About this course:
The purpose of this activity is to enable the learner to identify/understand the pathophysiology, transmission, signs and symptoms and treatment of these STIs: chlamydia, gonorrhea, herpes, HPV, syphilis, and trichomoniasis.
Course preview
Introduction
Sexually transmitted infections (STIs) are a serious public health problem. STIs can cause significant complications; many cases are untreated or never reported. The Centers for Disease Control and Prevention (CDC, 2018) note that there was a substantial and sustained increase in gonorrhea and syphilis from 2013 to 2017, and chlamydia was the most common condition reported to the CDC in 2017.
Chlamydia
Chlamydia trachomatis is a gram-negative bacterium, and the most common cause of STIs in the United States (Gaydos & Quinn, 2018). The CDC has estimated that there are two to three million new cases of C. trachomatis infection every year (Gaydos & Quinn, 2018). However, 50% of men and 80-90% of women who are infected are asymptomatic, or they may only have minimal symptoms, and the underreporting of C. trachomatis infections is significant (Gaydos & Quinn, 2018; Lang et al., 2018). Chlamydia trachomatis infection is more common in women than men, due in part to increased screening and detection in women, and young age is a risk factor for women and men (Gaydos & Quinn, 2018). Most infections occur in women 18 to 24 years old, and the highest reported rate of infection is in people 15 to 24 years of age (Gaydos & Quinn, 2018; Wiesenfeld, 2017).
Transmission
Chlamydia is transmitted by sexual contact with the anus, mouth, penis, or vagina, and C. trachomatis can be transmitted by virtually any type of sexual activity (CDC, 2016a). The rate of transmission from an infected person to an uninfected person is estimated to be approximately 70% (Wiesenfeld, 2017). The higher rate of infection in young women may be caused by cervical ectopy, a specific change in cervical anatomy that is common after puberty and into the early 20s (Hwang, Ma, & Moscicki, 2014; Wiesenfeld, 2017). There is evidence that suggests that autoinoculation from the vagina to the rectum can occur (Khosropour et al., 2019).
Risk Factors
Table 1 (below) lists factors that increase the risk of developing chlamydia

The evidence for oral contraceptive use as a risk factor for chlamydial infection is mixed and inconclusive (Deese, Pradhan, Goetz, & Morrison, 2018). The rate of recurrent infections can be as high as 20%, and recurrent chlamydial infections increase the risk of another infection, the risk of transmission, and the risk of an infection-related complication (Khosropour et al., 2019; Lang et al., 2018).
Signs and Symptoms
C. trachomatis infection can be the cause of multiple clinical syndromes (See Table 2 below). This module will focus on genitourinary tract infections.
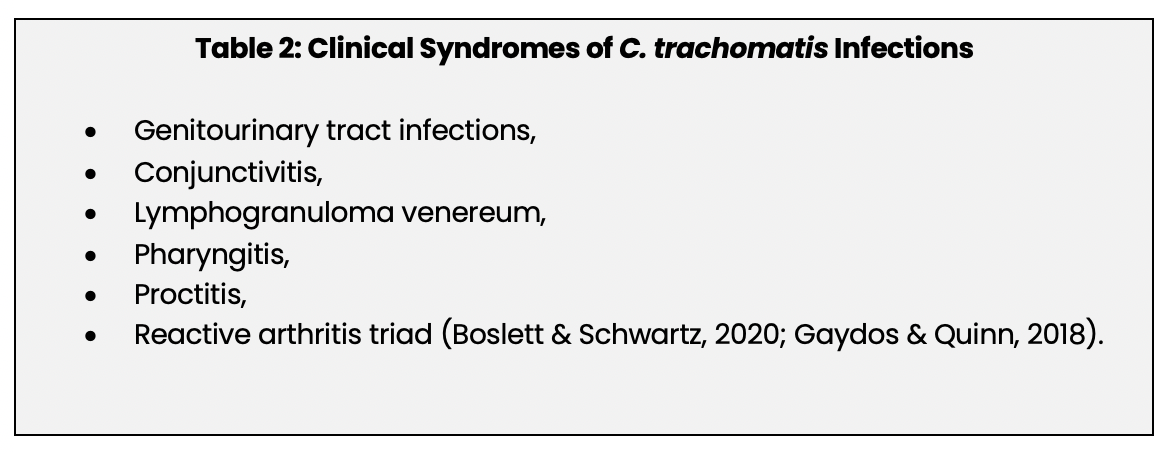
The incubation period for a chlamydial infection is approximately one to three weeks (Gaydos & Quinn, 2018). A C. trachomatis genitourinary tract infection in women typically begins in the cervix, causing a mucopurulent discharge (Boslett & Schwartz, 2018; CDC, 2016a; Whiteley, 2019). The urethra and the urinary tract may become infected as well, causing dysuria, pyuria, and urinary frequency (CDC, 2016a). Chlamydial infection can spread to the upper genital tract, i.e., fallopian tubes, ovaries, and uterus, putting the patient at risk for pelvic inflammatory disease (PID) (CDC, 2016a; Whiteley, 2019). Upper genital tract infection and PID may be asymptomatic or cause abdominal or pelvic pain. PID is a potentially serious complication and can lead to chronic pelvic pain, ectopic pregnancy, infertility, and permanent damage to the fallopian tubes or uterus (CDC, 2016a; den Heijer et al., 2019). A chlamydial infection significantly increases the risk of developing PID, and PID caused by chlamydia is the most important preventable cause of infertility (den Heijer et al., 2019).
Men who have a C. trachomatis genitourinary tract infection will usually present with urethritis. They may have a watery or mucoid discharge from the penis, and dysuria. Some patients will have no spontaneous discharge, but massaging the penis can produce drainage (CDC, 2016a). Epididymitis, with or without urethritis, can occur. This is an uncommon complication, but C. trachomatis infection is one of the two most common causes of acute epididymitis (Gaydos & Quinn, 2018; Levinson, Chin-Hong, Joyce, Nussbaum, & Schwartz, 2018). Acute epididymitis is characterized by pain and swelling of the epididymis, unilateral testicular pain, an enlarged, reddened scrotum, or a hydrocele (CDC, 2016a; Levinson et al., 2018). A C. trachomatis infection can also cause chronic prostatitis (Cai et al., 2017).
Perihepatitis, also known as Fitz-Hugh-Curtis syndrome, is an uncommon complication of chlamydial infection. It is an inflammation of the liver capsule and nearby areas of the peritoneum that occurs primarily in women (especially women who have a chlamydial infection complicated by PID), rarely in men, and is characterized by right upper quadrant abdominal pain, pleuritic pain, and normal liver enzymes (Takata et al., 2018; Whitely, 2019).
Screening
The CDC (2015; 2016a) recommends that all sexually active women under 25 years of age be screened once a year for chlamydia. Women over 25 should be screened annually if there are risk factors present. All pregnant women under 25 or at risk should be screened at the first prenatal visit and again during the third trimester, and any woman who has a sex partner who has an STI or who has signs/symptoms of a genitourinary tract infection should be evaluated for screening. Chlamydia is easily detected, and screening has been shown to reduce the risk of complications (CDC, 2015; 2016a).
Routine chlamydia screening for men is not recommended. Sexually active men who live in an area where chlamydia is highly prevalent and men who have sex with men (MSM) should be screened (CDC, 2015; 2016a).
Diagnosis
Nucleic acid amplification tests (NAATs) using urine or vaginal swabs are very sensitive, and they are the recommended method for diagnosing a chlamydial infection (CDC, 2016a; Gaydos & Quinn, 2018). The vaginal swab is the preferred test for women; the urine tests are preferred for men, and both may be self-collected by the patient (Gaydos & Quinn, 2018).
Treatment
The antibiotic regimens in the numbered list below are recommended for treating uncomplicated genitourinary chlamydial infection. Other regimens can be considered. Concurrent chlamydial and gonorrheal infections are common, so clinicians should consider using a regimen that is effective for treating both diseases (Gaydos & Quinn, 2018; Whitely, 2019).
- Azithromycin (Zithromax): One-time dose of 1 g.
- Doxycycline (Doxy 100): 100 mg, twice a day for seven days.
- Levofloxacin (Levaquin): 500 mg, once a day for seven days
- Ofloxacin (Floxin): 300 mg, twice a day for seven days.
- Tetracycline (Panmcyin): 500 mg, four times a day for seven days.
Directly observing the azithromycin (Zithromax) or the first dose of doxycycline (Doxy 100) improves adherence. Patients should not engage in sexual activity during treatment or for at least seven days afterwards (Fyle-Thorpe, 2019). Empiric treatment should be considered for a patient with signs/symptoms and risk factors for chlamydia or if the patient has recently been exposed to chlamydia, and non-infected partners should be offered treatment, as well (Gaydos & Quinn, 2018). There is no vaccine for chlamydia. Test of cure is recommended in pregnant patients three to four weeks after treatment (Workowski, Boland, & CDC, 2015).
Genital Herpes
Genital herpes is caused by infection with the herpes simplex virus, types 1 a
...purchase below to continue the course
Transmission
Transmission of HSV can occur during anal, oral, and vaginal sex, and by contact with herpetic lesions, oral secretions/saliva, mucosal surfaces, and cervical, genital, and urethral secretions. Transmission of HSV most commonly occurs when the infected person is asymptomatic and does not have visible lesions via viral shedding. Viral shedding is the recurrent presence of HSV on the skin or in secretions, happening on approximately 10.2% of days for asymptomatic herpes carriers (CDC, 2017a; Ryan, 2018a).
