About this course:
The purpose of this learning module is to provide an overview of the most common vascular access devices utilized in clinical practice for adult patients. The various guidelines regarding the care, maintenance, and assessment of central access devices will be highlighted. The indications and procedures for insertion and removal, benefits, risks, and complications in conjunction with clinical decision-making for best-practice standards and enhanced patient outcomes will be outlined. The interpretation and management of invasive monitoring and its role in clinical decision-making will be reviewed. This module endeavors to review and explain the most common central VADs and the necessary management and assessment skills required for safe clinical practice. The introduction and revision of these devices and their use require healthcare professionals (HCPs) to update their knowledge base continually to provide the latest evidence-based practice (EBP) standards. VAD insertion, management, and use is an essential component of routine patient care. Therefore, a thorough understanding of the numerous VADs utilized in clinical practice and their management and assessment is critical for providing quality care. Please refer to Part 1 of this series to review infection control techniques and peripheral access devices, including short peripheral IVs, midline catheters, intraosseous catheters, and arterial catheters.
Course preview
The purpose of this learning module is to provide an overview of the most common vascular access devices utilized in clinical practice for adult patients. The various guidelines regarding the care, maintenance, and assessment of central access devices will be highlighted. The indications and procedures for insertion and removal, benefits, risks, and complications in conjunction with clinical decision-making for best-practice standards and enhanced patient outcomes will be outlined. The interpretation and management of invasive monitoring and its role in clinical decision-making will be reviewed. This module endeavors to review and explain the most common central VADs and the necessary management and assessment skills required for safe clinical practice. The introduction and revision of these devices and their use require healthcare professionals (HCPs) to update their knowledge base continually to provide the latest evidence-based practice (EBP) standards. VAD insertion, management, and use is an essential component of routine patient care. Therefore, a thorough understanding of the numerous VADs utilized in clinical practice and their management and assessment is critical for providing quality care. Please refer to Part 1 of this series to review infection control techniques and peripheral access devices, including short peripheral IVs, midline catheters, intraosseous catheters, and arterial catheters.
Upon the completion of this module, the learner will be able to:
- describe the various types of central venous catheters (CVCs) and their indications for use, site selection, placement, care, and safety considerations
- describe the management, complications, and site-specific contraindications to the insertion of CVCs
- explain the interpretation and clinical guidance derived from invasive monitoring with central venous and pulmonary artery catheters, their placement, management, and role in patient care
Vascular access devices (VADs) are a core component of patient care widely utilized across various healthcare settings. A VAD is a hollow tube inserted into a vein or artery through the peripheral or central vasculature. VADs have diagnostic and therapeutic uses, including fluid replacement therapy, intravenous medications, blood products, nutrition, blood sampling, and hemodynamic monitoring (Nettina, 2019).
VADs are commonly divided into two categories: peripheral intravenous (PIV) catheters and central venous catheters (CVCs). CVCs are inserted into the central venous system and include peripherally inserted central catheters (PICCs). CVC insertion sites include the subclavian, internal or external jugular, or femoral veins. Over 5 million CVCs are placed each year in the US (Kornbau et al., 2015).
A CVC is commonly referred to as a central line. CVCs are indwelling devices inserted into a vein of the central vasculature. They consist of a thin, flexible tube inserted by a puncture directly through the skin into the intended large vein, often in the neck, chest, arm, or groin. The catheter tip is threaded through the vein until it empties into a large vein near the heart, allowing the treatment to be administered within seconds. Like midline catheters, the point where the central line leaves the skin is called the exit site. CVCs can contain 1-4 lumens. The end of each lumen must be covered with a cap when not used to prevent air entrainment into the central vasculature. Each lumen of the CVC is treated and managed as a separate catheter and must be flushed according to the defined institutional protocol. Multiple lumens allow for the safe administration of various medications simultaneously, including incompatible drugs (Centers for Disease Control and Prevention [CDC], 2019). CVCs are long-term devices that can remain in place for extended periods if adequately maintained, often for months to years. They are ideal for administering vesicant or irritant therapy (e.g., chemotherapy, vasopressors, parenteral nutrition), large rapid fluid boluses, blood products, antibiotics, stem cell transplantation, potassium chloride (KCl), and concentrated dextrose infusions. Central lines allow treatments to move quickly into the bloodstream (Lippincott, 2019).
Apart from catheters, central venous access can also be used for interventions such as extracorporeal therapies (e.g., renal replacement therapy/hemodialysis, plasmapheresis), central venous pressure (CVP) measurement, vena cava filter placement, venous thrombolytic therapy, venous angioplasty or stenting, pulmonary artery catheters (which will be outlined later), and cardiac pacemaker/defibrillator placement (Heffner & Androes, 2021). Unlike peripheral catheters, a CVC can also be used to draw blood. While CVCs may be used in the inpatient, outpatient, and community settings, they serve a chief role in intensive care units (ICUs) for acute or critical care resuscitation and complex infusion therapies and treatments (Leib et al., 2019). In addition, patients with a history of difficult peripheral venous access due to extensive prior PIV therapy, surgery, or previous tissue damage for whom vascular site selection is limited are also candidates for CVC placement (Nagelhout & Plaus, 2014). CVCs are inserted by specially trained and certified nurses, advanced practice nurses (APRNs), or physicians. Some CVCs require surgical placement in the operating room or interventional radiology, while others may be inserted at the bedside (McDiarmid et al., 2017).
The type of CVC inserted depends on the anticipated type and duration of therapy, patient complexity (e.g., comorbidities), and overall clinical status (e.g., diagnosis, condition). Site selection (see Table 1) is also based on the patient's age, local and surrounding vasculature conditions, the skin condition at the intended insertion site, history of prior venipunctures or access devices, and preference. To reduce central line-associated bloodstream infection (CLABSI) risk, clinicians should select the device with the smallest gauge and fewest lumens necessary to complete the prescribed therapy (Gorski et al., 2021). The most common types of CVCs are as follows:
- peripherally inserted central catheter (PICC)
- tunneled CVC
- non-tunneled CVC
- implantable port

Potential contraindications to CVC placement at a selected site include a skin infection over the intended insertion site, obstruction secondary to thrombus within the intended vein, or stenosis of the vein (Tse & Schick, 2019). The presence of a thrombus or stenosis of the vein may preclude placement and necessitate an alternative site. A history of surgical manipulation or trauma to the intended location is another potential contraindication. A cervical spine collar often contraindicates the placement of an internal jugular non-tunneled CVC, as a pelvic binder can contraindicate the insertion of a femoral line (Leib et al., 2019). The presence of a pacemaker or a hemodialysis catheter may preclude the use of a particular site, as well as proximity to a burn, wound, or tracheostomy (Heffner & Androes, 2021). A tunneled CVC placed within the internal jugular vein may be ideal for patients with chronic kidney disease (CKD), as upper extremity PICC and subclavian placement should be avoided if possible (Gorski et al., 2021).
Use of the subclavian vein may decrease the risk of infection compared to the jugular vein and is generally preferred for non-tunneled catheters. The femoral vein should be avoided whenever possible due to higher risks of infection, bleeding, and thrombosis, especially for ad
...purchase below to continue the course
CVC Placement
The Institute for Healthcare Improvement (IHI, 2004) recommends using a procedural checklist to ensure preparation and complete adherence to protocols during the placement of all CVCs (see Figure 1).
Figure 1
Example of CVC Checklist

PICC and non-tunneled percutaneous catheters are typically placed at the bedside, while tunneled catheters and implantable ports are inserted in an interventional suite or an operating room. While consent is implied in emergencies, informed consent should be obtained before the planned placement of any CVC in nonemergent circumstances. This discussion should include the planned procedure and its indications, potential complications, and associated corrective procedures to manage possible complications (e.g., chest tube placement for a pneumothorax). Patients should receive continuous cardiac rhythm monitoring and pulse oximetry during CVC placement, and supplemental oxygen should be immediately available at the bedside. Those at risk of respiratory compromise may require anesthesia and a controlled airway for safety during the procedure. The patient’s bed should be elevated to optimize the operator’s comfort during the placement procedure. Based on the insertion site, the patient should be positioned to facilitate access to the intended location and maximize the diameter of the intended vein. When placing a device in the subclavian or jugular veins, Trendelenburg positioning (head down) may help reduce the risk of air embolism. However, this position may be intolerable for critically ill patients or those with obesity (Heffner & Androes, 2021).
CVC placement is a sterile procedure that should be performed using aseptic technique, or surgical aseptic non-touch technique (ANTT), even in emergencies. Surgical ANTT requires the use of a sterile drape to cover the entire patient, a sterile ultrasound probe cover, a long-sleeved sterile gown, a surgical mask, sterile gloves, and a head covering (surgical cap). The operator should perform hand hygiene using surgical antiseptic wash before donning the proper personal protective equipment (PPE). A chlorhexidine-alcohol skin antiseptic solution should be used to scrub the site and allowed to dry before drape placement, as described by the CDC, IHI, and INS guidelines. For additional details regarding infection control guidelines, ANTT, hand hygiene, and PPE recommendations, please see Part 1 of this VAD series. Prophylactic antibiotic administration is not routine. Sedation may be required to facilitate patient comfort, ranging from a low-dose, short-acting benzodiazepine to more profound procedural sedation. Local anesthetics (e.g., topical lidocaine/prilocaine [EMLA] or subcutaneous 1% lidocaine) may also enhance patient comfort during the procedure. Bupivacaine (Marcaine) is a slightly longer-acting anesthetic that may provide pain relief for up to 12 hours for patients receiving a tunneled catheter or implantable port (Heffner & Androes, 2021).
Patency, site suitability, and anatomic variations can be assessed at the bedside before placement using ultrasound when available. This evaluation is critical for those with a history of prior deep vein thrombosis (DVT) or instrumentation in the proposed region. An ultrasound can be used (if available) for real-time guidance during placement. Compared to landmarks, ultrasound guidance during cannulation by experienced practitioners reduces the time to cannulation and the risk of complications. An ultrasound can also identify potential complications (e.g., guidewire malposition and pneumothorax) immediately (Heffner & Androes, 2021).
Although equipment and procedural details vary by institution, setting (bedside, interventional suite, or operating suite), and insertion site, the basic steps of non-tunneled CVC placement correspond with the Seldinger technique. The vein is cannulated using an introducer needle, micropuncture needle, or angiocatheter (Heffner & Androes, 2021). The operator should occlude the needle hub with their thumb when the guidewire is not in place to avoid air entrainment/embolism, which can occur if air is externally introduced into the systemic circulation (MCarthy et al., 2016). The guidewire is inserted through the needle or angiocatheter, and its position is confirmed via ultrasound or fluoroscopy. The guidewire should be inserted just beyond the anticipated catheter depth to avoid intracardiac advancement (Heffner & Androes, 2021). Guidewire depth should not exceed 16 cm to limit this risk (Young & Yuo, 2020). If a cardiac arrhythmia is detected, the guidewire should be withdrawn slightly until this resolves. The needle or angiocatheter is removed while the guidewire is carefully stabilized. A single stab incision may be required adjacent to the guidewire to introduce a tissue dilator or a coaxial dilator/sheath, which is inserted over the guidewire, keeping the guidewire’s position unchanged. Then, the tissue dilator is removed, leaving either a guidewire or a sheath to guide catheter insertion, depending on the particular kit. Sheaths are typically equipped with a side port that can be aspirated and irrigated to assess function. After being secured, the catheter, device, or pacemaker lead is introduced through the sheath, and the sheath is then removed. Alternately, the catheter can be threaded over the guidewire and held in place while the guidewire is removed. This procedure was originally termed the Seldinger technique (ST) and has since been adapted and termed the modified Seldinger (MST, Heffner & Androes, 2021; Stoker, 2009). A catheter/needle combination may also be inserted initially during an MST approach, allowing for the advancement of a catheter early, followed by needle removal. This catheter serves as the conduit for the guidewire, followed by a tissue dilator and, finally, the indwelling catheter (Song et al., 2018). Each port/access hub of the indwelling catheter should be checked for blood aspiration as well as saline flush (Heffner & Androes, 2021).
