased risk for a blood clot related to immobility, the patient may be started on anticoagulants. Muscle relaxants and/or antianxiety medications may be used for patients on ventilators to assist with muscle tension. This allows the ventilator to work against less resistance and makes the patient more comfortable and less anxious. Pain medications may be indicated for the treatment of pain and during various procedures that need to be performed (ALA, 2019b; Ignatavicius et al., 2018; NHLBI, 2019).
In a critically ill patient, nutrition can be a challenge. There should be a collaborative approach between the provider, dietary services, and nursing to maintain adequate nutrition. If the patient has a mild case of ARDS, oral intake could be possible with supplements as needed. In more critical cases, the patients will likely need enteral feedings or total parenteral nutrition. A discussion and decision will be made with the healthcare provider and the dietitian to maintain an appropriate diet that will assist in tissue support and repair. These patients could be on bed rest for several days or even weeks, so nutrition is essential to promote skin integrity and vital functions (Ignatavicius et al., 2018).
Additionally, physical and or occupational therapy should be included to assist in the care of these complex patients. Both disciplines evaluate the patient’s needs during the acute and recovery phases to prevent muscle wasting, loss of muscle strength, possible development of contractures as well as other concerns. Either physical or occupational therapy may assist with positioning to promote oxygenation and respiratory effort. Both specialty areas would work with the patient as they recover their strength and mobility as well as prepare for discharge (Ignatavicius et al., 2018; NHLBI, 2019).
Another type of treatment utilized in ARDS patients that are not responding to traditional ventilation and oxygenation methods would be extracorporeal membrane oxygenation (ECMO). Mainly, this is utilizing heart-lung bypass equipment to function for the patient like a lung that is external to the body. The ECMO helps to rid the body of excess carbon dioxide and to add oxygen to the patient's bloodstream. ECMO is used when other methods are not successful, and often while the patient is awaiting a lung transplant (ALA, 2019b; Ignatavicius et al., 2018; NHLBI, 2019).
Nursing Roles
The role of the nurse in the care and treatment of ARDS patients is multi-faceted and typically in an ICU. Nursing assesses the patient by collecting history and physical assessment data initially and ongoing. The nurse must carefully monitor vital signs, respiratory effort, oxygen saturation levels, and diagnostic studies, contacting the provider with remarkable findings. It is also essential that the nurse keeps the family updated within their scope of practice and patient confidentiality, ensuring not to violate HIPPA. The patient’s family may be anxious and apprehensive. Although the nurse will be busy caring for the patient, they must remember to integrate the family into patient care when possible and use open and honest communication (Ignatavicius et al., 2018).
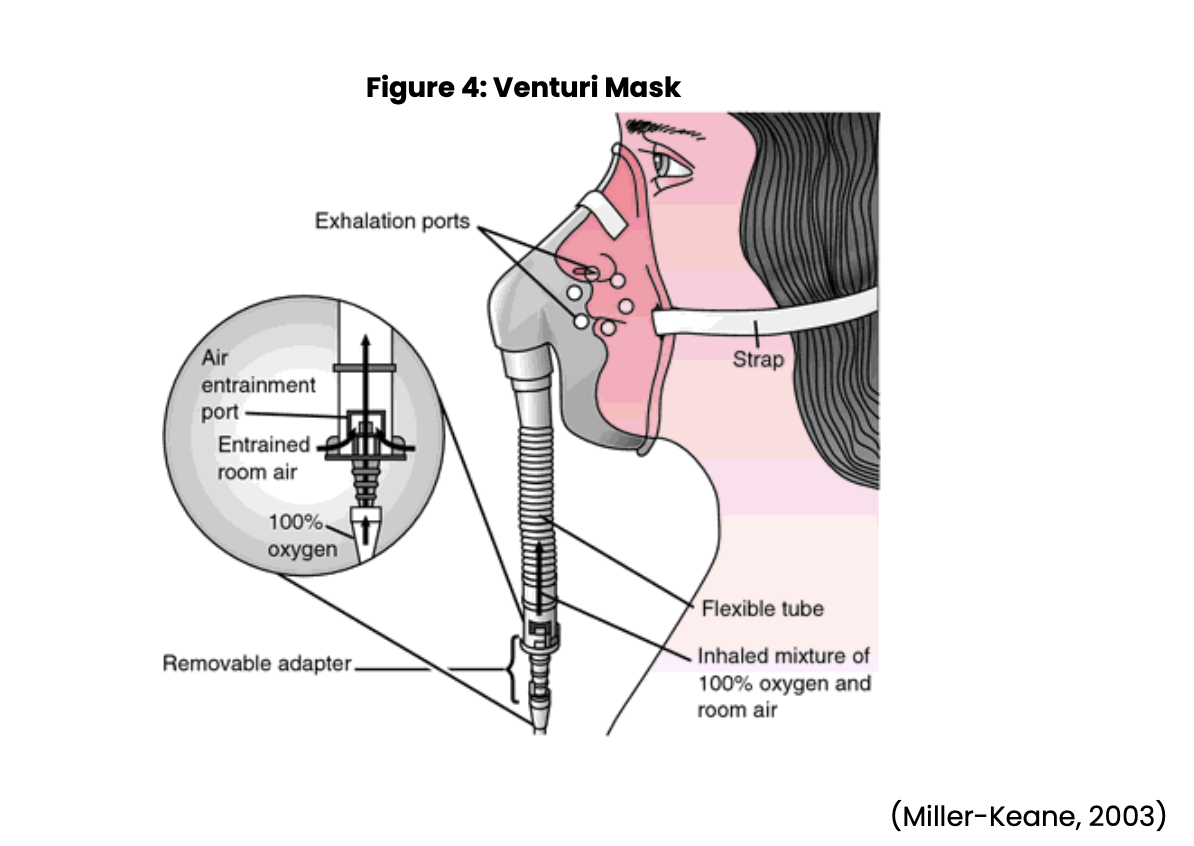
Nursing may also be involved in assisting with diagnostic procedures such as obtaining sputum specimens, collecting urine samples, and assisting with bronchoscopies. The nurse may also need to set up sterile fields for procedures, and monitor the patient following procedures. Nursing will need to watch for anticipated and unexpected events following these procedures. Treatment options include using supplemental oxygen as ordered and observing for the effectiveness of the oxygen. The nurse will need to be prepared to increase oxygen levels per provider order and change oxygen delivery devices based on arterial blood gases and/or oxygen saturation levels. Nursing should be aware of the various types of oxygen devices, flow rates, and the percent of oxygen they can deliver through each device. Oxygen devices include a nasal cannula (see Figure 1 below), simple face mask (see Figure 2 below), partial rebreather, nonrebreather mask (see Figure 3 below), venturi mask (see Figure 4 below), face tent, tracheostomy collar, and a T-piece (a T-shaped tube connected to an endotracheal tube [ETT] to deliver supplemental oxygen when mechanical ventilation is not required) (Ignatavicius et al., 2018; Wilkinson et al., 2016).
The ARDS patient may be placed on mechanical ventilation, so nursing must be knowledgeable and prepared to work with mechanical ventilators, intubation, and suctioning of the airway. Nursing must be familiar with different types of respirators and recognize what the standard settings mean, and how to troubleshoot the ventilator. Working with respiratory therapy will be essential to deliver the best care for the patient. It is imperative that the nurse suction as needed using a vigilant sterile technique (Ignatavicius et al., 2018; Wilkinson et al., 2016).
