About this course:
The purpose of this course is to outline the roles and responsibilities of the advanced practice nurse when caring for patients and families considering and then undergoing organ donation to serve as an educator and liaison during this complicated and emotionally difficult period. This course also satisfies the state of New Jersey’s requirement for all professional nurses to complete one continuing education course that covers organ and tissue donation and recovery designed to address clinical aspects of the donation and recovery process.
Course preview
The purpose of this course is to outline the roles and responsibilities of the APRN caring for patients and families considering and then undergoing organ donation to serve as an educator and liaison during this complicated and emotionally difficult period. This course also satisfies the state of New Jersey’s requirement for all professional nurses to complete one continuing education course that covers organ and tissue donation and recovery designed to address clinical aspects of the donation and recovery process.
At the completion of this activity, the learner should be prepared to:
- define the relevant terms and discuss the national and worldwide statistics regarding organ donation and its inherent value to society
- review eligibility criteria for the donation of tissue and organs
- recognize which organs and tissues are typically donated
- define the process of living organ donation, donation after circulatory death (DCD), and donation after brain death (DBD)
- illustrate how to engage with a donor’s family during interdisciplinary discussions regarding the donation of tissue and organs after a loved one has been deemed eligible
- explore the evidence-based recommendations regarding the assessment and care of a potential organ donor immediately prior to organ procurement
- discriminate between common myths and facts as well as barriers and facilitators of organ and tissue donation in order to better understand the process professionally as well as educate patients and their families
- identify some ethical concerns and research questions that remain to be answered in the field of transplant medicine
Definitions and Statistics
The dead-donor rule refers to a longstanding ethical principle forbidding organ removal if it will result in the death of the donor (Mezrich & Scalea, 2015; Rosenbaum, 2020).
The cold ischemia time refers to the time between organ retrieval and implant, during which period the organ is maintained at cold temperatures to preserve tissue integrity (Health Resources and Services Administration [HRSA], 2020a; Tullius & Rabb, 2018).
Donation after brain death (DBD) refers to the use of the American Association of Neurology (AAN) definition of brain death (irreversible coma due to a known cause, brainstem areflexia, and apnea) prior to consideration of eligibility for organ and tissue recovery (Lewis et al., 2020; Starr et al., 2020).
Donation after cardiac death (DCD) refers to the irreversible cessation of circulatory and respiratory functions due to cardiac arrest or severe, advanced heart disease prior to consideration of eligibility for organ and tissue recovery (Lewis et al., 2020; Nursing@Georgetown, 2018; Starr et al., 2020).
An early transplant refers to a transplant in a recipient shortly after organ failure (Lewis et al., 2020).
An imminent death donation is a process whereby patients with a terminal condition who are in the process of dying consent to having their organs donated prior to their death (Rosenbaum, 2020).
A preemptive transplant refers to a transplant in a recipient with chronic organ disease (e.g., chronic kidney disease [CKD]) but who is not yet in end-stage organ failure (e.g., requiring dialysis; Lewis et al., 2020).
A specified direct donation involves the donation of an organ to a specified recipient by a living donor within their family (Lewis et al., 2020).
A specified indirect donation involves the donation of an organ to a specified recipient by an unrelated living donor through an exchange program (Lewis et al., 2020).
An unspecified donation involves the anonymous living donation of an organ between a donor and recipient that are unknown (Lewis et al., 2020). Transplant centers and organ procurement organizations (OPOs) are obligated by HIPAA regulations to protect the identity of both parties. The only circumstance under which contact information for either party may be exchanged is if both parties agree to an exchange of contact information. More often, grateful recipients interested in thanking a donor or a donor’s family will typically correspond with a note that is delivered via an intermediary, such as the OPO or the transplant center itself (HRSA, 2020a).
The warm ischemia time is the time spent without blood flow while the organ is still inside the patient. In DCD, this time begins with the withdrawal of life support and extends until organ procurement/preservation in the operating room. This includes two consistent or predictable periods (the 5-minute waiting period and the time spent prepping the patient for surgical organ retrieval) as well as the unknown factor of how long after removal of artificial life support before cardiovascular death occurs. In DBD, this period is significantly shorter, as DBD patients typically remain on circulatory/ventilatory support up until the time of organ retrieval. In both circumstances, warm ischemia time is also accrued as the organ is being rewarmed and prepared for implant following organ transport to the recipient’s location (Hong et al., 2011; Mezrich & Scalea 2015; Serri & Marsolais, 2017).
Myths and Facts Regarding Organ Donation
The following are common myths concerning organ donation that the nurse should be aware of in order to better educate patients and families as they consider the possibility of donating their organs and tissue.
Myth: If an individual agrees to donate their organs, the medical team will not try to save their life.
Fact: The effort to save a patient’s life is never halted prematurely based on their organ donor status (Mayo Clinic Staff, 2019).
Myth: What if the patient is not really dead yet when they start to remove their organs?
Fact: Organ donors are tested more rigorously to determine that they are, in fact, dead and potentially able to donate their organs than other patients (Mayo Clinic Staff, 2019).
Myth: Organ donation is against the religious beliefs of many common religions.
Fact: As mentioned earlier, most major world religions support organ donation, including Roman Catholicism, Islam, most Protestant faiths, and most branches of Judaism (Mayo Clinic Staff, 2019).
Myth: Individuals under the age of 18 are too young to make this decision.
Fact: The ability to register as an organ or tissue donor under the age of 18 varies by state, and the final consent will be the responsibility of the legal parent or guardian. However, those under the age of 18 wishing to donate organs or tissues should discuss their wishes with their family so that they are aware of how they feel about it (Mayo Clinic Staff, 2019).
Myth: Organ and tissue donation eliminates the possibility of an open casket viewing or funeral.
Fact: Organ and tissue donation usually does not interfere with funeral arrangements, although the process may affect the timing slightly. Once clothed, organ donors have no visible signs of having undergone donation (Mayo Clinic Staff, 2019).
Myth: Older patients are often too old to donate their organs.
Fact: There is no specific age cutoff for organ donation, especially following brain death (Mayo Clinic Staff, 2019).
Myth: Many patients are too sick to donate their organs.
Fact: Very few medical conditions are contraindications for organ donation, and in many circumstances, there are some tissues or organs that may still be healthy enough for transplantation (Mayo Clinic Staff, 2019).
Myth: Many folks are interested in donating one of their kidneys to someone now, but they don’t have any family members in need, so they don’t have the opportunity.
Fact: Living kidney donations between friends or complete strangers can now be performed th
...purchase below to continue the course
Myth: Many individuals are afraid to donate one of their kidneys. Don’t people need both of their kidneys for themself?
Fact: The absolute risk of developing ESRD following the donation of a kidney is less than 1% (Thomas et al., 2019). Extensive testing is completed on living donors prior to donation to ensure that the donor is not seeking secondary gain, that their organs are healthy enough to be donated, and that they are healthy enough to live without their retrieved organ following the surgery (Mayo Clinic Staff, 2019).
Myth: Organs are given first to the rich, famous, and powerful.
Fact: UNOS uses medical algorithms to assess relative need and stratifies potential organ recipients based on numerous objective factors; a patient’s wealth, power, and celebrity are not considered as factors in these decisions (Mayo Clinic Staff, 2019).
