About this course:
The purpose of this activity is to enable the learner to define current standards for pressure injuries related to hospitalizations and medical devices and recognize risk factors along with prevention and management that promotes positive patient outcomes.
Course preview
Pressure Injuries from Medical Devices and Hospitalization
Learning Outcomes
Upon completion of this activity, participants should be able to:
- Define the incidence and prevalence of pressure injuries from hospitalization or medical devices.
- Review the pathophysiology of the skin concerning the incidence of pressure injuries.
- Understand pressure injury risk from medical devices.
- Discuss the impact of hospitalization on pressure injury risk.
- Explore National Patient Safety Goals related to pressure injuries.
- Consider management of pressure injuries based on current clinical guidelines.
- Explain how to reduce the occurrence of hospital-acquired pressure injuries.
The purpose of this activity is to enable the learner to define current standards for pressure injuries related to hospitalizations and medical devices and recognize risk factors along with prevention and management that promotes positive patient outcomes.
Introduction
Each year more than 2.5 million patients are affected by hospital-acquired pressure injuries (HAPI), formerly known as pressure ulcers or pressure sores, due to hospitalizations and medical device injuries (Health Research and Educational Trust, 2017). In 2016, the National Pressure Ulcer Advisory Panel (NPUAP, 2017) updated their terminology and staging definitions and published updated practice guidelines.
While incidence rates have fallen from 40.3% in 2010 to 30.9% in 2014, there are still a high number of HAPI cases in the US (Rondinelli et al., 2018). Up to 1 in 30 patients admitted to hospitals in the US will develop a pressure injury (Black & Maegley, 2019). The cost of these wounds can exceed $70,000 per incidence resulting in over $11 billion annually (California Hospital Patient Safety Organization [CHPSO], 2017). As noted, these injuries are costly in treatment, reimbursement, and associated patient mortality and morbidity (Padula et al., 2018). The Centers for Medicare and Medicaid deny payments for HAPI’s classified as Stage III or IV since they are considered preventable (NPUAP, 2017). Furthermore, hospitals can be penalized with a 1% reduction of their reimbursement from Medicare with elevated HAPI rates if they fall into the top 25% of hospitals across the country (Black & Maegley, 2019).
Prevention is vital to improving patient outcomes while avoiding the financial pitfalls associated with HAPI’s. Moreover, the price of human suffering and increased length of illness makes these types of injuries even more imperative to avoid. Pressure injuries, regardless of cause, are primarily preventable through assessment and ongoing observation to prevent tissue injury and further breakdown (NPUAP, 2017). This module will discuss the prevalence and risk factors for pressure injuries caused by medical devices or hospitalizations and review the current clinical guidelines for the management of these wounds.
Pathophysiology of the skin in relation to pressure injuries
It is crucial to understand the structures of human skin and its vulnerabilities to understand the implications of pressure and how injuries can quickly occur. The skin covers the entire outside of the body and is the largest organ, receiving a third of the body’s blood circulation. The skin protects against heat, light, chemical reaction, or physical action and is strong but pliable. The skin helps maintain body temperature, protects from infection, and gathers sensory information, allowing the individual to feel both pain and pleasurable stimuli. Vitamin D, fat, and water are stored in the skin as well (Peate & Glencross, 2015).
The skin is made up of three layers, the epidermis, dermis, and subcutaneous tissue (see Figure 1 below). The epidermis, which is the surface layer, is made up primarily of dead skin cells that continuously shed. The next layer, the dermis, holds the sweat and oil glands, nerve endings, and capillaries (small blood vessels) that are woven together by collagen. Collagen is a protein that provides nutrition and support to the skin cells. The nerves in this area transmit feelings of pain, pleasure, touch, and itch to the surface. Hair follicles also initiate in the dermis. Injury or destruction of the dermis or epidermis can lead to infection and further implications for the entire body (Peate & Glencross, 2015).
Figure 1: Structure of the Skin
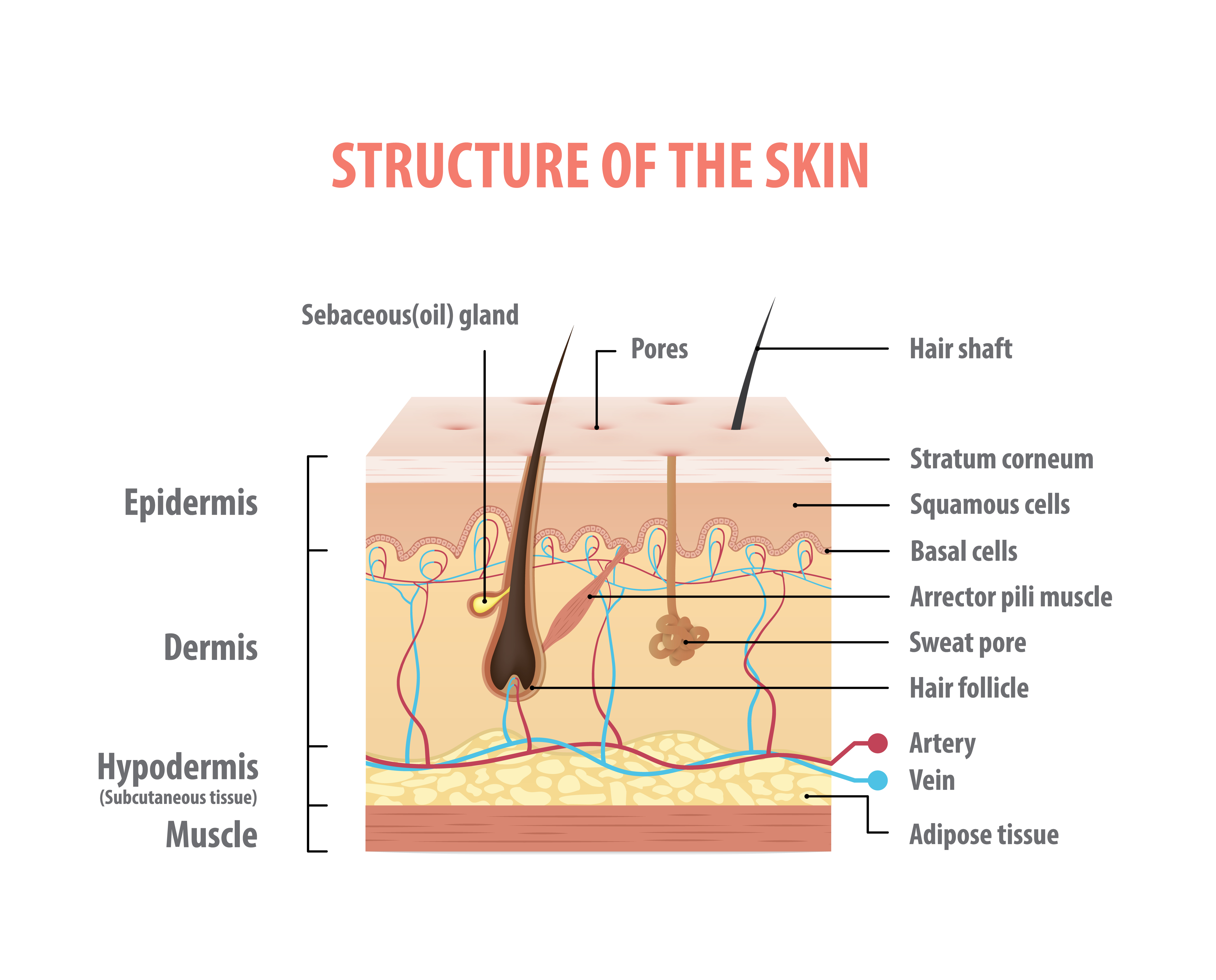
The final layer is the subcutaneous tissue or hypodermis and is comprised of fat and collagen, also housing larger blood vessels and nerves. It is significant in controlling body temperature and protecting from injury by acting as a cushion or shock absorber. If damaged or disrupted, the skin can self-heal remarkably fast. However, the skin is still prone to injury if there are prolonged insults such as friction, moisture, shearing force, or pressure (Peate & Glencross, 2015).