The catheter should be secured and covered with a transparent and semipermeable sterile dressing or gauze if the patient is diaphoretic, bleeding, or oozing (Heffner & Androes, 2021). The INS 2016 standards discuss the importance of chlorhexidine-impregnated dressings in reducing the infection risk for patients with CVC devices. The 2021 standards noted the benefits of chlorhexidine-impregnated dressings for use with short-term, non-tunneled CVC devices with the highest level of evidence (i.e., Level I). The new standards expand the usage recommendations for patients over 18 and various catheter types, including arterial, epidural, dialysis, and implanted ports (Gorski et al., 2021).
The securement of a non-tunneled CVC is challenging, and the 2021 Infusion Nurses Society (INS) guidelines recommend additional research to delineate the ideal securement method for these lines. However, until this research can be completed, the INS recommends using either tissue adhesive (TA) in combination with sutures or an integrated securement device (ISD) to secure non-tunneled CVC catheters (Gorski et al., 2021). According to the CDC (2017) and INS (Gorski et al., 2021), chlorhexidine-impregnated dressings with an FDA-cleared label that specifies a clinical indication are recommended to reduce CLABSI and protect the insertion site of short-term, non-tunneled CVCs. Sponges impregnated with chlorhexidine gluconate may be utilized in some facilities. Finally, the tip position of jugular and subclavian catheters should be confirmed with a chest x-ray before use, ideally in the lower superior vena cava (SVC), at the cavoatrial junction (CAJ), outside the right atrium, and above the pericardial reflection. Femoral catheter tips should be located just superior to the confluence of the iliac veins in the inferior vena cava (IVC) and above the level of the diaphragm (Gorski et al., 2021; Heffner & Androes, 2021). Figure 2 depicts the anatomy of the thoracic abdominal veins that are involved.
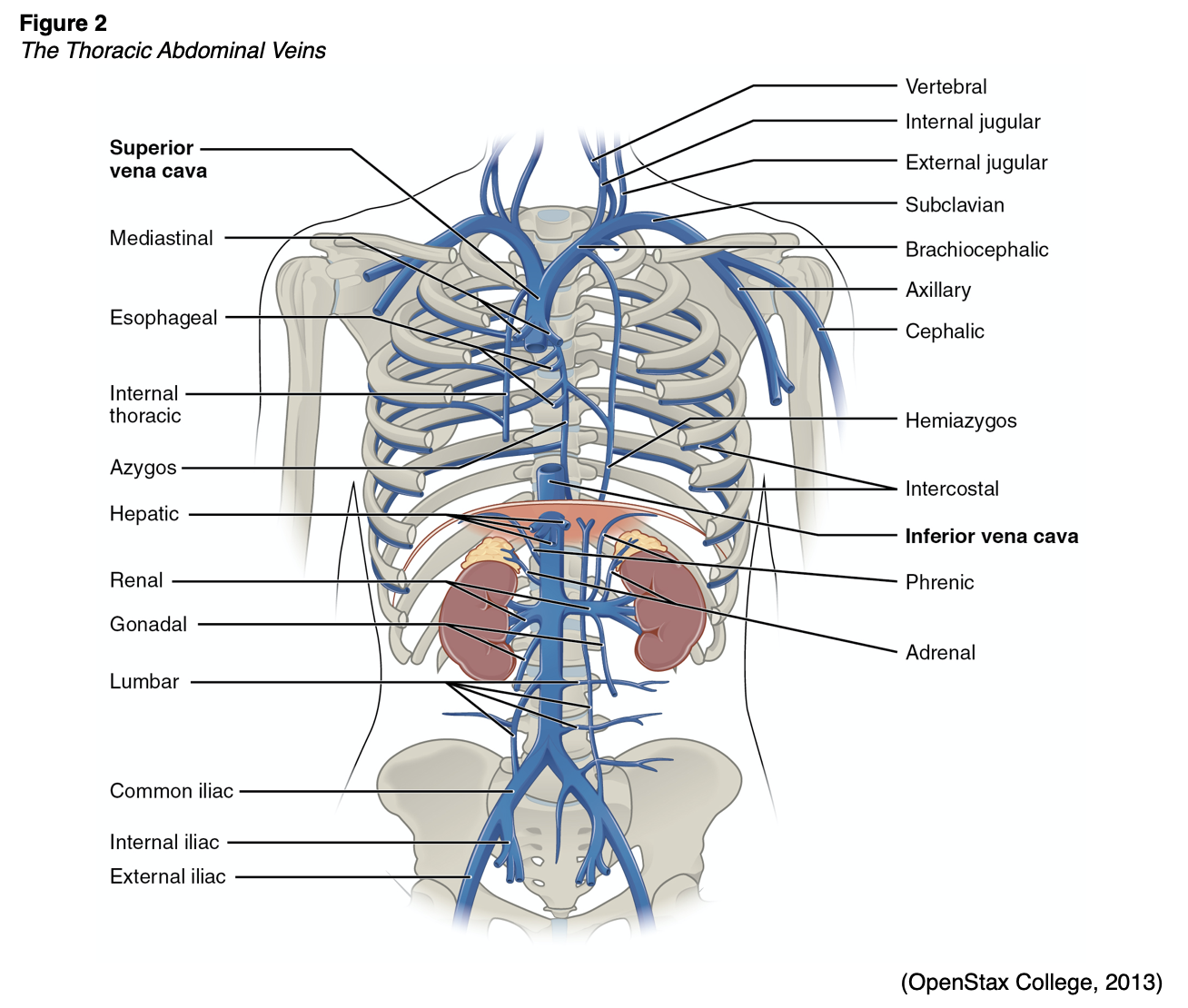
For most subclavian and jugular lines, the exit site should lie below the mid-clavicle at a position that does not interfere with clothing or upper extremity movement. Many tunneled catheters can be trimmed before or after tunneling, although hemodialysis catheters often come in fixed sizes and cannot be shortened. In these cases, the exit site is determined by the length of the catheter. Tunneled catheters are inserted using fluoroscopic guidance, although ultrasound may be used for pediatric patients. The procedure begins as described above. Once the guidewire is set and the guidewire exit site incision is made, the planned tunnel and catheter exit site should be anesthetized (if not already). Many tunneled CVC kits include a tunneling device attached to the most distal catheter lumen opening. An incision is made at the planned catheter exit site. The device should be used to tunnel from the catheter exit site to the guidewire exit site, creating a gentle curve and avoiding acute angulation. The tunneling device is then removed, and the remainder of the insertion mirrors the MST previously described. The catheter tip position should be assessed and adjusted as needed before securing the catheter, and the cuff should be placed at the catheter exit site (Heffner & Androes, 2021). Hemodialysis catheters should terminate at the mid-right atrium, as opposed to within the lower SVC. While tunneled catheters were historically sutured in place until the cuff became incorporated into the subcutaneous tissue, the 2021 INS guidelines recommend using a securement device (a subcutaneous anchor securement system [SASS], ISD, TA, or an adhesive securement device [ASD]) for both cuffed and non-cuffed tunneled catheters (Gorski et al., 2021).
As an alternative to the MST, the WAND device uses an accelerated Seldinger (AST). The WAND combines the needle, guidewire, dilator, and sheath into a single device. It purports to reduce the number of steps and the time required to cannulate, thereby improving insertion safety. The 21-gauge introducer needle is inserted until the “fast-flash” is identified, at which point the hub should be held still. The guidewire can then be advanced after disengaging the cap. The dilator collar is turned clockwise one-quarter turn, allowing the dilator/sheath to advance over the guidewire. This sheaths the needle tip and prevents needlestick injuries automatically, or it can be done manually by withdrawing the needle hub until it locks within the sheath/dilator hub. The dilator hub is then disengaged from the needle hub, allowing the needle, guidewire, and dilator to be removed as a single unit, leaving behind only the cannulated sheath within the vessel. Finally, the sheath is used to insert the device or indwelling catheter of choice (Stoker, 2009).
CVC care requires astute clinical judgment, assessment, and training to ensure competency in managing and maintaining each type of device. Central lines serve critical roles in restoring the health of acutely and critically ill patients. While CVC catheters are widely used clinically, they are associated with potentially life-threatening risks and complications. The primary responsibility of the HCP caring for those with CVCs is to safeguard patient care through the consistent use of EBP interventions and clinical assessment to reduce the risk for complications (Gorski et al., 2021). The line must be assessed daily for continued necessity and the potential for prompt removal. The line should be removed as soon as it is no longer clinically indicated. Daily CVC assessment should include, at minimum, the following components, which must be documented in a flowsheet in the patient's medical record:
- date, time, and insertion site
- date of the last needle, cap, and infusion supply changes
- radiographic confirmation of tip location if indicated
- daily review of line necessity, including the functionality of line, flush protocol, site appearance, blood return, and condition and appearance of potential site complications (CDC, 2017; Gorski et al., 2021; IHI, 2012).
VAD documentation should be comprehensive, occur promptly, and also include the following:
- type, length, and size of the device
- specific site preparation, infection control, and safety precautions as appropriate for the procedure
- number of attempts and patient tolerance of insertion
- type of stabilization device
- device discontinuation, date, condition, site appearance, dressing applied, the reason for removal, and patient response (Campagna et al., 2018; Gorski et al., 2021)
Individual care recommendations for various CVCs will follow.
Tunneled CVCs
A tunneled CVC is surgically implanted (typically in an interventional suite or operating room) in a central vein in the neck or chest and then subcutaneously tunneled to an exit site in the chest wall. Figure 3 demonstrates the vein entry site on the upper chest and the catheter exit site between the third and fourth intercostal spaces. The catheter should terminate in the SVC at the CAJ, which often lies roughly at the level of T5-7 (Kornbau et al., 2015). A Dacron cuff is positioned within the tunneled portion of the catheter, approximately 2 to 3 cm from the exit site. Tissue grows around the cuff to create a mechanical barrier against microorganisms and anchor the catheter in place (Gorski et al., 2021). The subcutaneous tunnel reduces the risk for infection by preventing organisms on the skin from reaching the catheter and, ultimately, the bloodstream (Nettina, 2019).

Tunneled CVCs are used for patients who require long-term access, generally for more than 30 days. Tunneled CVCs may also be ideal when a PICC line is not feasible for the patient (e.g., bilateral mastectomies with lymph node dissection). A tunneled CVC can be used for any medication infusion, including antibiotics, vesicants (e.g., chemotherapy), blood products, or extended parenteral nutrition (i.e., months or longer). The line may also be used for blood sampling, and it may have a single, double, or triple lumen (Gorski et al., 2021).