Myth: Organ donation is expensive.
Fact: The final efforts to resuscitate a patient at the end of their life may be expensive and are often misinterpreted as costs related to organ donation, but these are separate. The costs related to organ retrieval are incorporated into the cost of the organ transplant process for the recipient and are not charged to the donor (Mayo Clinic Staff, 2019). Living donations are similarly paid for using the insurance (or Medicare) coverage of the recipient. However, post-donation insurance coverage is oftentimes inadequate. Many organ donation advocates are working towards increased funding for potential living organ donors to cover the indirect costs of donation, such as lost wages, travel, and childcare during surgical recovery, through NLDAC and similar organizations. As mentioned earlier, this was one of the issues addressed within the 2019 executive order regarding American Kidney Health (HRSA, 2020a; Lewis et al., 2020; Thomas et al., 2019).
The History of Organ Donation in the United States
The National Organ Transplant Act was first passed in the United States in 1984 and established the Organ Procurement and Transplantation Network (OPTN). The act specifies that the network must be run by a private, non-profit organization under a federal contract. The United Network of Organ Sharing (UNOS, n.d.) was granted this federal contract in 1986 and has maintained it ever since. In the US, approximately 20 people die each day while awaiting an organ transplant (Mayo Clinic Staff, 2019). The US utilizes an opt-in model of consent for organ donation, meaning that organ donors are not assumed to have consented; Americans must register/otherwise legally express their consent for organ donation prior to the procedure. Spain utilizes an opt-out, or presumed consent, model for deceased organ donation (Childress, 2017). Only half of the US population is currently registered with their state or a national registry as a potential organ or tissue donor. Of those awaiting an organ transplant, nearly 83% are awaiting a kidney, 12.3% a liver, 3.4% a heart, while the rest are awaiting a pancreas, lungs, or intestines (Nursing@Georgetown, 2018). In 2017, more than half of the transplants performed in the US were kidney transplants (57%, or 34,700). Unfortunately, while the number of transplants nationwide has increased by 30% since 1998, the number of awaiting recipients on the waitlist has more than doubled (Tullius & Rabb, 2018).
Kidney disease currently affects 30 million Americans. In 2016, the estimated cost to manage Medicare patients with kidney disease totaled $114 billion. The federal government established an executive order in July of 2019 in attempt to further improve the kidney health of Americans. It was organized into three main goals:
- to prevent CKD and end-stage renal disease (ESRD) through enhanced diagnosis, early treatment, and incentivizing preventative care;
- to increase patient choice with affordable alternatives for the management of ESRD by encouraging high-value care and the development of an artificial kidney;
- to modernize organ recovery and transplantation and updating regulations in the US (Thomas et al., 2019).
The executive order requested changes in the payment model for Medicare patients with CKD to incentivize home dialysis and other alternative treatments and encouraged the biotechnology sector to develop models for an artificial kidney. It endorsed the development of more transparent, reliable, and objective metrics to measure OPOs and streamline the matching and delivery of donated organs, thereby reducing the discard rate (Trump, 2019).
In 2016, over 27,000 organs were donated from nearly 10,000 deceased donors, in addition to nearly 6,000 living organ donations (Childress, 2017). UNOS reports that in 2019, the country performed 39,718 transplants, an 8.7% increase from 2018. This is the 7th consecutive year in a row with an increase over the prior year. This includes over 30,000 transplants from deceased donors and 7,397 living donations. This constitutes a record number of living donors, beating the prior record set in 2004 (UNOS, n.d.) There are an estimated 30,000 tissue donors annually in the US (HRSA, 2020b). Despite all of this apparent success with organ donation in our country, there are over 108,000 patients currently on the waiting list for an organ donation of some sort (OPTN, n.d.). Nurses play various key roles throughout the organ donation process, including serving as a patient and family advocate, referring potential donors, providing family support, and providing comfort care to the donor (Dopson & Long-Sutehall, 2019).
Who Can Donate?
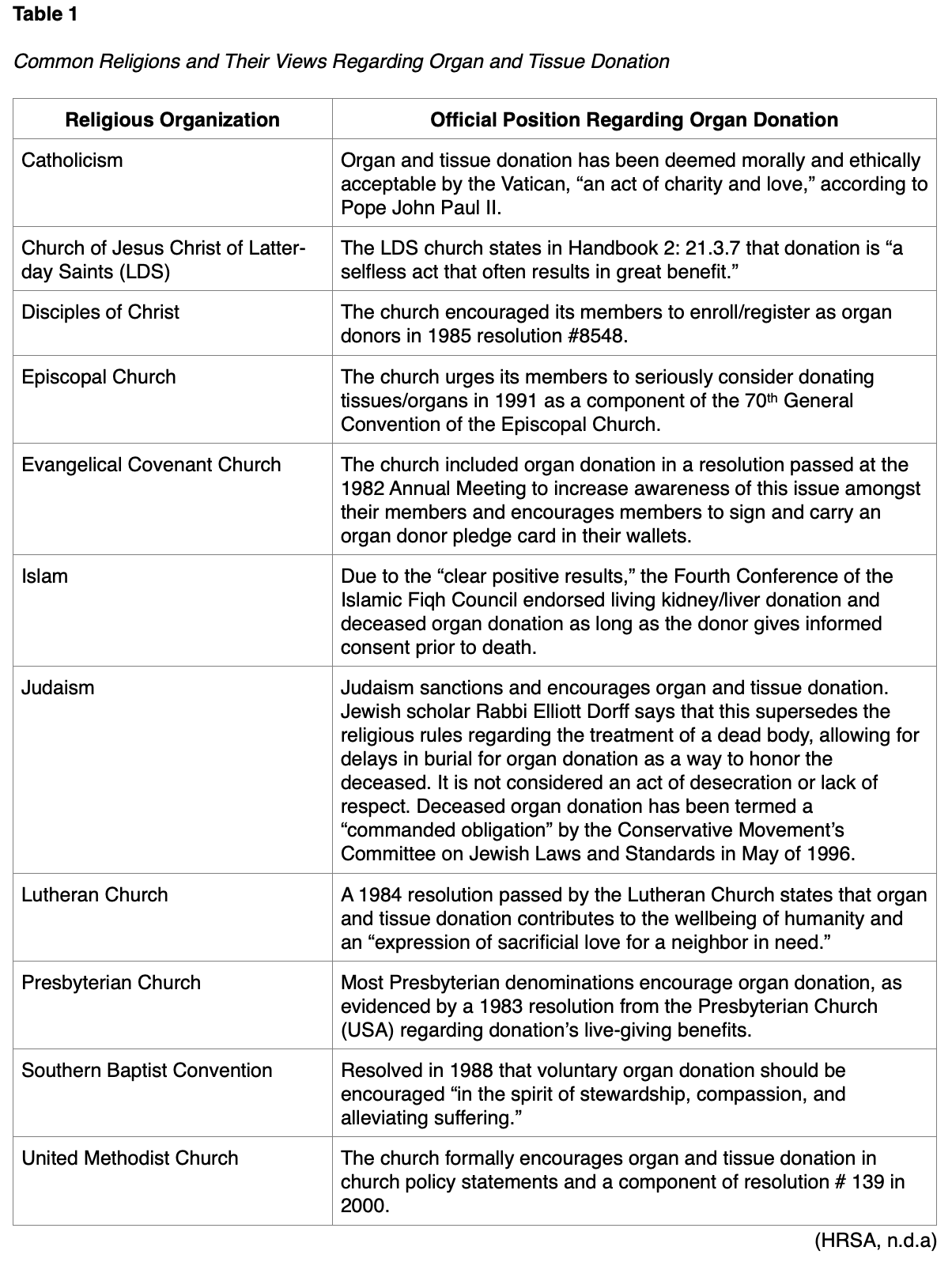
Most individuals can donate, with rare exceptions related to systemic infection (e.g., sepsis, bacteremia) and active cancer (HRSA, n.d.b; Rosenbaum, 2020). In specific circumstances, some organs and tissues can be donated despite these conditions. Age is not a disqualifying factor, as neonates and older adults (into their 90s!) have donated organs and tissues (HRSA, n.d.b). Organs donated from older donors carry an increased level of risk and somewhat decreased function, limiting viable donations to the kidneys and the liver in most older adults (Tullius & Rabb, 2018). Pediatric patients (under the age of 18) require informed consent from an adult guardian or parent to donate tissues/organs. While religious beliefs are a common concern amongst prospective donors, the vast majority of religions allow organ and tissue donation as a selfless gift of life. The specifics regarding a handful of common religious organizations and their views regarding organ donation are discussed in Table 1 (HRSA, n.d.b).
