About this course:
The purpose of this activity is to review and highlight the evidence-based care of patients diagnosed with SUD to facilitate the optimal care of these patients.
Course preview
The purpose of this activity is to review and highlight the evidence-based care of patients diagnosed with substance use disorder to facilitate the optimal care of these patients.
At the conclusion of this activity, the nurse should be prepared to:
- Define the terms and epidemiology associated with substance use disorder and addiction.
- Review the pathophysiology, risk factors, and protective factors related to substance use disorder and addiction in the US and worldwide.
- Highlight the screening tools and diagnostic criteria of substance use disorder and addiction.
- For the most common substances abused, elaborate on the intended effects, signs and symptoms of intoxication, and signs/symptoms of withdrawal.
- Evaluate the most effective and evidence-based treatment options available for substance use disorder and addiction, as well as pending research.
The National Institute on Drug Abuse (NIDA, 2018b) defines tolerance to medication as the gradual need over time for an increased dose of a particular medication to obtain a similar effect. The development of tolerance varies significantly from individual to individual and from medication to medication and is due to the brain's ability to adapt to its environment physically. It is a phenomenon that is not limited to pain medication or illicit drugs but is also seen in other specialties and circumstances. Physical dependence is the physiological adaptation to a medication that develops with consistent and regular use and is a component of addiction. The medication becomes necessary for normal homeostasis and functioning. This phenomenon typically correlates with opposing symptoms of withdrawal if that medication is no longer used. Addiction is a combination of physical dependence and compulsive drug-seeking behaviors despite significant negative repercussions. Misuse of prescription drugs is the ingestion or utilization of these medications in a manner, a dose, or by an individual other than prescribed. This includes taking someone else's medication or taking pain medication to induce feelings of euphoria instead of relieving pain. The medical terms substance abuse and substance dependence have been replaced in recent years with the term substance use disorder (SUD). This may refer to an individual who has become addicted to nicotine, alcohol, prescription medications, or illicit drugs (NIDA, 2018b). Separate from these, there also exists another phenomenon called pseudoaddiction, in which the patient becomes intensely fearful of being in pain. This is common in postoperative patients and usually manifests as clock-watching, asking to be awoken to receive pain medication, and hypervigilance with documenting and monitoring pain medications. Pseudoaddiction usually resolves with effective pain management treatments and the resolution of painful stimuli (in the postoperative patient, this is the healing of the surgical site). Psychological dependence occurs when the ingestion of medication becomes associated with the alleviation of discomfort, such as pain, anxiety, depression, etc. The presence of that drug then becomes a calming and reassuring presence in the patient’s life, similar to a comfort or security object (Hudspeth, 2016a).
Epidemiology
The Substance Abuse and Mental Health Services Administration (SAMHSA, 2019) conducts the National Survey on Drug Use and Health (NSDUH) regarding substance use in the US, which was last performed in 2018. This survey includes self-reported data on over 67,700 noninstitutionalized Americans over the age of 11 and is conducted across all 50 states and the District of Columbia. It does not include data on long-term facility residents, incarcerated individuals, or homeless individuals not currently residing in a homeless shelter. They estimate that in 2018, 47 million Americans smoked cigarettes (27.3 million on a daily basis), and roughly 12 million smoked cigars. These numbers are down when compared to statistics from 2002. They estimate that 139.8 million Americans used alcohol in the last month, including 67 million who report binge drinking, over 16 million who report heavy drinking, and over 2 million adolescents between the ages of 12 and 17. This last number has also decreased since 2002. Of those surveyed, 19.4% report illicit drug use in the past year, and 15.9% (an estimated 43.5 million) report marijuana use in the last year (an increase from previous surveys). Prescription pain reliever misuse in the past year was reported by 3.6% of respondents (a decrease from the 2015 and 2017 survey results), estimated at nearly 10 million Americans. Sixty three percent of these users report that they abused prescription pain medication to relieve pain, and 51% report that they obtained this medication from a relative or friend. They estimate usage in the last month of cocaine; prescription sedatives, tranquilizers, or other central nervous system (CNS) depressants; prescription stimulants; hallucinogens; methamphetamine (meth); and inhalants (see Table 1). Roughly 350,000 report using heroin in the last month, and over 800,000 in the previous year (SAMHSA, 2019). Many addiction experts theorize that there exists a public perception that prescription opioids and other CNS depressants are safer than illicit drugs because they can be prescribed and used therapeutically. They believe that this perception, in combination with their ease of access, is responsible for the relatively large number of individuals who abuse prescription opiates in comparison to the small number of individuals who abuse heroin (NIDA, 2018b). This is supported by the NSDUH results, which indicate that 94% of respondents associated weekly heroin use with overall great risk, 86% with weekly cocaine use, and 72% with daily tobacco smoking. Only 30% of the respondents related great risk with weekly marijuana use (SAMHSA, 2019). NIDA estimates that SUD costs the US approximately $600 billion annually. In contrast, the cost of incarcerating an individual is $24,000 annually, and the cost of providing outpatient medication-assisted treatment (MAT) for SUD with buprenorphine (Sublocade) and twice-weekly clinic visits for one year is only $6,552 per person (Moore, 2019).
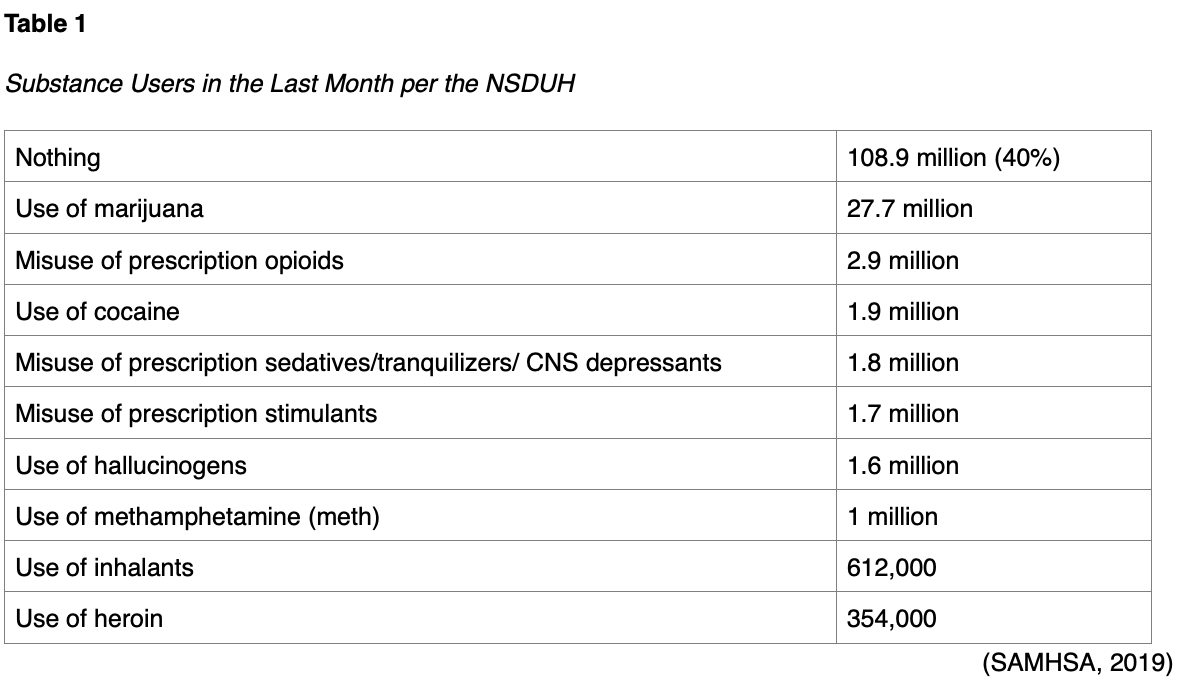
SAMHSA (2019) utilizes the diagnostic criteria for SUD from the 4th edition of the American Psychiatric Association's (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM-4) to estimate that 20.3 million Americans have verifiable SUD, roughly 75% of those based on alcohol use. Following alcohol, the most common drug of choice among those with SUD include marijuana (4.4 million) and prescription opioids (1.7 million). Adding a small percentage of users based on treatment in the past year for SUD, they estimate that just over 21 million Americans needed treatment for a SUD in 2018, despite only 3.7 million receiving said treatment. Of those over 17 million who needed treatment but did not receive it, less than one million perceived a need for treatment. In those who perceived a need, two out of five respondents cited not being ready to stop using, and a third cited lack of insurance coverage or financial resources to afford treatment. SAMHSA (2019) estimates that 9.2 million adults and over 350,000 adolescents have SUD in combination with another mental illness, which may be depression, anxiety, attention deficit hyperactivity disorder (ADHD), or various other psychological disorders. They found SUD to be more common in those with other mental health disorders (SAMHSA, 2019).
Pathophysiology
While the root cause of addiction is still not entirely understoo
...purchase below to continue the course
Long-term effects of substance use include decreased educational attainment, un- or underemployment, unstable housing, strained personal relationships, and criminal justice involvement. Aside from the financial, social, personal, professional, and legal consequences that may accompany substance use, the health consequences can broadly be broken down into short-term and long-term effects. Short-term health consequences include changes in appetite, wakefulness, heart rate, blood pressure, or mood; acute myocardial infarction; stroke; psychosis; and overdose/death. The long-term effects are varied and extensive but may include lung disease, heart disease, cancer, mental illness, HIV/AIDs, hepatitis, addiction, and impulsivity and poor decision making leading to increased risk of trauma/violence/injury (NIDA, 2017b).
