About this course:
The purpose of this course is to provide an overview of pancreatic cancer, its risk factors, clinical features, and treatment options to help nurses provide optimal care, patient education, and support throughout the disease trajectory.
Course preview
This module aims to provide an overview of pancreatic cancer, its risk factors, signs and symptoms, and treatment modalities to inform nursing practice and help nurses provide optimal care, patient education, and support throughout the disease trajectory.
By the completion of this learning activity, the nurse should be able to:
- discuss the epidemiology of pancreatic cancer in the US and risk factors for the development of the disease
- examine the anatomy of the pancreas, the pathophysiology leading to the development of pancreatic cancer, and pancreatic cancer subtypes
- discuss the signs and symptoms of pancreatic cancer, and review the possible treatment options, side effects, risks, and elements of patient education
According to the American Cancer Society (ACS, 2020a), approximately 57,600 people will be diagnosed with pancreatic cancer in 2020, and about 47,050 will die from the disease. Pancreatic cancer is one of the most challenging cancers to diagnose, as it shows little or no symptoms during the early stages, and there are no effective screening tests. It carries a very poor prognosis and is characterized by a high mortality rate, currently ranking as the 3rd leading cause of all cancer deaths in the United States. Given its grim prognosis, nurses must remain updated and informed on the clinical features of the disease, as well as its pertinent risk factors and treatment modalities, to provide optimal care, education, and support to patients and their families (ACS, 2020a; Siegel et al., 2020).
Epidemiology
The average lifetime risk for developing pancreatic cancer among the United States population is approximately 1.6% (1 in 64). Based on data from the Surveillance, Epidemiology, and End Results Program (SEER, 2020), the median age at diagnosis is 70. It is most frequently diagnosed in individuals aged 65 to 74 (30%), followed by those aged 75 to 84 (24.3%). The condition appears to be more common among men than women. Incidence rates are highest for Black males (16.9 per 100,000), followed by non-Hispanic White males (15.2 per 100,000), Black females (14.1 per 100,000), American Indian/Alaska Native males (12.9 per 100,000), and Hispanic males (12.5 per 100,000). Asian/Pacific Islanders have the lowest incidence rates among males (11 per 100,000), whereas American Indian/Alaska Natives rank the lowest among females (7.8 per 100,000). Mortality rates are highest among Black males and females, respectively (15 per 100,000 and 12 per 100,000). Mortality rates are lowest among Asian/Pacific Islander males and females, respectively (8.1 per 100,000 and 7.1 per 100,000). The 5-year survival rate for pancreatic cancer is dismal and depends on the stage at diagnosis. Localized pancreatic cancer (i.e., cancer that has not spread outside of the pancreas) has a 5-year survival rate of 37%, which declines to only 3% for those with distant metastases (i.e., cancer spreading to distant organs or sites outside the pancreas). Unfortunately, roughly 80% of pancreatic cancers are diagnosed at an advanced stage, accounting for the bleak survival rates. The median age at death is 72 years, with the highest percentage of deaths among those aged 65 to 74 (30.1%), followed by those aged 75 to 84 (26.9%; ACS, 2020a, 2020c; SEER, 2020).
Risk and Protective Factors
All individuals are at risk for pancreatic cancer, and the risk increases with age. Environmental, lifestyle choices, and genetic factors all serve important roles in the development of the condition. Although most pancreatic cancers are diagnosed in the absence of identifiable risk factors or family history, several influences can increase or decrease a person’s risk for pancreatic cancer. Table 1 describes the major risk and preventative factors (ACS, 2020b).

Germline and Somatic Gene Mutations
Germline (inherited) and somatic (acquired) mutations in cancer-causing oncogenes and tumor suppressor genes are common in pancreatic cancer. Inherited (or hereditary) mutations are passed along familial lines, directly from a parent to a child. These mutations are found in every cell of the body and increase the individual’s predisposition to developing pancreatic cancer. Unlike inherited mutations, somatic changes are not hereditary or passed to subsequent generations. Instead, somatic mutations occur during one’s lifetime due to environmental exposures, such as ultraviolet radiation from the sun, ionizing radiation, free radicals, carcinogens, or chemical exposures. Somatic mutations only occur only in specific cells within the body. An oncogene is a mutation associated with cancer development; it can be inherited or somatic. In their normal and non-mutated state, oncogenes are called proto-oncogenes, regulating healthy cell growth and division. When a proto-oncogene mutates into an oncogene, it becomes permanently activated (turned on), fueling unregulated cell growth and resultant cancer cells (CancerQuest, 2020; Yarbro et al., 2018).
RAS is a family of genes that include KRAS and NRAS genes, which comprise the most frequently mutated oncogene family in cancer. KRAS is cited as the most commonly activated oncogene in pancreatic cancer; present in more than 95% of pancreatic adenocarcinomas. In these cases, the KRAS protein becomes permanently activated, inducing continual cellular proliferation, invasion, and survival. Clinical research has demonstrated that KRAS mutation correlates with a poorer prognosis in patients with pancreatic cancer (Buscail et al., 2020; Waters & Der, 2018). Tumor suppressor genes are healthy genes that slow cell division, repair DNA errors, and induce apoptosis (programmed cell death). Under physiologic conditions, tumor suppressor genes regulate healthy cellular growth and division and prevent cells with mutated or damaged DNA from replicating, thereby avoiding tumor development. When tumor suppressor genes are inactivated (turned off), these processes continue in an unregulated manner. Consequently, DNA damage accumulates in cells, and they continue to divide in an uncontrolled way, leading to tumor growth (CancerQuest, 2020; Yarbro et al., 2018).
The most common tumor suppressor gene affected in pancreatic cancer is the p16 gene, which is inactivated in about 95% of cases. The p16 gene is also known as cyclin-dependent kinase inhibitor 2A (CDKN2A), and mutations in this gene occur in pancreatic cancer at a rate higher than nearly any other tumor type. P16 is one of the inhibitors for cyclin-dependent kinases, or CDK enzymes, which drive a cell’s progression through the division cycle. Individuals with CDNK2A/p16 mutations have a 20% increased risk of developing pancreatic cancer by age 75. The second most common is the p53 gene, inactivated in approximately 70% of pancreatic cancers. Since p53 plays a critical role in regulating DNA repair and cell division, it is a nicknamed “guardian of the genome” (MedlinePlus, 2020a, 2020e).
Inherited Risk
According to the American Society of Clinical Oncology (ASCO, 2020b), roughly 10% of pancreatic cancers are associated with inherited mutations in specific genes. Some of the most common genetic syndromes that predispose individuals to pancreatic cancer are described in this section.
Hereditary Pancreatitis (HP)
HP is a rare genetic syndrome characterized by recurring episodes of severe epigastric pain and hyperamylasemia (elevated serum amylase), with the first episode typically occurring before age 10. The condition is most commonly associated with a mutation in the PRSS1 gene, inherited in an autosomal dominant (AD) pattern. As demonstrated in Figure 1, in an AD inheritance pattern, a copy of the mutated gene in each cell
...purchase below to continue the course

Family Breast Cancer (BRCA1/2 gene mutations)
While mutations in BRCA1 and BRCA2 (BRCA1/2) genes are most commonly associated with an increased risk for breast cancer and ovarian cancer, they are also among the most common inherited causes of pancreatic cancer. Females with BRCA1/2 mutations have an increased risk for breast, ovarian, melanoma, and pancreatic cancer, whereas males with BRCA1/2 mutations have an increased risk for breast, prostate, melanoma, and pancreatic cancer. Everyone is born with BRCA1 and BRCA2 genes. In their non-mutated state, BRCA1/2 genes function as essential tumor suppressor genes that promote the healthy growth, development, and division of specific cells in the body. Mutations in these genes disrupt their normal functions, increasing the propensity toward cancer development. Mutations in BRCA1/2 genes follow an AD inheritance pattern. Mutations in the BRCA1/2 genes are the most common hereditary cause of familial pancreatic cancer, linked to a 2 to 6-fold higher risk of pancreatic cancer, a younger age of onset, and a more aggressive clinical course. Of the two gene mutations, the link with BRCA2 is better established and carries a higher risk for developing pancreatic cancer; found in 5 to 10% of cases, whereas BRCA1 is identified in about 1 to 2% of cases. Mutations in BRCA1/2 are about 10 times more common in those of Ashkenazi Jewish descent than the general US population. Among individuals of Ashkenazi Jewish descent with pancreatic cancer, BRCA2 mutations are found in up to 13.7% of cases (Centers for Disease Control and Prevention [CDC], 2019b; National Comprehensive Cancer Network [NCCN], 2020a, 2020b; Pilarski, 2019).
Peutz-Jeghers syndrome (PJS)
PJS is a rare disorder that affects an estimated 1 in 50,000 to 1 in 200,000 people. It is caused by a mutation in the STK11 gene (also called LKB1), which provides instructions for generating an enzyme called serine/threonine kinase 11, which functions as a tumor suppressor, regulating cellular growth and division. PJS is inherited in an AD pattern but can also occur as a new (de novo) mutation in 25 to 45% of affected individuals. It typically presents during childhood and is characterized by benign growths (polyps) in the digestive tract called ‘hamartomatous polyps.’ While these are noncancerous polyps, they can cause bleeding and problems with the bowel, including intestinal obstruction. Classic signs of PJS in children and young adults include freckles and pigmented spots on the skin and in the mouth, called mucocutaneous hyperpigmentation. However, these typically fade during puberty, and the condition often goes undiagnosed. PJS increases the risk for developing several types of cancers such as breast, colorectal (CRC), gastric, ovarian, testicular, lung, and many others. In the absence of appropriate surveillance, the lifetime risk for developing any type of cancer in these patients ranges from 85 to 95%. The lifetime risk for pancreatic cancer ranges from 11 to 35% (ASCO, 2020b; MedlinePlus, 2020d).