Once a potential donor has been identified, their risk for hepatitis C virus (HCV), HIV, and hepatitis B virus (HBV) transmission should be assessed. Those at increased risk include those who utilize IV recreational drugs and those who engage in high-risk sexual activities (multiple sexual partners, unprotected sexual intercourse, and men who have sex with men; CDC, 2019; WHO, 2019a, 2019b). If determined to be at increased risk, nucleic acid testing can be performed to test for the three infections. In recent years, advances in nucleic acid testing and antiviral treatment for HCV have allowed for donors who are at increased risk for the infection to be considered for organ and tissue donation. With informed consent, donors found to be positive for HCV can either donate organs to recipients already known to be infected with HCV or uninfected recipients willing to undergo antiviral treatment after receiving the organ. The antiviral treatment protocols for organ recipients are able to achieve undetectable levels of HCV in over 95% of recipients after 12-24 weeks of treatment. Similarly, donors found to be infected with HIV or HBV may still be permitted to donate organs to recipients with known HBV or HIV infection, assuming informed consent (Thomas et al., 2019; Tullius & Rabb, 2018).
What Can Be Donated?
Solid organs are most often donated by deceased donors, although living donors can donate one of their kidneys, one of their lungs, or a portion of their pancreas, liver, or intestines. Living donors can donate blood or platelets via venipuncture, or stem cells may be donated via bone marrow, umbilical cord blood, or peripheral blood (with pharmacological provocation using filgrastim [Neupogen]). Filgrastim (Neupogen) stimulates the proliferation, differentiation, and maturation of neutrophils, generating an increased number of mature circulating neutrophils. Deceased donors can potentially donate their kidneys, liver, lungs, heart, pancreas, and intestines. The hands and face were added to the organ transplant list in 2014. These vascularized composite allografts (VCAs) are rare and complicated procedures involving the grafting of bones, skin, muscle, nerves, and blood vessels. As of 2018, less than 200 VCAs had been performed worldwide. Deceased donors can also donate both of their corneas, the clear part of the eye covering the iris and pupil that may become damaged or scarred due to eye disease, injury, or congenital defects. After the donor’s organs have been retrieved, various tissues can also be donated within 24 hours of death and stored in tissue banks to restore vision, repair hearts, protect burns, replace veins, or repair damaged connective tissue and cartilage during various orthopedic surgical procedures. Some of the tissues that may be used include the donor’s heart valves, skin, bone, and tendons (HRSA, 2020a, 2020b).
Each organ that is donated is matched independently with the most suitable recipient, except for heart/lung and pancreas/kidney transplants, which may be matched as a set. The matching process is done through the OPTN by UNOS and in collaboration with local OPOs. The match is based on blood type, body size, the severity of the recipient’s medical condition, the geographic distance between the donor and the recipient, the length of time that the recipient has been on the waitlist, and the recipient’s immediate availability to undergo surgery (HRSA, 2020a).
Additional specific details are also considered with varying importance for each organ donated. When a heart or lung is being donated, the maximum cold ischemia time is 4 to 6 hours, although some experts cite 4 hours as the maximum time allowed for a donated heart. Potential heart recipients are rated with a status code, but physical location is also crucial due to the limited time constraints. Body size is also considered important for heart recipients (HRSA, 2020a; Tullius & Rabb, 2018). A heart transplant is the treatment of choice for patients with severe dilated or restrictive cardiomyopathy, end-stage heart disease secondary to coronary artery disease (CAD), valvular disease, or congenital heart disease. To be considered for a heart transplant, recipients typically have less than a 12-month life expectancy, age under 65 years, New York Heart Association (NYHA) class III or IV heart failure, normal or only slightly elevated pulmonary vascular resistance, no active infection, stable psychosocial status, and no active drug or alcohol use. Unfortunately, heart transplant recipients' 10-year survival rate is only 50%, as most develop diffuse plaque formation in their coronary arteries, termed coronary artery vasculopathy or CAV. This can be especially insidious, as heart transplant patients cannot sense pain in their transplanted organ and therefore do not present with angina as an early warning sign, such as is seen in native heart disease. This plaque formation can be prevented through active lifestyle choices and taking calcium channel blockers (CCB). Patient education regarding the importance of faithfully maintaining medication regimen adherence and psychosocial support in the form of support group participation and individual psychotherapy is crucial (Ignatavicius et al., 2018).
Similarly, lung recipients are matched based on laboratory results, underlying diagnosis, body size, and physical location. Potential liver recipients are scored using the model for end-stage liver disease (MELD) or pediatric end-stage liver disease (PELD) to indicate the severity of need. While the geographic distance between the donor and recipient is a major factor, a liver may remain viable despite a cold ischemia period of 8 to 12 hours, extending the potential region further than heart and lung donors. Kidneys are matched based on the blood type, the amount of time the recipient has been waiting, pediatric status (pediatric recipients are prioritized over adults), and body size. The donor is assigned a kidney donor profile index (KDPI) score, which is a numerical score based on ten key factors that collectively indicate the expected relative quality of the kidneys. Donors and recipients are also evaluated for lymphocytotoxic crossmatch (this must be negative for a match to occur) and the number of human leukocyte antigens (HLAs) in common between the pair. Since kidneys have the longest maximum cold ischemia window (24 to 36 hours), the geographic area is less concerning when matching kidney donors with potential recipients. Pancreas and intestine donations are rare. Pancreas donations are matched based on blood type and the amount of time the recipient has been on the waitlist and are often transplanted in conjunction with a kidney. The maximum cold ischemia time allowed for a pancreas donation is 12 to 18 hours. Intestines are matched based on the relative risk of graft versus host disease (GVHD) in the potential recipient as evidenced by ABO blood type due to the high risk associated with these donations. Additionally, the recipient of an intestine transplant must be slightly larger than the donor to allow for sufficient space for the organ placement, and the donor and recipient must align regarding their previous exposure to cytomegalovirus (CMV) and Epstein Barr virus (EBV). Intestine donations have a maximum cold ischemia time of 8 to 16 hours, although some sources establish a limit of 12 hours (HRSA, 2020a; Thomas et al., 2019; Tullius & Rabb, 2018). Organ donors and recipients may also be paired based on age and longevity. Older patients, those who have been on the waiting list for an extended period, or those with serious chronic comorbid conditions (e.g., diabetes mellitus) may be offered suboptimal or marginal quality organs. This is due to the fact that kidney transplant recipients have increased survival rates if they are transplanted after 1 year using an organ approved with expanded criteria, versus waiting for 3 years and then receiving a higher quality kidney based on the standard criteria (Tullius & Rabb, 2018).
