About this course:
Further your education on oncology prescribing, chemotherapy and other cancer treatments
Course preview
Objectives:
By completing this educational activity, the learner should be able to:
- Understand the difference between normal cell development and cancer cell development
- Discuss primary and secondary cancer prevention strategies
- Understand the goals of cancer therapy
- Examine patterns in cancer drug resistance
- Recognize the most common side effects of chemotherapy and discuss the nursing implications of each
- Identify differences between cytotoxic chemotherapy and other cancer treatments
- Understand the different routes in which cancer treatments may be administered
- Identify the signs of chemotherapy hypersensitivity reaction and nursing interventions
- Demonstrate understanding of the basic principles of safe handling, administration, storage and disposal of cytotoxic medications
- Describe the proper PPE required when handling or administering hazardous medications
- Cognize the structures of immune system and differentiate between innate and acquired immune system
- Describe how the immune system can be used to prevent, detect, and treat cancer
- Explain the side effects of cancer immunotherapy and evidence-based interventions to manage them
- Understand targeted therapies used in cancer treatment and the various mechanisms in which they work
- Differentiate between the side effects of targeted therapies, immune-based therapies, and hormonal therapies
- Discuss the critical teaching points of patients receiving intravenous and oral cancer treatments and the nurse’s role in the education process
- Understand the principles of chemoprevention and identify common chemo-preventative agents
Purpose
The purpose of this module is to provide an overview of the various oral and intravenous (IV) cancer treatment modalities, including chemotherapy, targeted agents, biologic and immune-mediated therapies, hormonal treatments, and chemoprevention.
Introduction
Cancer is a cluster of malignant diseases characterized by uncontrollable, abnormal cell growth, the ability to invade surrounding tissue and lymph nodes, and metastasize (spread) to distant locations within the body (Nettina, 2019). The definition of the term ‘cancer’ has evolved over the last several decades as the intensity of biological research has successfully enhanced the scientific understanding of cancer development and the methods in which cancers grow and spread (Yarbro, Wujcik, & Gobel, 2018). However, cancer research remains profoundly driven toward decorously answering the trillion-dollar question: “how do we correct the abnormal mechanisms that occur at the cellular level to prevent, eradicate, and control the disease?”
Scientific advancements and treatment breakthroughs have revolutionized the way in which cancer is managed; leading to innovative fields such as precision (or personalized) medicine, with the development of targeted therapies and immunotherapy. Precision medicine uses the genomic profiling of the patient’s tumor to identify genetic mutations that are unique to the tumor. This information allows healthcare providers to tailor cancer treatment to the patient’s tumor and select the treatment most likely to work. Targeted therapies block the growth and spread of cancer by interfering with specific genes, proteins, and/or blood vessels that allow cancer cells to replicate, grow, and spread (Yarbro et al., 2018). Immune-based therapies assist the body’s own defense system (the immune system) in identifying cancer cells and attacking them in the same manner it would for any other infection, virus, or other potential threat. Immunotherapy refers to a class of medications that include monoclonal antibodies, checkpoint inhibitors, cancer vaccines, and CAR T-cell therapy. Nevertheless, cytotoxic chemotherapy remains the most highly prevalent treatment option and is still considered the standard of care and first-line treatment for many types of cancers (Miliotou & Papadopoulou, 2018).
The National Comprehensive Cancer Network (NCCN, 2019) is an alliance of leading cancer centers and world-renowned experts devoted to cancer care, research, and education. Through rigorous clinical trial research, data compiled across institutions, and annual expert panel review, the NCCN provides evidence-based treatment guidelines for cancer according to cancer type, pathology, genetics, staging, inheritance patterns as well as several other specific features. The guidelines are widely utilized in cancer care and guide medical decision-making throughout the patient’s disease trajectory (NCCN, 2019). Despite the tremendous evolution and scientific advancements identifying more specific and highly effective, promising cancer treatments, drug resistance is one the major barriers to finding a cure for cancer (Yarbro et al., 2018). The question of how to cure this disease remains open-ended and unrequited, as the disease continues to expand and affect a significant portion of the population.
Incidence/Prevalence
According to the American Cancer Society (ACS, 2019), more than 1.7 million new cancers are expected to be diagnosed in the United States in 2019, with approximately 606,880 cancer-related deaths. These numbers translate to nearly 1,660 deaths per day. Cancer is the second most common cause of death in the U.S.; exceeded only by heart disease. There are an estimated 16.9 million cancer survivors in the U.S., which represents 5% of the population, and is projected to increase to 26.1 million by 2040 (Bluethmann, Mariotto, & Rowland, 2016).
Pathophysiology of Cancer
Cancer cells have distinct features in comparison to normal cells, such as the way they appear under a microscope, how they grow, replicate, and their overall function. The cell cycle is a five-stage process of cellular reproduction that occurs in both normal and cancerous cells. Gap 0, or G0 (quiescence) is the resting stage in which cells are temporarily out of the cell cycle. During this stage, cellular activity continues to occur except for reproduction. During Gap 1, or G1, ribonucleic acid (RNA) and protein synthesis occur. This stage is considered the gap between resting and DNA synthesis. Synthesis, or S, is when deoxyribonucleic acid (DNA)
synthesis occurs, as cellular DNA is duplicated in preparation for DNA division. Gap 2, or G2, encompasses further protein and RNA synthesis, as the cell constructs the mitotic apparatus. Finally, Mitosis, or M, is cellular division. See Figure 1 for a graphic depiction of the cell cycle (Yarbro et al., 2018).

Figure 1. The Cell Cycle
When cancer cells collect in one area, they develop into a malignant (or cancerous) tumor. Normal, healthy cells reproduce in an organized, controlled, and orderly manner as they mature into individual cells that serve specific functions and have predetermined lifespans. They undergo apoptosis, or programmed cell death, which is the process in which the body rids itself of unneeded cells. They do not divide when space or nutrients are limited and do not spread to other parts of the body where they do not belong (Yarbro et al., 2018). In contrast, cancer cells are less specialized, exhibit dysplasia (disorganized growth) and hyperplasia (increased size), as displayed in Figure 2. They have the ability to evade apoptosis, as they continue to divide and grow uncontrollably, even when space is crowded (Polovich, Olsen, & LeFebvre, 2014). Cancer cells i
...purchase below to continue the course
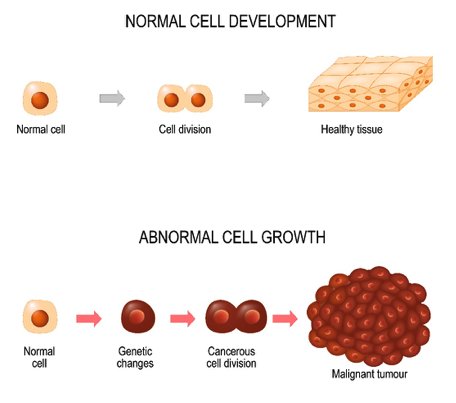
Figure 2. Normal cell development versus abnormal cell growth.
All cancer is inherently genetic, as all cancer cells carry genetic mutations that lead to unregulated cell division and growth (Ring & Modesitt, 2018). However, it is critical to understand the distinction between the term genetic and inherited, as these terms are not synonymous. Inherited (or hereditary) cancer occurs when a damaged gene with a high susceptibility to cancer is passed down through generations within a family (Nettina, 2019; Ring & Modesitt, 2018). In other words, a patient with a hereditary cancer is born with a genetic mutation and genetic predisposition to develop cancer at some point in their lives. A classic example of this are the BRCA1 and BRCA2 genes, which produce tumor suppressor proteins that help repair damaged DNA, ensuring proper stability and function of cellular genetic material (Ring & Modesitt, 2018). When either of these genes are mutated, DNA damage may not be restored properly, leaving cells vulnerable to developing additional genetic alterations that can lead to cancer (Ring & Modesitt, 2018). Inherited mutations in BRCA1/2 genes substantially increase the risk of breast and ovarian cancers and are also associated with heightened risk for several other cancers (Ring & Modesitt, 2018). A mutated BRCA gene can be inherited through a patient’s mother or father (Ring & Modesitt, 2018). Therefore, each child of a parent who carries the mutation has a 50% chance of inheriting the mutation (Ring & Modesitt, 2018). Despite the heightened media attention inherited cancers and BRCA mutations gained following the 2013 New York Times op-ed spotlighting celebrity superstar Angelina Jolie’s decision to have preventative mastectomy after learning she carried the genetic mutation, most cancers are not inherited and patients are often diagnosed without a family history of it (Liede et al., 2018).
Metastases, the secondary growth of the primary cancer in another organ, occurs after a cancerous cell detaches from the original tumor site, invades local tissue, and migrates through the lymphatics and/or blood vessels to another area of the body (Nettina, 2019). Over time, the cancerous cell replicates in the new area, creating a secondary tumor site (Nettina, 2019). The four most common sites that cancers metastasize to include the liver, lung, bone and central nervous system (Yarbro et al., 2018). A common misconception among patients with metastatic cancer is that they have developed a secondary cancer type, so vigilant patient and family education is often required to ensure accurate understanding (Yarbro et al., 2018). For example, a patient with breast cancer who develops metastases in the liver does not have liver cancer. Instead, the patient’s breast cancer cells have traveled to the liver, reproduced in the liver, and created a tumor, leading to a diagnosis of metastatic breast cancer, or ‘breast cancer with metastasis to the liver.’
Risk/Protective Factors
While the definitive cause of cancer is not completely understood, numerous factors are identified as increasing the risk for the disease and are generally distributed between two categories; modifiable and nonmodifiable. Some theories postulate that cancer may occasionally occur due to spontaneous transformation of the cell, where no causative agent is identified, but the majority credit cancer development as a process resulting from cell damage induced by outside influences, called carcinogens (Yarbro et al., 2018). Carcinogens are substances, radiation, or exposures that can damage genetic material (DNA) over the course of one’s lifetime, resulting in carcinogenesis, or the formation of cancer (Itano, 2016). A few examples of carcinogens include tobacco smoking, tanning beds, diesel exhaust, and ultraviolet radiation (Polovich et al., 2014). Age is the most outstanding risk factor for cancer, as incidence of cancer rises alongside age (Nettina, 2019). Other risk factors include exposure to chemicals, viruses, poor nutrition, diets high in fat, obesity, sedentary lifestyles, and excessive alcohol intake (Yarbro et al., 2018).
A substantial proportion of cancers can be thwarted through primary cancer prevention, which involves minimizing harmful exposures and reducing or omitting unhealthy lifestyle behaviors. American Cancer Society (ACS, 2019) researchers have determined that approximately 42% of newly diagnosed cancers in the United States are potentially avoidable, as they are directly correlated with tobacco use, obesity, sedentary lifestyle, and other modifiable behaviors. In fact, tobacco is the single greatest cause of cancer-related deaths and is attributed to more than 480,000 deaths annually with 42,000 deaths from secondhand smoke (Nettina, 2019). Skin cancers are largely due to excessive sun exposure and indoor tanning beds, and prevention strategies focus on the application of proper sunscreen, lightweight clothing and hats to shield oneself from direct exposure, reducing sunlight exposure during peak hours of the day when the ultraviolet rays are the strongest, and avoiding tanning beds altogether (Polovich et al., 2014). Infections and viruses are associated with an increased risk of certain forms of cancer, such as hepatitis B, hepatitis C, and hepatocellular cancer (Nettina, 2019). Cancers associated with the human papilloma virus (HPV) can be prevented through behavioral and lifestyle changes, as well as through vaccination (Nettina, 2019). According to the Centers for Disease Control and Prevention (CDC, 2019), 80% of people will get an HPV infection in their lifetime and approximately 14 million Americans become infected with HPV each year. HPV can cause cancers of the cervix, vagina, vulva, throat, tongue, and tonsils. The U.S. Food and Drug Administration (FDA) approved HPV vaccination, Gardasil9, can protect against over 90% of HPV cancers and genital warts (CDC, 2019).
Secondary cancer prevention involves partaking in activities such as screenings and testing to identify those at high-risk who require increased surveillance as compared to the general population (Yarbro et al., 2018). These measures can prevent cancer through the identification of precancerous lesions and by taking appropriate action prior to the cells developing into invasive cancer; or by undergoing interventions, such as prophylactic mastectomy in an otherwise healthy patient with a BRCA mutation to diminish the lifetime risk of breast cancer development (Yarbro et al., 2018). Screenings allow for the early detection of cancers when they are still treatable or potentially curable. Examples of cancer screening tests including colonoscopy, sigmoidoscopy, fecal occult blood testing (FOBT), mammography, Papanicolaou test (pap smear), prostate specific antigen (PSA), and digital rectal exam (DRE) (Yarbro et al., 2018). Some institutions are now offering cancer screening programs with low-dose spiral computed tomography (CT) scans to detect curable stage I lung cancer in patients who meet the designated criteria (Yarbro et al., 2018).
