About this course:
This module aims to provide an overview of sepsis, its risk factors, clinical features, best practices for diagnosis, and treatment to inform nursing practice and help nurses provide optimal care, patient education, and support improved patient outcomes and decreased mortality.
Course preview
This module aims to provide an overview of sepsis, its risk factors, clinical features, best practices for diagnosis, and treatment to inform practice and help nurses provide optimal care, patient education, and support improved patient outcomes and decreased mortality.
By the completion of this learning activity, the learner should be able to:
- discuss the pathophysiology of sepsis
- examine the risk factors of sepsis
- describe the clinical manifestations and management of sepsis
- define the complications related to sepsis or its management
- discuss the emerging research related to sepsis
Background
Sepsis is one of the oldest described medical conditions. The term sepsis is derived from the ancient Greek term for “decomposition” or “decay”. The first documented use was in Homer’s poems around 2,700 years ago. Hippocrates utilized the term around 400 BCE to describe how meat decays and swamps release decomposing gases. He also used the term to describe how infected wounds become purulent. The germ theory of disease was developed in the 1800s, which led to the recognition that sepsis originated from invading microorganisms. The first modern definition was attempted in the early 1900s. Still, 2,000 years passed before developing the hypothesis that the host response, not the pathogen, was responsible for sepsis. The term “blood poisoning” has been used for centuries, and it persists as a description for sepsis in the nonmedical population. Understanding the actual cause and pathophysiology of sepsis has led to better management of and treatment for the diagnosis (Berg & Gerlach, 2018; Gyawali et al., 2019).
Epidemiology
Today, sepsis is the most common cause of admissions to intensive care units (ICUs) in the US (Genga & Russell, 2017). It is also the most common cause of death in adults admitted to ICUs (Gauer et al., 2020). Sepsis and septic shock commonly occur in the United States and worldwide. Although the management of sepsis has improved, the condition’s incidence is increasing as more drug-resistant organisms emerge. This can also be attributed to patients being discharged from the hospital faster and possibly before manifestations of sepsis are apparent. Mortality rates from sepsis are high (Ignatavicius & Workman, 2016). In 2018, the global average mortality rate for patients with sepsis in an ICU was 37% (Martins et al., 2018). According to the Centers for Disease Control and Prevention (CDC), 1 in 3 patients who die in a hospital will die of sepsis. Every year, over 1.7 million Americans develop sepsis, and almost 270,000 of these patients die (CDC, 2020; Fay et al., 2020). Sepsis is the most expensive diagnosis treated in the US and accounted for more than $24 billion in healthcare costs in 2013 (Paoli et al., 2018).
Fay and colleagues (2020) performed a cohort study of US adults with sepsis and septic shock across the country. Most of the patients in the study were found to have sepsis onset outside of the hospital but had recent encounters with the healthcare system. According to the study, 42% of patients had received antimicrobials, chemotherapy, wound care, dialysis, or surgery in the month before developing sepsis (Fay et al., 2020).
Pathophysiology
Sepsis is a complex process that usually begins as a fungal or bacterial infection. Most of the time, sepsis results from a bacterial infection. Fungal infections more commonly occur in immunocompromised patients. Organisms that often cause sepsis include gram-negative (Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli) and gram-positive (Staphylococcus aureus and Streptococcus aureus) bacteria (Ignatavicius & Workman, 2016). Infections with antimicrobial-resistant organisms confer a higher risk of developing sepsis. Common antimicrobial-resistant organisms include vancomycin-resistant Enterococcus, carbapenem-resistant bacteria (i.e., Enterobacter, K. pneumoniae, E. coli, and Pseudomonas aeruginosa), methicillin-resistant Staphylococcus aureus (MRSA), and penicillin-resistant Streptococcus pneumoniae (Fay et al., 2020).
A sepsis infection progresses to a critical situation over several days. As the infection advances, the pathological changes occur faster and become more severe. Control and prevention of sepsis are easier to achieve early in the infectious process. Sepsis that is not recognized early with quick intervention contributes significantly to progression to septic shock and death (Ignatavicius & Workman, 2016).
An infection that is localized rarely leads to sepsis and shock. A healthy immune system and inflammatory response will confine and eliminate the invading organism when the invasion starts, preventing the infection from becoming worse or widespread. White blood cells (WBCs) around the infection will secrete cytokines that trigger local inflammation, summoning other WBCs to kill invading organisms. The inflammatory response also constricts small veins and dilates arterioles in the area to increase perfusion to the infected tissue. Capillary leakage will then occur, which allows plasma to enter the surrounding tissues and cause swelling. The size and severity of the infection will determine the duration of inflammation, but it typically subsides within a few days when the infection has been managed effectively. The inflammation in this response is limited only to the local area and will stop once it is no longer necessary. Systemic symptoms such as fevers, tachycardia, decreased oxygen saturation, and reduced urine output do not accompany a local infection (Ignatavicius & Workman, 2016).
Sepsis is the systemic manifestation of infection that occurs when certain organisms have entered the bloodstream. Widespread inflammation is triggered. This response is called systemic inflammatory response syndrome (SIRS). The organisms in the bloodstream will enter other body areas, leading to the systemic inflammatory response and extensive hormonal, tissue, and vascular changes. Oxidative stress further impairs tissue perfusion and oxygenation. The circulating WBCs produce cytokines, which result in widespread vasodilation and blood pooling. This leads to early signs of mild hypotension, low urine output, and tachypnea, resulting in decreased cardiac output. Body temperature may be elevated, but this varies depending on WBC function and the duration of the sepsis. A patient may have either a low grade or a high fever. Other patients may have a subnormal body temperature. SIRS is responsible for the fever and hypotension. Impaired oxygenation and tissue perfusion lead to decreased urine output and tachypnea. At this point, the patient usually presents with an elevated WBC count in their laboratory results as expected with a systemic infection (Ignatavicius & Workman, 2016). The cytokines also release lipoxygenase, leukotrienes, bradykinin, histamine, serotonin, and IL-2. The body opposes these with anti-inflammatory mediators like IL-4 and IL-10, which results in a negative feedback mechanism (Maggio, 2020).
Abnormal clotting and microthrombi formation in some organ capillaries cause hypoxia and reduce organ function. At this point, arteries and arterioles will dilate and decrease peripheral arterial resistance, so cardiac output increases. This stage is called “warm shock”. If sepsis is diagnosed and treated at this point, organ damage is reversible. If undiagnosed and not treated, poor capillary flow and capillary obstruction from the microthrombi decrease oxygen delivery to tissues and impairs the removal of carbon dioxide and other waste products. These toxic metabolites damage more cells and increase the production of inflammatory cytokines leading to worsened SIRS and a damaging cycle of hypoxia and poor perfusion that will progress without interventio
...purchase below to continue the course
Kaukonen and colleagues (2015) looked at patients with infection and organ failure and evaluated the SIRS criteria. Twelve percent of participants were identified as having SIRS-negative sepsis, demonstrating fewer than 2 of the SIRS criteria. Other studies have shown that many hospitalized patients meet SIRS criteria but never develop infections (Marik & Taeb, 2017).

Severe sepsis consists of the features described previously plus sepsis-induced organ dysfunction. All tissues are involved and considered hypoxic to some degree. At this point, some organs will be experiencing cell death and organ dysfunction. Microthrombi formation is widespread, and clots continue to form where they are not needed, expending the available platelets and clotting factor and resulting in a condition known as disseminated intravascular coagulation (DIC). The SIRS and cytokine release prompts capillary leakage, cell injury, and increased cell metabolism. Cellular damage reduces the body’s anti-clotting ability and triggers the formation of more clots, increasing DIC. Metabolism increases, which negatively affects the cellular uptake of oxygen. The stress response triggers the release of glucose from the liver, which causes hyperglycemia. The more severe a patient’s response is, the higher their blood glucose level will be (Ignatavicius & Workman, 2016).
Even though this stage is severe and can last 24 hours or more, it is still often unrecognized. One reason may be the body’s compensatory mechanisms. Blood pooling and capillary leakage stimulate the heart, elevating the heart rate and blood pressure and increasing cardiac output. The patient may have warm extremities, even with decreased tissue perfusion, and there will be little or no cyanosis. At this point, the patient’s WBC count is no longer elevated. Prolonged sepsis has exceeded the bone marrow’s ability to produce mature neutrophils and other WBCs. The patient will exhibit lower oxygen saturation, tachypnea, decreased or absent urine output, and changes in the level of consciousness and cognition. Mortality in patients who have reached this stage is much higher, but aggressive intervention can prevent septic shock (Ignatavicius & Workman, 2016).
Septic shock consists of multiple organ dysfunction syndrome (MODS) with organ failure and poor clotting with uncontrollable bleeding. The death rate among patients with septic shock is high, as the patient has entered sepsis-induced hypotension that does not respond to fluid resuscitation. Vasodilation and capillary leakage will continue. Cardiac contractility is poor because of cardiac ischemia. Severe hypovolemic shock and decreased cardiac function result from the blood’s inability to clot due to DIC. The clinical manifestations resemble late-stage hypovolemic shock (Ignatavicius & Workman, 2016).
Risk Factors and Comorbidities
Sepsis starts with an infection—most often pneumonia—that triggers a dysregulated host response. Other infections that commonly lead to sepsis include gastrointestinal, genitourinary, and skin and soft tissue infections, as well as other respiratory infections (Gauer et al., 2020).
Patients at exceptionally high risk for sepsis are those with a compromised immune system or a central venous access device. Central lines in place for even a short period will provide a point of entry for microorganisms and lead to central line-associated bloodstream infections (CLABSIs). Other conditions that predispose a patient to sepsis include malnutrition, large wounds, GI ischemia, invasive procedures, cancer, increased age, infections with resistant microorganisms, chemotherapy, alcoholism, diabetes mellitus, chronic kidney disease, transplants, hepatitis, and HIV or AIDS (Ignatavicius & Workman, 2016). Essentially patients with many underlying conditions are at risk for sepsis (Markwart et al., 2020).
Sepsis can complicate infections that a patient has acquired in the community. Up to 70% of sepsis cases are community-acquired. Between 5.7% and 19.1% of sepsis cases are related to healthcare-acquired infections. In some countries, such as Brazil, as many as 60% of sepsis cases result from hospital-acquired infections (Markwart et al., 2020). Postoperative sepsis is a complication of surgery. The patient-related risk factors associated with postoperative sepsis include preexisting conditions such as heart failure, diabetes, and chronic kidney disease. Male patients are also at a higher risk of postoperative sepsis. Surgery-related risk factors for post-surgical sepsis include emergency surgery, inpatient hospital stay, perioperative blood transfusions, and open surgery (Plaeke et al., 2020).
Rehospitalization is common in sepsis survivors. As many as 1 in 5 survivors are rehospitalized within 30 days of discharge. Risk factors for rehospitalization after sepsis include increased age, male gender, non-White race, multiple comorbidities, non-elective admissions, and hospitalizations prior to sepsis admission. Patients who are not discharged home following their sepsis admission also have an increased risk of readmission. Sepsis-specific risk factors include those with a gastrointestinal site of infection, infections from ESBL bacteria, increased severity of sepsis, and prolonged initial hospital length of stay. Other characteristics that elevate a patient’s rehospitalization risk include lower socioeconomic strata, lower hemoglobin on discharge, use of total parenteral nutrition (TPN), and tracheostomy at sepsis admission (Shankar-Hari et al., 2020).
