About this course:
The purpose of this module is to expand the APRN’s knowledge of the epidemiology, pathophysiology, risk factors, clinical manifestations, diagnosis, management, and complications related to thyroid dysfunction based on the most recent practice guidelines and up-to-date research.
Course preview
The purpose of this module is to expand the APRN’s knowledge of epidemiology, pathophysiology, risk factors, clinical manifestations, diagnosis, management, and complications related to thyroid dysfunction based on the most recent practice guidelines and up-to-date research.
Objectives
By the completion of this exercise, the APRN will be able to:
understand the normal anatomy and physiology of the thyroid gland and related endocrine organs
differentiate hyperthyroidism, hypothyroidism, and central hypothyroidism
discuss the epidemiology, pathophysiology, and risk factors of thyroid dysfunction
describe the clinical manifestations of thyroid dysfunction
understand the process for diagnosing thyroid dysfunction
explain the medical treatment of thyroid dysfunction
identify the potential complications related to thyroid dysfunction
The endocrine system is complex and works in tandem with the nervous system to maintain the delicate balance of homeostasis. Nurses need to understand both the function of the endocrine system and alterations in its function that can lead to the several pathologies discussed within this module. Disorders of the endocrine system contribute significantly to health care expenses each year. Dieleman and colleagues (2016) identified that more than $224.5 billion US dollars were spent in 2013 on endocrine disorders. Aside from the monetary costs, the loss of work, impaired quality of life, and ongoing personal disparities caused by endocrine disorders pose a significant burden to patients and their families. The estimated prevalence of endocrine disorders is over 5% of the adult population in the United States for each of the major diseases, including diabetes mellitus (DM), obesity, metabolic syndrome, osteoporosis, erectile dysfunction, dyslipidemia, and thyroiditis. Thyroid disorders and osteoporosis are the most common endocrine disorders in females, while erectile dysfunction and osteopenia are the most common disorders in males. DM occurs most often among patients who identify as an ethnic minority (Lonnemann, n.d.).
Anatomy and Physiology of the Endocrine System
The endocrine system consists of glands that produce and secrete hormones to regulate cell and organ activity, as well as the body’s growth, metabolism, sexual function, and development. These hormones serve as the body’s chemical messengers, which transfer information from one organ to another, coordinating functions between various parts of the body. The integral parts of the endocrine system include the hypothalamus, pituitary, thyroid, adrenals, pancreas, parathyroids, pineal body, and ovaries/testes. Each gland secretes a set of hormones that help regulate the body’s functions, much like a thermostat regulates the temperature in a building. The system regulates itself based on a feedback system that involves stimulating hormones and releasing hormones; it is responsible for maintaining a balance of hormone levels within the bloodstream. Releasing hormones are sent to the pituitary from the hypothalamus, prompting the pituitary to secrete various stimulating hormones. The stimulating hormones then signal the target glands to release hormones into the circulation. As the circulating level of the desired hormone from the target gland increases, the hypothalamus secretes less of the releasing hormone, and/or the pituitary gland decreases the secretion of the stimulating hormone. This process signals the target gland to decelerate its hormone secretion (Kemp, 2019). Figure 1 illustrates the endocrine system and its glands.
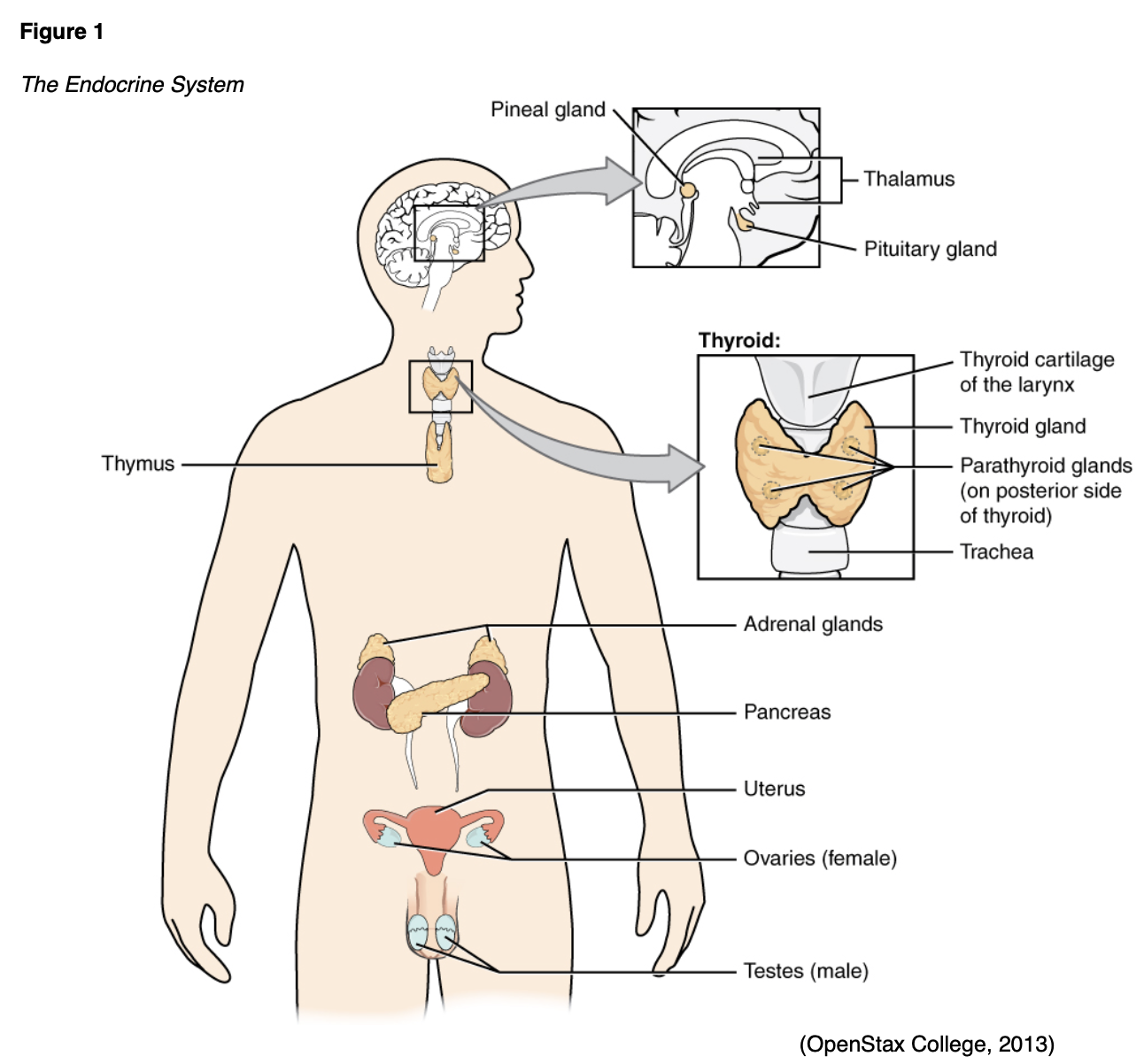
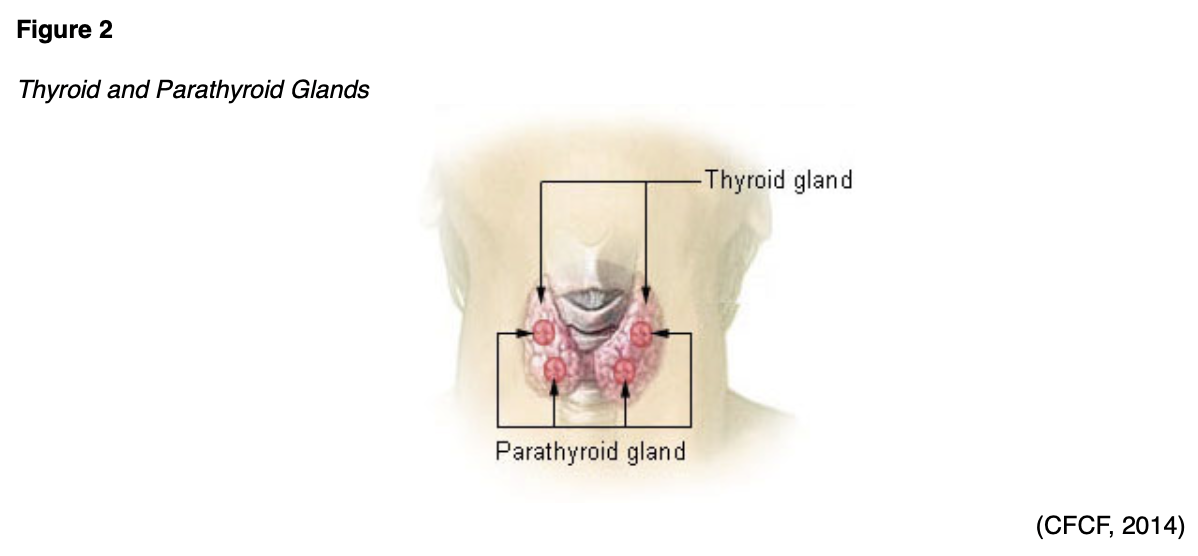
The thyroid gland is located at the anterior of the neck and produces hormones that regulate metabolism (see Figures 1 and 2).
In children, the thyroid supports bone growth, brain development, and nervous system development. In adults, it also helps maintain normal blood pressure (BP), heart rate (HR), muscle tone, and reproductive functions. Additionally, the thyroid gland regulates body temperature, metabolism, and calcitonin (American Thyroid Association [ATA], n.d.b). Specifically, metabolic rate, oxygen consumption, caloric needs, carbohydrate and fat metabolism, and brain and nervous system function are affected by thyroid hormones (Kelly, 2017). The thyroid gland produces three different hormones (see Figure 3 below). The follicular epithelial cells of the thyroid produce triiodothyronine (T3) and tetraiodothyronine or thyroxine (T4; Institute for Quality and Efficiency in Health Care [IQWiG], 2018). Iodine is essential for this hormone’s production. The human body does not produce endogenous iodine, so dietary iodine is an essential nutrient. After being absorbed through the bowel, iodine is directed to the thyroid gland to be utilized in hormone production (Taylor et al., 2018). T4 makes up 90% of the thyroi
...purchase below to continue the course
Figure 3
The Thyroid Feedback Loop
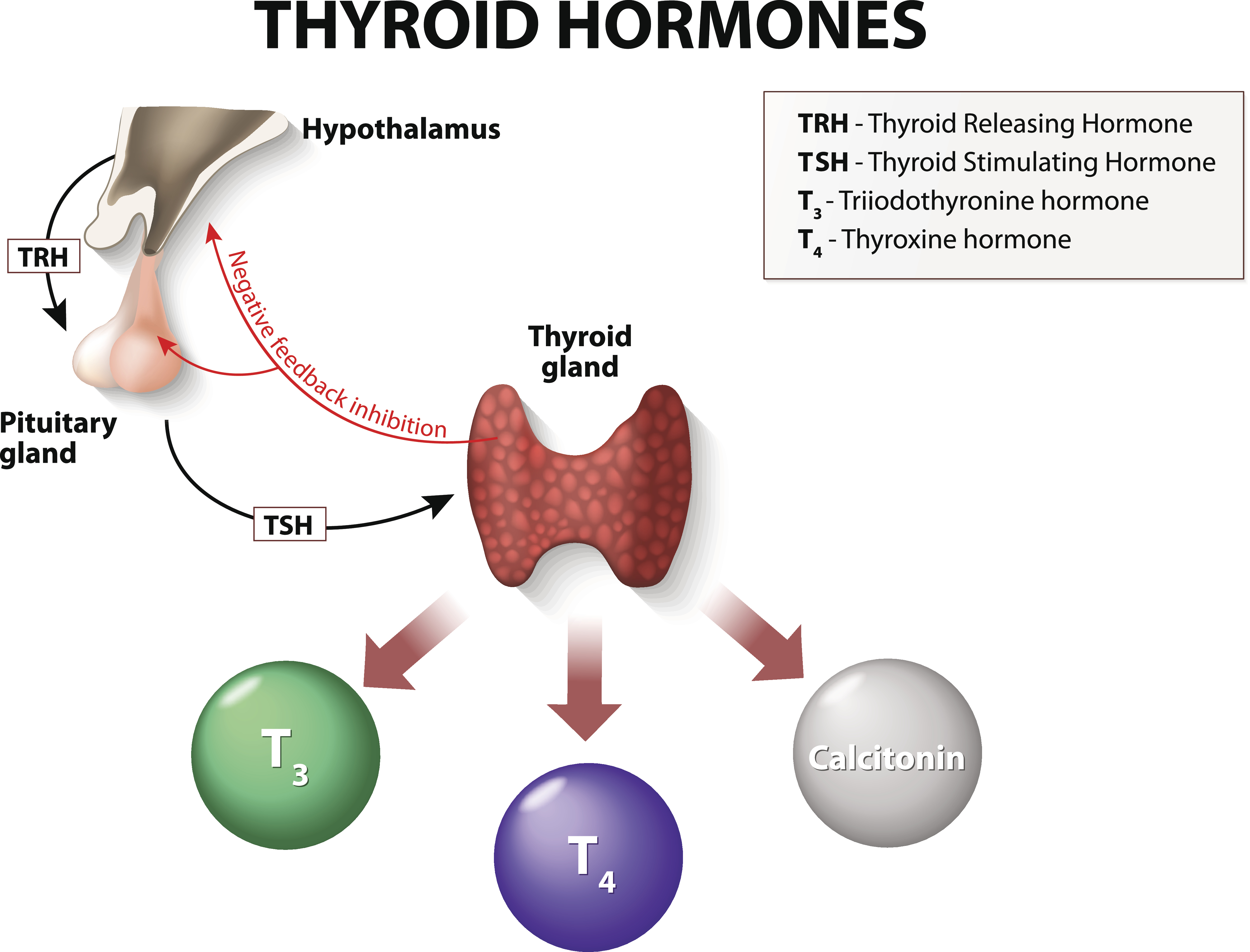
As demonstrated in Figure 3, the release rate of T3 and T4 is controlled by the anterior pituitary gland and hypothalamus, which act as sensory controllers. The process is initiated by the hypothalamus, which emits thyrotropin-releasing hormone (TRH). TRH prompts the release of thyroid-stimulating hormone (TSH) from the anterior pituitary gland. TSH is critical for modulating the release of T4 by the thyroid, which is then converted to T3. The pituitary constantly measures the amount of T3/4 and responds to changes to maintain an appropriate balance. The amount of TSH that the pituitary releases into the bloodstream depends on the amount of T4 that the pituitary perceives, as it functions on a negative feedback system. If the pituitary senses insufficient T4, it will boost TSH production, signaling the thyroid gland to produce more T4. Once T4 reaches an acceptable level within the blood, TSH production decreases (ATA, n.d.b).