Hereditary Nonpolyposis Colorectal Cancer (HNPCC)
HNPCC or Lynch syndrome (LS) is most commonly known for its high-risk association with CRC; however, it is also associated with an increased risk of various other types of cancers, including uterine (endometrial), ovarian, gastric, pancreatic, urothelial, glioblastoma, biliary tract, and small intestine. Individuals inherit LS in an AD pattern. In the United States, it is estimated that 1 in 279 individuals (1.2 million people) have a gene mutation associated with LS; however, most are undiagnosed since identification depends on a cancer diagnosis. Changes in the protein expression of MLH1, MSH2, MSH6, or PMS2 genes are most commonly found in LS. Under physiologic conditions, these genes are responsible for repairing any potential errors during DNA replication (the process during which DNA is copied in preparation for cell division); collectively, they are known as mismatch repair (MMR) genes. Since mutations in any of these genes impede the cell’s ability to repair the DNA replication errors, abnormal cells continue to divide. Over time, the accumulated DNA replication errors can lead to uncontrolled cell growth and an increased propensity for cancer development. Mutations in the MLH1 or MSH2 gene are associated with a higher risk (70 to 80%) of developing cancer than mutations in the MSH6 or PMS2 genes, which carry a lower risk (25 to 60%). While mutations in these genes predispose individuals to cancer, not all people with these mutations will develop cancerous tumors. The lifetime risk of pancreatic cancer in patients with LS varies but ranges from 1 to 6% (ASCO, 2020a; MedlinePlus, 2020c). The accumulated risk of pancreatic cancer in LS patients is around 3.7%, or an 8.6-fold increase compared with the general population (Bujanda & Herreros-Villanueva, 2017). Greater than 90% of LS-related cancers are mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H), meaning they lack expression of at least one of the MMR proteins. According to the NCCN guidelines (2020a), patients with MSI-H or dMMR should be referred to a genetic counselor to undergo formalized genetic testing. LS can only be confirmed by a specialized gene panel blood test (CDC, 2019a; NCCN, 2020a).
ATM Mutation
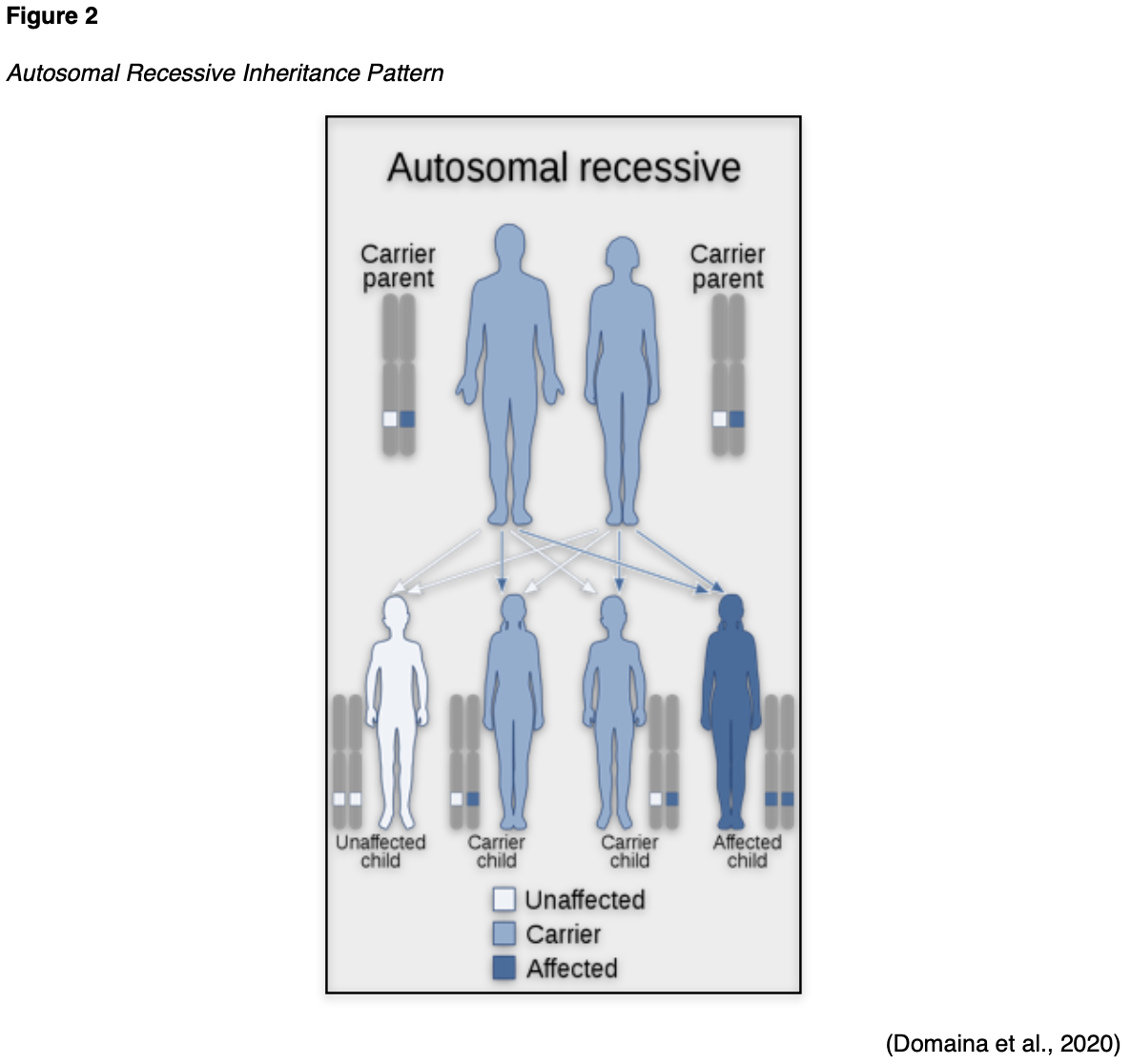
Advancements in technology have recently led to the discovery of new pancreatic cancer susceptibility genes such as the ataxia telangiectasia mutated (ATM) gene. The ATM gene provides instructions for generating serine/threonine kinase, an integral component of DNA repair. The ATM gene also plays an essential role in controlling the rate at which cells grow and divide and is necessary for the health and development of the nervous and immune systems. Ataxia-telangiectasia is a risk factor for pancreatic cancer as it is an immunodeficiency disease caused by mutations in the ataxia-telangiectasia gene (ATM). Ataxia-telangiectasia is an autosomal recessive (AR) disease of childhood that causes the immune system to break down, heightening susceptibility to illness. In AR disease, one abnormal gene is inherited from each parent, as the patient requires two copies of the mutation to be affected by the disease. A carrier has one abnormal gene (recessive) and one normal gene (dominant) and is clinically unaffected by the disorder. As demonstrated in Figure 2, two carriers have a 25% chance of having an unaffected child with two normal genes, a 50% chance of having an unaffected carrier, and a 25% chance of having an affected child. AR disorders are not typically seen in every generation of an affected family. Ataxia-telangiectasia is rare, occurring in only 1 out of 40,000 – 100,000 people worldwide. The condition confers increased cancer risk, most notably pancreatic and breast cancers. An association between ATM gene mutations and pancreatic cancer is evident, although the exact risks are not yet defined; additional research is needed to clarify this relationship (Pancreatic Cancer Action Network, 2019; US National Library of Medicine [NLM], 2020b).
Familial Atypical Multiple Mole Melanoma (FAMMM)
FAMMM is a rare AD disorder most commonly caused by an inherited mutation in the p16/CDKN2A gene associated with skin and eye melanomas. FAMMM is characterized by numerous (usually more than 50) melanocytic nevi (moles) of different shapes and sizes, as well as a family history of malignant melanoma. The family history typically includes one or more first or second-degree relatives with melanoma (Genetic and Rare Diseases, 2017). FAMMM increases the risk of pancreatic cancer 15 to 65 times relative to the general population, and the prognosis is very poor (My Gene Counsel, 2020; NCCN, 2020a, 2020b; Pancreatic Cancer Action Network, 2019).
Familial Adenomatous Polyposis (FAP)
FAP is known for its early onset of multiple gastrointestinal (GI) adenomas. Individuals with FAP tend to develop numerous benign polyps in their colon as early as their 20s and 30s. The number of polyps increases to thousands with advancing age, and unless the colon is removed, these polyps will develop into CRC. The condition is most commonly linked to pathogenic mutations in the APC gene, inherited in an AD pattern, and affects the cell’s ability to maintain healthy growth and function. Aside from CRC risk, individuals with mutations in the APC gene are at a 5-fold increased likelihood of developing pancreatic cancer than the general population (ACS, 2020a; NLM, 2020a).
PALB2 Gene Mutations
Mutations in the PALB2 gene may be inherited from either parent as it follows an AD inheritance pattern. PALB2 mutations are most notably associated with an increased risk for breast cancer but also carry an increased risk for pancreatic cancer; the exact risk is unknown at this time. Mutations in the PALB2 gene have been identified in approximately 3 to 4% of familial pancreatic cancer cases (My Gene Counsel, 2020; NCCN, 2020b).
Genetic Testing
DNA sequencing techniques can identify both germline and somatic mutations. Germline testing is required to determine if the mutation is inherited; these mutations can be identified by utilizing a saliva sample containing buccal cells or a peripheral blood sample. Genetic testing performed on the tumor specimen can identify genetic changes directly within the cancerous cells. This information helps identify treatment options likely to be the most effective (Mahon, 2020; NCCN, 2020a).