The Donation Process
A living organ donation involves the transplantation of an organ, usually a kidney or portion of a liver. This procedure was first performed between a pair of twins in 1954. For an optimal match of immunological factors and blood type, living donations are most often performed between family members. Organs procured via living donations are preferred over organs from deceased donors, as they are not subject to the tissue damage incurred from decreased blood flow, decreased oxygenation, and increased inflammatory proteins associated with the dying process (Mezrich &Scalea, 2015). The cost to the donor that is associated with a living organ donation can be a deterrent. The direct medical costs are covered by the recipient and their medical insurance provider. The National Living Donor Assistance Center (NLDAC) supports donors with funding for additional expenses, such as travel, lodging, and meals (HRSA, 2020a).
The first successful organ donation from a deceased donor was in the 1980s secondary to the development of cyclosporine (Sandimmune). The two currently available methods for deceased donation in the US include DCD and DBD (Mezrich & Scalea, 2015). These two distinct methods will be discussed in detail, as well as what differentiates the two. The initial regulations for determining death in a potential organ donor were addressed in the Uniform Determination of Death Act in 1980 by the National Conference of Commissioners on Uniform State Laws and adopted by both the American Medical Association (AMA) and the American Barr Association. Death was defined as “the irreversible cessation of circulatory and respiratory functions” or “of all functions of the entire brain” (Starr et al., 2020).
DCD refers to the donation of organs following the withdrawal of life support in patients who have suffered a severe neurological injury and are unable to survive without life support, yet do not officially meet the qualifications of brain death (Mezrich & Scalea, 2015). This often occurs after cardiac arrest or due to severe heart disease (Nursing@Georgetown, 2018). Research suggests performing a multimodal neurological prognostication after all instances of significant cardiac arrest, but not before an initial 72 hour waiting period. The American College of Cardiology (ACC) and the American Heart Association (AHA) both recommend that cardiac arrest patients be evaluated for the potential of organ donation. In 5-10% of cardiac arrest patients, the cerebral edema is so severe that they meet the criteria for DBD (see these below). Unfortunately, the DCD process in cardiac arrest patients typically precludes the use of the heart itself (unless the warm ischemia time is less than 30 minutes; Serri & Marsolais, 2017). In order for any of the organs to be viable for donation, the patient must expire within 120 minutes following the withdrawal of life support (Mezrich & Scalea 2015; Serri & Marsolais, 2017). In about one-third of cases, none of the available organs are viable for donation due to inflammatory and hypoxic tissue damage (Rosenbaum, 2020).
When the medical community in the US was standardizing a method for DBD, a universal definition of brain death had to be established first. A patient is defined as being in a persistent vegetative state if their brain no longer performs any cortical function, but the brainstem's function remains intact. Conversely, brainstem death involves a lack of brainstem reflexes despite a few cortical functions and hypothalamic integrity (such as osmoregulation). Finally, brain death or whole-brain death involves the biological death of the entire brain, with no cortical or brainstem functions remaining. The brainstem reflexes include:
- the corneal reflex causes the eyelid to blink after lightly touching the cornea
- the pupillary light reflex causes pupillary constriction with direct bright light
- the oculocephalic reflex causes rotation of the patient’s gaze in the opposite direction when the head is rotated to one side or the other briskly
- the oculovestibular reflex causes eye movement when 50 mL of ice water is infused into the patient’s ear canal
- the gag reflex causes throat constriction (a gag) after stimulation of the posterior pharynx with a spatula
- the cough reflex causes a cough after stimulation of the carina with a bronchial catheter
- the withdrawal reflex to noxious stimuli along the route of cranial nerves (Munakomi & Al Khalili, 2020).
DBD occurs commonly after trauma, stroke, aneurysm rupture, or due to brain tumor (Nursing@Georgetown, 2018). To qualify, the patient must satisfy the three diagnostic qualifications established by the American Association of Neurology (AAN) in 1995, which was last revised and reviewed in 2010. The three diagnostic criteria include a patient who is in a coma with a known underlying cause, the absence of any brainstem reflexes, and the presence of apnea (Starr et al., 2020; Wijdicks et al., 2010). The underlying cause of the patient’s coma should be established, and this can be done via the patient’s history, examination, imaging studies, and laboratory results. They also found insufficient evidence to determine the minimal observation period, but typically the patient is observed for several hours. Brainstem reflexes should be established using physical examination (Wijdicks et al., 2010). Patients with pentobarbital (Nembutal) in their system may have an unreliable physical exam (Neurocritical Care Society [NCS], 2017).
Finally, apnea testing should be performed by the attending physician or the institutions designated intensivist trained in such testing procedures. According to the AAN, apneic oxygenation diffusion testing to determine apnea is safe, there is insufficient evidence to recommend a particular technique. The AAN recommends that the medical team establish normothermia (at least 36 °C), normotension (systolic blood pressure [SBP] of at least 100 mm Hg), euvolemia, and eucapnia (PaCO2 35-45 mm Hg) prior to apnea testing. Patients who are hypoxic or with prior evidence of CO2 retention (history of chronic obstructive pulmonary disease [COPD] or obesity) may not perform as expected on apnea testing. Patients should not be given any central nervous system depressants or neuromuscular blockers prior to apnea testing. Severe electrolyte, acid/base, and endocrine abnormalities may also interfere with apnea testing and should be normalized prior to testing if possible. The patient should be oxygenated for 10 minutes prior to the start of the test with 100% O2 to establish a PaO2 greater than 200 mm Hg. The ventilatory should be reduced to 10 breaths per minute (bpm) with a reduced positive end-expiratory pressure (PEEP) of 5 cm H2O. If that patient’s pulse oximetry reading remains over 95%, the baseline blood gas panel should be drawn to establish the patient’s baseline PaO2/PaCO2, pH, bicarbonate, and base excess). The ventilator should be disconnected, and 100% O2 delivered at 6 L/min via an insufflation catheter placed through the endotracheal tube (ET tube) to the level of the carina. The patient should be observed for 8-10 minutes to assess for any indications of spontaneous respirations. The test should be aborted for a decrease in SBP below 90 mm Hg or a pulse oximetry reading under 85% for at least 30 seconds. In this scenario, the test can be restarted after repeating the preoxygenation step; the repeat testing should be performed using a T-piece, continuous positive airway pressure (CPAP) at 10 cm H2O, and 100% O2 delivered at12 L/min. Once the test can be completed successfully for 8-10 minutes with no indications of spontaneous respiration, the blood gas panel should be redrawn to prove that the PaCO2 level is greater than or equal to 60 mm Hg or increased from the baseline level by at least 20 mm Hg, which indicates a positive apnea test and confirms brain death. Alternately, the same test can be repeated for 10-15 minutes if the results are initially inconclusive, assuming the patient remains stable (Munakomi & Al Khalili, 2020; Wijdicks et al., 2010). The medical team should keep in mind that the underlying purpose of the apnea test is to confirm a lack of inhalation, not the increase in PaCO2. This level merely reassures the medical provider that the patient has sufficient stimulus for spontaneous respiration. Similarly, patients on ECMO may undergo the apnea test by decreasing the flow significantly enough to allow the PaCO2 to increase to 60 mm Hg, or via an ancillary test as described below (NCS, 2017).
