About this course:
The purpose of this course is to ensure that the advanced practice registered nurse (APRN) is cognizant of the most recent recommendations regarding the diagnosis and management of acute ischemic attacks within the brain, or strokes.
Course preview
The purpose of this course is to ensure that the nurse is cognizant of the most recent recommendations regarding the diagnosis and management of acute ischemic attacks within the brain, or strokes.
By the completion of this activity, the nurse will be able to:
- Recall vital statistics regarding stroke in the US and worldwide and the basics of cerebral vascular anatomy.
- Review the pathophysiology involved in both ischemic and hemorrhagic strokes.
- List the risk factors for stroke as well as methods for prevention.
- Explain the signs and symptoms of a stroke.
- Identify the components of a full stroke workup and acute treatment options, including surgical treatment for hemorrhagic strokes, the use of thrombolytics, and endovascular procedures.
- Discuss the rehabilitation that should occur following a stroke.
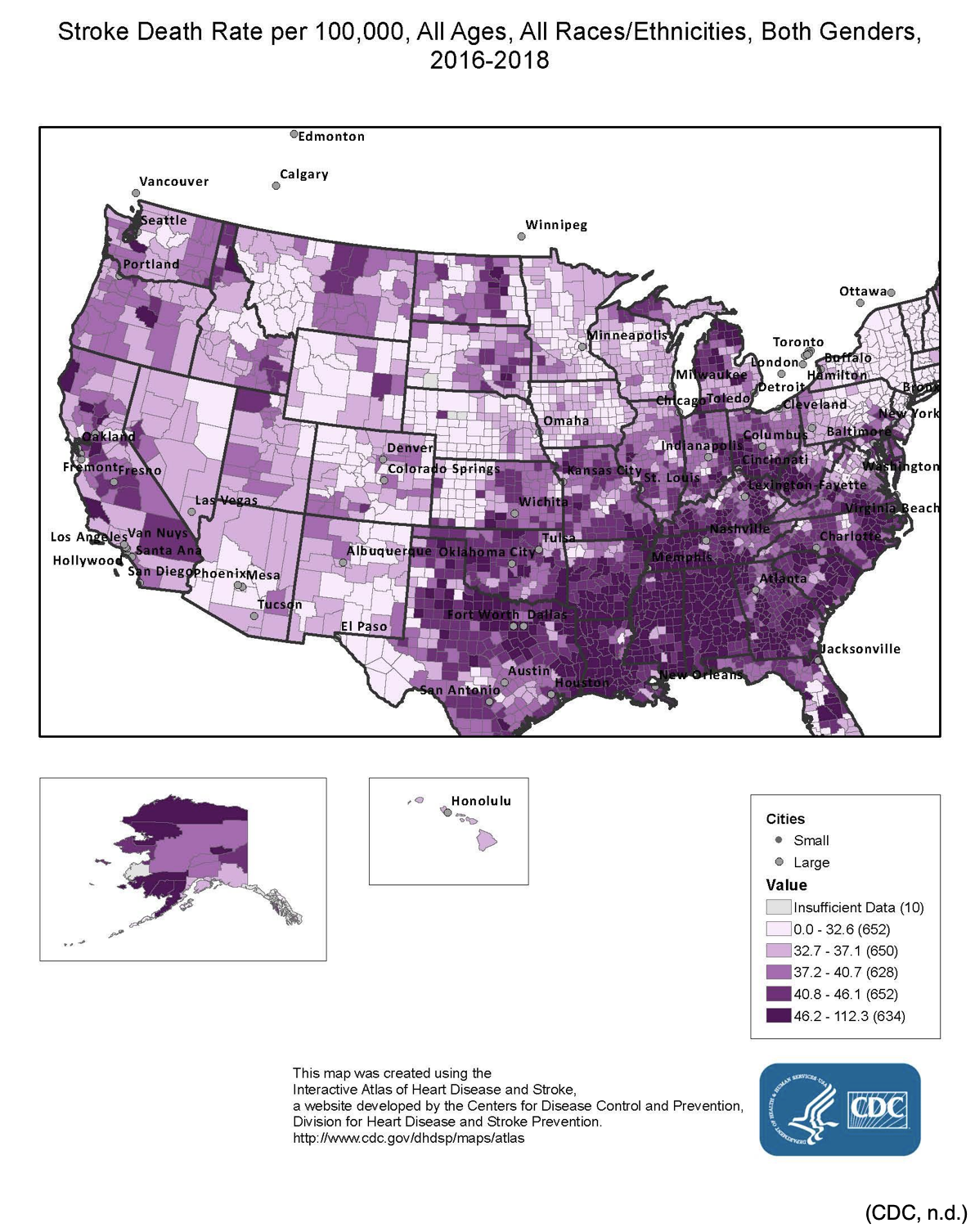
Globally, cardiovascular disease is the number one cause of death. In 2016, 31% of all global deaths, or an estimated 17.9 million people, died from cardiovascular diseases; 85% of these were due to heart attack or stroke. Over 75% of these deaths occurred in middle-to-low income countries (World Health Organization [WHO], 2017). Within the US adult population, roughly 7.2 million people self-report having had a stroke. Each year around 800,000 people in the US have a stroke, or around one person every 40 seconds nationwide. It is more common in African Americans and Native Americans/Alaskan Natives than European Americans, and more women die secondary to stroke than men. Stroke is the fifth leading cause of death in the US (Benjamin et al., 2018). In the US, more than 140,000 people die annually following a stroke, or about 1 in every 20 adult deaths. The good news is that the stroke death rate has declined in the US since at least the 1960s, decreasing by an impressive 35.8% between 2000 and 2010. This is largely related to improvements in risk reduction and enhanced primary prevention practices as well as technology to treat patients diagnosed with a stroke. The bad news is that the stroke death rate is not improving as quickly as it was prior to 2000, especially since 2013, and particularly among African American males. The estimated cost of stroke in the US is $34 billion annually, including the cost of healthcare services, medications, and lost productivity. There is a significant variation in mortality regionally across the US, with a much higher death rate seen in the "stroke belt" of the southeast (North and South Carolina, Georgia, Tennessee, Mississippi, Alabama, Louisiana, and Arkansas) (Bowen, 2016; Centers for Disease Control and Prevention [CDC], 2020; Winstein et al., 2016). See Figure 1 for mortality rates from stroke nationwide.
Figure 1
Stroke Death Rate per 100,000 from 2016-2018
Pathophysiology
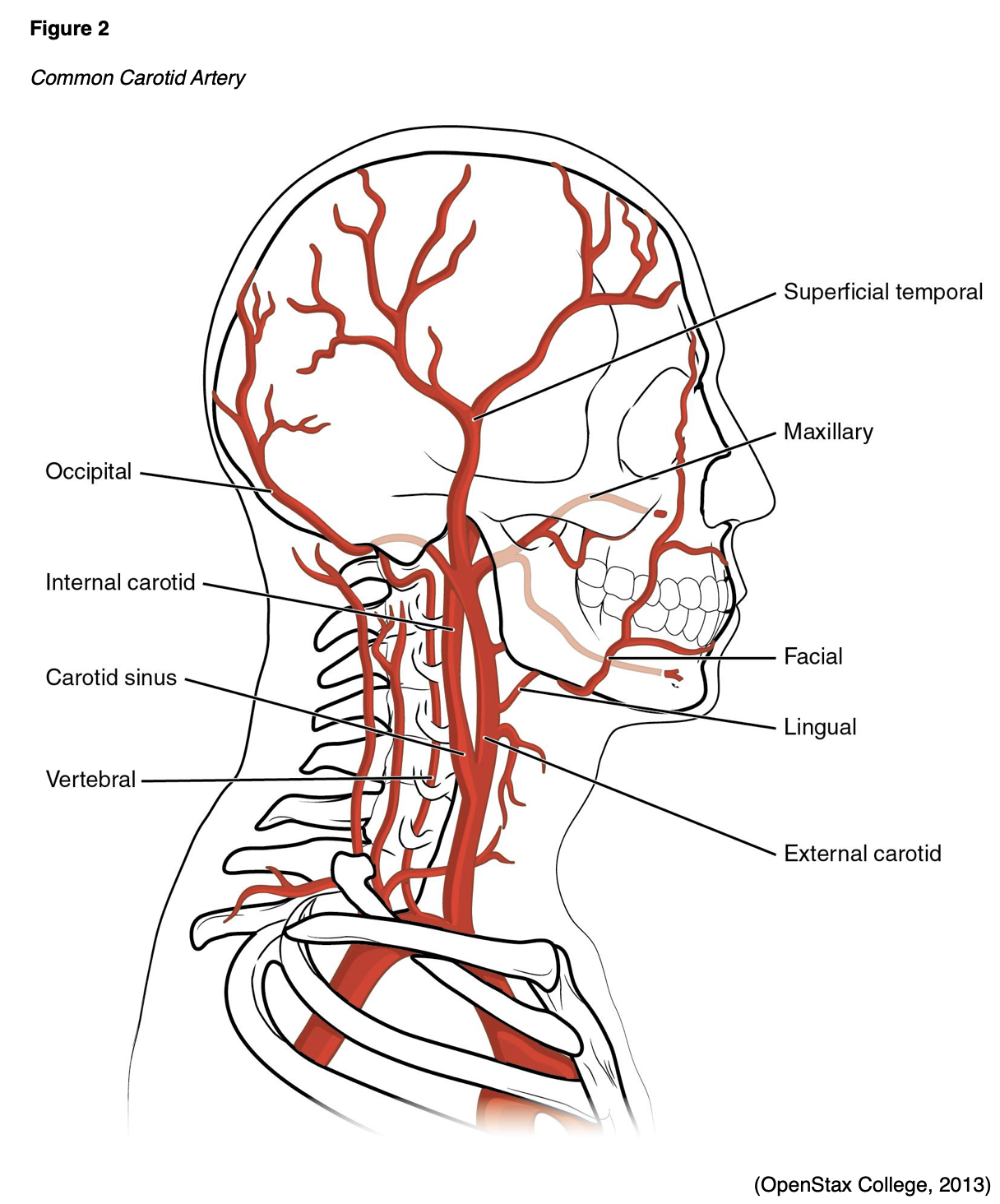
The anatomy of cerebral circulation starts with the common carotid arteries (Figure 2), which split into the external and internal carotid arteries (ICAs). The external carotid arteries supply the posterior scalp and face. The ICAs supply blood to roughly 80% of the cerebrum. From this, the anterior cerebral artery branches off to supply the superior portion of the frontal and parietal lobes. The ophthalmic artery and middle cerebral artery (MCA) also branch off of the ICA. The ophthalmic artery supplies the ocular orbit as well as some structures in the nose, face, and meninges. The MCA supplies the remainder of the lateral cortex, such as most of the frontal and parietal lobes, as well as the superior and medial portions of the temporal lobe, the basal ganglia, and the internal capsule (Franco, 2018).