Pancreatic Cancer Screening
Routine screening for pancreatic cancer is not recommended for the general population and is typically not recommended for asymptomatic, high-risk individuals. According to the NCCN (2020a) guidelines, individuals suspected of having a familial pancreatic syndrome or a high-risk genetic mutation should be referred to a genetic counselor. Individuals with a strong family history or those with specific known high-risk mutations (e.g., p16/CDKN2A) may opt to undergo heightened surveillance following a shared-decision making discussion with their clinician. The most common tests include computerized tomography (CT), magnetic resonance imaging (MRI), or endoscopic ultrasound (EUS). Test selection depends on patient risk factors, patient preference, and test availability. Nurses should ensure patients are appropriately counseled on the limitations and potential harms of screening, including false-positive and false-negative results. There is a high incidence of pancreatic abnormalities detected on screening tests (e.g., pancreatic cysts), and there are no clear evidence-based guidelines for the proper surveillance of these lesions. Positive (abnormal) or inconclusive results often subject patients to invasive procedures (e.g., biopsy), which are not without risks, such as bleeding and infection. Additionally, medical imaging is subject to error, and screening tests are often imperfect and costly (NCCN, 2020a, 2020b).
Pathophysiology
The pancreas is an organ of the digestive system that measures approximately 20 cm in length and is positioned in the back of the abdomen, directly behind the stomach. As displayed in Figure 3, the pancreas is divided into three sections: the head, body, and tail. The head is the widest portion of the pancreas, located on the abdomen’s far-right side in the duodenal curve (the beginning of the small intestine). The body is the central portion of the pancreas and is situated behind the duodenum. The tail is the narrow, left side of the pancreas that extends slightly upward and ends near the spleen (McCance & Heuther, 2019; Yarbro et al., 2018).

The pancreatic duct joins the biliary duct (from the liver) and extends the length of the pancreas. Blood is supplied to the pancreas by branches of the celiac and superior mesenteric arteries. The pancreas head drains venous blood through the portal vein, and the body and tail drain via the portal vein. The pancreas is unique in that it has both digestive (exocrine) and hormonal (endocrine) functions, serving essential metabolic roles as part of the digestive and the endocrine systems (Lumen Learning, n.d.; McCance & Heuther, 2019).
Exocrine Pancreas
The majority of the pancreas is comprised of exocrine cells, which contain two major types of epithelium: acinar and ductal, which cluster to form exocrine glands. Exocrine glands secrete enzymes into a network of ducts that release alkaline (bicarbonate-rich) fluids and other pancreatic enzymes to facilitate digestion. The acinar cells secrete the enzymes, and the ductal cells secrete the bicarbonate fluid. As displayed in Figure 4, the acinar cells are organized into spherical lobule-like structures surrounding secretory ducts. The secretions are called proenzymes at this point since they remain inactivated. They drain into a duct system that leads to the pancreatic duct before emptying into the common bile duct and being transported to the duodenum. Once the proenzymes enter the duodenum, they become activated by enterokinase, an enzyme secreted by the duodenal mucosa. Once activated, the pancreatic enzymes help break down carbohydrates, fats, proteins, and acids. Secretin is a substance that stimulates the acinar cells to release a bicarbonate-rich fluid to neutralize the stomach acids (chyme), which pass from the stomach into the duodenum. The pancreatic enzyme amylase digests carbohydrates, and the enzyme lipase digests triglycerides, cholesterol, and lipids. Amylase and lipase are used as markers of pancreatic inflammation (e.g., pancreatitis). Amylase may be measured in the urine or blood, whereas lipase is only measured in the blood (McCance & Heuther, 2019; Yarbro et al., 2018). A normal serum amylase level is 25–125 U/L, and a normal serum lipase level is 10–140 U/L (American Board of Internal Medicine [ABIM], 2020).

Endocrine Pancreas
The endocrine glands account for a much smaller percentage of the pancreas. The endocrine gland primarily consists of the islets of Langerhans, which produce and secrete hormones (glucagon and insulin) into the bloodstream. These hormones regulate a significant portion of the carbohydrate metabolism within the body by facilitating the formation and cellular uptake of glucose. The three types of hormone-secreting cells that comprise the islets of Langerhans include the following:
- alpha (α)-cells (secrete glucagon and comprise 15 to 20% of islet cells)
- beta (ß)-cells (secrete insulin and comprise 65 to 80% of islet cells)
- delta (D)-cells (secrete somatostatin and gastrin and comprise 3 to 10% of islet cells; Lumen Learning, n.d.; McCance & Heuther, 2019).
A major disorder of the endocrine pancreas is DM, a chronic disease impacting multiple body systems due to abnormal insulin production, impaired insulin utilization, or both. T1DM is characterized by the autoimmune destruction of the pancreatic ß-cells leading to the total absence of insulin production. There is typically a genetic predisposition compounded by exposure to a virus that contributes to this autoimmune condition. Autoantibodies to the islet cells cause a decrease in the normal function before other symptoms of T1DM appear. Patients with T1DM depend on external insulin for their survival since the hormone is not produced internally. In T2DM, insulin is either generated in insufficient quantities, used poorly by the tissues, or both. The most common risk factor for T2DM is obesity, especially abdominal adiposity. Those with TD2M are insulin-resistant and are managed with oral hypoglycemics (e.g., metformin [Glucophage]). If control of hyperglycemia is not achieved, patients with TD2M may eventually require external insulin (Lewis et al., 2014; Lumen Learning, n.d.).
Normal insulin metabolism occurs through the continuous release of insulin by the ß-cells in the islets of Langerhans of the pancreas (Figure 4). Insulin synthesis begins with its precursor, proinsulin. Enzymes break down proinsulin to produce insulin and C-peptide in equal amounts. This byproduct, C-peptide, is useful when assessing pancreatic ß-cell function as it can be measured in the urine and blood. The average amount of insulin secreted daily by a healthy adult is 40-50 U or 0.6 U/kg of body weight. Insulin acts as an anabolic or storage hormone in the body. The insulin secreted with food intake promotes glucose transport into the cells to be used for energy by unlocking receptor sites in the skeletal muscle and adipose tissue. Skeletal muscles and fatty tissue are considered insulin-dependent; the brain, liver, and blood cells do not depend on insulin and only require an adequate glucose supply for normal functioning. While liver cells (hepatocytes) are not insulin-dependent, they do have “insulin receptor sites that facilitate hepatic uptake of glucose and its conversion to glycogen” (Lewis et al., 2014, p. 1154).
As blood glucose (BG) increases after a meal or food intake, glucose is stored as glycogen in the liver and muscle tissue. Concurrently, insulin secretion inhibits gluconeogenesis (the production of glucose from non-sugar substances), enhances fatty tissue deposition, and increases protein synthesis. The reduced insulin that occurs overnight (or from fasting) causes the liver to release glucose, the muscles to release proteins, and the fatty tissue to release fat. Counter-regulatory hormones such as glucagon, epinephrine, growth hormone, and cortisol oppose the effects of insulin. They increase BG by stimulating the production of glucose and liver output and decreasing glucose movement into the cells. Insulin secretion is designed to maintain a stable BG level of 70-120 mg/dL. A healthy BG level is typically maintained by regulating the release of glucose for energy during periods of fasting, food intake, and the production and release of insulin and the counter-regulatory hormones (Lewis et al., 2014; Lumen Learning, n.d.). Less is understood about the function of pancreatic somatostatin and gastrin. Somatostatin is considered an essential hormone in carbohydrate, fat, and protein metabolism. It suppresses the release of other hormones generated in the pancreas and functions to maintain the homeostasis of nutrients. Gastrin helps to control the secretion of glucagon. All hormonal pancreatic secretions pass through the portal vein into the liver (McCance & Heuther, 2019).
For a detailed account of diabetes, refer to the Diabetes NursingCE course.
Pancreatic Cancer Subtypes
Pancreatic tumors develop in both the endocrine and exocrine glands and are grouped accordingly.
Exocrine Tumors
Exocrine tumors account for at least 90% of all pancreatic cancer diagnoses, with ductal adenocarcinoma comprising the vast majority. Exocrine tumors are outlined in Table 2 (Yarbro et al., 2018).

Precursor Lesions to Ductal Adenocarcinoma
There are three well-cited noninvasive precursor lesions to ductal adenocarcinoma: pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasm (MCN), and intraductal papillary mucinous neoplasms (IPMN). These tumors are usually small, cystic, and discovered incidentally. They are often benign when diagnosed but have a chance of becoming cancerous. The incidence increases with age but most commonly occurs in those aged 60 to 70 years. Many are managed conservatively with heightened surveillance (e.g., annual radiographic monitoring) and never progress to invasive cancer. Tumors with certain high-risk features, such as IPMNs arising from the main pancreatic duct, require surgical resection and monitoring for recurrence (NCCN, 2020b).
Endocrine Tumors
Endocrine tumors are rare; they account for less than 5% of all pancreatic tumors, with only 1,000 new cases in the US each year. They are typically referred to as islet cell tumors or pancreatic neuroendocrine tumors (PNETs). They denote cancer of the hormone-producing cells. PNETs may be functioning (meaning they generate hormones) or nonfunctioning (do not generate hormones). Most PNETs are functional (75%) and typically present with symptoms of hormone hypersecretion. PNETs are characteristically small, well-circumscribed tumors that are difficult to distinguish from healthy islet cells. They are not always malignant and may or may not produce symptoms. The presence of metastases is the most reliable criterion for establishing a malignant process. Table 3 outlines the three most common functional PNETs (National Cancer Institute [NCI], 2020; Yarbro et al., 2018).