If the patient’s apnea test cannot be performed, the AAN recommends older and more established ancillary testing to shorten the observation period, such as a 30-minute EEG, cerebral angiography, or a nuclear scintigraphy cerebral brain flow study. They found insufficient evidence to confirm the accuracy of newer ancillary tests such as magnetic resonance imaging (MRI), MR angiography (MRA), computed tomography angiography (CTA), somatosensory evoked potentials (SSEPs) via nasopharyngeal electrode recording, and bispectral index. Ancillary testing should not replace the clinical examination (Munakomi & Al Khalili, 2020; NCS, 2017; Wijdicks et al., 2010). The use of ancillary testing, even a CT scan with subsequent flow study, has not been sufficiently validated. Flow studies are considered more reliable in patients on pentobarbital (Nembutal) than EEG if ancillary confirmation is needed (NCS, 2017).
Discussions with Family
Federal and state laws require hospitals to contact the local assigned OPO after brain death has been established (HRSA, 2020a; Wijdicks et al., 2010). A consultation with the OPO is recommended before discussing or even mentioning the possibility of organ donation to a patient or family member; however, this possibility should be considered for every patient who might qualify to increase the overall rates of organ donation worldwide (Finger Lakes Donor Recovery Network, 2015; NCS, 2017; Shemie et al., 2017). Typically, the OPO will ask the medical team several initial screening questions about the patient and their medical status to determine if they are a potential donor, and then assign a representative to travel to the hospital directly to assist if the screening questions indicate a potential for donation. The OPO representative will search the state database of registered organ donors and the department of motor vehicle records regarding organ donor status. They will collect the medical and social history via the electronic medical record and confirm the information with the family when appropriate (HRSA, 2020a). Some OPOs allow the nurse or other trusted medical professional to deliver a premention to the family to gradually and carefully introduce the idea of organ donation to the family while awaiting the OPO representative’s arrival. This may include asking if the family is aware of the patient’s organ donation registration status or their advance directive specifications, or generic comments such as “many people in your situation see organ donation as a way to have something positive come out of tragedy.” This premention should not be done prematurely, however, as the family may become disappointed if they later determine that their loved one is not eligible to donate their organs. Once the OPO representative arrives, the care team should inform them regarding the patient’s current status, what the family has/has not been told, what their response was, who the primary decision-maker is within the family unit, and any specific religious or cultural considerations (Finger Lakes Recovery Network, 2015). This “team huddle” should include the OPO representative, the primary nurse, and the medical attending at a minimum. Other topics that should be discussed before meeting with the family include plans for the family meeting, such as who will be in the room and responsible for leading the conversation (Shemie et al., 2017).
When the conversation regarding organ donation is had with a potential donor family, this conversation must be separated in both time and space from the conversation regarding their loved one’s prognosis. For example, if life support will be withdrawn or if brain death has been confirmed, these conversations should happen separately from (and before) any conversation regarding organ donation. This concept is called decoupling. When decoupling does not occur, the consent rate for donation decreases by roughly one-third. Families must be allowed time to process and accept their loved one’s prognosis before being asked to discuss the next steps. For potential DBD donors, this means the family should fully understand the concept of neurological death. For potential DCD donors, this means that the family should have already discussed and accepted their loved one’s prognosis and made the difficult decision to withdraw support (Serri & Marsolais, 2017; Shemie et al., 2017). Studies indicate that the perceived skill and personal relationship of the healthcare provider with the family are important factors cited by families during the discussion/decision to donate organs of a deceased loved one. Trust must be established first. Organ donation conversations should be held in private, and the healthcare provider(s) present should be very knowledgeable regarding the donation process to quickly and confidently answer the family’s questions (Serri & Marsolais, 2017). An invitational forum in Canada regarding organ donation discussions with patient families determined that the medical team should be primarily focused during these conversations on being collaborative, compassionate, supportive, and informative regarding the process and inherent value of organ donation. These conversations are best had using a multidisciplinary team, and some recommend that a different group of providers should be present for the organ donation conversation than was present for prior conversations regarding prognosis and withdrawal of support. Information should be communicated in a clear, comprehensive, and comprehensible manner with a focus on sensitivity, compassion, caring, confidence, positivity, and family wellbeing. The medical team should outline the roles of various professionals, the step-by-step process of donation, and any impact on funeral arrangements (Shemie et al., 2017). For example, many families are relieved to learn that they are still able to proceed with an open casket funeral for their loved one following organ donation in almost all cases. The representative from the OPO will obtain the required informed consent from the patient’s legal next of kin following this conversation. They are also responsible for logistical details such as contacting UNOS, who manages the OPTN in the US, and arranging transport of the organs following procurement (HRSA, 2020a).
If the initial reaction from the family during the organ donation conversation is reluctance or hesitation, it is acceptable to sensitively explore and discuss the reasons underlying their feelings further. However, the medical team should avoid appearing apologetic, guarded, aggressive, or coercive when discussing organ donation with family members. If the prior discussion was had with an untrained staff member or led to a misunderstanding of the facts, then a second attempt may be made with a more experienced team and concrete action plan. A repeat attempt to discuss organ donation with a family may also be reasonable in situations where additional relevant clinical information is now available, the patient in question has since been confirmed as a registered organ donor, or advance directives with specific instructions regarding organ donation have since been located. Unconditional support should be provided to the patient’s family before, during, and after the donation process, regardless of whether or not they elect to provide consent (Shemie et al., 2017).
Assessment and Care of the Potential Organ Donor
OPOs follow more conservative criteria when assessing organs donated following cardiac death, which may reduce the number of viable organs for donation. Typically, this leads to the donation of just the kidneys; the donor’s lungs, liver, and pancreas are less commonly viable (healthy enough for transplantation; Tullius & Rabb, 2018). The average donation for a patient after DCD varies from 1.5-2.75 organs, versus 3-4 organs after DBD (Serri & Marsolais, 2017).