For children with sepsis, risk factors for increased mortality include younger ages, complex neurological conditions, infective endocarditis, immunodeficiency, HIV, burns, malignancy, and transplant status. African American and Hispanic pediatric patients also have higher mortality rates with sepsis (Thavamani et al., 2020). Low-birth-weight neonates are also considered a high-risk population (Markwart et al., 2020). Neonatal sepsis is classified as early or late. Early neonatal sepsis appears within the first 72 hours after birth, and late neonatal sepsis begins after 72 hours. Early neonatal sepsis is acquired before or during childbirth, so the pathogens are usually obtained from the mother’s genitourinary tract. Late neonatal sepsis occurs most often in infants who remain hospitalized. Risk factors for early neonatal sepsis include maternal Streptococcus agalactiae colonization. Mothers who do not undergo prophylactic antibiotic treatment have a 25-fold risk of having a newborn with early neonatal sepsis. Amniotic membrane rupture for over 18 hours and chorioamnionitis are also risk factors for early neonatal sepsis. Late neonatal sepsis more frequently occurs in low-birth-weight infants with long-term hospitalization or in late preterm or full-term infants who require prolonged hospitalization. Prematurity, invasive procedures, histamine-2 antagonists (H2 blockers), and prolonged antibiotic therapy are risk factors for late neonatal sepsis (Procianoy & Silveira, 2020).
Clinical Manifestations
Sepsis is characterized by confusion, unusual behavior, hypotension, and tachypnea. Many patients have non-specific symptoms and complain of generally feeling unwell (Dexter & Mortimore, 2020). Patients may have tachycardia, complain of extreme pain or discomfort, or experience shivering. The patient may complain of shortness of breath, feel cold, and develop clammy or sweaty skin (CDC, 2021). Neurological dysfunction happens in about half of sepsis cases admitted into the ICU, including confusion, delirium, drowsiness, seizures, and coma (Cavaillon et al., 2020). Many patients with sepsis will have fevers, but clinical manifestations are generally subtle, especially in older or immunocompromised patients (Gauer et al., 2020).
Clinical signs of early and late neonatal sepsis include apnea, difficulty breathing, cyanosis, fast or slow heart rate, poor perfusion, irritability, lethargy, hypotonia, seizures, vomiting, abdominal distension, food intolerance, gastric residue, hepatomegaly, unexplained jaundice, inability to regulate body temperature, petechiae, or purpura (Procianoy & Silveira, 2020).
Diagnosis
The diagnosis of sepsis has proven problematic to define. In the hopes of finding a consistent diagnosis for treatment and clinical trials, a formal definition was developed by the American College of Chest Physicians and the Society of Critical Care Medicine. This definition has been modified as the understanding of sepsis has become more precise. The first definition of sepsis, or Sepsis-1, was developed in 1991 and focused on the patient demonstrating symptoms of SIRS (fulfilling two or more of the SIRS criteria as seen in Table 1) as a response to infection. Sepsis-1 was defined as an infection or suspected infection that led to the onset of SIRS. Sepsis-2 criteria were defined in 2001 and are essentially unchanged from the original Sepsis-1 definition. The patient must satisfy at least two of the SIRS criteria and a suspected or confirmed infection. Sepsis-3 is the latest definition of sepsis published in 2016; it identifies life-threatening organ dysfunction caused by a dysregulated response to an infection. Severe sepsis is diagnosed when a patient meets the definition of sepsis with new-onset organ dysfunction (Marik & Taeb, 2017).
There is no single diagnostic test or screening tool for sepsis, nor does a single set of criteria identify sepsis with perfect accuracy. The Adult Sepsis Event (ASE) offers one method of retrospectively defining sepsis. The ASE captures the widest variety of patients with sepsis. To receive a diagnosis of sepsis, a patient must have demonstrated two components of criteria A and organ dysfunction(s) from criteria B, as summarized in Table 2 (CDC, 2018).
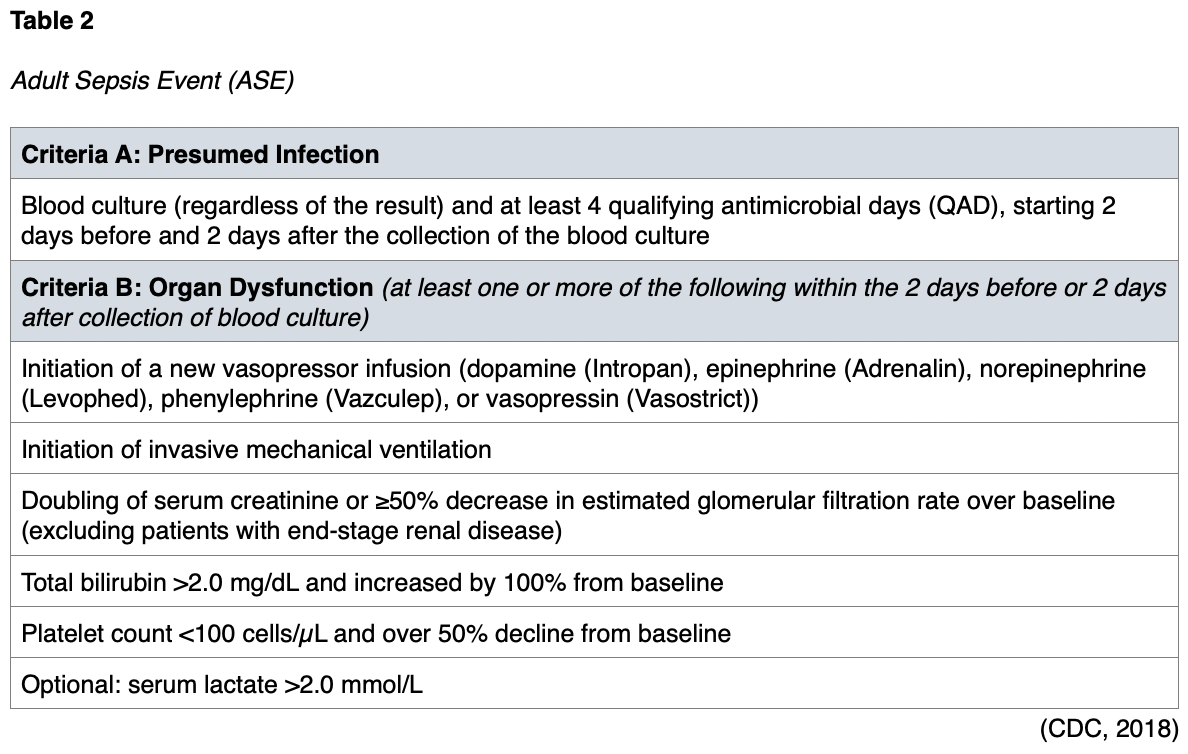
The ASE supports the surveillance of adult patients in an acute hospital setting and the tracking of sepsis incidence and outcomes within a facility. The resulting data can be used for quality-improvement initiatives or epidemiology and public health research. The ASE is not recommended for the surveillance of pediatric patients, as it has not been validated in this population (CDC, 2018).
Staging and Scoring
In addition to multiple definitions, many scoring and staging systems have been developed for sepsis. These scoring and staging systems identify the severity and predict morbidity and mortality in sepsis patients. While there are many scoring systems, this module will focus on the Sequential Organ Failure Assessment (SOFA) score; the Quick Sequential Organ Failure Assessment (qSOFA); the Predisposition, Infection, Response, and Organ dysfunction (PIRO) staging system; and the Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score.
The Society of Critical Care Medicine has compared the SIRS criteria to other methods of sepsis scoring or staging. The SOFA score was recommended by Marik and colleagues (2017) to assess the severity of organ dysfunction in a septic patient and predict patient mortality. The SIRS criteria do not have the predictive capacity of the SOFA score and are not an ideal scoring system for sepsis (Marik & Taeb, 2017).
SOFA is a simple scoring system that notes the number and severity of failure in 6 organ systems, consisting of the respiratory system, coagulative function, cardiovascular system, liver, kidneys, and neurological system. It was developed in 1994 to describe the degree of organ failure over time (Lambden et al., 2019). The score ranges from 0 to 24, and higher scores predict a higher possibility of mortality (Vafaei et al., 2019). The SOFA score should be calculated on admission before the initiation of treatment and then every 24 hours for daily monitoring of acute morbidity in ICU patients. Clinical guidelines define multiorgan dysfunction as acute changes in the SOFA score of 2 or more points due to the infection (CDC, 2018). While originally developed to measure morbidity and not outcomes, the developers acknowledged that measurements of morbidity are associated with predicting mortality (Lambden et al., 2019). The SOFA score criteria are listed in Table 3.

In addition to the SOFA score, the qSOFA was developed as a simplified method of identifying patients at risk of dying from sepsis (Marik & Taeb, 2017). Since the qSOFA does not require lab results, it may be more useful when evaluating patients with sepsis at the bedside (Lehman & Dendache, 2020). A qSOFA score greater than 2 points indicates multiorgan dysfunction. While the qSOFA score is more accessible, it is not accurate for early risk assessment; thus, it is not recommended as a screening tool for early sepsis (Marik & Taeb, 2017). The qSOFA is summarized below in Table 4.
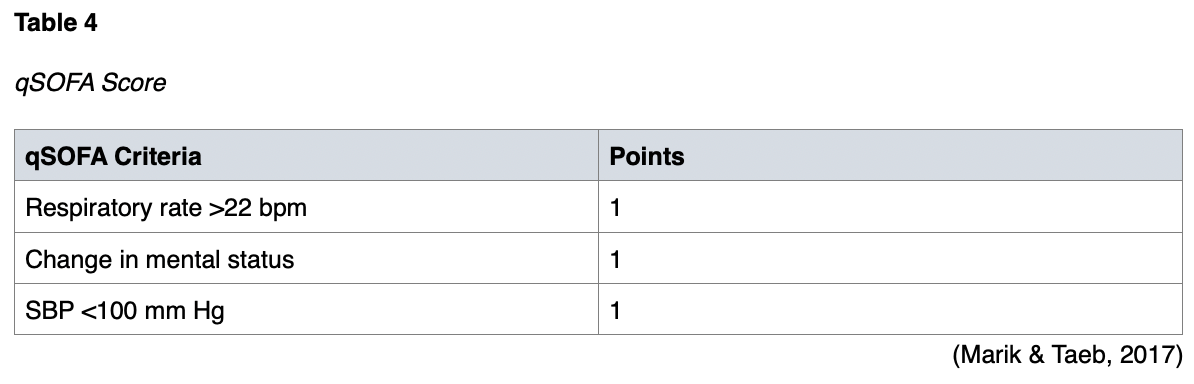
The PIRO system began in 2001. It was fully developed in 2008 to predict 28-day mortality, ICU admission, and the development of multiorgan dysfunction in septic patients. PIRO scores will be higher in patients admitted to the ICU. The respiratory system, pulse rate, and bands are evaluated, as well as organ dysfunction, SBP, and platelet count (Vafaei et al., 2019). The PIRO score is simple, making it more practical for busy emergency departments (EDs), as it is based on four main components that can be quickly assessed (Rathour et al., 2015). Since its development, three more PIRO scores have been introduced—the Moreno PIRO, Howell PIRO, and Rubulotta PIRO (Songsangjinda & Khwannimit, 2020). An example of the Howell PIRO criteria and scoring system is shown in Table 5.
