About this course:
The purpose of this course is to inform APRN practice and help clinicians recognize the risk factors and clinical features of uterine (endometrial) cancer to diagnose women as early as possible and provide optimal care, patient education, and support throughout the disease trajectory.
Course preview
The purpose of this module is to inform APRN practice and help clinicians recognize the risk factors and clinical features of uterine (endometrial) cancer to diagnose women as early as possible, improve clinical outcomes, and provide optimal care, patient education, and support throughout the disease trajectory.
By the completion of this learning activity, the APRN should be able to:
- discuss the epidemiology of uterine cancer in the US and risk factors for the development of the disease
- discuss the anatomy of the uterus and the pathophysiology leading to the development of uterine cancer
- identify the signs and symptoms, diagnostic workup, and uterine cancer subtypes
- Discuss the components of the diagnostic workup, uterine cancer subtypes, and the core components of cancer staging
- describe the management of uterine cancer, including an overview of treatment risks, side effects, and the elements of patient education
Uterine (endometrial) cancer is the most common gynecologic malignancy in the US. According to the American Cancer Society (ACS, 2020), approximately 65,620 women will be diagnosed with uterine cancer in 2020, and about 12,590 will die from the disease. The condition is commonly referred to as endometrial cancer (EC), as more than 92% of uterine cancers begin in the endometrium (the lining of the uterus). Historically a disease solely affecting postmenopausal women, this cancer has become increasingly prevalent in younger, premenopausal women. This increased incidence is linked to the obesity epidemic plaguing the nation. When diagnosed early and managed effectively, it is a highly treatable condition, as there are currently more than 600,000 survivors in the US. APRNs must remain informed on the disease’s clinical features and warning signs to expedite a timely diagnosis and reduce morbidity and mortality (American College of Obstetricians and Gynecologists [ACOG], 2018b; Centers for Disease Control and Prevention [CDC], 2019a; Siegel et al., 2020).
*Module disclaimer: Since the vast majority of uterine cancers are endometrial in origin, the information in this course refers to EC unless otherwise specifically stated.
Epidemiology
EC represents 3.6% of all new cancer diagnoses in the US. According to the latest data from the Surveillance, Epidemiology, and End Results Program (SEER, 2020), the annual age-adjusted incidence of EC is 27.8 per 100,000 people. Approximately 3.1% of women are affected during their lifetime. Most frequently diagnosed in postmenopausal women aged 55 to 64 years, the median age at diagnosis is 63 years. EC is slightly more common among non-Hispanic White women (NHW; 28.3 per 100,000) than non-Hispanic Black women (27.9 per 100,000). Between 2007 and 2016, the incidence rate increased by 1% per year among NHW and 2% per year among Black women. It is the sixth most common cause of cancer death among women in the US, with the highest deaths among women aged 65 to 74. The 5-year survival rate of localized EC (i.e., cancer that has not spread outside of the uterus) is 95%, declining to 17% for those with distant metastases (i.e., cancer spreading to distant organs or sites outside the uterus). Black women are more likely to be diagnosed with more aggressive EC and have reduced survival compared to NHW; the 5-year survival rates for NHW and Black women are 84% and 62%, respectively (SEER, 2020). Siegel and colleagues (2020) highlight improved cancer survival rates across almost all cancers over the last five decades, except for endometrial and cervical cancers. A lack of significant treatment advances for recurrent and metastatic disease accounts for the dismal survival.
Pathophysiology
The uterus, also known as the womb, is the hollow, pear-shaped organ that provides protection and support for the developing fetus. Positioned between the urinary bladder and the rectum, the uterus is a thick-walled and muscular structure comprised of three major parts: the fundus, uterine corpus (body), and cervix. The basic anatomy of the female reproductive system is displayed in Figure 1. The fundus is the curved uppermost region that serves as a connection point for the fallopian tubes. The corpus is the largest part of the uterus, responsible for the bulk of its size, and is the usual site of implantation. The cervix is the fibromuscular lower portion that connects the uterine cavity to the vagina (Ameer et al., 2020; McCance & Heuther, 2019).

The cervix has two narrow openings at each end, as demonstrated in Figure 2. It enables the passage of sperm into the uterine cavity through dilation of the external os (external orifice) and the internal os (internal orifice). During labor, the cervix opens (dilates) to allow for the passage of the neonate through the birth canal. The uterus often varies in size and shape depending on the woman’s reproductive phase and response to sex hormones. The average non-pregnant adult uterus measures 6 to 8 cm in length, 5 cm in width, and 2.5 cm in thickness, but during pregnancy, it can enlarge up to five times its size. The uterus is substantially larger in parous women (i.e., those who have given birth) than nulliparous women (i.e., those who have never carried a pregnancy to term). After menopause (the permanent cessation of menstruation), the uterus shrinks to its nulliparous size (Ameer et al., 2020; McCance & Heuther, 2019). According to ACOG (2020b), the average age of menopause is 51 years.

The uterus is comprised of three major inner layers: the endometrium, myometrium, and perimetrium, as outlined in Table 1 and shown in Figure 3.
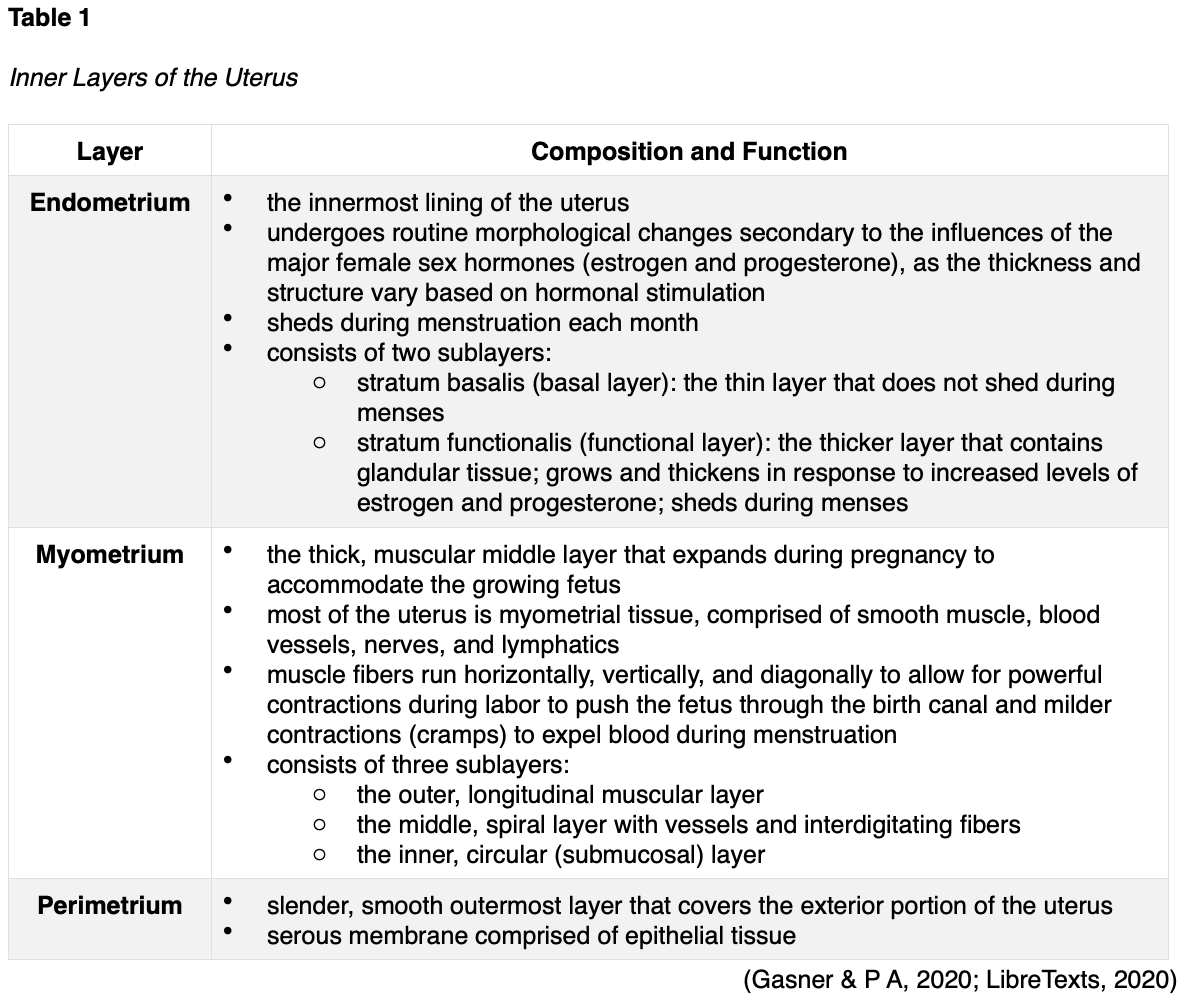
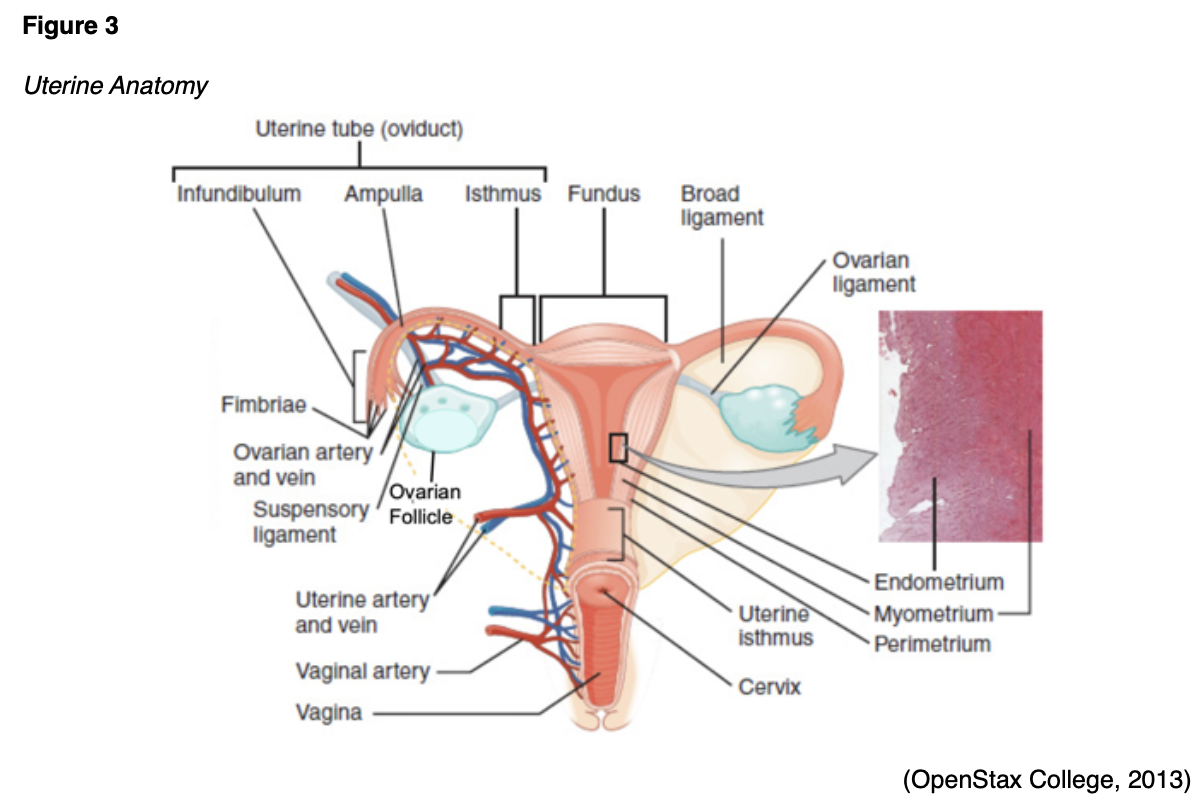
The uterine tubes (also called fallopian tubes) are ducts that transport the oocyte (immature egg cell) from the ovary to the uterus. Several ligaments uphold the uterus’s position within the pelvic cavity. The broad ligament is the primary support, extending laterally on both sides of the uterus and attaching to the pelvic wall. The round ligament is embedded within the broad ligament, extends downward to the vagina, and pulls the uterus forward to maintain its anteverted positioning. Most women have an anteverted uterus, which is tilted forward at the cervix toward the abdomen. The uterosacral ligaments extend from the posterior aspect of the cervix and vagina and support the uterus and pelvic organs posteriorly. The uterine artery is the main blood supply to the uterus, with a smaller blood supply from the ovarian artery. The uterine artery arises from the internal iliac artery and divides into ascending and descending branches, as demonstrated above in Figure 3. The ascending branch supplies the corpus and tubes. The descending (vaginal) branch supplies the cervix and upper vagina in conjunction with the vaginal artery, which arises on the lateral wall of the vagina (LibreTexts, 2020).