HSV-1 is the virus that also causes oral cold sores but can cause a genital infection by way of oral sex or sexual intercourse. Infection with HSV-1 is much more common than infection with HSV-2. Evidence suggests that HSV-2 is more easily transmitted from men to women than from women to men, while HSV-1 is equally transmittable between the genders (Ayoub, Chemaitelly, & Abu-Raddad, 2019).
Risk Factors
Factors that increase the risk of developing genital herpes are listed below in Table 3.

HSV infections are more common in people infected with HIV, and the infections in these patients are likely to be more severe (Katz, 2020; Omori et al., 2018). Condoms can help to prevent transmission of HSV, but the virus can also live in areas that are not covered by a condom (USDHSS, 2019).
Screening
Screening for HSV infection in people who do not have signs and symptoms of the disease is not recommended (CDC, 2017b; United States Preventive Service Task Force [USPSTF], 2016b). The reasons for this recommendation are listed below:
1. Making a diagnosis of herpes in asymptomatic people has not been shown to change sexual behavior.
2. The available serologic tests have low specificity and a high rate of false positives.
3. A false-positive test can cause considerable psychological stress.
4. Serologic tests for HSV-1 cannot determine if the patient has a genital or oral infection.
5. Genital herpes does not usually cause serious complications (CDC, 2017b; USPSTF, 2016b).
People who have signs and symptoms of genital herpes should be tested for the disease, and a patient who has had sex with someone who has genital herpes may consider serologic testing (CDC, 2017b).
Diagnosis
Genital herpes is diagnosed by testing a sample from a lesion or tissue for HSV DNA or by a viral culture. Both tests are used, but the molecular assays for HSV DNA like the polymerase chain reaction (PCR) test are preferred because they are more sensitive (CDC, 2017a; Spitzer, 2019). For patients who do not have lesions, serology tests can detect anti-HSV antibodies. However, the time from infection to a detectable antibody level can be two weeks to three months, so testing soon after exposure to HSV may result in a false negative (CDC, 2017a; LeGoff, Péré, & Bélec, 2014).
Signs and Symptoms: Initial Infection
The mean time between sexual contact and the first genital lesions in an initial infection is five days (Ryan, 2018a). Multiple lesions develop, and the typical progression begins with erythematous papules changing to vesicles which then change to pustules. Within three to five days, the pustules break and become painful ulcers that usually heal without scarring. Approximately one-third of patients have headache, fever, malaise, and myalgias. Vaginal or urethral discharge is common, and dysuria can occur if the lesions are near the urethra. For most patients, the signs and symptoms of an initial infection last about 12 days (Corey, 2018; Ryan, 2018a).
Signs and Symptoms: Recurrent Episodes
After the initial symptomatic episode, recurrent symptomatic episodes are pervasive, occurring in approximately 80-90% of all patients. It is not known what causes recurrent symptomatic episodes, but fever, emotional stress, exposure to sunlight, and ultraviolet radiation can precipitate an episode. The number and frequency of recurrences vary from person to person, and they vary in frequency and duration for each patient, as well (Corey, 2018; Ryan, 2018a). The average number of recurrences per year is four to five, and the number of recurrences usually decreases over time. Prior to a recurrence, the patient may be asymptomatic, or the recurrence may be preceded by a prodromal syndrome characterized by local paresthesias in the buttocks, the genitals, or the perineum. Vesicular lesions appear, the patient has mild systemic symptoms like itching or pain, and recurrent episodes last about two to five days. Then the systemic effects stop, and the lesions disappear (Ryan, 2018a). Genital herpes infections can cause encephalitis and acute and recurrent aseptic meningitis; these complications, particularly encephalitis, rarely occur (Gnann & Whitely, 2017; Noska, Kyrillos, Hansen, Hirigoyen, & Williams, 2016; Ryan, 2018a).
Treatment
Genital herpes is incurable, no vaccine can prevent HSV infection, and there no drugs available that can clear asymptomatic infections (CDC, 2017a; Ryan, 2018a). However, antiviral drugs acyclovir (Zovirax), famciclovir (Famvir), and valacyclovir (Valtrex) are used to treat initial and recurrent episodes and to suppress recurrent episodes. The initial symptomatic episode of genital herpes should be treated with antiviral therapy; treatment will reduce the duration and severity of the episode as well as the risk of complications (Corey, 2108; Ryan, 2018a; Workowski et al., 2015).
Antivirals for long-term suppression therapy has been shown to safely and effectively prevent recurrent episodes of genital herpes, shorten the duration and frequency of recurrent episodes, reduce asymptomatic shedding, and reduce the transmission of HSV-2 between sex partners (Corey, 2018; Gnann & Whitely, 2016; Groves, 2016; Ryan, 2018a; Workowksi et al., 2015). Acyclovir (Zovirax), famciclovir (Famvir), and valacyclovir (Valtrex) appear to be similarly effective in treating recurrences. The choice of drug is based on convenience, cost, the preference of the prescriber, and beginning treatment within 24 hours of the onset of symptoms will help to shorten the duration of symptoms by one to two days (Gnann & Whitely, 2016). Although antiviral therapy is effective for preventing recurrent episodes and limiting the duration and severity of recurrences, these beneficial effects only occur during treatment with the drugs (Workowski et al., 2015). Specific treatment regimens are outlined in Tables 4 and 5 below.


Human Papilloma Virus
The human papillomavirus (HPV) is the cause of condyloma acuminata, commonly called genital warts. HPV is the cause of almost every case of cervical cancer, and infection with HPV can also cause cancer of the anus, oropharynx, penis, vagina, and vulva (CDC, 2019a; Pagliaro, 2016). Human papillomavirus infection is one of the most common STIs. Approximately 50-75% of sexually active Americans will develop an HPV infection at some time during their life (Leung, Barankin, Leong, & Hon, 2018). There are an estimated 79 million Americans infected with HPV, mostly teen to early 20s (CDC, 2019a). Most HPV infections do not cause genital warts, and the infection clears spontaneously. Still HPV infections that do not clear are a significant risk for cervical cancer (Senkomago et al., 2019).
Transmission
HPV can be transmitted by anal, oral, and vaginal sex, by skin to skin contact, and by contact with fomites (CDC, 2019a; Leung et al., 2018). HPV can be transmitted when there are no visible genital warts, and genital warts may appear years after contact with an infected person (CDC, 2019a). It is assumed that the potential for HPV transmission from an asymptomatic infected person to an uninfected person is high (Hoffman et al., 2016).
Risk Factors
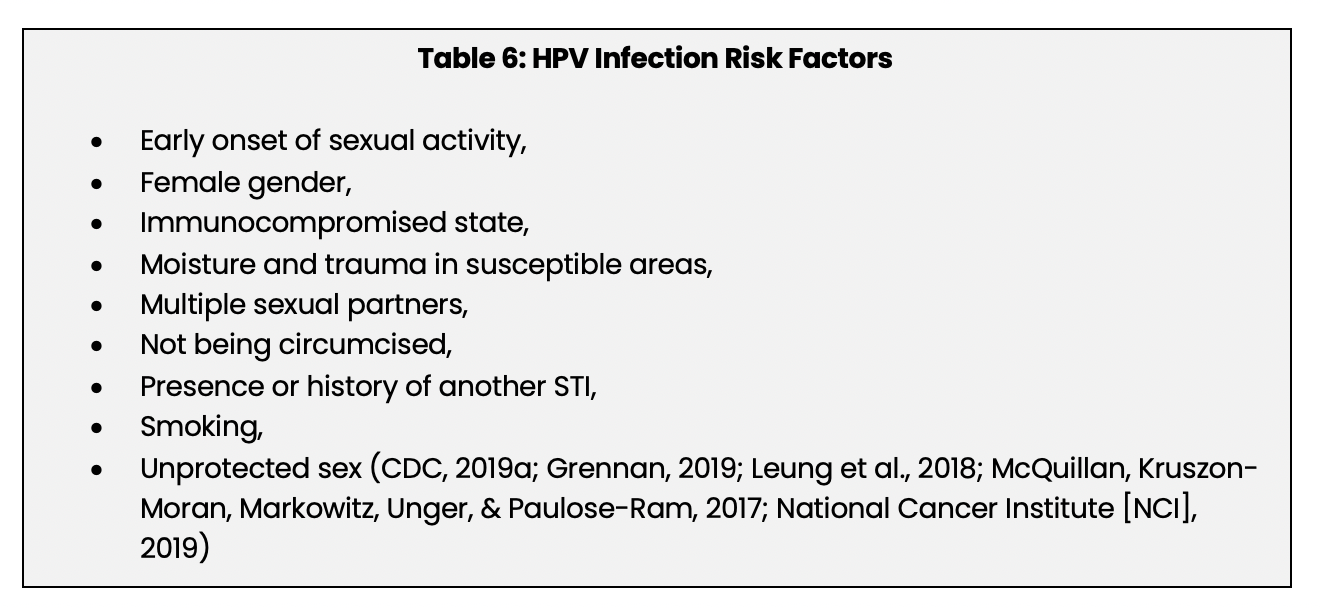
Factors that increase the risk of developing an HPV infection are listed below in Table 6:
Screening
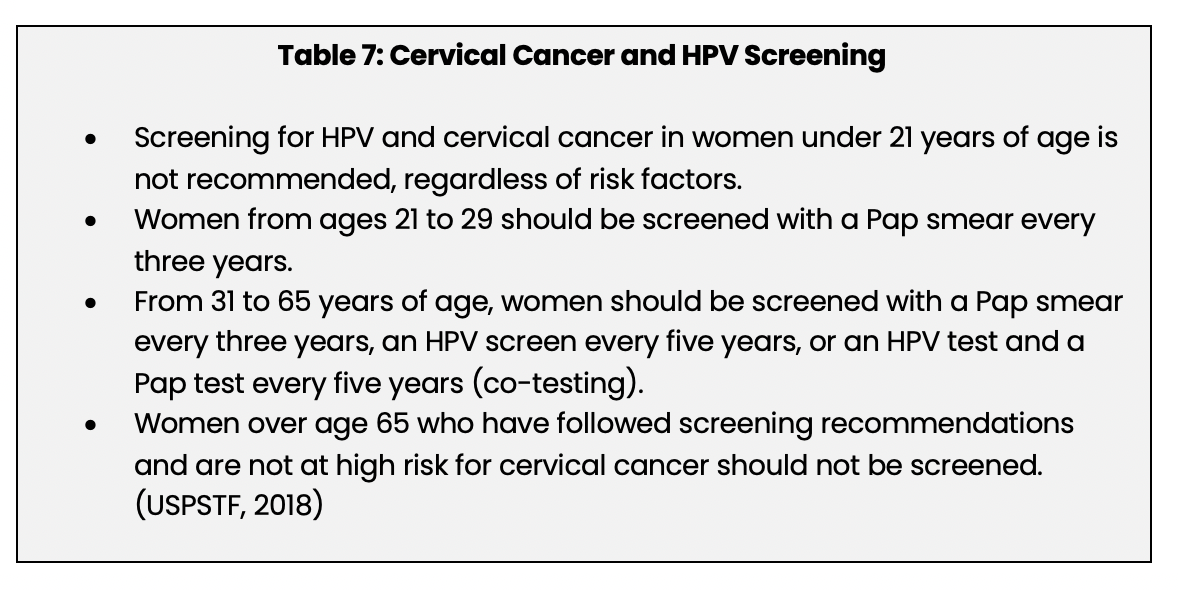
Screening for HPV is done in conjunction with screening for cervical cancer (USPSTF, 2018). The recommendations for screening developed by the USPSTF are outlined in Table 7 below. These recommendations are based on the benefits and risks of screening, and the full text can be viewed on the USPSTF website.