Goals of Cancer Therapy
There are four main goals of cancer therapy which include: prevention, cure, control, palliation (Yarbro et al., 2018). While prevention and cure are relatively transparent in their definitions, control refers to the extension of a patient’s life when cure is unlikely or impossible by preventing the growth of new cancer cells and reducing the size and impact of existing disease (Yarbro et al., 2018). Palliation focuses on comfort when cure and control of disease cannot be achieved (Yarbro et al., 2018). There are several key terms to describe the types of therapy prescribed for cancer patients which are important for nurses to understand. Neoadjuvant therapy is given to shrink a tumor so that the main treatment, usually surgical intervention, may not need to be as extensive (Itano, 2016). For instance, in breast cancer patients, neoadjuvant chemotherapy is given to shrink the tumor so that the breast surgeon may be able to perform a lumpectomy instead of a mastectomy. Adjuvant therapy is given after the primary treatment and aims to prevent recurrence and reduce micro-metastases (Itano, 2016). For potentially curative treatment regimens, maximum tolerated doses of drugs are delivered on a specific schedule to achieve greatest efficacy; or greatest cancer cell kill. Chemotherapy may also be used for palliation. Palliative therapy aims to relieve or delay cancer symptoms, focusing on comfort, symptom management, and improving quality of life (Itano, 2016). Therefore, with palliative intent, chemotherapy doses are often adjusted to minimize treatment-related toxicity. Chemoprevention is the use of selected pharmaceutical agents to prevent cancer in high-risk individuals (Itano, 2016). The most common example of chemoprevention is the use of tamoxifen (Soltamox), an oral selective estrogen receptor modulator, in women who are at high risk for the development of breast cancer. Myeloablation is the obliteration of bone marrow in preparation for stem cell or bone marrow transplantation with high-dose/intensive chemotherapy (Itano, 2016).
Drug Resistance
Drug resistance occurs when the cancer stops responding to the prescribed treatment and starts to grow again. It is a major barrier to finding a cure for cancer, as tumors develop the ability to resist the effects of chemotherapy agents (Wu et al., 2017). For instance, a patient may have an initial robust response to treatment for a period of time, but then the treatment stops working. Drug failure in cancers attributed to resistance can be due to a variety of causes and it is possible that more than one resistance mechanism can occur (Yarbro et al., 2018). Intrinsic resistance occurs when cancer cells are inherently resistant to cancer drugs, whereas acquired resistance is when cancer cells develop resistance after exposure to the drug as a consequence of the emergence of resistant cancer clones (Konieczkowski, Johannessen, & Garraway, 2019). In some cases, cancers can develop resistance to multiple drugs, called multiple drug resistance (MDR), resulting in minimal cell death and the growth of drug-resistant tumors (Wu et al., 2017). Cancer is very smart in its ability to adapt and mutate over time, particularly in response to treatment. While there are several proposed rationales for cancer drug resistance, it is most often multifactorial and a combination of issues. Some mechanisms include:
- Impaired metabolism.
- Insufficient dosing may lead to resistance caused by random mutations in cellular DNA. Chemotherapy may kill sensitive cells and leave behind cells that are resistant to treatment. The cells not killed by the chemotherapy mutate and become resistant to the drug.
- Gene amplification, in which a cancer cell may produce hundreds of copies of a particular gene which triggers an overproduction of protein rendering the drug ineffective.
- Drug efflux may occur, during which the cancer cells pump the drug out of the cell as fast as it is going in using a molecule called p-glycoprotein.
- Cancer cells may stop taking in the drugs because the protein that transports the drug across the cell wall stops working.
- Cancer cells may learn how to repair the DNA breaks caused by some cancer drugs.
- Cancer cells may develop a mechanism that inactivates the drug (Konieczkowski et al., 2019; Wu et al., 2017; Yarbro et al., 2018).
Concern for drug resistance is the primary reason why cancer drugs are often given in combination. Since cancer cells are shrewd in their division, replication, and spread mechanisms, combining therapies with varying mechanisms of action can reduce the incidence of developing resistance to any one drug (Konieczkowski et al., 2019). Further, as cancer progresses, it becomes increasingly keen by developing mutations. As a result, if a cancer becomes resistant to one drug or one group of drugs, it is much more likely that the cancer will become resistant to other drugs (Wu et al., 2017). This is why following appropriate evidence-based treatment guidelines is essential, as the best possible treatment protocol should be administered first (Yarbro et al., 2018).
Types of Therapies
Chemotherapy
Chemotherapy, also referred to as cytotoxic or antineoplastic therapy, encompasses a group of high-risk, hazardous drugs with the intent to destroy as many cancer cells with as minimal effect on healthy cells as possible (Yarbro et al., 2018). Premised on the concepts of cellular kinetics, chemotherapy generally works by interfering with the normal cell cycle, impairing DNA synthesis and cell replication, preventing cancer cells from dividing, multiplying, and forming into new cancer cells (Yarbro et al., 2018). The cancer’s response to chemotherapy is multifactorial, but largely depends on the cancer’s growth fraction, doubling time and tumor burden (Yarbro et al., 2018). The growth and size of a tumor is a product of the proportion of cells actively dividing (growth fraction), the length of the cell cycle (doubling time), and the rate of the cell loss (Yarbro et al., 2018). The higher the growth fraction, the greater the chemotherapy response, or greater cell kill (Yarbro et al., 2018). Cancers with smaller tumor burden are usually more sensitive to chemotherapy because they are small and vascular (Yarbro et al., 2018). As the tumor increases in size, the growth rate slows due to crowding of cells, poor vascularization from decreased blood flow, and limited nutrients (Yarbro et al., 2018). As a result, the number of cells actively dying decreases and treatments become less effective (Yarbro et al., 2018). Factors outside the characteristics of the tumor that influence response to chemotherapy is the patient’s general state of health and physical status, including performance status, comorbidities, age, and if they have received prior cancer treatment (Nettina, 2019). As a general rule, patients who are chemotherapy-naïve, or those who have never received chemotherapy before, tend to tolerate and respond to treatment with less toxicity and greater efficacy than those who have received prior therapies (Nettina, 2019).
Chemotherapy drugs are distributed throughout the body by the blood stream and have the potential to cause significant morbidity and mortality if not used correctly and cautiously (Konieczkowski et al., 2019). Chemotherapy may be used as a single agent or in combination with other drugs, but it is more commonly used in combination for greater efficacy against the cancer and to reduce the potential for drug resistance (Konieczkowski et al., 2019). The majority of chemotherapy dosing is based on the patient’s body surface area (mg/m2), although some agents are based on area under the curve (AUC), which refers to the amount of drug exposure over time or the total drug concentration in plasma over a period of time (Itano, 2016). While the most common route of chemotherapy administration is intravenous, it may also be administered via others routes, including oral, subcutaneous (injection), intramuscular (injection), intrathecal (directly into central nervous system), intravesicular (directly into the bladder by urinary catheter), or intraperitoneal (infused directly into the intraabdominal cavity) (Itano, 2016). Intravenous chemotherapy is most commonly administered in hospitals, outpatient infusion clinics, or by continuous infusion pumps that patients wear at home (Itano, 2016).
Specialized education, preparation, and training are required for oncology nurses who administer chemotherapy and other hazardous cancer medications to ensure a safe level of care for patients (Nettina, 2019). The Oncology Nursing Society (ONS, 2019a) offers the ONS/ONCC Immunotherapy Certificate Course and provides up-to-date evidence-based resources. The ONS also outlines competencies required for nurses to administer these agents, including in-depth knowledge of cancer medications and infusion therapy practices. The majority of accredited cancer centers and hospitals within the United States require oncology nurses to hold proper certification prior to administering these medications (ONS, 2019b). In addition, the American Society of Clinical Oncology's (ASCO) quality oncology practice initiative certification program also requires that hospitals, infusion centers, and physician practices comply with safety standards for chemotherapy administration (ASCO, 2019; Neuss et al., 2017).
Due to the ability of chemotherapy to cause severe irritation, damage, and injury to the veins and subcutaneous tissue, many patients have a central venous catheter (i.e. ‘port’ or ‘mediport’) placed, which is a small device that is surgically implanted under the skin, usually in the chest wall, to allow for easy access to the bloodstream (Kreidieh, Moukadem, & El Saghir, 2016). The port may be used to draw blood and infuse chemotherapy drugs (Kreidieh et al., 2016). Some chemotherapy medications, such as vesicants can only be given through a port, as they are too caustic to be given through a peripheral vein (Kreidieh, Moukadem, & El Saghir, 2016). Vesicants are drugs that can lead to severe soft tissue necrosis or formation of blisters when they leak or infuse outside the vein and into the soft tissue; called extravasation (Kreidieh, Moukadem, & El Saghir, 2016). Chemotherapy extravasation is manifested by a range of symptoms and severity varies according to the type, amount, and concentration of the drug (Kreidieh et al., 2016). Initial symptoms can present as an acute burning pain, swelling, at the infusion site, but can becoming increasingly severe in the hours, days, and weeks following the initial injury (Itano, 2016). Patients may develop blisters, which usually begin within 3 to 5 days, and may be followed by peeling or sloughing of the skin with invasion and destruction of deeper structures (Itano, 2016).Tissue necrosis usually occurs within 2 to 3 weeks (Itano, 2016). In the most severe cases, damage can reach tendons, nerves, and joints, leading to functional and sensory impairment of the area, disfigurement, or loss of the limb entirely (Kreidieh et al., 2016; Nettina, 2019). Some examples of chemotherapy vesicants include doxorubicin (Doxil), dactinomycin (Cosmegen), mitomycin C (Mutamycin, Mitosol), and the Vinca Alkaloids (Vinblastine, Vincristine, and Vinorelbine) (Olsen, LeFebvre, & Brassil, 2019). For those patients who receive chemotherapy through a peripheral intravenous (IV) site, nurses must remain hypervigilant to the appearance and function of the IV site before, during, and after the infusion, monitoring for erythema, swelling, and loss of blood return (Olsen et al., 2019). Nurses should counsel patients to report any pain, burning, or other abnormal sensations during the infusion (Olsen et al., 2019). There are specific guidelines surrounding the management of peripheral IV sites for chemotherapy, such as location, placement, monitoring parameters, and how often blood return must be evaluated (Itano, 2016). In general, all chemotherapy agents should be considered irritants, as they all have the potential to cause inflammation, pain or irritation (Itano, 2016; Kreidieh et al., 2016;).
The majority of chemotherapeutic agents are broad in their attack, meaning they kill normal, healthy cells in the body together with the cancer cells (Itano, 2016). According to Olsen, LeFebvre & Brassil (2019), agents are classified according to their biochemical activity, mechanism of action, and phase of action during the cell cycle, which is broken down into two major categories: cell cycle-specific and cell cycle-nonspecific Cell cycle-specific drugs exert cytotoxic effects on cells that are actively dividing at specific stages within the cell cycle. These drugs are not active against cancer cells during the resting phase (G0) and are schedule-dependent, meaning they are most effective if administered in divided doses or by continuous infusion. Continuous infusion may occur over 24 hours for up to 7 days and is primarily given at a slower rate, allowing the drug to reach more cancer cells when they are actively dividing and amenable to cell kill. Cell cycle-nonspecific drugs have a broader impact on cancer cells, as major cytotoxic effects are exerted on cells at any phase within the cell cycle; including G0. These agents are considered dose-dependent and are most effective when administered by bolus doses; as the number of cells affected is directly proportional to the amount of drug given. Table 1 displays the classification of the most common chemotherapy agents according to their mechanism of action and effect on the cell cycle.
Table 1. Classification of Chemotherapy Agents
Category | Chemotherapy Agents | Mechanism of Action | Effect on Cell Cycle |
Antimetabolites |
|
|
|
Alkylating Agents |
|
|
|
Antitumor Antibiotics |
|
|
|
Vinca Alkaloids |
|
|
|
Taxanes (Microtubule Agents) |
|
|
|
Podophyllotoxins |
|
|
|
Camptotecins |
|
|
|
(Itano, 2016; Pfeiffer, Gut, & Schwappach, 2018; Rose, DeVita, Lawrence, & Rosenberg, 2013).
Since cancer cells divide rapidly, chemotherapy is primed to target cells that divide rapidly, thereby impacting normal cells that divide quickly; such as the gastrointestinal tract, skin/hair cells, and bone marrow (Olsen et al., 2019). This explains why the most common chemotherapy side effects include bone marrow suppression, nausea, vomiting, diarrhea, hair loss, and mucositis (Itano, 2016) Several chemotherapy agents have dose-limiting toxicities, which are defined by the occurrence of severe toxicities and side effects during the systemic cancer therapy that are serious enough to lead to reduction of the dose, or discontinuation of the treatment altogether. (DeVita et al., 2015). Dose limiting toxicities require early recognition and intervention in order to minimize hospital admissions, readmissions, infections, and improve the patient’s survival (Olsen et al., 2019). There is a large range of acute and long-term toxicities associated with chemotherapy agents and the comprehensive list extends well beyond the range of this module, however the most common side effects will be discussed (Itano, 2016). Refer to Table 2 for an overview of the most common side effects of chemotherapy.
