About this course:
The purpose of this two-part educational series is to enhance clinical practice and improve patient outcomes by educating advanced practice registered nurses (APRNs) on the various types of diagnostic radiology imaging tests, ensuring satisfactory understanding of the appropriate indications for ordering each exam, as well as the risks, benefits, and important clinical considerations regarding the use of contrast media.
Course preview
This is a two-part educational series on diagnostic radiology. Part I will focus on the diagnostic imaging process, the components of imaging quality, a detailed account of several types of contrast agents, with regards to their clinical considerations, risks, monitoring parameters, and regimens for managing contrast allergies. Part II will proceed with an overview of radiation safety as pertaining to diagnostic imaging, radioactive contrast agents used in nuclear medicine, the various modalities of diagnostic radiology testing, including their indications for use and an overview of the appropriate use criteria, essential components of patient education, as well as contraindications to testing.
By the completion of this module, the APRN should be able to:
- Describe the background of radiology, outline the components of the medical imaging process, and characteristics that impact image quality.
- Review the different types of contrast media agents, with regards to their clinical considerations, including risks, contraindications, and monitoring parameters.
- Outline the signs and symptoms of allergic reactions to iodinated contrast, as well as the management and premedication regimens as guided by the American College of Radiology.
Diagnostic radiology refers to a sector of medicine that includes various kinds of medical imaging technologies widely utilized throughout the US healthcare system, with millions of patients undergoing imaging evaluations on a daily basis. These are non-invasive and minimally invasive medical procedures used to view the inside of the body as a means to assess, diagnose, monitor, and treat a wide array of medical conditions. Several of these tests also serve as the backbone of preventative medicine and routine heath maintenance, including cancer prevention, screening, and early detection. While diagnostic radiology is a valuable and critical component to proper diagnosis of numerous medical conditions, they are not without risks. Many of these tests are linked to potential harms that must be considered prior to ordering the test, as well as a high cost burden to patients and society. To promote patient safety and safeguard optimal outcomes, clinicians have an obligation to ascertain an appropriate understanding of each type of imaging modality, as well as a keen awareness of the clinical risks, benefits, and management of adverse events (Elsayes & Oldham, 2014).
Radiology is considered one of the most technologically advanced fields in medicine, dating back to 1895 when Wilhelm Conrad Rontgen first discovered the x-ray, using his wife as his patient. Subsequently, Henri Becquerel discovered radioactivity in 1896, followed by the discovery of radium in 1898 by Marie and Pierre Curie. The field has expanded exponentially over the last few centuries and continues to rely on the collaboration of scientists, medical physicists, radiologists, and imaging technologists. Medical physicists ensure the safe and optimal use of radiological imaging modalities in patients. Diagnostic radiologists are physicians who undergo specialized training in the analysis and interpretation of medical imaging to draw conclusions useful for the diagnosing, treating, and managing of acute and chronic medical conditions and injuries. Interventional radiologists are physicians who undergo specialized training in the use of medical imaging to guide minimally invasive surgical procedures that diagnose, treat, and cure many kinds of conditions. Radiology technologists, also referred to as radiographers, have specialized training in performing digital imaging procedures under the direct supervision of a nurse or physician. Radiographers are skilled in optimally positioning patients for imaging procedures to ensure the images obtained are accurate and of the highest quality, and they also serve important roles in patient education (Elsayes & Oldham, 2014).
Imaging Process
According to the International Atomic Energy Agency (IAEA), a radiograph, or medical image "is a pictorial representation of a measurement of an object or function of the body" (IAEA, 2014, p. 55). The overall objective of medical imaging is to make a particular area within the patient's body visible. Radiographs are created by sending an x-ray beam, which is generated by an x-ray tube, through a patient. As described in the most simplistic form, the image produced represents a shadow of the underlying structures that the x-ray beam passes through. There are five core components of the medical imaging process, which include the patient, the imaging system, the system operator (the radiographer), the resultant image, and the observer (the radiologist). Each medical imaging method is devised to reveal distinctive characteristics of the body, and the variability in imaging quality and visibility of structures can differ considerably. Some of the most common factors contributing to the inconsistencies in the resulting images include the quality and characteristics of the imaging equipment, the skill of the operator (including positioning and placement of the patient), specific patient characteristics (such as body habitus, prior surgeries, presence of scar tissue, or prior radiation therapy), as well as the timing of the imaging in relation to the injury or medical condition in question (IAEA, 2014).
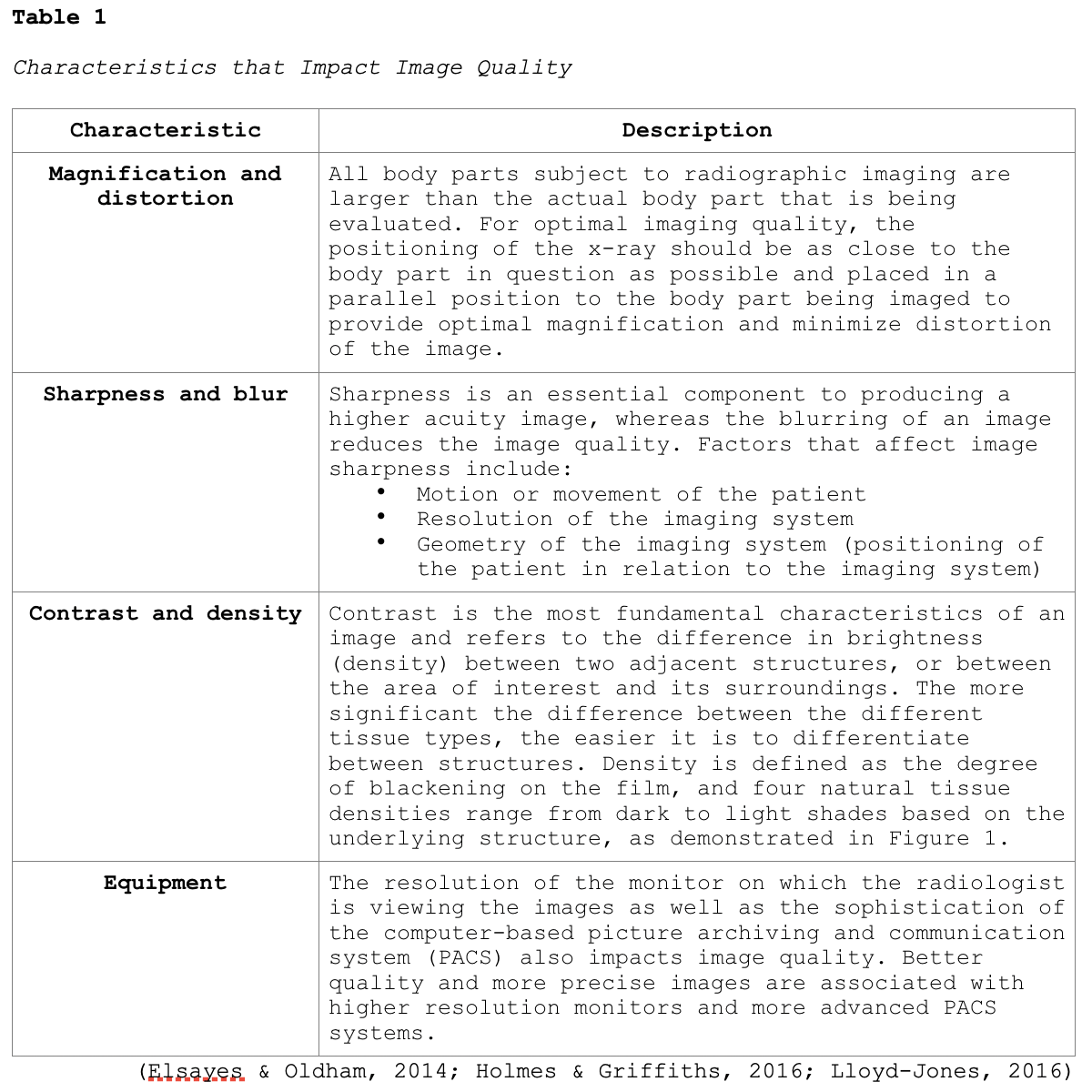
Imaging Quality
Obtaining high-quality images that are of optimal clarity is critical to draw precise conclusions. High-quality images enable radiologists to accurately visualize the structures of the body, evaluate the underlying injury or medical condition, and make accurate diagnoses. Poor quality images hinder the ability to accurately assess and evaluate the condition, leading to inconclusive or wrongful diagnoses. This often leads to the need for additional imaging tests, increasing the risks to the patient, contributing to delays in the diagnosis and treatment of the condition, and also adding to the high-cost burden to patients and society (Elsayes & Oldham, 2014).