- The HPV test uses a sample from the cervix, and there are multiple tests available for detecting HPV DNA/RNA that have been approved by the Food and Drug Administration (FDA) (Salazar, Duhon, Olsen, & Thrall, 2019).
- Women under 21: Cervical cancer is rare in women under 21, and screening does not appear to lower the risk in this patient population (American Society of Colposcopy and Cervical Pathology, 2017; USPSTF, 2018). HPV infections, including infections with high-risk HPV types, are common in this age group, but the majority of both low-risk and high-risk HPV infections are spontaneously cleared (NCI, 2019; USPSTF, 2018). Screening for HPV DNA is only FDA-approved for women over the age of 30 and only when used in conjunction with a Pap smear (NCI, 2019). Abnormal Pap smear results called low-grade squamous epithelial lesions (LSILs) are common in these young women. These LSILS and HPV infections usually resolve spontaneously; treating women who have these abnormalities may prompt clinicians to order further diagnostic testing and perform procedures that can permanently harm the cervix and adversely affect reproductive capability (NCI, 2019). Given this information, the USPSTF concluded that the benefits of routine screening for cervical cancer and HPV in women under 21 years of age do not outweigh the risks (USPSTF, 2018).
- Screening Intervals: The optimal cervical cancer screening intervals have not been established. A considerable amount of research has shown that for women who have normal examinations, cervical cancer screening done every two to three years compared to yearly screening does not increase the risk of developing cervical cancer (NCI, 2019).
- Pap smear alone, HPV testing alone, or co-testing: These tests, solely or in combination, are recommended for screening women aged 31 to 65. All three of these tests significantly reduce the incidence of cervical cancer and reduce mortality from the disease. Each test has specific strengths and weaknesses (e.g., higher sensitivity, more false positives), and there is some disagreement among professional organizations as to which ones to use, for whom, and how often (USPSTF, 2018). The CDC recommends that the patient and her clinician discuss which tests should be done (CDC, 2019).
- Women over age 65: The USPSTF noted that cervical cancer is rare in women over age 65 years who have had normal examinations, and the benefits of screening did not outweigh the potential harms (USPSTF, 2018).
- Abnormal test results: There are protocols for repeat testing, follow-up testing, and treatment if the Pap smear or HPV tests are abnormal but discussing these is beyond the scope of this module (USPSTF, 2018).
Infection with HPV is a primary cause of anal cancer (Kobayashi et al., 2019). There are no standard guidelines regarding screening for anal HPV infections, but the general approach is to screen high-risk groups (Apaydin, Fontenot, Shtasel, Mayer, & Keuroghlian, 2018; Brown & Ermel, 2018; Senkamago et al., 2019). People at risk for anal HPV infection and anal cancer include (but are not limited to), MSM, people infected with HIV, transgender persons, and anyone who has an STI (Apaydin et al., 2018; Morris, Crane, & Eng, 2016). Screening is done with an anal Pap smear and high-resolution anoscope (Apaydin et al., 2018).
Signs and Symptoms
Most HPV infections do not cause genital warts, and the infection is spontaneously cleared within several years (Senkamgo et al., 2019). Genital warts can appear on the cervix, penis, scrotum, skin of the groin, perineal area, urethra, vagina, and vulva. Oral sex can cause warts to appear in and around the mouth and in the oropharynx. The warts are typically small, 2-5 mm in diameter, usually but not always multiple, and they may be clustered in groups or be isolated. They can be brown, white, red, or flesh-colored; they may be smooth or rough and raised from, or even with, the surface of the skin. Subclinical warts cannot be seen (Brown & Ermel, 2018; Grennan, 2019). Genital warts are usually painless. Still, some patients complain of itching and irritation, and anal warts can become very large and cause bleeding and difficulty defecating (Foss et al., 2018; Grennan, 2019). Genital warts in the vaginal introitus can cause bleeding during delivery (Bornstein, 2019). Genital warts may grow and increase in number, and warts that are treated/removed often recur. The rate of recurrence after treatment/removal has been reported to be 35-67%, and the median number of reported recurrences in a 50.4-month follow-up period was as high as ten (Leung et al., 2018; Steben, 2019).
Diagnosis
HPV infections are typically diagnosed via routine screening methods previously discussed. If they occur, anogenital warts are usually diagnosed by a clinical examination. If the diagnosis is in doubt, a biopsy can be done, and if the warts are in an area that is difficult to visualize, a colposcopy is indicated (Pennycook & McCready, 2019).
Prevention: Vaccination
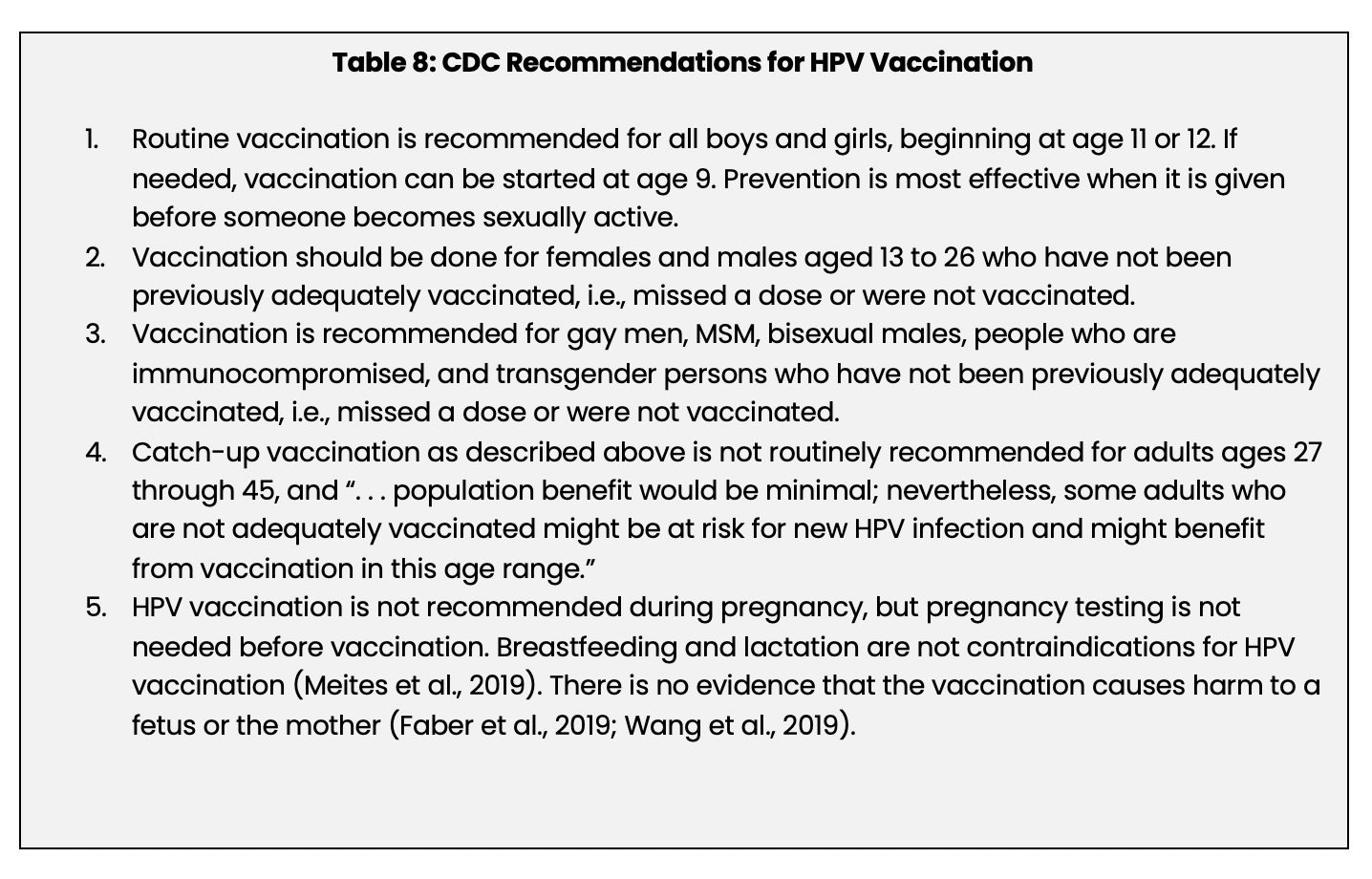
Vaccination is a safe and proven method of preventing anal, cervical, oropharyngeal, penile, vaginal, and vulvar cancers and anogenital warts caused by HPV (CDC, 2016c; Kaul et al., 2019; Meites et al., 2019). The CDC’s 2019 recommendations for HPV vaccination are listed in Table 8 below.
