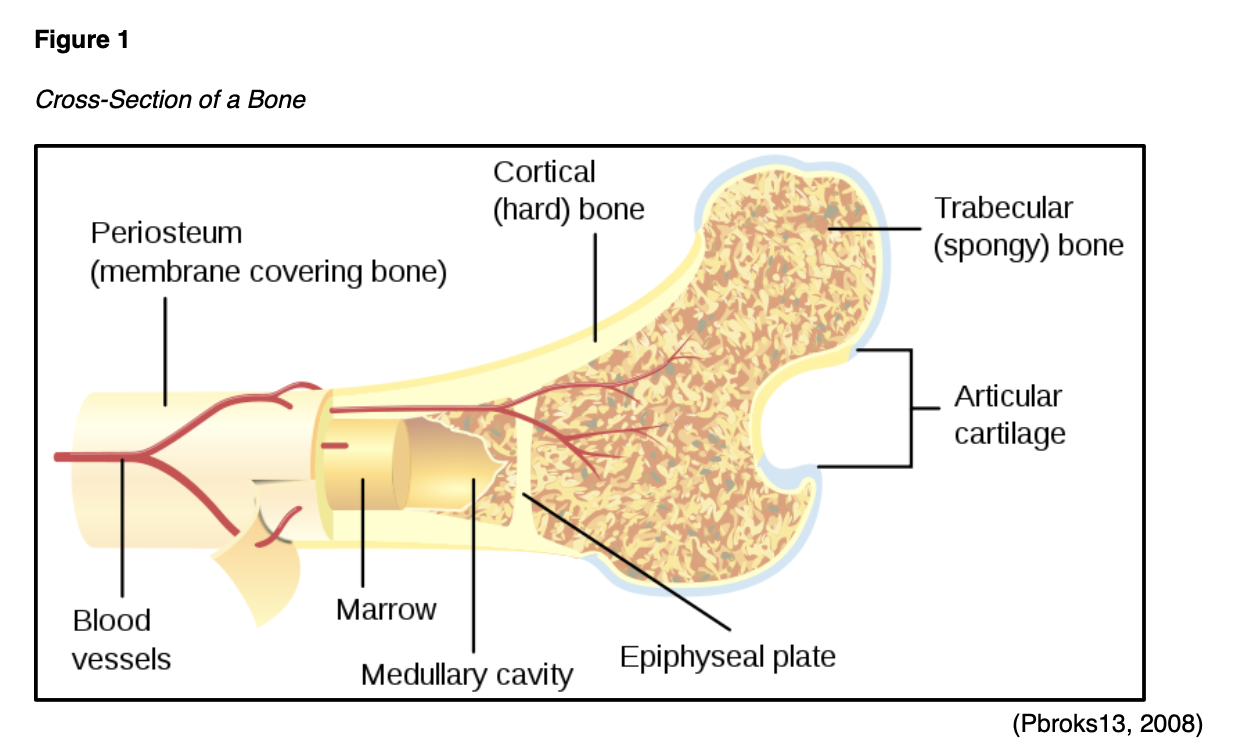
The purpose of this module is to provide an overview of osteoporosis, the diagnostic work up and clinical criteria for diagnosis, nonpharmacologic and pharmacologic treatment options, as well as a summary of evidence-based prevention and screening guidelines.
...purchase below to continue the course
steoclasts are the bone-destroying cells, as they aid in bone resorption by breaking down old or damaged bone to allow it to be renewed and replaced by osteoblasts. Osteoclasts are the abundant cell type found within mature bones (Ignatavicius & Workman, 2015).
Bone modeling is the formation and shaping of bone, which is most prominent during infancy and childhood (Katsimbri, 2017). During modeling, the resorption and formation of bone occur independently at various skeletal sites to generate significant changes in the bone's architecture (Kenkre & Bassett, 2018). Bone remodeling involves the resorption of old or damaged bone, followed by the formation and deposition of new bone material. This ongoing process occurs throughout life to ensure that bone maintains its strength and mineral composition (Rowe & Sharma, 2019). Bone remodeling is the predominant skeletal process during adulthood, except after a bone fracture, during which time a significant increase in bone modeling occurs in response to the injury (Katsimbri, 2017). It has two primary functions: (a) to repair micro-damage within the skeleton to maintain strength and ensure the relative youth of the skeleton, and (b) to supply calcium when needed from the skeleton to maintain serum calcium (Lindsay & Cosman, 2018). During remodeling, resorption and formation occur in close proximity so that overall bone volume and structure remain unchanged (Kenkre & Bassett, 2018). While bone remodeling occurs through the coordinated activity of the osteocytes, osteoblasts, and osteoclasts, it is also regulated by multiple hormones, including estrogens (in both genders), androgens, vitamin D, parathyroid hormone (PTH), and various types of growth factors (Lindsay & Cosman, 2018). The process of bone remodeling is demonstrated in Figure 2.