Table 2. Common Chemotherapy Side Effects
System | Side Effects |
Hematopoietic |
|
Integumentary |
|
Gastrointestinal |
|
Genitourinary |
|
Neurologic |
|
Cardiovascular |
|
Vascular |
|
Pulmonary |
|
Reproductive |
|
Endocrine |
|
Psychiatric |
|
Latent Effects |
|
(Olsen et al., 2019; Polovich et al., 2014).
Bone marrow suppression refers to three main hematopoietic consequences of chemotherapy: neutropenia (reduction in white blood cells), anemia (reduction in red blood cells), and thrombocytopenia (reduction in platelets) (DeVita et al., 2015). The patient is most likely to experience the chemotherapy nadir, the point where the blood counts are at their lowest, approximately 7-10 days after each chemotherapy dose (DeVita et al., 2015). When the body’s natural defense, the immune system, is suppressed due to chemotherapy, the patient is considered neutropenic and the ability to mount a response to everyday germs, bacteria or pathogens is very poor (Nettina, 2019). As a result, the patient is highly susceptible to acquiring illness and is at heightened risk for blood stream infection (bacteremia or sepsis) (Nettina, 2019). Neutropenia is defined by an absolute neutrophil count (ANC) of 1,500/mm3 or less and is a primary dose-limiting toxicity of chemotherapy (Nettina, 2019). The risk for infection is heightened when the ANC reaches levels under 500/mm3 (Nettina, 2019). The most common sign of infection in a neutropenic patient is a fever, and patients need to be monitored and treated promptly for fever or other signs of infection (Nettina, 2019). A fever in the setting of neutropenia is called febrile neutropenia, and this is a medical emergency, requiring prompt evaluation, work up, and the initiation of empiric antibiotics (Nettina, 2019). Patients should be counseled on ways to avoid infection, such as thorough handwashing, hygiene and avoiding those with illness (Nettina, 2019). Patients should also avoid eating raw meats, seafood, eggs, or unwashed vegetables when they are neutropenic due to risk for acquiring food-borne illness (Nettina, 2019).
Some providers may prescribe a colony-stimulating factor such as filgrastim (Neupogen®) or pegfilgrastim (Neulasta®) to help manage neutropenia (Polovich et al., 2014). These are injectable agents that stimulate the bone marrow to rapidly produce white blood cells, thereby reducing the risk of infection and neutropenic fever (Polovich et al., 2014). According to Olsen, LeFebvre, and Brassil (2019), there are specific parameters and indications for using a colony-stimulating agent and they are usually well-tolerated. The most common side effects include bone pain, particularly in the long bones such as the hips and sternum where normal bone marrow is naturally produced (Olsen et al., 2019). Patients may use over-the-counter pain relievers such as acetaminophen or nonsteroidal anti-inflammatory drugs for comfort, however most patients find antihistamines provide superior relief from symptoms (Olsen et al., 2019).
Box 1-1. Critical Consideration. Fever greater than 101°F (38.3°C) in a patient with an ANC less than 500/mm3 is a medical emergency requiring immediate intervention and administration of antibiotics, as the patient is at risk of sepsis, which can be fatal if not treated. Note: antibiotics should only be administered after the appropriate blood, wound, and/or urine cultures have been obtained. (Olsen et al., 2019).
Anemia is a common consequence of chemotherapy and generally becomes more significant with each successive dose of chemotherapy due to a cumulative effect as patients progress through treatment. (Olsen et al., 2019). In addition to a low hemoglobin and hematocrit as seen through bloodwork, patients may display symptoms of pallor, fatigue, low energy, chest pain, shortness of breath, and weakness (Nettina, 2019). Some patients may benefit from oral iron supplements, folic acid, and consuming an iron-rich diet. Others may require erythropoietin-stimulating agents such as epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp) or may need blood transfusions. Thrombocytopenia, or low platelet count, is an effect of chemotherapy caused by suppression of megakaryocytes in the blood, impairing the body’s ability to form a blood clot, thereby heightening the risk for bleeding (Polovich et al., 2014). Platelets stop bleeding by clumping and forming plaques in blood vessel injuries, such as cuts, lacerations and other wounds. The risk of bleeding is present when a patient’s platelet count falls below 50,000/mm3, high risk if their count falls below 20,000/mm3, and critical risk if their count falls below 10,000/mm3 (Nettina, 2019). Patients may require platelet transfusions if their count drops below 20,000/mm3. This is particularly dangerous in patients who are on anticoagulation therapy, as their risk of bleeding is already increased, and they require close monitoring and surveillance (Polovich et al., 2014). Signs of thrombocytopenia may include easily bruising, petechiae, epistaxis, gum bleeding, hematuria, rectal bleeding, wounds that won’t stop bleeding, or any other acute signs of bleeding (Nettina, 2019) It is critical to counsel patients regarding ways to prevent injury when their platelet counts are low, including avoiding shaving with razors, rectal suppositories, using dental floss, or participating in activities that place them at risk for injury (i.e. contact sports, skiing, horseback riding) (Olsen et al., 2019). Patients and caregivers must also be counseled on the risk for hemorrhage, paying particular attention to any acute head injuries (Itano, 2016). Patients with low platelet counts who are involved in a motor vehicle collision or any kind of trauma or fall where they hit their head are at increased risk for subarachnoid or intracerebral hemorrhage, or acute bleeding inside the skull (Itano, 2016). These patients always require emergent evaluation by a clinician and will often undergo some form of radiographic imaging of the head to rule out bleeding such as a computed tomography (CT) scan (Itano, 2016).
The gastrointestinal (GI) system is routinely impacted by chemotherapy, causing side effects such as nausea, vomiting, diarrhea, constipation, anorexia, and mucositis (Itano, 2016). These symptoms can lead to serious complications such as dehydration (fluid volume deficit), electrolyte disturbances, protein deficit, weight loss and cachexia (muscle wasting) (Itano, 2016). Anorexia, or loss of appetite, is often of multifactorial etiology in cancer patients receiving chemotherapy (Nettina, 2019). Patients often endure dysgeusia, or altered taste, as the chemotherapy changes the reproduction of taste buds, inducing absent or altered taste (Nettina, 2019). This can lead to inadequate nutrition and protein intake resulting in weight loss and cancer cachexia (Nettina, 2019). Mucositis refers to inflammation and ulceration of the mucous membranes lining the mouth and other parts of the GI tract (Itano, 2019). This condition commonly presents with initial oral burning and discomfort, followed by the disruption of open sores and ulcers throughout the oral cavity, which may extend all the way down the GI tract to the anus (Itano, 2019). Often associated with significant pain, thrush, and dysphagia (difficulty swallowing), mucositis can significantly impair oral intake. Consistent oral hygiene with baking soda-based oral rinses and normal saline solution is critical to promote healing, stimulate cell turnover, and avoid infection (Olsen et al., 2019). Patients should be encouraged to use oral agents to promote cleansing, debridement, and comfort, and mouthwashes that are alcohol-based should be avoided due to potential for irritating effects (Nettina, 2019). Painful mucositis can also be treated with oral solutions including anti-fungal suspensions (i.e. nystatin) and lidocaine (numbing analgesia) for comfort (Nettina, 2019). Nurses must monitor for diarrhea and/or constipation in patients on chemotherapy, as life-threatening diarrhea is associated with certain chemotherapeutic agents; particularly irinotecan (Camptosar) (Olsen et al., 2019). This drug has such a high risk of inducing severe diarrhea, that it has been nicknamed “I ran to the can” by oncology nurses for decades (Olsen et al., 2019). Diarrhea can be life-threatening due to associated fluid and electrolyte depletion, as electrolyte imbalance can induce life-threatening changes in cardiac function. In contrast, constipation can not only exacerbate existing anorexia and nausea, but can place patients at risk for bowel obstructions (Olsen et al., 2019). Nurses should assess and monitor patients for changes to bowel habits prior to each treatment (Itano, 2019).
According to DiVita, Lawrence and Rosenberg (2015), patterns of chemotherapy-induced nausea and vomiting include acute, delayed, and anticipatory nausea. They describe acute nausea as occurring 0 to 24 hours after chemotherapy administration, whereas delayed nausea often occurs several days after chemotherapy administration. Anticipatory nausea is described as a conditioned response generated from repeated association between treatment and prior nausea and vomiting episodes, and may even be trigged by entering the hospital environment, certain smells, or sounds (DeVita et al., 2015). Anticipatory nausea can and should be prevented with adequate antiemetic control. Prevention of nausea and vomiting is the goal, so the prescribed nausea management plan is directly correlated with the level of emetogenicity of the drug (DeVita et al., 2015). Each chemotherapeutic agent is classified according to their emetogenic (nausea) risk, ranked from low to high (ONS, 2019). Several major oncology groups have published consensus reports and evidence-based guidelines on the prevention of chemotherapy-induced emesis, such as NCCN, ONS, and ASCO (ONS, 2019; NCCN, 2019). Highly emetogenic drugs require enhanced anti-emetic control with dual or triple therapy targeting various nausea receptors (ONS, 2019; NCCN, 2019). Prior to starting any chemotherapy regimen, it is critical that the nurse performs a thorough assessment of the patient’s prior experiences with nausea so that nursing interventions can be tailored to meet the patient’s needs (ONS, 2019; NCCN, 2019). Identifying factors that stimulated nausea in the past can help prevent anticipatory nausea and also serve to ease the patients overall experience and outcome (ONS, 2019; NCCN, 2019). In general, all patients should be premedicated with an appropriate anti-emetic and have appropriate as needed anti-emetic agents prescribed for home (ONS, 2019; NCCN, 2019). The most common anti-emetic medication regimens include a first-generation serotonin receptor antagonists (i.e. ondansetron [Zofran]), an NK1 receptor antagonist (i.e. aprepitant [Emend]), and/or a first-generation antipsychotic drug (i.e. prochlorperazine [Compazine]) (ONS, 2019; NCCN, 2019). For severe nausea and vomiting uncontrolled by the prior, many clinicians will add corticosteroids (i.e. dexamethasone [Decadron]) for enhanced control (ONS, 2019; NCCN, 2019). Nurses must teach patients and families to response quickly to breakthrough nausea with as-needed medications before vomiting occurs (ONS, 2019; NCCN, 2019). Patients should also be counseled on benefit of consuming small, frequent meals with high calories instead of trying to consume three large meals daily (ONS, 2019; NCCN, 2019). For more information, refer to ONS guidelines for more evidence-based guidelines on controlling chemotherapy-induced nausea and vomiting (CINV) or NCCN guidelines for supportive care and antiemesis (ONS, 2019; NCCN, 2019).
Alopecia, or hair loss, deserves special attention because it can cause significant emotional distress to patients. Chemotherapy-induced hair loss generally begins as hair thinning, about 7-15 days after the first dose (Rose et al., 2013). It is due to damage to the dividing hair matrix cells, which causes the hair shaft to break at the follicular orifice or hair bulb (Rose et al., 2013). The degree of hair loss depends on the chemotherapy agent, the dose, and the schedule of administration (Olsen et al., 2019). Not all chemotherapy agents cause hair loss, so it is possible to progress through chemotherapy treatment without losing hair (Olsen et al., 2019). However, there are certain regimens that are known to cause complete hair loss; specifically, first line chemotherapy for breast cancer, ovarian cancer, and endometrial cancer (Olsen et al., 2019). Some agents that cause complete alopecia include higher doses of cyclophosphamide (Cytoxan), paclitaxel (Taxol), bleomycin (Blenoxane), dactinomycin (Cosmegan), topotecan (Hycamtin), and docetaxel (Taxotere); whereas much less hair loss is seen with agents such as 5-fluorouracil (Adrucil), etoposide (Vepesid), gemcitabine (Gemzar), methotrexate (Trexall), and ifosfamide (Ifex) (Rose et al., 2013). Patients should be counseled that their hair will begin to regrow within a few weeks following the cessation of chemotherapy, as permanent alopecia following chemotherapy is very rare (Rose et al., 2013). Novel treatments such as scalp hypothermia (i.e. “cold caps” or “scalp cooling”) worn during the infusion of certain alopecia-inducing chemotherapies have been shown to be effective in reducing alopecia (Rice et al., 2018). In fact, Gianotti and colleagues (2019) conducted a multicenter interventional study and found a 68% overall success rate of scalp cooling in the prevention of hair loss in women being treated for breast cancer. Using their methodology, severe hair loss was avoided in 89% of the women receiving taxane-based chemotherapy and in 78% of women receiving both anthracyclines and taxanes. They utilized cold caps and scalp cooling systems, which are tightly fitting, helmet-type hats filled with a gel coolant that’s chilled to between -15 to -40 degrees Fahrenheit. According to Gianotti and colleagues, work by narrowing the blood vessels beneath the skin of the scalp, reducing the amount of chemotherapy medicine that reaches the hair follicles. The cold is postulated to decrease the activity of the hair follicles, which slows down cell division and makes the follicles less affected by the chemotherapy (Gianotti et al., 2019).
