About this course:
The purpose of this learning module is to provide an overview of the most common vascular access devices (VADs) utilized in clinical practice for adult patients. The various guidelines regarding the care, maintenance, and assessment of peripheral access devices will be highlighted. The indications and procedures for insertion and removal, benefits, risks, and complications in conjunction with clinical decision-making for best-practice standards and enhanced patient outcomes will be outlined. This module endeavors to review and explain the most common peripheral VADs and the necessary management and assessment skills required for safe clinical practice. The introduction and revision of these devices and their use require healthcare professionals (HCPs) to update their knowledge base continually to provide the latest evidence-based practice (EBP) standards. VAD insertion, management, and use is an essential component of routine patient care. Therefore, a thorough understanding of the numerous VADs utilized in clinical practice and their management and assessment is critical for providing quality care. Please refer to Part 2 of this series to review central access devices and invasive monitoring (central venous pressure and pulmonary artery catheters).
Course preview
Upon the completion of this module, the learner will be able to:
- identify the different types of vascular access devices (VADs), the standards for insertion, and infection control measures following the 2021 Infusion Nurses Society (INS) Standards of Practice
- describe the various types of peripheral intravenous catheters (PIVs), their indications for use, site selection, placement, care, and safety considerations
- identify and manage complications for PIVs and patient-specific access by identifying contraindications for site-specific insertion
- review specialized VADs, including intraosseous and arterial catheters, and their indications for use, site selection, placement, care, and safety considerations, including complications and contraindications to insertion
- explain the interpretation and clinical guidance derived from arterial catheters
Vascular access devices (VADs) are a core component of patient care widely utilized across various healthcare settings. A VAD is a hollow tube inserted into a vein or artery through the peripheral or central vasculature. VADs have diagnostic and therapeutic uses, including fluid replacement therapy, intravenous medications, blood products, nutrition, blood sampling, and hemodynamic monitoring (Nettina, 2019).
VADs are commonly divided into two categories: peripheral intravenous (PIV) catheters and central venous catheters (CVCs). Short infusion catheters and midline catheters—the most common PIV catheters—are inserted into the upper extremity veins. In addition, specialized VADs include intraosseous catheters (IO) and arterial lines (Kornbau et al., 2015).
Standards of VAD Care
The Infusion Nurses Society (INS; Gorski et al., 2021) is an international organization established to advance the delivery of quality infusion therapy to patients through evidence-based standards, professional ethics, and education. The INS (Gorski et al., 2021) has published standards for intravenous (IV) therapy practices that apply to all patient care settings in which VADs are utilized. These practice standards seek to ensure that high-quality care is delivered for optimized patient outcomes. Hospitals and healthcare organizations utilize INS standards of care to establish institutional policies and clinical practice standards for the placement, management, and use of VADs. Therefore, this module will focus on the industry standards according to the INS Infusion Therapy Standards of Practice (Gorski et al., 2021). However, the learner should refer to their state's nurse practice act and institutional policies regarding specific practices.
VAD Selection
Device selection is based on individual patient factors, the indications for the VAD, and the duration of the prescribed therapy. Patient-specific characteristics such as age, comorbidities, and vascular integrity, in conjunction with therapeutic requirements, are essential factors to consider when selecting an optimal VAD, location, and size. Beyond the therapeutic purposes, the goal is to choose a device, size, and site that will promote vessel health and preservation while providing the necessary access required for the duration of the prescribed therapy (Campagna et al., 2018). Overall, the placement of a VAD is indicated for the administration of therapies that are not available or are less effective via a less invasive route. For example, blood products must be administered intravenously. Patients with severe nausea and vomiting can safely receive IV hydration and antiemetics via a PIV, despite being unable to take medications or fluids orally (Frank, 2020). Upper extremity sites carry a lower risk of infection than the lower extremities. A short PIV line should not be selected for therapy expected to last longer than 6 days (Kornbau et al., 2015).
Infection Control Guidelines
VADs disrupt the integrity of the skin, increasing the risk for infection with bacteria or fungi. Since the catheter provides a portal of entry and a direct pathway to the venous system, an infectious agent can quickly spread throughout the bloodstream, making the patient critically ill (Haddadin & Regunath, 2019). A breach in sterile technique during the insertion procedure can lead to an infection of the catheter or surgical site. An infection can develop if the line is not cared for adequately. Bloodstream infections can induce hemodynamic changes, leading to organ dysfunction and sepsis, which can be fatal (Centers for Disease Control and Prevention [CDC], 2017). Peripheral line-associated bloodstream infection (PLABSI), central line-associated bloodstream infection (CLABSI), and catheter-related bloodstream infection (CRBSI) are all categories of bloodstream infections (BSI) encountered in clinical practice. Since multiple catheter types can cause a CRBSI, the INS advises strict adherence to infection prevention measures during catheter insertion and all catheter management encounters. Infection control measures remain the most critical method for preventing catheter-associated infections (Gorski et al., 2021). BSI can lead to sepsis, shock, and death. The estimated cost of CLABSIs is more than $16,000 per infection, with a mortality rate of up to 25% (Kornbau et al., 2015).
Adherence to hand hygiene recommendations and aseptic techniques during all aspects of VAD insertion and care is critical for all healthcare providers. Hand hygiene should be performed using an alcohol-based rub or antimicrobial soap and water before direct contact with any VAD, including insertion and dressing changes and before and after patient contact. EBP standards have determined that the full antimicrobial effect is achieved when the skin antiseptic agent has completely dried before accessing the lines or applying the dressing. The use of antimicrobial soap and water is recommended when the hands are visibly soiled. (Gorski et al., 2021).
The INS 2016 standards discuss the importance of chlorhexidine-impregnated dressings in reducing the infection risk for patients with CVC devices. The 2021 standards noted the benefits of chlorhexidine-impregnated dressings for use with short-term, non-tunneled CVC devices with the highest level of evidence (i.e., Level I). In addition, the new standards expand the usage recommendations for patients over 18 and various catheter types, including arterial, epidural, dialysis, and implanted ports (Gorski et al., 2021).
The INS (Gorski et al., 2021) standards recommend active disinfection of all needleless connectors, ports, or hubs with a 70% isopropyl alcohol or alcohol-based chlorhexidine swab pad and air drying before access to reduce the introduction of intraluminal microbes. Passive disinfection with disinfecting caps is also acceptable. Both active and passive disinfection were associated with lower rates of catheter-associated bloodstream infection. However, 70% isopropyl alcohol swabs were less effective based on a meta-analysis of quasi-experimental studies (Gorski et al., 2021).
The INS (Gorski et al., 2021) recommends using catheter-associated skin injury (CASI) guidelines to describe any localized skin damage around the VAD site, including drainage and erythema below a dressing. Likewise, using a sterile alcohol-free skin barrier compatible with an antiseptic solution to protect at-risk skin is now recommended. The INS standards also address the global COVID-19 pandemic, highlighting the need to expand standard precautions (e.g., fit-tested certified N95 respirators and additional hand hygiene protocols) to prevent cross-contamination for HCP and patient safety (Gorski et al., 2021).
The aseptic non-touch technique (ANTT), a new term introduced in the INS 2021 guidelines, builds on the original concept of key-parts and key-site protection to maintain asepsis. As it relates to VADs, the entrance site of the VAD would be considered a key-site, and the key-parts of a VAD would include the syringe tip, the IV tubing spike,
...purchase below to continue the course
The importance of implementing EBP standards to improve healthcare quality prompted the Institute for Healthcare Improvement (IHI, 2012) to establish clinical practice bundles. Bundles are concise and straightforward guides intended to assist HCPs in delivering consistent and reliable care. These EBP interventions improve the processes of care and patient outcomes. There are several types of best-practice bundles for CVC care, which are revised and adapted to the specifics of each healthcare organization. While there are alterations based on institutional policies, an extensive literature review demonstrated that the vast majority are premised on five essential components of care geared toward preserving the integrity and preventing infection of central lines (IHI, 2012). These five components include:
- using proper hygiene and sterile contact barriers
- properly cleaning the patient’s skin
- finding the best vein possible for the IV
- checking every day for signs or symptoms of infection
- removing or changing the line only when needed
Since October 2008, the Centers for Medicare and Medicaid Services (CMS) no longer reimburse for hospital-acquired conditions, including CLABSI. In addition, the CDC published revisions to their 2011 Intravascular Catheter-Related Infections Guidelines in 2017. In partnership with several other accredited organizations, these guidelines determined the EBP standards for preventing CLABSI and other healthcare-associated infections. A comparison of the CDC, IHI, and INS guidelines shows predominantly consistent recommendations. Table 1 offers an overview of these critical aspects of VAD care (CDC, 2017; Gorski et al., 2021; IHI, 2012).