Clinical Manifestations
Pancreatic cancer remains one of the most challenging cancers to diagnose based on its anatomical location and subtle onset. The signs and symptoms of pancreatic cancer typically occur late in the illness, are vague, nonspecific, and often mimic other conditions. It is typical for the individual with pancreatic cancer to ignore initial symptoms for several months until jaundice (yellowing of the skin and eyes caused by a buildup of bilirubin) or other prominent signs present. Ominous signs usually develop late and only after invasion or obstruction of nearby tissue. Further, manifestations of the disease can also differ based on the tumor’s anatomical location (ACS, 2019; Yarbro et al., 2018).
Head of the Pancreas
When the tumor involves the pancreatic head, symptoms typically appear earlier than those involving the body or tail. Pancreatic head tumors tend to occlude the distal common bile duct, causing obstruction of the duct, inducing a constellation of symptoms including jaundice, weight loss, and pain. Jaundice with pain is much more common than painless jaundice and is the most common presenting symptom prompting patients with pancreatic head tumors to seek medical care. Interestingly, patients with pancreatic head tumors have a high incidence of depression and anxiety, which has been shown to predate the diagnosis by as many as 3.5 years. This psychological phenomenon was first identified in 1931. While the relationship is poorly understood, research has demonstrated a 2 to 3 times higher incidence of depression in patients with pancreatic cancer than other intra-abdominal cancers. The proposed rationale is that hormonal neuroendocrine substances circulate through the central nervous system. Other symptoms of pancreatic head tumors include diarrhea, indigestion, weakness, and anorexia (ACS, 2019; Yarbro et al., 2018; Yaskin, 1931).
Body of the Pancreas
Tumors in the pancreatic body produce signs and symptoms late in the course of illness, making it nearly impossible to detect early. The predominant presenting symptom of pancreatic body tumors is severe epigastric pain, intensifying about 3 to 4 hours after meals. The tumor compresses or displaces the stomach, inducing intense pain when the stomach is full; this pain is often accompanied by vomiting. The pain may be relieved by sitting up, leaning forward, or lying in the fetal position to alleviate the pressure on the stomach. In many cases, the tumor is palpable on abdominal examination and may be accompanied by splenomegaly (enlarged spleen) caused by tumor compression of the splenic vein. Tumors in the pancreatic body and tail do not induce jaundice but cause more pain and weight loss than those located in the pancreatic head (ACS, 2019; Yarbro et al., 2018).
Tail of the Pancreas
In addition to the characteristics described above, tumors in the pancreatic tail are known for their silent growth and insidious progression, typically diagnosed in advanced stages. The most common symptoms include left upper quadrant pain, generalized weakness, anorexia, and indigestion. In addition to splenomegaly, signs of portal hypertension and ascites may be present from thrombosis of the portal system or liver damage. Upper GI bleeding (GIB) may also present with advanced pancreatic tail tumors. Weight loss is often accompanied by cachexia and muscle wasting. Ominous physical examination findings suggesting metastatic pancreatic cancer include the following:
- left supraclavicular adenopathy (Virchow node)
- periumbilical adenopathy (Sister Mary Joseph nodes)
- deep metastases in the pelvis encircling the perirectal region (Blumer’s shelf; ACS, 2019; Yarbro et al., 2018).
PNETs
As noted earlier, PNETs do not always cause symptoms. Functional PNETs secrete excess hormones, which can induce an array of unusual manifestations. Different types of PNETs have different signs and symptoms (see Table 3; NCI, 2020).
Biopsy
The only way to confirm the diagnosis and establish the specific clinical features of the tumor is through a biopsy specimen. Most pancreatic biopsies are obtained using nonsurgical procedures; the two most common techniques include endoscopy ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP; NCCN, 2020b; RadiologyInfo.org, 2018, 2020).
EUS
EUS is a specialized imaging technique that generates detailed and high-resolution three-dimensional images of the pancreas and surrounding structures. It also facilitates the tissue sampling of the suspicious mass. EUS is performed to confirm the primary site of pancreatic cancer involvement and to obtain a tissue biopsy. An endoscope (a thin, tube-like instrument with a light on the end) is guided down the esophagus and through the stomach until it reaches the duodenum. At the end of the tube, an US device emits sound waves to generate images of the pancreas, blood vessels, bile ducts, and nearby tissues. A needle biopsy (fine-needle aspiration [FNA] or core needle biopsy) is taken from the tumor site during the procedure. EUS is an outpatient procedure performed in a hospital or same-day surgery center with the use of anesthesia. While complications from an EUS are rare, some of the most commonly cited complications include infection, pancreatitis, GIB, tearing, and adverse reactions from anesthesia (RadiologyInfo.org, 2018; 2020; Yousaf et al., 2020).
ERCP
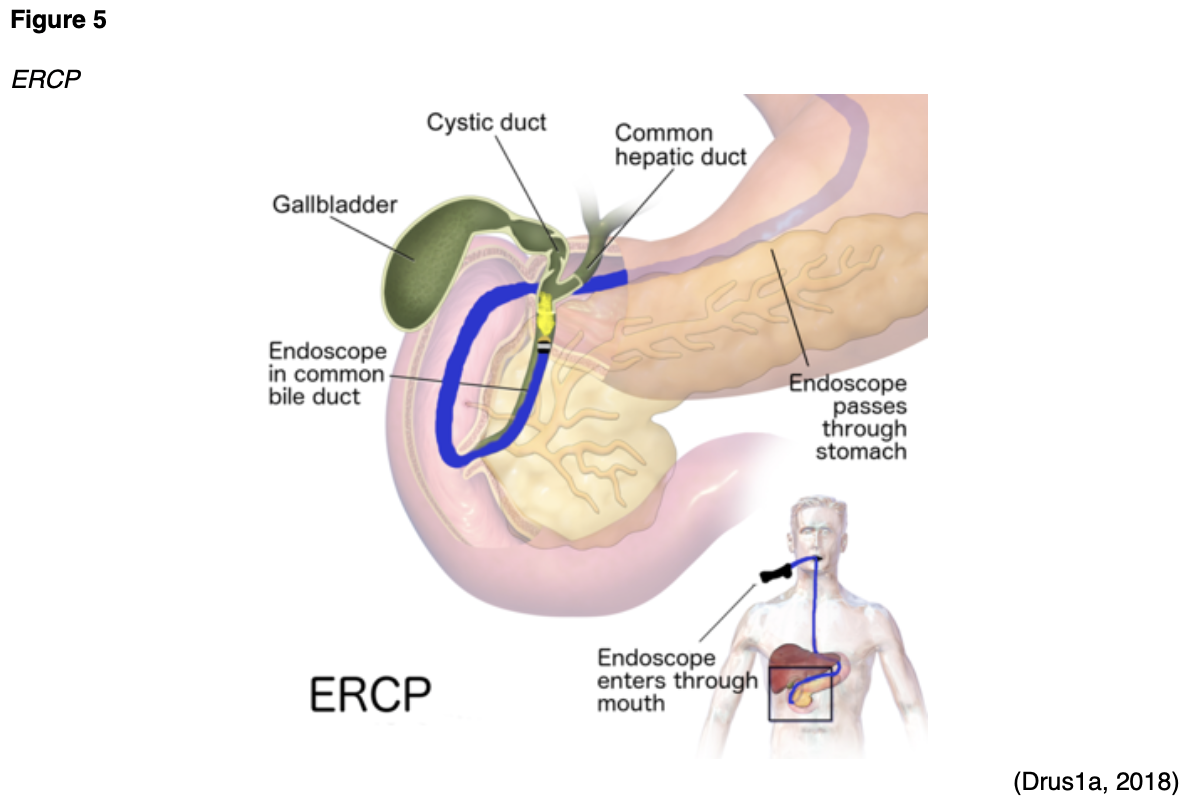
There are only a few differences between an EUS and an ERCP. A key difference is that the ERCP typically uses x-ray imaging and contrast, whereas an EUS does not. Most sources cite that an ERCP produces higher quality images than a standard EUS and allows for enhanced visualization of the organs and ducts. As demonstrated in Figure 5, during an ERCP, an endoscope is inserted through the esophagus into the duodenum. Air is inserted through the endoscope into the duodenum to make it easier to see the organs, and a catheter is inserted into the endoscope until it reaches the bile and pancreatic ducts. Next, contrast material is injected, and x-rays are taken to evaluate for gallstones, strictures, or blockages. Similar to an EUS, an FNA or core-needle biopsy can be taken. However, an ERCP additionally allows for more advanced therapeutics such as sphincterotomy or stenting procedures to manage identified problems. A sphincterotomy involves making a small incision to enlarge the bile duct or the opening of the pancreatic duct to improve the drainage or alleviate any blockage. Stenting involves placing a small plastic tube within a blocked or narrowed duct to improve drainage. Some stents are designed to pass through the intestine after a few weeks, whereas other stents must be removed or changed every 3 to 4 months. Permanent stents are typically comprised of metal. ERCP is the gold standard for alleviating biliary drainage in patients with biliary obstruction of the distal common bile duct, a common complication of pancreatic cancer. The most common ERCP complications include pancreatitis and cholangitis (inflammation of the bile duct system; Pancreatic Cancer Action Network, n.d.-a; RadiologyInfo.org, 2020).
