About this course:
This module aims to provide an overview of ovarian cancer, its risk factors, signs and symptoms, and treatment options to enrich nursing knowledge of the condition, patient education, and nursing practice.
Course preview
This module aims to provide an overview of ovarian cancer, its risk factors, signs and symptoms, and treatment options to enrich nursing knowledge of the condition, patient education, and nursing practice.
By the completion of this module, the nurse should be able to:
- discuss the epidemiology of ovarian cancer in the US and review the risk and protective factors for the development of the disease
- review the pathophysiology of ovarian cancer, identify the signs and symptoms, and ovarian cancer subtypes
- discuss treatment options for ovarian cancer, including surgery, chemotherapy, targeted therapy, immunotherapy, and hormonal therapy, as well as their most common side effects and components of patient education
Ovarian cancer is the second most common gynecologic malignancy in the US and leads to more deaths than any other female reproductive cancer. Ovarian cancer is a disease in which malignant (cancerous) cells originate within the ovaries, fallopian tubes, or peritoneum (primary peritoneal cancer [PPC]). These three cancers are grouped into one disease based on their anatomical proximity and similarities in risk factors and medical treatments. To simplify the terminology in this module, ovarian cancer describes all three cancers unless otherwise specified (Centers for Disease Control and Prevention [CDC], 2019b; National Comprehensive Cancer Network [NCCN], 2020).
Epidemiology
According to the American Cancer Society (ACS, 2020a), there will be an estimated 21,750 new cases and 13,940 deaths from ovarian cancer this year. While ovarian cancer is uncommon, accounting for only 1.2% of all new cancer diagnoses in the US, it ranks fifth among cancer-related deaths in women and is the most lethal gynecological malignancy. Based on data from the National Cancer Institute’s (NCI, 2020b) Surveillance, Epidemiology, and End Results Program (SEER), the median age at diagnosis is 63; it is most frequently diagnosed in women aged 55 to 64 (24.7%), followed by women aged 65 to 74 (23.1%). The average lifetime risk for developing ovarian cancer among the general US population is roughly 1 in 78, and the lifetime risk of dying from the disease is around 1 in 108. The median age at death is 70 years, with the highest percentage of deaths among women aged 65 to 74 (28.4%), followed by those aged 75 to 84 (24.4%). The 5-year overall survival rate for ovarian cancer is 48.6%, which drops to 30.2% for patients with distant metastases (i.e., cancer spread) at diagnosis. Incidence rates are highest for White women (11.7 per 100,000), followed by Hispanic (10.4 per 100,000), Asian/Pacific Islander (9.5 per 100,000), and Black women (9.1 per 100,000). American Indian/Alaskan Natives have the lowest incidence, with 8.4 per 100,000 women. Mortality rates are also highest for White women (6.9 per 100,000) and lowest for Asian/Pacific Islanders (4.4 per 100,000). Statistical analyses demonstrate an overall decline in new diagnoses and ovarian cancer-related deaths over the last decade. Between 2008 and 2017, the age-adjusted rates for new ovarian cancer diagnoses have decreased on average by 2.5% each year, and death rates have fallen by 2.3% per year between 2009 and 2018 (NCI, 2020b).
Risk and Protective Factors
All women are at risk for ovarian cancer, and the risk increases with age. Hormonal, environmental, and genetic risk factors all serve roles in the development of the condition. Although most women are diagnosed in the absence of identifiable risk factors, several influences can increase or decrease a person’s risk of developing the disease. Table 1 describes the major risk and preventative factors (Yarbro et al., 2018).


Inherited Risk
Up to 25% of ovarian cancers are part of familial cancer syndromes resulting from inherited mutations in specific genes. Approximately 3% of women with 2 or more first-degree relatives with ovarian cancer will have a hereditary cancer syndrome (Woo & Long, 2021). Mutations in BRCA1 and BRCA2 (BRCA1/2) genes commonly lead to hereditary breast and ovarian cancer syndrome (HBOC), a condition characterized by a heightened lifetime risk of developing breast cancer and ovarian cancer. Under physiologic conditions, BRCA1/2 genes function as tumor suppressor genes and promote the normal and healthy growth, development, and division of specific cells in the body. Mutations in these genes prevent them from working correctly, thereby increasing the propensity toward cancer development. Mutations in BRCA1/2 genes follow an autosomal dominant inheritance pattern in which a copy of the mutated gene in each cell is sufficient to increase the risk for developing cancer. Although ovarian cancer is a sex-dependent disease, the altered gene can be inherited from either parent. Each child of a parent with a BRCA1 or BRCA2 mutation has a 50% chance of inheriting the same gene mutation. Approximately 1 in 500 women in the US have a mutation in a BRCA gene. Mutations in BRCA1/2 are about 10 times more common in women of Ashkenazi Jewish descent than the general US population. According to the CDC (2020), about 10% of ovarian cancers (nearly 2,000 women per year) are attributed to inherited mutations in the BRCA1/2 genes. Patients with BRCA1/2 mutations are more likely to be diagnosed with high-grade serous ovarian cancer than other histologic subtypes (Temkin et al., 2018). Approximately 30 in 100 women with an inherited BRCA mutation will be diagnosed with ovarian cancer by 70 years of age. A mutation in the BRCA1 gene is more closely linked to ovarian cancer, posing a lifetime risk between 35% and 70%. Women with BRCA1 mutations tend to be diagnosed with ovarian cancer approximately a decade younger than women with ovarian cancer without an identifiable genetic mutation (Temkin et al., 2018). Women with a BRCA2 mutation have a 10% to 30% lifetime risk of developing ovarian cancer by age 70. For comparison, the lifetime risk of ovarian cancer among women in the general US population is under 2% (CDC, 2019a, 2020; NCCN, 2021).
Hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome, is most commonly known for its high-risk association with colorectal cancer; however, it is also associated with an increased risk of ovarian cancer and endometrial (uterine) cancer. The lifetime risk of ovarian cancer in women with HNPCC is 10%. Although changes in the MLH1, MSH2, MSH6, or PMS2 genes are most common, many different genes can cause this syndrome. Under physiologic conditions, these genes are responsible for repairing potential errors during DNA replication (the process during which DNA is copied in preparation for cell division); collectively, they are known as mismatch repair (MMR) genes. Since mutations in any of these genes impede the cell’s’ ability to repair DNA replication errors, abnormal cells continue to divide. Over time, these accumulated DNA replication errors can lead to uncontrolled cell growth and an increased propensity for cancer development (US National Library of Medicine [NLM], 2020a). MUTYH-associated polyposis (MAP) is a disorder caused by a mutation in the MUTYH gene, which prevents cells from remedying errors made during DNA replication. As these errors accumulate, more polyps develop, conferring an increased likelihood of cancer. Pathogenic mutations in the MUTYH gene are associated with a 6% to 14% lifetime risk for ovarian cancer with a median age at diagnosis of 51 years (Nielson et al., 2019). Additional genes linked to ovarian cancer with evolving clinical evidence include the following: ATM, BRIP1, NBN, STK11, RAD51C, RAD51D, and PALB2 (Kurian et
...purchase below to continue the course
Tumor Protein p53 (TP53)
Somatic (or acquired) mutations occur during a person’s lifetime due to environmental exposures, such as ultraviolet radiation from the sun, free radicals, carcinogen exposure, ionizing background radiation, and chemical exposure. Somatic mutations only occur in specific cells in the body. Unlike inherited mutations, these changes are not hereditary or passed to subsequent generations. TP53 gene mutations are common in ovarian cancer, occurring in at least half of diagnoses. Under healthy conditions, TP53 functions as a tumor suppressor gene, regulating cellular growth and division by preventing cells from growing and dividing too quickly or uncontrollably. TP53 prevents cells with mutated or damaged DNA from dividing, thereby avoiding the development of tumors. Mutations in TP53 impair its ability to control cell proliferation, and it becomes unable to trigger apoptosis in cells with mutated or damaged DNA. Consequently, DNA damage accumulates in cells, and they continue to divide in an uncontrolled way, leading to tumor growth (NLM, 2020b; Oien & Chien, 2016).