Chemotherapy-induced peripheral neuropathy (CIPN) can be a severe side effect commonly associated with certain chemotherapy agents such as the platinum agents (i.e. carboplatin (Paraplatin), oxaliplatin (Eloxatin), the taxanes (Paclitaxel, Docetaxel), vinca alkaloids (vincristine (Oncovin), vinblastine(Velban), thalidomide (Thalomid), and bortezomib (Velcade) (Brown, Sedhom, & Gupta, 2019). CIPN results from injury to the sensory and motor axons due to demyelination, which reduces nerve conduction velocity leading to the loss of deep tendon reflexes and symptoms of paresthesia (numbness and tingling), weakness, and burning pain (Brown et al., 2019). CIPN often initially affects the most distal points of the body, such as the fingertips and toes, and progressively moves up the extremities toward the midline as the damage progresses (Brown et al., 2019). Symptoms are usually symmetrical and in severe cases, patients may lose sensation of the fingers, hands, toes, and feet altogether, leading to dysfunction in ability to grasp or hold items, and gait disturbance including imbalance and falls (Brown et al., 2019). Brown, Sedhom and Gupta (2019) define CIPN as a complex topic, since no single unifying pathophysiologic process can be identified to explain the various neuropathies that occur after exposure to different chemotherapeutic agents. They view CIPN as dose-dependent and progressive while receiving treatment, but can also have a cascading effect after treatment ends; which is a phenomenon where symptoms become more prominent after discontinuation of the offending agent. Pain, sensory changes, and weakness that present during treatment generally lead to chemotherapy dose reductions, changes in treatment protocols, or termination of the therapeutic agent entirely (Brown et al., 2019). The morbidity associated with CIPN can lead to pronounced alterations in quality of life and loss of independence with activities of daily living (Brown et al., 2019). The etiology is not well established or understood, but CIPN can be severe, debilitating, and is often a dose-limiting toxicity of certain chemotherapies (Brown et al., 2019).
Currently there are no medications or supplements that have been shown to definitively prevent CIPN (Addington, & Freimer, 2016). Regular exercise, reducing alcohol use, and treating preexisting medical conditions (vitamin B12 deficiency) may reduce the risk of CIPN (Brown et al., 2019). Patients who are undergoing treatment with agents that cause neuropathy may be advised to take B vitamin supplementation prophylactically, but research confirming the benefit of this intervention in reducing the risk of neuropathy is not available (Addington & Freimer, 2016). Management for CIPN is equally complex and effective treatment options are limited (Addington, & Freimer, 2016). Pharmacologic treatment focuses on symptom relief, though many agents are not highly effective (Addington & Freimer, 2016) Over-the-counter pain medications, menthol creams, capsaicin cream, or lidocaine patches may be used for comfort (Addington & Freimer, 2016). Some patients may be prescribed medications such as gabapentin (Neurontin), which is an anti-convulsant/anti-epileptic agent that is also utilized to treat neuropathic pain. Some patients may find relief from selective serotonin-norepinephrine reuptake inhibitors (SSRI) such as duloxetine (Cymbalta) (Addington & Freimer, 2016). Patients with CIPN must be counseled on ways to avoid injury, such as wearing good, supportive, close-toed shoes and paying attention to home safety, such as using handrails on stairs and removing throw rugs (Olsen et al., 2019). Patients must also be mindful of water temperatures, they may become less sensitive to hot water, increasing their risk for burns when bathing or washing dishes (Olsen et al., 2019). Improvement in function and resolution of symptoms often occurs gradually over time, but in some cases, nerve damage may be permanent (Brown et al., 2019).
Chemotherapy-induced cardiotoxicity is a serious complication that limits the use of certain chemotherapy agents as they can eventually culminate in the development of life-threatening dysrhythmias, conduction disturbances, cardiomyopathies, pericarditis or myocarditis, and pericardial effusions (Angsutararux, Luanpitpong, & Issaragrisil, 2015). Anthracyclines including doxorubicin(Adriamycin), daunorubicin(Cerubidine), epirubicin(Ellence), and idarubicin (Idamycin), are some of the medications that most often induce cardiac effects (Angsutararux et al., 2015). According to Angsutararux et al., (2015) acute cardiotoxicities that occur within the course of treatment or immediately afterwards are normally reversible and generally manageable, however chronic cardiotoxicity may manifest for long periods of time, up to decades after the completion of treatment. They define chronic cardiotoxicity as serious and clinically significant, substantially affecting overall morbidity and mortality and requiring long-term management. The cumulative dose of doxorubicin is an important factor that dictates potential for cardiotoxicity; as the cumulative dose should not exceed 500 mg/m2 or the risk of congestive heart failure rises tremendously (Angsutararux et al., 2015; Olsen et al., 2019). Nurses must remain vigilant when administering cardiotoxic chemotherapy agents to ensure cumulative doses do not exceed 500mg/m2 (Angsutararux et al., 2015). Patients should undergo baseline cardiac evaluation with an echocardiogram or multigated acquisition (MUGA) scan to evaluate cardiac function and left ventricular ejection fraction (LVEF) prior to initiating cardiotoxic therapies, and then again at defined intervals or as clinically indicated (Angsutararux et al., 2015). Patients should also be monitored closely for any signs and symptoms of cardiac dysfunction such as dyspnea, shortness of breath, peripheral edema, fluid retention, chest pain (angina), lightheadedness, or so forth (Angsutararux et al., 2015). Early detection and immediate proper medication could reverse the condition in time that minimizes cardiotoxic effects (Angsutararux et al., 2015; (Olsen et al., 2019).).
Chemotherapy may induce reproductive alterations and consequences in both males and females, including sexual dysfunction, loss of libido, and psychological consequences associated with distorted body image (Itano, 2016). For instance, surgical intervention with orchiectomy (removal of testis in males with testicular cancer) or mastectomy (removal of breasts in females with breast cancer) may negatively impact one’s self-image and in turn, impair sexual health and
intimate relationships (Liede et al., 2018). Side effects of chemotherapy can equally induce other body image distortions secondary to alopecia, vaginal dryness from hormonal therapy, and erectile dysfunction (Rose et al., 2013). For females, chemotherapy can cause amenorrhea (permanent or temporary loss of menses), damage to the ovarian follicles, or cause ovarian fibrosis (Itano, 2016). It can also induce premature ovarian failure leading to infertility, and menopause, with associated hot flashes, night sweats, and weight gain (Itano, 2016). Males may endure low sperm counts, poor sperm motility, sterility, impotence, and erectile dysfunction (Itano, 2016) It is important to discuss these risk factors with patients prior to the initiation of treatment (Itano, 2016). Nurses have a responsibility to ensure patients are educated honestly and openly about the potential effects of chemotherapy; including the possibility of fertility loss. Patients and/or couples may desire fertility preservation for future family planning with cryopreservation of embryos or sperm. (Polovich et al., 2014).
While this may be a challenging topic to approach, it is essential that patients are educated on safe sex practices while undergoing cancer treatment (Itano, 2016). Patients and their partners must understand the importance of taking precautions to avoid pregnancy while receiving treatment, as chemotherapy can induce fetal harm and birth defects if the fetus is exposed during certain stages of development (Itano, 2016). Females must be educated that it is still possible to conceive despite not having regular menstrual cycles on chemotherapy, so it is critical to ensure proper precautions are being taken to avoid pregnancy during their treatment (Polovich et al., 2014). Patients should be advised to abstain from sexual intercourse within the first 24-48 hours after chemotherapy, due to the known presence of chemotherapy in bodily fluids (Polovich et al., 2014). Patients should then use a barrier method, such as a condom, to prevent any unnecessary exposure to partners via bodily fluids (Polovich et al., 2014). Finally, breastfeeding is contraindicated during chemotherapy treatment due to the exposure of the infant to the chemotherapy medications in breastmilk (Polovich et al., 2014).
Hypersensitivity Reactions
A hypersensitivity reaction (HSR) occurs when the immune system is over stimulated by a foreign substance (i.e. chemotherapy) and forms antibodies that cause an immune response (Nettina, 2019). Some chemotherapeutic agents commonly associated with HSR include paclitaxel (Taxol), L-asparaginase (Erwinaze), and bleomycin (Blenoxane) (Nettina, 2019).
- HSRs can occur during the initial chemotherapy infusion or after subsequent administrations of the same agent (Nettina, 2019).
- Most HSRs occur during the first 15 minutes of the infusion, but reactions may occur outside of this time frame as well (Nettina, 2019).
- Initial signs and symptoms can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, and anxiety (Nettina, 2019).
- However, symptoms may suddenly progress to life threatening hypotension, bronchospasm, angioedema (swelling of the oral cavity, lips and/or tongue), and anaphylaxis (Nettina, 2019).
- In these cases, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) may need to be administered by IV push or subcutaneous injection until emergency personnel arrive (Nettina, 2019).The risk of HSR can be reduced by pre-medicating patients with a combination of agents such as corticosteroids, antihistamines, acetaminophen, and/or h2-receptor antagonists (Nettina, 2019).
- Oncology nurses must remain vigilant for signs of HSR and ensure they are prepared to intervene immediately (Nettina, 2019).
- If an HSR is suspected, the nurse must first stop the infusion immediately, notify the health care provider, and monitor the patient closely (Nettina, 2019).
- Additional nursing interventions include monitoring vital signs, applying supplemental oxygen, administering normal saline and other emergency medications as indicated and according to institution policy or physician order (Nettina, 2019).
- Nurses should be familiar with their own institution’s specific chemotherapy hypersensitivity protocols and policies for further information and instruction (ONS, 2019b).
Safety and Exposure
In addition to patient safety, cytotoxic drugs can be equally hazardous to nurses and other health care workers, so it is critical to adhere to standards and practices of hazardous drug handling to minimize occupational exposure (ONS, 2019b). Exposure to hazardous medications are linked to increased risk for several types of malignancies, and exposure can occur through various sources, including workplace surface contamination (Polovich et al., 2014). According to the 2016 updated American Society of Clinical Oncology and Oncology Nursing Society chemotherapy administration safety standards as outlined by Neuss and colleages (2017), nurses must wear appropriate personal protective equipment whenever there is a risk of chemotherapy being released into the environment such as preparation or mixing of chemotherapy, spiking/priming IV tubing, administering the drug, and when handling body fluids or chemotherapy spills. These guidelines also describe hazardous drug handling as posing reproductive risks, so healthcare workers who are pregnant, breastfeeding, or trying to conceive must notify their employer, as these individuals should not be handling hazardous medications such as chemotherapy.) Neuss and colleagues (2017) also outline that chemotherapy medications must be mixed, spiked/primed under an approved filtered hood to reduce risk of aerosolized exposure). Gloves that have been tested for use with hazardous drugs are required and reuse of gloves is prohibited (Neuss et al., 2017). Nurses should wear disposable, lint-free gowns made of low-permeability fabric when administering chemotherapy and spill kits should be available in all areas where chemotherapy is stored, prepared, and administered (Neuss et al., 2017). Gloves and gowns should be discarded in leak-proof containers, which should be marked as contaminated or hazardous waste (Neuss et al., 2017). Linens or clothes contaminated with chemotherapy or bodily fluids from patients who have received chemotherapy within 48 hours should be contained in specially marked hazardous waste bags (Neuss et al., 2017). If any chemotherapy spills on clothes in the clinic, clothing should be thrown away or double-bagged in a plastic bag sealed for transport home. The clothing must be washed separately in hot water with regular detergent (Neuss et al., 2017). The Oncology Nursing Society (2019a; 2019b) has two standards that address education of nurses who administer and care for patients receiving chemotherapy, biotherapy, and immunotherapy agents. The standards support the Registered Nurse (RN) as the minimum appropriate licensure for nurses who administer chemotherapy and biotherapy. They recommend educational requirements for nurses which are the same regardless of treatment indications, clinical settings, routes of administration, and patient population. Due to the unique safety considerations of these drugs, specialized education is needed for all nurses who administer chemotherapy or other anti-cancer agents. ONS offers online courses for initial didactic preparation and knowledge maintenance for nurses who administer chemotherapy and immune-based treatments. However, each institution or practice must determine how it will assess nursing competence in performing various chemotherapy-related skills (ONS, 2019a; ONS, 2019b).