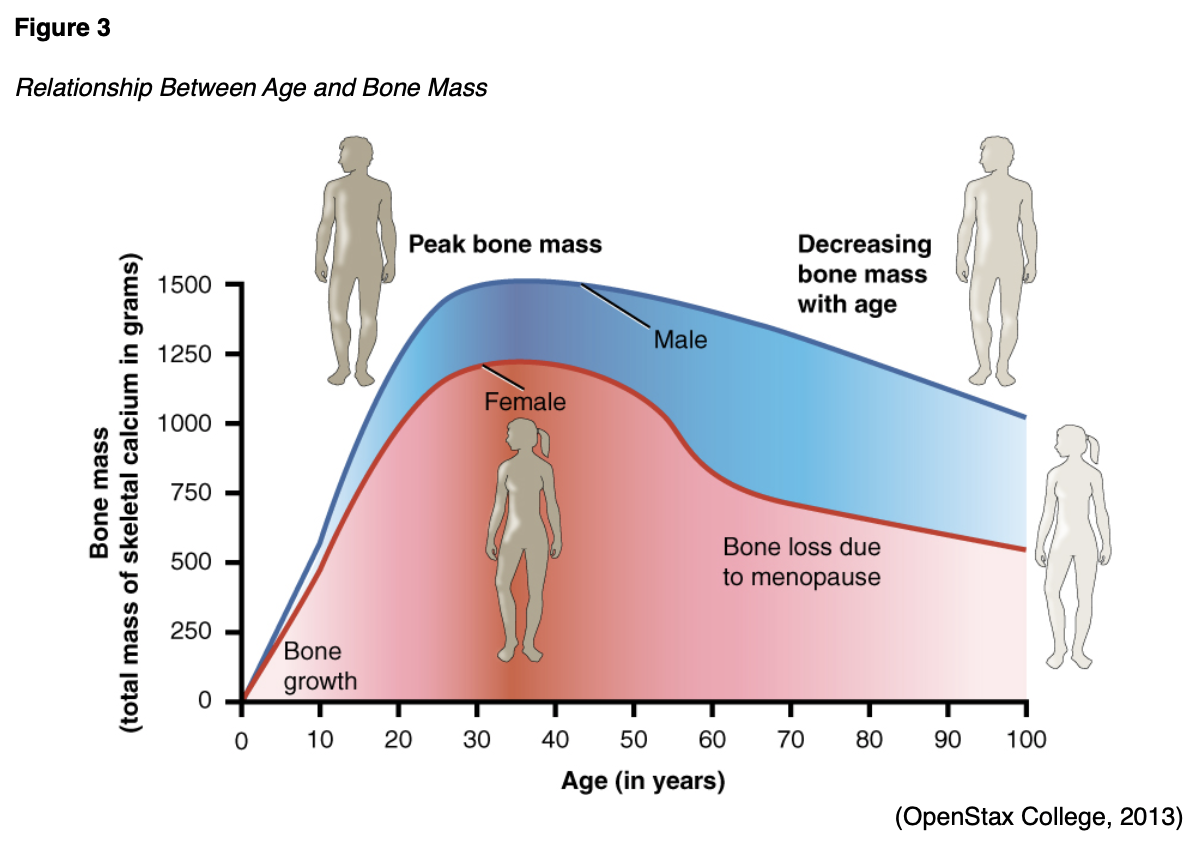
Throughout early childhood and adolescence, bone grows and forms faster than the body's ability to break down old bone. This process provides the basis for healthy, strong bones among younger age groups. The skeleton's BMD remains relatively constant after peak bone mass is achieved in young adulthood and until around age 30–35, at which time the resorption and formation processes become imbalanced. After this point there is a progressive decline in bone formation and a rise in bone resorption (Lindsay & Cosman, 2018). Figure 3 provides a graphic representation of the relationship between age and bone mass in men and women.
Pathophysiology
Osteopenia is the first stage of the disease, in which the BMD is lower than the normal range. If left untreated, osteopenia can develop into osteoporosis, which is a much more severe condition (Porter & Varacello, 2019). Loss of bone mass is caused by a weakening of the bony microvasculature, as the inner layer of spongy bone becomes brittle due to loss of calcium (AAFP, 2019). Osteoporosis affects both spongy and compact bone. It causes reduced bone density and structure of the spongy bone first, followed by thinning of the cortical bone after that. A comparison of the distinction between normal bone and osteoporotic bone is displayed in Figure 4.

Etiology
The etiology of bone loss and resulting osteoporosis can be primary or secondary, as there are a variety of extrinsic and intrinsic factors that can exaggerate the disease process (Lindsay & Cosman, 2018). Primary osteoporosis is associated with advancing age and age-related bone loss resulting from the continual deterioration of the trabeculae over time (Tu et al., 2108). An imbalance in bone resorption and bone remodeling drives the underlying mechanism of the disease, in which osteoclastic activity becomes greater than osteoblastic activity. Osteoporosis can be a consequence of the failure to reach a normal peak bone mass during formative years, an accelerated process of bone loss, or a combination of both factors (Porter & Varacello, 2019). Postmenopausal women experience a biological reduction of estrogen production, which fuels the progressive decline in BMD. In men, sex-hormone-binding globulin inactivates testosterone and estrogen as aging occurs, which also contributes to the decline in BMD over time. Secondary osteoporosis is primarily caused by comorbid health conditions, diseases, or as a result of medications that induce imbalances in calcium, vitamin D, and sex hormones (Tu et al., 2108).
Risk Factors
There are many risk factors and conditions that can affect bone density and contribute to the development of osteoporosis.
Nutrition
Achieving peak bone mass may be impaired by inadequate calcium intake during growth or deficiencies in calories, protein, and minerals, leading to increased risk of osteoporosis later in life. This includes patients with low BMI related to inadequate nutrition, which can be secondary to eating disorders such as anorexia nervosa or bulimia, or those living in poverty (Lindsay & Cosman, 2018). Calcium plays a vital role in maintaining bone integrity by decreasing bone turnover and slowing bone loss. Patients with calcium deficiency, such as those who do not ingest enough calcium through their diet or acquire it from dietary supplementation, are at risk for osteopenia and osteoporosis. Vitamin D deficiency can also result in bone disease and poor mineralization of calcium. The prevalence of vitamin D deficiency is higher among patients with intestinal malabsorption disorders, including those status post-bariatric surgery for weight loss, and those who are malnourished. While vitamin D deficiencies should be corrected, excessive supplementation of vitamin D should be avoided. When given in excess, vitamin D suppresses PTH secretion and promotes bone resorption. Further, vitamin D toxicity is harmful to bones and promotes bone loss (Mueller, 2017).