Risk for injury to the skin amplifies with specific conditions such as immobility, pressure from medical devices, poor nutrition that alters the tissue integrity, or decreased sensation allowing injuries to occur without awareness by the patient (Peate & Glencross, 2015).
HAPIs occur when there is “localized damage to the skin or underlying soft tissue usually over a bony prominence… The injury can present as intact skin or an open ulcer and may be painful. The injury occurs as a result of intense or prolonged pressure or pressure in combination with a shear” (Black & Maegley, 2019, p. 31). Approximately 70% of generic pressure wounds appear on the hips or buttocks, with an additional 15-25% occurring on the malleolar, heel, patellar and pretibial areas. The mechanism of injury is constant pressure on a specific area for an extended prior of time, impairing blood flow to soft tissues resulting in ischemia followed by necrosis. While the skin and underlying muscle can withstand pressure for brief periods, sustained pressures, even slight ones, can lead to these injuries. The compression from mattresses, wheelchair pads, bed rails, or other surfaces found in the patient's environment serves as the source for this pressure. Shearing forces against the tissues also cause injuries that extend deep into the skin's layers. Maceration from prolonged exposure to moisture due to incontinence or sweating can open the skin to further damage from friction, shearing, or pressure as well (Kirman, Geibel, & Kolaski, 2018).
While the skin is visible during inspection, the underlying muscle tissue is damaged first in pressure injuries. Muscle tissue requires increased oxygen and nutrients when compared to surface tissues. Only two hours of continuous pressure can induce damage to the underlying muscle and tissue, creating a wound in the shape of an inverted cone from the surface down to the muscle. Often the internal damage to the underlying layers of skin and muscle is much more significant than the visible tissue injury (Kirman et al., 2018).
Risk factors
Risk factors for generic HAPI
As with all types of prevention, the nurse and healthcare team must first identify the risk. A thorough assessment can determine the threat and alert the healthcare team to the potential for a HAPI. The injuries can occur in any part of the hospital, including the operating room, acute care setting, long-term care setting, pediatric unit, or critical care unit. However, the incidence of generic HAPI is highest in critical care, elderly care, and operating room settings with rates as high as 59%. A keen awareness and understanding of the various risks posed within each area of the healthcare system can guide quality imp
...purchase below to continue the course
Common risk factors for general HAPIs include:
- Neurological deficiencies such as paralysis or decreased sensation related to diabetic neuropathy,
- Immobility from bed rest or physical conditions,
- Decreased circulation secondary to diabetes or peripheral vascular disease,
- Poor nutritional status,
- Advanced age,
- Incontinence,
- Mental health conditions (CHPSO, 2017).
In a study by CHPSO (2017), pressure injuries not caused by medical devices were noted to be more common in older adult males. Over 40% of these injuries occurred in the inpatient general care areas of a hospital. A majority of these injuries were located within the coccyx area, followed closely by the sacrum and heels. These wounds were related to a lack of mobility and many of the other factors discussed above (CHPSO, 2017).
Risk factors for medical device-related pressure injury
A medical device-related pressure injury (MDRPI) is a pressure injury that results from the use of a device designed and applied for diagnostic or therapeutic purposes. The wound typically takes on the pattern or shape of the offending medical device and should be staged using the NPUAP staging system. Poorly-fitting devices or improperly-positioned fixation devices used to secure a medical device can render the patient vulnerable to resulting MDRPIs. The number of pressure injuries that are caused by medical devices is unknown. Many injuries caused by medical devices are included in reports related to the specific device rather than in pressure injury reports; thus, the number of MDRPIs may be far higher than is currently thought. These injuries are harder to determine risk, as well. While the skin assessment tools may be predictive of pressure injuries in general, they may not be predictive of MDRPIs (Jackson, Sarki, Betteridge & Brooke, 2019).
Similar to the generic risk factors listed above, the risk factors for MDRPI’s include impaired sensation, poor perfusion, altered tissue tolerance, poor nutritional status, or moisture under the device (NPUAP, 2014). Medical devices associated with pressure injuries include, but are not limited to, the following:
- Orthopedic devices such as immobilizers, cervical collars, or casts;
- Foley catheters or surgical drain tubing;
- Dialysis catheters;
- Central venous catheters and peripherally inserted central catheters (PICC) lines;
- Intermittent pneumatic compression devices;
- TED hose or compression stockings;
- Tracheostomy securement devices;
- Endotracheal tubes;
- Oxygen delivery devices;
- Pulse oximeter probes;
- Intra-aortic balloon pumps (NPUAP, 2014)
As noted earlier, MDRPIs can occur in any area within the hospital: acute-care, long-term care, intensive care, general medical, pediatric, trauma, and rehabilitation facilities (Jackson et al., 2019). Since there is a significant risk for MDRPIs among many patient populations, the nurse’s clinical judgment and visual inspection is vital to protect patients. According to a systematic review by Jackson et al. (2019), policies and procedures with a focus on the increased assessment of the medical device location are needed to prevent MDRPIs. Early intervention with repositioning of the device at regular intervals, adding protective layers between the skin and the medical device where possible, securing devices with appropriate tapes to prevent friction, and careful monitoring of moisture associated with the device may decrease the incidence or severity of injuries in the hospitalized patient. Surprisingly, a study reviewing hospitalized patient data collected through state-mandated reporting in Minnesota noted that 63% of reported MDRPI’s had no documentation of device removal at regular intervals for pressure relief, an inspection of the skin, or cleaning of the area. 74% of those same MDRPI's were at Stage 3 or higher on discovery (Demore & Ayello, 2017).