The two most common disorders of the thyroid gland are hyperthyroidism and hypothyroidism. Hyperthyroidism is an overactive state of the thyroid gland with hypersecretion of thyroid hormones. Hypothyroidism consists of a scarcity of thyroid hormones, which causes a general slowing of the metabolic rate. Hypothyroidism may be classified as primary (due to thyroid disease) or secondary/central (due to pituitary and/or hypothalamic dysfunction) (Kelly, 2017). Primary hypothyroidism is much more common than secondary/central hypothyroidism, accounting for over 95% of cases (Ross, 2019b; Yani, 2019).
Epidemiology and Etiology of Thyroid Disease
As estimated by the ATA (n.d.c), 20 million Americans have a type of thyroid dysfunction, and 60% of these individuals are unaware of their condition. One in eight women will develop a thyroid problem during her lifetime. Women are five to eight times more likely than men to develop thyroid problems. Thyroid dysfunction is a somewhat common condition; if left undiagnosed, it can have detrimental side effects and may lead to death (Taylor et al., 2018).
Hyperthyroidism is more common in men than hypothyroidism (Shoman, 2019). The vast majority of hyperthyroidism cases (75%) are related to Graves’ disease, an autoimmune condition of unknown etiology. Women are five times more likely to develop Graves’ disease than men. Hyperthyroidism occurs most frequently between the ages of 20 and 40 (Kelly, 2017). The annual incidence of Graves’ disease is approximately 0.5 per 1,000 people in the United States (Lee, 2020a). The prevalence of hyperthyroidism is just over 1% (Kravets, 2016). Hyperthyroidism increases an individual’s risk for thyroid malignancy; it is also a risk factor for myocardial infarction and ischemic stroke in females, especially those who are non-obese and over the age of 50 (Lee, 2020a).
Hypothyroidism, often referred to as thyroid hormone deficiency, is the most common type of thyroid dysfunction. It affects 1-2% of American adults, with a significant increase in prevalence between the ages of 40 and 50 years (ATA, n.d.a). Hypothyroidism is five to eight times more common in women than men, and as many as 4-10% of adults may have subclinical hypothyroidism (Ross, 2019b). The most common form of hypothyroidism in the US is primary hypothyroidism caused by atrophy of the thyroid gland—often secondary to autoimmune disease—that is termed Hashimoto’s thyroiditis (Kelly, 2017; Yani, 2019). Worldwide, the most common cause of hypothyroidism is iodine deficiency. Americans typically ingest sufficient iodine through iodized table salt, shellfish, eggs, soymilk, cow’s milk, and cheese (ATA, n.d.c). Primary deficiency can also be due to defective hormone synthesis (Kelly, 2017). Thyroiditis is typically characterized by a self-limiting and transient increase followed by a decrease in hormone levels (Mayo Clinic, 2020). Secondary or central hypothyroidism is caused by a failure of the anterior pituitary gland to secrete adequate amounts of TSH. This may be due to decreased levels of TRH (in hypothalamic dysfunction) or pituitary dysfunction (Yani, 2019). The condition occurs in approximately 1 in 20,000 to 80,000 people (Ross, 2019a). Decreased thyroid hormones during pregnancy or in early infancy lead to neonatal or congenital hypothyroidism (CH), which is sometimes referred to as cretinism (Willis, 2019). CH is the most common form of hypothyroidism in children, affecting 1 in 1,500 infants (Daniel, 2017).
Hyperthyroidism
Pathophysiology and Risk Factors
Hyperthyroidism is a hyperactive clinical syndrome of the thyroid gland characterized by thyrotoxicosis. Thyrotoxicosis is defined as an excess of T3 and T4 in the circulation. Due to increased T3 and T4 levels, the TSH should be low if the pituitary gland can appropriately sense excessive amounts of thyroid hormone in the body. Hyperthyroidism can develop due to thyroiditis (typically transient and self-limiting), excessive iodine intake, pituitary tumors, thyroid cancer, toxic adenoma, and toxic multinodular goiters (an enlarged gland with varying nodule sizes that show hyperplasia). However, the most common cause is Graves’ disease, an autoimmune condition. In this disease, the immune system reacts inappropriately by producing antibodies (most often thyroid-stimulating antibodies, TSab or TSI) that act as TSH receptor agonists and overstimulate the thyroid gland, causing a marked increase in T3 and T4. In addition to female patients, those with a personal or family history of other autoimmune disorders have an increased risk of Graves’ disease. The US has a relatively higher rate of Graves’ disease compared to other areas of the world, secondary to the fact that the US diet tends to be higher in iodine. Autoimmune thyroid disease occurs with lower frequency in African American patients (Kelly, 2017; Kravets, 2016; Lee, 2020a).
Thyroid nodules may be singular or multiple. Toxic adenoma, or Plummer disease, is caused by a somatic mutation in the TSH receptor of the GS alpha gene, typically resulting in a single thyroid nodule (Kelly, 2017; Kravets, 2016). It is responsible for roughly 3-5% of cases of thyrotoxicosis in the US (Lee, 2020a). Toxic multinodular goiter involves the expansion of clonogenic cells with an activating TSH receptor mutation, leading to multiple palpable nodules. This is the second most common cause of hyperthyroidism in the US, causing 15-20% of thyrotoxicosis cases (Kelly, 2017; Kravets, 2016). Toxic multinodular goiter occurs more commonly in areas of the world that are prone to iodine deficiency and typically presents in people over the age of 50 (Lee, 2020a). Drug-induced thyroiditis, hyperemesis gravidarum, postpartum thyroiditis, and subacute granulomatous thyroiditis are less common etiologies. Lithium (Lithobid, an antipsychotic medication), interferon-alpha (Intron A), and interleukin 2 (Proleukin) may cause a release of preformed thyroid hormones. Amiodarone (Cordarone, a class III antiarrhythmic medication) may cause an overproduction of thyroid hormones (thyrotoxicosis type 1) or a release of preformed thyroid hormones (thyrotoxicosis type 2). Hyperthyroidism can also be related to the administration of tyrosine kinase inhibitors or antiretrovirals. Rare etiologies include factitious thyrotoxicosis, metastatic follicular thyroid cancer, struma ovarii, trophoblastic tumors, germ cell tumors, and TSH-secreting pituitary adenomas (Kelly, 2017; Kravets, 2016).
Iodine ingestion can also contribute to the development of hyperthyroidism. Patients who regularly ingest an iodine-deficient diet often develop a nodular goiter. If these individuals move to a different area or adjust their diet to allow for sufficient iodine intake, the sudden increase in iodine ingestion can trigger thyrotoxicosis. Iodine, in these instances, may act as an immune stimulator, precipitating autoimmune thyroid disease. Although they do not prompt true hyperthyroidism, subacute thyroiditis and exogenous intake of excessive amounts of thyroid hormone can both cause thyrotoxicosis, mimicking the signs and symptoms of hyperthyroidism (Lee, 2020a). Subacute thyroiditis typically occurs in three phases, causing 4-10 weeks of thyrotoxicosis, then up to 2 months of mild hypothyroidism, and finally returning to a previously healthy state. Subacute granulomatous (painful) thyroiditis causes approximately 15% of cases of thyrotoxicosis in the US, as well as 10% of cases of hypothyroidism. Subacute granulomatous thyroiditis may be associated with a viral illness related to a post-infectious complication of influenza, adenovirus, mumps, or coxsackievirus. Silent (painless or subacute lymphocytic) thyroiditis is characterized by thyroid tissue damage causing an excessive release of previously formed thyroid hormones. It is most likely autoimmune, but this is not definitively known. As mentioned previously, certain medications can also trigger thyroiditis. Patients with subacute lymphocytic thyroiditis are more likely to develop goiters, and some develop permanent hypothyroidism. A third form of subacute thyroiditis occurs within a year of pregnancy and is termed subacute postpartum thyroiditis (Kelly, 2017; Kravets, 2016; Lee, 2020b).
Signs and Symptoms
Hyperactive thyroid hormone synthesis and secretion commonly cause tremors, anxiety, palpitations, fatigue (often due to insomnia), weight loss, polydipsia (increased urination), excessive perspiration, and heat intolerance. Approximately 25% of patients diagnosed with Graves’ disease develop exophthalmos (bilateral protrusion of the eyeballs). A goiter (enlarged thyroid gland) may also be palpable or evident upon inspection (De Leo et al., 2016; Kravets, 2016). This may also be termed Graves’ ophthalmopathy or orbitopathy and is likely a result of the body’s T-cells attacking the tissues in the orbital space. This leads to the thickening of the muscles and an increase in adipose and connective tissue volume around the eye, as the tissue in the retro-orbital space may share antigenic epitopes with thyroid follicular cells. Ophthalmopathy can present as periorbital edema, exophthalmos, or diplopia (Lee, 2020a). Figure 4 demonstrates exophthalmos and goiter.

A patient can develop a goiter with either hypothyroidism or hyperthyroidism, so additional testing should be performed to determine the type and cause (Kelly, 2017). Goiters related to Graves’ disease are typically smooth with a positive thrill to palpation and a positive bruit on auscultation (Kravets, 2016). Goitrogens are foods or medications that may cause a goiter to develop. See the list below for types of goitrogens.
Medications:
propylthiouracil (PTU)
methimazole (Tapazole)
large doses of iodine
sulfonamides
salicylates
P-aminosalicylic acid
lithium (Lithobid)
amiodarone (Cordarone)
Foods:
broccoli
brussels sprouts
cabbage
cauliflower
kale
mustard
peanuts
strawberries
turnips (Kelly, 2017)
Acropachy is a less common extrathyroidal manifestation. It is characterized by digital clubbing and edema of the fingers. Patients with autoimmune thyroid disease may develop myxedema, an altered physical appearance of the skin and subcutaneous tissues due to the accumulation of hydrophilic mucopolysaccharides (hyaluronic acid) in the dermis and surrounding tissues. Myxedema may manifest with periorbital (around the eyes) edema, facial puffiness, and a masklike face, or it may be pretibial (affecting the anterior lower legs). Many individuals with myxedema struggle with an altered self-image. Pretibial myxedema may not be confined to the pretibial area but may also affect the skin of the ankle, dorsum of the foot, knees, shoulder, elbows, or upper back (Gill, 2020; Kelly, 2017). Pretibial myxedema can cause nonpitting edema, erythema, and thickening of the skin. Patients rarely report associated pain or pruritis (Lee, 2020a). The presence of orbitopathy, pretibial myxedema, and thyroid acropachy is pathognomonic for Graves’ disease (Kravets, 2016). Men with thyroid dysfunction (hypothyroidism or hyperthyroidism) can develop low testosterone with erectile dysfunction or gynecomastia (Shoman, 2019). See Table 1 for other body systems that may be affected by hyperthyroidism.