Uterine Physiology
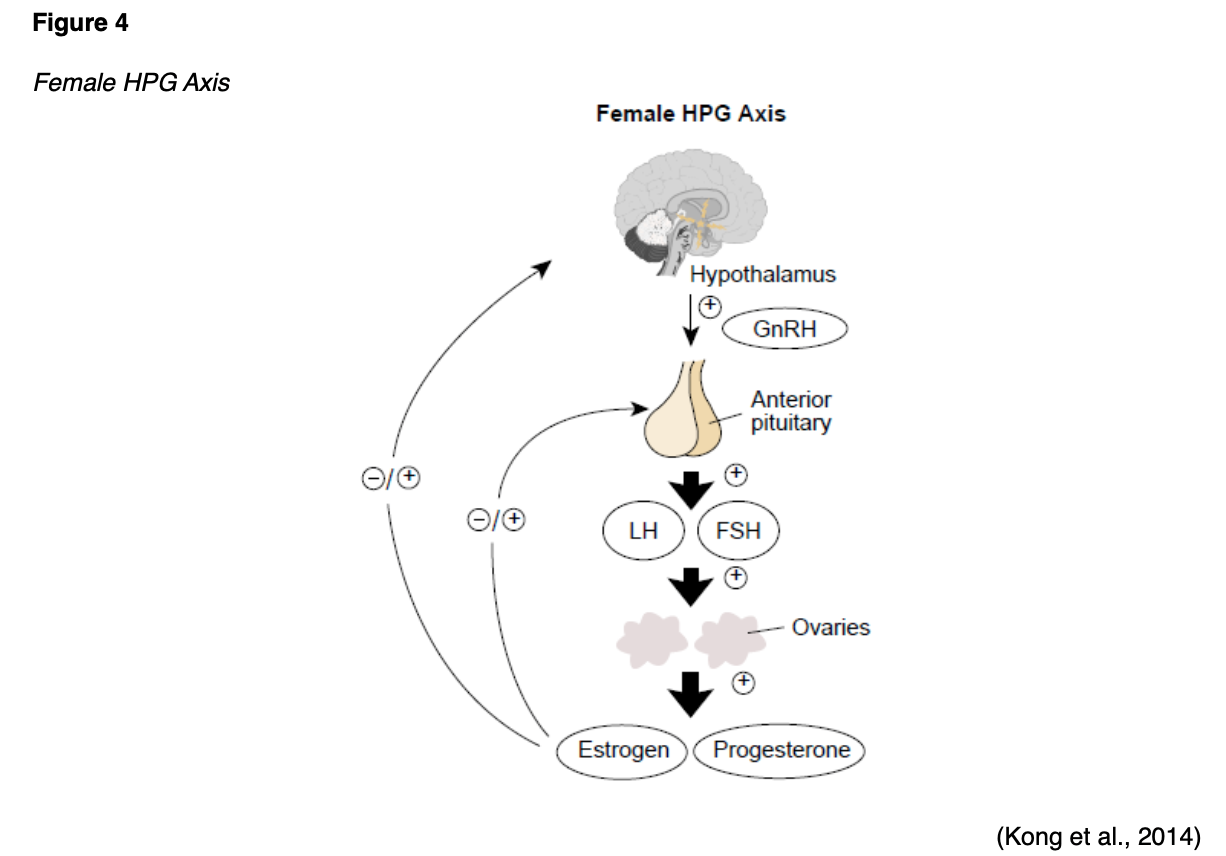
Aside from implantation, pregnancy, and labor, the uterus is also responsible for menstruation. These functions are primarily controlled by a balance between estrogen and progesterone as part of the hypothalamic-pituitary-gonadal (HPG) axis. The HPG axis is a tightly regulated feedback system between the hypothalamus, pituitary gland, and ovaries (see Figure 4). Hormonal changes during puberty signal the hypothalamus to rel
...purchase below to continue the course
Endometrial Hyperplasia
Endometrial hyperplasia (EH) is a pathological condition in which the endometrial cells crowd together, become unusually thick, and may become abnormal in appearance. Hyperplasia refers to the increase in the number of cells in an organ or tissue. These cells are not cancer; however, they can develop into dysplasia (abnormal precancerous cells). Dysplasia can be mild, moderate, or severe, depending on how abnormal the cells look under a microscope and how much of the tissue or organ is affected (Sobczuk & Sobczuk, 2017). This process is demonstrated in Figure 5.

EH is a well-established predisposing factor for EC. It is most commonly caused by excess estrogen with inadequate levels of progesterone to oppose it. This leads to the proliferation (overgrowth) of the endometrial glands, which become variably shaped and irregularly distributed. EH most commonly occurs in postmenopausal women due to ovulation cessation (since progesterone is not generated). However, it may also occur during the perimenopausal period (transition period to menopause) due to ovulation’s irregularity. If ovulation does not occur, progesterone is not secreted, and the endometrium is not shed, leading to an overgrowth of the endometrial lining (ACOG, 2018b, 2020b). The World Health Organization (WHO) last updated the classification of EH in 2014, which is comprised of two categories: benign hyperplasia without atypia and atypical hyperplasia/endometrial intraepithelial neoplasia (EIN). The absence or presence of atypia (abnormal appearing cells) is the most crucial feature of EH. Benign hyperplasia does not demonstrate any abnormal cells and carries a very minimal risk (about 1%) of progression to invasive cancer. In contrast, atypical EIN is marked by an overgrowth of atypical cells and is a precancerous condition. Without treatment, EIN will develop into EC (Sobczuk & Sobczuk, 2017).
Risk Factors
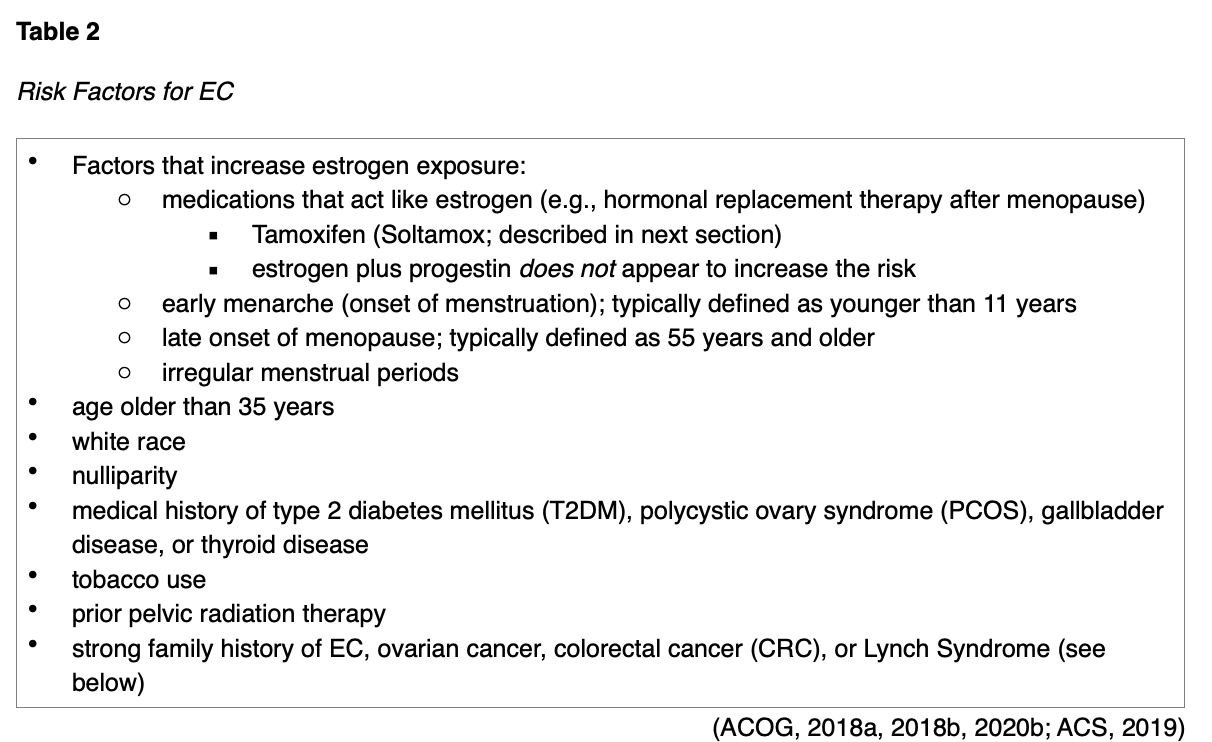
While some women with EC do not have any identifiable risk factors, the two most substantial risk factors include estrogen exposure and obesity. As described earlier, the balance of estrogen and progesterone plays a significant part in developing these conditions. Therefore, anything that increases endogenous (internal) or exogenous (external) estrogen exposure heightens risk (ACS, 2019). The most common risk factors are outlined in Table 2.
Tamoxifen
Tamoxifen (Soltamox) is the oldest hormonal treatment for breast cancer, initially approved by the US Food & Drug Administration (FDA) in 1977. It is a nonsteroidal antiestrogen agent that functions as a partial agonist by blocking estrogen receptors on cancer cells. It is also the most common agent used for chemoprevention in patients at high-risk for breast cancer and is highly effective. However, it carries an increased risk for EC as it has an estrogen-like effect on the uterus. Tamoxifen (Soltamox) increases the risk for EH, EC, and uterine sarcoma in postmenopausal women only. Premenopausal women are not at increased risk for these conditions. Several studies have found that the increased risk for developing EC is 2 to 3 times higher in postmenopausal women taking Tamoxifen (Soltamax) than in age-matched controls not taking the medication. The risk is time-dependent, meaning the risk increases with a longer duration of therapy. APRNs must counsel patients on these risks and the need to follow up with a gynecologist regularly. Women must report any abnormal vaginal bleeding, bloody vaginal discharge, staining, or spotting, the most common early warning signs of EH and EC. All women taking Tamoxifen (Soltamox) are at increased risk for thromboembolic events, such as stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE). Since thromboembolic events can lead to significant morbidity and mortality, resulting in life-threatening consequences, early identification and intervention are essential (ACOG, 2018c; FDA, 2018). The APRN must be well-versed in the most common signs and symptoms, which can vary depending upon the affected body part, as outlined in Table 3. APRNs are responsible for counseling patients regarding the risks associated with Tamoxifen (Soltamox) and provide adequate education on the monitoring precautions and immediate reporting of any suspicious symptoms (Longo, 2019).
