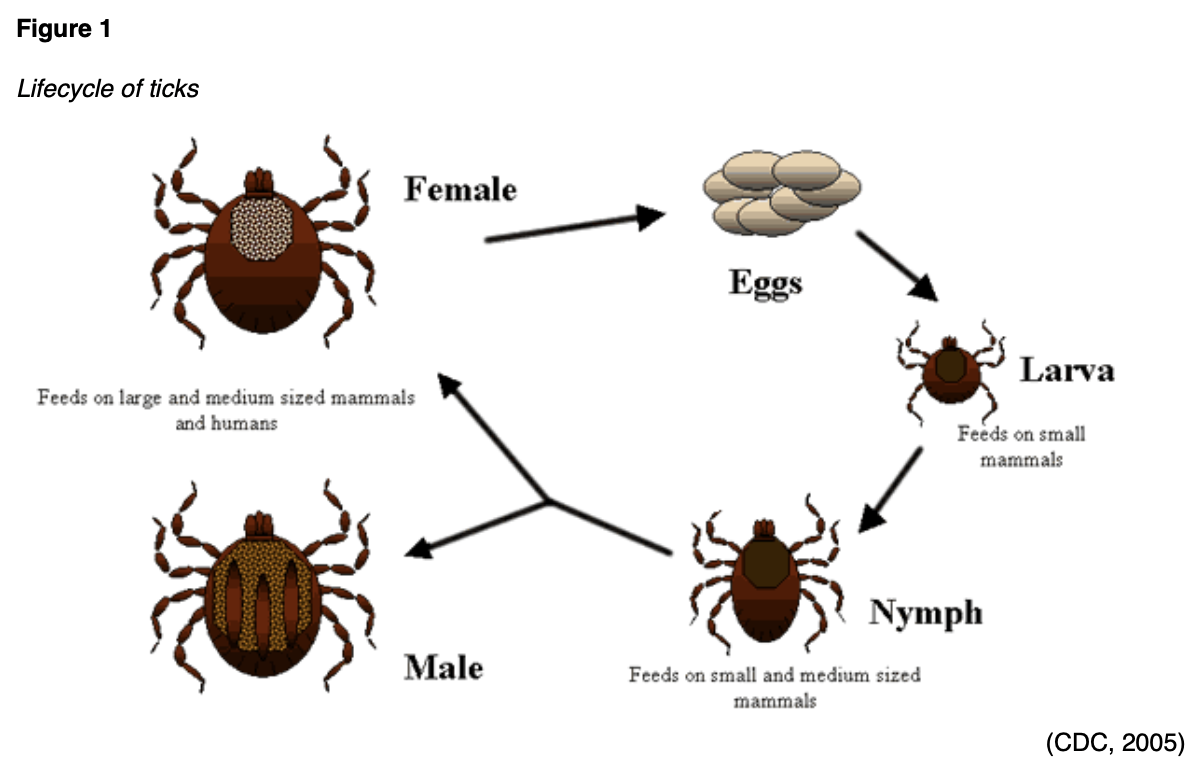
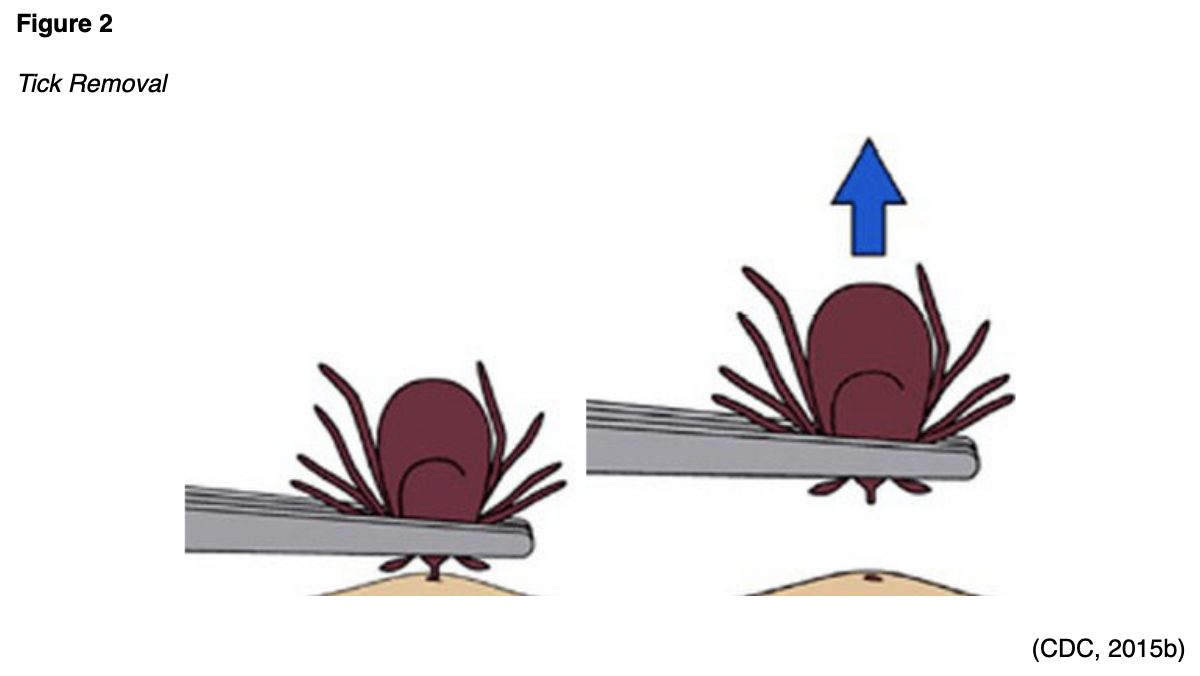
The purpose of this course is to examine the most common types of tickborne diseases prevalent throughout the US, reviewing the epidemiology, transmission, clinical presentation, diagnostic criteria, and treatment modalities from the CDC.