Immunotherapy
Immunotherapy, or biologic therapy, is a relatively novel sector of cancer treatment that stimulates the body’s own immune system to fight cancer (Sengupta, 2017). Immunotherapy has emerged as an important modality of cancer treatment, where intensive research is actively being conducted (Sengupta, 2017). The connection between cancer and the immune system was discovered nearly 100 years ago by Dr. William Coley, coined the father of immunotherapy, when he discovered that malignant tumors disappear in patients who contracted acute streptococcal infections (Yarbro et al., 2018). The goal of immunotherapy is to produce antitumor effects through the action of natural host defense mechanisms (Miliotou & Papadopoulou, 2018). It is based on the principle that the primary function of the immune system is to detect and eliminate any foreign pathogens that attempt to invade the body’s defense system (Miliotou & Papadopoulou, 2018). Immunotherapy strives to modify the patient’s own immune defenses to become more sensitive to cancer cells by learning how to identify, attack, and kill them; with the ultimate goal of promoting tumor regression (Miliotou & Papadopoulou, 2018). More recently, novel drugs known as checkpoint inhibitors have been developed to enable the immune system to more effectively harness disease fighting immune cells (T cells) against cancer (Miliotou & Papadopoulou, 2018). These are systemic treatments and they work very differently than chemotherapy as they are so specific in their action (Miliotou & Papadopoulou, 2018). Some forms of immunotherapy are so highly specialized that they only target a single receptor on the surface of tumor cells (Miliotou & Papadopoulou, 2018). Immunotherapies are frequently combined with other treatment modalities (Miliotou & Papadopoulou, 2018). The main types of immunotherapy currently being used to treat cancer include: some monoclonal antibodies, immune checkpoint inhibitors, and cancer vaccines (Sengupta, 2017). While immunotherapy represents one of the most promising new cancer treatment approaches since the development of chemotherapy, it is still not equally effective on all cancers at this stage in development (Sengupta, 2017). To understand how immunotherapy treatments work, it is critical to first ensure a proper understanding of how the immune system functions normally (ONS, 2019a; Nettina, 2019).
Overview of the Immune System
The immune system, the body’s defense system, is a collection of cells, tissues, and organs that work together to defend the body against attacks by “foreign” invaders such as microbes, virus, parasites, or other pathogens (Miliotou & Papadopoulou, 2018). The immune system strives to prevent invasion and protect against illness and infection by seeking out and destroying pathogens (Miliotou & Papadopoulou, 2018). The key to a healthy immune system is within its ability to distinguish between the body’s own cells (“self”) and foreign cells (“non-self”) (Miliotou & Papadopoulou, 2018). The cells of the immune system launch an attack when they encounter anything that appears foreign (Miliotou & Papadopoulou, 2018). Any substance capable of triggering an immune response is called an antigen (Rose et al., 2013). An antigen can be a microbe, such as a virus or bacteria, and all antigens carry marker molecules that identify them as foreign (Rose et al., 2013). The organs of the immune system are positioned throughout the body and they are called lymphoid organs because they house lymphocytes, the small white blood cells that are the key performers of the adaptive immune system (Miliotou & Papadopoulou, 2018). There are two main types of lymphocytes; B-cells and T-cells (Miliotou & Papadopoulou, 2018). B-cells work by secreting substances called antibodies into the body’s fluids, which serve to ambush antigens circulating in the bloodstream (Miliotou & Papadopoulou, 2018). They then hand off the baton to the T-cells, who have the job of attacking target cells (the infected cells) (Miliotou & Papadopoulou, 2018). An antigen matches an antibody much like a key matches a lock (Rose et al., 2013).
Mounting an Immune Response
There are two main types of immune response: innate (or nonspecific) and acquired (or adaptive) immunity (Nettina, 2019). Innate immunity is also known as natural immunity, as it is present from birth. It is considered the first line of defense against pathogens, and is a nonspecific form of immunity that is activated immediately and rapidly in response to invading pathogens (Nettina, 2019). Innate immunity is always present in the body, prepared to attack predators and does not generate immunologic memory (DeVita et al., 2015). In other words, the innate immune system has no memory of prior predators and responds nonspecifically each time a predator launches an attack (DeVita et al., 2015). It includes physical barriers (skin and mucus membranes), mechanical barriers (coughing and sneezing), chemical barriers (tears and sweat), inflammatory responses (production of specialized white blood cells such as neutrophils, macrophages and monocytes), complement activation, and the production of natural killer (NK) cells (large granular lymphocytes (DeVita et al., 2015). An example of innate immunity includes the development of redness and swelling around a wound caused by lymphocytes invading the wound, working to keep microbes out, and heal the injury before further damage or infection occurs (DeVita et al., 2015).
Adaptive immunity is the second line of defense and is highly specific in nature, as it responds individually to every pathogen it encounters (Nettina, 2019). Mediated by T-cells and B-cells, the adaptive immune system comes into action if innate immune mechanisms are somehow breached by an invading pathogen (DeVita et al., 2015). The adaptive immune system boasts immunologic memory and specificity, meaning it “remembers” prior attacks and has the ability to develop a repeat specified response (DeVita et al., 2015). An example of adaptive immunity is a vaccination, which leads to the creation of antibodies against a virus to prevent acquisition of the illness at a later time. If that particular virus tries to invade the body, the adaptive immune system is activated to quickly produce additional antibodies to address the infection, thereby preventing illness (DeVita et al., 2015). Due to the process of adaptation, the acquired immune system responds comparatively slower than the innate immune system. There are three types of adaptive immunity which include humoral immunity, cell-mediated immunity, and T-regulatory cells (Sasikumar & Ramachandra, 2018). Humoral immunity is mediated largely by B-cells and result in production of immunoglobulins (IGs) (Sasikumar & Ramachandra, 2018). Cell-mediated immunity is mediated by T-cells and their cytokine products. It does not involve an antibody, but instead includes Cytotoxic T-cells (usually CD8) and Helper T-cells (usually CD4). T-regulatory cells, also known as suppressor T cells, display the markers CD4 and CD25, and act to limit the activity of other immune effector cells (Sasikumar & Ramachandra, 2018). Ultimately, their major role is to prevent damage to normal tissues and limit the inflammatory response that can occur with infections (Sasikumar & Ramachandra, 2018).
When immune surveillance fails, cancerous tumors form. There are various schools of thought on the exact etiology of tumor escape mechanisms, but largely encompass the following:
- Altered Immunogenicity: Antigen expression on the tumor-cell surface is altered, allowing the antigen to go unrecognized by the humoral immunity system; or cell-mediated immune response can be blunted through cellular mutations.
- Antigen Modulation: Antibodies produced as part of the immune response cause antigens to enter the tumor cell or leave it completely.
- Immune Suppression: Cancer cells can produce substances that inhibit the body’s immune response.
- Acquired Deficiencies: Age or disease-associated alterations can occur, including alterations in apoptosis mechanisms and signaling defects.
- Immunologic Aging: Alterations in T-cell functions can cause a decline in the T-cell proliferation, thereby weakening the effect of the immune system.
- Tumors can evade the immune system by appearing similar to normal cells, thereby not setting off inflammatory or warning signals that they are foreign
(Miliotou & Papadopoulou, 2018; Sasikumar & Ramachandra, 2018).
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors work by blocking the receptors that cancer cells use to inactivate T-cells (Sasikumar & Ramachandra, 2018). When this signal is blocked, T-cells may be better able to differentiate between normal cells and cancer cells, thereby augmenting the immune system’s response to the cancer cells (Sasikumar & Ramachandra, 2018). Checkpoint inhibitors are presently categorized into two main groups: programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) inhibitors and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitors (Sasikumar & Ramachandra, 2018). By blocking tumor cells from inactivating T cells, these medications allow the T cells to remain active and fight the tumor cells (Sasikumar & Ramachandra, 2018).
PD-1 Inhibitors /PD-L1 Inhibitors
PD-1 is a checkpoint protein on T cells that normally acts as a type of “off switch” to keep the T cells from attacking other cells in the body (Sasikumar & Ramachandra, 2018). It does this when it attaches to PD-L1, a protein on some normal (and cancer) cells (Sasikumar & Ramachandra, 2018). When PD-1 binds to PD-L1, it signals the T cell to leave the neighboring cells alone (Sasikumar & Ramachandra, 2018). Some cancer cells have large amounts of PD-L1, which helps them evade immune attack (Naidoo et al., 2015). PD-1 is a transmembrane protein expressed on the surface of circulating T-cells, B-cells, and NK-cells and is used to recognize “self” antigens from “non-self” (Sasikumar & Ramachandra, 2018). The binding of PD-L1, an inhibitory ligand expressed on various cancer cells, to PD-1 receptors on immune effector cells inhibits the immune effector cells from attacking the tumor cells (Sasikumar & Ramachandra, 2018). PD-1 and PD-L1 inhibitors have been designed to prevent the formation of this complex and enable immune cells to attack tumor cells (Sasikumar & Ramachandra, 2018). Monoclonal antibodies that target either PD-1 or PD-L1 can block this binding and boost the immune response against cancer cells (Naidoo et al., 2015). Based on increasing treatment-response rates, PD-1 and PD-L1 inhibitors are being approved for various types of cancer, including melanoma, non–small cell lung cancer, urothelial cancer, and most notably, solid tumors with microsatellite instability-high (MSI-H) mutations and mismatch repair deficient (dMMR) mutations (Sasikumar & Ramachandra, 2018). The approval of pembrolizumab (Keytruda) in 2015 for MSI-H or dMMR solid tumors was the first time a drug has been approved based on a biomarker test for a trait and not an anatomical tumor site, which represents a significant shift in cancer treatment (Sasikumar & Ramachandra, 2018). Examples of drugs that target PD-1 (PD-1 inhibitors) include: Pembrolizumab (Keytruda), Nivolumab (Opdivo), and Cemiplimab (Libtayo) (Olsen et al., 2019). Examples of drugs that target PD-L1 (PD-L1 Inhibitors) include: Atezolizumab (Tecentriq), Avelumab (Bavencio), and Durvalumab (Imfinzi) (Olsen et al., 2019).
These drugs have been shown to be helpful in treating several types of cancer, including melanoma of the skin, non-small cell lung cancer, kidney cancer, bladder cancer, head and neck cancers, and Hodgkin lymphoma (Naidoo et al., 2015).PD-L1 inhibitors/PD-1 inhibitors are generally well tolerated; however, patients can potentially experience serious and possibly life-threatening autoimmune-related adverse effects (Naidoo et al., 2015).Though any organ system can be subjected to an autoimmune reaction, most commonly observed reactions are colitis, hepatitis, endocrinopathies (thyroid and adrenals), pneumonitis, and skin-rash progression to Stevens-Johnson syndrome (Naidoo et al., 2015). However, the most common side effects include fatigue, nausea, anorexia, cough, diarrhea, skin rash, and itching (Naidoo et al., 2015; Sasikumar & Ramachandra, 2018).
CTLA-4 Inhibitors
In addition to inhibition of the programmed cell death complex, other inhibitory modulators, such as the CTLA-4 have proven to be worthwhile targets in immunotherapy (Sasikumar & Ramachandra, 2018). Inhibitory effects of CTLA-4 occur in the priming phase of the immune response by interfering with signals required for T-cell activation (Sasikumar & Ramachandra, 2018). Binding of CTLA-4 to CD80/86 inhibits the activation of T-lymphocytes, resulting in a negative feedback signal that decreases immune response (Sasikumar & Ramachandra, 2018). The drug ipilimumab (Yervoy) induces T-cell activation by disabling the CTLA-4 feedback inhibition and allowing the immune system to activate (Sasikumar & Ramachandra, 2018). Ipilimumab (Yervoy) is currently FDA-approved for two distinct melanoma indications. It has been shown to demonstrate a synergistic effect with increased T-cell priming via CTLA-4 inhibition in the lymph nodes and cytotoxic activity via PD-1 inhibition in the tumor environment (Sasikumar & Ramachandra, 2018). Compared to drugs that target PD-1 or PD-L1, serious side effects are much more likely with these agents (Naidoo et al., 2015). Side effects of fatigue, skin rash, pruritis, and diarrhea are common (Naidoo et al., 2015). Clinically significant adverse effects are related to their ability to induce nonspecific inflammation throughout the body, leading to immune-mediated problems in the lung (pneumonitis), intestines (enterocolitis), liver (hepatitis), kidneys (nephritis), hormone-making glands (endocrinopathies including thyroiditis and hypophysitis), eyes (uveitis), as well as several other organs (Naidoo et al., 2015; Sasikumar & Ramachandra, 2018). Most immune-related events are reversible with immunosuppressive steroid treatment, but must be graded according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5 (2017) and managed per specific medication guidelines (Naidoo et al., 2015).
Other types of immunotherapy currently being explored include cancer vaccines and virus immunotherapy, in which viruses are used to deliberately infect the cancer cell, which in turn triggers an immune response against the infected cancer cell (Sengupta, 2017). These modalities are still largely in the clinical trial stage of development, with only minimal agents approved by the U.S. FDA at this point (Sengupta, 2017). An example of a cancer vaccine is Provenge, which is designed to treat certain forms of prostate cancer, however data regarding its effectiveness in improving life expectancy is limited and dubious (Sengupta, 2017).