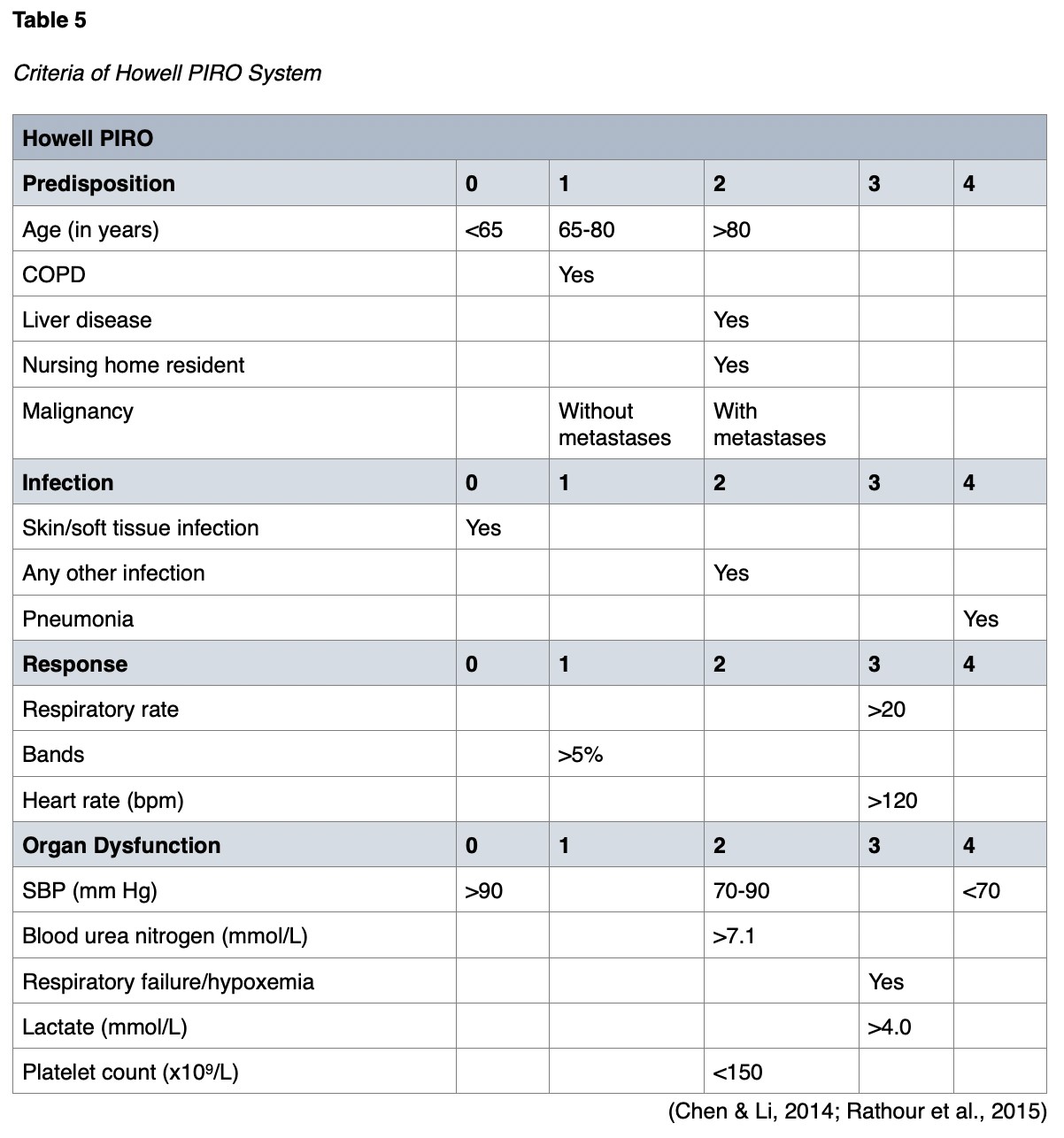
The APACHE II score was developed in 1985 and followed by the APACHE III score in 1991. These scoring systems are used in critically ill patients of any disease category admitted into the ICU. Both systems have been predictors of hospital mortality in sepsis patients. The APACHE III is more time-consuming and complex. The APACHE II is simpler and as effective as APACHE III at predicting hospital mortality in sepsis patients (Sadaka et al., 2017). In studies comparing PIRO and APACHE II, both scoring systems were predictors of 28-day mortality in sepsis patients. Still, the PIRO score was better at predicting ICU admission and multiorgan dysfunction (Chen & Li, 2014). The SOFA score was also more helpful for predicting mortality than the APACHE II in one study (Kim et al., 2013). However, other studies have demonstrated that the APACHE II performed better than the SOFA (Naqvi et al., 2016). The APACHE II scoring system is detailed in Figure 1.
The Surviving Sepsis Campaign App provides a screening tool for identifying patients with sepsis. The app allows users to set up alerts for the sepsis bundles and quickly access guidelines and the Surviving Sepsis website. The app is available for iOS and Android devices. The app also directs users to the Society of Critical Care Medicine’s content and website (Society of Critical Care Medicine, 2019).
Treatment and Management of Care
The management of sepsis requires prompt recognition, appropriate antimicrobial use, hemodynamic support, and infection control. The management of sepsis is moving away from strict protocols in favor of guidelines for individualized care (Dugar et al., 2020). Recommendations from the Surviving Sepsis Campaign Guidelines Committee are often used to guide the care of patients with sepsis and septic shock (Schmidt & Mandel, 2020).
Initial Management
Securing the patient’s airway, correcting hypoxemia, and establishing venous access for quick administration of fluids and antimicrobials are priorities in the initial management of sepsis and septic shock patients. The patient should have venous access established as soon as possible. Peripheral venous access may be sufficient, but many patients will require central venous access at some point. The administration of resuscitative fluids and antimicrobials should not be delayed while waiting on a central line (Schmidt & Mandel, 2020).
A history and examination, as well as laboratory and imaging studies, will often be obtained simultaneously while venous access is attained and the patient’s airway is stabilized. The following laboratory and imaging tests should be acquired within 45 minutes of presentation:
- complete blood counts with differential
- chemistries
- liver function tests
- coagulation studies, including D-dimer
- serum lactate and procalcitonin
- peripheral blood cultures
- urine sample
- tissue and wound cultures from suspected sources of infection
- arterial blood gas
- imaging of the suspected site of infection (chest x-ray, computed tomography [CT] of chest or abdomen; Schmidt & Mandel, 2020)
Cultures should not be drawn from indwelling or central vascular access catheters. These are often colonized with skin flora and will increase the likelihood of a false-positive culture result. If blood cultures were drawn from an intravenous line, a second specimen should be obtained from another peripheral venipuncture site (Schmidt & Mandel, 2020).
Fluid Resuscitation
Initial resuscitation should begin immediately. For resuscitation from sepsis-induced hypoperfusion, administer at least 30 mL/kg of IV crystalloid fluid within the first 3 hours. Beyond this, additional fluids should be given accordingly based on a reassessment of the patient’s hemodynamic status. Reassessment should include clinical examination and evaluation of at least heart rate, blood pressure, arterial oxygen saturation, respiratory rate, temperature, and urine output. The evaluation of any other available information or variables, including invasive and noninvasive monitoring, should be included (Rhodes et al., 2017). Fluid resuscitation should be administered in prescribed, rapidly infused boluses. The patient’s clinical response is assessed before and after each bolus. The patient should be evaluated for signs and symptoms of pulmonary edema. Intravenous therapy can be repeated until blood pressure and signs of tissue perfusion are acceptable, the patient demonstrates symptoms of pulmonary edema, or the care team decides fluid is not augmenting perfusion (Schmidt & Mandel, 2020).
Antimicrobials
According to current recommendations, hospitals and hospital systems should have a quality and performance-improvement program focused on sepsis. This program should include sepsis screenings for acutely ill and high-risk patients. Routine microbiologic cultures are obtained before initiating antimicrobial therapy in patients with suspected sepsis or septic shock. Appropriate microbiologic cultures should include at least two sets of blood cultures, including aerobic and anaerobic (Rhodes et al., 2017).
Intravenous antibiotics should be administered as soon as sepsis is diagnosed (within 1 hour of diagnosis). Each hour delay is associated with an increase in patient mortality. Studies have also shown an increase in organ injury with delays in antibiotic treatment. Delays in the initiation of antimicrobials are often related to organizational factors, such as the hospital drug-delivery chain. Quality-improvement initiatives, such as defined order sets for suspected sepsis, can address delays due to drug delivery. A patient factor that can delay the initiation of antimicrobial treatment is difficulty with vascular access. Intramuscular preparations of antimicrobials are available and can be used when vascular access cannot be established promptly. Empiric broad-spectrum antimicrobial therapy is recommended to cover more possible pathogens. Once the culture results are obtained and the pathogen is identified, treatment can be narrowed. Awaiting culture results causes a significant delay, spiking patient morbidity and mortality (Rhodes et al., 2017).
Another pathogen to consider is the Candida species, which leads to a fungal infection. Patients at risk for Candida infections include immunocompromised or those with prolonged invasive vascular devices, TPN, necrotizing pancreatitis, recent major surgery, extended broad-spectrum antibiotics therapy, a lengthy hospital or ICU stay, or recent fungal infection. Empiric use of antifungals is preferred in patients with severe illness, especially those with septic shock. An infectious disease specialist should be consulted. Outcomes for sepsis patients can be improved with the early involvement of infectious disease specialists (Rhodes et al., 2017).
Once the pathogen has been identified, the antimicrobial therapy should be narrowed to the most effective agent, as indicated by sensitivity results. Approximately one-third of patients diagnosed with sepsis do not have a diagnosed causative pathogen. If cultures are drawn after the initiation of antimicrobial therapy, this can affect culture and sensitivity results. Even if cultures are negative, antimicrobials should be de-escalated based on clinical improvement. If an infection is not found, antimicrobial therapy should be stopped to decrease the possibility that antimicrobial-resistant pathogens will develop. The decision to continue, reduce, or stop antimicrobial treatments should be made on a case-by-case basis using the best clinical judgment (Rhodes et al., 2017).
The most recent recommendations caution against sustained systemic antimicrobial administration for prophylaxis in patients with inflammatory, noninfectious states (e.g., severe pancreatitis or burn injuries). Avoiding systemic antimicrobial therapy when there is no suspected infection can minimize the likelihood of infection with an antimicrobial-resistant pathogen (Rhodes et al., 2017).
The measurement of procalcitonin levels is recommended to support the decision to shorten antimicrobial therapy in septic patients. Procalcitonin levels can also inform the decision to discontinue antibiotics in patients who had suspected sepsis but limited or no evidence of infection. The measurement of serum procalcitonin is used in many parts of the world during the diagnosis of acute infection, helping define the duration of antimicrobial therapy. The procalcitonin level directly correlates with the severity of illness (Vijayan et al., 2017). An algorithm for procalcitonin use in antibiotic therapy can be seen in Figure 2. Procalcitonin levels should be combined with clinical assessment data to base treatment decisions (Rhodes et al., 2017).

Intravascular Access Devices
Prompt removal of any intravascular access device that is a suspected source of sepsis is recommended after another vascular access has been established. Central venous catheters and other intravascular devices are a possible source of sepsis. Although implanted or tunneled catheter-related infections may be treated with prolonged antimicrobial therapy if removal is not feasible, catheter removal is preferred (Rhodes et al., 2017).