Treatment: Wart Removal
Genital warts can and have been treated with the following therapies:
- Caustics: Bichloroacetic acid and trichloroacetic acid (Tri-Clor).
- Cryotherapy.
- Immunomodulatory drugs: Imiquimod topical (Aldara), interferon injected into the warts, and oral cimetidine (Tagamet).
- Laser therapy.
- Podophyllin (Podocon), which destroys wart tissue by keratolysis.
- Surgical excision (Brown & Ermel, 2018; Leung et al., 2018).
There is conflicting evidence as to which treatment is more effective than the others at removing warts by the end of the treatment protocol. None of them are superior in terms of preventing recurrence. Although they can destroy and remove anogenital warts, there is no proof that they eliminate the HPV in the surrounding tissue. The choice of which treatment to use will depend on the clinician’s experience, the patient’s preference, and the location of warts, e.g., anal warts are usually removed with cryotherapy or surgical excision (Barton, Wakefield, O’ Mahony, & Edwards, 2019; Brown & Ermel, 2018; Leung et al., 2018).
Gonorrhea
Gonorrhea is caused by the Neisseria gonorrhoeae bacterium. N. gonorrhoeae infects the mucous membranes of the female and male reproductive tracts, i.e., the cervix, fallopian tubes, uterus, and urethra, as well as other mucous membranes of the eyes, mouth, rectum, and throat (CDC, 2016b). Gonorrhea is a common STI, and the CDC estimated that in 2018, there were approximately 820,000 new cases of the disease. That number is troubling, and the incidence of gonorrhea has increased 67% from 2013 to 2017 (CDC, 2018). N. gonorrhoeae has become resistant to most antibiotics (CDC, 2016b).
Transmission
Neisseria gonorrhoeae can be transmitted by anal, oral, and vaginal sexual contact (CDC, 2016b). Transmission of N. gonorrhoeae occurs more easily from men to women than from women to men. Transmission of N. gonorrhoeae after a single sexual contact occurs in 50-70% of all women and approximately 20% of all men (Ram & Rice, 2018; Ryan, 2018). About 20% of women are infected with N. gonorrhoeae after performing fellatio; infection in men and women from cunnilingus is rare. Transmission of N. gonorrhoeae from contact with infected environmental surfaces is rare (Ryan, 2018). Gonococcal conjunctivitis is rare. N. gonorrhoeae can be transmitted by ocular contact with semen, yet the infection usually occurs by autoinoculation in someone with urogenital gonorrhea (Bodurtha, Smith, Holzman, Manesh, & Perl, 2017; Ram & Rice, 2018).
Risk Factors
Factors that increase the risk of developing gonorrhea are listed below in Table 9:

Signs and Symptoms
The incubation period for urogenital gonorrhea is two to seven days. The symptoms of a gonococcal infection can be mild, and approximately 50% of infected women and 95% of infected men are asymptomatic; for those with symptoms, the nature of the signs and symptoms depend on gender and the site of the infection. The cervix is the most common site of infection in women, and the signs and symptoms may include abdominal pain, cervical inflammation, dysuria, menstrual abnormalities, urinary frequency, and mucopurulent vaginal discharge. In men, the urethra is the primary site of infection, and patients may have dysuria and a mucopurulent discharge (Ram & Rice, 2018; Ryan, 2018). If epididymitis develops, the patient has pain in the scrotum or testicles (CDC, 2016b).
Rectal gonorrhea can be caused by rectal intercourse and in women, autoinoculation from vaginal secretions. Therefore, in most women diagnosed with gonorrhea, the rectum is seldom the only infected site. Signs and symptoms of rectal gonorrhea may include bleeding, proctitis, pruritus, purulent discharge, and tenesmus (painful/ineffectual straining to pass stool or urine) (Ram & Rice, 2018; Ryan, 2018).
The incubation period of ocular gonorrhea is longer than that of urogenital gonorrhea, 3-19 days, and symptomatic ocular gonorrhea is characterized by chemosis, hyperemia, pain, and a swollen eyelid (Bodurtha et al., 2017; Ram & Rice, 2018).
Oropharyngeal gonorrhea is almost always present with a genital infection. As with the other types of gonorrhea, the patient may be asymptomatic. If symptomatic, the patient may have cervical adenitis and a sore throat (Ram & Rice, 2018; Ryan, 2018).
Complications of gonorrhea may include endometritis, epididymitis, PID, perihepatitis salpingitis, and tuboovarian abscess (Ram & Rice, 2018). Disseminated gonococcal infection (DGI) is one of the most potentially severe complications of gonorrhea and can occur with another gonorrheal infection or by itself. Between 0.5 to 3% of local gonorrheal infections may be complicated by DGI, and it is four times as common in women than in men, particularly in women who are menstruating. DGI is typically characterized by polyarthralgia, rash, and tenosynovitis and rarely, endocarditis, liver abscess, and meningitis (Burns & Graf, 2018; Lohani, Nazir, Tochamo, & Patel, 2016; Ram & Rice, 2018).
Screening
The CDC (2015) recommends screening these groups for gonorrhea routinely:
- All sexually active women under age 25, annually.
- Women over age 25 who have risk factors, annually.
- All pregnant women should be screened early during pregnancy if under 25 or at increased risk.
- All sexually active bisexual and gay men (MSM) should be screened at least annually (CDC, 2015).
However, the USPSTF concluded that there is “little direct evidence on the effectiveness of screening for gonorrhea in men or low-risk women. It previously found that screening for gonorrhea in all sexually active adults is inefficient because of its low prevalence in these groups. Moreover, the majority of genital gonococcal infections in men are symptomatic, which can result in more timely clinical presentation and lead to diagnosis and treatment that prevents serious complications.” (Lefevre, 2014, page 907).
Diagnosis
Infection with N. gonorrhoeae is diagnosed with NAAT of urine or a specimen collected by swabbing the urethra or the vagina (CDC, 2016b). Swabs of other areas can be used for the diagnosis of pharyngeal, rectal, and conjunctival infections. NAAT is not FDA-approved for these sites, but its sensitivity and specificity are superior to culture (Fyle-Thorpe, 2019).
Treatment
N. gonorrhoeae has become resistant to most antibiotics. The recommended treatment for an uncomplicated gonococcal infection of the cervix, pharynx, rectum, or urethra is a single 250 mg dose of ceftriaxone (Rocephin) given IM plus a single 1 g dose of azithromycin (Zithromax) given orally (PO). This combination effectively treats the N. gonorrhoeae as well as any potential chlamydia infection, as these two have a 1040% coinfection rate. Patients should not engage in sexual activity during treatment or for at least seven days afterwards (Fyle-Thorpe, 2019) (CDC, 2016b; Fyle-Thorpe, 2019; Ram & Rice, 2018). The CDC also recommends retesting to confirm a cure if symptoms persist (Workowski et al., 2015). An ocular gonococcal infection should be treated with saline irrigation and a single dose of ceftriaxone (Rocephin) IM (Ram & Rice, 2018). There is no vaccine for gonorrhea.
Syphilis
The Treponema pallidum bacterium causes syphilis. Syphilis is less common than chlamydia, genital warts, gonorrhea, and herpes, with 115,045 new cases reported in 2018 (CDC, 2017c). But, like gonorrhea, the incidence of syphilis has been increasing, and the CDC reported that from 2013 to 2017, primary and secondary syphilis diagnoses increased by 76% (CDC, 2018). The increase has occurred primarily in men and most of these cases are in bisexual or gay MSM; the rate of infection in these groups is much higher than in women or in men who only have sex with women (CDC, 2019b; Schmidt, Carson, & Jansen, 2019).
Syphilis is similar to other STIs in many ways, e.g., signs and symptoms, modes of transmission, but syphilis is unique among STIs because it is characterized by a latent stage that can last for years (CDC, 2017c, 2018). There is a rare form of the disease called tertiary syphilis in which the onset of symptoms may begin 10 to 30 years after the initial infection (CDC, 2017c).
