The purpose of this module is to provide an overview of the safe and effective use of medical marijuana and cannabinoids, outlining the various medicinal uses and pertinent prescribing information to safeguard patient care, improve outcomes, and uphold the practice of the nurse.
...purchase below to continue the course
ntiemetic treatments and for the treatment of anorexia associated with weight loss in patients with HIV/AIDs (FDA, 2020a; NCSL, 2020). Prior to prescribing these medications, clinicians are encouraged to assess the patient’s risk for abuse or misuse, as these agents may induce psychiatric and cognitive effects that can impair mental or physical abilities. Those with a history of substance abuse, dependence, or psychiatric history are at heightened risk for abuse, misuse, and adverse effects. The safety of these medications in the pediatric patient population has not yet been established (Smith et al., 2015).
In 2018, the FDA approved cannabidiol (Epidiolex) for the treatment of seizures associated with two severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients who are two years of age and older. Both of these conditions are rare, appear early in life, and can cause seizures that are difficult to control, which may be life-threatening. This is the first FDA-approved drug that contains a purified drug substance derived from marijuana. The efficacy of cannabidiol (Epidiolex) was evaluated through three randomized, double-blind, placebo-controlled clinical trials involving 516 patients with either Lennox-Gastaut syndrome or Dravet syndrome. Within these studies, when cannabidiol (Epidiolex) was taken alongside with other medications, it was shown to be effective in reducing the frequency of seizures when compared with placebo. The most common side effects include sedation, sleepiness, fatigue, weakness, malaise, and lethargy. Less common side effects include elevated liver enzymes, decreased appetite, diarrhea, skin rash, and insomnia. Aside from cannabidiol (Epidiolex), there are no other FDA-approved drug products that contain CBD (FDA, 2020a).
Outside of the US (Canada and Europe), nabiximols (Sativex) is a purified natural combination that includes equal parts of THC and CBD in an oromucosal spray formulation and is administered for the treatment of pain and spasticity (Schrot & Hubbard, 2016).
Medicinal Uses
The bulk of cannabis research is early and inconclusive. According to Whiting and colleagues (2018), there is moderate quality evidence based on existing studies to support the use of nabiximols (Sativex) or smoked THC (neither of which are currently FDA approved for use in the US) for chronic cancer or neuropathic pain. This determination is based on 28 studies reviewed, most of which were deemed moderate quality research. Of these studies, thirteen were conducted with nabiximols (Sativex), five with nabilone (Cesamet), four with smoked THC, and two with dronabinol (Marinol, Syndros). Twelve of these studies focused on neuropathic pain, with three premised on diabetic neuropathy, three on cancer pain, two on HIV neuropathy, and two on fibromyalgia (Whiting, et al., 2015). Regarding the treatment of acute pain with cannabinoids, there was one randomized placebo-controlled trial of smoked cannabis and dronabinol (Marinol, Syndros) in which researchers found decreased pain sensitivity and increased pain tolerance versus placebo, but this study was small (N=30) so more information is needed for definitive conclusions to be translated to larger populations. A similarly sized trial showed nabilone (Cesamet) to be superior to ibuprofen (Motrin) in the treatment of medication overuse headaches. Chronic, non-cancer pain was studied in four trials with inconsistent results (Mouhamed et al., 2018).
Cannabis use has been studied for numerous ailments reported by patients diagnosed with Multiple Sclerosis (MS). The American Academy of Neurology reviewed the literature related to patients with MS and rated the evidence for the use of oral cannabis extract (CBD or combination CBD/THC) in the treatment of patient-reported spasticity, central pain, and painful spasms an “A” (effective). They rated the treatment of the same symptoms with dronabinol (Marinol, Syndros), nabilone (Cesamet), or nabiximols (Sativex) a “B” (probably effective). In the treatment of MS-related bladder frequency, nabiximols (Sativex) treatment also received a rating of “B”. Oral cannabis extract was given a “B” rating for probably ineffective in the treatment of bladder complaints and tremors in MS patients. Nabiximols (Sativex) use in the treatment of tremors was given a “C” rating for possibly ineffective, and smoked cannabis was rated a “U” for insufficient evidence (Schrot & Hubbard, 2016). In a separate review of existing literature, fourteen studies were assessed to establish the use of dronabinol (Marinol, Syndros), nabilone (Cesamet), nabiximols (Sativex), or oral CBD/THC tablets to treat spasticity in 2,280 MS or spinal cord injury patients and were rated as “moderate” quality. In those studies, cannabinoids were associated with greater improvements in spasticity versus placebo but failed to reach statistical significance (Whiting, et al., 2015). Despite some of these positive findings, there is currently no evidence that medical cannabis has an overall effect on slowing the progression of MS (Mouhamed, et al., 2018).
