About this course:
This module offers an overview of pneumonia and its risk factors, clinical features, best practices for diagnosis and treatment to inform nursing practice, facilitate optimal care, patient education, and improved patient outcomes.
Course preview
This module offers an overview of pneumonia and its risk factors, clinical features, best practices for diagnosis and treatment to inform nursing practice, facilitate optimal care, patient education, and improved patient outcomes.
By the completion of this activity, the nurse will be prepared to:
- discuss the pathophysiology of pneumonia
- list the risk factors for pneumonia
- examine the classifications of pneumonia
- describe the signs and symptoms, diagnostic criteria, and management of pneumonia
- define the complications related to pneumonia and its management
- discuss various types of immunizations for pneumonia, including vaccine schedules, development, and research
Definitions
Alveoli: tiny air sacs at the end of the bronchioles (see Figure 1) that inflate with inspiration (inhalation) and deflate with expiration (exhalation). Gas exchange occurs in capillaries in the alveolar walls (Dugdale et al., 2020).
Atelectasis: alveolar collapse (Ignatavicius & Workman, 2015).
Bronchioles: small branches of the bronchi that end in alveoli. The trachea branches into the bronchi (see Figure 1), which divide into smaller branches known as bronchioles (Dugdale et al., 2020).
Consolidation: an x-ray finding that indicates a lack of air in the alveoli, as evidenced by grey or white shading in those areas. The alveoli may be filled with fluid (e.g., exudate, blood), cells (e.g., inflammation), or other materials (Ignatavicius & Workman, 2015).
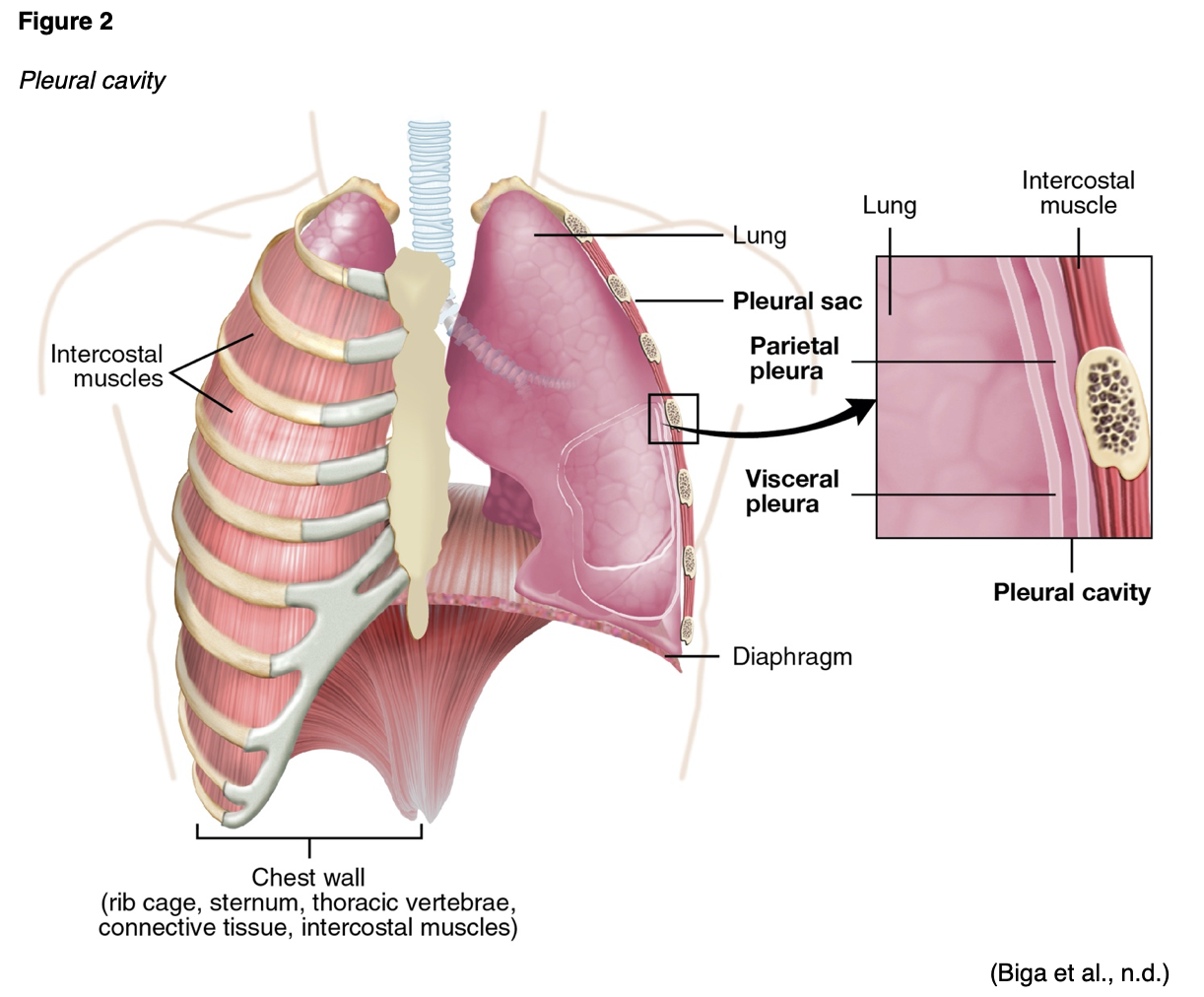
Empyema: a collection of purulent fluid in the pleural cavity (see Figure 2; Ignatavicius & Workman, 2015).
Immunogenicity: the ability of a vaccine to elicit immunity (Ignatavicius & Workman, 2015).
Interstitial spaces (interstitium): the tissue area in or around the alveolar wall where oxygen moves from the alveoli into the capillaries. Oxygen crosses the interstitial space and enters the bloodstream while carbon dioxide (CO2) crosses from the capillaries through the interstitial space into the lungs for expiration (Stanford Medicine, n.d.).
Pulmonary compliance: compliance refers to the ease with which an organ or tissue stretches. Pulmonary compliance is the extent of lung expansion (Desai & Moustarah, 2020).
Vital capacity: the maximum volume of air that can be expired following a full inspiration (David & Sharma, 2020).

Definition and Epidemiology of Pneumonia
Pneumonia is defined as an excess of fluid in the lungs resulting from an inflammatory process. The inflammation can be triggered by an invasion of an infectious organism or inspiration of an irritating agent, and it occurs in the alveoli, interstitial spaces, and bronchioles (Ignatavicius & Workman, 2015). The World Health Organization (WHO, 2019) has estimated that a lower respiratory tract infection is the most common infectious cause of death globally and the fourth leading cause of death. Three million deaths worldwide were attributed to lower respiratory tract infections in 2016 (WHO, 2019). In the US, between 2 and 5 million cases of pneumonia occur every year (Ignatavicius & Workman, 2015). An estimated 20 million hospitalizations for pneumonia occurred in the US between 2001 and 2014; in-hospital death occurred in 7.4% of these cases. Non-Hispanic Native Americans, Alaskan Natives, and non-Hispanic Black patients had the highest average rates of pneumonia-associated hospitalizations across all races and ethnicities. Total costs for pneumonia-associated hospitalizations in 2014 were over $84 billion (Hayes et al., 2017). The incidence of pneumonia is higher in older adults, hospitalized patients, nursing home residents, and those on mechanical ventilation (Ignatavicius & Workman, 2015).
Pathophysiology and Etiology
Pneumonia may be noninfectious or infectious. Noninfectious pneumonia can be due to the inspiration of toxic gases, chemicals, smoke, or the aspiration of water, food, fluid (including saliva), or vomit. The process of infectious pneumonia begins when an organism enters the airway mucosa and multiplies within the alveolar spaces. A person’s environment, contact with other people, invasive devices, medical equipment or supplies, and staff can spread organisms that cause pneumonia. Organisms that cause pneumonia include bacteria, mycoplasmas, viruses, fungi, rickettsia, protozoa, and helminths (parasites; Ignatavicius & Workman, 2015). Viral pathogens have been identified in 27% of cases, and bacterial pathogens have been identified in 14% (Grief & Loza, 2018). In adults, Streptococcus pneumoniae (S. pneumoniae) is the most common bacterial cause. Other causative bacteria include Staphylococcus aureus (S. aureus), Enterobacteriaceae, Mycoplasma pneumoniae (M. pneumoniae), Chlamydophila pneumoniae (C. pneumoniae), and Haemophilus influenzae type B (H. influenzae; Grief & Loza, 2018).
The severity of pneumonia and the extent of lung involvement depend on the patient’s immune response. Initial host mechanisms are in place to prevent infection, such as hair in the nasal passages and mucus in the nasopharynx and oropharynx. Alveolar epithelial cells produce surfactant A and D, proteins that opsonize bacteria. Alveolar macrophages are then able to engulf any bacteria that make it past these initial defenses. Bacteria can multiply quickly in immunocompromised patients. Alveolar macrophages initiate the inflammatory cascade, which leads to the release of cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and granulocyte colony-stimulating factor (G-CSF). Fluid collects in and around the alveoli, with few neutrophils and macrophages (types of white blood cells [WBCs]) initially. Chemotaxis (movement of motile WBCs) is driven by the inflammatory cytokine IL-8, while G-CSF accelerates neutrophil maturation (Sattar & Sharma, 2018).
Additional WBCs migrate to the infected area, which causes capillary leakage, edema, and exudate. As the lobe becomes consolidated with serous exudate and fibrin, the alveolar walls thicken. This consolidation and thickening reduce gas exchange and interfere with oxygenation. Red blood cells (RBCs) migrate into the alveoli as capillary leakage facilitates the spread of infection to other lung regions and hemoptysis (bloody sputum production) in some cases. Fibrin and tissue edema cause the lungs to stiffen, leading to reduced compliance and decreased vital capacity. Atelectasis further reduces the ability of the lungs to oxygenate the circulating blood, worsening hypoxemia (decreased arterial oxygen levels). The constitutional symptoms of pneumonia are primarily related to cytokine release, such as fever caused by the release of IL-1 and TNF. In lobar pneumonia, consolidation occurs in a segment or lobe of the lung. Bronchopneumonia consists of diffuse patches of consolidation around the bronchi. If the infection spreads into the pleural cavity, empyema results (see Figure 2). Septicemia results if the organism moves into the bloodstream (Ignatavicius & Workman, 2015; Sattar & Sharma, 2018).
Pneumonia is often categorized by setting, including community-acquired pneumonia (CAP), healthcare-associated pneumonia (HCAP), hospital-acquired pneumonia (HAP), or ventilator-associated pneumonia (VAP; Ignatavicius & Workman, 2015).
Community-acquired pneumonia (CAP): pneumonia contracted outside of a healthcare setting or within 4
...purchase below to continue the course
Healthcare-associated pneumonia (HCAP): pneumonia contracted from a healthcare facility such as a long-term care facility, skilled nursing facility, dialysis center, or outpatient clinic. HCAP most often occurs within 48 hours of hospital admission in a patient that meets at least one of the following conditions:
- a prior inpatient hospital admission for longer than 48 hours in the past 90 days
- resides in a skilled nursing or assisted-living facility
- has received intravenous (IV) therapy, wound care, antibiotics, or chemotherapy in the past 30 days
- has been seen at a hospital or dialysis clinic within the past 30 days (Ignatavicius & Workman, 2015; Sattar & Sharma, 2018)
*Note: This designation was introduced in the 2005 pneumonia guidelines but is no longer recommended for use as outlined in the 2019 guidelines from the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA).
Hospital-acquired pneumonia (HAP): pneumonia that begins or is diagnosed more than 48 hours after hospital admission in a non-intubated patient. The most common bacterial causes of HAP include methicillin-resistant S. aureus (MRSA) and P. aeruginosa (Ignatavicius & Workman, 2015; Sattar & Sharma, 2018).
Ventilator-associated pneumonia (VAP): pneumonia that begins or is diagnosed 48 to 72 hours after endotracheal intubation (Ignatavicius & Workman, 2015; Sattar & Sharma, 2018).
CAP occurs more often than HAP, particularly in the late fall and winter, as a complication of influenza (Ignatavicius & Workman, 2015). Pneumonia can also be designated as typical or atypical. Usual pneumonia organisms can be cultured on standard media or seen on a traditional Gram stain. It may be due to S. pneumoniae, S. aureus, Streptococcus pyogenes (i.e., group A Streptococcus or GAS), H. influenzae, Moraxella catarrhalis, anaerobes, or aerobic gram-negative bacteria. Atypical pneumonia cannot be cultured on standard media or viewed consistently on a Gram stain and may be caused by Legionella pneumophila (L. pneumophila), M. pneumoniae, C. pneumoniae, or Chlamydia psittaci (C. psittaci; Sattar & Sharma, 2018).
Risk Factors and Prevention
The risk factors for pneumonia vary slightly depending on the setting in which it was contracted. For CAP, risk factors include increased age, lack of pneumococcal vaccination for protection against S. pneumoniae, pneumococcal vaccination over 5 years ago, lack of influenza vaccination, chronic health conditions, immunodeficiency, recent exposure to a respiratory virus (e.g., influenza), tobacco or alcohol use, and exposure to secondhand smoke (Ignatavicius & Workman, 2015).
Risk factors for HAP include increased age; chronic lung disease; gram-negative colonization of the upper gastrointestinal tract; an altered level of consciousness; recent aspiration; the presence of an endotracheal tube (ETT), tracheostomy, or nasogastric (NG) tube; poor nutritional status; immunocompromised status; use of medications that increase gastric pH (e.g., histamine-2 antagonists [H2 blockers]) or alkaline tube feedings; and mechanical ventilation. VAP is considered a type of HAP that occurs in mechanically ventilated patients, and its risk factors are discussed later in this module (Ignatavicius & Workman, 2015).