As discussed above, when caring for a patient with ARDS, they may already have an active infection; thus, nursing must correctly use sterile technique when suctioning to decrease the risk of introducing additional organisms. Proper handwashing must always be utilized, and nurses who exhibit any signs of active illness should not be involved with the care of these patients. The concepts of safety and infection control are paramount in caring for these patients (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Medication administration will involve parenteral therapy as well as possible oral administration, depending on the ability of the patient to swallow. Nursing will need to be knowledgeable regarding the mechanism of action, anticipated outcomes, and possible adverse effects. Medication administration will involve the assessment of the patient before administration, evaluation at appropriate times, and clear and concise documentation. Depending on the patient's condition, they may require supplemental nutrition, so nursing must be aware of how to correctly administer parenteral and/or enteral nutrition, recognizing that this is another potential source of infection (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Nursing must work in collaboration with other specialty areas such as physical therapy, occupational therapy, respiratory therapy, and others in meeting the needs of the ARDS patient. The patient will need to be turned and repositioned frequently; the provider will determine the most appropriate position(s) for the patient but will likely involve being in the prone position for intervals. Some of the other disciplines may be involved in using equipment to assist in positioning the patient in the prone position to ensure safety. The nurse plays a vital role in supporting and providing care for the ARDS patient (Ignatavicius et al., 2018).
Mechanical Ventilation and Intubation
This type of respiratory support is used in patients with ARDS as well as other disorders. Patients who require intubation and mechanical ventilation are unable to appropriately complete gas exchange necessary to maintain life. This patient has failed attempts to support ventilation and gas exchange with supplemental oxygen. This is likely a short-term situation for patients who have had surgery with general anesthesia, or given sedatives. Patients with traumatic brain injury, chronic lung disease, or end-stage neuromuscular diseases may require long-term respiratory support (Ignatavicius et al., 2018; Wilkinson et al., 2016).
The nurse should determine if the patient has an advance directive as these often contain parameters for the implementation of mechanical ventilation. In some cases, patients or families may decide to suspend the advance directive and implement mechanical ventilation. The process should be explained to the patient and their family, and any questions they have should be discussed. In life-threatening situations, the educational component might come after the intubation and ventilation process has begun (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Prior to intubation and mechanical ventilation, the nurse should perform a full assessment of the respiratory system including rate, depth, and rhythm as well as auscultation of lung sounds, assessment for skin pallor and/or cyanosis, and pulse oximetry. An oral assessment should be completed for any open wounds, evidence of candidiasis or other possible infections, and any bleeding or abnormal odor. The nurse should continue to complete a full physical assessment if time allows prior to the intubation and implementation of mechanical ventilation. If time does not allow, the nurse can complete the remainder of the full assessment following the procedure. The nurse should carefully document their assessment findings. The provider will likely order arterial blood gases and other diagnostic studies, and the nurse should alert the team of the results when available (Ignatavicius et al., 2018; Wilkinson et al., 2016).
If a patient requires mechanical ventilation, they will need an artificial airway. The selection of an airway depends on multiple factors, including whether the need is short-term or long-term. If the patient requires short-term ventilation, the artificial airway can be provided by an ETT, but long-term ventilation (longer than 14 days) will likely require the placement of a tracheostomy tube. Utilization of an ETT for more than 14 days puts the patient at risk for damage to the vocal cords and trachea (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Placement of an artificial airway not only allows for ventilation and suctioning if needed, but also helps stabilize the airway. ETTs are inserted through the nose or mouth into the trachea (see Figure 5 below). Anesthesia providers and respiratory therapists usually perform intubations. Each patient will require a different size of tube based on their size, but an average size adult will use from a 7 to 9 mm ETT with pediatric and neonates requiring much smaller tubes. The tube may be inserted quickly under emergency conditions, or in a more controlled situation in a patient who is slowly getting worse (Ignatavicius et al., 2018; Wilkinson et al., 2016).
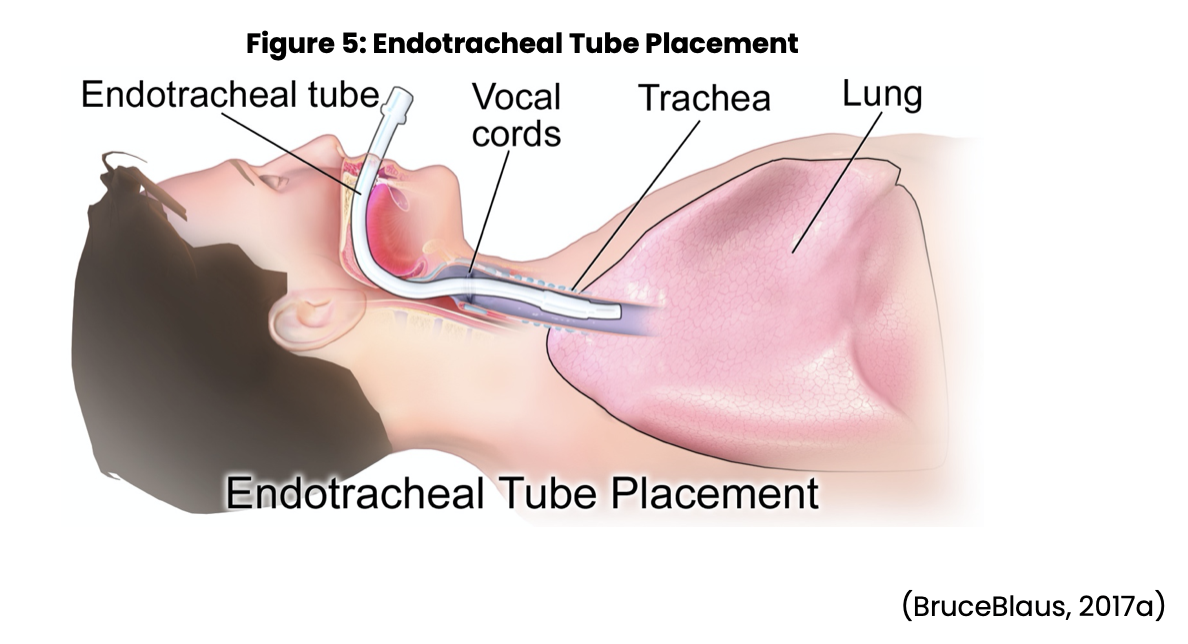
As the tube is being placed, nursing is responsible for monitoring the patient for any changes in vital signs or increasing signs of hypoxia or cardiac dysrhythmias. The nurse should also monitor time lapse during the insertion procedure, with a goal of 15-30 seconds for insertion of the tube. If the intubation lasts longer than 30 seconds, the patient must be ventilated with a manual resuscitation bag and supplemental oxygen to prevent further injury or damage. The patient must continue to receive oxygen to prevent hypoxia, and potential cardiac arrest. If necessary, the patient may need to be suctioned. If inserted correctly, the tip of the tube should be positioned approximately 2 cm above the tracheal carina (internal ridge at the bifurcation of the right and left bronchi). Once the ETT has been placed, an x-ray is taken to confirm the correct placement. The ETT has radiopaque lines that can be seen on x-ray and allow the provider to establish placement, and act as a guide for identifying the depth of the tube. The tube’s depth markings are assessed frequently to make sure the correct tube placement is maintained at the nares or mouth (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Once the placement is confirmed, the ETT needs to be sealed by inflating the cuff with air. When the cuff is inflated, the patient will not be able to talk; therefore, a different form of communication will be necessary. At this time, the ETT will need to be stabilized by two persons. The tube should be marked at the nares or the incisors where the tube touches. It is best to use a manufactured device to hold the tube in place. One person holds the patient's head as well as the ETT at the entry point while the other secures the tube into the device. Both nurses should verify that the tube has remained in the correct position and that the tube device is secure (Ignatavicius et al., 2018; Wilkinson et al., 2016).