Risk and Protective Factors
There are several risk factors for the development of SUD. As previously mentioned, half of the risk is genetic, as evidenced by a family history of addiction (Mayo Clinic, 2017; NIDA, 2019a). Most risks occur early in life, even during gestational development. The following increase the risk of SUD:
- Maternal smoking and alcohol use
- Having a “difficult temperament” during infancy
- Insecure attachment to a parent(s)
- Uncontrolled aggression as a toddler
- Lack of school readiness skills
- Poor self-regulation as a child
- Lack of classroom structure as a child
- Chronic stress (i.e., family poverty, serious parental illness, child maltreatment)
- Parental substance abuse
- A personal history of mental illness, especially early mental illness (i.e., anxiety, depression, ADHD)
- Academic failure
- Social/emotional difficulties
- History of trauma/abuse
- Peer pressure
- The use of certain high-risk substances (stimulants, cocaine, opioids; Mayo Clinic, 2017; NIDA, 2016).
Studies regarding IV heroin users have identified a list of risk factors specific to opioid users transferring to heroin use, which includes non-oral use of opioids (via sniffing/snorting, smoking, injection), developing opioid dependence, early initiation of opioid use (under age 16), and only using opioids to achieve feelings of euphoria instead to treat pain (Carlson et al., 2016; Mayo Clinic, 2017). Studies have also identified many protective factors against SUD, including:
- Good maternal nutrition
- A birth weight that is within normal limits
- A strong parent-child bond
- Improving behavior control during the preschool years
- School readiness skills
- Increased intelligence
- An easy temperament (flexible, positive mood)
- Parenting that is warm, consistent, and age-appropriate
- Parents that regularly listen to and communicate with their children
- Praise for accomplishments
- Parents that provide a good example and consistent rules and routines
- Opportunities for regular social interaction/after school programs and exercise (NIDA, 2016, 2019a).
Screening and Diagnosis
Screening for the risks of SUD should be performed prior to dispensing an opioid and as needed using a validated screening tool such as the Opioid Risk Tool (ORT), the Tobacco, Alcohol, Prescription Medication, and other Substance Use Tool (TAPS), the NIDA Drug Use Screening Tool: Quick (NMASSIST), CAGE Adapted to Include Drugs (CAGE-AID), Screening to Brief Intervention (S2BI), Brief Screener for Alcohol, Tobacco, and other Drugs (BSTAD), Diagnosis, Intractability, Risk, and Efficacy (DIRE) and others (NIDA, 2018a). The Screener and Opioid Assessment for Patients with Pain (SOAPP) and the Current Opioid Misuse Measure (COMM) can both be used to screen current opioid users. The SOAPP has four versions for chronic pain patients to assess their ability to self-manage their treatment. The COMM is a 17-item questionnaire which assesses for signs and symptoms of abuse, psychiatric disorders, evidence of willful dishonesty, provider visitation patterns, and medication noncompliance. Not all screening tools are appropriate for all patients; for example, the S2BI and BSTAD are intended for use with adolescent patients while most other tools are designed for adult patients (Hudspeth, 2016a; NIDA, 2018a).
There exist some red flags that a patient may be misusing or diverting their prescribed medications. These include rapid increases in the amount of medication needed, frequent/unscheduled refill requests, repeated dramatic stories about prescriptions or medication being lost or stolen, multiple visits with multiple providers or pharmacies, resistance to nonopioid and nonpharmacological treatments, or frequent after-hours calls to the on-call prescriber or trips to the emergency department (ED) resulting in prescriptions. Patients that repeatedly delay needed or planned surgeries and opt instead to treat an otherwise correctable condition with medications should be monitored closely (Hudspeth, 2016b).
The diagnosis of SUD is a clinical one based primarily on thorough patient history. The DSM-5 lists eleven different criteria for the diagnosis of SUD. Mild SUD is diagnosed when a patient has two to three criteria met, while moderate SUD is diagnosed when four to five are met, and severe SUD is diagnosed when six or more are met. Being aware of the signs and symptoms of addiction will allow the nurse to identify patients, coworkers, or even family/friends that may be struggling with addiction. The signs and symptoms of addiction may include:
- A need to use the substance on a regular basis
- Strong urges/cravings for the substance that eclipse other thoughts
- Tolerance, or needing more of the substance in order to create the same effect
- Using more of the substance or for longer than intended
- An obsession with protecting or maintaining a steady supply of the substance
- Spending time and money to obtain the substance without regard for the availability of those resources, multiple requests for money without explanation, reports of missing money or valuable personal items from those around the user
- Doing immoral/illegal/unethical things to obtain the substance
- Missing social/recreational activities and failing to fulfill family/professional obligations due to substance use
- Continuing to use the substance despite harm to the user’s relationships
- Engaging in high-risk behavior while under the influence of the substance, such as driving
- Trying/wanting to stop using the substance but not being able to
- Experiencing symptoms of withdrawal when not using the substance
- Lack of energy or motivation
- Changes in weight
- Red/bloodshot eyes
- Sudden change in appearance (lack of interest in clothes, grooming)
- Changes in behavior (sudden insistence on privacy, being secretive, different friends)
- Decrease in grades (adolescents) or performance at work (adults) (APA, 2013; Mayo Clinic, 2017)
TAPS can also be used as an assessment tool for adults whose screening is positive, as well as NMASSIST and Helping patients who drink too much: A clinician’s guide by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). CRAFFT or the Alcohol screening and brief intervention for youth: A practitioner’s guide should be used to assess further adolescents whose screening is positive for alcohol use (NIDA, 2018a).
Laboratory testing may be helpful: an elevated blood alcohol concentration may indicate acute intoxication as well as help to assess tolerance to alcohol, and a high (> 20 units) carbohydrate-deficient transferrin (CDT) or high-normal (> 35 units) gamma-glutamyltransferase (GGT) is found in at least 70% of heavy drinkers. Both CDT and GGT levels return to normal within days to weeks of abstaining from alcohol. A serum or urine toxicology screen would indicate recent or current usage of various substances (APA, 2013).
Common Substances of Abuse
For this activity, we will focus on eight of the ten substances that the APA (2013) includes in their definition for SUD (eliminating a discussion regarding caffeine and other substances), including alcohol; opioids; sedatives, hypnotics, and anxiolytics; stimulants; hallucinogens; tobacco, cannabis, and other inhalants. While caffeine is included in the DSM-5, and serious adverse effects are possible (including insomnia, diuresis, and cardiac arrhythmia), they are rare. The substances contained in the DSM-5's Other category are anabolic steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), nitrous oxide, antihistamines, betel nuts, kava, cathinone (bath salts), and others. While some of these substances may have severe adverse effects, their prevalence is likely significantly lower than the other substances as mentioned earlier, albeit based on minimal data. For these reasons, we will focus primarily on the eight categories listed above for now (APA, 2013).
CNS Depressants: Alcohol, Opioids, Sedative, Hypnotics, and Anxiolytics
The intended effects of prescription CNS depressants vary somewhat:
- Alcohol is used recreationally, with no intended medicinal effects;
- Opioids are intended to relieve pain;
- Most sedatives, anxiolytics, and hypnotics are intended to produce a sense of calm, relaxation, or induce sleep.
Some sedatives, such as sodium oxybate (Xyrem), are used to treat serious neurological disorders such as narcolepsy. Others may be manufactured and sold illicitly, such as gamma hydroxybutyrate (GHB, typically homemade) and flunitrazepam (Rohypnol or "roofie"), a benzodiazepine that is not approved for use in the US. Both are considered club drugs or "date rape" drugs, as they are often mixed with alcohol and used in sexual assault cases. All CNS depressants may cause symptoms such as drowsiness/sedation, ataxia (lack of coordination), mood changes (i.e., depression), dysarthria (slurred speech due to muscle weakness), difficulty concentrating, poor memory, decreased inhibition, dizziness/falls, tachypnea, hypotension, nystagmus (involuntary eye movements), and confusion (Mayo Clinic, 2017).
Alcohol
Alcohol use disorder (AUD) affects millions of Americans, costing $250 billion, and taking at least 88,000 lives annually (Pearson & Duff, 2019). Slightly more than half of the respondents to the NSDUH (SAMHSA, 2019) report some alcohol use in the last month, a number relatively unchanged since 2002. The survey found that nearly half of all alcohol users report binge drinking, defined as five or more drinks for men and four or more drinks for women on at least one day in the past month. Roughly 12% of all alcohol users report heavy drinking, defined as binge drinking on at least five occasions in the past month. Both heavy and binge drinking were reported most often in respondents between the ages of 18 and 25. Reports of alcohol use in the last month in the 12 to 17-year-old cohort have remained below 10% since 2015, as compared to 16-17% from 2002-2007. Unfortunately, roughly half of the adolescents who report alcohol use also report binge drinking (SAMHSA, 2019). In addition to those symptoms mentioned above for all CNS depressants, alcohol intoxication may present with an unsteady gait, inappropriate sexual or aggressive behavior, mood lability, impaired judgment, impaired social/occupational functioning, stupor or coma. Repeated heavy drinking can cause chronic health problems, including gastritis, stomach or duodenal ulcers, liver cirrhosis, pancreatitis, low-grade hypertension, cardiomyopathies, increased levels of triglycerides and low-density lipoproteins, peripheral neuropathy leading to muscle weakness, paresthesia, and decreased peripheral sensation, cognitive deficits, memory impairment, degenerative changes in the cerebellum, vitamin B deficiencies, and an increased risk of suicide. Wernicke-Korsakoff syndrome is a rare alcohol-induced CNS complication causing a severely impaired ability to encode new memory (APA, 2013).