Vasopressor and Inotropic Therapy
Vasopressor therapy is another fundamental component of sepsis or septic shock treatment. The goal of vasopressor therapy is to correct hypotension and improve organ perfusion. Norepinephrine (Levophed) is the first-line vasopressor recommended by the Surviving Sepsis Campaign (Shi et al., 2020). Other frequently used vasopressors include epinephrine (Adrenaline), vasopressin (Vasostrict), dopamine (Intropin), and dobutamine (Dobutrex). Norepinephrine (Levophed) is more potent than dopamine (Intropin) and may improve hypotension more effectively in patients with septic shock, resulting in reduced mortality and a lower risk of arrhythmias. Vasopressin (Vasostrict) or epinephrine (Adrenaline) can be added to norepinephrine (Levophed) with the intent to raise MAP to the desired level. Alternatively, vasopressin (Vasostrict) can be added to decrease the norepinephrine (Levophed) dosage. All patients requiring vasopressors should have an arterial catheter placed as soon as possible if resources are available. With patients in septic shock, measurements of blood pressure using a cuff can be inaccurate. An arterial cannula provides a more accurate measurement (Rhodes et al., 2017).
Glucocorticoid Therapy
Glucocorticoids are not recommended in routine use for all patients with sepsis but are frequently provided to patients with septic shock (Schmidt & Mandel, 2020). Intravenous hydrocortisone (Solu-Cortef) is not used in patients with septic shock if fluid resuscitation and vasopressors can restore hemodynamic stability. Several trials have shown elevated hyperglycemia and hypernatremia in patients with septic shock who receive hydrocortisone (Solu-Cortef) therapy. Routine labs should include measurements for these values in patients on hydrocortisone (Solu-Cortef) therapy (Rhodes et al., 2017).
Blood Products
For septic patients, red blood cell (RBC) transfusions should only occur once the hemoglobin concentration decreases to under 7.0 g/dL in adults unless there are extenuating circumstances (e.g., severe hypoxemia, acute hemorrhage, or myocardial ischemia). Erythropoietin should not be used to treat sepsis-associated anemia. Fresh frozen plasma is not recommended to correct clotting abnormalities when there is no bleeding or planned invasive procedure. Current recommendations indicate that fresh frozen plasma (FFP) should be transfused in patients with diagnosed deficiencies in coagulation factors in the presence of active bleeding. Prophylactic platelet transfusions should occur when counts are below 10,000/mm3(10x109/L), even if there are no signs of apparent bleeding. If the patient has a significant risk for bleeding and the counts are under 20,000/mm3(10x109/L), prophylactic platelet transfusions are appropriate. A platelet count over 50,000/mm3(10x109/L) is recommended for cases of active bleeding, surgery, or invasive procedures. Intravenous immunoglobulin is not recommended in patients with sepsis. Antithrombin—an abundant anticoagulant circulating in plasma—is also not recommended for treatment in sepsis patients. The drop in antithrombin plasma activity at the beginning of sepsis can result in DIC. Trials with antithrombin for adult patients with sepsis have not demonstrated beneficial results with patient mortality, as antithrombin was associated with an increased risk of bleeding (Rhodes et al., 2017).
Oxygenation and Mechanical Ventilation
All patients with sepsis should receive continuous monitoring with pulse oximetry with supplemental oxygen. The patient’s condition may require intubation and mechanical ventilation due to the increased work of breathing (Schmidt & Mandel, 2020).
For mechanical ventilation, a target tidal volume of 6 mL/kg of predicted body weight is recommended, in contrast to 12 mL/kg in adult patients with sepsis-induced acute respiratory distress syndrome (ARDS). An upper limit goal for plateau pressures of 30 cm H20 is recommended over higher plateau pressures for adults with sepsis-induced ARDS. Current guidelines recommend higher positive end-expiratory pressure (PEEP) over lower PEEP for adults with sepsis-induced moderate to severe ARDS (Rhodes et al., 2017). Raising PEEP in ARDS may open lung units to promote gas exchange. Transient increases in transpulmonary pressure are designed to open collapsed, airless alveoli. These are called recruitment maneuvers and include temporary increases in airway pressure during mechanical ventilation. During tidal ventilation, some collapsed alveoli are adjacent to alveoli that are still inflated. When the alveoli are inflated, injuries can occur between the tissue. The cyclic collapse and re-expansion of each breath cause shear-induced injury. Furthermore, some alveoli remain inflated through the cycle and can become overinflated. The trauma of each cycle of expansion can cause cytokine release and contribute to further multiorgan failure and mortality. Recruitment maneuvers open collapsed lung tissue, and PEEP is used to prevent a cyclic collapse. Recruitment maneuvers are recommended for adult patients with sepsis-induced severe ARDS. Opening the atelectatic alveoli improves gas exchange. Care should be taken to prevent the overdistention of aerated lung units, leading to ventilator-induced lung injury and transient hypoxemia. The use of sustained continuous positive airway pressure (CPAP) has been shown to improve survival and reduce severe hypoxia. Recruitment maneuvers combined with higher levels of PEEP can be beneficial in patients with severe hypoxemia. Any patient who receives this therapy should be monitored closely, and the recruitment maneuvers can be discontinued if the patient’s condition deteriorates. Recruitment can also occur without performing recruitment maneuvers by removing mucous plugs with suction or bronchoscopy, chest physiotherapy, and proning (placing the patient in the prone position; Chacko & Rani, 2009).
Prone instead of supine positioning can be beneficial for adult patients with sepsis-induced ARDS and a PaO2/FiO2ratio below 150. Prone positioning during the first 36 hours of intubation for 16 or more hours a day has been shown to improve survival in these patients due to improved oxygenation and lung compliance. Proning can be associated with accidental dislodgement of the endotracheal tube and an increased likelihood of pressure injuries, as prone positioning limits the repositioning options to prevent pressure injuries (Rhodes et al., 2017).
Neuromuscular blocking agents (NMBAs) should be used for under 48 hours in adults with sepsis-induced ARDS and a PaO2/FiO2ratio below 150. NMBAs improve chest wall compliance and expansion, prevent respiratory desynchrony, and reduce peak airway pressure. Reduced oxygen consumption may also result from muscle paralysis by decreasing the work of breathing and the blood flow to respiratory muscles. Continuous or intermittent sedation should be minimized for sepsis patients who are mechanically ventilated (Rhodes et al., 2017). Succinylcholine (Anectin) and rocuronium (Zemuron) are often used when a rapid effect is desired. Succinylcholine (Anectin) is the only depolarizing NMBA available. It functions by competing with acetylcholine at the postsynaptic nicotinic receptors. It causes skeletal muscle fasciculations immediately after administration and can lead to malignant hyperthermia and transient hyperkalemia. It is typically reversed by administering an acetylcholinesterase inhibitor, such as neostigmine (Bloxiverz) or edrophonium (Enlon; Renew et al., 2020). Nurse-directed protocols incorporating a sedation scale improve patient outcomes and decrease sedative use. Another method used to reduce sedation use is daily sedation interruption. Beta-2 agonists are also not recommended to treat patients with sepsis-induced ARDS without bronchospasms. The routine use of a pulmonary artery catheter is also not recommended in patients with sepsis-induced ARDS. When these patients are ready for weaning from mechanical ventilation, spontaneous breathing trials and weaning protocols should be used (Rhodes et al., 2017). Please see the NursingCE course on The Basics of Ventilator Weaning for additional information on weaning patients from mechanical ventilation.
Sepsis patients who are mechanically ventilated should have the head of their bed kept between 30 and 45 degrees to decrease the risk of aspiration and prevent the development of ventilator-associated pneumonia. The risk of developing ventilator-associated pneumonia is high in patients receiving enteral feedings. Patients should only be laid flat when necessary for procedures, hemodynamic measurements, or during episodes of hypotension; however, patients should not receive enteral feedings while supine (Rhodes et al., 2017).
Blood Glucose
A protocol should be utilized to manage blood glucose in ICU patients with sepsis. Insulin should be initiated when two consecutive blood glucose levels are greater than 180 mg/dL. Blood glucose levels should be monitored every 1 to 2 hours until glucose levels and insulin infusion rates are stable. Once stable, the patient’s blood glucose can be monitored every 4 hours in patients receiving continuous insulin infusions. Capillary blood glucose levels may not accurately depict arterial or plasma glucose levels; therefore, arterial blood rather than capillary blood is recommended for point-of-care testing in patients with arterial catheters (Rhodes et al., 2017).
Dialysis
Renal replacement therapy can assist patients with sepsis and acute kidney injury. There are multiple modes of renal support that can be used to manage critically ill patients with renal failure. These include conventional intermittent hemodialysis (IHD), prolonged intermittent renal replacement therapy (PIRRT), and continuous renal replacement therapy (CRRT). A rapid solute clearance and ultrafiltration are used in IHD during brief treatments lasting 3 to 5 hours. The continuous therapies remove fluid gradually, with solute clearance happening over prolonged times. PIRRT treatments usually last between 8 and 16 hours and provide solute clearance faster than CRRT. CRRT and PIRRT are often used in hemodynamically unstable patients, but preference may vary with the facility. The indications for renal replacement therapy are volume overload, metabolic acidosis, hyperkalemia, hyponatremia, hyperphosphatemia, encephalopathy, pericarditis, and persistent or progressive acute renal injury. The standard thresholds for initiating renal replacement therapy include a pH less than 7.1 to 7.2 or a serum bicarbonate level less than 12 to 15 mmol/L. Renal replacement therapy may be needed earlier in mechanically ventilated patients to prevent severe acidemia that results from the combination of metabolic and respiratory acidosis (Tandukar & Palevsky, 2019).
Bicarbonate therapy (i.e., sodium bicarbonate) can improve hemodynamics and reduce vasopressor requirements in patients experiencing hypoperfusion-induced lactic acidemia with a pH greater than 7.15. Acutely ill patients with sepsis should also receive venous thromboembolism prophylaxis with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH; Rhodes et al., 2017). LMWHs, such as enoxaparin (Lovenox) and dalteparin (Fragmin), are subcutaneous medications that work by inhibiting thrombin and factor Xa. Treatment with both pharmacological and mechanical prophylaxis is not recommended (Schunemann et al., 2018).
Stress Ulcer Prophylaxis
Stress ulcers often develop in the gastrointestinal tract of critically ill patients. These ulcers can be associated with significant morbidity and mortality. Patients at the highest risk for gastrointestinal ulcers include those who are on mechanical ventilation for over 48 hours and those with impaired clotting. Stress ulcer prophylaxis is essential in patients with sepsis and risk factors for gastrointestinal bleeding. Proton pump inhibitors or H2 blockers are recommended. The routine monitoring of gastric residual volume is not recommended unless the patient has a feeding intolerance or is at high risk for aspiration (Rhodes et al., 2017).
Nutrition
Sepsis patients who can tolerate enteral feedings should receive early enteral nutrition. Parenteral nutrition, either alone or in conjunction with enteral nutrition, should be avoided when possible. Parenteral nutrition is invasive and associated with complications such as an increased risk for infection. Vomiting, aspiration of gastric contents, or high gastric residual volumes are seen with enteral feeding intolerance. This intolerance is more common in patients with gastroparesis, diabetes, or those receiving sedatives or vasopressors. During the first 7 days, the initiation of intravenous glucose and advancement of enteral feedings as tolerated are recommended in patients with enteral feeding intolerance. Prokinetic agents such as metoclopramide (Reglan) are recommended in patients with feeding intolerance. A post-pyloric feeding tube is an option for nutritional support in patients with feeding intolerance or at high risk of aspiration. These patients may require parenteral nutrition to meet their nutritional goals (Rhodes et al., 2017).