The NPUAP (2014) identified in their Clinical Practice Guideline that mucosal tissue is particularly vulnerable to pressure injuries. These injuries can occur in the “oral mucosa, gastrointestinal tract, nasal passages, urinary tract, tracheal lining, and vaginal tract" (NPUAP, 2014, p. 9). Pressure in these areas can lead to ischemia and ulceration due to the delicate nature of the tissue and the ambient moisture involved. Medical devices frequently associated with these injuries are oxygen tubing, urinary catheters, and endotracheal tubes. As anatomically possible, pressure relief should be implemented prior to the patient enduring an injury. Upon initial identification of a pressure injury, the medical device should be removed or changed. Due to the histologic characteristics of mucosal tissue that do not allow healthcare providers to distinguish partial and full-thickness tissue loss, these wounds are not staged (NPUAP, 2014).
Current skin assessment tools may not identify the risk from medical devices as their focus is on risk factors such as nutritional status, age, or illness. Additionally, patients may be at risk for an MDRPI even where these factors are not present. Injuries from medical devices can occur in any patient without proper assessment, prevention, or early intervention. In the systematic review, Jackson et al. (2019) recognized that the development of new tools to identify risk or actual injury from medical devices might be needed, considering the limitations of current skin assessment tools. A robust exploration into quality improvement initiatives should be considered by healthcare organizations that include higher levels of vigilance in assessment, staff involved product selection, and efficient reporting strategies. Quality improvement initiatives should focus on a more definitive recognition of population or situational risk associated with medical devices (Jackson et al., 2019).
Even though all patients are at risk for pressure injuries, the NPUAP (2014) Clinical Practice Guidelines for Medical Device Related Pressure Ulcers note further risks that should be considered in populations using medical devices. Older adults and children have an increased risk of pressure injury when a medical device is involved. The guidelines note that an adult with a medical device is 2.5 times more likely to develop a pressure injury than those without a medical device. “Children hospitalized at least 24 hours who had an external medical device had up to a 40% chance of developing a pressure injury related to the device” (NPUAP, 2014, p. 10).
In critically ill patients requiring multiple medical devices, the risk of injury increases dramatically. The critically ill patient may be too sick to reposition the devices themselves. Furthermore, nurses and hospital staff may overlook the potential skin injury or may not identify damage beneath the dressings, tubing, or tape used to secure the device. These patients are often unable to communicate discomfort or sensory alterations as well (Emergency Care Research Institute, 2014).
Patients with mobility limitations or those confined to the bed or chair have a higher risk of pressure injury development. Available risk tools examine risk variables including but not limited to:
- Mobility/activity related to ADL’s,
- Activity descriptors such as chairfast or bedfast,
- Factors affecting mobility including weakness or paralysis,
- Increased friction or shear on a subscale of a risk assessment tool,
- Decreased activity on a subscale of a risk assessment tool,
- Interface pressures (NPUAP, 2017).
Perfusion and oxygenation have a direct relationship to the risk of developing a pressure injury. Vascular disease, hypertension, diabetes, circulatory conditions, smoking or edema cause decreased circulation to the skin and increase the risk of tissue ischemia leading to injury as well as a reduced ability to heal (NPUAP, 2017).
Skin moisture is a risk factor for pressure injury development across the literature. While a certain amount of hydration is a positive aspect of skin condition and function, excess moisture impacts the vulnerability of the skin by affecting the mechanical properties and barrier function of the skin. Indicators that increase the risk include but are not limited to:
- Skin moisture,
- Fecal or urinary incontinence,
- High moisture subscale on a risk assessment tool,
- An indwelling urinary catheter (NPUAP, 2017).
A nutritional assessment can offer vital information regarding future and current risk for skin injuries, including pressure injuries. Nutritional deficits are associated with reduced tolerance of the skin, abnormal morphology of the tissues, altered physiology, altered ability for repair, and transport/thermal properties. Each of these factors can increase the risk of pressure injury. Specific risk variables to assess are:
- Food intake descriptors,
- Low weight or recent weight loss,
- Low body mass index (BMI),
- Malnutrition,
- Decreased arm measurements,
- Nutrition scale assessment (NPUAP, 2017)
Further factors that impact skin integrity and the risk of pressure injury include:
- Advancing age,
- Decreased sensory perception,
- Altered hematological measures,
- Elevated or reduced body temperature,
- Diminished general health status (NPUAP, 2017).
The elderly patient has the highest rate of pressure injuries, even though pediatric through adult patients can develop them as well. The NPUAP (2017) makes note that advanced age alone is a variable, but risk increases with co-morbidities such as mobility/activity, skin status, perfusion and oxygenation, or skin moisture (NPUAP, 2017).
Decreased sensory perception occurs with spinal cord injuries or other neurological disorders. Much like the elderly patient, these individuals have the highest risk where co-morbidities exist. Co-existing factors such as loss of sensation, lack of response, or other deficits will increase the likelihood of pressure injuries in these patients (NPUAP, 2017).
Altered hematological measures that are of particular concern include lymphocytopenia, decreased albumin, decreased hemoglobin, or an elevated C-reactive protein. Additional concerns are urea and electrolyte imbalances. A creatinine level above 1 mg/dL is associated with a higher risk of pressure injury development. The variables of these levels could be related to overall blood loss to severe malnutrition, yet each may lead to increased risk for tissue injury (NPUAP, 2017).
An increase or significant decrease in body temperature can lead to an increased risk for injury to the skin as it “affects the susceptibility and tolerance of the skin by affecting physiology and repair; and transport and thermal properties.” The patient with fever or hypothermia for an extended time is at increased risk (NPUAP, 2017, p. 54).
Finally, poor mental or physical health status can lead to pressure injury. Risk variables in this category include:
- Chronic wounds,
- Medication use,
- Chronic illnesses including AIDS, diabetes, or respiratory conditions,
- Mental health conditions including dementia,
- Acute injuries,
- Surgical recovery,
- Extended hospitalizations (NPUAP, 2017).
Skin assessment tools
The elements of a comprehensive skin tool includes skin temperature, color, moisture, turgor, and integrity. There are many tools available to the healthcare team, yet the most common include the Braden and Norton Scales. Both scales have high levels of validity and reliability to identify high-risk patients (AHRQ, 2017b).
The Braden scale (Braden & Bergstrom, 1988), Norton scale (Seniors Health Knowledge Network, n.d.) or another skin assessment tool should be used to measure risk for skin injury as soon as possible after admission. The Braden scale consists of six categories that apply a score ranging from 1-4 in each category. Categories include Sensory Perception, Moisture, Activity, Mobility, Nutrition, and Friction & Shear. Total scores may range from 6-23. The lower the score, the higher the risk for a pressure injury. A score of 18 or less indicates an at-risk status for the development of a pressure injury. A low score in a single category indicates a higher risk in that specific area to take into consideration when developing a plan of care. The nurse should keep in mind that patients could potentially have a low-risk score, yet have an increased risk in a single category; thus, a review of the information may give opportunities to avoid injury (Braden & Bergstrom, 1988; NPUAP, 2017).