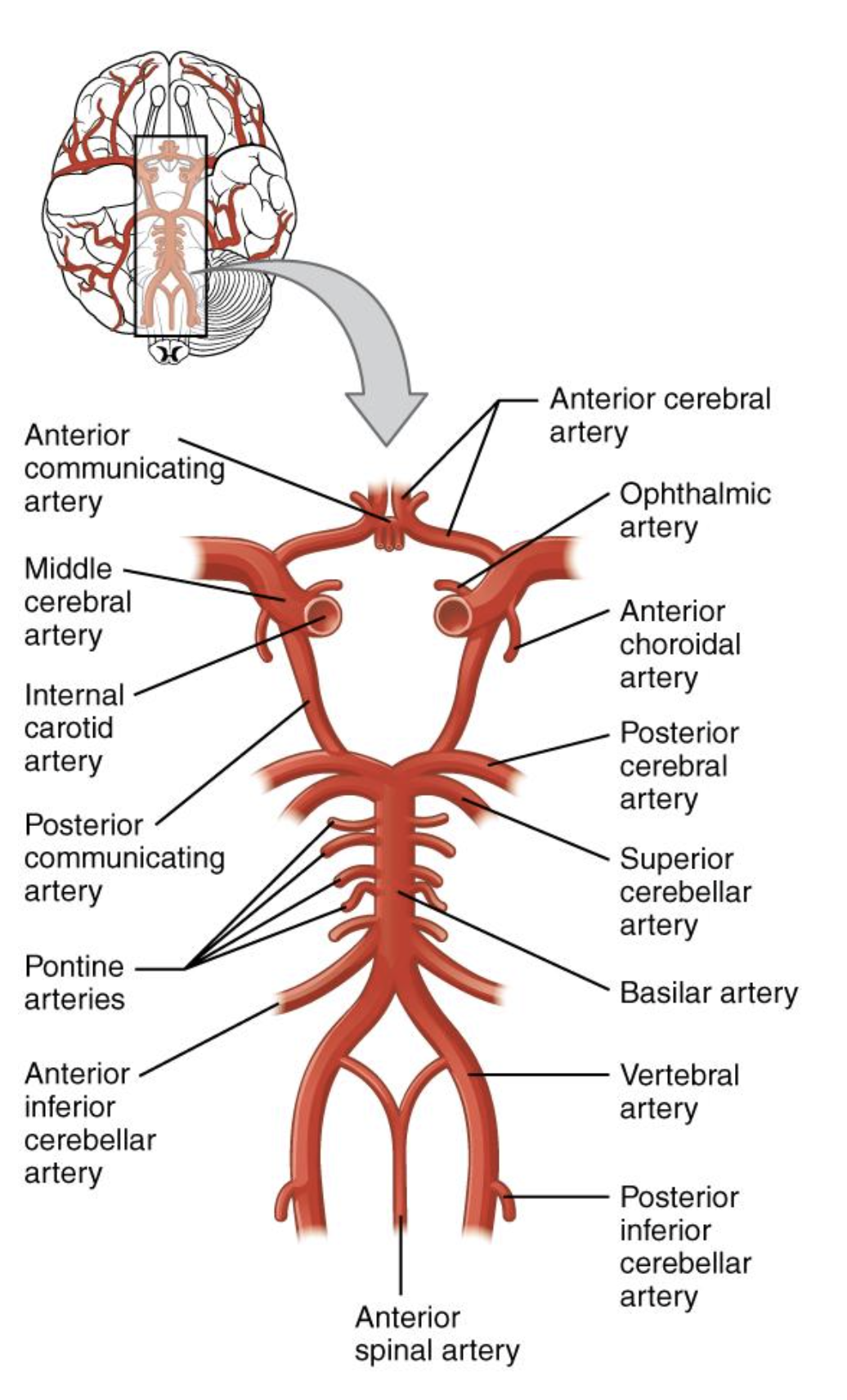
Posterior circulation starts with the vertebral arteries, which branch off of the subclavian arteries, and travel up through the transverse foramina of the cervical vertebra and through the foramen magnum to supply blood to the cranial bones and meninges and converge to form the basilar artery. The basilar artery supplies blood to the pons, cerebellum, and inner ear. The basilar artery splits again into the posterior communicating artery to form the Circle of Willis (Figure 3), which surrounds the pituitary gland, although only about 20% of the population has a complete arterial circle. The posterior cerebral artery then branches off to supply the inferior and medial portions of the temporal and occipital lobes, the midbrain, and the thalamus (Franco, 2018).
Figure 3
Circle of Willis
(OpenStax, 2016)
There are two main types of stroke: an ischemic stroke in which blood flow to an area is reduced to critical levels or stopped completely, and a hemorrhagic stroke in which there is abnormal bleeding into an area. Ischemic strokes (Figure 4) can either be caused by a thrombus, which is a blood clot that forms in an artery that supplies blood to the brain, or an embolus, which is a blood clot, plaque, or fatty tissue that travels from somewhere else in the body (such as the heart or the peripheral vasculature) and becomes lodged in and blocks an artery that supplies blood to the brain. In both types of ischemic stroke, the plaque or clot keeps oxygen-rich blood from getting to a portion of the brain, and neurons that are deprived of oxygen start to die within minutes. Ischemic stroke is most commonly caused by atherosclerosis, a condition in which a fatty substance called plaque builds up along the walls of arteries. This plaque formation hardens and narrows arteries, and the plaque can crack or rupture, attracting platelets and eventually leading to the formation of blood clots. If a plaque formation breaks off into the artery, this can then create an embolus downstream. Atrial fibrillation (Afib) is an irregular rhythm that allows for pooling of blood in the atrium of the heart, which increases the risk of embolic stroke if a blood clot forms there and is then expelled and travels up to the brain (National Heart, Lung, and Blood Institute [NHLBI], n.d.).

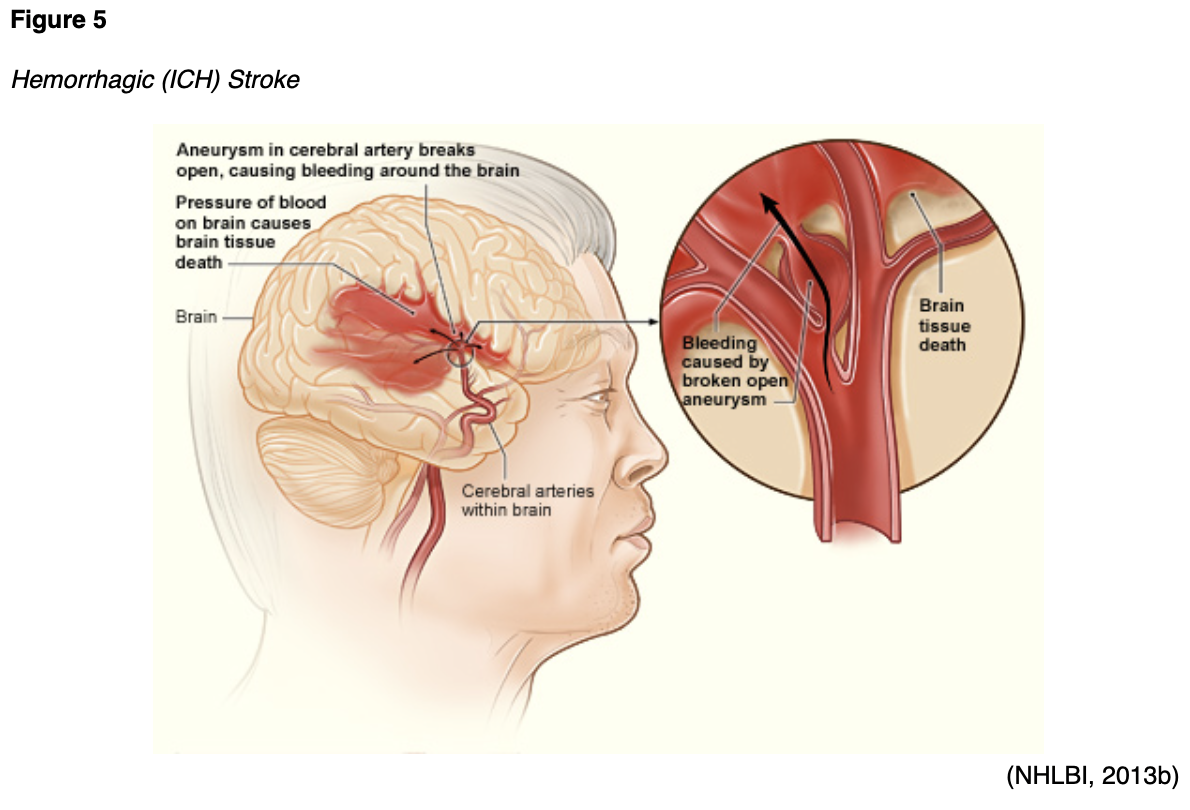
A hemorrhagic stroke (Figure 5) can either be caused by bleeding into the parenchyma of the brain (intracerebral hemorrhage [ICH]) or in the space around the brain between the inner and middle membranes (subarachnoid hemorrhage [SAH]). In both types of hemorrhagic stroke, the blood causes swelling and cellular damage in the surrounding brain tissue as well as increased pressure within the skull, which is a closed container. SAHs are often caused by a ruptured aneurysm, which is a weakened bulge in an artery. ICHs may be caused by hypertension, trauma, or an arteriovenous malformation (AVM), which is an abnormal clustered connection of blood vessels between arteries and veins (NHLBI, n.d.). Hemorrhagic strokes may also be caused by medications, coagulopathy, sympathomimetic drugs of abuse, and cerebral amyloid angiopathy. Ischemic strokes account for 87%, while ICHs account for 10%, and SAHs account for 3% of all strokes (Benjamin et al., 2018; Franco, 2018).
Risk Factors and Prevention
Stroke has both non-modifiable and modifiable risk factors. Non-modifiable factors include age, sex, race, the presence of brain aneurysms o
...purchase below to continue the course
Primary Prevention
Given the large number of modifiable risk factors, prevention should be the first step in treating stroke. Primary prevention refers to treating and controlling modifiable risk factors as a means of preventing a patient's first stroke. This means actively and appropriately managing hypertension, as well as diabetes, Afib, hypercholesterolemia, and any other risk factors. Following a heart-healthy lifestyle, which includes regular exercise, and a healthy diet (a diet high in fiber, fruits, vegetables, whole grains, and lean meat and low in saturated fats, trans fats, sodium, added sugar and alcohol) can help to lower the risk of stroke. This may also include a weight loss program to maintain a healthy body weight. Smoking cessation also decreases the risk of stroke, along with numerous other detrimental effects of smoking. Appropriately managing stress can also decrease the risk of stroke (Franco, 2018; NHLBI, n.d.). The AHA (2017) published a guideline on the importance of self-care for stroke and other cardiovascular disease prevention. Self-care is defined as a process “whereby individuals and families maintain health through health-promoting practices and managing illness” (p. 2). They break this down into three general categories: self-care maintenance, which includes maintaining physical and emotional stability; self-care monitoring, which includes observing oneself for changes in signs or symptoms (also called "body listening"); and self-care management, which includes appropriately responding to signs or symptoms if and when they occur. Other than the previously mentioned individual-level prevention techniques discussed, stroke prevention programs should also assess and optimize positive family support as well as community-level prevention methods such as access to medical services, healthy food, open spaces, a safe environment, healthy air, regular physical activity, and social cohesion (Riegel et al., 2017).