Withdrawal symptoms related to AUD typically present 4-12 hours after the reduction of intake, peak during the second day, and improve by the fourth or fifth day. Common symptoms include anxiety, headache (HA), depression, fatigue, irritability, mood swings, nightmares, and dysfunctional thinking. Less common withdrawal symptoms may consist of autonomic hyperactivity (i.e., sweating, tachycardia with pulse above 100 bpm, dilated pupils), hand tremor, insomnia, poor appetite, and nausea/vomiting. Delirium tremens (DT), a severe form of alcohol withdrawal, may present with transient hallucination or illusions, fever, psychomotor agitation, confusion, and seizures. The associated sleep disturbances, anxiety, and autonomic dysfunction may persist at lower intensities for months. In severe cases, alcohol withdrawal can be life-threatening, but symptoms are severe in only about 10% of cases, and seizures are seen in less than 3% (APA, 2013; MedlinePlus, 2019).
Alcohol use is incredibly risky during pregnancy. Ten percent of pregnant women between the ages of 18 and 44 report alcohol use in the last month. Fifty percent of nonpregnant women report alcohol use in the previous month, even though most women are unaware that they are pregnant for at least the first four to six weeks of their pregnancy. The exposure in utero to alcohol may lead to the development of fetal alcohol spectrum disorder (FASD), which includes several biological, psychological, and physiological variants. This may consist of fetal alcohol syndrome (FAS), congenital disabilities, as well as neurodevelopment and neurobehavioral disorders. They are all associated with significant morbidity and even mortality. Consequences of maternal alcohol use may include low birth weight, poor coordination, hyperactivity, impulsivity, inattentiveness, poor memory, learning and intellectual disabilities, speech/language delays, fine/gross motor delays, vision/hearing problems, as well as cardiac, renal, or skeletal malformations. These infants are more likely to be unemployed, incarcerated, or victims of violence later in life. Universal screening of all women of reproductive age via screening, brief intervention, and referral to treatment (SBIRT) may help identify those most at risk sooner to reduce the prevalence and improve the identification of FASDs. Experts advocate for this screening to be completed as part of the annual wellness exam using a validated tool such as AUDIT by the World Health Organization. A score of 8 or higher on this scale indicates risk potential and warrants further assessment. The AUDIT-C is a condensed version with just three questions, asking about how often the patient consumes alcohol, how many alcoholic drinks they typically consume in a day, and how often they have six or more alcoholic beverages on one occasion. A score of three or higher on this scale indicates a potential for AUD in women. After delivery, alcohol metabolites can be detected using an in-hospital fatty acid ethyl esters analysis of the meconium fluid (Albrecht et al., 2019).
Opioids
It is estimated that as many as 100 million Americans suffer from chronic pain (NIDA, 2018b). Opioids are a group of controlled substance analgesics that are commonly prescribed for moderate to severe pain control. Opioids function by binding to mu-opioid receptors (agonists) that are spread diffusely throughout the central nervous system (CNS). By attaching to a receptor in the CNS, they reduce or block the pain signal to the brain, thereby altering pain perception and response to pain. Opioids can also affect receptors in the respiratory and gastrointestinal tract, so beyond their clinical uses for pain management, they are occasionally used to treat diarrhea and cough. Opioids are classified as agonists, partial agonists, and mixed agonist-antagonists. Opioid antagonists may be used to counteract the adverse effects of opioids (Berger et al., 2013).
Opioid agonists are drugs that produce a maximum biologic effect between receptor binding and response (Berger et al., 2013). Opioids vary in the ratio of their analgesic potency and their potential for respiratory depression. According to the US Department of Health and Human Services (USDHHS, 2019), the most common opioid agonists prescribed for moderate or severe pain include:
- Hydrocodone (Vicodin, Lortab, Norco): found only in combination products, usually combined with acetaminophen; may be oral tablet formulation or liquid cough syrup;
- Oxycodone (Roxicodone, Oxycontin): available as immediate-release (IR) or extended-release (ER) formula, fast onset, available in oral tablet or solution form;
- Morphine sulfate (Roxanol, MS Contin): available as an IR or ER formula, as well as parenteral and oral formulations;
- Oxymorphone (Opana, Opana ER): available as an IR or ER formula, long half-life;
- Hydromorphone (Dilaudid, Exalgo ER): derivative of morphine, but with a faster onset; available as an oral tablet, liquid, suppository, and parenteral formulations; available as an IR or ER formula (USDHHS, 2019).
They are classified into the following categories: naturally occurring alkaloids (derived from the opium poppy plant), synthetic (human-made), and semi-synthetic forms. These and other common types of opioid agonists are listed according to each of these categories in Table 2.

Fentanyl (Duragesic) is a highly lipid-soluble opioid that can be administered through various parenteral administration modalities and is 50 to 100 times more potent than morphine sulfate (Roxanol, MS Contin). Similarly, heroin is a naturally derived product of morphine, but it is not approved for medicinal use within the US. Methadone (Dolophine) is a long-acting opioid that has particular importance in neuropathic pain. It has a prolonged plasma half-life that allows for a once every 8-hour dosing schedule. However, prescribers must be able to provide evidence of appropriate education to manage this drug. The half-life of methadone (Dolophine) is significantly longer than morphine (8 to 59 hours), is considerably cheaper than other medications, and is widely dispensed in substance use disorder clinics as a component of medication-assisted treatment (MAT) for opioid-dependent patients. Since it is long-acting, it can delay the opioid withdrawal symptoms that patients experience when taking short-acting opioids. Therefore, it allows time for detoxification (Hudspeth, 2016b).
Partial opioid agonists are opioids that have a submaximal response between receptor binding and response. They cause less conformational change and receptor activation than full agonists. At low doses, they provide similar effects to full agonists, but when the amounts are increased, the analgesic activity plateaus. In other words, they have a ceiling effect. The continued increase in dosing of these medications often leads to increased adverse effects without providing additional pain relief. Examples of partial agonists include tramadol (Ultram) and buprenorphine (Sublocade). Tramadol (Ultra) is a synthetic analog of codeine with a low affinity for opioid receptors that is commonly used to treat moderate to severe pain. It has a dual mechanism of action and poses less abuse potential than other opioids. Adverse effects include dizziness, vertigo, nausea, constipation, and headache (Berger et al., 2013). Buprenorphine (Sublocade) is a semi-synthetic partial agonist used in MAT to treat opioid use disorders (OUD). It has unique pharmacological properties that help to reduce the potential for misuse and diminish the effects of physical dependency on opioids, including withdrawal symptoms and cravings. Similar to opioids, it can generate effects such as euphoria and respiratory depression when administered at low to moderate doses; however, these effects are weaker than with other opioid agonists. Therefore, buprenorphine (Sublocade) increases patient safety in the event of an overdose. The opioid effects of buprenorphine (Sublocade) increase with each dose until they level off with moderate amounts, even if further dosing increases. This "ceiling effect" helps to lower the risk of misuse, dependency, and side effects. Some of the most common side effects of buprenorphine (Sublocade) include nausea, vomiting, constipation, muscle cramps, inability to sleep, cravings, distress, irritability, and fever (SAMHSA, 2018).
An opioid agonist-antagonist refers to an opioid with mixed actions. These drugs produce different activities at different receptors, acting on one opioid receptor to create a response, and binding to another receptor to prevent a response. Medications in this class demonstrate varying activity depending on the receptor that is targeted and the dose. Some examples of agonist-antagonists include pentazocine/naloxone (Talwin) and butorphanol (Stadol). Pentazocine/naloxone (Talwin) is used to treat moderate to severe pain and contains two medications. It acts by binding to and activating specific opioid receptors while simultaneously blocking the activity of other opioid receptors. The naloxone component of the drug helps to prevent misuse of the medication. Pentazocine (Talwin) is not available in the US as a single agent. Butorphanol (Stadol) is a mixed opioid agonist-antagonist available as a spray for the treatment of migraine headaches. Similar to partial agonists, agonist-antagonist opioids have a ceiling effect. Therefore, they offer lower analgesic efficacy and heightened risk for psychotomimetic or psychotogenic effects, or symptoms of psychosis, such as hallucinations and delirium (Berger et al., 2013).
Other than the aforementioned signs/symptoms listed for all CNS depressants, opioid intoxication causes signs and symptoms such as a reduced sensation of pain, agitation, constricted or pinpoint pupils, and lack of awareness of surroundings (Mayo Clinic, 2017). They may also cause respiratory depression, nausea/vomiting, HA, fatigue, pruritus, and urinary retention. These drugs can induce euphoria, especially when taken in higher doses than prescribed or ingested via snorting or injection, putting them at increased risk for abuse and addiction. Long term, the patient may also exhibit constipation, a runny nose/nasal lesions (if snorting medication), needle/track marks (if injecting), drug tolerance, and hyperalgesia, which is increased sensitivity to pain caused by damage to nociceptors or peripheral nerves (Mayo Clinic, 2017; US Food & Drug Administration [FDA], 2018). A severe opioid overdose will eventually present with loss of consciousness, and if anoxia develops, pupillary dilation. Withdrawal may develop within 6-12 hours of short-acting opioids such as heroin, peak within one to three days, and resolve within a week. With long-acting opioids such as methadone, withdrawal symptoms may take two to four days to begin. Signs and symptoms of withdrawal from opioids may include nausea, vomiting, diarrhea, dysphoric mood, muscle aches, rhinorrhea/lacrimation, sweating, dilated pupils, piloerection (involuntary erection of body hairs), yawning, fever, and insomnia. The patient may report feeling anxious or restless. Chronic symptoms of anxiety, dysphoria, anhedonia (lack of pleasure), and insomnia may last for weeks to months (APA, 2013). The Clinical Opiate Withdrawal Scale (COWS) is an 11-item scale that can be used to assess common symptoms of withdrawal (NIDA, 2018a).