The Norton scale was created in 1962 in England and has been in use since. It is used primarily for the elderly patient in a long-term care setting. The scale is easy to use and effective in recognizing the risk of pressure injuries or other skin concerns. The Norton scale has five categories, including Physical Condition, Mental Condition, Activity, Mobility, and Incontinence. Within each category, the patient is given a score of 1-4 based on characteristics defined by the scale. For example, the Activity category includes ambulant (4), walks with help (3), chair bound (2) or bedfast (1) (Seniors Health Knowledge Network, n.d.). The individual score in each category is totaled to determine the patient’s overall risk. See Table 1 below to see how this tool interprets the overall risk score:
Table 1: Interpretation of Risk in the Norton Scale
Greater than 18 | Low risk |
Between 14 and 18 | Medium risk |
Between 10 and 14 | High risk |
Less than 10 | Very high risk |
(Seniors Health Knowledge Network, n.d.)
The Braden scale is considered to be more precise than the Norton scale due to the broader number of factors related to skin condition and risk within each category (Seniors Health Knowledge Network, n.d.).
The acronym HALT has been used to support decision making with pressure injury risk in combination with a scale:
- History
- Assess co-morbidities, medications
- Look at the skin
- Touch the skin (AHRQ, 2017b)
The NPUAP (2017) suggests that a full skin assessment using a validated assessment tool such as the Braden or Norton scale is completed as soon as possible, but no more than eight hours after admission. In contrast, the National Database of Nursing Quality Indicators (NDNQI, 2018) suggests the skin assessment is completed within 24 hours of admission to identify any preexisting injuries along with an assessment of risk for future injury. The nurse should also remember that risk can exist without a high score on the skin assessment tool. Any change in condition or acuity that could increase the risk requires a repeated risk assessment. Along with each risk assessment, a comprehensive skin assessment with documentation should be completed and maintained in the medical record. While there is no universally accepted risk assessment, each facility should have a structured risk assessment that is consistently applied. Each institution should have a risk-based prevention plan for patients identified at-risk for the development of pressure injuries. The physical assessment and skin risk assessment tool results determine the risk for pressure injury (NPUAP, 2017).
A head-to-toe skin assessment is critical to determine risk or identify existing issues with skin integrity and should include considerations for darkly pigmented skin, impaired tissue integrity, discoloration, or erythema. The presence of pre-existing pressure injuries indicates a higher risk for the future development of pressure injuries. Further indicators of risk related to skin status is a previous history of pressure ulcer or any alterations in skin condition (NPUAP, 2017).
As the acuity of illness increases and the length of hospital stay increases, the opportunity for injury to the skin also rises. Maintaining nutritional status, managing medical needs, and increasing mobility are all actions that reduce the risk of injury. Further, the identification of risk allows for early intervention upon discovery of pressure injuries that may improve outcomes. Once an injury has occurred, staging skin injuries is essential to direct the most appropriate management based on evidence and current guidelines (NPUAP, 2017).
Prevention Recommendations
The NPUAP guidelines recommend that medical devices are selected and adjusted to an individual to produce the least amount of damage from pressure or shearing forces. For instance, nurses should use the softest device or securement products that promote the least amount of friction or shearing force for any tubing such as an endotracheal tube, urinary catheter, or tracheostomy tube. Medical devices such as helmets, halo vests, or restrictive devices should fit correctly to avoid excessive pressure. All devices should be applied according to the manufacturer’s specifications to prevent unintended injury and liability to the facility. The focus should be on securing any medical device appropriately and using padding or cushioning against the tissue where possible (NPUAP, 2014).
Recommendations for the assessment of skin surrounding and or beneath a medical device include:
- Increased frequency of skin assessments, observing for an increase in edema or irritation.
- Education for the family and patient on risk related to the medical device should include recognizing and reporting injuries or concerns.
- If an injury occurs, classify the MDRPI using the International NPUAP/EPUAP Pressure Classification system except for mucosal pressure injuries (NPUAP, 2014, p. 12).
Prevention recommendations include:
- Remove devices as quickly as appropriate for the medical condition.
- Maintain skincare under the medical device, keeping it clean and dry.
- Reposition the patient regularly to decrease pressure from the medical device.
- Reposition or rotate the position of the medical device regularly when possible to avoid ongoing pressure to the same area.
- Provide cushioning or supportive devices to decrease pressure to the underlying tissue.
- Prophylactic dressings for pressure such as a soft foam silicone dressing may reduce the risk of skin breakdown. The NPUAP guidelines have further information on the ideal characteristics of prophylactic dressings, which include the ability to assess skin under the dressing; ease of removal and reapplication; location of the device on the patient’s body; or ability to manage body fluids around the dressing (NPUAP, 2014).
National Patient Safety Goals and pressure injuries
The Joint Commission endorses the National Patient Safety Goals (NPSGs), which are a set of standards addressing the highest priority patient safety issues to implement changes to patient safety in all healthcare settings. Goal 14 of the NPSG is to “prevent healthcare-associated pressure ulcers (decubitus ulcers)” (Agency for Healthcare Research and Quality [AHRQ], 2017a, p. 8). This goal notes that each patient's risk should be identified, and action should be taken to address any pressure ulcers discovered during the skin assessment. The goal notes that most pressure ulcers, can be avoided through preventative measures and damage can be decreased if injuries are identified early, at Stage I. The healthcare organization is expected to have a written plan to identify risk and prevent pressure injuries; perform initial skin assessment that identifies risk for pressure injuries; use a Braden or Norton scale to validate risk assessment; reassess at specified intervals as determined by the organizational policies; take actions appropriate for the risks identified during the evaluation that prevents injury to the patient and protects from “external mechanical forces” (AHRQ, 2017a, p. 8). Also, there should be education for staff that identifies risk factors for pressure injuries and prevention techniques as defined by the organization based on the latest evidence and guidelines (AHRQ, 2017a).
A training program for hospitals is available on the AHRQ website called Preventing Pressure Ulcers in Hospitals Toolkit (AHRQ, 2017b). The training materials offer the latest guidelines in pressure injury prevention based on a two-year pilot project which reduced the rate of HAPI-Stage II injuries and then sustained the lower rates of injury throughout the project. The toolkit has five modules that define the steps to reduce the rate of pressure ulcers through education, prevention, and assessment, including measuring the success and follow-up. All training materials, including written materials and videos, are available on the AHRQ website. Training materials do not require specific permissions and are available for use by institutions (ARHQ, 2017b).
Staging Pressure Injuries
The NPUAP updated its staging system for pressure inures in 2016 (see Table 2 and Figure 2 below). The system has four main stages of pressure injury ranging from 1 to 4. The stages are not considered progressive and do not follow a standard regression from 4 to 1, followed by complete healing. The system is used to describe a wound at a specific time of assessment and meant as a communication tool among healthcare providers and various disciplines to drive the plan of care (NPUAP, 2016b).