Figure 6
Primary Stroke Prevention
 Secondary Prevention
Secondary Prevention
One in four stroke survivors has another stroke within five years, and the risk of a stroke within 90 days of a transient ischemic attack (TIA) is as high as 17% (CDC, 2018). Secondary prevention refers to preventing a recurrent stroke after an initial stroke or TIA. The American Stroke Association (ASA) and AHA (2018) publishes early management guidelines for acute ischemic stroke patients. They recommend management of hypertension and the use of antiplatelet medications for a few months, as well as anticoagulant medications in those with Afib and a suspected or confirmed embolic stroke (Powers et al., 2018).
Evaluating cholesterol levels, screening for obstructive sleep apnea (OSA), assessing antiphospholipid antibodies, or performing echocardiograms in all acute stroke patients is not recommended for secondary prevention if the stroke is presumed to be atherosclerotic in origin. Cholesterol levels may be obtained after a stroke to assess the effectiveness of a previously prescribed statin medication. High-intensity statin therapy is recommended in patients aged 75 and under following an acute stroke; moderate intensity therapy should be considered for patients over the age of 75, based on the individual risks and benefits. Lifestyle and diet modification should be attempted in these patients as well. If a patient is suspected or known to have had a stroke in the carotid territory and may be a candidate for stent placement or carotid endarterectomy (CEA), noninvasive imaging of the cervical vessels is typically performed within 24 hours of admission. However, the potential benefit of urgent or emergent CEA procedures in the acute phase following a stroke is not well established. Finally, secondary prevention measures after a stroke should include managing or treating any medical conditions discussed above as a primary prevention measure, such as smoking cessation and a heart-healthy lifestyle (Powers et al., 2018).
Many of the secondary prevention measures discussed here are also core/primary stroke clinical performance measures (CPMs) published by the Joint Commission, including the use of antiplatelet medication, anticoagulants, and lipid-lowering statin medications for secondary prevention following a stroke (The Joint Commission, 2018b). A specific secondary prevention measure that has been tested with some favorable results in stroke patients is repetitive bilateral arm ischemic preconditioning (BAIPC). This method aims to improve cerebral perfusion and thus decrease the risk of stroke recurrence. In a study published by Meng et al. (2015), a group of 58 patients over the age of 80 were enrolled in the study, which randomized them into a test or control group. The test group was instructed to perform five cycles twice daily of bilateral arm ischemia lasting five minutes per cycle for 180 days starting about seven days following either a stroke or TIA while the control group was given a sham exercise to perform. The test group (n=30) had a total of two recurrent infarcts and seven TIAs during the study period, while the control group (n=28) had eight infarcts and 11 TIAs during the same period of time. The test group also showed decreased levels of c-reactive protein (CRP), interleukin-6, leukocytes, and a decreased platelet aggregation rate; they also developed increased tissue plasminogen activator (tPA) compared to the control group participants (Meng et al., 2015).
Stroke Signs and Symptoms
Signs and symptoms of a stroke can develop very quickly (i.e., minutes) or more slowly (i.e., over hours or days). They will vary depending on the area of the brain that has been affected. These symptoms will last for at least 24 hours. Symptoms of a stroke that resolve spontaneously in less than 24 hours, and usually in one to two hours, are defined as TIAs. Common signs and symptoms of a stroke include:
- Weakness, usually on one side of the body;
- Paralysis or numbness of the face, arms, or legs, usually on one side of the body;
- Confusion;
- Difficulty speaking or understanding speech;
- Vision changes in one or both eye(s);
- Difficulty breathing;
- Dizziness;
- Difficulty walking or unexplained fall(s);
- Loss of balance and/or coordination;
- Loss of consciousness;
- Sudden and severe headache (usually indicates a hemorrhagic stroke) (CDC, 2020; NHLBI, n.d.).
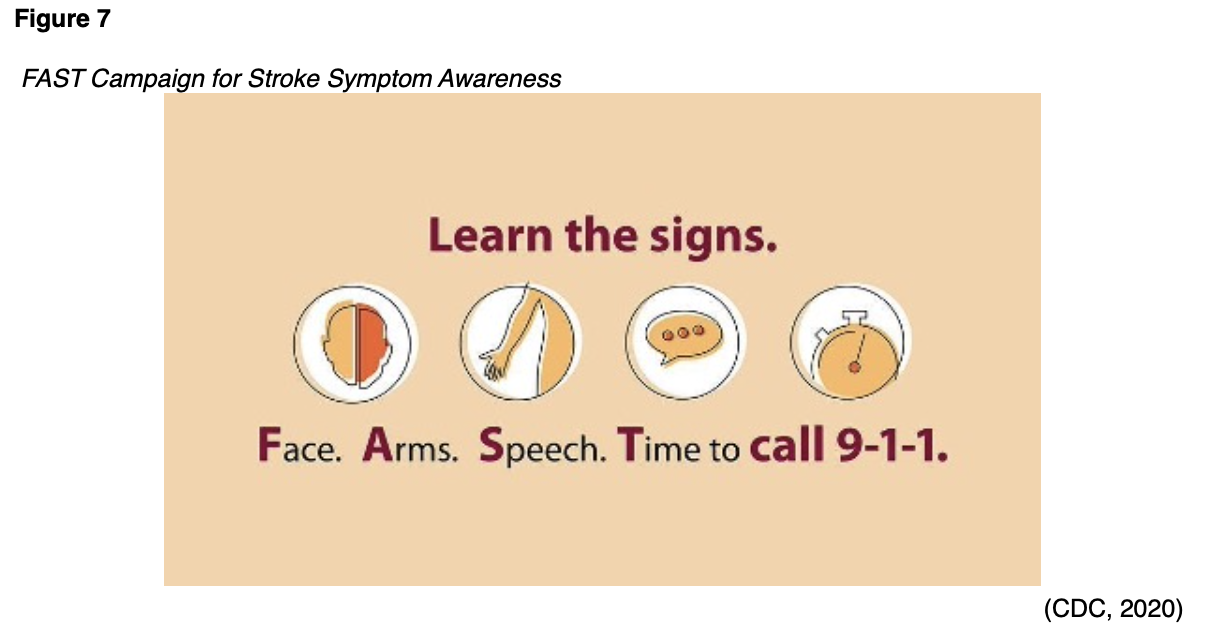
The importance of public education campaigns such as FAST (face, arms, speech, time to call 911) that target stroke education cannot be overstated (see Figure 7). The general public has a long-held false belief that strokes are not medical emergencies worthy of initiating the emergency management system (EMS). Patients should all be made aware that when signs or symptoms of a stroke are seen, the only acceptable response is to call 911 for ambulance transport to a hospital equipped to manage acute strokes (CDC, 2018; NHLBI, n.d.). Aphasia is a common stroke symptom that is defined as difficulty understanding speech, speaking, reading, or writing language. It affects up to one-third of all stroke patients and is usually seen in strokes affecting the left side of the brain. Unfortunately, 30-40% of those diagnosed with aphasia symptoms immediately following a stroke develop chronic difficulties (Palmer, 2015). Brainstem strokes that affect the pons are also called pontine strokes and account for 10% of all ischemic strokes. If severe enough, these can cause "locked-in syndrome", which is a complete lack of control of motor function with the exception of the eyes, but maintained cognitive function (Franco, 2018).
Diagnosis and Management
Initial Management
Acute evaluation and treatment for stroke symptoms typically start with EMS providers. The ASA (2018) guidelines state that EMS providers should have a stroke assessment system in place with treatment protocols as well as notification of the nearest stroke center hospital in hopes of delivering comprehensive specialized stroke care that also incorporates rehabilitation. The primary objectives of prehospital care are airway management, cardiovascular support, and transport to the closest facility prepared to care for acute stroke patients (Powers et al., 2018).
Hospitals can be given designations by the Joint Commission as an acute stroke ready hospital (ASRH), a primary stroke center (PSC), a thrombectomy-capable stroke center (TSC), or a comprehensive stroke center (CSC). Each certification has designated requirements, and hospitals must apply and be reviewed for initial certification as well as continued certification or renewal.
- An ASRH must have an acute stroke team available 24 hours a day, initial evaluation by an emergency department (ED) physician, nurse practitioner (NP) or physician’s assistant (PA), a neurologist available in person or via telemedicine 24 hours a day, neurosurgical services available within three hours, a transfer protocol established with at least one PSC or CSC, the ability to administer IV tPA and then transfer the patient safely to another facility, and document that they meet three inpatient and two outpatient stroke clinical performance measures (CPMs).
- A PSC must have all of the above, plus an initial evaluation by an ED physician, a designated unit or group of beds for acute care of stroke patients, the ability to manage a patient after receiving IV tPA, neurosurgical services within two hours with 24/7 operating room availability for neurosurgical services, and document that they meet eight core stroke CPMs.
- A TSC must have all of the above, as well as provide evidence that they have completed at least 15 thrombectomies over the last 12 months, a dedicated neurointensive care unit or beds available 24/7, magnetic resonance imaging (MRI), computed tomography angiography (CTA), magnetic resonance angiography (MRA), and catheter angiography available 24/7, ability to perform and manage mechanical thrombectomies and intra-arterial (IA) thrombolytics, and document that they meet an additional five ischemic hemorrhagic comprehensive stroke CPMs.
- A CSC must demonstrate all of the above, as well as provide evidence that they have treated at least 20 SAH cases caused by aneurysm annually, including 15 endovascular coiling or microsurgical clipping procedures, managed IV thrombolytic therapy at least 25 times annually, neurologist and neurosurgical services accessible 24/7 in person with a written call schedule, ability to perform stenting of carotid arteries or CEA procedures, and document that they meet a total of eight core and ten comprehensive stroke CPMs.
Each category also has specific education requirements for the providers, nurses, and staff caring for acute stroke patients. Certification reviews range from one reviewer for one day in ASRHs to two reviewers for two days in CSCs (The Joint Commission, 2018a).
Otherwise, the emergency care of a stroke patient also includes circulatory assessment (with chest compressions if necessary), airway assessment (and management if necessary), and breathing assessment (with ventilatory support if necessary) just as in any other medical emergency. A full history and physical exam, including a comprehensive neurological exam, should be completed quickly on any suspected stroke patient upon arrival to the ED. This includes a history of risk factors as well as current signs or symptoms and exactly when the symptoms began (if known). The physical and neurological exam should include evaluation of the patient's alertness, comprehension, coordination, balance, sensation, strength, speech, and vision. A quick check for an audible carotid bruit should also be included (NHLBI, n.d.). A neurologist is available 24/7 in a CSC hospital, but oftentimes the initial neurology consult may be completed via telemedicine in other lower-level hospitals, even PSC and TSC hospitals (The Joint Commission, 2018a).