Diagnosis
There are no universal recommendations for screening of thyroid disease in adults, but the ATA suggests screening patients at 35 years of age and every 5 years thereafter, particularly in high-risk groups such as:
pregnant women,
women over 60 years of age,
patients with autoimmune disease such as type 1 DM (T1DM),
patients with a history of radiation to the neck or brain,
patients with other hypothalamus or pituitary hormone deficiencies, and
patients with a prior history of TBI or pituitary/hypothalamus surgery (Orlander, 2019; Ross, 2019a).
The US Preventive Services Task Force (USPSTF, 2015) found insufficient evidence regarding screening asymptomatic patients. The American Academy of Family Physicians suggests periodic screening for older women, while the American College of Physicians suggests that screening women over the age of 50 may be indicated. It may be prudent to consider screening asymptomatic patients who fall under a high-risk category, such as those with a goiter on examination, a history of autoimmune disease, previous radioactive iodine ablation, previous radiation therapy to the head or neck, a family history of thyroid disease, or current use of medication(s) that may impair thyroid function (Ross, 2019b).
A full history and physical examination should be performed to evaluate a patient for potential hyperthyroidism. Some symptoms of hyperthyroidism may manifest in patients with elevated estrogen levels, such as pregnant patients or those receiving hormone replacement therapy. The TSH and T4 levels in these individuals are typically within the expected reference range. This possible explanation for the patient’s symptoms should be ruled out in female patients (Kravets, 2016). In addition to the history and physical examination, a serum TSH level should be performed to establish a diagnosis. According to the USPSTF (2015), TSH is considered the first-line screening test for patients with suspected thyroid dysfunction. The level of circulating TSH in the blood helps determine if the thyroid is functioning normally, overactive, or underactive. If the TSH is low, the thyroid is likely producing too much T3/T4, raising the clinical suspicion for hyperthyroidism or secondary hypothyroidism. T4 can be measured as total T4 or free (FT4). Total T4 measures both the free and the bound hormone available, whereas FT4 assesses the T4 hormone that is freely circulating in the blood and available for use. FT4 is more commonly performed since it provides the best insight into the severity of an abnormal TSH level. FT4 is most accurate when performed in conjunction with a TSH level, so these tests are usually ordered together (ATA, 2019; BCGuidelines, 2018; Snyder, 2020).
A thyroid panel typically consists of three main tests: TSH, FT4, and free T3 (T3, Free) or total T3, as seen in Table 2 (American Board of Internal Medicine [ABIM], 2019). Free T3 is less reliable and not clinically indicated in suspected thyroid disease. The total T3 test is reserved for identifying hyperthyroidism or determining its severity, as patients with an overactive thyroid have elevated T3 levels. Reverse T3 is another thyroid test that is less commonly performed. It measures inactive thyroid hormone, and it is also only indicated in the evaluation of patients with suspected hyperthyroidism (ATA, 2019).
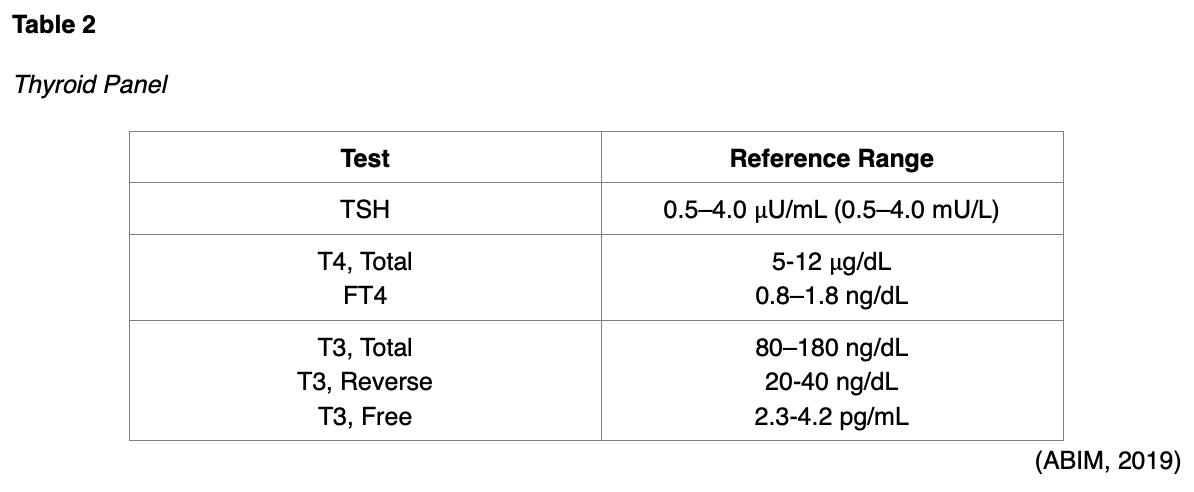
If not performed concurrently as a panel of tests, the TSH should be drawn first; if TSH is low, FT4 and T3 should be added to determine the degree of hyperthyroidism (ATA, n.d.b). The reference range for total T3 is lower in adults over the age of 50 (40-181 ng/dL; Kelly, 2017). When interpreting the FT4, the APRN must consider several conditions that are commonly associated with transient elevations of the FT4, such as:
amphetamine abuse
high altitude exposure
selenium deficiency
hyperemesis gravidarum
acute psychosis
estrogen withdrawal (DeGroot, 2016)
The most common thyroid conditions classified by TSH and FT4 values are demonstrated in Table 3 (ATA, n.d.b). The USPSTF recommends that multiple tests over 3 to 6 months should be performed to confirm abnormal results (USPSTF, 2015).
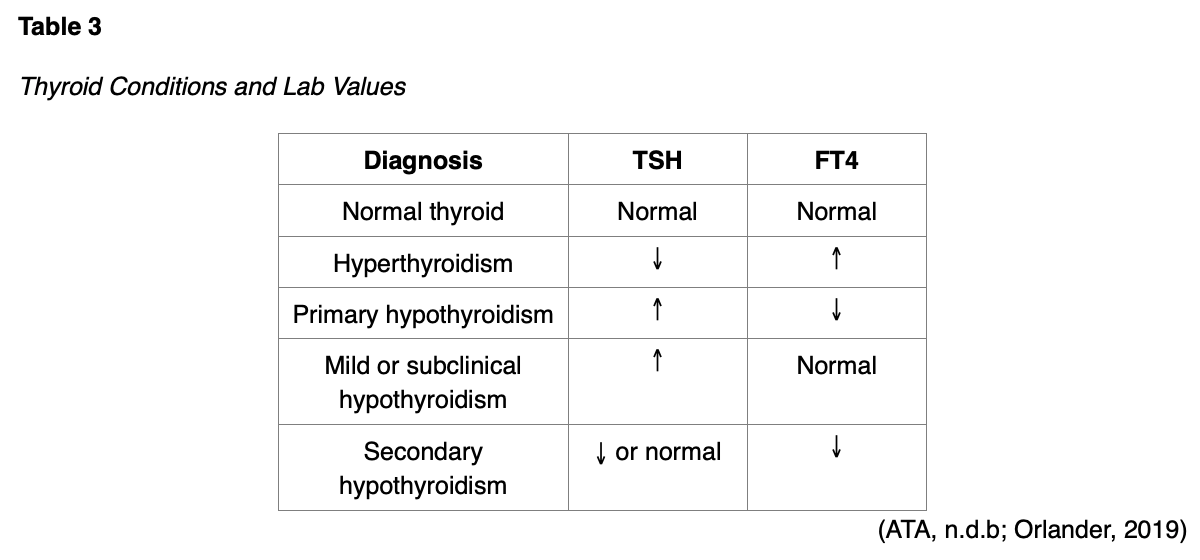

If both the TSH and FT4 are elevated, the rare presence of a TSH-secreting pituitary adenoma should be considered. A complete blood count (CBC) and hepatic panel should also be completed at baseline, especially in those diagnosed with Graves’ disease (Kravets, 2016). An electrocardiogram (ECG) may be indicated if the patient’s heart rate is elevated and may demonstrate sinus tachycardia or dysrhythmias (Kelly, 2017; Lee, 2020a).
Thyroid antibody tests are a separate subtype of thyroid function test; they assess for the presence of thyroid antibodies. Thyroid peroxidase antibody, otherwise called antithyroid peroxidase antibody (TPO), is one of the most common antibody tests currently used in clinical practice. It is performed to determine if thyroid disease is autoimmune, such as Graves’ disease. Once a patient is known to be TPO antibody positive, repeat analysis is not indicated (ADA, n.d.c; MedlinePlus, 2020). The presence of thyroid-stimulating immunoglobulin (TSI) or TSH-receptor-thyrotropin receptor antibody (TRAb) strongly supports a diagnosis of Graves’ disease (DeGroot, 2016). These tests can determine the underlying etiology of hyperthyroidism in patients who cannot undergo a radioactive iodine uptake or a thyroid scan (Kravets, 2016).
If a patient presents with a thyroid nodule or goiter, a thyroid ultrasound should be performed. This is not necessary if there is no palpable nodule or goiter (Kravets, 2016). An ultrasound determines if a nodule is cystic (fluid-filled) or solid and measures the size of the nodule. If thyroid cancer is suspected, an ultrasound-guided fine needle biopsy may be performed (ATA, n.d.c).
Thyroid Scintigraphy and Radioactive Iodine Uptake Test
If the patient’s laboratory tests indicate hyperthyroidism, as evidenced by decreased TSH and elevated FT4, imaging with a nuclear medicine scan is indicated (Kravets, 2016). Nuclear medicine imaging differs from conventional diagnostic imaging as it is capable of visualizing how the body is functioning at the cellular and molecular levels. Nuclear imaging uses small quantities of radioactive tracers (radiotracers) to diagnose and treat disease. The radiotracers are most commonly injected into a vein but may also be taken orally or inhaled. The radiotracer travels through the body, releasing energy in the form of gamma rays that are absorbed by specific tissues and organs. It is then detected by the external scanning device to provide information on organ function and cellular activity (Society of Nuclear Medicine & Molecular Imaging [SNMMI], n.d.). The radiotracers are comprised of molecules that are bonded tightly to a radioactive atom, and these molecules vary greatly depending on the purpose of the scan (National Institute of Biomedical Imaging and Bioengineering [NIBIB], 2019). Radiotracers must meet US Food & Drug Administration (FDA) standards for safety due to radiation exposure. The use of radioactive materials for nuclear medicine is regulated by the Nuclear Regulatory Commission (NRC), the FDA, and individual states to ensure the safety of patients, healthcare professionals, and the general public. Each nuclear medicine imaging test uses a specific radioactive agent (SNMMI, n.d.).
There are two types of nuclear medicine imaging tests of the thyroid: thyroid scintigraphy (also called a thyroid scan) and radioactive iodine uptake (RAIU) test. Both scans use a small amount of radioactive iodine, usually in the form of sodium iodide-123 (I-123), as the thyroid gland is the only tissue within the body that absorbs and holds onto iodine. The radiation emitted by I-123 is harmless to thyroid cells, and it can be detected externally through thyroid scanning. In rare instances, sodium iodide-131 (I-131 or Iodotope) may be used with RAIU scans, but I-131 destroys thyroid cells and is commonly reserved for the treatment of thyroid disorders such as overactive thyroid, thyrotoxicosis, and thyroid cancers. The American College of Radiology (ACR, 2019) states that it is safe to use radioactive iodine in patients who report iodinated contrast allergies or seafood allergies, as the reaction is to the compound containing iodine and not the iodine itself (ACR, 2019). I-123 and I-131 readily cross the placenta, and their use should be avoided in pregnancy. If a thyroid scan is essential, the radioactive isotope technetium 99m is recommended (American College of Obstetricians and Gynecologists, 2017). Otherwise, an ultrasound is the recommended and safer imaging alternative for pregnant patients with suspected thyroid disease (Kravets, 2016).