Pathophysiology
The ovaries and fallopian tubes are primary components of the female reproductive system. The ovaries, or female gonads, are a pair of organs located in the pelvis in a region called the ovarian fossa. The ovaries appear on either side of the uterus, which is the hollow, pear-shaped organ that carries a fetus. The ovaries are attached to the uterus by the ovarian ligament, which is embedded within the broad ligament. Figures 1 and 2 demonstrate the anatomical structures and positioning of the female reproductive system (McCance & Heuther, 2019).
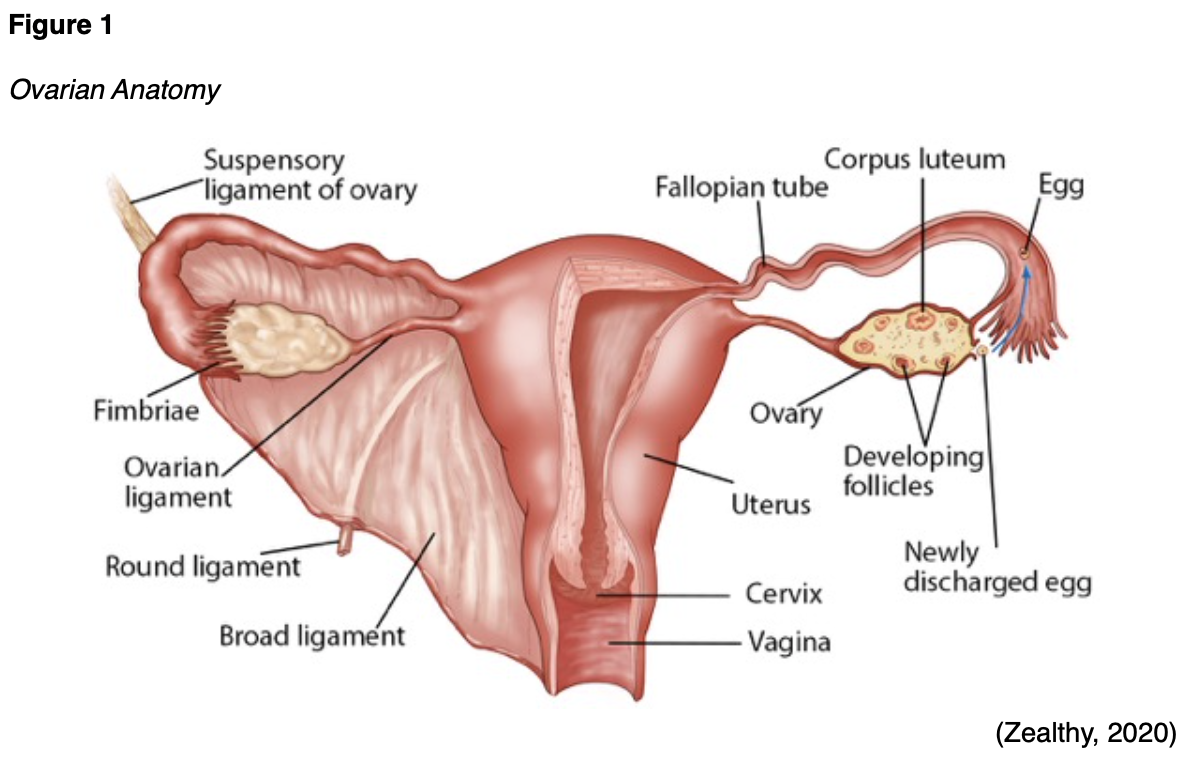
The ovaries serve two primary functions: secretion of the female sex hormones and development and release of the female ova (or egg). Oocytes are immature egg cells that undergo development and maturation within the ovarian follicles. The ovaries are comprised of three major types of cells; epithelial, germ, and stromal. The tunica albuginea is a thin layer of dense connective tissue covering the ovary’s outermost aspect. The cortex appears directly beneath the tunica albuginea and consists of a framework of tissues called the ovarian stroma. The cortex forms the bulk of the ovary and nurtures the follicles at various stages of maturation. The medulla is the middle layer of the ovary; it is comprised of loose connective tissues, elastic fibers, and neurovascular structures, including the lymph and blood vessels. The hilum is the ovary’s innermost layer and provides the entry point for the blood vessels and nerves (Lumen Learning, n.d.; McCance & Heuther, 2019).

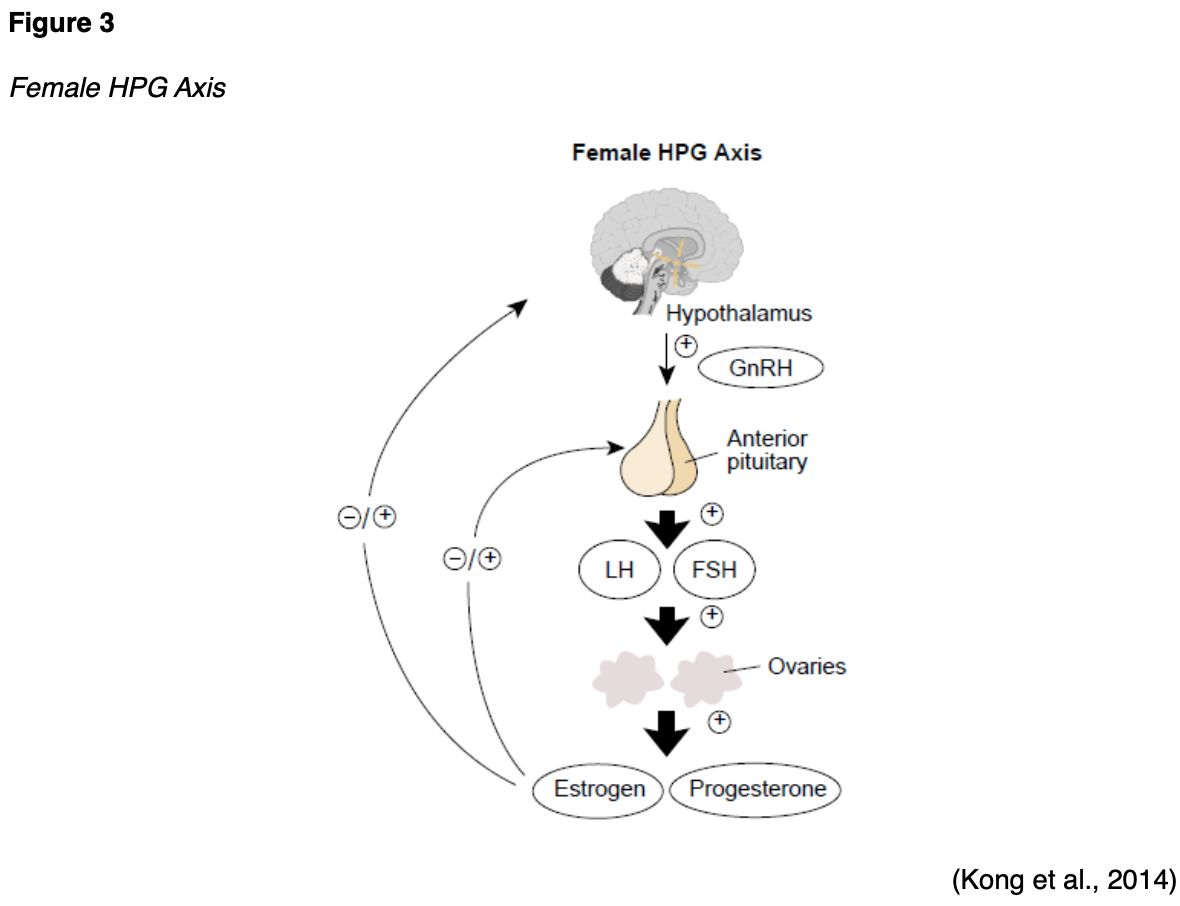
The hypothalamic-pituitary-gonadal (HPG) axis is a tightly regulated feedback system that controls female sex hormones and reproduction. As displayed in Figure 3, the HPG forms a complex interplay between the organs of the female endocrine system, including the hypothalamus, pituitary gland, and ovaries. During puberty, increasing levels of gonadotropin-releasing hormone (GnRH) are released by the hypothalamus. In response, the anterior pituitary produces follicle-stimulating hormone (FSH) and luteinizing hormone (LH), prompting the ovaries to secrete increasing sex hormones, primarily estrogen and progesterone, along with a smaller quantity of testosterone (Lumen Learning, n.d.; McCance & Heuther, 2019).