Lifestyle Factors
Aside from ensuring adequate nutrition, other lifestyle factors are noted to increase one's risk for osteoporosis and osteoporotic fractures. Some of the most common include:
- Excess alcohol consumption;
- Excess caffeine consumption;
- Sedentary lifestyle including a lack of physical activity and weight-bearing activity;
- Tobacco use (Porter & Varacello, 2019).
Gender
Women are at greater risk for osteoporosis than men for several reasons, such as:
- Women have a lower bone mass overall;
- Women absorb less calcium;
- Women tend to live longer;
- Bone loss accelerates after menopause as estrogen levels decline (NOF, 2020).
Since the ovaries produce estrogen, which contributes to bone strength and development, bone loss occurs when the ovarian function declines during menopause. Accelerated bone loss may occur if the ovaries are removed by surgery or if their activity is suppressed by chemical-induced menopause, such as through the use of hormonal treatment or other medications that suppress ovary function. Prevention of this expected bone loss is possible with hormone replacement therapy (HRT) during the time of natural menopause. However, HRT is controversial due to the potential for a heightened risk of estrogen-based cancers such as breast cancer, uterine cancer, or ovarian cancer. Therefore, HRT is contraindicated for many women (NOF, 2020).
Genetic/Hereditary Factors
- Females who are of European, Asian, or Hispanic/Latina descent;
- History of hip fracture in a biological parent, which is associated with a twofold increased risk of hip fracture in women, regardless of BMD (Lewiecki et al., 2019b);
- Family history of osteoporosis; peak bone mass is often lower among individuals with a family history of osteoporosis (Lindsay & Cosman, 2018).
Medical Conditions
Some of the most common types of health conditions and their treatments that are known to accelerate bone loss and increase the risk for osteoporosis include the following:
- Chronic illness such as rheumatoid arthritis and cardiovascular disease
- Malnutrition and poor diet
- Cancer and cancer treatment, particularly:
- Breast cancer
- Prostate cancer
- Leukemia
- Lymphoma
- Multiple myeloma
- Endocrine disorders
- Diabetes mellitus, type II
- Hyperparathyroidism
- Hyperthyroidism
- Cushing’s syndrome (accelerates bone loss through excess glucocorticoid production)
- Thyrotoxicosis
- Vitamin D deficiency
- Hormonal disorders
- Irregular menstrual cycles
- Premature menopause
- Low levels of testosterone and estrogen in males and females
- Neurologic disorders
- Stroke
- Parkinson’s disease
- Multiple sclerosis
- Gastrointestinal Disorders
- Inflammatory bowel disease
- Weight loss surgery such as gastric bypass or adjustable gastric band
- Celiac disease
- Psychiatric disorders
- Eating disorders (anorexia nervosa, bulimia)
- Other conditions
- AIDS/HIV
- Chronic obstructive pulmonary disease (COPD), including emphysema
- Female athlete triad disorder, which includes loss of menstrual periods, an eating disorder, and excessive exercise
- Chronic kidney disease
- Liver disease, including biliary cirrhosis
- Organ transplantation
- Scoliosis (NOF, 2020).
*Please note that this is not an exhaustive list.
Medications
Medications can be equally detrimental to the bones, even when they are required for the treatment of another condition. Steroids are often crucial in treating a wide range of conditions, such as asthma, COPD, skin rashes, and allergies. They are often prescribed for the vast majority of inflammatory disorders to reduce inflammation, such as Crohn's disease. They are also used alongside other medicines to treat cancer and autoimmune conditions such as rheumatoid arthritis and lupus. Glucocorticoids and corticosteroids are considered the most common cause of medication-induced osteoporosis (Lindsay & Cosman, 2018). They work very similarly to certain innate hormones and have significant anti-inflammatory effects on various organ systems within the body. They induce profound and varied metabolic effects and modify the body's immune response to stimuli. Steroids can also be used as replacement therapy for patients with adrenocortical deficiency (IBM Micromedex Solutions, 2020).
BMD has been found to decline rapidly within only three to six months of starting steroid therapy at a dose of greater than or equal to 5 mg prednisone or equivalent per day (American Association of Clinical Endocrinologists/American College of Endocrinology [AACE/ACE], 2016). When administered over extended periods of time and at higher doses, steroids have an even greater impact on bone loss and the development of severe osteoporosis (Tu et al., 2018). In addition, other types of immunosuppressive agents used to prevent transplant rejection, such as tacrolimus (Prograf) and cyclosporine (Restasis), are also linked to bone loss and heighten the risk for osteoporosis (Tu et al., 2018).
Aside from steroids, there are several other types of medications that are associated with bone loss. Some of the most common include:
- Excessive doses of thyroid hormone, which can accelerate bone remodeling and lead to bone loss;
- Aluminum-containing antacids, such as tums;
- Proton pump inhibitors such as esomeprazole (Nexium), omeprazole (Prilosec), and lansoprazole (Prevacid);
- Many types of cytotoxic drugs (chemotherapy);
- Specific types of anticonvulsants, such as phenytoin (Dilantin) and phenobarbital (Luminal);
- Heparin;
- Lithium (Lithobid);
- Medroxyprogesterone acetate (Depo-Provera), which is used for contraception;
- Methotrexate (Trexall);
- Thiazolidinediones, including pioglitazone (Actos) and rosiglitazone (Avandia);
- Hormonal blocking therapies:
- Aromatase inhibitors such as anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin);
- Gonadotropin-releasing hormone (GnRH) medications such as leuprolide acetate (Lupron) and goserelin (Zoladex);
- Tamoxifen (Nolvadex) when used in premenopausal women;
- Androgen deprivation therapies: flutamide (Eulexin), bicalutamide (Casodex), or nilutamide (Anandron) (Lindsay & Cosman, 2018; Tu et al., 2018).