Table 2: NPUAP Pressure Injury Staging
Stage 1 Pressure Injury: Non-blanchable erythema of intact skin | Intact skin with a localized area of non-blanchable erythema, which may appear differently in darkly pigmented skin. Presence of blanchable erythema or changes in sensation, temperature, or firmness may precede visual changes. Color changes do not include purple or maroon discoloration; these may indicate deep tissue pressure injury. |
Stage 2 Pressure Injury: Partial-thickness skin loss with exposed dermis | Partial-thickness loss of skin with exposed dermis. The wound bed is viable, pink or red, moist, and may also present as an intact or ruptured serum-filled blister. Adipose (fat) is not visible, and deeper tissues are not visible. Granulation tissue, slough, and eschar are not present. These injuries commonly result from adverse microclimate and shear in the skin over the pelvis or behind the heel. This stage should not be used to describe moisture associated skin damage (MASD) including incontinence associated dermatitis (IAD), intertriginous dermatitis (ITD), medical adhesive related skin injury (MARSI), or traumatic wounds (skin tears, burns, abrasions). |
Stage 3 Pressure Injury: Full-thickness skin loss | Full-thickness loss of skin, in which adipose (fat) is visible in the ulcer and granulation tissue and epibole (rolled wound edges) are often present. Slough or eschar may be visible. The depth of tissue damage varies by anatomical location; areas of significant adiposity can develop deep wounds. Undermining and tunneling may occur. Fascia, muscle, tendon, ligament, cartilage, or bone are not exposed. If slough or eschar obscures the extent of tissue loss, this is an Unstageable Pressure Injury. |
Stage 4 Pressure Injury: Full-thickness skin and tissue loss | Full-thickness skin and tissue loss with exposed or directly palpable fascia, muscle, tendon, ligament, cartilage or bone in the ulcer. Slough or eschar may be visible. Epibole, undermining or tunneling often occurs. Depth varies by anatomical location. If slough or eschar obscures the extent of tissue loss, this is an Unstageable Pressure Injury. |
Unstageable Pressure Injury: Obscured full-thickness skin and tissue loss | Full-thickness skin and tissue loss in which the extent of tissue damage within the ulcer is not confirmed because it is obscured by slough or eschar. If slough or eschar is removed, a Stage 3 or Stage 4 pressure injury will be revealed. Stable eschar (i.e. dry, adherent, and intact without erythema or fluctuance) on the heel or ischemic limb should not be softened or removed. |
Deep Tissue Pressure Injury (DTPI): Persistent non-blanchable deep red, maroon or purple discoloration | Intact or non-intact skin with localized area of persistent non-blanchable deep red, maroon, purple discoloration or epidermal separation revealing a dark wound bed or blood-filled blister. Pain and temperature change often precede skin color changes. Discoloration may appear differently in darkly pigmented skin. This injury results from intense or prolonged pressure and shear forces at the bone-muscle interface. The wound may evolve rapidly to reveal the actual extent of tissue injury or may resolve without tissue loss. If necrotic tissue, subcutaneous tissue, granulation tissue, fascia, muscle, or other underlying structures are visible, this indicates a full thickness pressure injury (Unstageable, Stage 3, or Stage 4). Do not use DTPI to describe vascular, traumatic, neuropathic, or dermatologic conditions. |
(NPUAP, 2016b)
Figure 2: Stage 1-4 Pressure Injuries

See Table 3 below for further definitions related to pressure injuries from the NPUAP 2016 Staging Guidelines:
Table 3: NPUAP Staging Guidelines
MDRPI: This describes an etiology. | MDRPIs result from the use of devices designed and applied for diagnostic or therapeutic purposes. The resultant pressure injury generally conforms to the pattern or shape of the device. The injury should be staged using the aforementioned NPUAP staging system. |
Mucosal Membrane Pressure Injury | Mucosal membrane pressure injury is found on mucous membranes with a history of a medical device in use at the location of the injury. Due to the anatomy of the tissue, these ulcers cannot be staged. |
(NPUAP, 2016b)
Management of pressure injuries
The NPUAP (2017) Clinical Practice Guideline of interventions for the treatment of pressure ulcers examine wound bed preparation. This is a clinical concept, which is a holistic, systematic approach to evaluating and treating wounds that allows a natural progression toward wound healing (NPUAP, 2017). The goal of wound bed preparation is "to promote a well-vascularized wound bed, free from non-viable tissue and excess exudate, and with a reduced bacterial burden and reduced edema, that is optimal for the development of healthy granulation tissue," (NPUAP, 2017, p. 11)
The acronym TIME describes the four components of wound care related to wound bed preparation.
- Tissue management,
- Infection and inflammation control,
- Moisture balance,
- Epithelial edge advancement (NPUAP, 2017, p. 11)
Wound healing facilitated by routine assessment and wound care driven by TIME will remove the barriers that delay normal healing in chronic wounds. Debridement of necrotic tissue and the associated bacterial and cellular burden serve to stimulate healthy tissue growth. Wound cleansing removes remnants of old dressings and decreases the number of bacteria in the wound bed. If there are further particles or devitalized tissue, debridement is needed. Cleansing should be gentle to avoid damaging new tissue growth. Literature does not identify a specific cleansing solution or process for routine wound cleansing. For immunocompromised patients or if the wound enters a sterile body cavity, aseptic technique with sterile products should be utilized. For clean pressure injuries or those without any signs of infection, tap water or normal saline is recommended for wound cleansing. For wounds that have confirmed or suspected infection, cleansing solutions that contain an antimicrobial should be used. Wounds that have sinus tracts or tunneling should be cleansed with caution as some of the solutions could be retained in the wound, causing further injury (NPUAP, 2017).
The wound cleansing should include sufficient pressure to adequately cleanse the wound but avoid damaging tissue or driving bacteria further into the wound. All cleansing solution should be contained and disposed of properly to avoid cross-contamination. The surrounding skin should also be cleansed, taking particular care for wounds on the coccyx or perineal areas (NPUAP, 2017).
When debridement is needed, many factors should be considered. The procedure can be excruciating, and pain management should be part of the plan of care. Devitalized tissue that is thick or leathery and discolored including yellow, green, tan, grey, brown or black, should be debrided to promote healing. Bacteria grows in necrotic tissue and further delays wound healing. However, the patient's overall condition and ability to tolerate the debridement is part of the decision regarding this aspect of wound care. Indications for debridement may include the presence of biofilm (collection of one or more types of organisms that form a slimy build-up), delayed wound healing, or failure to respond to standard wound care. Methods of debridement in pressure injuries may utilize conservative sharp instruments such as scalpels or other surgical instruments; mechanical debridement, wound irrigation, low-frequency sound or ultrasonic mist; biological debridement such as larval therapy with sterile maggots; or enzymatic debriding agents (NPUAP, 2017).