For pneumonia prevention among pediatric patients, all vaccinations should be encouraged, especially S. pneumoniae, H. influenzae type b, and pertussis. Yearly influenza vaccines are recommended, and parents and caretakers should be immunized against influenza and pertussis (Metlay et al., 2019). CAP prevention in adults can be accomplished via smoking cessation, annual influenza vaccination, and pneumococcal vaccination administration per the current guidelines when appropriate (Ramirez, 2020).
Diagnosis
No individual part of a patient’s history or physical examination can serve as a definitive finding to diagnose pneumonia. A combination of multiple findings is required. Typically, chest imaging should demonstrate new or worsening infiltrates combined with a clinical picture of pneumonia (e.g., fever, dyspnea, and a productive cough; Ramirez, 2020).
History
A patient history for suspected pneumonia should include all potential risk factors, documenting the patient’s age, diet, exercise habits, sleep routine, social environment, use of a tracheostomy or NG tube, and current or historical tobacco, alcohol, and recreational drug use. The interviewer should inquire about past respiratory illnesses and recent exposure to individuals with known or suspected influenza or pneumonia. Patients should always be asked when they last received an influenza and pneumococcal vaccine (Ignatavicius & Workman, 2015). Patients should also be asked about recent travel, as L. pneumophila, Blastomyces dermatitidis, Coccidioides, Hantavirus, Middle East respiratory syndrome, and avian influenza often cause pneumonia in national and international travelers. Recent travel aboard a cruise ship may indicate L. pneumophila (Grief & Loza, 2018).
Eliciting a patient history of risk factors, behaviors, and environmental exposures can help establish potential pathogens and etiologies. A history of asthma, chronic obstructive pulmonary disease (COPD), immunodeficiency, and smoking can indicate an H. influenzae infection. Contaminated air conditioners and water systems can harbor L. pneumophila, causing pneumonia, often referred to as Legionnaire’s disease. Crowded spaces—such as jails, dorms, and shelters—can expose a person to S. pneumoniae, Mycobacterium tuberculosis (M. tuberculosis, or TB), M. pneumoniae, and Chlamydia trachomatis (C. trachomatis). Animals can expose patients to Coxiella burnetii, particularly cats, sheep, and cattle. Birds like chickens, turkeys, and ducks can expose patients to C. psittaci (Sattar & Sharma, 2018). In patients with chronic respiratory disease, the interviewer should assess for and document respiratory equipment used within the home, including the cleaning and storing of this equipment (Ignatavicius & Workman, 2015). S. aureus and Enterobacteriaceae affect more patients in intensive care units (ICUs; Grief & Loza, 2018).
Clinical Manifestations
Clinical manifestations often include generalized symptoms such as fevers or fatigue, but many patients will also demonstrate findings that indicate damage to the pulmonary tissue (Sattar & Sharma, 2018). For patients who present with acute fever or chills and coughing, pneumonia should be a differential diagnosis. The cough may or may not be productive. For patients who present with coughing, the probability of pneumonia is only 5%. If they do not have changes in blood pressure (BP), heart rate (HR), or respiratory rate (RR), the probability drops to 1% (Grief & Loza, 2018). Patients with pneumonia may report chest pain or discomfort (pleuritic chest pain may be sharp, stabbing, burning, intense, or sudden and occurs with respiration, especially inspiration), dyspnea, and increased or bloody sputum production. They may present with coughing and tachypnea (an increased RR above 18 breaths/minute). Pleuritic chest discomfort is due to inflammation of the parietal pleura. Tachypnea and dyspnea often result from the stimulation of chemoreceptors in pneumonia, but anxiety and pain can also contribute to changes in the patient’s RR. Coughing is due to fluid accumulation in the receptors of the trachea, bronchi, and bronchioles. Purulent, bloody, or rust-colored sputum is due to the inflammatory process, which causes fluid from the pulmonary capillaries and RBCs to move into the alveoli. Patients with severe coughing may complain of chest muscle weakness (Grief & Loza, 2018; Ignatavicius & Workman, 2015).
Vital signs should be compared to the patient’s baseline values. Hypotension is common in patients with pneumonia due to vasodilation and dehydration, especially in older adults. Fevers manifest when pyrogens are released by phagocytes and cause the hypothalamus to increase body temperature above 100.4 °F (38 °C). The patient may report associated myalgias (muscle aches) and chills. Tachycardia (an increased HR above 100 beats/minute) is often seen in patients with pneumonia; a rapid, weak pulse can indicate hypoxemia, dehydration, or possible sepsis or shock. Cardiac tissue hypoxia can cause dysrhythmias (Ignatavicius & Workman, 2015). The patient may report anorexia (loss of appetite) and headaches (Grief & Loza, 2018; Ignatavicius & Workman, 2015). A decreased body temperature below 95 °F (35 °C) or bradycardia (HR below 60 beats/minute) may also indicate pneumonia, sepsis, or shock (Sattar & Sharma, 2018). Oxygen saturation (SpO2 or pulse oximetry) should be used to assess patients for hypoxemia (Ignatavicius & Workman, 2015).
Individuals with pneumonia may appear flushed, anxious, or cyanotic. Each patient’s breathing pattern should be carefully observed, as patients may demonstrate abnormal breathing patterns and accessory muscle use. Labored breathing occurs in response to decreased lung compliance. Hypoxic patients may feel uncomfortable in a reclining position and often request to sit upright. They will sometimes lean forward while sitting and place their hands on their knees, referred to as the tripod position. On auscultation of the lungs, rales (i.e., short, high-pitched, intermittent crackles most often heard in the lung bases) can be heard when fluid is in the alveolar and interstitial areas. Breath sounds may also be diminished. Wheezing (i.e., a high-pitched, coarse whistling sound) may be heard if exudate, mucus, or inflammation narrows the airways. Over areas of density or consolidation, bronchial breath sounds (i.e., high-pitched and hollow or tubular, as would typically be heard over the central airways) may be heard due to sound transmission from the trachea. A pleural friction rub (a raspy, grating, or creaky sound due to inflammation within the pleural cavity) may be audible. Egophony can be assessed by asking the patient to say the letter “e” while auscultating with a stethoscope. The sound being audibly altered, becoming more high-pitched, nasal, and sounding more like an “a”, indicates potential consolidation. Similarly, whispered pectoriloquy could be assessed by asking the patient to whisper “99” several times. If the patient can be heard clearly, this indicates potential consolidation. Tactile fremitus (i.e., palpable vibration of the chest wall with breathing) will be increased over pneumonia-affected regions, and chest percussion will be flat or dulled over consolidated areas. Chest expansion may be diminished or asymmetrical during inspiration (Ignatavicius & Workman, 2015; Sattar & Sharma, 2018). Tracheal deviation and lymphadenopathy may be evident on the exam. Confusion manifests earlier in older patients with pneumonia (Sattar & Sharma, 2018). Patients with nursing home residency, immunocompromise, and advanced age often display fewer symptoms of pneumonia, so this condition should be suspected even without overt symptoms (Grief & Loza, 2018).
Atypical pneumonia often prompts both pulmonary and generalized clinical manifestations. Some clinical manifestations that can aid in identifying etiology include the following (Sattar & Sharma, 2018):
- Rigors (excessive shivering due to a steep rise in body temperature and often accompanied by a feeling of cold and profuse sweating) and rust-colored sputum are seen more often with pneumococcal pneumonia (caused by S. pneumoniae).
- P. aeruginosa or H. influenzae are associated with green sputum.
- K. pneumoniae is associated with “red currant jelly” sputum.
- Foul-smelling and bad-tasting sputum or a recent history of dental illness is associated with anaerobic bacteria.
- Legionnaire’s disease will often cause alterations in mental status and gastrointestinal symptoms and is associated with bradycardia.
- Aspiration pneumonia is associated with an impaired gag reflex.
- Nocardia, a Gram-positive bacterium found in the water and soil, will often cause cutaneous nodules.
- Bullous myringitis (bullae or vesicles found on the tympanic membrane) can accompany Mycloplasma.
Pneumonia Severity Scale
Pneumonia severity scales assist in determining disease severity and optimal treatment setting and predicting patient mortality. The most common options are the Pneumonia Severity Index (PSI) and the CURB-65 (Ignatavicius & Workman, 2015). Both scales reasonably predict 30-day mortality (Murillo-Zamora et al., 2018). The PSI is more accurate and better validated (Ramirez, 2020). The PSI coalesces a patient’s demographics, comorbidities, physical examination, and laboratory values, yielding a score that determines the severity of pneumonia (Ignatavicius & Workman, 2015). The patient’s score is then used to assign them a risk class that determines inpatient or outpatient treatment (Patel & Makwana, 2021). The CURB-65 relies on laboratory values, age, vital signs, and the presence of confusion to determine risk and recommend treatment setting/level of care (Ignatavicius & Workman, 2015).
Imaging Assessments
The most common diagnostic tool used for pneumonia is a chest x-ray. However, pneumonia-related changes may not be seen until 2 or more days after clinical manifestations occur. Consolidation creates an opaque area of increased density on a chest x-ray. The increased density can affect a lung segment, an entire lobe, an entire lung, or both lungs. A chest x-ray is essential for a pneumonia diagnosis for older adults since clinical manifestations can be vague. Examples of pneumonia shown via chest x-ray appear in Figures 3 and 4 (Ignatavicius & Workman, 2015).
If a patient with pneumonia has symptoms that improve in 5 to 7 days of treatment, follow-up chest x-rays are not routinely recommended, as reimaging does not have clinical significance (Metlay et al., 2019). Maughan and colleagues (2014) found that 11.4% of emergency department patients with a negative chest x-ray had conclusive evidence of pneumonia on computed tomography (CT) scan. However, this is not the current standard of practice (Ramirez, 2020).

For all children receiving inpatient care for pneumonia, 2-view chest x-rays with posteroanterior and lateral views should be obtained. While routine chest x-rays are not necessary to confirm all suspected pneumonia diagnoses in children in the outpatient setting, 2-view chest x-rays should be obtained for pediatric patients with hypoxemia or respiratory distress (Bradley et al., 2011).
Laboratory Assessments
For patients with confirmed or suspected mild pneumonia, sputum and blood cultures are not recommended for routine outpatient use (Metlay et al., 2019). Sputum is often obtained in the inpatient setting for Gram stain, culture, and sensitivity testing. The offending organism is not identified in many cases. A sputum sample can be obtained easily from patients who can cough into specimen containers. Unfortunately, these specimens are contaminated with upper airway organisms. Patients who are extremely ill or unable to cough for collection may need suctioning to obtain a specimen using a sputum trap to obtain a sample (Ignatavicius & Workman, 2015). There are several steps that the medical team can take to optimize the quality of a sputum sample. The specimen should be obtained before antibiotic administration. The mouth should be rinsed before expectoration. The patient should avoid eating or drinking for 1-2 hours before expectoration, and the specimen should be inoculated onto the culture media immediately after collection (Boruchoff & Weinstein, 2021).
The 2019 ATS/IDSA guidelines recommend Gram staining and culturing (both blood and sputum cultures) for hospitalized patients with severe CAP and risk factors for MRSA and P. aeruginosa. This recommendation also extends to all patients with HAP. Sputum and blood cultures are not routinely recommended for all patients managed in the hospital (Metlay et al., 2019).
Blood cultures are not routinely performed for children with pneumonia who are fully immunized and receiving outpatient care. Blood cultures should be obtained when a child fails to demonstrate clinical improvement with treatment or has progressive symptoms or clinical deterioration after starting antibiotic therapy. For children admitted for inpatient treatment of suspected bacterial pneumonia that is moderate to severe, blood cultures and sputum cultures should be obtained. If a child improves on appropriate antimicrobial therapy, a positive blood culture should not prevent discharge, especially if a follow-up visit with a healthcare provider is scheduled. Repeat blood cultures are unnecessary if the child demonstrates clear clinical improvement unless the blood culture showed bacteremia due to S. aureus. Testing (e.g., urine antigen) for pneumococcal infections is not recommended in children, as false-positive results are common (Bradley et al., 2011).
Fiber optic bronchoscopy for microbiologic testing can be considered for hospitalized and severely ill patients if the potential benefits of this testing (i.e., confirmation of microbiologic diagnosis) outweigh the risks (e.g., bleeding, pneumothorax, need for intubation, bronchospasm). A bronchoscopy specimen should be sent for aerobic culture, L. pneumophila culture, fungal stain/culture, and testing for respiratory viruses (e.g., influenza, adenovirus, parainfluenza, RSV, and human metapneumovirus; Ramirez, 2020). This specimen may be obtained via bronchoalveolar lavage, routine brushing, washing, or protected specimen brushing with a double-sheathed catheter to minimize contamination. It is considered most useful in patients with an underlying etiology of TB, P. jirovecii, fungal or viral pathogens, or noninfectious cases (e.g., malignancy; Boruchoff & Weinstein, 2021).
More invasive tests rarely performed on a patient with pneumonia include transtracheal aspiration and direct needle aspiration (Ignatavicius & Workman, 2015). Transtracheal aspiration involves using a large (15g) needle to access the trachea by entering caudally approximately 1 cm below the cricoid cartilage. The access needle is then replaced with a catheter attached to a syringe. The patient is encouraged to cough while suction is applied to the attached syringe. A sterile saline wash can also be injected to obtain a sample for culture/sensitivity testing (Mohanty et al., 2017). Thoracentesis may assist patients who are suffering from accompanying pleural effusions (Ignatavicius & Workman, 2015).