As previously stated, cannabis and cannabinoid use in the treatment of epilepsy has also been studied, particularly in special populations of pediatric refractory epilepsy syndromes that tend to respond poorly to existing antiepileptic drugs (AEDs). Although there are multiple case reports regarding the use of CBD as an antiepileptic, only a small number of placebo-controlled clinical trials exist. This first, published in 1980 in the Journal Pharmacology, evaluated 15 patients, eight of which received CBD orally for 8-18 weeks at a dose of 2-300 mg daily. Four of the eight CBD patients had significant resolution of their seizures, three had partial resolution, and all tolerated the medication well. Only one placebo patient noted improvement. Within this study, three of the CBD-treated patients had measurable electroencephalographic (EEG) improvements (Santos et al., 2015). The American Academy of Neurology and Cochrane reviews completed in 2014 both rated the evidence for cannabis use in the treatment of epilepsy limited, inconclusive, or insufficient. In 2015, Rosenberg and colleagues evaluated four primary clinical trials to examine the efficacy and safety of CBD in seizures. Two of these studies showed partial antiseizure effect with CBD, and two showed no statistically significant effect. However, these studies were small, with insufficient blinding or randomization and incomplete data sets. A parent survey of 117 children with intractable seizures reported an 85% perceived reduction in seizures with the use of plant-derived enriched CBD (Schrot & Hubbard, 2016). Mouhamed and colleagues (2018) assessed treatment with THC to be too broad for therapeutic purposes but found positive findings when they reviewed five trials published between 2013 and 2018 studying the use of CBD in the treatment of drug resistant epilepsy in children or young adults (three of which were referenced above in the section on cannabidiol [Epidiolex]). These studies showed a more significant reduction in frequency of atonic and partial seizures, followed by reductions in tonic/tonic-clonic seizures, with minimal reports of adverse effects. Of note, these studies did not utilize CBD as a replacement to the subjects’ AED, but as an adjunct. In contrast, Whiting et al. (2015) found studies that reported seizures as a possible reported adverse effect of cannabinoid treatment
Cannabinoids have been tested for the treatment of various other neurological disorders and complaints, including anxiety, Parkinson’s disease, Tourette's Syndrome (TS), cervical dystonia, Huntington’s disease, schizophrenia, sleep disorders, depression, psychosis, and opioid or other substance use disorder. The American Society for Experimental Neurotherapeutics reviewed the use of CBD for the treatment of anxiety disorders. They identified preclinical evidence that strongly supports the use of CBD in the treatment of general anxiety disorder (GAD), panic disorder, social anxiety disorder (SAD), obsessive compulsive disorder (OCD), and post-traumatic stress disorder (PTSD). Human studies they reviewed supported use in healthy adults between 3-600 mg which acutely decreased induced anxiety without affecting baseline levels. CBD was also shown to decrease anxiety in patients with SAD, and acutely enhances fear extinction, which may prove useful in the treatment of PTSD. However, these studies all utilized acute dosing and small sample sizes, so further studies would need to be done to extend the patient population tested, establish independent replication, and determine if chronic use is as effective and safe in other anxiety disorders (Blessing et al., 2015). Mouhamed and colleagues (2018) found studies on patients with PTSD showed consistently decreased frequency of nightmares when treated with nabilone (Cesamet) or 5mg THC in oil, and several case reports of decreased tic severity and frequency in TS when given CBD/THC daily. The American Academy of Neurology review in 2014 assessed oral cannabis extracts such as dronabinol (Marinol, Syndros) and nabilone (Cesamet) as “probably ineffective” for the treatment of Parkinson’s disease and tremors, and nabiximols (Sativex) as “possibly ineffective” for tremors. They found insufficient evidence supporting the