Diagnostic Work-Up
A thorough history taking, medical evaluation, and laboratory assessment are vital to identify risk factors and estimate the risk of breaking a bone. A comprehensive medical history, including current or prior use of any medications that promote bone loss, as well as details of the patient's medical, surgical, social, and family history that may heighten their risk is a required first step. The nurse should inquire about the patient's diet and intake of any dietary supplementations, evaluating for any potential nutritional deficiencies. Activity level, lifestyle habits, exercise patterns, and alcohol or tobacco use should also be assessed. On physical examination, some patients may have evidence of height loss due to compression fractures of the vertebral bodies. Other tests that may be used to evaluate bone health but are not used to diagnose osteoporosis include biochemical marker tests, x-rays, vertebral fracture assessments (VFAs), and bone scans (NOF, 2020).
Screening
Since patients with osteoporosis are generally asymptomatic, screening is critical to identifying patients at risk. The US Preventive Services Task Force (USPSTF, 2018) recommends routine osteoporosis screening with bone measurement testing in women 65 years of age and older, and in postmenopausal women younger than 65 years who are at increased risk of osteoporosis. For postmenopausal women younger than 65 years who have at least one risk factor, the USPSTF recommends the use of a clinical risk assessment tool as a means of determining which patients should be screened with bone measurement testing. Regarding males, the USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis; therefore, routine screening is not advised at any age (USPSTF, 2018). However, the NOF (2019) recommends bone measurement testing in all men 70 years of age and older, as well as for men aged 50 through 69 years if they have clinical risk factors.
Clinical risk should be evaluated using a formal clinical risk assessment tool. There are many tools available, such as the Simple Calculated Osteoporosis Risk Estimation (SCORE), the Osteoporosis Risk Assessment Instrument (ORAI), or the Osteoporosis Self-Assessment Tool (OST). These tools are considered moderately accurate at predicting osteoporosis risk (USPSTF, 2018). The ORAI is a validated tool developed by the World Health Organization (WHO). It provides a ten-year probability of a major fracture and is useful in both men and women. It takes into consideration the individual's BMI, certain risk factors, and some causes of secondary osteoporosis (Lewiecki et al., 2019b). The Fracture Risk Assessment Tool (FRAX), estimates the 10-year probability of a hip fracture when DXA bone density test is unknown or not performed (USPSTF, 2018).
Dual-Energy X-Ray Absorptiometry (DXA)
According to the WHO, the gold standard bone density test is the dual-energy x-ray absorptiometry (DXA) scan of the central skeleton, which includes the hip and lumbar spine (Porter & Varacallo, 2019). This is a low-level x-ray radiographic imaging test that measures the BMD to diagnose osteopenia or osteoporosis and assess fracture risk. BMD is most commonly measured at the spine, hip, and wrist levels. The degree of bone loss is calculated and classified according to the defined diagnostic criteria (CDC, 2019a). The DXA scan is a quick, noninvasive, and painless test. As demonstrated in Figure 5, the patient is instructed to lie or sit down for less than 10 minutes while the machine scans the body. The test exposes the patient to a small amount of radiation, which is less than the amount associated with a chest x-ray (American Society of Obstetricians and Gynecologists [ACOG], 2018). Quantitative ultrasound (QUS) is another test that may be used for osteoporosis diagnosis. While QUS is considered a low-cost and readily accessible alternative to DXA, it measures density in bones that are not at high risk and does not correlate well to standard DXA scan results. Data is still limited on its accuracy, as the vast majority of osteoporosis literature and clinical research is premised on the use of the DXA scan. Therefore, it is not as useful in diagnosis or treatment decisions and is not utilized widely in clinical practice (Hans & Balm, 2017).

DXA Results Interpretation
DXA test results are reported as a T-score for each site measured, which is a number comparing the patient's BMD level to that of a healthy young adult with ethnicity and gender-matched controls. The WHO separates those T-scores into four categories: normal, low bone mass (osteopenia), osteoporosis, and severe (established) osteoporosis (Mueller, 2017). A T-score of 0 indicates that the BMD is equal to that of a healthy young adult, a negative T-score indicates that the bones are thinner than normal, and a positive T-score denotes that the bones are stronger than normal. The difference between a patient's BMD and the normal range is measured in units referred to as standard deviations. The more standard deviations below 0, denoted by negative numbers, the lower the BMD, the more severe the osteoporosis, and the higher the risk of fracture. The WHO classifies osteoporosis as a BMD that is 2.5 standard deviations below normal. Treatment is usually recommended when the T-score is -2.5 or lower to prevent fractures. Refer to Table 1 for a comparison of T-scores and the associated BMD level (NIAMS, 2018). Figure 6 is an example of a DXA test result report for a patient with osteopenia of the lumbar spine.

Once osteopenia or osteoporosis has been identified, the patient should undergo additional testing, including laboratory evaluation of their renal and thyroid function, as well as 25-hydroxyvitamin D, magnesium, and calcium levels. Hypocalcemia is a prominent manifestation of magnesium deficiency as magnesium is required for the mobilization of calcium from the bone. These patients require magnesium replacement therapy in addition to calcium supplementation (Mueller, 2017). The laboratory evaluation should include a complete blood count (CBC); comprehensive metabolic panel (CMP), intact PTH; phosphate; and a 24-hour urine collection for calcium, sodium, and creatinine. For patients receiving thyroid hormone or if there is a concern for a thyroid disorder, thyroid-stimulating hormone (TSH) should also be measured. In patients with clinical suspicion for a malabsorption disorder, celiac antibodies should be evaluated. For those patients in which there is suspicion for multiple myeloma, serum and urine protein electrophoresis testing should ensue (AACE/ACE, 2016).