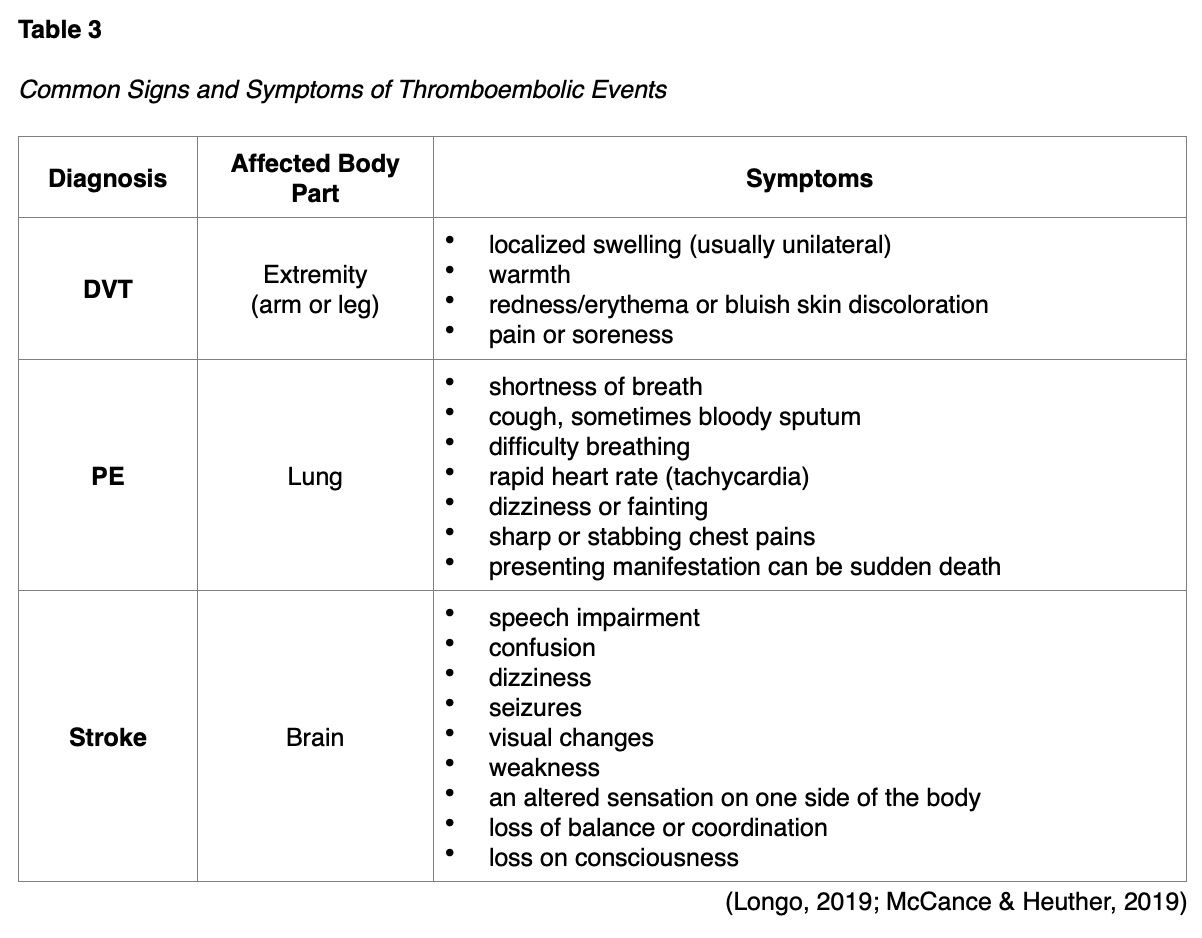
For information regarding the diagnosis and management of thromboembolic events, refer to the following NursingCE courses:
- Blood Clotting and Bleeding Disorders for APRNs (5 ANCC credits)
- Stroke for APRNs (2.5 ANCC credits)
- Venous Thromboembolism (3 ANCC credits)
Lynch Syndrome (LS)
LS, also called hereditary non-polyposis colorectal cancer, is a common hereditary risk factor for CRC that also increases the risk for EC. In the US, it is estimated that 1 in 279 individuals (1.2 million people) have a gene mutation associated with LS; however, most are undiagnosed since identification depends on a cancer diagnosis. In addition to CRC and EC, additional LS-related cancers include gastric, ovarian, pancreas, urothelial, glioblastoma, biliary tract, and small intestine cancers. Changes in the protein expression of MLH1, MSH2, MSH6, or PMS2 genes are most commonly found in LS. Under physiologic conditions, these genes are responsible for repairing any potential errors during DNA replication (the process during which DNA is copied in preparation for cell division); collectively, they are known as mismatch repair (MMR) genes. Since mutations in any of these genes impede the cell’s ability to repair the DNA replication errors, abnormal cells continue to divide. Over time, the accumulated DNA replication errors can lead to uncontrolled cell growth and an increased propensity for cancer development. Mutations in the MLH1 or MSH2 gene are associated with a higher risk (70 to 80%) of developing cancer than mutations in the MSH6 or PMS2 genes, which carry a lower risk (25 to 60%). While mutations in these genes predispose individuals to cancer, not all people with these mutations will develop cancerous tumors (US National Library of Medicine [NLM], 2020).
Individuals inherit LS in an autosomal dominant pattern, which means one inherited copy of each cell’s altered gene is sufficient to increase cancer risk. Women with LS are at higher lifetime risk (up to 60%) for EC. In a 2019 systematic review and meta-analysis examining 53 studies, Ryan and colleagues determined that the prevalence of LS in EC patients is approximately 3%, similar to that of CRC patients (Ryan et al., 2019). The NCCN (2020a) guidelines advise that patients who meet any of the following criteria should be evaluated for LS:
- known LS in the family
- diagnosis of EC or CRC under the age of 50
- a first-degree or second-degree relative with LS-related cancer diagnosed under the age of 50
- more than one first-degree or second-degree relatives with LS-related cancer diagnosed at any age
- in relatives with LS but without a diagnosis of EC, an annual endometrial biopsy is recommended to assess for cancer (CDC, 2019b; NCCN, 2020a; 2020b).
Immunohistochemistry (IHC) and microsatellite instability (MSI) are screening tests performed to help identify patients at higher risk for LS. The NCCN guidelines on LS (2020a) added a new recommendation statement encouraging the universal screening of all CRCs and ECs for mismatch repair deficiency (dMMR) to “maximize sensitivity for identifying individuals with LS and to simplify care processes” (LS-A). Greater than 90% of LS-related cancers are MSI-H or lack expression of at least one of the MMR proteins; therefore, MMR testing helps guide if the patient should be tested for LS. While MMR deficiency can be reported as mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H), they have the same meaning (NCCN, 2020a, 2020b). All patients with MSI-H or dMMR should be referred to a genetic counselor to undergo formalized genetic testing. LS can only be confirmed by a specialized gene panel blood test (CDC, 2019b). According to Ryan and colleagues (2019), diagnosing LS in EC patients is critical. It allows for the testing and early diagnosis of relatives who may also be affected by the condition, thereby reducing morbidity and mortality associated with LS-related cancers (Ryan et al., 2019).
Obesity
Roughly 60% of EC cases in the US are attributed to obesity and, therefore, are potentially preventable. Worldwide, obesity is responsible for up to 80% of all cases (Moore & Brewer, 2017; Onstad et al., 2016). Obesity, a well-known major global health challenge, is defined by a body mass index (BMI) of 30.0 or higher. In the US, obesity has become an epidemic. Based on data from the CDC’s National Center for Health Statistics, from 2017 to 2018, the age-adjusted prevalence of obesity in adults across the US was 42.4%. Despite its adverse effects on health, obesity is expected to rise substantially over the next several decades (CDC, 2020; Connor et al., 2017). EC was one of the first malignancies linked to obesity, dating back to 1966 when an epidemiological study proposed that weight reduction was the most practical preventative measure for the disease (Wynder et al., 1966). According to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR, 2018), there is compelling evidence that obesity throughout adulthood increases the risk of at least 12 different tumor types. Of these 12 tumor types, EC has the strongest link to obesity (Onstad et al., 2016). In a meta-analysis of 26 studies by the WCRF/AICR (2018), for every 5 kg/m2 increase in BMI, there was a 50% increase in the risk of developing uterine cancer. A 2015 meta-analysis of 40 studies involving more than 32 million women revealed a direct association between increased BMI and EC risk; this relationship’s strength increases incrementally alongside rising BMI (Jenabi & Poorolajal, 2015).
The mechanisms underlying obesity as a core driver of EC are premised partly on the increased amount of circulating estrogen carried in adipose tissue, thereby igniting a series of molecular mechanisms contributing to the pathogenesis of EC. As noted earlier, the ovaries stop making estrogen after menopause, but a small amount of estrogen is generated in adipose tissue. Therefore, estrogen supplied in adipose tissue has a significant impact on postmenopausal women. An excess of adipose tissue leads to an overproduction of estrogen (hyperestrogenism), unopposed by progesterone. Even small amounts of circulating estrogens are not adequately counterbalanced in postmenopausal women. Obesity also induces insulin resistance, increased bioavailability of steroid hormones, and inflammation, collectively generating a metabolic state that drives tumorigenesis (Constantine et al., 2019; Onstad et al., 2016; Papatla et al., 2016). Once diagnosed with EC, obesity leads to poorer long-term health outcomes and negatively impacts the treatment course. Bouwman et al. (2015) found that women with obesity have higher perioperative complications, such as an increased risk for infection, more antibiotic use, and higher rates of wound complications. Several studies have demonstrated a significant association between obesity and an increase in all-cause mortality as compared to normal-weight counterparts. Survivors with a higher BMI are found to have decreased physical and social functioning, reduced quality of life, increased risk for morbidity, and higher death rates (Koutoukidis et al., 2015). Despite the clear evidence linking EC and obesity, there is limited public awareness of this relationship. Studies have demonstrated that at least half of women diagnosed with EC were unaware of the impact that obesity has on cancer risk (Onstad et al., 2016).
Risk Reduction and Prevention
Unfortunately, there are currently no routine screening tests to identify EC. Aside from lifestyle choices geared toward maintaining a healthy BMI, additional factors that protect against or lower the risk of EC include pregnancy, oral contraceptive pills (OCPs), and the use of specific intrauterine devices (IUDs). Since hormonal balance shifts toward progesterone during pregnancy, multiparous women (those having several pregnancies) are at lower risk for EC (ACS, 2019). OCP use is associated with a 30% risk reduction of EC when compared to no OCP use. Researchers have proposed that OCPs effectively lower the risk of EC by suppressing endometrial cell proliferation and regulating the balance of the female sex hormones. The risk reduction is strengthened with OCP use over a more extended period, with the lowest risk among those who have taken OCPs for greater than 10 years. The protective effects of long-term OCP use persist for at least 10 years after OCPs are discontinued. According to a population-based cohort study of more than 100,000 predominantly postmenopausal women, the risk reduction for EC was specifically pronounced in long-term OCP users who were also smokers, obese, or rarely exercised (Michels et al., 2018). According to the ACOG (2018b), all women who take estrogen after menopause should take progesterone to counteract the excess estrogen. If menses are irregular, OCPs (or alternate forms of progesterone) may be recommended to help balance hormones. Women who have used an intrauterine device (IUD) seem to have a lower chance of getting EC; however, information regarding the protective effects of IUDs is primarily limited to those that do not contain hormones (National Cancer Institute [NCI], 2018). The benefit of IUD use as a risk reduction strategy for EC is less clear than OCP use. However, progesterone-only releasing IUDs (i.e., levonorgestrel [Mirena]) have demonstrated efficacy in managing complex atypical hyperplasia or early-grade ECs. Studies have shown that levonorgestrel (Mirena) IUD therapy for the conservative treatment of these conditions has resulted in a return to normal histology in most patients. Therefore, IUD use may have a beneficial effect as they are increasingly utilized to treat EH (Navdeep et al., 2018; NCI, 2018).