At this time, the nurse should assess the patient for symmetry: equal breath sounds in both lungs as well as bilateral chest rise and fall. Documentation indicating the lung sounds and the level of the tube should then be completed if the patient is stable. Nursing should also make sure the patient is comfortable, with all needs met as well as a call light available. Essential nursing care requires continuous monitoring of vital signs, ETT positioning, and frequent respiratory assessments (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Tracheostomy Tubes
If the provider determines that the need for mechanical ventilation will be long-term, then the patient will require a tracheostomy (see Figure 6 below). Education should be provided to the patient regarding the placement procedure, advantages, and disadvantages before the procedure and obtaining the consent. If this is an emergency, the provider will briefly explain the process but may not have time to go into detail. The patient will have routine preoperative care, and then anesthesia will insert an ETT. The surgeon will make a tracheotomy incision and then create the stoma or opening into the trachea, which is referred to as the tracheostomy. Anesthesia will remove the ETT once the trachea is open, which allows for the placement of the tracheostomy tube. The tube will need to be secured with sutures and either tracheostomy ties or another type of securement device. As with an ETT, the patient will need to have a chest x-ray to confirm exact placement (Ignatavicius et al., 2018; Wilkinson et al., 2016).
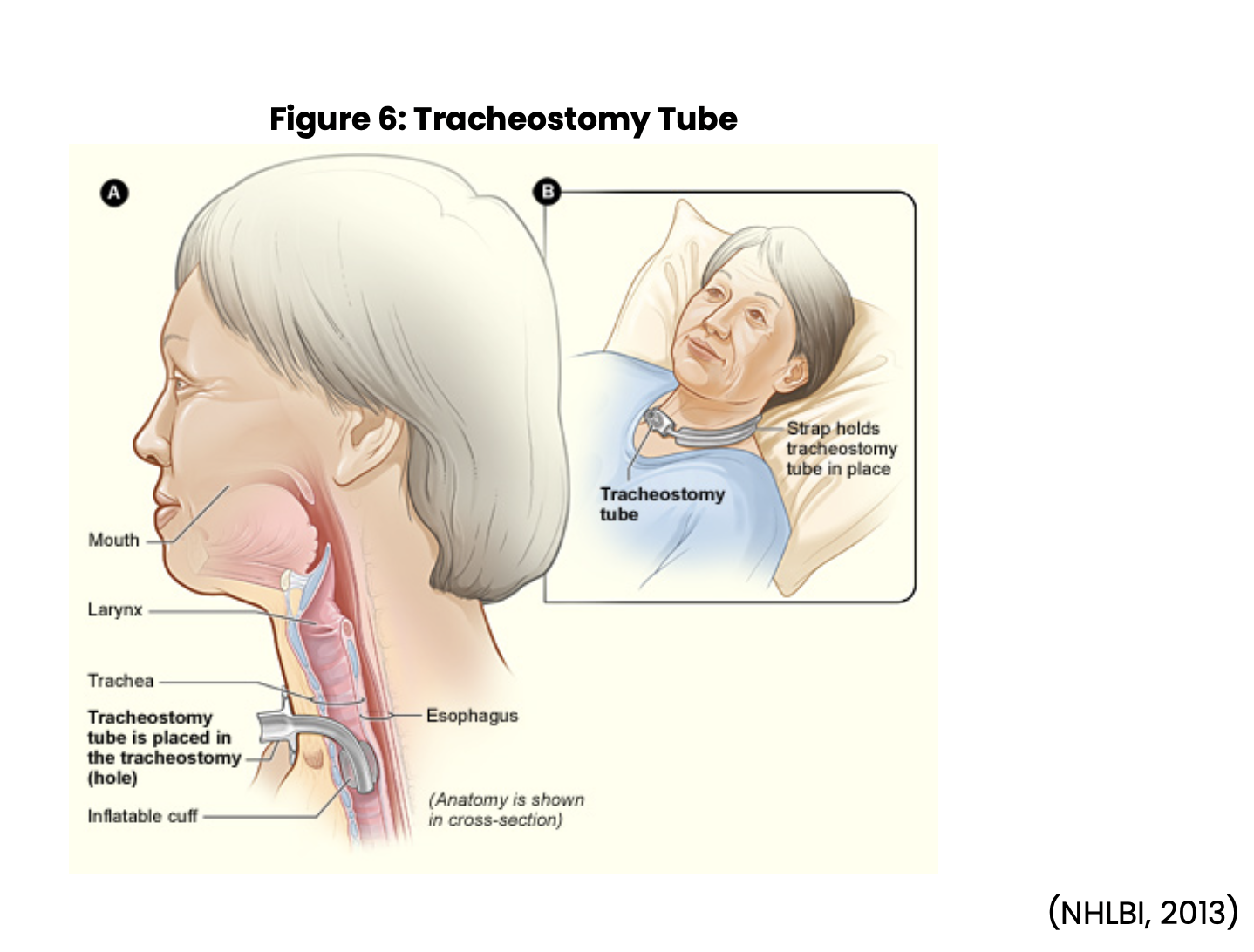
Nursing postoperative care includes assessing for, and maintaining a patent airway, monitoring vital signs, observing for signs of bleeding, and completing respiratory assessments frequently. In a patient with a new tracheostomy, minor bleeding will likely occur, but heavier bleeding should be reported to the provider. Nursing will want to assess the tracheostomy tube to ensure proper positioning and patency are maintained. The nurse may need to suction the cannula or provide tracheostomy care frequently at first. Respiratory therapy and nursing may work in collaboration to provide care such as oxygen therapy, assisting the patient to cough and deep breath, and nebulizer treatments if required. Any change in respiratory status, secretions, or vital signs must be documented and reported to the provider immediately to identify postoperative complications early (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Another concern in the postoperative period is an infection of the surgical site. Sterile technique should always be utilized during the postoperative period for tracheostomy care and suctioning to reduce the risk of infection. As nursing is providing care, it is imperative to assess for signs of infection, which could include swelling, inflammation, or excessive/purulent drainage coming from around, or in the tracheostomy. The patient may also be febrile or have other changes in other their vital signs. Prior to or during tracheostomy care, the patient may complain of pain, or burning at the site, which should be assessed. A split gauze dressing specifically made for stoma sites should be applied after inspecting the site. It is not recommended to use standard 4x4 gauze and cut it to fit as the small fibers or threads can enter the open surgical site and lead to infection. It is important to change the dressing as needed when it becomes soiled with blood or drainage to prevent the moist dressing from becoming a receptacle for infection (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Nursing should be familiar with the various types of tracheostomy tubes that are utilized currently in their facility. Tracheostomy tubes vary in physical design and the type of material that is utilized, which is usually plastic or metal. Some tubes may have a cuff and can be single or double lumen. The provider decides on the type of tube to use based on the client's needs. A patient who will be on a mechanical ventilator or has dysphagia will most likely have a cuffed tube. If a patient can perform self-care, the best option is a tracheostomy tube that has an inner cannula. This type of tube allows the patient to remove the inner cannula for cleaning and is easier to maintain patency. If the tracheostomy tube has an inner cannula, this should be changed regularly per the institution’s protocols and procedures (Ignatavicius et al., 2018; Wilkinson et al., 2016).