Carbohydrate Antigen 19-9 (CA 19-9)
Tumor markers are substances, or proteins, secreted by cancer or by the body’s response to cancer’s presence. Tumor markers are also generated in smaller quantities by healthy cells, and therefore, healthy people can have small amounts of tumor markers in their blood. While tumor markers are considered nonspecific and are not beneficial when used in isolation, they can help evaluate treatment response and cancer recurrence. Although no tumor marker is sufficiently sensitive or specific to be considered 100% reliable and accurate for the screening of pancreatic cancer, CA 19-9 is the most specific tumor marker for the condition (Yarbro et al., 2018). According to the ABIM (2020), a normal CA 19-9 level is 0–37 U/mL.
Pancreatic Cancer Staging
Cancer stage at diagnosis guides treatment options and strongly influences overall survival. The American Joint Committee on Cancer’s (AJCC, 2018) Tumor, Node, Metastasis (TNM) System is the universal pancreatic cancer staging system. As cited within the NCCN (2020b) guidelines, the AJCC describes specific characteristics to assign stages I through IV, as demonstrated in Figure 6. Cancer staging reflects the cell type, tumor grade, anatomical location of the tumor, and extent of malignancy. Within the TNM staging system, T denotes the size of the tumor, and if it has grown into nearby tissue, N refers to the presence of cancer in the lymph nodes, and M indicates if cancer has metastasized to other parts of the body beyond the origin site. The most common patterns of initial spread for pancreatic cancers include the liver, peritoneum, regional lymph nodes, and Virchow nodes (AJCC, 2018; NCCN, 2020b; Yarbro et al., 2018).

Tumor Grade
Tumor grade measures how different the cancer cells look in comparison to healthy cells under the microscope. It is based on cell differentiation and varies from low-grade (grade 1) to high-grade (grade 3). Grade 1 is well-differentiated and appears similar to healthy cells, whereas grade 3 is poorly differentiated (i.e., does not resemble healthy cells) and most aggressive (NCCN, 2020b; Yarbro et al., 2018).
Treatment Modalities
Pancreatic cancer is difficult to treat due to its biological nature and advanced stage at diagnosis. Treatment for pancreatic cancer is usually multifactorial, involving combined modalities, and primarily depends on the cancer stage (Yarbro et al., 2018). The NCCN (2020b) provides evidence-based treatment guidelines for pancreatic cancer according to histopathological findings, genetics, staging, and other specific features. The guidelines are widely utilized in cancer care and guide medical decision-making throughout the patient’s disease trajectory. Due to the aggressive biology of pancreatic cancer and the advanced stage at diagnosis for the majority, treatment is chronic, and most patients experience periods of remission and relapse. Many patients receive maintenance therapy, and all need close monitoring and surveillance after treatment to monitor for recurrence. This section will provide an overview of the most common treatment strategies (NCCN, 2020b).
Surgery
The role of surgery in pancreatic cancer is limited to tumors confined to the pancreas without any evidence of spread to distant lymph nodes or other organs. The surgical technique varies based on the tumor’s location and its relationship to surrounding blood vessels. The surgical goals are to optimize the quality of life, completely excise the tumor, obtain long-term control of the cancer, and reduce morbidity (NCCN, 2020b). The risks and side effects of surgery depend on the size and degree of cancer invasion, the extent of surgery, and the structures removed. All surgeries and invasive procedures are accompanied by risks, such as adverse reactions to anesthesia, bleeding, blood clots, fistula formation (an abnormal connection between two hollow spaces within the body), bowel and bladder injury, infection, and life-threatening sepsis (Yarbro et al., 2018).
Pancreatoduodenectomy (Whipple Procedure)
A Whipple procedure is one of the most common surgeries performed to manage pancreatic tumors confined to the pancreatic head. It is a complex surgery in which the head of the pancreas, gallbladder, duodenum, a portion of the stomach, and surrounding lymph nodes are removed, and the remaining part of the pancreas and digestive organs are reconnected. Alternatively, some patients may undergo a pylorus-preserving Whipple, a modified version of the procedure in which the entire stomach and the stomach valve (pylorus) are kept in place. Patients usually require hospitalization for at least a week following a Whipple to allow for close monitoring as the body adapts to the significant changes. The most common postoperative complication is delayed gastric emptying (gastroparesis), a motility disorder in which the stomach does not empty food as quickly as possible. Gastroparesis induces nausea, vomiting, bloating, abdominal cramping, anorexia, and symptoms typically improve within 7 to 10 days. If gastroparesis continues, patients may require nutrition support in the form of a feeding tube or total parenteral nutrition (TPN) intravenously. The most serious complication is an abdominal infection caused by a pancreatic leak (leakage in the area that the pancreas connects to the intestine) or fistula. These complications occur in about 10% of cases and require timely antibiotic administration to minimize morbidity and mortality. Long-term GI effects are common; many experience ongoing diarrhea, flatus, and stomach cramping requiring dietary adjustments. Most patients require the long-term and potentially permanent use of exogenous pancreatic enzymes to facilitate proper digestion after a Whipple (Mirrielees et al., 2020; Pancreatic Cancer Action Network, n.d.-b).
Distal Pancreatectomy with En-bloc Splenectomy
In rare cases, pancreatic body or tail tumors are detected early enough to be considered curable, usually as an incidental finding in patients undergoing workup for an unrelated issue. In these patients, distal pancreatectomy with splenectomy has historically been the most common procedure performed in which the pancreas and spleen are removed (Yarbro et al., 2018). The spleen serves vital immunologic and hematologic functions, such as filtering the blood of debris. Excising the spleen increases the risk of infection, hypercoagulability, diabetes, and other hematological complications. Therefore, in recent years, many surgeons have advocated for spleen-preserving distal pancreatectomy, which carries a lower incidence of postoperative complications (NCCN, 2020b; Sun et al., 2017.
Radiation Therapy
Radiation therapy is a type of localized treatment that delivers a precisely measured amount of high-energy, highly focused rays of ionizing radiation to the tumor while providing as little injury as possible to surrounding tissue. Radiation causes cellular damage to cancer cells, leading to biological changes in the DNA, rendering cells incapable of reproducing or spreading. All healthy cells and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed; however, healthy cells can repair themselves and remain functional. The total dose of radiation is hyper-fractionated, which means it is delivered to the tumor in small, divided doses, or fractions, rather than all at once. Hyper-fractionation allows healthy cells a chance to recover between treatments. The total number of fractions (doses) administered depends on the tumor size, location, reason for treatment, patient’s overall health, performance status, goals of therapy, as well as consideration of any other concurrent therapies the patient is receiving (ACS, 2020c; Nettina, 2019).
External Radiation
External beam radiation therapy (EBRT) delivers radiation from a source outside the body and is the most common type of radiation therapy used for pancreatic cancer. Traditionally, radiation beams were only able to match the tumor’s height and width, exposing more healthy tissue to the consequences of radiation. Over recent decades, 3-D conformational radiation therapy (3D-CRT) became the mainstay of EBRT for many solid tumors, including pancreatic cancer. 3D-CRT is credited with the ability to reshape the radiation beam to match the shape of the tumor. Further advancements in imaging technology have led to more precise treatment mechanisms that allow even more of the radiation beam to reach the tumor. Intensity-modulated radiation therapy (IMRT) is a newer, highly conformal form of radiation that further reduces unintended exposure to healthy tissues. While 3D-CRT and IMRT are very similar in that they both target the tumor while sparing healthy tissue, IMRT allows for modulation of the radiation beam’s intensity, delivering a higher radiation dose to a precise location. The enhanced targeting technology of IMRT allows for the delivery of higher radiation doses to the site of disease, thereby enhancing clinical outcomes and limiting side effects. Stereotactic body radiation therapy (SBRT) is a technique in which extremely high biological doses of radiation are administered over a few short treatments. The target area is affected to a higher degree over a shorter period with minimal impact on healthy tissue. In pancreatic cancer treatment, the role of SBRT continues to evolve as it has been shown to improve cancer-related pain and quality of life. SBRT has a relatively favorable toxicity profile with minimal systemic effects and therefore is currently reserved for patients who are not candidates for systemic therapy, such as the elderly, those with poor performance status, or multiple complex medical comorbidities (ACS, 2020c; NCCN, 2020b; Rosati & Herman, 2017).
Radiation Side Effects
Radiation side effects depend on the specific area(s) of the body exposed and the dose received. Superficial skin irritation at the site where the EBRT beams aim is common and can include redness, blistering, and sunburn. GI symptoms are common due to the tumor’s anatomical location and the impact of the radiation beams on surrounding tissues and structures. Common symptoms of GI toxicity may include nausea, vomiting, diarrhea, anorexia, bowel incontinence, abdominal pain, bloating, gastroparesis, heartburn, and esophagitis. Systemic effects may include fatigue, weakness, dehydration, scarring, fibrosis, and adhesion formation (the tissues impacted by radiation stick together; ACS, 2020c).
Systemic Therapy
According to the NCCN (2020b), systemic therapy is used in all stages of pancreatic cancer. Systemic therapy includes intravenous and oral chemotherapy, targeted therapy, and immunotherapy.