Signs and Symptoms
The most common sign of EH and EC is abnormal uterine bleeding (AUB). AUB may include bleeding during the menstrual cycle that is heavier or lasts longer than usual, spotting between periods, and postmenopausal bleeding (PMB). AUB includes menstrual cycles that are shorter than 21 days (counting from the first day of the menstrual period to the first day of the next menstrual period) or unusually heavy and prolonged menstrual bleeding characterized by blood clots larger than a quarter and lasting more than 7 days. PMB is the most ominous sign of EC and should always raise clinical suspicion and prompt further workup. Other symptoms concerning for EC may include watery or blood-tinged vaginal discharge, pelvic pain, dyspareunia (painful intercourse), dysuria (painful urination), and urinary frequency. Patients can also experience abdominal bloating and distention, early satiety, and associated weight loss (CDC, 2019a; Chi et al., 2017).
A significant warning sign of EH and EC is a thickened endometrial lining, particularly in postmenopausal women. The endometrium changes in appearance and thickness throughout the menstrual cycle. In premenopausal women, there is significant variation in the endometrium thickness based on different stages of the menstrual cycle; thickness can range from 2 mm to 16 mm. The endometrium in postmenopausal women should be smooth, homogeneous, and less than 5 mm. Endometrial abnormalities are detected using transvaginal ultrasound (TVUS) or magnetic resonance imaging (MRI). Abnormal signs include endometrial thickness greater than 5 mm, edema (fluid), and irregularity of the lining with heterogeneous enhancement (Tokhi & Weerakkody, 2020).
Differential Diagnosis
Aside from EH and EC, there are several other uterine conditions that the APRN should consider in the differential diagnosis of patients presenting with AUB. The International Federation of Gynecology and Obstetrics (FIGO) generated a universally utilized and widely adopted acronym to classify the underlying etiologies of AUB called PALM-COEIN (see Figure 6).
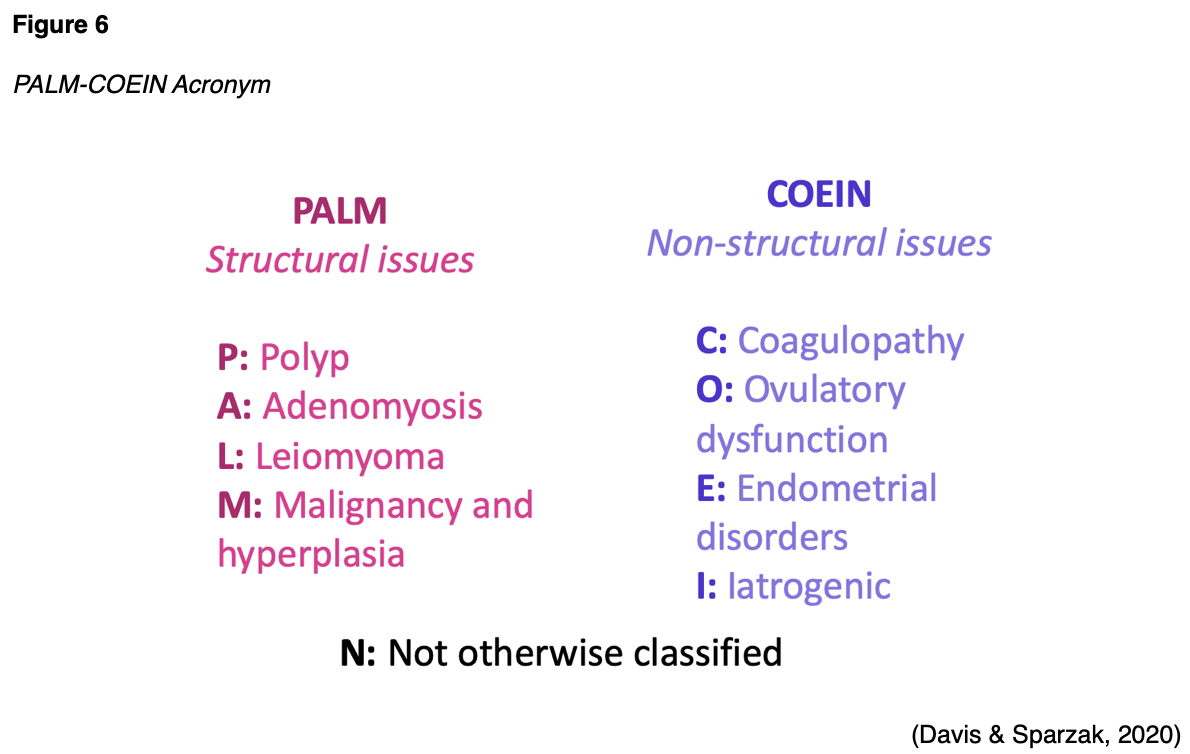
While one or more of the above conditions can contribute to AUB, the five most common non-cancerous uterine pathologies that may induce AUB are described in Table 4.

Diagnostic Workup
According to the ACOG Committee on Gynecologic Practice (ACOG, 2018a), PMB requires prompt and efficient evaluation. The committee supports TVUS as the initial evaluation of PMB and as part of the diagnostic workup of AUB in menstruating women. TVUS is a safe, noninvasive imaging modality that uses soundwaves to generate images of the internal structures within the vagina and pelvis. It is capable of evaluating and measuring the thickness of the endometrium. As demonstrated in Figure 7, a small probe (transducer) is placed directly inside the vagina. The transducer produces soundwaves at very high frequencies, which exceed the human hearing threshold and are used to generate images on a computer. Images are captured in real-time, which allows for the evaluation of the structures and movement of the body’s internal organs (National Institute of Biomedical Imaging and Bioengineering, 2016).

Abnormalities identified on TVUS and/or persistent bleeding should prompt histologic evaluation of the endometrium as the essential next step. The only way to definitively diagnose EC is by evaluating the endometrial cells under a microscope. An endometrial biopsy (EMB) is the most common procedure to obtain a sample of the endometrial tissue. An EMB is usually performed in the outpatient setting by a gynecologist or gynecologic oncologist. It is a very accurate and straightforward procedure in which a thin, rod-like tool is inserted (uterine sound) through the cervix to induce dilatation of the external os. The uterine sound is removed, and a thin, flexible catheter is inserted through the cervical opening into the uterus. The catheter is rotated, and a small amount of the endometrial tissue is removed using suction. The procedure typically takes less than 15 minutes and causes discomfort and pelvic cramping similar to menstrual cramps. While an EMB is a very safe and effective procedure, rare but possible complications may include bleeding, infection, and perforation of the uterine wall (the tip of an instrument passes through the uterine wall creating a hole; Chi et al., 2017).
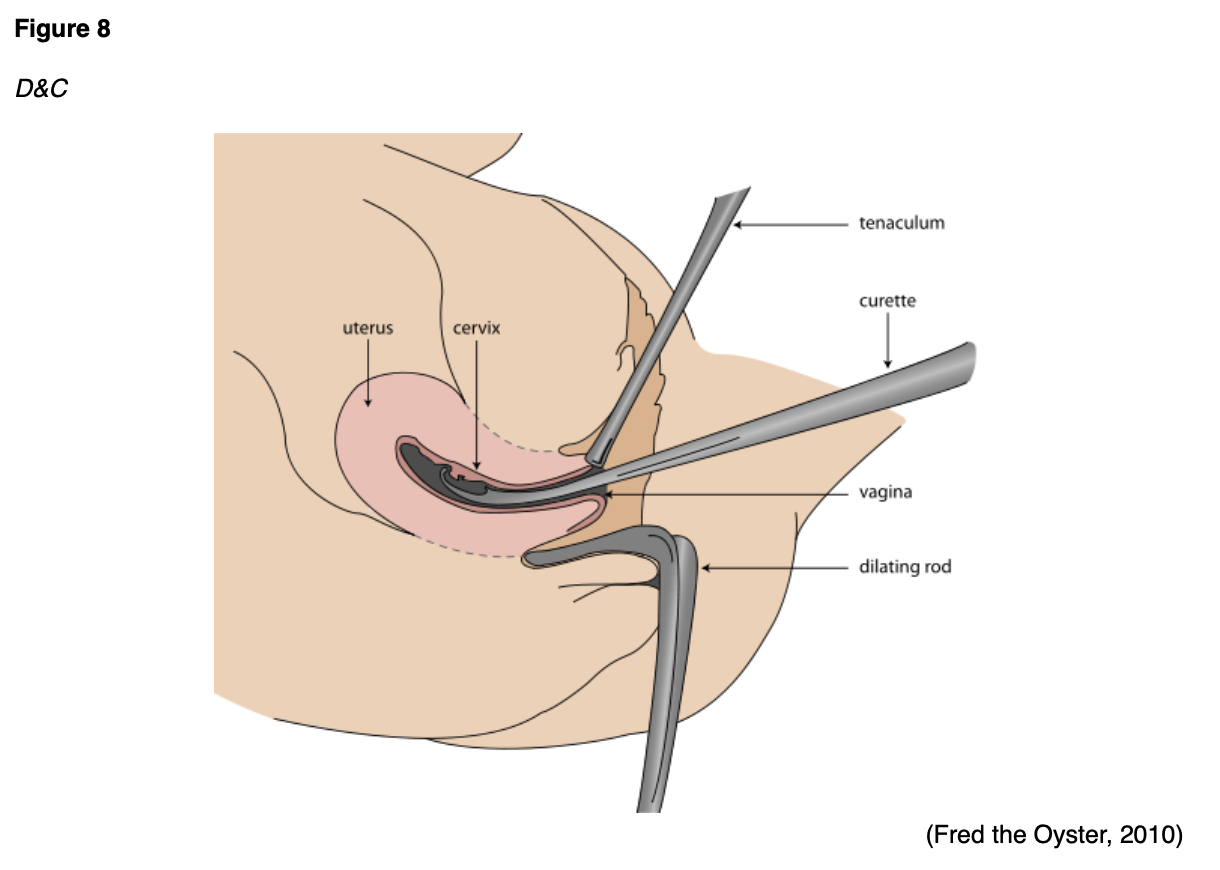
Alternatively, endometrial tissue can be obtained by dilation and curettage (D&C) with or without a hysteroscopy. If the EMB did not provide enough tissue or if the results are unclear, D&C is the next step in the diagnostic workup. A D&C is a surgical procedure typically performed in an outpatient surgery center or hospital under some type of sedation. Like an EMB, the cervix is dilated, and a thin instrument (curettage) is inserted into the uterus to remove tissue, as shown in Figure 8 (ACOG, 2020a; Chi et al., 2017).