There should always be a new tracheostomy set and insertion kit at the patient’s bedside that is the same brand, type, and size (or one size smaller). Nurses working with new tracheostomy patients should be prepared to change or replace the outer cannula if indicated (i.e., the tube becomes plugged and the nurse is unable to suction it out or the tube becomes dislodged). To replace a tracheostomy tube, the nurse should position the head by extending the neck and secure the airway by using a clamp to hold back any tissue. Make sure to place the obturator into the new tracheostomy tube and check the cuff for leaks. If the old tracheostomy tube is still in place, remove any ties/sutures, deflate the cuff, and gently remove the tube. Insert the new tube into the patient’s trachea quickly with the curve pointing down. Once the tracheostomy tube is in the trachea, remove the obturator and secure the tube. Inflate the cuff and ventilate the patient if needed. Once the tube is secured and the patient is settled, the nurse should assess for any signs of bleeding or trauma to the site and complete a full respiratory assessment. If the nurse is unable to replace the tube, they should maintain the airway and breathing with rescue methods and notify the provider and respiratory therapy for assistance. Nursing may also need to activate the emergency system within your facility for additional support (Ignatavicius et al., 2018; Wilkinson et al., 2016).
A tracheostomy cuff is an internal balloon that wraps around the outside of the tracheostomy tube. When it is inflated, the cuff puts pressure on the trachea and creates a seal around the cannula, and airflow is contained in the tracheostomy tube. There is an external balloon often referred to as the pilot balloon. A syringe of air is attached to a valve at the end of the pilot balloon, which allows air to be injected into the balloon, flow through the thin tube, and into the internal cuff. This inflates the cuff and the pilot balloon simultaneously, allowing healthcare workers to visualize and palpate the external balloon to monitor for cuff inflation. When assessing the air in the pilot balloon, the nurse must recognize that air in the pilot balloon indicates air is present in the internal cuff, but it does not indicate the amount of air present in the cuff (Ignatavicius et al., 2018; Wilkinson et al., 2016).
There are also fenestrated tracheostomy tubes. When the inner cannula is in place, this closes the fenestration and is recognized as a double lumen. If the patient is attempting to speak or cough, the inner cannula can be removed, the cuff deflated, and a stopper-like device can be attached. Because the inner cannula is gone, this allows air to come in through the fenestrated part of the tube, which helps the patient to cough and speak. Some patients adapt to this quickly while others become anxious, and have trouble breathing. If a patient has trouble when the stopper is applied, the nurse should assess whether the type, size, or placement needs to be adjusted. Fenestrated tubes come with cuffs or can be cuffless. It is imperative to deflate the cuff before applying the stopper to ensure the patient has a patent airway (Ignatavicius et al., 2018; Wilkinson et al., 2016).
When caring for a patient with a tracheostomy tube, consideration must be given to the potential complications. A cuffed tracheostomy tube puts pressure on the lining of the trachea, which can cause impaired tissue integrity. To prevent damage, the pressure exerted should range between 14 and not exceed 20 mmHg or between 20 and 30 cm of water. The recommendation is to use less than 25 cm of water for the best outcomes, unless there are special circumstances and it is indicated by the provider or respiratory therapy (Ignatavicius et al., 2018; Wilkinson et al., 2016).
There are two ways to measure the cuff pressure, and best practice suggests that cuff pressure should be checked at least once per shift, or at least every eight hours at home. This may be done more frequently in a newly established tracheostomy. The nurse can check the cuff pressure with the minimal leak technique, or a pressure cuff inflator. The minimal leak technique can be used anytime, but it is convenient to complete after tracheostomy suction and care are completed. Using a 10 ml syringe attached to the valve on the pilot balloon, start to withdraw the air while auscultating with a stethoscope on the patient’s neck near the tracheostomy site. The nurse should hear a pronounced rush of air as the pilot balloon deflates. The sound of rushing air is the seal between the internal cuff and the trachea being broken. The nurse re-inflates the pilot balloon while listening for the air going into the cuff. When air is no longer auscultated, this will indicate the seal is again in place. The nurse should withdraw 1 ml of air to decrease the risk of over-inflation. The cuff pressure can also be measured with a pressure cuff inflator or pressure manometer. The manometer is attached to the pilot tube and the pressure is read on the gauge. If the pressure is too high or too low, it can be adjusted by inflating or deflating with the manometer (Ignatavicius et al., 2018).
Nursing care of a tracheostomy patient should also focus on monitoring and maintaining adequate nutrition, hydration, and oxygen levels. The nurse should ensure the secured and stable positioning of the tube and limit suction to the amount that is needed to keep the patient’s airway patent. Warmed humidity can help keep secretions thinner and add moisture to the tissue (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Tracheostomy patients require suctioning to decrease secretions in the cannula, and to keep the tube patent. Nursing needs to monitor the patient closely for the need to suction with each interaction. The patient should also notify the nurse if they feel the need to be suctioned and are able to communicate. All staff should be responsible for monitoring patients that have artificial airways and mechanical ventilators. Some signs that a patient needs to be suctioned are:
- obvious secretions coming from the tube,
- increasing shortness of breath,
- dyspnea,
- wheezing, or other adventitious breath sounds,
- increasing respiratory effort,
- decreased oxygen saturation,
- increased respiratory and heart rate, and/or
- appearing restless or agitated.
Suctioning should remove the secretions from the tube, decrease the respiratory and heart rate to an appropriate rate, increase oxygen saturation, improve lung sounds, and cause the patient to feel better (Ignatavicius et al., 2018; Wilkinson et al., 2016).
An assessment should be completed prior to suctioning and should include auscultation of lung sounds, oxygen saturation, respiratory and heart rate, presence of secretions, and distress of the patient. If the patient is on mechanical ventilation, PIP should be assessed prior to suctioning. Suctioning can be painful, especially deep suction, and should be done as quickly and efficiently as possible. Emotional support should be provided to the patient in addition to good communication to explain what is going to be done and when it will be done (Ignatavicius et al., 2018; Wilkinson et al., 2016).
If the assessment indicates the need for suction, the nurse begins with handwashing and donning personal protective equipment (PPE) as warranted by the situation. This may include sterile gloves, protective eyewear, as well as other PPE. When performing tracheostomy suction, there are various opinions about using a sterile technique, modified sterile technique, or clean technique based on the client scenario. In most facilities, sterile technique is utilized for patients that have a new tracheostomy or are highly susceptible to infection. Modified sterile technique is frequently used for patients that have an established tracheostomy with normal risk for infection, and no history of complications with their tracheostomy. With this technique, the nurse still uses a sterile suction catheter and supplies but could use clean gloves instead of sterile. This is becoming a more widely used method for suction. The final technique is a clean technique that is more prevalent for patients and families providing care in their own homes. This technique utilizes clean gloves and clean supplies. If the patient is doing their own suction and care, some argue that there is no need for the use of gloves. This final technique has limited situations for valid use. If a nurse is performing suction in a home, they should wear clean or sterile gloves to prevent infection in both the client and themselves (Ignatavicius et al., 2018).
A suction kit, or necessary supplies based on the technique that will be used should be at the patient’s bedside. For a normal-sized adult with a standard tracheostomy, a 12-14 French suction catheter should be used. The suction catheter should not be larger than half the size of the lumen of the tracheostomy tube. Using a suction catheter that is too large can cause damage and decrease the oxygen level. As the nurse is preparing their supplies, they should explain to the patient what will be done, and what they might experience. Test the suction tubing for the appropriate level of suction. Occluding the suction tubing, if functioning and set properly, should cause the pressure gauge to increase to between 80 to 120 mmHg. The sterile field and supplies from the suction kit should be set up and easily accessible. In some patients, hyperventilation with or without supplemental oxygen may be required prior to suctioning, and some may need additional ventilation immediately following the suctioning. If indicated, the patient should be hyperventilated prior to suctioning with three to four inhalations using a manual resuscitation bag. If hyperventilation is not necessary, the nurse should ask the patient to take three or four deep breaths prior to suctioning. Monitor the patient’s oxygen saturation level, heart rate, and level of distress while suctioning (Ignatavicius et al., 2018; Wilkinson et al., 2016).