A thyroid scan may be performed to obtain additional information about any structural abnormalities within the gland, such as nodules, masses, or inflammation. According to the ACR (2019), thyroid scans are useful in, but not limited to, the evaluation of the following:
size and location of thyroid tissue;
the cause of overt and subclinical thyrotoxicosis;
suspected focal masses or diffuse thyroid disease;
clinical laboratory tests suggestive of abnormal thyroid function;
the function of thyroid nodules detected on clinical examination or other imaging examinations;
congenital thyroid abnormalities, including ectopia; and
differentiating hyperthyroidism from other forms of thyrotoxicosis (ACR, 2019, p.2).
When undergoing a thyroid scan, the I-123 is either injected into a vein within 30 to 60 minutes of the scan or administered orally as a pill or liquid. With oral administration, the I-123 must be given approximately 4 to 6 hours before the scan to allow the radioactive iodine to reach and saturate the thyroid gland. The thyroid scan is painless, and patients are usually positioned lying flat (supine) on an examination table with their head tilted back to extend their neck. A scanner will take images of the thyroid from at least three different angles, and the patient will be asked to lie still. It takes about 30 minutes to complete a thyroid scan (RadiologyInfo.org, 2019).
An RAIU scan is performed to evaluate the function of the gland or determine the etiology of hyperthyroidism, most commonly to differentiate Graves’ disease from other forms of hyperthyroidism (Kelly, 2017). It can also help guide treatment for patients who have thyroid cancer. The RAIU uses a specialized probe to measure how much tracer the thyroid gland absorbs. In most cases, the RAIU scan is performed alongside a thyroid scan to determine if the radiotracer is evenly spread in the gland. According to the ACR (2019), while the RAIU scan does have overlapping indications with the thyroid scan, it is considered most useful in the following situations:
differentiating hyperthyroidism from other forms of thyrotoxicosis (e.g., subacute or chronic thyroiditis and thyrotoxicosis factitial [exogenous thyrotoxicosis]) and
assessing the necessity and calculating I-131 sodium iodide administered activity for patients to be treated for hyperthyroidism (ACR, 2019, p.2).
The RAIU scan requires administration of the radioactive iodine in liquid or capsule form, and scanning occurs at 4 to 6 hours after radiotracer administration and again at 24 hours. The administered dose for adults is typically 0.2 to 0.4 millicuries (mCi, or 7.4-14.8 megabecquerels [MBq]). For pediatric patients, the dose is typically weight-based at 0.0075 mCi/kg (0.28 MBq/kg) within a range of 0.027-0.3 mCi (1-11 MBq). The patient is usually seated in an upright position, and a small device called a radioactive detector (uptake probe) is placed against the patient’s neck. The uptake probe takes measurements of radioactive iodine uptake, and a gamma camera records pictures of the thyroid gland. Both instruments detect and record the distribution of the radioactive material within the thyroid. The RAIU test usually takes several minutes. For patients who undergo both thyroid and RAIU scanning, oral administration is preferred, as this negates the need for a second dose of the radiotracer (ACR, 2019; RadiologyInfo.org, 2019).
Agents that contain iodine can decrease iodine uptake in the thyroid gland and lead to inaccurate test results. This includes many commonly used supplements, over-the-counter agents, and certain prescription medications. Comprehensive medication reconciliation should be performed, and patients should discontinue thyroid hormones, antithyroid medications, and anything else that contains iodine. The period for which medication should be discontinued before the scan varies (e.g., levothyroxine [Synthroid] must be discontinued for 4 to 6 weeks, and iodine-containing cough syrups should be discontinued for 2 weeks; ACR, 2019). Please see Table 4 for medications that should be withheld prior to scanning and the timing recommended by the ACR:

Other iodine-based products that need to be discontinued include but are not limited to iodized salt and multivitamins (ACR, 2019).
Patients should be advised to consume a low-iodine diet, avoiding the highest sources of dietary iodine (e.g., salt, grains, cereals, fish, poultry, and milk products) in the 1 to 2 weeks leading up to the scan (ATA, 2020). Following the test, the majority of radioactive material is cleared from the body within 1-2 days. No special precautions need to be taken since I-123 is harmless to thyroid cells (ACR, 2019). A patient with Graves’ disease may have a highly elevated diffuse radioactive uptake of 35-95%, compared to a normal result of 3-16% (at 6 hours) and 8-25% (at 24 hours). While a moderately elevated uptake (25-60%) that appears homogenous indicates Graves’ disease, an elevated uptake with a nodular appearance indicates either a toxic multinodular goiter (if found in multiple areas) or toxic adenoma (if found in a concentrated area). If the scan indicates reduced radiotracer uptake, this may indicate thyroiditis (<2%) or hyperthyroidism due to ectopic thyroid hormone production or exogenous hormone intake (Kelly, 2017; Kravets, 2016; Lee, 2020a; Wisse, 2020).
Management
Cases of subacute thyroiditis are typically self-limiting, resolving spontaneously within 6 months, and do not require specialized treatment. Supportive treatment may be recommended. Symptomatic treatment with a beta-blocker such as propranolol (Inderal) or atenolol (Tenormin) may be prescribed to decrease adrenergic symptoms of tachycardia, nervousness, and irritability by inhibiting the sympathetic nervous system in any patient with hyperthyroidism (Kelly, 2017; Kravets, 2016). For a patient with hyperthyroidism related to amiodarone (Cardarone), the medication should not be discontinued unless it can be stopped safely due to the risk of related cardiovascular complications. Treatment varies by type. For those with type 1, which involves the overproduction of thyroid hormones, treatment should consist of a thionamide (an antithyroid medication). Type 2, which is characterized by thyroid tissue destruction, requires treatment with corticosteroids. For a patient with hyperthyroidism related to other medications (e.g., lithium [Lithobid], interferon alfa (Intron A), tyrosine kinase inhibitors, or antiretrovirals), the offending medication should be safely tapered, discontinued, and replaced with an equivalent therapy (Kravets, 2016).
Treatment options for other etiologies of hyperthyroidism are complex, including radioactive iodine (RAI) ablation, pharmacological therapy with a thionamide, and surgical intervention. Treatment choice should be based on the patient’s underlying pathology, any contraindications to a particular treatment modality, the severity of the disease, and patient preference. Treatment is typically advocated in those with a TSH < 0.1 μU/mL (0.1 mU/L). RAI ablation therapy has been the most common treatment utilized in the US for hyperthyroidism due to Graves’ disease. RAI ablation is usually performed in an outpatient setting (Kravets, 2016, USPSTF, 2015). A study by Wong and colleagues (2018) found that treatment with a single calculated RAI dose was effective in over 90% of 316 hyperthyroid patients with Graves’ disease. RAI is administered as sodium iodide (I-131 or Iodotope) in a solution or capsule form (Ross, 2020). RAI can aggravate orbitopathy related to Graves’ disease, so this is considered a relative contraindication to RAI therapy in Graves’ disease patients, especially those who smoke. RAI is typically considered the treatment of choice for patients with toxic adenoma or toxic multinodular goiter unless it is causing compressive symptoms. The advantages of RAI therapy include the elimination of pharmacological side effects and perioperative risks. For 3 months leading up to the RAI administration, patients should avoid exposure to large amounts of nonradioactive iodine (e.g., iodinated contrast, amiodarone [Cordarone]; Kravets, 2016). For a week prior to RAI administration, patients should also be counseled to avoid supplements that contain iodine. Pregnancy should be ruled out within 48 hours of RAI administration. Breastfeeding patients should not be treated with RAI for 6 to 12 weeks after weaning (Ross, 2020).
RAI inhibits the release of T3 and T4 into the bloodstream by damaging thyroid tissue. It may be used in conjunction with a thionamide to achieve a euthyroid state or given short-term before surgery. RAI also decreases vascularity to the thyroid gland, improving surgical safety when given preoperatively. Patients can develop iodine toxicity if it is not properly dosed, which may involve buccal mucosa edema, excessive salivation, skin reactions, or nausea and vomiting. The patient should notify their medical team immediately if these symptoms occur (Kelly, 2017). Underdosing can lead to inadequate results, and patients should be counseled on the possibility of future recurrence after successful treatment with RAI ablation (Kravets, 2016). For this reason, higher doses are preferred, aiming for a hypothyroid state in order to limit the probability of treatment failure in lieu of a euthyroid state. The dose may be fixed or calculated using μCi or MBq per gram (g) of thyroid tissue based on 24-hour radioiodine uptake. Dosing for patients diagnosed with Graves’ disease ranges from 150 to 200 μCi/g [5.9 to 7.4 MBq/g]. For fixed dosing, the ATA guidelines suggest 10-15 mCi (370-555 MBq) for adults with Graves’ disease. Typically, adults with toxic adenomas or toxic multinodular goiters require a higher dose of 200 μCi/g (7.4 MBq/g) to treat effectively (Ross, 2020).
It may take 6 to 18 weeks for the maximum desired effect of RAI to occur. Patients may experience a temporary exacerbation of hyperthyroidism symptoms immediately after RAI administration. This can be alleviated by an oral thionamide, as methimazole (Tapazole) may return thyroid function to a normal range faster. Pretreatment with methimazole (Tapazole) for 4 to 6 weeks may be especially beneficial for patients with severe symptoms of hyperthyroidism, severe thyrotoxicosis (two to three times the upper limit of normal), comorbidities that increase their vulnerability, and patients over the age of 60 or 65. Methimazole (Tapazole) should be withheld for 3 to 5 days prior to RAI administration and restarted 3 to 5 days following treatment. Then, this treatment can be continued for 4-18 weeks after RAI administration to manage hyperthyroidism symptoms while monitoring thyroid function tests and goiter size and awaiting the RAI to take effect fully (Ross, 2020). The patient’s TSH and FT4 should be reassessed approximately 4-8 weeks following RAI administration and every 4-6 weeks thereafter if levels remain elevated. Patients who develop early symptoms or laboratory indications of hypothyroidism should begin thyroid hormone replacement. Pregnancy should be avoided for 6 months following RAI administration (Kravets, 2016).
Patients receiving RAI may develop thyroiditis or parotitis (inflammation of the parotid salivary gland). These complications may manifest with hoarseness, dry mouth, and throat irritation. The APRN should instruct the patient to gargle with salt water and take frequent sips of water for relief (Kelly, 2017). Approximately 80% of patients develop post-treatment hypothyroidism within 2-6 months of treatment and require lifelong thyroid hormone replacement therapy. Patients should be counseled regarding this risk, including hypothyroidism symptoms and when to report them. Radiation exposure to the patient and others is another risk that should be discussed with patients prior to treatment selection and administration (Kelly, 2017; Kravets, 2016). The APRN should also review strategies with the patient to prevent exposing others to radiation. Home precautions include:
using a private toilet,
flushing two or three times after each use,
washing laundry separately,
minimizing the time spent handling food while cooking for others, and
avoiding contact with pregnant females and children for 7 days post-treatment (Kelly, 2017).