Bacteria is present on all surfaces of the skin, and when the skin integrity is breached, bacteria present on the wound surface can enter the wound. If the bacteria presence increases to the point that wound damage occurs, then infection is said to be present. Healthy individuals can usually avoid developing an infection due to the response of their immune system defense, yet an immunocompromised patient will have a decreased ability to fight the bacteria. "The number of bacteria and their effect on the patient are categorized as: contamination, colonization, critical colonization/topical infection, local infection, regional spreading infection/cellulitis and sepsis," (NPUAP, 2017, p. 22). Microorganisms may multiply, invade, or damage tissues in or around the wound bed, delay healing, and may cause systemic responses. If a pressure ulcer is not healing due to the bacteria in the wound bed, an infection is present. Biofilms may be the source of wound infection, delaying healing. The biofilms must be removed by debridement and prevented from reforming by the use of antiseptics and antimicrobial dressings (NPUAP, 2017).
Other signs or symptoms of infection are:
- Erythema that extends past the wound edges;
- Induration of the tissue;
- Increasing or change in pain or warmth;
- Purulent drainage;
- Increasing wound size;
- Systemic reactions including fever, malaise, or lymph node enlargement; confusion or anorexia (particularly in the older adult) (NPUAP, 2017).
For proper management of a wound infection, wound cultures may be required to determine the appropriate treatment based on the type of organism involved. If a biofilm is recognized, a tissue biopsy may be required. Notify other members of the healthcare team with signs and symptoms of infection. The healthcare team would include dieticians, physicians, including vascular specialists, or wound care specialists. Poor nutritional status, lack of glycemic control, certain medications, and inadequate circulation are possible causes of an infection. Any deficits in these areas should be further explored and managed by the healthcare team (NPUAP, 2017).
Treatment for infected wounds could include systemic antibiotics, topical antiseptics, silver Silvadene, medical-grade honey, or topical antibiotics. Limit topical antibiotics except in particular circumstances where the benefits outweigh the risk of side-effects and resistance (NPUAP, 2017).
Wound dressings should be selected based on the following:
- Ability to keep the wound bed moist,
- Need to address bacterial burden,
- Type and amount of wound exudate,
- Condition of the tissue in the wound bed,
- Status of the surrounding skin,
- Ulcer stage and location,
- Presence of tunneling or undermining,
- Goals of the patient and the healthcare team (NPUAP, 2017).
Types of dressings
Use hydrocolloid dressings for non-infected Stage I through Stage IV pressure injuries, such as Stage II wounds located in areas that the dressing will not roll or melt. These dressings are moisture-retentive and used to protect wounds with a small to moderate amount of drainage (NPUAP, 2017; Smith & Caple, 2019).
Use transparent film dressings when the wound requires autolytic debridement, in patients who are not immunocompromised. Hydrocolloids can function as a secondary dressing with alginates or wound fillers that need to remain in the wound bed for an extended time. Do not use hydrocolloids with excessive exudate in wounds. Do not cover enzymatic debriding agents, gels, or ointments with hydrocolloid dressings (NPUAP, 2017).
Use hydrogel dressing on minimally draining and painful wounds. The most common types of hydrogels are amorphous hydrogels or sheet hydrogels. Use amorphous hydrogel dressings on injuries that are not infected but are granulating. Gravity-dependent body areas such as the lower legs benefit from amorphous hydrogel dressings. Hydrogel sheets can be used to treat dry bed wounds and are often used on non-moving or nondependent body surfaces (NPUAP, 2017).
Utilize alginate dressings for wounds with concurrent treatment for an infection. The alginate dressing should be gently removed and irrigated first to aid in removal. If the alginate dressing is dry, leave in place for extended periods. These dressings have minimal antimicrobial properties and should not be used as a primary treatment for infected wounds (NPUAP, 2017).
Choose foam dressings for heavy drainage from Stage II or III pressure injuries. These dressings absorb heavy exudate and act as a wick to pull drainage from the wound bed to the surface of the dressing. These dressings promote moisture evaporation and facilitate drainage removal from the wound bed and skin (NPUAP, 2017).
Use silver-impregnated dressings in wounds that are clinically infected or heavily colonized with bacteria. Discontinue the silver-impregnated dressing as the infection subsides. The NPUAP (2017) cautions that "topical silver products should not be used on patients with silver sensitivities. Silver may have toxic properties, especially to keratinocytes and fibroblasts; the extent of the toxicities has not been fully described" (p. 41).
The NPUAP (2017) guidelines suggest the use of medical-grade honey-impregnated dressings in the treatment of Stage II and III pressure injuries. This treatment compares to alginates, hydrocolloids, silver, and advanced topical treatments (NPUAP, 2017).
Healthcare providers may use cadexomer iodine dressings for excessive wound drainage. However, these should be used with extreme caution and avoided altogether in individuals with renal failure, thyroid disorders, or known iodine sensitivity. Women who are breastfeeding or pregnant should not use cadexomer iodine dressings. The risk of systemic absorption is high (NPUAP, 2017).
Gauze dressings have been a staple in wound care for decades, but in the current guidelines they have limited use. Gauze dressings can cause further tissue damage in open pressure injuries, particularly if they’re removed dry. The 2017 NPUAP guideline offers a caution to avoid the use of wet-to-dry dressings in pressure injuries. Use gauze dressings when other forms of moisture-retentive dressings are not available but maintain moistness while on the wound. Further, the gauze dressing can be utilized as cover dressings or for loosely filling in deep pressure wounds as packing. Only use saline-moistened gauze when other forms of moisture-retentive dressings are not available. Multiple layers of gauze should not be used to control drainage as they can serve as a source of infection. Several studies have shown that other forms of dressings have higher success rates with wound healing, and the use of gauze dressings can promote infection or delayed wound healing (NPUAP, 2017).
Silicone dressings are used as a wound contact layer to promote atraumatic dressing changes or to prevent periwound tissue injury around fragile tissue as they will not chemically interact with the wound and are easy to remove (NPUAP, 2017).
Incorporate collagen matrix dressings as a treatment for Stage III and IV pressure injuries. The application of collagen has shown to promote wound healing in at least one study, but the cost of the product is high (NPUAP, 2017).
Finally, the guidelines mention composite dressings that contain various types of dressings within one product. There is no significant data that supports the use of composite products due to cost; however, the ease of use can be a positive aspect for the patient and caregiver (NPUAP, 2017).
Other treatment options
Biophysical agents can be used to deliver treatment substances to the wound bed, including oxygen therapy delivered via hyperbaric pressure. Electromagnetic spectrum (EMS) is an energy source that impacts living tissue. Thermal radiation, ultraviolet light, laser, or electrical stimulation could improve healing in pressure injuries. Healthcare providers may consider EMS for Stage II through Stage IV pressure injuries. Use caution with EMS for patients with fever, bleeding, seizures, or dehydration. Pregnancy, pacemakers, or organ transplants are contraindications for this type of therapy (NPUAP, 2017).