With sterile gloves on and with the sterile suction catheter, the nurse will access the tracheostomy tube by inserting the catheter into the tube until feeling resistance. While inserting the catheter, it is essential not to apply any suction as this could cause damage to the mucosa. The nurse should then pull the suction catheter back approximately 0.5 in and apply the suction. Suction should be applied continuously as the catheter is gently rotated and withdrawn. It will take a while after suctioning for the patient to settle as suctioning temporarily decreases available oxygen and, therefore, the saturation rate. For this reason, the nurse should suction tracheostomy patients thoroughly but quickly and not more often than is necessary. Suctioning should take no more than 10 to 15 seconds. The patient may need hyperoxygenation again until the pulse and oxygen saturations reach the patient's baseline. This process may need to be repeated up to three times total. Suctioning can irritate the mucosal lining of the trachea, which can produce more secretions, so limiting suction attempts is recommended unless patency is a concern. If necessary, the nurse should suction the mouth and provide oral care at this time as well. As with all patient care, the nurse should conclude by removing the gloves and completing hand hygiene. The nurse should also complete an evaluation of how the patient tolerated the procedure and the outcome and document accordingly in the patient’s medical record (Ignatavicius et al., 2018; Wilkinson et al., 2016).
In addition to regular suctioning, tracheostomy patients require routine care for their stoma and tube apparatus. Depending on the type of device that is used to secure the tracheostomy tube, this will also need to be changed at intervals or as it becomes soiled. The nurse should gather a tracheostomy tube care kit and other supplies prior to getting started with tracheostomy care. Arrange the supplies and set up the sterile field after completing hand hygiene and explaining the procedure to the patient. Don the appropriate PPE other than gloves, which are typically contained in the kit. The old tracheostomy dressing and any secretions that are present should be removed. If present, remove the inner cannula and replace it if disposable. If not disposable, the cannula should be cleaned with half-strength peroxide on the inside and outside and rinsed with sterile saline. The stoma and faceplate should be cleaned and rinsed using sterile cotton swabs and half-strength peroxide. While cleaning the stoma, the nurse should assess for any swelling, erythema, odor, or drainage. The skin should be rinsed well and dried. The ties or device securing the tracheostomy tube should be checked. If soiled or wet, it should be changed. Make sure to attach and secure the new ties or device before removing the old ones. Only one finger should be able to fit between the securing device and the patient's neck. The nurse should complete hand hygiene and evaluate the patient, making sure all needs have been met. Complete all documentation of the tracheostomy care and relevant information (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Types of Ventilators and Settings
Once an airway is established with either an ETT or tracheostomy tube, the patient can be managed on mechanical ventilation. The mechanical ventilator is used to improve gas exchange and to assist or provide effective breathing for the patient. In most cases, the patient has either an acute or chronic disease that is causing the ineffective breathing and impaired gas exchange, so the goal is to use mechanical ventilation until the lungs improve and can provide effective breathing and better gas exchange on their own. In some patients with significant damage or progressive disease, weaning off of the mechanical ventilator may not be possible (Ignatavicius et al., 2018).
There are many types of ventilators available for patients; the provider will choose one based on the individual patient’s needs, how long the ventilator will be needed, and how severe the condition is. Most of the ventilators being used today are positive-pressure ventilators: upon inhalation, the ventilator forces air into the patient’s lungs to expand the chest. There are three types of positive-pressure ventilators: pressure-cycled, time-cycled, or volume cycled (Ignatavicius et al., 2018).
A pressure-cycled ventilator has a preset airway pressure. The ventilator will conduct air into the lungs until that preset pressure is obtained. These ventilators are indicated for short-term use, such as in a postoperative patient, or when respiratory therapy needs to conduct some intervention. A more recent variation of the pressure-cycled ventilator is the bi-level positive airway pressure (Bi-PAP) that provides a preset inspiratory pressure but also integrates expiratory pressure as well. A time-cycled ventilator works in the same manner, but instead of a preset pressure, it is based on a preset time for forcing air into the lungs. A volume-cycled ventilator is preset to force air into the lungs until a preset volume is achieved. In the first two types of ventilators, the tidal volume varies, but in this type of ventilator, the tidal volume is preset. There are also microprocessor ventilators that are controlled by a built-in computer that allows for more flexibility with settings. This type of ventilator is indicated for a patient that has severe lung disease, or is ready to attempt the weaning process, but a long weaning process is anticipated (Ignatavicius et al., 2018; Wilkinson et al., 2016).
When working with ventilators, there are a variety of modes that the nurse will need to be familiar with. The most common modes include assist-control, synchronized intermittent mandatory ventilation (SIMV), and Bi-PAP. In assist control, both the tidal volume and the rate are preset for the patient. This mode allows the patient to take breaths on their own, but if the patient does not take a breath independently, the ventilator will trigger and provide a breath. Additionally, if the patient makes an effort to inhale, the ventilator provides assistance to the preset tidal volume. It is important to monitor the patient’s respiratory rate and effort when on assist control; if the patient increases their rate on their own, and the ventilator is also delivering breaths, the patient can develop respiratory alkalosis related to hyperventilation. The nurse would want to assess why the patient is increasing their rate, which could be related to fever, pain, and other etiologies (Ignatavicius et al., 2018; Wilkinson et al., 2016).
SIMV is a mode that can be used for patients who are dependent on the ventilator, or who are trying to wean from the ventilator. Similar to assist-control above, the tidal volume and rate are preset in SIMV. This mode will allow the patient to take breaths on their own but does not assist with completing a patient-triggered breath. If the patient does not initiate their own breath, the ventilator will establish a pattern, or the ventilator can be adjusted for the weaning process. This allows for coordination of ventilator breaths and patient breaths (Ignatavicius et al., 2018).
Bi-PAP is noninvasive and done externally. Instead of the patient needing an ETT or tracheostomy tube, the patient is supported with a face mask or a smaller mask for the nose. The most common reason this mode is a patient with sleep apnea or who is exhibiting signs of potential respiratory failure. It allows for temporary noninvasive treatment while the patient recovers, sleeps, or awaits transition to an invasive type of ventilation (Ignatavicius et al., 2018).
Nursing, in collaboration with respiratory therapy and the provider, will monitor the ventilator settings. There are many different components that need to be monitored, and the nurse should understand what the settings indicate. Most patients today needing mechanical ventilation will likely be maintained on a volume-cycled ventilator with universal controls and settings. The nurse will monitor each of the following settings: tidal volume, respiratory rate, fraction of inspired air (FiO2), peak airway inspiratory pressure, continuous positive airway pressure, positive end-expiratory pressure (PEEP), flow rate, and others as determined by respiratory therapy and the exact type of ventilator being utilized (Ignatavicius et al., 2018; Wilkinson et al., 2016).
- The tidal volume can be measured on either inhalation or exhalation and is the total volume of air with each breath. This volume should be in the range of 6-8 ml per kilogram of body weight.
- The rate is the number of breaths that have been set for the ventilator to deliver per minute, with an average of 10-14 breaths per minute.