Management
The goal of treatment is to prevent fractures, improve bone density, and reduce morbidity and mortality. This can be achieved by increasing bone mass at maturity, preventing subsequent bone loss, or by restoring BMD following bone loss. The importance of nonpharmacological methods to both prevent and manage the disease should be emphasized, as outlined in Table 2.

Pharmacological Treatment
The primary objective of pharmacological treatment of osteopenia is to slow the progression of the disease to prevent osteoporosis, whereas in osteoporosis, the goal is to reduce the risk of fracture. Treatment recommendations are based on specific patient characteristics, such as gender, degree of fracture risk, comorbid diseases, medications, and severity of the disease (Tu et al., 2018). According to the USPSTF (2018), there is adequate evidence that drug therapies provide at least a moderate benefit in reducing fractures in postmenopausal women with osteoporosis. Patients with a T-score of -2.5 or less should be offered pharmacologic treatment (Porter & Varacallo, 2019). The AACE/ACE provides evidence-based guidelines and recommendations for the management of osteoporosis; they strongly recommend pharmacologic therapy for patients with “a history of fragility fracture of the hip or spine; a T-score of –2.5 or lower in the spine, femoral neck, total hip, or 33% radius; or a T-score between –1.0 and –2.5 if the FRAX 10-year probability for major osteoporotic fracture is at least 20% or the 10-year probability of hip fracture is at least 3%” (AACE/ACE, 2016, p.18).
Pharmacological interventions include both dietary supplementation and prescription medications. Medications that improve BMD and decrease fracture risk in patients with osteoporosis may be used to prevent or treat the condition. Deficiencies in calcium, vitamin D, and magnesium should be corrected first with replacement therapy. Medications to treat osteoporosis are categorized as either antiresorptive or anabolic. Antiresorptive medications primarily decrease the rate of bone resorption, while anabolic medications aim to increase bone formation. Medications that decrease bone resorption include bisphosphonates, estrogen agonist/antagonists [EAAs] such as raloxifene (Evista), estrogens, calcitonin, and denosumab (Prolia). Several of these medications have overlapping indications for the prevention and/or treatment of osteoporosis (Lindsay & Cosman, 2018). Some types of anabolic agents include teriparatide (Forteo), romosozumab-aqqg (Evenity), and abaloparatide (Tymlos) (NOF, 2020).
Patients who are at lower or moderate fracture risk, such as younger postmenopausal women with no prior fractures and only moderately low T-scores, are typically started on oral agents. Combination therapy is not advised due to limited evidence on the efficacy of combining medications, as well as the heightened potential for increased toxicities, adverse effects, and cost. Injectable agents such as teriparatide (Forteo), denosumab (Prolia), or zoledronic acid (Reclast, Zometa) can be used as initial therapy for those patients who have the highest fracture risk (i.e., advanced age, frailty, glucocorticoid use, low T-score, a history of hip or multiple vertebral fractures, or increased fall risk). Patients who may also benefit from injectable medications are those who have upper gastrointestinal (GI) conditions and are not able to tolerate the adverse effects commonly associated with oral bisphosphonate therapy. Patients with dementia or those who have difficulty remembering to take their medications may also benefit from injectable medication alternatives (Lindsay & Cosman, 2018).
The AACE/ACE (2016) clinical practice guidelines list the following four medications as having evidence for "broad spectrum" anti-fracture efficacy with regards to vertebral, hip, and nonvertebral fracture risk reduction.
- Alendronate (Fosamax), a bisphosphonate
- Risedronate (Actonel), a bisphosphonate
- Zoledronic Acid (Reclast, Zometa), a bisphosphonate
- Denosumab (Prolia), a RANKL inhibitor
In patients with no prior fragility fractures or those who are considered to be of moderate fracture risk, these medications are recommended as first-line options for patients who meet criteria for pharmacologic therapy. As previously mentioned, denosumab (Prolia), zoledronic acid (Reclast, Zometa) and teriparatide (Forteo) are also recommended for first-line use in patients who have endured a prior fragility fracture or those who are considered to be of high fracture risk; alendronate (Fosamax) and risedronate (Boniva) may be considered as secondary alternatives in high-risk patients (AACE/ACE, 2016).
Bisphosphonates
Bisphosphonates are the most widely used medications for treating osteoporosis. They inhibit the action of osteoclast cells, decreasing bone turnover, and increasing bone density (Payne et al., 2017). There are four main bisphosphonates, which include alendronate (Fosamax), ibandronate (Boniva), risedronate (Actonel), and zoledronic acid (Reclast, Zometa). Alendronate (Fosamax) has been shown to reduce the rate of hip, vertebral, and wrist fractures by 50%, whereas risedronate (Actonel) has been found to reduce vertebral and nonvertebral fractures by 40% over three years. Zoledronic acid (Reclast, Zometa) reduces the rate of vertebral fractures by 70% and hip fractures by 40% over three years (Porter & Varacallo, 2019).
Before starting bisphosphonate therapy, patients must be fully educated on the associated potential risks. Patients must be well-informed on the importance of monitoring and reporting any potential effects to the prescriber. In addition, the nurse should educate patients on how to minimize the adverse effects of oral therapies, such as reducing esophageal irritation by taking the medication with a full glass of water and not lying down for at least 30 minutes after taking the medication (USPSTF, 2018). The most critical patient education points are described in Table 3.