A complete blood count (CBC) should be obtained in hospitalized patients to assess for an elevated WBC count. A high WBC count (leukocytosis) is a common finding among patients with pneumonia, except older adults (Ignatavicius & Workman, 2015). Arterial blood gas (ABG) analysis determines the patient’s baseline arterial oxygen and carbon dioxide levels. Serum lactate levels are recommended to evaluate for possible sepsis. Serum electrolytes, blood urea nitrogen (BUN), and creatinine levels should be used to assess fluid status and organ function (Ignatavicius & Workman, 2015). Labs such as an erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), or serum procalcitonin are often elevated in pneumonia patients (Ramirez, 2020). Empiric antibiotic therapy should be initiated in patients with clinical features and radiographic findings consistent with pneumonia, regardless of serum procalcitonin results (Metlay et al., 2019). These labs also indicate the clinical response to therapy in patients with moderate to severe pneumonia who require hospitalization. Testing recommendations for pediatric pneumonia patients include a CBC for more severe cases (Bradley et al., 2011).
Patients presenting with weight loss, persistent coughing, night sweats, and hemoptysis should be tested for M. tuberculosis. Patients who immigrated from countries with TB outbreaks, reside in homeless shelters, inject illicit drugs, or have HIV should also be considered high risk for TB (Grief & Loza, 2018). Some bacteria will demonstrate specific biochemical evidence found in laboratory evaluation. An example of this is L. pneumophila, which can present with hyponatremia and microhematuria (Sattar & Sharma, 2018). Immunocompromised patients should be tested for opportunistic infections such as Pneumocystis jirovecil, fungal or parasitic infections, and less common viral pathogens (e.g., cytomegalovirus; Ramirez, 2020).
During influenza season, patients with CAP or suspected CAP should be tested for influenza with a rapid molecular assay. The rapid molecular assay (i.e., nucleic acid amplification test [NAAT]) is preferred over a rapid influenza diagnostic test (i.e., antigen test). During periods of lower influenza activity in a community, influenza testing can be considered but is not recommended. Testing for influenza and other respiratory viruses should be used routinely to evaluate children with suspected pneumonia. For children with positive influenza tests, antibacterial therapy is not necessary unless a bacterial coinfection is suspected. Antiviral agents should be used according to the current guidelines for pediatric influenza patients (Bradley et al., 2011; Metlay et al., 2019).
Management and Treatment
The medical management and treatment of pneumonia depend on the type and severity of pneumonia. Most of the time, pneumonia can be treated in the outpatient setting, especially CAP. As previously outlined, a severity assessment tool can determine whether the patient needs outpatient or inpatient treatment. For patients who cannot be treated in the outpatient setting, there are guidelines and recommendations for inpatient treatment (Metlay et al., 2019).
Pneumonia Treatment for Infants and Children
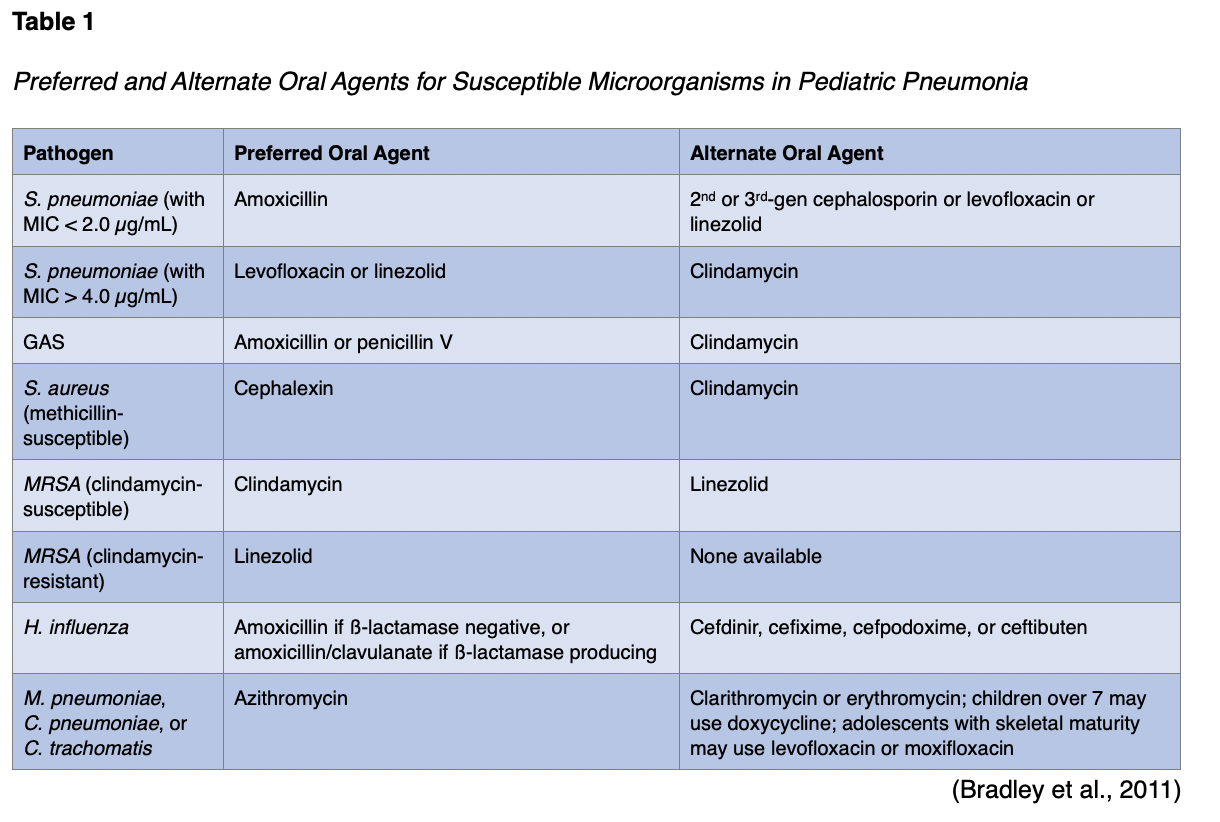
Antimicrobial therapy is not always required for preschool-aged pediatric patients with pneumonia, as these children often experience pneumonia caused by viral pathogens. If children need antibiotic therapy, amoxicillin (Amoxil) should be used for healthy, fully immunized infants, children, and adolescents with mild to moderate pneumonia, as shown in Table 1. It provides appropriate coverage for S. pneumoniae, which is the most common bacterial pathogen. Alternatives include second- or third-generation cephalosporins such as cefpodoxime (Vantin), cefuroxime (Zinacef), and cefprozil (Cefzil). Oral levofloxacin (Levaquin) or linezolid (Zyvox) can also be used. Macrolide antibiotics (e.g., azithromycin [Zithromax], erythromycin [Erythrocin], and clarithromycin [Biaxin]) should be used to treat children with pneumonia that may be caused by atypical pathogens such as M. pneumoniae, C. pneumoniae, and C. trachomatis. Treatment courses for antibiotics lasting 10 days have been studied the most, but shorter periods may be effective in those with mild disease (Bradley et al., 2011).
Pulse oximetry should be monitored in all children with pneumonia. The guidelines recommend hospitalization in pediatric patients with atypical or highly virulent pathogens (MRSA), infants with bacterial CAP less than 6 months old, or moderate to severe CAP based on clinical factors such as respiratory distress and hypoxemia (SpO2 < 90%). They also recommend hospital-based care if there is concern regarding the caregiver’s ability to provide sufficient supervision and comply with the prescribed treatment plan. Admission to an ICU is recommended for children with pneumonia who require positive pressure ventilation (either invasive or noninvasive), have impending respiratory failure (a SpO2 < 92% on 0.5 [50%] fraction of expired oxygen [FiO2]), sustained tachycardia, hypotension, altered mental status related to hypercarbia or hypoxemia, or a need for vasopressor support to maintain perfusion. The severity scores described within this activity should not be used as the sole determining factor regarding the optimal treatment setting for pediatric patients. Still, they may be included as a component of the decision-making process along with clinical, laboratory, and imaging findings (Bradley et al., 2011).
If IV antibiotics are required for a healthy, fully immunized pediatric patient due to lack of clinical improvement in the first 24-72 hours, the preferred treatment for S. pneumoniae is ampicillin (Omnipen) or penicillin. Alternatives include ceftriaxone (Rocephin) or cefotaxime (Claforan). These third-generation cephalosporins should be used for pediatric patients who are not fully immunized, live in areas with a high rate of penicillin resistance, have life-threatening infections, or develop empyema. Combination therapy with a macrolide given either orally or parenterally may be indicated, along with a ß-lactam antibiotic (e.g., -cillins, cephalosporins, and carbapenems), for pediatric patients with M. pneumoniae or C. pneumoniae. Vancomycin (Vancocin) or clindamycin (Cleocin) should be administered with ß-lactam therapy (e.g., cefazolin [Ancef], oxacillin [Bactocill]) for infections caused by S. aureus. As previously mentioned, treatment courses for antibiotics lasting 10 days have been studied the most. Infections caused by MDR pathogens may require more prolonged treatment. Pediatric patients who are receiving appropriate therapy should show clinical and laboratory improvements within 48 to 72 hours. For pediatric patients who show deterioration after admission and no improvement within 48 to 72 hours, further investigation is needed to determine the reason for their lack of response to therapy and whether higher-level care is indicated (Bradley et al., 2011). See Table 2 for recommended antibiotic agents for susceptible microorganisms.
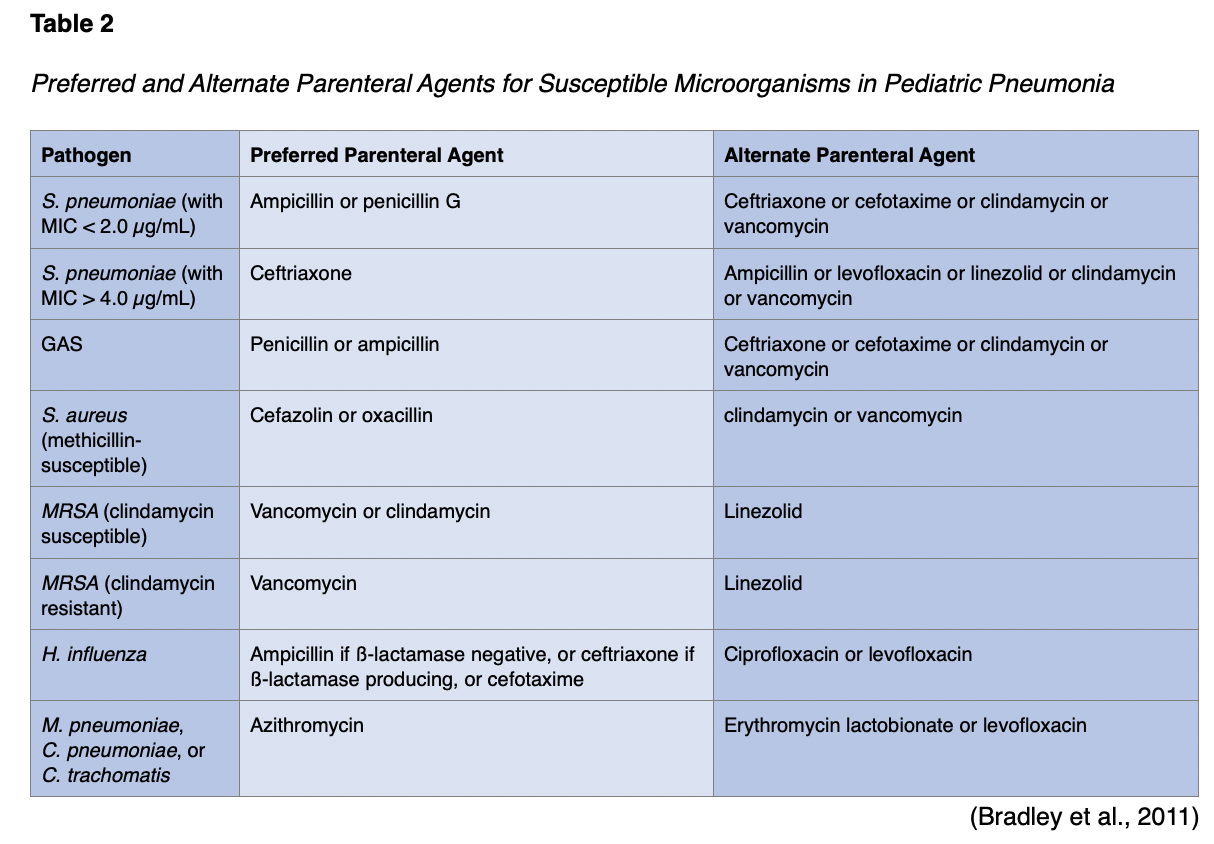
For children with moderate to severe pneumonia during influenza season and suspected influenza virus infection, influenza antiviral therapy should be administered as soon as possible. Oseltamivir (Tamiflu) is recommended in liquid or tablet form and is typically weight-based for pediatric patients. Other medications include zanamivir (Relenza), amantadine (Symmetrel), and rimantadine (Flumadine). Amantadine (Symmetrel) and rimantadine (Flumadine) are only recommended for treatment or prophylaxis during influenza seasons in which most influenza A strains are determined to be susceptible to adamantine (Symmetrel) due to a rapid emergence of resistance. For patients who require adamantane (Symmetrel) therapy, a course of about 7 days is suggested, or until signs and symptoms have resolved for 24 to 48 hours. Early antiviral treatment provides the maximum benefit, so treatment should not be delayed while awaiting the confirmation of a positive influenza test result (Bradley et al., 2011).
Repeat chest x-rays should be performed to assess the progression of pneumonia or the development of complications. Repeat cultures may be needed to identify whether the original pathogen persists or has developed resistance to the chosen antimicrobial; this step can also elucidate a new secondary infection. For seriously ill children on mechanical ventilation, a percutaneous lung aspiration or open lung biopsy may be required if previous cultures did not indicate a microbiologic etiology (Bradley et al., 2011).