Ovarian follicles are tiny fluid-filled sacs composed of granulosa cells, theca cells, and oocytes, varying according to each oocyte’s’ maturation stage. Folliculogenesis (the maturation of ovarian follicles) involves several growth factors and receptors. This process relies primarily on the HPG axis, as coordinated interactions between the granulosa and theca cells are necessary for estrogen synthesis. Granulosa cells form alongside the maturing follicle, as demonstrated in Figure 4. FSH has a high affinity for granulosa cells, promoting follicle growth and maturation by synthesizing androgens (testosterone) and estrogen. Theca cells consist of connective tissue and blood vessels that surround the ovarian follicle. They produce estradiol from androgens in response to LH. Estradiol is an estrogen steroid hormone that binds to tissues in the body, serving as the body’s primary estrogen source during reproductive years. Estradiol influences secondary sex characteristics such as breast growth, body shape, muscle mass, fat deposition, body hair, and bone health and regulates the menstrual cycle. It also serves a vital role in pregnancy. When the maturation process is complete, the oocyte becomes an ova. The pituitary gland releases a surge of LH, thereby stimulating the follicle’s’ rupture and release of the ova (Holesh et al., 2020; Lumen Learning, n.d.).

The ovaries typically alternate releasing a mature egg every month; however, if an ovary is dysfunctional or absent, the other ovary will take over. The ovaries change in size and appearance throughout the patient’s lifetime. Their size and appearance depend on the individual’s’ menstrual status. Menopause denotes menstruation’s permanent cessation in females with an intact uterus, and it is defined as 12 consecutive months without a menstrual cycle (Holesh et al., 2020). According to the Office on Women’s Health (OWH, 2019), the average age for menopause in the US is 52 years, ranging between 45 and 58 years. In premenopausal women (women of reproductive age who are menstruating), each ovary is comparable in size and shape to an almond, measures 3 to 5 cm long, and weighs 4 to 8 g. Normal ovarian volume starts to decrease after the age of 30. During menopause, reproductive hormone levels decline, egg production ceases, and the ovaries shrink in size as they undergo progressive involution (OWH, 2019).
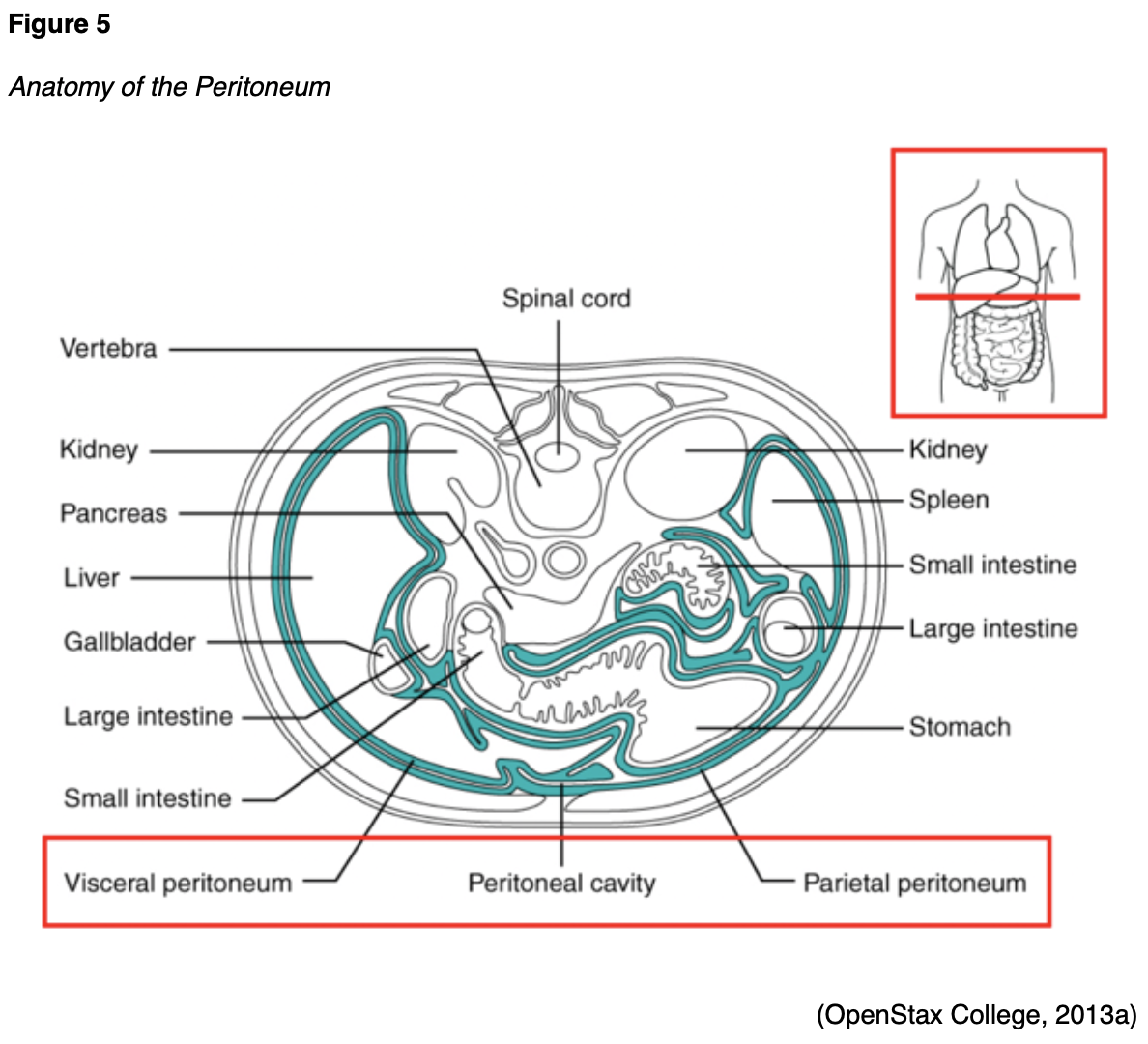
The fallopian tubes are a pair of long, slim conduits on either side of the uterus that transport eggs from the ovaries to the uterus. The tubes attach to the uterus and curve up and over the ovaries, as shown in Figure 1. Each tube is 8 to 12 cm in length and 1 cm in diameter; they flare into a bell shape at the ovarian end, which is called the infundibulum. The infundibulum contains fimbriae, which are swaying fringes that create a current to draw the ova into them. Once the ova enter the fallopian tube, cilia and peristalsis gradually propel them toward the uterus. The omentum is a layer of adipose (fatty) tissue that covers the intestines like an apron and is subdivided into the greater omentum (which hangs over the stomach) and the lesser omentum (which hangs over the liver). The peritoneum is a cellophane-like, moist sheet of tissue covering the surface of the organs in the abdomen and pelvis. As demonstrated in Figure 5, it provides support and protection to the organs and consists of 2 layers: the visceral peritoneum and the parietal peritoneum. The visceral peritoneum is the inner layer of tissue lining the organ. The parietal peritoneum is the outer layer that adheres to the abdominal cavity wall. The space between the layers is called the peritoneal cavity, which contains a small quantity of lubricating fluid (McCance & Heuther, 2019).
Ovarian Cancer
Many types of ovarian cancer begin in the distal portions of the fallopian tubes and spread to the ovary. Emerging research has found the fallopian tubes may be the source of many hereditary ovarian cancers. PPC appears to start in the cells lining the fallopian tubes, subsequently spreading to the ovaries (ACS, 2020b). As a disease, ovarian cancer is characterized by rapid growth, disseminated metastasis, and malignant ascites. Ascites involves the abnormal buildup of fluid within the abdominal or pelvic cavity. Without an anatomic barrier around the primary tumor, cancer cells easily infiltrate the peritoneal cavity, making the peritoneum and omentum the most common sites of initial metastases. Metastases often occur by direct extension: cancer penetrates and ruptures the ovarian capsule, thereby permitting the invasion of surrounding structures. Ovarian cancer cells uniquely can survive after detaching from the primary tumor by forming multicellular spheroids (spherical cellular aggregates). The spheroids can float in ascitic fluid, making the intraperitoneal cavity a predominant metastasis site. The continuous circulation of free peritoneal fluid facilitates the widespread dissemination of cancerous cells, a process referred to as peritoneal seeding. As the disease progresses, additional ascites accumulates, facilitating the spread of the cancer cells to more distant sites in the abdomen (Motohara et al., 2018). Ovarian cancer cells can also travel to distant sites in the body through the lymphatics, most commonly the pelvic or aortic lymph nodes. Common metastatic disease sites include the liver, pleura (serous membrane lining the lungs), diaphragm, intestines, spleen, brain, bladder, and skin (Mitra, 2016; Yarbro et al., 2018). Although PPC tends to spread along the pelvic and abdomen cavity surface, the precise origin is difficult to determine (ACS, 2020b).