In addition to optimizing VAD care, patient selection and risk stratification are essential in CLABSI prevention. Herc and colleagues (2017) performed a retrospective model-based study to establish CLABSI risk factors, estimating an individual’s risk before PICC placement. Their proposed model performed well and could inform patient selection and surveillance practices for high-risk groups, although it should first be validated for clinical practice. Their model, the Michigan PICC-CLABSI (MPC) score, assigns points for the presence of:
- hematological cancer (3 points)
- a CLABSI in the last 3 months (2 points)
- placement of a multi-lumen PICC (2 points)
- ongoing chemotherapy for a solid tumor/cancer (2 points)
- receipt of parenteral nutrition (1 point)
- another CVC at the time of PICC placement (1 point; Herc et al., 2017)
The most common pathogens found in CRBSI are S. aureus and S. epidermidis (Kornbau et al., 2015). Outside of pulmonary artery catheters, non-cuffed, femoral, and multi-lumen lines have the highest infection rate. Arterial catheters have a slightly higher infection rate than cuffed or tunneled CVCs and PICCs, and short PIVs have a somewhat higher rate than midline catheters (Jacob & Gaynes, 2021). Evidence supports the use of antimicrobial-impregnated catheters to prevent infection. The availability of alternative venous access and the severity of the illness should be considered when deciding whether a potentially infected VAD should be removed. Two blood cultures should be drawn from two different sites and sent for culture, along with the catheter tip (if removed) for a suspected infection. Broad-spectrum antibiotics should be started until the organism and effective antibiotics can be identified, at which time antibiotic treatment should be appropriately focused (Kornbau et al., 2015). A CRBSI that persists despite 48-72 hours of appropriate antimicrobial coverage should prompt the removal of the infected VAD (Gorski et al., 2021). Replacement of a temporary catheter over a guidewire for a patient with bacteremia is not recommended, as the existing skin tract is often colonized. Prophylactic catheter replacement at scheduled intervals has not been shown to reduce infection rates, and replacing functioning VADs without evidence of complications is unnecessary (IHI, 2012). Most guidelines recommend replacing VADs based on clinical indications, not a predetermined time frame. However, the risk for infection increases in short PIVs after 3-4 days of dwell time, arterial catheters after 4-6 days, and CVCs after 6 days. As a result, guidelines regarding the routine replacement of various VADs do not exist. Most research encourages close observation of VADs for indications of complications/malfunction, replacement when clinically indicated, and immediate removal when no longer required. The exception to this recommendation is the replacement of all emergently-inserted catheters as soon as possible (or within 48 hours of placement) as aseptic technique cannot be confirmed. Most research recommends replacing IV infusions sets at 4- to 7-day intervals to reduce the risk of infection, except as indicated for chemotherapeutics, blood products, inotropes, and lipid infusions (Jacob & Gaynes, 2021).
Documentation
Regardless of the type of VAD utilized, documentation is a critical component of practice. Documentation should be comprehensive, occur promptly, and include all of the following:
- type, length, and size of the device
- date and time of insertion and the number of attempts
- type of stabilization device
- patient tolerance of insertion
- identification of insertion site location
- radiographic confirmation of tip location if indicated
- condition and appearance of potential site complication
- specific site preparation, infection control, and safety precautions as appropriate for the procedure
- device discontinuation, date, condition, site appearance, dressing applied, the reason for removal, and patient response (Campagna et al., 2018; Gorski et al., 2021)
Peripheral VADS
Short PIV Catheters
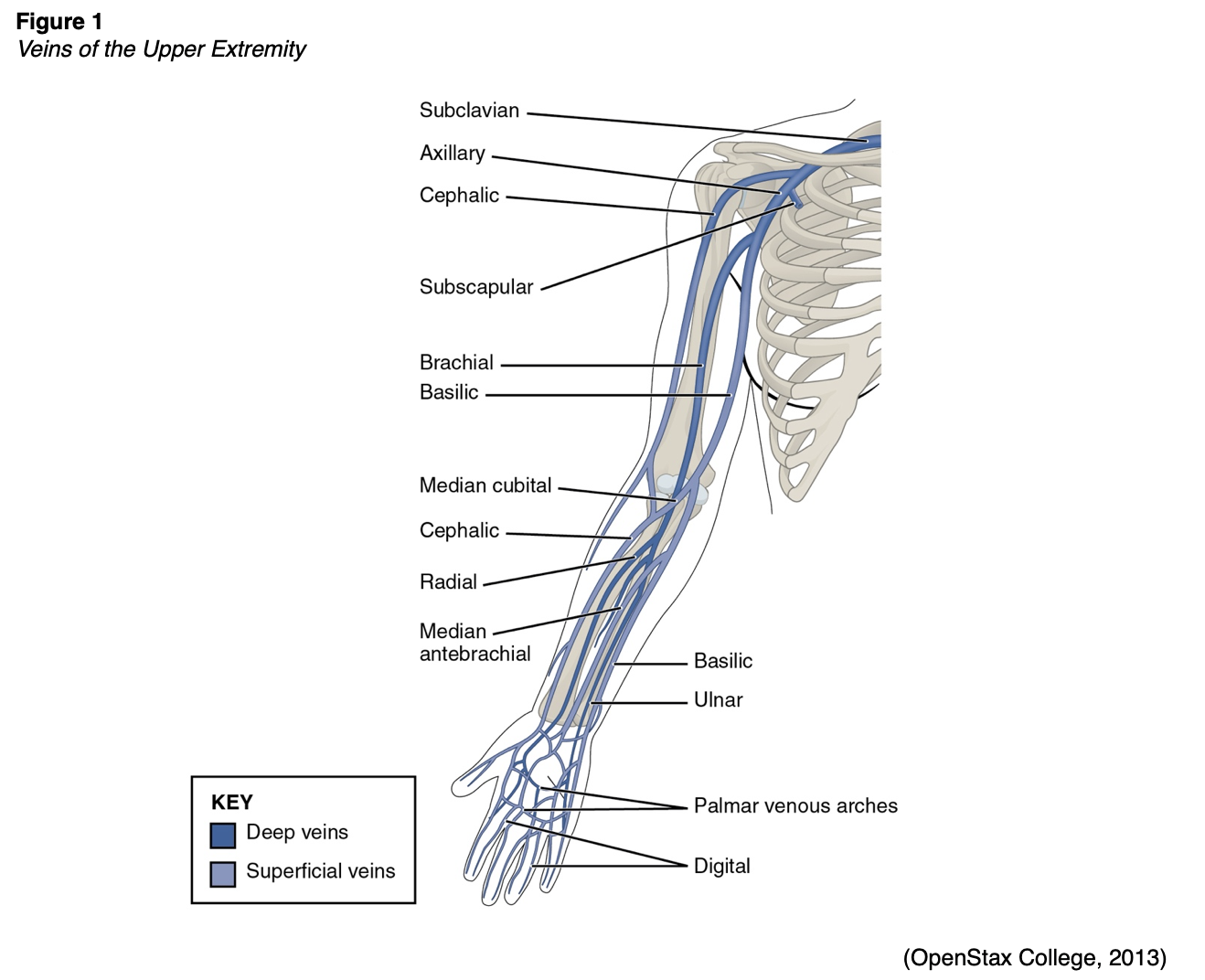
Short (or standard) PIV catheter insertion is one of the most common clinical procedures for hospitalized patients; virtually all hospitalized patients have at least one PIV inserted per hospital stay (Alexandrou et al., 2018). PIVs are short-length catheters (under 3 inches or 7.5 cm) intended for short-term therapy. They are inserted into the small veins in the dorsal and ventral surfaces of the upper extremities, including the metacarpal, cephalic, basilic, and median veins (Gorski et al., 2021). Refer to Figure 1 for an illustration of the veins within the upper extremity. The specific duration of these PIV catheters remains controversial and is undetermined at this time. Infusion standards have historically recommended that PIVs for adult patients are rotated every 72 to 96 hours. According to the CDC (2017) guidelines, there is no need to replace PIV catheters more frequently than every 96 hours for asymptomatic adults. Short PIVs may remain until removal is clinically indicated, with definitive timelines deferred to institutional policy (CDC, 2017).
Catheter Size
PIV catheters are available in a variety of sizes, or gauges (G), ranging from 24G (smallest) to 14 G (largest; Gorski et al., 2021). PIV gauges are universally color-coded. Table 2 provides a general overview of PIV gauges and their common uses (Pedagogy Online Learning Systems, 2016).

Catheter size impacts device functionality. Among adult hospitalized patients, catheters of 18G or larger showed increased rates of thrombosis and phlebitis, and catheters of 22G or smaller had higher rates of dislodgment, occlusion, and infiltration. Per the INS guidelines, the smallest gauge that can accommodate the prescribed therapies and meet the patient's needs should be selected. The potential needs of the patient must be considered when selecting a catheter size. Notwithstanding these recommendations, choosing a smaller size for long-term therapy when a larger size is attainable potentially subjects the patient to dislodgement or occlusion, requiring another venipuncture and the risks and discomfort associated with this procedure (Gorski et al., 2021).
Site Placement
The site of PIV placement is premised on the clinical judgment of the HCP, who must consider individual patient factors and the clinical situation. The INS recommends the placement of the PIV in an area of non-flexion, such as the forearm, to provide stability and reduce patient discomfort (Gorski et al., 2021). Emergently, PIV access can be established in the antecubital fossa if needed, but this is not the first choice for nonemergent access. The nondominant arm should be used if possible (Frank, 2020). The HCP should start distally with the dorsal surface of the hand and progress proximally to preserve peripheral access (Alexandrou et al., 2018). The use of the most distal site for VAD insertion affords additional proximal sites for future or repeated cannulation. The choice of a proximal site as the initial site of insertion negates the use of a distal location in the future due to the potential risk of extravasation of administered fluids or medications from the initial site. The reverse is far less likely to occur unless multiple catheters are inserted into the same limb along the same venous network. Lower-extremity access (greater saphenous or dorsal metatarsal veins) should only be considered if upper-extremity access is unavailable (Frank, 2020). A short PIV may be temporarily placed in the external jugular in emergency scenarios without alternatives through collaboration with a licensed provider (Frank, 2020; Gorski et al., 2021). Veins in the foot may be considered for neonates and infants who are nonambulatory, and scalp veins are considered a last resort (Gorski et al., 2021).