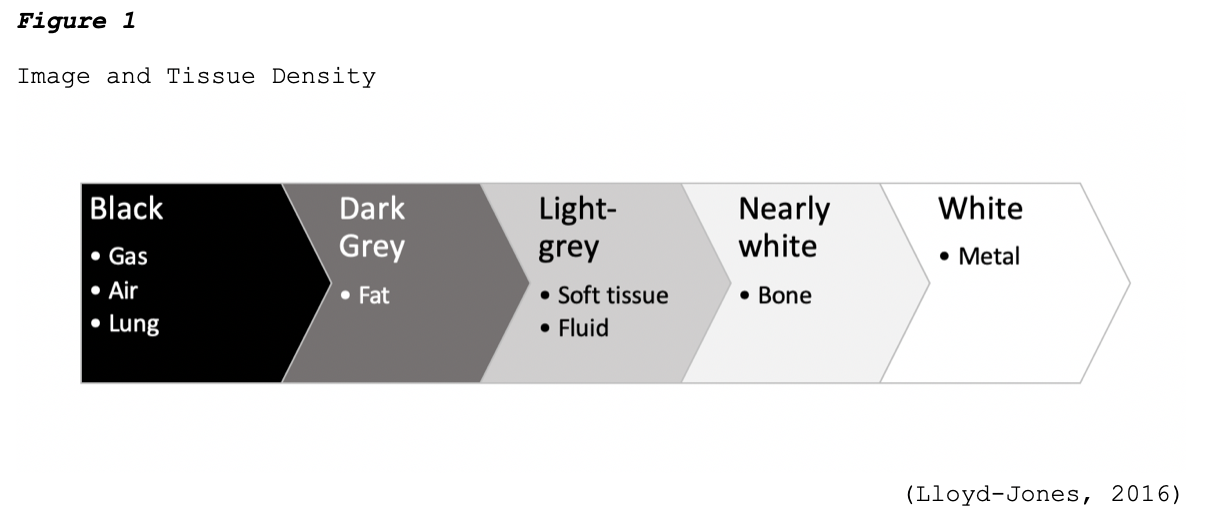
One key aspect of imaging quality is the signal-to-noise ratio, where signal refers to the information obtained from the part of the body being imaged, and noise refers to anything that hinders the ability to access this information. Higher quality radiographic images have high signal levels when compared to noise, which allows for structures within the body to be seen clearly. Images that are of low quality have a poor signal-to-noise ratio, or in other words, the signal level is similar to or less than the noise level, which causes the structures to become obliterated. Imaging artifact is a commonly reported contributor to poor imaging quality. An artifact is a visual anomaly or any feature which appears in an image that is not present within the original imaged portion of the body. Artifacts are a misrepresentation of structures that can obscure underlying structures and simulate pathology (Holmes & Griffiths, 2016). Additional characteristics that impact the quality of medical images are highlighted in Table 1.
Contrast Media in Medical Imaging
For various types of diagnostic radiology tests, substances called contrast media are administered to temporarily cha
...purchase below to continue the course

Contrast agents can be administered orally, intravenously (IV), intra-arterially (IA), or instilled directly into body cavities such as the urinary tract or gastrointestinal (GI) tract, including rectally through an enema. Less commonly, contrast can also be administered intrathecally (Spampinato et al., 2017). While contrast agents are used to enhance medical images, and their value is widely recognized, they are still considered pharmaceutical agents and are not without risks. Each type of contrast agent has its unique properties, side effect profile, risks, and are subject to specific monitoring and clinical decision-making with regards to individual patient factors. Adverse effects from contrast media agents can range from minor side effects to life-threatening clinical emergencies. Therefore, clinicians must be well versed on the potential risks these agents pose before prescribing them and ascertain a keen awareness of the signs of impending adverse reactions as a means to respond promptly and effectively to reduce morbidity and mortality (ACR, 2020).
Iodinated Contrast Agents
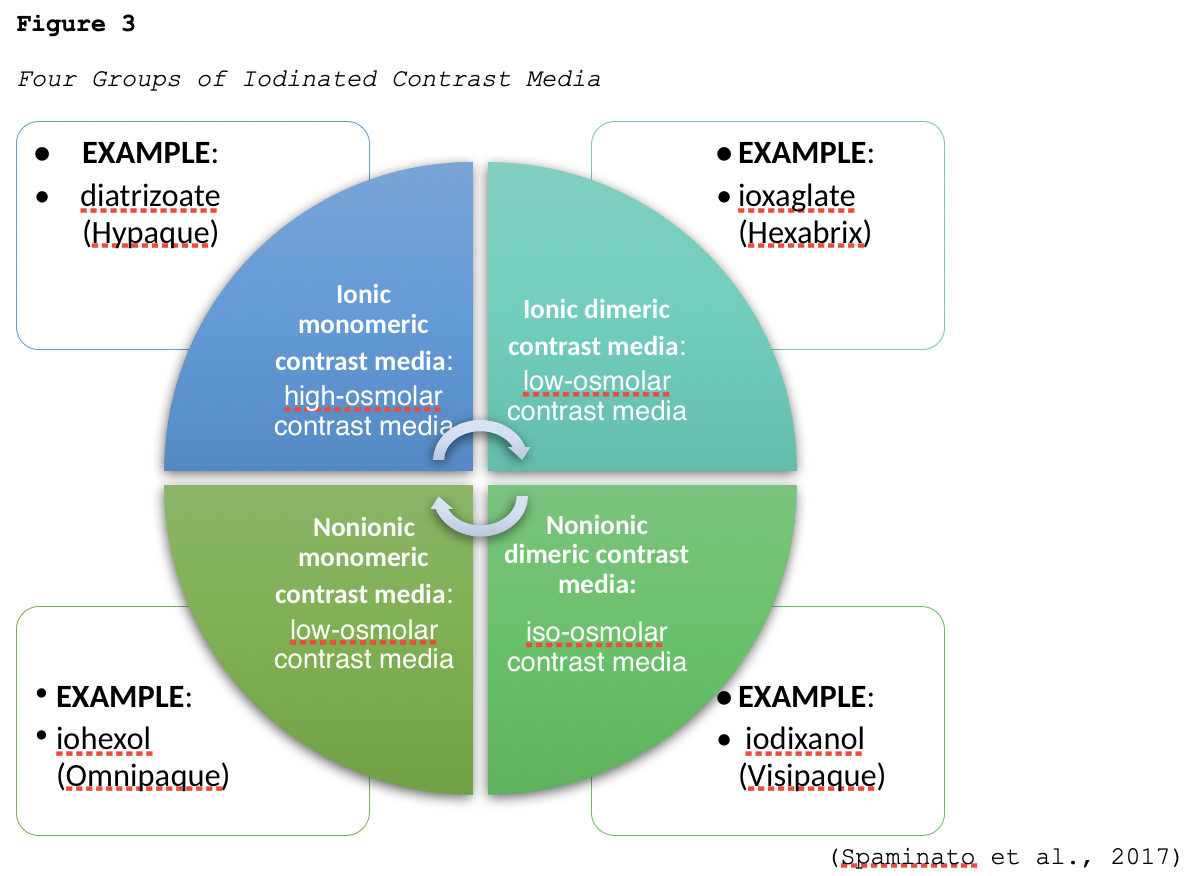
Iodine is a naturally occurring chemical element that is found in many types of contrast agents. Iodine-based or iodinated contrast agents (ICAs) enhance x-ray and CT images and are most commonly administered by injection through veins, arteries, or other body cavities. ICAs are the most widely utilized category of contrast agents, with millions of doses of ICAs administered each year in the US. Dating back to their initial development in the 1920s, their uses have expanded significantly over the last several decades, as well as their composition and chemical structuring (Spampinato et al., 2017). There are a few different types of ICAs available, and they are grouped according to their osmolarity (high, low, or iso-), ionicity (ionic or nonionic), and the number of benzene rings (monomer or dimer), as demonstrated in Figure 2 (ACR, 2020).

Ionic compounds dissociate (dissolve) into positively and negatively charged particles when entering a solution, whereas nonionic compounds do not dissociate. Osmolality is the concentration of particles dissolved in a fluid where higher osmolality denotes that there are more particles in the serum, and lower osmolality means the serum is more diluted and contains fewer particles. Osmolarity is the concentration of a solution expressed as the total number of solute particles per liter. The main difference between osmolarity and osmolality is that osmolarity is a measure calculated considering the volume of a solution whereas osmolality is calculated considering the mass of a solution. These concepts are essential to understand with regards to contrast agents, as the osmolality of the agent affects the incidence of side effects. Initial ICAs were ionic compounds with high osmolality. Still, they had toxic profiles and were accompanied by a high rate of allergic reactions in patients who received these high-osmolality agents, which led to the development of safer, nonionic, and lower osmolality ICAs. In current practice, nonionic low or iso-osmolar preparations are considered the gold standard for intravenous contrast injections and are used nearly exclusively. Nonionic contrast media cause less discomfort (burning) with intravascular administration and are associated with fewer adverse reactions when compared with ionic agents. Further, low-osmolar contrast is associated with significantly lower rates of acute allergic and adverse reactions when compared to high-osmolar agents. The four classes of iodinated contrast media are grouped according to ionization, and an example of each type is listed in Figure 3.
While the currently utilized nonionic and low-osmolar or iso-osmolar ICAs are considered safe and effective when administered correctly, there remain important risks and clinical considerations that must be contemplated before prescribing. Clinicians must balance the potential risks of contrast media with the diagnostic benefits, which is premised on a multitude of factors, including:
- the probability and necessity of an accurate diagnosis;
- alternative methods of diagnosis;
- risk of misdiagnosis;
- expectations about kidney function recovery;
- allergic reaction risk and risk for other adverse reactions (Davenport et al., 2020).
One of the most concerning and well-established risks associated with ICAs is contrast-induced nephropathy (CIN) in patients with impaired renal function. This condition is characterized by an acute kidney injury (AKI), or an acute worsening in renal function within 48 hours following intravenous contrast administration. The ACR and National Kidney Foundation (NKF) published a consensus statement on the use of intravenous contrast media in patients with kidney disease. The statement reveals that the actual risk for contrast-induced AKI is uncertain primarily due to a lack of control groups sufficient to separate contrast-induced AKI (AKI caused by the contrast media) and contrast-associated AKI (AKI coincident to contrast media administration). The consensus statement discloses concerns that the risk for contrast-induced AKI has been overstated and does not apply to all populations; however, it offers specific recommendations based on risk factors and the staging of the patient's underlying renal disease. Table 3 displays the Kidney Disease Improving Global Outcomes (KDIGO) staging criteria for AKI and chronic kidney disease (CKD) to be used for clinical decision-making regarding the use of intravenous ICAs (Davenport et al., 2020).