The management of a patient status-post brain death before organ donation can be complicated. Brain death typically leads to a systemic inflammatory response, a catecholaminergic discharge, and diabetes insipidus (DI) with hypovolemia due to the sudden decrease in antidiuretic hormone (ADH) production. The advanced practice nurse must focus on maintaining the patient’s blood pressure to perfuse their organs and avoid hypotension, with a goal mean arterial pressure of at least 70 mm Hg. The patient’s urine output must also be monitored while maintaining euvolemia, with a goal of at least 0.5 cc/kg/hour of urine output. DI typically presents with large amounts of dilute urine output combined with dehydration (i.e., increased serum osmolality and hypotension). There are no specific recommendations regarding the use of vasopressors or inotropes to maintain perfusion; however, vasopressin (Vasostrict) is preferred for managing DI in these patients if it does develop. These measures will help protect the kidneys and ensure adequate perfusion of the patient’s other organs. To protect the lungs, the medical team should avoid hypervolemia and utilize lung-protective ventilation techniques (tidal volume of 6-8 cc/kg) and lung recruitment strategies as needed. Based on observational data only (currently no randomized controlled trial data), the use of insulin, corticosteroids, and thyroid hormones is commonly incorporated to correct hormonal imbalances. Various tests may be performed to assess the health and viability of the patient’s organs before procurement. If the donor is over the age of 40 or has multiple cardiac risk factors, an echocardiogram and/or cardiac angiography may be performed. A bronchoscopy may be indicated to assess the patient’s anatomy and airway clearance. Additional laboratory or imaging studies may be recommended to further assess the health and functioning of the liver, kidneys, and pancreas. Despite the numerous considerations required to optimize potential organ donors following brain death, the primary advantage is that these patients may remain on cardiovascular life support throughout the entire process. These patients are transported to the operating room to retrieve the organs without any significant period of warm ischemia (Serri & Marsolais, 2017; Tullius & Rabb, 2018).
Patients that may have the potential to donate organs following cardiac death are managed similar to the considerations mentioned above, with a primary focus on maintaining euvolemia, hemodynamic stability, and utilizing lung-protective ventilation techniques. As these patients may retain some brain function, they may not require DI or hormonal imbalance management (Serri & Marsolais, 2017). As previously stated, the health and viability of a donor’s organs following cardiac death are directly related to the length of time between the withdrawal of cardiovascular and ventilatory support, the declaration of cardiovascular death, and the surgical procurement of the organs, or the warm ischemia time (Mezrich & Scalea 2015; Serri & Marsolais, 2017). The health of the patient’s organs is optimized if there are less than 240 minutes (4 hours) between the initial withdrawal of life support and organ retrieval/preservation (Dopson & Long-Sutehall, 2019). Often, patients over the age of 65 will not be able to successfully donate healthy organs following cardiac death, while DBD donors may be significantly older (Tullius & Rabb, 2018).
Following procurement, tissue biopsies may be performed to assess the organ viability, especially regarding kidneys. Additionally, recently developed ex-vivo perfusion techniques have improved the health and viability of transplanted organs by reducing the risk of tissue damage during the period of cold ischemia (Thomas et al., 2019). Machine perfusion devices are being researched and developed, such as lung perfusion devices, to continue ex vivo optimization, assess, and potentially increase the use of marginal quality organ donors. These perfusion devices are being developed for liver, kidney, and heart donations as well. The use of extracorporeal support prior to donation and its potential impact on tissue health is also being explored (Serri & Marsolais, 2017). These technologies may be especially crucial if attempting to successfully procure the organs of older donors, which typically have more strict limitations regarding the cold ischemia time between retrieval and placement. Early research indicates that normothermic perfusion may be the most effective for lung and liver tissue, while cold perfusion techniques appear to be beneficial for kidney assessment and transport. An obvious disadvantage of these technological advancements is their additional cost, which can be significant (Tullius & Rabb, 2018).
Barriers and Facilitators to Organ Donation
The barriers and facilitators to increasing the number of healthy organs transplanted are numerous, varied, and at times difficult to accurately identify and characterize. Experts in the field advocate for an increase in national public health campaigns educating the public about the importance and safety of living kidney donations. An unexpected yet potential facilitator of living organ donation is social media and technology. MatchingDonors.com is an internet site first developed in 2004 where those in need of an organ and those willing to donate a kidney or a portion of their liver are connected electronically. As previously stated, additional financial support for living donors would also serve as a facilitator, funding hardships such as lost wages and childcare for living donors. Spain, which is regarded as the world leader in organ transplantation, strives for early identification of potential donors, has developed broad eligibility criteria regarding viable organs for transplantation, and instituted nationwide training for healthcare professionals regarding family communication surrounding organ donation (Lewis et al., 2020; Thomas et al., 2019). Spain also utilizes nurses as transplantation coordinators in certain specialized transplant centers across the country. In a recent qualitative study, these nurse coordinators describe their role as distinctly different from traditional medical surgical bedside nursing. This confers a sense of pride that they are functioning professionally as a component of the organ donation system. The role requires additional specialized training, excellent communications skills, and the ability to manage a multidisciplinary team and navigate stressful situations or crises. The study participants agreed that this is not a role for a novice nurse and identified that the position often involves a heightened level of emotional stress and a poor work/life balance due to the unpredictable and extended work hours required (Fernández-Alonso et al., 2020).
In prior studies, nurses have cited concerns regarding the donation of organs following cardiac death. Respondents noted that they felt the mandatory 5-minute observation period for asystole was insufficient (14%), that the patient would suffer or experience pain (11%), or that there would be legal repercussions (8%). A recent qualitative study of pediatric ICU nurses in the UK identified the following major barriers to discussing the option of organ donation with patients’ families: a lack of knowledge and resources regarding the organ donation process, assumptions about the parent/guardian’s views regarding organ donation, and a general reluctance to engage in sensitive conversations with patients’ families. Less commonly reported barriers included moral or religious objection, organ donation not being in the best interest of the patient, undue burden on the medical staff, discomfort discussing a “taboo topic,” and the fear of being perceived as insensitive or inappropriate by the family. The positive personal and professional attitude towards organ donation and the quality of the nurse/family/patient relationship were both facilitators of discussing organ donation with families that were consistent throughout the study. The nurses participating in the study specified an increased level of comfort and willingness to approach families regarding the possibility of donation if they were given the following support:
- written information regarding organ donation to review and/or distribute
- simulation sessions to practice having sensitive, challenging, and uncomfortable conversations
- annual updates from the institution’s transplant coordinator regarding any changes in the donation process/policy, resources for families, and typical/commonly asked questions, and how best to respond (Dopson & Long-Sutehall, 2019).
Despite this clear indication that additional education and information would increase healthcare providers’ comfort level when discussing organ donation with patient families, a 2016 study in Germany found that 96% of healthcare professionals (both nurses and doctors) felt they were adequately informed regarding the signs of brain death. Further, 92% reported that they felt sufficiently informed regarding the legal and regulatory aspects of organ donation and transplantation (Hvidt et al., 2016). Organ donation programs should strive to foster healthy, cooperative, and mutually beneficial partnerships with their regional OPO. Organ donation discussions that are led by formally trained and experienced facilitators are more successful. The recent invitational forum in Canada identified the following qualities and characteristics that are essential for leaders of effective organ donation discussions:
- a good communicator, listener, and facilitator with high emotional and cultural intelligence;
- open, honest, collaborative, and patient;
- self-aware, non-judgmental;
- comfortable in dealing with families in crisis;
- trained and experienced in dealing with conflict;
- compassionate;
- passionate and knowledgeable about organ donation;
- confident in their ability to be successful;
- able to work well with various personalities (Shemie et al., 2017, p. 6).