Chemotherapy
Chemotherapy, also referred to as cytotoxic or antineoplastic therapy, encompasses a group of high-risk, hazardous drugs with the intent to destroy as many cancer cells with as minimal effect on healthy cells as possible. Premised on the concepts of cellular kinetics, chemotherapy generally works by interfering with the normal cell cycle, impairing DNA synthesis and cell replication, preventing cancer cells from dividing, multiplying, and forming into new cancer cells. Surgery for pancreatic cancer may or may not be preceded by neoadjuvant chemotherapy or followed by adjuvant therapy. Neoadjuvant chemotherapy is administered to shrink the tumor so that surgical intervention may not need to be as extensive. In pancreatic cancer, neoadjuvant chemotherapy may shrink the tumor enough so that the surgeon can perform a Whipple or other surgical resection of the tumor. Adjuvant therapy is given following surgery and aims to eradicate any micro-metastases and prevent cancer recurrence. Micro-metastases are a small collection of cancer cells too tiny to be identified on imaging scans that have detached from the original tumor and spread to other parts of the body. The danger with micro-metastases is that they can group together and form additional cancerous tumors within the body. Chemoradiation (concurrent chemotherapy and radiation therapy) is another common treatment that is typically administered after systemic chemotherapy. Chemotherapy acts as a radiosensitizer, thereby rendering cancer cells more vulnerable to the toxic effects of radiation. Palliative chemotherapy aims to relieve or delay cancer symptoms, enhance comfort, reduce symptom burden, and improve quality of life. Therefore, with palliative intent, chemotherapy doses are often adjusted to minimize treatment-related toxicity (Nettina, 2019; Yarbro et al., 2018).
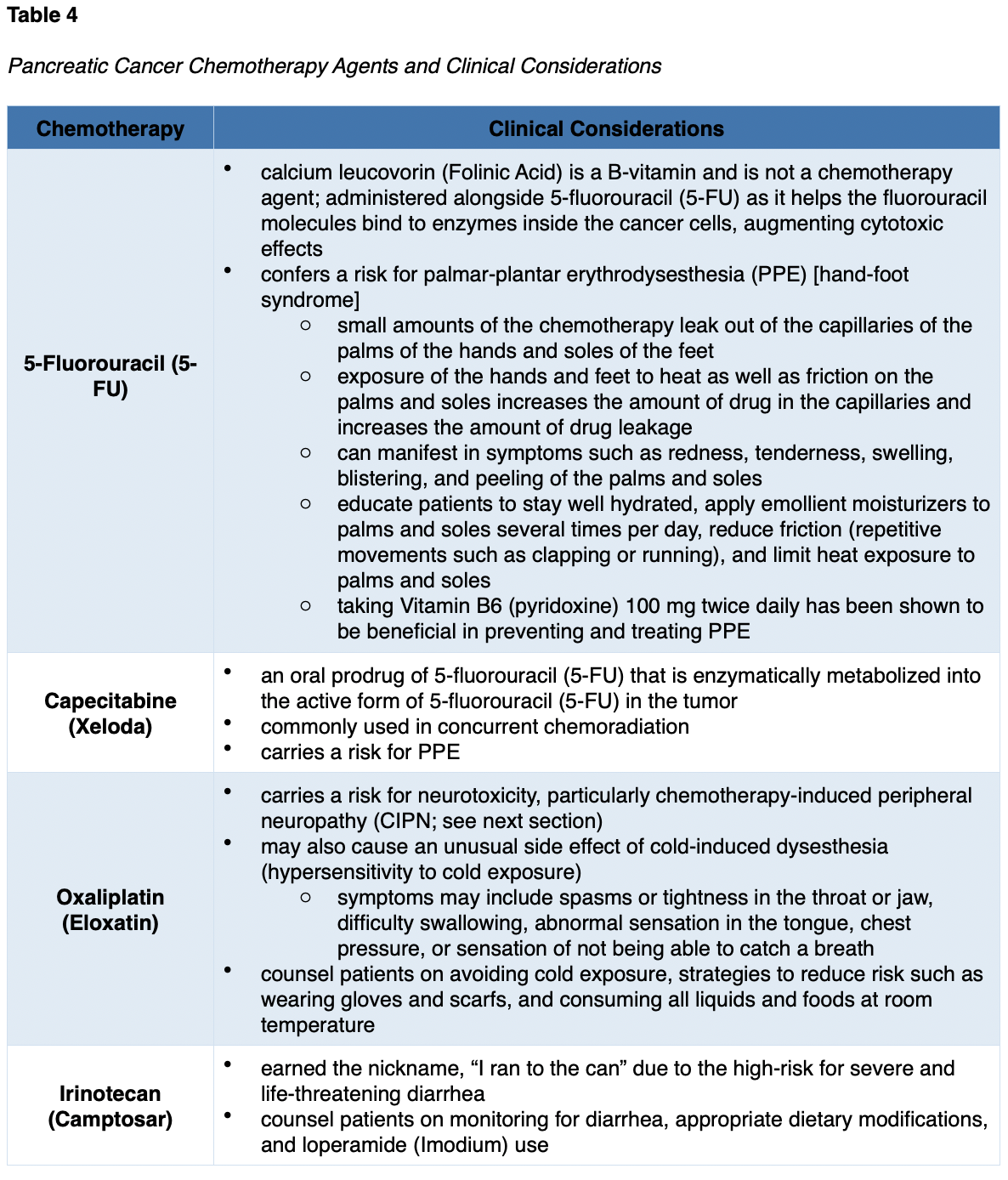
There is a wide range of chemotherapeutic agents used in pancreatic cancer, and they are usually given in combinations of two or three drugs. Some of the most common chemotherapy agents used for pancreatic cancer include the following:
- 5-fluorouracil (5-FU)
- capecitabine (Xeloda)
- irinotecan (Camptosar)
- oxaliplatin (Eloxatin)
- gemcitabine (Gemzar)
- cisplatin (Platinol)
- albumin-bound paclitaxel (Abraxane; NCCN, 2020b).
Chemotherapy Side Effects
The side effects of chemotherapy vary based on the drug type, dosage, duration of treatment, and specific patient factors. As a group, the most common side effects include lowering of the blood counts (anemia, thrombocytopenia, neutropenia), fatigue, nausea, anorexia, alopecia (hair loss), mucositis (mouth sores), diarrhea, skin changes, and peripheral neuropathy (damage to the sensory nerves). Table 4 reviews some of the unique side effects and key considerations associated with each agent (Olsen et al., 2019; Yarbro et al., 2018).

Bone Marrow Suppression. Bone marrow suppression refers to three main hematopoietic consequences of chemotherapy: neutropenia (reduction in white blood cells), anemia (reduction in red blood cells), and thrombocytopenia (reduction in platelets).
Neutropenia. When the body’s natural defense, the immune system, is suppressed due to chemotherapy, the patient is considered neutropenic; the ability to mount an immune response to everyday germs, bacteria, or pathogens is poor. As a result, the patient is highly susceptible to illness and is at heightened risk for life-threatening bloodstream infection (bacteremia or sepsis). Neutropenia is defined by an absolute neutrophil count (ANC) of 1,500/mm3 or less and is the primary dose-limiting toxicity of chemotherapy. The most common sign of infection in a neutropenic patient is a fever. Febrile neutropenia is a medical emergency requiring prompt evaluation, workup, and the initiation of empiric antibiotics. Nurses must counsel patients on strategies to avoid infection, such as thorough handwashing, hygiene, and avoiding others who are ill. Patients should also avoid eating raw meats, seafood, eggs, or unwashed vegetables when they are neutropenic due to the risk of acquiring foodborne illnesses (Nettina, 2019; Olsen et al., 2019).
Anemia. Anemia is a common consequence of chemotherapy and generally becomes more significant with each successive dose of chemotherapy due to a cumulative effect as patients progress through treatment. In addition to low hemoglobin and hematocrit as seen through bloodwork, patients may display pallor, fatigue, low energy, chest pain, shortness of breath, and weakness. Some patients may benefit from oral iron supplementation, folic acid, and consuming an iron-rich diet. Others may require erythropoietin-stimulating agents such as epoetin alfa (Procrit, Epogen) or darbepoetin alfa (Aranesp), or blood transfusions (Nettina, 2019; Olsen et al., 2019).
Thrombocytopenia. Thrombocytopenia is a consequence of chemotherapy that heightens the risk of bleeding. Platelets impede bleeding events by clumping and forming plaques in blood vessel injuries, such as cuts, lacerations, and other wounds. The risk of bleeding is present when a patient’s platelet count falls below 50,000/mm3, high risk if their count falls below 20,000/mm3, and critical risk if their count falls below 10,000/mm3 (Nettina, 2019). Patients may require a platelet transfusion if their count drops below 20,000/mm3. This is particularly dangerous in patients on anticoagulation therapy, as their risk of bleeding is already increased. Signs of thrombocytopenia may include bruising, petechiae, epistaxis, gum bleeding, hematuria, or rectal bleeding. Nurses serve an important role in counseling patients on strategies to prevent injury, such as avoiding shaving with razors, rectal suppositories, dental floss, or participating in activities that place them at risk for injury (i.e., contact sports, skiing, horseback riding; Olsen et al., 2019).