Ovarian cancer is classified into type I and type II. Type I cancers tend to be early stage, low-grade tumors that generally progress from benign precursor lesions (i.e., endometriosis). These cancers tend to remain indolent or grow slowly. In contrast, most ovarian cancers are type II, characterized by a more aggressive nature and rapid growth rate. Type II cancers are primarily diagnosed at advanced stages and account for most ovarian cancer-related deaths. They usually originate in the fallopian tube or the ovary’s surface epithelium and are commonly associated with BRCA1/2 genetic mutations (Salazar et al., 2018; Yarbro et al., 2018).
Epithelial Ovarian Cancer
The most common type of invasive ovarian cancer is epithelial cell tumors, which collectively account for at least 90% of cases and include PPC and fallopian tube carcinomas. Epithelial ovarian tumors occur in 5 major subtypes, as demonstrated in Table 2 (Salazar et al., 2018; Yarbro et al., 2018).

Less common types of ovarian cancer include germ cell tumors, sex-cord stromal cell tumors, and small cell carcinoma of the ovary.
Germ Cell Tumors
Germ cell tumors start from the cells that form the ova, and most cases are benign. Mature teratomas are the most common type of benign germ cell tumors and are usually treated surgically. Malignant ovarian germ cell tumors account for only about 5% of ovarian cancers. Unlike epithelial ovarian cancers, these characteristically occur in adolescents and premenopausal women. They are often confined to an ovary and are usually curable. Immature teratomas are malignant tumors that primarily develop in females under the age of 18. These can metastasize (spread) to other areas in the body. Dysgerminomas are the most common type of malignant germ cell tumors. They usually affect females under the age of 30 and are cured with surgery; under 25% of cases require additional treatment following surgery (ACS, 2020b; Ovarian Cancer Research Alliance [OCRA], 2020; Yarbro et al., 2018).
Sex-Cord Stromal Cell Tumors
Ovarian sex-cord stromal tumors are rare, accounting for under 2% of all malignant ovarian tumors. These tumors originate in the stroma cells that hold the ovary together and produce the female sex hormones. The most common subtype is the granulosa cell tumor. Patients with stromal tumors typically present with signs of hormonal production like hirsutism (excessive coarse hair growth on unexpected areas of the body such as the face or chest) and virilization. Virilization is a condition in which a female develops features of increased masculinization. Some of the most common symptoms include baldness, acne, deepening of the voice, increased libido (sex drive), decreased size of the breasts, and menstrual irregularities, including cessation of menses (ACS, 2020b; OCRA, 2020; Yarbro et al., 2018).
Small Cell Carcinoma
Small cell carcinoma of the ovary (SCCO) is a rare type of ovarian cancer, accounting for 0.1%. SCCO typically affects young women, as the median age of diagnosis is 23 years, and approximately 70% of patients have hypercalcemia (OCRA, 2020). Hypercalcemia of malignancy (HCM) is a complex metabolic disorder resulting from the destruction of bone and/or elevated renal absorption of calcium. HCM most commonly occurs in breast cancer, squamous cell lung cancer, and multiple myeloma. Symptoms of HCM are usually vague and can include anorexia, nausea, vomiting, constipation, malaise, polyuria, polydipsia, lethargy, and confusion. Depending on the severity, HCM is a life-threatening emergency. HCM is usually treated with initial aggressive fluid administration, followed by loop diuretics and bisphosphonate therapy. Bisphosphonates are bone-modifying agents that lower the patient’s calcium level by inhibiting osteoclasts and stabilizing the bone matrix by binding to calcium phosphate (Klemencic & Perkins, 2019).
Signs and Symptoms
Under 20% of ovarian cancers are detected in the early stages. Due to the ovaries’ anatomical location, deep within the pelvic cavity, it remains challenging to detect the condition in an early stage. An ovarian tumor often grows undetected until it becomes large enough to cause symptoms or be detected on a pelvic exam. Most patients with early stage ovarian cancer are asymptomatic. When symptoms do occur, they are often vague and nonspecific. Some of the most common symptoms include abdominal pain, bloating, pelvic pressure, bloating, low back discomfort, constipation, and fatigue. According to the National Ovarian Cancer Coalition [NOCC], symptoms are much more common in advanced disease when the tumor growth creates pressure on the bladder or rectum, and ascites develops. These processes can lead to persistent abdominal pain and distention, heartburn, early satiety, nausea, weight loss, urinary frequency or urgency, menstrual cycle irregularities, or pain with intercourse (NOCC, n.d.; Woo & Long, 2021).
CA-125
The CA-125 is a tumor marker for most ovarian cancers. Tumor markers are substances or proteins that may be produced by cancer or by the body’s response to cancer’s presence, but they are also made in smaller quantities by healthy cells. Tumor markers can be measured in the bloodstream or less commonly in the urine. While they are considered nonspecific and are not of significant value in isolation, they can be beneficial for establishing a baseline during the diagnostic workup, evaluating response to treatment, and detecting disease recurrence. Each tumor marker is specific to a different type of disease process (Yarbro et al., 2018). CA-125 is present on the surface of most ovarian cancer cells, and a blood test can measure the amount of CA-125 protein in the bloodstream. However, not all women with ovarian cancer will have elevated CA-125 levels. Small quantities of CA-125 are produced by healthy tissues and some other cancer types (American Association of Clinical Chemistry, 2020). CA-125 levels can rise in a variety of benign conditions and they can fluctuate with menstrual cycles (Yarbro et al., 2018). According to the American Board of Internal Medicine (2020), the normal CA-125 level is below 35 U/mL.
Ovarian Cancer Staging
The cancer stage at diagnosis guides treatment options and strongly influences overall survival. There are detailed staging systems for ovarian cancer: the International Federation of Gynecology and Obstetrics (FIGO, 2014) system and the American Joint Committee on Cancer’s (AJCC, 2017) Tumor, Node, Metastasis (TNM) staging system, 8th edition. Both systems are essentially the same and describe specific characteristics to assign stages I through IV as demonstrated in Figure 6. Cancer staging reflects the cell type, tumor grade, anatomical location of the tumor, and extent of malignancy. Within the TNM staging system, T denotes the size of the tumor and whether it has grown into nearby tissue, N refers to the presence of cancer in the lymph nodes, and M indicates if cancer has metastasized to other parts of the body beyond the origin site. Tumor grade measures how different the cancer cells look in comparison to healthy cells under the microscope. It is based on cell differentiation and varies from low-grade (grade 1) to high-grade (grade 3). Grade 1 is well-differentiated and appears similar to healthy cells, whereas grade 3 is poorly differentiated (i.e., does not resemble healthy cells) and aggressive (AJCC, 2017; FIGO, 2014; NCCN, 2020; Yarbro et al., 2018).
 Treatment Modalities
Treatment Modalities
Treatment for ovarian cancer is usually multifactorial, involving combined modalities, and primarily depends on the cancer stage. Due to the aggressive biology of ovarian cancer, treatment is chronic, and most patients experience periods of remission and relapse. Many require maintenance therapy, and all patients need close monitoring and surveillance after treatment is completed to monitor for recurrence. This section will provide an overview of the most common treatment strategies (NCCN, 2020).
Surgery
Surgery is the mainstay treatment for most ovarian cancers. When ovarian cancer is diagnosed early, standard therapy includes surgical staging, cytoreduction, hysterectomy, and bilateral salpingo-oophorectomy (BSO). The procedure may or may not include an omentectomy (removal of all or part of the omentum) and lymphadenectomy (removal of 1 or more lymph nodes to evaluate for cancer; NCCN, 2020).