The risks for opioid use during pregnancy are further increased. The repeated cycles of opioids cause repeated periods of withdrawal, which can result in reduced placental function and neonatal abstinence syndrome (NAS). This increases the risk for maternal infection, malnutrition, poor prenatal care, violence, and incarceration; it can lead to restricted growth, preterm labor, fetal convulsions, and in severe cases, death. NAS results in withdrawal signs/symptoms in the newborn after delivery, such as tremors, diarrhea, fever, irritability, seizures, and difficulty feeding (NIDA, 2017c).
Sedatives/Anxiolytics/Hypnotics/Tranquilizers
As previously mentioned, the NSDUH found roughly 1.8 million current (past month) users of prescription sedatives, tranquilizers, hypnotics, and anxiolytics in 2018 (SAMHSA, 2019). Many of these medications are prescribed for anxiety disorders, insomnia, and similar conditions requiring medication to induce relaxation, reduce heart rate, reduce blood pressure, and induce sleep. The most common of these, benzodiazepines (BZDs), include medications such as diazepam (Valium), clonazepam (Klonopin), lorazepam (Ativan), chlordiazepoxide (Librium) and alprazolam (Xanax). They may be used for panic attacks or, despite warnings to the contrary, to treat chronic anxiety. Some may be used to treat seizures or as a muscle relaxant or a sleep aid. BZDs function by binding to benzodiazepine receptors and enhancing the effects of gamma-aminobutyric acid (GABA), our primary inhibitory neurotransmitter. They carry a high risk for tolerance, dependence, and addiction, and for that reason are only recommended for short term or acute use. Non-benzodiazepine sleep medications (hypnotics) include zolpidem (Ambien), eszopiclone (Lunesta), and zaleplon (Sonata). These drugs affect the same GABA type A receptors but have a different chemical structure than BZDs, decreasing the risk of dependence and improving their side effect profile. Barbiturates, such as phenobarbital (Luminal), pentobarbital (Nembutal), secobarbital (Seconal) and mephobarbital (Mebaral) are potent sedatives/hypnotics typically reserved for emergent seizure disorder treatment and procedural sedation. They alter the sensory cortex, cerebellar, and motor activities in the brain. Initial adverse effects include drowsiness and ataxia, but as tolerance develops, these symptoms may dissipate (NIDA, 2018b). In addition to the list of intoxication symptoms apparent with all CNS depressants mentioned previously, intoxication with these drugs may also cause irritability and increase the risk of falls (Mayo Clinic, 2017). Severe intoxication can lead to respiratory depression and coma (APA, 2013).
Acute withdrawal from BZDs and barbiturates can be life-threatening, resulting in seizures and other complications (NIDA, 2018b). Within several hours to a few days, symptoms such as autonomic hyperactivity (sweating, tachycardia), tremor, insomnia, nausea/vomiting, transient hallucinations, psychomotor agitation, anxiety, and seizures may develop. Seizures may be seen in as many as 20-30% of those going through untreated withdrawal from these medications. The duration of withdrawal symptoms varies based on the half-life of the offending substance. Substances that last 10 hours or less, such as lorazepam (Ativan) and temazepam (Restoril) typically peak on the second day and improve by day five. Medications with longer half-lives such as diazepam (Valium) may not produce withdrawal symptoms for a week, peak during the second week, and improve during the third or fourth week. Chronic symptoms of withdrawal may continue for months (APA, 2013).
Stimulants
Prescription stimulants may be used to treat conditions such as attention-deficit hyperactivity disorder (ADHD), narcolepsy (a sleep disorder), severe depression, and chronic diseases that cause fatigue, such as multiple sclerosis (MS). They help improve alertness, attention, and energy level. Adverse effects include tachycardia, hypertension, and tachypnea. Previously, stimulants were used for a number of other conditions, but as the risk for addiction became evident, their use has been reduced. Dextroamphetamine (Dexedrine, Adderall) and methylphenidate (Ritalin, Concerta) enhance the effects of norepinephrine and dopamine in the brain. The dopamine enhancement, when augmented, can result in a feeling of euphoria. The effects of norepinephrine include hypertension, tachycardia, constriction of blood vessels, increased blood glucose, and bronchodilation. The public perception of the relative safety and benign nature of these medications has likely contributed to their misuse and abuse in the last two decades. Patients and their caregivers/parents should be adequately educated regarding the potential risks of nonprescription drug use, such as unsafe driving, unsafe sexual activities, and a progression to illicit substances. Non-therapeutic stimulants include cocaine and methamphetamine (meth) (NIDA, 2018b; Via, 2019).
Stimulant use may produce rapid/rambling speech, hallucination, irritability, anxiety, and impaired judgment (Mayo Clinic, 2017). Stimulant intoxication presents with many of the physical signs indicated above. Tachycardia or bradycardia may be present, along with pupillary dilation, hypo- or hypertension, sweats, chills, nausea/vomiting, weight loss, psychomotor agitation/retardation, weakness, respiratory depression, chest pain, confusion, seizures, dyskinesias, dystonias, and coma (APA, 2013). Chronic use may lead to feelings of hostility, paranoia, psychosis, arrhythmias, hyperthermia, cardiovascular failure, and seizures. These symptoms, especially hypertension and arrhythmias, may also present acutely if prescription stimulants are combined with certain over the counter decongestants (NIDA, 2018b). If stimulants are snorted, nasal congestion, epistaxis, or ulceration of the mucous membranes may be seen. If the medication is being smoked, it often results in mouth sores, gum disease, and tooth decay (often referred to as meth mouth; Mayo Clinic, 2017).
Stimulant withdrawal presents with fatigue, dysphoric mood, hyper- or insomnia, and bradycardia is common. Patients may also report vivid, unpleasant dreams, increased appetite, anhedonia, and psychomotor retardation or agitation. These symptoms typically present within a few hours to days of last use and are accompanied by cravings for the drug (APA, 2013; NIDA, 2018b; Mayo Clinic, 2017).
Hallucinogens
Recreational hallucinogens include 3,4-methylenedioxymethamphetamine (MDMA, ecstasy, or molly), ketamine (Special K), D-lysergic acid diethylamide (LSD), and phencyclidine (PCP; Mayo Clinic, 2017). Less common hallucinogens include phenylalkylamines (similar to MDMA) such as mescaline (peyote) and 2,5-dimethoxy-4-methylamphetamine (DOM), indoleamines such as 4-phosphoryloxy-N,N-dimethyltryptamine (psilocybin, psilocin, or magic mushrooms) and N,N-dimethyltryptamine (DMT), morning glory seeds (an ergoline similar to LSD), dextromethorphan (DXM), Salvia divinorum, jimsonweed, 251-NBOMe, and others. Hallucinogens can be ingested via pills or liquid, chewed, brewed into tea, snorted, injected, inhaled/smoked, or absorbed via the oral mucosa. Most hallucinogens function by affecting the neurotransmitter serotonin (APA, 2013; NIDA, 2019b). Substituted cathinones, frequently referred to as bath salts, are eaten, snorted, inhaled, or injected and create effects similar to MDMA and a stimulant such as cocaine. They are highly addictive (Mayo Clinic, 2017).
Hallucinogen use may present with hallucinations, paranoia, dilated pupils, chills, sweating, tremors, muscle cramping, teeth clenching, heightened or altered sense of sight, sound, and taste, impulsive behavior, emotional lability, dry mouth, nausea, ataxia, blurred vision, tachycardia/palpitations, hyperthermia, and hypertension. These symptoms typically appear within 20-90 minutes of ingestion and may last anywhere from 15 minutes (synthetic DMT) to 12 hours (LSD). PCP, ketamine, DXM, and other similar dissociative agents can also cause dissociation, or a feeling of being separated from the body, ataxia, aggression/violence, muscle rigidity, nystagmus, decreased perception of pain, dysarthria, impaired judgment, intolerance to loud noise (hyperacusis), seizures, and coma. Dissociative agents function by affecting the action of glutamate. Life-threatening overdoses are rare with hallucinogens, but seizures, coma, and fatalities have been reported with 251-NBOMe and/or PCP use. Due to poor judgment and contamination, users of hallucinogens are also at increased risk for injury and accidental poisoning. Substituted cathinones will typically present with many of the hallucinogen symptoms mentioned above, as well as symptoms of stimulants such as euphoria, increased sociability, increased energy, heightened sex drive, and psychotic/violent behavior. Users may experience loss of muscle control, panic attacks, and delirium (APA, 2013; Mayo Clinic, 2017; NIDA, 2019b).
Long term, hallucinogen use leads to permanent mental alterations, especially in perception, and can create flashbacks. Persistent psychosis, which includes visual disturbances, mood changes, paranoia, and disorganized thinking, has been reported. Hallucinogen persisting perception disorder (HPDD) is the terminology given to subsequent hallucinations or flashbacks, which may occur days or years after drug use. Persistent psychosis and HPDD are seen more often in patients with a history of mental illness but can be seen in anyone. Dissociative agents may lead to speech problems, weight loss, memory loss, chronic anxiety/depression, and suicidality. Repeated use of LSD can lead to tolerance, and PCP users may develop an addiction and subsequent withdrawal, with reports of symptoms such as cravings, HAs, and sweating (APA, 2013; Mayo Clinic, 2017; NIDA 2019b).
Tobacco, Cannabis, and Other Inhalants
As previously mentioned, according to the NSDUH, nearly 50 million (17%) of Americans smoked cigarettes (27.3 million on a daily basis), and roughly 12 million smoked cigars in 2018. These numbers are down when compared to statistics from 2002. Nearly as many people (43.5 million) report marijuana use in the last year, 27.7 million in the last month, both of which are increasing (SAMHSA, 2019).