Pediatric patients can be eligible for discharge if they have demonstrated an overall clinical improvement in activity level, appetite, and absence of fever for at least 12 to 24 hours. Patients should have a consistent SpO2 over 90% on room air for at least 12 to 24 hours and a stable or returned-to-baseline mental status. For children who had a chest tube, discharge is appropriate once the chest tube has been removed for 12 to 24 hours with no evidence of deterioration. Patients should not have increased work of breathing, sustained tachypnea, or tachycardia to be eligible for discharge. Documentation should indicate that the patient has tolerated their home antimicrobial and oxygen regimens before release. Parents should demonstrate an ability to comply with the prescribed antibiotic regimen before discharge. Any issues or concerns about home care, ability to comply with therapy, or availability for follow-up care should be addressed before release (Bradley et al., 2011).
Outpatient parenteral antibiotic therapy is appropriate in patients who no longer require skilled nursing care but continue to need ongoing parenteral treatment. The therapy can be offered through a pediatric home health program or daily intramuscular (IM) injections in an outpatient facility. Oral treatment is preferred to parenteral outpatient therapy when possible (Bradley et al., 2011).
Pneumonia Treatment for Adults
For healthy adult patients without comorbidities who are receiving outpatient treatment, monotherapy is recommended with amoxicillin (Amoxil), doxycycline (Vibramycin), or a macrolide antibiotic such as azithromycin (Zithromax) or clarithromycin (Biaxin) if the local pneumococcal resistance is below 25%. If the patient has comorbidities such as chronic disease of the heart, lung, liver, or kidneys; diabetes mellitus; alcoholism; cancer; or asplenia, combination therapy with either amoxicillin/clavulanate (Augmentin) or a cephalosporin plus either a macrolide or doxycycline (Vibramycin) is recommended. Alternately, monotherapy in patients with comorbidities with a respiratory fluoroquinolone (e.g., levofloxacin [Levaquin], ciprofloxacin [Cipro], moxifloxacin [Avelox], gemifloxacin [Factive]) is advised. Patients with comorbidities are more vulnerable to poor outcomes when initial antibiotic therapy has failed. Combining a ß-lactam or a cephalosporin with a macrolide or doxycycline (Vibramycin) helps target possible MDR organisms, such as S. pneumoniae common in patients with comorbidities. Current treatment recommendations outline antibiotic options but do not specify a preference order. However, monotherapy using amoxicillin (Amoxil) in those without comorbidities and monotherapy using a fluoroquinolone in those with comorbidities were both strong recommendations, while the remaining regimens were conditional (Metlay et al., 2019). Table 3 summarizes adult outpatient treatment recommendations.
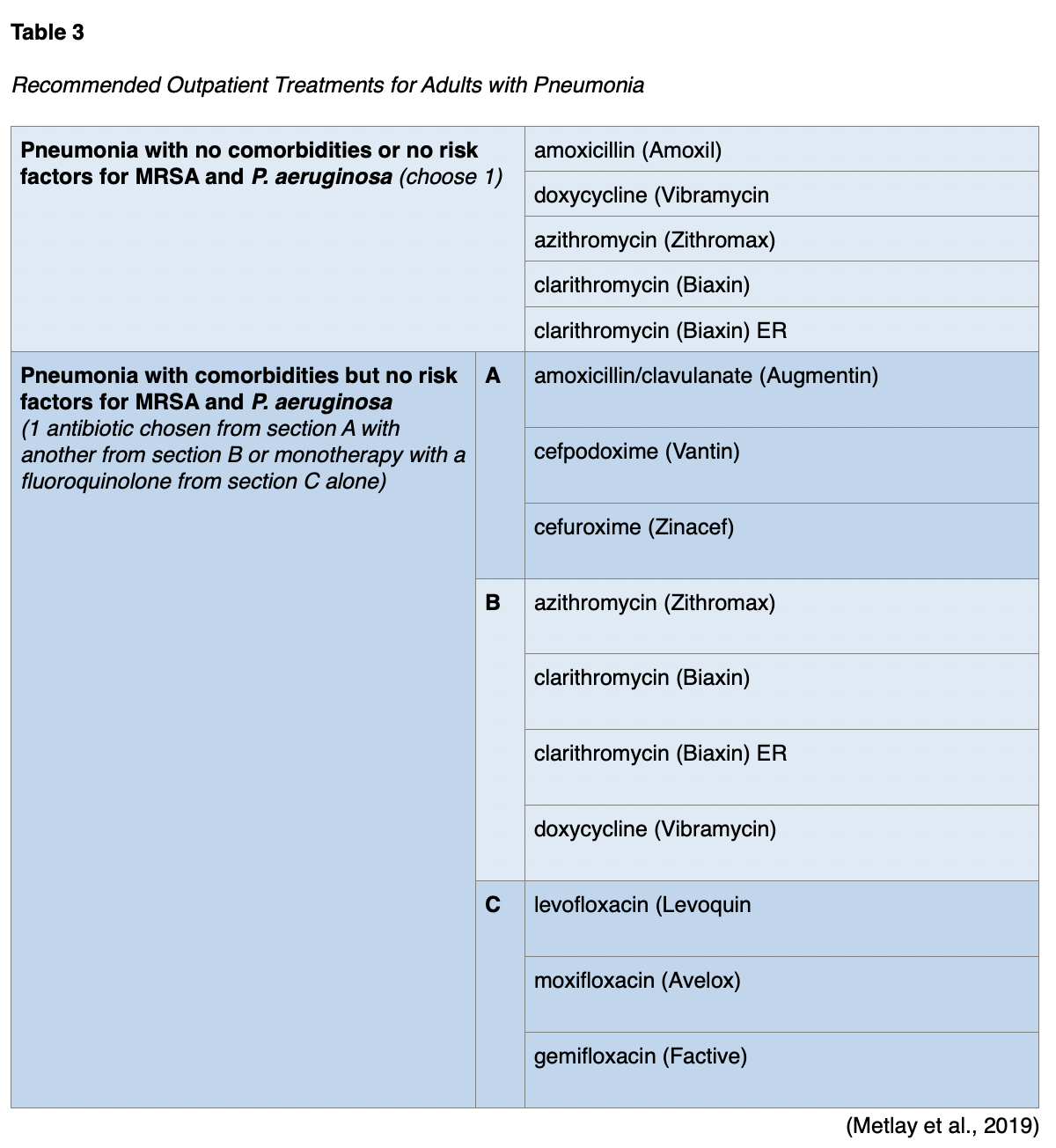
Patients with CAP being treated as an outpatient should be seen for follow-up in the office 2 days after diagnosis/starting therapy to assess for symptom improvement and the potential development of complications (Sattar & Sharma, 2018). During an ordinary course of pneumonia, tachycardia and hypotension should start to improve in 2 days. Fevers, oxygenation, and tachypnea should improve in 3 days. The patient will typically report feeling better within 3 to 5 days of treatment. Coughing, fatigue, and resolution of infiltrates on chest x-ray may take 2-4 weeks or longer to improve, even in mild pneumonia (Gronthoud, 2021).
The decision to admit a patient for treatment should be based on a validated severity score described above. Those with a PSI class of III or more or a CURB-65 score of 2 or more should be admitted. Generally, a patient diagnosed with pneumonia with a SpO2 < 92% on room air requires admission to the hospital (Ramirez, 2020). Those with respiratory failure requiring mechanical ventilation or sepsis requiring vasopressor support should be admitted to an ICU, as these are the major criteria for severe pneumonia established by the ATS/IDSA. The minor criteria, of which three are required for classification as severe pneumonia, includes:
- RR > 30 breaths/minute
- PaO2/FiO2 ratio < 250
- multilobar infiltrates on chest imaging
- confusion/disorientation
- uremia (BUN > 20 mg/dL)
- leukopenia (WBC < 4,000 cells/µL)
- thrombocytopenia (platelet count < 100 x 103/µL
- hypothermia (core temperature < 36 °C/96.8 °F)
- hypotension requiring aggressive fluid resuscitation (Metlay et al., 2019)
Healthcare providers should rely on more than clinical severity to determine the need for inpatient treatment. A patient’s comorbidities and psychosocial conditions should also be considered, as well as their ability to maintain oral intake, history of substance abuse, comorbidities, cognition, and functional status (Metlay et al., 2019). For patients with pneumonia receiving inpatient treatment, combination therapy is recommended (e.g., a ß-lactam and a macrolide). If combination therapy is not used, monotherapy with a respiratory fluoroquinolone is recommended. An alternative for those unable to tolerate macrolides or fluoroquinolones is combination therapy with a ß-lactam antibiotic and doxycycline (Vibramycin). The recommended options for inpatient pneumonia target the most likely pathogens that cause pneumonia. Separate recommendations are made for those with a history of or are at risk for infection with MRSA or P. aeruginosa. The highest risk factors for these organisms are a prior diagnosed infection with documented cultures, a recent hospitalization, or recent use of parenteral antibiotics. The treatment options for MRSA include vancomycin (Vancocin) or linezolid (Zyvox; Metlay et al., 2019). Treatment options for P. aeruginosa include piperacillin-tazobactam (Zosyn), cefepime (Maxipine), ceftazidime (Fortaz), aztreonam (Azactom), meropenem (Merrem), or imipenem (Primaxin) (Metlay et al., 2019). IV antibiotics should be started as soon as pneumonia is determined to be the appropriate diagnosis, ideally within 4 hours (Ramirez, 2020). In a landmark study, Houck and colleagues (2004) retrospectively assessed the data regarding the timing of the initial antibiotic dose in Medicare patients admitted with CAP. They found reduced mortality and length of stay when the initial dose of antibiotics was administered within 4 hours of admission. Table 4 summarizes inpatient recommendations for adults with nonsevere CAP, while Table 5 summarizes adults with severe CAP.


Patients with aspiration pneumonia should not receive routine anaerobic coverage added unless a lung abscess or empyema is suspected or diagnosed. Routine corticosteroids are not recommended for patients with pneumonia. They should not be used routinely for adults with severe CAP or influenza pneumonia but are advised for those with pneumonia and septic shock per the Surviving Sepsis guidelines. For adults with pneumonia who test positive for influenza, antiviral treatment with Oseltamivir (Tamiflu) is recommended (Metlay et al., 2019).
Pneumonia Bundles
For patients admitted with CAP, the British Thoracic Society (BTS, 2016) proposes a care bundle consisting of four areas to improve care outcomes. The priorities include patient safety, timely antimicrobial prescription, prompt oxygen administration, and a chest x-ray. The CAP bundle is appropriate for patients admitted for inpatient treatment of lower respiratory tract infection with new infiltrates on chest x-ray and no hospital admission within the last 10 days. These patients should have an Sp02 assessment completed within 1 hour of admission. The goal for patients over the age of 16 is a SpO2 of 94% to 98%. If a patient needs supplemental oxygen, it should be prescribed and administered within 1 hour of admission. The BTS bundle recommends that all patients admitted with suspected CAP undergo a chest x-ray. The chest x-ray should be completed quickly enough to allow the diagnosis to be confirmed and antibiotics to be prescribed within 4 hours. The BTS recommends completing a CURB-65 score for all patients diagnosed with pneumonia via chest x-ray. IV antibiotics should be administered for patients with a CURB-65 score equal to or greater than 3. Antibiotics should be ordered and administered within 4 hours of hospital admission (BTS, 2016). Similarly, ventilator bundles have been developed to reduce VAP incidence and are discussed below (Ignatavicius & Workman, 2015; Klompas et al., 2014).
Ventilator-Associated and Hospital-Acquired Pneumonia
HCAP was initially featured in the 2005 ATS/IDSA guidelines as a separate clinical diagnosis requiring specific procedures that differed from CAP. The independent treatment recommendations for HCAP were made because of the likelihood of pathogens that are not susceptible to standard initial antibiotic therapy, chiefly MRSA and P. aeruginosa. A significant increase in empiric, broad-spectrum antibiotics resulted but did not lead to improvements in patient outcomes. Many studies since that time have shown that some factors used to define HCAP do not predict a high rate of MDR pathogens. Instead, the 2019 ATS/IDSA guidelines recommend enhanced knowledge of local epidemiology and early identification of risk factors for MDR pathogens such as MRSA and P. aeruginosa. Treatment for those who previously would have been categorized as HCAP should follow the antibiotic regimen outlined previously for those with CAP at increased risk of MRSA or P. aeruginosa colonization (Kalil et al., 2016; Metlay et al., 2019).
VAP is a common, preventable, and costly complication of mechanical ventilation. Implementing prevention measures for aspiration and oral bacterial translocation to the lower respiratory tract is necessary (Ignatavicius & Workman, 2015). HAP and VAP account for 22% of all hospital-acquired infections. About 10% of patients receiving mechanical ventilation experience VAP. While all-cause mortality estimates for VAP patients range from 20-50%, the direct mortality rate for VAP was recently estimated at 13%. Recent studies also estimate that VAP prolongs the length of stay by 11.5 to 13.1 days, extends ventilation time by 7.6 to 11.5 days, and is associated with a cost of approximately $40,000 per patient. About half of HAP patients develop severe complications, such as respiratory failure, septic shock, empyema, renal failure, or pleural effusion (Kalil et al., 2016).
An ETT or tracheostomy tube bypasses the body’s normal filtering process and provides direct access for pathogens to enter the lower respiratory system. An artificial airway will be colonized with bacteria within 48 hours, provoking pneumonia development (Ignatavicius & Workman, 2015). The most common causative organisms for bacterial HAP/VAP include gram-positive cocci (e.g., S. pneumoniae, H. influenzae, S. aureus [either methicillin-susceptible or resistant]) and gram-negative bacilli (e.g., Escherichia coli, K. pneumoniae, P. aeruginosa, Acinetobacter, or Enterobacteriaceae). Sensitivity testing should be performed consistently with bacterial cultures, as MDR organisms associated with VAP are of concern (Klompas, 2020a; Sattar & Sharma, 2018).