Transmission
Syphilis is transmitted during anal, oral, and vaginal sex, by direct contact with a syphilitic lesion, i.e. a chancre, condylomata lata, or an infected area of mucous membrane (CDC, 2017c; Lukehart, 2018; Ryan, 2018). Transmission of syphilis has been reported to occur in >50% of sexual contacts with an infected person who has s syphilitic lesion (Ryan, 2018).
Risk Factors
Factors that increase the risk of developing syphilis are listed in Table 10 below.
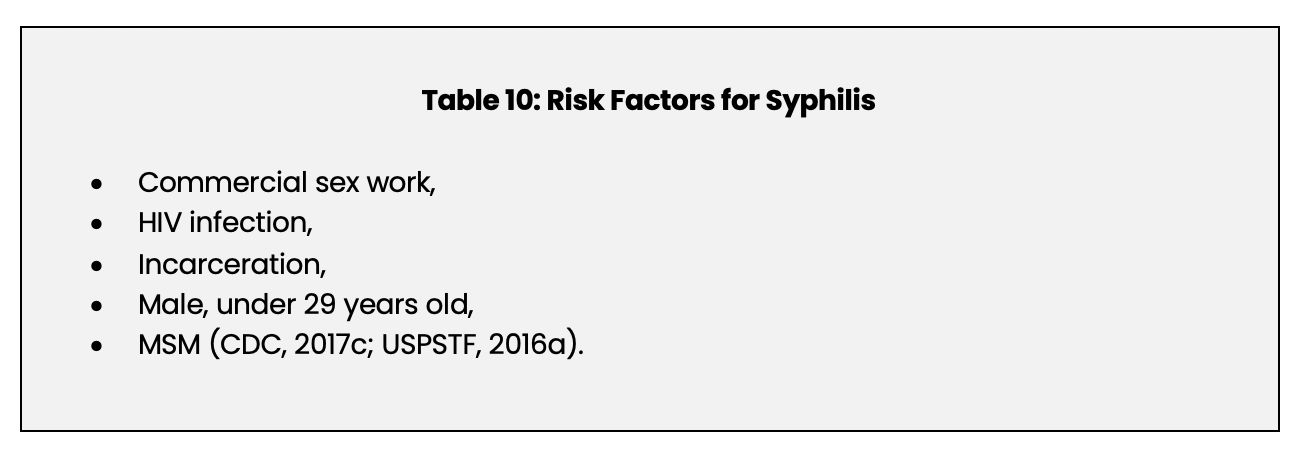
An untreated syphilis infection can increase the risk of transmitting and acquiring an HIV infection (CDC, 2017c). Co-infection with HIV and syphilis has been reported to be five times higher in MSM than in HIV infected men who do not have sex with men (USPSTF, 2016a).
Signs and Symptoms
There are four stages of syphilis infection: primary, secondary, latent, and tertiary. Primary syphilis occurs after the incubation period, lasting for 3-90 days, and is characterized by the development of the primary syphilitic lesion, the chancre. The chancre begins as a papule on the genital area, the cervix, the labia, the mouth, or the anal area, and then progresses to an ulcer. The chancre heals spontaneously in four to six weeks (Lukehart, 2018; Ryan, 2018).
Approximately one-third of patients will develop secondary syphilis. Secondary syphilis begins two to eight weeks after the chancre develops. This stage is characterized by lymph node enlargement, fever, malaise, and a mucocutaneous maculopapular rash on the extremities, face, palms, soles of the feet, and trunk. Patients may also develop lesions called condylomata lata, a wart-like erosion on the mucous membranes. The signs and symptoms of secondary syphilis will disappear in several days or weeks, and the infection may be cleared. Still, in approximately two-thirds of all patients, syphilis will then enter the latent phase (Ryan, 2018).
Latent syphilis is often asymptomatic and divided into two categories: early and late. Early latent syphilis is defined as up to one year after the initial infection, during which the patient is infectious (Harmon & Robertson, 2019). Late latent syphilis is defined as at least one year after the infection, and during this time, the patient is not infectious. Although usually asymptomatic, if latent syphilis persists over years, some patients may develop occasional episodes of systemic symptoms; these relapses become progressively less severe over time (Harmon & Robertson, 2019; Ryan, 2018).
Tertiary syphilis develops in approximately 15 to 33% of infected and untreated patients. The onset of signs and symptoms can occur from 3-30 years after the initial infection in otherwise healthy patients; in patients who are immunocompromised, tertiary syphilis onset can be within months. Tertiary syphilis causes cardiovascular, ocular, and neurologic damage as well as granulomatous lesions called gummas. Cardiovascular complications of tertiary syphilis are very rare but may occur 10-40 years after the infection; they can include aortic aneurysm, aortitis, aortic regurgitation, and arterial stenosis. Ocular syphilis can cause decreased visual acuity and blindness. Gummas are granulomatous inflammatory lesions that can affect any organ and any bone or cartilage but are typically found on the skin. Gummas can be microscopic or several centimeters in diameter, and although they may be painless, they can also ulcerate and become necrotic (Harmon & Robertson, 2019; Lukehart, 2018; Merson & Shehu, 2019).
Neurologic complications of syphilis are complex. Although they can be a prominent part of tertiary syphilis, they can occur at any time during a syphilis infection: during the first few weeks after an infection or many years later. The signs and symptoms are quite varied. They could include behavioral changes, coordination and sensory deficits, dementia, hearing loss, meningitis, and paralysis. Asymptomatic infection of the cerebral spinal fluid is possible as well (Harmon & Robertson, 2019; Lukehart, 2018; Merson & Shehu, 2019). Neurologic complications of syphilis are more common in patients also infected with HIV (Lukehart, 2018).
Screening
The USPSTF recommends screening for syphilis in asymptomatic, non-pregnant adolescents and adults who have a high risk for contracting the disease. High-risk individuals were identified based on population surveillance data, and they include:
- MSM,
- people who are infected with HIV,
- anyone involved in commercial sex work,
- males under 29 years of age,
- anyone who because of ethnicity, geographical location, or race has a high risk (Cantor, Pappas, Daeges, & Nelson, 2016).
The optimal screening interval has not been determined (Cantor et al., 2016).
All pregnant women should be screened for syphilis at the first prenatal visit. If the patient has a high risk for developing syphilis or if she lives in an area where the disease is endemic, she should be screened again during the third trimester (Workowski et al., 2015).
Diagnosis
Screening is a two-step process to detect antibodies in the blood, not the Treponema palladium organism. First, a nontreponemal test is done; this can be either the venereal disease research lab (VDRL) or the rapid plasma regain (RPR) (Cantor et al., 2016). These tests detect antibodies that have been produced in response to cellular damage caused by syphilis (Harmon & Robertson, 2019) If positive, these tests should be followed by a treponemal antibody test for confirmation, which detects antibodies to T. palladium. This process reveals past exposure or a current infection (Cantor et al., 2016). The same tests should be used to diagnose syphilis in a patient who is infected with HIV, but there have been reports of testing inaccuracies in this patient population, e.g., false negatives. If an HIV-infected patient has the signs and symptoms of syphilis but testing is inconclusive or negative, other diagnostic tests should be considered (Workowski et al., 2015).
Treatment
There is no vaccine to prevent syphilis. Patients who have primary, secondary, or early latent syphilis should be treated with one dose of 2.4 million units of penicillin G benzathine (Bicillin L-A) IM. Other forms of penicillin should not be used as a substitute (Harmon & Robertson, 2019; Merson & Shehu, 2019). The treatment for tertiary syphilis or late latent syphilis is three doses of 2.4 million units of penicillin G benzathine (Bicillin L-A) IM given weekly. Pregnant women can be treated with two weekly doses. If the patient has a penicillin allergy, a penicillin desensitization program can be initiated (Workowski et al., 2015). The treatment for neurosyphilis is 18 to 24 million units of intravenous penicillin G sodium daily for 10-14 days. This may be administered as 3-4 million units every 4 hours or 24 million units as a continuous infusion over 24 hours. Alternatively, 10-14 days of 2.4 million units of penicillin G procaine IM once daily plus probenecid 500 mg PO four times a day may be used (Harmon & Robertson, 2019; Workowski et al., 2015).
Between 50-75% of patients who have early syphilis develop the Jarisch-Herxheimer reaction from penicillin. The Jarisch-Herxheimer reaction begins 4 to 12 hours after the injection, and is characterized by fever, headache, and myalgias. The reaction is short-lived, lasting approximately 24 hours, and resolves without sequelae; in pregnant women, it may precipitate labor (Whitely, 2019).
Trichomoniasis
Trichomoniasis is an STI that is caused by the protozoan parasite Trichomonas vaginalis. The CDC (2017d) has estimated that approximately 3.7 million people in the United States are infected with Trichomonas vaginalis. Still, trichomoniasis is not a reportable disease and many people who are infected are asymptomatic, so the actual number of cases is likely to be higher (CDC, 2017d). The cure rate for trichomoniasis is very high, but reinfection is common, and the disease is easily transmitted. One study found that 72% of men whose female partners were infected became infected. Therefore, treatment of all sexual partners is extremely important (Alessio & Nyirjesy, 2019; CDC, 2017d; Rosenberger & Fisk, 2017).
Transmission
Trichomonas vaginalis can infect the cervix, genital tract, prostate, vagina, or urethra, and it is transmitted during penile-vaginal intercourse and from vagina to vagina contact (Alessio & Nyirjesy, 2019; CDC, 2017d; Ryan, 2018b). There is no evidence that T. vaginalis is transmitted by anal or oral sex, and male-to-male transmission appears to be uncommon (Van Gerwen & Muzny, 2019; Workowski et al., 2015). There is evidence that T. vaginalis can be transmitted by infected fomites and (possibly) infected water (Kissinger, 2015).