Prolonged use of uninterrupted bisphosphonate therapy that extends beyond three to five years places patients at higher risk for atypical femur fractures (AFFs). An AFF is a fracture of the femoral shaft in patients on current or prior treatment with bisphosphonate therapy (Portal & Varacallo, 2019). The most commonly affected area of the femur includes the sub-trochanteric and diaphyseal regions along the lateral cortex. Before starting therapy, patients must be counseled to seek care for any sudden onset of thigh discomfort immediately. Any patient on bisphosphonate therapy who presents with thigh discomfort should be educated to discontinue all weight-bearing activity until radiographic imaging rules out a fracture. Full-length femur and hip radiographs (x-rays) should be obtained, as thigh pain can be indicative of an impending AFF, and bisphosphonate therapy should be immediately discontinued. In many cases, a medullary nail is placed to provide fixation of the fracture and to allow for healing. Rehabilitation programs are often necessary for those who have had a complete fracture (Adler, 2018; Portal & Varacallo, 2019).
Patients should be counseled on the potential for the rare but serious adverse effect of medication-related osteonecrosis of the jaw (MRONJ) that increases with prolonged use of bisphosphonate therapy. MRONJ, formerly referred to as bisphosphonate-related osteonecrosis of the jaw (BRONJ), is a chronic condition that affects the oral cavity, leading to mucosal ulceration and refractory exposure of underlying necrotic bone. The mechanism by which bisphosphonate therapy causes MRONJ is not entirely understood but is likely due to a combination of factors, such as decreased bone remodeling, impairments in wound healing, and an antiangiogenic effect that is thought to induce ischemic changes followed by necrosis. When the blood supply to the area is diminished or lost, local traumatic insult ensues with subsequent necrosis (Payne et al., 2017). Symptoms of MRONJ may include pain at the affected site, presence of a periodontal pocket, abscess, infection, or numbness of the lower lip (Kishimoto et al., 2019).
If MRONJ is suspected, bisphosphonate therapy should be discontinued immediately, and the patient should be referred to an oral surgeon for evaluation regarding the need for surgical intervention. Nonsurgical management of MRONJ is aimed at improving symptoms and avoiding progression of the condition. Some treatment options include antimicrobial mouth rinses, pain control, antibiotics, and nutritional support. Local debridement of the exposed bone may be performed for disinfection and cleaning, or to reduce sharp bone edges and diminish soft tissue irritation (Kishimoto et al., 2019). Before starting any bisphosphonate or antiresorptive therapy, all patients must be fully informed and counseled on the importance of oral hygiene and routine dental care. It is advised that patients undergo regular dental prophylaxis and dental examination every six months. Prevention of MRONJ is possible in many cases, when proper care and dental treatment is performed. Nurses must also warn patients of the heightened risk for MRONJ with any dental extractions or dental implants. Patients should be advised to alert their dentist that they are taking bisphosphonate therapy (American Association of Oral and Maxillofacial Surgeons, 2014).
Selective Estrogen Receptor Modulators (SERMs)
Some types of SERMs, such as raloxifene (Evista) and conjugated estrogens/bazedoxifene (Duavee), have estrogen activity in the bone, which helps prevent bone loss, improve BMD, and decrease the risk of vertebral fracture. Conjugated estrogens/bazedoxifene (Duavee) is approved by the US Food and Drug Administration (FDA) for the prevention of postmenopausal osteoporosis only, whereas raloxifene (Evista) is approved for the prevention and treatment of osteoporosis in postmenopausal women (Rosen et al., 2019).
Raloxifene (Evista) is a SERM that acts as an agonist to estrogen receptors on bone cells to reduce osteoclast resorption. It is prescribed as an oral tablet taken once daily for the treatment or prevention of osteoporosis. It is contraindicated in women of childbearing potential and those who have had a prior deep venous thrombosis (DVT) or other venous thromboembolism (VTE), as it is associated with a nearly threefold increase in the occurrence of VTE. Patients should be counseled to immediately stop taking raloxifene (Evista) and seek emergency care if any other rare adverse effects occur, such as hemoptysis (coughing up blood), change in speech, vision, or coordination, pain or numbness in the chest, arm, or leg, as well as unexplained shortness of breath. The most common side effects are milder and less severe. These may include hot flashes, joint pains, nausea, dizziness, leg cramps, headache, and increased sweating, Once the medication is stopped, the benefits are lost within one to two years (AACE/ACE, 2016).
Denosumab (Prolia)
Denosumab (Prolia, Xgeva) is a fully human monoclonal antibody whose antiresorptive effects differ from bisphosphonates. It works by reducing "the differentiation of precursor cells into mature osteoclasts and decreases the function and survival of activated osteoclasts" (AACE/ACE, 2016, p. 20). Denosumab (Prolia) is considered a medication of choice for patients with renal insufficiency. Still, it is not recommended for dialysis patients or those with stage 5 kidney disease due to the high risk of hypocalcemia. Denosumab (Prolia) is injected subcutaneously once every six months and is approved for both male and female patients. Once denosumab (Prolia) therapy is initiated, discontinuation is not advised unless clinically necessary. Clinical trial data have demonstrated that if denosumab (Prolia) is stopped after two years of use, BMD declines back to baseline values (AACE/ACE, 2016). This medication also carries a risk for MRONJ and AFF at nearly the same rates as bisphosphonate therapy (Adler; 2018; Kishimoto et al., 2019).