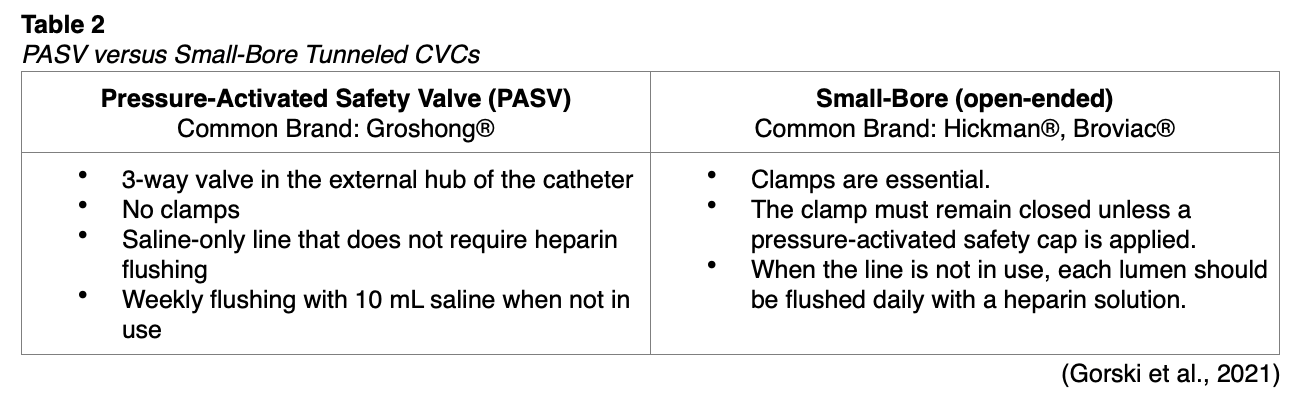
There are two main types of tunneled CVCs: small-bore (open-ended, such as Hickman® or Broviac®) and pressure-activated safety valve (PASV, closed-ended, such as Groshong®). The functionality is similar to non-valved and valved PICC lines (Nettina 2019). Small-bored tunneled CVCs require clamps to prevent the backflow of blood. These clamps must remain closed unless a pressure-activated safety cap is applied to the end of the line, and each lumen should be flushed daily with a heparin solution to maintain patency (Gorski et al., 2021).
The Groshong® tunneled CVC is a closed-ended system with one or more patented 3-position PASVs near the closed catheter tip (hub). The PASVs serve as the gatekeepers for infusion of fluids or medications and aspiration of blood, allowing fluids to flow in or out but closing when not in use. They restrict blood from backflowing and reduce the risk for air embolism by remaining closed when not in service. The valves also maintain catheter patency and reduce the need for heparin flushing with using clamps. This system is safer and more cost-effective than open-ended catheters due to the reduction in catheter maintenance, as they only need to be flushed once weekly with normal saline when not in use (Gorski et al., 2021).
When aspirating from a PASV, negative pressure is created by pulling back on an attached 10 mL syringe, causing the valve to open inward and allowing specimen retrieval. Conversely, positive pressure caused by gravity, an infusion pump, or injecting with a syringe will open the valve outward and allow fluids to infuse into the catheter. Whenever the catheter luminal pressure returns to normal, the valve closes. After blood is aspirated or a medication is infused into the catheter, it must be flushed with 0.9% sodium chloride in a 10 mL syringe to clear the lumen and allow the valve to return to its normal closed position (Gorski et al., 2021). Table 2 compares the main differences between the two types of lines.
Contraindications to Tunneled CVCs
Insertion of a tunneled CVC is contraindicated in patients with active septicemia due to the risk for colonization of the device. Insertion is contraindicated in patients with severe coagulopathy or thrombocytopenia due to the risk of hemorrhage from the target vessel. Placement should be avoided in patients with an elevated INR (i.e., > 1.5) or a platelet count below 50,000/µL (i.e., < 50 x 109/L) if possible (Clark et al., 2016). Non-tunneled catheter placement tends to allow easier monitoring for potential bleeding and may be a safer option for patients with coagulopathy (Heffner & Androes, 2021).
Pros and Cons of Tunneled CVCs
Tunneled CVCs have lower infection rates when compared to non-tunneled CVCs. Patients require fewer venipunctures, and the devices can remain in place for years (CDC, 2017). Tunneled CVCs place restrictions on certain activities. Patients are advised to avoid swimming and contact sports when the catheter is in place. Tunneled CVCs are often inserted and removed surgically in the operating room under conscious or local sedation (Nettina, 2019).
Care of Tunneled CVCs
Before a subclavian or jugular tunneled CVC can be used, an x-ray must be performed and read by a licensed provider to confirm the correct placement of the catheter (Gorski et al., 2021). Many facilities do not confirm catheter tip placement in femoral catheters, and alternatives to radiography for tip placement confirmation include ultrasound imaging, echocardiography, or intracavitary electrocardiography (Heffner & Androes, 2021). The INS recommends that an initial sterile dressing should be placed on the site following insertion. This dressing should be changed 24 hours following line insertion, every 7 days thereafter, and when visibly dirty, wet, or soiled. Once the insertion site has completely healed, usually about 21 days following insertion, the line may be left open to the air and uncovered. The site does not require routine dressings in outpatient and community settings based on EBP guidelines. When parenteral nutrition is prescribed, a dedicated line must be allotted for these infusions and labeled. This dedicated lumen should not be used for other medications, infusions, or therapies due to an increased risk of occlusion and infection (Gorski et al., 2021).
As noted, valved tunneled CVC lines are considered saline-only lines. When a line is not in use, it should be flushed with 10 mL of 0.9% sodium chloride once per week. By contrast, each lumen of small-bore catheters must be flushed with a heparinized solution daily. Catheters should be aspirated to assess blood return and patency before each use. The blood return should be brisk. Patency should be checked before administering any drug or infusion, and the line should also flush easily and without resistance. Syringes smaller than 10 mL should never be used to flush any type of tunneled CVC. The INS recommends that a 10 mL syringe (or larger) be used to withdraw blood samples or inject into any tunneled CVC. (Gorski et al., 2021).
Strict handwashing is essential before handling the catheter. Clean gloves should be worn when accessing a tunneled catheter, even once it has fully healed following insertion. Clean technique must also be used while retrieving blood cultures, changing a needle-free access device, and connecting or disconnecting infusion lines. The cap on small-bore tunneled CVCs should be changed every 7 days using aseptic technique. The patient or caregiver can be trained to care for the site, including performing cap changes and flushes (CDC, 2017; Gorski et al., 2021). Lines should be monitored for signs of potential infection such as erythema, edema, pain or tenderness, drainage, fluid pocket in the subcutaneous tunnel, induration at the exit site or over the pocket, as well as signs of systemic illness such as a fever, chills, rigors, lethargy, disorientation, and confusion. A tunnel infection is an indication for removing a tunneled CVC (Gorski et al., 2021).
Removal of Tunneled CVCs
When properly maintained and free from infection, tunneled CVCs may remain in place indefinitely (CDC, 2017; Gorski et al., 2021). According to the 2021 INS guidelines, a licensed independent practitioner should remove a tunneled CVC as soon as it is no longer required, although the specific details outlining the removal of a tunneled CVC are subject to institutional policy. Catheter removal is a surgical procedure, and sterile technique must be maintained throughout the process to reduce infection risk. It is essential to ensure the complete removal of the subcutaneous cuff. Incomplete removal of the cuff and retention of any products place the patient at risk for subcutaneous abscess and delayed wound healing. INS guidelines recommend the use of fluoroscopy and ultrasound guidance to verify cuff location and facilitate surgical removal (Gorski et al., 2021). As with a PICC line, the patient should be placed supine or in the Trendelenburg position with the entry site below the heart to optimize the CVP. Conscious patients should exhale or perform a Valsalva maneuver during removal. Manual pressure should be applied for 3-10 minutes or until bleeding stops, and a temporary sterile pressure dressing should be applied to the site. The patient should remain supine for 30 minutes following removal (Heffner & Androes, 2021; McCarthy et al., 2016).
Non-Tunneled CVCs
Non-tunneled CVCs are small-bore catheters inserted percutaneously (i.e., can be done at the bedside) through the subclavian vein of the upper chest or the neck's jugular veins, as demonstrated in Figure 4. The catheter exits the skin in the vicinity of the venous cannulation site, which may be the jugular, subclavian, or femoral vein. Non-tunneled CVCs vary in length from 15 to 25 cm and can have a single, double, triple, or quadruple lumen. Catheter placement is performed using sterile ANTT and the MST as previously described (Chopra et al., 2019).
The jugular site is associated with fewer mechanical complications during insertion, but the risk of infection and thrombosis increase with dwell time. A lower internal jugular insertion site is associated with improved securement and is recommended in pediatrics and neonates due to reduced risk of infection and thrombosis. The femoral vein is more easily accessed with ultrasound but is associated with a higher infection risk. Finally, the axilla-subclavian approach is associated with a reduced risk of symptomatic DVT and infection but an increased risk of mechanical complications during insertion (e.g., pneumothorax). This site should be avoided in patients with CKD (Gorski et al., 2021).
Non-tunneled CVCs are intended for short-term and temporary use, usually 5 to 10 days. They are most commonly used in emergent, trauma, and critical care settings in patients with limited peripheral access. They are not appropriate for home care or ambulatory clinic settings. The line can be used for continuous infusion therapy of medications, parenteral nutrition, vesicants (i.e., chemotherapy), high-dose potassium or other electrolyte replacement, blood products, antibiotics, and other intermittent treatments. Non-tunneled CVCs also allow for hemodynamic monitoring of patients who are critically ill via CVP. These catheters tolerate large volume infusions if needed (Nagelhout & Plaus, 2014). Non-tunneled CVCs may be secured to prevent migration or dislodgment with an ISD or using sutures and TA. Subclavian or jugular catheters should terminate in the SVC, and a chest x-ray must confirm proper placement before use (Gorski et al., 2021; Nagelhout & Plaus, 2014). Many facilities do not ensure catheter tip placement in femoral catheters, and alternatives to radiography for tip placement confirmation include ultrasound imaging, echocardiography, or intracavitary electrocardiography (Heffner & Androes, 2021).

Contraindications to Non-Tunneled CVCs
There are few absolute contraindications to non-tunneled CVCs, and recommendations are based on the targeted insertion site and patient factors. Coagulopathies merit careful consideration. Placement of a non-tunneled CVC is generally avoided for patients with an INR greater than 2.0 or a platelet count below 50,000/µL (< 50 x 109/L). However, values outside of these ranges are not absolute contraindications. Emergency access may justify non-tunneled catheter placement for clinically unstable or coagulopathic patients (Lee & Ramaswamy, 2018). The safe use of non-tunneled catheters in emergent circumstances despite coagulopathy has been documented. The subclavian site should be avoided for these patients due to the inability to compress the site easily. The use of ultrasound guidance by an experienced clinician reduces the number of attempts and complication rates. If time allows, the clinician may consider the administration of platelets or fresh frozen plasma to patients with a platelet count below 20,000/µL (< 20 x 109/L) before CVC placement (Heffner & Androes, 2021).
Pros and Cons of Non-Tunneled CVCs
Advantages of non-tunneled CVCs include the ability for bedside insertion under ultrasound guidance. Non-tunneled CVCs are designed for temporary, short-term use and can be placed in the setting of systemic infection (Lee & Ramaswamy, 2018). Non-tunneled CVCs cause the majority of CRBSIs. Therefore, they should be removed as soon as feasible to reduce infection risk, morbidity, and associated mortality (CDC, 2017). The use of multi-lumen catheters increases the risk of thrombosis, infiltration, and other complications. In addition, multi-lumen catheters cannot infuse fluids or blood products as quickly as single-lumen catheters (Chopra et al., 2019). Table 1 outlines the clinical considerations associated with various CVC insertion sites.