Tobacco
Despite the well-known effects of smoking and the use of tobacco and nicotine-containing products (see Figure 1), usage persists in large numbers, largely due to the highly addictive nature of nicotine.
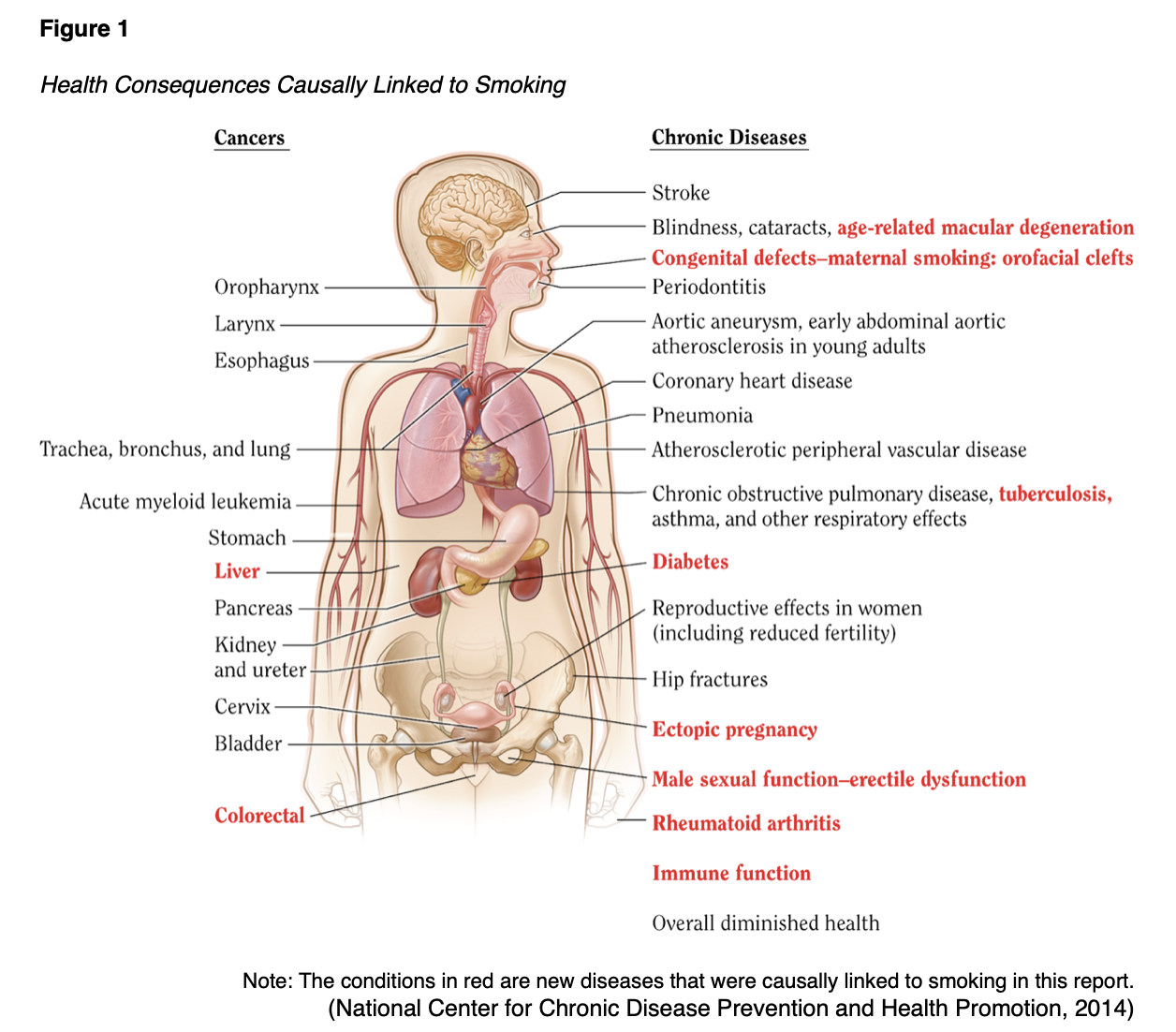
Most adolescents in the US experiment with tobacco, approximately 3% report current (past month) use by age 18, and most of these young adults become daily smokers (SAMHSA, 2019). Initiation after the age of 21 is rare. Patients may report nausea and dizziness initially with tobacco use, but these symptoms resolve quickly as tolerance develops. Tobacco intoxication is not a phenomenon, but tobacco withdrawal may present within 24 hours of cessation with symptoms such as irritability, anxiety, lack of mental focus/concentration, increased appetite (especially for salty or sweet foods), restlessness, depressed mood, constipation, dizziness, and insomnia. Some smokers also report an increase in coughing and sore throat immediately after quitting. Withdrawal symptoms typically peak after two to three days and resolve within two to three weeks (APA, 2013).
Cannabis
The terms cannabis or marijuana refer to drugs produced from plants belonging to the genus Cannabis (Whiting et al., 2015). Three specific species have been identified, Cannabis sativa, C. indica, and C. ruderalis. Ancient Chinese emperors suggested that cannabis be used for medicinal purposes over 4000 years ago. It was listed in the US Pharmacopeia from 1851-1942 but was then classified as dangerous and without significant medical use by the United Nations (UN) Single Convention on Narcotic Drugs in 1961 (Schrot & Hubbard, 2016). In 1970, cannabis was categorized by the US Drug Enforcement Agency’s (DEA) Comprehensive Drug Abuse Prevention and Control Act (Controlled Substances Act) as a Schedule I drug in the US, meaning it has a high potential for abuse and no accepted medicinal use, making any further research significantly more complicated to conduct (Bridgeman & Abazia, 2017; Rosenberg et al., 2015). The two most biologically active phytocannabinoids, Δ 9 -tetrahydrocannabinol (THC) and cannabidiol (CBD), have a direct effect on the endocannabinoid receptors, CB1 and CB2. While THC is responsible for the majority of the drug's psychoactive properties, CBD is thought to be non-addictive, non-psychoactive, and potentially contains anti-inflammatory, antioxidant, and neuroprotective properties. The primary mechanism of action for CBD is largely unknown and continues to be studied, as it is believed to not be primarily mediated via the CB1 and CB2 receptors due to CBD's low affinity (Schrot & Hubbard, 2016). Currently, there exist comprehensive medical marijuana access laws in 33 states as well as Washington DC, Guam, Puerto Rico, and US Virgin Islands and 13 additional states allow the use of "low THC, high CBD" products for medical reasons in specific situations, despite cannabis's continued classification as a Schedule I drug by the federal government. There are currently 14 states and territories that have legalized adult-use of cannabis, which allows for recreational use without medical certification. There are no marijuana access laws currently in Idaho, South Dakota, Nebraska, and Kansas (National Conference of State Legislatures, 2020)
Cannabis effects may vary depending on how it is ingested (smoking, eating, inhaling/vaping). Onset is within minutes when smoked and typically lasts three to four hours; when ingested orally, the onset is delayed, and symptoms are often prolonged. Symptoms associated with marijuana use may include:
- Euphoria
- Heightened perception (visual, auditory, or taste)
- Tachycardia
- Hypertension
- Dry mouth
- Conjunctival injection
- Ataxia, slowed reaction time
- Difficulty concentrating
- Loss of short-term memory
- Anxiety/paranoia
- Increased appetite with exaggerated cravings for certain foods (“munchies”; APA, 2013; Mayo Clinic, 2017).
Patients may also present with a distinct odor on their clothing and yellowing of the fingertips, similar to tobacco smokers. Long-term use may result in decreased mental acuity, performance issues at work/school, and a reduction in extracurricular interests or friends. Synthetic cannabinoids, referred to as K2 or Spice, may be sprayed on dried herbs and smoked or made into an herbal tea. It may create many of the aforementioned cannabis symptoms, as well as hallucinations, vomiting, and confusion (Mayo Clinic, 2017) Despite its low perceived threat (only 30% of survey respondents reported a great risk related to weekly marijuana use), up to 30% of cannabis users have a dysfunctional disorder, and the NSDUH estimates roughly 4.4 million meet the diagnostic criteria for SUD based on their use of cannabis (NIDA, 2019a; SAMHSA, 2019). A longitudinal study of 35,000 adults found a significant association between marijuana use and subsequent SUD (alcohol, nicotine, cannabis, or some other drug), even after adjusting for common risk factors such as age and sociodemographic data (Blanco et al., 2016).
Symptoms of withdrawal may develop within a week (usually 24-72 hours) of abstinence, and include depressed mood, irritability, aggression/anger, anxiety, insomnia, unpleasant dreams, weight loss/decreased appetite, restlessness, abdominal pain, tremors, sweating, fever/chills, fatigue, and HA. These symptoms may last one to two weeks, although sleep disturbance may persist for up to 30 days (APA, 2013).
Inhalants
An estimate of inhalant use from the NSDUH is just over 600,000 report use in the last month and about 2 million report use in the last year. The rate of inhalant use was highest among adolescents age 12-17 at 2.7% (SAMHSA, 2019). This term typically refers to volatile hydrocarbons, toxic gases found in glues, fuels, and paints. This may include paint thinner, correction fluid, felt tip markers, gasoline, and household cleaners/aerosol products (Mayo Clinic, 2017). Symptoms of intoxication may vary depending in the specific substance, but typically include:
- Dizziness
- Nystagmus
- Ataxia/unsteady gait
- Dysarthria
- Diminished reflexes/psychomotor retardation
- Tremor
- Muscle weakness
- Blurred vision/diplopia (double vision)
- Euphoria/intoxication
- Decreased inhibition
- Combativeness/belligerence
- Nausea/vomiting
- Arrhythmia
- Lethargy, stupor, or coma (APA, 2013).