CIPN. CIPN is a common side effect of many types of chemotherapy used to treat pancreatic cancer, such as cisplatin (Platinol), albumin-bound paclitaxel (Abraxane), and oxaliplatin (Eloxatin). CIPN results from the demyelination of sensory and motor axons. Patients experience reduced nerve conduction velocity, leading to the loss of deep tendon reflexes and paresthesia (numbness and tingling), weakness, and burning pain. Initially, CIPN often affects the body’s most distal points, such as the fingertips and toes, and progresses proximally toward the midline. In severe cases, patients may lose all sensation in the fingers, hands, toes, and feet; this can cause significant disability, such as the inability to grasp or hold items and gait disturbance, including imbalance and falls. CIPN is a complex topic since no single pathophysiologic process explains the various neuropathies that occur following chemotherapy exposure. CIPN is dose-dependent and progressive while a patient is receiving treatment. Pain, sensory changes, and weakness that manifest during treatment generally lead to chemotherapy dose reductions, changes in treatment protocols, or entirely termination of the therapeutic agent. CIPN can also have a cascading effect after treatment ends, whereby symptoms become more prominent after discontinuation of the offending agent. CIPN is challenging to manage as it does not respond well to conventional treatments. OTC analgesics, menthol creams, capsaicin cream, or lidocaine patches may offer comfort, but most are ineffective. Gabapentin (Neurontin), an anticonvulsant/anti-epileptic agent, has demonstrated limited efficacy but may cause intolerable side effects of weight gain, depression, sedation, and heightens suicide risk. Some patients may find relief from selective serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine (Cymbalta). Nurses should also counsel patients on strategies to avoid injury (e.g., wearing supportive shoes) and promote home safety (e.g., using handrails on stairs and removing throw rugs). Patients must be mindful of water temperatures due to decreased sensitivity to hot water, increasing their risk for burns when bathing or washing dishes. Improvement in function and resolution of symptoms often occurs gradually over time, but nerve damage may be permanent (Brown et al., 2019; Olsen et al., 2019).
Targeted Therapy
Poly ADP-Ribose Polymerase (PARP) Inhibitor
The PARP enzyme serves a critical role in cell growth, cell regulation, and the repair of healthy cells and cancer cells. It fixes DNA damage in cancer cells, essentially helping cancer cells repair themselves and survive. PARP inhibitors interfere with the PARP enzyme, hindering cancer cells with a BRCA1/2 mutation from repairing DNA damage, thereby inducing cell death. PARP inhibitors have transformed the treatment of BRCA–mutant cancers. On December 27, 2019, the US Food & Drug Administration (FDA) approved olaparib (Lynparza) for maintenance treatment in patients with pancreatic cancer with a germline BRCA1/2 mutation. The most common side effects include anemia, neutropenia, fatigue, nausea, diarrhea or constipation, anorexia, and arthralgias. Olaparib (Lynparza) is also associated with a rare risk (less than 1.5%) of myelodysplastic syndrome (MDS, a bone marrow failure disorder) or acute myeloid leukemia (AML, a type of blood cancer). It carries a slight risk (under 1%) of pneumonitis and embryo-fetal toxicity. There are several significant drug interactions, particularly antifungal medications and certain antibiotics. Nurses should ensure that they properly counsel patients about the potential drug interactions and notify their provider before starting any new medications. Patients should also be advised to avoid grapefruit and Seville oranges, as these can increase the drug's effects and toxicity (FDA, 2019a; NCCN, 2020b; Olsen et al., 2019).
Immunotherapy
Pembrolizumab (Keytruda)
Immunotherapy is a novel group of cancer treatments that stimulate the immune system to recognize and destroy cancer cells. Immunotherapy aims to produce antitumor effects by modifying the actions of the body’s natural host defense mechanisms to become more sensitive to cancer cells. Immune-based treatments work differently than chemotherapy as they are highly specialized in their activity. The role of immunotherapy in the treatment of pancreatic cancer is less advanced than other diseases, and clinical research is ongoing. Pembrolizumab (Keytruda) is the only immune-based agent currently approved for use in pancreatic cancers. It is FDA-approved for metastatic pancreatic tumors that are MSI-H or dMMR. Pembrolizumab (Keytruda) is a humanized monoclonal antibody that binds with high affinity to the programmed cell death-1 (PD-1)/PD-ligand 1 (PD-L1). PD-1 is a transmembrane checkpoint protein expressed on the surface of circulating immune cells. PD-1 normally acts as a type of “off switch” to keep the immune cells from attacking other cells in the body. When PD-1 binds to PD-L1, it signals the T-cell to leave the neighboring cells alone. Some cancer cells have large amounts of PD-L1, which helps them evade immune attack. PD-1 and PD-L1 inhibitors have been designed to prevent the formation of this complex and enable immune cells to continue attacking tumor cells. Drugs that target either PD-1 or PD-L1 are designed to block this binding and boost the immune response against cancer cells. In clinical trials, pembrolizumab (Keytruda) demonstrated promising and durable antitumor activity in patients with PD-L1-positive pancreatic cancer, offering a clinically meaningful and viable treatment strategy (Mehnert et al., 2020; Miliotou & Papadopoulou, 2018; NCCN, 2020b).
While pembrolizumab (Keytruda) is typically well-tolerated, all immunotherapy drugs carry boxed warnings for immune-mediated adverse reactions (irAEs), which can be fatal if left untreated. An autoimmune response can impact any organ system, inducing nonspecific inflammation throughout the body. Nursing care of the patient receiving immunotherapy requires cautious triage and continuous meticulous assessment to identify signs of potential immune-related adverse effects (irAEs). Timely diagnosis is critical to ensure prompt response and reduce morbidity, as most irAEs are reversible with immunosuppressive corticosteroids. Patient education is vital. Nurses must teach patients and caregivers about the importance of self-assessment and to immediately report any symptoms. With pneumonitis, symptoms can range from mild cough and dyspnea to severe shortness of breath and life-threatening hypoxia. GI toxicity can range from mild diarrhea and abdominal cramping to severe colitis, which can be fatal if not managed. Skin toxicity may present initially as mild pruritus or dermatitis and can progress to Stevens-Johnson syndrome, characterized by a painful systemic red rash that leads to blistering and sloughing of the skin’s top layer. Life-threatening endocrinopathies can cause an abundance of symptoms that may vary widely, such as extreme weakness, excessive fatigue or lethargy, electrolyte disturbances, thyroid inflammation, and pituitary dysfunction (NCCN, 2020b; Olsen et al., 2019; Sasikumar & Ramachandra, 2018).
Anti-Tumor Therapy for Functioning PNETs
Aside from surgical excision and chemotherapy, directed treatment strategies are recommended to control the unique hormonal effects of PNETs. Gastric hypersecretion caused by gastrinoma tumors can be managed with anti-secretory medications, such as proton pump inhibitors (PPIs; omeprazole [Prilosec], pantoprazole [Protonix], and lansoprazole [Prevacid]). These medications effectively suppress acid secretion, thereby reducing gastric distress, burning, and diarrhea. Somatostatin analogs such as octreotide (Sandostatin) and lanreotide (Somatuline Depot) are synthetic versions of somatostatin that suppress and decelerate hormone production. These injectable agents are primarily administered to palliate symptoms. However, clinical trials have demonstrated the antitumor effects of octreotide (Sandostatin) and lanreotide (Somatuline Depot) in advanced PNETs, suggesting they also help control the disease. The most common adverse reactions include abdominal pain, musculoskeletal pain, muscle spasm, nausea, vomiting, headache, injection site reactions, hyperglycemia, hypertension, and cholelithiasis (FDA, 2019b; NCCN, 2020b; Pavel et al., 2017).
Everolimus (Afinitor) is a relatively novel oral agent that inhibits the mammalian target of rapamycin (mTOR) pathway. The mTOR pathway is a component of a complex intracellular signaling mechanism that serves as a critical regulator of cell physiology in various cancers. Linked to multiple cellular and physiological functions involved in cellular growth, proliferation, and survival, mTOR is used as a drug target in several types of cancers (NCCN, 2020b; Pavel et al., 2017). The most frequent side effect is oral mucositis, inflammation, irritation, swelling, and ulceration of the oral mucosa and lips, which can develop as early as two weeks after starting the medication. Nurses should counsel patients on the benefits of good oral hygiene practices to maintain the integrity of the oral mucosa, such as using a soft-bristled toothbrush and avoiding alcohol-based mouthwashes, hot liquids, and any foods that can cause oral irritation or dryness. Additional reported side effects include fatigue, anorexia, skin rash, diarrhea, and increased serum cholesterol levels. Nurses should also be aware that everolimus (Afinitor) is associated with a risk of angioedema in patients taking concomitant angiotensin-converting enzyme (ACE) inhibitors and should perform a comprehensive medication reconciliation at each visit (FDA, 2018).
For an enhanced understanding of chemotherapy, immunotherapy, and targeted cancer treatments, refer to the following NursingCE courses:
- Oncology Nursing Part 2: Chemotherapy and Oncologic Emergencies.