use of cannabinoids in TS, cervical dystonia, and Huntington’s disease. Whiting et al. (2015) identified positive but low-quality evidence for the use of THC capsules in TS. The 2013-2014 Cochrane review found limited/inconclusive evidence for the use of cannabinoids in the treatment of schizophrenia or cannabis use disorder. Schrot and Hubbard (2016) identified a systematic review from 2015 using plant-derived CBD in the treatment of psychosis that showed “potential” as an antipsychotic but suggested a large randomized clinical trial in order to support regular clinical use. Whiting et al. (2015) identified only low-quality evidence showing no benefit for the use of CBD in psychosis treatment. When the current published evidence was assessed by Mouhamed, et al (2018), their conclusions were that CBD increased quality of life (QOL) scores in Parkinson’s patients, and may help in the treatment of schizophrenia, but that THC increased symptoms of psychosis. Similarly, a 2015 systematic review of cannabinoids in the treatment of substance abuse/addiction showed “potential” (Schrot & Hubbard, 2016). Due to its therapeutic effects on mood, anxiety, sleep and pain, CBD has been established as “potentially beneficial” in the treatment of opioid use disorder and addiction (Hurd, et al., 2015). Whiting et al. (2015) identified positive but low-quality evidence for the use of nabilone (Cesamet) or nabiximols (Sativex) in sleep disorders. What they deemed as very low-quality evidence suggested no benefit from the use of nabiximols (Sativex) in the treatment of depression (Whiting et al., 2015). Unfortunately, Mouhamed and colleagues (2018) found “mixed results” regarding cannabinoids in the treatment of opioid use disorder. When considering cannabinoids in the treatment plan for neurological disorders, it is important to keep in mind that nearly all of the studies reviewed list anxiety, dysphoria, psychosis, hallucinations, paranoia, and possible perceptual changes as potential adverse effects of cannabinoid products which contain THC.
The use of cannabinoids in cancer patients has been moderately successful when used to treat cancer-related pain; however, studies have used smoked THC and nabiximols (Sativex) only, neither of which are currently FDA approved, as outlined earlier within this module. Otherwise, there remains low-quality evidence that dronabinol (Marinol, Syndros) is effective for nausea and vomiting related to chemotherapy agents, despite FDA-approval for this indication (Whiting, et al., 2015). The National Cancer Institute (NCI, 2020) reports a beneficial effect of the cannabinoids dronabinol (Marinol, Syndros) and nabilone (Cesamet) when used for nausea and vomiting related to chemotherapy but found inconclusive evidence on the use of smoked/inhaled cannabis for chemotherapy-related nausea and vomiting (NCI, 2020). According to the American Cancer Society (ACS), there is potential evidence based on in vitro studies that THC and CBD can slow the growth and potentially even cause cell death in certain types of cancer cells. In preclinical trials, cannabinoids reduced the spread of some form of cancers. While early clinical trials of cannabinoid use in cancer patients have proven safe, they have not yet proven to be effective in curing or helping to control the disease (ACS, 2017). A study completed in Israel assessed the potential effect of THC and CBD in neuroblastoma, the most common extracranial solid tumor in pediatric patients. The in vitro portion of the study indicated that both THC and CBD had antitumor activity, reducing the viability and invasiveness of the tumor cells and in some cases inducing apoptosis. CBD was the more effective of the two compounds. In the in vivo portion of their study, neuroblastoma tumors were induced in non-obese diabetic immunodeficient mice and the mice were then treated for 14 days with either THC, CBD, pure ethanol (control), or were untreated. CBD slowed the growth of the tumors, as did THC, but to a lesser degree. While the exact mechanisms for how these drugs produced the observed effects is yet to be fully understood, the authors understandably suggested larger, more in-depth studies in the future to further explore this potential (Fisher et al., 2016).