Teriparatide (Forteo)
Teriparatide (Forteo) is an anabolic recombinant form of PTH that stimulates the osteoblasts to generate more bone. It is not indicated for use in the prevention of osteoporosis. Currently, teriparatide (Forteo) is the only medication approved by the FDA for the treatment of osteoporosis in both males and females that stimulates bone formation.
It is indicated to increase bone mass in male or female patients at high risk for fracture or have failed or been intolerant to prior pharmacologic therapies. It is a daily subcutaneous injection. The potential side effects include nausea, orthostatic hypotension, leg cramps, and rarely hypercalcemia and hypercalciuria. A serum calcium level should be drawn approximately 16 hours after administration. The drug comes with a boxed warning regarding a rare but potentially heightened risk for osteosarcoma; however, this has only been seen in rats. When treatment with teriparatide (Forteo) is stopped, BMD has been shown to decline quickly during the following year; however, fracture reduction may persist for one or two years. Teriparatide (Forteo) is not approved for use for greater than two years duration (AACE/ACE, 2016).
Calcitonin (Miacalcin, Fortical)
Calcitonin (Miacalcin, Fortical) is a synthetic hormone approved by the FDA for the treatment of osteoporosis in postmenopausal women. Calcitonin slows the breakdown of bone and helps increase bone density in the spine. Limitations for the use of calcitonin (Miacalcin, Fortical) are related to its efficacy. It produces minimal improvements in BMD within the spine but has not demonstrated efficacy in improving BMD at other skeletal sites. Calcitonin (Miacalcin, Fortical) is available in injectable and nasal spray recombinant formulations. It is dosed at 200 IU intranasally once daily or as a 100 IU subcutaneous injection every other day. The most common side effects of nasal calcitonin include rhinitis, epistaxis, headache, and back pain. Injectable calcitonin is associated with hypersensitivity reactions. Some patients experience flushing of the face and hands, urinary frequency, nausea, and skin rash (AACE/ACE, 2016).
Treatment Duration and Follow-Up
The duration of treatment varies depending on the class of medication, the patient's response to therapy, tolerance to the medication, and other specific factors such as cost and access to the medication. However, medications such as teriparatide (Forteo) and some other types of hormonal-based therapies generally require close follow-up treatment with another agent once stopping the medication. If not immediately followed up with another treatment, bone mass is often rapidly lost (Porter & Varacalla, 2019). Once pharmacologic therapy for osteoporosis is initiated, BMD should be evaluated with a DXA scan once every two years to monitor response to treatment (NOF, 2020).
Nonpharmacologic Interventions
The nurse should advise all patients on the importance of consuming a healthy diet that includes adequate amounts of calcium and vitamin D. The Institute of Medicine (IOM) “recommends dietary calcium intake should be limited to 1,000 mg daily for men 50 to 70 years of age and 1,200 mg daily for women 51 years of age and older and for men 71 years of age and older" (Tu et al., p.95). Patients who are unable to meet the daily recommended calcium and vitamin D quantities through their diet should be advised to take calcium 500 to 600 mg with vitamin D3 supplementation twice daily. As noted in Table 2, to ensure optimal absorption, calcium supplementation should not exceed 600 mg per dose, irrespective of the preparation (Mueller, 2017). Patients with osteoporosis should be educated on the importance of low-intensity exercise. They should be instructed to engage in 120 to 300 minutes of at least moderate-intensity aerobic or weight-bearing activity each week. This type of exercise can reduce the risk of hip fracture and build bone mass. Performing balance and muscle-strengthening activities can also help to reduce the risk of falls in older adults (CDC, 2018). Table 4 summarizes other beneficial nonpharmacologic interventions.

Risk Reduction and Prevention
Many interventions can be taken throughout the lifespan to keep bones healthy and strong and prevent osteoporosis development, which mirror nonpharmacological interventions for the treatment of osteoporosis outlined above and listed in Table 4. Particularly important aspects of prevention are adequate calcium intake and physical activity during adolescence and young adulthood. Building strong bones during childhood and adolescence helps to prevent osteoporosis later in life. Table 5 outlines the critical patient counseling points on steps to take to reduce the risk of falls, which significantly heightens the risk for bony fractures. Clinical issues that increase fall risk in patients with osteoporosis include difficulty with balance or gait, orthostatic hypotension, lower extremity weakness, poor vision or hearing, and cognitive impairment (NIAMS, 2019).

References
Adler, R. A. (2018). Atypical femoral fractures: Risks and benefits of long-term treatment of osteoporosis with anti-resorptive therapy. Management of Endocrine Disease, 178(3), R81-R87. https://doi.org/10.1530/EJE-17-1002
American Academy of Family Physicians. (2019). AAFP conditions A-Z. Stat!Ref Online Electronic Medical Library. https://online.statref.com/document/ZEHQxHwd2nQ4KgCIzBhuwj!!