In severe cases, brain damage and death may be possible. Symptoms typically resolve within a few minutes to hours, and a withdrawal syndrome is not typically seen. The patient may also present with an unexplained rash around the mouth and nose (APA, 2013; Mayo Clinic, 2017). The abuse of nitrous oxide (laughing gas) is included within the NSDUH definition of inhalants but is considered separately by the APA in the DSM-5 as “other”. It is used therapeutically as an anesthetic for medical and dental procedures as well as commercially as a propellant in products such as whipped cream dispensers (termed whippets). Chronic heavy use may result in myeloneuropathy, spinal cord subacute combined degeneration, peripheral neuropathy, and psychosis (APA, 2013; SAMHSA, 2019).
Effective Management/Treatment
Overdose or Acute Withdrawal Management
As mentioned above, acute alcohol, BZD, and barbiturate withdrawal, and specifically severe alcohol withdrawal with DTs and barbiturate withdrawal, can be life-threatening. These patients should undergo medically supervised detoxification and managed with tapered doses of benzodiazepines (i.e., chlordiazepoxide [Librium]) and potentially barbiturates to limit the symptoms of withdrawal and avoid seizures, which typically improve after four to five days (APA, 2013; MedlinePlus, 2019; NIDA, 2018b). In the case of benzodiazepine overdose, there exists a benzodiazepine antagonist, flumazenil (Romazicon), that can be administered intravenously by emergency medical personnel (NIDA, 2018b).
As many as 130 people die daily from opioid overdose in the US (Moore, 2019). Naloxone (Narcan) is the most widely used opioid antagonist, as it is FDA-approved for the use of an opioid overdose. It is highly effective in reversing an opioid overdose and respiratory depression and is dispensed in intravenous (IV), intramuscular (IM), subcutaneous (SC), and intranasal (IN) formulations. When administered intravenously, effects begin almost instantly and last for about an hour. Still, the dose should be titrated to achieve the reversal of respiratory depression without full reversal of the analgesic effects. Rapid infusion of the medication should be avoided to reduce the risk of hypertension, tachycardia, nausea, and vomiting. Vital signs should be monitored, especially respirations, and naloxone administration repeated until the manifestations of opioid toxicity have subsided (Theriot & Azadfard, 2019). With IM and SC administration, the drug takes effect in two to five minutes and lasts for several hours. These administration routes are most commonly used by emergency medical services (EMS) personnel and civilians responding to opioid overdose in the community. According to the National Emergency Medical Services Information System, the rate of EMS naloxone administration events increased 75.1%, from 573.6 to 1,004.4 administrations per 100,000 EMS events from 2012-2016, which corresponds well with 79.7% increase in opioid overdose mortality during those same years (Cash et al., 2018). Many EMS personnel are moving toward intranasal administration, which is viewed as providing important advantages over injection when responding to overdose patients in the community. The safety profile of intranasal formulations appears to be no different from the injection formulation in the treatment of opioid overdose. Krieter and colleagues (2016) compared the pharmacokinetic properties of intranasal naloxone (2-8 mg) delivered in low volumes (0.1-0.2 mL) to the approved (0.4 mg) IM dose. All doses of intranasal naloxone resulted in plasma concentrations and areas under the curve greater than observed following the intramuscular dose, and the time to reach maximum plasma concentrations was not different following intranasal and intramuscular administration (Krieter et al., 2016).
Naloxone (Narcan) has become increasingly available to the public, as the prescribing and dispensing of it has become a central part of the public health response to the opioid overdose epidemic. In addition, the majority of states now allow naloxone to be purchased from a pharmacy without a prescription. Prescribers are advised to consider prescribing a concurrent prescription for naloxone (Narcan) for patients who are at high risk for intentional or accidental overdose. Those considered to be at the highest risk include patients who meet any of the following criteria:
- Doses of > 90 MME/day;
- Chronic opioid use;
- Concurrent use of benzodiazepines;
- Personal history of substance abuse/opioid misuse disorders;
- Current treatment for substance abuse/opioid misuse disorders;
- Family history of substance abuse;
- Patients being treated with opioids who live in isolated or rural areas;
- Patients with chronic respiratory disease;
- HIV/AIDS;
- Chronic renal, hepatic, or cardiac disease;
- Current or past history of depression or other serious mental health conditions (Webster, 2017).
Serious adverse effects of naloxone (Narcan) are rare, and usually, the benefit of using it for an overdose outweighs the risk for adverse effects. However, it can induce acute opioid withdrawal symptoms such as tachycardia, agitation, vomiting, body aches, and convulsions. Naloxone has no reversal effect on tramadol (Ultram), alcohol, or other CNS depressants such as benzodiazepines (CDC, 2018).
Long-Term Management
Ideally, SUD treatment should consist of detoxification, counseling, and medications, and in reality, may require multiple attempts before full recovery is achieved (NIDA, 2018b). It should also include effective treatment for any comorbid mental health conditions other than addiction as well as long-term follow up to prevent and manage any relapses. Legal services, educational/vocation services, infectious disease screening/referrals, and case management should also be components (see Figure 2; NIDA, 2019c).

Brief intervention (BI) is an appropriate engagement of most SUD patients. It encourages the patient to recognize the problem with substance use and engage them in positive change. It typically lasts 5-15 minutes and consists of brief advice via motivational interviewing techniques. The FRAMES acronym is one commonly used technique:
- Feedback of risk
- Encouraging responsibility for change
- Advice
- A menu of options
- Therapeutic empathy
- Enhancing self-efficacy (Pearson & Duff, 2019).
BI is a component of SBIRT, an evidence-based method of screening patients and briefly providing education and/or motivation to change, with an associated referral for additional treatment. SBIRT is meant to be brief, universal, comprehensive, targeted, and performed outside of a substance abuse treatment setting. Once granted permission to discuss alcohol/tobacco/drug use by the patient, SBIRT should begin with a validated screening tool to assess risk, which provides the basis for the feedback of risk (step #1 above). The brief counseling session should include an exploration of ambivalence, enhance motivation, and facilitate shared goal setting (if applicable) by first defining healthy patterns of use/behavior and then drawing a clear distinction with the patient's current use/behavior in a nonjudgmental manner. The discussion should be collaborative, with questions regarding how the patient perceives any benefits and potential risks of their substance use. Abstinence is not a goal, but a question regarding whether or not the patient is interested in and ready to change their behavior is appropriate. If the answer is yes, the provider may ask the patient to gauge their motivation using a 1-10 scale. A patient who is unwilling or not yet ready to make a behavioral change should be treated with respect, dignity, and simply reassessed at a later date. A comprehensive substance-related history should be completed if the above screening tools are positive, with questions regarding onset, duration, characteristics, precipitating factors, other substances used, and withdrawal symptoms (Albrecht et al., 2019).
Goal setting is associated with improved outcomes, as are nonpharmacological lifestyle changes such as establishing a consistent sleep schedule, exercising regularly, avoiding triggers (situations, people, or places associated with the substance of abuse), engaging in a new hobby/interest to prevent boredom and occupy time, and spending time with supportive friends and family members. Both inpatient and outpatient treatment programs should be explored/considered, both of which should consist of some combination of sessions with an addictions counselor, behavior therapies, group therapies, and psychotherapy (Pearson & Duff, 2019). For the treatment of most SUDs, cognitive behavioral therapy (CBT) has been proven effective. CBT attempts to modify the patient's unhealthy thought and behavior patterns as well as develop strategies to manage cravings and avoid triggers. Some forms of therapy also provide incentives for abstinence. Behavioral treatments may involve the individual, the family, or take place in a group setting. The goal is to improve relationships and functionality at work, home, and in the community. Motivational incentives (contingency management) designed to use positive reinforcement to encourage abstinence may also be used. In 2018, reSET, the first mobile application designed as an adjunct for SUD outpatient treatment, was approved for marketing by the FDA for patients struggling with addiction to alcohol, marijuana, and stimulants, including cocaine. Opioid addiction was added to the platform in 2018, designed to be used along with buprenorphine (Sublocade) for a remote-style MAT (NIDA, 2018b, 2019c).
Alcohol and Opioid Use Disorder
Pharmacological treatment for moderate to severe AUD may consist of one of three FDA-approved medications, naltrexone (Vivitrol, Revia), acamprosate (Campral), and disulfiram (Antabuse). Naltrexone (Vivitrol, Revia) is an opioid antagonist that is used to treat substance use disorders, namely AUD and OUD. It is also used to treat postoperative respiratory depression due to the use of opioids during the operative period. When used for OUD or AUD, its purpose is to prevent euphoria and decrease the desire/craving to use opioids or alcohol. The patient should be opioid-free for seven to ten days before beginning the medication to prevent opioid withdrawal syndrome, making this option difficult for some. It is available as a long-acting injection every four weeks. Despite this convenient delivery method, and the fact that it can be prescribed by any licensed prescriber, it is used less commonly than methadone (Dolophine) and buprenorphine (Sublocade) for the treatment of OUD (Moore, 2019; Theriot & Azadfard, 2019). Abstinence from alcohol prior to starting naltrexone (Vivitrol, Revia) is associated with better outcomes in the treatment of AUD but is not required. Naltrexone (Vivitrol, Revia) use is contraindicated in patients with liver failure. Adverse effects include nausea, vomiting, HA, dizziness, insomnia, depression, and muscle cramps. Acamprosate (Campral) is also approved as a first-line treatment for moderate to severe AUD, functioning as an N-methyl-D-aspartate (NMDA) antagonist, an excitatory neurotransmitter. It appears to function better if started four to seven days after abstaining from alcohol, but this is not required. Adverse effects include anxiety, stomach upset, HA, and muscle weakness. (Pearson & Duff, 2019).