Other treatments that have been used include omega-3 fatty acids and intravenous selenium. These measures are not recommended due to insufficient evidence. Similarly, arginine supplementation can lead to vasodilation and hypotension and is not recommended in patients with sepsis. Glutamine levels are reduced in patients with sepsis, and exogenous supplementation can improve the gut mucosa. The clinical significance of these findings and their benefit for sepsis patients have not been established (Rhodes et al., 2017).
Monitoring
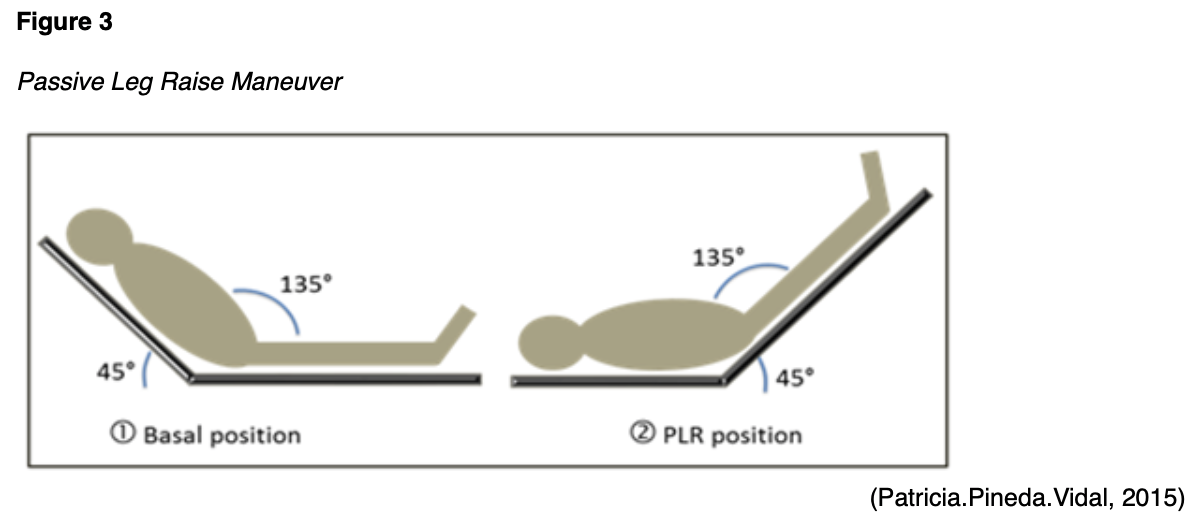
All patients with sepsis should receive close monitoring for improved MAP, urine output, heart rate, respiratory rate, temperature, pulse oximetry, skin color, and mental status. Common target measurements are MAP >65 mm Hg and urine output >0.5 mL/kg per hour. A target range for MAP (e.g., 60 to 70 mm Hg as recommended) can be used instead of a specific number. If a central venous catheter is being used to monitor fluid status and vital signs, a CVP target of 8 to 12 mm Hg and ScvO2 >70% can be used to determine whether fluid management is adequate. An increase in cardiac output in response to the passive leg-raising (PLR) maneuver can also predict fluid responsiveness. The maneuver should begin with the patient in the recumbent position. The patient’s trunk is lowered simultaneously as her bilateral lower extremities are raised (as shown in Figure 3). Then, cardiac output should be assessed directly (not just blood pressure) for one minute. Cardiac output can be assessed by echocardiography, esophageal Doppler, arterial pulse contour analysis, or contour analysis of the volume clamp-derived arterial pressure while performing the maneuver (Schmidt & Mandel, 2020).
Serum lactate levels may be followed every 6 hours until the value has fallen to normal levels (i.e., 0.5-1 mmol/L, termed lactate clearance). Studies are unclear on the benefits of this approach, as lactate can be a poor marker of tissue perfusion. Also, epinephrine (Adrenalin) can increase aerobic lactate production and make the use of lactate clearance-guided resuscitation impossible. Current recommendations consider lactate unhelpful in determining the restoration of perfusion, with the following exception: a rising serum lactate level should prompt a reevaluation of the adequacy of perfusion, as elevated lactate levels can indicate poor perfusion and increased mortality (Schmidt & Mandel, 2020).
Arterial blood gases should be followed to determine gas exchange and the presence of acidosis, pulmonary edema, and complications such as pneumothorax, ARDS, or venous thromboembolism. Routine laboratory values should be evaluated, with particular attention paid to the platelet count, serum chemistries, and liver function tests. These may be performed every 6 hours until the values reach baseline. If hyperchloremia occurs, the intravenous solution may need to be switched to a low-chloride or buffered solution (Schmidt & Mandel, 2020).
Sepsis Bundles
The Surviving Sepsis Campaign has developed bundles, which are lists of interventions and treatments that promote improved outcomes when implemented together. In 2008, the Surviving Sepsis Campaign created the severe sepsis 3-hour resuscitation bundle and the 6-hour septic shock bundle. These interventions are to be completed in 3 or 6 hours, depending on the bundle chosen. In 2018, these bundles were combined into a single hour-1 bundle (Gyawali et al., 2019). The hour-1 bundle is designed to encourage nurses and providers to obtain blood cultures, administer empiric antimicrobials, start fluid resuscitation, measure lactate levels, and initiate vasopressor therapy quickly if clinically indicated. The hour-1 bundle’s goal is to have interventions begin in the first hour after a patient’s sepsis diagnosis. While the interventions should be initiated in the first hour, they are not expected to be completed during the first hour. The hour-1 bundle includes the following interventions:
- measure lactate level or remeasure if the initial lactate was elevated
- obtain blood cultures before initiating antimicrobials
- administer broad-spectrum antimicrobials
- initiate the rapid administration of 30 mL/kg crystalloid intravenous fluids for hypotension or lactate levels greater than 4 mmol/L
- begin vasopressor therapy in patients who are hypotensive during or after fluid resuscitation to maintain MAP greater than 65 mm Hg (Society of Critical Care Medicine, 2019)
The hour-1 bundle requires nurses and providers to act quickly when sepsis or septic shock is recognized in a patient. The time to treatment should be minimized, as sepsis and septic shock are medical emergencies. The patient should be monitored for a clinical response to the interventions, and their sepsis status should be communicated in hand-offs (Society of Critical Care Medicine, 2019).
Goals of Care
The goals of care and the prognosis should be discussed with the patient and their family. Patients with sepsis and multiorgan system failure have a high mortality rate, and some who survive may have a poor quality of life. The treatment goals for a septic patient in the ICU should be realistic, even though the outcome for these patients may be difficult to predict (Rhodes et al., 2017). End-of-life planning is essential, and palliative care should be discussed early and implemented when appropriate, especially if the patient was experiencing declining health before the diagnosis of sepsis (Prescott & Angus, 2018). The goals of care should be established and discussed with the patient and their family as early as possible but no later than 72 hours after ICU admission (Rhodes et al., 2017). Even though patients can experience decreased quality of life, long-term sepsis survivors often report being satisfied with their quality of life and state they would undergo ICU treatment again. In this context, patient-specific conversations and goals of care are needed (Prescott & Angus, 2018).
Special Populations
Sepsis is the fourth leading cause of maternal death in the US (Bridwell et al., 2019). The best way to manage sepsis in pregnancy is unknown, but most experts recommend the same guidelines outlined above (Schmidt & Mandel, 2020). The regular physiological changes that occur during pregnancy complicate the evaluation and management of sepsis (Bridwell et al., 2019). The healthcare team should be aware of the altered hemodynamics of pregnancy when evaluating patient data and establishing goals (Schmidt & Mandel, 2020).
Physiologic anemia of pregnancy results from an increase in plasma volume without a proportional increase in red blood cell count, impairing the oxygen supply to tissues. Pregnant patients have an increased risk of aspiration due to delayed gastric emptying and elevation of the diaphragm, complicating intubation. The elevated diaphragm causes decreased residual volume, decreased oxygenation, and a faster rate of desaturation. Pregnancy can also increase WBC count. Preeclampsia can further increase a person’s WBC count, which complicates the diagnosis of infection. The increased heart rate and cardiac output during pregnancy can elevate the risk of hypoperfusion and may mask the signs of sepsis. Increased clotting factors and von Willebrand factor elevate pregnant patients’ risk of DIC and venous thromboembolic disease. Ureteral dilation increases the risk of pyelonephritis, but the increased renal plasma flow and glomerular filtration rate during pregnancy may mask renal injury in sepsis (Bridwell et al., 2019).