Targeted Agents
In 2003, the completion of the Human Genome Project marked a dramatic shift in the understanding of cancer and other diseases (National Human Genome Research Institute [NHGRI], 2014). Project researchers mapped the entire human genetic code and discovered that every human cell is made up of 20,000 to 30,000 genes. As a result, the past decade has been one of exploration into novel approaches to treating cancer and new drug discovery (NHGRI, 2014). Targeted agents are drugs that are formulated to attack specific parts of cancer cells, with the intent of preventing tumor development or to shrink existing tumors (Sengupta, 2017). There are numerous proteins located on the cellular membranes called growth factor receptors, which connect the external and internal cellular environments and are essential for cell growth and development (Sengupta, 2017). Alterations in genes lead to changes in these cellular proteins, stimulating the disruption of normal processes, inducing malfunction, and subsequently sanctioning cancer growth (Sengupta, 2017). The development of specialized drugs that block these growth factor receptors has been a tremendous part of cancer research throughout the last few decades (Sengupta, 2017). Targeted therapies impede the specific proteins that promote cancer growth through unique and distinctive pathways (Sengupta, 2017). Each type of targeted therapy works a little differently, but all generally interfere with the ability of the cancer cells to grow, divide, repair and/or communicate with other cells, as depicted in Figure 3 (Sengupta, 2017). Some targeted therapies focus on the external components and function of the cancer cell, whereas others use small molecules that can actually enter into the cell and disrupt the function of the cells, causing them to die (Sengupta, 2017). Others target receptors that are on the outside of the cell (Sengupta, 2017). In summary, targeted therapies can function by any of the following mechanisms: (a) block or turn off chemical signals that tell the cancer cell to grow and divide, (b) alter proteins within the cancer cells so the cells die, (c) starve the tumor by cutting off blood supply and by preventing the formation of new blood vessels, (d) help the immune system to destroy cancer cells, (e) carry toxins or poison to the cancer cells directly, to kill them without harming healthy, normal cells (f) starve cancer of the hormones it needs to grow (Bar-Zeev et al., 2017; Sengupta, 2017).
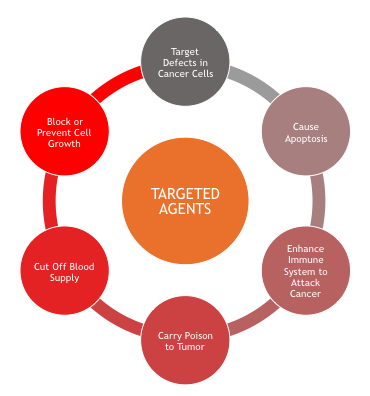
Figure 3. Targeted Agents Mechanism of Action (Sengupta, 2017).
By directing their effects at tumor cell growth through specific targets, these therapies are considered less toxic to normal cells and tissues than traditional chemotherapy agents (Olsen et al., 2019; Sengupta, 2017). However, the downside to targeted therapies is that they contain the potential for cancer cells to become resistant to them, as they block specific pathways of cancer growth (Sengupta, 2017). Since cancer cells are very astute, they are able develop many pathways of growth, thereby rendering the blocking of one pathway less likely to be effective as a sole agent (Olsen et al., 2019). Therefore, targeted therapies are purported to have greatest efficacy when combined with other cancer treatments, such as chemotherapy or radiation (Sengupta, 2017). However, while combination therapy has been deemed more effective, it does promote increased toxicity and potential for side effects (Sengupta, 2017). Further, despite major advancements within the field of targeted agents, they are generally not considered a replacement for traditional therapies at this point in time (Sengupta, 2017). While there are various types of targeted therapies, they are generally grouped into two broad categories: monoclonal antibodies and small molecule inhibitors (Olsen et al., 2019).
Monoclonal antibodies
Therapies that target receptors are also known as monoclonal antibodies. Some monoclonal antibodies are combined with toxins, chemotherapy drugs, and radiation (Olsen et al., 2019). Once these monoclonal antibodies attach to targets on the surface of cancer cells, the cells take up the cell-killing substances, causing them to die. Cells that don’t have the target will not be harmed (Sengupta, 2017) Antibodies are part of the adaptive immune system. Normally, the body creates antibodies in response to an antigen (such as a protein in a germ) entering the body (Olsen et al., 2019). The antibodies attach to the antigen in order to mark the antigen for destruction by the body's immune system (Sengupta, 2017). In the laboratory, scientists analyze specific antigens on the surface of cancer cells (target) to determine a protein to match the antigen. Then, using protein from animals and humans, scientists work to create a special antibody that will attach to the target antigen like a key fits a lock (Sengupta, 2017). This technology allows treatment to target specific cells, causing less toxicity to other, healthy cells (Sengupta, 2017). Monoclonal antibody therapy can be done only for cancers in which antigens (and the respective antibodies) have been identified. Monoclonal antibodies work on cancer cells in the same way natural antibodies work, by identifying and binding to the target cells, and then alerting other cells in the immune system to the presence of the cancer cells (Sengupta, 2017)
Monoclonal antibodies may be used alone or in combination with chemotherapy and are administered intravenously (Olsen et al., 2019).The way in which monoclonal agents are named provide clues into how they work (Olsen et al., 2019). The ending letters (called the stem) of the name indicates what family the drug is from and how the drug works to kill cancer cells (Olsen et al., 2019). Monoclonal antibodies always end with the stem “-mab”. The “-mab” family is used when receptor targets are overexpressed on the outside of cancer cells (Olsen et al., 2019). Monoclonal antibodies have an additional layer of classification within their ‘sub stem’, which represents how the drug was comprised (Olsen et al., 2019). Murine monoclonals, drugs ending in “-momab” (i.e. ibritumomab [Zevalin]), are composed of 100% mouse components and include conjugated antibodies, which are agents that are physically attached to antitumor agents such as radioisotopes, chemotherapy drugs, toxics or other biologic agents (Olsen et al., 2019). Chimeric monoclonals, drugs ending in “-ximab” (i.e. ituximab [Rituxan]), are primarily made of mouse protein with a (lesser) human protein component and therefore have a higher risk of hypersensitivity reaction among patients due to the exposure to the mouse component of the drug (Olsen et al., 2019). Humanized mouse monoclonals, drugs ending in “-zumab” (i.e. bevacizumab [Avastin]) include a small percentage of mouse components (or other protein) and the rest is human in origin. These medications generally induce less-severe allergic reactions (Olsen et al., 2019). Finally, human monoclonal antibodies, drugs ending in “-mumab” (i.e. panitumumab [Vectibix]) are fully human and reactions to these medications are rare. Some monoclonal antibodies boast additional information in their naming classification that designates the main target of the drug (Olsen et al., 2019). For example, the “li” in ipilimumab identifies the immune system as the target, where as the “tu” in rituximab indicates the target is the tumor and the “ci” in bevacizumab designates the circulatory system (Olsen et al., 2019). Table 3 displays an overview of several monoclonal antibodies categorized according to class, target, and mechanism of action (Olsen et al., 2019).Of note, the targets listed within Table 3, column 4 are receptors on the cell in which the drug has been specially designed to attack as a means of blocking cellular signals, thereby disrupting, or deactivating cancer growth (Olsen et al., 2019). Two very common targets of monoclonal antibodies include Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) (Olsen et al., 2019).
Table 3. Categories of Monoclonal Antibodies
Category and Composition | Stem | Examples | Target | Mechanism of Action |
Murine: All mouse components | -momab | Ibritumomab (Zevalin) | CD20
|
|
Tositumomab (Bexxar) | CD20 |
| ||
Chimeric: Primarily made of mouse protein with a (lesser) human protein component | -ximab | Rituximab (Rituxan) | CD20 |
|
Cetuximab (Erbitux) | Anti-EGFR |
| ||
Humanized: include a small percentage of mouse (or other protein) and the rest is human in composition | -zumab | Bevacizumab (Avastin) | Anti-VEGF |
|
Traztumab (Herceptin) | Anti-HER2 |
| ||
Alemtuzumab (Campath) | CD52 |
| ||
Human: Fully human Note: due to their composition as fully human agents, most drugs in this class will also fall within category of immunotherapy as described in the prior section | -mumab | Panitumumab (Vectibix) | EGFR |
|
(Olsen et al., 2019; Polovich et al., 2014).
As stated earlier, hypersensitivity reactions are common in many kinds of monoclonal antibodies, with the greatest risk among the Murine and Chimeric groups due to the large mouse composition of these drugs (Olsen et al., 2019). Conjugate monoclonal antibodies present the highest risk for bone marrow suppression and additional side effects since they are physically attached to a toxic or poisonous agent (Olsen et al., 2019). Hypersensitivity symptoms can present in the same manner as chemotherapy hypersensitivity reactions and may be mild with facial flushing, pruritus, and hives, or may progress to severe anaphylaxis requiring epinephrine (Olsen et al., 2019). Other potential adverse effects of monoclonal antibodies include dyspnea and wheezing, fever and chills, headache, rash, nausea and vomiting, tachycardia, and bleeding (Olsen et al., 2019). Since many patients endure rigors, shaking chills and fevers during the infusion, it is recommended that patients be pre-medicated with acetaminophen and an antihistamine (Olsen et al., 2019). The nurses must remain vigilant and attentive to patients receiving infusions with monoclonal antibodies, as rigors require interruption of the infusion and may require medication with agents such as meperidine (Demerol) or corticosteroids (Olsen et al., 2019). Many institutions have policies outlining the importance of slowly titrating the infusion rate of all monoclonal antibodies to reduce the risk of rigors, chills, and fevers (Olsen et al., 2019). Slowing down the infusion rate can also reduce the risk of prolonged rigors (Olsen et al., 2019).
CAR (Chimeric Antigen Receptor) T-Cell Therapy
According to Miliotou and Papadopoulou (2018), CAR T-cell therapy is a novel, but highly complex form of immunotherapy in which T-cells are isolated from a patient and genetically engineered to express a chimeric antigen receptor that recognizes tumor-specific antigen. They explain that the compound is then injected back into the patient and the CAR T-cells induce cancer cell death. They report that there are only a couple of types of CAR T-cell therapies approved by the U.S. FDA to date. One is for the treatment of aggressive, relapsed, or refractory B-cell acute lymphocytic leukemia, and another is for aggressive, relapsed, or refractory non-Hodgkin’s lymphoma (Miliotou & Papadopoulou, 2018). These agents target a receptor called CD19, which is found on the cell surface in these types of cancers and many other hematological malignancies (Miliotou & Papadopoulou, 2018). Miliotou & Papadopoulou (2018) emphasize that this therapy poses the risk of multiple potential toxicities that the nurse must be prepared to recognize and respond to quickly. They describe two of the major immune-related adverse effects associated with CAR T-cell therapy as cytokine release syndrome (CRS) and neurotoxicity. Cytokines are small protein molecules that are generally activated by a stimulus and induce response by binding to specific receptors and thus affect the growth and differentiation of white blood cells (Miliotou & Papadopoulou, 2018). They also regulate immune and inflammatory responses through enhancing cytotoxic activity by secreting additional mediators and amplifying the immune response when stimulated (Miliotou & Papadopoulou, 2018). CRS is characterized by symptoms of fever, nausea, vomiting, diarrhea, tachycardia, hypotension, tachypnea, and rash (Miliotou & Papadopoulou, 2018). In general, these symptoms occur approximately 1-14 days after the treatment is administered and is a byproduct of cell lysis (Miliotou & Papadopoulou, 2018). As cancer cells die, they release cytokines into the body which stimulate the above symptoms. Some patients may require hospitalization for supportive care, but most are managed on an outpatient basis (Miliotou & Papadopoulou, 2018). Complications of CRS may include renal dysfunction requiring dialysis, acute respiratory distress syndrome, or a decline in cardiac ejection fraction or stroke volume (Miliotou & Papadopoulou, 2018). A rare, but severe effect that has been reported with these medications is disseminated intravascular coagulation (DIC), which is a potentially life-threatening condition in which blood clots form in small blood vessels throughout the body in combination with associated bleeding (Miliotou & Papadopoulou, 2018). These blood clots can block blood flow and induce harm to major organs such as the kidneys and liver. Neurotoxicity may present in patients as delirium, confusion, seizures, tremors, encephalopathy, incontinence, and paralysis (Miliotou & Papadopoulou, 2018). The onset of these symptoms is unique and considered biphasic, as symptoms generally occur concurrently with fevers or sedating medication during phase 1 (days 0-5) or phase 2 (after day 5). Symptoms are usually reversible and last about 2-4 days but have been noted to persist for a few weeks (Miliotou & Papadopoulou, 2018).