Weekly or biweekly visits are encouraged to assess medication effects and treatment plan adherence, provide ongoing support, and discuss drinking/craving patterns. The patient’s overall health, mental status, family/social activities, work status, and legal status should be assessed. Relapses are common, and the nurse should be certain that the patient is aware of this fact. The nurse should encourage the patient to continue their efforts and view the relapse as a “stepping-stone to wellness” and not as a failure or defeat (Pearson & Duff, 2019).
MAT is an example of a holistic, patient-centered approach to the care of patients with SUD. Medication, counseling, and behavioral therapies are combined, resulting in improved survival rates, extended treatment periods, decreased illicit opioid use and criminal activity, and reduced risk of contracting an infectious disease (HCV, HBV, HIV) or developing an abscess. The three medications FDA-approved for OUD include buprenorphine (Sublocade), methadone (Dolophine), and naltrexone (Vivitrol, Revia). Opponents to MAT claim that the treatment simply replaces one drug with another (Moore, 2019). The use of MAT in incarcerated patients improves post-release outcomes (NIDA, 2018b).
The partial opioid agonist buprenorphine (Sublocade) was first approved for use by the FDA in 2002 when used in combination with counseling and behavioral therapies to treat OUD. It has a half-life of approximately 24 hours. It may cause mild euphoria in some patients but is designed with the aforementioned ceiling effect to limit the abuse potential. It may also cause respiratory depression when combined with BZDs or other CNS depressants. There are a few types of buprenorphine approved for use by the FDA, which include a monthly subcutaneous injection (Sublocade), a subdermal implant that lasts for six months (Probuphine), buprenorphine/naloxone (Bunavail, Suboxone) sublingual or buccal film, and buprenorphine/naloxone (Zubsolv) sublingual tablets. The combination products are used most often, as the addition of naloxone effectively blocks attempts at misuse. The naloxone component, an opioid antagonist, is not well absorbed through the oral mucosa. However, if the tablets/films are crushed or melted for injection or any other delivery method, the naloxone component blocks the effects of all opioids. Buprenorphine is not without risk, as some patients may divert the drug or combine it with a BZD to augment the euphoric effects, a potentially lethal combination. Random UDTs may help screen for and identify patients that are combining other medications or those that are diverting/selling their prescription and not taking it at all. Consistent use of available prescription drug monitoring programs (PDMPs) will also help the APRN identify patients that are obtaining prescriptions elsewhere. There are also reports of significant difficulty obtaining a supply of the medication, as many pharmacies do not want to consistently carry large stockpiles of the medication, in combination with a system-wide drug shortage. Overall, overdoses are rare, and some studies indicate that buprenorphine (Sublocade) is six times safer than methadone (Dolophine). With regards to dosing, it is a long-acting agent and requires only daily dosing for many patients (Moore, 2019; SAMHSA, 2018)
Buprenorphine (Sublocade) treatment does not require close monitoring in the highly structured environment of methadone (Dolophine) based clinics. It can be prescribed by trained and certified physicians and advanced practice providers in an office setting, as it is considered safe and effective when taken as prescribed (Moore, 2019; SAMHSA, 2018). Studies indicate that starting buprenorphine (Sublocade) in an ED immediately after the initial treatment for an opioid overdose is more effective than a referral or BI (NIDA, 2018b). A treatment duration of two years is recommended by most clinical experts (Moore, 2019).
As previously mentioned, methadone (Dolophine) is a long-acting lipophilic synthetic opioid agonist that is commonly used in MAT for the treatment of OUD. Its half-life may vary from 8-59 hours, and steady-state plasma levels may take three to five days to achieve. (Villegas & Thielet, 2020). Unfortunately, methadone is also highly addictive and is therefore strictly regulated. The Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS) ensures that providers are well educated and counsel and educate patients regarding the risks included in the medication guide. Methadone (Dolophine) is typically only dispensed in designated opioid treatment programs (OTPs). Patients with a history of opioid abuse for at least one year, or who are currently at high risk due to pregnancy or recent release from prison or a similar institution qualify for MAT with methadone (Dolophine; Villegas & Thielet, 2020).
Risks of methadone (Dolophine) include the risk of overdose and respiratory depression seen with all opioid agonists. Of the over 17,000 opioid-related deaths confirmed in 2016, over 3,000 involved methadone (Dolophine). The risk of respiratory depression may occur later and persist longer due to methadone’s pharmacokinetics and are highest at initiation and after dosing escalations. Methadone (Dolophine) also carries a risk of QTc prolongation. The initial dose of methadone should be supervised by medical personnel and should not exceed 40 mg. Maintenance dosing will vary from patient to patient but is typically 60-120 mg. The appropriate dose should eliminate cravings for outside opioids and block the effects of any outside opioids if taken with little to no sedation effects. If currently taking another opioid, a patient can be transitioned onto methadone. The MAT should also include social services and counseling. Patients should be counseled on the importance of avoiding any other CNS depressants while on methadone (Dolophine) such as alcohol and benzodiazepines (Villegas & Thielet, 2020).
Harm reduction programs are another treatment alternative. These programs focus on decreasing the negative consequences of drug use, especially of injecting drugs. Most programs provide clean, unused needles in an attempt to prevent needle sharing, and thus prevent the transmission of HCV and HIV. Programs provide education, health promotion, and other prevention methods to people who inject drugs (PWID). These programs may function on a 1:1 exchange of used for unused needles, or a needs-based negotiation model, providing a certain number of syringes based on the individual’s usage along with sharps containers for safe disposal. Some programs include other equipment (cookers, cotton, alcohol pads, tourniquets. Some provide condoms, naloxone kits/prescriptions, and laboratory testing/screening for HCV and HIV. Programs often offer counseling and referrals to treatment if requested. A key to the success of these programs is a nonjudgmental and bias-free atmosphere and a special focus on flexibility, mobility, and privacy (Barker & Galeota, 2018).
A percutaneous nerve field stimulator device, the NeuroStim System-2 (NSS-2) Bridge, was recently granted an indication by the FDA for the treatment of opioid withdrawal symptoms. It is placed behind the ear, where it provides electrical pulses to reduce the effects of acute opioid withdrawal. In 2018, iofexidine (Lucemyra) was granted FDA approval for acute opioid withdrawal syndrome. (up to 14 days) It functions as a centrally acting alpha-2 adrenergic agonist, binding to adrenergic receptors and decreasing norepinephrine release. Adverse effects may include bradycardia, hypotension, CNS depression, and QTc prolongation (NIDA, 2019c).
Stimulants
As with the abuse of most therapeutically prescribed medications, experts regarding the prevention of stimulant abuse cite the importance of consulting and regularly monitoring the state’s PDMP. Additionally, studies indicate that the prompt, appropriate, and long-term treatment for ADHD benefits the patient’s mental wellbeing and decreases the risk of substance abuse rather than increasing it, as their mental health needs are being adequately met. The Coalition to Prevent ADHD Medication Misuse is an online resource that launched in 2016 to provide healthcare providers and others with information on how to discuss and prevent the misuse and diversion of prescription stimulants (Via, 2019). Prescription stimulants should be tapered to ease withdrawal symptoms. Behavioral therapies are effective for the treatment of stimulant misuse, but currently, there are no FDA-approved medications for the treatment of stimulant addiction (NIDA, 2018b). Studies are currently ongoing regarding possible pharmacological adjuncts. One such product, N-acetylcysteine (NAC or Acetadote, a glutamatergic agent), may be helpful in the treatment of cocaine addiction by helping to correct glutamate dysregulation (McClure et al., 2014).
Hallucinogens
Long term effects of hallucinogen abuse may require a multimodal treatment approach. Antidepressant or antipsychotic medications may be helpful in improving mood and treating symptoms of psychosis, along with behavioral therapy to assist patients in dealing with confusion or fear related to visual disturbances (NIDA, 2019b).
Tobacco
The treatment of tobacco/nicotine dependence has a number of options, but unfortunately, none of the options have perfect or even remarkably good outcomes. As with most addictions, a combination of behavioral therapy and medication have a higher rate of success in quitting. Initial interventions may include brief advice from a trusted healthcare provider, a telephone helpline, text messages, or printed pamphlets/materials to be reviewed later. Public health campaigns to increase taxes and reduce the nicotine content within products sold may also lead to a reduction in tobacco addiction rates across the country. Patients should be reminded that, according to the Surgeon General, smoking cessation at any age improves health and quality of life, along with reducing the risk of premature death and adding years/decades to their life. Options for the treatment of tobacco dependence include:
- Behavioral counseling, in four to eight sessions, performed by a smoking cessation expert based on CBT, motivational interviewing, and/or mindfulness. Initial contact may be via a telephone support line with trained counselors.
- Technology use may help support cessation efforts through text messaging, interactive web-based offerings, and social media support.
- Nicotine replacement therapy (NRT) provides a method of tapering down the amount of nicotine to limit withdrawal symptoms and cravings. Current products in the US include patches, gum, or lozenge (over the counter for people 18 years and older) or a nasal spray or inhaler with a prescription; more effective when used along with behavioral treatment and when sustained-release and immediate-release options are combined (versus monotherapy).
- Prescription medications such as bupropion (Zyban) or varenicline (Chantix)
- Bupropion (Zyban) functions by inhibiting the reuptake of norepinephrine and dopamine and appears to be as effective as NRT.
- Varenicline (Chantix) functions by stimulating the alpha-4 beta-2 nicotinic receptor to a lesser degree than nicotine and appears to be slightly more effective than NRT monotherapy and bupropion (Zyban).