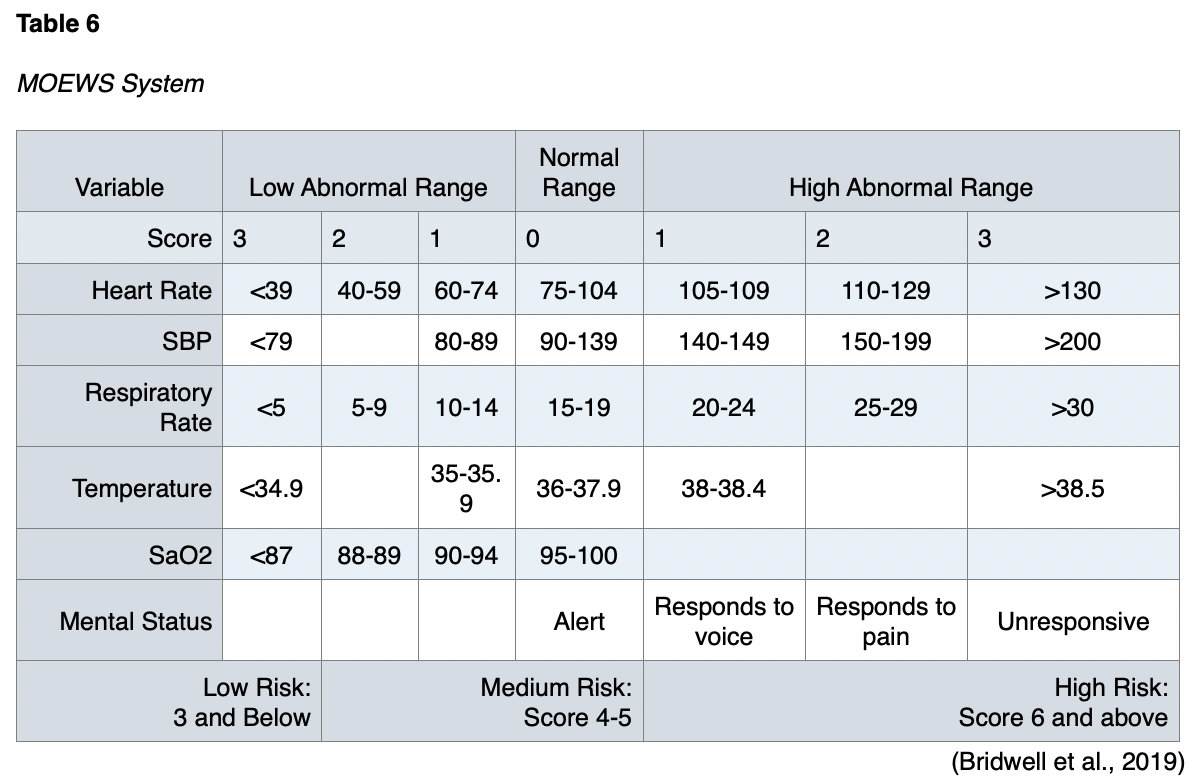
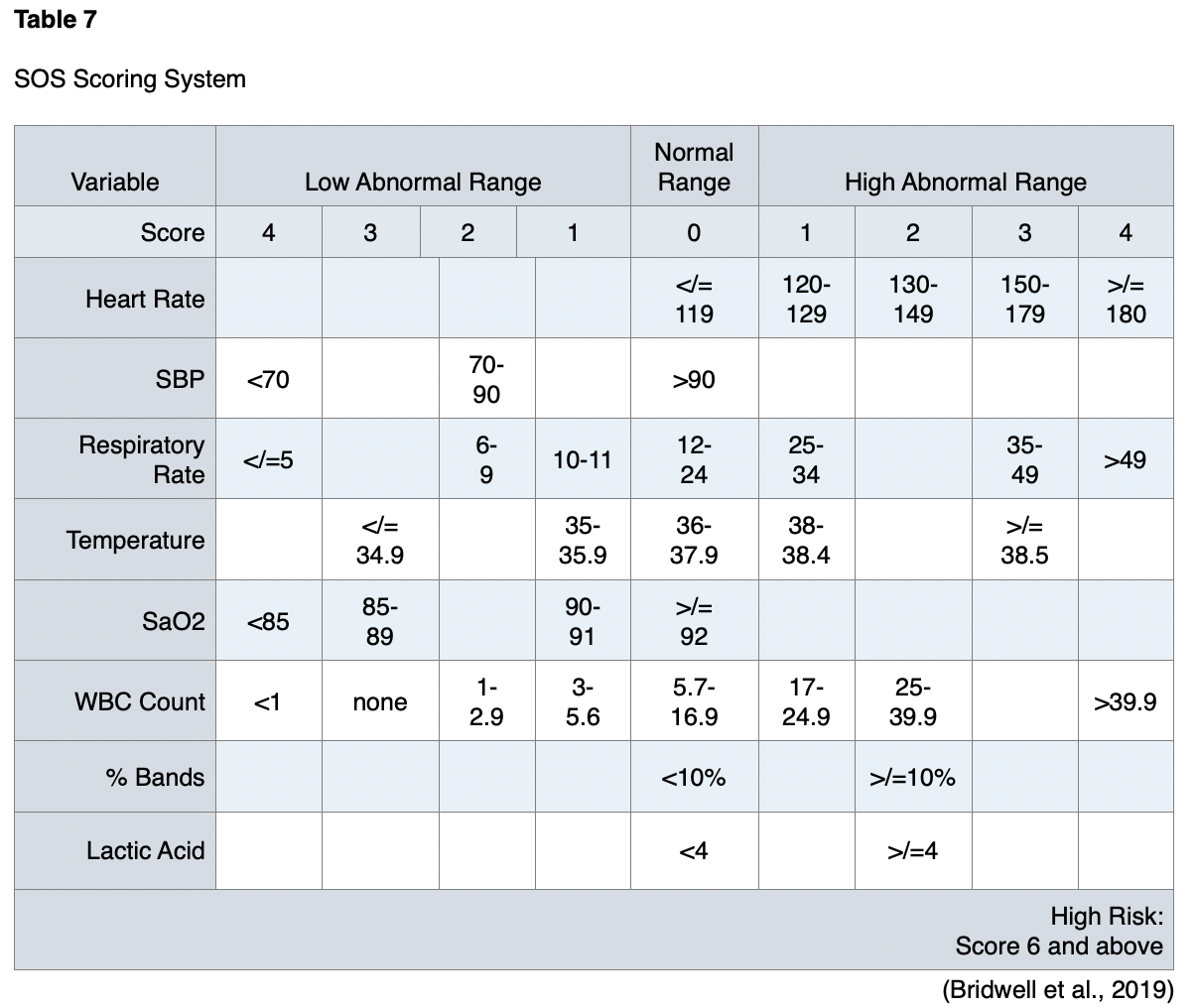
Pregnancy is usually an exclusion criterion for clinical trials and studies of sepsis. Pregnancy was an exclusion criterion in the studies that established SOFA and qSOFA, so no studies have validated the use of these scoring systems in pregnant populations. The scoring systems used in pregnant patients with sepsis are the Modified Early Warning Score (MOEWS) and the Sepsis in Obstetrics Score (SOS; Bridwell et al., 2019). The MOEWS system is outlined in Table 6, and the SOS is summarized in Table 7.
Sepsis is also a leading cause of morbidity, mortality, and healthcare resource consumption for children worldwide. Across the globe, approximately 1.2 million cases of childhood sepsis happen each year. Mortality for pediatric patients with sepsis ranges from 4% to 50% and depends on the severity of illness, risk factors, and geographic location. The majority of pediatric patients who die from sepsis suffer from shock or MODS. Many of these deaths occur during the initial 48-72 hours of treatment. The Surviving Sepsis Campaign updated the International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children in 2020. Before this, the definition and criteria for sepsis in children were based on findings in adult sepsis at the time, with modifications for physiology based on age and maturation of the patient. There have been attempts to change the pediatric definition to match that of Sepsis-3 for adults, but no official update has occurred; therefore, the 2005 definition still applies to pediatric sepsis. This definition consists of the following:
- two or more SIRS criteria
- confirmed or suspected infection
- cardiovascular dysfunction, ARDS, or two or more non-cardiovascular organ system dysfunctions
These pediatric guidelines apply to all patients from greater than or equal to 37 weeks of gestation at birth to 18 years of age with sepsis or septic shock (Weiss et al., 2020).
The guidelines for treating septic shock and sepsis-associated organ dysfunction in children are similar to the recommendations for adults. The Surviving Sepsis Campaign has published the Initial Resuscitation Algorithm for Children that provides systemic screening for sepsis in pediatric patients and offers care guidance for settings with and without intensive care services. The algorithm has been endorsed by many professional organizations, including the American Academy of Pediatrics (Society of Critical Care Medicine, n.d.). The algorithm can be seen in Figure 4.

Health Promotion and Prevention
The best management for sepsis and septic shock is prevention. It is crucial to evaluate all patients’ risks for sepsis. This is especially important for older patients, as the death rate in patients over 65 is nearly double the rate of younger adults. Using medical and surgical asepsis as appropriate during invasive procedures is paramount. Patients should have intravenous access lines and indwelling urinary catheters removed as soon as they are no longer required, and patients on ventilators should be weaned as soon as possible. Sepsis complicates many conditions that bring patients to acute care settings, so it should always be considered a possibility. Nurses are the healthcare professionals who have the most contact with a patient, so the early detection of sepsis is a significant nursing responsibility. Using protocols to identify patients in the ED can identify early sepsis on admission and improve the timing for treatment initiation. Patients discharged home after invasive procedures should be taught manifestations of local infection and early sepsis. Using a thermometer, they should take their temperature twice a day or when feeling unwell. Patients should also be taught to take antibiotics as prescribed for the entire course (Ignatavicius & Workman, 2016).
Surveillance methods are recommended for administrative, program, and clinical practices for quality-improvement projects. A pre-assessment of a facility’s sepsis practices helps interpret the surveillance results and a basis to create recommendations for improving patient care. Administrative and program procedures to review include the following:
- sepsis education and awareness campaigns
- sepsis compliance efforts
- ED, hospital ward, or ICU sepsis teams
- presence of sepsis code teams
- antibiotic stewardship and systematic antibiotic practices
- use of electronic health record prediction tools, alerts, order sets, and protocols related to sepsis
- sepsis billing and coding practices (CDC, 2018)
The following clinical practices should be reviewed:
- ED sepsis diagnosis and triage practices
- hospital ward or ICU screening practices
- presence of and compliance with sepsis protocols
- clinical trial or quality improvement initiatives
- referral or transfer of sepsis patients to other hospitals (CDC, 2018)
Hand hygiene is one of the most effective infection-prevention measures. The World Health Organization emphasizes hand hygiene during five periods:
- before contact with the patient
- before a procedure is performed
- after the risk of exposure to biological fluids
- after contact with a patient
- after contact with areas near a patient (Procianoy & Silveira, 2020)
Complications
Of the patients who survive sepsis and are discharged from the hospital, half recover fully, a third will die in the following year, and one-sixth of the patients will have severe persistent impairments. These patients will develop an average of 1-2 new functional limitations, such as the inability to perform activities of daily living like bathing or dressing independently. They also have triple the prevalence of moderate to severe cognitive impairment and a high rate of mental health problems, including anxiety, depression, and post-traumatic stress disorder. Around 40% of the patients will be rehospitalized within 90 days of being discharged. Patients who survive sepsis have an increased risk of recurrent infections, acute renal failure, and new cardiovascular events. There are many reasons for the deterioration of health after sepsis, such as a rapid progression of preexisting, chronic health conditions, residual organ damage, and impaired immune function. Patients who had poor health before developing sepsis and those who experienced severe sepsis have higher rates of complications after hospital discharge (Prescott & Angus, 2018).
Patients commonly contract hospital-acquired infections while being treated for sepsis. A nosocomial infection usually develops in the late phase of sepsis, particularly in the lungs. The development of this nosocomial infection is not related to the primary site of infection from which sepsis developed. Instead, the main contributing factor is likely sepsis-related immunosuppression from depressed cytokine responses and lymphocyte apoptosis. Reactivation of dormant viral infections has also been documented. Risk factors for the development of nosocomial infections include increased age, high illness severity scores, an extended ICU stay, and respiratory insufficiency. Central lines and endotracheal intubation also increase the risk of nosocomial infections after sepsis (Denstaedt et al., 2018). Encephalopathy and a depressed level of consciousness are other frequent complications of sepsis (Schmidt & Mandel, 2020). Patients may acquire neurological damage related to sepsis in various ways, including cerebral ischemia, metabolic changes, and brain inflammation leading to long-term impairments in memory, attention span, and verbal fluency. Thus, their ability to return to work or school may be limited (Prescott & Angus, 2018). Patients who survive sepsis often enter a state of chronic critical illness driven by persistent inflammation, immunosuppression, and catabolism syndrome. The pathophysiology of these conditions is not entirely understood (Loftus et al., 2017). After suffering from sepsis, other symptoms reported by patients include pain, numbness, visual disturbances, hair loss, dental problems, and problems with their fingernails. Amputation due to gangrene in a limb is an extreme complication of sepsis that results from cardiovascular shock, circulatory dysfunction, or high dosages of vasopressors (Prescott & Angus, 2018). Nerve conduction can become impaired due to a loss of axonal fibers during sepsis, leading to critical illness neuropathy. A similar mechanism can also induce a sepsis-related myopathy. In this context, paired neuropathy and myopathy are called ICU-acquired weakness and can worsen patient outcomes. ICU-acquired weakness may not improve for up to a year after discharge or become permanent (Cavaillon et al., 2020).
Patients who survive sepsis have reported a lower quality of life in comparison with the average population. These patients often cannot resume their prior roles and activities. If they were employed before developing sepsis, only 43% return to work within a year. Only 33% of patients who lived at home before developing sepsis can return to living independently within 6 months of discharge. For the family members of those surviving sepsis, their role often shifts to that of a caregiver. One study of spouses caring for their partners after sepsis found that rates of depression in the spouses increased by 14% (Prescott & Angus, 2018).