Angiogenesis Inhibitors
Blood vessels carry oxygen and nutrients to tissue that are necessary for growth and survival (Nettina, 2019). In cancer, tumors need blood vessels in order to grow and spread. Angiogenesis is a process that occurs in both healthy and cancerous tissue to form new blood vessels by sending out signals (Olsen et al., 2019).. Angiogenesis is largely modulated through Vascular Endothelial Growth Factor Receptors (VEGF), which, as described earlier,are signal proteins that are produced to stimulate angiogenesis in both normal cells and cancer cells (Olsen et al., 2019). Anti-angiogenesis is the process of stopping the formation of new blood vessels by blocking the VEGF-receptors (Olsen et al., 2019). Angiogenesis inhibitors, or VEGF-inhibitors, are drugs that target the blood vessels that supply oxygen to the tumor cells, ultimately causing the cells to starve by cutting off their nutrient supply (Olsen et al., 2019).. VEGF-inhibitors such as bevacizumab (Avastin) work by cutting off blood supply to the cancer by interfering with the VEGF receptor, as without blood supply, tumors stay small and eventually die due to starvation Olsen et al., 2019).. Other types of angiogenesis inhibitors include interferon (Extavia) and thalidomide (Thalomid) (Olsen et al., 2019). The side effects of VEGF inhibitors can be severe and include bleeding, headache, hypertension and proteinuria (protein spilling out in the urine due to increased pressure in the kidneys) (Olsen et al., 2019).. Patients require strict blood pressure monitoring to ensure that treatment is only administered if blood pressure is less than 140/90 (Olsen et al., 2019).. Many patients require concurrent treatment with anti-hypertensives to maintain this range. A urinalysis must also be completed to assess for proteinuria prior to each dose, which is graded on a scale of 0-3+ (Olsen et al., 2019). Patients with protein of 2+ or greater on a simple urine dipstick test should undergo further assessment with a 24-hour urine collection, and treatment withheld for proteinuria ≥2 grams per 24 hours (Olsen et al., 2019).. Treatment may resume when proteinuria is <2 grams per 24 hours, but should be discontinued permanently in patients who develop the rare side effect of nephrotic syndrome (Olsen et al., 2019).
Given the risk for hemorrhage and delayed wound healing, it is contraindicated for patients to receive a VEGF-inhibitor within 6 weeks of any surgical procedure (preoperatively or postoperatively) (Olsen et al., 2019). There is a risk of fistula development, which is an abnormal connection between two hollow spaces within the body, such as blood vessels, intestines, or other organs. VEGF-inhibitors also carry a black box warning of bowel perforation, which is a hole in the lining of the intestines. Therefore, Olsen et al. (2019) recommend patients are counseled on the importance of monitoring for any of the following symptoms, which can vary and may come on slowly or rapidly:
- Abdominal pain, which is often severe and diffuse
- Severe abdominal cramping
- Bloating
- Nausea/Vomiting
- Rectal Bleeding
- Fever/chills (Olsen et al., 2019).
Small molecule inhibitors
Small molecule drugs are pills or capsules that are taken orally and work on specific pathways within the cancer (Bar-Zeev et al., 2017). Similar to monoclonal antibodies, the generic naming of small molecule inhibitors is strategically devised to provide clues into how these medications work (Bar-Zeev et al. 2017). Small molecule inhibitors end with the stem “-ib” and since this family of drugs targets processes inside the cell, they must be small enough in molecular weight to enter the cell and interfere with proteins on both the inside and outside of the cell. Some examples include: Tyrosine kinase inhibition—sub stem “-tinib” (i.e. imatinib [Gleevac]), proteasome inhibition—“-zomib” (i.e. bortezomib [Velcade]), and cyclin-dependent kinase inhibition (CDK 4/6)—“-ciclib” (i.e. abemaciclib (Verzenio) (Bar-Zeev et al., 2017). Currently, there are several small molecule inhibitors approved by the U.S. FDA and on the market for cancer therapy (Bar-Zeev et al., 2017). The most common molecular targets include tyrosine kinase inhibitors (TKIs), proteasome inhibitors, apoptosis targets, and matrix metalloproteinases (MMPs) and heat shock proteins (HSPs) (Bar-Zeev et al., 2017). Another important class of small molecule inhibitors that warrant mention are the ADP-ribose polymerase (PARP) inhibitors. These drugs block PARP, a protein that has a critical role in cell growth, regulation and repair, which help cancer cells repair themselves and survive; causing cancer cell death (Olsen, LeFebvre & Brassil, 2019). PARP-inhibitors have revolutionized the way in which BRCA–mutation positive cancers, such as ovarian cancer and breast cancers, are treated (Ring & Modesitt, 2018). The role of BRCA mutations in hereditary cancer development was described earlier in this module within the section entitled, “Pathophysiology of Cancer.” Table 4 displays some of the small molecule inhibitors used for cancer treatment, although this is not a comprehensive list.
Table 4. U.S. FDA-Approved Small Molecular Inhibitors for Cancer Therapy
Molecular Target | Drug | Specific Target/Pathway | Cancer Type |
Tyrosine Kinase Inhibitors (TKIs) | Erlotinib (Tarceva) Gefitinib (Iressa) |
|
|
Lapatinib (Tykerb) |
|
| |
Imatinib (Gleevac) |
|
| |
Everolimus (Afinitor) |
|
| |
Ruxolitinib (Jafaki) |
|
| |
Pazopanib (Votrient) |
|
| |
Regorafenib (Stivarga) |
|
| |
Vemurafenib (Zelboraf) |
|
| |
Crizotinib (Xalkori) |
|
| |
Proteosomes | Carfilzomib (Kyprolis) |
|
|
Bortezomib (Velcade) |
|
| |
Cyclin-dependent kinase inhibitors (CDK 4/6) | Palbociclib (Ibrance) Abemaciclib (Verzenio) |
|
|
ADP-ribose polymerase (PARP) Inhibitors | Olaparib (Lynparza) Rucaparib (Rubraca) Niraparib (Zejula) |
|
|
(Bar-Zeev et al., 2017; Olsen et al., 2019; Sasikumar & Ramachandra, 2018; Wu, Nielsen, & Clausen, 2015).
The side effect profile of these agents is distinctly different from cytotoxic chemotherapy (Bar-Zeev et al., 2017). Oncology nurses should be aware of the toxicities and management of adverse effects. While some of them can induce bone marrow suppression (i.e. palbociclib and abemaciclib), these drugs are generally not associated with as much risk for infection and febrile neutropenia as cytotoxic chemotherapies are (Bar-Zeev et al., 2017). The most common toxicities are dermatologic reactions, such as redness, swelling, pain, pins-and-needles on palms of hands and soles of feet, dermatitis and other skin rashes, pruritis, xerostomia (dry mouth), skin discoloration, hair thinning, and nail changes (Bar-Zeev et al., 2017). They can induce toxic effects on the liver and kidneys, so routine laboratory monitoring and clinical assessment are essential (Bar-Zeev et al., 2017).
Hormonal Therapy
Some cancers require certain hormones to grow (DeVita et al., 2015). Hormone therapies are a type of targeted therapy that primarily follow two basic mechanisms of action: (a) prevent the body from producing the hormones that drive cancer growth, and (b) prevent the hormones from reaching and acting on the cancer cells (DeVita et al., 2015). The most common hormone-dependent cancers include breast and prostate, but other potentially hormone-driven cancers include endometrial (uterine), kidney, and ovarian cancers (Itano, 2016). Adverse effects of hormone treatment depend on the type of drug, and can differ between men and women, but generally include: hot flashes, night sweats, loss of libido, weight gain, vaginal dryness/atrophic vaginitis, joint aches or pains, mood changes, weight gain, and thinning or weakening of the bones (osteopenia or osteoporosis) (Olsen et al., 2019). Men may additionally experience impotence (inability to have or maintain an erection), shrinking of the testicles, and gynecomastia (enlargement of breast tissue) (Olsen et al., 2019). Patients on hormonal therapy should be counseled on the importance of following a calcium-rich diet with at least 1,200 mg of dietary calcium daily. Patients who are unable to get this recommended amount of calcium in their diet should consider calcium supplementation (Olsen et al., 2019).
Table 5. Hormonal Therapies for Cancer Treatment
Category | Drug | Cancer Type(s) | Mechanism of Action |
Selective Estrogen Receptor Modulators (SERM) | Tamoxifen (Soltamox) |
|
|
Steroidal Antiestrogens / Selective Estrogen Receptor Degrader (SERD) | Fulvestrant (Faslodex) |
|
|
LH Luteinizing Hormone-Releasing Hormone (LHRH) Analog | Goserelin (Zoladex) Leuprolide (Lupron) |
|
|
LHRH Antagonists | Degarelix (Firmagon®) |
|
|
Aromatase Inhibitors (AI) | Letrozole (Femara) Anastrazole (Armidex) Exemestane (Aromasin) |
|
|
Anti-Androgens | Bicalutamide (Casodex) |
|
|
Androgen Receptor Blockers | Abiraterone (Zytiga) Enzalutamide (Xtandi) |
|
|
(DeVita et al., 2015; Olsen et al., 2019)
Oral Cancer Drugs
An oral cancer drug is any medication taken by mouth (in liquid, tablet, or capsule formulation) to treat cancer (Olsen et al., 2019). Advancement in cancer treatment has led to the development of many new oral agents to treat cancer, which offer the convenience of taking the medication at home, with less time spent traveling to and from the doctors’ offices and clinics (Olsen et al., 2019).. However, despite the popular misconception that oral cancer drugs are less toxic, the contrary is true and they are equally as strong and effective as intravenous or injected drugs (Olsen et al., 2019).. There has been a surge in the development of novel oral treatments, many of which are not classified as cytotoxic chemotherapy based on their mechanism of action, but still require the same special precautions and patient education. Therefore, the term oral cancer medication has largely replaced oral chemotherapy and will be used throughout this section (Olsen et al., 2019).
There are several special considerations with oral cancer treatments, as they pose unique safety challenges compared to traditional intravenous therapies. One of the most challenging issues with oral cancer treatment involves poor adherence, which has been consistently reported to have a substantial impact on the success of the treatment, side effects and toxicity, as well as patient safety. The NCCN (2019) established a task force to explore the impact of increased utilization of oral chemotherapy. They concluded that safety issues stem from a lack of checks and balances in administration, risk of patient noncompliance, lack of monitoring techniques, and a shift to patient management of oral chemotherapy (NCCN, 2019). Oral cancer medications are prescribed at defined intervals based on the mechanism of action of the drug, the drug’s half-life (the amount of time it takes for 50% of the drug to be excreted from the body), and side effect profiles (Weingart, Zhang, Sweeney, & Hassett, 2018). It is crucial that patients are educated to consistently take the medication as prescribed to ensure a constant level of the drug remains in the body to kill the cancer cells (Weingart et al., 2018). Even a slight increase or decrease in the dose level can be harmful and/or impact the drug’s efficacy, or can lead to increased side effects (Weingart et al. 2018). More is not always better when it comes to cancer treatment and medications can become dangerous if not taken exactly as prescribed (Weingart et al., 2018). Patients must be counseled not to crush, chew, or split oral cancer pills, as this can affect how the medication works (Olsen et al., 2019). Establishing a routine can help keep patients on track with their medication dosing schedule (Olsen et al., 2019). Some strategies may include: pill boxes that are filled each week, setting pill reminders on smartphones or tablets, enrolling in electronic medication reminders through a pharmacy, or using a simple paper pill diary, marking down when the pill was taken to avoid overdosing (Olsen et al., 2019).
Oral cancer drugs can be highly expensive and may not be covered fully by insurance and prescription plans (Elsaid, Gargullo & Collins, 2015). The financial burden is one of the most common reasons for noncompliance with oral medications among cancer patients (Elsaid et al., 2015). Patients should be encouraged to speak to their healthcare provider if they are having difficulty affording their medication prior to stopping (Elsaid et al., 2015). Some manufacturers offer co-pay assistance programs or have grants to fund free-drug/compassionate use (Elsaid et al., 2015). Some states have passed laws that require insurance companies to cover oral cancer medications in the same way they would cover intravenous cancer treatments (Elsaid et al., 2015).
Safe Medication Handling
Oral cancer drugs are potentially hazardous and require special precautions to stay safe when handling medications; especially for caregivers (Olsen et al., 2019). As described by the 2016 American Society of Clinical Oncology and Oncology Nursing Society chemotherapy administration safety standards, nurses should educate patients and caregivers on drug safety prior to oral cancer medications being prescribed (Neuss et al., 2017). These recommendations are summarized according to the administration standards with the bullet points listed below (Neuss et al., 2017).
General Safety Guidelines
- Keep cancer drugs in their original packaging until used or placed within the daily pill box.
- Do not mix chemotherapy medications with other medications in the pill box; they should always remain separate from other medications.
- Perform hand hygiene (soap and water) before and after handling all medications, even if wearing gloves.
- Be cautious not to let the medication come in contact with household surfaces (such as countertops, tables). If they do, clean the surface thoroughly to prevent contact with traces of the drug.
- Store oral cancer medications in a cool, dry place, away from excess heat or sunlight.