Some therapies, such as vesicants (e.g., chemotherapy), should never be infused through a hand, wrist, or antecubital vein (Alexandrou et al., 2018). Hand veins should only be utilized for short-term therapy (i.e., under 24 hours) due to increased failure rates with longer dwell times (Gorski et al., 2021). VADs should not be placed in the veins of an upper extremity on the same side as a previous breast surgery with axillary lymph node dissection, in the setting of lymphedema, or with a known deep vein thrombosis (DVT) due to heightened risks for infection and thrombotic complications. Additional contraindications include the presence of a hemodialysis catheter (e.g., an arteriovenous fistula [AVF]), current or recent infection (e.g., cellulitis), fracture, burn injury, or neuromuscular dysfunction related to a central nervous system injury (e.g., hemiparesis, hemiplegia). These contraindications apply to PIVs, midline catheters, and PICC lines (Nettina, 2019). An upper extremity PIV is only relatively contraindicated in a limb with neuromuscular dysfunction (Frank, 2020). Refer to Figure 1 for an overview of the interconnectedness of the vasculature.
Placement of a PIV
Implied consent is typically considered acceptable for PIV placement, but the procedural steps and risks should be discussed with the patient in nonemergent situations. The patient should be warm and calm, as cold and anxiety lead to vasoconstriction and make PIV placement more challenging. Excessive hair should be clipped, not shaved. A topical anesthetic (1-2 g of lidocaine per 10 cm2) can be used if required, and sufficient time should be given for the medication to take effect (30-60 minutes). The selected limb should be extended on a stable surface slightly below the level of the heart to enhance venous dilation. A rubber tourniquet can be placed 5-10 cm proximal to the intended site. The vein can be gently tapped or stroked (i.e., proximal to distal) to enhance dilation further, or the patient can alternately clench and relax their fist on that side. Finally, a warm compress can be placed over the intended site for several minutes. The site should be palpated, and access should be planned. Hand hygiene should be performed, and the appropriate PPE should be donned for standard ANTT (clean gloves, protective eyewear). Unlike CVC placement, surgical ANTT is not recommended for PIVs unless indicated by institutional policy (Frank, 2020).
Several options can assist with locating a vein, such as infrared light (e.g., VeinViewer) or an LED light at a specified frequency that highlights deoxygenated blood (e.g., Veinlite LED). A Doppler ultrasound can visualize veins larger than 2 mm. PIV placement should not be attempted in the proximal (upper) arm without ultrasound guidance due to iatrogenic arterial or nerve injury risk. The site should then be scrubbed with chlorhexidine or alternative skin cleanser per institutional protocol and not touched after being cleaned. The limb can be stabilized with the nondominant hand, using the thumb to gently pull on the intended vein without excessive pressure, which may unintentionally collapse the vein. The access catheter should be held in the dominant hand between the forefinger and thumb, ensuring the bevel is facing upward. The needle should be inserted at a 10-30° angle with the skin in a slow, continuous motion. Once a flash of blood in the chamber is visualized, the angle of the catheter should be lowered, and the tip advanced another 1-2 mm to ensure the entire catheter tip—not just the needle tip—has been inserted into the lumen of the vein. Next, the catheter should be advanced into the vein using the forefinger of the dominant hand without moving the needle hub. The needle should be retracted, the tourniquet removed, and the catheter secured. The insertion device should be disposed of safely in a sharps container. An IV infusion set or syringe can be attached to the catheter. Fluids and medication should infuse easily without discomfort, resistance, or swelling at the site (Frank, 2020). After two unsuccessful attempts to cannulate a PIV by the same HCP, the task should be escalated to a clinician with a higher skill level, or an alternate form of access should be considered (Gorski et al., 2021).
If resistance is encountered as the operator attempts to advance the catheter, it may have advanced too far through the posterior wall of the vein, in which case it should be withdrawn slightly until another flash of blood appears. Alternately, the needle may have entered the vein, but the catheter has not, in which case the needle should be advanced slightly before advancing the catheter. Resistance when advancing the catheter may be related to a valve or tortuous portion of the vein. This may be addressed by instilling sterile saline from a small syringe into the catheter while advancing. An appropriately placed PIV should provide steady venous blood flow with no swelling at the puncture site. Swelling at the site may indicate an extravenous placement. This catheter should be removed, pressure should be applied, and a dressing should be placed over the area. Arterial cannulation is characterized by bright red pulsatile blood flow, which produces a waveform if a transducer is attached. If a second attempt needs to be made within the same vein or extremity, this should be done proximal to the first attempt (Frank, 2020).
Securing the PIV to limit movement is recommended to reduce the risk of inadvertent dislodgement and thrombophlebitis (Alexandrou et al., 2018; Frank, 2020). Proper securement decreases patient reports of pain, fear, and anxiety related to device replacement, in addition to reducing healthcare costs. Tissue adhesive (TA) such as cyanoacrylate can be applied to seal the insertion site and bond the catheter hub to the skin, which is then covered by a transparent dressing (e.g., Opsite, Tegaderm). TA should be reapplied with each dressing change and offers immediate hemostasis at the insertion site. It may also prolong the interval until the first dressing change. Alternately, PIVs may be secured with an integrated securement device (ISD, e.g., SorbaView), which combines a transparent dressing with built-in securement technology. PIV catheters should not be secured using non-sterile tape, suture material, or a rolled bandage (Gorski et al., 2021). A splint may be required for PIVs placed along a joint line or in the patient’s dominant hand to limit motion (Frank, 2020).
Care of PIVs
The PIV site must be assessed at least once per shift for adult patients. Proper assessment involves monitoring for signs of malfunction, infection, displacement, or pain. Best practice guidelines recommend the prompt removal of symptomatic devices, such as when phlebitis or other complications are suspected, as well as when the catheter is no longer required (Alexandrou et al., 2018). PIVs should be assessed regularly for redness, edema, tenderness/pain, or resistance to flushing. Pain, swelling, and a slow infusion may indicate extravasation of fluid (Frank, 2020). Accurate documentation regarding the insertion, maintenance, and removal of PIVs in the medical record is considered best practice and is the policy of most healthcare facilities. PIVs have limitations regarding therapeutic usage. Short PIVs are not appropriate for continuous vesicant therapy, parenteral nutrition, infusions with a pH below 5 or above 9, or infusions with an osmolality over 900 mOsm/L (Gorski et al., 2021; Nettina, 2019). PIVs being used for intermittent therapy (i.e., not continuous infusion) should be flushed with 2-10 mL of sterile saline after every medication administration or at least every 4-12 hours (Frank, 2020).
Removal of PIVs
As previously mentioned, PIV removal is performed when clinically indicated and not on a predetermined timeline. While removal procedures vary by institution, the nurse should collect the required supplies, perform hand hygiene, and don clean gloves. The dressing and any securement device should be removed while stabilizing the catheter in place. A sterile 2x2 gauze can be placed over the entry site as the catheter is withdrawn slowly and steadily. Pressure should be held on the entry site after removal for 2-3 minutes, and a gauze/tape or band-aid should be applied for 12-24 hours to ensure bleeding has stopped. All equipment should be disposed of safely, hand hygiene should be repeated, and the procedure should be documented in the patient’s chart (Thomas, 2018).
Midline (PIV) Catheters
A midline catheter is a deep peripheral catheter intended for intermediate-term therapy (more than 5 but fewer than 28 days). These catheters are inserted 1.5 inches proximal to the antecubital fossa into the basilic, cephalic, or brachial veins. Veins in the leg (e.g., saphenous, popliteal, or femoral) may also be considered for pediatric or neonate patients. The tip should then terminate below the inguinal crease. Scalp veins may be used as a last resort for pediatric patients with the tip located in the neck above the thorax (Adams et al., 2016; Gorski et al., 2021). Midline catheters vary in length, ranging from 3 to 8 inches (8 to 20 cm). They extend up the arm with the proximal tip resting just distal to the axillary arch. Ideally, they should be used for a maximum of 2 weeks (Gorski et al., 2021). Midline catheters should be considered for patients who need intermediate-term IV therapy when standard PIV access is difficult.
Midline catheters do not dwell in the central circulation. They offer several advantages over central lines and standard PIV catheters. A midline catheter reduces the need for repeated venipunctures for patients with difficult peripheral venous access and poses significantly lower complication rates than CVCs. Midline catheters are associated with decreased risks of infection and catheter-related thrombosis while allowing for prolonged use. Some midline catheters are power-injectable and can tolerate high flow rates, allowing for the administration of iodinated contrast for enhanced radiographic studies. This use depends on the type of device inserted and institutional policy. Midline catheters are functionally similar to a PICC line (Adams et al., 2016). Xu and colleagues (2016) found in a retrospective study of 367 patients that midline catheters resulted in more numerous yet less severe (i.e., non-life-threatening) complications than PICC lines, which led to fewer yet more serious complications. Figure 2 shows the key distinctions between a midline catheter and a PICC line.