Antithyroid medications (or thionamides) include propylthiouracil (PTU) and methimazole (Tapazole). These medications reach the thyroid gland via active transport, where they limit the synthesis of T3 and T4 by inhibiting thyroid peroxidase (De Leo et al., 2016; Kravets, 2016). Indications for antithyroid drugs are Graves’ disease (especially in young patients), pregnant patients with a diagnosis of hyperthyroidism, and patients needing to establish a euthyroid state before RAI or surgical intervention. Propylthiouracil (PTU) inhibits the peripheral conversion of T4 to T3 in addition to inhibiting the production of thyroid hormones, thus achieving a euthyroid state more quickly than other drugs. However, it must be taken three times a day (Kelly, 2017). The initial dosing is 300 to 400 mg/day PO divided every 8 hours, but most patients can be maintained on a final dose of 100 to 150 mg/day PO divided every 8 hours (Epocrates, n.d.d). Methimazole (Tapazole) is dosed at 5-20 mg PO TID initially, but maintenance dosing is 5-15 mg PO once daily (Epocrates, n.d.c). For this reason, methimazole (Tapazole) is used for the chronic treatment of hyperthyroidism in most patients, and propylthiouracil (PTU) is reserved for women in the first trimester of pregnancy, patients presenting in thyroid storm or crisis, and those with a methimazole (Tapazole) allergy or sensitivity (Lee, 2020a). Clinical improvement typically happens within 1 to 2 weeks of initiating drug therapy, but optimal results do not occur for 4 to 6 weeks. Between 20% and 40% of patients with Graves’ disease will experience spontaneous remediation after 6-15 months of treatment. However, patients should be cautioned that abruptly discontinuing the medication can cause regression of their hyperthyroidism (Kelly, 2017). Propylthiouracil (PTU) is safe during the first trimester of pregnancy, but methimazole (tapazole) may lead to birth defects and should be avoided in the first trimester. Thionamides may control hyperthyroidism in patients with toxic adenoma or multinodular goiter but do not induce remission in these patients; therefore, they are not the ideal treatment choice. Potential benefits of pharmacological treatment include no exposure to radiation, no perioperative risks, and no risk of permanent hypothyroidism. If they are using a pharmacological agent, patients should be counseled about the potential risks, such as agranulocytosis, hepatotoxicity (boxed warning associated with propylthiouracil [PTU]), and rash. FT4 and total T3 should be reassessed 4 weeks after starting a thionamide and then every 4-8 weeks until the patient is stable. Once they are stable, labs should be repeated every 3 months, and treatment should continue for at least 12-18 months. If the patient’s TSH is back within the reference range at this point, a taper should be considered. Thyroid function should continue to be monitored every 1 to 3 months for a year. Relapse occurs in 30% to 70% of patients with Graves’ disease, generally within the first year (Kravets, 2016).
Surgical thyroidectomy may be indicated for those with tracheal compression from a nodule/goiter, an inadequate response to thionamide therapy, or thyroid cancer. Patients are typically treated with RAI or a thionamide before surgery to achieve a euthyroid state (Kelly, 2017). High doses of oral potassium iodide (SSKI, ThyroShield) can suppress the release of thyroid hormone as a short-term therapy, such as 10-14 days before surgical thyroidectomy (Lee, 2020a). The preferred surgical intervention is a subtotal thyroidectomy (see Figure 5).
Figure 5
Thyroidectomy

Generally, the goal of surgery is to remove 90% of the thyroid. If a subtotal or partial thyroidectomy is performed, a patient can live in a euthyroid state without needing additional treatment. Endoscopic or robotic assistance is preferred for patients with small benign nodules and a healthy BMI due to smaller incisions leading to a faster recovery and less postoperative pain (Kelly, 2017). Patients should be counseled regarding the potential benefits (e.g., lack of medication side effects, absence of radiation exposure, and decreased risk of recurrence) and risks (e.g., anesthesia reaction, as well as others discussed below) of surgery. For these reasons, surgery is typically reserved for those with compressive symptoms or contraindications to RAI or thionamides (Kravets, 2016).
Postoperative complications following thyroidectomy are uncommon, affecting 1-3% of patients (De Leo et al., 2016). The patient should be monitored closely for airway obstruction. Laryngeal stridor may occur due to excess edema, hematoma, hemorrhage, or inflammation at the surgical site. A suction set and a tracheostomy kit should be readily available at the patient’s bedside. Thyroidectomy procedures also carry an associated risk for damage to the recurrent laryngeal nerve, leading to voice changes (most likely hoarseness) or respiratory distress (Kravets, 2016).
Postoperative assessments for indications of hemorrhage and tracheal compression should be ordered at least every 2 hours for the first 24 hours. Symptoms may include frequent swallowing, choking, a saturated dressing, edema, dyspnea, or irregular breathing. Patients should remain in a semi-Fowler’s position, supporting the head and neck with pillows to avoid creating tension on the suture lines. Vital signs and calcium levels should be monitored frequently. The parathyroid glands may be partially removed or unintentionally damaged during a thyroidectomy, causing hypocalcemia. Severe hypocalcemia can lead to tetany, a series of involuntary muscle spasms, which potentially worsen laryngeal stridor (Kelly, 2017). The patient should be monitored for signs of hypocalcemia, such as:
a positive Trousseau’s sign (carpopedal spasms that occur when a blood pressure cuff is inflated higher than the patient’s systolic pressure)
a positive Chvostek’s sign (twitching of the facial muscles when the facial nerve is percussed over the cheek)
tingling around the mouth
tingling in the extremities
muscle twitching (tetany)
Calcium gluconate should be administered IV to treat any hypocalcemia with tetany (Kelly, 2017). Ten mL of 10% IV solution contains 1 g of calcium gluconate contains 90 mg (4.5 mEq) of elemental calcium; 1-2 g can be given over 5-10 minutes, or an infusion can be dosed at 5.4-21.5 mg/kg/hour (Epocrates, n.d.a). If the absence of complications, the patient typically can walk within a few hours of surgery. Oral fluids should be allowed as tolerated. A soft diet should begin the day after surgery. Postoperatively, the patient should be instructed to monitor for signs and symptoms of hypothyroidism and alert the medical team if these develop. Weight and caloric intake should be monitored to prevent weight gain. Education should be provided on appropriate iodine intake, which often equates to one serving of seafood weekly or regular use of iodized salt. Regular exercise is also essential to stimulate thyroid function (Kelly, 2017).
The 2016 ATA guidelines for hyperthyroidism/thyrotoxicosis management in children state the following:
Children with Graves’ disease should be treated with methimazole (Tapazole), RAI therapy, or thyroidectomy. RAI therapy should be avoided in very young children (under 5 years). RAI in children is acceptable if the activity is over 150 μCi/g (5.55 MBq/g) of thyroid tissue and for children between ages 5 and 10 years if the calculated RAI administered activity is under 10 mCi (370 MBq). Thyroidectomy should be chosen when definitive therapy is required, the child is too young for RAI (under the age of 5), and surgery can be performed by a high-volume thyroid surgeon. (Ross et al., 2016, p. 1369)
In 2017, the ATA released guidelines regarding the management of thyroid disease for women before pregnancy, during pregnancy, and in the postpartum period. These recommendations include the following:
Avoid scintigraphy or radioiodine uptake determination during pregnancy.
If a decreased serum TSH is detected in the first trimester, a medical history, physical examination, and measurement of maternal FT4, TSab, and total T3 should be performed to aid in determining the etiology of thyrotoxicosis.
Appropriate management of gestational transient thyrotoxicosis and/or hyperemesis gravidarum should include supportive therapy, dehydration management, and hospitalization if needed, but antithyroid drugs are not recommended. Beta-adrenergic blockers can be considered if necessary (Alexander et al., 2017).
The APRN should educate patients with hyperthyroidism regarding:
reporting potentially important side effects, such as elevated temperature and pharyngitis (which may indicate agranulocytosis or fatigue), weakness, loss of appetite, abdominal pain, bruising, itching, or yellowing of the skin or eyes;
decreasing physical activity until their condition is controlled to avoid cardiopulmonary stress in elderly or otherwise high-risk patients;
the importance of keeping all appointments with the interdisciplinary team, including the ophthalmologist, endocrinologist, dietician, and primary care physician or APRN (Lee, 2020a).
Complications
Approximately 50% of patients with Graves’ disease will develop mild signs and symptoms of thyroid eye disease, and another 5% will develop severe ophthalmopathy. Less severe cases should be treated with tight-fitted sunglasses that should be worn whenever the patient is outside, as well as saline drops as needed for comfort. If the eyelid does not fully close due to exophthalmos and the cornea is exposed at night, the patient will likely report irritation and tearing when awake. Treatment with saline gel or drops and covering the eyelids overnight with paper tape or goggles may keep the eyes moist and decrease discomfort. If severe orbital edema exists, optic nerve compression can occur, risking the loss of color vision and the development of orbital pain. These patients should be referred to an ophthalmologist with experience treating hormonal eye conditions for additional management. High-dose glucocorticoids, orbital decompression surgery, and ocular radiation therapy may be necessary (Lee, 2020a).
Thyroid storm (also called acute thyrotoxicosis or thyrotoxic crisis) is an acute, rare complication of hyperthyroidism that occurs when excessive amounts of T3, T4, and calcitonin are released into circulation. This can result from trauma or increased stress in a patient with preexisting Graves’ disease, toxic adenoma, or toxic multinodular goiter. Patients are also at risk during or immediately following a thyroidectomy due to the manipulation of the thyroid gland (Kelly, 2017; Kravets, 2016). Patients with hyperthyroidism can develop thyrotoxicosis following exposure to iodinated contrast, although this is a rare complication. The ACR (2020) does not recommend the restriction of contrast medium solely based on a history of hyperthyroidism but makes the following recommendations for two particular circumstances:
“In patients with acute thyroid storm, iodinated contrast medium exposure can potentiate thyrotoxicosis; in such patients, iodinated contrast medium should be avoided. Corticosteroid premedication in this setting is unlikely to be helpful;
In patients considering RAI ablation or in patients undergoing RAIU imaging of the thyroid gland, administration of iodinated contrast medium can interfere with the uptake of the treatment or diagnostic dose. If iodinated contrast medium was administered, a washout period is suggested to minimize this interaction. The washout period is ideally 3-4 weeks for patients with hyperthyroidism, and 6 weeks for patients with hypothyroidism” (ACR, 2020, p.7).
Symptoms of thyroid storm mirror those of hyperthyroidism but to a greater degree, such as heart failure, extreme tachycardia, hypertension, vomiting, diarrhea, severe hyperthermia, excessive perspiration, shock, neurocognitive changes (agitation, restlessness, confusion), coma, and possibly death. The APRN should be familiar with the signs and symptoms of thyroid storm, as early initiation of treatment is crucial to avoid fatality (Daniel, 2017; Kelly, 2017). A thyroid storm can result in death within 2 hours if untreated (Hopper, 2015). Thyroid storm is diagnosed clinically, and the Burch-Wartofsky point scale (BWPS) developed in 1993 can help the APRN assess a patient objectively. The BWPS assigns points for thermoregulatory dysfunction (based on hyperthermia), central nervous system disturbance (as evidenced by agitation, delirium, psychosis, seizure, or coma), gastrointestinal-hepatic dysfunction (as evidenced by nausea, vomiting, diarrhea, abdominal pain, or jaundice), cardiovascular dysfunction (as evidenced by tachycardia), congestive heart failure (as evidenced by pedal edema, rales, or pulmonary edema), atrial fibrillation, and precipitant history. A score under 25 is unlikely to represent a thyroid storm, a score of 25-44 indicates an impending storm, and a score over 45 indicates a thyroid storm (Kravets, 2016).
The treatment of thyroid storm consists of initial supportive therapies to manage the patient’s airway, oxygenation (supplemental oxygen to maintain an oxygen saturation over 95%), dehydration (IV fluid resuscitation), and hyperthermia (a cooling blanket and antipyretics). Salicylates (e.g., aspirin [Bayer]) should be avoided as they may increase free T4/T3 levels (Kravets, 2016). Acetaminophen (Tylenol) and ibuprofen (Motrin, Advil) may be administered to reduce the body temperature further for patients with severe hyperthermia (Hopper, 2015). Thionamides should be administered to inhibit thyroid hormone synthesis. Both methimazole (Tapazole) and propylthiouracil (PTU) can be administered orally, rectally, or via a nasogastric tube. Methimazole (Tapazole) can also be administered via IV and should be dosed at 20-40 mg every 8 hours. Propylthiouracil (PTU) should be dosed at 200 to 400 mg every eight hours (Kravets, 2016). Propylthiouracil (PTU) also inhibits the peripheral conversion of T4 to T3 and may achieve a euthyroid state more quickly than other drugs (Kelly, 2017). A saturated solution of potassium iodide (SSKI, ThyroShield) should be given orally (5 gtts every 6 hours) at least 1 hour after the thionamide to inhibit the release of any previously formed T3/T4. The beta-blockers esmolol (Brevibloc, 50-100 µg/kg/min IV), propranolol (Inderal, 60-80 mg PO every 4 hours), or metoprolol (Lopressor, 5-10 mg IV every 2-4 hours) should be administered to decrease the patient’s heart rate. Diltiazem (Cardizem) can also be administered IV to patients in whom beta-blockers are contraindicated. Hydrocortisone (Solu-Cortef) 100 mg IV every 8 hours should be given to patients in order to decrease the conversion of circulating T4 to T3. Finally, the underlying cause of the thyroid storm should be evaluated and managed (Kravets, 2016).