Guidelines recommend the use of supplemental oxygen in acute stroke care only if the patient’s oxygen saturation is less than 94%. Hypotension and hypovolemia should be corrected to help support optimal organ function and maintain systemic perfusion. Blood pressure should be carefully lowered to below 185/110 prior to the initiation of IV fibrinolytic therapy using labetalol (Trandate), nicardipine (Cardene), clevidipine (Cleviprex), or similar. If no fibrinolytic therapy or endovascular procedures are possible, there is no benefit to initiating antihypertensives within the first 48-72 hours after the initial stroke as long as blood pressure is below 220/120. If above 220/120, it might be reasonable to lower the blood pressure 15% in 24 hours. Sources of hyperthermia (temperature above 100.4 °F [38 °C]) should be identified and corrected; antipyretics should be given to lower the temperature to normal range. Hyperglycemia should be treated, with a goal of maintaining a range of 140-180 mg/dL; hypoglycemia below 60 mg/dL should also be corrected. Poorly managed blood glucose levels can lead to poor outcomes in stroke patients. In fact, the only laboratory test recommended prior to the administration of IV tPA in an acute stroke patient is a serum blood glucose check (Powers et al., 2018).
A class I (strong) recommendation from the ASA guidelines (2018) is that hospitals develop a stroke protocol for patient evaluation and treatment by a trained and qualified Stroke Team. They also provide a moderate recommendation that telestroke evaluation, a telehealth evaluation with a trained neurologist experienced in the remote diagnosis of stroke, can improve triage and accurate decision making for IV tPA in stroke patients. A weaker recommendation based on nonrandomized data is that IV tPA administration within a telestroke network is just as beneficial and safe as when it is done within a stroke center hospital (Powers et al., 2018). Teleneurology has been shown to be a cost-effective solution to provide stroke neurology expertise to every patient, regardless of where they may present to obtain care. It has been shown to significantly improve both short-term and long-term clinical outcomes and decrease disparities in quality of care (Bowen, 2016).
Diagnosis
An initial evaluation of a potential ischemic stroke patient should also include a rating using a stroke severity scale, such as the National Institutes of Health Stroke Scale (NIHSS) (Powers et al., 2018). The NIHSS was first developed in 1983 to assess the neurological deficits present post-stroke, modified last in 1992, and takes approximately seven minutes to complete. It includes 11 items and produces a final score between 0 and 42. The higher the score, the worse the deficits. It assesses the patient's level of consciousness, gaze palsy, visual fields, facial palsy, bilateral upper extremity strength, bilateral lower extremity strength, limb ataxia, sensation, extinction/neglect/inattention, dysarthria, and language. A provider should be certified and trained to administer the NIHSS properly (Scheinfeld et al., 2016). Hemorrhagic stroke patients should also be evaluated with specialized tools. For SAH patients, ASA (Hemphill et al., 2015) and the Joint Commission (2018b) recommend the Hunt and Hess Scale for initial evaluation; for ICH patients, the ICH score is recommended. These should be completed prior to any surgical intervention to serve as a baseline measure and help inform prognosis as well as facilitate effective communication between providers and facilities regarding patient severity and condition. The Hunt and Hess Scale provides a score that ranges from 1 (asymptomatic, mild headache, slight nuchal rigidity) to 5 (decerebrate posturing, coma). The ICH score takes into account the patient's Glasgow coma score (GCS), age, the volume of ICH, and whether there exists an infratentorial origin or an intraventricular hemorrhage on imaging. These are then combined into a numerical value ranging from 0 to 6, with a higher score indicating a worse prognosis or higher expected mortality rate (Hemphill et al., 2015; The Joint Commission, 2018b).
If a stroke is suspected after a complete history and physical exam, the next step in evaluation is typically imaging studies. We will briefly discuss the imaging studies commonly ordered in stroke patients, as well as their positive and negative features. Regarding imaging studies, the ASA guidelines (2018) recommend all suspected stroke patients undergo diagnostic brain imaging, usually in the form of a noncontrast computed tomography (CT) scan of the head. Ideally, at least 50% of stroke patients should receive this within 20 minutes of arrival at the ED. They strongly recommend a noninvasive intracranial vascular study during the initial imaging evaluation of a stroke patient if they are believed to be a candidate for endovascular treatment as long as this does not delay IV tPA administration. A CT scan of the head without contrast is the most common imaging study ordered initially in suspected stroke patients. This study is very quick, widely available, and allows the radiologist and neurologist to reliably rule out hemorrhage. Disadvantages of a noncontrast CT are the high dose of radiation delivered during the exam, the lack of reliability in detecting an early infarct core (brain tissue identified on imaging as having both decreased blood flow and blood volume), and the variable ability to detect a stroke via CT scan depending on the expertise level of the radiologist, the size and acuity of the stroke, and the window and level settings (Franco, 2018; Powers et al., 2018). A noncontrast head CT is also used to determine a patient’s Alberta Stroke Program Early CT Score (ASPECTS), which in turn is used as a criterion for certain treatment options and can help predict stroke severity and functional independence. Scores range from 0-10; a lower score indicates a more pervasive and severe stroke. For more accuracy, perfusion or diffusion-weighted studies can also be used to calculate a patient’s ASPECTS (Schroder & Thomalla, 2017). Recently, mobile CT scanners have been constructed to potentially reduce time to treatment by up to 42 minutes. These look like large ambulances equipped with a CT scanner inside that function by scanning potential stroke patients en route to the hospital. CT angiography (CTA) creates a three-dimensional (3D) computer-generated reconstruction of a patient's entire intra- and extracranial circulation from the aorta to the Circle of Willis secondary to a timed, rapid injection of iodinated IV contrast in less than 60 seconds. The exposure to contrast is not without risk, however, and patients with a known allergy to iodine or iodinated contrast or renal insufficiency (glomerular filtration rate [GFR] less than 30) should not receive iodinated contrast. Despite this, the ASA guidelines state that serum creatinine is not necessary to assess before performing a CTA on an acute stroke patient with no history of renal impairment. CT perfusion studies use the same process as the CTA above, but with the addition of specialized computer software that then determines the mean transit time to establish a penumbra (an area of brain tissue at risk for hypoxic damage but still currently salvageable if treated quickly, as it has decreased blood flow but normal blood volume on imaging). The disadvantages of a CT perfusion study include the same aforementioned radiation exposure and contrast administration as well as the expensive software needed to process the images. According to the ASA (2018), perfusion imaging should also not delay IV tPA administration. In fact, the ASA found no benefit to performing CT perfusion images in acute stroke patients that are within the six-hour time window from symptom onset for the purpose of patient selection for mechanical thrombectomy procedure. These imaging studies are strongly recommended in acute stroke patients with large vessel occlusions in the anterior circulation that are between 6-24 hours from symptom onset as they may be crucial in treatment decision making in this group (Franco, 2018; Powers et al., 2018).
MRI scans of the brain are also commonly used to evaluate stroke patients. They are considered by some to be radiographically superior to CT scans in the acute phase. Additionally, they do not expose the patient to any radiation and are recommended by the American College of Radiology. However, they take longer than CT scans to complete and thus have the risk of delaying treatment. MRI machines are also generally less available than CT scanners. The small area inside the machine commonly causes patients discomfort and anxiety related to claustrophobia, and there are several contraindications to MRI scans (i.e., the presence of certain aneurysm clips, pacemakers, defibrillators, stimulators, and shrapnel or other metal fragments in the eyes). They are recommended in cases of uncertain diagnosis, considered more sensitive in TIA cases, and the American Academy of Neurology officially recommends an MR diffusion-weighted study in the first 12 hours after stroke symptoms develop as they find them more useful than CT scans. However, the general consensus is that if obtaining an MRI scan will significantly delay treatment, then a CT scan is preferred. If it is unknown whether a patient has MRI-compatible aneurysm clips, a pacemaker, metal eye fragments, or other contraindication to receiving an MRI scan, an x-ray of the head, chest, and abdomen can be done to help determine eligibility (Franco, 2018).
Management of Hemorrhagic Stroke Patients
The ASA (Hemphill et al., 2015) has published guidelines regarding the treatment of ICH. A baseline severity score (ICH score if depressed level of consciousness, or NIHSS if the patient is conscious) and rapid neuroimaging to define the problem are just as important for hemorrhagic stroke patients as they are in ischemic stroke patients. Vital signs, as well as a complete history and targeted physical exam, will help direct immediate care. A severely hypertensive patient (SBP 150-220) with a hemorrhagic stroke needs antihypertensive medication to safely reduce their SBP to 140 to help limit the bleeding, with vigilant control of blood pressure going forward. Coagulation factors and platelet count should be ordered to assess for coagulation factor deficiency, thrombocytopenia, or other abnormalities so these things can be addressed. Coagulation factor deficiencies should be treated with factor replacement therapy and thrombocytopenia with platelets. Patients taking vitamin K antagonists (i.e., warfarin [Coumadin]) with elevated INR should receive vitamin K as well as fresh frozen plasma (FFP) or other therapy to replace vitamin K–dependent factors. In patients taking newer anticoagulation medications such as dabigatran (Pradaxa), apixaban (Eliquis), or rivaroxaban (Xarelto) without clear reversal agents, the ASA recommends evaluation of the activated partial thromboplastin time (aPTT) and prothrombin time (PT) and consultation with a hematologist. All ICH patients should receive treatment with sequential compression devices (SCDs) to prevent deep vein thrombosis (DVTs) starting the day of hospital admission. Antiepileptic drugs (AEDs) are recommended in patients with clinical seizures or those with a change in mental status who are found to have electrographic seizures on EEG. ICH patients should be cared for in an intensive care unit (ICU) or a dedicated stroke unit with physicians and nurses trained in their specialized care. If deteriorating neurologically, patients with cerebellar hemorrhage and subsequent brainstem compression and/or hydrocephalus related to ventricular obstruction should have spinal fluid diversion with a ventriculostomy or lumbar drain and/or surgical removal of the hemorrhage as soon as possible (Hemphill et al., 2015). If an AVM is found to be the cause of the patient’s stroke, the AVM can be removed surgically, or a liquid tissue adhesive can be injected into the AVM to block blood flow and stop the bleeding. Radiation can also be used to treat AVMs if they are found non-emergently and are not actively bleeding (Franco, 2018; NHLBI, n.d.; NINDS, 2020).