Following a thorough review of several large, well-controlled research studies, the ACR and NKF report the following findings:
- Contrast-associated AKI
- The risk for contrast-associated AKI increases with each stepwise increase in CKD stage with risk percentages as follows:
- 5% risk with eGFR ≥ 60;
- 10% risk with eGFR 45-59;
- 15% risk with eGFR 30-44;
- 30% risk with eGFR less than 30 mL/min/1.73 m2 ;
- Risk factors for contrast-associated AKI include: eGFR, diabetes mellitus, exposure to other nephrotoxic agents, hypotension, hypovolemia, congestive heart failure, and impaired kidney perfusion;
- There was no clinically significant difference in the risk of contrast-associated AKI between types of contrast administered;
- The risk for contrast-associated AKI increases with each stepwise increase in CKD stage with risk percentages as follows:
- Contrast-induced AKI
- The risk of contrast-induced AKI is cited as substantially less than contrast-associated AKI. Still, the authors caution that the actual risk remains undetermined in those patients with severe kidney disease. Several large controlled observational studies have demonstrated no evidence of contrast-induced AKI regardless of the CKD stage.
- The risk percentages of contrast-induced AKI are as follows:
- 0% risk with eGFR ≥ 45;
- 0-2% risk with eGFR 30-44;
- 0-17% risk with eGFR less than 30 mL/min/1.73 m2
- There are limited studies linking patient-related factors to contrast-induced AKI, and the primary risk factor was found to be eGFR, without any other risk factors confirmed;
- No studies have directly compared the risk for contrast-induced AKI between the various types of contrast. Still, there is no clinically significant difference in risk based on the type of contrast use (Davenport et al., 2020).
Table 4 summarizes some of the key recommendations for clinicians, as put forth in the consensus statement for the use of intravenous ICA in patients with CKD, as well as those at risk for AKI (Davenport et al., 2020).

Patients with renal disease are not the only ones who are at risk for complications from ICAs. Patients with type II diabetes, or non-insulin-dependent diabetes mellitus, who are taking metformin (Glucophage), an oral anti-hyperglycemic agent, as well as other biguanides, are at risk for a significant adverse reaction known as lactic acidosis. Lactic acidosis is characterized by a buildup of lactic acid in the bloodstream (greater than 2 mmol/L), which can lead to the development of severe symptoms including extreme exhaustion, muscle cramps, body weakness, abdominal pain, diarrhea, headache, jaundice, and breathing changes. Since the kidneys are primarily responsible for excreting metformin (Glucophage), patients who are taking this medication who have underlying renal insufficiency are at heightened risk for this severe complication. Therefore, the US Food & Drug Administration (FDA, 2016), recommends the discontinuation of metformin (Glucophage) and metformin-containing products, 24 hours prior to ICA administration in the following patient situations:
- In patients with an eGFR between 30 and 60 mL/minute/1.73 m2;
- In patients with a history of liver disease, alcoholism, or heart failure;
- In patients who will be administered intra-arterial ICA (FDA, 2016).
According to the ACR (2020), patients taking metformin (Glucophage) who have no evidence of AKI and have an eGFR of >/=30 mL/min/1.73 m2 do not need to discontinue metformin (Glucophage) before or after ICA administration. There is no obligatory need to re-evaluate the patient's renal function following the test or procedure. However, for patients who are known to have AKI or severe CKD (stage 4 or 5), the metformin (Glucophage) should be temporarily discontinued at the time of or before the procedure. The FDA (2016) and ACR (2020) similarly concur that for patients who meet the criteria to withhold the metformin (Glucophage), their renal function (eGFR) must be reassessed 48 hours after the imaging procedure and before resuming the metformin (Glucophage). Further, patients with severe cardiac disease, such as those with symptomatic congestive heart failure, angina, pulmonary hypertension, cardiac arrhythmias, or severe but compensated cardiomyopathy, may also be at risk of a cardiac event. While the ACR (2020) determines that these patients are at modestly increased risk, they do not advise restricting ICAs solely based on the patient's cardiac status. However, the risks and benefits should be considered individually, and the ACR clarifies several common misconceptions regarding the use of ICAs in the following patient groups:
- Beta-blockers: Patients on beta-blockers do not need to discontinue their medication(s) before contrast administration;
- Sickle-cell trait/disease: Contrast should not be restricted in patients with sickle-cell trait/disease, as there is no evidence that iodinated contrast increases the risk for acute sickle crisis;
- Pheochromocytoma: There is no evidence that contrast use increases the risk of hypertensive crisis in patients with pheochromocytoma;
- Myasthenia gravis: There is a questionable relationship between the use of iodinated contrast and exacerbations of myasthenia gravis due to limited studies in a small number of patients. It remains controversial whether iodinated contrast should be considered a relative contraindication in these patients;
- Hyperthyroidism: Patients with hyperthyroidism can develop thyrotoxicosis following exposure to iodinated contrast, although it is a rare complication. The ACR (2020) does not recommend the restriction of contrast medium solely based on a history of hyperthyroidism, however, makes the following recommendations for two particular circumstances:
- "In patients with acute thyroid storm, iodinated contrast medium exposure can potentiate thyrotoxicosis; in such patients, iodinated contrast medium should be avoided. Corticosteroid premedication in this setting is unlikely to be helpful;
- In patients considering radioactive iodine therapy or in patients undergoing radioactive iodine imaging of the thyroid gland, administration of iodinated contrast medium can interfere with uptake of the treatment and diagnostic dose. If iodinated contrast medium was administered, a washout period is suggested to minimize this interaction. The washout period is ideally 3-4 weeks for patients with hyperthyroidism, and 6 weeks for patients with hypothyroidism" (ACR, 2020, p.7).
Allergic Reactions
ICAs pose a risk for allergic reactions, which can have variable presentations ranging from mild to severe, and delayed, as outlined in Table 5. Patients can also have an allergic reaction at initial exposure to the ICA, mediated by anaphylaxis mechanisms, in which symptoms develop within minutes of exposure. Anaphylactic reactions are usually unpredictable but are the most clinically significant reactions to ICAs, as they involve the release of histamine and other biologic mediators. Most reactions occur within the first 5 to 60 minutes following ICA administration. However, delayed reactions can occur between 1 hour and 1 week following the injection. Delayed reactions are more common in young adults, women, and patients with a history of a contrast allergy, and they tend to be cutaneous in nature (Beckett et al., 2015).

While ICA reactions are sporadic and often unpredictable, there are some risk factors associated with an increased risk of reaction to ICA. The ACR (2020) advises that prior allergic-like or unknown type of reactions to the same class of contrast medium is considered the most significant risk factor for predicting future adverse events. Additional risk factors for ICA allergic-like reactions include the following:
- Patients with a prior allergic reaction (or an unknown-type of reaction) to an iodinated contrast agent are at an approximately 5-fold increased risk of developing a future allergic-like reaction with subsequent exposure to contrast;
- Patients with unrelated allergies are at a 2- to 3- fold increased risk for an allergic-like reaction;
- Patients with a history of asthma are at increased risk for allergy and may be more likely to develop bronchospasm, but those with well-controlled asthma may not be at increased risk;
- Patients with multiple allergies (such as dermatitis and urticaria) have at least a 3-fold increased risk for a severe reaction to contrast media;
- Patients with mild allergies (such as seasonal allergies) are at unclear risk (ACR, 2020; Beckett et al., 2020).
Despite popular misconception, it is now well-established and confirmed that there is no link between shellfish allergy and allergy to ICAs. Patients with shellfish or povidone-iodine (Betadine) allergies are at no higher risk from iodinated contrast than patients with other allergies. Furthermore, there is no cross-reactivity between the different classes of contrast; therefore, a patient with an ICA allergy is not at heightened risk for a gadolinium-based allergy (ACR, 2020; Beckett et al., 2015).