...purchase below to continue the course
applicators, consumers, and the environment. According to the CDC and FIFRA, when N, N-diethyl-meta-toluamide (DEET) products are used as directed on the manufacturer's label, they are not considered to be harmful, and only in rare cases are they associated with skin irritation (EPA, 2017).
The CDC (2019f) also recommends treating clothing and other outdoor gear such as hiking boots, sneakers, backpacks, and so forth with products containing 0.5% permethrin. According to the US Department of Health and Human Services (USDHHS, 2018) Tick-Borne Disease Working Group, clothing treated with permethrin has been shown to provide extended protection from blacklegged tick and lone star tick bites. Following outdoor exposure, individuals are encouraged to perform a full body check, examining their clothing and bodies for ticks. One risk reduction strategy that is associated with reduced risk of acquiring LD and other common types of TBDs is to remove clothing immediately and shower within two hours of outdoor exposure. A full-body skin check should be performed using a hand-held or full-length mirror for proper inspection of all body parts. Common hiding spots for ticks on the human body include the following areas: under the armpits, back of the knees, between the legs, around the waist, inside the belly button, and in and around the ears. Ticks also like to hide throughout the scalp, in and around the hair. Individuals are advised to wash clothing in hot water immediately following outdoor exposure. Tumble drying clothing on high heat for at least 10 minutes (or until fully dry) helps to kill ticks that may be hiding on clothing. The water temperature must be hot for maximum effect, as cold and medium temperature water will not kill ticks (CDC, 2019f).
Aside from clothing, another highly common portal for the transmission of ticks is on household pets, which can then later attach themselves to humans. Since dogs are particularly vulnerable to tick bites and TBDs, owners should carefully examine dogs for ticks following each outdoor exposure. Essential areas to assess include in and around the dog's ears, around the tail and eyelids, under the collar and front legs, as well as between the back legs and toes. However, tick bites can be even more challenging to detect on dogs due to their heavy coats of hair and fur obscuring visualization of many areas of the dog's skin. Therefore, the CDC also advises owners to use tick preventative products on their dogs to reduce the chances that a tick bite will make the dog ill. Similar to humans, if a tick is identified on a dog, it is essential to remove it as soon as possible (CDC, 2019h).
Further, individuals can take preventative measures to reduce tick burden in the yard, such as with the use of pesticides, to aid in a tick-safe zone to minimize the risk for tick bites in both humans and dogs. Before spraying pesticides, the CDC (2019g) recommends that users check with their local health or agricultural officials regarding the best time to spray pesticides in the designated geographical area, the appropriate type of pesticide to use, as well as the regulations regarding pesticide application on residential properties. Furthermore, additional landscape recommendations include mowing the lawn frequently, removing leaf litter, creating barriers (such as fences) to prevent deer or raccoons from entering the yard, and clearing tall grasses or bushes around the home (CDC, 2019g).
Tick Bite Prophylaxis
It is not recommended that antibiotic treatment be used following a tick bite as a means to prevent TBDs, such as anaplasmosis, babesiosis, RMSF, or other rickettsial diseases. However, according to the Infectious Disease Society of America (IDSA), in geographic areas that are highly endemic for LD, a single dose of doxycycline (Doryx) may be appropriate as a means of antimicrobial prophylaxis in non-pregnant adults and children older than eight years of age. As per the IDSA recommendations, to prescribe antimicrobial prophylaxis the estimated time of attachment must be 36 hours or greater, prophylaxis must be started within 72 hours of tick removal, and LD must be common in the county or state in which the affected individual lives or recently traveled to (CDC, 2018d).
Lyme Disease
LD is the most common and frequently diagnosed TBD, with about 300,000 reported cases in the US each year. The number of counties in the US that are considered to be of high incidence for LD has increased by over 300% in the Northeastern states and by nearly 250% in North-Central states (USDHHS, 2018). Approximately 10 to 20% of patients with LD experience chronic and disabling symptoms, and direct costs to the US healthcare system reach $1.3 billion per year (USDHHS, 2018). LD is primarily transmitted by the blacklegged tick (Ixodes scapularis) or the western blacklegged tick (Ixodes pacificus). The blacklegged tick (Ixodes scapularis) is otherwise known as the deer tick and transmits the spirochete borrelia burgdorferi and B. mayonii, both of which are known to cause LD. These ticks are the culprit for the majority of LD infections in the US, are most prevalent in the warmer months, and as demonstrated in Figure 3, they are widely spread through the eastern US. The western blacklegged tick (Ixodes pacificus) is found along the Pacific coast, primarily in northern California, although rates of infection are usually low (~1%) in adults. (CDC, 2019c).