Risk Factors
Factors that increase the risk for developing trichomoniasis are listed below in Table 11.
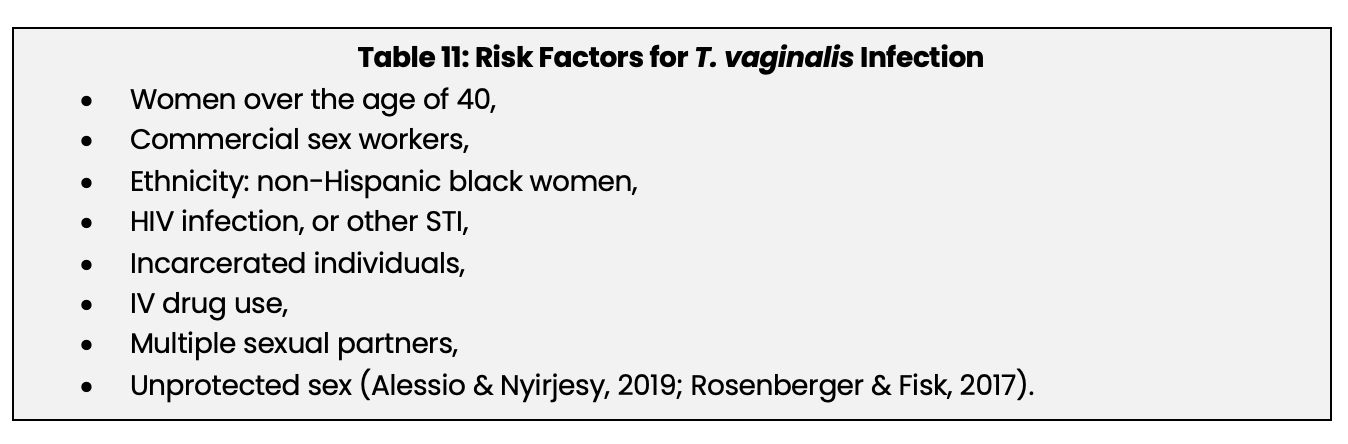
Signs and Symptoms
The incubation time of T. vaginalis is 3-28 days, but the symptoms can occur after that time frame (CDC, 2017d; Kissinger, 2015; Rosenthal, 2020). Approximately 70% of infected persons are asymptomatic, and asymptomatic infections are more common in men than in women (CDC, 2017d; Rosenthal, 2020; Ryan, 2018b). Infected women may develop cervical and vaginal inflammation, a copious, malodorous vaginal discharge, dyspareunia, dysuria, lower abdominal pain, pruritis, or vulvar-vaginal discomfort (CDC, 2017d; Rosenberger & Fisk, 2017; Rosenthal, 2020). Infected men may develop dysuria, lower abdominal pain, pruritis, a urethral discharge, or urinary frequency (Alessio & Nyirjesy, 2019). In men, a T. vaginalis infection is usually cleared in less than 10 days, but for women, it may persist. Up to 50% of women can become asymptomatic carriers for months or years (Kissinger, 2015; Rosenberger & Fisk, 2017). Reinfection that occurs several months after treatment occurs in approximately one of every five patients (CDC, 2017d). Complications of trichomoniasis include cervical dysplasia, infertility, PID, low birth weight, preterm birth, or vaginal abscess; men may develop balanoposthitis, decreased sperm motility, epididymitis, or prostatitis (Alessio & Nyirjesy, 2019; Patel, Gaydos, Packman, Quinn, & Tobian, 2018; Rosenberger & Fisk, 2017; Whitely, 2019). Trichomoniasis vaginalis infection increases the risk of acquiring and transmitting HIV (CDC, 2017d; Patel et al., 2018). A 2019 review and meta-analysis concluded that infection with T. vaginalis increases a woman’s risk of developing an HIV infection by 50% (Masha, Cools, Sanders, Vaneechoutte, & Crucitti, 2019). No information was located on the relationship between T. vaginalis infection and HIV in men.
Screening
Screening for T. vaginalis is recommended annually for women who are infected with HIV, but there are no other populations for whom routine screening is recommended (Van Gerten & Muzny, 2019; Workowski et al., 2015). The CDC does recommend that providers consider screening high-risk women (CDC, 2015).
Diagnosis
A urine sample or swab of fluid/secretions from the urethra or vagina may be used to test for T. vaginalis RNA or antigens, and the tests are highly sensitive and specific (Alessio & Nyirjesy, 2019; Workowski et al., 2015).
Treatment
As previously stated, all known partners should be treated simultaneously, and patients should wait for seven days after treatment before resuming sexual intercourse. The standard recommended treatment for asymptomatic and symptomatic patients infected with T. vaginalis is a single 2 g dose of either metronidazole (Flagyl) or tinidazole (Tindamax), given PO (Alessio & Nyirjesy, 2019; Rosenberger & Fisk, 2017; Ryan, 2018b). This regimen is very effective; the cure rates are 90-95% for metronidazole (Flagyl) and 86-100% for tinidazole (Tindamax) (Alessio & Nyirjesy, 2019). Women who are HIV-positive should be given metronidazole 500 mg PO twice a day for seven days (Rosenberger & Fisk, 2017; Workowski et al., 2015). Metronidazole (Flagyl) can be taken during pregnancy, but tinidazole (Tindamax) should not (Rosenberger & Fisk, 2017). Treatment for resistant strains is empirical, and it generally involves using higher doses of the same medications. Patients should not drink alcohol while they are taking metronidazole (Flagyl) or tinidazole (Tindamax) or for 24 hours after taking metronidazole (Flagyl) or 72 hours after taking tinidazole (Tindamax). The combination of these drugs and alcohol can cause a disulfiram-like reaction with nausea, vomiting, dizziness, headache, flushing, etc. (Alessio & Nyirjesy, 2019). There is no vaccine that prevents infection with T. vaginali
Nursing Considerations
Assessment of sexual health is important. The CDC (Workowski et al., 2015) recommends an interview technique for assessing the risk of STIs using questions that address the following categories, the five Ps:
Partners
- Who do you have sex with? Men, women, or men and women?
- How many sex partners have you had in the past two months?
- How many sex partners have you have in the past 12 months?
- Do you know if, or is it possible that, any of the sex partners you have had in the past 12 months had sex with someone else?
Practices
- What type of sex have you recently had: anal, oral, or vaginal? Describe the type of sex you have.
- Do you ever use condoms or another form of protection? If so, do you use them all the time or sometimes?
- If you don’t use condoms or another form of protection, why not?
Prevention
- What do you do to prevent becoming pregnant?
Protection
- How do you protect yourself from getting STIs?
Past History
- Have you ever had an STI? Have you had sex with someone who had an STI?
- Do you inject drugs?
- Have you ever traded sex for drugs or money? (Workowski et al., 2015)
Patients who have an STI should be notified as soon as possible. Clinicians may prefer to speak to the patient in person or by phone, but some patients, particularly patients aged 15 to 24, may prefer notification by text message (Sieving, Gerwitz O’Brien, Saftner, & Argo, 2019).
Preventing transmission of STIs is critically important, and part of this effort involves reporting the STI to the health department, notifying sexual partners of the potential infection, and treating sexual partners. Each state and county health department have specific requirements for which STIs must be reported and regarding partner notification (Sieving et al., 2019). Sexual partners can be treated directly by a clinician or by a process called expedited partner therapy (EPT). With EPT, the patient who has been diagnosed and treated for an STI is either given the appropriate medication or a prescription for the appropriate medication to deliver to the possibly-infected partner. The CDC has endorsed EPT, especially for chlamydia and gonorrhea (Nemeth & Schillinger, 2019). EPT is legal in 44 states, potentially allowable in five additional states, and prohibited in South Carolina. Research indicates that EPT reduces the incidence of STIs (Weiss et al., 2019).
References
Agyemang, E., Magaret, A.S., Selke, S., Johnston, C., Corey, L., & Wald, A. (2018). Herpes simplex virus shedding rate: Surrogate outcome for genital herpes recurrence frequency and lesion rates, and phase 2 clinical trials end point for evaluating efficacy of antivirals. Journal of Infectious Diseases, 218(11), 1691-1699. doi: 10.1093/infdis/jiy372.
Alessio, C., & Nyirjesy, P. (2019). Management of resistant trichomoniasis. Current Infectious Disease Reports, 21(9), 31. doi: 10.1007/s11908-019-0687-4.
American Society of Colposcopy and Cervical Pathology. (2017). ASCCP Five things physicians and patients should question. Retrieved from http://www.choosingwisely.org/wp-content/uploads/2017/02/ASCCP-5things-List_NewLogoUpdated_100319.pdf
Apaydin, K.Z., Fontenot, H.B., Shtasel, D.L., Mayer, K.H., & Keuroghlian, A.S. (2018). Primary care provider practices and perceptions regarding HPV vaccination and anal cancer screening at a Boston community health center. Journal of Community Health, 43(4), 792-801. doi: 10.1007/s10900-018-0486-0.
Ayoub, H.H., Chemaitelly, H., & Abu-Raddad, L.J. (2019). Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: Model-based predictions. BMC Medicine, 17(1), 57. doi: 10.1186/s12916-019-1285-x.