Safe Medication Disposal
- Never discard cancer medications in the trash, place down the drain, or flush down the toilet
- Ask a healthcare provider or pharmacist where to return unused medication, such as at a doctor’s office or pharmacy
- Empty pill bottles may be put in household trash but do not recycle them. Before throwing away, remove the label completely.
- Never reuse cancer medication pill bottles
Exposure to household contacts
- Whenever possible, the patient should handle the medication themselves.
- If anyone (other than the patient) comes in contact with oral cancer pills, they should be advised to wash with soap and water immediately. If any rash, irritation, or skin changes develop, they should contact the oncology office.
- Caregivers should transfer the medication into a cup or spoon. If they need to pick up the medication with their hand, they are advised to wear disposable gloves when handling medications to prevent any unnecessary exposure (i.e. absorption via the skin).
- Gloves should never be re-used, but instead should be discarded in household trash after one use, and trash should be double-bagged if there is contact with bodily fluids.
- In general, it takes approximately 48 hours for the body to excrete most cancer drugs. During this timeframe, a small amount of the medication can come out in the patient’s urine, stool, vomit, or blood. For patients who are continuously receiving oral cancer medication, there will be small amounts of drug in these body fluids at all times. Special precautions must be taken to protect caregivers and other household contacts.
- Caregivers should wear disposable gloves when handling any body fluids from patients taking oral (or intravenous) cancer drugs.
- Items soiled with body fluids should be kept in plastic bags until washed
- These items should be washed separately from other laundry in hot water
- Pregnant caregivers should be very cautious not to come in contact with oral cancer medications or bodily fluids
- Low-pressure toilets should be double-flushed after each use by people on oral cancer medications.
- Toilet lid should always be closed prior to flushing the toilet.
- If any fluids splash from the toilet, the surface should be wiped down with disinfectant cleaner
- Take precautions to ensure pets do not drink from the toilet
(Neuss et al., 2017; Olsen et al., 2019)
Box 1-2. Critical Consideration. It is crucial that patients are properly educated about the oral treatment plan, the proper administration schedule, storage, potential side effects, drug and food interactions, and when and how to contact their provider (Neuss et al., 2017).
Chemoprevention
Chemoprevention is the use of natural or synthetic substances to reduce the risk of developing cancer or to reduce the risk that a cancer will recur (Nettina, 2019). Only a few agents have been shown to decrease the risk of cancer in high-risk individuals, namely hormonal therapies used in breast cancer such as tamoxifen (Nolvadex) and raloxifene (Evista) (Rose et al., 2013). Tamoxifen (Nolvadex) was the first chemopreventive agent to be approved by the U.S. FDA for risk reduction of breast cancer (Pfeiffer et al., 2018). Tamoxifen (Nolvadex) is a selective estrogen receptor modulator (SERM) that has been widely used in the treatment of premenopausal women who are already diagnosed with breast cancer to prevent recurrence (Pfeiffer et al., 2018). More recent clinical trials have shown that Tamoxifen (Nolvadex) may reduce the risk of breast cancer by nearly 50% in women who are at high-risk but have not yet been diagnosed (Pfeiffer et al., 2018). Tamoxifen (Nolvadex) does carry a risk of rare, yet severe adverse effects of uterine sarcoma and venous thromboembolism/deep venous thrombosis (VTE/DVT) (Olsen et al., 2019). Therefore, these risks must be taken into consideration and patients must be counseled on these risks and must monitor for unilateral extremity swelling or postmenopausal bleeding (Olsen et al., 2019). Raloxifene (Evista) was initially approved for the treatment of osteoporosis but was later found to have similar impact as chemoprevention for breast cancer, without the risk of uterine sarcoma or VTE/DVT (Olsen et al. , 2019).
Third-generation aromatase inhibitors such as exemestane (Aromasin) and anastrozole (Arimidex) have been shown to reduce the risk of estrogen receptor (ER) positive breast cancer in postmenopausal women (Olsen et al., 2019). Adverse effects include joint aches and bone thinning (osteopenia or osteoporosis) (Olsen et al., 2019). Imiquimod (Aldara) cream, a topical agent, has been approved for the prevention of nonmelanoma skin cancer (Olsen et al., 2019). When applied to actinic keratosis, which are premalignant skin lesions, this agent helps to diminish the progression of the lesions to skin cancer (Olsen et al., 2019). These medications are often prescribed to patients after genetic counseling and evaluation of hereditary risk patterns for cancer based on family history and other risk factors (Olsen et al., 2019) Other domains of chemoprevention include:
- Aspirin— may decrease the risk of colorectal cancer.
- Metformin—may decrease the risk of breast, colon, endometrial, and possibly other cancers.
Statins—may reduce the risk of prostate, lung, colorectal, and breast cancers (Nettina, 2019)
Closing Thoughts
While the premise of this educational module was to focus on oncology prescribing and provide an overview of cancer treatments, there are several other cancer treatment modalities that were not reviewed, but are equally significant and warrant remark. Clinical trials are an essential component to cancer care and cancer research, as they comprise a large sector of the field of oncology. Without clinical trials there would be no new drug development and advancements in cancer care, such as the novel, cutting-edge field of immunotherapy. Supportive therapies and radiation therapy are two other subdivisions of cancer treatment which are equally extensive and merit discussion in future, separate activities.
References
Addington, J., & Freimer, M. (2016). Chemotherapy-induced peripheral neuropathy: An
update on the current understanding. F1000 Research, 5, 1466. doi: 10.12688/f1000research.8053.1
American Cancer Society. (2019). Cancer Factors & Figures 2019. Retrieved from
https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
American Society of Clinical Oncology. (2019). Quality Oncology Practice Initiative
(QOPI). Retrieved from https://practice.asco.org/quality-improvement/quality-programs/quality-oncology-practice-initiative
Angsutararux, P., Luanpitpong, S., & Issaragrisil, S. (2015). Chemotherapy-induced
cardiotoxicity: Overview of the roles of oxidative stress. Oxidative Medicine and Cellular Longevity, 2015, 1-13. doi: 10.1155/2015/795602
Bar-Zeev, M., Livney, Y. D., & Assaraf, Y. (2017). Targeted nanomedicine for cancer
therapeutics: Towards precision medicine overcoming drug resistance. Drug Resistance Updates, 31, 15-30. doi: 10.1016/j.drup.2017.05.002
Bluethmann, S. M., Mariotto, A. B., Rowland, J. H. (2016). Anticipating the “silver tsunami:
Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology Biomarkers Prev, 25(7), 1029-1036. doi: 10.1158/1055-9965
Brown, T. J., Sedhom, R., & Gupta, A. (2019). Chemotherapy-induced peripheral
neuropathy. JAMA Oncology, 5(5),750. doi: 10.1001/jamaoncol.2018.6771
DeVita, V. T., Lawrence, T. S., & Rosenberg, S. A. (2015). DeVita, Hellman, and
Rosenberg’s cancer principles & practices of oncology. (10th ed.). Philadelphia, PA: Wolters Kluwer Health
Elsaid, K. A., Gargullo, S., & Collins, C. M. (2015). Chemotherapy e-prescribing:
opportunities and challenges. Integrated Pharmacy Research and Practice, 4, 39-48. doi: 10.2147/IPRP.S84232
Gianotti, E., Razzini, G., Bini, M., Crivellaro, C., Righi, A., Darecchio, S., …Artioli, F.
(2019). Scalp cooling in daily clinical practice for breast cancer patients undergoing curative chemotherapy: A multicenter interventional study. Asia Pac J Oncol Nurs, 6(3), 277-282. doi: 10.4103/apjon.apjon_4_19
Itano, J. K. (2016). Core curriculum for oncology nursing. (5th ed.). (J. Brant, F. Conde, & M.
Saria, Eds.). St. Louis, MO: Elsevier
Konieczkowski, D., Johannessen, C. M., & Garraway, L. A. (2019). A convergence-based
framework for cancer drug resistance. Cancer Cell, 33(5), 801-815. doi: 10.1016/j.ccell.2018.03.025
Kreidieh, F. Y., Moukadem, H. A., & El Saghir, N. S. (2016). Overview, prevention and
management of chemotherapy extravasation. World Journal of Clinical Oncology, 7(1), 87-97. doi: 10.5306/wjco.v7.i1.87
Liede, A., Cai, M., Crouter, T. F., Niepel, D., Callaghan, F., & Evans, D. G. (2018). Risk-
reducing mastectomy rates in the US: A closer examination of the Angelina Jolie effect. Breast Cancer Research and Treatment, 171(2), 435-442. doi: 10.1007/s10549-018-4824-9
Miliotou, A. N., & Papadopoulou, L. C. (2018). CAR T-cell therapy: A new era in cancer
immunotherapy. Current Pharm Biotechnology, 19(1), 5-18. doi: 10.2174/1389201019666180418095526.
Naidoo, J., Page, D. B., Li, B. T., Connell, L. C., Schindler, K., Lacouture, M. E.,
…Wolchok, J. D. (2015). Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of Oncology, 26(12), 2375-239. doi: 10.1093/annonc/mdv383
National Comprehensive Cancer Network. (2019). NCCN Guidelines for Treatment
of Cancer by Site. Retrieved from https://www.nccn.org/
National Institute of Health, National Human Genome Research Institute.
(2014, April). The Human Genome Project. Retrieved from https://www.genome.gov/human-genome-project
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice. (11th ed.). Philadelphia,
PA: Wolters Kluwer.
Neuss, M. N., Gilmore, T. R., Belderson, K. M., Billett, A. L., Conti-Kalchik, T., Harvey, B.
E., …Polovich, M. (2017). 2016 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards,
including standards for pediatric oncology. Oncology Nursing Forum, 44(1), 1-13. doi: 10.1200/JOP.2016.017905
Olsen, M., LeFebvre K., & Brassil, K. (2019). Chemotherapy and Immunotherapy Guidelines
and Recommendations for Practice. (1st Ed.). Pittsburgh, PA: Oncology Nursing Society
Oncology Nursing Society. (2019a). ONS/ONCC chemotherapy immunotherapy
certificate course. Retrieved from https://www.ons.org/courses/onsoncc-chemotherapy-immunotherapy-certificate-course
Oncology Nursing Society. (2019b). Oncology Nursing Society. Retrieved from
https://www.ons.org
Pfeiffer, Y., Gut, S. S., & Schwappach, D. (2018). Medication safety in oncology care:
Mapping checking procedures from prescription to administration of chemotherapy. Journal of Oncology Practice, 14(4), e201-e210. doi: 10.1200/JOP.2017.026427
Polovich, M., Olsen, M., & LeFebvre, K. (2014). Chemotherapy and Biotherapy Guidelines
and Recommendations for Practice (4th Ed.). Pittsburgh, PA: Oncology Nursing Society
Rice, B. A., Ver Hoeve, E. S., DeLuca, A. N., Esserman, L. J., Rugo, H. S., & Melisko, M. E.
(2018). Registry study to assess hair loss prevention with the penguin cold cap in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat, 167(1),117-122. doi: 10.1007/s10549-017-4506-z.
Ring, K. L., & Modesitt, S. C. (2018). Hereditary cancers in gynecology, Obstetrics and
Gynecology Clinics, 45(1), 155-173. doi: 10.1016/j.ogc.2017.10.011
Rose, M. G., DeVita, V. T., Lawrence, T. S., & Rosenberg, S. A. (Eds.). (2013). Lippincott’s
primary care: Oncology in primary care (1st ed.). Philadelphia, PA: Lippincott Williams & Williams
Sasikumar, P. G., & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors
targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. doi: 10.1007/s40259-018-0303-4.
Sengupta, S. (2017). Cancer nanomedicine: Lessons for immune-oncology. Trends Cancer,
3(8), 551-560. doi: 10.1016/j.trecan.2017.06.006.
U.S. Department of Health and Human Services, Centers for Disease Control and
Prevention. (2019, April). HPV Cancers. Retrieved from https://www.cdc.gov/hpv/parents/cancer.html
U.S. Department of Health and Human Services, National Institute of Health, National Cancer Institute. (2017, November). Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
Weingart, S. N., Zhang, L., Sweeney, M., & Hassett, M. (2018). Chemotherapy medication
errors. Lancet Oncology, 19(4), PE191-E199. doi: 10.1016/S1470-2045(18)30094-9.
Wu, G., Wilson, G., George, J., Liddle, C., Hebbard, L., & Quao, L. (2017). Overcoming
treatment resistance in cancer: Current understanding and tactics. Cancers Letters, 387, 69-76. doi: 10.1016/j.canlet.2016.04.018
Wu, P., Nielsen, T. E., & Clausen, M. H. (2015). FDA-approved small-molecule kinase
inhibitors, Trends in Pharmacological Sciences, 36(7), 422-429. doi: 10.1016/j.tips.2015.04.005
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and
practice. (8th ed.). Burlington, MA: Jones & Bartlett Learning.