The incubation period of LD can range from 3 to 30 days following a bite by an infected tick. Early-stage symptoms may include erythema migrans (EM), the classic bullseye rash, which is a ring-like rash with central clearing that slowly expands its perimeter as demonstrated in Figure 4. The rash occurs in up to 80% of infected persons, begins at the site of the tick bite about seven days following a bite, and expands gradually over several days. It may be warm to the touch but is rarely associated with pruritus or skin lesions. Other early symptoms may include flu-like syndromes such as fatigue, headache, fever, arthralgia, myalgias, and lymphadenopathy. These symptoms may develop, persist, and progress for days or months following the tick bite, leading to the disseminated (later) stage of the condition. The disseminated stage can be characterized by multiple secondary annular rashes, and progressive symptoms affecting various body systems, especially the musculoskeletal, neurologic, and cardiac systems. Rheumatologic and musculoskeletal manifestations of LD may include Baker's cysts, severe joint pain, redness, and swelling that most prominently affect the large joints, but also migratory arthritis and effusion in one or more joints. If these symptoms are left untreated, patients can experience chronic or recurrent episodes of arthritis in the joints. Cardiac manifestations include conduction abnormalities, myocarditis, pericarditis, shortness of breath, and dizziness. Neurologic manifestations may consist of Bell's palsy (paralysis of one side of the face), inflammation of the brain and spinal cord (meningitis, encephalopathy, encephalitis), and nerve pain, including peripheral neuropathy (CDC, 2018d).

There are several types of testing available for LD; however, the CDC (2019k) endorses a two-step antibody testing protocol, which can be performed using the same blood sample. The testing evaluates for immunoglobulin M (IgM) or immunoglobulin G (IgG) LD antibodies in the serum sample using a sensitive enzyme immunoassay (EIA) or immunofluorescence assay, followed by a western immunoblot assay for specimens yielding positive or equivocal results (CDC, 2019k). On routine laboratory testing, patients with LD may demonstrate elevated erythrocyte sedimentation rates (ESR) and mild hepatic transaminases (CDC, 2018d).
The preferred first-line treatment for LD in adults is antibiotic therapy with doxycycline (Doryx). When treatment is initiated early in the course of the disease, patients usually recover quickly and completely within two to four weeks. The standard treatment regimens for uncomplicated LD are as follows:
Pharmacological Management of Adults
- doxycycline (Doryx) 100 mg by mouth twice daily for 10 to 21 days (preferred regimen);
- cefuroxime axetil (Ceftin) 500 mg by mouth twice daily for 14 to 21 days; or
- amoxicillin (Amoxil) 500 mg by mouth three times per day for 14 to 21 days;
Pharmacological Management of Children
- amoxicillin (Amoxil) 50 mg/kg/day by mouth, divided into three doses for 14 to 21 days;
- doxycycline (Dorxy) 4mg/kg/day by mouth, divided into two doses for 10 to 21 days;
- cefuroxime axetil (Ceftin) 30 mg/kg/day by mouth, divided into two doses for 14 to 21 days (CDC, 2018d).
For more complex LD cases, such as those with facial palsy or when meningitis is suspected, a lumbar puncture may be required to evaluate the cerebral spinal fluid (CSF) for the presence of infection. Intravenous antibiotic therapy may be required for these patients, as well as those with other severe symptoms, including cardiac involvement, or those with manifestations of late LD, such as Lyme arthritis that does not respond to oral antibiotic therapy. Intravenous antibiotics are recommended only if the arthritis does not improve with oral treatment and is typically prescribed for up to 28 days. Ceftriaxone (Rocephin) is the preferred antibiotic for neurologic LD in the US and is administered at 2 g IV daily for 14–28 days (CDC, 2019d).
Post-treatment Lyme disease syndrome (PTLDS) is manifested by a persistent constellation of symptoms, including fatigue, headache, joint pain, musculoskeletal aches, and cognitive difficulties that can last for a significant period, usually more than six months, following the completion of treatment. Symptoms can range from mild to severe, and the etiology and management of these symptoms are poorly understood. There is, unfortunately, no treatment proven effective for PTLDS, and efforts are focused on managing symptoms (Hu, 2019).
Anaplasmosis
The pathogen, Anaplasma phagocytophilum, causes anaplasmosis. It is primarily transmitted through the Western blacklegged tick (Ixodes pacificus) and the blacklegged tick (Ixodes scapularis), both of which are also known to transmit LD. This is why coinfection with anaplasmosis and LD is not uncommon. Therefore, anaplasmosis is more common in the geographic areas in which LD is endemic, such as the Pacific coast of the US, particularly northern California, and throughout the eastern US (CDC, 2019c). The incubation period is 5 to 14 days, and symptoms usually begin within one to two weeks following an infected tick bite. Early signs and symptoms are generally mild to moderate and commonly include any or several of the following: fever, chills, rigors, severe headache, myalgias, malaise, nausea, vomiting, diarrhea, and anorexia. Patients may experience a skin rash, although this is relatively rare, occurring in less than 10% of cases. Therefore, the presence of a rash in a patient with suspected anaplasmosis may be an indication that the patient has a coinfection with LD. Clinical manifestations of late-stage illness may include hypoxia and respiratory failure, organ failure, bleeding problems, and death. Given the heightened risk for morbidity and mortality with late-stage illness, treatment with appropriate antibiotic therapy should be not be delayed in patients who present with clinical signs suggestive of anaplasmosis. Diagnosis is often made based on the signs and symptoms of the condition and is later confirmed with definitive diagnosis through laboratory testing. Early recognition and timely initiation of treatment are essential. Routine laboratory work typically indicates anemia, thrombocytopenia, leukopenia, and mild to moderate elevations in hepatic transaminases. Blood-smear microscopy may indicate the presence of morulae in the cytoplasm of granulocytes. Polymerase chain reaction (PCR) amplification of DNA extracted from a whole blood specimen is the most sensitive during the first week of illness. A negative PCR result does not rule out anaplasmosis. An indirect immunofluorescence antibody (IFA) assay for IgG using A. phagocytophilum antigen is the standard serologic test for diagnosis of anaplasmosis. These assays should be collected two to four weeks apart to evaluate for seroconversion (change from negative to positive) (CDC, 2019a).