- The FiO2 is the amount of oxygen the patient is receiving via the ventilator. This is typically based on the patient’s overall condition and their arterial blood gases. The oxygen must be warmed, and humidity must be added to preserve the mucosal lining of the respiratory tract.
- The peak airway inspiratory pressure (PIP) is the amount of pressure that is required to deliver the tidal volume. The PIP is impacted by the compliance of the lung tissue and is therefore subject to change as the patient’s condition improves or declines. It is essential for nurses to watch this level as it indicates resistance in the airway or ventilator. The patient’s tubing may be occluded, and if the pressure gets too high, the high-pressure alarm will sound on the ventilator. A high-pressure alarm must be responded to and corrected quickly.
- The continuous positive airway pressure (CPAP) is a setting that is used for patients that have their own respiratory effort. This setting allows the ventilator to deliver positive airway pressure during the respiratory cycle to attempt to keep the alveoli open and prevent collapse. This is an important tool for the patient who has started the weaning process.
- The PEEP is an essential setting in the prevention of atelectasis and is used to help treat hypoxemia that has not responded to other methods. The PEEP applies pressure (5-15 cm of water) during the exhale cycle to keep the lungs inflated to prevent collapse.
- Flow rate indicates how quickly the breath is being delivered. An average flow rate is approximately 40 L/min (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Any patient on a ventilator will require ventilator checks to maintain critical settings. Each facility will establish the procedure for ventilator checks and how often they should be performed. Initially, the ventilator checks could be as frequently as every 15 minutes to one hour and then every two hours. The ventilator checks may also be assigned to respiratory therapy. The frequency of ventilator checks is set by provider order and facility policy. Ventilator checks involve checking the mode of operation, temperature, humidity, as well as the components listed above. The nurse should remove any condensation from the tubing during the check. When checking the ventilator, the nurse should check the airway for patency and proper position and ask the patient how they are feeling. Oral cares should be done either at every ventilator check or as indicated by facility policy, as this is essential to decrease the risk of ventilator-acquired pneumonia. All staff should be aware of the alarm system on the ventilators and should respond immediately when a ventilator alarm sounds. Alarms on the ventilator should never be turned off and must be in working condition (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Care of Ventilator Patients and Complications
As a patient is supported on a ventilator in the ICU, they may develop anxiety, depression, and other mental health concerns. Nursing needs to work closely with the patient and family to answer questions and to provide not only physical care but emotional support as well. When alarms do sound, always assess the patient first to try and establish why the alarm is sounding, and then move to the ventilator. High-pressure alarms typically indicate the tubing is being pinched, there are excess secretions, the patient is anxious, the patient is coughing, or the airway is impacted by wheezing or spasm. Low-pressure alarms typically indicate the tubing may have come apart or developed a hole, a connection is loose, or the cuff is leaking. Nurses working with ventilators should be very knowledgeable about what each setting indicates and what to do if something is not working appropriately. It is also imperative to work with respiratory therapy and the provider to give the patient the best possible care (Ignatavicius et al., 2018).
The ventilated patient needs to be monitored frequently with careful documentation completed. The assessment should include vital signs, oxygen saturation, lung sounds to indicate if the patient is making any respiratory effort on their own, monitoring the weaning process if indicated, and overall observations. The provider will order arterial blood gases as needed, and nursing will need to monitor the results, and contact the provider and/or respiratory therapy as indicated. The ongoing monitoring is crucial to evaluate if the patient is improving or maintaining their oxygenation and gas exchange. As the condition warrants, the patient will likely need consults and treatment with physical, occupational and/or speech therapy, increased activity, and progress towards the weaning process if possible (Ignatavicius et al., 2018).
Patient care while on a ventilator will also include assessment of the artificial airway, either the ETT or tracheostomy tube, and the need for suction. The patient may express the need to be suctioned, have obvious secretions, or the ventilator may indicate increased pressure. The insertion site and placement should also be checked at frequent intervals: at least every four hours or per facility policy. The nurse should monitor the ventilator patient for any adverse signs but also for signs of improvement, especially noting if the patient is working against the ventilator or taking more breaths than previous. The nurse must also consider the patient’s psychosocial and emotional needs. Communication will likely be at least temporarily impaired, so speech therapy should provide communication tools or assist the tracheostomy patient with therapy as the condition of the patient warrants (Ignatavicius et al., 2018; Wilkinson et al., 2016).
Patients maintained on mechanical ventilation can develop many complications, sometimes referred to as ventilator-associated or ventilator-acquired events (VAE). These events indicate the patient has deteriorated regarding their ability to maintain oxygenation. There are three tiers of associated events: ventilator-associated condition (VAC), infection-related ventilator-associated complication (IVAC), and ventilator-associated pneumonia (VAP). In VAC, the patient has hypoxemia that has lasted more than two days, as evidence by increased daily minimum PEEP or FiO2. If this hypoxemia develops concurrently with a fever as a result of some type of infection that requires antibiotic therapy, it is referred to as IVAC. If blood work reveals positive gram stains or positive cultures in a patient diagnosed with IVAC, it is now considered VAP (Ignatavicius et al., 2018).
Infection in the ventilator-dependent patient can be a serious complication, and every effort must be made to decrease the risk of infection. Artificial airways make these patients vulnerable because of the ease of access via the devices for bacteria to descend into the airway rather quickly. Some research indicates that ETT or tracheostomy tubes are colonized with bacteria within 48 hours following insertion. These bacteria (along with bacteria that can be aspirated from the stomach, spread from staff not using appropriate hand hygiene, and introduced via inappropriate technique when suctioning or performing cares) can lead to pneumonia and other infections which increase the patient’s risk of illness and death (Ignatavicius et al., 2018).
Strict adherence to handwashing and the use of sterile technique, as indicated by facility policy, is recommended to prevent VAE. Besides using strict infection control, aspiration precautions should be in place for all patients on a ventilator, such as elevating the head of the bed to at least 30° to prevent aspiration. Oral care, as previously discussed, is essential for general health but also decreases the risk of infection. Continued monitoring of respiratory secretions, and appropriate suctioning and cares are essential to maintain infection control (Ignatavicius et al., 2018).
Multiple body systems can be impacted by mechanical ventilation. Some common cardiac complaints include hypotension and a reduction in the cardiac output, which leads to fluid retention. Nursing should monitor vital signs, intake, output, and daily weights to evaluate fluid changes as well as attempt to maintain appropriate hydration (Ignatavicius et al., 2018).
Trauma to the respiratory system in a variety of forms is one of the concerns with long-term use of ventilators. Traumatic events include:
- Barotrauma, which is damage to the lung tissue caused by positive pressure.
- Volutrauma, which results from too much volume being used and is typically more pronounced in one lung.
- Atelectrauma, which is a shearing injury that occurs to the alveoli from opening and closing.
- Boitrauma, which is damage to the alveoli that arises from an inflammatory response. This can also result in pneumothorax and subcutaneous emphysema and is common in patients who have ARDS (Ignatavicius et al., 2018).
Another injury that can arise from prolonged ventilator use includes ventilator-associated lung injury or ventilator-induced lung injury. This results in a loss of surfactant, inflammation, fluid escape, and edema of the lungs. Using low tidal volume and moderation of PEEP levels reduces the risk of lung trauma or injury for all ventilator patients, especially patients with ARDS or other acute lung injuries. It is also common to see acid-base imbalances in patients on ventilators. If a patient is struggling with acid-base concerns, respiratory therapy should be consulted to adjust the ventilator settings and the provider may adjust fluids and electrolytes to regain balance. It is imperative for electrolyte balance to be monitored and maintained as many electrolytes play an important role in muscle function (Ignatavicius et al., 2018).