References
American Board of Internal Medicine. (2020). ABIM laboratory test reference ranges - January 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American Cancer Society. (2019). Signs and symptoms of pancreatic cancer. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/signs-and-symptoms.html
American Cancer Society. (2020a). Key statistics for pancreatic cancer. https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html
American Cancer Society. (2020b). Pancreatic cancer risk factors. https://www.cancer.org/cancer/pancreatic-cancer/causes-risks-prevention/risk-factors.html
American Cancer Society. (2020c). Radiation therapy side effects. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation/effects-on-different-parts-of-body.html
American Cancer Society. (2020d). Survival rates for pancreatic cancer. https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html
American Joint Committee on Cancer. (2018). AJCC cancer staging form supplement: AJCC cancer staging manual, eighth edition. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Cancer%20Staging%20Form%20Supplement.pdf
American Society of Clinical Oncology. (2020a). Lynch syndrome. https://www.cancer.net/cancer-types/lynch-syndrome
American Society of Clinical Oncology. (2020b). Pancreatic cancer: Risk factors. https://www.cancer.net/cancer-types/pancreatic-cancer/risk-factors
American Society of Clinical Oncology. (2020c). Peutz-Jeghers syndrome. https://www.cancer.net/cancer-types/peutz-jeghers-syndrome
Brown, T. J., Sedhom, R., & Gupta, A. (2019). Chemotherapy-induced peripheral neuropathy. JAMA Oncology, 5(5),750. https://doi.org/10.1001/jamaoncol.2018.6771
BruceBlaus. (2013). Pancreas anatomy [image]. https://commons.wikimedia.org/wiki/File:Blausen_0699_PancreasAnatomy2.png
Bujanda, L., & Herreros-Villanueva, M. (2017). Pancreatic cancer in Lynch syndrome patients. Journal of Cancer, 8(18), 3667-3674. http://doi.org/10.7150/jca.20750
Buscail, L., Bournet, B., & Cordelier, P. (2020). Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nature Reviews Gastroenterology & Hepatology, 17, 153-168. https://doi.org/10.1038/s41575-019-0245-4
CancerQuest. (2020). Cancer genes. https://www.cancerquest.org/cancer-biology/cancer-genes
Cancer Research UK. (2014a). Stage I pancreatic cancer [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_T1_cancer_of_the_pancreas_CRUK_246.svg
Cancer Research UK. (2014b). Stage II pancreatic cancer [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_T2_cancer_of_the_pancreas_CRUK_254.svg
Cancer Research UK. (2014c). Stage III pancreatic cancer [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_T4_cancer_of_the_pancreas_CRUK_267.svg
Cancer Research UK. (2014d). Stage IV pancreatic cancer [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_pancreatic_cancer_that_has_spread_(M_staging)_CRUK_179.svg
Centers for Disease Control and Prevention. (2019a). Genetic testing for Lynch syndrome. https://www.cdc.gov/genomics/disease/colorectal_cancer/testing_lynch.htm
Centers for Disease Control and Prevention. (2019b). Hereditary breast cancer and BRCA genes. https://www.cdc.gov/cancer/breast/young_women/bringyourbrave/hereditary_breast_cancer/index.htm
Domaina. (2012). Autosomal dominant inheritance pattern [image]. https://commons.wikimedia.org/wiki/File:Autosomal_dominant_-_en.svg
Domaina, Kashmiri, & SUM1. (2020). Autosomal recessive inheritance pattern [image]. https://en.wikipedia.org/wiki/File:Autosomal_recessive_-_en.svg
Drus1a. (2018). ERCP [image]. https://commons.wikimedia.org/wiki/File:ERCP.png
Genetic and Rare Diseases. (2017). Familial atypical multiple mole melanoma syndrome. https://rarediseases.info.nih.gov/diseases/9281/fammm-syndrome#ref_13155
Lewis, S. Dirksen, S., Heitkemper, M., & Bucher, L. (2014). Medical-surgical nursing: Assessment and management of clinical problems. Elsevier/Mosby.
Lumen Learning. (n.d.). Overview of pancreatic islets. Retrieved December 28, 2020, from https://courses.lumenlearning.com/boundless-ap/chapter/the-pancreas/
Mahon, S. (2020). Germline and somatic mutations: What is the difference? https://voice.ons.org/news-and-views/germline-and-somatic-mutations-what-is-the-difference
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children. (8th ed.). Elsevier.
MedlinePlus. (2020a). CDKN2A gene: Cyclin dependent kinase inhibitor 2A. https://medlineplus.gov/genetics/gene/cdkn2a/
MedlinePlus. (2020b). Hereditary pancreatitis. https://medlineplus.gov/genetics/condition/hereditary-pancreatitis/#synonyms
MedlinePlus. (2020c). Lynch syndrome. https://medlineplus.gov/genetics/condition/lynch-syndrome/
MedlinePlus. (2020d). STK11 gene. https://medlineplus.gov/genetics/gene/stk11/
MedlinePlus. (2020e). TP53 gene: Tumor protein p53. https://medlineplus.gov/genetics/gene/tp53/#conditions
Mehnert, J. M., Bergsland, E., O’Neil, B. H., Santoro, A., Schellens, J. H. M., Cohen, R. B., Boi, T., Ott, P. A., Pishvaian, M. J., Puzanov, I., Aung, K. L., Hsu, C., Le Tourneau, C., Hollebecque, A., Elez, E., Tamura, K., Gould, M., Yang, P., Stein, K., & Piha-Paul, S. A. (2020). Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer, 126(13), 3021-3030. https://doi.org/10.1002/cncr.32883
Miliotou, A. N., & Papadopoulou, L. C. (2018). CAR T-cell therapy: A new era in cancer immunotherapy. Current Pharm Biotechnology, 19(1), 5-18. https://doi.org/10.2174/1389201019666180418095526.
Mirrielees, J. A., Weber, S. M., Abbott, D. E., Greenberg, C. C., Minter, R. M., & Scarborough, J. E. (2020). Pancreatic fistula and delayed gastric emptying are the highest-impact complications after Whipple. Journal of Surgical Research, 250, 80-87. https://doi.org/10.1016/j.jss.2019.12.041
My Gene Counsel. (2020). The genetics of pancreatic cancer. https://www.mygenecounsel.com/the-genetics-of-pancreatic-cancer/
National Cancer Institute. (2020). Pancreatic neuroendocrine tumors (islet cell tumors) treatment (PDQ®)-Health professional version. https://www.cancer.gov/types/pancreatic/hp/pnet-treatment-pdq
National Comprehensive Cancer Network. (2020a). NCCN clinical practice guidelines in oncology (NCCN guidelines®): Genetic/familial high-risk assessment for breast, ovarian, and pancreatic, version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
National Comprehensive Cancer Network. (2020b). NCCN clinical practice guidelines in oncology (NCCN guidelines®): Pancreatic adenocarcinoma, version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice (1st ed.). Oncology Nursing Society.
OpenStax College. (2013). Pancreas [image]. https://commons.wikimedia.org/wiki/File:1820_The_Pancreas.jpg
Pancreatic Cancer Action Network. (n.d.-a). Endoscopic retrograde cholangiopancreatography (ERCP). Retrieved December 20, 2020, from https://www.pancan.org/facing-pancreatic-cancer/diagnosis/endoscopic-retrograde-cholangiopancreatography-ercp/
Pancreatic Cancer Action Network. (n.d.-b). Whipple procedure (pancreaticoduodenectomy). Retrieved December 20, 2020, from https://www.pancan.org/facing-pancreatic-cancer/treatment/treatment-types/surgery/whipple-procedure-pancreaticoduodenectomy/
Pancreatic Cancer Action Network. (2019). Genetics: Risk factor of pancreatic cancer. https://pancreaticcanceraction.org/about-pancreatic-cancer/risk-factors-of-pancreatic-cancer/genetics-risk-factor-of-pancreatic-cancer/
Pavel, M. E., Baudin, E., Oberg, K. E., Hainsworth, J. D., Voi, M., Rouyrre, N., Peeters, M., Gross, D. J., & Yao, J. C. (2017). Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: Final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann Oncology, 28(7), 1569-1575. https://doi.org/10.1093/annonc/mdx193
Pilarski, R. (2019). The role of BRCA testing in hereditary pancreatic and prostate cancer families. American Society of Clinical Oncology Educational Book, 39, 79-86. https://doi.org/10.1200/EDBK_238977
RadiologyInfo.org. (2018). General ultrasound. https://www.radiologyinfo.org/en/info.cfm?pg=genus
RadiologyInfo.org. (2020). Pancreatic cancer. https://www.radiologyinfo.org/en/info.cfm?pg=pancreatic-cancer
Rosati, L. M., & Herman, J. M. (2017). Role of stereotactic body radiotherapy in the treatment of elderly and poor performance status patients with pancreatic cancer. Journal of Oncology Practice, 13(3), 157-166. https://doi.org/10.1200/JOP.2016.020628
Sasikumar, P. G. & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. https://doi.org/10.1007/s40259-018-0303-4.
Sun, N., Lu, G., Zhang, l., Wang, X., Gao, C., Bi, J., & Wang, X. (2017). Clinical efficacy of spleen-preserving distal pancreatectomy with or without splenic vessel preservation: A meta-analysis. Medicine, 96(48), e8600. http://doi.org/10.1097/MD.0000000000008600
Surveillance, Epidemiology, and End Results Program. (2020). Cancer stat facts: Pancreatic cancer. https://seer.cancer.gov/statfacts/html/pancreas.html
US Food & Drug Administration. (2018). Highlights of prescribing information: Afinitor®(everolimus). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022334s040,203985s013lbl.pdf
US Food & Drug Administration. (2019a). FDA approves olaparib for gBRCAm metastatic pancreatic adenocarcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-gbrcam-metastatic-pancreatic-adenocarcinoma
US Food & Drug Administration. (2019b). Highlights of prescribing information: Somatuline® depot (lanreotide) injection. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/2019/08/30162316/Somatuline_Depot_Full_Prescribing_Information_7.22.19.pdf
US National Library of Medicine. (2020a). Familial adenomatous polyposis. https://ghr.nlm.nih.gov/condition/familial-adenomatous-polyposis
The US National Library of Medicine. (2020b). What are the different ways in which a genetic condition can be inherited? https://ghr.nlm.nih.gov/primer/inheritance/inheritancepatterns
Waters, A. M., & Der, C. J. (2018). KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harbor Perspectives in Medicine, 8(9), 1-17. https://doi.org/10.1101/cshperspect.a031435
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice (8th ed.). Jones & Bartlett Learning.
Yaskin, J. C. (1931). Nervous symptoms as earliest manifestations of carcinoma of the pancreas. JAMA, 96(20), 1664-1668. https://doi.org/10.1001/jama.1931.02720460010003
Yousaf, M. N., Chaudhary, F. S., Ehsan, A., Suarez, A. L. Muniraj, T., Jamidar, P., Aslanian, H. R., & Farrell, J. J. (2020). Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterology, 7(1), e000408. https://doi.org/10.1136/bmjgast-2020-000408