Risk Factors and Prevention
Mechanical ventilation is the primary risk factor for HAP, especially if it is indicated for acute respiratory distress syndrome (ARDS; Klompas, 2021). Risk factors for VAP may be separated into three categories: host-related, device-related, and personnel-related. Host-related risk factors include previous hospitalization and pre-existing conditions (e.g., immunosuppression, chronic lung diseases such as COPD, ARDS, malnutrition, chronic renal failure, anemia, and Charlson Comorbidity Index). Host-related factors also involve the patient’s body positioning, increased age, decreased level of consciousness, multiple or prolonged intubations, receipt of blood transfusions, and certain medications (e.g., sedatives, opioids, muscle relaxants, broad-spectrum antibiotics, glucocorticoids, and agents that elevate the gastric pH [H2 blockers, PPI]). Supine positioning facilitates pulmonary aspiration and should be avoided, as secretions will pool above the cuff of the ETT (Klompas, 2021; Klompas et al., 2014). Additional risk factors for HAP/VAP include aspiration, recent chest or abdominal surgery, multiple trauma, paralysis, the number of central venous access devices, or the presence of an intracranial pressure monitor (Klompas, 2021). Other factors that increase a patient’s risk for aspiration include dysphagia, altered mental status, drug abuse/alcoholism, gastroesophageal reflux, and seizure disorders (Sattar & Sharma, 2018).
Device-related risk factors come from the equipment that is being used during ventilation. VAP is a risk for all patients using mechanical ventilation, and rates increase the longer a patient is intubated. Other device-related factors include ETTs, frequent ventilator circuit changes, and NG or orogastric tubes. Low-pressure cuffs can allow micro-aspiration of fluids or leakage of bacteria into the trachea, so a cuff pressure of 20 to 30 cm H2O should be maintained. Drainage of subglottic secretions and the application of positive end-expiratory pressure (PEEP) may also reduce the risk of aspiration. Orogastric and NG tubes disrupt the gastroesophageal sphincter and provoke reflux, which increases a patient’s risk of VAP (Klompas, 2021; Osti et al., 2017).
Personnel-related risk factors include insufficient handwashing, not changing gloves between patient contacts, and not wearing appropriate personal protective equipment when caring for patients with MDR infections. Poor handwashing before suctioning or manipulating the ventilator circuit increases the likelihood of cross-contamination between patients (Osti et al., 2017).
Infection can be prevented through strict adherence to infection control, such as handwashing and aseptic technique during suctioning and caring for ETTs or tracheostomy tubes. Ventilator bundles are order sets designed to prevent VAP. These ventilator bundles usually include orders to:
- keep the head of the bed elevated to at least 30°
- perform oral care per agency policy, usually brushing teeth every 8 hours and using an antimicrobial rinse such as chlorhexidine every 2 hours
- prevent aspiration
- maintain pulmonary hygiene, which includes chest physiotherapy, postural drainage, and turning and positioning (Ignatavicius & Workman, 2015)
The most recent VAP prevention guidelines published by the Society for Healthcare Epidemiology of America (SHEA) and IDSA (Klompas et al., 2014) recommend avoiding intubation whenever possible via noninvasive ventilation methods, minimizing transport of ventilated patients, using weaning protocols to extubate ventilated patients more efficiently, minimizing sedation (e.g., use sparingly, with daily sedation vacations in adults and spontaneous breathing trials without sedation in adults and pediatrics), maintaining the physical conditioning of critically ill and ventilated adults, minimizing pooling of secretions at the ETT cuff, elevating the head of the bed in adults and pediatrics, and maintaining ventilator circuits (i.e., changing circuits when visibly soiled or malfunctioning). In ventilated neonates, the recommended positioning to reduce VAP risk is the lateral recumbent or reverse Trendelenburg positions, and daily sedation vacations/spontaneous breathing trails are not recommended. Combining these prevention measures into a bundle may be a practical method of enhancing HAP/VAP prevention efforts, although the evidence regarding their effectiveness is mixed (Klompas et al., 2014).
ETTs that can drain subglottic secretions continuously or intermittently have been developed to avoid pooled secretions that may lead to aspiration. They are more expensive and not consistently available but are recommended if available in adults expected to require more than 48 hours of mechanical ventilation (Klompas, 2021). ETTs with subglottic drainage ports are not recommended for use in ventilated neonates or younger pediatric patients. The use of ultrathin polyurethane ETT cuffs or automated control of ETT cuff pressure may decrease the VAP rate. Still, the low-quality evidence was insufficient to determine if this impacts the duration of mechanical ventilation, length of stay, or mortality. Similarly, saline instillation before tracheal suctioning may decrease the rate of VAP, but the low-quality evidence was insufficient to determine if this reduces the duration of mechanical ventilation or length of stay or improves mortality (Klompas et al., 2014).
Oral care is also a component of VAP prevention, although policies vary regarding timing, products, and application methods. VAP prevention efforts initially targeted the prevention of aspiration; however, many studies showed that oral care improvement contributed to VAP reduction significantly. Even though oral care may contribute to VAP prevention, a best-practice protocol has not yet been identified. Some oral care methods include toothbrushing or sponge swabs, and products consist of a chlorhexidine rinse or a sodium chloride solution. Most recommended oral care frequencies range from twice daily to every 2 hours (Ignatavicius & Workman, 2015). In neonates, the guidelines recommend regular oral with sterile water. In pediatrics, regular oral with a toothbrush or gauze is recommended over the use of chlorhexidine. Routine oral care with chlorhexidine in ventilated adults may decrease the rate of VAP, but the moderate-quality evidence was insufficient to determine if this impacts the duration of mechanical ventilation, length of stay, or mortality. Similar assessments were made by the 2014 guidelines regarding mechanical tooth brushing (Klompas et al., 2014).
Probiotics may decrease the rate of VAP, but the moderate-quality evidence was insufficient to determine if this impacts the duration of mechanical ventilation, length of stay, or mortality. Selective oral or digestive decontamination with nonabsorbable antibiotics was reviewed, but the data on the associated risks (increase in antimicrobial resistance) and benefits were deemed insufficient. The 2014 pneumonia prevention guidelines recommend against silver-coated ETTs, kinetic beds, and prone positioning due to a lack of impact on the duration of mechanical ventilation, length of stay, or mortality. They also recommend against stress ulcer prophylaxis, early tracheotomy, monitoring residual gastric volumes, early parenteral nutrition, and closed/in-line endotracheal suctioning in adults due to a lack of evidence that these reduce the VAP rate. Closed/in-line suctioning may prevent VAP in neonates (Klompas et al., 2014).
Clinical Manifestations and Diagnosis
A patient with VAP will demonstrate similar clinical manifestations as other forms of pneumonia. VAP diagnosis is confirmed using a chest x-ray, clinical assessment, and lab results (including sputum culture and sensitivity; Ignatavicius & Workman, 2015). The 2005 diagnostic criteria for HAP/VAP were essentially unchanged in the 2016 guidelines. HAP must occur 48 hours or more after admission, while VAP is pneumonia occurring more than 48 hours after intubation. Clinical criteria for the diagnosis of HAP or VAP include the presence of new lung infiltrates on imaging in addition to two of the three following indicators that the infiltrates are related to an infectious organism:
- purulent sputum
- elevated body temperature (above 100.4 °F/38 °C)
- leukocytosis or leukopenia (ATS & IDSA, 2005; Kalil et al., 2016; Klompas, 2020a).
These clinical criteria can be used to initiate empirical antibiotic treatment (see below). A reevaluation of this decision based on semiquantitative lower respiratory tract cultures and serial clinical evaluations should occur within 3 days. A bacteriologic approach relies on quantitative lower respiratory secretion cultures (obtained via endotracheal aspiration, protected specimen brush [PSB], or bronchoalveolar lavage [BAL]). This method is less sensitive but more specific, with an increased risk of false-negative findings, especially in patients with a recent (within 24-72 hours) antibiotics change. For this reason, all cultures should be obtained before any planned changes in antibiotic regimen. Alternately, a modified clinical pulmonary infection score (CPIS) may be used. This score combines clinical, radiographic, and physiological (PaO2/FiO2) data for a subjective score. The modified CPIS does not rely on culture data. A CPIS score of 6 or less for 3 consecutive days is an objective method to identify patients at low risk for early discontinuation of empiric treatment for HAP. Regardless of the diagnostic approach utilized, all patients with suspected VAP should have extrapulmonary sources of infection excluded (e.g., urinary tract infection) and a lower respiratory sample sent for culture. Initiation of treatment should not be delayed for diagnostic testing in patients with suspected HAP or VAP who are clinically unstable (ATS & IDSA, 2005).
The Centers for Disease Control and Prevention (CDC) defines the presence of a ventilator-associated condition (VAC) by at least 2 days of stable or decreasing daily minimum PEEP or FiO2 followed by a 2-day increase of at least 3 cm H2O in PEEP or at least 0.20 points (20%) FiO2. Infectious VAC (IVAC) is defined as VAC in addition to indicators of an infectious process, such as an abnormal body temperature (below 36 °C/96.8 °F or above 38 °C/100.4 °F) or white blood cell (WBC) count (less than 4,000 or greater than 12,000 cells/µL). Possible VAP is defined as evidence of IVAC in combination with purulent pulmonary secretions based on Gram stain or pulmonary culture results indicating a pathogen. Probable VAP is defined as evidence of IVAC in addition to Gram stain and quantitative or semiquantitative growth of a pathogen based on specified laboratory thresholds. These definitions are meant to be used for surveillance and quality improvement purposes and not for diagnosis and treatment at the bedside (CDC, 2021c; Klompas et al., 2014).
Treatment of HAP/VAP
Patients with HAP and mechanically ventilated patients with VAP should be cultured using noninvasive methods (e.g., spontaneous expectoration, sputum induction, endotracheal aspiration) rather than invasive methods (e.g., bronchoscopy). Hospitals should regularly create and disseminate local antibiograms to inform empiric regimens with the distribution of pathogens and their susceptibilities within specified units of their facility. After the initial dosing regimen described below, antibiotics should be dosed based on pharmacokinetic and pharmacodynamic data, not the manufacturer’s prescribing information. Susceptibility cultures should be used to focus or de-escalate antibiotic therapy once available. A total of 7 days of antibiotics treatment is recommended for both VAP and HAP if the patient is clinically stable, using procalcitonin levels and clinical criteria (not CPIS) to determine timing beyond that (Kalil et al., 2016). Oral agents are often started once the patient is stable clinically and able to tolerate PO medications (Klompas, 2020b).
All empiric regimens for VAP or HAP should include coverage for S. aureus, P. aeruginosa, and other gram-negative bacilli, as shown in Table 6. For patients with VAP, an active agent for MRSA should be included in ICUs where the prevalence of methicillin resistance is unknown or > 10-20%, in the presence of risk factors for MDR organisms, and patients with a prior history of MRSA colonization. Patient factors that increase the risk of MDR pathogens include IV antibiotic administration in the previous 90 days, current septic shock, the presence of ARDS before VAP onset, 5 days of hospitalization before VAP onset, and acute renal replacement therapy before VAP onset. For HAP patients, risk factors for MRSA mirror some of the factors listed above (e.g., IV antibiotic administration in the previous 90 days, hospitalization on a unit where the prevalence of methicillin resistance is > 20% or unknown a prior history of MRSA infection) and include those considered at increased risk of mortality (i.e., requiring ventilatory support due to HAP/septic shock). Options for empiric MRSA coverage in these patients include vancomycin (Vancocin) or linezolid (Zyvox; Kalil et al., 2016).
Otherwise, empiric methicillin-sensitive S. aureus (MSSA) coverage should be provided by piperacillin-tazobactam (Zosyn), cefepime (Maxipine), levofloxacin (Levaquin), imipenem (Primaxin), or meropenem (Merrem). This recommendation applies to VAP or HAP patients who are not at-risk for MDR pathogens and HAP patients not at increased risk of mortality (Kalil et al., 2016).
Empiric coverage for VAP and HAP should also include two antipseudomonal antibiotics in patients at increased risk. This condition applies to VAP patients at increased risk of MDR colonization (as described above), with a prior history of colonization with MDR P. aeruginosa, or in units where the prevalence of resistance amongst gram-negative cultures is > 10%. Risk factors increasing the risk of P. aeruginosa infection in HAP patients include prior use of IV antibiotics in the last 90 days, structural lung disease (e.g., bronchiectasis, cystic fibrosis), a Gram stain with predominantly gram-negative bacilli, or an increased risk of mortality. Typically, this would include a ß-lactam-based agent (i.e., as described above in row 3, Table 4 and row 2, Table 5) and a non- ß-lactam-based agent (e.g., ciprofloxacin [Cipro, levofloxacin [Levaquin], amikacin [Amikin], gentamicin [Gentak, tobramycin [Nebcin], colistin [Coly Mycin M], or polymyxin B). Patients not at increased risk can be given a single agent (Kalil et al., 2016). The healthcare team should remain alert regarding the risk of nephrotoxicity and ototoxicity associated with aminoglycosides and significant nephrotoxicity associated with polymixins (polymyxin B and colistin [Coly Mycin M]; Klompas, 2020b). In a patient with VAP due to gram-negative bacilli that are only susceptible to aminoglycosides (e.g., amikacin [Amikin], gentamicin [Gentak], or tobramycin [Nebcin]) or polymixins, the guidelines recommend both systemic and inhaled antibiotics. Once susceptibility information is known, monotherapy with a single effective agent is acceptable in clinically stable patients, avoiding aminoglycoside monotherapy. Combination therapy with two antibiotics should be continued in those patients with VAP or HAP related to P. aeruginosa if they remain in septic shock or clinically unstable (Kalil et al., 2016).