Barton, S., Wakefield, V., O' Mahony, C., & Edwards, S. (2019). Effectiveness of topical and ablative therapies in treatment of anogenital warts: A systematic review and network meta-analysis. BMJ Open, 9(10), e027765. doi: 10.1136/bmjopen-2018-027765.
Bornstein, J. (2019). Chapter 40: Benign disorders of the vagina & vulva. In: Alan H. DeCherney, Lauren Nathan, Neri Laufer & Ashley S. Roman (Eds). Current diagnosis & treatment: Obstetrics & gynecology, (12th ed.). New York, NY: McGraw-Hill Education [Online Edition].
Boslett, B.A., & Schwartz, B.S. (2020).Chlamydia trachomatis infections. In: Maxine A. Papadakis, Stephen J. McPhee, & Michael W. Rabow (Eds). Current medical diagnosis and treatment 2020. New York, NY: McGraw-Hill Education [Online edition].
Brown, D.R., & Ermel, A. (2018). Chapter 193: Human papillomavirus infections. In: J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, & Joseph Loscalzo (Eds). Harrison’s principles of internal medicine, (20th ed.). New York, NY: McGraw-Hill Education [Online edition].
Cai, T., Pisano, F., Nesi, G., Magri, V., Verze, P., Perletti, G., . . . Bartoletti, R. (2017). Chlamydia trachomatis versus common uropathogens as a cause of chronic bacterial prostatitis: Is there any difference? Results of a prospective parallel-cohort study. Investigative and Clinical Urology, 58(6), 460-467. doi: 10.4111/icu.2017.58.6.460.
Cantor, A.G., Pappas, M., Daeges, M., & Nelson, H.D. (2016). Screening for syphilis: Updated evidence report and systematic review for the US Preventive Services Task Force. Journal of the American Medical Association, 315(21), 2328-37. doi: 10.1001/jama.2016.4114.
The Centers for Disease Control and Prevention. (2015). Screening recommendations and considerations referenced in treatment guidelines and original sources. Retrieved from https://www.cdc.gov/std/tg2015/screening-recommendations.htm
The Centers for Disease Control and Prevention. (2016a). Chlamydia - CDC Fact Sheet (Detailed). Retrieved from https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm.
The Centers for Disease Control and Prevention. (2016b). Gonorrhea- CDC fact sheet (Detailed Version). Retrieved from https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm. b
The Centers for Disease Control and Prevention. (2016c). HPV vaccine recommendations. Retrieved from https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.htm.
The Centers for Disease Control and Prevention. (2017a). Genital Herpes – CDC fact sheet. Retrieved from https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm.
The Centers for Disease Control and Prevention (2017b). Genital Herpes: Genital Herpes Screening Questions. Retrieved from https://www.cdc.gov/std/herpes/screening.htm.
The Centers for disease Control and Prevention. (2017c). Syphilis. CDC Fact sheet (Detailed). Retrieved from https://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm.
The Centers for Disease Control and Prevention. (2017d). Trichomoniasis - CDC Fact Sheet. Retrieved from https://www.cdc.gov/std/trichomonas/STDFact-Trichomoniasis.htm.
The Centers for Disease Control and Prevention. (2018). New CDC analysis shows steep and sustained increases in STDs in recent years. Retrieved from https://www.cdc.gov/media/releases/2018/p0828-increases-in-stds.html.
The Centers for Disease Control and Prevention. (2019a). Human papilloma virus (HPV)– fact sheet. Retrieved from https://www.cdc.gov/std/hpv/stdfact-hpv.htm.
The Centers for Disease Control and Prevention. (2019b). Sexually transmitted disease surveillance. Retrieved from https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report.html.
Corey, L. (2018). Chapter 187: Herpes simplex virus infections. In: J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo & Joseph Loscalzo (Eds). Harrison’s Principles of Internal Medicine, (20th ed.). New York, NY: McGraw-Hill Education [Online edition].
Deese, J., Pradhan, S., Goetz, H., & Morrison, C. (2018). Contraceptive use and the risk of sexually transmitted infection: systematic review and current perspectives. Open Access Journal of Contraception, 9, 91-112. doi: 10.2147/OAJC.S135439.
den Heijer, C.D.J, Hoebe, C.J.P.A., Driessen, J.H.M., Wolffs, P., van den Broek, I.V.F., Hoenderboom, B.M., . . . Dukers-Muijrers , N.H.T.M (2019). Chlamydia trachomatis and the risk of pelvic inflammatory disease, ectopic pregnancy, and female infertility: A retrospective cohort study among primary care patients. Clinical Infectious Diseases, 69(9), 1517-1525. doi: 10.1093/cid/ciz429.
Faber, M.T., Duun-Henriksen, A.K., Dehlendorff, C., Tatla, M.K., Munk, C., & Kjaer SK. (2019). Adverse pregnancy outcomes and infant mortality after quadrivalent HPV vaccination during pregnancy. Vaccine, 37(2), 265-271. doi: 10.1016/j.vaccine.2018.11.030.
Foss, H.E., Blank, J.J., Lundeen, S.J., Peterson, C.Y., Ludwig, K.A., & Ridolfi, T.J. (2018). Race is associated with burden of anal condyloma and need for operative intervention. Journal of Surgical Research, 232, 629-634. doi: 10.1016/j.jss.2018.07.020.
Fyle-Thorpe, O. (2019) Chlamydia and gonorrhea: An update. The Journal for Nurse Practitioners, 15(6), 424-428. Doi: 10.1016/j.nurpra.2018.12.027
Gaydos, C.A., & Quinn, T.C. (2018). Chapter 184: Chlamydial infections. In J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, & Joseph Loscalzo (Eds). Harrison’s Principles of Internal Medicine, (20th ed.) New York, NY: McGraw-Hill Education. [Online edition].
Gnann, J.W. Jr, & Whitley. R. J. (2016). Genital herpes. New England Journal of Medicine, 375(7), 666-674. doi: 10.1056/NEJMcp1603178.
Gnann, J. W., Jr, & Whitley, R. J. (2017). Herpes simplex encephalitis: An update. Current Infectious Diseases Reports, 19(3), 13. doi: 10.1007/s11908-017-0568-7.
Grennan, D. (2019). Genital warts. Journal of the American Medical Association, 321(5), 520. doi: 10.1001/jama.2018.20181.
Groves, M.J. (2016). Genital herpes: A review. American Family Physician, 93(10), 928-934.
Harmon, E.D., & Robertson, E.W. (2019). Syphilis: A growing concern. Nurse Practitioner, 44(8), 21-28. doi: 10.1097/01.NPR.0000558159.61349.cb.
Hoffman, B.L., Schorge J.O., Bradshaw, K.D, Halvorson, L.M., Schaffer, J.L., & Corton, M. M. (2016). Chapter 29: Preinvasive lesions of the lower genital tract. Williams Gynecology, (3rd ed.). New York, NY; McGraw-Hill Education [Online edition].
Hwang, L.Y., Ma, Y., & Moscicki, A.B. (2014). Biological and behavioral risks for incident Chlamydia trachomatis infection in a prospective cohort. Obstetrics and Gynecology, 124 (5):954-960. doi: 10.1097/AOG.0000000000000429.
Katz, M.H. (2020). 31-04: HIV infection & AIDS: Complications. In: Maxine A. Papadakis, Stephen J. McPhee & Michael W. Rabow (Eds). Current medical diagnosis and treatment 2020. McGraw-Hill Education: New York, NY. [Online edition].
Kaul, S., Do, T.Q.N., Hsu, E., Schmeler, K.M., Montealegre, J.R., & Rodriguez, A.M. (2019). School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Research, 22,(8), 100-189. doi: 10.1016/j.pvr.2019.100189.
Khosropour, C.M., Soge, O.O., Suchland R, Leipertz, G., Unutzer, A., Pascual, R., . . . Golden, M.R. (2019). Recurrent/intermittent vaginal and rectal chlamydial infection following treatment: A prospective cohort study among female sexually transmitted disease clinic patients. Journal of Infectious Diseases, 220(3), 476-483. doi: 10.1093/infdis/jiz113.
Kissinger, P. (2015). Epidemiology and treatment of trichomoniasis. Current Infectious Disease Reports, 17(6), 484. doi: 10.1007/s11908-015-0484-7.
Kobayashi, T., Sigel, K., Kalir, T., MacLeod, I.J., Liu, Y., & Gaisa, M. (2019). Anal cancer precursor lesions in HIV-infected persons: Tissue human papillomavirus type distribution and impact on treatment response. Diseases of the Colon and Rectum, 62(5), 579-585. doi: 10.1097/DCR.0000000000001307.
Lang, A. S., An der Heiden, M., Jansen, K., Sailer, A., Bremer, V., & Dudareva, S. (2018). Chlamydia trachomatis laboratory sentinel team. Not again! Effect of previous test results, age group and reason for testing on (re-)infection with Chlamydia trachomatis in Germany. BMC Infectious Diseases, 18(1):424. doi: 10.1186/s12879-018-3323-2.
LeFevre, M.L., on behalf of the U.S. Preventive Services Task Force. (2014). Screening for Chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 161(12), 902-10. doi: 10.7326/M14-1981.
LeGoff, J., Péré, H., & Bélec. L. (2014). Diagnosis of genital herpes simplex virus infection in
the clinical laboratory. Virology Journal, 12(11), 83. doi: 10.1186/1743-422X-11-83.
Leung, A.K., Barankin, B., Leong, K.F., & Hon, K.L. (2018). Penile warts: An update on their evaluation and management. Drugs in Context, 19(7), 212-563. doi: 10.7573/dic.212563.