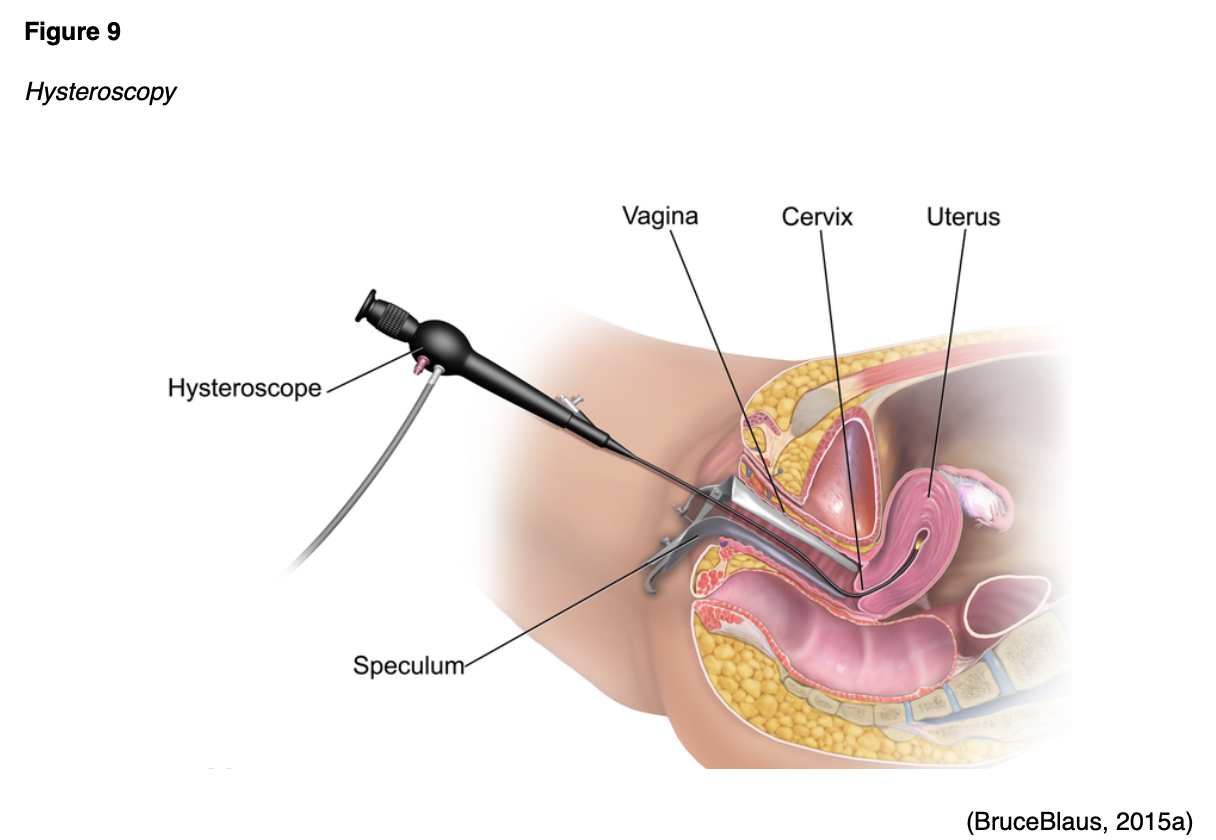
A hysteroscopy is an examination of the uterine cavity using a thin tube with a light at the end called a hysteroscope (see Figure 9). A biopsy may be performed for any abnormalities identified. Although rare, complications following a D&C or hysteroscopy are the same as an EMB and can include bleeding, infection, or perforation of the uterus (ACOG, 2020a; Chi et al., 2017).
APRNs must ensure patients are educated on post-procedure care. It is normal to have some mild cramping and vaginal spotting for a few days following an EMB or D&C. Symptoms are typically well-controlled with over-the-counter analgesics such as anti-inflammatories (e.g., ibuprofen [Motrin, Advil]) or acetaminophen (Tylenol). Many patients find adequate relief from nonpharmacological analgesics, such as the application of heat or ice. Patients should not place anything inside the vaginal for at least 3 days after the procedure; this includes avoiding the use of tampons, douching, and abstaining from sexual intercourse (ACOG, 2020a; Chi et al., 2017).
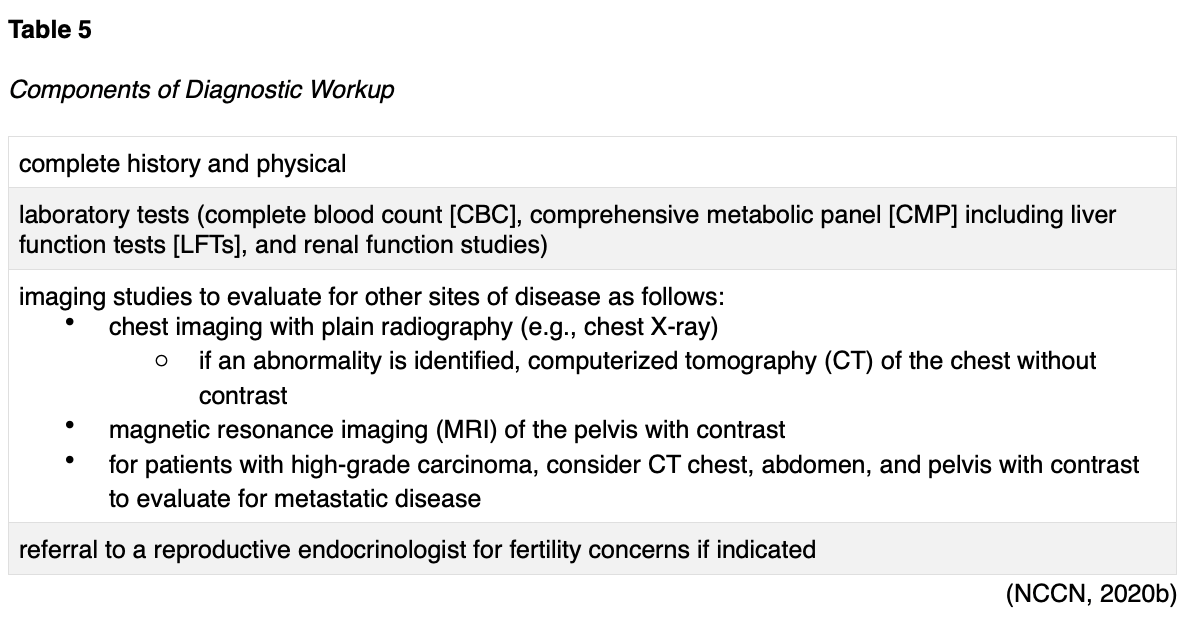
Once EC is diagnosed, the NCCN (2020b) guidelines endorse the diagnostic workup components as outlined in Table 5.
Cancer Subtypes
Approximately 95% of all EC are adenocarcinomas, which is a cancer of the glandular cells. EC is classified into two major groups: Type I and Type II. Type I tumors are most common and account for 80% of all ECs. These tend to be early-stage, low-grade tumors that typically develop from precursor lesions (e.g., EH). These are linked to excess estrogen (are estrogen-dependent), obesity, and are more likely to develop in younger women. They are typically less aggressive, do not commonly spread to other tissues, and usually carry a favorable prognosis. Endometrioid adenocarcinoma is the most common Type I tumor. Type II ECs are more likely to develop in older women. They are primarily diagnosed at advanced stages and are usually high-grade tumors (grade 3). They are not linked to excess estrogen, are more aggressive, faster-growing, and more likely to spread. Type II tumors carry a poorer prognosis, are more likely to recur, and are associated with higher mortality, accounting for at least 40% of EC-related deaths. Type II subtypes include papillary serous carcinoma, clear cell carcinoma, and carcinosarcoma. They are more likely to grow and spread outside the uterus and require more aggressive treatment (Chi et al., 2017; Feinberg et al., 2019; NCCN, 2020b).
Uterine Sarcoma
Uterine sarcoma is a very rare type of uterine cancer that develops in the myometrium or connective tissues rather than the endometrium. It accounts for less than 8% of all uterine cancer diagnoses and is more aggressive. It tends to spread to other parts of the body, most commonly the lungs, more rapidly than EC subtypes. Uterine sarcoma is more aggressive and harder to treat. Sarcomas include three major subtypes as follows:
- uterine leiomyosarcoma (LMS): most common type of uterine sarcoma, forms in the myometrium and accounts for up to 4% of uterine cancers
- endometrial stromal sarcoma (ESS): develops in the connective tissue that supports the endometrium, accounts for less than 2% of all uterine cancers, and typically grows slowly
- undifferentiated uterine sarcoma (UUS): rare subtype; similar to ESS but is more aggressive; grows and spreads more rapidly; accounts for less than 2% of all uterine cancers (Chi et al., 2017; NCCN, 2020b).
Cancer Staging
The cancer stage at diagnosis guides treatment options and strongly influences overall survival. There are detailed staging systems for cancer: The FIGO system and the American Joint Committee on Cancer’s (AJCC) Tumor, Node, Metastasis (TNM) staging system, 8th edition. Both systems are essentially the same and describe specific characteristics to assign stages I through IV, as outlined in Table 6 and demonstrated in Figure 10. Cancer staging reflects the cell type, tumor grade, anatomical location of the tumor, and extent of malignancy. Within the TNM staging system, T denotes the size of the tumor, and if it has grown into nearby tissue, N refers to the presence of cancer in the lymph nodes, and M indicates if cancer has metastasized to other parts of the body beyond the origin site. Tumor grade measures how different the cancer cells look in comparison to healthy cells under the microscope. It is based on cell differentiation and varies from low-grade (grade 1) to high-grade (grade 3). Grade 1 is well-differentiated and appears similar to healthy cells, whereas grade 3 is poorly differentiated (i.e., does not resemble healthy cells) and aggressive (NCCN, 2020b; Yarbro et al., 2018).


Treatment of Endometrial Hyperplasia
There is not a standard of care treatment for EH. Treatment depends on the patient’s age, presence of risk factors, desire for future fertility, and the type and extent of EH. As noted earlier, many cases of EH can be effectively managed conservatively with synthetic progesterone. Progesterone can be administered in various preparations, such as oral or injectable agents, vaginal creams, or in the form of an IUD (i.e., levonorgestrel [Mirena]). The dose and duration of therapy also depend on the patient’s clinical situation. Treatment with progesterone can cause vaginal bleeding, similar to a menstrual period. Since EH can progress to EC, the favored treatment for women with atypical hyperplasia/EIN is hysterectomy (removal of the uterus) unless future fertility is desired. Hysterectomy is the standard treatment for those who do not respond to progesterone therapy (Chandra et al., 2016; NCCN, 2020b).
Cancer Treatment
The optimal cancer treatment depends on various factors, such as the pathologic features, cancer stage, plans for fertility, patient preference, age, and medical history. Treatment is often multimodal, with several therapies combined and administered simultaneously (concurrently) or sequentially. Since many cases require specialized surgical care, all patients with suspected EC or uterine sarcoma should be promptly referred to a gynecologic oncologist. This section will provide a synopsis of the most common evidence-based treatment strategies (NCCN, 2020b).
Surgery
When the cancer is early-stage and limited to the uterus, surgical intervention is typically indicated. Total hysterectomy and bilateral salpingo-oophorectomy (TH/BSO) with surgical staging (lymph node assessment) is the most common treatment option for Type I ECs. Patients who desire fertility preservation should be promptly referred to a fertility expert. Progesterone-based therapy may be offered initially to select women with early-stage and low-grade disease; however, this requires close monitoring with EMB or D&C every 3 to 6 months. TH/BSO should be encouraged in those who demonstrate signs of disease progression on progesterone-based therapy (NCCN, 2020b).