Research regarding the orexin and glutamate signaling systems are the focus of ongoing research to develop additional medications to treat tobacco and nicotine addiction, as well as studies reviewing the effect of N-acetylcysteine (NAC or Acetadote, a glutamatergic agent) and the development of a nicotine vaccine. Repetitive transcranial magnetic stimulation (rTMS) is also being researched for nicotine dependence treatment (NIDA, 2020).
Cannabis and Other Inhalants
The occurrence of cannabis use disorder is not common but does occur in 1 in 11 adults who have ever used it. The aforementioned behavioral treatments, as well as supportive and adjunctive components of SUD treatment for other substances, are effective for those with cannabis or inhalant dependence. There are no medications currently approved for the treatment of SUD specific to these substances (NIDA, 2019a). N-acetylcysteine (NAC or Acetadote, a glutamatergic agent) has shown promising results when used in adolescents, but results in adults were less promising. In 300 cannabis-dependent adults over 12 weeks, just 22% submitted clean urine samples (NIDA, 2017a).
Conclusion
While this activity was by no means a complete and thorough discussion of any and all substances that can be used inappropriately or lead to addiction, it is instead meant to serve as a general and varied overview. For a more detailed discussion of tobacco dependence treatment, please see the NursingCE activity entitled Tobacco Dependence Treatment. For a more detailed discussion of cannabis, please see the NursingCE activity entitled Medical Marijuana and Cannabinoids. For more information regarding the safe prescribing of some of the medications discussed here such as opioids and benzodiazepines, please see the NursingCE activities entitled Safe and Effective Prescribing of Controlled Substances, The Nurses Role in the Opioid Epidemic, Mental Health Pharmacology and Pain Management Part III: Opioid Administration and Monitoring. For information on the appropriate use and potential misuse of anabolic steroids, please see the NursingCE activity entitled Anabolic Steroids.
References
Albrecht, S. A., Kameg, B. A., Puskar, K. R., Lewis, E. L., & Mitchell, A. M. (2019). Fetal alcohol spectrum disorders: Implications for primary care nurse practitioners. The Journal for Nurse Practitioners, 15(8), 550-552. https://doi.org/10.1016/j.nurpra.2019.05.012
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental health disorders, DSM-5 (5th ed.). American Psychiatric Publishing.
Barker, K. L., & Galeota, E. Y. (2018). Shining a LIGHT into drug darkness. The Journal for Nurse Practitioners, 14(6), 463-469. https://doi.org/10.1016/j.nurpra.2017.12.004
Berger, A. M., Shuster, J. L., & Von Roenn, J. H. (2013). Principles and practices of palliative care and supportive oncology (3rd ed.). Lippincott Williams & Wilkins.
Blanco C., Hasin D. S., Wall M. M., Florez-Salamanca, L., Hoertel, N., Wang, S., Kerridge, B. T., & Olfson M. (2016). Cannabis use and risk of psychiatric disorders: Prospective evidence from a US national longitudinal study. JAMA Psychiatry, 73(4), 388–395. https://doi.org/10.1001/jamapsychiatry.2015.3229
Bridgeman, M. B., & Abazia, D. T. (2017). Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharmacy and Therapeutics, 42(3), 180–188. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312634/#__ffn_sectitle
Carlson, R. G., Nahhas, R. W., Martins, S. S., & Daniulaityte, R. (2016). Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: A natural history study. Drug and Alcohol Dependence, 160, 127–134. https://doi.org/10.1016/j.drugalcdep.2015.12.026
Cash, R. E., Kinsman, J., Crowe, R. P., Rivard, M. K., Faul, M., & Panchal, A. R. (2018). Naloxone administration frequency during emergency medical service events — United States, 2012–2016. MMWR. Morbidity and Mortality Weekly Report, 67(31), 850-853. https://doi.org/10.15585/mmwr.mm6731a2
The Centers for Disease Control and Prevention. (2018). Using naloxone to reverse opioid overdose in the workplace: Information for employers and workers. https://www.cdc.gov/niosh/docs/2019-101/
Hudspeth, R. S. (2016a). Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. The Journal for Nurse Practitioners, 12(3), 141-148. https://doi.org/10.1016/j.nurpra.2015.10.012
Hudspeth, R. S. (2016b). Safe opioid prescribing for adults by nurse practitioners: Part 2. Implementing and managing treatment. The Journal for Nurse Practitioners, 12(4), 213-220. https://doi.org/10.1016/j.nurpra.2015.11.032
Krieter, P., Chiang, N., Gyaw, S., Skolnick, P., Crystal, R., Keegan, F., Aker, J., Beck, M., & Harris, J. (2016). Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. Journal of Clinical Pharmacology, 56(10), 1243-1256. https://www.doi.org/10.1002/jcph.759
Mayo Clinic. (2017). Substance use disorder (Drug addiction). https://www.mayoclinic.org/diseases-conditions/drug-addiction/symptoms-causes/syc-20365112
McClure, E. A., Gipson, C. D., Malcolm, R. J., Kalivas, P. W. & Gray, K. M. (2014). Potential role of N-acetylcysteine in the management of substance abuse disorders. CNS Drugs, 28(2), 95-106. https://doi.org/10.1007/s40263-014-0142-x.
MedlinePlus. (2019). Alcohol withdrawal. https://medlineplus.gov/ency/article/000764.htm
Moore, D. J. (2019). Nurse practitioners’ pivotal role in ending the opioid epidemic. The Journal for Nurse Practitioners, 15(5), 323-327. https://doi.org/10.1016/j.nurpra.2019.01.005
National Center for Chronic Disease Prevention and Health Promotion (2014). The health consequences of smoking- 50 years of progress: A report of the surgeon general. https://www.ncbi.nlm.nih.gov/books/NBK179276/
National Conference of State Legislatures. (2020). State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
National Institute on Drug Abuse. (2016). Principles of substance abuse prevention for early childhood. https://www.drugabuse.gov/publications/principles-substance-abuse-prevention-early-childhood
National Institute on Drug Abuse. (2017a). After showing promise for cannabis-using adolescents, N-acetylcysteine falters in adult study. https://www.drugabuse.gov/news-events/nida-notes/2017/09/after-showing-promise-cannabis-using-adolescents-n-acetylcysteine-falters-in-adult-study
National Institute on Drug Abuse. (2017b). Health consequences of drug misuse. https://www.drugabuse.gov/related-topics/health-consequences-drug-misuse
National Institute on Drug Abuse. (2017c). Treating opioid use disorder during pregnancy. https://www.drugabuse.gov/treating-opioid-use-disorder-during-pregnancy
National Institute on Drug Abuse. (2018a). Chart of evidence-based screening tools and assessments for adults and adolescents. https://www.drugabuse.gov/nidamed-medical-health-professionals/tool-resources-your-practice/screening-assessment-drug-testing-resources/chart-evidence-based-screening-tools
National Institute on Drug Abuse. (2018b). Misuse of prescription drugs. https://www.drugabuse.gov/publications/misuse-prescription-drugs/overview
National Institute on Drug Abuse. (2019a). Genetics and epigenetics of addiction. https://www.drugabuse.gov/publications/drugfacts/genetics-epigenetics-addiction
National Institute on Drug Abuse. (2019b). Hallucinogens. https://www.drugabuse.gov/publications/drugfacts/hallucinogens
National Institute on Drug Abuse. (2019c). Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction
National Institute on Drug Abuse. (2020). Tobacco, nicotine, and e-cigarettes: What are treatments for tobacco dependence? https://www.drugabuse.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/what-are-treatments-tobacco-dependence
Pearson, C., & Duff, E. (2019). Beyond brief intervention: Pharmacological management of alcohol use disorder. The Journal for Nurse Practitioners, 15(9), 627-30. https://doi.org/10.1016/j.nurpra.2019.05.015
Rosenberg, E. C., Tsien, R. W., Whalley, B. J., & Devinsky, O. (2015). Cannabinoids and epilepsy. Neurotherapeutics, 12(4), 747-768. https://doi.org/10.1007/s13311-015-0375-5
Schrot, R. J., & Hubbard, J. R. (2016). Cannabinoids: Medical implications. Annals of Medicine, 48(3), 128-141. https://doi.org/10.3109/07853890.2016.1145794
Substance Abuse and Mental Health Services Administration. (2018). Buprenorphine. https://www.samhsa.gov/medication-assisted-treatment/treatment/buprenorphine
Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No PEP19-5068, NSDUH Series H-54). https://www.samhsa.gov/data/report/2018-nsduh-annual-national-report
Theriot, J., & Azadfard, M. (2019). Opioid antagonists. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK537079/
US Department of Health and Human Services. (2019). Pain management best practices inter-agency task force report: Updates, gaps, inconsistencies, and recommendations. https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
US Food & Drug Administration. (2018). Drug safety and availability – Medication guides. https://www.fda.gov/Drugs/DrugSafety/ucm085729.htm
Via, K. D (2019). Preventing the next epidemic: Prescribed stimulant abuse. The Journal for Nurse Practitioners, 15(3), 232-35. https://doi.org/10.1016/j.nurpra.2018.12.012
Villegas, S. C., & Thielet, N. (2020) Methadone prescribing caveats for chronic pain and opioid use disorder. The Journal for Nurse Practitioners, 16(3), 176-80. https://doi.org/10.1016/j.nurpra.2019.12.017
Whiting, P. F., Wolff, R. F., Deshpande, S., Nisio, M. D., Duffy, S., Hernandez, A. V., Keurentjes, J. C., Lang, S., Misso, K., Ryder, S., Schmidlkofer, S., Westwood, M., & Kleijnen, J. (2015). Cannabinoids for Medical Use: A systematic review and meta-analysis. Journal of the American Medical Association, 313(24), 2456-2473. https://doi.org/10.1001/jama.2015.6358