Implications for Nursing
The nurse’s role in managing sepsis is not well defined in existing guidelines. Nurses have reported deficits in their ability to recognize and respond to patients with suspected sepsis. The development and implementation of nurse-inclusive sepsis guidelines have been suggested to address the deficits identified in the nurse’s role in sepsis care (Harley et al., 2019). In multiple studies, nurses were authorized to initiate order sets for lactate levels and blood cultures or begin fluid boluses when sepsis was suspected. Nurse-directed care that promotes sepsis’s early identification and treatment reduced the in-hospital sepsis mortality rate (Ferguson et al., 2019; Moore et al., 2019).
Most of the research on sepsis focuses on patients in ED and ICU settings; however, many patients develop sepsis while on general medical-surgical units in hospitals. Nurses in all hospital settings must be familiar with the early warning signs of sepsis to improve survival rates (Creed & Spiers, 2020).
Screening and early recognition of sepsis in at-risk patients can prevent further progression of the sepsis cycle. Nurses should know their facility’s screening tools and protocols for treatment to facilitate early recognition and rapid initiation of treatment. In addition to improved survival, earlier treatment may also result in fewer long-term complications (Prescott & Angus, 2018). Furthermore, infection prevention can be accomplished by encouraging patients to manage their chronic conditions. Nurses should always follow hand hygiene recommendations and medical and surgical asepsis protocols as recommended (CDC, 2020).
To prevent complications in patients who survive sepsis, nurses should identify any new physical, cognitive, and mental problems and refer these patients for appropriate treatment. Long-term medications should be reviewed and adjusted based on organ functioning and the patient’s condition. Changes in renal function, weight, and fluid balance often occur, potentially altering the dosage and suitability of previous medications. Patients should be evaluated for treatable conditions to prevent secondary infections or rehospitalization, such as aspiration, heart failure, or renal failure. If a patient had poor health before sepsis and experienced deterioration of their condition, hospice care might be an option to consider with the family (Prescott & Angus, 2018).
Because of the multiple complications and changes a patient may experience after sepsis, early referrals to subspecialists and ancillary services may be needed. Various factors affect each patient’s ability to follow up and carry out treatment plans, such as weakness, cognitive impairment, loss of income, and caregiver availability. These should all be considered when developing discharge plans. Referrals to address the most significant problems may be prioritized, and then additional referrals can be made. Patients and their caregivers should be educated about sepsis and any possible complications they may experience, and they should be offered peer support resources. The Society of Critical Care Medicine recently developed in-person, online, and telephone-based support groups for patients and their families after surviving critical illness (Prescott & Angus, 2018).
ICU diaries are nonmedical accounts of the patient’s hospitalization. They are written by nurses and family members and have been shown to reduce post-traumatic stress disorder symptoms when given to patients and caregivers a month after their ICU stay (Prescott & Angus, 2018).
To prevent reinfection and readmission, nurses should counsel patients about their risk of infection and recurrent sepsis. Patients should be encouraged to receive all appropriate vaccinations. The nurse should explain the early signs and symptoms and encourage the patient to seek medical care early for suspected infection. Patients should recognize the early indications that infection has progressed to sepsis, including decreased urine output, changes in the level of consciousness, cyanosis, and mottling skin. Nurses may need to schedule telephone or in-person follow-up appointments to monitor patient improvement after discharge (Prescott & Angus, 2018).
Since many patients experience a worsening of chronic conditions after an episode of sepsis, nurses should perform a medication review of diuretics, beta-blockers, and angiotensin-converting enzyme inhibitors. Finally, nurses should teach patients the importance of regular laboratory testing, such as chemistry panels to monitor renal and hepatic function (Prescott & Angus, 2020).
Emerging Research
Since 2004, The Surviving Sepsis Campaign has developed international guidelines for treating sepsis and septic shock. The Surviving Sepsis Campaign sought to reduce sepsis mortality by over 25% over 5 years. In the first 5 years of using sepsis bundles worldwide, this mortality reduction was reached, and patient outcomes were improved. The Surviving Sepsis Campaign’s most recent guideline updates occurred in 2017, informing the treatment recommendations in this module. Since the dissemination of the original sepsis bundles in 2004, hospitals and groups worldwide have used this model to produce comparable projects. In Spain, the Edusepsis Group was developed and led to similar results. A German group recently published data from a hospital-supported quality improvement program to reduce sepsis mortality. These projects used standardized protocols and control instruments (e.g., standard operating procedures and checklists). New York State required hospitals to implement a 3-hour bundle, mandating blood cultures before antibiotics, serum lactate measurements, and an infusion of broad-spectrum antibiotics. Following the implementation of this bundle, they found that a 1-hour delay in the diagnosis of sepsis can increase mortality by 4%. Based on this data, the Surviving Sepsis Campaign developed the hour-1 bundle. Studies that validate or refute these most recent suggestions are not available (Berg & Gerlach, 2018).
The CDC’s Division of Healthcare Quality Promotion has developed tools for tracking sepsis, such as the Hospital Toolkit for Adult Sepsis Surveillance. These help healthcare facilities assess the incidence of adult sepsis in their facilities. The CDC is also working with partners and other federal agencies to develop ways to improve early sepsis detection and treatment. The Get Ahead of Sepsis initiative promotes the early recognition and treatment of sepsis by educating patients, healthcare professionals, and the general public. Get Ahead of Sepsis also collaborates with partners, consumer groups, and clinical organizations to improve antibiotic prescribing and responsible use. Finally, the CDC encourages infection control in the community and healthcare settings to stop infections before they lead to sepsis (CDC, 2020).
Many studies utilize various lab values in an algorithm for the early diagnosis of sepsis. One method uses the neutrophil-lymphocyte ratio to predict sepsis and mortality among critically ill patients in intensive care units (Martins et al., 2018).
Vitamin C and thiamine have shown promising results in reducing hospital mortality in sepsis patients. In the Memphis Veterans Affairs Medical Center, a vitamin C protocol was developed for patients with sepsis or septic shock symptoms in the ICU. The Veterans Affairs Medical Center’s study compared patient outcomes for those treated with intravenous vitamin C, thiamine, and hydrocortisone with patients who received intravenous hydrocortisone alone. The SOFA score was calculated on the initial day and for the next 3 days. No difference in hospital mortality was noted, and the length of hospitalization did not differ between the groups. However, the duration in the ICU was significantly shorter in the treatment group compared to the control (Mitchell et al., 2020).
In a Swedish study, ICU admissions that satisfied the Sepsis-3 criteria were identified and divided into a low CRP group and a high CRP group. The mortality rate was higher in the higher CRP group as well as the duration of ICU and hospital admission. An initial CRP level greater than 100 mg/L at admission was associated with increased 30-day mortality, increased risk of ICU admission, and prolonged hospitalization (Koozi et al., 2020).
Animal studies have shown a link between sepsis and long-term psychological dysfunction. Improvements in cognitive dysfunction after sepsis have been achieved with treatments such as anti-HMGB-1 antibody administration or electroacupuncture (Cavaillon et al., 2020).
One key question is asked in sepsis research: should the research studies focus on saving lives or improving the quality of life for patients who have survived? Enhancing the quality of life for survivors has gained attention in recent years, as 70% of patients with sepsis will suffer post-sepsis physical, psychological, or cognitive impairments after discharge from the hospital. Contributing factors that decrease quality of life in survivors of sepsis include underlying frailty and multiple comorbidities; severity of sepsis illness; lack of early rehabilitation; and the use of corticosteroids, sedatives, and paralyzing agents during treatment. The goal of ongoing research is to identify interventions for reversible conditions that can improve recovery for sepsis patients. Others are attempting to identify factors that can predict the likelihood of survival at an early stage, such as biochemical, molecular, and physiological prognosticators. Predicted survivors could be identified for research focusing on time to recovery and quality-of-life improvement after critical illness. Alternately, research can focus on improved treatment in patients who are not prognosed to survive. Studies also target brain-protective therapies; avoiding prolonged benzodiazepines and adequate pain treatment are being researched for this purpose. Physiological light cycles, cognitive stimulation, and early mobilization may also be helpful. Existing drugs such as metformin (Glucophage) and minocycline (Dynacin) have shown protective effects on the brain in early research (Cavaillon et al., 2020).
References
Berg, D., & Gerlach, H. (2018). Recent advances in understanding and managing sepsis. F1000Research, 7(F1000 Faculty Rev), 1570. https://doi.org/10.12688/f1000research.15758.1
Bridwell, R. E., Carius, B. M., Long, B., Oliver, J. J., & Schmitz, G. (2019). Sepsis in pregnancy: Recognition and resuscitation. The Western Journal of Emergency Medicine, 20(5), 822–832. https://doi.org/10.5811/westjem.2019.6.43369
Cavaillon, J., Singer, M., & Skirecki, T. (2020). Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Molecular Medicine, 12, e10128. https://doi.org/10.15252/emmm.201810128
Chen, Y., & Li, C. (2014). Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: A cohort study. Critical Care, 18(74). https://doi.org/10.1186/cc13832
Centers for Disease Control and Prevention. (2018). Hospital toolkit for adult sepsis surveillance. https://www.cdc.gov/sepsis/pdfs/Sepsis-Surveillance-Toolkit-Aug-2018_508.pdf
Centers for Disease Control and Prevention. (2020). Sepsis clinical information: Surveillance and epidemiology. https://www.cdc.gov/sepsis/clinicaltools/index.html
Centers for Disease Control and Prevention. (2021). Sepsis: What is sepsis? https://www.cdc.gov/sepsis/what-is-sepsis.html
Creed, F., & Spiers, C. (2020). Care of the acutely ill adult: A essential guide for nurses, 2nd edition. Oxford University Press.
Denstaedt, S., Singer, B., & Standiford, T. (2018). Sepsis and nosocomial infection: Patient characteristics, mechanisms, and modulation. Frontiers in Immunology, 9. https://doi.org/10.3389/fimmu.2018.02446
Dugar, S., Choudhary, C., & Duggal, A. (2020). Sepsis and septic shock: Guideline-based management. Cleveland Clinic Journal of Medicine, 87(1), 53-64. https://doi.org/10.3949/ccjm.87a.18143
Fay, K., Sapiano, M., Gokhale, R., Dantes, R., Thompson, N., Katz, D., Ray, S., Wilson, L., Perlmutter, R., Nadle, J., Godine, D., Frank, L., Brousseau, G., Johnston, H., Bamberg, W., Dumyati, G., Nelson, D., Lynfield, R., DeSilva, M., . . . Epstein, L. (2020). Assessment of health care exposures and outcomes in adult patients with sepsis and septic shock. Journal of the American Medical Association Network Open, 3(7). https://doi.org/10.1001/jamanetworkopen.2020.6004
Ferguson, A., Coates, D., Osborn, S., Blackmore, C., & Williams, B. (2019). Early, nurse-directed sepsis care. American Journal of Nursing, 119(1), 52-58. https://doi.org/.1097/01.NAJ.0000552614.89028.d6
Gauer, R., Forbes, D., & Boyer, N. (2020). Sepsis: Diagnosis and management. American Family Physician, 101(7), 409-418. https://www.aafp.org/afp/2020/0401/p409.html
Genga, K., & Russell, J. A. (2017). Update of sepsis in the intensive care unit. Journal of Innate Immunity, 9(5), 441-455. https://doi.org/10.1159/000477419
Gyawali, B., Ramakrishna, K., & Dhamoon, A. S. (2019). Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Medicine, 7. https://doi.org/10.1177/2050312119835043
Harley, A., Johnston, A., Denny, K., Keijzers, G., Crilly, J., & Massey, D. (2019). Emergency nurses’ knowledge and understanding of their role in recognizing and responding to patients with sepsis: A qualitative study. International Emergency Nursing, 43, 106-112. https://doi.org/10.1016/j.ienj.2019.01.005
Ignatavicius, D., & Workman, L. (2016). Medical-surgical nursing: Patient-centered collaborative care (8th ed.). Elsevier, Inc.