Surgical Risks and Side Effects
The risks and side effects of surgery depend on the size and degree of cancer invasion, the extent of surgery, and the structures removed. All surgeries and invasive procedures are accompanied by risks, such as adverse reactions to anesthesia, bleeding, blood clots, fistula formation (an abnormal connection between two hollow spaces within the body), bowel and bladder injury, infection, sexual dysfunction, and life-threatening sepsis. APRNs must counsel women on the side effects of surgical menopause and infertility following TH/BSO. The loss of fertility can negatively impact interpersonal relationships, quality of life, psychological health, and emotional wellbeing. The APRN serves a vital role in helping patients acclimate to these life-altering changes by facilitating healthy coping, addressing concerns, and referring patients to appropriate support groups or therapists (NCCN, 2020b; Nettina, 2019; Yarbro et al., 2018).
Radiation Therapy
Radiation therapy is a localized treatment that delivers a precisely measured amount of high-energy, focused ionizing radiation to the tumor while causing as little injury as possible to the surrounding tissue. Radiation causes cellular damage to cancer cells, leading to biological changes in the DNA and rendering cells incapable of reproducing or spreading. All healthy cells and cancer cells are vulnerable to the effects of radiation and may be injured or destroyed; however, healthy cells can repair themselves and remain functional. The total dose of radiation is hyper-fractionated, which means it is delivered to the tumor in small, divided doses (i.e., fractions) rather than all at once. Hyper-fractionation gives healthy cells a chance to recover between treatments. The total number of fractions (doses) administered depends on the tumor size, location, reason for treatment, patient’s overall health, performance status, and any other treatments the patient is receiving. Radiation therapy plays a central role in treating EC and can be delivered externally or internally; many patients receive both. The most common types of radiation used for EC include external beam radiation therapy (EBRT) and brachytherapy (NCCN, 2020b; Nettina, 2019)
EBRT
EBRT delivers radiation from a source outside the body and is a common type of radiation therapy used for EC. Traditionally, radiation beams could only match the tumor’s height and width, exposing more healthy tissue to the consequences of radiation. Further advancements in imaging technology have led to more precise treatment mechanisms that allow even more of the radiation beam to reach the tumor. Intensity-modulated radiation therapy (IMRT) is a newer, highly conformal form of radiation that modulates the radiation beam’s intensity. IMRT delivers a higher radiation dose to a precise location, reducing unintended exposure to healthy tissues, enhancing clinical outcomes, and limiting side effects. In EC, IMRT helps minimize radiation exposure to the bowel and other critical structures, especially among patients who have undergone a TH/BSO (NCCN, 2020b; Nettina, 2019).
Brachytherapy
Brachytherapy is a form of internal radiation therapy that plays a critical role in the treatment of EC. It is commonly administered after EBRT is completed or used in patients who are not surgical candidates. Following TH/BSO, most women receive brachytherapy to the upper part of the vagina (i.e., the vaginal cuff). This provides an added boost of radiation to the most common cancer recurrence site. Brachytherapy involves implanting a wire or catheter into the body within or near the tumor. The radioactive material is placed inside a cylinder (or applicator) and subsequently positioned directly at the vaginal cuff. This direct positioning protects nearby structures such as the bladder and rectum from excess radiation exposure (Chi et al., 2017). An example of brachytherapy is demonstrated in Figure 11.
Brachytherapy may be delivered using low-dose-rate (LDR) or high-dose-rate (HDR) for EC. LDR brachytherapy typically requires hospitalization for several days, during which time the patient is confined to an isolated, radiation-safe room to protect others from exposure to the radiation. Specific LDR treatments involve the placement of radioactive rods, which require the patient to be confined to a bed to prevent dislodgment of the applicator for a defined period (usually 1-3 days). HDR brachytherapy is much more common than LDR as it is performed on an outpatient basis. Each treatment lasts only minutes, and hospitalization and bedrest are not indicated. The treatment is delivered in a radiation-shielded room to protect others from exposure, and patients are not considered “radioactive” following the treatment. They can safely go about their regular routines and lifestyles without potentially exposing others (Nettina, 2019).

Radiation Side Effects
Radiation side effects depend on the specific area(s) of the body exposed and the dose received. The urinary system, bowel, and genitalia are most commonly affected by radiation for EC. Bladder dysfunction may manifest as dysuria, hematuria, acute kidney injury, hydronephrosis, or incontinence. The most common side effect of brachytherapy is changes to the internal lining of the vagina as the radiation can cause mild burns. This may lead to vaginal dryness, atrophy (drying and thinning of the vaginal walls), atrophic vaginitis (inflammation and dryness of the vaginal tissue), vaginal agglutination (fusion and fibrosis of the vaginal walls), and recurrent yeast infections. Sexual dysfunction is likely, particularly dyspareunia, decreased libido, and postcoital (after intercourse) vaginal spotting. If the ovaries are within the radiation field, patients may experience a permanent loss of ovarian function. Systemic effects can include fatigue, weakness, and dehydration (Nettina, 2019; Yarbro et al., 2018).
Chemotherapy
The treatment of high-grade and advanced stage EC (Stage III and IV) and uterine sarcomas typically requires systemic therapy in the form of chemotherapy. Chemotherapy, also referred to as cytotoxic or antineoplastic therapy, encompasses a group of high-risk, hazardous drugs with the intent to destroy as many cancer cells with as minimal effect on healthy cells as possible. Chemotherapy generally works by interfering with the normal cell cycle, impairing DNA synthesis and cell replication, preventing cancer cells from dividing, multiplying, and forming new cancer cells. There are several chemotherapeutic agents used in EC, and they are usually administered in combinations of two or three drugs. The drug selection depends on the cancer’s stage, its subtype, and if the intent of treatment is curative or palliative. Chemotherapy may be administered following surgery, which is called adjuvant therapy. Adjuvant therapy is given to eradicate any micro-metastases. Micro-metastases are a small collection of cancer cells too tiny to be identified on imaging scans that have detached from the original tumor and spread to other parts of the body. The danger with micro-metastases is that they can grow and develop into additional cancerous tumors throughout the body. Adjuvant therapy aims to prevent cancer recurrence (Nettina, 2019; Yarbro et al., 2018).
The most common treatment regimens recommended by the NCCN (2020b) guidelines are outlined in Table 7. Of note, due to the aggressive nature of carcinosarcomas, there is a separate category for further management following the failure of first-line preferred treatment for this particular subtype. The NCCN (2020b) strongly recommends clinical trial participation for all patients with uterine sarcoma due to the condition’s aggressiveness, its inadequate response to chemotherapy, and its high-risk for mortality (NCCN 2020b).

Chemotherapy Side Effects
The majority of chemotherapeutic agents are broad in their attack, meaning they kill normal, healthy cells in the body together with the cancer cells. As a result, they pose a wide array of several side effects, which can also vary based on the specific agent. The side effects of chemotherapy vary based on the drug type, dosage, duration of treatment, and specific patient factors. Since cancer cells divide rapidly, chemotherapy is primed to target cells that divide rapidly. This means that they also impact normal cells that divide quickly, like those in the GI tract, skin/hair cells, and bone marrow. As a group, the most common side effects include lowering of the blood counts (anemia, thrombocytopenia, neutropenia), fatigue, nausea, anorexia, alopecia (hair loss), diarrhea, skin changes, and peripheral neuropathy (damage to the sensory nerves). Alopecia (hair loss) deserves special attention because it can cause significant emotional distress to women. Chemotherapy-induced hair loss generally begins with hair thinning, which occurs about 7-15 days after the first dose. This effect results from damage to the dividing hair matrix cells, which causes the hair shaft to break at the follicular orifice or hair bulb. While the degree of hair loss depends on the chemotherapy agent, dose, administration schedule, paclitaxel (Taxol), and docetaxel (Taxotere) are well-known for inducing alopecia. APRNs should reassure women that their hair typically begins to regrow within a few weeks following the cessation of chemotherapy, as permanent alopecia following chemotherapy is rare (Olsen et al., 2019).
Cisplatin (Platinol) is a moderate-to-highly emetogenic agent that induces both acute and delayed nausea. Poorly controlled chemotherapy-induced nausea and vomiting (CINV) are associated with unfavorable treatment compliance, impairing survival. Aprepitant (Emend) is approved to reduce CINV associated with cisplatin (Platinol) therapy. Available in oral and intravenous preparations, aprepitant (Emend) is a neurokinin-1 (NK-1) receptor antagonist that blocks substance P/neurokinin 1 in the brain. It is used in combination with a 5-hydroxytryptamine type 3 (5HT3) receptor antagonist (e.g., ondansetron [Zofran] or palonosetron [Aloxi]) and a corticosteroid (e.g., dexamethasone [Decadron]) for therapy. Patients require aggressive hydration before and after the administration of cisplatin (Platinol) to manage associated nephrotoxicity and protect the renal system from injury (Brown et al., 2019; Olsen et al., 2019).
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of cisplatin (Platinol), carboplatin (Paraplatin), paclitaxel (Taxol), and docetaxel (Taxotere). It is often the dose-limiting toxicity (DLT) of these agents. DLTs are severe toxicities and side effects that are serious enough to warrant a dose reduction or discontinuation of the treatment. CIPN results from demyelination of the sensory and motor axons. Patients experience reduced nerve conduction velocity, leading to the loss of deep tendon reflexes, paresthesia (numbness and tingling), weakness, and burning pain. CIPN initially affects the body’s most distal points, such as the fingertips and toes, and moves proximally toward the midline as the damage progresses. In severe cases, patients may lose all sensation in the fingers, hands, toes, and feet; this can cause significant disability, such as the inability to grasp or hold items and gait disturbance, leading to imbalance and falls. CIPN is a complex topic since no single pathophysiologic process has been identified to explain the various neuropathies that occur following exposure to these chemotherapy agents. CIPN is dose-dependent and progressive during treatment but also can have a cascading effect after treatment ends. During this cascading phenomenon, symptoms become more prominent after discontinuation of the offending agent. Pain, sensory changes, and weakness that manifest during treatment generally lead to chemotherapy dose reductions, changes in treatment protocols, or discontinuation of the agent. The morbidity associated with CIPN can lead to a pronounced decline in quality of life and independence with activities of daily living (Brown et al., 2019).
Currently, no medications or supplements are effective in preventing CIPN. Exercising regularly, reducing alcohol use, and treating preexisting medical conditions (vitamin B12 deficiency) may reduce the risk of CIPN. Management for CIPN is complex, and effective treatment options are limited. Pharmacologic treatment focuses on symptom relief, although many agents are not highly effective. Some patients describe relief from over-the-counter pain medications, menthol creams, capsaicin cream, or lidocaine patches. Gabapentin (Neurontin), an anticonvulsant/anti-epileptic agent, is commonly prescribed with some effect.
Other patients report relief from selective serotonin-norepinephrine reuptake inhibitors (SNRIs) like duloxetine (Cymbalta). The American Society of Clinical Oncology (ASCO) released an updated statement on the management of CIPN, and new recommendations support the use of duloxetine (Cymbalta) as the only agent with appropriate evidence to support its use in patients with established painful CIPN, although the degree of benefit is limited (Loprinzi et al., 2020). APRNs must counsel patients on ways to avoid secondary injury through wearing supportive shoes and paying attention to home safety, such as using handrails on stairs and removing throw rugs. Patients must also be mindful of water temperatures, as they may become less sensitive to hot water, increasing their risk of burns when bathing or washing dishes. An improvement in function and resolution of symptoms often occur over time, but nerve damage may be permanent (Brown et al., 2019).