To assess success, medical centers and donation coordinators should consider using the rate of family consent as an objective measure of success. Family surveys are an equally insightful method of obtaining more subjective and open-ended feedback regarding the effectiveness of organ donation discussions within a facility or institution (Shemie et al., 2017).
Ethical Questions that Remain
Transplant medicine inherently contains several pertinent ethical considerations, such as:
- Is death being hastened for the sake of donated organs?
- Is the donor or family well-informed?
- Is the recipient well-informed?
- Are both the recipient (and donor, if a live donation) prepared with adequate care awaiting their discharge following the procedure?
- Are their ulterior motives underlying the donation (Nursing@Georgetown, 2018)?
The dead-donor rule has been in effect since the beginning of transplant medicine. Imminent death donation, or live donation prior to planned withdrawal (LD-PPW), continues to provoke ethical discussions amongst organ transplant experts. These patients are anesthetized after being given the opportunity to bid farewell to their family members, and their organs are removed in an operating room under general anesthesia, making the official cause of death organ donation. While performed in other countries and generally considered to produce the healthiest and highest quality organs for transplantation, this process has not been sanctioned by the OPTN (Mezrich & Scalea, 2015; OPTN, 2016). An ethics committee review in 2016 cited ethical concerns; specifically, patients with a severe neurological injury who would be unable to provide informed consent of their own accord were of concern to the committee members. The stress of this ethical dilemma on the surrogate decision-maker was felt to be unreasonable by the committee. One member of the committee described this as a political, not an ethical, problem. The committee cited “potential risks that are too great at this time based on the responses and substantial concern from the nine other committees, lack of community support, and substantial challenges.” There exists no data with which to determine if LD-PPW would lead to an effective increase or decrease in the number of organs available for transplantation. Currently, any surgical program that proceeds with this method of donation in the US could be held legally liable for accelerating the death of a patient, even with a signed consent form. In Canada, where physicians have recently been granted legal immunity to facilitate a comfortable and somewhat hastened death by terminally ill patients, transplant teams continue to abide by the “5-minute no touch” rule prior to initiating the organ and tissue recovery process (OPTN, 2016; Rosenbaum, 2020).
It seems that public opinion in the US may be less divided than those in medicine regarding these ethical questions. In a 2015 survey including more than 1,000 Americans, 85% expressed intention to donate their organs following death, 61% signed an organ donor card, and 71% agreed that a patient in an irreversible coma (a vignette describing a patient who suffers a severe neurological injury leading to a complete lack of cortical activity, reliant on life support) should be allowed to donate their organs with consent, even if it meant causing their death to do so. Respondents also agreed (67%) that they would want to donate their organs in this scenario. However, opinions collected during this survey were somewhat complex. Most (69%) of respondents indicated that it was somewhat or very important for a potential donor to be “dead” prior to organ donation. When asked to define the term death, the two most common responses chosen were “dead means dead” and “scientifically dead- the body does not function as a whole, biologically” (Nair-Collins et al., 2015).
Unfortunately, the ethical concerns regarding the lack of equity in access to medical care amongst communities of color extend into the transplant medicine world as well. In 2019, more than half (67,000) of the patients on the organ donation waiting list were people of color (Lewis et al., 2020). Individuals of color, including African Americans, Asian Americans, Pacific Islanders, Native Americans/Alaskan Natives, and Hispanic Americans, have higher rates of certain chronic diseases that affect their vital organs; this increases the need for organ transplantation in these groups. Certain blood types are more common amongst individuals of color. Since organs must be matched based on the donor and the recipient’s blood type, it is vital that people of color register as organ donors. This community's need is higher than within the general population (Mayo Clinic Staff, 2019). Strategies suggested to address this inequity include increased efforts in communities of color to increase public awareness and public education regarding organ donation and improved primary care access to reduce the secondary need for organ donation. While an increase in organ allocation efficiency is still warranted, a transition from a location-based allocation system to a need-based system several years ago did improve the equity of organs being donated (Lewis et al., 2020).
Additional ethical considerations regarding the use and allocation of organs from older donors are also ongoing. To reduce the waste of donated and potentially viable organs that are considered of marginal quality, Eurotransplant has begun a pilot program whereby organs from donors over the age of 65 are transplanted into recipients within the same age group in lieu of being discarded. Five years after initiation, this model doubled the number of older kidneys transplanted and reduced the waiting time for a kidney transplant by more than 1 year. Similarly, they are exploring the potential benefit of dual kidney transplants, whereby one recipient receives both of the donor’s kidneys, which previously would have been deemed suboptimal and potentially discarded. The strict use of regulatory benchmarks by national agencies such as UNOS may serve as a deterrent to such experimental considerations in the US out of concern regarding regulatory consequences if the outcomes are inferior (Tullius & Rabb, 2018). Certainly, the fear regarding the scrutiny, backlash, and public relations debacle that would result following the death of a donor that was involved in a living organ donation, and the resulting impact this could have on the entire transplant program, deters many programs from exploring alternative and innovative ways to screen, recruit, and optimize patients willing and interested in donating their organs (Mezrich & Scalea, 2015).
Future Research to Optimize Organ Donation and Reduce Transplant Need
Most research in the field of transplantation is focused on how to optimize the tissue health of donated organs prior to, during (see prior discussion regarding ex vivo perfusion techniques), or after the transplant process. Enhanced early identification of potential organ donors and the expansion of the currently existing organ donor criteria are strategies that should be evaluated to improve the organ shortage across the country. Public awareness and education are vital to obtaining consent, as research consistently indicates that donation consent rates are directly related to the public’s level of understanding (Lewis et al., 2020). The usefulness of mild hypothermia is being explored in managing potential organ donors immediately following brain death declaration. The potential role of immunosuppressants and antioxidants in donors prior to organ procurement is being studied. Ex vivo perfusion techniques allow more time for organ and tissue assessment before placement. Immediately following reperfusion, significant tissue injury occurs related to inflammation, apoptosis, epigenetic changes, and oxidative stress. Immune therapies targeted at reducing or limiting this damage are ongoing but are struggling to overcome the small number of patients enrolled in their clinical trials. Alternately, stem cells, anti-inflammatory T cells, and regenerative agents are being explored to enhance the repair process following this initial tissue damage. Pharmaceutical companies are exploring methods to enhance the effectiveness and reduce the adverse effects of immunosuppressive agents given to organ recipients (Tullius & Rabb, 2018).
In 2017, the National Academies of Sciences, Engineering, and Medicine (NASEM) convened an expert panel to address the national imbalance in the number of organs needed versus those available for transplant. This expert panel concluded that future research efforts should focus primarily on organ donor interventions (i.e., research focused on organs prior to transplantation). As the primary step in this process, the panel suggested developing, assessing, and then disseminating methods to discuss donor intervention research to prospect donors in order to optimize consent. They recommended that the Uniform Anatomical Gift Act be amended to clarify the patient’s wishes regarding donation for the sake of donor intervention research to identify and respect patients’ wishes. This registry should be accessible to regional OPOs and automatically encompass registration information from state motor vehicle departments. For recipients, the expert panel recommended clinical and research informed consent before accepting an organ that was subjected to research interventions. They recognize that this requirement may present a barrier to efficient research, especially if individual institutional review board (IRB) approval is required. They recommend a centralized and collaborative approach to donor intervention research oversight and monitoring with three affiliated but independent structures to offset this barrier. These three structures would include a single central IRB; an oversight committee to provide monitoring and prioritize, review, implement, and track research; and a data and safety monitoring board. Beyond donor intervention research, the expert panel also concluded that a single, unified, and secure donor registry would facilitate and simplify the process of obtaining legal consent for organ donation (Childress, 2017).