A midline catheter is usually inserted in the patient's nondominant arm via ultrasound guidance, which decreases the risk of cannulation failure, arterial puncture, and hematoma. Surgical ANTT is used when placing a midline catheter in most institutions (Gorski et al., 2021; Nettina, 2019). First, the vein is cannulated using an introducer needle, micropuncture needle, or angiocatheter (Heffner & Androes, 2021). The operator should occlude the needle hub with their thumb when the guidewire is not in place to avoid air entrainment/embolism, which can occur if air is externally introduced into the systemic circulation (MCarthy et al., 2016). Next, the guidewire is inserted through the needle or angiocatheter, and its position is confirmed via ultrasound or fluoroscopy (Heffner & Androes, 2021). The needle or angiocatheter is removed while the guidewire is carefully stabilized. A single stab incision may be required adjacent to the guidewire to introduce a tissue dilator or a coaxial dilator/sheath, which is inserted over the guidewire, keeping the guidewire’s position unchanged. Then, the tissue dilator is removed, leaving either a guidewire or a sheath to guide catheter insertion, depending on the particular kit. The catheter is then threaded over the guidewire (or through the sheath) and held in place while the guidewire (or sheath) is removed. This procedure was originally termed the Seldinger technique (ST) and has since been adapted and termed the modified Seldinger (MST, Heffner & Androes, 2021; Stoker, 2009). A catheter/needle combination may also be inserted initially during an MST approach, allowing for the advancement of a catheter early, followed by needle removal. This catheter serves as the conduit for the guidewire, followed by a tissue dilator and, finally, the indwelling catheter (Song et al., 2018).
A chest x-ray is not required for catheter tip verification following insertion. The midline is anchored to the skin with a securement device to reduce the risk of inadvertent dislodgement (Nettina, 2019). The INS recommends securing midline and other long PIVs using a method such as TA, an ISD, an adhesive securement device (ASD), or a subcutaneous anchor securement system (SASS). TA and ISD procedures mirror those described for short PIVs. An ASD (e.g., StatLock) anchors the catheter to the skin. A SASS contains flexible feet placed beneath the skin to stabilize the catheter at the insertion site. Both the ASD and SASS should be covered with a transparent dressing. ASDs should be replaced with dressing changes per the manufacturer’s guidelines, while a SASS will remain in place during dressing changes (Gorski et al., 2021). The exit site should be covered with a transparent dressing to facilitate adequate assessment. Most midline catheters need to be replaced every 28 to 30 days, although some midline catheters can remain for longer durations (Nettina, 2019). The device's manufacturing guidelines and institutional policies should be referenced for a specific replacement timeline (Gorski et al., 2021).
Care of Midline Catheters
The care of midline catheters includes measuring and documenting arm circumference before insertion and while the catheter is in situ as clinically indicated to monitor for an increased circumference of the extremity due to edema, which can signal a DVT. The measurement should be taken about 10 cm above the antecubital fossa. Basic flushing protocols include the use of 10-20 mL of 0.9% preservative-free saline solution following each infusion of medication. The line should be flushed every 12 hours when used for intermittent infusions. Since many midline catheters are equipped with a valve system to prevent the backflow of blood, they do not require heparin flushes to maintain patency (Gorski et al., 2021). The transparent dressing should be changed weekly or sooner if it becomes visibly soiled, loose, or damaged (Nettina, 2019).
Limitations of Midline Catheters
Midline catheters are not suitable for continuous vesicant therapy, parenteral nutrition, or the administration of certain types of antibiotics, such as erythromycin (Erythrocin), vancomycin (Vancocin), or nafcillin (Penicillin). Dextrose concentrations greater than 10% are contraindicated, as well as infusions with a pH below 5 or over 9 or with an osmolality greater than 600 mOsm/L (Nettina, 2019). A midline catheter should not be placed in patients with a history of thrombosis, hypercoagulable blood clotting disorders, or currently decreased venous flow. Additionally, blood samples should not be drawn from a midline catheter. HCPs and caregivers should avoid performing any blood pressure assessments or venipunctures on an extremity with an indwelling midline catheter. As with other VADs, midline catheters should not be placed in an arm following axillary lymph node dissection or in the presence of lymphedema. Since midline catheters and PICC lines are placed in similar locations, documentation in the medical record must indicate which type of line has been inserted. Appropriate and clear documentation can prevent confusion between a midline catheter and a PICC device and inappropriate use (Gorski et al., 2021).
Extended-Dwell PIV Catheters
Extended-dwell PIVs are similar to midline catheters; they are FDA-approved for a dwell time of 29 days. Unlike midline catheters, they are shorter in length, ranging from 6 cm to 15 cm. They are considered ideal alternatives for patients with difficult peripheral venous access and require extended IV therapy (Bahl et al., 2019). They are typically placed within the cephalic, basilic, or median veins of the forearm without crossing into the antecubital fossa (Gorski et al., 2021). Extended-dwell PIVs are instrumental in emergency department settings, as they can be placed at the bedside by specially trained IV nurses (Bahl et al., 2019).
Complications Associated with PIVs
PIVs are associated with high complication rates, including insertion difficulty, phlebitis, infiltration, occlusion, dislodgment, and PLABSI. Several studies have demonstrated that up to 90% of PIVs malfunction before therapy is completed and are removed (Alexandrou et al., 2018). Catheters placed during emergencies are more prone to complications (Frank, 2020). When an initial catheter fails, vascular access often becomes problematic, compromising patient care and safety. The use of ultrasound guidance with VAD insertion has improved insertion success and reduced premature catheter failure (Bahl et al., 2019; Kornbau et al., 2015). As is the case for other VADs, the number of unsuccessful attempts is the most accurate predictor of complications. The experience level of the HCP placing the VAD also strongly influences immediate complication rates. Immediate complications for any VAD include bleeding or hematoma due to venous or arterial injury. Arterial injury is the most crucial to identify immediately to limit bleeding. The pulsatile flow of blood is characteristic of arterial insertion but may be less evident in a hypotensive or critically ill patient (Kornbau et al., 2015). Refer to Table 3 for an overview of the most common PIV complications.

Careful assessment of any VAD before and during each flush and medication administration may help prevent or identify complications early and allow for prompt intervention. If a complication is suspected, alternative access should be explored and established if required. A supervising physician should be notified of suspected medication extravasation and details thoroughly documented in the patient’s chart. This documentation should include the affected site, symptoms, medication and amount (approximate), and any treatments provided. Most institutions are equipped with protocols regarding symptomatic treatment (e.g., cold or warm compresses) and antidote therapies (if available) that should be administered based on the situation. The administration set should be detached, and any residual medication should be aspirated from the catheter hub before removing the VAD. Infection control guidelines should be followed for prevention and management purposes. Risk factors for VAD thrombosis include a history of prior venous thromboembolism (VTE), acute critical illness, presence of coagulopathy, recent surgery or trauma, extremes of age, pregnancy, oral contraceptive use, and certain chronic conditions (e.g., end-stage renal disease, diabetes, irritable bowel syndrome, and cancer). Insertion sites in the upper extremity typically confer a higher risk of thrombosis when compared to the internal jugular or subclavian (Gorski et al., 2021; Lippincott Nursing Center, 2019).
The smallest catheter size should be used for the shortest time with the fewest lumens required. Early mobilization and adequate hydration also reduce the risk of VTE for all patients. The 2021 INS standards recommend against removing a venous catheter based only on the presence of a DVT. However, if the VTE is confirmed with imaging studies, treatment should commence as soon as possible per the current VTE management guidelines. A VAD that appears occluded should be inspected for any areas of obvious crimping. For an upper extremity device, the patient should be instructed to maintain a straight arm during infusions to avoid internal occlusions. The insertion site should be examined for potential catheter migration, and the possibilities of medication precipitate (i.e., combining incompatible medications or the infusion of a high-precipitate drug such as furosemide [Lasix] in 5% glucose solution or amphotericin [Amphocin] in 0.9% normal saline) or thrombus formation should be considered. Facility protocols regarding flushing and locking VADs should also be carefully followed. An occluded PIV is often removed and replaced with a new access point, but in extreme situations with limited access options, fibrinolytic treatment may be considered per facility standards (Gorski et al., 2021; Lippincott Nursing Center, 2019).
Thrombophlebitis occurs in up to 15% of patients with a PIV. This risk is mitigated by using the upper extremity veins (avoiding the lower extremity sites described above), minimizing catheter movement through proper securement and splinting, using the smallest catheter size appropriate for the therapy required, and removing the catheter when no longer needed. Rarely, PIVs have also been associated with septic discitis, venous air embolism, pneumocephalus, skin necrosis, bacteremia, compartment syndrome, nerve/tendon/artery injury, and venous aneurysm (Frank, 2020).
Nerve damage is also possible with PIV insertion and should be considered. For example, the cephalic vein (see Figure 1) at the wrist may traverse near the superficial radial nerve. The median nerve is at risk when cannulating the wrist's volar (inner) portion, and the interosseous nerves and antebrachial nerves are at risk when a PIV or PICC line is inserted at or just proximal to the antecubital fossa (Gorski et al., 2021).
Specialized Vascular Access Devices
Intraosseous (IO) Cannulation
Intraosseous cannulation provides access to the vasculature located within the long bones and is generally reserved for critically ill patients who require rapid access for stabilization in emergent situations (Petitpas et al., 2016). Among adults, IO cannulation may be used in battlefield settings and cases of trauma, hemorrhage, or cardiac arrest when IV access is not available or unable to be obtained rapidly. Adults usually require a 15G needle (2.5 cm long) for IO access. There are three categories of IO devices: manual, impact-driven, and drill-powered needles (Gorski et al., 2021). Figure 3 displays an example of an IO needle insertion device, and Figure 4 demonstrates the placement of an IO catheter.