Complications
Bacterial pneumonia creates many complications, most commonly coagulopathy, exacerbation of pre-existing comorbidities, multiorgan failure, respiratory failure, and sepsis (Sattar & Sharma, 2018). Other complications include:
- cavitation
- destruction of lung parenchyma
- empyema
- pulmonary abscess
- lung fibrosis
- meningitis
- death (Sattar & Sharma, 2018)
Complications due to pneumonia chiefly affect children, older adults, and patients with comorbidities. Damage to the kidneys, liver, and heart can happen due to hypoxia secondary to pneumonia (National Heart, Lung, and Blood Institute [NHLBI], 2019). When an abscess forms and perforates the bronchial wall, tissue necrosis (or necrotizing pneumonia) results (Ignatavicius & Workman, 2015). Necrotizing pneumonia is hard to treat, potentially requiring surgery to remove the purulent drainage. Sepsis can occur if the pathogen from the lungs gets into the blood and causes a systemic inflammatory response (NHLBI, 2019).
The treatment used for pneumonia can also lead to iatrogenic complications. While all antimicrobials increase the risk of C. difficile infection, fluoroquinolones and broad-spectrum cephalosporins are the most common culprits. Cefepime (Maxipime) and imipenem (Primaxin) increase the risk of seizures in those with renal insufficiency. Fluoroquinolones can lead to QT prolongation, tendinitis/tendon rupture, and neurotoxicity (Klompas, 2020b).
Non-Resolving Pneumonia
Non-resolving pneumonia can result from MDR organisms, patient factors, or other antibiotic contributions. Pneumonia resolves more slowly in some patients than in others. In these cases, it can be challenging to differentiate between non-resolving pneumonia and a slower recovery process. As previously mentioned, most pneumonia symptoms are expected to resolve within 3-5 days of treatment. Coughing and fatigue may take up to 2 weeks or longer to improve, and chest x-rays may take a month or longer to show an improvement in infiltrates, even in mild pneumonia (Gronthoud, 2021).
Several common factors contribute to non-resolving pneumonia. First, some pathogens (e.g., TB) are challenging to treat. Second, host factors (e.g., increased age, immunodeficiency, airway disease, cancers, alcoholism, or tobacco use) can complicate the disease course. Third, complications of pneumonia can create a non-resolving case. Other causes include a malignancy or an airway obstruction due to malignancy (Gronthoud, 2021).
If a patient has difficult-to-treat or non-resolving pneumonia, MDR organisms or atypical pathogens should be considered and ruled out. The patient should be asked again about recent travel. Patients in the Mississippi area are at risk for Histoplasma, and those in the rest of the southern US are at risk for coccidioidomycosis. Rare pathogens may affect patients with cavitary lesions or allogeneic bone marrow transplants. Patients with HIV, immune deficiencies, asplenia, alcoholism, seizures, poor oral hygiene, a history of aspiration, and COPD have increased host-related factors for non-resolving pneumonia. They should be monitored for effusions, empyema, and lung abscesses. Pulmonary aspergillosis should be considered if the patient is immunocompromised (e.g., AIDS, severe neutropenia, or chronic corticosteroid use). Noninfectious causes of non-resolving pneumonia include pulmonary embolisms, malignancy, sarcoidosis, pulmonary vasculitis syndromes, eosinophilic pneumonia, and alveolar damage (Gronthoud, 2021).
If a patient is slowly improving, they should be carefully monitored for 4 to 8 weeks. A more aggressive diagnostic approach should be initiated if no resolution or improvement occurs or if the illness progresses. An extensive medical history and physical examination should be repeated (Gronthoud, 2021).
Sepsis
Sepsis starts with an infection—most often pneumonia—that triggers a dysregulated host response (Gauer et al., 2020). Pneumonia patients should be monitored closely for indications of sepsis, and SpO2 should be evaluated with every vital sign assessment. The systemic inflammatory response syndrome (SIRS) criteria for sepsis include suspected or diagnosed infection with at least two of the following:
- temperature >101 °F (38.3 °C) or <96.8 °F (36 °C)
- heart rate >90 beats per minute
- respiratory rate >20 breaths per minute
- arterial hypotension (systolic blood pressure [SBP] <90 mm Hg, mean arterial pressure [MAP] <70 mm Hg)
- arterial hypoxemia (partial pressure of arterial oxygen [PaO2]/fraction of inspired oxygen [FiO2] <300)
- absent bowel sounds
- decreased capillary refill or presence of mottling
- unexplained change in mental status
- significant edema or positive fluid balance
- abnormal WBC count (>12,000 cells/µL or <4,000 cells/µL)
- normal WBC count with >10% bands
- platelet count (<100 x103/µL)
- international normalized ratio (INR) >1.5 or activated partial thromboplastin time (aPTT) >60
- plasma CRP >2 standard deviations above normal
- elevated lactic acid (lactate) level (>2.0 mmol/L)
- plasma procalcitonin >2 standard deviations above normal
- urine output <0.5 mL/kg/hr for 2 hours despite adequate fluid resuscitation
- creatinine increase >0.5 mg/dL
- elevated total bilirubin (>4 mg/dL)
- hyperglycemia (plasma glucose >140 mg/dL or 7.7 mmol/L) in absence of diabetes
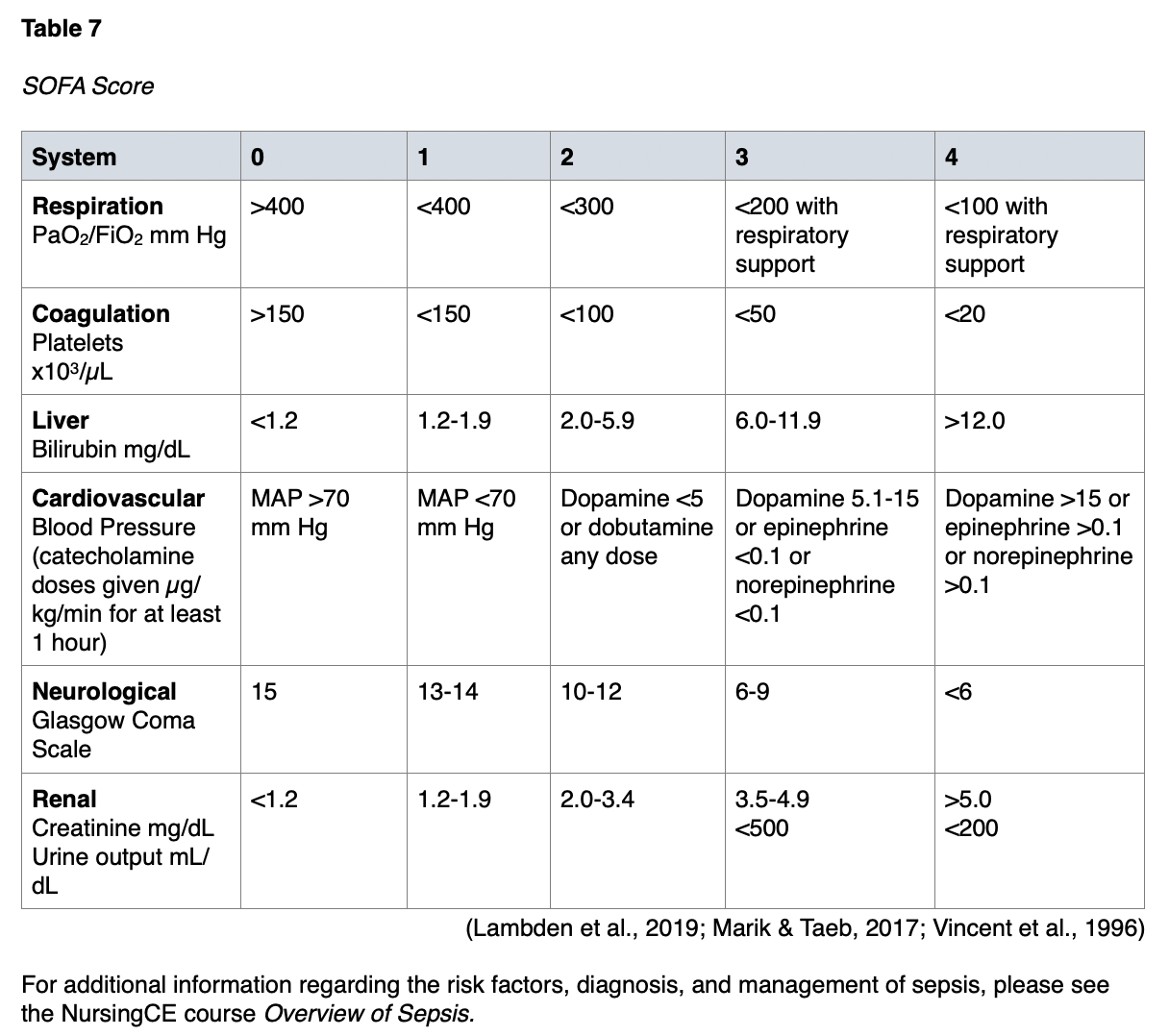
The international Sepsis-3 consensus defines sepsis as “life-threatening organ dysfunction caused by a dysregulated host response to infection” (p. 1). They suggest operationalizing organ dysfunction by a Sequential (sepsis-related) Organ Failure Assessment (SOFA) score that increases by 2 or more points (Marik & Taeb, 2017; Singer et al., 2016). SOFA is a simple scoring system that notes the number and severity of failure in 6 organ systems, consisting of the respiratory system, coagulative function, cardiovascular system, liver, kidneys, and neurological system. It was developed in 1994 to describe the degree of organ failure over time (Lambden et al., 2019). The score ranges from 0 to 24, and higher scores predict a higher possibility of mortality (Vafaei et al., 2019). The SOFA score should be calculated on admission (i.e., before initiating treatment) and then every 24 hours for daily monitoring of acute morbidity in ICU patients. Clinical guidelines define multiorgan dysfunction as acute changes in the SOFA score of 2 or more points due to the infection (CDC, 2018). While initially developed to measure morbidity and not outcomes, the developers acknowledge that measurements of morbidity are associated with predicting mortality (Lambden et al., 2019). The SOFA score criteria are listed in Table 7.
Health Promotion and Prevention
Handwashing is a prevention technique that applies at home and in healthcare settings. Patients with respiratory symptoms should visit a healthcare provider for a fever over 24 hours, a sickness that persists for over a week, or worsening symptoms. Respiratory therapy equipment should be decontaminated and changed per recommendations. Sterile water should be used in place of tap water for gastrointestinal tubes. Patients should be screened for aspiration risk, and aspiration precautions should be initiated as indicated. Thorough assessments and the use of patient care bundles in the ICU environment can reduce a ventilated patient’s risk of VAP. Patients who smoke and are diagnosed with pneumonia should also receive smoking cessation education (Ignatavicius & Workman, 2015).
Vaccination
Vaccination for pneumococcal disease has been available since 1983, prompting a decline in CAP caused by pneumococcus in the United States. S. pneumoniae is a gram-positive anaerobic bacterium with over 90 serotypes. It is the most common cause of CAP and may also cause otitis media, meningitis, and bacteremia. The pneumococcal vaccines are divided into whole-cell and subunit types. Whole-cell vaccines consist of live attenuated and inactivated forms. Subunit types include polysaccharide, conjugate, and protein-based vaccines. The pneumococcal polysaccharide vaccine (PPV) and pneumococcal conjugate vaccine (PCV) are currently available. A weakened form of the pathogen is used in a live attenuated vaccine. These are usually more cost-effective than other forms of vaccination, but the live vaccine strain can revert to its virulent type and cause severe disease in rare cases. Whole-cell live attenuated vaccines provide superior protection against various pneumococcal serotypes. An inactivated vaccine uses pathogens that were treated with chemicals or physical processes. These are safer than live attenuated vaccines (Kim et al., 2017).
A polysaccharide (subunit) vaccine uses the polysaccharide capsule from encapsulated bacteria. The polysaccharide interacts with B cells and directly induces antibodies without a T-cell response. The PPV includes polysaccharides from 23 serotypes of pneumococcal bacteria, responsible for 85% to 90% of pneumococcal infections worldwide (WHO, 2019). While PPV23 (Pneumovax 23) vaccination does not prevent CAP or reduce its incidence, it does alleviate its severity. It is effective against invasive pneumococcal disease (IPD). The PPV23 (Pneumovax 23) vaccine is usually given as a one-time dose in adults over the age of 65; however, some experts have suggested that older patients and those with chronic health problems might benefit from a second vaccination at least 5 years after their first dose (Kim et al., 2017).