Hypothyroidism
Pathophysiology and Risk Factors
Hypothyroidism risk is increased in people with reduced size at birth or during childhood (Ross, 2019b). Hashimoto’s disease is characterized by chronic, autoimmune thyroiditis (Chaker et al., 2017). The body’s immune cells attack the thyroid, causing inflammation, which eventually leads to decreased hormone production (Mayo Clinic, 2020). Eventually, the patient develops a significantly decreased T3/T4 and an elevated TSH (Ledesma & Lawson, 2018). Patients with preexisting autoimmune diseases such as T1DM and celiac disease are more likely to develop Hashimoto’s hypothyroidism (ATA, n.d.c). Sex, family history, previous radiation exposure, and age are also risk factors for the development of this condition. It most commonly affects middle-aged women (Mayo Clinic, 2020). The two most common antibodies that cause thyroid dysfunction are thyroid peroxidase and thyroglobulin (ATA, n.d.c).
As mentioned in the section on hyperthyroidism, certain medications often affect thyroid function. Hypothyroidism can develop due to the destruction of the thyroid gland from certain drugs. Amiodarone (Cordarone) has a high iodine content and can destroy the thyroid gland, blocking T3, T4, and calcitonin production (Ross, 2018). Approximately 14% of patients taking amiodarone (Cordarone) develop hypothyroidism (Chaker et al., 2017). Lithium (Lithobid) may cause hypothyroidism by blocking thyroid hormone synthesis. Approximately 50% of patients taking it develop a goiter, usually within the first 2 years (Surks, 2019). Interferon-alpha (Intron A) and interleukin 2 (Proleukin) can also block the production of thyroid hormones (ATA, n.d.a).
Iatrogenic hypothyroidism may develop due to damage to the thyroid gland in response to radiation therapy, surgery, or the overuse of RAI ablation for hyperthyroidism. As previously mentioned, iodine deficiency is a cause of hypothyroidism that is significantly more common outside of the US. Without iodine, the thyroid cannot produce thyroid hormones. Subacute thyroiditis typically leads to mild transient hypothyroidism that does not require treatment, although some patients with subacute lymphocytic thyroiditis go on to develop permanent hypothyroidism (ATA, n.d.a; Lee, 2020b).
Congenital hypothyroidism (CH) develops during infancy due to a lack of thyroid hormone in fetal or neonatal life. In the US, all infants are screened for thyroid dysfunction at birth (Kelly, 2017). CH is typically due to an anatomical defect in the thyroid gland, a metabolic dysfunction of the thyroid, or iodine deficiency (Daniel, 2017).
Secondary hypothyroidism results from a failure to stimulate normal thyroid function and not the thyroid itself. Secondary hypothyroidism occurs due to hypothalamic or pituitary disease or trauma, resulting in diminished TRH or TSH secretion (ATA, n.d.a). Central hypothyroidism may also be associated with deficiencies in other pituitary hormones, such as antidiuretic hormone (ADH), oxytocin, prolactin (PRL), follicle-stimulating hormone (FSH), luteinizing hormone (LH), growth hormone (GH), and adrenocorticotropic hormone (ACTH; Duke Health, 2018). It may result from a tumor, an infection, an infarction, or a TBI causing damage to the hypothalamus or pituitary gland. Iatrogenic causes that may affect pituitary/hypothalamus function include previous radiation therapy or surgical trauma (Ross, 2019a; Shahid et al., 2020).
Regardless of the underlying pathology, a reduced circulating level of T3 and T4 leads to a slowed metabolic rate, reduced oxygen consumption, decreased oxidation of nutrients for energy, and decreased body heat (ATA, n.d.a).
Signs and Symptoms
The initial signs and symptoms of hypothyroidism directly relate to the slowing of the metabolic processes discussed above. Symptoms may be vague and nonspecific, with extreme fatigue as the most common initial indication (ATA, n.d.a). Clinical manifestations diverge depending on the severity and extent of thyroid deficiency, as well as the patient’s age at the time the deficiency is diagnosed (Chaker et al., 2017). The development of Hashimoto’s thyroiditis is gradual, so patients may be asymptomatic (ATA, n.d.c). Patients with Hashimoto’s disease may present with a goiter (DeGroot, 2016). The gradual development of symptoms is common unless hypothyroidism occurs after a thyroidectomy, after RAI ablation, or as a result of thionamide medications. The most common symptoms of hypothyroidism include weight gain, cold intolerance, lethargy, fatigue, dry and flaky skin, hoarseness, and constipation (Chaker et al., 2017). Additional symptoms are demonstrated in Figure 6. Typically, patients with central hypothyroidism have milder symptoms than patients with primary disease (Ross, 2019a).
Figure 6
Signs and Symptoms of Hypothyroidism
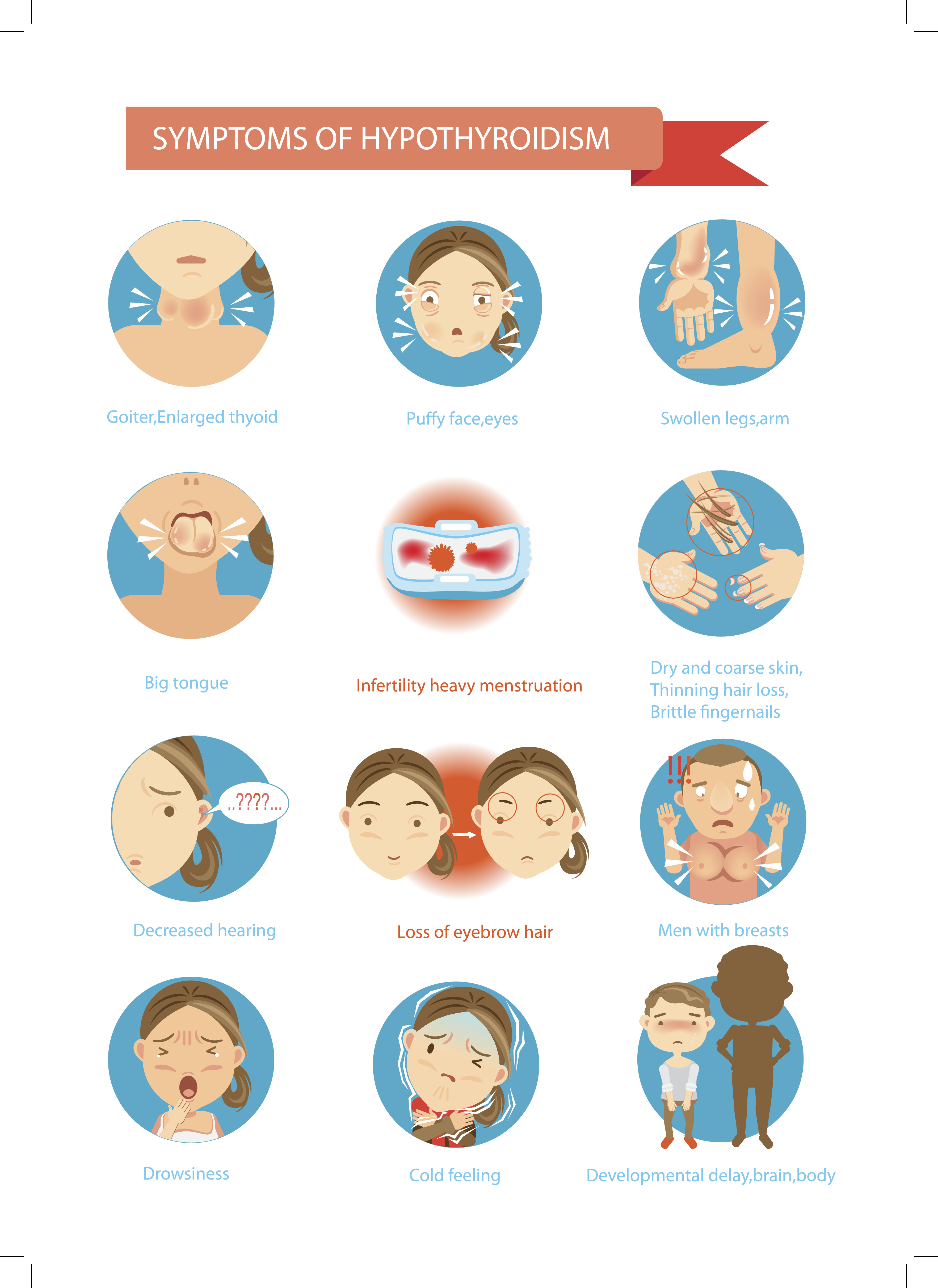
Patients may develop cognitive and personality changes; in geriatric patients, these symptoms may be attributed to aging. The new onset of these symptoms in a geriatric patient warrants an evaluation of thyroid function (Kelly, 2017). Patients with preexisting cardiac disease have a higher risk of cardiac complications. Kidney function may also be affected, leading to a reduced glomerular filtration rate (Chaker et al., 2017). In addition to the most common manifestations, patients with a diagnosis of hypothyroidism may also have specific organ-related manifestations (see Table 5).

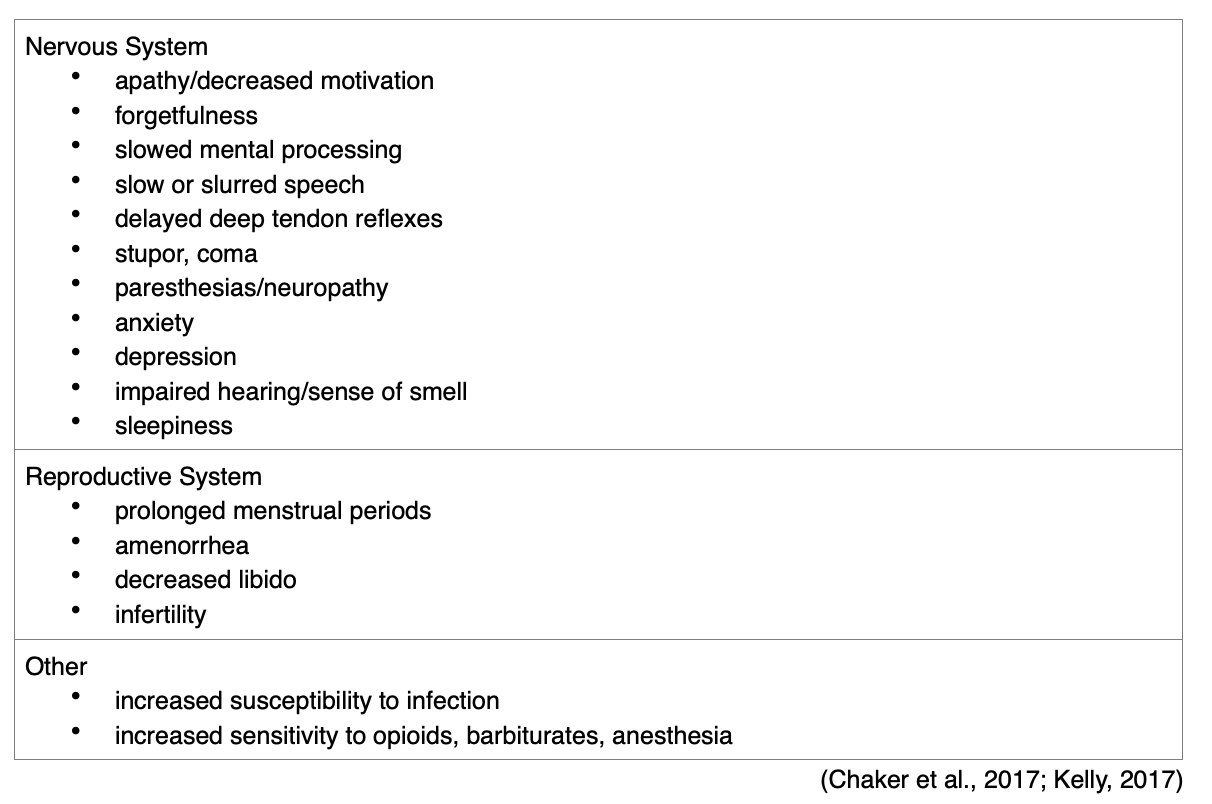
Babies with CH are typically born at term or post-term. If the condition is not recognized at birth and treated effectively, infants with CH typically present with:
jaundice;
hoarse crying;
respiratory issues;
decreased physical activity;
a large anterior fontanelle;
failure to thrive (poor feeding, inadequate weight gain/growth);
developmental delays;
constipation or decreased stools;
hypotonia;
course facial features;
umbilical hernia;
skin that is mottled, pale, dry, and cool;
goiter;
macroglossia (enlarged tongue); or
atrial or ventricular septal defects (Daniel, 2017).