The most IO common insertion sites for adults are the proximal tibia and humerus. The sternum should be avoided, as it is too thin to accommodate the needle and could lead to pneumothorax and impede resuscitation. Before placing an IO device, the extremity should be restrained, and the skin should be prepped using aseptic technique with > 0.5% chlorhexidine in alcohol solution, povidone-iodine, or 70% alcohol. Catheter insertion must be performed by a specially trained nurse or licensed clinician (Petitpas et al., 2016).
Care of IO Devices
The IO site must remain covered with a sterile dressing, and the device should be stabilized and secured to prevent movement out of the bone. Proper placement of the IO device can be confirmed by assessing needle positioning and the ability to flush at least 5 mL of 0.9% normal saline easily without signs of infiltration. The ability to aspirate blood or bone marrow may also be used to confirm needle placement; however, aspiration may be difficult for some patients, especially those experiencing severe dehydration. Needle placement and the surrounding site should be assessed frequently and reevaluated before using the device, especially when highly irritating agents or large volumes will be infused (Gorski et al., 2021).
The same doses of IV fluids and medications that can be infused through a PIV may be infused through an IO device. However, an infusion pump is often required for rapid flow rates. IO devices become clogged or clotted with bone marrow more quickly than with PIV access. Clogging can be avoided by maintaining a connection to a continuous flow of IV fluids or frequent flushing to prevent occlusion. IO catheters should only be used during the immediate trauma or resuscitation period while the patient is stabilized. IO catheter dwell time should be limited to a maximum of 24 hours (Gorski et al., 2021).
Complications of IO Cannulation
Complications associated with IO devices are uncommon but can include local infection at the site, infiltration, extravasation, catheter dislodgment, and compartment syndrome. Compartment syndrome is a condition in which increased tissue pressure in a confined anatomic space decreases circulation to the area, leading to tissue hypoxia and pain. HCPs must monitor for discoloration, mottling, coldness, or swelling of the extremity. Compartment syndrome is a medical emergency requiring immediate intervention. Failure to perform a fasciotomy to release the localized pressure resulting in tissue hypoxia can result in permanent damage, and amputation of the limb may be required. Rarely, IO devices can lead to other serious complications such as fat emboli, iatrogenic fracture, and osteomyelitis. The risk for osteomyelitis, or severe infection of the bone, increases when the dwell time extends beyond 24 hours (Gorski et al., 2021).
Contraindications to IO Insertion
The INS lists the following absolute contraindications to IO placement:
- compartment syndrome in the extremity
- previously used IO site or a recently failed IO attempt
- fracture adjacent to the intended site
- prior orthopedic surgery or implanted orthopedic hardware
- infection, severe burns, open wounds, or tissue necrosis at or near the intended site
- local vascular compromise
- bone disease such as osteoporosis or osteogenesis imperfecta (Gorski et al., 2021)
Arterial Catheters
Arterial lines are distinct from PIVs and CVCs in several ways. An arterial line is a thin, flexible tube placed into an artery and is most commonly used in operating rooms and ICU settings. Arterial lines are primarily inserted for hemodynamic monitoring (i.e., beat-to-beat blood pressure monitoring) and frequent laboratory testing or blood sampling. They provide easy access for routine blood samples to monitor oxygen saturation and carbon dioxide levels in critically ill patients. Arterial blood pressure (mean arterial pressure, or MAP) is a measurement of the pressure exerted on the walls of the arteries, which directly affects the perfusion of oxygen and nutrients to the tissues and the removal of waste products (Butterworth et al., 2013; Nagelhout & Plaus, 2014).
Selection Site for Artery Cannulation
The radial artery is the most common site of arterial catheter placement in adults due to its accessibility (i.e., secondary to its superficial location) and collateral flow. The ulnar artery is usually not chosen because it is much deeper and tends to be torturous. Although the radial and ulnar arteries ultimately join together to provide flow to the hand, the ulnar artery carries greater blood flow, and damage from insertion could result in a loss of function up to and including loss of digits or the hand. The brachial artery is large and easy to palpate, but its presence in the antecubital fossa leads to an increased risk of catheter kinking. The brachial artery should not be used in pediatric patients due to a lack of collateral flow. The axillary artery is rarely used because of the risk of nerve damage from hematoma formation or traumatic cannulation. The femoral artery conveys an increased risk of infection and hematoma formation. Finally, the dorsalis pedis and posterior tibial artery are a long distance from the aorta, often leading to distorted waveforms in adults; however, they may be appropriate for pediatric patients (Butterworth et al., 2013; Gorski et al., 2021). Like venous catheters, arterial catheterization (and arterial puncture for one-time sampling) is contraindicated in the presence of insufficient collateral or peripheral circulation, local infection, thrombus, or abnormal anatomy due to previous surgery (e.g., radial artery harvesting), congenital malformation, trauma, burn injury, aneurysm, stent placement, AVF, or vascular graft. It is also contraindicated in severe peripheral artery disease or active Raynaud’s syndrome of the intended artery. Arterial catheters are also contraindicated in those with a platelet count below 50,000/µL (50 x 109/L). The use of therapeutic anticoagulation is a relative contraindication, but an antiplatelet agent is not (Theodore, 2020; Weiner et al., 2017).
Collateral circulation should be confirmed before cannulation using the original or modified Allen’s test. This test involves elevating the wrist with a closed fist to allow the blood to drain from the hand; the investigator then compresses the ulnar and radial arteries. Next, the hand is lowered, opened, and observed as the pressure is removed from the ulnar artery only. Healthy blood flow from the ulnar artery and an intact superficial palmar arch is indicated by a return to pink from the initial white in the patient’s palm. In the original Allen’s test, the process is repeated while only releasing the radial artery pressure. The same test can be performed on the dorsalis pedis artery to assess collateral flow with the posterior tibialis. Alternatives include doppler flow, finger pulse plethysmography, and measurement of the arterial pressure of the thumb, but these are rarely used clinically (Theodore, 2020).
Techniques for Arterial Cannulation
An arterial line can be inserted at the bedside by a specially trained nurse, licensed practitioner, or physician. Similar to central line placement, the insertion of an arterial line is a sterile procedure requiring appropriate skin antisepsis with a chlorhexidine-alcohol solution. The practitioner is advised to wear a cap, mask, sterile gloves, and eyewear, and a small, sterile drape is used. A 20G catheter is recommended for adults and simplifies blood sampling and vigorous waveform interpretation (Gorski et al., 2021). A local anesthetic may be used for conscious patients. Typically, 0.5-1 mL of 1% to 2% lidocaine without epinephrine is injected subcutaneously, creating a dermal papule for enhanced patient comfort (Theodore, 2020).
The INS recommends ultrasound guidance to increase first-attempt success when placing arterial catheters (Gorski et al., 2021). Ultrasonography can increase first-attempt success and decrease the failure risk when used for real-time guidance throughout the placement procedure. However, ultrasound guidance does not reduce the time needed for insertion or improve the overall success rate. Either B-mode or color duplex and both transverse (i.e., short axis) or longitudinal views can be utilized. Most clinicians opt for B-mode (due to fewer artifacts) and combination or transverse views. Ultrasonography may reduce the risk of hematoma or aneurysm by identifying pseudoaneurysms, AV fistulas, or atheroma (i.e., plaque deposit along an artery wall). It may also reduce the risk of damage to adjacent structures, especially when attempting to avoid injury to the brachial plexus during axillary catheterization (Theodore et al., 2020).
There are two generally accepted techniques for arterial line insertion: (a) direct cannulation and (b) through-and-through technique. In direct cannulation, the operator’s nondominant hand palpates the artery as the dominant hand inserts and then manipulates the catheter. Direct cannulation is typically performed with the assistance of a guidewire to prevent sheering of the artery and thread the catheter through the arterial ("pressure”) side of the vasculature. As the name implies, direct cannulation involves puncturing the artery directly and threading the guidewire through the cannulated vessel. An intravascular catheter with an inner needle should be inserted at a 30° to 45° angle and advanced slowly until pulsatile blood flow is observed. With an integral guidewire (most common), the angle should be decreased after pulsatile blood flow is noted so that the needle/guidewire/catheter is nearly parallel to the skin and artery. If pulsatile flow ceases, the entire unit should be advanced slightly until flow returns. If the pulsatile flow continues, the guidewire should be advanced with the dominant hand. The catheter is then advanced over the needle-guidewire before removing the needle-guidewire and securing the catheter. If a separate guidewire is utilized, the catheter should be advanced slightly after the pulsatile flow is first observed to ensure that the catheter tip is within the artery’s lumen. The intravascular catheter is then stabilized with the operator’s nondominant hand while the needle is removed. If pulsatile blood flow is not observed, the catheter should be withdrawn slightly until pulsatile flow returns. Next, the guidewire is inserted through the catheter into the artery’s lumen, well beyond the catheter’s tip. The catheter is advanced along the guidewire into the artery before removing the guidewire and securing the catheter. Performing direct cannulation without the aid of a guidewire is only recommended for experienced operators. The process mirrors the integral guidewire steps described above for the initial puncture. After the angle of the needle-catheter unit, it should be advanced another 1-2 mm, observing for continued pulsatile flow. This ensures that the catheter tip is now within the artery lumen, as the needle tip extends beyond the catheter tip by 1-2 mm. If pulsatile blood flow stops, the needle may be slowly withdrawn, allowing blood flow to resume. The catheter may also need to be withdrawn slightly if the unit has punctured the back wall of the artery, allowing the catheter to re-enter the lumen and advance within the artery. If the pulsatile flow continues as the needle-catheter unit is initially advanced, the outer catheter alone is then advanced into the artery over the needle without the aid of a guidewire. The needle is removed slowly, and the catheter is secured (Butterworth et al., 2013; Nagelhout & Plaus, 2014; Theodore et al., 2020).