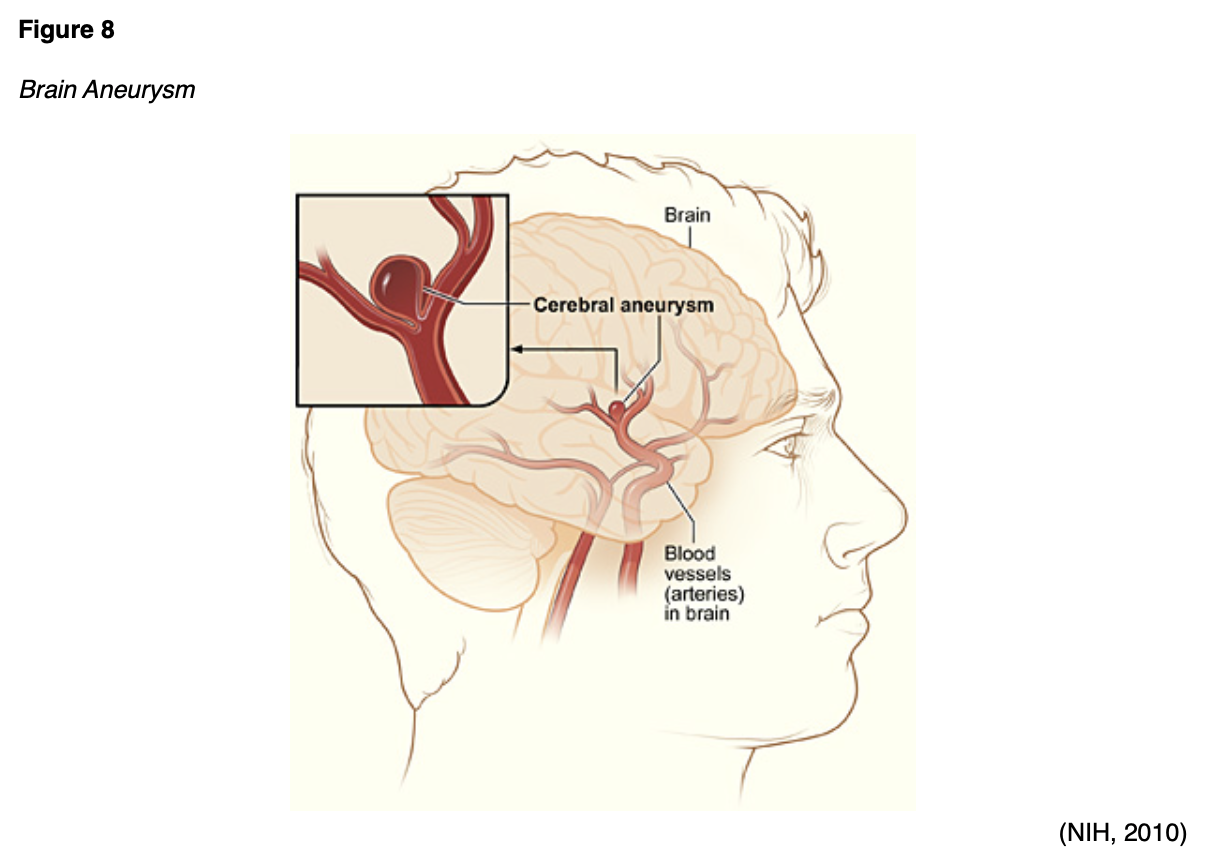
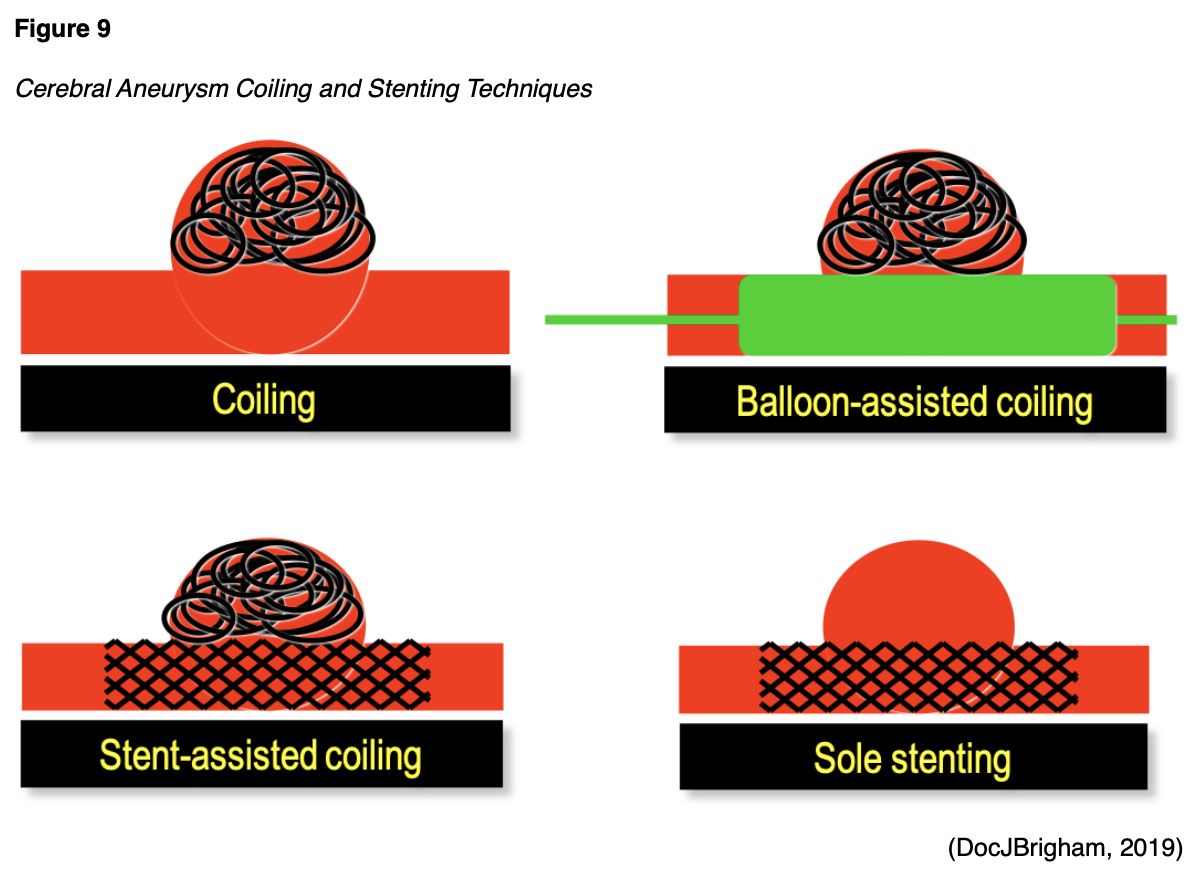
SAHs are most commonly associated with an aneurysm (Figure 8) that has ruptured or is leaking. An aneurysm may be treated surgically using microvascular clips placed at the base or neck of the aneurysm to prevent future leaking by blocking it off from blood flow. The risks are high for this procedure, as it requires open brain surgery to place the clips, but there is less risk of recurrence and repeat procedures in the future if done properly. Coil embolization (Figure 9) uses an access catheter placed in the groin to embolize an aneurysm with detachable platinum coils. It is less invasive than clipping, but may need to be repeated in the future and is more likely to lead to the complication of cerebral vasospasms postoperatively (Franco, 2018; NHLBI, n.d.; NINDS, 2020). Three large randomized, prospective studies have compared both techniques. Of these, the International Subarachnoid Aneurysm Trial (ISAT) found a statistically significant improvement in survival rates at 12 months with coiling and has thus strongly influenced the surgical patterns in the last 15 years since its initial publication in the early 2000s. The most recent guidelines published by the ASA regarding aneurysmal SAH also recommend coiling over clipping when both are feasible. These procedures can also be done preventatively, depending on the location of the aneurysm. Larger aneurysms in the posterior circulation are more prone to rupture, and surgeons are more likely to recommend prophylactic surgical treatment if these are found non-emergently. The severity of the hemorrhage and the patient's age remain the two most important prognostic factors in aneurysmal SAH patients (Grasso et al., 2017). A third surgical option for larger and more difficult to treat aneurysms is placing a stent-like flow diversion device (Figure 9) via an endovascular catheter (NINDS, 2020).
Fibrinolytic Therapy and Thrombectomy Devices for Ischemic Strokes
IV tPA was first approved for therapeutic use in 1995 by the US Food and Drug Administration (FDA) and remains only approved for intravenous use (Spiotta et al., 2015). As previously stated, the patient’s blood glucose should be checked prior to the initiation of IV tPA and corrected if necessary. Baseline troponin and an electrocardiogram (ECG) are recommended, but not required, and should not delay administration. IV tPA is dosed at 0.9 mg/kg with a max of 90 mg and given over 60 minutes. The initial bolus should account for 10% of the patient's total dose and be given over the first minute. Treatment is time-dependent and thus should be initiated as soon as possible. In acute stroke patients that are within three hours of symptom onset, IV fibrinolytic therapy with tPA is recommended in patients that are at least 18 years old with severe or mild but disabling stroke symptoms present. There is also a strong recommendation based on level B evidence that acute stroke patients within three to four-and-a-half hours of initial symptom onset that otherwise meet criteria should be treated with IV tPA as well. Additional criteria are suggested for patients within the three- to four-and-a-half-hour window in addition the criteria mentioned above (Powers et al., 2018).
Risks and benefits of IV thrombolytic therapy should be reviewed with the patient and family or caregivers extensively, and staff and providers should be prepared and fully capable of managing any emergent adverse effects such as bleeding or angioedema. Treatment should be stopped, and a head CT obtained immediately if the following symptom changes occur during or after IV tPA administration:
- severe headache,
- acute hypertension,
- nausea/vomiting, or
- a worsening or changed neurological exam (Powers et al., 2018).
IV tenecteplase (TNKase) may be used as an alternative in patients with no major intracranial occlusion and only minor neurological impairment. It is recommended to avoid other concurrent treatments that could cause bleeding issues during administration, such as nasogastric (NG) tubes, urinary catheters, and intra-arterial pressure catheters if they can be safely avoided. IV tPA is contraindicated in patients with acute intracranial hemorrhage on CT scan, a history of severe head trauma in the last three months, symptoms consistent with infective endocarditis or aortic arch dissection, or coagulopathy. IV tPA may be potentially harmful in patients with a history of acute ischemic stroke in the last three months, any history of ICH, signs/symptoms of SAH, a structural GI malignancy, a recent bleeding event within 21 days, use of a thrombin inhibitor/factor Xa inhibitor within the previous 48 hours, an intra-axial intracranial neoplasm, or intracranial/intraspinal surgery in the last three months (Powers et al., 2018).
In 2004, the Mechanical Embolus Removal in Central Ischemia (Merci) retriever device (Figure 10) became the first device cleared by the FDA for mechanical thrombectomy in acute stroke patients. It utilizes a corkscrew wire/suture tip to remove the clot en bloc, leading to improved clearance of large vessel occlusions. Revascularization rates range from 48-68%, and up to 36% of patients in initial studies reported a modified Rankin score (mRS) of 2 or less at 90 days. The Outreach distal access catheter (DAC) was approved in 2010 and provides buttressing access for Merci and similar devices. It provides more stable access and increased aspiration power (Spiotta et al., 2015).