The UNOS information hotline, 888-TXINFO1 (888-894-6361), can be contacted for additional details regarding the process of organ donation, recovery, and transplantation (HRSA, 2020a).
References
Centers for Disease Control and Prevention. (2019). HIV transmission. https://www.cdc.gov/hiv/basics/transmission.html
Childress, J. F. (2017). Organ donor research: Overcoming challenges, increasing opportunities. JAMA, 318(22), 2177–2178. https://doi.org/10.1001/jama.2017.16442
Dopson, S., & Long-Sutehall, T. (2019). Exploring nurses’ knowledge, attitudes, and feelings towards organ and tissue donation after circulatory death within the paediatric intensive care setting in the United Kingdom: A qualitative content analysis study. Intensive and Critical Care Nursing, 54, 71–78. https://doi.org/10.1016/j.iccn.2019.07.004
Fernández-Alonso, V., Palacios-Ceña, D., Silva-Martín, C., & García-Pozo, A. (2020). Facilitators and barriers in the organ donation process: A qualitative study among nurse transplant coordinators. International Journal of Environmental Research and Public Health, 17(21). https://doi.org/10.3390/ijerph17217996
Finger Lakes Donor Recovery Network. (2015). Who should talk to families about donation? http://www.donorrecovery.org/healthcare-professionals/partners-newsletters/partners-newsletter-spring-2015/best-practices-who-should-talk-to-families-about-organ-donation-collaborative-approach-with-finger-lakes-donor-recovery-network/
Health Resources and Services Administration. (n.d.a.) Religion and organ donation. Retrieved December 20, 2020, from https://www.organdonor.gov/about/donors/religion.html
Health Resources and Services Administration. (n.d.b). Who can donate? Retrieved December 20, 2020, from https://www.organdonor.gov/about/donors.html
Health Resources and Services Administration. (2020a). How organ donation works. https://www.organdonor.gov/about/process.html
Health Resources and Services Administration. (2020b). What can be donated? https://www.organdonor.gov/about/what.html
Hong, J. C., Yersiz, H., Kositamongkol, P., Xia, V. W., Kaldas, F. M., Petrowsky, H., Farmer, D. G., Lipshutz, G., Markovic, D., Hiatt, J., & Busuttil, R. W. (2011). Liver transplantation using organ donation after cardiac death: A clinical predictive index for graft failure-free survival. JAMA Archives of Surgery, 146(9), 1017-1023. https://doi.org/10.1001/archsurg.2011.240
Hvidt, N. C., Mayr, B., Paal, P., Frick, E., Forsberg, A., & Büssing, A. (2016). For and against organ donation and transplantation: Intricate facilitators and barriers in organ donation perceived by German nurses and doctors. Journal of Transplantation. https://doi.org/10.1155/2016/3454601
Ignatavicius, D. D., Workman, L., & Rebar, C. R. (2018). Medical-surgical nursing: Concepts for interprofessional collaborative care (9th ed.). Elsevier.
Lewis, A., Koukoura, A., Tsianos, G.-I., Gargavanis, A. A., Nielsen, A. A., & Vassiliadis, E. (2020). Organ donation in the US and Europe: The supply vs. demand imbalance. Transplantation Reviews, 100585. https://doi.org/10.1016/j.trre.2020.100585
Mayo Clinic Staff. (2019). Organ donation: Don’t let these myths confuse you. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/organ-donation/art-20047529?p=1
Mezrich, J. D., & Scalea, J. (2015, March 16). As they lay dying: Two doctors say it’s far too hard for terminal patients to donate their organs. The Atlantic. https://www.theatlantic.com/magazine/archive/2015/04/as-they-lay-dying/386273/
Munakomi, S., & Al Khalili, Y. (2020). Brainstem death. StatPearls. http://www.ncbi.nlm.nih.gov/books/NBK551584/
Nair-Collins, M., Green, S. R., & Sutin, A. R. (2015). Abandoning the dead donor rule? A national survey of public views on death and organ donation. Journal of Medical Ethics, 41(4), 297–302. https://doi.org/10.1136/medethics-2014-102229
Neurocritical Care Society. (2017). Brain death toolkit. https://www.neurocriticalcare.org/education/digital-education/brain-death-toolkit?mod=article_inline
Nursing@Georgetown. (2018). Give and take: A nurse’s role in organ transplantation. https://online.nursing.georgetown.edu/blog/give-take-nurses-role-organ-transplantation/
Organ Procurement and Transplantation Network. (n.d.). Data. Retrieved December 28, 2020, from https://optn.transplant.hrsa.gov/data/
Organ Procurement and Transplantation Network. (2016). Ethical considerations of imminent death donation. https://optn.transplant.hrsa.gov/governance/public-comment/ethical-considerations-of-imminent-death-donation/
Rosenbaum, L. (2020). Altruism in extremis: The evolving ethics of organ donation. New England Journal of Medicine, 382(6), 493–496. https://doi.org/10.1056/NEJMp2000048
Serri, K., & Marsolais, P. (2017). End-of-life issues in cardiac critical care: The option of organ donation. Canadian Journal of Cardiology, 33(1), 128–134. https://doi.org/10.1016/j.cjca.2016.10.017
Shemie, S. D., Robertson, A., Beitel, J., Chandler, J., Ferre, E., Evans, J., Haun, M., Torrance, S., & Participants, on behalf of the E. C. with F. of P. D. (2017). End-of-life conversations with families of potential donors: Leading practices in offering the opportunity for organ donation. Transplantation, 101(5S), S17. https://doi.org/10.1097/TP.0000000000001696
Starr, R., Tadi, P., & Pfleghaar, N. (2020). Brain death. StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK538159/
Thomas, E., Milton, J., & Cigarroa, F. G. (2019). The advancing American kidney health executive order: An opportunity to enhance organ donation. JAMA, 322(17), 1645–1646. https://doi.org/10.1001/jama.2019.14500
Trump, D. J. (2019). The executive order on advancing American kidney health. The White House. https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/
Tullius, S. G., & Rabb, H. (2018). Improving the supply and quality of deceased-donor organs for transplantation. New England Journal of Medicine, 378(20), 1920–1929. https://doi.org/10.1056/NEJMra1507080
United Network of Organ Sharing. (n.d.). UNOS fast facts. Retrieved December 28, 2020, from https://unos.org/about/fast-facts/
United States Food and Drug Administration (2018). Highlights of Neupogen prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103353s5194lbl.pdf
World Health Organization (2019a). Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
World Health Organization (2019b). Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
Wijdicks, E. F. M., Varelas, P. N., Gronseth, G. S., & Greer, D. M. (2010). Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology, 74(23), 1911–1918. https://doi.org/10.1212/WNL.0b013e3181e242a8