Ifosfamide (Ifex) is a highly-emetogenic agent that requires premedication similar to cisplatin (Platinol) to prevent CINV. It carries a unique side effect profile of hemorrhagic cystitis since it is primarily excreted through the renal system. Hemorrhagic cystitis is a diffuse inflammatory condition of the urinary bladder that induces bleeding from the bladder mucosa, ranging from microscopic hematuria (blood in urine) to bright red, exsanguinating hematuria. Patients are concomitantly prescribed several doses of mesna (Mesnex) at defined intervals. Mesna (Mesnex) acts as a cytoprotectant (bladder protectant) and can prevent hemorrhagic cystitis. Other signs of hemorrhagic cystitis include dysuria (painful urination), frequency, and urgency. Since ifosfamide (Ifex) is one of the few agents that can cross the blood-brain barrier, it can cause neurotoxic effects. APRNs must monitor patients for neurotoxicity, which may manifest as somnolence, confusion, hallucinations, and depressive psychoses. Rare neurologic toxicities include seizures, ataxia, weakness, neuropathies, and encephalopathy (diffuse disease of the brain that alters brain function or structure). The risk can be mitigated by the concomitant administration of dexamethasone (Decadron) or mannitol (Osmitrol) to reduce cerebral edema. Methylene blue (Urolene Blue) is an inhibitor of nitric oxide that is used to treat ifosfamide (Ifex)-induced encephalopathy (IIE). While the exact mechanism is poorly understood, it is proposed that methylene blue (Urolene Blue) counteracts some of the abnormal metabolic pathways cited in IIE. Symptoms of IIE and other types of ifosfamide (Ifex)-induced neurotoxicity most commonly present during the drug administration but may also develop over a few days. Patients with hypoalbuminemia (low serum albumin levels) and renal dysfunction are at higher risk for these neurotoxic effects (MedlinePlus, 2017; Olsen et al., 2019).
Chemotherapy-induced cardiotoxicity is a serious complication that limits certain chemotherapy agents and can lead to life-threatening dysrhythmias, conduction disturbances, cardiomyopathies, pericarditis, or myocarditis, and pericardial effusions. Doxorubicin (Adriamycin) and epirubicin (Ellence) are among the most common offenders. Acute cardiotoxicities that occur within the treatment period are generally reversible and manageable. However, chronic cardiotoxicity may occur up to decades after completing treatment. The cumulative dose of doxorubicin (Adriamycin) is an essential factor that dictates the potential for cardiotoxicity. The cumulative dose should not exceed 500 mg/m2, or the risk of congestive heart failure rises tremendously. APRNs must remain vigilant when prescribing cardiotoxic chemotherapy agents to ensure cumulative doses do not exceed this threshold. Patients must undergo baseline cardiac evaluation with an echocardiogram or multigated acquisition (MUGA) scan to evaluate cardiac function and left ventricular ejection fraction (LVEF) before initiating cardiotoxic therapies. These cardiac function tests are performed at defined intervals and as clinically indicated. Patients must be monitored closely for any signs and symptoms of cardiac dysfunction such as dyspnea, shortness of breath, peripheral edema, fluid retention, chest pain (angina), lightheadedness, and so forth. Early detection and immediate management can reverse the condition and minimize cardiotoxic effects. Liposomal doxorubicin (Doxil) is doxorubicin (Adriamycin) encapsulated in a closed lipid sphere (liposome). Liposomal doxorubicin (Doxil) carries a lower risk for cardiotoxicity and is a common alternative to doxorubicin (Adriamycin) in patients who have underlying cardiac dysfunction (Olsen et al., 2019).
Hypersensitivity Reactions to Chemotherapy
A hypersensitivity reaction (HSR) occurs when the immune system becomes overstimulated by a foreign substance and creates antibodies, igniting an immune response. HSRs are most prominently associated with paclitaxel (Taxol), docetaxel (Taxotere), and carboplatin (Paraplatin). HSR risk can be lowered by pre-medicating patients with corticosteroids, antihistamines, and acetaminophen (Tylenol). HSRs can occur during the initial chemotherapy infusion or after subsequent administrations of the same agent. Paclitaxel (Taxol) is well-known for its risk of nearly immediate acute HSR, whereas carboplatin (Paraplatin) more commonly induces an HSR after several cycles. The majority of cases of HSR occur during the first 15 minutes of the infusion. Initial signs and symptoms can include hives, urticaria, pruritis, swelling, back pain, facial flushing, rhinitis, abdominal cramping, chills, hypotension, and anxiety. Patients may require supplemental oxygen, fluid resuscitation, or other emergency medications as indicated. For life-threatening symptoms such as bronchospasm, angioedema (swelling of the oral cavity, lips, or tongue), or anaphylaxis, epinephrine 0.1-0.5 mg (1:10,000 solution for adult patients) is advised (Nettina, 2019).
For a more detailed review of chemotherapy agents, their side effects, prescribing indications, and monitoring, refer to the “Oncology Prescribing” NursingCE course.
Targeted Therapy
Bevacizumab (Avastin) is a humanized monoclonal antibody that binds to and inhibits the activity of human vascular endothelial growth factor (VEGF) with its receptors, thereby blocking proliferation and formation of new blood vessels that supply tumor cells. VEGF is a signaling protein that stimulates angiogenesis (the formation of new blood vessels) in healthy and cancerous cells. Blood vessels carry oxygen and nutrients to the tissue, supporting growth and survival. Thus, tumors need blood vessels to grow and spread. Anti-angiogenesis is the process of inhibiting the formation of new blood vessels by blocking the VEGF receptors. Angiogenesis inhibitors (i.e., VEGF inhibitors) target the blood vessels that supply oxygen to the tumor cells, ultimately causing them to starve by cutting off their nutrient supply. VEGF inhibitors such as bevacizumab (Avastin) sever the blood supply to cancer cells by interfering with the VEGF receptor, so tumors stay small and eventually starve. While the NCCN (2020b) guidelines cite that bevacizumab (Avastin) may be used in patients with EC (not uterine sarcomas) who have progressed on prior cytotoxic chemotherapy, it is still awaiting FDA-approval for this indication. Studies have demonstrated that adding bevacizumab (Avastin) to carboplatin (Platinol) and paclitaxel (Taxol) as a first-line treatment increases progression-free survival and patient outcomes in EC. However, additional clinical investigation is warranted (NCCN, 2020b; Rose et al., 2017). Bevacizumab (Avastin) is generally well-tolerated. Potential side effects include bleeding events, headaches, hypertension, and proteinuria (protein spilling in the urine due to increased pressure in the kidneys). Many patients require concurrent treatment with antihypertensives due to the medication’s elevation of blood pressure. Bevacizumab (Avastin) is contraindicated within 6 weeks of surgery (preoperatively or postoperatively) due to an increased risk for major bleeding events, delayed wound healing, and fistula formation. It also carries a black box warning for bowel perforation (a hole in the intestines). Patients should report any sudden onset of severe and diffuse abdominal pain, bloating, nausea, vomiting, or rectal bleeding (Olsen et al., 2019).
Immunotherapy
The role of immunotherapy in the treatment of EC and uterine sarcomas is less advanced than other diseases, and clinical research is ongoing. Currently, pembrolizumab (Keytruda) is the only agent used in this setting; it is approved for the treatment of metastatic EC and uterine sarcomas that are MSI-H or dMMR. Pembrolizumab (Keytruda) is a humanized monoclonal antibody that binds with high affinity to PD-1, thereby preventing its interaction with PD-L1 and PD-L2. In the phase II KEYNOTE-158 clinical trial, pembrolizumab (Keytruda) demonstrated promising and durable antitumor activity in patients with PD-L1-positive EC cancer, offering a clinically meaningful and viable treatment strategy. In 2019, the FDA approved pembrolizumab (Keytryuda) for use in patients with advanced PD-L1–positive EC and uterine sarcomas with disease progression or recurrent disease following first-line chemotherapy. Pembrolizumab (Keytruda) has demonstrated long-term durable responses in this population with minimal side effects. It is well-tolerated; the most common side effects include fatigue, nausea, anorexia, coughing, diarrhea, skin rash, and itching. However, patients may experience severe and possibly fatal autoimmune-related adverse effects. Although any organ system can be affected, the most common reactions include colitis, hepatitis, endocrinopathies (thyroid and adrenal glands), pneumonitis, and skin rash, including Stevens-Johnson syndrome (Chan et al., 2020; Sasikumar & Ramachandra, 2018).
Hormonal Therapy
Hormone therapies are a targeted treatment strategy for patients with estrogen-dependent ECs. These medications prevent the body from producing the hormones that drive cancer growth or prevent the hormones from reaching and acting on the cancer cells. Since estrogen is a major driver in many EC subtypes, hormone-blocking agents are used to shrink or slow the cancer growth. Aside from the use of progesterone-releasing agents for early-stage and low-grade ECs described earlier, there are several agents that may be used as maintenance therapy in the adjuvant setting. Fulvestrant (Faslodex) is an intramuscular injection that binds to estrogen receptors, downregulating estrogen in cancer cells, and blocking estrogen throughout the body. Three oral aromatase inhibitors (AIs) are commonly used in postmenopausal women: anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin). Anastrazole (Arimidex) and letrozole (Femara) are selective nonsteroidal agents that bind to and inhibit the aromatase enzyme. Exemestane (Aromasin) is a steroidal aromatase inactivator that binds irreversibly to the aromatase enzyme, thereby inactivating it. Adverse effects typically include hot flashes, night sweats, loss of libido, weight gain, vaginal dryness/atrophic vaginitis, joint aches or pains, mood changes, weight gain, and thinning or weakening of the bones (osteopenia or osteoporosis). Due to the impact of hormonal therapy on bone thinning, patients should be counseled on the importance of a calcium-rich diet with at least 1,200 mg of dietary calcium daily. Patients who are unable to get this recommended amount of calcium in their diet should consider calcium supplementation. APRNs should also counsel patients on the importance of engaging in weight-bearing exercises for bone health. Exercise can also help reduce the severity of the joint aches and pains commonly associated with these medications (Olsen et al., 2019).
References
Ameer, M. A., Fagan, S. E., Sosa-Stanley, J. N., & Peterson, D. C. (2020). Anatomy, abdomen and pelvis, uterus. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470297/
American Cancer Society. (2019). Causes, risk factors, and prevention. https://www.cancer.org/cancer/endometrial-cancer/causes-risks-prevention.html
American Cancer Society. (2020). Key statistics for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html
American College of Obstetricians and Gynecologists. (2018a). ACOG committee opinion: The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstetrics & Gynecology, 131(5), e124-e129.