Chemotherapy
Chemotherapy, also called cytotoxic or antineoplastic therapy, refers to a group of high-risk, hazardous medications that destroy cancer cells throughout the body. Chemotherapy works by interfering with the normal cell cycle, impairing DNA synthesis and cell replication to prevent cancer cells from dividing and multiplying. The majority of chemotherapy agents attack broadly (i.e., they kill normal, healthy cells in the body together with the cancer cells). Chemotherapy is classified according to biochemical activity, mechanism of action, and phase of action during the cell cycle. Chemotherapy agents are divided into two categories: cell cycle-specific and cell cycle-nonspecific. Cell cycle-specific drugs exert cytotoxic effects on cells that are actively dividing at specific stages within the cell cycle. These drugs do not act against cancer cells during the resting phase (G0) and are schedule-dependent, so they are most effective when administered in divided doses or by continuous infusion. Cell cycle-nonspecific drugs have a broader impact on cancer cells, as their cytotoxic effects affect cells at any phase in the cell cycle, including G0. These agents are considered dose-dependent and are most effective when administered by bolus doses, as the number of cells affected is directly proportional to the amount of drug given (Olsen et al., 2019).
Chemotherapy serves a prominent role in treating ovarian cancer and can be used at various time points during the disease trajectory. Neoadjuvant chemotherapy intends to reduce the tumor burden in preparation for surgery. Adjuvant chemotherapy (chemotherapy after surgery) aims to decrease cancer recurrence risk, reduce micro-metastases, and eradicate any remaining cancer cells. Palliative chemotherapy (or recurrence therapy) treats recurrent cancer, aiming to relieve or delay cancer symptoms, enhance comfort, reduce symptom burden, and improve quality of life. IV platinum-based chemotherapy agents (i.e., carboplatin [Paraplatin] or cisplatin [Platinol]) are highly effective against ovarian cancer. They are usually first-line treatment for ovarian cancer unless contraindicated and are usually combined with a microtubule agent such as paclitaxel (Taxol) or docetaxel (Taxotere). Some of the most common chemotherapy agents for ovarian cancer include the following:
- Alkylating agents
- disrupt DNA, prevent DNA replication, and are cell cycle-nonspecific
- carboplatin (Paraplatin)
- cisplatin (Platinol)
- ifosfamide (Ifex)
- cyclophosphamide (Cytoxan)
- disrupt DNA, prevent DNA replication, and are cell cycle-nonspecific
- Microtubule agents
- inhibit cell division and are cell cycle-specific (acting on the M-phase)
- paclitaxel (Taxol)
- docetaxel (Taxotere)
- inhibit cell division and are cell cycle-specific (acting on the M-phase)
- Antitumor antibiotics
- bind to DNA, unwind DNA helix, and are cell cycle-specific (acting on the S-phase)
- doxorubicin (Adriamycin)
- liposomal doxorubicin (Doxil; Nettina, 2019; NCCN, 2020; Olsen et al., 2019; Yarbro et al., 2018)
- bind to DNA, unwind DNA helix, and are cell cycle-specific (acting on the S-phase)
Chemotherapy Side Effects
The side effects of chemotherapy vary based on the drug type, dosage, duration of treatment, and specific patient factors. Since cancer cells divide rapidly, chemotherapy is primed to target cells that divide rapidly, thereby also impacting normal cells that divide quickly (e.g., in the GI tract, skin/hair cells, and bone marrow). As a group, the most common side effects include lowering of blood counts (anemia, thrombocytopenia, neutropenia), fatigue, nausea, anorexia, alopecia (hair loss), diarrhea, skin changes, and peripheral neuropathy (damage to the sensory nerves). Alopecia, or hair loss, deserves special attention because it can cause significant emotional distress to women. Chemotherapy-induced hair loss generally begins as hair thinning, about 7-15 days after the first dose. It results from damage to the dividing hair matrix cells, which causes the hair shaft to break at the follicular orifice or hair bulb. While the degree of hair loss depends on the chemotherapy agent, the dose, and the administration schedule, paclitaxel (Taxol) and docetaxel (Taxotere) are well-known for inducing alopecia. Nurses should reassure patients that their hair typically begins to regrow within a few weeks following the cessation of chemotherapy, as permanent alopecia following chemotherapy is rare (Olsen et al., 2019).
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of carboplatin (Paraplatin), cisplatin (Platinol), paclitaxel (Taxol), and docetaxel (Taxotere). It is often the dose-limiting toxicity (DLT) of these agents. DLTs are severe toxicities and side effects that are serious enough to warrant a reduction in the dose or discontinuation of the treatment. CIPN results from the demyelination of sensory and motor axons. Patients experience reduced nerve conduction velocity, leading to the loss of deep tendon reflexes and paresthesia (numbness and tingling), weakness, and burning pain. Initially, CIPN often affects the body’s most distal points, such as the fingertips and toes, and progresses proximally toward the midline. In severe cases, patients may lose all sensation in the fingers, hands, toes, and feet; this can cause significant disability, such as the inability to grasp or hold items and gait disturbance, including imbalance and falls. CIPN is a complex topic since no single pathophysiologic process explains the various neuropathies that occur following exposure to chemotherapy agents. The morbidity associated with CIPN can prompt pronounced reductions in quality of life and independence with activities of daily living (Brown et al., 2019).
Currently, no medications or supplements are effective in preventing CIPN. Regular exercise, reduced alcohol use, and treatment of preexisting medical conditions (vitamin B12 deficiency) may reduce the risk of CIPN. Management of CIPN is complex, and effective treatment options are limited. Pharmacologic treatment focuses on symptom relief, although many agents are not highly effective. Over-the-counter pain medications, menthol creams, capsaicin cream, or lidocaine patches may offer comfort. Some patients may be prescribed medications such as gabapentin (Neurontin), an anticonvulsant/anti-epileptic agent that also treats neuropathic pain. Some patients may find relief from selective serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine (Cymbalta). Patients with CIPN must be counseled on ways to avoid injury (e.g., wearing supportive shoes) and promote home safety (e.g., using handrails on stairs and removing throw rugs). Patients must also be mindful of water temperatures due to decreased sensitivity to hot water, increasing their risk for burns when bathing or washing dishes. Improvement in function and resolution of symptoms often occur gradually over time, but nerve damage may be permanent (Addington & Freimer, 2016; Brown et al., 2019).
Hypersensitivity Reactions to Chemotherapy
A hypersensitivity reaction (HSR) happens when the immune system becomes overstimulated by a foreign substance and creates antibodies, provoking an immune response. HSRs are commonly associated with several chemotherapy agents that are used widely in ovarian cancer treatment, such as paclitaxel (Taxol), docetaxel (Taxotere), and carboplatin (Paraplatin). HSR risk can be reduced by premedicating patients with corticosteroids, antihistamines, and/or acetaminophen (Tylenol). HSRs can occur during the initial chemotherapy infusion or after subsequent administrations of the same agent. Paclitaxel (Taxol) is well-known for its risk of a nearly immediate, acute HSR, whereas carboplatin (Paraplatin) more commonly induces an HSR after several cycles. The majority of HSRs occur during the first 15 minutes of the infusion. Initial signs and symptoms can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, hypotension, and anxiety. Patients may require supplemental oxygen, fluid resuscitation, or other emergency medications as indicated. For life-threatening symptoms like bronchospasm, angioedema (swelling of the oral cavity, lips, or tongue), or anaphylaxis, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) may be required (Nettina, 2019).