Care of Non-Tunneled CVCs
The CDC (2017) and INS (Gorski et al., 2021) recommend chlorhexidine-impregnated dressings for short-term, non-tunneled CVCs. Dressings on short-term CVCs should be changed every 7 days (if transparent) or 2 days (if gauze; Heffner & Androes, 2021). Catheters should be assessed for continued need daily, as prompt removal is strongly advised when the catheter is no longer necessary to reduce potential complications. The risks of infection and thrombosis rise with increased dwell time. INS (Gorski et al., 2021) standards advise that non-tunneled CVCs should be monitored closely for complications and the presence of any of the following signs and symptoms, which must be reported and documented immediately:
- pain or tenderness in unusual locations of neck, chest, or upper abdomen
- erythema or blanching at the insertion site
- changes in skin temperature at or surrounding the insertion site
- edema
- sudden or unusual respiratory and neurological changes
- leaking of fluid or purulent drainage from the puncture site
- resistance when flushing
- absence of brisk blood return
- changes in catheter function associated with arm position changes
- signs of systemic illness, such as elevated body temperature, chills, or rigors (Gorski et al., 2021)
Removal of Non-Tunneled CVCs
Removal of non-tunneled catheters should be performed per organizational policies (Gorski et al., 2021). The line should never be forcibly removed if met with resistance, as catheter fracture and embolization may occur. Immediately after removing a non-tunneled line, the catheter tip should be examined to ensure it was removed fully intact (Nagelhout & Plaus, 2014). As previously described for other CVCs, the patient should be supine or in the Trendelenburg position to prevent air entrainment and instructed to exhale or do a Valsalva maneuver during removal. Manual pressure should be applied to the site for 3-10 minutes following removal or until bleeding stops, and a temporary sterile pressure dressing should be applied. The patient should remain supine for 30 minutes following removal (Heffner & Androes, 2021; McCarthy et al., 2016).
Peripherally Inserted Central Catheters
First described by Hoshal in 1975, a PICC is a CVC inserted in the basilic, cephalic brachial, or median cubital veins in the upper arm and terminates in the lower segment of the SVC. A PICC is indicated for long-term access; the dwell time varies from weeks to months, but PICCs can remain in place for more than a year with proper care. PICCs are usually chosen for patients requiring IV therapy ranging from a week to a year. INS guidelines recommend placing a PICC line early during treatment before veins are damaged from multiple venipunctures and infusions. Nearly all infusion therapies and medications can be administered through a PICC, and the line may also be used for laboratory blood draws (Gorski et al., 2021).
Herc and colleagues (2017) performed a retrospective model-based study to establish CLABSI risk factors, estimating an individual’s risk before PICC placement. Their proposed model performed well and could inform patient selection and surveillance practices for high-risk groups, although it should first be validated for clinical practice. Their model, the Michigan PICC-CLABSI (MPC) score, assigns points for the presence of:
- hematological cancer (3 points)
- a CLABSI in the last 3 months (2 points)
- placement of a multi-lumen PICC (2 points)
- ongoing chemotherapy for a solid tumor/cancer (2 points)
- receipt of parenteral nutrition (1 point)
- another CVC at the time of PICC placement (1 point; Herc et al., 2017)
PICC sizes range from 2F to 6F with a catheter length of 40 to 60 cm. The catheter is measured and cut to the patient's size at the time of insertion. PICC lines are available with single, double, and triple lumens; however, multi-lumen PICC lines have twice the complication rate as single-lumen catheters (Paje et al., 2019). Therefore, as with all intravenous insertions, INS guidelines recommend inserting the fewest lumens and the smallest lumen required for the prescribed therapy (Gorski et al., 2021).
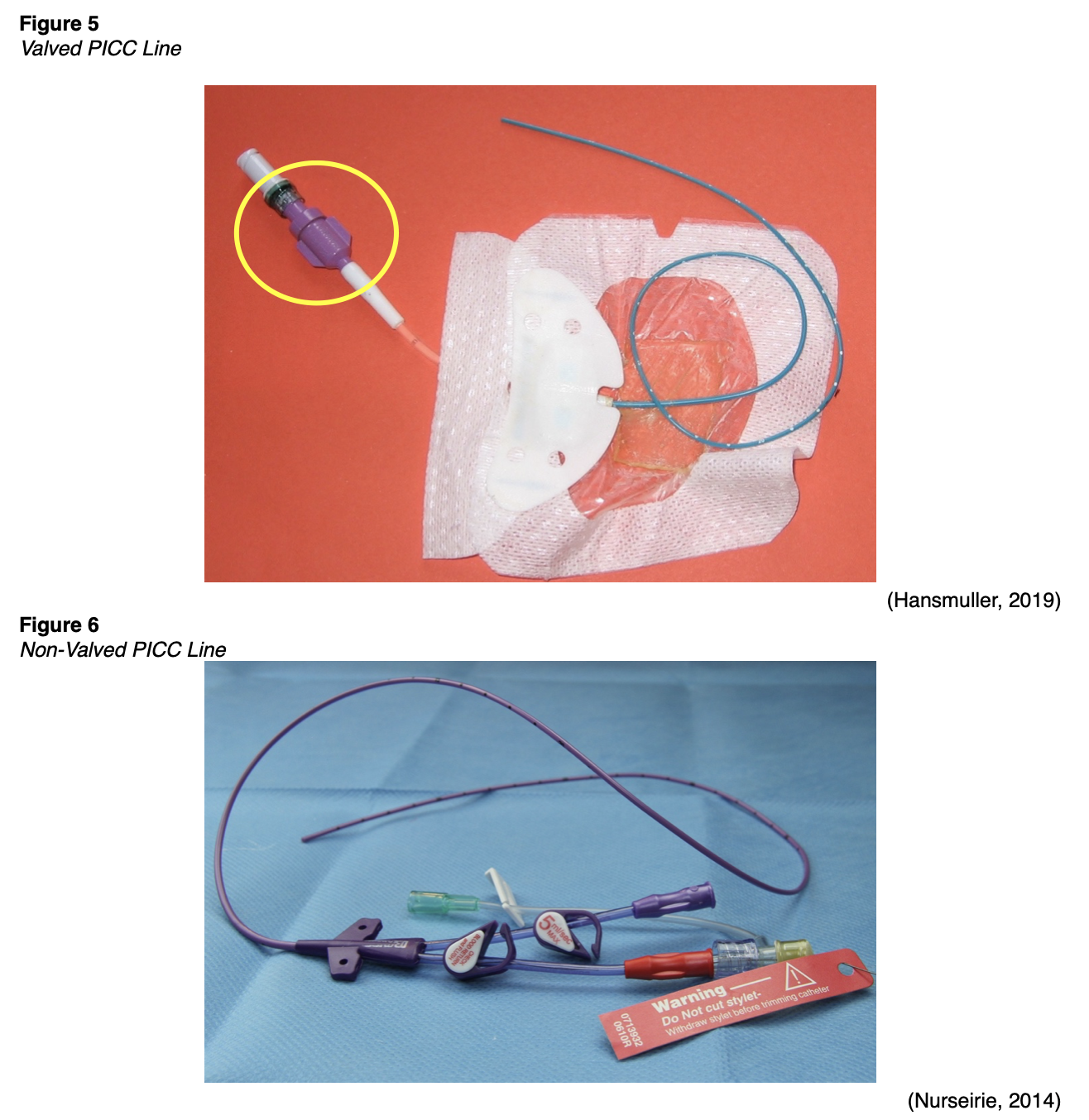
There are two basic types of PICC lines: valved and non-valved (open-ended). Valved PICCs are more common and have a PASV located at the external end of the catheter, which is called the hub. The PASV is a slit at the end of the tube that opens when blood is withdrawn or when fluid is infused and self-seals when the line is not in use. The PASV prevents the backflow of blood into the catheter and negates the need for clamps. Valved PICCs are considered saline-only lines, as they do not require heparin flushing to ensure patency (Gorksi et al., 2021).
Open-ended PICC lines, like small-bore CVCs, do not have a valve or a slit in the tubing. The end of the PICC tubing remains open and requires a clamp to close. The line should be clamped when not in use to prevent blood from backing into the tubing (Gorksi et al., 2021). Figure 5 displays a valved PICC with a PASV hub on the end of the catheter (yellow circle). Figure 6 is a photograph of an open-ended PICC with clamps.
Pros and Cons of PICC Lines
Anesthesia is not required to insert or remove PICC lines, making insertion less complicated, less expensive, and less risky for patients. PICC lines are inserted by specially trained PICC-certified nurses, APRNs, or physicians at the bedside. The catheter size of PICC lines is typically smaller, and the insertion site (i.e., the upper arm) eliminates the risk of pneumothorax or injury to the vessels in the neck associated with other CVCs. PICC lines reduce the need for multiple venipuncture and IV sticks, thereby enhancing patient satisfaction compared with PIVs. PICC lines contribute to decreased length of hospital stay, as they allow for IV therapy in non-acute settings, such as home care, skilled nursing facilities, and hospice (Caprara, 2017; Kornbau et al., 2015).
When properly cared for, PICC lines may pose lower infection rates than other CVCs, although recent studies indicate similar infection rates (Caprara, 2017; Kornbau et al., 2015). There remains a lack of large RCTs comparing PICC lines with other CVCs. Most potential complications associated with PICC lines mirror those discussed above for CVCs in general, such as malposition, bleeding at the insertion site, AV fistula, arrhythmia, and nerve damage. Nerve damage typically causes radiating electrical pain upon insertion or other nerve symptoms, such as paresthesia, tingling, or numbness. Specifically, PICC lines have led to reported cases of Horner's syndrome, which is inflammation of the cervical sympathetic nerves resulting in asymmetrical eyelid drooping, pupillary constriction, and a lack of facial sweating (Kornbau et al., 2015; Lippincott Nursing Center, 2019).
A significant complication associated with PICC lines is an increased risk of venous thromboembolism, particularly DVT. PICC lines carry a substantial risk of upper extremity DVTs compared to other central lines and are also associated with an increased risk of central vein stenosis (Chopra et al., 2019). Careful consideration should be given before inserting a PICC line in cancer patients due to the hypercoagulability that often accompanies cancer or critically ill patients due to the increased risk of CLABSI in hospitalized patients (Gorski et al., 2021).
National guidelines strongly recommend that PICC line insertion should be avoided for patients with advanced CKD who may require hemodialysis in the future. An autogenous arteriovenous fistula (AVF) is the most common VAD used for long-term hemodialysis. Research demonstrates higher rates of AVF failure in veins into which PICC lines or other types of indwelling vascular catheters have been inserted (Paje et al., 2019). PICC lines should not be placed in the veins of an upper extremity on the same side as a previous breast surgery with axillary lymph node dissection, in the setting of lymphedema, or with a known DVT due to heightened risks for infection and thrombotic complications. Additional contraindications include the presence of a hemodialysis catheter (e.g., AVF), current or recent infection (e.g., cellulitis), fracture, burn injury, or neuromuscular dysfunction related to a central nervous system injury (e.g., hemiparesis, hemiplegia). These contraindications apply to PIVs and PICC lines (Nettina, 2019). PICC lines are contraindicated in an extremity affected by a newly implanted pacemaker or defibrillator (Gorski et al., 2021; Leib et al., 2019).
Clinically, PICC lines pose particular challenges related to catheter occlusion. A catheter placement slightly distal to or at the antecubital fossa may result in occlusion of the line and discontinuation of the infusion therapy. If the patient’s arm does not remain straight, flow within the catheter may be obstructed. Movement by the patient can necessitate constant repositioning and clearing the line, resulting in therapy delays and resource loss due to the vigilance required (Lippincott Nursing Center, 2019).