Doxycycline (Doryx) is the recommended treatment for all patients with suspected anaplasmosis infections, including both adults and children, and it is the most effective at preventing complications when started early in the course of the illness. Adults should receive a dose of 100 mg twice daily, either orally or intravenously, for 10-14 days. Children weighing less than 100 pounds should receive a dose of 2.2 mg/kg, twice daily, up to a maximum of 100 mg per dose, for 10-14 days. Doxycycline (Doryx) is a broad-spectrum antibiotic derived from the tetracycline class, which is a group of antibiotics that are associated with staining of permanent teeth in young children. However, when doxycycline (Doryx) is prescribed at the recommended dose and duration required to treat anaplasmosis, there has been no evidence that is causes staining of permanent teeth when given before the age of eight (CDC, 2018d).
Rocky Mountain Spotted Fever (RMSF)
RMSF belongs to a group of related conditions known as spotted fever group rickettsioses. RMSF is caused by the pathogen Rickettsia rickettsii and is transmitted by the Rocky Mountain wood tick (Dermacentor andersoni), the American dog tick (Dermacentor variabilis), and the Brown dog tick (Rhipicephalus sanguineus). The Rocky Mountain wood tick (Dermacentor andersoni) is found within the Rocky Mountain states and southwestern Canada in elevations of 4,000 to 10,500 feet. The American dog tick (Dermacentor variabilis), sometimes referred to as the wood tick, is widely dispersed in areas east of the Rocky Mountains and found in isolated areas along the Pacific Coast. The Brown dog tick (Rhipicephalus sanguineus) is found worldwide, as demonstrated in Figure 5, but primarily transmits RMSF in the southwestern portion of the US and along the US-Mexico border. While dogs are the primary host of the Brown dog tick, it can also directly bite humans or other mammals (CDC, 2019c).

RMSF can be transmitted after only 2 to 20 hours of being bitten by an infected tick, with symptoms developing within 3 to 12 days. Early symptoms include high fever, severe headache, malaise, myalgias, nausea, vomiting, anorexia, and edema around the eyes and on the back of the hands. A rash usually appears between two and four days following the onset of the fever, although nearly 10% of patients with RMSF never develop a rash. The rash is initially maculopapular, with small, flat, pink, non-pruritic macules on the wrists, forearms, and ankles, but eventually spreads to the trunk, palm, and soles. Around day six or later, the rash usually changes to a petechial rash, which is considered a sign of progression to severe disease. Late symptoms, such as those occurring around a week after the initial onset, may include an altered mental status and respiratory compromise. In the most severe cases, damage to blood vessels may lead to extremity necrosis requiring amputation, cerebral edema, coma, and multiorgan system damage (CDC, 2019i).
A definitive diagnosis of RMSF is based on laboratory testing. However, due to the potential for rapid disease progression and the severity of the condition, antibiotic therapy should not be delayed in patients presenting with clinical signs concerning for RMSF. The goal is to start antibiotic therapy before the development of the petechial rash, as this is more likely to prevent fatal outcomes, particularly when treatment is started within the first five days of symptom onset. In early illness, routine laboratory results are often within normal limits, but as the condition progresses, thrombocytopenia, elevated hepatic transaminases, and hyponatremia are common. Detection of DNA in a skin biopsy specimen of a rash lesion by PCR assay, or through a whole blood specimen during the acute phase, can definitely diagnose RMSF. Some state health departments offer new pan-Rickettsia and R. rickettsia-specific PCR assays. Otherwise, diagnosis can be confirmed via demonstration of a four-fold change (typically rise) in IgG-specific antibody titer by IFA assay in paired serum samples. The first sample should be collected within the first week of illness, and the second sample should be collected two to four weeks later. However, antibody titers are frequently negative in the first 7-10 days of illness. A third option is the immunohistochemical (IHC) staining of the organism from a skin or tissue biopsy specimen (CDC, 2018d, 2019i).
Similar to anaplasmosis and LD, doxycycline (Doryx) is the first-line, mainstay treatment for RMSF in patients of all ages. The duration of therapy should continue for at least three days after the fever subsides and until evidence of clinical improvement. The CDC (2018d) recommends that doxycycline (Doryx) is continued for at least five to seven days. Patients with RMSF usually respond well to treatment, and rarely are there long-term health complications or persistent infections. However, a small subset of patients who recover from the illness may have permanent damage to the extremities, which may be manifested as neuropathy, paralysis, or amputation (CDC, 2019i).
Babesiosis
Babesiosis is caused by the microscopic parasite, Babesia microti, which infects red blood cells (RBCs) and can cause severe disease. Babesia microti is most commonly carried by the blacklegged tick (Ixodes scapularis) and is found in the Northeast and upper areas of the Midwest, particularly New England, New York, New Jersey, Wisconsin, and Minnesota (CDC, 2019c).