Treatment of Allergic Reactions
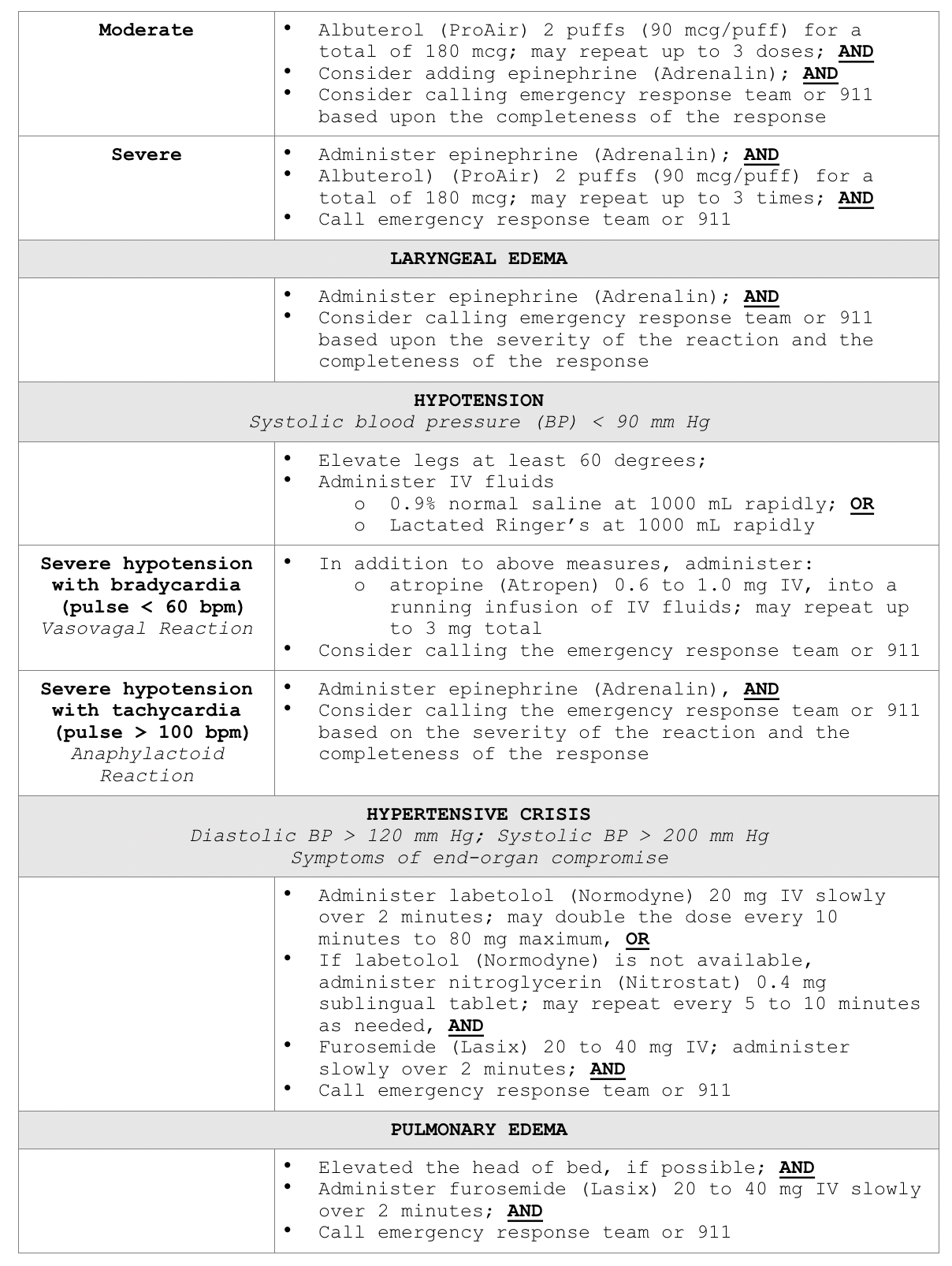
According to ACR (2020), all facilities in which ICAs are injected must be equipped with emergency supplies, and staff must be trained in basic life support in the event of a life-threatening allergic reaction. While the majority of allergic reactions are mild, self-limiting, and do not progress in severity, they can sometimes become more severe. Therefore, it is advised that any patient who is displaying signs of reaction should be observed for a minimum of 20 to 30 minutes, or longer if needed, to ensure clinical stability and recovery. Mild reactions may require symptomatic treatment, but often do not require any treatment at all. Moderate reactions are usually not life-threatening, and severe reactions are rare but can be potentially fatal. Severe reactions require prompt recognition and action by qualified healthcare providers to prevent morbidity and death (ACR, 2020; Beckett et al., 2015).
For all types of reactions, clinicians should ensure intravenous access is maintained, observe vital signs, and assess the patient's appearance. The ACR (2020), lists the following five immediate assessments that should be performed upon recognition of a potential allergic reaction:
• What is the patient's general appearance?
• Can the patient speak? How does their voice sound?
• What is the quality of the patient's breathing?
• What is the patient's pulse?
• What is the patient's blood pressure? (ACR, 2020).
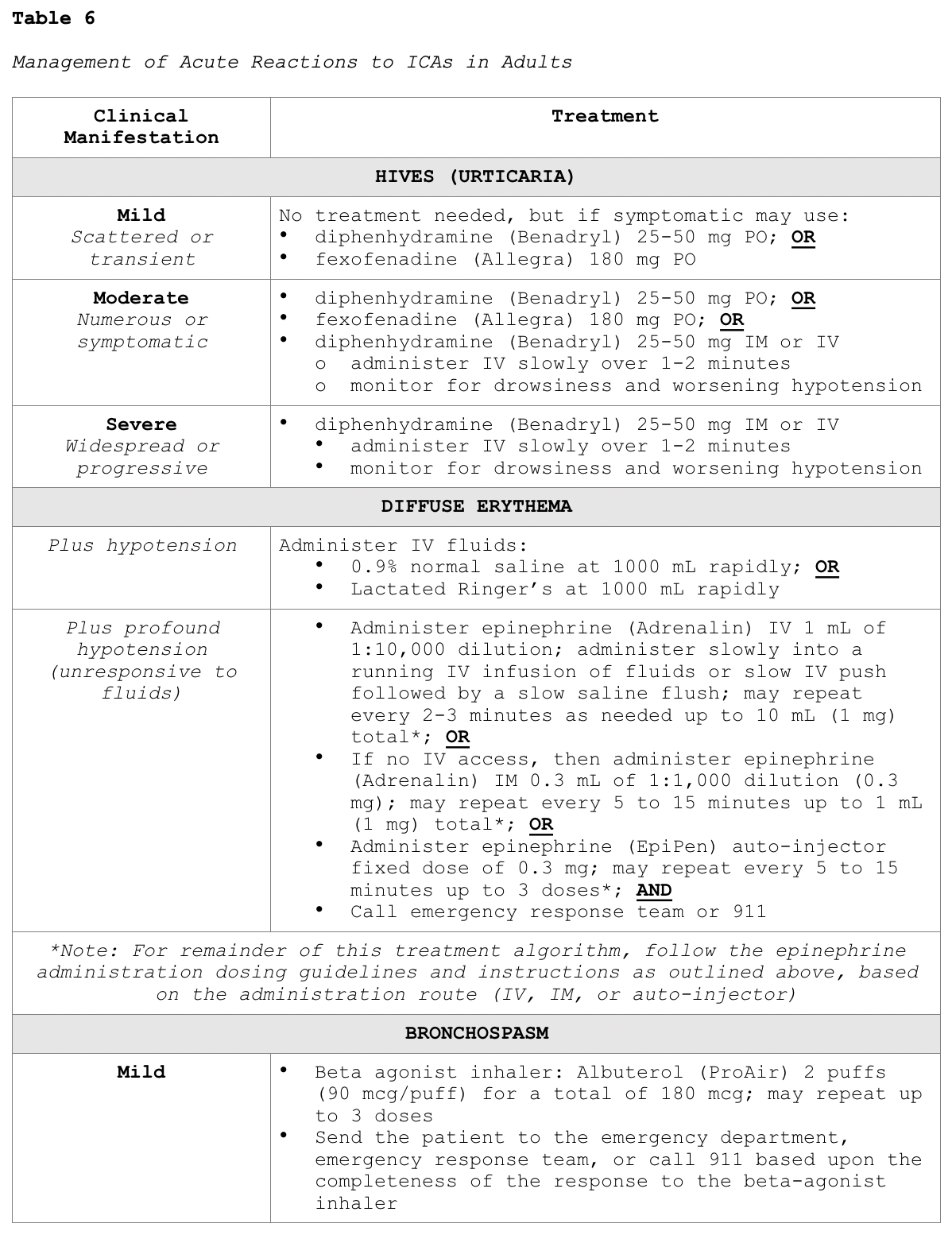
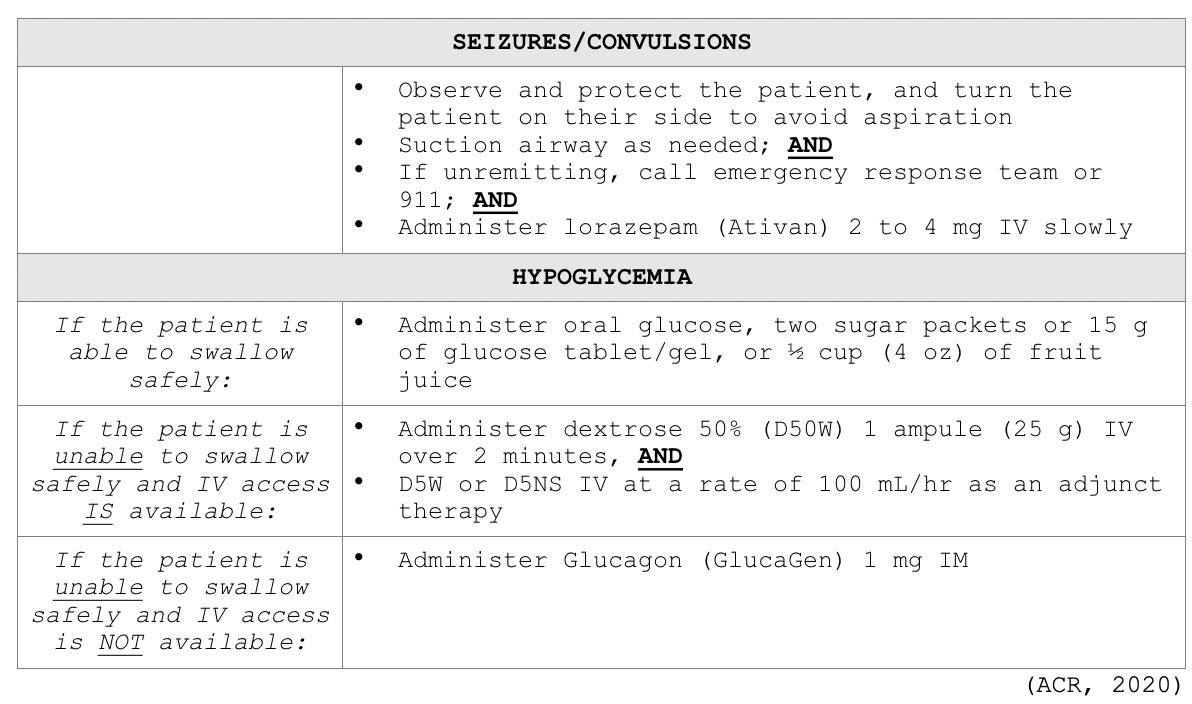
Clinicians responding to these patients are advised to prioritize a focused assessment, evaluate the patient's level of consciousness, the appearance of their skin, quality of phonation, lung auscultation, blood pressure, and heart rate. These key assessment points help quickly determine the severity of the reaction so that effective treatment can be promptly and successfully administered. In free-standing outpatient facilities, staff should be trained on how to activate emergency response systems and understand when to elevate the care, such as by calling 911. These facilities should also be equipped with necessary equipment including but not limited to oxygen, an automated external defibrillator (AED), suction equipment, and medications. In addition to preserving IV access, monitoring vital signs and delivering supplemental oxygen by mask as needed (at a rate of 6 to 10 L/min), Table 6 provides a concise account of the guidelines of additional interventions for managing various types of acute reactions to ICAs in adult patients (ACR, 2020).