PICC Placement
PICC line insertion is a sterile procedure that requires formal training. Most PICC lines are inserted by vascular teams led by specially trained PICC-certified nurses. PICC lines are inserted at the bedside or in interventional radiology under fluoroscopy guidance (Gorski et al., 2021). The preferred site for PICC insertion is the middle third of the upper arm (Sabado & Pittiruti, 2020). The right basilic vein is the largest, straightest, and, therefore, the most frequently chosen. It also travels in a more superficial position, making cannulation easier. In addition, it has fewer valves, better hemodilution, and a shallower angle of insertion. The cephalic vein is smaller than the basilic, may be tortuous in some patients, and tends to have a sharper insertion angle. The brachial vein is large yet runs close to the brachial artery and median nerve and deeper than the basilic. While typically prominent in the antecubital fossa, the median cubital vein is prone to mechanical phlebitis and occlusion due to bending at the elbow (Gonzalez & Cassaro, 2020). For pediatric and neonatal patients, the axillary, temporal, posterior auricular, popliteal, and saphenous veins may also be considered (Gorski et al., 2021). If available, an ultrasound should be used to evaluate the access site. Dynamic (real-time) ultrasound guidance can guide venipuncture, direct tip navigation, and scan for complications following cannulation. This guidance is especially crucial when directing the catheter into the brachiocephalic vein and avoiding the brachial artery and median nerve (Sabado & Pittiruti, 2020).
Materials should be gathered, and informed consent should be obtained, as with other CVCs. The patient's upper arm circumference should also be measured and documented to assess possible swelling later (Sabado & Pittiruti, 2020). The measurement should be 10 cm above the antecubital fossa (Gorski et al., 2021). The patient should be well hydrated, as with other venous access devices, to enhance vein location. A tourniquet is applied, and the insertion site is marked. The length of the catheter required should be measured from the insertion site to the mid-right mid-clavicular line and down to the third intercostal space. Hand hygiene is performed, and the upper arm is cleansed with chlorhexidine or alcohol. PPE should be donned (mask, face shield, hair cover, gown, and sterile gloves), and the patient should be draped. The insertion site can be anesthetized as described previously, and the vein should be relocated using ultrasound. The MST is most often used for the placement of a PICC catheter. The needle is used to access the vein until blood is aspirated, the guidewire is placed through the needle, and the needle is then removed. Guidewire placement should be confirmed with an ultrasound. A scalpel is used to enlarge the insertion site to accommodate the dilator and introducer, which enters via the guidewire. The guidewire and dilator are removed, leaving only the introducer in place. The catheter is inserted through the introducer to the predetermined length, and then the introducer is removed (Gonzalez & Cassaro, 2020).
According to the 2021 INS guidelines, PICC lines should be stabilized using a securement device (SASS, ISD, TA, or ASD) similar to tunneled CVCs. Sutures increase the risk of infection and are associated with needlestick injuries. While a single RCT and several small observational studies have indicated that SASS may be more effective than ASD, additional studies are needed to differentiate the risks and benefits between the securement methods. The National Institute for Clinical Excellence (NICE) in the UK also recommends using SASS for patient safety and cost/benefit reasons, especially with catheters expected to remain for longer than 15 days. A chest x-ray is required to confirm the catheter tip placement in the lower segment of the SVC before the PICC line is used. Clinicians should avoid placing the catheter tip in veins distal to the SVC due to the increased risk of thrombosis (Gorski et al., 2021).
Care of PICC Lines
Aseptic technique is required at each encounter, and all clinicians who utilize a PICC line should be appropriately trained and competent in its use. Since the PICC is placed within a large vessel, blood return should be brisk. The PICC line requires regular flushing with a 10 mL syringe, the minimum size syringe that can safely be used with this device. Smaller syringes may cause excessive pressure within the device, leading to catheter fracture. The line should be flushed at least daily (most institutional protocols calling for every 12 hours) with 10 mL of 0.9% sodium chloride to maintain patency and prevent the line from clotting when not in use. It must also be flushed following any infusion, bolus injection, or blood withdrawal to clear any residue. Flushing helps reduce the buildup of fibrin and platelets. Blood present in the catheter lumen contributes to the risk of infection (Gorski et al., 2021). A transparent dressing should cover the PICC insertion site and the hub at all times to ensure infection control. Dressing changes are recommended every 7 days or if the dressing becomes soiled, wet, loose, or dirty (Nettina, 2019). Additionally, PICC lines should be wrapped or secured before bathing and showering to preserve dressing integrity and reduce the infection risk (Gorski et al., 2021). Extension tubing should remain clamped and secured to the patient's arm (Nettina 2019). A tubular or sleeve gauze or mesh is recommended for tubing securement instead of a rolled bandage (Gorski et al., 2021).
As noted earlier, open-ended PICC lines should remain clamped when the catheter is not in use (Nettina 2019). The patient's arm circumference should be measured before the PICC insertion and when clinically indicated to assess for the presence of edema, which could indicate a DVT. Patients should be counseled to avoid heavy lifting, which can lead to catheter dislodgement or lumen occlusion. PICC lines should routinely be evaluated for infiltration and extravasation, which require immediate intervention to prevent morbidity (Gorski et al., 2021). Signs of infiltration or extravasation include leakage at the insertion site, firmness, blistering, or surrounding edema (Lippincott Nursing Center, 2019).
PICC Line Removal
The removal of any central line, including a PICC, should be considered in the following circumstances:
- sepsis
- suppurative (septic) thrombophlebitis
- endocarditis
- bloodstream infection that continues despite 48–72 hours of adequate antimicrobial coverage
- infection with a resistant or challenging to eradicate pathogen (Gorski et al., 2021)
PICC lines should be promptly removed when they are no longer essential (CDC, 2017). The patient should be placed in a supine or Trendelenburg position for removal unless contraindicated. This position minimizes the risk of air embolism, which is a rare complication of PICC line removal. The occlusive dressing and securement device should be removed while the end of the catheter is stabilized. Next, the catheter should be withdrawn with gentle yet firm pressure while the patient exhales, hums, or performs a Valsalva maneuver. If resistance is encountered, a more experienced professional or a physician should be contacted for assistance. The catheter should never be forcibly removed if met with resistance, as there is a potential for catheter fracture and embolization. A catheter fragment retained within the vein may require endovascular removal to avoid infection, thrombosis, and migration. Once removed, the catheter tip should be examined to ensure it is fully intact, and documentation should indicate that the tip of the catheter was observed upon removal. An occlusive sterile dressing with antibiotic ointment should be applied and firmly held with pressure on the site for a minimum of 2 minutes or until bleeding subsides. The site should be monitored for 48 hours to detect any signs of post-infusion phlebitis, bleeding, or infection. If the patient is being discharged or if the access line is being removed in an outpatient setting, the patient and any available caregivers should receive written instructions regarding monitoring for infectious signs and symptoms (erythema, edema, warmth, increased bleeding, or purulent drainage) and whom to contact if these occur (Gorski et al., 2021; McCarthy et al., 2016).
Implantable VADs (Ports)
An implantable port is a central VAD surgically placed (typically in an interventional suite or operating room) into a subcutaneous pocket of the anterior chest wall, about one inch beneath the collarbone (see Figure 7). The device may also be implanted in the abdomen or upper arm, but these sites are less commonly used. This device is referred to as a port-a-cath or mediport and is inserted under local anesthesia with or without IV sedation by a surgeon or an interventional radiologist. The port consists of a thin, flexible catheter that is attached to a reservoir. The catheter is threaded into the central venous system via the subclavian or jugular vein. The tip of the catheter resides within the SVC. The reservoir can be made of plastic, stainless steel, or titanium and is about the size of a quarter. The port is covered with a self-sealing silicone septum that can withstand multiple needle punctures. Ports have a single or double lumen, and they may also be power-injectable to withstand high-speed injections (Lippincott, 2019).
Accessing the port requires a specialized needle called a non-coring (Huber) needle. The Huber needle is inserted perpendicular to the reservoir (see Figure 8). Since the device is implanted below the skin, it is palpated to identify the placement of the Huber needle. Accessing the port with a needle is required to administer fluids and medications or to perform blood sampling. Ports are used for long-term infusion therapy and are associated with a low risk of infection. Aside from tunneled CVCs, implantable ports are the most common CVC chosen for chemotherapy administration, including continuous vesicant administration. An implanted port is similar to a tunneled catheter, except that the port is not visible due to its placement beneath the subcutaneous tissue. Ports do not require external CVC maintenance care or impede daily activities such as swimming and contact sports when not accessed (CDC, 2019).

Contraindications to Implantable Ports
Contraindications to the insertion of implantable VADs include bacteremia (i.e., positive blood cultures) and clinical sepsis. Port insertion is also contraindicated in severe uncorrected coagulopathies, such as increased bleeding time and severe thrombocytopenia. Relative contraindications may include burns or trauma at the intended site. INS guidelines recommend the upper extremity as a potential alternative site for patients in whom chest ports cannot be implanted (Gorski et al., 2021).
Pros and Cons of Implantable Ports
There are several benefits to implantable ports, particularly when the patient's peripheral veins have been damaged due to recurrent needlesticks and IV placement. Ports can eliminate the need for multiple needlesticks, reducing discomfort and fear for patients who require long-term therapies and ensuring adequate vascular access for necessary treatments. Since ports are hidden beneath the skin, they are considered the most cosmetically appealing central lines (Lippincott, 2019). Importantly, when used intermittently with proper aseptic technique during needle access, ports have a lower incidence of CLABSI than other chest-accessed central lines. However, continuous port access is associated with infection rates comparable to other long-term CVCs (CDC, 2019; Gorski et al., 2021). In addition, implantable ports require less routine catheter care than other devices, such as PICC lines. Furthermore, ports minimally restrict patient activity, as patients can bathe, shower, and swim when the port is not accessed (Lippincott, 2019).
Some disadvantages of implantable ports include the port insertion process, which requires a minor surgical procedure in the operating room or interventional radiology. Medication delivery requires needle access, which can be uncomfortable or stressful for the patient. The most common complications associated with implantable ports include infiltration secondary to improper insertion, dislodgment of the needle, occlusion, thrombus, infection, catheter fracture, and catheter migration. Abscess formation around an implantable port is an indication for removal. Additionally, institutional protocols may prohibit the access of devices that have not been accessed for an extended period due to the increased risk of dislodging a clot into the vasculature (Gorski et al., 2021).
Placement of Implantable Ports
Implantable ports are inserted using fluoroscopic guidance—the initial steps in the process mirror those described above for other CVCs. After guidewire placement, the intended subcutaneous pocket area is anesthetized and then cauterized to the fascia level. The patency and function of the port hub should be assessed before implantation by flushing with sterile saline. The port is placed into the pocket and its size/position adjusted as needed. The catheter is then tunneled from the pocket to the guidewire exit site if needed (e.g., jugular vein access), avoiding any acute angulation. As with tunneled catheters, the tissue dilator/sheath combination is inserted over the guidewire using fluoroscopic guidance. The tissue dilator is then removed, the catheter is advanced through the sheath, and the sheath is peeled away. Catheter tip position should be assessed and adjusted as needed. The catheter should be trimmed if required and attached to the port hub. The port is then placed into the pocket and sutured with three fixation points into the surrounding fascia. The subcutaneous and skin tissue is sutured closed, the port should then be accessed, aspirated, and irrigated to confirm proper functioning. A sterile dressing should then be applied (Heffner & Androes, 2021). The INS standards recommend chlorhexidine-impregnated dressings for patients over 18 with implanted ports (Gorski et al., 2021).
Care of Implantable Ports
Ports can safely remain in place for months to years, but they require routine care so that they do not get blocked or clogged. While implantable ports may be used immediately following placement, HCPs must first ensure that catheter tip placement has been confirmed radiographically. This confirmation is generally attained at the time of port insertion. Accessing the port is a sterile procedure and requires aseptic technique. Ports that are power-injectable (i.e., can be used for CT contrast injection) require an access needle equipped for power injection to ensure that the tubing and connections do not rupture or separate under the infusion pressure (Lippincott, 2019). Adherence to aseptic technique includes protocols governed by national guidelines and institutional policy. According to INS (Gorski et al., 2021) guidelines, accessing an implanted port requires adherence to the following steps:
- Hand hygiene should be performed before examining or touching the site.