Levinson, W., Chin-Hong, P., Joyce, E.A., Nussbaum, J., & Schwartz, B. (2018). Chapter 74: Pelvic infections. In: Review of medical microbiology & immunology: A guide to clinical infectious diseases, (15th ed.). New York, NY: McGraw-Hill Education [Online edition].
Lohani, S., Nazir, S., Tachamo, N., & Patel, N. (2016). Disseminated gonococcal infection: An unusual presentation. Journal of Community Hospital Internal Medicine Perspectives, (3), 31841. doi: 10.3402/jchimp.v6.31841.
Lukehart, S.A. (2018). Chapter 177: Syphilis. In: J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, & Joseph Loscalzo (Eds). Harrison’s principles of internal medicine, 20th ed. New York, NY: McGraw-Hill Education;2018. [Online edition].
Masha, S.C., Cools, P., Sanders, E.J., Vaneechoutte, M., & Crucitti, T. (2019). Trichomonas vaginalis and HIV infection acquisition: A systematic review and meta-analysis. Sexually Transmitted Infections, 95(1), 36-42. doi: 10.1136/sextrans-2018-053713.
McQuillan, G., Kruszon-Moran, D. Flagg, E.W., & Paulose-Ram, R. (2018). Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief No. 304. Retrieved from https://www.cdc.gov/nchs/products/databriefs/db304.htm.
McQuillan, G., Kruszon-Moran, D., Markowitz, L.E., Unger E.R., & Paulose-Ram, R. (2017). Prevalence of HPV in adults aged 18–69: United States, 2011–2014. NCHS Data Brief No. 280. Retrieved from https://www.cdc.gov/nchs/data/databriefs/db280.pdf
Meites, E., Szilagyi, P.G., Chesson, H.W., Unger, E.R., Romero, J.R., & Markowitz. L.E. (2019). Human papillomavirus vaccination for adults: Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and Mortality Weekly Report, 68(32), 698-702. doi: 10.15585/mmwr.mm6832a3.
Merson, J.R., & Shehu, M. (2019). Syphilis. Journal of the Association of Physician Assistants, 32(5), 59-60. doi: 10.1097/01.JAA. 0000554749.77547.b1.
Morris, V., Crane, C.H., & Eng, C. (2016). Chapter 25: Anal Cancer. In: Hagop M. Kantarjian & Robert A. Wolff. (Eds). The MD Anderson manual of medical oncology, 3rd ed. New York, NY: McGraw-Hill Education;2016. [Online edition].
National Cancer Institute. (2019). Cervical cancer screening (PDQ®)–Health professional version. Retrieved from https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq
Nemeth, S.V., & Schillinger, J.A. (2019). Overcoming the challenges of studying expedited partner therapy in the real world. Sexually Transmitted Diseases, 46(10), 693-696. doi: 10.1097/OLQ.0000000000001047.
Noska, A., Kyrillos, R., Hansen, G., Hirigoyen, D., & Williams, D.N. (2016). The role of antiviral therapy in immunocompromised patients with herpes simplex virus meningitis. Clinical Infectious Diseases, 60 (2), 237-242. doi: 10.1093/cid/ciu772.
Omori, R., Nagelkerke, N., & Abu-Raddad, L.J. (2018). HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sexually Transmitted Infections, 94 (5), 372-376. doi: 10.1136/sextrans-2017-053336.
Pagliaro, L.C. (2016). Chapter 38: Penile cancer. In: Hagop M. Kantarjian & Robert A. Wolff. (Eds). The MD Anderson manual of medical oncology, 3rd ed. New York, NY: McGraw-Hill Education;2016. [Online edition].
Patel, E.U., Gaydos, C.A., Packman, Z.R., Quinn, T.C., & Tobian, A.A.R.. (2018). Prevalence and correlates of trichomonas vaginalis infection among men and women in the United States. Clinical Infectious Diseases, 67 (2), 211-217. doi: 10.1093/cid/ciy079.
Pennycook, K.B., & McCready, T.A. (2019). Condyloma acuminatum. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Ram, S., & Rice, P.A. (2018). Chapter 151: Gonococcal infections. In: J. Larry Jameson, Anthony S. Fauci, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, & Joseph Loscalzo (Eds). Harrison’s principles of internal medicine, 20th ed. New York, NY: McGraw-Hill Education;2018. [Online edition].
Rosenberger, K.D., & Fisk, C. (2017). A missed diagnosis of trichomoniasis. Nurse Practitioner, 42(2), 1-4. doi: 10.1097/01.NPR.0000511777.44250.20.
Rosenthal, P.J. (2020). 35-12: Trichomoniasis. In: Maxine A. Papadakis, Stephen J. McPhee, Michael W. Rabow (Eds). Current medical diagnosis and treatment 2020. New York, NY: McGraw-Hill Education;2020. [Online edition].
Ryan, K.J. (2018a). Chapter 14: Herpes viruses. Sherris medical microbiology, 7th ed. New York, NY: McGraw-Hill Education. [Online edition].
Ryan, K.J. (2018b). Chapter 53: Sarcomastigophora -The flagellates. Sherris medical microbiology, 7th ed. New York, NY: McGraw-Hill Education. [Online edition].
Salazar, K.L., Duhon, D.J., Olsen, R., & Thrall, M. (2019). A review of the FDA-approved molecular testing platforms for human papillomavirus. Journal of the American Society of Cytopathology, 8(5), 284-292. doi: 10.1016/j.jasc.2019.06.001.
Sieving, R.E., Gewirtz O'Brien, J.R., Saftner, M.A., & Argo, T.A. (2019). Sexually transmitted diseases among US adolescents and young adults: Patterns, clinical considerations, and prevention. Nursing Clinics of North America, 54(2), 207-225. doi: 10.1016/j.cnur.2019.02.002.
Senkomago, V., Henley, S.J., Thomas, C.C., Mix, J.M., Markowitz, L.E, & Saraiya M. (2019). Human papillomavirus-attributable cancers - United States, 2012-2016. MMWR Morbidity and Mortality Weekly Report, 68 (33), 724-728. doi: 10.15585/mmwr.mm6833a3.
Schmidt, R., Carson, P.J., & Jansen, R.J. (2019). Resurgence of syphilis in the United States: An assessment of contributing factors. Infectious Diseases (Auckl), 16 (12), 1178633719883282. doi: 10.1177/1178633719883282.
Spitzer, E.D. (2019). Chapter 5: Infectious diseases. In: Michael Laposata (Ed). Laposata's laboratory medicine: Diagnosis of disease in the clinical laboratory, 3rd ed. New York, NY: McGraw-Hill Education;2019. [Online edition].
Steben, M. (2019). A very common intimate concern: "Will my genital warts ever stop recurring?" Journal of Infectious Diseases, 219(5), 682-684. doi: 10.1093/infdis/jiy610.
Takata, K, Fukuda H, Umeda K, Yamauchi R, Fukuda S, Kunimoto H, . . . Sakisaka, S. (2018). Fitz-Hugh-Curtis syndrome in a man positive for Chlamydia trachomatis. Clinical Journal of Gastroenterology, 11(4), 338-342. doi: 10.1007/s12328-018-0829-5.
U.S. Department of Health and Human Services. (2019). Gonorrhea. Retrieved from https://www.hhs.gov/opa/reproductive-health/fact-sheets/sexually-transmitted-diseases/gonorrhea/index.html
US Preventive Services Task Force. (2016a). Screening for syphilis infection in nonpregnant adults and adolescents. US Preventive Services Task Force recommendation statement. Journal of the American Medical Association, 315(21), 2321-2327. doi:10.1001/jama.2016.5824
US Preventive Services Task Force. (2016b). Serologic screening for genital herpes infection. US Preventive Services Task Force recommendation statement. Journal of the American Medical Association, 316(23), 2525-2530. doi:10.1001/jama.2016.16776.
US Preventive Services Task Force. (2018). Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Journal of the American Medical Association, 320(7), 674-686. doi: 10.1001/jama.2018.10897.
Van Gerwen, O.T., & Muzny, C.A. (2019). Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Research, 20, 8. doi: 10.12688/f1000research.19972.1.
Wang, A., Liu, C., Wang, Y., Yin, A., Wu, J., Zhang, C., . . . Huang, Y. (2019). Pregnancy outcomes after human papillomavirus vaccination in periconceptional period or during pregnancy: A systematic review and meta-analysis. Human Vaccines & Immunotherapeutics. 7 1-9. doi: 10.1080/21645515.2019.1662363.
Whiteley, G.E. (2019). Chapter 45: Sexually transmitted diseases & pelvic infections. In Alan H. DeCherney, Lauren Nathan, Neri Laufer & Ashley S. Roman (Eds). Current diagnosis & treatment: Obstetrics & gynecology, 12th ed. New York, NY: McGraw-Hill Education;2019. [Online Edition].
Wiesenfeld, H.C. (2017). Screening for Chlamydia trachomatis infections in women. New England Journal of Medicine, 376(8), 765-773. doi: 10.1056/NEJMcp1412935.
Weiss, K.M., Jones, J.S., Katz, D.A., Gift, T.L., Bernstein, K., Workowski, K., . . . Jenness, S.M. (2019). Epidemiological impact of expedited partner therapy for men who have sex with men: A modeling study. Sexually Transmitted Diseases, 46(11): 697-705. doi: 10.1097/OLQ.0000000000001058.
Workowski, K.A., Bolan, G.A., & the Centers for Disease Control and Prevention. (2015). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep, 64 (RR-03), 1-137.