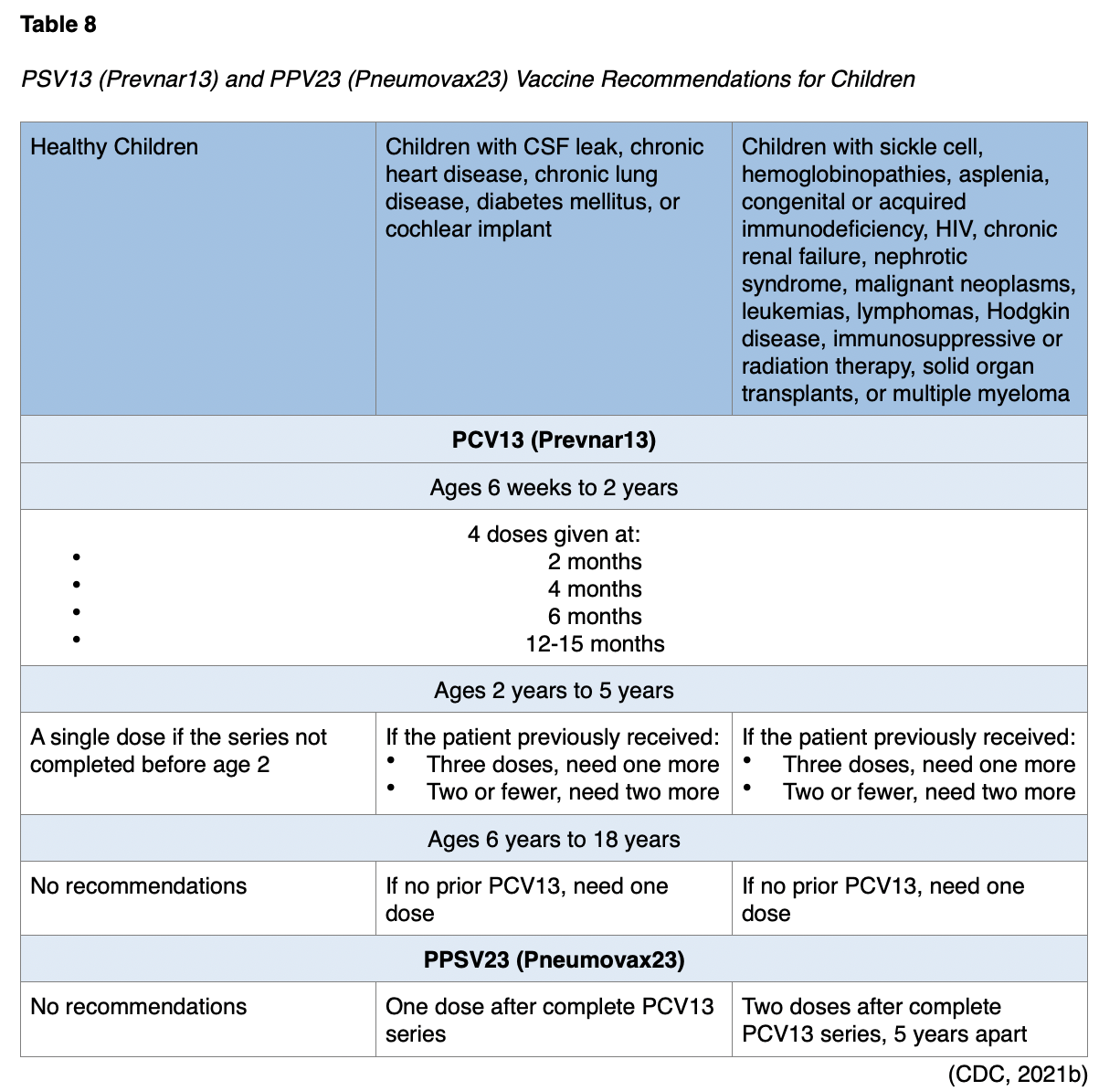
Children must be at least 2 years old to receive the PPSV23 (Pneumovax23) vaccine. Patients ages 6 to 18 who have never had a PPSV23 (Pneumovax23) dose should receive a single dose of PPSV23 (Pneumovax23) at least 8 weeks after completing all recommended PCV13 (Prevnar13) doses. For children with chronic heart disease, chronic lung disease, and diabetes mellitus who have no history of a PPSV23 (Pneumovax23) vaccination, a dose of PPSV23 (Pneumovax23) is needed at least 8 weeks after completing all recommended PCV13 (Prevnar13) doses (CDC, 2021b).
Children ages 2-5 years with a CSF leak or a cochlear implant with no history of PPSV23 (Pneumovax23) should receive a dose at least 8 weeks after a PCV13 (Prevnar13) vaccine. Children with sickle cell disease, other hemoglobinopathies, asplenia, congenital or acquired immunodeficiency, HIV, chronic renal failure, nephrotic syndrome, malignant neoplasms, leukemias, lymphomas, Hodgkin disease, immunosuppressive or radiation therapy, solid organ transplants, or multiple myeloma should receive a dose of PPSV23 (Pneumovax23) at least 8 weeks after any previous PCV13 (Prevnar13) doses and then again 5 years later. Those ages 6 to 18 also need a PPSV23 (Pneumovax23) at least 8 weeks after their last PSV13 (Prevnar13) dose. A second dose can be repeated 5 years later. A summary of PSV13 (Prevnar13) and PPV23 (Pneumovax23) vaccine recommendations for children appears in Table 8 (CDC, 2021b).
The CDC recommends a dose of PPSV23 (Pneumovax23) for all adults ages 65 years and older. The PPSV23 (Pneumovax23) dose should be administered after the PCV13 with at least a year between doses. If a patient receives the PPSV23 (Pneumovax23) vaccine before 65, they should repeat the vaccine at least 5 years later when they are over 65. For immunosuppressed patients age 65 and over, a single dose of PPSV23 (Pneumovax23) should be given at least 5 years after their most recent dose of PPSV23 (Pneumovax23). Only a single dose of PPSV23 (Pneumovax23) should be given to healthy patients 65 or older (CDC, 2021a).
For patients ages 19 to 64 years with chronic heart conditions, lung disease, liver disease, diabetes, alcoholism, or a history of smoking, a single dose of PPSV23 (Pneumovax23) should be administered. Immunosuppressed patients and those with a CSF leak or a cochlear implant age 19 or older (e.g., immunodeficiency, HIV, chronic renal failure, nephrotic syndrome, leukemia, lymphoma, Hodgkin disease, generalized malignancy, radiation therapy, solid organ transplant, multiple myeloma, or asplenia), PCV13 (Prevnar13) should be given first, followed by PPSV23 (Pneumovax23) at least 8 weeks later. A repeat dose of PPSV23 (Pneumovax23) should be given at least 5 years later. The CDC also recommends PPSV23 (Pneumovax23) for smokers aged 19 to 64 (CDC, 2021a).
A conjugate (subunit) vaccine uses polysaccharide antigens conjugated with carrier proteins. Unlike the polysaccharide vaccine, the conjugate vaccine can elicit a T-cell response, creating superior immunogenicity and longer-lasting immunity. The pneumococcal conjugate vaccine with seven polysaccharides (PCV7) is commercially available as Prevnar. The pneumococcal conjugate vaccine with ten polysaccharides (PCV10) is called Synflorix. Finally, the polysaccharide vaccine with 13 polysaccharides (PCV13) is known as Prevnar 13. Because of the different serotypes included in these vaccines, some work better for specific populations than others. PCV7 (Prevnar) has demonstrated protective effects against IPD, pneumonia, and otitis media. PCV7 (Prevnar) can also protect HIV-infected adults from pneumococcal infection. PCV13 (Prevnar13) has decreased the incidence of pneumococcal pneumonia in children because it contains the two serotypes that cause about half of the childhood pneumococcal cases. Unfortunately, PCV13 (Prevnar13) is more expensive than the PPV23 (Pneumovax23) vaccine (Kim et al., 2017).
The CDC recommends PCV13 (Prevnar13) for children under 2 years of age. It should be given to infants in a series of four doses. The minimum age to receive PCV13 (Prevnar13) is 6 weeks, but the series is typically given at 2 months, 4 months, 6 months, and 12 through 15 months. Children who miss shots or start the series late should receive the vaccine based on the CDC’s catch-up recommendations. The number of doses and their intervals will depend on a child’s age at the start of vaccination. For children with sickle cell disease, other hemoglobinopathies, asplenia, congenital or acquired immunodeficiency, HIV, chronic renal failure, nephrotic syndrome, malignant neoplasms, leukemia, lymphoma, Hodgkin disease, immunosuppressive or radiation therapy, solid organ transplants, or multiple myeloma, the recommends that they also receive PPSV23 (Pneumovax23) at least 8 weeks after their last PSV13 (Prevnar13) dose. A second dose should be repeated 5 years later (CDC, 2021b). A summary of the CDC’s PSV13 (Prevnar13) and PPV23 (Pneumovax23) recommendations for children and adolescents appears in Table 8.
Pneumococcal pneumonia remained a considerable burden in the elderly population despite the use of PPSV23 (Pneumovax23). As a result, immunization with both the PPV23 (Pneumovax23) and PCV13 (Prevnar13) vaccines is available for older adults. This combination helps overcome the disadvantages of individual vaccines (Kim et al., 2017). The CDC suggests that healthcare professionals discuss the risks and benefits of getting the PCV13 (Prevnar13) vaccine with healthy adult patients 65 and older. If a patient elects to receive both vaccines, the PCV13 (Prevnar13) should be given first, and the provider should wait at least a year before administering the PPSV23 (Pneumovax23). A single PCV13 (Prevnar13) dose is recommended for adults over 65 with immunosuppression, a CSF leak, or a cochlear implant. For patients 19 or older with immunosuppression (e.g., immunodeficiency, HIV, chronic renal failure, nephrotic syndrome, leukemia, lymphoma, Hodgkin disease, generalized malignancy, radiation therapy, solid organ transplant, multiple myeloma, or asplenia), a single dose of PCV13 should be given (CDC, 2021a).
H. influenzae type B is a type of bacteria that causes many different infections, including pneumonia (CDC, 2020a). The CDC recommends the H. influenzae type B (Hib) vaccine for all children under 5. The Hib vaccine is recommended at age 2 months, 4 months, 6 months (depending on the brand ActHib, Hiberix, or Pentacel), with a booster at 12 to 15 months. The Hib vaccine brand PedvaxHib is a two-dose series with the third booster between 12 and 15 months. Older children and adults may not need a Hib vaccine. These vaccines are contraindicated in people who have experienced anaphylaxis or another severe reaction to a previous Hib dose, those who are allergic to any of the vaccine components, and those under the age of 6 weeks. For a patient with a moderate to severe acute illness with or without fever, the provider and the patient should weigh the risks and benefits of vaccination (CDC, 2020b). Adults with asplenia or sickle cell disease should receive a Hib vaccine if they did not receive it as a child. If an elective splenectomy is being performed, the patient should get a dose at least 14 days before the scheduled procedure. A three-dose H-flu series should be given 4 weeks apart, starting 6 to 12 months after a successful hematopoietic stem cell transplant regardless of the patient’s Hib vaccination history (CDC, 2021a).
Pneumonia is a common coinfection with influenza, especially in the older adult population. Consequently, all patients should be encouraged to receive the seasonal influenza vaccination annually (Ignatavicius & Workman, 2015; Kim et al., 2017).
Vaccine Development and Emerging Research
In addition to the currently available vaccines, several potential vaccines for pneumonia are being investigated or undergoing clinical trials. A live attenuated mucosal vaccine is being studied. Inactivated whole-cell pneumococcal vaccines provide adequate immunity, as demonstrated in studies with reduced colonization and higher survival rates. Intranasal and subcutaneous (SQ) inactivated pneumococcal vaccines are being studied (Kim et al., 2017).
Protein-based vaccines, also known as recombinant protein vaccines, are purified protein antigens produced in bacteria. The protein elicits antibody production, thereby protecting recipients from disease. Such a vaccine using a pneumococcal surface protein is in development, as well as various other forms using specific protein antigens. These can be given intranasally, IM, SQ, and intraperitoneally (Kim et al., 2017).
Respiratory pathogens like pneumococcal bacteria enter the body and begin colonization in the mucosal surfaces, such as the nose. As a result, the mucosal immune system acts as the primary physical barrier against respiratory diseases. Secretory IgA—a primary factor of mucosal immunity—can be induced by vaccines to capture microbes or block microbial adherence to and invasion of the mucosa. These vaccines also trigger local IgG production. For these reasons, the induction of both the mucosal and systemic immune response provides superior protection against respiratory diseases such as pneumococcal pneumonia. Injectable vaccines are less effective than mucosal vaccines in generating a mucosal immune response. Mucosal vaccines, such as cholera, polio, and influenza, offer many advantages, but few are available. The mucosal immune system reacts to these vaccines as if they were true pathogens, degrading the proteins and impeding the secretory (e.g., IgA) immune response (Kim et al., 2017).
Patient Education
Patients should receive education on their risk factors for pneumonia, especially those over 65, with chronic respiratory conditions, or limited mobility. Available vaccinations, including the yearly influenza vaccine and pneumococcal vaccines, should be discussed with patients. Each patient should be encouraged to avoid crowded places during flu and holiday seasons and individuals with upper respiratory symptoms. They should also be taught how to cough, turn, and breathe deeply, especially in the context of mobility problems. Incentive spirometry should be provided when available. If a patient uses at-home respiratory equipment, the proper methods to clean and store the equipment should be demonstrated. Patients should receive education and support for smoking cessation and be counseled to avoid indoor pollutants like dust, smoke (firsthand or secondhand), and chemicals such as aerosol sprays. Proper nutrition and fluid intake are also crucial aspects of patient teaching (Ignatavicius & Workman, 2015).
Patient education is needed when discharged after treatment for pneumonia. All prescribed medications should be reviewed with the patient or responsible family members. The completion of all anti-infective medicines as prescribed, such as antibiotics or antifungals, should be emphasized. The patient should be advised to notify their healthcare provider if they experience fevers, chills, persistent cough, dyspnea, hemoptysis, increased sputum production, wheezing, chest discomfort, or increased fatigue. Patients recovering from pneumonia should be instructed to get plenty of rest and increase activity gradually as tolerated (Ignatavicius & Workman, 2015).