The gastrointestinal system is primarily affected by the stress of the illness, and the associated ventilator status. This stress can result in irritation of the mucosal lining and a stress ulcer, which in turn can impair the nutritional status of the patient. Impaired integrity of the gastrointestinal tract further increases the risk of systemic infection. It is common for providers to order gastrointestinal medications prophylactically when a patient is intubated. These medications could include antacids, histamine-2 receptor antagonists such as ranitidine (Zantac), or proton pump inhibitors such as omeprazole (Prilosec). Sucralfate (Carafate) is considered a miscellaneous gastrointestinal medication that covers and protects the ulcer site that could be prescribed. Prevention of stress ulcers is key, as malnutrition can further complicate the patient’s overall condition (Ignatavicius et al., 2018).
Malnutrition contributes to difficulty weaning from the ventilator secondary to poor muscle tone and function not only of the respiratory muscles but of the diaphragm as well. This leaves the patient with decreased ability to expand and contract the lungs successfully. When patients need to exert more energy to breathe, they become fatigued quicker, resulting in a problematic weaning process or leaving the patient dependent on the ventilator. It is essential to start the patient on appropriate caloric intake within 48 hours of intubation. The type of nutrition will need to be individualized based on the patient’s condition and their ability to swallow. Patients with proper ability to swallow can be started on oral intake but may require supplements. Nutrition may be enteral or parenteral in patient unable to swallow effectively. Depending on the underlying etiology, patients may need enteral feedings or supplements that are designed for specific conditions such as chronic obstructive pulmonary disease (COPD). In COPD, the patient needs a formula with fewer carbohydrates and more fat, such as Pulmocare or others (Ignatavicius et al., 2018).
Along with respiratory muscle health, maintaining overall muscle health is equally important in the care of this patient. Activity prescribed by the provider should be strictly adhered to, and nursing needs to cooperate with physical therapy to increase activity or provide range of motion to maintain strength and muscle function (Ignatavicius et al., 2018).
The Weaning Process
The goal of placing a patient on a ventilator is to maintain oxygenation and gas exchange and ultimately to wean the patient off. The process of weaning can vary from short and uncomplicated to long with multiple complications. The decision to attempt the weaning process should be made collaboratively by the nurse, provider, and respiratory therapist as well as input from other departments. There are multiple ways to attempt to wean a patient from a ventilator; three common methods are SIMV, the T-piece technique, and pressure support ventilation. The healthcare team will decide which process to use and proceed with the weaning process (Ignatavicius et al., 2018).
In SIMV, as described previously, the patient can breathe on their own between breaths delivered by the ventilator. The rate is usually started at approximately 12 breaths per minute and is decreased as the patient makes progress until the patient is taking more breaths independently than the ventilator is providing. The T-piece technique allows the patient to be taken off the ventilator to breathe on their own for short periods of time, usually five to ten minutes. When off the ventilator, the patient is supported by a T-piece that connects over their airway or by a CPAP to deliver oxygen. The patient will continue to wean as they can maintain oxygenation off the ventilator for longer periods of time. Some patients will wean off the ventilator during the day and go back on the ventilator at night until they can support independent breathing. In pressure support ventilation weaning, the pressure of each inhalation is decreased gradually, or the number of breaths provided per minute is decreased until the patient has regained the ability to support full inhalations and an appropriate number of breaths. In any weaning method, the patient will be considered successfully weaned when they are independently breathing, and able to support appropriate oxygenation and gas exchange (Ignatavicius et al., 2018).
Research
In April of 2019, Scheunemann et al. published a discussion in JAMA regarding care in ICUs. The intention was to evaluate if providers and patient advocates were keeping the patient’s values and preferences in mind when making important treatment decisions. The study involved the evaluation of recorded conferences held between providers and the persons making decisions for the critically ill. The patients that were studied were not able to speak for themselves, had been diagnosed with ARDS, and had at least a 50% predicted mortality risk while in the hospital. These recorded conferences were analyzed by coders for what the patients had previously indicated about types of treatments they would want, what their values were regarding healthcare, and what they would possibly choose in the given situation. They found that most of these conferences did not contain enough information about the possible prognosis and patient outcome and did not integrate enough discussion about the patient’s values and preferences regarding their health care. The study suggests that families, patient proxies, and providers need to be better prepared in these conversations to ensure appropriate decisions are made regarding the care and treatments that are implemented (Scheunemann et al., 2019).
ECMO has been utilized and studied for many decades and has been indicated as a treatment option for ARDS, but there has been controversy about when this treatment is appropriate. This type of life support has been used in persons with respiratory failure requiring the use of mechanical ventilation despite significant complications. With improvements in technology, ECMO has been revaluated. ECMO involves the withdrawal of venous blood via a cannula in the right femoral vein that is slowly advanced towards the right atrium. A pump draws the deoxygenated blood into a device known as the oxygenator. Room air and oxygen are delivered into the fibers of the oxygenator where gas exchange will take place, producing oxygenated blood and removing the carbon dioxide. The oxygenated blood is returned to the right atrium via a cannula that is inserted into the right jugular vein (Brodie, Slutsky & Combes, 2019).
This type of treatment can be used to support the lungs or cardiac system and is known by multiple names, including ECMO. There have been many recorded complications, which include hemorrhage, infarcts, seizures, pneumothorax, cardiac arrhythmia, cardiac tamponade, infections, and more. There is a need for further research and advances in technology to improve the function while decreasing the potential complications. There is a need for continued use of this type of treatment in acute respiratory failure, and patients who need the support for the right side of the heart. Other technologies are being studied that may ultimately change the way people are treated, where they are treated, and potentially could change lives (Brodie et al., 2019).
Aoyama et al. (2019) focused on patients who were diagnosed with moderate to severe ARDS using lung-protective ventilation versus other therapeutic interventions and what the outcomes would be. They looked at prone positioning and ECMO, and how their use would impact the mortality rate. Nine different treatment options were evaluated using a 28-day mortality rate in 7,753 patients. In patients who had moderate ARDS, using a prone position reduced the 28-day mortality rate significantly compared to patients who only had lung-protective ventilation. Patients who were diagnosed with severe ARDS and started on ECMO also had a significantly lower mortality rate. This study supports the continued use of lung-protective ventilation, prone positioning, and the need to further explore the use of ECMO earlier in the treatment of ARDS (Aoyama et al., 2019).
CCRN
In 1969, the American Association of Cardiovascular Nurses was formed to educate nurses in cardiac care. A few years later, in 1971, the association changed its name to the American Association of Critical-Care Nurses (AACN, 2019c). The association focuses on assisting nurses in giving the best care possible to critically ill patients. With that goal at the forefront in 1974, the first annual conference known as the National Teaching Institute & Critical Care Exposition (NTI) was started. The next year, the AACN Certification Corporation was founded to provide specialty certification for critical care nurses who were providing care for patients who were acutely or critically ill. The certification included care for patients across the lifespan. Their journal, Critical Care Nurse, was published in 1980 to provide continued information to their nurses. This was followed by a quarterly journal, Advanced Critical Care, published in 1989. In 1992 the American Journal of Critical Care was published, which provided a higher level of information. In 1999, the organization added the Critical Care Nurse Specialist certification for nurses educated at the graduate level who care for critically ill patients across the lifespan. Since that time, other subspecialty certifications have been added. In 2019 the AACN celebrates fifty years of supporting and educating critical care nurses (AACN, 2019c).