A lateral knee radiograph may indicate a distal femoral epiphysis, which suggests prenatal hypothyroidism. If left untreated, the child may develop dystrophy of the bones and muscles, spasticity, gait abnormalities, inadequate growth, mutism, and mental deficiencies (Daniel, 2017; Willis, 2019). Patients with chronic, severe hypothyroidism may develop myxedema, which may appear as periorbital (around the eyes) edema, facial puffiness, or a masklike face (Kelly, 2017).
Diagnosis
In addition to the history and physical examination, a serum TSH level should be performed to establish a diagnosis of hypothyroidism as described previously for hyperthyroidism. If not performed concurrently as a panel of tests, a TSH level should be drawn first (ATA, n.d.b). If TSH is high, the thyroid gland is likely not producing enough T3/T4, which would raise the clinical suspicion for primary hypothyroidism. Conversely, if TSH is low, the thyroid may be producing too much T3/T4, raising clinical suspicion for hyperthyroidism or secondary hypothyroidism (ATA, 2019; BCGuidelines, 2018; Snyder, 2020). In most cases, the TSH level should be repeated for confirmation if it is initially abnormal, along with an FT4 if it has not already been done (ATA, n.d.b; Ross, 2019b). T3 testing is not clinically useful in patients with hypothyroidism, as both TSH and FT4 are typically abnormal earlier in this condition than the T3 level. Even patients with severe hypothyroidism may present with a T3 level that is within normal limits (ATA, 2019). Please refer to Table 2 for common reference ranges for thyroid function tests. In primary hypothyroidism, the patient’s TSH is typically elevated, and their thyroid hormone levels are lower than expected. With secondary hypothyroidism, the TSH level is typically normal or slightly decreased, and the T4 level often decreases (Ross, 2019b). For the most common thyroid conditions classified by TSH and FT4 values, please refer to Table 3 (ATA, n.d.b). The differential diagnoses that should be considered or ruled out in a patient with an elevated TSH level include recent recovery from a nonthyroidal illness (if recently recovered, consider repeating labs in 4-6 weeks), a TSH-secreting pituitary adenoma (these patients typically present with elevated TSH and FT4 levels), primary adrenal insufficiency, or a rare resistance to TSH or T3/T4 (Ross, 2019b). A CBC and metabolic profile should also be completed. Patients with hypothyroidism may exhibit:
anemia, especially macrocytic anemia;
dilutional hyponatremia;
hyperlipidemia and elevated triglycerides; and
elevations of transaminases, creatinine kinase, and alkaline phosphates (Orlander, 2019; Ross, 2019b).
An ECG may be indicated and may demonstrate sinus bradycardia, flat or inverted T-waves, or low-voltage QRS complexes (ATA, n.d.a; Hopper, 2015). In addition, adrenal function must be fully assessed via a fasting morning serum cortisol level, potentially requiring an ACTH stimulation test, prior to initiating corrective treatment for secondary hypothyroidism. Both conditions (central hypothyroidism and adrenal insufficiency) often occur simultaneously, and the correction of hypothyroidism without first initiating treatment for adrenal insufficiency may trigger a life-threatening adrenal crisis (Ross, 2019a). Thyroid scanning using technetium-99m, I-123, or an ultrasound can help provide information about the etiology of the disease, as previously described in the section regarding hyperthyroidism (Daniel, 2017).
Thyroid antibody tests—TPO, as explained earlier—are performed to determine if thyroid disease is autoimmune, such as in Hashimoto’s disease. In patients with suspected Hashimoto disease, anti-thyroglobulin antibody (Tg) testing may also be performed to confirm the diagnosis, as these patients typically have high levels of TPO and Tg (ADA, n.d.c; MedlinePlus, 2020). The presence of TSI or TRAb strongly supports a diagnosis of Graves’ disease (DeGroot, 2016).
Management
Monotherapy for most cases of hypothyroidism should begin with a low dose of levothyroxine (Synthroid), which is a synthetic form of T4, in those with a TSH > 10 μU/mL (10 mU/L; Haker et al., 2017, USPSTF, 2015). The typical initial dose for an adult with mild to moderate hypothyroidism is 50-75 µg/day, although a younger adult may tolerate starting at the goal dose, and an older adult may be started at 25 µg. The goal of treatment is to replace the hormone that is no longer being made by the thyroid. Clinical benefits should occur within 5 days of initiating the medication and level off in about 4 weeks (Orlander, 2019). Optimal results from levothyroxine (Synthroid) may take up to 8 weeks (Kelly, 2017). Initial dosing and pediatric dosing may also be calculated using body weight. Most adult patients under the age of 50 with minimal endogenous thyroid function require between 1.6 and 1.8 µg/kg of body weight or an average maintenance dose of 100-200 µg daily. There is some discrepancy about whether this should be calculated using actual or ideal body weight. The maximum dose is 200 µg daily. Levothyroxine should be taken first thing in the morning on an empty stomach (Orlander, 2019; Skidmore-Roth, 2015).
A patient’s TSH level should be rechecked 6 to 10 weeks after treatment initiation or dose changes if they have been diagnosed with primary hypothyroidism. Levothyroxine (Synthroid) dosing should be adjusted every 4 to 8 weeks based on the patient’s TSH level. Once stabilized, patients should have their TSH checked at least annually thereafter. The APRN should consider adding exogenous T3 in the form of liothyronine (Cytomel) if symptoms persist despite a stable TSH level within the desired range (Kelly, 2017; Orlander, 2019). This may be especially helpful for patients who have undergone total thyroidectomy. A combination product with levothyroxine/liothyronine (Armour Thyroid) may add convenience for patients requiring both hormones. However, patients should be cautioned about naturally occurring inconsistencies leading to fluctuations in hormone levels with the combination product, as it is derived from the thyroid of pigs (Mayo Clinic, 2020).
Dosing for secondary hypothyroidism should be weight-based initially and then adjusted to keep the patient’s FT4 in the upper normal reference range per the treatment guidelines from the ATA (Snyder, 2019). The FT4 and the patient’s report of symptoms should be the primary indicators for dose adjustments in central hypothyroidism, as the TSH level is not recommended for continuous monitoring of maintenance therapy (Ross, 2019a). Treatment with glucocorticoids for adrenal insufficiency should begin before or concurrently with thyroid hormone replacement but not after (Snyder, 2019).
Special dosing considerations apply to certain patient populations. Children may require more frequent TSH level assessments and dosing changes as they grow to avoid mental and physical complications (Orlander, 2019). Patients with thyroid cancer often require a levothyroxine (Synthroid) dose of 2.1- 2.7 µg/kg. Adults under the age of 50 may only require 25-50 µg daily (Orlander, 2019; Skidmore-Roth, 2015). Patients with ischemic heart disease should start at a quarter to half of the suggested adult starting dose (Orlander, 2019). Pregnant patients should be universally screened for signs and symptoms of thyroid dysfunction and previous use of thyroid medications. Pregnant patients diagnosed with hypothyroidism should be treated with levothyroxine (Synthroid) based on the patient’s TSH level, as shown in Table 6. Serum TSH should be drawn every 4 weeks during the first 20 weeks of pregnancy in those newly diagnosed with hypothyroidism. Monitoring in the second half of pregnancy should be based on the patient’s symptoms and prior laboratory testing consistency. Those who were previously diagnosed and on stable levothyroxine (Synthroid) dosing prior to pregnancy should be instructed to take two additional doses per week spread several days apart (e.g., Sunday and Thursday) once pregnancy is confirmed (Alexander et al., 2017; Orlander, 2019).

The primary risk of levothyroxine is overdosing or underdosing. If too much thyroid hormone is taken, signs and symptoms of hyperthyroidism will result. If too little is taken, the clinical manifestations of hypothyroidism will persist. During annual clinical evaluations, a patient on levothyroxine (Synthroid) therapy should be evaluated for the classic symptoms of hypothyroidism or hyperthyroidism (Orlander, 2019).
The APRN should explain to patients that levothyroxine (Synthroid) is not a cure but a lifelong treatment requirement. It is absorbed in the small intestine and should be administered on an empty stomach—typically in the morning before breakfast—as a single, daily dose. Oral antacids, iron, or calcium supplements should be separated by at least 4 hours from levothyroxine (Synthroid) administration. The APRN should counsel patients to promptly report any neurologic excitability or cardiac symptoms (including palpitations) (Skidmore-Roth, 2015). Patients should be counseled regarding:
the importance of maintaining lifelong thyroid hormone replacement and consistent follow-up care;
avoiding cold temperatures;
performing proper skincare/hygiene, avoiding perfumed soaps and body wash, and using fragrance-free lotion to moisturize their skin daily to prevent skin breakdown and lesions;
managing constipation by gradually increasing physical activity, dietary fiber, OTC stool softeners, adequate hydration, and developing regular bowel patterns;
monitoring for signs and symptoms of bleeding or blood loss in patients taking anticoagulants, as thyroid hormone replacement increases this risk;
monitoring heart rate daily and digoxin levels periodically in patients taking digoxin (Lanoxin), as thyroid hormone replacement may interact with this medication and decrease or increase circulating digoxin levels;
informing all healthcare providers of their condition and all current medications to avoid potential interactions;
discussing the use of any over-the-counter (OTC) medications, including herbal and other supplements, with their health care team for approval prior to starting to avoid products that may interact negatively with their thyroid medications
avoiding commercial weight loss products;
maintaining a balanced diet (ATA, n.d.a; Kelly, 2017).
Infants are treated with oral levothyroxine (Synthroid) that is initially dosed at 10-15 µg/kg/day or 50 µg daily. Pills should be crushed in a small amount of liquid (breastmilk, formula, or water) and administered orally via syringe. Dosing should be adjusted slowly to avoid thyrotoxicosis. In infants, this may present with perspiration, agitation, restlessness, loss of consciousness, and hypertension, in addition to the previously described symptoms of acute thyrotoxicosis. Toddlers can be allowed to chew pills (Daniel, 2007).
Complications
If not properly managed, the potential complications of hypothyroidism include:
bleeding tendencies;
benign intracranial hypertension;
atherosclerosis, decreased peripheral circulation, ischemic heart disease, heart failure, cardiomegaly, and other cardiac complications;
deafness;
infertility;
GI dysfunction, such as pernicious anemia, achlorhydria (lack of hydrochloric acid), megacolon, or obstruction;
iron-deficiency anemia;
psychiatric/mood disorders;
myxedema crisis/coma (ATA, n.d.a; Hopper, 2015).
Myxedema coma was first described in the late 1900s as a result of long-standing, untreated hypothyroidism. It is now a rare complication, yet it remains a medical emergency with a mortality rate of 40% (Chaker et al., 2017). Myxedema crisis and coma typically occur in patients with undiagnosed hypothyroidism. These effects may also be precipitated by infection, certain types of drugs (e.g., opioids, barbiturates, and sedatives), extreme cold exposure, or trauma (Kelly, 2017). Patients who develop myxedema crisis will typically have altered cognition, progressive lethargy, bradycardia, bradypnea, hypoglycemia, decreased cardiac output, and hypothermia (body temperature under 95°F). These manifestations can progress to multisystem organ failure, coma, and death if immediate treatment with IV thyroid hormone therapy is not initiated (Chaker et al., 2017; Hopper, 2015). The APRN should be familiar with the signs of myxedema crisis, as the early initiation of treatment is crucial to avoid fatality (Kelly, 2017). Additional testing to diagnose myxedema can include:
random serum cortisol to assess adrenal function (if this test is within the normal range, an ACTH stimulation test is needed to assess function);
sodium level (typically indicates hyponatremia with low serum osmolality);
serum creatinine level (usually elevated); and
serum glucose (expected hypoglycemia) (Eledrisi, 2018).
Diagnostic tools such as the one developed by Chiong and colleagues (2015) can screen for myxedema coma. This tool incorporates six criteria, which are:
heart rate,
body temperature,
Glasgow coma scale score,
TSH level,
free thyroxine index (FTI), and
precipitating factors like infection, medication noncompliance, furosemide (Lasix) use, cold exposure, hypoglycemia, GI bleeding, heart failure, hypercapnia, or a cerebrovascular event (Chiong et al., 2015).