Intraperitoneal (IP) Chemotherapy
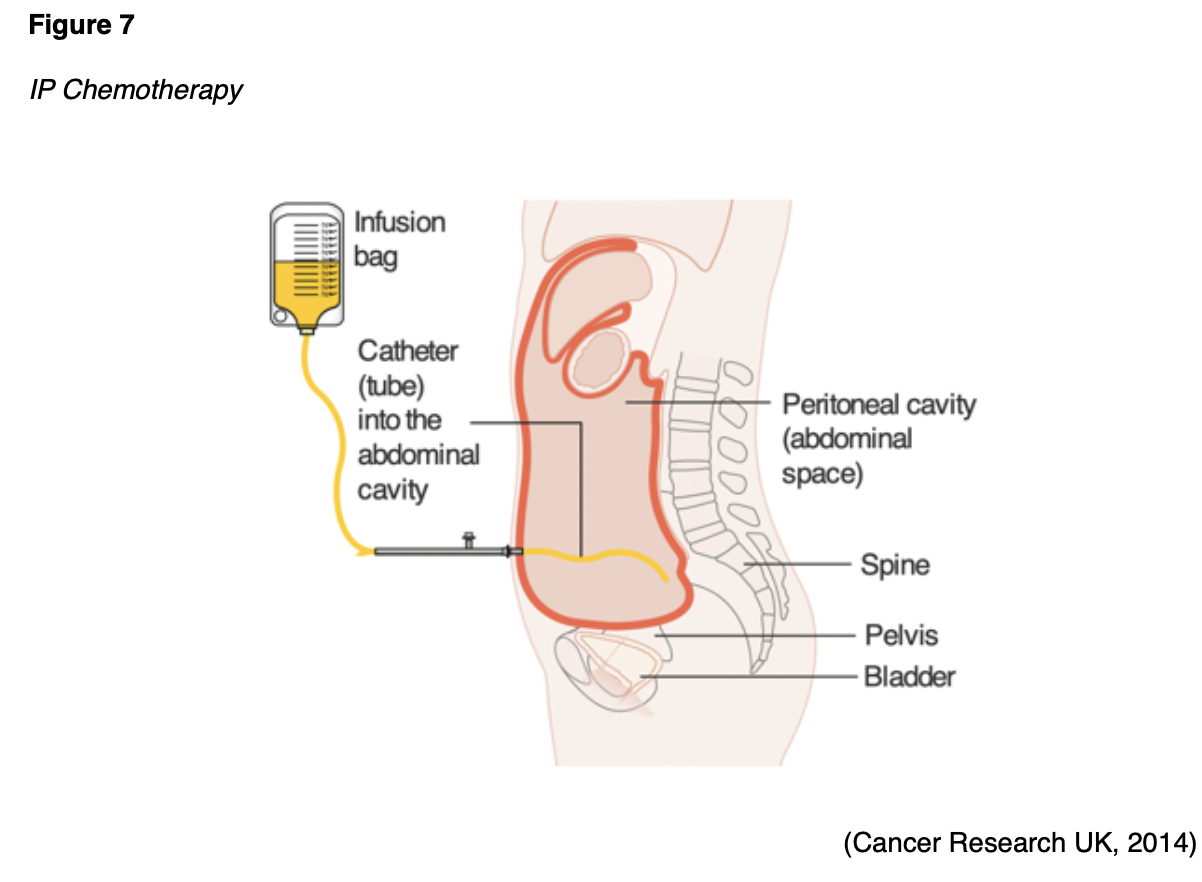
Chemotherapy for ovarian cancer may also be administered through the IP route. While IV chemotherapy is more common, a select subset of patients may benefit from IP chemotherapy. As demonstrated in Figure 7, IP chemotherapy is given through an implanted catheter and access port inserted into the peritoneal space. The medication is instilled directly into the port to target cancer within the peritoneal cavity. Several studies have demonstrated that the frequency and severity of toxic side effects are significantly higher in patients receiving IP compared to IV chemotherapy. Common side effects include abdominal pressure, bloating, urinary frequency, nausea, vomiting, and anorexia. IP chemotherapy carries a risk of peritonitis (inflammation of the peritoneum), which can be life-threatening and lead to sepsis. IP chemotherapy is also associated with prolonged bone marrow suppression and higher renal toxicity compared to IV chemotherapy (Eoh et al., 2017).
Targeted Therapy
Targeted therapies refer to a few classes of novel treatment modalities that function by attacking specific parts of cancer cells to prevent tumor development or shrink existing tumors. Numerous proteins are located on the cellular membranes; these growth factor receptors connect the external and internal cellular environments and are essential for cell growth and development. Targeted agents can block or turn off chemical signals that tell cancer cells to grow and divide. They can also starve the tumor by cutting off blood supply and preventing the formation of new blood vessels or by carrying toxins or poison directly to the cancer cells, thereby killing them without harming healthy cells (Sengupta, 2017). A significant portion of ovarian cancer research throughout the last decade has focused on the development of specialized drugs that block growth factor receptors. These drugs have revolutionized treatment options for patients living with advanced and metastatic ovarian cancer by fighting against drug resistance and increasing survival. The next section provides an overview of the most common types of targeted agents that treat ovarian cancer (Masoud & Pages, 2017; NCCN, 2020).
Anti-Angiogenesis Agents
Vascular endothelial growth factor (VEGF) is a signaling protein that stimulates angiogenesis (the formation of new blood vessels) in healthy and cancerous cells. Blood vessels carry oxygen and nutrients to the tissue for growth and survival. Tumors need blood vessels to grow and spread. Anti-angiogenesis is the process of inhibiting the formation of new blood vessels by blocking the VEGF receptors. Anti-angiogenesis agents (VEGF inhibitors) cut off blood supply to cancer cells by interfering with the VEGF receptor; tumors stay small and eventually starve (Olsen et al., 2019). Bevacizumab (Avastin) is a humanized monoclonal antibody that binds to and inhibits the activity of human VEGF to its receptors, thereby blocking the proliferation and formation of new blood vessels that supply tumor cells. The side effects of bevacizumab (Avastin) include bleeding, headaches, hypertension, and proteinuria (protein spilling into urine due to increased pressure within the kidneys). Nurses should educate patients on the risk of hypertension while on treatment with VEGF-inhibitors. Patients may require blood pressure management with antihypertensive medications or may need an adjustment to their current antihypertensive regimen. Patients should also be counseled on ways to reduce their blood pressure through compliance with medications, dietary adjustments such as following a heart-healthy diet high in fiber, fruits and vegetables, and low in sodium, and engaging in regular cardiovascular exercise. VEGF-inhibitors are contraindicated within 6 weeks of surgery (preoperatively or postoperatively) due to an increased risk for major bleeding events, delayed wound healing, and fistula (an abnormal connection between two hollow spaces within the body). VEGF-inhibitors carry a boxed warning for bowel perforation (a hole in the intestines). Nurses must ensure patients are aware of this rare but serious side effect. Patients must report any sudden onset of severe and diffuse abdominal pain, an abdomen that is unusually firm or hard to touch, bloating, nausea, vomiting, or rectal bleeding (Olsen et al., 2019).
Poly ADP-Ribose Polymerase (PARP) Inhibitors
The PARP enzyme serves a critical role in cell growth, cell regulation, and the repair of healthy cells and cancer cells. It fixes DNA damage in cancer cells, essentially helping cancer cells repair themselves and survive (Olsen et al., 2019). PARP inhibitors interfere with the PARP enzyme, hindering cancer cells with a BRCA1/2 mutation from repairing DNA damage, thereby inducing cell death. Currently, there are 3 PARP inhibitors approved to treat BRCA1/2-mutant ovarian cancers: olaparib (Lynparza), rucaparib (Rubraca), and niraparib (Zejula). The most common side effects include anemia, neutropenia, fatigue, nausea, diarrhea or constipation, anorexia, and arthralgias. These agents are also associated with a rare risk (under 1.5%) of myelodysplastic syndrome (MDS, a bone marrow failure disorder) or acute myeloid leukemia (AML, a type of blood cancer). They carry a slight risk (under 1%) of pneumonitis and embryo-fetal toxicity. Nurses should educate patients on reporting all new medications, as PARP inhibitors have several drug interactions with commonly prescribed antibiotics and antifungal agents. Patients should also be educated on avoiding grapefruit and Seville oranges, as they can increase PARP inhibitors’ effects and lead to toxicity (NCCN, 2020; Olsen et al., 2019; Ring & Modesitt, 2018).