- Don sterile gloves and mask.
- Perform site antisepsis before port access:
- The preferred skin antiseptic agent is >0.5% chlorhexidine in an alcohol solution.
- For those with contraindications to chlorhexidine, appropriate alternatives include:
- tincture of iodine,
- povidone-iodine, or
- 70% alcohol.
- The HCP should scrub back and forth for 30 seconds.
- The skin antiseptic agent must be allowed to fully dry (2 minutes) to allow for maximal effect before port access.
- The smallest-gauge non-coring needle that will accommodate the prescribed therapy should be used.
- A sterile dressing covering both the non-coring needle and the access site should remain in place as long as the port is accessed.
- Perform hand hygiene after examining the site and any direct contact.
Like most central access devices, a syringe size smaller than 10 mL should never be used to flush a port, as smaller syringes can create excessive pressure within the device and lead to catheter fracture. Each lumen of the port must be managed separately and flushed accordingly. The port should be flushed and aspirated for the presence of brisk blood return before each infusion or administration of medication to confirm correct needle placement and catheter function. As with other CVC devices, failure to obtain blood flow through the port site requires evaluation before use and potentially repositioning the access needle. The port should also be flushed after each infusion to clear the catheter lumen and reduce the catheter's risk of occlusion. Ports accessed with a needle must be flushed daily if they are not infused with continuous IV fluids. If the port remains in continual use, the dressing and non-coring needle should be changed at least every 5 to 7 days or when visibly soiled. When not in use, implanted ports do not require exit-site care or dressings over the site and should be flushed once every 4 to 8 weeks or according to institutional policy (Gorski et al., 2021).
Following the final infusion or medication, the port should be flushed and locked with preservative-free 0.9% sodium chloride and 5 mL of heparin 10 units/mL in a pre-filled syringe before removing the needle or de-accessing the port. This heparin-lock solution reduces the risk of catheter occlusion (Lippincott, 2019). Many ports require needle access and flushing once every 4 to 8 weeks or according to the institutional policy when not in use. Additionally, some patients are placed on low-dose warfarin (Coumadin) at < 3 mg/day to prevent thrombosis of the port (Gorski et al., 2021). HCPs must continually monitor the site for any abnormalities at the insertion site—such as redness, swelling, warmth, tenderness, or pain—indicating an infection (Lippincott, 2019).
Troubleshooting Common Port Problems
HCPs caring for patients with implanted ports should be trained in managing and troubleshooting common port problems. The inability to flush a port or obtain a brisk blood return is commonly reported, and the etiology can vary. The port needle may not be correctly placed, the catheter may be lodged against the vessel wall, or a fibrin sheath may have developed at the catheter tip. Interventions to resolve some of the most common port problems include the following:
- ensure correct needle placement within the port and advancement of the needle through the septum
- reposition the patient or ask the patient to cough, raise their arms, lay back, sit up, or take a deep breath, as these maneuvers can help dislodge the catheter from the vessel wall
- re-access the port with a new needle
- use a fibrinolytic agent to dissolve a suspected clot per facility protocol
- assess for signs of potential catheter rupture, such as localized swelling, erythema, or acute pain at the site (Gorski et al., 2021)
The potential for catheter rupture during and after power injection can lead to extravasation, catheter fragment emboli, and the subsequent need for port removal and replacement.
Removal of Implantable VADs
According to the 2021 INS guidelines, implanted vascular access ports should be removed in the operating room or interventional radiology department with a minor sterile procedure. The skin should be prepped with appropriate antisepsis. Immediately following removal, the catheter should be examined to ensure it has been removed fully intact. If any piece of the catheter is retained within the vein, it will require removal through endovascular techniques to reduce the risk of infection, thrombosis, and migration of the catheter fragment. An occlusive dressing should be applied, and pressure should be held on the site until bleeding subsides. Bleeding is typically minimal with implantable port removal, and excessive bleeding should prompt the potential re-exploration of the port site (Gorski et al., 2021).
Complications of CVCs
The potential complications for all VADs are listed in Table 3. These can be limited with the consistent use of imaging modalities to guide CVC placement. Dynamic ultrasound is typically indicated during puncture and wire placement, and fluoroscopic guidance of the wires and catheter is recommended, if available. The incidence of arterial puncture varies from 3.7% to 12% of central venous access procedures. If an arterial puncture is recognized immediately upon needle insertion, the needle can be removed, and direct (but nonocclusive) pressure should be held for 15 minutes continuously. External pressure is easier to apply at the femoral and jugular sites and more challenging when accessing the subclavian vein (Young & Yuo, 2020).
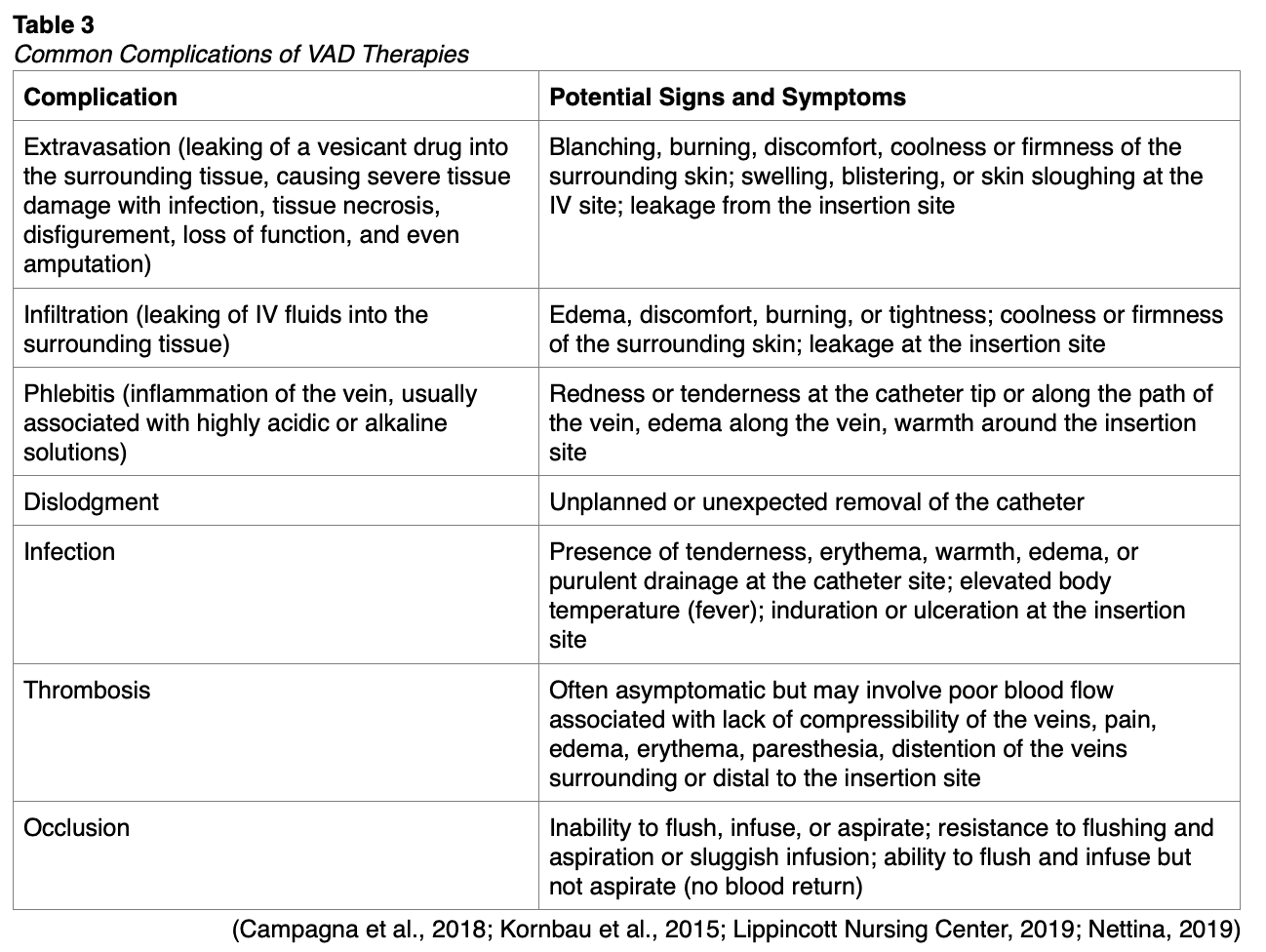
Arterial puncture is more common with femoral site placement and less common with subclavian. If arterial placement is suspected after the guidewire has been placed, a single-lumen catheter should be placed over the guidewire and temporarily connected to a pressure transducer to assess venous versus arterial waveforms. If a catheter is inadvertently placed into the arterial system, there is some debate regarding how to proceed. Historically, the recommendation was to remove the catheter immediately, hold pressure to limit bleeding for several minutes, and perform follow-up imaging to assess for any continued bleeding, arteriovenous (AV) fistula, or pseudoaneurysm. This technique is still acceptable for femoral artery catheterization but is accompanied by a risk for hematoma, false aneurysm, airway obstruction, and stroke. As a result, recent evidence has suggested the safer alternative with carotid, or subclavian artery cannulation is to leave the misplaced catheter undisturbed and proceed with surgical or endovascular removal and repair of any damage as soon as possible. This is also the case for most tunneled (hemodialysis, apheresis) catheters, which are often larger than 12 French. Image-guided placement (fluoroscopy or ultrasound) followed by confirmatory imaging of newly placed CVCs is paramount to preventing and identifying misplaced catheters quickly. Similarly, any significant venous injury to a major vessel (subclavian, jugular) warrants emergent surgical repair. While typically not life-threatening, hematomas can foster microorganism growth, develop into an abscess, or lead to hemothorax or hemomediastinum. A carotid puncture can obstruct the airway and become life-threatening if it is not recognized early. Manual pressure held at the insertion site can help limit hematoma formation in patients with coagulopathies, favoring a compressible insertion site (internal jugular or femoral vein). Congenital anomalies (persistence of the left-sided vena cava) should be recognized, and consideration should be made for alternative placement (right-sided) in these cases if appropriate. The presence of additional devices, such as IVC filters, should also be noted before placement to avoid entanglement (Butterworth et al., 2013; Kornbau et al., 2015; Nagelhout & Plaus, 2014; Young & Yuo, 2020). Due to the anatomic location of the thoracic duct, placing a non-tunneled CVC in the internal jugular or subclavian increases the risk of lymphatic injury (Nagelhout & Plaus, 2014).
Pulmonary complications associated with CVC placement are rare. Pneumothorax is most seen with subclavian and, to a lesser degree, internal jugular access sites. Underlying risk factors that increase this risk include lung disease, operator inexperience, and setting (Young & Yuo, 2020). A larger catheter size or repeated attempts at placement increase the risk of subclavian catheter placement and injury to the parietal pleura resulting in pneumothorax or pneumomediastinum. Although high-flow oxygen treatment may be sufficient, a pneumothorax coupled with hemodynamic instability and hypoxia indicates the need for chest tube placement. Lymphatic injury to the thoracic duct is also possible and termed chylothorax (or chylopericardium; Butterworth et al., 2013; Kornbau et al., 2015; Lippincott Nursing Center, 2019; Nagelhout & Plaus, 2014). Patients with a pre-existing pneumothorax should have a CVC placed only on the side of the pneumothorax to prevent a bilateral pneumothorax. This is likewise necessary for patients undergoing lung surgery who require presurgical or post-surgical placement of a CVC. The CVC must be placed on the ipsilateral (same) side as the surgery to prevent the non-surgical, fully inflated lung from suffering a pneumothorax leading to bilateral pneumothoraces (Nagelhout & Plaus, 2014).