The through-and-through technique mirrors the initial steps described above, except the needle is advanced further, "going through" the other side of the vessel wall. The needle catheter is then retracted until pulsatile flow is achieved, indicating the catheter is now back in the vessel's lumen. The guidewire is then advanced beyond the catheter, and the catheter is slid into place over the guidewire (Butterworth et al., 2013; Nagelhout & Plaus, 2014).
Following needle removal, the artery should be compressed manually proximal to the catheter to limit bleeding while the pre-flushed arterial tubing is connected to the catheter. The site should be covered with a transparent, sterile, occlusive dressing to ensure adequate assessment of the insertion site and the catheter secured with a securement device per institutional protocols (Butterworth et al., 2013; Theodore et al., 2020). The INS standards recommend chlorhexidine-impregnated dressings for patients over 18 with arterial access devices (Gorski et al., 2021).
Arterial lines cannot be used for medication administration. Injectable drugs can lead to severe tissue damage and require amputation of the limb if administered into an artery rather than a vein (Butterworth et al., 2013). Additionally, administering some drugs directly into the arterial system can result in severe systemic consequences up to and including death. Therefore, it is essential to label all arterial lines properly. Two nurses should check before administering any drug through an IV line in the same limb as an arterial line to avoid accidental injection into the arterial line (Pierre et al., 2021).
Complications of Arterial Lines
While arterial lines are considered relatively safe, complications are possible. Complications associated with arterial line placement include hematoma, bleeding, vasospasm, arterial thrombosis, embolization of a thrombus, pseudoaneurysm, skin necrosis, infection, nerve damage, necrosis of the extremities or digits, and unintentional intraarterial injection. Arterial lines can pose similar risks as other vascular devices, including infiltration, occlusion, and catheter migration. Life-threatening hemorrhage can ensue (arterial bleeding) if accidental catheter disconnection occurs. (Butterworth et al., 2013). Occlusion and hematoma are the most common complications of radial catheters, although peripheral neuropathy is also possible. Femoral artery catheters convey a risk of hematoma, the most reported complication for this site, specifically retroperitoneal hematoma. Axillary catheters can cause brachial plexopathy, and brachial site catheters can damage the median nerve (Theodore et al., 2020).
Thrombosis is a common complication of arterial line placement and more closely associated with the narrow vessels of the distal circulation than in the larger central arteries. Patients with pre-existing hypercoagulable states, such as those with advanced malignancies, generally have a higher risk of thrombosis (Weiner et al., 2017). The incidence of thrombosis correlates directly with the dwell time, increased length, and gauge of the arterial catheter. Other risk factors include low cardiac output, peripheral artery disease, and vasospastic disorders (e.g., Raynaud’s syndrome). Signs of thrombosis include a loss of distal pulses, a lost or dampened arterial waveform, or peripheral digits that appear cyanotic (Theodore et al., 2020).
Embolism can also occur due to dislodgment of a thrombus at the catheter site leading to extremity ischemia. Emboli are primarily associated with peripheral catheters placed at the radial and brachial locations, although catheters near the carotid artery (e.g., axillary catheters) may lead to cerebral emboli. Therefore, distal pulses should be monitored closely, and flushes should be performed manually with the lowest pressure needed instead of prolonged or high-powered flushes with the system flush valve. If a clot is suspected in the catheter tip, the catheter should be replaced. Signs and symptoms of embolization vary with collateral circulation and the size of emboli but typically create distal ischemia (Theodore et al., 2020).
Another possible complication is air embolism. An arterial air embolism can lead to ischemia or infarction of any organ not supplied with sufficient collateral flow. While less common than venous air embolism due to the relatively higher intravascular pressure of the arterial system, even a tiny air volume can be detrimental. In a primate model, 2 mL of air injected into the radial artery resulted in cerebral air emboli that were clinically significant (Theodore et al., 2020). The lines should be flushed before establishing a connection with the arterial catheter, and all air must be removed from the pressure bag to prevent air emboli. The procedure to manage arterial air emboli differs from Durant’s maneuver described above for venous air emboli. The flush should be stopped immediately to avoid any additional air from entering the system. The system can be turned vertically to allow air bubbles to rise, and the rotating hemostatic valve should be fully opened to allow the arterial pressure to dispel the air. The patient should be kept in the supine position while high-flow oxygen and the rapid response or code blue system are initiated to obtain assistance in resuscitating the patient (McCarthy et al., 2016).
Arterial catheters can cause vasospasm in up to 57% of cases. Risk factors include female sex, diabetes, and the size of the catheter in relation to the diameter of the vessel. The signs and symptoms of vasospasm include pain in the extremity, decreased arterial pressure, severe damping of the arterial waveform, a loss of arterial pulse, or a significant decrease in pulse oximetry signal quality distal to the cannulation site. Unintentional intra-arterial injection of medication is also a potential sequela and warrants vigilant care to ensure that medications are constantly being injected into the proper (venous) line. This may lead to end-organ ischemia, tissue damage, or necrosis. Uncommonly, arterial dissection, pseudoaneurysm, and AV fistula can occur due to arterial cannulation. Dissection should be watched for closely (i.e., monitoring the waveform), as it can lead to occlusion and distal ischemia. Although rare (incidence < 0.1%), pseudoaneurysm manifests as a pulsatile mass, typically after local site bleeding or hematoma formation. This is generally secondary to multiple cannulation attempts, larger catheter size, and catheter infection (Theodore et al., 2020).
The risk of infection in arterial catheters is typically lower than in CVCs. Infections most often affect the insertion site and appear more frequently in femoral catheters. Other risk factors include poor aseptic technique, insertion via surgical cut-down, and longer dwell time (> 4 days). Prevention techniques include those described above for CVCs (Theodore et al., 2020).
Blood draws/sampling from an arterial catheter require an additional 3-12 mL of initial blood to be wasted to avoid contamination of the sample with saline or heparin. Iatrogenic blood loss can be limited by sampling from the port closest to the catheter insertion site. Alternately, intra-arterial blood gas monitoring with a fluorescent optode eliminates the need to withdraw blood from the patient if this is the primary indication for blood sampling (Theodore et al., 2020).
Care of Arterial Lines
The insertion site and areas distal to insertion must be monitored closely and frequently for warmth, loss of sensation, capillary refill, and pulses. HCPs should ensure that patients who have femoral arterial catheters wear anti-embolic compression stockings to reduce the risk of thrombosis. Arterial catheters are not routinely replaced or relocated to a new site at any specific or defined interval, except for catheters placed during an emergency (i.e., without standard sterile precautions). The catheter is changed only for an infection, malfunction, or another complication. However, the maximum dwell time for femoral lines should be 5 days and 7 days for other sites. A sterile, transparent dressing should be changed when it becomes soiled, wet, or loose. The continued need for the catheter should be reassessed daily, and the catheter should be promptly removed when it is no longer required. Disposable transducers are generally replaced at 96-hour intervals, along with the associated tubing, continuous flush device, and flush solutions. HCPs should refer to manufacturing equipment and institutional policy for definitive timeframes. Arterial catheters should be flushed, typically with sterile saline solution, at defined intervals per institutional policy to maintain patency (Theodore et al., 2020). Arterial catheters are often attached to a continuous infusion of normal saline (potentially with 1-2 units/mL of added heparin, although this is unnecessary per the existing evidence), infused at 1-3 mL/hour to maintain patency. For this reason, blood draws from arterial lines typically require an initial waste of the first 1-3 mL of blood withdrawn to prevent lab errors (Pierre et al., 2021).
Arterial Line Monitoring
Continuous monitoring with an intra-arterial catheter is the gold standard for determining a patient’s blood pressure (or MAP). It is more accurate than non-invasive cuff pressure measurements for patients with shock, cardiac arrhythmias, severely increased systemic vascular resistance due to vasoconstrictive medications, or significantly decreased systemic vascular resistance due to distributive shock. Invasive arterial monitoring also reduces discrepancies in patients with extreme hypotension and hypertension compared to non-invasive cuff monitoring (Theodore et al., 2020).