Immunotherapy
Immunotherapy is a novel group of cancer treatments that stimulate the immune system to recognize and destroy cancer cells. Immunotherapy can produce antitumor effects by modifying the body’s natural host defense mechanisms, making them more sensitive to cancer cells. Immune-based treatments work differently than chemotherapy via highly specialized activity. Immune checkpoint inhibitors block the receptors that cancer cells use to inactivate immune cells (specifically T-cells). When this signal is blocked, T-cells can better differentiate between healthy cells and cancer cells, thereby augmenting the cancer cells’ immune response. Checkpoint inhibitors occur in 2 categories: 1 programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1) inhibitors and 2 cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitors. PD-1 is a transmembrane checkpoint protein expressed on the surface of circulating immune cells. PD-1 usually acts as an “off switch” to keep the immune cells from attacking other cells in the body. When PD-1 binds to PD-L1, it signals the T-cell to leave the neighboring cells alone. Some cancer cells have large amounts of PD-L1, which helps them evade immune attack. PD-1 and PD-L1 inhibitors prevent this complex formation and enable immune cells to continue attacking tumor cells. Monoclonal antibodies that target PD-1 or PD-L1 can block this binding and boost the immune response against cancer cells. Pembrolizumab (Keytruda) is a humanized monoclonal antibody that binds with high affinity to PD-1, thereby preventing its interaction with PD-L1 and PD-L2 (Sasikumar & Ramachandra, 2018).
The role of immunotherapy in the treatment of ovarian cancers is less advanced than for other diseases, and clinical research is ongoing. Microsatellite instability (MSI) occurs in about 20% of endometrioid ovarian cancers. Therefore, new guidelines suggest that microsatellite testing should be performed in all newly diagnosed endometrioid ovarian cancers (Fraune et al., 2020). ASCO recommends that all women with clear cell, endometrioid, or mucinous ovarian cancer should be tested for mismatch repair deficiency (dMMR). MMR deficiency can be reported as microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), but these groups have the same meaning (Konstantinopoulos et al., 2019). Currently, pembrolizumab (Keytruda) is the only agent used in this setting for metastatic ovarian tumors that are MSI-H or MMR-deficient (dMMR). Pembrolizumab (Keytruda) is generally well-tolerated; common side effects include fatigue, nausea, anorexia, coughing, diarrhea, a skin rash, and itching. However, patients may experience severe and possibly fatal autoimmune-related adverse effects (irAEs). Nursing care of the patient receiving immunotherapy requires cautious triage and continuous meticulous assessment to identify signs of potential irAE, as a timely diagnosis is critical to a prompt response and reduced morbidity. Patient education regarding the importance of self-assessment and the immediate reporting of any symptoms is vital. With pneumonitis, symptoms can range from mild cough and dyspnea to severe shortness of breath and life-threatening hypoxia. Gastrointestinal toxicity can range from mild diarrhea and abdominal cramping to severe colitis, which can be fatal if not managed. Skin toxicity may present initially as mild pruritus or dermatitis and can progress to Stevens-Johnson syndrome (SJS). SJS is characterized by a painful systemic red rash that leads to blistering and sloughing of the skin’s top layer. Life-threatening endocrinopathies can cause an abundance of varied symptoms, such as extreme weakness, excessive fatigue or lethargy, electrolyte disturbances, thyroid inflammation, and pituitary dysfunction (NCCN, 2020; Olsen et al., 2019; Sasikumar & Ramachandra, 2018; Sengupta, 2017).
For a more detailed review of the principles of chemotherapy, immunotherapy, and specific nursing implications, refer to the following NursingCE courses:
- Oncology Nursing Part 2: Chemotherapy and Oncologic Emergencies (5 ANCC contact hours)
- Oncology Medication Administration for LPNs and RNs (7 ANCC contact hours)
Hormonal Therapy
Hormonal treatments block certain hormones from reaching cancer cells or prevent the body from producing these hormones. Estrogen and estrogen receptors drive many types of ovarian cancers, making them amenable to hormone-blocking treatments to shrink or slow their growth. The three major types of hormonal therapy used in ovarian cancer treatment include selective estrogen receptor modulators (SERM), luteinizing hormone-releasing hormones (LHRH), and aromatase inhibitors (AI). These agents are outlined in Table 3 (Nettina, 2019; NCCN, 2020).

The most common adverse effects of hormonal therapies include hot flashes, night sweats, loss of libido, weight gain, joint aches or pains, mood changes, thinning or weakening of the bones (osteopenia or osteoporosis), and atrophic vaginitis (atrophy, loss of elasticity, dryness, and resulting irritation of the vaginal mucosa). Nurses should counsel patients on the importance of following a calcium-rich diet (at least 1,200 mg of calcium daily) and engaging in weight-bearing exercises to build strong bones (Olsen et al., 2019).
Screening and Early Detection
Extensive research has focused on identifying screening tests for ovarian cancer without success. The need for a reliable method for the early detection of ovarian cancer among asymptomatic women remains a prominent issue and the subject of ongoing research. The US Preventive Services Task Force (USPSTF, 2018), ACS (2020b), and Society of Gynecologic Oncology (SGO, 2017) recommend against screening for ovarian cancer in asymptomatic women without a known high-risk hereditary cancer syndrome due to unnecessary harms associated with screening; such as false-positive results leading to unnecessary diagnostic testing, psychological harm, and surgical interventions. Instead, the ACS (2020b) recommends that all women should undergo routine health maintenance with an annual physical exam, including a pelvic examination with a bimanual rectovaginal exam. Ovarian cancer screening guidelines for high-risk women are more complex and less fluid than those available for average-risk women. The ACS (2020b), USPSTF (2018), SGO (2017), and the American College of Obstetricians and Gynecologists (ACOG, 2017) collectively endorse that all women with a family history suspicious for an inherited BRCA1/2 gene mutation should be referred to a genetic counselor. Nurses serve a vital role in assisting patients with identifying their risk factors and coordinating necessary referrals to genetic counselors and specialists (Peshkin & Isaacs, 2020).
References
Addington, J., & Freimer, M. (2016). Chemotherapy-induced peripheral neuropathy: An update on the current understanding. F1000 Research, 5, 1466. https://doi.org/10.12688/f1000research.8053.1
American Association of Clinical Chemistry. (2020). CA-125. https://labtestsonline.org/tests/ca-125#
American Board of Internal Medicine. (2020). ABIM laboratory test reference ranges – January 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American Cancer Society. (2020a). Key statistics for ovarian cancer. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
American Cancer Society. (2020b). Ovarian cancer. https://www.cancer.org/cancer/ovarian-cancer.html
American College of Obstetricians and Gynecologists. (2017). Clinical management guidelines for obstetricians-gynecologists: Hereditary breast and ovarian cancer syndrome. Obstetrics & Gynecology, 130(3), e110-e126. https://www.sgo.org/wp-content/uploads/2012/09/PB-182.pdf
American Joint Committee on Cancer. (2017). AJCC cancer staging form supplement: AJCC cancer staging manual (8th ed.). https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf
Babic, A., Sasamoto, N., Rosner, B. A., Tworoger, S. S., Jordan, S. J., Risch, H. A., Harris, H. R., Rossing, M. A., Doherty, J. A., Fortner, R. T., Chang-Claude, J., Goodman, M. T., Thompson, P. J., Moysich, K. B., Ness, R. B., Kjaer, S. K., Jenson, A., Schildkraut, J. M., Titus, L., . . . & Cramer, D. W. (2020). Association between breastfeeding and ovarian cancer risk. JAMA Oncol, 6(6), e200421. https://doi.org/10.1001/jamaoncol.2020.0421