American College of Obstetricians and Gynecologists. (2018b). Endometrial hyperplasia. https://www.acog.org/womens-health/faqs/endometrial-hyperplasia
American College of Obstetricians and Gynecologists. (2018c). Tamoxifen and uterine cancer. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2014/06/tamoxifen-and-uterine-cancer.pdf
American College of Obstetricians and Gynecologists. (2020a). Dilation and curettage. https://www.acog.org/womens-health/faqs/dilation-and-curettage
American College of Obstetricians and Gynecologists. (2020b). The menopause years. https://www.acog.org/womens-health/faqs/the-menopause-years
Bouwman, F., Smits, A., Lopes, A., Das, N., Pollard, A., Massuger, L., Bekkers, R., & Galaal,
K. (2015). The impact of BMI on surgical complications and outcomes in endometrial cancer surgery—An institutional study and systemic review of the literature. Gynecologic Oncology, 139(2), 369-376. https://doi.org/10.1016/j.ygyno.2015.09.020
Brown, T. J., Sedhom, R., & Gupta, A. (2019). Chemotherapy-induced peripheral neuropathy. JAMA Oncology, 5(5),750. https://doi.org/10.1001/jamaoncol.2018.6771
BruceBlaus. (2015a). Hysteroscopy [image]. https://commons.wikimedia.org/wiki/File:Hysteroscopy.png
BruceBlaus. (2015b). Transvaginal ultrasound [image]. https://commons.wikimedia.org/wiki/File:Vaginal_Ultrasound.png
Cancer Research UK. (2014a). Brachytherapy [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_the_position_of_the_applicators_for_internal_radiotherapy_for_cervical_cancer_CRUK_344.svg
Cancer Research UK. (2014b). Stage IA and IB [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_1A_and_1B_cancer_of_the_womb_CRUK_196.svg
Cancer Research UK. (2014c). Stage IIIA, IIIB, and IIIC [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_3A_to_3C_cancer_of_the_womb_CRUK_224.svg
Cancer Research UK. (2016a). Stage II [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_2_cancer_of_the_womb_CRUK_206-ar.png
Cancer Research UK. (2016b). Stage IVA and IVB [image]. https://commons.wikimedia.org/wiki/File:Diagram_showing_stage_4A_and_4B_cancer_of_the_womb_CRUK_234-ar.png
Centers for Disease Control and Prevention. (2019a). Basic information about uterine cancer. https://www.cdc.gov/cancer/uterine/basic_info/index.htm
Center for Disease Control and Prevention. (2019b). Genetic testing for Lynch syndrome. https://www.cdc.gov/genomics/disease/colorectal_cancer/testing_lynch.htm
Center for Disease Control and Prevention. (2020). Prevalence of obesity and severe obesity among adults: United States, 2017-2018. https://www.cdc.gov/nchs/products/databriefs/db360.htm
Chan, J. K., Lakomy, D. S., McDonald, Y., & Kapp, D. S. (2020). Long-term durable responses after pembrolizumab immunotherapy for recurrent, resistant endometrial cancer. Gynecologic Oncology Reports, 33, 1-3. https://doi.org/10.1016/j.gore.2020.100581
Chandra, V., Kim, J. J., Benbrook, D. M., Dwivedi, A., & Rai, R. (2016). Therapeutic options for management of endometrial hyperplasia. Journal of Gynecologic Oncology, 27(1), e8-e33. http://dx.doi.org/10.3802/jgo.2016.27.e8
Chi, D. S., Berchuck, A., Dizon, D. S., & Yasher, C. M. (2017). Principles and practice of gynecologic oncology. (7th ed.). Lippincott Williams & Wilkins.
Connor, E., Raker, C., Clark, M., & Stuckey, A. (2017). Obesity risk awareness in women with endometrial cancer. Archives of Gynecology and Obstetrics, 295(4), 965-969. https://doi.org/10.1007/s00404-017-4301-4
Constantine, G. D., Kessler, G., Graham, S., & Goldstein, S. R. (2019). Increased incidence of endometrial cancer following the women’s health initiative: An assessment of risk factors. Journal of Women’s Health, 28(2). https://doi.org/10.1089/jwh.2018.6956
Danielah67. (2017). Progression of normal cells to cancer cells [image].
https://commons.wikimedia.org/wiki/File:Shkzf.jpg
Davis, E., & Sparzak, P. B. (2020). Abnormal uterine bleeding. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK532913/
Feinberg, J., Albright, B., Black, J., Lu, L., Passarelli, R., Gysler, S., Whicker, M., Altwerger, G., Menderes, G., Hui, P., Santin, A. D., Azodi, M., Silasi, D. A., Ratner, E. S., Litkouhi, B., & Schwartz, P. E. (2019). Ten-year comparison study of type 1 and type 2 endometrial cancers: Risk factors and outcomes. Gynecologic and Obstetric Investigation, 84, 290-297. https://doi.org/10.1159/000493132
Fred the Oyster. (2010). D&C [image]. https://commons.wikimedia.org/wiki/File:Dilation_and_curettage.svg
Gasner, A., & P. A., A. (2020). Physiology, uterus. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK557575/
Jenabi, E., & Poorolajal, J. (2015). The effect of body mass index on endometrial cancer:
a meta-analysis. Public Health (Elsevier), 129(7), 872-880. https://doi.org/10.1016/j.puhe.2015.04.017
Kong, L., Zhang, T., Tang, M., & Wang, D. (2014). Female HPG axis [image]. https://commons.wikimedia.org/wiki/File:Hypothalamic%E2%80%93pituitary%E2%80%93gonadal_axis_in_females.png
Koutoukidis, D. A., Knobf, M. T., & Lanceley, A. (2015). Obesity, diet, physical activity, and
health-related quality of life in endometrial cancer survivors. Nutrition Reviews, 73(6), 399-408. https://doi.org/10.1093/nutrit/nuu063
LibreTexts. (2020). 14.3: Anatomy and physiology of the female reproductive system. https://bio.libretexts.org/Courses/Lumen_Learning/Book%3A_Biology_of_Aging_(Lumen)/14%3A_The_Reproductive_System/14.03%3A_Anatomy_and_Physiology_of_the_Female_Reproductive_System
Longo, D. L. (2019). Harrison’s hematology and oncology. (3rd ed.). McGraw-Hill Education.
Loprinzi, C. L., Lacchetti, C., Bleeker, J., Cavaletti, G., Chauhan, C., Hertz, D. L., Kelley, M. R., Lavino, A., Lustberg, M. B., Paice, J. A., Schneider, B. P., Smith, E. M. L., Smith, M. L., Smith, T. J., Wagner-Johnston, N., & Hershman, D. L. (2020). Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology, 38(28), 3325-3348. https://doi.org/10.1200/JCO.20.01399
Lumen Learning. (n.d.). Boundless anatomy and physiology: The female reproductive system. Retrieved November 27, 2020, from https://courses.lumenlearning.com/boundless-ap/chapter/the-female-reproductive-system/
McCance, K. L., & Heuther, S. E. (2019). Pathophysiology: The biologic basis for disease in adults and children (8th ed.). Elsevier.
MedlinePlus. (2017). Mesna. https://medlineplus.gov/druginfo/meds/a613013.html
Michels, K. A., Pfeiffer, R. M., Brinton, L. A., & Trabert, B. (2018). Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncology, 4(4), 516-521. https://doi.org/10.1001/jamaoncol.2017.4942
Moore, C., & Brewer, M. (2017). Endometrial cancer: Is this a new disease? American Society of
Clinical Oncology Education Book, 37, 435-442. https://doi.org/10.14694/EDBK_175666
Munro, M. G., Critchley, H. O. D., & Fraser, I. S. (2018). The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Obstetrics & Gynecology, 143, 393-408. https://doi.org/10.1002/ijgo.12666
Mysid. (2006). Anatomical location of the internal and external os [image]. https://commons.wikimedia.org/wiki/File:Gray1167.svg
National Cancer Institute. (2018). Oral contraceptives and cancer risk. https://www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet#r23
National Comprehensive Cancer Network. (2020a). NCCN clinical practice guidelines in oncology (NCCN guidelines®): Lynch syndrome, version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
National Comprehensive Cancer Network. (2020b). NCCN clinical practice guidelines in oncology (NCCN guidelines®): Uterine neoplasms, version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
National Institute of Biomedical Imaging and Bioengineering. (2016). Ultrasound. https://www.nibib.nih.gov/science-education/science-topics/ultrasound
Navdeep, Pal., Broaddus, R. R., Urbauer, D. L., Balakrishnan, N., Milbourne, A., Schmeler, K. M., Meyer, L. A., Soliman, P. T., Ramirez, P. T., Ramondetta, L., Bodurka, D. C., & Westin, S. N. (2018). Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstetrics & Gynecology, 131(1), 109-116. https://doi.org/10.1097/AOG.0000000000002390
Nettina, S. M. (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Olsen, M., LeFebvre, K., & Brassil, K. (2019). Chemotherapy and immunotherapy guidelines and recommendations for practice (1st ed.). Oncology Nursing Society.
Onstad, M. A., Schmandt, R. E., & Lu, K. H. (2016). Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. Journal of Clinical Oncology, 34(35), 4225-4230. https://doi.org/10.1200/JCO.2016.69.4638
OpenStax College. (2013). Uterine anatomy [image]. https://commons.wikimedia.org/wiki/File:Figure_28_02_06.JPG
Papatla, K., Huang, M., & Slomovitz, B. (2016). The obese endometrial cancer patient:
How do we effectively improve morbidity and mortality in this patient population? Annals of Oncology, 27(11), 1988-1994. https://doi.org/10.1093/annonc/mdw310
Rose, P. G., Ali, S., Moslemi-Kebria, M., & Simpkins, F. (2017). Paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma. International Journal of Gynecologic Cancer, 27(3), 452-458. https://ijgc.bmj.com/content/27/3/452
Ryan, N. A. J., Glaire, M. A., Blake, D., Cabrera-Dandy, M., Evans, D. G., & Crosbie, E. J. (2019). The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genetics in Medicine, 21, 2167-2180. https://doi.org/10.1038/s41436-019-0536-8
Sasikumar, P. G., & Ramachandra, M. (2018). Small-molecule immune checkpoint inhibitors targeting PD-1/PDL1 and other emerging checkpoint pathways. BioDrugs, 35(5), 481-497. https://doi.org/10.1007/s40259-018-0303-4.
Siegel, R. L., Miller, K. D., & Jemal, A. (2020). Cancer statistics, 2020. CA Cancer Journal for Clinicians, 70, 7-30. https://doi.org/10.3322/caac.21590
Sobczuk, K., & Sobczuk, A. (2017). New classification system of endometrial hyperplasia WHO 2014 and its clinical implications. Menopause Review, 16(3), 107-111. https://doi.org/10.5114/pm.2017.70589
Surveillance, Epidemiology, and End Results Program. (2020). Cancer stat facts: Uterine cancer. https://seer.cancer.gov/statfacts/html/corp.html
Tokhi, Y., & Weerakkody, Y. (2020). Endometrial thickness. https://radiopaedia.org/articles/endometrial-thickness?lang=us
US Food & Drug Administration. (2018). Highlights of prescribing information: Soltamox® (tamoxifen citrate). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
US National Library of Medicine. (2020). Lynch syndrome. https://ghr.nlm.nih.gov/condition/lynch-syndrome#:~:text=Inheritance%20Pattern,sufficient%20to%20increase%20cancer%20risk
World Cancer Research Fund/American Institute for Cancer Research. (2018). Body fatness and weight gain and the risk of cancer. https://www.wcrf.org/sites/default/files/Body-fatness-and-weight-gain_0.pdf
Wynder, E., Escher, G., & Mantel, N. (1966). An epidemiological investigation of cancer of the endometrium. Cancer, 19(4), 489–520. https://doi.org/10.1002/1097-0142(196604)19:4%3C489::aid-cncr2820190406%3E3.0.co;2-w.
Yarbro, C. H., Wujcik, D., & Gobel, B. H. (Eds.). (2018). Cancer nursing: Principles and practice (8th ed.). Jones & Bartlett Learning.