American Association of Clinical Endocrinologists and American College of Endocrinology (2016). Clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocrine Practice, 22(Suppl 4), 1-42. https://doi.org/10.4158/EP161435.GL
American Association of Oral and Maxillofacial Surgeons. (2014). Position paper: Medication-related osteonecrosis of the jaw-2014 update. https://www.aaoms.org/docs/govt_affairs/advocacy_white_papers/mronj_position_paper.pdf
American Nurses Association. (n.d.). Advanced practice registered nurse (APRN). Retrieved January 5, 2020 from https://www.nursingworld.org/practice-policy/workforce/what-is-nursing/aprn/
American Society of Obstetricians and Gynecologists. (2018). Osteoporosis. https://www.acog.org/Patients/FAQs/Osteoporosis?IsMobileSet=false
BruceBlaus. (2014). DXA bone density scan [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Blausen_0095_BoneDensitometryScan.png
BruceBlaus. (2016). Normal bone and osteoporosis [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Osteoporosis_Locations.png
Cancer Research UK. (2014). The process of bone remodeling [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Diagram_showing_bone_remodelling_Fig_CRUK_112.svg
The Centers for Disease Control and Prevention. (2016). Hip fractures among older adults. https://www.cdc.gov/HomeandRecreationalSafety/Falls/adulthipfx.html
The Centers for Disease Control and Prevention. (2018). Physical activity and health. https://www.cdc.gov/physicalactivity/basics/pa-health/index.htm
The Centers for Disease Control and Prevention. (2019). Does osteoporosis run in your family? https://www.cdc.gov/genomics/disease/osteoporosis.htm
Hans, D., & Balm, S. (2017). Quantitative ultrasound (QUS) in the management of osteoporosis and assessment of fracture risk. Journal of Clinical Densitometry, 20(3), 322-333. https://doi.org/10.1016/j.jocd.2017.06.018
IBM Micromedex Solutions. (2020). Prednisone. https://www.micromedexsolutions.com/micromedex2/librarian/CS/9D6EF8/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/F2078F/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.DoIntegratedSearch?SearchTerm=PREDNISONE&UserSearchTerm=PREDNISONE&SearchFilter=filterNone&navitem=searchALL#
Ignatavicius, D. D., & Workman, L. (2015). Medical-surgical nursing: Patient-centered collaborative care (8th ed.). Elsevier.
Jmarchn. (2015). DXA scan results: Osteopenia of the lumbar spine [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:DXA_Lumbar_vertebral_column_Osteopenia_es.png
Katsimbri, P. (2017). The biology of normal bone remodeling. European Journal of Cancer Care, 26(6). https://doi.org/10.1111/ecc.12740
Kenkre, J. S., & Bassett, J. (2018). The bone remodeling cycle. Annals of Clinical Biochemistry, 55(3), 308-327. https://doi.org/10.1177/0004563218759371
Kishimoto, H., Noguchi, K., & Takaoka, K. (2019). Novel insight into the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Japanese Dental Science Review, 55(1), 95-102. https://doi.org/10.1016/j.dsr.2018.09.002
Lewiecki, E. M., Ortendahl, J. D., Vanderpuye-Orgle, J., Grauer, A., Arellano, J., Lemay, J., Harmon, A. L., Broder, M. S., & Singer, A. J. (2019a). Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus (WOA), 3(9), 1-7. https://doi.org/10.1002/jbm4.10192
Lewiecki, E. M., Rosen, C. J., Schmader, K. E., & Mulder, J. E. (2019b). Osteoporotic fracture risk assessment. UpToDate. https://www.uptodate.com/contents/osteoporotic-fracture-risk-assessment?search=Osteoporotic%20%20fracture%20risk%20assessment.&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
Lindsay, R. & Cosman, F. (2018). Osteoporosis. In J. L. Jameson, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, & J. Loscalzo (Eds.), Harrison's principles of internal medicine (20th ed.). McGraw-Hill Education.
Mueller, C. M. (2017). The ASPEN adult nutrition support core curriculum. (3rd ed.). American Society for Parenteral and Enteral Nutrition.
National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2018). Bone mass measurement: What the numbers mean. https://www.bones.nih.gov/health-info/bone/bone-health/bone-mass-measure#c
National Institute of Arthritis and Musculoskeletal and Skin Diseases. (2019). Osteoporosis. https://www.niams.nih.gov/health-topics/osteoporosis#tab-overview
National Osteoporosis Foundation. (n.d.). Osteoporosis fast facts. Retrieved January 5, 2020 from https://cdn.nof.org/wp-content/uploads/2015/12/Osteoporosis-Fast-Facts.pdf
National Osteoporosis Foundation. (2019). Medicare cost of osteoporotic fractures. https://static1.squarespace.com/static/5c0860aff793924efe2230f3/t/5d76b949deb7e9086ee3d7dd/1568061771769/Medicare+Cost+of+Osteoporotic+Fractures+20190827.pdf
National Osteoporosis Foundation. (2020). What is osteoporosis and what causes it? https://www.nof.org/patients/what-is-osteoporosis/
OpenStax College. (2013). The relationship between age and bone mass [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:615_Age_and_Bone_Mass.jpg
Payne, K. F., Goodson, A. M., Tahim, A. S., Rafi, I., & Brennan, P. A. (2017). Why worry about bisphosphonate-related osteonecrosis of the jaw? A guide to diagnosis, initial management, and referral of patients. British Journal of General Practice, 67(660), 330-331. https://doi.org/10.3399/bjgp17X691565
Pbroks13. (2008). Cross-section of a bone [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Bone_cross-section.svg
Porter, J. L., & Varacallo, M. (2019). Osteoporosis. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK441901/
Rosen, H. N., Rosen, C. J., & Mulder, J. E. (2019). Selective estrogen receptor modulators for prevention and treatment of osteoporosis. UpToDate. https://www.uptodate.com/contents/selective-estrogen-receptor-modulators-for-prevention-and-treatment-of-osteoporosis
Rowe, P., & Sharma, S. (2019). Physiology, bone remodeling. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK499863/
Tu, K. N., Lie, J. D., Wan, C. K., Cameron, M., Austel, A. G., Nguyen, J. K., Van, K., & Hyun, D. (2018). Osteoporosis: A review of treatment options. Pharmacy & Therapeutics, 43(2), 92-104. https://www.ncbi.nlm.nih.gov/pubmed/29386866
US Preventative Services Task Force. (2018). Final recommendation statement: Osteoporosis to prevent fractures: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/osteoporosis-screening1