Figure 10
Merci Device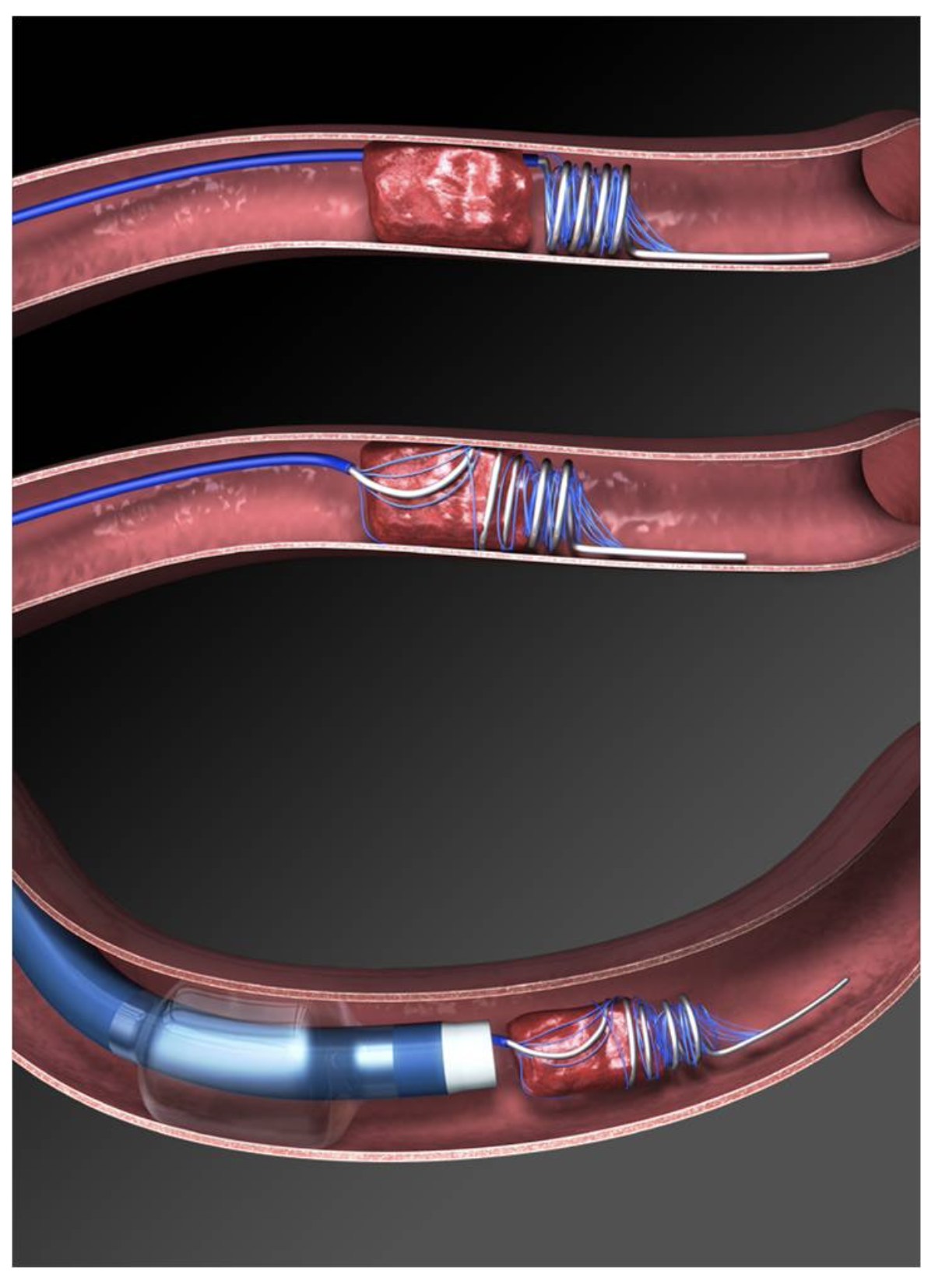
(Neilbarman, 2008)
Then, in 2008 the Penumbra aspiration system was introduced. The Penumbra macerates the clot with a separator, which is repeatedly advanced into and withdrawn from the clot, all under direct suction. It utilizes a relatively large-bore catheter versus Merci’s microcatheter. Studies of the Penumbra indicate revascularization rates between 82-87%, with up to 41% of patients reporting an mRS of 2 or less at 90 days. The Penumbra studies also report slightly lower complication rates than the Merci (Spiotta et al., 2015).
Finally, the most recent development in thrombectomy devices is the stent retriever device, such as the Solitaire and Trevo Pro. These are now the most commonly used, and they are based on the earlier partially deployed stents, such as the Enterprise. These stents are removable, negating the need for long-term dual antiplatelet therapy (DAPT). The stents are opened from within the center of the thrombus, and suction is applied during retrieval; alternatively, they can be used with the Penumbra system for retrieval. Studies have shown the recanalization rates are superior to the Merci device (61% and 86% for Solitaire and Trevo respectively), as are the mRSs (mRS of 2 or less at 90 days, 36% and 40% for Solitaire and Trevo respectively). Future research is looking at the use of ultrasound vibration to facilitate thrombolysis, designed to be used with intra-arterial tPA (Spiotta et al., 2015). The ASA does not recommend devices to augment cerebral blood flow, neuroprotective agents such as magnesium, or transcranial near-infrared laser therapy (Powers et al., 2018).
One of the largest advantages of a mechanical thrombectomy procedure is that it has been proven safe in acute stroke patients within six hours of symptom onset. ASA guidelines (2018) stipulate that in order to qualify for mechanical thrombectomy with a stent retriever, an acute stroke patient should meet all of the following criteria:
- mRS pre-stroke of 1 or less (no significant disability present),
- occlusion of the internal carotid artery or segment 1 of the MCA,
- NIHSS score of 6 or greater,
- at least 18 years of age,
- ASPECTS of 6 or greater (Powers et al., 2018).
With all of these treatment options for acute ischemic stroke available, the choice of how to treat a patient is oftentimes difficult to assess. In an effort to ascertain the most reliable and safe treatment available, numerous studies have been done to prove both the efficacy and safety of stent retrievers for mechanical thrombectomy in acute ischemic stroke patients. In 2015, five randomized clinical trials were all published in the same year, attempting to establish the superiority of mechanical thrombectomy procedures for selected patients with acute ischemic strokes. A meta-analysis of 1,287 patients from these five studies was conducted and concluded that in cases of proximal occlusions of either of the anterior arteries (MCA or the ICA), a combination of medical treatment and thrombectomy procedure led to significantly less disability at 90 days versus standard medical treatment alone. This difference was even present in three high-risk groups of patients: those over the age of 80, those more than 300 minutes (five hours) out from symptom onset, and those patients that were determined ineligible for IV tPA treatment. Regarding safety, the five studies showed no significant increase in mortality rate when combined; they also found no significant increase in the risk of parenchymal hematoma or symptomatic ICH following thrombectomy procedures. One note, the meta-analysis and many of these studies were at least partially funded by Covidien/Medtronic, the company that manufactures the Solitaire thrombectomy device, leading to some healthy skepticism regarding their inherent bias (Goyal et al., 2016). Another group combined the above five studies in another meta-analysis to determine the ideal timing in which to consider thrombectomy procedure. Their analysis determined that the benefits of thrombectomy procedure versus medical treatment alone diminished after just over seven hours (seven hours and 18 minutes, to be exact) from symptom onset to anticipated arterial puncture (representing the beginning of the endovascular procedure) (Saver et al., 2016).
The following year, two studies were published regarding whether or not IV tPA was a necessary adjunct to thrombectomy procedures or simply an added expense. Another meta-analysis, this time of 13 studies, some including multiple trials, was published to review cases of thrombectomy alone (n=236) versus IV tPA followed by thrombectomy (n=681). They concluded that within the thrombectomy group, 45.8% of patients reported functional independence at 90 days, versus 49% in the combination group. Although this difference was statistically non-significant, the authors found it important. Mortality rates were 12.5% in the combination group versus 17.4% in the thrombectomy-only group, which was a significant difference. They also found a higher rate of successful recanalization in the combination group as well as less "passes" required during the procedures, and no increased risk of symptomatic ICH. The authors commented on the fact that the trials used for this analysis did not randomize patients to receive the IV tPA treatment and suggested further randomized controlled clinical trials in the future (Mistry et al., 2017). Another group analyzed two older studies, the SWIFT trial and the STAR trial published in 2012 and 2013, respectively, to explore the same question. This pooled analysis used the records of 291 patients who had undergone mechanical thrombectomy with the Solitaire device within eight hours of symptom onset, 160 of which had also received IV tPA treatment prior to the procedure. When comparing the combination patients against the thrombectomy-only patients, the authors found a decreased risk of symptomatic ICH (1.1% vs. 3.8%) and an increased rate of functional independence at 90 days (57.7% versus 47.7%) in the combination group, but these findings did not reach the level of statistical significance. The authors did comment that vasospasm was more common in the combination group who received IV tPA (Coutinho et al., 2017).
While the level of benefit is uncertain, the ASA found that it is reasonable to also proceed with thrombectomy in “carefully selected patients” with an occlusion in segment 2 or 3 of the MCA, the anterior cerebral artery, vertebral artery, basilar artery, the posterior cerebral artery, or those patients with a pre-stroke mRS of more than 1, an ASPECTS under 6, or an NIHSS under 6. If the patient presents with symptoms present for 6-16 hours, they also recommend thrombectomy procedure if the patient is able to meet DAWN or DEFUSE3 criteria, or 16-24 hours if the patient meets the DAWN criteria (Powers et al., 2018).
Post Procedure Care
After administration of IV tPA, the patient should be monitored closely for changes in neurological status, with blood pressure checks and a neurological exam every 15 minutes for the first two hours, then every 30 minutes for six hours, then hourly until 24 hours after initial administration of the medication. Neuroimaging should be repeated approximately 24 hours following the administration of thrombolytics prior to starting an oral antithrombotic agent. A blood pressure of less than 180/105 should be maintained for at least 24 hours following either administration of IV fibrinolytic or an endovascular thrombectomy. Blood pressure over this during the first 24 hours should be treated to avoid hemorrhagic conversion. In patients not treated with endovascular procedures or IV tPA, blood pressure may be allowed to increase to as high as 220/120 for the first 48-72 hours after an acute ischemic stroke. Over this, it is reasonable to attempt to medicate to decrease the blood pressure by 15% within 24 hours. Vasodilatory agents are not recommended. If the blood pressure consistently remains over 140/90, it is safe and reasonable to initiate or restart an antihypertensive prior to discharge. Cardiac monitoring is recommended for at least 24 hours to rule out atrial fibrillation or another arrhythmia. In stroke patients with a diagnosis of Afib, anticoagulation therapy should be started within 4-14 days. Regular skin assessments and optimal skincare (consistent turning, good hygiene, specialized mattresses/wheelchair cushions) are important to maintain skin integrity in stroke patients that may have limited mobility. A dysphagia (difficulty swallowing) screen should be done prior to allowing any oral intake in a stroke patient, preferably done by a speech and language therapist (SLT). An enteral diet should be started within seven days of admission. If the patient has significant dysphagia, then a nasogastric (NG) tube (for short-term dysphagia) or a percutaneous endoscopic gastrostomy (PEG) tube (for longer-term dysphagia) should be placed to achieve this. Nutritional supplementation and oral hygiene are reasonable. Daily acetylsalicylic acid (ASA, aspirin), adequate hydration, and SCDs are recommended in immobile patients to reduce the risk of DVT. For non-cardioembolic strokes, urgent anticoagulation is not recommended, but as previously mentioned, DAPT is recommended for the first 21 days (usually started with 24-48 hours if no tPA, 48-72 hours if the patient received tPA), followed by clopidogrel (Plavix) alone until 90 days. The ASA finds no benefit to prophylactic antibiotics, and routine urinary catheter placement should be avoided. Seizures, if they occur in acute stroke patients, should be treated as they would otherwise, with an AED chosen based on the patient’s individual situation. AEDs should not be given prophylactically (Powers et al., 2018).