Premedication Regimen for Patients with an ICA Allergy
While well-controlled studies and definitive data are not available on the efficacy of premedication in preventing an allergic reaction in high-risk patients, the ACR (2020) believes that premedication does reduce the likelihood of a reaction in high-risk patients receiving low-osmolality iodinated contrast medium. While there is no formal consensus on the exact premedication regimen, oral premedication is preferable to IV premedication due to lower cost, increased convenience, and greater evidence support in the literature. The majority of data exists on the use of oral methylprednisolone (Medrol Dosepak) or oral prednisone (Deltasone), with or without oral diphenhydramine (Benadryl). The ACR (2020) supports the use of the below premedication regimens in patients who are at high risk and at the discretion of the ordering clinicians.
Standard 12- or 13-hour premedication regimen:
- For use in outpatient, emergency department, and inpatient settings for patients at high risk in whom the use of medication is not anticipated to delay care decisions or treatment adversely.
- Regimen #1: prednisone (Deltasone) 50 mg PO at 13 hours, 8 hours, and 1 hour before contrast administration, plus diphenhydramine (Benadryl) 50 mg PO 1 hour before contrast administration, OR
- Regimen #2: methylprednisolone (Medrol Dosepak) 32 mg PO 12 hours and 2 hours before contrast administration, and diphenhydramine (Benadryl) 50 mg PO may be added as in regimen #1, OR
- Regimen #3: (for patients who are unable to take oral medication) hydrocortisone (Cortef) 200 mg IV at 13 hours, 8 hours, and 1 hour before contrast administration, plus diphenhydramine (Benadryl) 50 mg IV 1 hour before contrast administration (ACR, 2020).
Accelerated IV premedication regimen:
- For use in outpatient, emergency department, and inpatient settings for patients at high risk in whom the use of 12- or 13-hour premedication is anticipated to delay care or treatment adversely.
- Regimen #1: (usually 4-5 hours in duration) methylprednisolone sodium succinate (Solu-Medrol) 40 mg IV or hydrocortisone sodium (Solu-Cortef) 200 mg IV immediately, and then every 4 hours until contrast administration, plus diphenhydramine (Benadryl) 50 mg IV 1 hour before contrast administration.
- Regimen #2: (usually 4-5 hours in duration) dexamethasone sodium sulfate (Decadron) 7.5 mg IV immediately, and then every 4 hours until contrast administration, plus diphenhydramine (Benadryl) 50 mg IV 1 hour before contrast administration. This is the preferred regimen for patients with an allergy to methylprednisolone (Solu-Medrol).
- Regimen #3: methylprednisolone sodium succinate (Solu-Medrol) 40 mg IV or hydrocortisone sodium succinate (Solu-Cortef) 200 mg IV, plus diphenhydramine (Benadryl) 50 mg IV, each 1 hour before contrast administration. This regimen has no evidence of efficacy but may be considered in emergent situations where there are no alternatives (ACR, 2020).
Special Considerations for Patients who are Pregnant or Breastfeeding
The effects of ICAs on the human embryo and fetus remain incompletely understood at this time. Studies have demonstrated that ICA can cross the placenta and enter the fetus in measurable amounts, but the effects are unknown, and long-term data are lacking. The ACR (2020) does not recommend routine screening for pregnancy before ICA administration. The FDA supports this recommendation, and most ICAs are category B medications. The literature base regarding the safe use of ICA in patients who are breastfeeding is also limited; however, many studies have demonstrated that the routine dose of ICA absorbed by the infant's gut from ingested breast milk is extremely low. While the ACR states that available data suggest it is safe for the mother and infant to continue breastfeeding after receiving ICA, the mother should be allowed to make an informed decision after full disclosure of the available information. For mothers who opt to abstain from breastfeeding temporarily, the recommendation is to express and discard breastmilk for a period of 12 to 24 hours following ICA administration. There is no benefit to refrain from breastfeeding beyond 24 hours (ACR, 2020). The American College of Obstetricians and Gynecologists (2017) Committee on Obstetric Practice recommends that iodinated contrast should only be used if absolutely required to obtain essential additional diagnostic information that will affect the care of the fetus or mother during the pregnancy. The ACOG also supports the continuation of breastfeeding without interruption following the use of iodinated contrast materials (ACOG, 2017).
Gadolinium
Gadolinium-based contrast agents (GBCAs) are the primary form of contrast used to enhance the quality of magnetic resonance imaging (MRI) scans. Administered intravenously, GBCAs work by altering the magnetic properties of neighboring water molecules, thereby enhancing the quality of the MRI images. MRI is distinct from other radiology imaging exams as they do not operate using x-rays or ionizing radiation. Instead, they function on a high strength magnetic field, magnetic field gradients, radio waves, and a computer to generate images to demonstrate if a disease process, pathological condition, or injury is present. The first GBCA was approved for use by the FDA in 1988, and there are currently several types of MRI contrast agents available for use. Similar to ICAs reviewed in the prior section, osmolarity and viscosity of the GBCAs can also vary widely and are generally higher for the ionic than the non-ionic agents. Gadolinium belongs to a class of metal ions, and if used alone, it is toxic to humans. Gadolinium present in GBCAs has undergone a process called chelation, in which other chemical ions are mixed with the gadolinium to prevent it from harming the body. The majority of GBCAs used in the US are chelates of gadolinium, have a positive magnetic susceptibility, and function to produce an increased signal on the resulting images. The GBCA is injected into the patient before the MRI and is primarily eliminated from the body via the kidneys, with a small amount of liver excretion (Ibrahim et al., 2020).
GBCAs are generally well-tolerated and are much less likely to produce an allergic reaction than ICAs. The incidence of adverse events is low, ranging from 0.07% to 2.4%, and most reactions are mild and physiologic, including coldness, warmth, pain at the injection site, nausea, vomiting, headache, paresthesia, and dizziness. Allergic-like reactions are uncommon, ranging from 0.004% to 0.7%, and symptoms are generally very mild and transient; usually limited to hives, other skin reactions, or itchy eyes (RadiologyInfo.org, 2018). While manifestations of allergic-like reactions to GBCA can be similar to those of ICAs, they are rare, with life-threatening anaphylactic reactions occurring at a low rate of 0.001% or 0.01%. For example, in a survey of 20 million administered doses of GBCA, there were only 55 severe reactions (Davenport et al., 2013). While the ACR (2020), acknowledges that fatal reactions to GBCAs are possible, they are extraordinarily rare. When managing GBCA-related allergic reactions, clinicians should follow the same ICA guidelines outlined previously in Table 6 (ACR, 2020).
In 2015, the FDA published a safety alert as residual gadolinium has been found within the brain of patients who received multiple doses of GBCAs throughout their lifetime. Research has demonstrated that gadolinium deposits appear to occur preferentially within specific areas of the brain, even in the absence of clinically evident disease, and in the setting of intact blood-brain barriers. The etiology of this remains unclear, and it currently remains a relatively undefined clinical phenomenon, as no adverse health effects have been revealed; investigations are ongoing (ACR, 2020). Corresponding to the same clinical decision-making regarding the role of ICA use, clinicians must also balance the potential risks of GBCA with the diagnostic benefits, which is also premised on a multitude of patient-specific factors such as:
- the probability and necessity of an accurate diagnosis;
- alternative methods of diagnosis;
- risk of misdiagnosis;
- the clinical benefit of the diagnostic information against the unknown, yet potential risk of gadolinium deposition in the brain;
- expectations regarding the patient's risk for the development of nephrogenic systemic fibrosis (NSF) (Davenport et al., 2020).
NSF is a rare but potentially life-threatening complication unique to GBCAs. It is a systemic condition characterized by thickening of the skin, organs, and other tissues in patients with severe preexisting renal disease. The initial symptoms typically include skin thickening and pruritus, which can then rapidly progress to contractures and joint immobility. In severe cases, the condition can be fatal. The time between GBCA administration and the onset of NSF is usually within days to months. However, there have been rare case reports of symptoms appearing years following the last GBCA exposure. NSF seems to have first appeared around 2000, but radiology guideline changes did not take place until 2006, at which time clinicians began to withhold GBCAs in patients with AKI or severe CKD when the GFR is <30mL/min/1.73m2. While this led to a decline in the incidence of NSF cases, further investigation demonstrated the risk for NSF was higher in specific types of GBCAs. As a result, modern-day radiology algorithms group GBCAs based on their reported associations with NSF in vulnerable patient populations, as outlined in Table 7 (ACR, 2020).