The incubation period of babesiosis can range from 1 to 9 weeks. While the clinical manifestations may develop several weeks following exposure, some individuals may develop symptoms many months later. Babesiosis can vary dramatically in severity, ranging from asymptomatic infection to a life-threatening condition (CDC, 2018d). Some individuals may experience mild flu-like symptoms such as fever, chills, headache, body aches, anorexia, abdominal pain, nausea, or generalized fatigue. In contrast, others may endure more serious effects, including significant destruction to their RBCs, causing hemolytic anemia, jaundice, hepatosplenomegaly, and dark urine. Less commonly, patients may develop a sore throat, cough, photophobia, and emotional lability. Those at heightened risk for becoming severely ill include the elderly, those without a spleen, the immunocompromised (such as from cancer, organ transplant, or HIV), or those who have other serious, chronic conditions such as diabetes or liver, cardiac, or renal disease (CDC, 2018a). For patients who are acutely ill, routine laboratory testing may demonstrate decreased hematocrit (due to hemolytic anemia), thrombocytopenia, elevated levels of liver enzymes, blood urea nitrogen (BUN), and creatinine. Elevated hepatic transaminase levels have been reported. Urine sampling may show proteinuria, hematuria, and hemoglobinuria. Babesia parasites can be detected by light-microscopic examination of peripheral blood smears in acutely ill patients or positive Babesia (or B. microti) PCR analysis. IFA testing using B. microti parasites as antigen detects antibodies in 88-96% of patients with B. microti infection, but the presence of IgG antibodies does not reliably distinguish between acute and prior infection (CDC, 2017, 2018d)
While the majority of asymptomatic individuals do not require treatment, ill patients are treated with a combination of two medications for a duration of at least 7 to 10 days. Adult patients with mild to moderate babesiosis should be given atovaquone (Mepron) 750 mg by mouth twice daily plus azithromycin (Zithromax) daily for a total of 7 to 10 days of combined therapy, Those with severe illness may be treated with IV clindamycin (Cleocin) plus quinine (Qualaquin) by mouth (CDC, 2019e). In addition to the above definitive treatments, acutely ill patients often require supportive care therapies such as antipyretics or blood transfusions. Critically ill patients may require vasopressors, mechanical ventilation, or dialysis (CDC, 2019e). Complications of untreated babesiosis can include hypotension, thrombocytopenia, disseminated intravascular coagulation (DIC), which can lead to blood clots and bleeding, malfunction of vital organs, and death (CDC, 2018a).
Ehrlichiosis
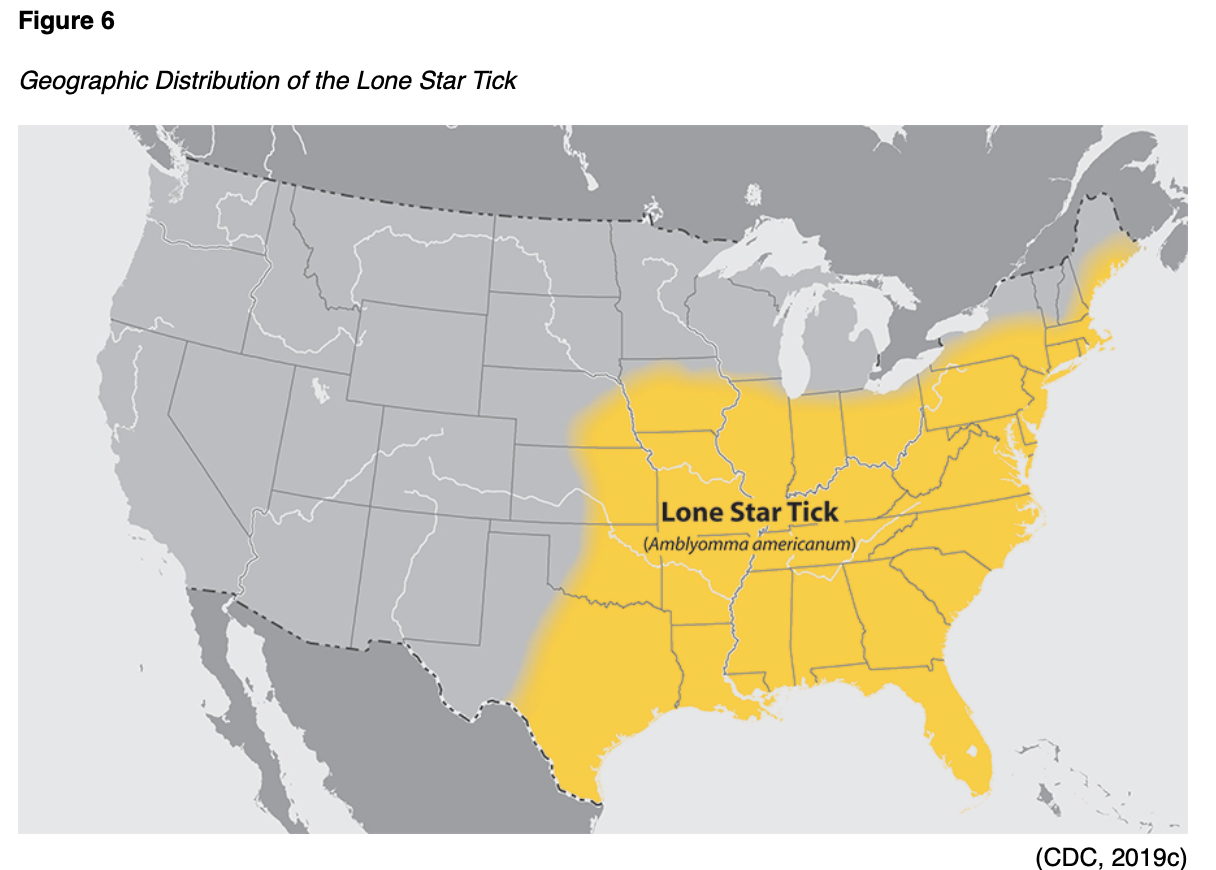
Ehrlichiosis can be caused by the pathogen, Ehrlichia muris eauclairensis, most commonly carried by the blacklegged tick (Ixodes scapularis), or by Ehrlichia chaffeensis and Ehrlichia ewingii, which are carried by the Lone star tick (Amblyomma americanum). As demonstrated in Figure 6, the Lone star tick (Amblyomma americanum) is widely distributed in the southeastern and eastern US and is considered a very aggressive tick that acquired its name from the classic white dot on its back. Individuals who are bit by the Lone star tick can develop skin irritation, redness, and discomfort at the site of the bite, but these symptoms are considered a local reaction. They do not necessarily indicate an infection (CDC, 2019c).