Other alternative therapies may include laser, infrared, or ultraviolet phototherapy to treat pressure injuries. These are not routine treatments and are typically added to other treatment modalities to decrease healing time. Bacterial burden reduces in Stage III and IV wounds with the addition of ultraviolet light as adjunctive therapy (NPUAP, 2017).
Acoustic energy or ultrasound has been utilized to assist with wound debridement. Ultrasonic spray is not recommended as a routine treatment. Avoid the use of ultrasonic spray near prostheses, electronic implanted devices such as pacemakers, over the lower back or uterus in pregnant women or on the hands or head/face. Low-frequency ultrasound can be used for debridement of soft necrotic tissue and as an adjunct treatment where an infection is present (NPUAP, 2017).
Negative pressure wound therapy is a method of applying suction or negative pressure to the wound bed. This treatment promotes healing of the wound by removing excess drainage, stimulating vascularization, and supporting the closure of wound edges or margins. Negative pressure is applied to create mechanical stress that promotes growth factor expressions, angiogenesis (formation of new blood vessels), and granulation tissue growth. The negative pressure helps to open the capillary beds and draw blood to the area of the wound. Additionally, the negative pressure aids in healing by reducing edema and bacterial colonization and providing a moist wound bed that promotes healing. Healthcare providers use negative pressure wound with Stage III or IV pressure injuries. It may also be used to prepare a wound bed for skin grafts. An adverse reaction of negative pressure wound therapy is pain, and the nurse should manage the pain prophylactically. Do not use this treatment in individuals with a risk of bleeding as life-threatening hemorrhage could result (Kaur, Midha, Giri & Mohanty, 2019; NPUAP, 2017).
Hyperbaric oxygen therapy (HBOT) may be used to promote wound healing, but due to a lack of evidence, it is not recommended for routine use. Wounds that are not healing are typically hypoxic and increasing oxygen tension or pressure by various methods can stimulate healing. HBOT promotes muscle and nerve regeneration by stimulating angiogenesis (Kaur et al., 2019; NPUAP, 2017).
Hyperbaric oxygen therapy treatments are typically performed on limbs placed in a limb-encasing device or a full-body chamber. Contraindications for HBOT are asthma, claustrophobia, COPD, eustachian tube dysfunction, pacemaker, high fever, epidural pain pump, pregnancy, seizures, and upper respiratory infections. Absolute contraindications would include a pneumothorax, severe respiratory disease, recent chemotherapy, and other drugs including disulfiram (Antabuse) or mafenide (Sulfamylon) (Kaur et al., 2019; NPUAP, 2017).
Pain control
Pain is an expected side effect of pressure injuries, and many patients report the pain they endure during wound care is as painful as the initial injury (Chester et al., 2016). Depending on the severity and location of the wound, the pain may or may not be a significant problem. Factors that increase pain are depth of the wound, structures involved, infection, and other injuries or conditions that co-exist with the wound. The nurse should complete a pain assessment to determine all aspects of the pain, including location, exacerbating and relieving factors, quality of pain, and severity based on a numerical pain scale. It is important to pre-medicate the patient prior to debridement or other wound care that may stimulate pain. Ensure that medication is given according to the expected onset of action and duration to ensure coverage during dressing changes or procedures such as debridement.
Administration of pain medications is important along with other considerations that may decrease the intensity and length of pain:
- Distractions may help to decrease pain including watching television, playing games, or reading materials.
- The nurse should space out care to give the patient uninterrupted rest time in a quiet environment.
- Reduce anxiety levels with music therapy or other guided imagery techniques.
- The nurse should help position the patient for comfort during any procedures or dressing changes.
- The nurse should limit the number of dressing changes where possible, leaving the dressings in place for longer periods whenever possible.
- The nurse should help determine dressing types that avoid adherence to the wound bed or tapes and adhesives that pull at the wound.
- Allow the patient to choose times for treatments or dressing changes to create a feeling of independence and decrease anxiety.
- Consider alternative therapies such as hypnosis to reduce pain (Chester et al., 2016).
While pain medications including opioids may be used during dressing changes and for wound debridement, careful consideration of the adverse risk for addiction should be openly discussed with the patient and healthcare team to determine the best options for positive outcomes (Chester et al., 2016).
Changing the outcomes: Reducing pressure injuries
Severe pressure injuries are considered "never events," which is a term describing a critical medical error that should never happen. Other injuries that fall into this category are wrong-site surgeries, medication errors, or those that result in serious injury that could lead to death or substantial disability (AHRQ, 2019).
Various interventions have been discussed in the literature to reduce the number of HAPI’s in today’s clinical setting, including intervention bundles. A bundle intervention is a “structured way of improving the processes of care and patient outcomes; a small, straightforward set of evidence-based practices-generally three to five-that, when performed collectively and reliably, have been proven to improve patient outcomes” (Institute for Healthcare Improvement [IHI], 2019). An example of a bundled intervention developed for patients at risk for pressure injuries is called the SKIN bundle. After recognizing the threat, the nurse or healthcare team implements treatment such as the SKIN bundle, which is a series of interventions to be implemented. The acronym “SKIN” stands for Surface selection, Keep turning patients, Incontinence management, and Nutrition. By applying the SKIN bundle, the bedside nurse is given the responsibility for pressure injury prevention, making them the first line of defense. Interventions such as the SKIN bundle help bring the focus on everyone caring for the patient as a team to decrease the risk and prevent injuries from occurring (IHI, 2019).
Regardless of the acronym, aspects of an intervention bundle or policies and procedures of the facility, those responsible for the patient’s care should work to utilize the tools available for assessment and prevention. Nurses should be familiar with the latest guidelines for pressure injury prevention and work to implement evidence-based care for their patients (IHI, 2019). Most agree that a multi-pronged approach to pressure injury prevention is preferred. Organizations typically adopt multiple strategies to decrease the risk from medical devices and the risk of pressure injuries during hospitalization and a recognition of each person’s role to promote patient safety. The IHI (2019) further identifies six key steps that aid in healthcare facilities meeting their goals to prevent pressure injuries, which duplicates the guidelines from other national organizations, including NPUAP (2017). The steps are:
- Conduct a pressure ulcer assessment for all patients.
- Use a Braden scale or other approved tool to measure risk.
- Reassess risk for all patients daily.
- Inspect the skin of at-risk patients daily.
- Manage moisture, including incontinence.
- Optimize nutrition/hydration.
- Minimize pressure (IHI, 2019).
Individual organizations must determine the methods that work best for them based on the guidelines and statistical data comprised of their patient population data. Budget, workforce, and resources in addition to national guidelines drive the policies and methods for patient care (NPUAP, 2017).