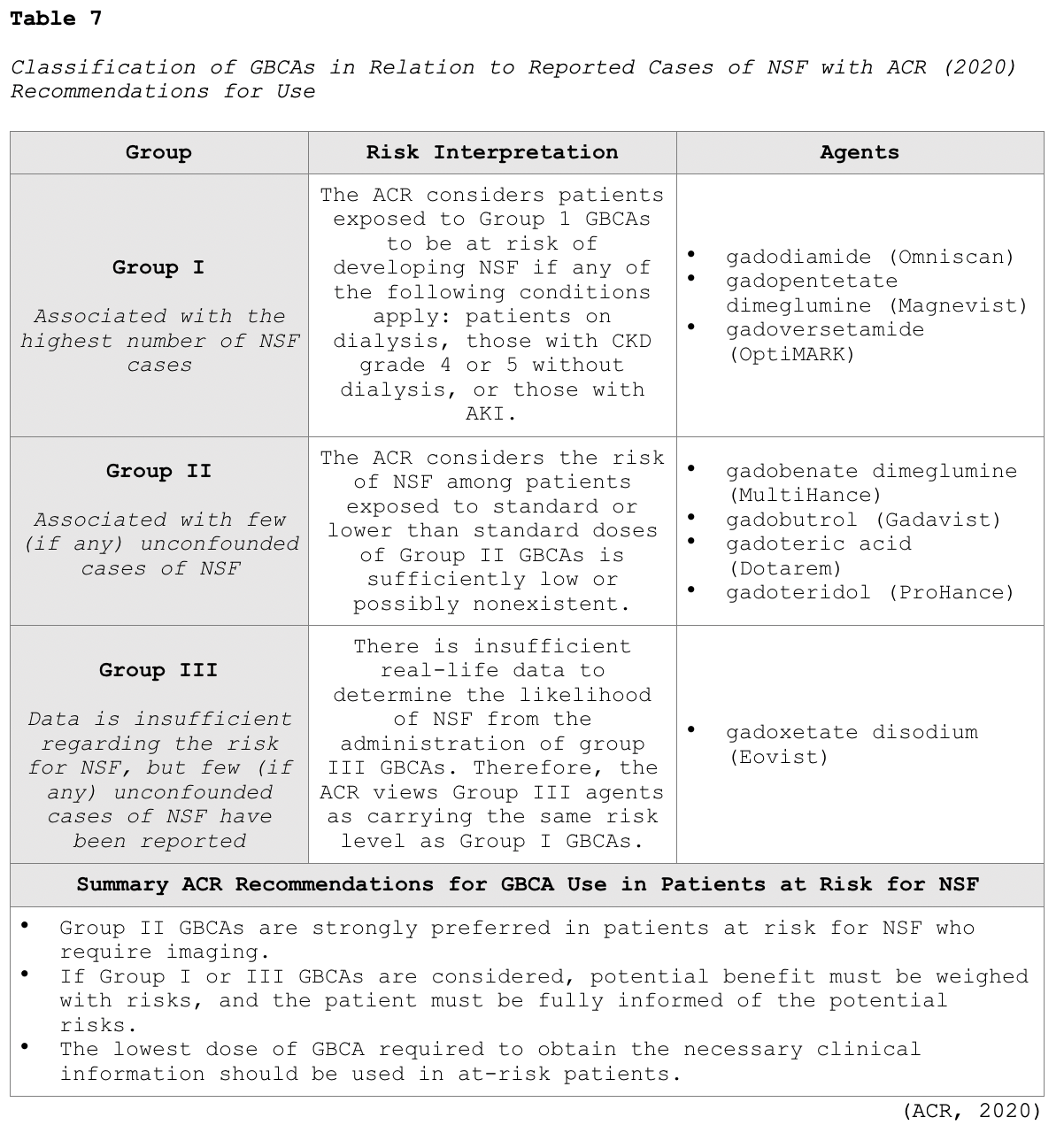
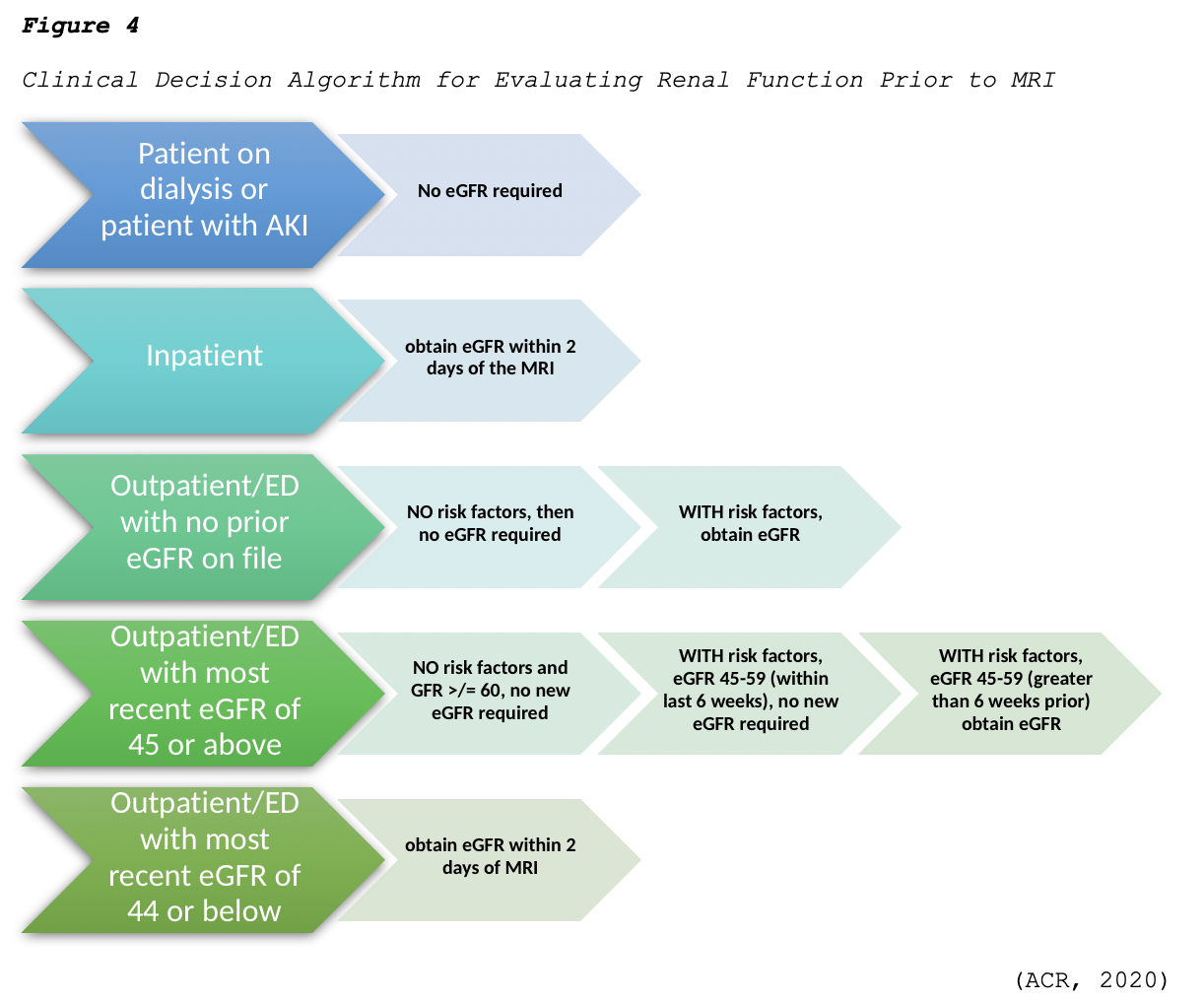
The ACR (2020) advises clinicians to follow the algorithm demonstrated in Figure 4 regarding the evaluation of renal function before GBCA administration. Regarding this clinical decision-making algorithm, a history of renal disease is considered a risk factor, as well as any of the following:
- Prior or current dialysis;
- Renal transplantation;
- Single kidney;
- Kidney surgery;
- Renal cancer;
- Hypertension requiring medical therapy;
- Diabetes mellitus (ACR, 2020).
The ACR (2020) makes several recommendations regarding the use of GBCAs in the following patient groups:
- Patients with CKD 4 of 5: Group I GBCAs are contraindicated and Group II GBCAs should be used instead;
- Patients with AKI: Group I GBCAs should be avoided if possible, and Group II agents are preferred;
- Patients on metformin (Glucophage): it is not necessary to discontinue metformin (Glucophage) prior to GBCA administration when the standard dose of gadolinium is being used;
- Sickle cell disease/trait: the risk to patients with sickle cell disease at approved dosages is very low or nonexistent, and there is no reason to withhold these agents from these patients when their use is indicated (ACR, 2020).
Special Considerations for Patients who are Pregnant or Breastfeeding
There are no known adverse effects reported in fetuses when standard doses of GBCAs have been administered to pregnant women; however, similar to ICAs, the data is limited, and long-term studies are lacking. There are no reported cases of NSF related to GBCAs in pregnant women. However, gadolinium chelates can accumulate in the amniotic fluid, which suggests a potential risk for NSF in the mother or fetus. Since it remains very unclear how GBCAs can affect the fetus, the ACR (2020) advises that these agents are administered with caution in pregnant patients. GBCAs should only be used if it is considered critical, and the potential benefits justify the potentially unknown risks to the fetus; the lowest possible dosage to achieve diagnostic results should be used. Studies have demonstrated that only a small percentage of GBCA is excreted into breast milk. Therefore, the ACR (2020) believes it is safe for the mother and infant to continue breastfeeding after receiving GBCA. However, the mother should be allowed to make an informed decision after full disclosure of the available information. For mothers who opt to abstain from breastfeeding temporarily, the recommendation is to express and discard breastmilk for a period of 12 to 24 hours following GBCA administration. There is no benefit to refrain from breastfeeding beyond 24 hours (ACR, 2020). The ACOG (2017) Committee on Obstetric Practice recommends that the use of gadolinium contrast is limited and should be reserved only if it significantly improves the predicted diagnostic outcomes for the fetus or mother. The ACOG also recommends that breastfeeding is not be interrupted following gadolinium administration (ACOG, 2017).