A single dose of 200-500 µg (4 µg/kg) of IV levothyroxine (Synthroid) should be administered at the time of myxedema crisis diagnosis to avoid coma, followed by 50-100 µg daily (1.2 µg/kg/d) until the patient can tolerate medications by mouth. It should be diluted to 100 µg/mL with 0.9% sodium chloride (NaCl), mixed well, and given through a three-way stop cock at a maximum rate of 100 µg/minute. Levothyroxine (Synthroid) is incompatible with the majority of other IV medications and should not be added to another infusion. The patient should be monitored closely for changes in vital signs, chest pain, tachycardia, neurological hyperexcitability, and ongoing thyroid laboratory values. If the patient is taking an anticoagulant, their prothrombin time (PT) should also be monitored, as levothyroxine (Synthroid) can interact and cause excessive bleeding (Epocrates, n.d.b; Jonklaas et al., 2014; Skidmore-Roth, 2015). IV glucocorticoid therapy should be added to the initial therapy for myxedema crisis (Jonklaas et al., 2014). Yasir and colleagues (2020) have suggested an initial dose of 1000 mg of methylprednisolone (Medrol). Once the patient can tolerate medications by mouth, levothyroxine (Synthroid) dosing should continue at the standard daily oral replacement rate (1.6 µg/kg/day) or adjusted based on their TSH results. Additionally, liothyronine (Cytomel) may be given in addition to levothyroxine (Synthroid). A 5-20 µg loading dose of liothyronine (Cytomel) followed by a 2.5-10 µg maintenance dose every 8 hours is recommended; lower doses are recommended for smaller or older patients or those with a history of coronary artery disease or arrhythmias (Jonklaas et al., 2014).
Future Research/Directions
Over the past century, thyroid research studies have found that patients with hypothyroidism are prone to overtreatment. According to McAninch and Bianco (2016), several patients in early trials were given large doses of replacement therapy, resulting in adverse effects (e.g., angina and psychosis). Dose reductions corrected these adverse effects (McAninch & Bianco, 2016). According to the ATA (n.d.c), thyroid research funding has not increased in the last 20 years.
References
Alexander, E. K., Pearce, E. N., Brent, G. A., Brown, R. S., Chen, H., Dosiou, C., Brobman, W. A., Laurberg, P., Lazarus, J. H., Mandel, S. J., Peeters, R. P., & Sullivan, S. (2017). 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid, 27(3), 315-389. https://doi.org/10.1089/thy.2016.0457
American Board of Internal Medicine. (2019). ABIM laboratory test reference ranges- January 2019. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf
American College of Obstetricians and Gynecologists. (2017). ACOG committee opinion: Guidelines for diagnostic imaging during pregnancy. Obstetrics & Gynecology, 130(4), e210-e216. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2017/10/guidelines-for-diagnostic-imaging-during-pregnancy-and-lactation.pdf
American College of Radiology. (2019). ACR-SNMMI-SPR practice parameter for the performance of scintigraphy and uptake measurements for benign and malignant thyroid disease. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Thy-Scint.pdf
American College of Radiology. (2020). ACR manual on contrast media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
American Thyroid Association. (n.d.a). Hypothyroidism (underactive). Retrieved July 16, 2020, from https://www.thyroid.org/hypothyroidism/
American Thyroid Association. (n.d.b). Thyroid function tests. Retrieved May 29, 2020, from https://www.thyroid.org/thyroid-function-tests/
American Thyroid Association. (n.d.c). Thyroid information. Retrieved July 15, 2019, from https://www.thyroid.org/thyroid-information/
American Thyroid Association. (2019). Thyroid function tests FAQ. https://www.thyroid.org/wp-content/uploads/patients/brochures/thyroid_function_tests_faq.pdf
American Thyroid Association. (2020). Low iodine diet. https://www.thyroid.org/low-iodine-diet/
BCGuidelines. (2018). Thyroid function testing in the diagnosis and monitoring of thyroid function disorder. https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/bc-guidelines/thyroid-function-testing.pdf
Chaker, L., Bianco, A. C., Jonklaas, J., & Peeters, R. P. (2017). Hypothyroidism. The Lancet, 390, 1550-1562. https://doi.org/10.1016/s0140-6736(17)30703-1
CFCF. (2014). Thyroid and parathyroid. [Image]. https://commons.wikimedia.org/wiki/File:Illu_thyroid_parathyroid.jpg
Daniel, M. S. (2017). Congenital hypothyroidism. https://emedicine.medscape.com/article/919758-overview#a1
DeGroot, L. J. (2016). Diagnosis and treatment of Graves’ disease. Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK285548/
De Leo, S., Lee, S. Y., & Braverman, L. E. (2016). Hyperthyroidism. Lancet, 388, 906-918. https://doi.org/10.1016/s0140-6736(16)00278-6.
Dieleman, J. L., Baral, R., Birger, M., Bui, A. L., Bulchis, A., Chapin, A., Hamavid, H., Horst, C., Johnson, E. K., Joseph, J., Lavado, R., Lomsadze, L., Reynolds, A., Squires, E., Campbell, M., DeCenso, B., Dicker, D., Flaxman, A. D., Gabeert, R., . . . Murray, C. J. L. (2016). US spending on personal health care and public health., 1996-2013. JAMA, 316(24), 2627-2646. https://doi.org/10.1001/jama.2016.16885
Duke Health. (2018). Thyroid disorders in children. https://www.dukehealth.org/pediatric-treatments/pediatric-endocrinology/thyroid-disorders-children
Eledrisi, M. S. (2018). Myxedema coma or crisis differential diagnoses. https://emedicine.medscape.com/article/123577-differential
Epocrates. (n.d.a). Calcium gluconate. Retrieved October 30, 2020, from https://online.epocrates.com/results?query=calcium%20gluconate
Epocrates. (n.d.b). Levothyroxine. Retrieved November 5, 2020, from https://online.epocrates.com/results?query=levothyroxine
Epocrates. (n.d.c). Methimazole. Retrieved November 5, 2020, from https://online.epocrates.com/results?query=methimazole
Epocrates. (n.d.d). Propylthiouracil. Retrieved November 5, 2020, from https://online.epocrates.com/results?query=propylthiouracil
Gill, R. S. (2020). Pretibial myxedema. https://emedicine.medscape.com/article/1103765-overview
Hopper, P. D. (2015). Understanding medical surgical nursing (5th ed.). FA Davis.
Institute for Quality and Efficiency in Health Care. (2018). How does the thyroid gland work? https://www.ncbi.nlm.nih.gov/books/NBK279388/
Jonklaas, J., Bianco, A. C., Bauer, A. J., Burman, K. D., Cappola, A. R., Francesco, S. C., Cooper, D. S., Kim, B. W., Peeters, R. P., Rosenthal, M. S., & Sawka, A. M. (2014). Guidelines for the treatment of hypothyroidism. Thyroid, 24(12). https://doi.org/ 10.1089/thy.2014.0028
Kelly, K. A. (2017). Endocrine problems. In Medical surgical nursing assessment and management of clinical problems (10th ed., pp. 1162-1171). Elsevier.
Kemp, S. (2019). Anatomy of the endocrine system. https://www.emedicinehealth.com/anatomy_of_the_endocrine_system/article_em.htm
Kravets, I. (2016). Hyperthyroidism: Diagnosis and treatment. American Family Physician, 93(5), 363–370.
Lee, S. L. (2020a). Hyperthyroidism and thyrotoxicosis. https://emedicine.medscape.com/article/121865-overview
Lee, S. L. (2020b). Subacute thyroiditis. https://emedicine.medscape.com/article/125648-overview
Li, C. Y., & Yu, S. F. (2008). Exopthalmos [image]. https://commons.wikimedia.org/wiki/File:Cherubism.jpg
Lonnemann, E. (n.d.). Metabolic/endocrine disorders. Retrieved July 16, 2020, from https://www.physio-pedia.com/Metabolic/Endocrine_Disorders
Mayo Clinic. (2020). Hashimoto's disease. https://www.mayoclinic.org/diseases-conditions/hashimotos-disease/symptoms-causes/syc-20351855
McAninch, E. A., & Bianco, A. C. (2016). Correction: History and future of treatment of hypothyroidism. Annals of Internal Medicine, 164(5), 376. https://doi.org/10.7326/m15-1799.
MedlinePlus. (2020). Thyroid antibodies. https://medlineplus.gov/lab-tests/thyroid-antibodies/
Myupchar.com. (2019). Goiter [image]. https://commons.wikimedia.org/wiki/File:A_woman_suffering_from_Goiter.png
National Institute of Biomedical Imaging and Bioengineering. (2019). What is nuclear medicine? https://www.nibib.nih.gov/sites/default/files/Nuclear%20Medicine.pdf
OpenStax College. (2013). The endocrine system. [Image]. https://commons.wikimedia.org/wiki/File:1801_The_Endocrine_System.jpg
Orlander, P. R. (2019). Hypothyroidism. https://emedicine.medscape.com/article/122393-overview
RadiologyInfo.org. (2019). Thyroid scan and uptake. https://www.radiologyinfo.org/en/info.cfm?pg=thyroiduptake
Ross, D. S., Burch, H. B., Cooper, D. S., Greenlee, C., Laurberg, P., Maia, A. L., Rivkees, S. A., Samuels, M., Sosa, J. A., Stan, M. N., & Walter, M. A. (2016). 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid, 26(10). 1343-1421. http://doi.org/10.1089/thy.2016.0229
Ross, D. S. (2018). Amiodarone and thyroid dysfunction. UpToDate. https://www.uptodate.com/contents/amiodarone-and-thyroid-dysfunction
Ross, D. S. (2019a). Central hypothyroidism. UpToDate. https://www.uptodate.com/contents/central-hypothyroidism
Ross, D. S. (2019b). Diagnosis of and screening for hypothyroidism in nonpregnant adults. UpToDate. https://www.uptodate.com/contents/diagnosis-of-and-screening-for-hypothyroidism-in-nonpregnant-adults
Ross, D. S. (2020). Radioiodine in the treatment of hyperthyroidism. UpToDate. https://www.uptodate.com/contents/radioiodine-in-the-treatment-of-hyperthyroidism
Shahid, Z., Asuka, E., & Singh, G. (2020). Physiology, hypothalamus. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK535380/
Shoman, M. (2019). An overview of thyroid disease in men. https://www.verywellhealth.com/thyroid-disease-in-men-3886166
Skidmore-Roth, L. (2015). Mosby’s drug guide for nursing students. Elsevier/ Mosby.
Snyder, P. J. (2019). Treatment of hypopituitarism. UpToDate. https://www.uptodate.com/contents/treatment-of-hypopituitarism
Snyder, P. J. (2020). Clinical manifestations of hypopituitarism. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-of-hypopituitarism
Society of Nuclear Medicine & Molecular Imaging. (n.d.). Fact sheet: What is nuclear medicine and molecular imaging? Retrieved on April 17, 2020 from https://www.snmmi.org/AboutSNMMI/Content.aspx?ItemNumber=5648
Surks, M. I. (2019). Lithium and the thyroid. UpToDate. https://www.uptodate.com/contents/lithium-and-the-thyroid
Taylor, P. N., Albrecht, D., Scholz, A., Gutierrez-Buey, G., Lazarus, J. H., Dayan, C. M., & Okosieme, O. E. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nature Reviews Endocrinology, 14(5), 301–316. https://doi.org/10.1038/nrendo.2018.18
U.S. Preventive Services Task Force. (2015). Screening for thyroid dysfunction: Recommendation statement. American Family Physician, 91(11). 790A-790F. https://www.aafp.org/afp/2015/0601/od1.pdf
Willis, L. M. (2019). Professional guide to diseases (11th ed.). Wolters Kluwer.
Wisse, B. (2020). Radioactive iodine uptake. https://medlineplus.gov/ency/article/003689.htm
Wong, K. K., Shulkin, B. L., Gross, M. D., & Avram, A. M. (2018). Efficacy of radioactive iodine treatment of graves’ hyperthyroidism using a single calculated 131I dose. Clinical Diabetes and Endocrinology, 4(1). https://doi.org/10.1186/s40842-018-0071-6
Yani, H. (2019). Differential diagnosis of thyrotoxicosis. Journal of Endocrinology & Metabolism, 9(5), 127-132. https://doi.org/10.14740/jem600
Yasir, M., Goyal, A., Bansal, P., & Sonthalia, S. (2020). Corticosteroid adverse effects. [StatPearls]. https://www.ncbi.nlm.nih.gov/books/NBK531462/