Due to the presence of CB1 receptors in the GI tract, certain GI effects have been credited to and therapeutically tested in cannabinoids, such as nausea and vomiting. Four studies were reviewed for appetite stimulation and weight gain, included 255 participants, and compared dronabinol (Marinol, Syndros) with placebo, megestrol acetate (Megace), and one study also compared cannabis. Trials all showed improved weight gain with dronabinol (Marinol, Syndros) or cannabis versus placebo, but the study comparing megestrol acetate (Megace) found less weight gain with dronabinol (Marinol, Syndros). Combining dronabinol (Marinol, Syndros) with megestrol acetate (Megace) did not seem to have additive benefit (Whiting, et al., 2015). Further, megestrol acetate (Megace) is a synthetic progesterone that is associated with an increased risk for blood clots and cardiac infarction, thereby making it a riskier adjunctive medication in the oncologic setting (OncoLink, 2019). According to Mohammed et al. (2018), the cannabinoid products performed at least as well and sometimes better than ondansetron (Zofran), but had higher rates of adverse effects. Two trials evaluated THC in postoperative nausea and vomiting and found no significant difference compared to placebo and unacceptable adverse effects reported. In patients diagnosed with inflammatory bowel disease (IBD), self-medicating users reported decreased abdominal pain, nausea, diarrhea, and joint pain and increased appetite with cannabis use, but there is a lack of reliable clinical evidence as these are the results of self-report observations and not premised on data compiled from clinical trials. The use of cannabis in IBD patients also correlated to higher rates of surgical procedures, leading the authors to question if the cannabis could be masking disease activity and/or progression (Mouhamed, et al., 2018).
Adverse Effects and Patient Counseling
Potential adverse effects of cannabinoids vary greatly depending on the composition of the individual product being evaluated. In epilepsy studies, adverse effects of CBD were reported as fatigue/somnolence, diarrhea, and decreased appetite, while cannabis and THC cause nausea, dizziness, weakness, behavioral/mood changes, hallucinations, suicidal ideations, fatigue, and feelings of intoxication (Rosenberg et al., 2015). In addition to the effects listed above, Schrot and Hubbard (2016) also list anxiety, dysphoria, psychosis, impairment of memory, coordination, judgment, attention and/or perception, tachycardia, hypertension, increased cardiac output, redness of conjunctiva, increased appetite, dryness of mouth, and increased risk of being involved in a motor vehicle accident as potential acute adverse effects of THC/cannabis. Chronic use also carries the risk for memory and cognitive deficits, psychosis, respiratory and immune system effects that increase the risk for infections, airway inflammation and bronchitis, social dysfunction, difficulty in school for adolescents, decreased job performance, increased unemployment, decreased income levels, and decreased satisfaction with life. While the addiction potential is lower for cannabis than other illicit substances, there does exist a roughly 9% risk for physical/psychological addiction in adults. A Lancet study in 2007 ranking controlled substances for a “mean harm score” ranked cannabis #11 out of 20 (Schrot & Hubbard, 2016). A 2015 meta-analysis in JAMA reviewed acute adverse effects published in 62 studies and corroborated the aforementioned effects, as well as complaints of euphoria, paranoia, and seizures (Whiting, et al., 2015). According to multiple large studies, there does not appear to exist a clear epidemiological link between marijuana and lung cancer, such as that seen with tobacco users. It remains inconclusive and a topic for further exploration, as “heavy use” of cannabis (more than 50 times in a lifetime) may carry some increased risk of testicular, prostate, or squamous cell carcinomas (NCI, 2020).
As with any treatment plan in medicine, especially one that may include a medication that carries with it risk of adverse effects, full disclosure of risks to the patient, extensive counseling, and screening are key to upholding safety and ensuring positive outcomes. Risk factors for addiction include genetic inheritance (at least 50%) and comorbid psychiatric disorder. In adolescents, risk factors also include peer usage and academic difficulties in school (Schrot & Hubbard, 2016).