The AACN is guided by a mission, vision and four values, which include: accountability, innovation, leadership, and collaboration. The organization is governed by a board of directors consisting of 15 members. There is also an 11-member board that is responsible for the certification and renewal process that now includes 12 additional specialty and sub-specialty certifications. There is also a nominating committee that reviews the criteria to serve on the various boards and the standards for potential candidates. Nurses can review the current benchmarks and make a nomination if they know someone that might be suitable for a position on one of the boards (AACN, 2019a).
As patients’ needs change, there has been an increasing level of care required by nurses. The certification process for those who work with patients who are critically ill is one way to continue to strive towards better patient care and positive outcomes. Seeking certification allows the nurse to validate their level of knowledge and to develop that knowledge further. It enables the nurse to become a leader in their profession, improve practice standards, and in some cases, advance their career or obtain promotions because they have demonstrated a desire to be the best nurse they can be and to put patient care at the forefront (AACN, 2019b).
The certified critical-care registered nurse (CCRN) certification is awarded to nurses who are working with acutely and or critically ill patients upon successful completion of an exam. Nurses who are considering becoming certified must meet the criteria for eligibility before starting the certification process. The criteria include:
- a current, unencumbered RN license issued in the United States,
- evidence of practice as an RN or advanced practice registered nurse (APRN) in direct care of patients who are acutely or critically ill for 1,750 hours in a two-year period with 875 hours accrued in the year preceding application, or
- evidence of practice as an RN or APRN in direct care of patients who are acutely or critically ill for 2000 hours in a five-year period with 144 hours accrued in the year preceding application (AACN, 2019d).
The practice hours have some specific requirements, which include that they must be completed in a United States or Canada-based facility for acute critical care nursing. Practice hours in another country would need to meet specific criteria, and the applicant would want to contact AACN. The number of hours listed above must be spent either providing direct care or acting as a supervisor of other nurses or nursing students and must be verifiable by a supervisor or a colleague (AACN, 2019d).
For the nurse seeking certification, there are a variety of options offered by AACN. The CCRN can be obtained in adult, pediatric, or neonatal care. The CCRN-K certifications indicate knowledge instead of direct care. This would be a person who influences or directs care but is not involved in direct patient care such as a manager, supervisor, or nurse administrator. The CCRN-E certification is for nurses that work in a teleICU. Multiple other certifications can be acquired through the AACN that involve acute or critical care in a variety of settings for RNs, APRNs, and clinical nurse specialists with specific criteria that would need to be met (AACN, 2019d).
Once the nurse decides to become certified, the AACN website can be referenced for more details and a step-by-step process outline. There are resources available to help prepare for the certification exam on the AACN website and others. There are handbooks, test banks with practice questions, seminars, and more to help the nurse become more confident and prepared to take the exam. Once the nurse becomes certified, the certification will need to be renewed with specific required criteria depending on the type of certification. Specific continued competency requirements usually include the option of retaking the initial examination or supplying evidence of a combination of practice hours and nursing professional development courses. These courses are available through the AACN and independent sites such as NursingCE.com, and the renewal process is done through the AACN website (AACN, 2019d).
References
American Association of Critical-Care Nurses. (2019a). About the American Association of Critical-Care Nurses. Retrieved from https://www.aacn.org/about-aacn
American Association of Critical-Care Nurses. (2019b). Board Certification. Retrieved from https://www.aacn.org/certification?tab=First-Time%20Certification
American Association of Critical-Care Nurses. (2019c). Complete History of AACN. Retrieved from https://www.aacn.org/AboutAACN/CompleteHistoryAACN
American Association of Critical-Care Nurses. (2019d). Get Certified. Retrieved from https://www.aacn.org/certification/get-certified/ccrn-adult
American Lung Association. (2019a). ARDS Symptoms, Causes and Risk Factors. Retrieved from https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/ards/symptoms-causes-risk-factors.html
American Lung Association. (2019b). Diagnosing and Treating ARDS. Retrieved from https://www.lung.org/lung-health-and-diseases/lung-disease-lookup/ards/diagnosing-and-treating-ards.html
Aoyama, H., Uchida, K., Aoyama, K., Pechlivanoglou, P., Englesakis, M., & Yamada, Y. (2019). Assessment of therapeutic interventions and lung-protective ventilation in patients with moderate to severe acute respiratory distress syndrome a systematic review and network meta-analysis. JAMA Network Open, 2(7), e198116. Doi 10.1001/jamanetworkopen.2019.8116
Brodie, D., Slutsky, A., & Combes, A. (2019) Extracorporeal life support for adults with respiratory failure and related indications. JAMA, (6), 557-568. Doi:10.1001/jama. 2019.9302.
BruceBlaus. (2017a). Endotracheal Tube Placement [image]. Retrieved from https://commons.wikimedia.org/wiki/File:Endotracheal_Tube.png
BruceBlaus. (2017b). Nasal Cannula [image]. Retrieved from https://commons.wikimedia.org/wiki/File:Nasal_Cannula_(Child).png
Grossman, S., & Porth, C. (2014) Porth’s pathophysiology concepts of altered health states (9th ed.). Philadelphia, PA: Wolters Kluwer Lippincott Williams & Wilkins.
Heilman, J. (2014a). Nonrebreather Mask [Image]. Retrieved from https://commons.wikimedia.org/wiki/File:NRBer.JPG
Heilman, J. (2014b). Simple Mask [Image]. Retrieved from https://commons.wikimedia.org/wiki/File:Simple_face_mask.jpg
Ignatavicius, D., Workman, M., & Rebar, C. (2018) Medical-surgical nursing concepts for interprofessional collaborative care (9th ed.). St. Louis, MO: Elsevier.
Marshall, J.C., Bosco, L., Adhikari, N. K., Connolly, B., Diaz, J. V., Dorman T., … Zimmerman, J. (2017). What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. Journal of Critical Care, (37), 270-276. doi: 10.1016/j.jcrc.2016.07.015
Miller-Keane Encyclopedia and Dictionary of Medicine, Nursing, and Allied Health. (2003). Venturi Mask [image]. Retrieved from https://medical-dictionary.thefreedictionary.com/Venturi+mask
National Heart, Lung, & Blood Institute. (2013). Tracheostomy Tube [image]. Retrieved from https://commons.wikimedia.org/wiki/File:Tracheostomy_NIH.jpg
National Heart, Lung, & Blood Institute. (2019). Acute Respiratory Distress Syndrome. Retrieved from https://www.nhlbi.nih.gov/health-topics/acute-respiratory-distress-syndrome
Ranieri, M., Rubenfeld, G., Thompson, B., Ferguson, N., Caldwell, E., Fan, E., & Slutsky, A. (2012). Acute respiratory distress syndrome the berlin definition. JAMA Network., 307(23), 2526-2533 doi:10.1001/jama.2012.5669
Scheunemann, L. P., Ernecoff, N. C., Buddadhumaruk, P., Carson, S. S., Hough, C. L., Curtis, J. R.,…White, D. B. (2019). Clinician-family communication about patients’ values and preferences in intensive care units. JAMA Internal Medicine, (5), 676-684. doi: 10.1001/jamainternmed.2019.0027
Wilkinson, J. M., Treas, L. S., Barnett, K. L., & Smith, M. H. (2016). Fundamentals of nursing: thinking, doing, and caring. (3rd ed., Vol. 1 & 2). Philadelphia, PA: F.A. Davis Company.
Complete your ICU training with our Intensive Care Unit Part 2 Nursing CE Course for 3 ANCC contact hours.