Case Study
The nurse is caring for Mrs. Mitchell, a frail, 82-year-old woman. Mrs. Mitchell was admitted to the hospital from an assisted living facility for surgery to repair a bowel obstruction. Her surgery concluded 49 hours ago. She currently has a nasogastric (NG) tube set to low suction. All of her medications are administered through the NG tube, including warfarin (Coumadin), cimetidine (Tagamet), and lisinopril (Zestril). Mrs. Mitchell receives oxygen at 1 liter per minute via nasal cannula at home while sleeping. She currently has an indwelling urinary catheter and a saline-locked peripheral IV. She reports increased weakness, fatigue, chest pain that is not relieved by IV opioids and refuses physical therapy participation. Most of her time each day has been spent in bed. Her most recent vital signs are as follows: BP 100/72, HR 98, oral temperature 100.5 °F, RR 24, and SpO2 92%. Her most recent lab results include WBC 6,000, BUN/creatinine 29/1.4, sodium 146, and lactic acid 5mmol/L. Upon pulmonary assessment, the nurse auscultates diminished breath sounds and dull percussion over the right upper lobe. Mrs. Mitchell has no accessory muscle use and denies dyspnea.
What risk factors does Mrs. Mitchell have for developing pneumonia?
Mrs. Mitchell’s advanced age, residence in an assisted living facility, recent surgery, NG tube placement, use of an H2 blocker (Tagamet), use of home respiratory equipment, and decreased mobility are all risk factors for pneumonia.
What physical findings, vital signs, and laboratory results suggest possible pneumonia?
Physical assessment findings consistent with pneumonia include decreased breath sounds and dull percussion. The patient reports chest pain, increased weakness, and fatigue. Mrs. Mitchell’s BP is on the lower end of normal and should be compared to her baseline. Her RR is high, and her SpO2 is low. She has a mild temperature elevation; however, 49 hours after surgery, this could be due to the inflammatory process. Mrs. Mitchell’s WBC is normal, but this often occurs in older adults with pneumonia. Her BUN/creatinine and sodium level are slightly elevated. These findings indicate possible dehydration.
With which classification of pneumonia would Mrs. Mitchell most likely be diagnosed?
Mrs. Mitchell lives in an assisted living facility, which puts her at risk for MDR HAP. She has developed symptoms over 48 hours after admission following an invasive abdominal operation, so she would most likely be diagnosed with HAP.
One day later, Mrs. Mitchell’s NG tube is removed. Her diet is advanced to ice chips and clear liquids. The nurse notices that when Mrs. Mitchell drinks liquids, she repeatedly swallows and coughs. What is the medical team most concerned about currently? What action should they take?
Mrs. Mitchell’s coughing and frequent swallowing with thin liquids suggest aspiration. She should receive a referral for a swallow function study with a speech pathologist. Thin liquids should be withheld until the study can be performed.
Another day passes, and Mrs. Mitchell becomes more fatigued. Her family is concerned that she is not acting like herself. A chest x-ray is obtained, but the results are not conclusive for pneumonia. What should be done at this time?
Chest x-ray findings for elderly adults may be inconclusive for pneumonia, especially early in the process. Sputum and blood cultures should be considered, and a CBC and lactic acid should be repeated. Broad-spectrum antibiotics should be initiated after sputum and blood cultures are collected until culture sensitivities are available.
Mrs. Mitchell begins IV levofloxacin (Levaquin) and shows an improvement in symptoms. She is being prepared for discharge back to her long-term care facility. She is no longer tachypneic, and her SpO2 is consistently higher than 94%. The patient reports feeling slightly better. What should be done in preparation for discharge?
Her dosage of IV antibiotics should be changed to an equivalent oral dosage in preparation for discharge for a total duration of 7 days. If Mrs. Mitchell tolerates the change and her condition continues to improve, she can be discharged home safely on the oral regimen.
When should Mrs. Mitchell be instructed to follow up for a repeat chest x-ray?
If Mrs. Mitchell’s symptoms improve after 5-7 days of treatment and continue to improve after discharge, a follow-up chest x-ray is not recommended. Further, chest x-rays may not show improvement for a month or longer, even for mild pneumonia. While Mrs. Mitchell does not need a follow-up chest x-ray, she should undergo an evaluation after completing the antibiotic course.
References
American Thoracic Society & Infectious Disease Society of America. (2005). Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory Critical Care Medicine, 171, 388-416. https://doi.org 10.1164/rccm.200405-644ST
Biga, L. M., Dawson, S., Harwell, A., Hopkins, R., Kaufmann, J., LeMaster, M., Matern, P., Morrison-Graham, K., Quick, D. & Runyeon, J. (n.d.). Pleural cavity [image] in Anatomy and physiology. OpenStax/Oregon State University. Retrieved May 5, 2021, from https://open.oregonstate.education/aandp/
Boruchoff, S. E., & Weinstein, M. P. (2021). Sputum cultures for the evaluation of bacterial pneumonia. UpToDate. Retrieved May 7, 2021, from www.uptodate.com/contents/sputum-cultures-for-the-evaluation-of-bacterial-pneumonia
Bradley, J. S., Byington, C. L., Shah, S. S., Alverson, B., Carter, E. R., Harrison, C., Kaplan, S. L., Mace, S. E., McCracken, G. H., Jr, Moore, M. R., St Peter, S. D., Stockwell, J. A., & Swanson, J. T. (2011). The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America, Clinical Infectious Diseases, 53(7), e25–e76. https://doi.org/10.1093/cid/cir531
British Thoracic Society. (2016). Adult community-acquired pneumonia: CAP care bundle. https://www.brit-thoracic.org.uk/document-library/quality-improvement/adult-cap/cap-care-bundle/
Centers for Disease Control and Prevention. (2018). Hospital toolkit for adult sepsis surveillance. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Aug-2018_508.pdf
Centers for Disease Control and Prevention. (2020a). Haemophilus influenzae disease (including Hib). https://www.cdc.gov/hi-disease/about/types-infection.html
Centers for Disease Control and Prevention. (2020b). Haemophilus influenzae disease vaccination. https://www.cdc.gov/hi-disease/vaccination.html
Centers for Disease Control and Prevention. (2021a). Immunization schedules: Adult immunization schedule. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html?CDC_AA_refVal
Centers for Disease Control and Prevention. (2021b). Immunization schedules: Child and adolescent immunization schedule. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
Centers for Disease Control and Prevention. (2021c). Ventilator-associated event. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf
David, S., & Sharma, S. (2020) Vital capacity. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK541099/
Desai, J. & Moustarah, F. (2020). Pulmonary compliance. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK538324/
Dugdale, D. C., Zieve, D., Conaway, B., & the A.D.A.M. editorial team. (2020). Gas exchange. MedlinePlus. https://medlineplus.gov/ency/anatomyvideos/000059.htm
Gauer, R., Forbes, D., & Boyer, N. (2020). Sepsis: Diagnosis and management. American Family Physician, 101(7), 409-418. https://www.aafp.org/afp/2020/0401/p409.html
Grief, S., & Loza, J. (2018). Guidelines for the evaluation and treatment of pneumonia. Primary Care: Clinics in Office Practice, 45(3), 485-503. https://doi.org/10.1016/j.pop.2018.04.001
Gronthoud, F. A. (2021). Practical clinical microbiology and infectious diseases: A hands-on guide. Taylor & Francis Group. http://dl1.tarjomac.com/Medical-Microbiology/TPC606161.pdf#page=290
Hayes, B., Haberling, D., Kennedy, J., Varma, J., Fry, A., & Vora, N. (2017). Burden of pneumonia-associated hospitalizations: the United States, 2001-2014. Chest, 153(2), 427-437. https://doi.org/10.1016/j.chest.2017.09.041
Houck, P. M., Bratzler, D. W., Nsa, W., Ma, A., & Bartlett, J. G. (2004). Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Journal of the American Medical Association, 164(6), 637-644. https://doi.org/10.1001/archinte.164.6.637
Ignatavicius, D. D., & Workman, M. L. (2015). Medical-surgical nursing: Patient-centered collaborative care. (8th ed.). Elsevier
Kalil, A. C., Metersky, M. L., Klompas, M., Muscedere, J., Sweeney, D. A., Palmer, L. B., Napolitano, L. M., O'Grady, N. P., Bartlett, J. G., Carratalà, J., El Solh, A. A., Ewig, S., Fey, P. D., File, T. M., Jr, Restrepo, M. I., Roberts, J. A., Waterer, G. W., Cruse, P., Knight, S. L., & Brozek, J. L. (2016). Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Diseases: An official publication of the Infectious Diseases Society of America, 63(5), e61–e111. https://doi.org/10.1093/cid/ciw353
Kim, G., Seon, S., & Rhee, D. (2017). Pneumonia and Streptococcus pneumoniae vaccine. Archives of Pharmacal Research, 40, 885–893. https://doi.org/10.1007/s12272-017-0933-y
Klompas, M. (2020a). Epidemiology, pathogenesis, microbiology, and diagnosis of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved May 12, 2021, from https://www.uptodate.com/contents/epidemiology-pathogenesis-microbiology-and-diagnosis-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Klompas, M. (2020b). Treatment of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved May 13, 2021, from https://www.uptodate.com/contents/treatment-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Klompas, M. (2021). Risk factors and prevention of hospital-acquired and ventilator-associated pneumonia in adults. UpToDate. Retrieved May 12, 2021, from https://www.uptodate.com/contents/risk-factors-and-prevention-of-hospital-acquired-and-ventilator-associated-pneumonia-in-adults
Klompas, M., Branson, R., Eichenwald, E. C., Greene, L. R., Howell, M. D., Lee, G., Magill, S. S., Maragakis, L. L., Priebe, G. P., Speck, K., Yokoe, D. S., Berenholtz, S. M., & Society for Healthcare Epidemiology of America. (2014). Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology, 35(8), 915–936. https://doi.org/10.1086/677144
LadyofHats & Jmarchn. (2007). The complete anatomy of the respiratory system [image]. https://commons.wikimedia.org/wiki/File:Respiratory_system_complete_en.svg
Lambden, S., Laterre, P., Levy, M., & Francois, B. (2019). The SOFA score- Development, utility, and challenges of accurate assessment in clinical trials. Critical Care, 23(374). https://doi.org/10.1186/s13054-019-2663-7
Marik, P. E., & Taeb, A. M. (2017). SIRS, qSOFA, and new sepsis definition. J Thorac Dis, 9(4):943-945. https://doi.org/10.21037/jtd.2017.03.125
Maughan, B. C, Asselin, N. Carey, J. L., Sucov, A., & Valente, J. H. (2014). False-negative chest radiographs in emergency department diagnosis of pneumonia. Rhode Island Medical Journal. http://www.rimed.org/rimedicaljournal/2014/08/2014-08-20-cont-maughan.pdf
Metlay, J., Waterer, G., Long, A., Anzueto, A., Brozek, J., Crothers, K., Cooley, L., Dean, N., Fine, M., Flanders, S., Griffin, M., Metersky, M., Musher, D., Restrepo, M., & Whitney, C. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. American Journal of Respiratory and Critical Care Medicine, 200(7), 45-67. https://doi.org/10.1164/rccm.201908-1581ST
Mohanty, A., Kabi, A., Mohanty, A. P., Gupta, P. & Gupta, P. (2017). Diagnostic usefulness of transtracheal aspiration in lower respiratory tract infections. International Journal of Research in Medical Sciences, 5(12), 5203-5206. http://dx.doi.org/10.18203/2320-6012.ijrms20175097
Murillo-Zamora, E., Medina-González, A., Zamora-Pérez, L., Vázquez-Yáñez, A., Guzmán-Esquivel, J., & Trujillo-Hernández, B. (2018). Performance of the PSI and CURB-65 scoring systems in predicting 30-day mortality in healthcare-associated pneumonia, Medicina Clínica, 150(3), 99-103. https://doi.org/10.1016/j.medcle.2017.12.003.
National Heart, Lung, and Blood Institute. (2019). Health topics: Pneumonia. https://www.nhlbi.nih.gov/health-topics/pneumonia
Osti, C., Wosti, D., Pandey, B., & Zhao, Q. (2017). Ventilator-associated pneumonia and role of nurses in its prevention. Journal of Nepal Medical Association, 56(208), 461-468.
Patel, V., & Makwana, S. (2021). Comparative study of pneumonia severity index (PSI) & CURB-65 score in assessing severity of community-acquired pneumonia. Indian Journal of Applied Basic Medical Sciences, 23(1), 61-71. https://doi.org/10.48165/ijabms.2021.23107
Ramirez, J. A. (2020). Overview of community-acquired pneumonia in adults. UpToDate. Retrieved May 7, 2021, from www.uptodate.com/contents/overview-of-community-acquired-pneumonia-in-adults
Sattar, S., & Sharma, S. (2018). Bacterial pneumonia. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK513321/
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., Bellomo, R., Bernard, G. R., Chiche, J. D., Coopersmith, C. M., Hotchkiss, R. S., Levy, M. M., Marshall, J. C., Martin, G. S., Opal, S. M., Rubenfeld, G. D., van der Poll, T., Vincent, J. L., & Angus, D. C. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA, 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Stanford Medicine. (n.d.). Understanding ILD. Retrieved March 14, 2021, from http://med.stanford.edu/ild/patient-resources/understanding-ild.html
Vafaei, A., Heydari, K., Hashemi-Nazari, S., Izadi, N., & Zadeh, H. (2019). PIRO, SOFA, and MEDS scores in predicting one-month mortality of sepsis patients: A diagnostic accuracy study. Archives of Academic Emergency Medicine. 7(1).
Vincent, J., Moreno, R., Takala, J., Willatts, S., De Mendonça, A., Bruining, H., Reinhart, C., Suter, P., & Thijs, L. (1996). The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Medicine, 22(7), 707-10. https://doi.org/10.1007/BF01709751
von Dadelszen, P. (Ed). (n.d.). Composite atlases. The Global Library of Women’s Medicine. Retrieved March 14, 2021, from https://www.glowm.com/atlas-page/atlasid/chestXray.html#
World Health Organization. (2019). Pneumonia. https://www.who.int/news-room/fact-sheets/detail/pneumonia