Regarding the counseling of patients within states that allow recreational use of cannabis for adults, the American Journal of Public Health developed some evidence-based recommendations for safe cannabis use. These are identified as the Lower-Risk Cannabis Use Guidelines (LRCUG) and could be utilized by nurses as a framework for counseling patients who elect to use cannabis recreationally. Their ten major recommendations are listed below in Table 2; these guidelines are intended to reduce the overall risk of adverse effects from cannabis use. They are not a guarantee against adverse effects in any particular patient, and instead strive to inform patients who may be willing to modify their use so as to reduce some of the health risks (Fischer, et al., 2017).

Prescriptive Authority
To qualify for a cannabis license, patients must both have an authorized clinician certify that they have a qualifying medical condition and then obtain their license from the state. Medical cannabis patient registries have been devised to monitor these patients as an added layer of regulation; however, the programs are governed by each individual state and there is tremendous variation between programs. For instance, California collects minimal or no data in voluntary registries, whereas states such as Arizona collect and publish detailed reports. There is also great variability regarding what is designated as a qualifying patient condition and an authorized prescriber, as these are additionally subjected to state regulations (Boehnke et al., 2019). Some states/territories allow APRNs to certify that a patient qualifies to use medical marijuana due to the presence of a qualifying medical condition. According to Act 21-565, Medical Marijuana Omnibus Amendment Act of 2016, this does not confer prescriptive authority, as marijuana is listed as a Schedule I controlled substance by the DEA. Instead, designated clinicians in these states simply have the authority to recommend the use of the substance as described by each state’s law based on the presence of a qualifying condition (Phillips, 2018). A few of the most common qualifying conditions are listed in Table 3, but this list is not comprehensive, and nurses are encouraged to refer to individual state policy for an accurate list of qualifying diagnoses in the designated state of interest (Leafly, 2020).

The American Nurses Association (ANA) has openly supported the use of medical marijuana since 1996. In a 2016 revised position statement, the ANA recognizes that marijuana is illegal and is classified as a Schedule I drug under federal law. Despite this, the ANA emphasizes support for the following:
- “Scientific review of marijuana’s status as a federal Schedule I controlled substance and relisting marijuana as a federal Schedule II controlled substance for purposes of facilitating research;
- Development of prescribing standards that includes indications for use, specific dose, route, expected effect and possible side effects, as well as indications for stopping a medication;
- Establishing evidence-based standards for the use of marijuana and related cannabinoids;
- Protection from criminal or civil penalties for patients using therapeutic marijuana and related cannabinoids as permitted under state laws;