While E. muris eauclairensis and E. ewingii have no reported deaths, E. chaffeensis is considered the most serious and is linked to fatalities. The incidence of E. chaffeensis increases with age. However, the highest rates of fatality are among children under ten years, and adults 70 years and older. The incubation period for ehrlichiosis is 5 to 14 days. The clinical presentation is similar to several other types of TBDs, exhibiting the classic early symptomology of fevers, chills, malaise, headache, myalgias, nausea, vomiting, diarrhea, and anorexia. Other symptoms may include altered mental status, confusion, and skin rash. The rash begins about five days after the onset of symptoms and is more common in children, affecting up to 60% of children and less than 30% of adults. More commonly associated with E. chaffeensis pathogen, the rash is generally non-pruritic, spares the face, and can range from maculopapular to petechial in presentation. Severe illness can develop if treatment is not initiated promptly, leading to serious manifestations such as meningitis, meningoencephalitis, acute respiratory distress syndrome, liver or renal failure, septic shock-like syndromes, and coagulopathies. Those at increased risk for severe illness include the elderly and the very young, as well as those with living immunocompromised conditions or taking immune-suppressing medications (CDC, 2019b).
Confirmation of the diagnosis is premised on laboratory testing. However, the CDC (2018d) supports the early initiation of antibiotic therapy in patients with high clinical suspicion for the illness. Early findings (during the first week of the disease) include thrombocytopenia, absolute leukopenia, and mild-to-moderately elevated hepatic transaminase on laboratory results. Anemia is also reported in about 50% of cases but is usually seen later in the illness. Microscopic examination of peripheral blood smear during the first week of illness may demonstrate morulae (microcolonies of Ehrlichia) in the cytoplasm of white blood cells and is highly suggestive of the diagnosis, but this method should not be relied upon solely due to its relative insensitivity. PCR amplification performed on whole blood specimen is the most sensitive during the first week of the illness. The standard serologic test for diagnosis of ehrlichiosis is an IFA assay for IgG performed on paired samples two to four weeks apart to demonstrate fourfold seroconversion. Antibody titers are usually negative during the first week of the illness. Culture isolation and IHC assays of Ehrlichia species are only available at specialized laboratories (CDC, 2018d, 2019b).
Doxycycline (Doryx) is the first-line treatment for ehrlichiosis in both adults and children. According to the CDC (2019b), patients who are treated early in the disease trajectory with the appropriate course of doxycycline (Dobryx) usually recover rapidly and completely. Treatment duration is advised to continue until at least three days after the fever subsides, and until evidence of clinical improvement. Typically, patients are treated for five to seven days. While the CDC (2019b) acknowledges that patients who experience a more severe illness trajectory may require IV antibiotics, they do not offer guidelines delineating which IV antibiotics are appropriate.
Tickborne Relapsing Fever (TBRF)
TBRF is caused by the pathogens Borreilia hermsii and B. turicatae and is most commonly transmitted through infected "soft ticks" that belong to the genus Ornithodoros such as O. hermsi ticks, O. parkeri ticks, and O. turicata ticks. These ticks are most commonly found within the mountainous regions of the Western parts of the US. O. hermsi is responsible for the most cases of TBRF in the US and are most commonly found at high altitudes of 1500 to 8000 feet, where they feed on tree squirrels and chipmunks. O. parkeri and O. turicata are generally found at lower altitudes in the Southwest region, where they dwell in caves and the burrows of squirrels, prairie dogs, and owls.
TBRF is more common in the summer months when people are vacationing in the mountains and sleeping in rustic cabins. It is also known to occur in the winter when fires are started to warm the cabin, activating ticks that are hiding in the walls and woodwork. The incubation period of TRBF is about seven days, and the classic triad of symptoms include high fever (i.e., 103 °F), muscle and joint aches, and headache. These symptoms follow a distinct pattern of febrile episodes that last about three days, followed by a seven-day afebrile period, followed by another three-day period of fever, as demonstrated in Figure 7. If not appropriately treated with antibiotics, this pattern can recur several times. Less commonly, patients may develop nausea, vomiting, a macular or petechial rash, jaundice, hepatosplenomegaly, and photophobia (CDC, 2015a).

Mildly increased serum bilirubin and mild to moderate thrombocytopenia may be present on routine blood work along with elevated ESR and slightly prolonged prothrombin time (PT) and partial thromboplastin time (PTT). With regards to diagnosing TBRF, organisms are best detected in the blood by microscopy or culture that is obtained during the febrile period of the illness. Spirochetes are more readily detected by microscopy in symptomatic, untreated patients early in the course of infection. Borrelia spirochetes may be identified on the peripheral blood smear, as they often reach high concentrations (>106 spirochetes/ml). erologic testing is not standardized, and results may vary based on the laboratory. If obtained early in infection, results may be falsely negative, so it is essential to also obtain a serum sample during the convalescent period (at least 21 days after symptom onset) (CDC, 2018e).