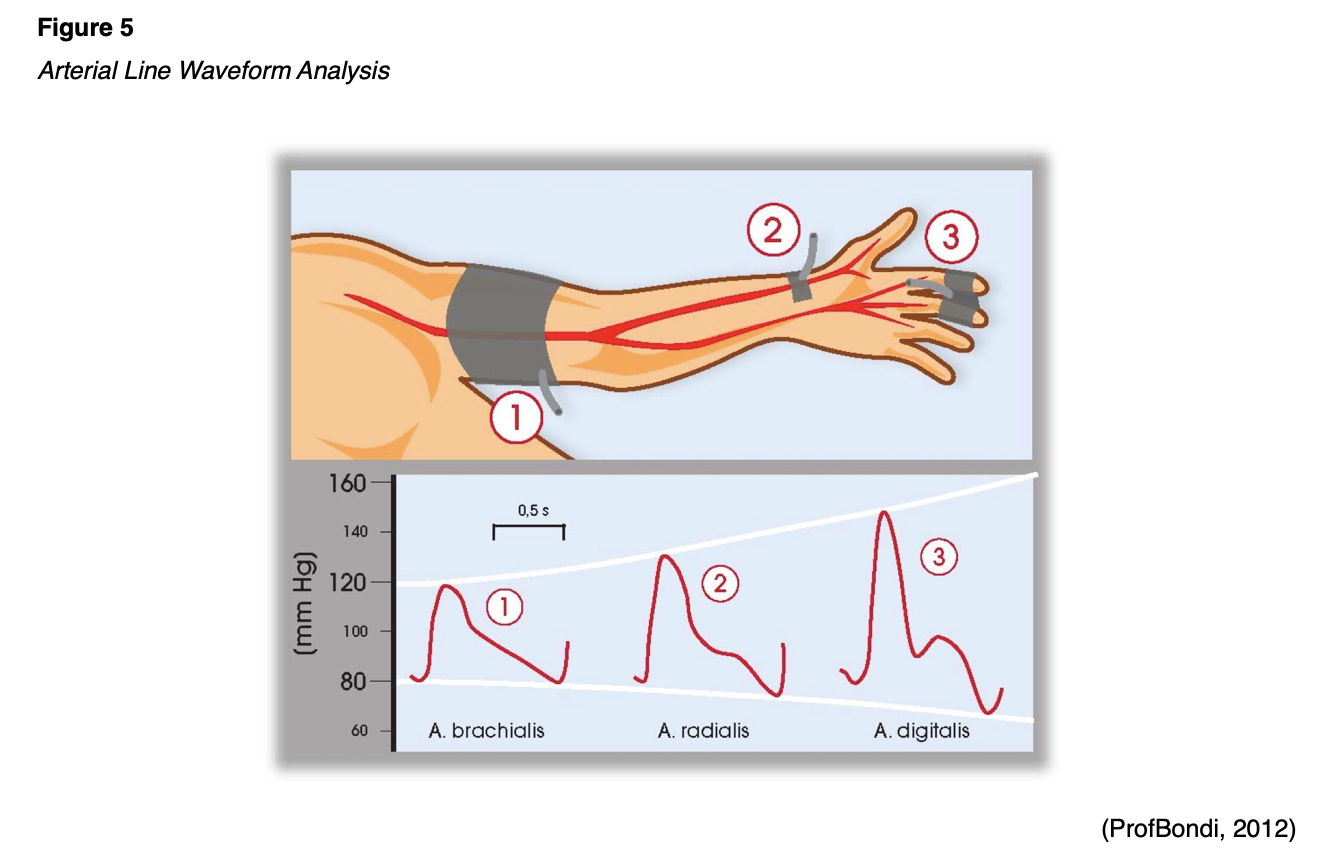
The arterial waveform reflects blood pumping from the left ventricle into the aorta during systole, followed by the diastolic peripheral runoff. The initial ascent in the waveform corresponds with the ventricular ejection. The dip or depression halfway down the systolic decline in the second half of the waveform is referred to as the dicrotic notch or incisura; this section of the waveform represents the closure of the aortic valve and the start of diastole. The remainder of the waveform is the primary determinant of left ventricular blood flow. The waveform provides the patient’s peak systolic pressure, nadir diastolic pressure, pulse pressure (i.e., the difference between the systolic and diastolic pressures), MAP (i.e., the average of the area under the curve over several cardiac cycles), left ventricular contractility (i.e., reflected in the slope of the systolic upstroke), and resistance in the arterial tree (i.e., as indicated by the slope of the diastolic decline/runoff). Pathology in the ascending aorta or aortic valve may lead to changes in the arterial waveform. Elevated pulse pressure may indicate age-associated vascular stiffness. A decrease in pulse pressure from the patient’s baseline reflects hypovolemia, decreased stroke volume, or increased systemic vascular resistance, while a relative increase reflects the reverse conditions. Left ventricular failure results in pulsus alterans, cardiac tamponade leads to pulsus paradoxus, aortic regurgitation is indicated by pulsus bisferiens or water hammer pulse, and aortic stenosis results in anacrotic pulse, pulsus parvus, or pulsus tardus (Theodore et al., 2020). The specifics of these waveforms are beyond the scope of this activity.
The waveform is affected by the site of catheter placement. There is an exaggeration of systolic pressure, a wider pulse pressure, a steeper systolic upstroke, a lower diastolic BP, and a lower/later dicrotic notch as the pressure wave moves peripherally through the arterial tree. This effect is due to the reduced diameter and elasticity of the peripheral vessels, wave reflections off the peripheral branch points and walls, and gravity. Therefore, blood pressure measurements for patients with known peripheral vascular disease may differ significantly across the extremities; the higher value is generally used (Theodore et al., 2020). An arterial catheter’s placement on the pressurized side of the vasculature requires the catheter to be attached to a transducer system with pressure tubing, a pressure bag of fluid (to prevent backflow), and a pressure monitoring cable linked to a bedside or centralized cardiac monitor. A mechanical signal received by the transducer is converted to a waveform on the monitor. Figure 5 demonstrates a typical arterial line waveform based on placement.
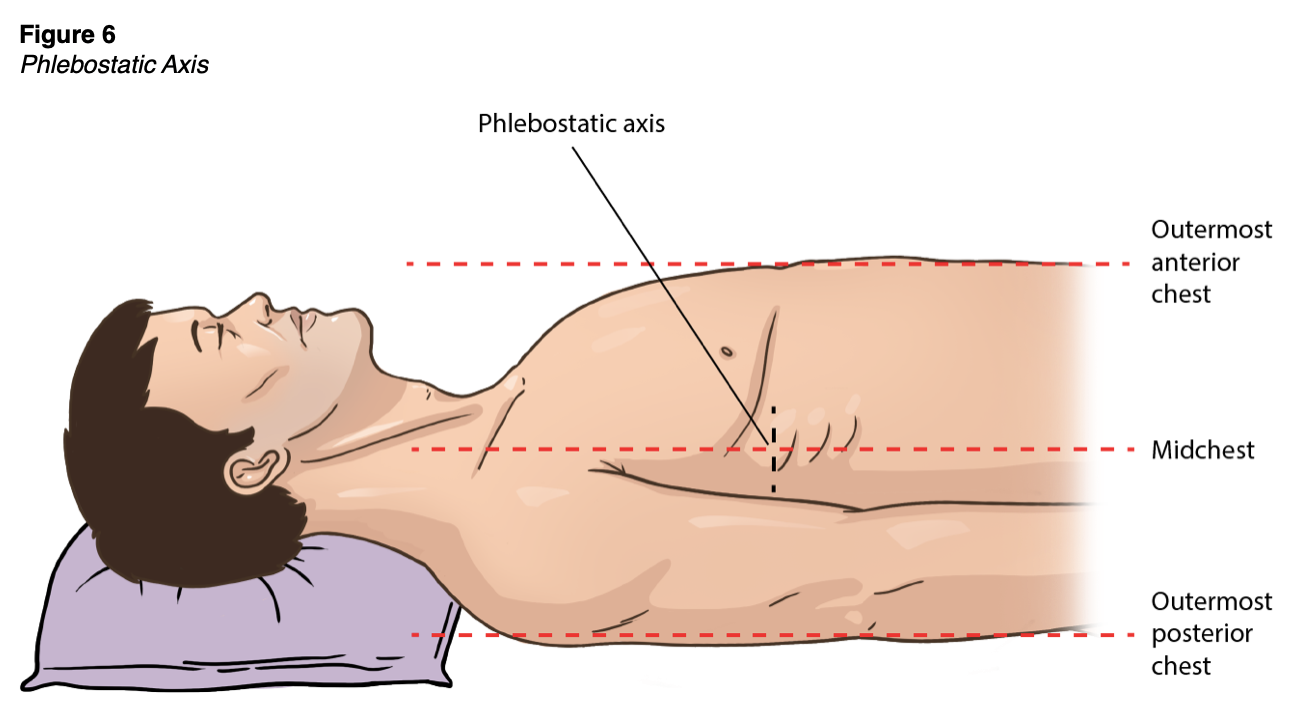
Before monitoring, the transducer must be zeroed. This process can be performed by turning the stopcock adjacent to the transducer off to the patient but open to the air, exposing it to the ambient atmospheric pressure. The ZERO button should be selected on the bedside monitor. The stopcock should then be closed to air and adjusted to the proper position (Theodore et al., 2020). Arterial line monitoring requires accurate transducer placement, which should be placed at the phlebostatic axis (as shown in Figure 6), aligned with the base of the right atrium. This point is at the fourth intercostal space, which in most adults is just inferior to the nipple line, at the mid-diameter of the anterior-posterior chest wall. Failure to position the transducer properly leads to imprecise blood pressure monitoring and potentially improper patient care. An arterial line transducer positioned above the phlebostatic axis will result in artificially lower blood pressure readings, and a transducer placed too low will result in higher readings (Butterworth et al., 2013). If the patient is lying in the left or right lateral decubitus position, the transducer can be placed mid-sternum. If blood pressure accuracy is in question or the electronic pressure monitoring cable becomes disconnected, this zeroing procedure should be repeated. Extra tubing and stopcocks may reduce the monitor's accuracy and should be avoided (this is referred to as damping). Air bubbles or clots in the system typically do not affect MAP accuracy but may create a subtle decrease in the displayed systolic pressure and a narrowed pulse pressure. Flushing the catheter to remove the air or clot often resolves this artifact (Theodore et al., 2020).