Future opportunities
Future opportunities to promote healing in pressure injuries include but are not limited to biological dressings comprised of animal materials, human skin cells, plant materials, synthetic materials, or a mixture of any of these. Examples are skin substitutes, xenografts, allografts, or collagen dressings. At this time, there is not sufficient evidence to support their use. Blood is drawn from the patient and centrifuged to create platelet-rich plasma (PRP) and then administered to the wound. PRP may present opportunities for escalated wound healing. Current investigations for the healing of pressure injuries include recombinant platelet-derived growth factor, basic fibroblast growth factor, granulocyte-macrophage colony-stimulating factor, or bone marrow nuclear cells. Stage III and IV pressure wounds with delayed wound healing may benefit from a recombinant platelet-derived growth factor. These treatments are in early stages of use and not first line treatments. For chronic wounds that have delayed healing, these treatments demonstrate opportunities for improvement or complete healing (NPUAP, 2017).
Summary
Pressure injuries are costly to both the organization and patient suffering. Litigation is at an all-time high for pressure injuries, and care for these patients is not reimbursable (IHI, 2019). Education for nurses, doctors, and the entire healthcare team is needed to ensure that all members are aware of the risk, appropriate intervention, and the overall cost to the organization. It is easy to provide evidence to support even costly interventions when compared to the cost of even one pressure injury. Nurses must be advocates that promote appropriate staffing, preventative devices, and other tools that may be needed to deliver safe and effective care to decrease the risk of pressure injuries (NPUAP, 2017).
References
Agency for Healthcare Research and Quality Patient Safety Network. (2017a). National patient safety goals effective January 2017. Retrieved from https://www.jointcommission.org/assets/1/6/NPSG_Chapter_NCC_Jan2017.pdf
Agency for Healthcare Research and Quality Patient Safety Network. (2017b). Pressure injury prevention in hospitals training program. Retrieved from https://www.ahrq.gov/professionals/systems/hospital/pressureinjurypxtraining/index.html
Agency for Healthcare Research and Quality Patient Safety Network. (2019). Never events. Retrieved from https://psnet.ahrq.gov/primers/primer/3
Black, J. & Maegley, J. (2019). Help-u to prevent HAPI: A change project to attain zero HAPIs. MEDSURG Nursing, 28(1), 31-47.
Braden, B. & Bergstrom, N. (1988). Braden Scale for Predicting Pressure Sore Risk. Retrieved from http://www.bradenscale.com/images/bradenscale.pdf
California Hospital Patient Safety Organization (2017). By the numbers: CHPSO pressure injury data. Retrieved from https://www.chpso.org/post/numbers-chpso-pressure-injury-data
Chester, S. J., Stockton, K., De Young, A., Kipping, B., Tyack, Z., Griffin, B., … Kimble, R. M. (2016). Effectiveness of medical hypnosis for pain reduction and faster wound healing in pediatric acute burn injury: study protocol for a randomized controlled trial. Trials, 17(1), 223. doi:10.1186/s13063-016-1346-9.
Delmore, B.A., & Ayello, E. A. (2017). Pressure injuries caused by medical devices and other objects: A clinical update. AJN, 117(12) 36-45. doi: 10.1097/01.NAJ.0000527460.93222.31
Emergency Care Research Institute (2014). Medical devices’ role in causing pressure ulcers. Retrieved from https://www.ecri.org/components/PSOCore/Pages/PSONav0814.aspx?tab=2#Lessons
Health Research and Educational Trust (2017). Pressure Ulcers. Retrieved from http://www.hret-hiin.org/topics/pressure-ulcers.shtml
Institute for Healthcare Improvement (2019). What is a bundle? Retrieved from http://www.ihi.org/resources/Pages/ImprovementStories/WhatIsaBundle.aspx
Jackson, D., Sarki, A.M., Betteridge, R., & Brooke, J. (2019). Medical device-related pressure ulcers: a systematic review and meta-analysis. International Journal of Nursing Studies, 92, 109-120.
Kaur, A., Midha, S., Giri, S., & Mohanty, S. (2019). Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells International, 1–20. doi:10.1155/2019/1286054.
Kirman, C., Geibel, J., Kolaski, K. (2018). Pressure injuries (pressure ulcers) and wound care. Retrieved from https://emedicine.medscape.com/article/190115-overview#a1
National Database of Nursing Quality Indicators (2018). Admission skin and pressure injury risk assessment. Retrieved from https://members.nursingquality.org/ndnqipressureulcertraining/Module3/PressureULcerSurveyGuide_7.aspx
National Pressure Ulcer Advisory Panel (2014). Prevention and treatment of pressure ulcers: Medical device related pressure ulcers-an extract from the clinical practice guideline. Retrieved from http://www.internationalguideline.com/static/pdfs/04-NPUAP-EPUAP-PPPIA%20MDRPU%20Extract%20of%20the%20CPG%202017.pdf
National Pressure Ulcer Advisory Panel (2016a). National Pressure Ulcer Advisory Panel (NPUAP) announces a change in terminology from pressure ulcer to pressure injury and updates the stages of pressure injury [news release]. Retrieved from http://www.npuap.org/national-pressure-ulcer-advisory-panel-npuap-announces-a-change-in-terminology-from-pressure-ulcer-topressure-injury-and-updates-the-stages-of-pressure-injury.
National Pressure Ulcer Advisory Panel (2016b). NPUAP Pressure Injury Stages. Retrieved from https://cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/npuap_pressure_injury_stages.pdf
National Pressure Ulcer Advisory Panel (2017). Prevention and treatment of pressure ulcers: Clinical practice guideline. Retrieved from http://www.internationalguideline.com/static/pdfs/NPUAP-EPUAP-PPPIA-CPG-2017.pdf
Padula, W.V., Pronovost, P.J., Makic, M.B.F., Wald, H.L., Moran, D., Mishra, M.K., & Meltzer, D. (2019). Value of hospital resources for effective pressure injury prevention: A cost-effectiveness analysis. British Medical Journal of Quality and Safety, 28(2), 132-141. DOI: 10.1136/bmjqs-2017-007505
Peate, I. & Glencross, W. (2015). Wound Care at a Glance. Malden, MA: Wiley-Blackwell.
Rondinelli, J., Zuniga, S. Kipnis, P., Kawar, L., Liu, V., & Escobar, G., (2019). Hospital-acquired pressure injury: Rick-adjusted comparisons in an integrated healthcare delivery system. Nursing Research. 67(1):16–25. DOI: 10.1097/NNR.0000000000000258.
Seniors Health Knowledge Network (n.d.) The Norton pressure sore risk assessment scale scoring system. Retrieved from https://shrtn.on.ca/sites/default/files/clinical-resources/Norton_presure_sore_risk_assessment_scale.pdf
Smith, N. R., & Caple, C. R. (2019). Wound Dressings: Hydrocolloid -- Applying. CINAHL Nursing Guide. Cinahl Information Systems, a division of EBSCO Information Systems.