Kaukonen, K., Bailey, M., Pilcher, D., Cooper, D., & Bellamo, R. (2015). Systemic inflammatory response syndrome criteria in defining severe sepsis. New England Journal of Medicine, 372(17), 1629-38. https://doi.org/10.1056/NEJMoa1415236
Kim, Y., Yeo, J., Kang, M., Lee, J., Cho, K., Hwang, S., Hong, C., Lee, Y., & Kim, Y. (2013). Performance assessment of the SOFA, APACHE II scoring system, and SAPS II in intensive care unit organophosphate poisoned patients. Journal of Korean Medical Science, 28(12), 1822-1826. https://doi.org/10.3346/jkms.2013.28.12.1822.
Koozi, H., Lengquist, M., & Frigyesi, A. (2020). C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. Journal of Critical Care, 56, 73-79. https://doi.org/10.1016/j.jcrc.2019.12.009
Lambden, S., Laterre, P., Levy, M., & Francois, B. (2019). The SOFA score- Development, utility, and challenges of accurate assessment in clinical trials. Critical Care, 23(374). https://doi.org/10.1186/s13054-019-2663-7
Lehman, B., & Dandache, P. (2020). Disease management: Sepsis. Cleveland Clinic Center for Continuing Education. Retrieved March 1, 2021, from http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/infectious-disease/sepsis/
Loftus, T., Mira, J., Ozrazgat-Baslanti, T., Ghita, G., Wang, Z., Stortz, J., Brumback, B., Bihorac, A., Segal, M., Anton, S., Leeuwenburgh, C., Mohr, A., Efron, P., Moldawer, L., Moore, F., & Brakenridge, S. (2017). Sepsis and critical illness research center investigators: Protocols and standard operating procedures for a prospective cohort study of sepsis in critically ill surgical patients. BMJ Open, 7(7). https://doi.org/10.1136/bmjopen-2016-015136
Maggio, P. (2020). Sepsis and septic shock. Merck Manual. Retrieved April 21, 2021, from https://www.merckmanuals.com/professional/critical-care-medicine/sepsis-and-septic-shock/sepsis-and-septic-shock
Markwart, R., Saito, H., Harder, T., Tomczyk, S., Cassini, A., Fleischmann-Struzek, C., Reichert, F., Eckmanns, T., & Allegranzi, B. (2020). Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systemic review and meta-analysis. Intensive Care Medicine, 46(8), 1536-1551. https://doi.org/10.1007/s00134-020-06106-2
Martins, E., Silveira, L., Viegas, K., Beck, A., Junior, G., Cremonese, R., & Lora, P. (2018). Neutrophil-lymphocyte ration in the early diagnosis of sepsis in an intensive care unit: A case-control study. Revista Brasileira de Terapia Intensiva, 31(1), 63-70. https://doi.org/10.5935/0103-507X.20190010
Mitchell, A., Ryan, T., Gillion, A., Wells, L., & Muthiah, M. (2020). Vitamin C and thiamine for sepsis and septic shock. The American Journal of Medicine, 133(5), 635-638. https://doi.org/10.1016/j.amjmed.2019.07.054.
Moore, W., Vermuelen, A., Taylor, R., Kihara, D., & Wahome, E. (2019). Improving 3-hour sepsis bundled care outcomes: Implementation of a nurse-driven sepsis protocol in the emergency department. Journal of Emergency Nursing, 45(6), 690-698. https://doi.org/10.1016/j.jen.2019.05.005
Monnet, X. & Teboul, J. (2015). Passive leg raising: Five rules, not a drop of fluid! Critical Care, 19(18). https://doi.org/10.1186/s13054-014-0708-5
Naqvi, I., Mahmood, K., Ziaullaha, S., Kashif, S., & Sharif, A. (2016). Better prognostic marker in ICU: APACHE II, SOFA, or SAP II! Pakistan Journal of Medical Science, 32(5), 1146-1151. https://doi.org/10.12669/pjms.325.10080
Paoli, C., Reynolds, M., & Crouser, E. (2018). Epidemiology and costs of sepsis in the United States – An analysis based on timing of diagnosis and severity level. Critical Care Medicine, 46(12). https://doi.org/10.1097/CCM.0000000000003342
Patricia.Pineda.Vidal. (2015). Passive leg raising test [image]. https://commons.wikimedia.org/wiki/File:Passive_Leg_Raising_test.png
Plaeke, P., DeMan, J., Coenen, S., Jorens, P., DeWinter, B., & Hubens, G. (2020). Clinical- and surgery-specific risk factors for postoperative sepsis: A systematic review and meta-analysis of over 30 million patients. Surgery Today, 50, 427-439. https://doi.org/10.1007/s00595-019-01827-4
Prescott, H., & Angus, D. (2018). Enhancing recovery from sepsis: A review. Journal of the American Medical Association, 319(1), 62-75. https://doi.org/10.1001/jama.2017.17687
Procianoy, R., & Silveira, R. (2020). The challenges of neonatal sepsis management: The challenges in managing neonatal sepsis. Journal of Pediatrics, 96(1), 80-86. https://doi.org/10.1016/j.jped.2019.10.004
Rathour, S., Kumar, S., Hadda, V., Bhalla, A., Sharma, N., & Varma, S. (2015). PIRO concept: Staging of sepsis. Journal of Postgraduate Medicine, 61(4), 235-242. https://doi.org/10.4103/0022-3859.166511
Renew, J., Ratzlaff, R., Hernandez-Torres, V., Brull, S., & Prielipp, R. (2020). Neuromuscular blockade management in the critically ill patient. Journal of Intensive Care, 8(37). https://doi.org/10.1186/s40560-020-00455-2
Rhodes, A., Evans, L., Alhazzani, W., Levy, M., Antonelli, M., Ferrer, R., Kumar, A., Sevransky, J., Sprung, C., Nunnally, M., Rochwerg, B., Rubenfeld, G., Angus, D., Annane, D., Beale, R., Bellinghan, G., Bernard, G., Chiche, J., Coopersmith, C., . . . Dellinger, R. (2017). Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Critical Care Medicine. 45(3), 486-552. https://doi.org/10.1097/CCM.0000000000002255.
Sadaka, F., EthmaneAbouElMaali, C., Cytron, M., Fowler, K., Javaux, V., & O’Brien, J. (2017). Predicting mortality in patients with sepsis: A comparison of APACHE II and APACHE III scoring systems. Journal of Clinical Medical Research. 9(11), 907-910. https://doi.org/10.14740/jocmr3083w
Saez, L. (2012). APACHE II [image]. https://commons.wikimedia.org/wiki/File:Apache_II.png#/media/File:Apache_II.png
Schmidt, G., & Mandel, J. (2020). Evaluation and management of suspected sepsis and septic shock in adults. Retrieved March 3, 2021, from UpToDate. https://www.uptodate.com/contents/evaluation-and-management-of-suspected-sepsis-and-septic-shock-in-adults/print
Schünemann, H. J., Cushman, M., Burnett, A. E., Kahn, S. R., Beyer-Westendorf, J., Spencer, F. A., Rezende, S. M., Zakai, N. A., Bauer, K. A., Dentali, F., Lansing, J., Balduzzi, S., Darzi, A., Morgano, G. A., Neumann, I., Nieuwlaat, R., Yepes-Nuñez, J. J., Zhang, Y., & Wiercioch, W. (2018). American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Advances, 2(22), 3198–3225. https://doi.org/10.1182/bloodadvances.2018022954
Shankar-Hari, M., Saha, R., Wilson, J., Prescott, H., Harrison, D., Rowan, K., Rubenfeld, G., & Adhikari, N. (2020). Rate and risk factors for rehospitalization in sepsis survivors: systematic review and meta-analysis. Intensive Care Medicine, 46, 619–636. https://doi.org/10.1007/s00134-019-05908-3
Shi, R., Hamzaoui, O., De Vita, N., Monnet, X., & Teboul, J. (2020). Vasopressors in septic shock: Which, when, and how much? Annals of Translational Medicine. 8(12), 794. https://doi.org/10.21037/atm.2020.04.24
Society of Critical Care Medicine. (n.d.). Surviving sepsis campaign: Pediatric patients. Retrieved February 16, 2021, from https://www.sccm.org/SurvivingSepsisCampaign/Guidelines/Pediatric-Patients
Society of Critical Care Medicine. (2019). Guidelines and bundles: Adult patients. https://www.sccm.org/SurvivingSepsisCampaign/Guidelines/Adult-Patients
Songsangjinda, T., & Khwannimit, B. (2020). Comparison of severity score models based on different sepsis definitions to predict in-hospital mortality among sepsis patients in the Intensive Care Unit. Medicina Intensiva, 44(4), 226-232. https://doi.org/10.1016/j.medine.2018.12.007
Tandukar, S., & Palevsky, P. M. (2019). Continuous renal replacement therapy: Who, when, why, and how. Chest, 155(3), 626–638. https://doi.org/10.1016/j.chest.2018.09.004
Thavamani, A., Umapathi, K., Dhanpalreddy, H., Khatana, J., Chotikanatis, K., Allareddy, V., & Roy, A. (2020). Epidemiology, clinical and microbiologic profile and risk factors for inpatient mortality in pediatric severe sepsis in the United States from 2003 to 2014: A large population analysis. The Pediatric Infectious Disease Journal, 39(9), 781-788. https://doi.org/10.1097/INF.0000000000002669
Vafaei, A., Heydari, K., Hashemi-Nazari, S., Izadi, N., & Zadeh, H. (2019). PIRO, SOFA, and MEDS scores in predicting one-month mortality of sepsis patients: A diagnostic accuracy study. Archives of Academic Emergency Medicine. 7(1).
Vijayan, A., Vanimaya, Ravindran, S., Saikant, R., Lakshmi, S., Kartik, R., & Manoj, G. (2017). Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. Journal of Intensive Care, 5(51). https://doi.org/10.1186/s40560-017-0246-8
Vincent, J., Moreno, R., Takala, J., Willatts, S., De Mendonça, A., Bruining, H., Reinhart, C., Suter, P., & Thijs, L. (1996). The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Medicine, 22(7), 707-10. https://doi.org/10.1007/BF01709751
Weiss, S., Peters, M., Alhazzani, W., Agus, M., Flori, H., Inwald, D., Nadel, S., Schlapbach, L., Tasker, R., Argent, A., Brierley, J., Carcillo, J., Carrol, E., Carroll, C., Cheifetz, I., Choong, K., Cies, J., Cruz, A., De Luca, D., . . . Tissieres, P. (2020). Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatric Critical Care Medicine, 21(2), 52-106. https://doi.org/10.1097/PCC.0000000000002198