Symptomatic ICH (sICH) occurs in 2-7% of acute ischemic stroke patients after receiving IV tPA treatment. This diagnosis is based on a combination of radiological findings as well as neurological exam changes. They typically occur within 36 hours of tPA administration. The neurological exams following an acute ischemic stroke treated with IV tPA are key, with thorough and complete documentation of any changes in the patient’s NIHSS. Once identified, the patient should be moved immediately to an ICU or back to the stroke unit if not already there. Management includes cardiovascular/respiratory support if needed, blood pressure management (ideal range varies and is still unclear), close neurological monitoring, prevention of hematoma expansion, and treatment of elevated intracranial pressure (ICP) and other complications that arise from the hemorrhage including seizures, as with any spontaneous ICH (see the previous section on management of ICH; Yaghi et al., 2017).
Other common complications following an acute stroke include cerebellar or cerebral edema, which may lead to obstructive hydrocephalus, especially in cerebellar infarcts. Ventriculostomy is the treatment of choice in this instance, according to the ASA guidelines, and potentially a decompressive craniectomy if necessary. If short-term spinal fluid diversion is needed postoperatively (as is common in SAH patients), a lumbar drain could also be considered. Patients with large supratentorial infarcts are also at increased risk for cerebral edema leading to increased ICP. This risk should be communicated with the patient and family and treatment and care options discussed early on. Measures should be taken to reduce the risk for edema, and patients should be monitored closely for signs or symptoms. Osmotic therapy and brief moderate hyperventilation (PCO2 target 30-34) are medical treatments that are reasonable for cerebral or cerebellar edema, but hypothermia, barbiturates, and corticosteroids should be avoided. Major infarcts should be transferred to a care facility with neurosurgical expertise if needed. Decompressive craniectomy is also reasonable to reduce mortality in stroke patients under the age of 60 with a unilateral MCA infarct with continued neurological deterioration for 48 hours after acute stroke. Over the age of 60, a craniectomy “may be considered” as it has still been shown to reduce mortality by as much as 50% in this older group but has less favorable outcomes on functional recovery. The ASA specifically recommends stroke patient education, provided to both the family and the patient, as well as allowing the patient the opportunity to talk about the impact of the illness on their lives (Powers et al., 2018). As previously found, many of these ASA guidelines are also primary stroke CPMs published by the Joint Commission (2018b).
A common complication following surgical treatment for an aneurysm and resultant SAH is vasospasm and secondary or delayed cerebral ischemia. Cerebral vasospasm occurs in 70% of the patients following aneurysmal SAH and leads to symptomatic brain ischemia in 30% of these cases. Oral nimodipine (Nimotop, Nymalize), a dihydropyridine calcium channel antagonist, should be started after surgery (60 mg every 4 hours) for a total of 21 days, after which the risk of this particular complication decreases. Previously, prevention of this complication was also accomplished via “Triple-H” therapy, which included hypervolemia, hemodilution, and induced hypertension. All but the induced hypertension have been studied individually and fallen out of favor as they have failed to show any significant benefit and may even carry risk. Induced hypertension and euvolemia is the current recommendation for vasospasm prevention, with isotonic crystalloid the intravascular fluid of choice. Cerebral vasospasm monitoring using transcranial dopplers is also reasonable for patients at risk or with a known history. Treatments currently being investigated include magnesium, statins, endothelin receptor antagonists, tirilazad, epoetin alfa (Procrit), and glyburide (DiaBeta, Glucovance). While these options all show promise in preclinical testing, none have shown a significant impact in phase III trials (Grasso et al., 2017).
Finally, a note regarding teamwork, protocols, and workflow optimization is important to include here as a final umbrella that affects all stages of care for stroke patients. The ASA recommends multiple times throughout their latest guidelines that hospitals and providers develop organized protocols and designate teams in order to provide "comprehensive specialized" care to stroke patients. While these recommendations may not be as interesting to focus on as new technology, devices, and pharmaceuticals, they are exceedingly important. A German hospital conducted a study that was published in 2018 regarding the effect of two significant changes to the manner in which they care for acute stroke patients. The first change was to provide 24-hour, on-site neuroradiological service. This provided some improvement in their outcomes but was not statistically significant. It was only after they instituted extensive workflow optimization and documentation of procedure times did they begin to see significant improvements in their door-to-image, image-to-puncture, and door-to-revascularization times, especially in patients presenting for care outside of normal business hours. And after all, time is tissue (Nikoubashman et al., 2018).
In addition to the aforementioned NIHSS and other stroke scales used to initially evaluate a patient, two other stroke scales are commonly utilized later to assess any residual or chronic deficits. The mRS was originally introduced in 1957 by Dr. John Rankin. It is an interview-style evaluation that can be completed in person or over the phone. It is traditionally performed 90 days after the stroke in most cases and takes approximately two minutes to complete. Scores range from 0, which indicates no residual symptoms, to a maximum score of 6, which indicates the patient is deceased as a direct result of the stroke. An mRS of 2 or less indicates a patient who is functionally independent. Another commonly used disability tool is the Barthel Index (BI), which was first published in 1965 and is used in a similar manner to the mRS. It is either an observational or interview-style tool that can be completed with a family member or caregiver. It assesses a patient’s ability to complete ten different activities of daily living (ADLs) with a total score ranging from 0-100. The BI includes feeding, bed to chair and back, personal care/hygiene, getting on or off the toilet, bathing, walking, going up or down the stairs, dressing, control of bladder, and control of bowels. A higher score correlates with a greater level of functional independence (Scheinfeld et al., 2016).
Rehabilitation
After a stroke, there are two primary mechanisms for brain recovery. The first happens early and automatically as the circulation to an area is restored, and the edema recedes. The second is termed neuroplasticity and involves neurons in the brain reorganizing their structure, function, and interconnections. While damaged neurons in the central nervous system often die and are not able to regenerate, undamaged axons have been shown to grow new nerve endings to connect to other undamaged neurons, called neuro-cortical sprouting. Also, stroke patients may be able to train their brains to perform tasks by using previously latent functional pathways. This learning is stimulated primarily by highly repetitive practice. Researchers are investigating ways in which this could potentially be augmented with things like medications or transcranial magnetic stimulation (Palmer, 2015).

The ASA found that roughly 70% of Medicare patients diagnosed with acute stroke utilize Medicare-covered post-acute care. About 32% are sent to skilled nursing facilities for rehabilitation, 22% to inpatient rehabilitation facilities (either free-standing or within an acute care hospital), and 15% are discharged directly home with home health care. This means that 30% of all stroke patients covered by Medicare get no post-acute rehabilitation at all, a number that has increased since the 1990s (Winstein et al., 2016). Prior to discharge, all stroke patients should be screened for depression and appropriately treated if present. Additionally, a formal assessment of functional ADLs, instrumental ADLs (iADLs), communication skills, and functional mobility by a clinician with expertise in rehabilitation should be completed. If they qualify, all stroke patients should receive early rehabilitation in an environment with organized interprofessional stroke care at an intensity commensurate with their tolerance and anticipated benefit. High-dose therapy within 24 hours of stroke should be avoided. Fluoxetine (Prozac, or another selective serotonin reuptake inhibitor [SSRI]) use during stroke rehabilitation to enhance motor recovery in the absence of confirmed depression is not yet well established (Powers et al., 2018; Winstein et al., 2016). The ASA Rehabilitation Guidelines (2016), recommend that stroke patients remain in an inpatient setting for their rehabilitation if they require therapy interventions from multiple disciplines (physical therapy [PT] and occupational therapy [OT] to address moderate to severe motor or sensory deficits, OT and SLT to address cognitive deficits, and SLT to address communication deficits) as well as skilled nursing care for:
- bowel or bladder impairment,
- existing or increased risk for skin breakdown,
- impaired bed mobility,
- dependence for basic ADLs,
- inability to manage medications independently, or
- increased risk for nutritional deficits.
Inpatient rehabilitation candidates should also have a documented need for daily physician contact to help manage:
- significant or multiple medical comorbidities;
- complex rehabilitation issues such as bowel/bladder incontinence, spasticity, or orthotics;
- acute illness; or
- pain management issues (Winstein et al., 2016).
A rehabilitation unit for acute stroke patients should consist of a comprehensive, evidence-based, and multidisciplinary program that focuses on prevention of complications, medical management, and the rehabilitation of sensorimotor impairments, upper extremity activities, cognitive or communication deficits, and transitions of care to home or to the community. It involves a sustained and coordinated effort from a team that includes the patient, the patient's family, caregiver(s) and friends, physicians, NPs, PAs, nurses, PTs, OTs, SLTs, recreational therapists, psychologists, nutritionists, social workers, and others. Communication and coordination are extremely important in these environments. A PT-guided balance training program is paramount to safety if there is poor balance or an increased risk of falls documented on the patient's physical therapy or nursing assessments or simply a decrease in the patient's balance confidence or a fear of falls secondary to the acute stroke. When the patient is appropriate for discharge back home, an individually tailored exercise program to gradually improve cardiovascular fitness is helpful for secondary stroke prevention (Winstein et al., 2016).
A meta-review of 13 systematic reviews found that when self-care was included as a component of a rehabilitation program post-stroke, it results in short-term (less than one year) improvements in ADLs and a decrease in the risk of dependence and death. A separate meta-analysis of six trials of self-care programs found that this intervention increases the quality of life and self-efficacy after stroke (Riegel et al., 2017). Other complementary treatments, such as acupuncture, are also being tested. A meta-analysis of eight randomized controlled trials was completed, including 399 patients with post-stroke spasticity treated with acupuncture versus sham procedures in one study, and acupuncture plus PT versus PT alone in the other seven studies. The analysis, unfortunately, shows no statistically significant effect on clinical outcomes (as tested by the modified Ashworth Scale [mAS]) or physiologic outcomes (as tested by the H-reflex/M-response ratio) except in two trials, which were able to show a significant improvement after the first visit only (Park et al., 2014). Dysphagia is another common chronic sequela of stroke. A systematic review of 58 studies involving over 6,000 patients who underwent acupuncture treatment for their dysphagia showed that the acupuncture group was superior to the control with moderate heterogeneity, and the efficacy rate of acupuncture was three times that of the control group with no heterogeneity (Ye et al., 2017).
Technology is also playing a role in increasing availability and decreasing the cost of post-stroke care and rehabilitation services. When stroke patients with chronic aphasia are treated with constraint-induced aphasia therapy (CIAT, also known as intensive language action therapy or ILAT), even in a computer-based format in their own home, it may help improve communication skills. Computer software can be personalized to an individual patient's needs, and with the development of better speech recognition software to help with word choice and pronunciation, speech therapy patients can be initially trained and then tracked remotely. Exercises can then be adjusted as the patient improves. All of this happens as the patient is able to practice and work at home instead of the increased time and cost of patients traveling for their speech therapy appointments (Palmer, 2015).