Barium Sulfate
Barium sulfate contrast agents (BSCAs) are the preferred agents for evaluating and visualizing the GI tract and can only be administered through the GI tract, which includes the oral and rectal routes (ACR, 2020). They are comprised of a suspension of insoluble barium sulfate particles that are not absorbed by the gut and are ideal for illuminating the GI tract since they have excellent coating properties of the GI mucosa, as demonstrated in Figure 5. They are the most common type of orally administered contrast material and enhance the visualization of the GI tract in CT scans of the abdomen and pelvis and specific types of MRI scans. They are also commonly used for fluoroscopic studies, such as an upper gastrointestinal (UGI) series or a small bowel follow-through (SBFT) examination. BSCAs are also used in patients undergoing GI studies that can be performed via nasogastric tubes, as well as via the rectal route during fluoroscopic studies evaluating the colon (RadiologyInfo.org, 2018).
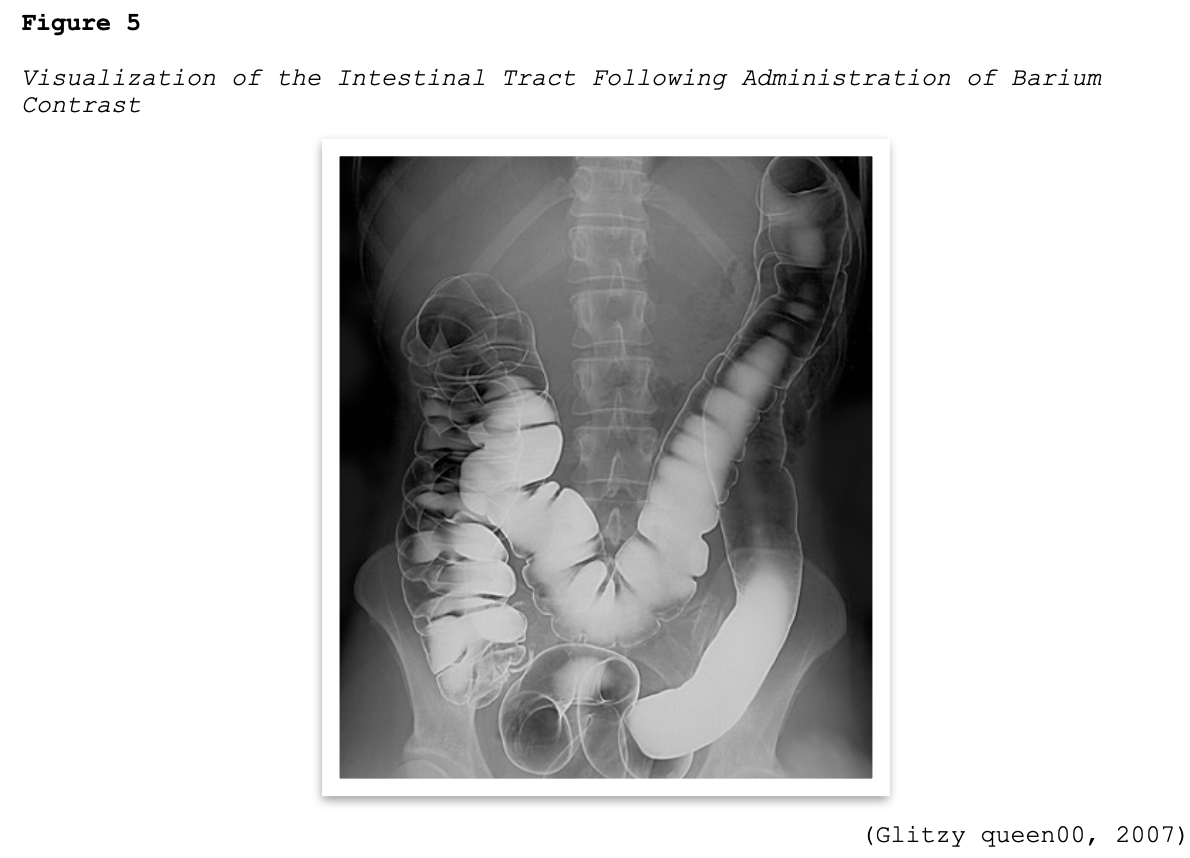
Patients scheduled to undergo a scan using an oral BSCA should be advised to remain nothing by mouth (NPO) for four to eight hours before the exam, depending on the type of scan and the institution's policy. If the contrast material is to be administered rectally, the patient will usually require a colon preparation geared toward cleansing out the colon before the exam (RadiologyInfo.org, 2018).
While the ACR (2020) states explicitly that there are no absolute contraindications for the use of BSCAs, they recommend to avoid their use in patients who are suspected or known to have bowel perforations or prior allergy to barium (ACR, 2020). Patients who have a suspected fistula, paralytic ileus, or a history of cystic fibrosis (which is associated with increased risk for blockage in the small bowel) are considered to be at greater risk for having an adverse reaction to or complication from BSCA administration (Cutler, 2016). Barium sulfate is generally well tolerated when administered orally, although some patients describe a mildly unpleasant taste. When barium sulfate is administered through the rectum using an enema, patients may experience temporary abdominal fullness and mild discomfort. It is generally recommended to ensure the BSCA is warmed to room temperature, which helps enhance patient tolerability and reduce spasms of the colon (RadiologyInfo.org, 2018).
Adverse reactions to BSCAs are rare and are almost always mild in presentation. The most common symptoms include nausea, vomiting, abdominal cramping, or discomfort, which can occur during or after the examination. Patients can have vasovagal reactions, which are more common with rectal administration of contrast after the colon is distended. Following the completion of a BSCA procedure, patients should be instructed to increase oral fluid intake to help facilitate and expedite the removal of the contrast material from the body, which is expelled through the feces. Patients should be educated that their stool may be white for a few days until all the contrast material is eliminated. It is common for some patients to be experience changes in their routine bowel habits for the first 12 to 48 hours, such as diarrhea or constipation. Barium enemas are also used therapeutically in some instances to remove feces from the bowel. This may be performed for patients with severe constipation or blockages, as means to prevent surgical intervention and reduce the risk for bowel perforation (RadiologyInfo.org, 2018).
Allergic-like reactions to BSCA are very uncommon, with an incidence rate of 1 in 750,000 examinations. If an allergic-like reaction does occur, the manifestations are usually very mild and limited to transient rashes, urticaria, pruritus, or mild bronchospasm. Moderate to severe allergic reactions are exceedingly rare, occurring in about 1 out of 2.5 million patient exposures. Clinical manifestations of moderate to severe allergic reactions may include more extensive dermatologic changes, respiratory symptoms, and vascular events such as hypotension. Rarely, angioedema of the stomach and small bowel, and toxic epidermal necrolysis (TEN) have been described (ACR, 2020).
Direct barium toxicity has been reported in patients who received oral and rectal BSCAs, although this is an infrequent occurrence. The etiology is based on the concept that any barium that dissociates from the stable barium compound may be absorbed through the bloodstream and lead to toxicity. Symptoms of barium toxicity usually present rapidly and are limited to the GI tract, including watery diarrhea, nausea, and vomiting. The treatment is limited to supportive therapy, rehydration, and managing any electrolyte disturbances associated with fluid volume deficit (ACR, 2020).
Microbubble Contrast Agents
Microbubble contrast agents (MCAs) are most commonly used to enhance the images of ultrasonography, particularly exams of the heart. MCAs are microscopic-sized, highly compressible gas-filled microbubbles that are coated with a phospholipid or protein outer layer (Wang et al., 2018). MCAs have a high degree of "echogenicity", or the ability to reflect ultrasound waves; structures with higher echogenicity will appear brighter than surrounding areas on the resulting ultrasound images (RadiologyInfo.org, 2018). These tiny bubbles of gas can be safely injected intravenously through a peripheral or central access line, or instilled into hollow structures, such as the urinary bladder. Once injected, they allow for the transient improvement in ultrasound visualization, capturing differences between the gas in the microbubbles and the surrounding tissues of the body. They enhance the ultrasound signal from blood, and improve the visualization of blood flow without subjecting the patient to several risks associated with other forms of contrast media (ACR, 2020).
These agents should be administered through slow intravenous infusion or bolus injection. Since the microbubbles dissolve rapidly, usually within 15 minutes, the ACR (2020) recommends real-time assessment with images obtained over a 10-minute period, before the microbubbles spontaneously rupture and dissolve. As the microbubbles dissolve, they release a gas that is mostly eliminated by the lungs through exhalation. This is considered a useful and practicable alternative to ICAs or GBCAs for patients with AKI, CKD, or allergies (RadiologyInfo.org, 2018).
According to the ACR (2020), there are currently three MCAs that are FDA-approved for IV administration in adults undergoing echocardiography to improve visualization of the left ventricular cavity and the endocardial borders. These three agents include perflutren lipid microspheres (Definity), sulfur hexafluoride lipid-type A microspheres (Lumason; SonoVue), and perflutren protein-type A (Optison). Sulfur hexafluoride lipid-type A microspheres (Lumason) is additionally approved for liver imaging, and for imaging of the pediatric urinary tract for voiding ultrasonography. While their current FDA-approved indications are limited to the above, these agents have also been successfully used off-label to assess the following:
- to evaluate blood flow in tumors;
- to differentiate between benign cysts and solid tumors in the kidney;
- for assessing for blood perfusion in organs;
- to assess for abnormalities in the heart;
- in the assessment of thrombosis, such as in myocardial infarction;
- to evaluate for bowel wall inflammation or inflammatory activity in inflammatory bowel disease and Crohn's disease, as well as liver and kidney masses;
- to detect endoleaks following abdominal aortic aneurysm repair;
- to differentiate between an abscess and a phlegmon;
- to guide ultrasound-guided interventions and ablative therapies (ACR, 2020; RadiologyInfo.org, 2018).