Trauma or hematoma development can lead to damage to the recurrent laryngeal nerve, brachial plexus, or phrenic nerve, requiring 6-12 months for a full recovery. Nerve damage is most often indicated by reports of electrical-type pain at insertion or reports of paresthesia, tingling, burning, or numbness after insertion. Phrenic nerve involvement may also lead to respiratory difficulty. Iatrogenic tracheal punctures have been reported but are typically insignificant clinically unless the patient is mechanically ventilated. If the endotracheal tube or tracheostomy cuff is punctured, a consequent air leak may necessitate a tube change (Butterworth et al., 2013; Kornbau et al., 2015; Lippincott Nursing Center, 2019; Nagelhout & Plaus, 2014).
Air embolism can occur when inserting, flushing, or removing a CVC. Emboli arise due to the open connection between the air and the vascular system and the pressure gradient in place secondary to the low CVP found in the SVC. This pressure gradient is increased by hypovolemia and during inspiration. An estimated 200-300 cc (or 3-5 mL/kg) of air may be lethal to an adult, but the rate and route of entry are also factors. An air embolism can be prevented by ensuring adequate hydration and placing the patient in the Trendelenburg position to increase venous pressure when possible during insertion. The operator should also occlude the needle hub with their thumb when the guidewire is not in place. Air emboli are most common in subclavian lines due to the intrathoracic pressure changes associated with breathing, which may be prevented by avoiding placement during inspiration. The subcutaneous route to a jugular catheter should be sufficient in length, as this reduces the risk of an air embolism during removal. All catheter hubs should be capped (occluded) at all times, and all air should be removed from catheters, syringes, administration sets, and needleless connectors. During removal, the patient should again be placed in the Trendelenburg position with the entry site below heart level to ensure adequate CVP. The patient should perform a Valsalva maneuver during removal, or the removal should be timed during active expiration. Symptoms are typically non-specific and may include dyspnea, coughing, tachypnea, wheezing, chest pain, tachyarrhythmia, hypotension, and neurological dysfunction. Patients may also present with sudden cardiopulmonary or neurological symptoms. Mechanically ventilated patients often present with a significant drop in end-tidal CO2 on capnography. If air embolism is suspected, the first step is to stop any additional air from entering the patient’s venous system. The patient should be placed on high-flow oxygen and rolled into the left lateral decubitus and Trendelenburg position (Durant’s maneuver), which helps localize any air to the right atrium/lung and prevent it from entering the pulmonary artery and causing hypoxia. This maneuver is ineffective for patients with a patent foramen ovale or other anatomical abnormality (e.g., previous pneumonectomy). The rapid response or code blue system should be initiated if the patient is unresponsive or hemodynamically unstable. Fluid boluses and adrenergic agents should be used as indicated clinically. Small amounts of air (1-2 mL) may be self-resolving and managed with supplemental oxygen and slightly increased systemic blood pressure. Large emboli may require hyperbaric oxygen therapy to reduce the gas volume or extracorporeal membrane oxygenation (ECMO) in the ICU. In some cases, cardiac catheterization has been performed to aspirate the air (Butterworth et al., 2013; Kornbau et al., 2015; Lippincott Nursing Center, 2019; McCarthy et al., 2016, Nagelhout & Plaus, 2014; Young & Yuo, 2020).
Cardiac complications typically involve contact with the guidewire. Brief contact with the right atrium may prompt arrhythmia, such as premature atrial or ventricular contractions. Prolonged contact with the AV node may lead to supraventricular tachycardia. Knowledge and awareness of guidewire depth and imaging during placement may help prevent arrhythmia, while telemetry monitoring allows for early recognition. Direct valvular or myocardial perforations (usually the tricuspid valve or the right ventricle) should be evaluated immediately using echocardiography if the patient is hemodynamically stable, and surgical intervention planned accordingly (Butterworth et al., 2013; Kornbau et al., 2015; Nagelhout & Plaus, 2014). Guidewire depth should not exceed 16 cm to limit this risk (Young & Yuo, 2020).
As discussed previously regarding PIV complications, device dysfunction with VADs may occur at any time and include infection or thrombosis. Infection is treated with catheter removal or systemic antibiotics if catheter removal is precluded. In addition to the signs and symptoms outlined in Table 3, thrombosis of a CVC can cause SVC syndrome with associated head and neck swelling. Thrombosis is least common in the subclavian location and most likely in femoral lines and among cancer patients. Prophylactic thrombolytic treatment is not recommended. Small thrombi (< 3 cm) can be treated with catheter removal, while larger or infected thrombi may require thrombolytics or surgical intervention to remove them safely. In addition, a fibrin sheath may develop with the first week after device placement, occluding the port openings and necessitating the use of fibrinolytic agents (e.g., 2 mg alteplase [Cathflo Activase] IV once) per facility protocol. A sheath is usually indicated by resistance when flushing the catheter and poor or absent blood return. Catheter fractures are more common in subclavian lines in place for longer. Fracture is often related to forceful flushing or flushing with an inappropriate syringe (smaller than 10 mL). Signs and symptoms of a catheter rupture include extravasation/infiltration of fluid, the development of an air embolism, or occult internal bleeding. Catheter embolization may lead to arrhythmia, sepsis, endocarditis, or cardiac perforation. Removal may be performed by intervention radiology via the endovascular route or surgically, or the patient can be observed if they are asymptomatic and nonmobile. Venous stenosis has been reported with chronic central venous access. This risk is higher in patients with previous cannulation, prior infection, and a longer catheter dwell time. Typically, patients are asymptomatic, but stenting may be required for symptomatic or severe cases of venous stenosis (Kornbau et al., 2015; Lippincott Nursing Center, 2019).
Hemodynamic Monitoring
CVP Monitoring
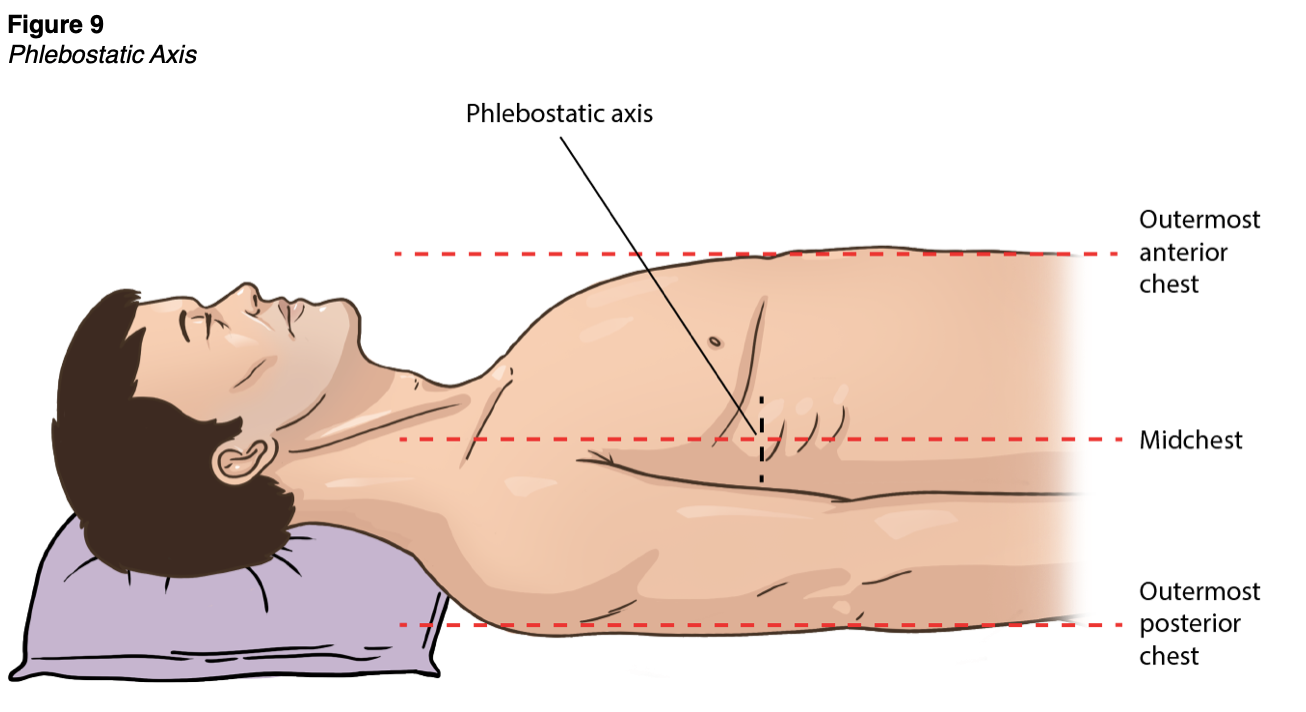
CVC placement is indicated not only for the intravenous administration of exogenous agents but also for monitoring CVP, the aspiration of air emboli, and the insertion of transcutaneous pacing leads. CVP is used clinically to assess a patient's fluid status and right-sided heart function. CVP is achieved by connecting a port of the CVC to a transducer, producing a waveform and numerical value to guide fluid management, primarily for critically ill patients (Butterworth et al., 2013). A transducer or amplifier attaches to a subclavian or internal jugular CVC. The transducer must first be zeroed by opening the stopcock to atmospheric pressure. The transducer should then be aligned to the horizontal plane of the tricuspid valve or the phlebostatic axis (i.e., the fourth intercostal space), which in most adults is just inferior to the nipple line, at the mid-diameter of the anterior-posterior chest wall (see Figure 9). A low CVP indicates hypovolemia or venous vasodilation, which decreases venous return. Increased CVP occurs in patients with heart failure due to decreased contractility, valve dysfunction, and arrhythmias. Elevated CVP may also affect ventilated patients with excessive positive end-expiratory pressure (PEEP), which increases pulmonary arterial resistance. CVP monitoring is typically used to guide fluid resuscitation for critically ill patients. For example, current sepsis guidelines recommend a target CVP of 8-12 mm Hg for critically ill patients receiving fluid boluses (Shah & Louis, 2020).
Pulmonary Artery Catheters
Pulmonary artery catheters (PACs or Swan-Ganz catheters) are typically inserted in an ICU, an operating room, or a cardiac catheterization lab. They generally are indicated for patients with known or suspected pulmonary hypertension, severe cardiogenic or unexplained shock, or unexplained dyspnea to assess the pulmonary artery pressure, pulmonary wedge (artery occlusion) pressure, right atrial or ventricular pressure, cardiac output, cardiac index, systemic vascular resistance, pulmonary vascular resistance, and mixed oxyhemoglobin saturation (SvO2). Pulmonary wedge pressure is often used to assess left heart function, including the mitral valve, ventricular filling, and atrial pressure. Routine PAC use has dropped as other technologies have become available, although they may still be helpful to guide fluid resuscitation for certain patients. Other than contraindications described above for other VADs, PACs are contraindicated without consent, during cardiopulmonary bypass, and in patients with right-sided ventricular assist devices. Significant coagulopathy, thrombocytopenia, and electrolyte or acid-base disturbances are relative contraindications (Weinhouse, 2019).
The most common site is the right internal jugular vein, followed by the left subclavian. Alternatives include the subclavian, femoral, or antecubital veins. Imaging is typically used for guidance (fluoroscopy when accessing via the antecubital or femoral veins, ultrasonography when using the jugular or subclavian). If the patient has an implanted pacemaker in their subclavian, then the contralateral side can be utilized for PAC access. An existing CVC can be exchanged for a PAC using a sterile guidewire of adequate length in patients with sufficient alternative venous access. Resuscitation and telemetry equipment should be available at the bedside before the procedure, along with imaging equipment and PAC supplies. Local anesthetic (1%-2% lidocaine) and intravenous sedatives (e.g., midazolam [Versed]) may be given before the procedure, and the patient should be placed in the Trendelenburg position (for jugular or subclavian access) or supine (for femoral or antecubital access; Weinhouse, 2019).