If the patient has chronic residual deficits, many find that even after the acute phase, they may still benefit from therapies months or years later. A combination treatment of four weeks of repetitive facilitative exercises (RFEs) and orthotics was tested within a physical therapy office on 27 stroke patients that were at least five months and an average of 35 months post-stroke. While this was a small sample size, all measures showed significant improvement after treatment. RFEs combine high repetition rate and neurofacilitation, where trained therapists use muscle spindle stretching and skin-generated reflexes to help the patient activate and move an affected limb (Tomioka et al., 2017).
References
Benjamin, E.J., Virani, S.S., Callaway, C.W., Chamberlain, A.M., Chang, A.R., Cheng, S., Chiuve, S. E., Cushman, M., Delling, F. N., Deo, R., de Ferranti, S. D., Ferguson, J. F., Fornage, M., Gillespie, C., Isasi, C. R., Jimenez, M. C., Jordan, L. C., Judd, S. E., Lackland, D., Lichtman, J. H.,… Muntner, P. (2018). Heart disease and stroke statistics—2018 Update: A report from the American Heart Association. Circulation,137, e67–e492. https://doi.org/10.1161/CIR.0000000000000558
Bowen, P. (2016). Early identification, rapid response and effective treatment of acute stroke: utilizing teleneurology to ensure optimal clinical outcomes. MedSurg Nursing, 25(4) 241-243.
The Centers for Disease Control and Prevention. (n.d.) Interactive atlas of heart disease and stroke. Retrieved on May 27, 2020 from https://nccd.cdc.gov/DHDSPAtlas/Default.aspx
The Centers for Disease Control and Prevention. (2020). Stroke education materials for health professionals. https://www.cdc.gov/stroke/materials_for_professionals.htm
Coutinho, J. M., Liebeskind, D. S., Slater, L., Nogueira, R. G., Clark, W., Dávalos, A., Bonafe, A., Jahan, R., Fischer, U., Gralla, J., Saver, J. L. & Pereira, V.M. (2017). Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: A pooled analysis of the SWIFT and STAR studies. JAMA Neurology, 74, 268–274. https://doi.org/10.1001/jamaneurol.2016.5374
DocJBrigham. (2019). Stenting coils [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:StentingCoils.png
Franco, J. (2018). Imaging and treatment of strokes. Radiologic technology, 89(6), 549-568.
Goyal, M., Menon, B. K., Zwam, W. H., Dippel, D. W., Mitchell, P. J., Demchuk, A. M., Dávalos, A., Majoie, C. B. L. M., van der Lugt, A., de Miquel, M. A., Donnan, G. A., Roos, Y. B. W. E. M., Bonafe, A., Jahan, R., Diener, H.-C., van den Berg, L. A., Levy, E. I., Berkhemer, O. A., Pereira, V. M., Rempel, J.,… Jovin, T. G. (2016). Endovascular thrombectomy after large vessel ischaemic stroke: A meta-analysis of individual patient data from five randomized trials. The Lancet, 387(10029), 1723-1731. https://doi.org/10.1016/s0140-6736(16)00163-x
Grasso, G., Alafaci, C., & Macdonald, R. L. (2017). Management of aneurysmal subarachnoid hemorrhage: State of the art and future perspectives. Surgical Neurology International, 8, 11. https://doi.org/10.4103/2152-7806.198738
Hemphill, J.C. III, Greenberg, S.M., Anderson, C.S., Becker, K., Bendok, B.R., Cushman, M., Fung, G. L., Goldstein, J. N., Macdonald, R. L., Mitchell, P. H., Scott, P. A., Selim, M. H., & Woo, D. (2015). Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 46, 1-29. https://doi.org/10.1161/STR.0000000000000069.
The Joint Commission. (2018a). Facts about Joint Commission stroke certification. https://www.jointcommission.org/facts_about_joint_commission_stroke_certification/
The Joint Commission. (2018b). Specifications manual for Joint Commission national quality measures. https://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx.
Meng, R., Ding, Y., Asmaro, K., Brogan, D., Meng, L., Sui, M., Shi, J., Duan, Y., Sun, Z., Yu, Y., Jia, J., & Ji, X. (2015). Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics, 12(3), 667-77. https://doi.org/10.1007/s13311-015-0358-6.
Mistry, E. A., Mistry, A. M., Nakawah, M. O., Chitale, R. V., James, R. F., Volpi, J. J., & Fusco, M. R. (2017). Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients. Stroke, 48, 2450–2456. https://doi.org/10.1161/STROKEAHA.117.017320.
National Heart, Lung, and Blood Institute. (n.d.). Stroke. Retrieved May 27, 2020, from https://www.nhlbi.nih.gov/health-topics/stroke
National Heart, Lung, and Blood Institute. (2013a). Hemorrhagic stroke [image]. https://commons.wikimedia.org/wiki/File:Stroke_hemorrhagic.jpg
National Heart, Lung, and Blood Institute. (2013b). Ischemic stroke [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Stroke_ischemic.jpg
National Institutes of Health. (2010). Cerebral aneurysm [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Cerebral_aneurysm_NIH.jpg
National Institutes of Health. (2020). Stroke. MedlinePlus. https://medlineplus.gov/stroke.html
National Institute of Neurological Disorders and Stroke (n.d.). Stroke materials: Infographics. Retrieved May 26, 2020 from https://www.stroke.nih.gov/materials/infographics.htm
National Institute of Neurological Disorders and Stroke. (2020). Cerebral aneurysms fact sheet (NIH Publication No. 18-NS-5506). https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Cerebral-Aneurysms-Fact-Sheet#7
Neilbarman. (2008). Merci device [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Merci_L5.jpg
Nikoubashman, O., Schürmann, K., Othman, A. E., Bach, J-P., Wiesmann, M., & Reich, A. (2018). Improvement of endovascular stroke treatment: A 24-hour neuroradiological on-site service is not enough. BioMed Research International, 2018, 1-8. https://doi.org/10.1155/2018/9548743.
OpenStax. (2016). Circle of willis [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:1314_Circle_of_WillisN.jpg
OpenStax College. (2013). Common carotid artery [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:2122_Common_Carotid_Artery.jpg
Palmer, R. (2015). Innovations in aphasia treatment after stroke: Technology to the rescue. British Journal of Neuroscience Nursing, 38-42. https://doi.org/10.12968/bjnn.2015.11.Sup2.38.
Park, S. W., Yi, S. H., Lee, J. A., Hwang, P. W., Yoo, H. C., & Kang, K. S. (2014). Acupuncture for the treatment of spasticity after stroke: A meta-analysis of randomized controlled trials. Journal of Alternative and Complementary Medicine, 20(9), 672-82. https://doi.org/10.1089/acm.2014.0097.
Powers, W.J., Rabinstein, A.A., Ackerson, T., Adeoye, O.M., Bambakidis, N.C., Becker, K., Biller, J., Brown, M., Demaerschalk, B. M., Hoh, B., Jauch, E. C., Kidwell, C. S., Leslie-Mazwi, T. M., Ovbiagele, B., Scott, P. A., Sheth, K. N., Southerland, A. M., Summers, D. V., & Tirschwell, D.L. (2018). Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 49, e46–e99. https://doi.org/10.1161/STR.0000000000000158.
Riegel, B., Moser, D. K., Buck, H. G., Dickson, V. V., Dunbar, S. B., Lee, C. S., Lennie, T. A., Lindenfeld, J., Mitchell, J. E., Treat-Jackson, D. J., & Webber, D. E. (2017). Self-care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American Heart Association. J Am Heart Assoc, 6, e006997. https://doi.org/10.1161/JAHA.117.006997.
Saver, J. L., Goyal, M., van der Lugt, A., Menon, B. J., Majoie, C. B. L. M., Dippel, D. W., Campbell, B. C., Nogueira, R. G., Demchuk, A. M., Tomasello, A., Cardona, P., Devlin, T. G., Frei, D. F., du Mesnil de Rochemont, R., Berkhemer, O. A., Jovin, T. G., Siddiqui, A. H., van Zwam, W. H., Davis, S. M., Castano, C.,… Hill, M. D. (2016). Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 316(12), 1279-89. https://doi.org/10.1001/jama.2016.13647.
Scheinfeld, M. H., Erdfarb, A. J., Krieger, D. A., Bhupali, D. & Zampolin, R. L. (2016). A radiologist’s guide to the clinical scales used in the 2015 Endovascular Stroke Trials and the Revised American Heart Association/American Stroke Association Guidelines for Endovascular Stroke Treatment. Emerg Radiol 23, 497-501. https://doi.org/10.1007/s10140-016-1420-3.
Schröder, J. & Thomalla, G. (2017). A critical review of Alberta Stroke Program Early CT Score for evaluation of acute stroke imaging. Frontiers in Neurology, 7, 245. https://doi.org/10.3389/fneur.2016.00245.
Spiotta, A. M., Chaudry, M. I., Hui, F. K., Turner, R. D., Kellogg, R. T. & Turk, A. S. (2015). Evolution of thrombectomy approaches and devices for acute stroke: A technical review. J NeuroIntervent Surg, 7, 2–7. https://doi.org/10.1136/neurintsurg-2013-011022.
Tomioka, K., Matsumoto, S., Ikeda, K., Uema, T., Sameshima, J. I., Sakashita, Y., Kaji, T., & Shimodozono, M. (2017). Short-term effects of physiotherapy combining repetitive facilitation exercises and orthotic treatment in chronic post-stroke patients. Journal of Physical Therapy Science, 29(2), 212-215. https://doi.org/10.1589/jpts.29.212.
Wändell, P., Carlsson, A. C., Holzmann, M., Ärnlöv, J., Johansson, S. E., Sundquist, J., & Sundquist, K. (2016). Association between antithrombotic treatment and hemorrhagic stroke in patients with atrial fibrillation-a cohort study in primary care. European Journal of Clinical Pharmacology, 73(2), 215-221. https://doi.org/10.1007/s00228-016-2152-8.
Winstein, C.J., Stein J., Arena, R., Bates, B., Cherney, L.R., Cramer, S.C., Deruyter, F., Eng, J. J., Fisher, B., Harvey, R. L., Lang, C. E., MacKay-Lyons, M., Ottenbacher, K. J., Pugh, S., Reeves, M. J., Richards, L. G., Stiers, W., & Zorowitz, R.D. (2016). Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 47, e98–e169. https://doi.org/10.1161/STR.0000000000000098.
World Health Organization. (2017). Cardiovascular diseases (CVDs) fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
Yaghi, S., Willey, J. Z., Cucchiara, B., Goldstein, J. N., Gonzales, N. R., Khatri, P., Kim, L. J., Mayer, S. A., Sheth, K. N., & Schwamm, L. H. (2017). Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 48, e343–e361. https://doi.org/10.1161/STR.0000000000000152.
Ye, Q., Xie, Y., Shi, J., Xu, Z., Ou, A., & Xu, N. (2017). Systematic review on acupuncture for treatment of dysphagia after stroke. Evidence-based Complementary and Alternative Medicine: eCAM, 2017, 6421852. https://doi.org/10.1155/2017/6421852.