MCAs are considered to have a reasonably favorable safety profile, as the rate of adverse events is less than that of ICAs or GBCAs. The ACR (2020) cites a severe reaction rate of 0.01% (n=8) in a large investigational study involving more than 78,000 doses conducted in 2008. Of these, only four were anaphylactoid reactions, and there were no fatalities reported (Wei et al., 2008). Adverse events are usually mild and physiologic. The most common symptoms include headache, flushing, warmth, nausea, and altered taste, and the majority of severe reactions occur within 30 minutes of MCA administration. The risk for severe cardiopulmonary reactions is increased in patients who have a preexisting and unstable cardiac condition, such as an acute myocardial infarction or congestive heart failure. There is no reported renal toxicity with MCAs. MCAs should not be used intra-arterially, and are contraindicated in patients with prior allergic reactions to these agents (ACR, 2020).
Special Considerations for Patients who are Pregnant or Breastfeeding
No well-controlled studies evaluating the risks of MCAs when administered to pregnant women are available. Therefore, the ACR (2020) recommends only using these agents in pregnant women when necessary and when the prospective benefits outweigh the unknown, but potential risks for the fetus. For women who are breastfeeding, they should be counseled on the temporary pumping and discarding of breastmilk for up to 24 hours following administration (ACR, 2020).
Saline Contrast Media
Agitated saline (salt water) is considered a distinct type of contrast that falls within the MCA family, as it is administered through intravenous injection and provides air microbubble contrast in the right side of the heart. Similar to the above MCAs, saline air bubbles are transient, and they diffuse into the lungs. This technique is useful for examining the right side of the heart and identifying intracardiac or extracardiac (pulmonary arteriovenous) shunts.; particularly right to left shunts. Data are lacking on the side effect and safety profile of saline-based microbubble contrast injections, and most information is limited to case studies. There have been reports of ischemic complications, including transient ischemic attack and stroke (Ahmed et al., 2020). An outdated, yet still clinically relevant study from 2009, describes five case reports of cerebral ischemic events (two TIAs and three ischemic strokes) occurring within five minutes of administration of agitated saline contrast. While the incidence rate of complications appears to be relatively low, additional well-controlled studies are necessary to draw definitive conclusions (Romero et al., 2009).
Gaseous Contrast Agents
Gaseous agents (such as oxygen [02] and carbon dioxide [CO2]) can also be used as contrast agents in specific types of imaging tests. Similar to MCAs used in ultrasonography, these agents are also absorbed rapidly by the body and eliminated through exhalation. Research is limited on these contrast agents, and the ACR (2020) manual of contrast media omits any reference to or discussion of these agents entirely. While air and oxygen can be dangerous due to the risk for gas emboli during specific procedures, CO2 does not pose a risk of gas emboli and can be used with relative safety. CO2 has the most research data supporting its clinical benefits and use as an intravascular contrast agent. It is used as an alternative to ICA or GBCAs in patients with renal dysfunction or allergies to ICA. It is inexpensive, readily available, has a low molecular weight when compared to ICAs, and is less viscous than blood. These characteristics make it an ideal agent to image small collateral vessels and a wide variety of vascular beds and chambers, as it displaces the blood in the vessels and acts as a negative contrast agent. However, administering CO2 as a contrast agent requires extreme care as it is a colorless, odorless, and significantly compressible gas. It is also less sensitive and specific than ICAs or GBCAs (Ali et al., 2017; Gupta et al., 2020).
Carbon dioxide has been successfully used as a contrast agent in the following clinical situations:
- angiography in patients with peripheral arterial disease who are at risk for CIN;
- aortic aneurysm repairs;
- aortography;
- renal artery angiography;
- inferior vena cava imaging;
- portal vein imaging;
- splenoportography;
- oncologic tumor embolization procedures;
- upper extremity venography;
- assessment of gastrointestinal bleeding (Ali et al., 2017; Gupta et al., 2020).
Ali and colleagues (2017) describe CO2 contrast as relatively safe with minimal complications across a systematic review of available studies. However, they recommend that the use of CO2 is avoided in cerebral, spinal, and cardiac procedures due to the potential risk of ischemia to vital organs. Further, relative contraindications include the presence of cardiac septal defects, pulmonary AV malformations, pulmonary hypertension, and severe emphysema due to the risk for potential reflux of the gas into the thoracic aorta, which has been noted to precipitate a transient loss of consciousness in isolated case studies (AIi et al., 2017).
Please proceed to the NursingCE.com course entitled “Part II: Diagnostic Radiology for APRNs” for the conclusion of this two-part educational series.
References
Ahmed, H., Manning, W. J., & Downey, B. C. (2020). Contrast echocardiography: Contrast agents, safety, and imaging techniques. UpToDate. https://www.uptodate.com/contents/contrast-echocardiography-contrast-agents-safety-and-imaging-technique
Ali, F., Mangi, M. A., Rehman, H., & Haluski, E. (2017). Use of carbon dioxide as an intravascular contrast agent: A review of current literature. World Journal of Cardiology, 9(9), 715-722. https://doi.org/10.4330/wjc.v9.i9.715
American College of Obstetricians and Gynecologists. (2017). ACOG committee
opinion: Guidelines for diagnostic imaging during pregnancy. Obstetrics & Gynecology, 130(4), e210-e216. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2017/10/guidelines-for-diagnostic-imaging-during-pregnancy-and-lactation.pdf
American College of Radiology. (2020). ACR manual on contrast media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
Beckett, K. R., Moriarity, A. K., & Langer, J. M. (2015). Safe use of contrast media: What the radiologist needs to know. Radiographics, 35(6), 1738-1750. https://doi.org/10.1148/rg.2015150033
Cutler, S. (2016). Chapter 28: Contrast media. https://radiologykey.com/contrast-media-2/
Davenport, M. S., Dillman, J. R., Cohan, R. H., Hussain, H. K., Khalatbari, S., McHugh, J. B., & Ellis, J. H. (2013). Effect of abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine on rate of allergic-like reactions. Radiology, 266(3), 773-782. https://doi.org/10.1148/radiol.12120253
Davenport, M. S., Perazella, M. A., Yee, J., Dillman, J. R., Fine, D., McDonald, R. J., Rodby, R. A., Wang, C. L., & Weinreb, J. C. (2020). Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American college of radiology with the national kidney foundation. Radiology, 294(3), 660-668. https://doi.org/10.1148/radio.2019192094
Elsayes, K. M., & Oldham, S. A. A. (2014). Introduction to diagnostic radiology. McGraw-Hill Education.
Glitzy queen00. (2007). Visualization of the intestinal tract with following administration of barium contrast [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Human_intestinal_tract,_as_imaged_via_double-contrast_barium_enema.jpg
Gupta, A., Dosekun, A. K., & Kumar, V. (2020). Carbon dioxide-angiography for patients with peripheral arterial disease at risk of contrast-induced nephropathy. World Journal of Cardiology, 12(2), 76-90. https://doi.org/10.4330/wjc.v12.i2.76
Holmes, K., & Griffiths, M. (2016). Chapter 14: Image quality. https://radiologykey.com/image-quality/
Ibrahim, M. A., Hazhirkarzar, B., & Dublin, A. B. (2020). Magnetic resonance imaging (MRI) gadolinium. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK482487/
International Atomic Energy Agency. (2014). Diagnostic radiology physics: A handbook for teachers and students. IAEA Publications. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1564webNew-74666420.pdf
Llyod-Jones, G. (2016). Basic of x-ray physics. https://www.radiologymasterclass.co.uk/tutorials/physics/x-ray_physics_densities
RadiologyInfo.org. (2018). Contrast materials. https://www.radiologyinfo.org/en/pdf/safety-contrast.pdf
Romero, J. R., Frey, J. L., Schwamm, L. H., Demaerschalk, B. M., Chaliki, H. P., Parikh, G., Burke, R. F., & Babikian, V. L. (2009). Cerebral ischemic events associated with ‘bubble study’ for identification of right to left shunts. Stroke, 40(7), 2343-2348. https://doi.org/10.1161/STROKEAHA.109.549683
Spampinato, M. W., Abid, A., & Matheus, M. G. (2017). Current radiographic iodinated contrast agents. Magnetic Resonance Imaging Clinics of North America, 25(4), 697-704. https://doi.org/10.1016/j.mric.2017.06.003
US Food & Drug Administration. (2016). FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. https://www.fda.gov/media/96771/download
Wang, S., Hossack, J. A., & Klibanov, A. L. (2018). Targeting of microbubbles – contrast agents for ultrasound molecular imaging. J Drug Target, 26(5-6), 420-434. https://doi.org/10.1080/1061186X.2017.1419362
Wei, K., Mulvagh, S. L., Carson, L., Davidoff, R., Gabriel, R., Grimm, R. A., Wilson, S., Fane, L., Herzog, C. A., Zoghbi, W. A., Taylor, R., Farrar, M., Chaudhry, F. A., Porter, T R., Irani, W., & Lang, R. M. (2008). The safety of Definity and Optison for ultrasound image enhancement: A retrospective analysis of 78,383 administered contrast doses. Journal of American Society of Echocardiography, 21(11),1202-1206. https://doi.org/10.1016/j.echo.2008.07.019