The treatment of TBRF is primarily antibiotics, using tetracycline (Sumycin) or erythromycin (Erythrocin). Ceftriaxone (Rocephin) may be used in adults with central nervous system involvement. Erythromycin (Erythrocin) is recommended in children (CDC, 2018d), Patients treated for TBRF should be monitored closely for Jarisch-Herxheimer reactions during the first two to four hours of antibiotic therapy. A Jarisch-Herxheimer reaction is a systemic response believed to be caused by the release of endotoxin-like substances into the bloodstream as a result of antibiotic treatment causing cellular lysis. As the bacterial cells are lysed or broken up and destroyed, endotoxin-like substances are dispersed throughout the bloodstream. When this occurs, a temporary inflammatory response ensues within the body leading to symptoms of fevers, chills, rigors, hypotension, tachycardia, and diaphoresis. The reaction usually lasts less than four hours, but may persist up to 24 hours, and is most commonly associated with the administration of tetracycline antibiotics. It is usually not seen with subsequent dosing of the same antibiotic and is most often a self-limiting condition that resolves without any significant morbidity. However, rare complications can include cardiovascular collapse, acute respiratory distress syndrome requiring intubation, and death. Treatment is limited to the management of symptoms; however, the use of acetaminophen (Tylenol), corticosteroids, and non-steroidal anti-inflammatory (NSAIDs) do not appear to modify the symptoms or the patient outcomes (Butler, 2017).
Tularemia
Tularemia is caused by the pathogen Francisella tularensis, and this can be transmitted by the American dog tick (Dermacentor variabilis), the Lone star tick (Amblyomma americanum), or the Rocky Mountain wood tick (Dermacentor andersoni). It is widely distributed east of the Rocky Mountains, southwestern Canada from elevations of 4,000 to 10,500 feet, as well as in discrete areas on the Pacific Coast. It carries an average incubation period of three to five days, but the range can extend up to 21 days. Tularemia is also referred to as "rabbit fever" or "deer fly fever," as rabbits, hares, and rodents are particularly susceptible to the illness (CDC, 2018d).
Tularemia can be difficult to diagnose as its clinical presentation can vary, and it is not solely a tickborne illness. People can become infected by several modes of transmission, such as skin contact with infected animals, drinking contaminated water, laboratory exposure, or inhaling contaminated agricultural or landscaping dust and aerosols. Symptoms can be mild to life-threatening and vary based on how the bacteria enter the body. General symptoms can include chills, headache, fatigue, malaise, myalgia, anorexia, sore throat, chest discomfort, cough, abdominal pain, nausea, and diarrhea. The six main forms of tularemia and their associated symptomology are outlined in Table 2. However, all forms of illness are accompanied by a fever, which can range from mild to severe, but not above a temperature of 104 °F (CDC, 2018f).

The diagnosis of tularemia is made through clinical suspicion based on signs and symptoms, travel history, and blood and culture results. In routine laboratory results, the leukocyte count and ESR may be normal or elevated along with thrombocytopenia, hyponatremia, elevated hepatic transaminases, elevated creatinine phosphokinase. Cultures may indicate isolation of F. tularensis from clinical specimen. Diagnosis may also be made via detection of F. tularensis in a clinical specimen by direct immunofluorescence assay (DFA) or PCR assay, a f our-fold or greater change in serum antibody titer to F. tularensis antigen between acute and convalescent specimens, or a single positive antibody titer to F. tularensis antigen (CDC, 2018b, 2018d).
Tularemia is treated with antibiotic therapy for 10 to 14 days. Streptomycin, which is only available in the generic formulation, is considered first-line therapy due to efficacy and the Food and Drug Administration (FDA) approval. Gentamicin (Genoptic) is an acceptable alternative; however, it is noted to have a lower success rate, and is not FDA-approved for this indication. Both streptomycin and gentamicin (Genoptic) require dose adjustments for patients with renal insufficiency. Ciprofloxacin (Cipro) and other fluoroquinolones are also not FDA-approved for the treatment of tularemia but have shown good efficacy in humans. Doxycyline (Doryx) may also be used for a duration of 14-21 days (CDC, 2018b, 2018d).
Powassan Virus Disease (POW)
POW originates from a family of viruses known as Flavivirdae, and the genus Flavivirus comprises more than 50 viral species worldwide. Tickborne flaviviruses (TBFVs) are more commonly found in subtropical areas of the Northern Hemisphere and less prominently in subtropical regions of Africa and Australia (Kemenesi & Banyai, 2019). POW is the only tickborne flavivirus known to occur in the US, commonly carried by the blacklegged tick (Ixodes scapularis) (CDC, 2019c). It is related to some mosquito-borne viruses such as the West Nile virus, and although it is rare, most cases in the US occur in the northeast and the Great Lakes geographic region during the height of tick season: between late spring and mid-fall (Fatmi et al., 2017).
The incubation period is one to four weeks. Initial signs and symptoms of the illness may include fever, headache, vomiting, and generalized weakness. While some people can experience very mild or no symptoms at all, others can be inflicted with severe disease, including encephalitis or meningitis. Symptoms of encephalitis and meningitis can include severe headache with nausea or vomiting, anorexia, confusion or difficulty concentration, photophobia, seizures, lethargy, altered mental status, seizures, aphasia, paresis, movement disorders, or cranial nerve palsies. Diagnosis is primarily made through IgM antibody testing available at the CDC and some state health departments, as commercial testing for POW is limited. PCR testing may detect viral RNA in acute CSF specimens or tissue, but sensitivity is inconclusive, and this method is not advised to rule out the diagnosis. Cross reaction with other flaviviruses, such as West Nile or Dengue, can occur. There is no specific antiviral treatment for POW currently available, so those affected should receive supportive care therapies for symptom management (CDC, 2018b, 2018d).