- Exemption from criminal prosecution, civil liability, or professional sanctioning, such as loss of licensure or credentialing, for health care practitioners who discuss treatment alternatives concerning marijuana or who prescribe, dispense, or administer marijuana in accordance with professional standards and state laws.” (ANA, 2016, p. 1-2).
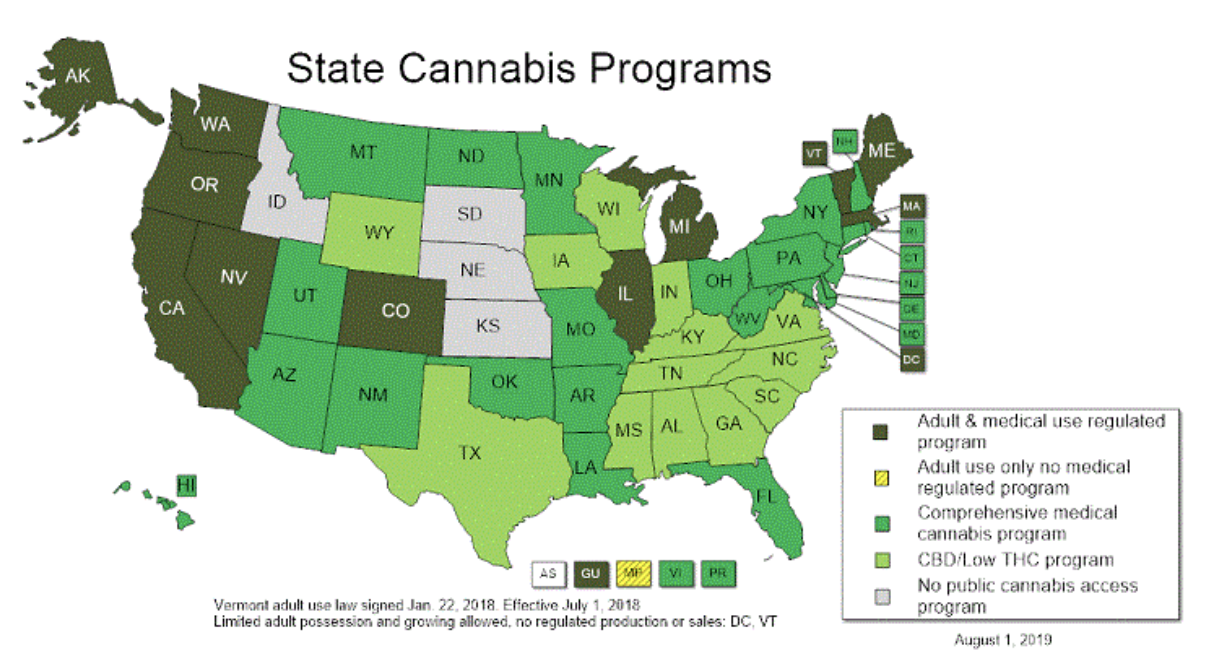
(NCSL, 2020)
Future Research
Regarding the future of cannabis and cannabinoid use in medicine, many researchers feel that the future of cannabis in medicine is not likely with cannabis as a whole, but instead in distilling and purifying the most therapeutic components to ensure a reliable, pure, and consistent product that has the ability to stand up to the strict standards put forth by the FDA. This may include products containing CBD that may function as antipsychotics, antiepileptics, or treatments for addiction. Other suggestions for future research include a CB1 antagonist for obesity or addiction treatment, a synthetic CB2 agonist as an anti-inflammatory in the treatment of scleroderma, or a mixture of cannabinoids for the treatment of diabetes or metabolic syndrome. Alternatives for pain medications could also explore drugs that inhibit the enzymatic degradation of the endogenous receptor agonists anandamide or 2-AG (Schrot & Hubbard, 2016). Larger randomized clinical trials are suggested on the use of cannabinoids for weight gain in HIV/AIDs patients, depression, sleep disorders, anxiety disorders, psychosis, glaucoma, and TS. Between 50-99% of the study participants discussed in this review were European American, and thus future research should also aim to broaden our understanding of any potential variability in effect amongst different races (Whiting, et al., 2015). The general consensus amongst the medical community is that the use of cannabinoids and the manipulation of the endocannabinoid system holds immense medical potential, but at the current time additional research is needed to confirm in whom and how exactly this should be carried out for optimal benefit and minimal risk.
References
American Cancer Society. (2017). Marijuana and cancer. https://www.cancer.org/treatment/treatments-and-side-effects/complementary-and-alternative-medicine/marijuana-and-cancer.html
American Nurses Association. (2016). Therapeutic use of marijuana and related cannabinoids. https://www.nursingworld.org/~49a8c8/globalassets/practiceandpolicy/ethics/therapeutic-use-of-marijuana-and-related-cannabinoids-position-statement.pdf
Blessing, E. M., Steenkamp, M. M., Manzanares, J., & Marmar, C. R. (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics,12(4), 825-836. https://doi.org/10.1007/s13311-015-0387-1
Boehnke, K. F., Gangopadhyay, S., Clauw, D. J., & Haffajee, R. L. (2019). Qualifying conditions of medical cannabis license holders in the United States. Health Aff (Millwood), 38(2), 295-302. https://doi.org/10.1377/hlthaff.2018.05266
Bridgeman, M. B., & Abazia, D. T. (2017). Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharmacy and Therapeutics, 42(3), 180–188. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312634/#__ffn_sectitle
Chaker, A. (2018). Cannabis comes to your coffee and candy—but is it legal? Wall Street Journal. https://www.wsj.com/articles/cannabis-comes-to-your-coffee-and-candybut-is-it-legal-1536761536
Fischer, B., Russell, C., Sabioni, P., Brink, W. V., Foll, B. L., Hall, W., Rehm, J., & Room, R. (2017). Lower-risk cannabis use guidelines: A comprehensive update of evidence and recommendations. American Journal of Public Health, 107(8), E1-E12. https://doi.org/10.2105/ajph.2017.303818a