PACs are placed under surgical ANTT conditions using a large sterile field, full barrier precautions described above, and 2% chlorhexidine for skin antisepsis. Arterial puncture is prevented by using image guidance during the initial needle insertion. The introducer is a large-bore (8.5 French), short, central venous catheter typically inserted using the MST. The introducer has a side-arm extension for medication or fluid administration and a hemostatic valve on the operator’s end through which the PAC is placed. Inadvertent arterial puncture should be managed by removing the needle and holding pressure for 10-15 minutes. Once the introducer is in place, positioning can be confirmed using a pressure waveform transducer. Alternatively, a blood gas analysis or the presence of dark red blood lacking pulsatile flow may be utilized if pressure transduction is not available. A chest x-ray is often completed to confirm that a pneumothorax has not occurred. Then, the introducer can be secured in place if desired (Weinhouse, 2019).
The field is resterilized before placement of the PAC, including the hub of the introducer. The PAC ports should be flushed and capped (except the thermistor port), and the balloon and transducer condition should be inspected to ensure their integrity. The PAC is then zeroed by opening the system to atmospheric air to establish zero pressure. The air-fluid interface of the catheter stopcock or the transducer stopcock should be placed at the phlebostatic axis (see Figure 9) for referencing (or leveling). To evaluate placement and function, the PAC transducer tip should be held at heart level, which should correlate to a pressure reading of 0 mm Hg. The tip can be extended straight up 30 cm, which should increase the pressure on the monitor to 22 mm Hg (Weinhouse, 2019).
The patient is placed supine while the PAC is advanced through the introducer. It is advanced through the SVC, cardiac chambers, and pulmonary artery (PA) while the pressure at its tip is transduced. The balloon may only be inflated when the pressure transducer indicates that the tip is in the SVC or right atrium. It should be inflated as the catheter is advanced further until the pulmonary capillary wedge pressure (PCWP) waveform is identified. The catheter is graduated to allow the operator to visualize the inserted depth. For the jugular or subclavian veins, the right atrium (RA) is usually 20 cm beyond the insertion site, followed by the right ventricle (RV) at 30 cm, the PA at 40 cm, and the PCWP after 50 cm. The risk of arrhythmias is greatest while the catheter tip is in the RV, and therefore advancement should not be stalled at this point in the process. If the catheter tip coils inside the RV, the catheter should be withdrawn back into the RA, the balloon should be deflated and reinflated, and another attempt can be made through the RV. After passing through the PA, the catheter tip is advanced until a decrease in pressure and a change in the waveform are seen, signifying the PCWP (Weinhouse, 2019).
If the catheter is left in position for continued monitoring, the protective sleeve should be attached to the introducer hub and secured with an antiseptic dressing. A chest x-ray can be obtained to confirm the catheter tip position daily. The site should be checked daily, and dressing changes need to be performed per institutional policy. Sterile technique is required to inject medications into PAC ports, adjust the catheter positioning, or connect tubing. PACs should be left in place for as short a period as possible. With the patient in the Trendelenburg position, the balloon is deflated when the PAC is withdrawn. Withdrawal should occur during expiration for a spontaneously ventilated patient and during inspiration for a patient receiving positive pressure ventilation to prevent air emboli. Other potential complications include structural damage to vessels, the heart, valvular damage, and arrhythmias. After catheter removal, the introducer may be used as a CVC, replaced with a new CVC, or withdrawn. If it is removed, the securement device should be removed first, and the patient placed in the Trendelenburg position. As above, the introducer should be removed during expiration/inspiration (depending on breathing mechanism) while pressure is held at the site to limit bleeding for 1-2 minutes. A sterile dressing should be placed over the area and observed over the next several days (Weinhouse, 2019).
Please refer to Part 1 of this series to review infection control techniques and peripheral access devices, including short peripheral IVs, midline catheters, intraosseous catheters, and arterial catheters.
References
BruceBlaus. (2013). Non-tunneled central venous access device [Image]. Wikimedia. https://upload.wikimedia.org/wikipedia/commons/6/60/Blausen_0181_Catheter_CentralVenousAccessDevice_NonTunneled.png
BruceBlaus. (2016a). Tunneled central venous access device [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Central_Venous_Access_Device_(Tunneled).png
BruceBlaus. (2016b). Venous access port [Image]. Wikimedia. https://en.m.wikipedia.org/wiki/File:Venous_Access_Port_Catheter.png
Butterworth, J. F., Mackey, D. C., & Wasnick, J. D. (2013). Morgan & Mikhail’s Clinical Anesthesiology (5th ed.). McGraw Hill.
Campagna, S., Gonella, S., Zerla, P. A., Corona, G., Correggia, T., Mussa, B., & Dimonte, V. (2018). The risk of adverse events related to extended-dwell peripheral intravenous access. Infection Control & Hospital Epidemiology, 1-3. https://www.vulturenews.net/wp-content/uploads/2018/04/risk_of_adverse_events_related_to_extendeddwell_peripheral_intravenous_access.pdf
Cancer Research UK. (2014). Needle access of implantable port [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Diagram_showing_an_implantable_port_under_the_skin_CRUK_100.svg
Caprara, J. (2017). PICC versus midline. Home Healthcare Now, 35(10), 575-575. https://www.nursingcenter.com/journalarticle?Article_ID=4388386&Journal_ID=2695880&Issue_ID=4388241
Centers for Disease Control and Prevention. (2017). Guidelines for the prevention of intravascular catheter-related infections, 2011. https://www.cdc.gov/infectioncontrol/guidelines/bsi/index.html
Centers for Disease Control and Prevention. (2019). Frequently asked questions about catheters. https://www.cdc.gov/hai/bsi/catheter_faqs.html
Chopra, V., Davidson, I., & Collins, K. (2019). Peripherally inserted central catheter (PICC)-related venous thrombosis. UpToDate. Retrieved February 15, 2021, from https://www.uptodate.com/contents/peripherally-inserted-central-catheter-picc-related-venous-thrombosis
Clark, E., Kappel, J., MacRae, J., Dipchand, C., Hiremath, S., Kiaii, M., Lok, C., Moist, L., Oliver, M., & Miller, L. M (2016). Practical aspects of non-tunneled and tunneled hemodialysis catheters. Canadian Journal of Kidney Health and Disease, 3, 1-9. https://doi.org/10.1177/2054358116669128
Gonzalez, R., & Cassaro, S. (2020). Percutaneous central catheter. Statpearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK459338/
Gorski, L. A., Hadaway, L., Hagle, M. E., Broadhurst, D., Clare, S., Kleidon, T., Meyer, B. M., Nickel, B., Rowley, S., Sharpe, E., & Alexander, M. (2021). Infusion therapy standards of practice, 8th Edition. Journal of Infusion Nursing, 44(1S), S1-S224. https://doi.org/10.1097/NAN.0000000000000396
Hansmuller. (2019). Valved PICC line [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:Peripherally_inserted_central_catheter_(PICC_or_PIC_line)_after_use_in_a_patient_for_two_months_2018_The_Netherlands.jpg
Heffner, A. C., & Androes, M. P. (2021). Overview of central venous access in adults. UpToDate. Retrieved April 23, 2021, from https://www.uptodate.com/contents/overview-of-central-venous-access-in-adults
Herc, E., Patel, P., Washer, L., Conion, A., Flanders, S., & Chopra, V. (2017). A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Control Hospital Epidemiology, 38(10), 1155-1166. https://doi.org/10.1017/ice.2017.167
Institute for Healthcare Improvement. (2004). Central line procedural checklist. http://www.ihi.org/resources/Pages/Tools/CentralLineInsertionChecklist.aspx
Institute for Healthcare Improvement. (2012). How-to guide: Prevent central line-associated bloodstream infections (CLABSI). http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventCentralLineAssociatedBloodstreamInfection.aspx
Kornbau, C., Lee, K. C., Hughes, G. W., & Firstenberg, M. S. (2015). Central line complications. International Journal of Critical Illness & Injury Science, 5(3),170-178. https://doi.org/10.4103/2229-5151.164940
Lee, K. A., & Ramaswamy, R. S. (2018). Intravascular access devices from an interventional radiology perspective: Indications, implantation techniques, and optimizing patency. Transfusion, 58, 549-557.
Leib, A. D., England, B. S., & Kiel, J. (2019). Central line. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK519511/#_article-19125_s10_
Lippincott. (2019). Lippincott nursing procedures. (8th ed.). Lippincott Williams & Wilkins.
Lippincott Nursing Center. (2019). Complications of central vascular access devices. https://www.nursingcenter.com/getattachment/Clinical-Resources/nursing-pocket-cards/Complications-of-Central-Vascular-Access-Devices/Complications-of-Central-Vascular-Access-Devices_January-2019.pdf.aspx
McCarthy, C. J., Behravesh, S., Naidu, S. G., & Oklu, R. (2016). Air embolism: Practical tips for prevention and treatment. Journal of Clinical Medicine, 5(11), 93. https://doi.org/10.3390/jcm5110093
McDiarmid, S., Scrivens, N., Carrier, M., Sabri, E., Toye, B., Huebsch, L., & Fergusson, D. (2017). Outcomes in a nurse-led peripherally inserted central catheter program: A retrospective cohort study. CMAJ Open, 5(3), E535-E539. https://doi.org/10.9778/cmajo.20170010
Nagelhout, J. J., & Plaus, K. L. (2014). Nurse Anesthesia (5th ed.). Elsevier Saunders.
Nettina, S. M. (Ed.). (2019). Lippincott manual of nursing practice (11th ed.). Wolters Kluwer.
Nurseirie. (2014). Non-valved (open ended) PICC line [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:PICC_line.jpg
OpenStax College. (2013a). Thoracic abdominal veins [Image]. Wikimedia. https://commons.wikimedia.org/wiki/File:2132_Thoracic_Abdominal_Veins.jpg
Paje, D., Rogers, M., Conlon, A., Flanders, S., Bernstein, S., & Choppra, V. (2019). Use of peripherally inserted central catheters in patients with advanced chronic kidney disease: A prospective cohort study. Annals of Internal Medicine, 171(1), 10-18. https://doi.org/10.7326/M18-2937
Sabado, J. J., & Pittiruti, M. (2020). Principles of ultrasound-guided venous access. UpToDate. Retrieved May 21, 2021, from https://www.uptodate.com/contents/principles-of-ultrasound-guided-venous-access
Shah, P., & Louis, M. A. (2020). Physiology: Central venous pressure. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK519493/
Song, I. K., Kim, E. H., Lee, J. H., Jang, Y. E. Kim, H. S., & Kim, J. T. (2018). Seldinger vs modified Seldinger techniques for ultrasound-guided central venous catheterization in neonates: A randomized controlled trial. Paediatric Anesthesia, 121(6), 1332-1337. https://doi.org/10.1016/j.bja.2018.08.008
Stoker, R. (2009). Accelerated Seldinger technique: A faster, safer method for diagnostic and interventional procedures. Managing Infection Control, 32–36.
Tse, A., & Schick, M. (2019). Central line placement. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470286/
Weinhouse, G. L. (2019). Pulmonary artery catheters: Insertion technique in adults. UpToDate. Retrieved April 23, 2021, from https://www.uptodate.com/contents/pulmonary-artery-catheters-insertion-technique-in-adults
Young, M. P., & Yuo, T. H. (2020). Overview of complications of central venous catheters and their prevention. UpToDate. Retrieved May 21, 2021, from https://www.uptodate.com/contents/overview-of-complications-of-central-venous-catheters-and-their-prevention