Arterial Line Removal
An arterial catheter should be removed by a specially trained nurse or practitioner, depending on institutional policy. The Trendelenburg position is not necessary as when removing CVCs. However, the supine position is recommended for patients with a femoral catheter to maintain adequate pressure at the site. Before removal, coagulation factors such as INR, partial thromboplastin time (PTT), platelet count, and any ongoing medications that may affect coagulation or platelet function should be assessed. Aseptic technique is used to remove arterial catheters, and PPE (i.e., a face mask with a shield, a gown, and clean, non-sterile gloves) should be worn to protect from splashing blood. The catheter should be flushed, or blood should be aspirated into the catheter before removal. The site should be cleaned with chlorhexidine in alcohol solution, and a 4x4 dressing should be used to apply pressure over the puncture site. The catheter should be removed slowly in a steady movement. Pressure should be applied at the artery and skin puncture sites for at least 5 (radial artery site) to 10 minutes (femoral site) or until bleeding subsides. Compression time should be extended (i.e., 10-20 minutes) in patients with impaired coagulation. Larger catheter sizes may also increase the compression time required for bleeding to subside. If oozing continues, pressure should be held for an additional 5 minutes and then reassessed. Failure to maintain adequate pressure can result in hematoma formation and potential arterial bleeding at the insertion site. Once the bleeding subsides, a sterile dressing should be placed. A patient with a femoral catheter should lay flat (i.e., no hip flexion) for up to 2 hours after catheter removal. Distal pulses should be checked every 15 minutes to assess for hematoma or extremity ischemia. After removal, the catheter should be inspected as instructed above for other VADs. If catheter fracture is suspected, pressure should be held proximal to the puncture site to prevent embolization until surgical consultation can be obtained (Gorski et al., 2021; Theodore et al., 2020).
Please refer to Part 2 of this series to review central access devices and invasive monitoring (central venous pressure and pulmonary artery catheters).
References
Adams, D. Z., Little, A., Vinsant, C., & Khandelwal, S. (2016). The midline catheter: A clinical review. Journal of Emergency Medicine, 51(3), 252-258. https://doi.org/10.1016/j.jemermed.2016.05.029
Alexandrou, E., Ray-Barruel, G., Carr, P. J. Frost, S. A., Inwood, S., Higgins, N., Lin, F., Alberto, L., Mermel, L., Rickard, C. M., & OMG Study Group. (2018). Use of short peripheral intravenous catheters: Characteristics, management, and outcomes worldwide. Journal of Hospitalist Medicine, E1-E7. https://doi.org/10.12788/jhm.3039
Bahl, A., Hang, B., Brackney, A., Joseph, S., Karabon, P., Mohammad, A., Nnanabu, I., & Shotkin, P. (2019). Standard long IV catheters versus extended dwell catheters: A randomized comparison of ultrasound-guided catheter survival. American Journal of Emergency Medicine, 37(4), 715-721. https://doi.org/10.1016/j.ajem.2018.07.031
BruceBlaus. (2016). PICC catheter vs. midline catheter [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:PICC_vs._Midline_Catheter.png
Butterworth, J. F., Mackey, D. C., & Wasnick, J. D. (2013). Morgan & Mikhail’s Clinical Anesthesiology (5th ed.). McGraw Hill.
Campagna, S., Gonella, S., Zerla, P. A., Corona, G., Correggia, T., Mussa, B., & Dimonte, V. (2018). The risk of adverse events related to extended-dwell peripheral intravenous access. Infection Control & Hospital Epidemiology, 1-3. https://www.vulturenews.net/wp-content/uploads/2018/04/risk_of_adverse_events_related_to_extendeddwell_peripheral_intravenous_access.pdf
Centers for Disease Control and Prevention. (2017). Guidelines for the prevention of intravascular catheter-related infections, 2011. https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html
Frank, R. L. (2020). Peripheral venous access in adults. UpToDate. Retrieved April 29, 2021, from https://www.uptodate.com/contents/peripheral-venous-access-in-adults
Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. (2021). Infusion therapy standards of practice, 8th Edition. Journal of Infusion Nursing, 44(1S), S1-S224. https://doi.org/10.1097/NAN.0000000000000396
Haddadin, Y., & Regunath, H. (2019). Central line-associated bloodstream infections (CLABSI). StatPearls [Internet], https://www.ncbi.nlm.nih.gov/books/NBK430891/
Heffner, A. C., & Androes, M. P. (2021). Overview of central venous access in adults. UpToDate. Retrieved April 23, 2021, from https://www.uptodate.com/contents/overview-of-central-venous-access-in-adults
Herc, E., Patel, P., Washer, L., Conion, A., Flanders, S., & Chopra, V. (2017). A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Control Hospital Epidemiology, 38(10), 1155-1166. https://doi.org/10.1017/ice.2017.167
Institute for Healthcare Improvement. (2012). How-to guide: Prevent central line-associated bloodstream infections (CLABSI). http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventCentralLineAssociatedBloodstreamInfection.aspx
Jacob, J. T. & Gaynes, R. (2021). Intravascular catheter-related infection: Prevention. UpToDate. Retrieved May 20, 2021, from https://www.uptodate.com/contents/intravascular-catheter-related-infection-prevention
Kornbau, C., Lee, K. C., Hughes, G. W., & Firstenberg, M. S. (2015). Central line complications. International Journal of Critical Illness & Injury Science, 5(3),170-178. https://doi.org/10.4103/2229-5151.164940
Lippincott Nursing Center. (2019). Complications of central vascular access devices. https://www.nursingcenter.com/getattachment/Clinical-Resources/nursing-pocket-cards/Complications-of-Central-Vascular-Access-Devices/Complications-of-Central-Vascular-Access-Devices_January-2019.pdf.aspx
McCarthy, C. J., Behravesh, S., Naidu, S. G., & Oklu, R. (2016). Air embolism: Practical tips for prevention and treatment. Journal of Clinical Medicine, 5(11), 93. https://doi.org/10.3390/jcm5110093
Michaellast.ban. (2015). Intraosseous devices [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Io-device.jpg
Nagelhout, J. J., & Plaus, K. L. (2014). Nurse Anesthesia (5th ed.). Elsevier Saunders.
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
OpenStax College. (2013). Veins of the upper extremity [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:2134_Thoracic_Upper_Limb_Veins.jpg
Pedagogy Online Learning Systems. (2016). Peripheral IV catheter chart. https://www.pedagogyeducation.com/PedagogyEducation/media/Resources/Posters/Peripheral-IV-Catheters-Chart.pdf
Petitpas, F., Guenezan, J., Vendeuvre, T., Scepi, M., Oriot, D., & Mimoz, O. (2016). Use of intraosseous access in adults: A systemic review. Critical Care, 20(102), 1-9. https://doi.org/10.1186/s13054-016-1277-6
Pierre, L., Pasrija, D., & Keenaghan, M. (2021). Arterial Lines. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK499989/
ProfBondi. (2012). Arterial line waveform analysis [Image]. Wikimedia. https://upload.wikimedia.org/wikipedia/commons/f/f5/Arterial_Pathway.jpg
Senior Airman Peter Reft. (2015). Intraosseous device placement [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Service_members_conduct_casualty-receiving_training_on_board_USNS_Mercy_during_Pacific_Partnership_2015_150622-F-YW474-042.jpg
Song, I. K., Kim, E. H., Lee, J. H., Jang, Y. E. Kim, H. S., & Kim, J. T. (2018). Seldinger vs modified Seldinger techniques for ultrasound-guided central venous catheterization in neonates: A randomized controlled trial. Paediatric Anesthesia, 121(6), 1332-1337. https://doi.org/10.1016/j.bja.2018.08.008
Stoker, R. (2009). Accelerated Seldinger technique: A faster, safer method for diagnostic and interventional procedures. Managing Infection Control, 32–36.
Theodore, A. C. (2020). Arterial blood gases. UpToDate. Retrieved April 20, 2021, from https://www.uptodate.com/contents/arterial-blood-gases
Theodore, A. C., Gilles, C., & Dalton, A. (2020). Intra-arterial catheterization for invasive monitoring: Indications, insertion techniques, and interpretation. UpToDate. Retrieved April 22, 2021, from https://www.uptodate.com/contents/intra-arterial-catheterization-for-invasive-monitoring-indications-insertion-techniques-and-interpretation
Thomas, M. (2018). Peripheral intravenous (IV) device management. The Royal Children's Hospital Melbourne. https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Peripheral_Intravenous_IV_Device_Management/
Weiner, R., Ryan, E., & Yohannes-Tomicich, J. (2017). Arterial line monitoring and placement. In J. M. Oropello, S. M. Pastores, & V. Kvetan (Eds.). Critical care. McGraw-Hill Education.
Xu, T., Kingsley, L., DiNucci, S., Messer, G., Jeong, J. H., Morgan, B., Shutt, K., & Yassin, M. H. (2016). Safety and utilization of peripherally inserted central catheters versus midline catheters at a large academic medical center. American Journal of Infection Control, 44(12), 1458–1461. https://doi.org/10.1016/j.ajic.2016.09.010