Fisher, T., Golan, H., Schiby, G., PriChen, S., Smoum, R., Moshe, I., Peshes-Yaloz, A., Castiel, A., Waldman, D., Gallily, R., Mechoulam, R., & Toren, A. (2016). In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Current Oncology, 23(Suppl 2), S15–S22. https://doi.org/10.3747/co.23.2893
Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., & Jutras-Aswad, D. (2015). Early phase in the development of cannabidiol as a treatment for addiction: Opioid relapse takes initial center stage. Neurotherapeutics, 12(4), 807-815. https://doi.org/10.1007/s13311-015-0373-7
Leafly. (2020). Qualifying conditions for medical marijuana by state. https://www.leafly.com/news/health/qualifying-conditions-for-medical-marijuana-by-state
Mouhamed, Y., Vishnyakov, A., Qorri, B., Sambi, M., Frank, S. S., Nowierski, C., Lamba, A., Bhatti, U., & Szewczuk, M. (2018). Therapeutic potential of medicinal marijuana: An educational primer for health care professionals. Drug, Healthcare and Patient Safety, 10, 45-66. https://doi.org/10.2147/dhps.s158592
National Cancer Institute. (2020). Cannabis and cannabinoids (PDQ®)-Health professional version. https://www.cancer.gov/about-cancer/treatment/cam/hp/cannabis-pdq
National Conference of State Legislatures. (2020). State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
OncoLink. (2019). About: Megestrol (Megace®, Megace-ES®). https://www.oncolink.org/cancer-treatment/oncolink-rx/megestrol-megace-r-megace-es-r
Phillips, S. J. (2018). 30th annual APRN legislative update. The Nurse Practitioner, 43(1), 27-54. https://ruralprep.org/wp-content/uploads/2018/02/30th-APRN-Legislative-Update.pdf
Rosenberg, E. C., Tsien, R. W., Whalley, B. J., & Devinsky, O. (2015). Cannabinoids and epilepsy. Neurotherapeutics, 12(4), 747-768. https://doi.org/10.1007/s13311-015-0375-5
Santos, R. G., Hallak, J. E., Leite, J. P., Zuardi, A. W., & Crippa, J. A. (2015). Phytocannabinoids and epilepsy. Journal of Clinical Pharmacy and Therapeutics, 40(2), 135-143. https://doi.org/10.1111/jcpt.12235
Schrot, R. J., & Hubbard, J. R. (2016). Cannabinoids: Medical implications. Annals of Medicine, 48(3), 128-141. https://doi.org/10.3109/07853890.2016.1145794
Smith, L.A., Azariah F, Lavender, V.T., Stoner, N.S., & Bettiol, S. (2015). Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database of Systematic Reviews, (11), CD009464. https://doi.org/10.1002/14651858.CD009464.pub2
US Department of Health and Human Services. (2015). Announcement of revision to the Department of Health and Human Services guidance on procedures for the provision of marijuana for medical research as published on May 21, 1999. https://www.federalregister.gov/documents/2015/06/23/2015-15479/announcement-of-revision-to-the-department-of-health-and-human-services-guidance-on-procedures-for
US Food & Drug Administration. (2020a). FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
US Food & Drug Administration. (2020b). FDA regulations of cannabis and cannabis-derived products, including cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd#farmbill
Whiting, P. F., Wolff, R. F., Deshpande, S., Nisio, M. D., Duffy, S., Hernandez, A. V., Keurentjes, J. C., Lang, S., Misso, K., Ryder, S., Schmidlkofer, S., Westwood, M., & Kleijnen, J. (2015). Cannabinoids for Medical Use: A systematic review and meta-analysis. Journal of the American Medical Association,313(24), 2456-2473. https://doi.org/10.1001/jama.2015.6358