References
Butler, T. (2017). The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: A review of recent cases and our understanding of pathogenesis. American Journal of Tropical Medicine and Hygiene, 96(1), 46-52. https://doi.org/10.4269/ajtmh.16-0434
The Centers for Disease Control and Prevention. (n.d.). Guidelines for DEET insect repellent use. Retrieved March 7, 2020 from https://www.cdc.gov/malaria/toolkit/DEET.pdf
The Centers for Disease Control and Prevention. (2005). Lifecycle of ticks [image]. https://commons.wikimedia.org/wiki/File:Life_cycle_of_ticks_family_ixodidae.PNG
The Centers for Disease Control and Prevention. (2007). Erythema migrans rash in Lyme disease [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Erythema_migrans_-_erythematous_rash_in_Lyme_disease_-_PHIL_9875.jpg
The Centers for Disease Control and Prevention. (2015a). Tickborne relapsing fever. https://www.cdc.gov/relapsing-fever/index.html
The Centers for Disease Control and Prevention. (2015b). Tick removal [image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Tickremoval.png
The Centers for Disease Control and Prevention. (2017). Babesiosis. https://www.cdc.gov/dpdx/babesiosis/index.html
The Centers for Disease Control and Prevention. (2018a). Babesiosis. https://www.cdc.gov/parasites/babesiosis/disease.html
The Centers for Disease Control and Prevention. (2018b). For clinicians: Diagnostic testing for Tularemia. https://www.cdc.gov/tularemia/clinicians/index.html
The Centers for Disease Control and Prevention. (2018c). Record number of tickborne diseases reported in U.S. in 2017. https://www.cdc.gov/media/releases/2018/s1114-record-number-tickborne-diseases.html
The Centers for Disease Control and Prevention. (2018d). Tickborne diseases of the United States: A reference manual for healthcare providers. (5th ed.). https://www.cdc.gov/ticks/tickbornediseases/TickborneDiseases-P.pdf
The Centers for Disease Control and Prevention. (2018e). Tickborne relapsing fever: Information for clinicians. https://www.cdc.gov/relapsing-fever/clinicians/index.html
The Centers for Disease Control and Prevention. (2018f). Tularemia signs and symptoms. https://www.cdc.gov/tularemia/signssymptoms/index.html
The Centers for Disease Control and Prevention. (2019a). Anaplasmosis. https://www.cdc.gov/anaplasmosis/index.html
The Centers for Disease Control and Prevention. (2019b). Ehrlichiosis – Information for healthcare providers. https://www.cdc.gov/ehrlichiosis/healthcare-providers/index.html
The Centers for Disease Control and Prevention. (2019c). Geographic distribution of ticks that bite humans. https://www.cdc.gov/ticks/geographic_distribution.html
The Centers for Disease Control and Prevention. (2019d). Lyme disease treatment. https://www.cdc.gov/lyme/treatment/index.html
The Centers for Disease Control and Prevention. (2019e). Parasites - Babesiosis: Resources for health professionals. https://www.cdc.gov/parasites/babesiosis/health_professionals/index.html#tx
The Centers for Disease Control and Prevention. (2019f). Preventing tick bites. https://www.cdc.gov/ticks/avoid/on_people.html
The Centers for Disease Control and Prevention. (2019g). Preventing ticks in the yard. https://www.cdc.gov/ticks/avoid/in_the_yard.html
The Centers for Disease Control and Prevention. (2019h). Preventing ticks on your pets. https://www.cdc.gov/ticks/avoid/on_pets.html
The Centers for Disease Control and Prevention. (2019i). Rocky mountain spotted fever (RMSF). https://www.cdc.gov/rmsf/index.html
The Centers for Disease Control and Prevention. (2019j). Tick bite: What to do. https://www.cdc.gov/ticks/pdfs/FS_TickBite-508.pdf
The Centers for Disease Control and Prevention. (2019k). Updated CDC recommendation for serologic diagnosis of lyme disease. https://www.cdc.gov/mmwr/volumes/68/wr/mm6832a4.htm?s_cid=mm6832a4_w
Cowles, J. (2018). Amazing arachnids. Princeton University Press.
Eickhoff, C., & Blaylock, J. (2017). Tickborne diseases other than Lyme in the United States. Cleveland Clinic Journal of Medicine, 84(7), 555-567. https://doi.org/10.3949/ccjm.84a.16110
Fatmi, S. S., Zehra, R., & Carpenter, D. O. (2017). Powassan virus- A new reemerging tick-borne disease. Frontiers in Public Health, 5(342), 1-12. https://doi.org/10.3389/fpubh.2017.00342
Hu, L. (2019). Patient education: Lyme disease treatment (beyond the basics). UpToDate. https://www.uptodate.com/contents/lyme-disease-treatment-beyond-the-basics#H6
Kemenesi, G., & Banyai, K. (2019). Tick-borne flavivirus, with a focus on Powassan Virus. Clinical Microbiology Reviews, 32(1), 1-29. https://doi.org/10.1128/CMR.00106-17
US Department of Health and Human Services. (2018). Tick-borne disease working group: 2018 report to congress. https://www.hhs.gov/sites/default/files/tbdwg-report-to-congress-2018.pdf
US Environmental Protection Agency. (2017). DEET. https://www.epa.gov/insect-repellents/deet#product