Brown, T. J., Sedhom, R., & Gupta, A. (2019). Chemotherapy-induced peripheral neuropathy. JAMA Oncology, 5(5),750. https://doi.org/10.1001/jamaoncol.2018.6771
Cancer Research UK. (2014). IP chemotherapy [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_how_you_have_chemotherapy_into_the_abdomen_CRUK_158.svg
Centers for Disease Control and Prevention. (2019a). BRCA gene mutations. https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/brca_gene_mutations.htm
Centers for Disease Control and Prevention. (2019b). Ovarian cancer. https://www.cdc.gov/cancer/ovarian/index.htm
Centers for Disease Control and Prevention. (2020). The BRCA1 and BRCA2 genes. https://www.cdc.gov/genomics/disease/breast_ovarian_cancer/genes_hboc.htm
Eoh, K. J., Lee, J., Nan, E. J., Kim, S., Kim, Y. T., & Kim, S. W. (2017). Long-term survival analysis of intraperitoneal versus intravenous chemotherapy for primary ovarian cancer and comparison between carboplatin- and cisplatin-based intraperitoneal chemotherapy. Journal of Korean Medical Science, 32(12), 2021-2028. https://doi.org/10.3346/jkms.2017.32.12.2021
Facing Our Risk of Cancer Empowered. (2020). Ovarian, fallopian tube, and primary peritoneal cancer risk management. https://www.facingourrisk.org/info/risk-management-and-treatment/ovarian-cancer-risk-factors
Fraune, C., Rosebrock, J., Simon, R., Hube-Magg, C., Makrypidi-Fraune, G., Kluth, M., Buscheck, F., Hoflmayer, D., Schmalfeldt, B., Muller, V., Wolber, L., Witzel, I., Paluchowski, P, Wilke, C., Heilenkotter, U., von Leffern, I., Clauditz, T. S., Wilczak, W., Sauter, S., & Burandt, E. (2020). High homogeneity of MMR deficiency in ovarian cancer. Gynecologic Oncology, 156(3), 669-675. https://doi.org/10.1016/j.ygyno.2019.12.031
Holesh, J. E., Bass, A. N., & Lord, M. (2020). Physiology, ovulation. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK441996/
International Federation of Gynecology and Obstetrics. (2014). FIGO ovarian cancer staging. https://www.sgo.org/wp-content/uploads/2012/09/FIGO-Ovarian-Cancer-Staging_1.10.14.pdf
Klemencic, S., & Perkins, J. (2019). Diagnosis and management of oncologic emergencies. West J Emerg Medicine, 20(2), 316-322. https://doi.org/10.5811/westjem.2018.12.37335
Kong, L., Zhang, T., Tang, M., & Wang, D. (2014). Female HPG axis [image]. https://commons.wikimedia.org/wiki/File:Hypothalamic%E2%80%93pituitary%E2%80%93gonadal_axis_in_females.png
Konstantinopoulos, P. A., Norquist, B., Lacchetti, C., Armstrong, D., Grisham, R. N., Goodfellow, P. J., Kohn, E. C., Levine, D. A., Liu, J. Y., Lu, K. H., Sparacio, D., & Annunziata, C. M. (2019). Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guidelines. Journal of Clinical Oncology, 38(11), 1222-1245. https://doi.org/10.1200/JC0.19.02960
Kurian, A. W., Hughes, E., Handorf, E. A., Gutin, A., Allen, B., Hartman, A., & Hall, M. J. (2017). Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequences results in women. JCO Precision Oncology, 1, 1-12. https://doi.org/10.1200/PO.16.00066
Lumen Learning. (n.d.). Boundless anatomy and physiology: The female reproductive system. Retrieved October 10, 2020, from https://courses.lumenlearning.com/boundless-ap/chapter/the-female-reproductive-system/
Masoud, V., & Pages, G. (2017). Targeted therapies in breast cancer: New challenges to fight against resistance. World Journal of Clinical Oncology, 8(2), 120-134. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5385433/
Mayo Clinic. (2020). Prophylactic oophorectomy: Preventing cancer by surgically removing your ovaries. https://www.mayoclinic.org/tests-procedures/oophorectomy/in-depth/breast-cancer/art-20047337
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). Elsevier.
Mitra, A. K. (2016). Ovarian cancer metastasis: A unique mechanism of dissemination. https://www.intechopen.com/books/tumor-metastasis/ovarian-cancer-metastasis-a-unique-mechanism-of-dissemination
Motohara, T., Masuda, K., Morotti, M., Zheng, Y., E-Sahhar, S., Chong, K. Y., Wietek, N., Alsaadi, A., Karaminejadranjbar, M., Hu, Z., Artibani, M., Gonzalez, L. S., Katabuchi, H., Saya, H., & Ahmed, A. A. (2018). An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene, 38, 2885-2898. https://doi.org/10.1038/s41388-018-0637-x
National Cancer Institute. (2018). Oral contraceptives and cancer risk. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet
National Cancer Institute. (2020a). Ovarian, fallopian tube, and primary peritoneal cancer prevention (PDQ®). https://www.ncbi.nlm.nih.gov/books/NBK65937
National Cancer Institute. (2020b). SEER cancer stat facts: Ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html
National Comprehensive Cancer Network. (2020). NCCN clinical practice guidelines in oncology (NCCN guidelines®) ovarian cancer including fallopian tube cancer and primary peritoneal cancer: Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
National Comprehensive Cancer Network. (2021). NCCN clinical practice guidelines in oncology (NCCN guidelines®) genetic/familial high-risk assessment: Breast, ovarian, and pancreatic: Version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Nielson, M., Infante, E., & Brand, R. (2019). MUTYH polyposis. GeneReviews. https://www.ncbi.nlm.nih.gov/books/NBK107219/#
Office on Women’s Health. (2019). Menopause. https://www.womenshealth.gov/menopause
Oien, D. B., & Chien, J. (2016). TP53 mutations as a biomarker for high-grade serous ovarian carcinoma: Are we there yet? Translational Cancer Research, 5(Suppl 2), S264-S268. https://doi.org/10.21037/tcr.2016.07.45
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice (1st ed.). Oncology Nursing Society.
OpenStax College. (2013a). Anatomy of the peritoneum [image]. https://commons.wikimedia.org/wiki/File:2403_The_PeritoneumN.jpg
OpenStax College. (2013b). Stages of folliculogenesis [image]. https://commons.wikimedia.org/wiki/File:Figure_28_02_04.JPG
Ovarian Cancer Research Alliance. (2020). Types of ovarian cancer. https://ocrahope.org/patients/about-ovarian-cancer/types-ovarian-cancer/#epithelial-ovarian-tumors-primary-peritoneal-tumors-fallopian-tube-tumors
Peshkin, B. N., & Isaacs, C. (2020). Genetic testing and management of individuals at risk of hereditary breast and ovarian cancer syndromes. UpToDate. https://www.uptodate.com/contents/genetic-testing-and-management-of-individuals-at-risk-of-hereditary-breast-and-ovarian-cancer-syndromes
Salazar, C., Campbell, I. G., & Gorringe, K. L. (2018). When is “type I” ovarian cancer not “type I”? Indications of an out-dated dichotomy. Frontiers in Oncology, 8(654), 1-9. https://doi.org/10.3389/fonc.2018.00654
Sasikumar, P. G. & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. https://doi.org/10.1007/s40259-018-0303-4.
Sengupta, S. (2017). Cancer nanomedicine: Lessons for immune-oncology. Trends Cancer, 3(8), 551-560. https://doi.org/10.1016/j.trecan.2017.06.006.
Society of Gynecologic Oncology. (2017). Committee opinion no. 716: The role of the obstetrician and gynecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstetric Gynecol, 130(3), e149-e149. https://doi.org/10.1097/AOG.0000000000002299
Temkin, S. M., Bergstrom, J., Samimi, G., & Minasian, L. (2018). Ovarian cancer prevention in high risk women. Clin Obstet Gynecol, 60(4), 738-757. https://doi.org/10.1097/GRF.0000000000000318.
US National Library of Medicine. (2020a). Lynch syndrome. https://ghr.nlm.nih.gov/condition/lynch-syndrome
US National Library of Medicine. (2020b). TP53 gene: Tumor protein p53. https://medlineplus.gov/genetics/gene/tp53/#conditions
US Preventive Services Task Force. (2018). Screening for ovarian cancer: US Preventive Task Force recommendation statement. JAMA, 319(6), 558-594. https://doi.org/10.1001/jama.2017.21926
Woo, J., & Long, J. (2021). Ovarian cancer & ovarian tumors. In Papadakis M. A., McPhee S. J., & Rabow M. W. (Eds.), Current medical diagnosis & treatment 2021. McGraw-Hill. https://accessmedicine-mhmedical-com.gshremote.senylrc.org/content.aspx?bookid=2957§ionid=249381517
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice (8th ed.). Jones & Bartlett Learning.
Zealthy. (2020). Female reproductive anatomy [image]. https://commons.wikimedia.org/wiki/File:Image_of_an_Ovary_-_English.jpg